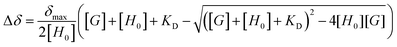Unveiling the role of pyrylium frameworks on π-stacking interactions: a combined ab initio and experimental study†
Reyes
Núñez-Franco
 a,
Gonzalo
Jiménez-Osés
a,
Gonzalo
Jiménez-Osés
 ab,
Jesús
Jiménez-Barbero
ab,
Jesús
Jiménez-Barbero
 abc,
Francisca
Cabrera-Escribano
abc,
Francisca
Cabrera-Escribano
 d and
Antonio
Franconetti
d and
Antonio
Franconetti
 *a
*a
aCIC bioGUNE, Basque Research and Technology Alliance (BRTA), 48160, Derio, Spain. E-mail: afranconetti@cicbiogune.es
blkerbasque, Basque Foundation for Science, 48009 Bilbao, Spain
cDepartment of Organic Chemistry II, Faculty of Science & Technology, University of the Basque Country, 48940 Leioa, Bizkaia, Spain
dDepartamento de Química Orgánica, Facultad de Química, Universidad de Sevilla, C/Profesor García González 1, 41012 Sevilla, Spain
First published on 24th September 2021
Abstract
A multidisciplinary study is presented to shed light on how pyrylium frameworks, as π–hole donors, establish π–π interactions. The combination of CSD analysis, computational modelling (ab intitio, DFT and MD simulations) and experimental NMR spectroscopy data provides essential information on the key parameters that characterize these intereactions, opening new avenues for further applications of this versatile heterocycle.
Introduction
Pyrylium heterocycle (Fig. 1) is an aromatic cation discovered more than a century ago.1 However, this six-membered ring remained underestimated for years, thus inducing the idea that the golden age of this ancient salt had already passed. Recent breakthroughs, however, have established a successful present far from single green shoots. | ||
| Fig. 1 (a) Structure of unsubstituted pyrylium cation and (b) structure of synthesized 2,4-di-(4-methylphenyl)-4-(4-fluorophenyl) tetrafluoroborate (1). | ||
The different chemical and physical properties of pyrylium-based scaffolds have been exploited in multiple fields. For instance, pyrylium compounds with extended conjugation have recently been applied as fluorophores, since they display photostability, good quantum yields and low cytotoxicity in targeting mitochondria organelles.2 From a chemical perspective, the electrophilic C2/C6 positions allow modification of the polysaccharide chitosan, introducing a fluorescent function on its backbone.3 Similarly, the C–N activation of alkylamines4 and the activation of low nucleophilic aminoheterocycles also take advantage of this reactivity.5 In addition, the excellent photoredox behaviour of pyrylium derivatives6 has provided an alternative metal-free photocatalyst for ring-opening metathesis polymerization (ROMP) reactions.7
Pioneering work by Schlüter and co-workers reported the X-ray structure of a new 2D polymeric material based on a [2+2]-cycloaddition between two styrylpyrylium structures.8 The crystal-to-crystal synthesis for obtaining this covalent stacked material sparked a new hot topic in material science because the pyrylium core can be easily transformed, thus increasing its applicability.9 The stability between its layers is presumably underpinned by van der Waals interactions,10 thus it has also been referred to as a cation-π-controlled crystal.11
Besides this evidence, a feature of pyrylium derivatives not to be overlooked is their accompanying anions which have been proposed to establish anion–π interactions with their cationic partners.12
Nowadays, non-covalent interactions are widely understood to be driving entities in supramolecular chemistry, particularly in molecular recognition processes. At first glance, each molecule or scaffold has interactive properties and spontaneously organizes itself with complementarity. The ways in which these molecules interact differ in their directionality, strength or cooperative effects. For this reason, this field has been rapidly developed and the current knowledge has tremendously expanded in the last decades. Beyond hydrogen and halogen bonds or π–π stacking, a plethora of new noncovalent interactions covering σ– and π–hole families,13 such as tetrel,14 chalcogen,15 coinage16 or spodium bonds,17 have blossomed.
In general, crystallographic structures are the reference sources for obtaining valuable information about these intermolecular interactions. In these densely packed environments, molecules are highly ordered, displaying multiple contacts at distances shorter than ∑Rvdw (±0.2 Å) and strong cooperativity effects.18 The combination of solid-state structural analyses and theoretical calculations affords a precise characterization of the energetic and geometric features of the relevant interactions. However, another situation arises in solution, where non-covalent interactions are often weaker and highly dynamic. In addition, the surrounding solvent molecules may significantly tune the pre-organization of the acceptor and donor moieties, thus modulating their final intermolecular association. The current standard methodology to experimentally study these interactions with high accuracy is based on molecular balances and has been applied to understand cation-π interactions involving pyridinium systems.19
To the best of our knowledge, experimental and/or computational studies of intermolecular interactions involving pyrylium frameworks as main players are scarce. The precise type of interaction should have molecular consequences (strength, directionality and cooperativity) and supramolecular features (reactivity and 2D interlayer distance).
To address these shortcomings, herein we report a computer-aided study to support the growing interest in pyrylium⋯arene interactions. Different X-ray structures were retrieved from the Cambridge Structural Database (CSD) and analyzed. Additionally, a systematic ab initio study was carried out to dissect the influence of substituents and fully characterize this interaction. Finally, data in solution (NMR) were recorded for a synthetic pyrylium tetrafluoroborate to provide a full picture of the molecular recognition event.
Results and discussion
CSD analysis
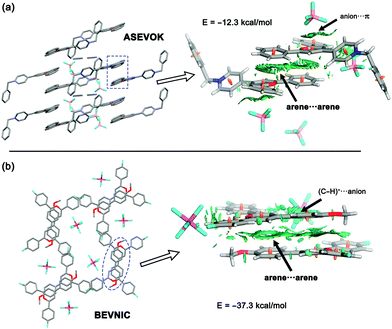
The solid-state architectures of pyrylium compounds account for their ability to self-assemble into near-planar π-stacked helical motifs.20 In most cases, these complexes participate in a four-ring antiparallel dimerization pattern displaying centroid-to-centroid distances in the range of 3.44 to 4.16 Å. In addition, analysis of the crystallographic structures also revealed that the anions are placed inside the formed channels or pores. In this context, two feasible arrangements for anions have been previously described: (a) anion–π contacts and (b) (C–H)+⋯anion.12 To obtain a more meaningful interpretation, an NCIplot index analysis21 was carried out for refcodes ASEVOK (Fig. 2a) and BEVNIC (Fig. 2b) to qualitatively characterize the weak and strong contacts between the pyrylium layers. These two structures cover the aforementioned possibilities of the anion locations (ASEVOK and BEVNIC for anion–π and (C–H)+⋯anion, respectively).Although the cation–π interaction has been invoked as one of the intermolecular motifs stabilizing these channels, according to the sign(λ2)ρ value, the NCIplots computed for these X-ray structures suggest a weak van der Waals interaction (green colour) as expected for arene⋯arene contact. In addition, NCIplots also highlight the intermolecular CH/π contacts between other substituents. The attractive nature of the combined interactions was confirmed by their large interaction energies (ΔEint = −12.3 and −37.3 kcal mol−1 for ASEVOK and BEVNIC, respectively).
Theoretical study
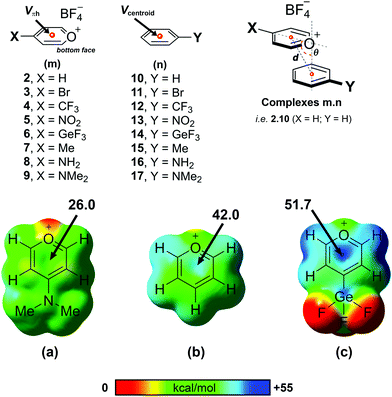
From a computational perspective, it should be noted that the pyrylium cation has an unusual behaviour. In the presence of its anion, the pyrylium shows two different faces. The first one is involved in anion recognition12 and is essentially locked for participating in another intermolecular contact, whereas the second one is available to establish additional interactions. Molecular electrostatic potential (MEP) surfaces of the pyrylium cation (Pyry+) and pyrylium tetrafluoroborate (Pyry-BF4, 2) shed light on this behaviour. The positive region of electrostatic potential (Vπh, Fig. 3) perpendicular to the molecular plane is defined as a π–hole.22 The MEP value corresponding to this π–hole for the Pyry+ cation was +128.0 kcal mol−1, while complex 2 shows a decrease in π–hole intensity (Vs,πh +42.0 kcal mol−1, Fig. 3b). However, the ability of its bottom face to act as an excellent π–hole donor was retained better than that of hexafluorobenzene (HFB, Vπh = +19.1 kcal mol−1).The influence of different substituents with electron-donating and electron-withdrawing properties on the geometry and energetic features of BF4–Pyry⋯π contacts was computationally analysed. For this purpose, we selected para-substituted pyrylium molecules as π–hole donors and mono-substituted aromatic rings as “electron-rich” molecules using a high-level ab initio MP2(fc)/def2-TZVP method. Despite four stable geometries being feasible (I–IV, Fig. S1, ESI†), the whole study was carried out only on geometry I (antiparallel-displaced structure) as it is the most similar to those found in the CSD.
As a first step, we carried out a MEP study at the π–hole in complexes 2–9 (Table 1 and Fig. 3). The substituent attached to the pyrylium core (X) has a significant influence on the Vπh, following the trend NMe2 < NH2 < Me < H < Br < CF3 < NO2 < GeF3 ≪ Pyry+. As expected, electron-withdrawing substituents increased the intensity of Vπh up to 9.7 kcal mol−1 whereas electron-donating substituents decreased it by 16.0 kcal mol−1 (see Table S1 for comparison with Pyry+, ESI†). The opposite behaviour is observed for the Vcentroid values in complexes 10–17. This suggests that the pyrylium core acts as an electron-deficient aromatic ring (similar to 2,4,6-trifluoro-s-triazine, Vπh +42.5 kcal mol−1).
| Compound | V πh | Compound | V centroid | σ p |
|---|---|---|---|---|
| 2, X = H | +42.0 | 10, Y = H | −16.8 | 0.00 |
| 3, X = Br | +43.5 | 11, Y = Br | −9.7 | 0.23 |
| 4, X = CF3 | +49.3 | 12, Y = CF3 | −5.8 | 0.54 |
| 5, X = NO2 | +50.6 | 13, Y = NO2 | −1.4 | 0.78 |
| 6, X = GeF3 | +51.7 | 14, Y = GeF3 | −0.8 | 0.97 |
| 7, X = Me | +38.6 | 15, Y = Me | −18.3 | −0.17 |
| 8, X = NH2 | +30.4 | 16, Y = NH2 | −21.3 | −0.66 |
| 9, X = NMe2 | +26.0 | 17, Y = NMe2 | −22.0 | −0.83 |
Ab initio calculations show that the BSSE-corrected interaction energies (EBSSE) for complexes 2.10–9.17 (Table S2, ESI†) are moderately strong, ranging from −9.4 to −3.2 kcal mol−1. The most stable complexes contained electron-withdrawing groups attached to the pyrylium moiety. For instance, complex 6.17 (X = GeF3; Y = NMe2) is the most stable dimer and 8.14 (X = NH2; Y = GeF3) is the weakest one.
The electrostatic nature of such intermolecular interactions was further analysed using Hammett parameters σp, which reflect the inductive/mesomeric effects of para-substituents. Thus, by plotting the individual Hammett constants (σp)23vs. the calculated interaction energies EBSSE for each interacting pair, the electronic fingerprint of the pyrylium⋯arene interaction was obtained (Fig. 4a). To account for the combined influence of both para-substituted moieties on EBSSE (Fig. 4b), the mixed σp(XY) parameter24 [σp(XY) = −1.45 × σp(X) + 2.41 × σp(Y)] was defined using coefficients obtained from multiple linear regression (see ESI†). A negative sign of βx (−1.45) indicates an inverse correlation between the electron-donating properties of the para-substituents in the pyrylium fragment and the interaction energy, while a positive sign of βy (2.41) shows a direct correlation. The fact that the absolute value of βy outweighs βx in these complexes points toward a larger influence of arene substitution on the strength of the interaction energy.
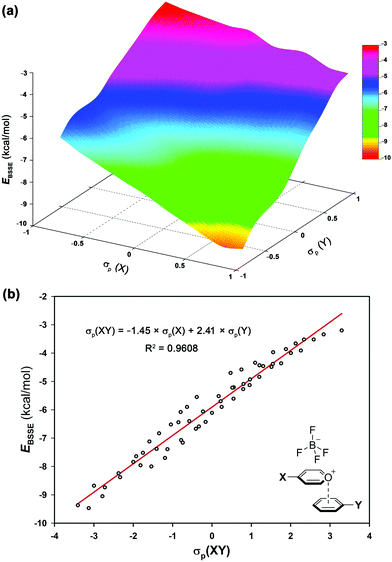 | ||
| Fig. 4 (a) Electronic fingerprint of π-stacking interaction involving pyrylium frameworks and (b) Hammett's plot based on mixed σp(XY) of complexes 2.10–9.17. | ||
An assessment in which pyrylium templates were linked to arenes was carried out and compared with related cation–π and π–π interactions. Three descriptors (EBSSE, d and θ, Fig. 3) were selected to characterize three systems: (a) Pyry+⋯π, (b) complex 2.10 (X = Y = H) and (c) benzene dimer (Fig. S2, ESI†). The most stable system proved to be Pyry+⋯π (ca. −11.1 kcal mol−1), a model for cation–π interaction. On the other hand, complex 2.10 is more stable than the benzene dimer (ΔΔEBSSE = 2.4 kcal mol−1). The trend Pyry+ > 2.10 > benzene also suggests an excellent π–hole donor instead of a canonical cation. In agreement with this trend, the centroid-to-centroid distance in complex 2.10 is slightly larger (Δd = 0.07 Å) than that in Pyry+⋯π but equally short compared to the benzene dimer (Δd = −0.07 Å). However, both 2.10 and Pyry+ complexes have the same angle between the two aromatic rings (tilt angle, θ = 102.0°) whereas the angle is closer to 90° for benzene⋯benzene stacking. An energy decomposition analysis (LMO-EDA, Fig. S3, ESI†) was also applied to separate the interaction energy into individual contributions. The quantitative analysis was mainly focused on electrostatic (Eelec) and dispersion (Edisp) terms. As expected, a dominant role of the dispersion term was obtained for the benzene⋯benzene dimer. In addition, the smallest electrostatic component was also obtained in this system. The opposite situation was found for Pyry+⋯π, which is clearly dominated by Eelec. On the other hand, complex 2.10 displayed intermediate behaviour with similar Eelec and Edisp terms.
Instead of the ordered environment found in X-ray architectures, the association between molecules in solution becomes more tangled because of solvation. Upon binding, solvent molecules are released to the bulk, often followed by an energetic penalty and an entropic benefit whose net consequences to the association free energy are difficult to predict.25 Hence, particularly when charged species are involved, the net stabilization might be smaller than the values calculated in the gas phase. In addition, the main driving forces toward binding (electrostatic and dispersion) might be altered in solution. In some cases, dispersion and electrostatic interactions are attenuated but still significant.26
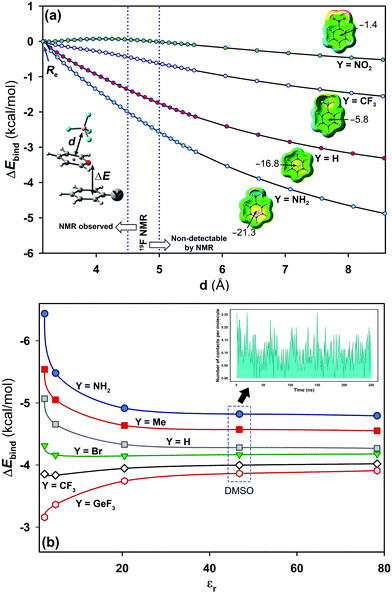
As a jumping-off point, MD simulations (250 ns) were carried out to test the behaviour of pyrylium tetrafluoroborate 1 in DMSO, which is known to better solvate cations than anions. Theoretical distances from the centroid of all pyrylium rings to the centroid of all tetrafluoroborates were calculated. It was found that the most frequent distance between the pyrylium centroids and the nearest BF4 anion is 9.9 ± 0.2 Å (Fig. S4, ESI†). MD simulations revealed that the closest pyrylium centroid⋯ BF4 contact is, on average, 4.7 ± 1.2 Å. This distance is still within the boundary for subsequent NMR detection based on NOE experiments (up to 5 Å), but the calculated ratio of these ionic pairs (shorter than 5 Å) is only around 3% (Fig. 5b, inset). These geometrical findings should modify the role of the pyrylium⋯BF4 interaction. For this reason, interaction energies were adapted (Fig. 5a). At large distances, these systems should evolve towards cation-π interaction. In this situation, electron-donating substituents stabilize (in the gas phase) the system (ΔE4.5Å = −2.1 kcal mol−1, complex 2.16), while the interaction energy slightly dropped with electron-withdrawing ones (ΔE4.5Å = +0.05 kcal mol−1, complex 2.13).
Solvent effects were included in the PCM continuum solvation model for complexes 2.10–2.16 (Fig. 5b). The results showed a dependency of the interaction energy on the dielectric constant (εr).27 At large dielectric constant values (εr > 20.5, acetone), the electrostatic component vanishes and dispersion forces dominate in solution. A representative situation is found with complex 2.14 (X = H; Y = GeF3). In the gas phase, this compound exhibits weak strength which is slightly increased with polar solvents. The opposite effect was observed for complex 2.16 (X = H; Y = NH2). For this complex, the interaction energy suffers a significant penalty at large dielectric values (ca. 1.5 kcal mol−1).
Experimental study
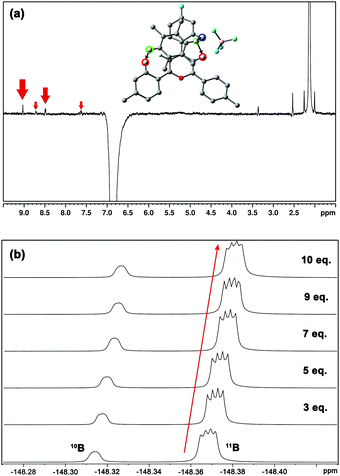
The experimental behaviour of these systems was analysed using DMSO (εr = 46.8) and acetone (εr = 20.5) as aprotic solvents. We prepared compound 1 (Fig. 1b) to study the interactions in solution. This compound displays a moderate Vπh (+23.2 kcal mol−1), is stable enough in solution (attached phenyl rings at C-2 and C-4 positions) and bears NMR observable atoms (1H and 19F). We then prepared the symmetric pyrylium tetrafluoroborate molecule by following a previous synthetic methodology.12a,c Briefly, the Lewis acid BF3·Et2O catalyses the reaction between methyl acetophenone and 4-fluorobenzaldehyde, affording the desired compound (1) in a 29% yield. One of the most important features of this kind of compound is the singular chemical shift of the aromatic H3/H5 protons at 9.03 ppm (DMSO-d6).
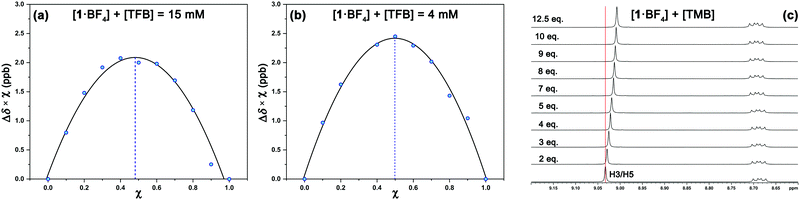
1H NMR experiments were employed to monitor the chemical shift perturbation of this signal upon binding with tetramethylbenzene (TMB) and tetrafluorobenzene (TFB). First, we applied the continuous variation method, commonly referred to as a Job's plot, to determine the stoichiometry of 1·BF4⋯arene complexes (Fig. 6). Despite this method's limitations, especially for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest stoichiometries,28 it is suitable for our outcomes since we obtained a 1
2 host–guest stoichiometries,28 it is suitable for our outcomes since we obtained a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 equilibrium between the complexes studied at two sets of concentrations and solvents ([1·BF4] + [Arene] = 4 mM and 15 mM, for deuterated acetone and DMSO, respectively).
1 equilibrium between the complexes studied at two sets of concentrations and solvents ([1·BF4] + [Arene] = 4 mM and 15 mM, for deuterated acetone and DMSO, respectively).
The increasing number of equivalents of TMB provokes a slight upfield shift (Δδ = 27 ppb, Fig. 6c) of the signal assigned to the H3/H5 protons. This effect agrees with the observed shielding of protons in spatial proximity to an aromatic ring.29,30 Theoretical calculations provide a meaningful interpretation (Fig. S5, ESI†). Starting from different initial geometries, the calculated minimum of the PES corresponds to π–π stacking with the BF4 anion displaying the aforementioned (C–H)+⋯anion contact, a binding mode commonly found in the CSD. This geometry (Fig. 7a, inset), with near-planar C2 and C6 phenyl rings, is in agreement with the intermolecular contacts between TMB and pyrylium protons (mainly H3/H5 and H2/H6a) obtained by selective 1D NOESY experiments (Fig. 7a). The calculated binding constant for this association, Ka, was estimated to be 2.91 ± 0.55 M−1. The calculated ΔG for this π⋯π association (−0.63 kcal mol−1) is in agreement with the ΔΔG obtained from DFT calculations (−0.70 kcal mol−1; see ESI,† for more details).
Finally, monitoring the chemical shift of the BF4− anion by 19F-NMR,31 we observed an analogous variation in chemical shift for the fluorine-coupled 10B and 11B isotope signals (Fig. 7b). The theoretical distance of anion⋯TMB in the ternary complex anion-Pyry-π was too large (d = 7.02 Å) to be detected by NMR. Therefore, the observed variations in 19F chemical shifts also support the proposed structure.
Conclusions
This systematic study highlights the ability of pyrylium frameworks to act as excellent π–hole donors to establish π–π stacking interactions. The pyrylium tetrafluoroborates behave as exacerbated arenes instead of canonical cations. In fact, an excellent correlation between interaction energies and mixed Hammett constants σp(XY) was found. In addition, our computational modelling in solution points to the possibility of switching their aromatic (dispersive) or cationic (electrostatic) character depending on dielectric constant (εr) values.The experimental NMR-based results allowed us to propose feasible π–π stacking geometries similar to those found in the crystallographic structures. The additional stabilization of this geometry was also estimated and correlates with those obtained from DFT calculations. Moreover, 19F NMR data suggest a straightforward arene⋯anion contact through the (C–H)+⋯anion arrangement.
Given that this study covers all environments where the applicability of pyrylium complexes is currently significant, the obtained results suggest a bright future for this versatile heterocycle in supramolecular chemistry.
Experimental
Synthesis of 2,4-di-(4-methylphenyl)-4-(4-fluorophenyl)pyrylium tetrafluoroborate (1)
To a solution of 4-fluorobenzaldehyde (432 μL, 4.03 mmol) and 4-methylacetophenone (1.62 mL, 12.09 mmol), BF3·Et2O (1.49 mL, 12.09 mmol) was added. The reaction mixture was heated at 80 °C. Monitoring of the reaction (TLC, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Hexane
1 Hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AcOEt) showed that the reaction was completed after 16 h. The solvent was evaporated and the product was then recrystallized from acetone to obtain compound 1 (523 mg, 29%) as an orange solid (see ESI,† for characterization data).
AcOEt) showed that the reaction was completed after 16 h. The solvent was evaporated and the product was then recrystallized from acetone to obtain compound 1 (523 mg, 29%) as an orange solid (see ESI,† for characterization data).
Binding stoichiometries and NMR titration experiments
The determination of stoichiometry for the interaction of compound 1 with methylated (TMB) and fluorinated (TFB) benzene analogues was carried out using Job's method.32 The aggregation propensity of compound 1 was overcome by changing the concentration of NMR samples. For Job's plot analysis, the concentrations [1·BF4] + [Arene] were 15 mM (for DMSO-d6) and 4 mM (for acetone-d6). Keeping the concentration constant, the molar fraction χ of each component in the mixture was varied from 0 to 1, with increments of 0.1 for each prepared sample. In all cases, the molar fraction χ = 1 was assigned to the solution containing just the host (compound 1).NMR titrations were carried out in DMSO-d6 at 300 K. Increasing amounts (ranging from 1–12.5 equivalents) of the appropriate benzene analogue were prepared in different NMR tubes containing a constant concentration [1·BF4] of 20 mM. The titration isotherms were fitted to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model (Fig. S6, ESI†) using Thordarson's equation33 as follows.
1 binding model (Fig. S6, ESI†) using Thordarson's equation33 as follows.
Spectra were acquired on a Bruker Avance 500 MHz spectrometer equipped with a 5 mm BBFO broadband probe head incorporating a z-gradient coil. Acetone-d6 or DMSO-d6 containing 0.1% TMS was used as the solvent. Spectra were acquired using a high-power 901 pulse of 14.8 ms and a recycle delay of 5 s. The 19F chemical shifts were referenced indirectly to CCl3F (Ξ = 94.094011).34
Theoretical methods
The geometries and energies of larger systems, for example, the interactions of compound 1 with different benzene analogues or crystallographic structures, were obtained at the PBE041-D342/def2-TZVP level of theory. The minimal nature of interactions involving this compound was confirmed by frequency analysis calculations. For these compounds, NCIplot isosurfaces43 were computed using cubefiles (high quality grid) generated from the Multiwfn v. 3.8 program.44 The color scheme for studying weak non-covalent interactions depends on the value of the reduced density gradient. In general, the colour codes are red (repulsive, ρ+cut), and blue (attractive, ρ−cut), while yellow-green surfaces indicate weak repulsive and weak attractive interactions, respectively.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Agencia Estatal de Investigación (Spain) for financial support (projects RTI2018-094751-B-C21, RTI2018-099592-B-C22 and CTQ2016-78703) and Severo Ochoa Excellence Accreditation SEV-2016-0644. A. F. acknowledge the Spanish Ministerio de Ciencia e Innovación (Spain) for a Juan de la Cierva contract (IJC2019-042061-I).Notes and references
- Y. Li, H. Wang and X. Li, Chem. Sci., 2020, 11, 12249 RSC.
- X. Chen, L. Yan, Y. Liu, Y. Yang and J. You, Chem. Commun., 2020, 56, 15080 RSC.
- A. Franconetti, L. Contreras-Bernal, R. Prado-Gotor and F. Cabrera-Escribano, RSC Adv., 2015, 5, 74274 RSC.
- C. H. Basch, J. Liao, J. Xu, J. J. Piane and M. P. Watson, J. Am. Chem. Soc., 2017, 139, 5313 CrossRef CAS.
- (a) Y. Ma, Y. Pang, S. Chabbra, E. J. Reijerse, A. Schnegg, J. Niski, M. Leutzsch and J. Cornella, Chem. – Eur. J., 2020, 26, 3738 CrossRef CAS PubMed; (b) D. Moser, Y. Duan, F. Wang, Y. Ma, M. J. O’Neill and J. Cornella, Angew. Chem., Int. Ed., 2018, 57, 11035 CrossRef CAS.
- M. A. Miranda and H. Garcia, Chem. Rev., 1994, 94, 1063 CrossRef CAS.
- P. Lu, V. K. Kensy, R. L. Tritt, D. T. Seidenkranz and A. J. Boydston, Acc. Chem. Res., 2020, 53, 2325 CrossRef CAS PubMed.
- R. Z. Lange, G. Hofer, T. Weber and A. D. Schlüter, J. Am. Chem. Soc., 2017, 139, 2053 CrossRef CAS.
- R. Z. Lange, K. Synnatschke, H. Qi, N. Huber, G. Hofer, B. Liang, C. Huck, A. Pucci, U. Kaiser, C. Backes and A. D. Schlüter, Angew. Chem., Int. Ed., 2020, 132, 5732 CrossRef.
- F. Hu, W. Hao, D. Mücke, Q. Pan, Z. Li, H. Qi and Y. Zhao, J. Am. Chem. Soc., 2021, 143, 5636 CrossRef CAS PubMed.
- S. Yamada, Coord. Chem. Rev., 2020, 415, 213301 CrossRef CAS.
- (a) A. Franconetti, R. Núñez-Franco, G. de Gonzalo, J. Iglesias-Sigüenza, E. Álvarez and F. Cabrera-Escribano, ChemPhysChem, 2018, 19, 327 CrossRef CAS PubMed; (b) D. Quiñonero, Molecules, 2015, 20, 11632 CrossRef PubMed; (c) A. Franconetti, L. Contreras-Bernal, S. Jatunov, M. Gómez-Guillén, M. Angulo, R. Prado-Gotor and F. Cabrera-Escribano, Phys. Chem. Chem. Phys., 2014, 16, 18442 RSC.
- (a) J. S. Murray, P. Lane, T. Clark, K. E. Riley and P. Politzer, J. Mol. Model., 2012, 18, 541–548 CrossRef CAS PubMed; (b) P. Politzer, J. S. Murray and P. Lane, Int. J. Quantum Chem., 2007, 107, 3046–3052 CrossRef CAS; (c) T. Clark, M. Henneman, J. S. Murray and P. Politzer, J. Mol. Model., 2007, 13, 291–296 CrossRef CAS PubMed.
- (a) A. Franconetti and A. Frontera, Chem. – Eur. J., 2019, 25, 6007 CrossRef CAS; (b) A. Bauzá, S. K. Seth and A. Frontera, Coord. Chem. Rev., 2019, 384, 107 CrossRef; (c) A. Frontera and A. Bauzá, Chem. – Eur. J., 2018, 24, 16582 CrossRef CAS PubMed; (d) A. Bauzá, T. J. Mooibroek and A. Frontera, Chem. Rec., 2016, 16, 473 CrossRef; (e) A. Bauzá, T. J. Mooibroek and A. Frontera, Angew. Chem., Int. Ed., 2013, 52, 12317 CrossRef; (f) J. S. Murray, P. Lane and P. Politzer, J. Mol. Model., 2009, 15, 723–729 CrossRef CAS PubMed.
- (a) A. Bauzá and A. Frontera, ChemPhysChem, 2020, 21, 26 CrossRef; (b) A. Franconetti, D. Quiñonero, A. Frontera and G. Resnati, Phys. Chem. Chem. Phys., 2019, 21, 11313 RSC; (c) R. Shukla and D. Chopra, Phys. Chem. Chem. Phys., 2016, 18, 13820 RSC; (d) S. P. Thomas, K. Satheeshkumar, G. Mugesh and T. N. Guru Row, Chem. – Eur. J., 2015, 21, 6793 CrossRef CAS PubMed; (e) J. S. Murray, P. Lane and P. Politzer, Int. J. Quantum Chem., 2008, 108, 2770 CrossRef CAS; (f) J. S. Murray, P. Lane, T. Clark and P. Politzer, J. Mol. Model., 2007, 13, 1033–1038 CrossRef CAS PubMed.
- (a) M. N. Piña, A. Frontera and A. Bauzá, J. Phys. Chem. Lett., 2020, 11, 8259 CrossRef PubMed; (b) J. H. Stenlid, A. J. Johansson and T. Brinck, Phys. Chem. Chem. Phys., 2018, 20, 2676 RSC; (c) A. Frontera and A. Bauzá, Chem. – Eur. J., 2018, 24, 7228 CrossRef CAS PubMed; (d) J. H. Stenlid and T. Brinck, J. Am. Chem. Soc., 2017, 139, 11012 CrossRef CAS PubMed.
- (a) R. M. Gomila, A. Bauzá, T. J. Mooibroek and A. Frontera, CrystEngComm, 2021, 23, 3084 RSC; (b) A. Buzá, I. Alkorta, J. Elguero, T. J. Mooibroek and A. Frontera, Angew. Chem., Int. Ed., 2020, 59, 17482 CrossRef PubMed; (c) I. Alkorta, J. Elguero and A. Frontera, Crystals, 2020, 10, 180 CrossRef CAS.
- M. K. Corpinot and D.-K. Bučar, Cryst. Growth Des., 2018, 18, 6404 CrossRef.
- (a) S. Yamada, N. Yamamoto and E. Takamori, J. Org. Chem., 2016, 81, 11819 CrossRef CAS; (b) S. Yamada, N. Yamamoto and E. Takamori, Org. Lett., 2015, 17, 4862 CrossRef CAS PubMed.
- R. A. Wiscons, M. Zeller and J. C. Rowsell, Cryst. Growth Des., 2016, 16, 2201 CrossRef CAS.
- (a) F. Pecatti, J. Chem. Inf. Model., 2020, 60, 6 CrossRef; (b) J. Contreras-García, E. R. Johnson, S. Keinan, R. Chaudret, J.-P. Piquemal, D. N. Beratan and W. Yang, J. Chem. Theory Comput., 2011, 7, 625 CrossRef.
- (a) A. Bauzá, A. Frontera and T. J. Mooibroek, Chem. – Eur. J., 2019, 25, 13436 CrossRef PubMed; (b) A. Franconetti, A. Frontera and T. J. Mooibroek, CrystEngComm, 2019, 21, 5410 RSC; (c) A. Bauzá, T. J. Mooibroek and A. Frontera, Chem. Commun., 2015, 51, 149 RSC.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165 CrossRef CAS.
- A. Franconetti and A. Frontera, Dalton Trans., 2019, 48, 11208 RSC.
- (a) F. Würthner, J. Org. Chem., 2021 DOI:10.1021/acs.joc.1c00625; (b) M. D. Driver, M. J. Williamson, J. L. Cook and C. A. Hunter, Chem. Sci., 2020, 11, 4456 RSC.
- (a) L. Montalvillo-Jiménez, A. G. Santana, F. Corzana, G. Jiménez-Osés, J. Jiménez-Barbero, A. M. Gómez and J. L. Asensio, J. Am. Chem. Soc., 2019, 141, 13372 CrossRef; (b) L. Yang, J. B. Brazier, T. A. Hubbard, D. M. Rogers and S. L. Cockrift, Angew. Chem., Int. Ed., 2016, 55, 912 CrossRef CAS; (c) E. Jiménez-Moreno, A. M. Gómez, A. Bastida, F. Corzana, G. Jiménez-Oses, J. Jiménez-Barbero and J. L. Asensio, Angew. Chem., Int. Ed., 2015, 54, 4344 CrossRef; (d) E. Jiménez-Moreno, G. Jiménez-Osés, A. M. Gómez, A. G. Santana, F. Corzana, A. Bastida, J. Jiménez-Barbero and J. L. Asensio, Chem. Sci., 2015, 6, 6076 RSC.
- Y. Liu, A. Sengupta, K. Raghavachari and A. H. Flood, Chem, 2017, 3, 411 CAS.
- D. B. Hibbert and P. Thordarson, Chem. Commun., 2016, 52, 12792 RSC.
- (a) G. Platzer, M. Mayer, A. Beier, S. Brüschweiler, J. E. Fuchs, H. Engelhardt, L. Geist, G. Bader, J. Schörghuber, R. Lichtenecker, B. Wolkerstorfer, D. Kessler, D. B. McConnell and R. Konrat, Angew. Chem., Int. Ed., 2020, 132, 14971 CrossRef; (b) A. Gimeno, S. Delgado, P. Valverde, S. Bertuzzi, M. A. Berbís, J. Echavarren, A. Lacetera, S. Martín-Santamaría, A. Surolia, F. J. Cañada, J. Jiménez-Barbero and A. Ardá, Angew. Chem., Int. Ed., 2019, 58, 7268 CrossRef CAS PubMed.
- The references provided at ref. 28 are examples on CH/π interactions on inside a protein binding pocked. However, they are suitable to understand the behaviour of acidic protons participating in non-covalent contacts.
- (a) J. D. Martínez, A. I. Manzano, E. Calviño, A. de Diego, B. Rodriguez de Francisco, C. Romanò, S. Oscarson, O. Millet, H.-J. Gabius, J. Jiménez-Barbero and F. J. Cañada, J. Org. Chem., 2020, 85, 16072 CrossRef PubMed; (b) J. D. Martínez, A. S. Infantino, P. Valverde, T. Diercks, S. Delgado, N.-C. Reichardt, A. Ardá, F. J. Cañada, S. Oscarson and J. Jiménez-Barbero, Pharmaceuticals, 2020, 13, 179 CrossRef PubMed.
- (a) J. S. Renny, L. L. Tomasevich, E. H. Tallmadge and D. B. Collum, Angew. Chem., Int. Ed., 2013, 52, 11998 CrossRef CAS PubMed; (b) K. Hirose, J. Inclusion Phenom. Macrocyclic Chem., 2001, 39, 193 CrossRef CAS; (c) P. Job, Ann. Chim., 1928, 9, 113 CAS.
- P. Thordarson, Chem. Soc. Rev., 2011, 40, 1305 RSC.
- (a) R. K. Harris, E. D. Becker, S. M. Cabral de Menezes, R. Goodfellow, P. Granger, R. E. Hoffman and K. W. Zilm, Pure Appl. Chem., 2008, 80, 59 CAS; (b) R. K. Harris, E. D. Becker, S. M. Cabral de Menezes, R. Goodfellow and P. Granger, Pure Appl. Chem., 2001, 73, 1795 CAS.
- M. J. Frisch, M. Head-Gordon and J. A. Pople, Chem. Phys. Lett., 1990, 166, 275 CrossRef CAS.
- (a) F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297 RSC; (b) F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 1057 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- S. F. Boys and F. Bernardi, Mol. Phys., 1970, 19, 553 CrossRef CAS.
- P. Su and H. Li, J. Chem. Phys., 2009, 131, 014102 CrossRef PubMed.
- M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. Su, T. L. Windus, M. Dupuis and J. A. Montgomery Jr, J. Comput. Chem., 1993, 14, 1347 CrossRef CAS.
- (a) J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed; (b) J. P. Perdew, M. Ernzerhof and K. Burke, J. Chem. Phys., 1996, 105, 9982 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- (a) J. Contreras-García, E. R. Johnson, S. Keinan, R. Chaudret, J.-P. Piquemal, D. N. Beratan and W. Yang, J. Chem. Theory Comput., 2011, 7, 625 CrossRef PubMed; (b) E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. Yang, J. Am. Chem. Soc., 2010, 132, 6498 CrossRef CAS PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580 CrossRef CAS PubMed.
- D. A. Case, K. Belfon, I. Y. Ben-Shalom, S. R. Brozell, D. S. Cerutti, T. E. Cheatham, III, V. W. D. Cruzeiro, T. A. Darden, R. E. Duke, G. Giambasu, M. K. Gilson, H. Gohlke, A. W. Goetz, R. Harris, S. Izadi, S. A. Izmailov, K. Kasavajhala, A. Kovalenko, R. Krasny, T. Kurtzman, T. S. Lee, S. LeGrand, P. Li, C. Lin, J. Liu, T. Luchko, R. Luo, V. Man, K. M. Merz, Y. Miao, O. Mikhailovskii, G. Monard, H. Nguyen, A. Onufriev, F. Pan, S. Pantano, R. Qi, D. R. Roe, A. Roitberg, C. Sagui, S. Schott-Verdugo, J. Shen, C. Simmerling, N. R. Skrynnikov, J. Smith, J. Swails, R. C. Walker, J. Wang, L. Wilson, R. M. Wolf, X. Wu, Y. Xiong, Y. Xue, D. M. York and P. A. Kollman, 2020, AMBER 2020, University of California, San Francisco Search PubMed.
- J. Wang, R. M. Wolf, J. W. Caldwell, P. A. Kollman and D. A. Case, J. Comput. Chem., 2004, 25, 1157 CrossRef CAS PubMed.
- T. Fox and P. A. Kollman, J. Phys. Chem. B, 1998, 102, 8070 CrossRef CAS.
- H. C. Andersen, J. Chem. Phys., 1980, 72, 2384 CrossRef CAS.
- T. A. Andrea, W. C. Swope and H. C. Andersen, J. Chem. Phys., 1983, 79, 4576 CrossRef CAS.
- S. Miyamoto and P. A. Kollman, J. Comput. Chem., 1992, 13, 952 CrossRef CAS.
- T. Darden, D. York and L. Pedersen, J. Chem. Phys., 1993, 98, 10089 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures and tables, experimental data and Cartesian coordinates. See DOI: 10.1039/d1cp02622d |
| This journal is © the Owner Societies 2022 |