Investigation of perfluoroalkyl substances in proglacial rivers and permafrost seep in a high Arctic watershed†
John
MacInnis
 *a,
Amila O.
De Silva
*a,
Amila O.
De Silva
 *b,
Igor
Lehnherr
*b,
Igor
Lehnherr
 c,
Derek C. G.
Muir
c,
Derek C. G.
Muir
 b,
Kyra A.
St. Pierre
b,
Kyra A.
St. Pierre
 d,
Vincent L.
St. Louis
d,
Vincent L.
St. Louis
 e and
Christine
Spencer
b
e and
Christine
Spencer
b
aDepartment of Chemistry, Memorial University, St. John's, NL A1B 3X7, Canada. E-mail: john.macinnis@mun.ca
bAquatic Contaminants Research Division, Environment and Climate Change Canada, Burlington, ON L7S 1A1, Canada. E-mail: amila.desilva@canada.ca; derek.muir@canada.ca; christine.spencer@canada.ca
cDepartment of Geography, Geomatics and Environment, University of Toronto, Mississauga, ON L5L 1C6, Canada. E-mail: igor.lehnherr@utoronto.ca
dInstitute for the Oceans and Fisheries, University of British Columbia, Vancouver, BC V6T 1Z4, Canada. E-mail: k.stpierre@oceans.ubc.ca
eDepartment of Biological Sciences, University of Alberta, Edmonton, AB T6G 2E9, Canada. E-mail: vince.stlouis@ualberta.ca
First published on 15th December 2021
Abstract
We measured perfluoroalkyl substances (PFAS) in proglacial rivers and along a non-glacial freshwater continuum to investigate the role of snow and ice melting in their transport and fate within the Lake Hazen watershed (82° N). PFAS concentrations in glacial rivers were higher than those in surface waters of Lake Hazen, suggesting melting glacial ice increased PFAS concentrations in the lake. Stream water derived from subsurface soils along a non-glacial (permafrost thaw and snowmelt) freshwater continuum was a source of PFAS to Lake Hazen. Lower concentrations were found downstream of a meadow wetland relative to upstream locations along the continuum, suggesting PFAS partitioning into vegetation and soil as water flowed downstream towards Lake Hazen. Our estimations indicate that total PFAS inputs from glacial rivers and snowmelt were 1.6 kg (78%) and 0.44 kg (22%), respectively, into Lake Hazen, totalling 2.04 kg, and the output of PFAS from Lake Hazen was 0.64 kg. A positive net annual change of 1.4 kg indicates PFAS had notable residence times and/or net storage in Lake Hazen.
Environmental significancePerfluoroalkyl substances (PFAS) are anthropogenic chemicals of prominent concern, owing to their persistence and ubiquity in the environment, including the Arctic. The Arctic has been responding rapidly to climate change, accelerating snow and ice melt. It is important to understand the effect of climate warming-induced melting in the Arctic because snow and ice are repositories for PFAS and other contaminants, whose meltwaters are transported to recipient freshwater ecosystems, and eventually, the Arctic Ocean. We show that glacial ice melt, accelerated by climate change, is the primary source of PFAS to Lake Hazen, the world's largest High Arctic lake. These results provide insight into the role of climate change on the accelerated release of contaminants from glacial ice in the Arctic. |
Introduction
Perfluoroalkyl substances (PFAS) are anthropogenic chemicals used in surface treatments (e.g., for stain, oil, and water repellency), aqueous-film-forming foams (AFFF), as aids in fluoropolymer manufacturing, and mist suppressants during electroplating, among others.1 The environmental persistence and bioaccumulative properties of several PFAS, such as long-chain perfluoroalkyl carboxylic acids (PFCA, seven or more perfluorinated carbons) and perfluoroalkyl sulfonic acids (PFSA, six or more perfluorinated carbons) have resulted in regulated production of these chemicals.2,3The detection of PFAS in freshwater and terrestrial ecosystems in the High Arctic is attributed to long-range and local transport to these environments.4–8 For instance, within Svalbard, Skaar et al.5 reported low total PFAS concentrations (ΣPFAS) in water from the proglacial Lake Linnévatnet (1.4 ± 0.2 ng L−1) and high ΣPFAS concentrations 100 km away in Ny-Ålesund (113–119 ng L−1 in run-off waters) due to impacts by local fire-fighting training in 2016. Cabrerizo et al.6 reported similar findings in Arctic Canada (Nunavut) in 2015–2016, where ΣPFAS concentrations (0.20–2.0 ng g−1 dw) in soils around two lakes on uninhabited Melville Island were low relative to those around Meretta Lake (9.52 ng g−1 dw) on Cornwallis Island, which was influenced by local airport activity. The occurrence of PFAS in diverse environmental media in the Arctic underscores the potential for permafrost and glaciers to store PFAS, providing an impetus for understanding their release to recipient ecosystems in response to increased glacial ice melting and permafrost thawing promoted by climate warming.
Our prior work reported PFAS in the Lake Hazen watershed, focusing on snowpack variability, snowmelt, and water column stratification.9 Key data gaps remaining in discerning sources of PFAS to Lake Hazen, are the role of glaciers and permafrost. The glaciers surrounding the northwestern shore of Lake Hazen are melting at an increasing rate.10,11 The enhanced melting and accompanying mass loss of glaciers have hydrological consequences in Lake Hazen, such as increasing lake water levels and freshwater discharge to the Arctic Ocean.10,11 Glacial meltwaters transport sediments, nutrients, and other contaminants10–13 to and within Lake Hazen. Our earlier studies emphasize a need to quantify glacial meltwaters as a source of PFAS to Lake Hazen. First, we identified exponentially increasing PFAS fluxes into a Lake Hazen sediment core from 1963 to 2011,14 coincident with the glacial meltwater discharge rates, suggesting increasing PFAS fluxes in Lake Hazen sediments were driven by climate warming. Secondly, seasonal water column profiles in the late summer ice-free period indicated PFAS concentrations were well mixed in the lake, which was suggestive of dense and turbid glacial meltwaters.9
Here, we investigate the delivery of PFAS via proglacial rivers and along a freshwater continuum fed by snowmelt and permafrost thaw. We hypothesize PFAS can be released from subsurface soils in the Lake Hazen watershed. Climate warming has deepened the soil active layer in the Lake Hazen watershed,10 a consequence of which is the thawing of soils that were once perennially frozen (i.e., permafrost) and the melting of ice contained within those soils (for a detailed depiction of the active layer and permafrost thawing, see Section S1 and Fig. S1 in the ESI†). We advance knowledge on the transport and fate of PFAS in glacial and ground ice melt and their relevance to recipient freshwater Arctic systems and ultimately to the oceans. Specifically, we provide the first quantitative assessment of PFAS in proglacial rivers in the High Arctic of Canada and insights into PFAS partitioning within a non-glacierized region of the Lake Hazen watershed.
Materials and methods
Study area
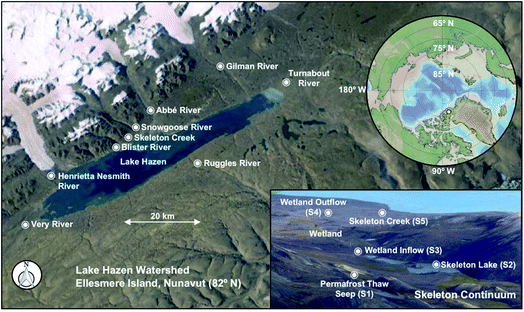
The Lake Hazen watershed is located within Quttinirpaaq National Park on northern Ellesmere Island, Nunavut, Canada (81.816° N, 70.700° W, Fig. 1). Lake Hazen is the largest lake by volume (51.4 km3) north of the Arctic Circle, with a maximum depth of 267 m,15 and is supplied primarily by hydrological inputs from melting snow and 11 glaciers, amounting to ∼12% and 87% of total hydrological inputs to the lake in 2015, respectively.11 The rivers that we sampled on the north shore of Lake Hazen are 6 of the 11 rivers that travel from land terminating outlet glaciers of the Northern Ellesmere Icefield (Fig. 1). Glacial rivers flow from early June until the end of August and first receive snow meltwaters from the landscape and higher altitudes in the glacierized area followed by glacial ice meltwaters in years with high melt, as in 2015. For perspective, glacial runoff was 0.979 km3 and 0.291 km3 in 2015 and 2016, respectively.11 Snowmelt from higher altitudes in the glacierized area may integrate atmospheric PFAS deposition if snow meltwaters coalesce from different time periods (e.g., mixing of meltwaters from 2013 and 2014 snowpacks). Similarly, it is anticipated that high melt years will result in the coalescence of glacial meltwaters, integrating historically archived PFAS. The Ruggles River is the only outflow of Lake Hazen, receiving integrated hydrological inputs from the lake (i.e., waters derived from precipitation, snowmelt and glacial rivers and creeks, landscape snowmelt, and permafrost thaw).Within the Lake Hazen watershed exists innumerable, small non-glacierized sub-catchments. These sub-catchments receive snowmelt from the landscape and stream waters derived from subsurface soils that integrate precipitation, snowmelt, and soil active layer/permafrost thaw (i.e., the thawing of soils and melting of ice contained within those soils, Section S1 and Fig. S1†), but are minor contributors to the hydrological budget of Lake Hazen (<1%). We sampled one of these representative sub-catchments, hereafter referred to as the Skeleton Continuum, located near the Lake Hazen base camp, along the northwestern shore of Lake Hazen (Fig. 1). During the spring and summer, hydrological inputs along the Skeleton Continuum are supplied primarily by snowmelt from the landscape, beginning at the end of May through to early June, and later may be fed by waters stored in soils through to the end of August. Snowmelt from the landscape and stream water (i.e., discharged to the surface from subsurface soils via seeps) enters Skeleton Lake, which drains into two small downstream ponds, a riparian and meadow wetland, and finally, the sparsely vegetated Skeleton Creek before discharging into Lake Hazen (Fig. 1).16 Further details related to the study area can be found in Section S2 and Table S1.†
Sampling
Glacial rivers and non-glacial creeks were sampled in the Lake Hazen watershed during snowmelt (3 June 2012, 23 May to 1 June 2014) and during the summer period dominated by melt from the glacierized area (7 to 31 July 2015). The sampling of the freshwater continuum occurred between 9 July and 1 August 2015 at five sites (Fig. 1). Samples were collected directly from an upland seep located down-elevation of a talus slope along the side of Mount McGill (S1).17 Surface water samples were also collected from the southeastern bank of Skeleton Lake (S2), wetland inflow (S3) and wetland outflow (S4), as well as Skeleton Creek (S5) at approximately 0.2 km before its outlet into Lake Hazen (Fig. 1). S2 was also sampled during an ice-covered period on 15 May 2013 from two depths (1.5 and 3 m) using a Niskin water sampler, while S5 was sampled during a snowmelt period from 23 May to 1 June 2014. Surface water was collected from Lake Hazen on 22 July 2015. Water was collected in pre-cleaned 1000 mL polypropylene bottles at all sites, typically dipping from shore, and subsequently stored at ∼5 °C until shipped to the Canada Centre for Inland Waters (Burlington, Ontario) for analysis.A portable weather station (Campbell Scientific) was mounted onshore at the Lake Hazen base camp for hourly temperature and wind speed measurements during the summer sampling period. The mean daily temperature in July 2015 was above 4 °C and daily maximum temperatures were as high as 17 °C (Fig. S2†).
Sample extraction
Water samples were extracted for 19 PFAS, including C4–C14 and C16 PFCA, C4, C6–C8, and C10 PFSA, perfluoro-4-ethyl-cyclohexane sulfonic acid (PFECHS), and perfluorooctane sulfonamide (FOSA) using a weak anion exchange solid-phase extraction method. Neutral FOSA was eluted using methanol, and anionic PFCA and PFSA were eluted using 0.1% ammonia in methanol.14,18,19 Extracts were concentrated to dryness and reconstituted in 0.5 mL of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solid-phase extraction-cleaned water
1 solid-phase extraction-cleaned water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol. Further details on chemicals and QA/QC are presented in Sections S3 and S4 and Tables S2 and S3.†
methanol. Further details on chemicals and QA/QC are presented in Sections S3 and S4 and Tables S2 and S3.†
Instrument and data analysis
PFAS in water samples were analyzed using liquid chromatography-tandem mass spectrometry (Table S4†).14,18,19 Particulate carbon and nitrogen were analyzed according to an amended EPA Method 440.0 at the accredited Biogeochemical Analytical Service Laboratory at the University of Alberta using a CE-440 elemental analyzer (Exeter Analytical, Inc.).For PFAS, limits of detection (LOD) and quantitation (LOQ) corresponded to concentrations yielding an instrument response with signal-to-noise ratios of 3 and 10, respectively (Table S3†).9,14 The sum of PFAS (ΣPFAS) corresponds to concentrations equal to or greater than the LOD. PFAS with concentrations below the LOD were not assigned a numerical value for ΣPFAS calculations or statistical analyses. Kruskal–Wallis and post hoc Mann–Whitney U-tests and Spearman rank (rs) correlations were performed using StatPlus:mac (V6) to compare PFAS concentrations, with a critical p-value set to 5%.
Results and discussion
PFAS in rivers and creeks from the Lake Hazen watershed
A homologous series of PFAS (C4–C14 PFCA, C4, C6–C8 PFSA, and FOSA) were detected in rivers and creeks in the Lake Hazen watershed (Fig. 2, 3, and S3, Table S5†). PFCA with chain lengths of C4, C6–C9 had 100% detection frequency, while long-chain PFAS (C12–C14 PFCA and C7 PFSA) were only detected in rivers and creeks during the May 2014 period. PFBA was by far the most abundant PFAS in rivers and creeks, with concentrations ranging from 1.1–12 ng L−1 and 0.62–3.3 ng L−1 during snowmelt periods (May 2014 and June 2012) and glacial ice melting periods (July 2015), respectively, accounting for 43–83% of ΣPFAS concentrations. ΣPFAS concentrations were comparable at Skeleton Creek (2.5–21 ng L−1, median 8.1 ng L−1), Blister River (10–15 ng L−1, median 11 ng L−1), and Snowgoose River (5.4–15 ng L−1, median 15 ng L−1) during May 2014 and June 2012 periods (8.9–9.4 ng L−1), as expected given the same landscape snowmelt water source (Fig. 2a). For perspective, the median ΣPFAS concentration in Lake Hazen snowpacks was 6.7 ng L−1 and ranged from 3.3 to 30 ng L−1 during June 2014. ΣPFAS concentrations during the July 2015 period were highest in order of Blister (2.4–4.1 ng L−1), Turnabout (2.4 ng L−1), Very (1.8 ng L−1), Snowgoose (1.1–1.9 ng L−1), Henrietta Nesmith (1.3 ng L−1), and Abbé (1.1 ng L−1) Rivers. The variability in ΣPFAS concentrations in rivers during the July 2015 period could suggest that rivers received different water sources from their glacierized areas, including glacial melt and snowmelt from higher elevations. Lower PFAS concentrations in rivers during July 2015 relative to May 2014/June 2012 (Mann–Whitney U test, p < 0.001) could be due to dilution effects. For example, the hydrological contributions from melting glaciers were much higher than those from snowmelt to the water budget of Lake Hazen (0.979 km3via glacial ice melt and 0.067 km3 snowmelt in July 2015 vs. 0.017 km3 snowmelt in May 2014),11 providing a rationale for lower PFAS concentrations in rivers during 2015 than those during 2014 and 2012. However, PFAS concentrations in glacial rivers were higher than those in the water column of Lake Hazen (0–250 m depths) during the July 2015 period (Mann–Whitney U test, p < 0.001), suggesting melting glaciers were increasing PFAS concentrations in the lake. Chen et al.20 noted similar findings, where glacial rivers in the Tibetan Plateau were implicated in the enhanced delivery of PFAS to Lake Nam Co. ΣPFAS concentrations in glacial rivers from the Lake Hazen watershed during 2015 were similar to those reported in the glacier-fed Qugaqie River in the Tibetan Plateau during 2017 (0.7–1.6 ng L−1)20 and meltwaters from the Lake Linnévatnet catchment (1.1–4.2 ng L−1) on Svalbard in 2015.5 A comparison of PFAS concentrations in river, creek, and lake water in the Lake Hazen watershed with other freshwater systems in the Arctic can be found in Table S5.†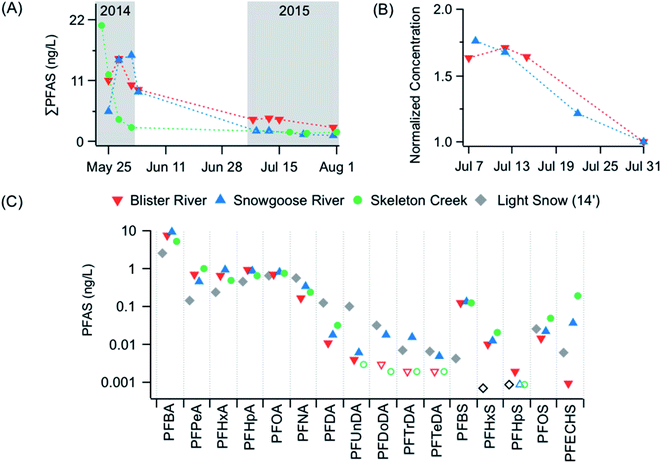 | ||
| Fig. 2 (A) Total PFAS concentrations (ng L−1) in river and creek water during snowmelt from 23 May to 1 June 2014 and 3 June 2012, and during glacial ice melting from 7 to 31 July 2015. River concentrations during the 2012 snowmelt period are represented by the symbols outside of the grey-shaded area in panel (A). (B) Normalized Total PFAS concentration during July 2015 at Blister and Snowgoose Rivers. Concentrations in each river were normalized to total PFAS concentrations on 31 July. (C) Comparison of median concentrations in river and creek water in 2014 with those in light snowpacks9 from the ice surface of Lake Hazen in 2014. Concentrations below the LOD are denoted by unfilled symbols. | ||
PFAS were detected in the Ruggles River outflow of Lake Hazen, including C4, C6–C9 PFCA, and PFOS. PFBA (0.44 ng L−1) and PFOA (0.066 ng L−1) were most abundant, collectively accounting for 85% of the ΣPFAS concentration (0.59 ng L−1). The ΣPFAS concentration in the Ruggles River was comparable to those in surface waters from Lake Hazen (0.32 ng L−1, Fig. S3†). Similar observations were reported in a recent study of mercury (Hg) across the watershed, where concentrations of methylmercury (MeHg) and total Hg were similar in surface waters of Lake Hazen and the mouth of the Ruggles River.11
The PFAS composition profiles in glacial rivers are generally consistent with those in the water column of Lake Hazen during the ice-free period and the Ruggles River outflow in 2015 (Table S5†). PFAS concentrations are well mixed in the lake during the ice-free period, consistent with the mixing of PFAS by underflows promoted by the delivery of turbid glacial meltwaters.9 Interestingly, PFBS and PFDA were detected frequently in glacial rivers but not in the water column of Lake Hazen during the ice-free period or the Ruggles River outflow in 2015 (Table S5†). This could suggest these congeners partitioned to biota (e.g., Arctic char) and/or were transported as sediments to the bottom of the lake, consistent with their occurrence in a Lake Hazen sediment core.14
Rivers and creeks were sampled regularly during May 2014 and July 2015 periods to understand temporal changes in PFAS concentrations. The magnitude of ΣPFAS concentrations was similar at Skeleton Creek, Blister River, and Snowgoose River during snowmelt in May 2014 (Kruskal–Wallis Test, p = 0.79). However, temporal changes in PFAS concentrations varied spatially within the high-frequency sampling in May 2014. For example, ΣPFAS concentrations had very slight changes in Blister River with an increase from 11.0 to 15.4 ng L−1 from 25 to 28 May, followed by 10.1 ng L−1 on 1 June. Over the same period, ΣPFAS concentrations increased from 5.4 to 15.7 ng L−1 in Snowgoose River and decreased from 20.9 to 2.5 ng L−1 in Skeleton Creek (Fig. 2a). These temporal changes in ΣPFAS concentrations could be related to differences in snowmelt dynamics within non-glacierized and glacierized areas of the watershed. For example, Skeleton Creek receives snowmelt from its non-glacierized catchment, whereas Blister River and Snowgoose River receive various snowmelt inputs from different elevations within the glacierized area of their watersheds. This could explain the absence of decreasing ΣPFAS concentrations at Blister River and Snowgoose River if these systems received delayed inputs of snow meltwaters from higher elevations that were enriched in PFAS (i.e., an ionic pulse).21
The weekly sampling during July 2015 indicated that ΣPFAS concentration trends were similar at Blister and Snowgoose Rivers. For instance, ΣPFAS concentrations decreased in both rivers by a factor of 1.6–1.8 (Fig. 2b). Differences in ΣPFAS concentrations were observed during the May–June period in 2014 relative to July 2015 (Fig. 2a), which could be attributed to differences in hydrological sources (i.e., snow versus glacial ice melt inputs). These observations nevertheless highlight the importance of seasonal melting as a source of PFAS in tributaries within the Lake Hazen watershed.
Mass transfer of PFAS in the Lake Hazen watershed
A recent study demonstrated that using an annual mass balance model, Lake Hazen was a sink for total Hg (THg) primarily derived from glacial rivers.11 By analogy, Lake Hazen could store PFAS, considering PFAS were consistently detected throughout its water column9 and sediments.14Here, we adapted the approach used by St. Pierre et al.11 to estimate net PFAS inputs by glacial rivers (Section S5, Table S6, Fig. S4†) and snowmelt into Lake Hazen and the output of PFAS by the Ruggles River according to:
| ΔPFAS = ∑inputsPFAS − outputPFAS |
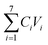 ). Snowmelt is based on the product of the average PFAS concentrations in snowpacks from the Lake Hazen region in 2013 (kg km−3)9 and the annual volume of snowmelt runoff (from the glacier-free landscape and lake surface) to the lake for 2015 (0.127 km3).11 The output of PFAS by the Ruggles River is based on the product of PFAS concentrations and the annual volume of runoff in the Ruggles River for 2015.11
). Snowmelt is based on the product of the average PFAS concentrations in snowpacks from the Lake Hazen region in 2013 (kg km−3)9 and the annual volume of snowmelt runoff (from the glacier-free landscape and lake surface) to the lake for 2015 (0.127 km3).11 The output of PFAS by the Ruggles River is based on the product of PFAS concentrations and the annual volume of runoff in the Ruggles River for 2015.11
Using this approach, the estimated total input of PFAS was 1.6 kg by glacial rivers and 0.44 kg by snowmelt into Lake Hazen, totalling 2.04 kg, whereas the discharge of PFAS from the Ruggles River was 0.64 kg (Table S7†). These results indicate a positive net change accumulation of 1.4 kg in Lake Hazen during the summer of 2015. On a congener basis, PFBA had a much higher net change accumulation in Lake Hazen than those of other PFAS (Table S7†), driven by inputs from glacial rivers, representing 84% of the total PFBA input into the lake. The net accumulation of PFBA (and other PFAS with high water solubilities) in Lake Hazen is likely attributable to sustained melting inputs from snow and glaciers over time together with the long residence time of water in the lake (25 years).10
A limitation of this study is only one Ruggles River data point is used to estimate the output. However, the net estimate for ΣPFAS obtained using this limited data set would suggest Lake Hazen sediments may act as a long-term sink for approximately 36% of the PFAS inputs, assuming the measured ΣPFAS sediment flux in 2015 is representative of that in 2011 (0.51 kg).14 While the estimated net accumulation is dominated by PFBA, which was not found in Lake Hazen sediments, PFBA was the dominant congener in the Lake Hazen water column during the glacier melting period in 2015.9 The implication of these data is PFAS have significant residence times and/or storage in Lake Hazen. Lake Hazen sediments will likely continue acting as a long-term sink for select PFAS congeners (i.e., with low water solubilities), while the concentrations of other PFAS (e.g., PFBA) may increase in the Lake Hazen water column as it continues receiving hydrological inputs over time until a steady-state is established.
PFAS inputs by glacial rivers into Lake Hazen were higher than those reported in Lake Nam Co on the Tibetan Plateau.20 For example, glacial runoff accounted for 27% (0.49 kg) of the 1.81 kg annual input of PFAS into Lake Nam Co,20 whereas glacial rivers accounted for 78% (1.6 kg) of the 2.04 kg annual input into Lake Hazen. Furthermore, the catchment area-normalized PFAS input by glacial rivers in Lake Hazen (7516 km2, 2.1 × 10−4 kg km−2) is approximately 5-times higher than that of Lake Nam Co (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 610 km2, 4.6 × 10−5 kg km−2).20 The contrast observed with our study is reflective of differences in hydrological inputs because Lake Nam Co receives less glacial meltwater runoff than Lake Hazen. Lake Nam Co was supplied by 0.365 km3 of glacial runoff20 compared to 0.979 km3 in Lake Hazen11 during 2015, accounting for 16% and 87% of hydrological inputs to each lake, respectively.
610 km2, 4.6 × 10−5 kg km−2).20 The contrast observed with our study is reflective of differences in hydrological inputs because Lake Nam Co receives less glacial meltwater runoff than Lake Hazen. Lake Nam Co was supplied by 0.365 km3 of glacial runoff20 compared to 0.979 km3 in Lake Hazen11 during 2015, accounting for 16% and 87% of hydrological inputs to each lake, respectively.
Our analysis for PFAS are also in contrast to the estimated 95% of THg inputs by glacial rivers (16.4 kg) stored within Lake Hazen.11 St. Pierre et al.11 noted that Lake Hazen was a sink for Hg due to the enhanced delivery of particles via glacial rivers and turbid underflows, which transported particle-bound Hg to the bottom of the lake. The differences in mass balance for Hg versus PFAS were likely due to differences in physicochemical properties because many PFCA and PFSA targeted in this study have high water solubilities and are less likely to partition to particles in the water column and accumulate in lake sediments than metal species such as Hg(II) and MeHg. However, PFAS with lower water solubilities and higher log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc, such as long-chain PFAA (e.g., C10–C14 PFCA), may have similar environmental fates as particle-bound Hg in Lake Hazen. It is also important to consider that differences between PFAS and Hg may be expected due to differences in the underlying sources of these contaminants to Lake Hazen (i.e., proglacial soils versus glacial ice). These observations highlight the importance of glacial meltwater as a vector and source of contaminants to Lake Hazen.
Koc, such as long-chain PFAA (e.g., C10–C14 PFCA), may have similar environmental fates as particle-bound Hg in Lake Hazen. It is also important to consider that differences between PFAS and Hg may be expected due to differences in the underlying sources of these contaminants to Lake Hazen (i.e., proglacial soils versus glacial ice). These observations highlight the importance of glacial meltwater as a vector and source of contaminants to Lake Hazen.
Unique PFAS signatures along the Skeleton Continuum
To better understand non-glacial inputs, an investigation of the Skeleton Continuum was conducted because it is broadly representative of the many smaller, non-glacierized catchments across the watershed. The concentrations and detection frequencies of PFAS along the Skeleton Continuum are shown in Fig. 3, 4, and S5 and Table S8.† A seasonal comparison of PFAS in the Skeleton Lake water column during ice-covered and ice-free periods is shown in Fig. S6 and discussed in Section S6.†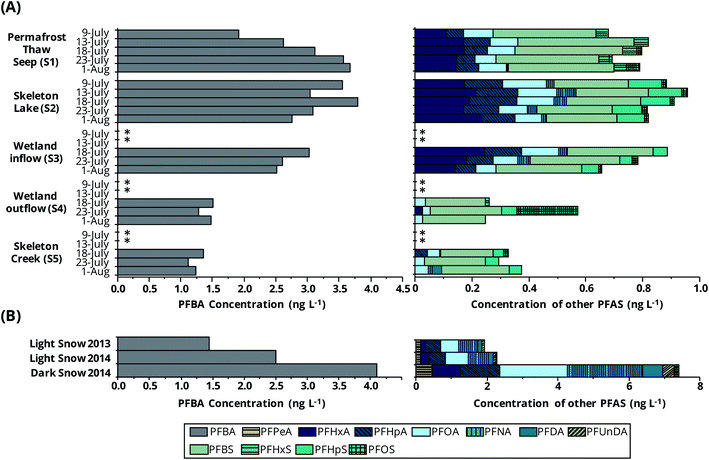 | ||
| Fig. 3 Concentrations (ng L−1) of PFAS along the Skeleton Continuum (A) from July 9 to August 1 2015 and in Lake Hazen snow9 from May 2013 and June 2014 (B). * indicates sampling was not conducted because water was not flowing through sites. | ||
PFBA, PFBS, and PFOA were detected at all sites along the continuum. Other PFAS were specific to zones within and closest to S1. For example, PFHxA, PFHpA, and PFOS were detected at S1, S2, and S3 with detection frequencies corresponding to 60 to 100% but were only present in 0 to 33% samples further downstream (Table S8†). PFNA and PFHpS were only detected frequently at the downstream sites, S2, S3, and S5.
Maximum PFAS (Σ and individual) concentrations along the continuum generally occurred at S1 and S2. PFAS profiles were dominated by PFBA and PFBS, with concentration ranges of 1.1–3.8 ng L−1 and 0.18–0.41 ng L−1, respectively, accounting for 69–86% and 5–15% of the ΣPFAS concentrations, respectively. ΣPFAS concentrations were of the same magnitude at S1, S2, and S3 (medians 3.3–4.0 ng L−1, Kruskal–Wallis test, p = 0.25). However, concentrations in the upstream sites along the Skeleton Continuum (sites S1–S3) were higher than those at downstream sites (S4 and S5, Mann–Whitney U test, p < 0.001). For example, median concentrations at downstream sites were approximately a factor of 2 lower than those measured at the upstream sites. ΣPFAS concentrations along the Skeleton Continuum (1.4–4.7 ng L−1) were much higher than those in waters from Lake A (Ellesmere Island, Nunavut, 0.027–0.456 ng L−1),7 and waters from Lake Hazen during the same sampling period (0–10 m, 0.26–0.31 ng L−1).9 ΣPFAS concentrations in waters from S1–S3 were similar to those in light snowpacks from the Lake Hazen region (Fig. 4). ΣPFAS concentrations in waters from S1–S3 were also similar to those at Blister River during July 2015 (2.4–4.1 ng L−1, Mann–Whitney U Test, p = 0.91), further suggesting the importance of non-glacial inputs within that watershed (e.g., snowmelt from higher elevations within the glacierized area).
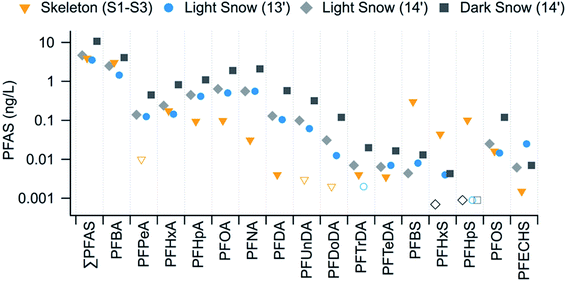 | ||
| Fig. 4 Comparison of median PFAS concentrations in water from upstream sites (S1–S3) along the Skeleton Continuum with those in snowpacks from the ice surface of Lake Hazen in 2013 and 2014.9 Concentrations below the LOD are denoted by unfilled symbols. | ||
PFAS congener profiles were notably different along the Skeleton Continuum than those found in snowpacks9 from the Lake Hazen region (Fig. 4 and Table S8†). In particular, snowpacks from the ice surface of Lake Hazen in 2013 and 2014 consistently had higher median concentrations of C5 and C7–C12 PFCA and PFECHS, whereas sites S1–S3 showed higher median concentrations of PFBS, PFHxS, and PFHpS (Fig. 4 and Table S8†). These observations would suggest the Skeleton Continuum was impacted by different sources than those influencing Lake Hazen snowpacks in 2013 and 2014.
The composition profile of PFAS along the Skeleton Continuum provided evidence of unique hydrological and atmospheric sources to small non-glacierized sub-catchments within the Lake Hazen watershed. For instance, PFHxS was only detected frequently at S1 but not in the recipient S2–S3 or S5 (Table S8†), whereas PFHpA and PFOA were both frequently detected in S1 to S3. Reported sorption coefficients for these PFAS are similar with the organic carbon-normalized sorption coefficient (Koc) corresponding to 50 ± 2.2 for PFHxS, 50 ± 1.3 for PFHpA, and 107 ± 4.5 for PFOA.22 Thus, it is unlikely that the absence of PFHxS within S2 was due to removal via sorption and sedimentation. Furthermore, PFHpA and PFOA concentrations were not correlated to other particulate parameters within S2 over the sampling period (Fig. S7†). The S2 site was supplied by multiple seeps and snowmelt inputs from the landscape. In contrast, PFHxA and PFHpA, which were frequently detected in Lake Hazen snowpacks,9 were found at higher concentrations in S2 than S1 (Mann–Whitney U test, p ≤ 0.03), while higher detection frequencies were observed for other PFAS (i.e., PFNA, PFTrDA, PFHpS, FOSA) at S2. These observations indicated that integrated hydrological inputs from seeps and snowmelt resulted in higher detection frequencies and concentrations of PFAS in S2 relative to the single S1 seep sampled in this study (Table S8†). PFHpS was not detected in snowpacks,9 sediments,14 or the water column of Lake Hazen,9 but was found in S2 and further downstream along the Skeleton Continuum. PFHpS correlates with PFBA, PFHpA, and PFOA along the continuum (rs ≥ 0.75, p ≤ 0.02, Table S9†), suggesting these congeners may be derived from a common source. The sites along the Skeleton Continuum were ice-free from mid-June until late August, facilitating the direct atmospheric deposition of aerosol particles and gases.
The source of PFHxS to the Skeleton Continuum is interesting because it was not a dominant PFAS in Lake Hazen snowpacks, with detection frequencies ranging from 0–11% (Table S8†).9 A recent report from the Norwegian Environment Agency indicated that PFHxS may be present in AFFF, food-contact paper, weather-proofing additives, and cleaning agents.23 Intentional production and use of PFHxS as an additive has not been disclosed24 but it is a known impurity in some PFAS technical mixtures,25 including historical AFFF formulations.26 While it is unknown if direct emissions from the use of PFHxS-containing products were sources of PFHxS in the Lake Hazen watershed over time, the long-range atmospheric transport of aerosols and volatile precursors (e.g., perfluorohexane sulfonamide, FHxSA) is a possible pathway for PFHxS, despite low detection frequencies in the Lake Hazen sediments14 and snowpacks,9 because neutral PFSA precursors have been measured in the Arctic atmosphere.27,28
In comparison, PFBS was prevalent throughout the Skeleton Continuum, much more than in glacial rivers, but not in light snowpacks from the Lake Hazen area in 2013 and 2014![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (Table S8†). PFBS was detected frequently in dark snowpacks enriched with light-absorbing particles in 2014, possibly due to the role of atmospheric particulate matter (e.g., particle-mediated transport and/or scavenging); however, PFBS concentrations in dark snowpacks were much lower than those along the Skeleton Continuum (Mann–Whitney U test, p < 0.001, Table S8†).9 PFBS was detected in the Lake Hazen sediment core, with a deposition rate doubling time of 7.7 years from 1963 to 2011.14 These results suggest that PFAS fluxes in Lake Hazen sediment are attributable to enhanced climate warming-induced glacier melt over the past 10–15 years. Similarly, climate warming-induced soil active layer deepening may account for the occurrence of PFBS and PFHxS at S1 (Fig. S1†). Warmer temperatures could promote the release of archived PFAS from ice contained in active layer/permafrost soils. The origin of water at S1 may also reflect integrated inputs from permafrost and snowmelt infiltrated into soils during 2015. This could explain both PFBS and PFHxS profiles at S1 and the consistency of ΣPFAS concentrations at S1 with Lake Hazen snowpacks.9
9 (Table S8†). PFBS was detected frequently in dark snowpacks enriched with light-absorbing particles in 2014, possibly due to the role of atmospheric particulate matter (e.g., particle-mediated transport and/or scavenging); however, PFBS concentrations in dark snowpacks were much lower than those along the Skeleton Continuum (Mann–Whitney U test, p < 0.001, Table S8†).9 PFBS was detected in the Lake Hazen sediment core, with a deposition rate doubling time of 7.7 years from 1963 to 2011.14 These results suggest that PFAS fluxes in Lake Hazen sediment are attributable to enhanced climate warming-induced glacier melt over the past 10–15 years. Similarly, climate warming-induced soil active layer deepening may account for the occurrence of PFBS and PFHxS at S1 (Fig. S1†). Warmer temperatures could promote the release of archived PFAS from ice contained in active layer/permafrost soils. The origin of water at S1 may also reflect integrated inputs from permafrost and snowmelt infiltrated into soils during 2015. This could explain both PFBS and PFHxS profiles at S1 and the consistency of ΣPFAS concentrations at S1 with Lake Hazen snowpacks.9
Alternatively, it is possible that snowmelt during 2015 was a primary source and/or vector for the occurrence of PFBS and PFHxS at S1 through the action of remobilizing atmospheric deposition from snowpacks and soils in the Skeleton catchment. The remobilization of atmospheric deposition from soils is a plausible mechanism, according to observations of exposed landscape in the Lake Hazen region due to low snow accumulation11 and PFAS concentrations in snowpacks9 and snowmelt-fed rivers in May 2014 (Fig. 2c). For example, PFBS concentrations were higher in the snowmelt-fed Skeleton Creek, Blister River, and Snowgoose River than those in snowpacks from the Lake Hazen region in May 2014 (Mann–Whitney U test, p < 0.001, Fig. 2c), while PFHxS was only detected in one snowpack sample in 2014 (Table S8†). This suggested these rivers are receiving: (1) integrated hydrological inputs (i.e., mixing of snow meltwaters); (2) different atmospheric deposition than snowpacks; or (3) meltwaters that entrained atmospheric deposition from soils.
PFAS composition profiles along the Skeleton Continuum highlight that water samples in this region are affected by complex sources and processes. For instance, samples in this study likely reflect integrated inputs from various hydrological (i.e., snowmelt, precipitation, and permafrost thaw) and atmospheric sources in this region. Furthermore, Lake Hazen snowpacks were not analyzed in 2015, limiting source allocation along the continuum. Although we are unable to confirm specific sources or mechanisms along the continuum, this analysis provides insights into the role of permafrost thaw as a possible source of historically archived PFAS and the processes affecting the transport and fate of PFAS in subsurface soil environments in the Arctic, such as the elution of PFAS from melting ice in permafrost soils and the transport of meltwaters through subsurface soils to recipient freshwater systems.
Transport and partitioning of PFAS along the Skeleton Continuum
PFAS profiles along the Skeleton Continuum provide insight into the transport and partitioning behavior of PFAS. The occurrence of PFAS at S1 indicated that water from S1 was a source of PFAS; however, the low detection frequency of long-chain PFCA (i.e., C9–C14) was attributed to the enhanced hydrophobic interactions with subsurface soils. This also provided a rationale for the delayed delivery of PFOS to S1 (Fig. 3). Similar observations were reported in the Krycklan Catchment, where the mobility of PFUnDA and PFDoDA within a groundwater system was limited by PFAS partitioning within soils.29 Although we are unable to confirm this hypothesis, it is conceivable that the delivery of PFOS (and other PFAS) to S1 could be delayed, particularly in comparison to other PFAS with higher water solubilities and lower Koc. For example, if the ice in permafrost soils was a source of PFBA and PFOS, it is expected that the initial ice meltwaters from those soils will be enriched in PFBA relative to PFOS, based on preferential elution behavior (i.e., water solubility), and it is likely that PFBA in meltwater will show a lesser tendency to partition to subsurface soils in comparison to PFOS, which has a higher Koc. Therefore, the delayed delivery of PFOS to S1 could be a result of ice melting behavior together with enhanced partitioning with subsurface soils. However, it is challenging to assign interpretations to the temporal variations in PFAS concentrations at S1, as they may not necessarily reflect the release of PFAS from permafrost soils, but instead an integrated PFAS profile representative of multiple hydrological sources and processes (e.g., subsurface partitioning with soils).The C6–C8 PFCA and C4, C6 PFSA concentrations varied similarly over time at site S1 during July (Fig. S7†). These observations suggested that subsurface transport and partitioning were similar for these PFAS, which is consistent with similarity in log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc.22 In contrast, a distinct profile was observed for PFBA, such that concentrations increased from 1.9 ng L−1 to 3.7 ng L−1 during 9 July to 1 August sampling period (Fig. 3). This distinctive trend could be related to the progressive melting of ice contained in permafrost soils during the summer, enhancing PFBA concentrations at S1 due to its relatively higher water solubility and lower log
Koc.22 In contrast, a distinct profile was observed for PFBA, such that concentrations increased from 1.9 ng L−1 to 3.7 ng L−1 during 9 July to 1 August sampling period (Fig. 3). This distinctive trend could be related to the progressive melting of ice contained in permafrost soils during the summer, enhancing PFBA concentrations at S1 due to its relatively higher water solubility and lower log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc.22
Koc.22
Overall, PFAS concentrations and detection frequencies were comparable at S1, S2, and S3, suggesting limited PFAS partitioning to soils or lake/pond sediments across these sites (Table S8,†Fig. 3). However, lower PFAS concentrations and detection frequencies were typically observed at downstream sites S4 and S5, consistent with a recent study, where lower MeHg concentrations were measured at S4 and S5 in comparison with upstream concentrations (sites S2 and S3), resulting from soil partitioning, demethylation, and/or the settling of particle-bound MeHg in the wetland.17 Analogously, it is possible lower PFAS concentrations at S4 and S5 could be attributed to the partitioning of PFAS with plants and soils in the meadow wetland. Müller et al.30 reported uptake and depuration kinetics of PFAS in vegetation using a hydroponic plant model system. In that study, concentrations in plant roots at equilibrium were high and corresponded to 317 ± 16 ng g−1 PFNA, 771 ± 35 ng g−1 PFDA, 766 ± 68 ng g−1 PFOS, and 275 ± 14 ng g−1 PFBA; however, faster uptake and depuration kinetics were reported for PFNA, PFDA, and PFOS relative to PFBA.30 This was attributed to the enhanced translocation ability of PFBA throughout the plant system due to its high water solubility.30 This would suggest plant uptake could contribute to the removal and storage of PFAS from waters at S4, and reinforces the distinction between the transport and fate dynamics of PFAS and MeHg in the Lake Hazen watershed, as the latter is more susceptible to particle partitioning and accumulating in soils.17 However, lower concentrations and detection frequencies of other PFAS at S4 with higher Koc could also be attributed to soil partitioning and/or the settling of particle-bound PFAS in the meadow wetland. The utility of constructed wetlands to remove and store PFAS from waters in the environment has been studied as a remediation approach.31,32 We are unable to preclude the possibility that high flow conditions (i.e., dilution) contributed to lower PFAS concentrations at downstream sites (S4 and S5), as water discharge rates were not measured. However, we believe this is unlikely because this non-glacial area receives little precipitation and is not known to have seeps, according to field observations at the time of sampling.
Conclusions and future outlook
The results in this study provide unique insights into the sources, as well as the post-depositional transport and fate of PFAS in High Arctic freshwater ecosystems in Canada. This study demonstrates that snowmelt and glacial ice melt are important sources of PFAS to Lake Hazen. Comparing the net PFAS accumulation in Lake Hazen with an earlier sediment PFAS inventory14 indicates Lake Hazen sediments may only act as a long-term sink for a proportion of inputs, suggesting PFAS concentrations will continue to increase in its water column until a steady state is established. Although large, deep proglacial lakes are rare in the Arctic, the mass balance approach used here, based on a concerted assessment of inflow, outflow, and sediment inventories, can be extended to other landlocked lake systems to better understand the transport and fate dynamics of PFAS and other contaminants within those environments. The composition profiles of PFAS along the Skeleton Continuum suggest this region receives inputs from the atmosphere, snowmelt, and ice contained in permafrost soils. The attenuation of PFAS in downstream sites along the Skeleton Continuum suggests plants and soils in High Arctic wetlands remove and store PFAS from water, which may have important implications for mitigating PFAS exposure in Arctic biota inhabiting these regions, such as juvenile Arctic char.33 It is expected that continued climate warming in the Lake Hazen watershed will enhance permafrost and glacial inputs of PFAS into Lake Hazen and the export of PFAS to Arctic marine waters.While our study fills knowledge gaps on the importance of proglacial rivers as sources of PFAS to Lake Hazen, the role of melting glaciers on the transport and fate of PFAS in the watershed is not fully understood. Our earlier studies indicate that the delivery of glacial meltwaters creates underflows in Lake Hazen, mixing PFAS in the water column of the lake9 and promoting sedimentation.14 However, further research is required to discern the role of melting glaciers as a source or vector (i.e., entrainment of particle-bound PFAS from proglacial river channels) of PFAS in the Lake Hazen watershed. Our results also underscore the importance of permafrost soils as repositories of contaminants in the Arctic. While permafrost thaw contributes little to the water budget of Lake Hazen, it may become an important source of historically archived contaminants to freshwater and marine ecosystems in other Arctic regions with ubiquitous permafrost and high ground ice content, particularly under the influence of climate warming. Future research in the Lake Hazen watershed should focus on (1) the monitoring of PFAS along proglacial river transects (from glaciers to Lake Hazen) and along glaciers (e.g., meltwater streams), (2) exploring local PFAS sources, (3) the occurrence of PFAS in permafrost and wetland soils and vegetation in the meadow wetland, and (4) the monitoring of emerging PFAS (e.g., perfluoroalkyl ether carboxylic and sulfonic acids) and other organic pollutants of concern in the Lake Hazen watershed.
Conflicts of interest
The authors declare no competing interests.Acknowledgements
We would like to thank Jessica Serbu (University of Alberta, UA), Craig Emmerton (UA), Charlie Talbot (ECCC), Lisa Szostek (UA), and Pieter Aukes (University of Waterloo) for their assistance with sample collection, and Alex Gardner (NASA Jet Propulsion Laboratory, California Institute of Technology) for modelling the glacial runoff data. This project was funded by the Northern Contaminants Program (Indigenous and Northern Affairs Canada), the Natural Science and Engineering Research Council (NSERC), Polar Continental Shelf Program (Natural Resources Canada), and the W. Garfield Weston Foundation. NSERC and Vanier (KSP) are acknowledged for postgraduate scholarships.References
- R. C. Buck, J. Franklin, U. Berger, J. M. Conder, I. T. Cousins, P. de Voogt, A. A. Jensen, K. Kannan, S. A. Mabury and S. P. J. van Leeuwen, Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins, Integr. Environ. Assess. Manage., 2011, 7, 513–541 CrossRef CAS PubMed.
- Z. Wang, J. M. Boucher, M. Scheringer, I. T. Cousins and K. Hungerbühler, Toward a comprehensive global emission inventory of C4–C10 perfluoroalkanesulfonic acids (PFSAs) and related precursors: focus on the life cycle of C8-based products and ongoing industrial transition, Environ. Sci. Technol., 2017, 51, 4482–4493 CrossRef CAS PubMed.
- Z. Wang, I. T. Cousins, M. Scheringer, R. C. Buck and K. Hungerbühler, Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: production and emissions from quantifiable sources, Environ. Int., 2014, 70, 62–75 CrossRef CAS PubMed.
- L. W. Y. Yeung, C. Dassuncao, S. Mabury, E. M. Sunderland, X. Zhang and R. Lohmann, Vertical profiles, sources, and transport of PFASs in the Arctic Ocean, Environ. Sci. Technol., 2017, 51, 6735–6744 CrossRef CAS PubMed.
- J. S. Skaar, E. M. Ræder, J. L. Lyche, L. Ahrens and R. Kallenborn, Elucidation of contamination sources for poly- and perfluoroalkyl substances (PFASs) on Svalbard (Norwegian Arctic), Environ. Sci. Pollut. Res., 2019, 26, 7356–7363 CrossRef CAS PubMed.
- A. Cabrerizo, D. C. G. Muir, A. O. De Silva, X. Wang, S. F. Lamoureux and M. J. Lafrenière, Legacy and emerging persistent organic pollutants (POPs) in terrestrial compartments in the High Arctic: sorption and secondary sources, Environ. Sci. Technol., 2018, 52, 14187–14197 CrossRef CAS PubMed.
- J. Veillette, D. Muir, D. Antoniades, J. M. Small, C. Spencer, T. N. Loewen, J. A. Babaluk, J. D. Reist and W. F. Vincent, Perfluorinated chemicals in meromictic lakes on the northern coast of Ellesmere Island, High Arctic Canada, Arctic, 2012, 65, 245–256 CrossRef.
- G. L. Lescord, K. A. Kidd, A. O. De Silva, M. Williamson, C. Spencer, X. Wang and D. C. G. Muir, Perfluorinated and polyfluorinated compounds in lake food webs from the Canadian High Arctic, Environ. Sci. Technol., 2015, 49, 2694–2702 CrossRef CAS PubMed.
- J. J. MacInnis, I. Lehnherr, D. C. G. Muir, K. A. S. Pierre, V. L. S. Louis, C. Spencer and A. O. De Silva, Fate and transport of perfluoroalkyl substances from snowpacks into a lake in the High Arctic of Canada, Environ. Sci. Technol., 2019, 53, 10753–10762 CrossRef CAS PubMed.
- I. Lehnherr, V. L. S. Louis, M. Sharp, A. Gardner, J. Smol, S. Schiff, D. Muir, C. Mortimer, N. Michelutti, C. Tarnocai, K. S. Pierre, C. Emmerton, J. Wiklund, G. Köck, S. Lamoureux and C. Talbot, The world's largest High Arctic lake responds rapidly to climate warming, Nat. Commun., 2018, 9, 1–9 CrossRef CAS PubMed.
- K. A. S. Pierre, V. L. S. Louis, I. Lehnherr, A. S. Gardner, J. A. Serbu, C. A. Mortimer, D. C. G. Muir, J. A. Wiklund, D. Lemire, L. Szostek and C. Talbot, Drivers of mercury cycling in the rapidly changing glacierized watershed of the High Arctic's largest lake by Volume (Lake Hazen, Nunavut, Canada), Environ. Sci. Technol., 2019, 53, 1175–1185 CrossRef PubMed.
- K. A. S. Pierre, V. L. S. Louis, I. Lehnherr, S. L. Schiff, D. C. G. Muir, A. J. Poulain, J. P. Smol, C. Talbot, M. Ma, D. L. Findlay, W. J. Findlay, S. E. Arnott and A. S. Gardner, Contemporary limnology of the rapidly changing glacierized watershed of the world's largest High Arctic lake, Sci. Rep., 2019, 9, 1–15 Search PubMed.
- Y. Sun, A. O. De Silva, K. A. St Pierre, D. C. G. Muir, C. Spencer, I. Lehnherr and J. J. MacInnis, Glacial melt inputs of organophosphate ester flame retardants to the largest High Arctic lake, Environ. Sci. Technol., 2020, 54, 2734–2743 CrossRef CAS PubMed.
- J. J. MacInnis, I. Lehnherr, D. C. G. Muir, R. Quinlan and A. O. De Silva, Characterization of perfluoroalkyl substances in sediment cores from High and Low Arctic lakes in Canada, Sci. Total Environ., 2019, 666, 414–422 CrossRef CAS PubMed.
- G. Köck, D. Muir, F. Yang, X. Wang, C. Talbot, N. Gantner and D. Moser, Bathymetry and sediment geochemistry of Lake Hazen (Quttinirpaaq National Park, Ellesmere Island, Nunavut), Arctic, 2012, 65, 56–66 CrossRef.
- C. A. Emmerton, V. L. S. Louis, I. Lehnherr, J. A. Graydon, J. L. Kirk and K. J. Rondeau, The importance of freshwater systems to the net atmospheric exchange of carbon dioxide and methane with a rapidly changing high Arctic watershed, Biogeosciences, 2016, 13, 5849–5863 CrossRef CAS.
- S. Varty, I. Lehnherr, K. S. Pierre, J. Kirk and V. Wisniewski, Methylmercury transport and fate shows strong seasonal and spatial variability along a High Arctic freshwater hydrologic continuum, Environ. Sci. Technol., 2021, 55, 331–340 CrossRef CAS PubMed.
- J. J. MacInnis, K. French, D. C. G. Muir, C. Spencer, A. Criscitiello, A. O. De Silva and C. J. Young, Emerging investigator series: a 14-year depositional ice record of perfluoroalkyl substances in the High Arctic, Environ. Sci.: Processes Impacts, 2017, 19, 22–30 RSC.
- H. M. Pickard, A. Criscitiello, C. Spencer, M. J. Sharp, D. C. G. Muir, A. O. De Silva and C. J. Young, Continuous non-marine inputs of per- and polyfluoroalkyl substances to the high Arctic: a multi-decadal temporal record, Atmos. Chem. Phys., 2018, 18, 5045–5058 CrossRef CAS.
- M. Chen, C. Wang, X. Wang, J. Fu, P. Gong, J. Yan, Z. Yu, F. Yan and J. Nawab, Release of perfluoroalkyl substances from melting glacier of the Tibetan Plateau: insights into the impact of global warming on the cycling of emerging pollutants, J. Geophys. Res.: Atmos., 2019, 124, 7442–7456 Search PubMed.
- M. M. Plassmann, T. Meyer, Y. D. Lei, F. Wania, M. S. McLachlan and U. Berger, Laboratory studies on the fate of perfluoroalkyl carboxylates and sulfonates during snowmelt, Environ. Sci. Technol., 2011, 45, 6872–6878 CrossRef CAS PubMed.
- J. Fabregat-Palau, M. Vidal and A. Rigol, Modelling the sorption behaviour of perfluoroalkyl carboxylates and perfluoroalkane sulfonates in soils, Sci. Total Environ., 2021, 801, 149343 CrossRef CAS PubMed.
- Norwegian Environment Agency, Investigation of Sources to PFHxS in the Environment Investigation of Sources to PFHxS in the Environment Final Report, Oslo, Norway, 2018 Search PubMed.
- J. M. Boucher, I. T. Cousins, M. Scheringer, K. Hungerbühler and Z. Wang, Toward a comprehensive global emission inventory of C4–C10 perfluoroalkanesulfonic acids (PFSAs) and related precursors: focus on the life cycle of C6- and C10-based products, Environ. Sci. Technol. Lett., 2019, 6, 1–7 CrossRef CAS.
- J. P. Benskin, A. O. De Silva and J. W. Martin, Isomer profiling of perfluorinated substances as a tool for source tracking: A review of early findings and future applications, Reviews of Environmental Contamination and Toxicology: Perfluorinated Alkylated Substances, 2010, pp. 111–154 Search PubMed.
- L. A. D'Agostino and S. A. Mabury, Certain perfluoroalkyl and polyfluoroalkyl substances associated with aqueous film forming foam are widespread in Canadian surface waters, Environ. Sci. Technol., 2017, 51, 13603–13613 CrossRef PubMed.
- F. Wong, M. Shoeib, A. Katsoyiannis, S. Eckhardt, A. Stohl, P. Bohlin-Nizzetto, H. Li, P. Fellin, Y. Su and H. Hung, Assessing temporal trends and source regions of per- and polyfluoroalkyl substances (PFASs) in air under the Arctic Monitoring and Assessment Programme (AMAP), Atmos. Environ., 2018, 172, 65–73 CrossRef CAS.
- Z. Xie, Z. Wang, W. Mi, A. Möller, H. Wolschke and R. Ebinghaus, Neutral Poly-/perfluoroalkyl Substances in Air and Snow from the Arctic, Sci. Rep., 2015, 5, 1–6 Search PubMed.
- M. Filipovic, H. Laudon, M. S. McLachlan and U. Berger, Mass balance of perfluorinated alkyl acids in a pristine boreal catchment, Environ. Sci. Technol., 2015, 49, 12127–12135 CrossRef CAS PubMed.
- C. E. Müller, G. H. Lefevre, A. E. Timofte, F. A. Hussain, E. S. Sattely and R. G. Luthy, Competing mechanisms for perfluoroalkyl acid accumulation in plants revealed using an Arabidopsis model system, Environ. Toxicol. Chem., 2016, 35, 1138–1147 CrossRef PubMed.
- Y.-C. Chen, S.-L. Lo and Y.-C. Lee, Distribution and fate of perfluorinated compounds (PFCs) in a pilot constructed wetland, Desalin. Water Treat., 2012, 37, 178–184 CrossRef CAS.
- T. Yin, H. Chen, M. Reinhard, X. Yi, Y. He and K. Y. H. Gin, Perfluoroalkyl and polyfluoroalkyl substances removal in a full-scale tropical constructed wetland system treating landfill leachate, Water Res., 2017, 125, 418–426 CrossRef CAS PubMed.
- R. N. Sinnatamby, J. A. Babaluk, G. Power, J. D. Reist and M. Power, Summer habitat use and feeding of juvenile Arctic charr, Salvelinus alpinus, in the Canadian High Arctic, Ecol. Freshw. Fish, 2012, 21, 309–322 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Additional details on QA/QC parameters, study areas, permafrost dynamics, correlation analysis, and PFAS concentration data. See DOI: 10.1039/d1em00349f |
| This journal is © The Royal Society of Chemistry 2022 |