 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Insights into the solution structure of the hydrated uranyl ion from neutron scattering and EXAFS experiments†
Samuel J.
Edwards
 a,
Daniel T.
Bowron
*b and
Robert J.
Baker
a,
Daniel T.
Bowron
*b and
Robert J.
Baker
 *a
*a
aSchool of Chemistry, University of Dublin Trinity College, Dublin 2, Ireland. E-mail: bakerrj@tcd.ie
bISIS Pulsed Neutron and Muon Source, Science and Technology Facilities Council, Rutherford Appleton Laboratory, Harwell Oxford, Didcot, Oxfordshire OX11 OQX, UK. E-mail: Daniel.bowron@stfc.ac.uk
First published on 22nd August 2022
Abstract
The solution structure of 1.0 M Uranyl Chloride has been determined by the EPSR modelling of a combination of neutron scattering and EXAFS data. The experimental data show an equilibrium in solution between [UO2(H2O)5]2+ and [UO2Cl(H2O)4]+ with a stability constant of 0.23 ± 0.03 mol−1 dm−3. A much smaller fraction of the neutral [UO2Cl2(H2O)3] ion is also observed. The data also show, for the first time in solution, that the uranyl ion is a very poor hydrogen bond acceptor, but the coordinated waters show enhanced hydrogen bond ability compared to the bulk water.
The fundamental chemistry of the actinide elements1 has been mapped out over the past 40 years. However, there are still gaps in fundamental properties such as the hydration and solution structures in water2 that are important for applications in areas as diverse as thermochemical modelling and environmental chemistry. This information is more challenging to gain for the actinides as rich redox chemistry is observed, including disproportionation, radiolysis (for the later actinides) and hydrolysis. As the actinides are generally highly charged species, hydrolysis form condensation reactions of various nuclearities occur with bridging oxo or hydroxo groups. This is pH dependent and typically low pH values are required to prevent this; a typical example is the speciation diagram of uranyl(VI) ions where at a pH of >3 hydrolysis products are formed which depends also on concentration and temperature. Even more complex behaviour is displayed by plutonium as disproportionation affords different solution structures. Characterizing these solution state structures is experimentally challenging to do as reproducible techniques are limited. Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy is the most popular methodology and whilst there are reasonably good correlations with bond distances across sometimes very different conditions, determination of coordination numbers is more problematic. And it has been suggested that this is due to the correlation of coordination numbers with both the amplitude reduction factor and the Debye–Waller factor.3 Some authors also note that double electron excitations in the actinide L3 edge spectra can also complicate accurate determination of coordination numbers.4 Scattering techniques such as WAXS, LANS and HEXS have intermittently been used.2
A further demonstration of the importance of understanding the solution structures of actinides is in thermodynamic modelling for environmental applications pertaining to nuclear storage for long timescales. Previous thermodynamic data were derived from potentiometric of extraction experiments (macroscopic approaches), where structural evidence of some postulated complexes are missing. The well-known uranyl aqueous chemistry is still missing unequivocal spectroscopic evidence. As an example, the number of water molecules coordinated to the uranyl ion from the majority of EXAFS experiments are 5 (with a typical error of ±10%), but HEXS analysis gives a non-integer 4.61(7). For comparison the solid-state structure gives a pentagonal bipyramidal structure with 5 coordinated water molecules. Theoretical approaches also have their place in this discussion and numerous studies have been devoted to actinide water complexes. The hydrogen bond ability of the –yl oxygen has also been interrogated based on structural and vibrational spectroscopy, but no conclusive evidence has been reported that directly probes this in solution. Theory has been used previously in this arena5 and we have recently reported that U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Oyl to H–OH is significantly weaker than O LP to H–OU (σ* = 23.7 kJ mol−1vs. 128.5 kJ mol−1).6
Oyl to H–OH is significantly weaker than O LP to H–OU (σ* = 23.7 kJ mol−1vs. 128.5 kJ mol−1).6
As the coordination chemistry of the aqua complexes obviously involves light elements, a probe for these would be complimentary to the X-ray techniques that depend on the heavy metal centres. Thus, in this report, we utilize neutron diffraction in solution to probe the bulk coordination of the uranyl aqua compound; in addition, the published EXAFS data allows a subtle but important change to the model that also includes the local information to generate a robust structural model for the solution structure of the simple [UO2]2+ ion. Moreover, given the potential for equilibria to exist in solution which is even more difficult for EXAFS to fully interrogate, we believe that the identification of these inner or outer sphere chloride complexes would constitute an advance as both HEXS and EXAFS rely on probing the uranium centre directly. We believe these results represent the first examples of using neutron diffraction to complement and enhance the study of in-solution actinide complexes and hope to spur the traditional actinide chemistry community to draw the use of neutrons into their tool-chest of characterization techniques.
To gain detailed insight into the solution structure and ion-pairing in the investigated 1.0 M aqueous solution of UO2Cl2, we have combined insight gained from neutron diffraction with isotopic substitution (NDIS) and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.7 The NDIS measurements performed on solutions prepared using H2O, D2O and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of H2O and D2O, provide a comprehensive picture of the bulk solution structure, whilst data obtained from earlier EXAFS spectroscopy8 studies provided important local structure information on the short-range chemical environments of the uranyl ions that helped constrain and enhance the detailed insights derived from the final models we have developed. Taken individually, the detailed interpretation of each experimental technique requires significant assumptions, that unfortunately can erroneously bias the resulting understanding of the solution structure. Specifically, at the 1.0 M concentration used for this study, the collected neutron diffraction patterns only contain contributions from the ion-related atomic pair correlations at a level of typically 1% or less (Table 1), whilst in contrast the EXAFS spectra are blind to the interface between the direct hydration shell of the uranyl ion and the bulk solution that can significantly impact the local molecular organization of the near-neighbour environment. To circumvent these difficulties, we have undertaken our study using the technique of Empirical Potential Structure Refinement (EPSR).9 In this approach the EXAFS data have first been utilized to establish a robust model for the uranyl ion environment, that has subsequently been refined into a data-consistent model of the bulk system structure using the isotopically enhanced neutron diffraction data. In this fashion the structural results we report here, are consistent with both bulk solution and local chemical information.10
1 mixture of H2O and D2O, provide a comprehensive picture of the bulk solution structure, whilst data obtained from earlier EXAFS spectroscopy8 studies provided important local structure information on the short-range chemical environments of the uranyl ions that helped constrain and enhance the detailed insights derived from the final models we have developed. Taken individually, the detailed interpretation of each experimental technique requires significant assumptions, that unfortunately can erroneously bias the resulting understanding of the solution structure. Specifically, at the 1.0 M concentration used for this study, the collected neutron diffraction patterns only contain contributions from the ion-related atomic pair correlations at a level of typically 1% or less (Table 1), whilst in contrast the EXAFS spectra are blind to the interface between the direct hydration shell of the uranyl ion and the bulk solution that can significantly impact the local molecular organization of the near-neighbour environment. To circumvent these difficulties, we have undertaken our study using the technique of Empirical Potential Structure Refinement (EPSR).9 In this approach the EXAFS data have first been utilized to establish a robust model for the uranyl ion environment, that has subsequently been refined into a data-consistent model of the bulk system structure using the isotopically enhanced neutron diffraction data. In this fashion the structural results we report here, are consistent with both bulk solution and local chemical information.10
| Pair correlation | Pair correlation scattering weight % (1.0 M UO2Cl2 in D2O) |
|---|---|
| OW–OW | 8.6 |
| OW–HW | 39.4 |
| OW–U | 0.4 |
| OW–OU | 0.6 |
| OW–Cl | 1.0 |
| HW–HW | 45.3 |
| HW–U | 1.0 |
| HW–OU | 1.3 |
| HW–Cl | 2.2 |
| U–U | 0.0 |
| U–OU | 0.0 |
| U–Cl | 0.0 |
| OU–OU | 0.0 |
| OU–Cl | 0.0 |
| Cl–Cl | 0.0 |
The end-product of the EPSR procedure is a three-dimensional atomistic model of the solution, built to match the known chemical stoichiometry and component density of the system. The naming convention applied to the various atomic components and used to specify the pair correlation terms in the structural analysis, is OW and HW for the oxygen and hydrogen sites of the water molecules, U and OU for the uranium and oxygen sites of the uranyl ion, and Cl for the charge balancing chloride ions. Within the model, the interaction of each of these atomic components is initially governed by a series of Lennard-Jones and Coulomb charge interaction parameters (Table 2), which are subsequently perturbed using iteratively derived empirical potential terms that are generated from the difference between the neutron diffraction patterns calculated in the model and the experimental data, as the refinement process proceeds. The structure refinement of the uranyl ion solution was initially attempted using a fully unconstrained system in which the water molecules, uranyl, and chloride ions were incorporated into the model and allowed to refine under a standard Monte Carlo simulation procedure. Comparisons made between the model-calculated EXAFS spectra and the experimental signals highlighted that this approach was unsuitable, as the Monte Carlo algorithm unavoidably generated too much local disorder in the ion environment and led to unphysical spectral parameters if agreement between the model and experimental EXAFS functions was to be achieved. To resolve this difficulty, we adjusted the way the uranyl ions were incorporated into the model. Instead of introducing the uranyl ions as fully uncoordinated ionic species, we loosely pre-bound two water molecules in the equatorial plane of the uranyl ion at a rU–OW distance of 2.41 Å, and with these water molecules separated by an OW–U–OW angle of 144°. These two water molecules were then further constrained by an imposed preference for them to maintain an OU–U–OW angle of 90°. This structure remained flexible throughout the refinement, but the long-term average configuration of these bound molecules within the model would tend towards these geometric constraints. These mild requirements were found to provide just enough influence on the level of local disorder in the local ion environments to deliver equivalence between the model-derived and experimentally determined EXAFS spectra, and importantly with physically reasonable values for the theoretical parameters that underpin the signal calculation i.e. S02, the spectral inelastic loss parameter. Adding further restraints afforded a worse fit to the EXAFS data. Data from the two fitting processes are included in the ESI (Fig. S1–S5†).
| Atom | ε (kJmol−1) | σ (Å) | M (amu) | Q (e) |
|---|---|---|---|---|
| OW | 0.65 | 3.16 | 16 | −0.8476 |
| HW | 0.0 | 0.0 | 2 | 0.4238 |
| U | 0.125 | 3.52 | 238 | 6.0 |
| OU | 0.65 | 4.3 | 16 | −2.0 |
| Cl | 0.419 | 4.38 | 36 | −1.0 |
Having close correspondence to both the isotopically distinguished neutron scattering data, and the X-ray absorption spectrum, the refined model now allows us to confidently interrogate the solution structure of the uranyl ion under our measurement conditions. It's notable that no hydrolysis or high nuclearity clusters were observed. The most important result is the conclusive determination of the coordination number in the equatorial plane i.e. the hydration number, and the influence of both 1st and 2nd shell solvation and the bond lengths (Table 3). Examining the plot in Fig. 1, clearly highlights an equilibrium in solution with 3, 4 and 5 coordinated water molecules and concomitantly 0, 1 and 2 chlorides in the coordination sphere of the uranyl ion. The average water coordination (4.6 ± 0.6) is consistent with the HEXS data that had non-integer coordination numbers {4.61(7)}. The U–Ow bond length is 2.42 A, consistent with the EXAFS literature, whilst angular data can also be extracted (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U–OW = 90° and O–U–O = 68 and 145°). The non-integer value from the HEXS study was ascribed to an equilibrium and this is confirmed in our results. To corroborate this, we see no evidence of a uranium compound with only 4 equatorial ligands.
U–OW = 90° and O–U–O = 68 and 145°). The non-integer value from the HEXS study was ascribed to an equilibrium and this is confirmed in our results. To corroborate this, we see no evidence of a uranium compound with only 4 equatorial ligands.
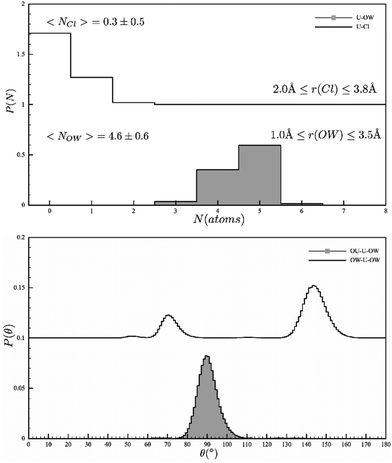 | ||
| Fig. 1 EPSR models of the Uranyl coordination sphere (top) the coordinated Cl and water and (bottom) the angular description of the water ions. | ||
| Bond | Distance (Å) |
|---|---|
U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
1.77 |
| U–Ow 1st shell | 2.42 |
| U–Cl | 2.82, 4.8 |
| U–Ow 2nd shell | 4.15, 4.58 |
Turning to the U–Cl environment, we see two bond lengths at 2.82 A and 4.8 A corresponding to a coordinated and ion-separated halide. The U–Cl bond length is also consistent with literature data (e.g. 2.81 A).11 The model also allows us to interrogate the second hydration sphere. This has proven to be important in calculations to reproduce the non-integer experimental values reported from the X-ray scattering experiments.12 HEXS13 and NMR14 measurements report 10 or 14 water molecules in the second shell, but our model puts this higher at 20.9 ± 2.3. The discrepancy likely comes about because neutron diffraction is intrinsically more sensitive to water positions. Finally, the hydration sphere of the uncoordinated chloride shows a broad range of coordinated waters with a mean of 5.3 (Fig. S7†).
As the model allows us to separate the components in the equilibrium, we can trivially calculate ion pair stability constants. Given that 71% of our initial 1 M solution is [UO2Cl]+, using the equilibrium shown in eqn (1) and (2), the ion pair concentrations are [UO22+] = 0.71 M, [UO2·Cl]+ = 0.27 M, [UO2·2Cl] = 0.02 M and [Cl–] = 1.69 M. Thus βUO2Cl = 0.23 ± 0.03 mol−1 dm−3 and βUO2Cl2 = 0.04 mol−1 dm−3. This is somewhat lower, but still within the range of literature values e.g. 0.79 mol−1 dm−3 from calorimetry15 or 1.5(10) mol−1 dm−3 from HEXS data.16 However, given the small values of these formation constants, the advantages of extracting them from the neutron diffraction data over conventional methods are obvious.
| UO2(aq)2+ + Cl(aq)− ⇌ UO2Cl(aq)+ | (1) |
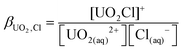 | (2) |
A final point of interest is that our model allows us to interrogate the hydrogen bonding in the solution phase (Fig. 2). Our model points to a U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯HOH distance of 2.05 Å which is indicative of a weak hydrogen bond and is the first conclusive evidence for this assumption; other reports rely on vibrational spectroscopy generally in the solid state.17 Conversely, the coordinated water molecules show a peak at 1.66 Å, indicating very strong hydrogen bonding (bulk water in our measurements was 1.75 Å, Fig. S8†). To conclude, the combined EPSR modelling of the neutron diffraction and EXAFS has produced a model that is consistent with bulk and local structure information but importantly circumvents the traditional limitations of direct EXAFS analysis of disordered systems and crucially allows for more realistic incorporation of structural disorder. This study confirms the picture of a well-defined five-fold equatorial coordination of water to the uranyl ions, but clearly shows this is in equilibrium with [UO2Cl(H2O)4]+ and [UO2Cl2(H2O)3]. The bond lengths and angles are readily extracted, and our model allows for the estimation of ion-pair stability coefficients. Furthermore, the results confirm the weak hydrogen bond acceptor chemistry of the uranyl oxygen atoms and the strong hydrogen bond donor character of the equatorial water molecules bound to the uranyl ions. We reiterate that to probe hydration behaviour of the actinides, the use of neutron diffraction in solution offers an unparalleled probe and we will report elsewhere on further examples of thorium and uranium complexes where the hydration numbers are only known from EXAFS data.
O⋯HOH distance of 2.05 Å which is indicative of a weak hydrogen bond and is the first conclusive evidence for this assumption; other reports rely on vibrational spectroscopy generally in the solid state.17 Conversely, the coordinated water molecules show a peak at 1.66 Å, indicating very strong hydrogen bonding (bulk water in our measurements was 1.75 Å, Fig. S8†). To conclude, the combined EPSR modelling of the neutron diffraction and EXAFS has produced a model that is consistent with bulk and local structure information but importantly circumvents the traditional limitations of direct EXAFS analysis of disordered systems and crucially allows for more realistic incorporation of structural disorder. This study confirms the picture of a well-defined five-fold equatorial coordination of water to the uranyl ions, but clearly shows this is in equilibrium with [UO2Cl(H2O)4]+ and [UO2Cl2(H2O)3]. The bond lengths and angles are readily extracted, and our model allows for the estimation of ion-pair stability coefficients. Furthermore, the results confirm the weak hydrogen bond acceptor chemistry of the uranyl oxygen atoms and the strong hydrogen bond donor character of the equatorial water molecules bound to the uranyl ions. We reiterate that to probe hydration behaviour of the actinides, the use of neutron diffraction in solution offers an unparalleled probe and we will report elsewhere on further examples of thorium and uranium complexes where the hydration numbers are only known from EXAFS data.
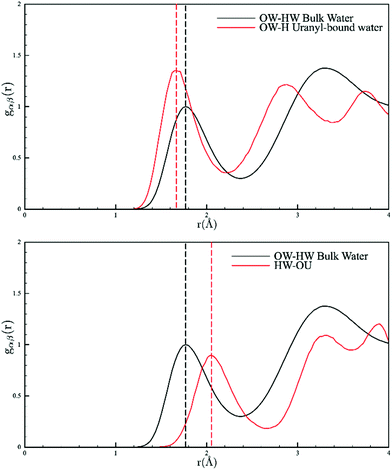 | ||
| Fig. 2 Pair distribution functions of the solvent water molecules showing the distances between the –yl⋯OH2 (top) and U–OH2⋯OH2 (bottom). Bulk water is shown in black and coordinated water in red. | ||
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Experiments at the ISIS Neutron and Muon Source were supported by a beamtime allocation RB1910235 from the Science and Technology Facilities Council. Data is available here: https://doi.org/10.5286/ISIS.E.RB1910235. We also thank Dr Marek Jura and Dr James Taylor for assistance with sample preparation at ISIS.References
- The Chemistry of the Actinides and Transactinides, ed. L. R. Morss, N. M. Edelstein and J. Fuger, Springer, Dordrecht, 2011 Search PubMed.
- K. E. Knope and L. Soderholm, Solution and Solid-State Structural Chemistry of Actinide Hydrates and Their Hydrolysis and Condensation Products, Chem. Rev., 2013, 113, 944–994 CrossRef CAS PubMed.
- J. Chaboy and S. Díaz-Moreno, Ab Initio X-ray Absorption Spectroscopy Study of the Solvation Structure of Th(IV), U(IV), and Np(IV) in Aqueous Solution, J. Phys. Chem. A, 2011, 115, 2345–2349 CrossRef CAS PubMed.
- C. Hennig, Evidence for double-electron excitations in the L3-edge x-ray absorption spectra of actinides, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 035120 CrossRef; C. Hennig, C. Le Naour and C. Den Auwer, Double photoexcitation involving 2p and 4f electrons in L3-edge X-ray absorption spectra of protactinium, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 235102 CrossRef.
- Recent review: P. D'Angelo and R. Spezia, Hydration of Lanthanoids(III) and Actinoids(III): An Experimental/Theoretical Saga, Chem. – Eur. J., 2012, 18, 11162–11178 CrossRef PubMed.
- R. J. Baker and J. A. Platts, Non-covalent interactions of uranyl complexes: a theoretical study, Phys. Chem. Chem. Phys., 2018, 20, 15380 RSC.
- D. T. Bowron and S. Diaz-Moreno, Using synchrotron X-ray and neutron methods to investigate structural aspects of metal ion solvation and solution structure: An approach using empirical potential structure refinement, Coord. Chem. Rev., 2014, 277–278, 2–14 CrossRef CAS.
- P. G. Allen, J. J. Bucher, D. J. Shuh, N. M. Edelstein and T. Reich, Investigation of Aquo and Chloro Complexes of UO22+, NpO2+, Np4+, and Pu3+ by X-ray Absorption Fine Structure Spectroscopy, Inorg. Chem., 1997, 36, 4676–4683 CrossRef CAS PubMed.
- A. K. Soper, Partial structure factors from disordered materials diffraction data: An approach using empirical potential structure refinement, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 104204 CrossRef.
- (a) D. T. Bowron and S. Diaz-Moreno, Local Structure Refinement of Disordered Material Models: Ion Pairing and Structure in YCl3 Aqueous Solutions, J. Phys. Chem. B, 2007, 111, 11393–11399 CrossRef CAS PubMed; (b) D. T. Bowron, Experimentally consistent atomistic modeling of bulk and local structure in liquids and disordered materials by empirical potential structure refinement, Pure Appl. Chem., 2008, 80, 1211–1227 CrossRef CAS.
- M. Åberg, On the Structures of the Predominant Hydrolysis Products of Uranyl(VI) in Solution, Acta Chem. Scand., 1970, 24, 2901–2915 CrossRef.
- K. E. Gutowski and D. A. Dixon, Predicting the energy of the water exchange reaction and free energy of solvation for the uranyl ion in aqueous solution, J. Phys. Chem. A, 2006, 110, 8840–8856 CrossRef CAS PubMed.
- L. Soderholm, S. Skanthakumar and J. Neuefeind, Determination of actinide speciation in solution using high-energy X-ray scattering, Anal. Bioanal. Chem., 2005, 383, 48–55 CrossRef CAS PubMed.
- M. Aberg, D. Ferri, J. Glaser and I. Grenthe, Structure of the hydrated dioxouranium(VI) ion in aqueous solution. An X-ray diffraction and 1H NMR study, Inorg. Chem., 1983, 22, 3986–3989 CrossRef.
- A. E. Martell, R. M. Smith and R. J. Motekaitis, Critically Selected Stability Constants of Metal Complexes Data-base Version 5.0, NIST Standard Reference Data, Gaithersburg, MD, 1998 Search PubMed.
- L. Soderholm, S. Skanthakumar and R. E. Wilson, Structural Correspondence between Uranyl Chloride Complexes in Solution and Their Stability Constants, J. Phys. Chem. A, 2011, 115(19), 4959–4967 CrossRef CAS PubMed.
- See for example: S. Fortier and T. W. Hayton, Oxo ligand functionalization in the uranyl ion (UO22+), Coord. Chem. Rev., 2010, 254, 197–214 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental data and results of EPSR modelling. See DOI: https://doi.org/10.1039/d2dt02535c |
| This journal is © The Royal Society of Chemistry 2022 |
