Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Removal of 293 organic compounds in 15 WWTPs studied with non-targeted suspect screening†
Michael S.
McLachlan
 *a,
Zhe
Li
*a,
Zhe
Li
 a,
Lisa
Jonsson
a,
Lisa
Jonsson
 a,
Sarit
Kaserzon
a,
Sarit
Kaserzon
 b,
Jake W.
O'Brien
b,
Jake W.
O'Brien
 b and
Jochen F.
Mueller
b
b and
Jochen F.
Mueller
b
aDepartment of Environmental Science, Stockholm University, SE-106 91 Stockholm, Sweden. E-mail: michael.mclachlan@aces.su.se
bThe University of Queensland, Queensland Alliance for Environmental Health Sciences (QAEHS), 20 Cornwall Street, Woolloongabba, QLD 4102, Australia
First published on 13th May 2022
Abstract
Understanding how contaminant breakthrough in wastewater treatment plants is influenced by chemical structure and treatment technology is important for protecting the aquatic environment. In order to assess this question, consistent contaminant breakthrough measurements are required for a large number of chemicals. Using direct injection UHPLC-Orbitrap-MS/MS with data-dependent non-target data acquisition followed by suspect screening against a library of >7000 compounds with exact mass and MS2 spectra, we quantified the removal of 293 chemicals in 15 WWTPs with widely varying treatment technology. Principle component analysis showed a clear and consistent influence of treatment technology on contaminant breakthrough. Log breakthrough was significantly correlated with log TSS and log BOD in treated effluent for 71% and 68% of the chemicals, respectively. Chemicals were identified which could be used as indicators of the standard of wastewater treatment. Furthermore, chemicals were identified that could be used to predict the breakthrough of groups of other chemicals. A high degree of correlation was found for the breakthrough of different groups of chemicals, which suggests that the data could be used to develop models describing how chemical structure influences breakthrough or removal efficiency. Non-targeted suspect screening is a useful method for generating consistent WWTP breakthrough data for large numbers of chemicals.
Water impactUnderstanding the relationship between chemical structure and WWTP breakthrough is essential for designing chemicals that can be used sustainably and for building WWTPs to a standard that protects the environment. To achieve this understanding large internally consistent datasets are required. This paper shows how such datasets can be generated with modern analytical techniques. |
Introduction
Wastewater treatment plants (WWTPs) act as filters to restrict leakage of society's waste into the natural environment. A range of treatment technologies are used in WWTPs depending on the nature of the wastewater, local conditions and the availability of resources to build and operate the facilities. The effectiveness of WWTPs is typically monitored using bulk indicators of effluent water quality such as total suspended solids (TSS), biological oxygen demand (BOD), and the total nitrogen and phosphorus content. This reflects the traditional focus of WWTPs, namely the treatment of human waste. However, with the discovery of the prevalence of many organic contaminants such as antibiotics1 and pharmaceuticals2 in treated WWTP effluent over the past 25 years, interest has grown in understanding the effectiveness of WWTPs at removing specific organic chemicals. In Switzerland, this has resulted in the implementation of advanced wastewater treatment with ozonation or activated carbon, and an average removal efficiency requirement of 80% for trace organic contaminants in the WWTP.3During the last 20 years a tremendous amount was learned about the behavior of organic contaminants in WWTPs. Sorption, biodegradation and volatilization are the primary elimination processes in plants with conventional treatment, whereby volatilization is only relevant for volatile substances. Photodegradation can also play a role when maturation lagoons or wetlands are part of the treatment.4 Advanced treatment technologies such as treatment with activated carbon and ozonation can further enhance the elimination of some substances.5–7 Many studies have looked at removal between WWTP influent and effluent, but some studies have quantified removal during primary and secondary treatment separately.8,9 Extensive work has been done to understand the effectiveness of advanced treatment methods,7,10–12 and integrated assessment strategies have been developed that go beyond chemical removal to include transformation product formation, different kinds of biological activity, and antibiotic resistant genes.13 In addition to the influence of treatment technology, the influence of environmental variables such as season has been studied.9,14,15
There has been less progress in developing a broad understanding of the influence of chemical structure on contaminant removal, most particularly during biodegradation. Data for a large number of chemicals is required to develop predictive models of the influence of chemical structure on removal that have a reasonably large applicability domain. The chemical-rich studies measuring WWTP removal efficiency often report data for just 20–50 substances,14,16–18 with a few notable exceptions reporting upwards of 100.11 In an interesting recent development, non-target screening using high-resolution accurate mass spectrometry has been used to evaluate the removal of chemical features in WWTPs.19,20 However, this work does not alleviate the need for chemical-rich data sets because it does not provide information on chemical structure. To overcome the limited chemical domain of individual studies, one can combine data sets from different studies.21,22 However, variability in conditions between studies introduces uncertainty in the relative removal efficiency of different chemicals.
In this work the goal was to generate a dataset of measured treatment efficiency for a much larger number of chemicals in a collection of WWTPs with a broad range of treatment technologies. We did this using a non-targeted suspect screening method that we have employed successfully to quantify organic contaminant breakthrough in WWTPs.23 We use the data to explore whether there are systematic relationships between wastewater treatment technology and organic contaminant breakthrough, and to what extent contaminant breakthrough can be predicted using bulk indicators of treatment efficiency. Furthermore, we identify chemicals that can serve as indicators of the successful application of a given wastewater treatment technology, and we identify groups of chemicals for which contaminant breakthrough is highly correlated.
Methods
Sample collection
The samples were collected on August 9, 2016 as part of the Australian national wastewater monitoring program (SewAus 2016). In this program samples were collected from municipal WWTPs across Australia during census week. WWTP operators were provided with detailed sampling instructions to optimize sampling based on their specific sampling capabilities,24 and completed a sampling protocol as well as a questionnaire on the characteristics of the WWTP and the operating conditions during the sampling period.25 15 WWTPs were selected to represent a diversity of treatment technologies ranging from lagoons to advanced treatment with ozonation and microfiltration (Table 1). All WWTPs were operating under dry flow conditions.| SITE | Treatment standard | BOD5a | Phosphorousa | Nitrogena | TSSa |
|---|---|---|---|---|---|
| a In mg L−1. b Did not meet inclusion criterion of a hydraulic residence time ≤ 25 h. c Ozone concentration 8 mg L−1 before biological media filter, 5 mg L−1 after biological media filter. | |||||
| Primary treatment only or anaerobic lagoon | |||||
| S10 | Primary sedimentation | 96 | 2.9 | 44.9 | 57 |
| S66b | High rate anaerobic lagoon | 6 | 5.4 | 6.7 | 18 |
| Trickling filter | |||||
| S39 | Primary clarification, trickling filter | 20 | 7 | 24 | 32 |
| S53 | Primary clarification & trickling filter in parallel with activated sludge with extended aeration (equal capacities), disinfection (chlorination) | 14.5 | 4.9 | 17.9 | 20 |
| Activated sludge (AS) | |||||
| S25 | Activated biofilter tower followed by activated sludge | 32 | 1.7 | 22.5 | 22 |
| S67b | Primary clarification, combination of activated sludge and lagoons | 6 | 7.9 | 23 | 15 |
| Activated sludge with nitrification/denitrification (AS-N/DN) | |||||
| S2 | Activated sludge with nitrification/denitrification A2O (4 lines) and oxidation ditch (2 lines), disinfection (chlorination) | 2.5 | 0.56 | 3.2 | 5.2 |
| S5 | Primary sedimentation, activated sludge with nitrification/denitrification (5-stage Bardenpho bioreactor) | 2.5 | 5.2 | 5 | 10 |
| S11 | Activated sludge with nitrification/denitrification (oxidation ditch) | 2.5 | 2.4 | 3.1 | |
| S24 | Activated sludge with nitrification/denitrification (modified Johannesburg), submerged membrane filtration | 3.2 | 0.12 | 3.4 | 3.4 |
| S40 | Activated sludge with nitrification/denitrification (modified Johannesburg), UV-disinfection | 3 | 0.11 | 1.8 | 4 |
| Advanced treatment – filtration | |||||
| S9 | Primary sedimentation, activated sludge with nitrification/denitrification (modified Ludzack–Ettinger (MLE) process), filter coal/sand filtration, disinfection (chlorination) | <2 | 0.06 | 12.3 | 0.7 |
| S29 | Sequence batch reactor, filter coal/sand filtration | <5 | 1.6 | 6.6 | 2 |
| S51 | Activated sludge with nitrification/denitrification (Denipho), granular activated carbon filtration, microfiltration, UV-disinfection | <2 | <0.05 | 1.44 | <2 |
| Advanced treatment – ozonation | |||||
| S1 | Primary sedimentation, activated sludge, ozonationc, biological media filtration, UV disinfection, chlorination | 6 | 7.8 | 13 | 0.5 |
WWTP influent and effluent samples were collected over 24 h with autosamplers using either flow proportional or time proportional sampling (with the exception of S66, which supplied a grab sample for the effluent). The samples were transferred to high density polyethylene (HDPE) bottles that had been pre-rinsed with methanol and MilliQ water, frozen at −20 °C, shipped frozen to the Queensland Alliance of Environmental Health Sciences laboratories at the University of Queensland, Australia, and later after preparation to Stockholm University, where they were stored at −18 °C until analysis.
Chemicals
Isotope-labeled standards (see Table S1†) were purchased from Novachem, VIC, Australia. LC/MS-grade acetonitrile, methanol and sodium hydroxide were purchased from VWR (Stockholm, Sweden). LC/MS-grade formic acid and sulfuric acid were purchased from Sigma-Aldrich (Steinheim, Germany). Milli-Q water was produced by a Milli-Q Integral Water Purification System (Merck Millipore, Stockholm, Sweden).Sample preparation
The samples were prepared and analysed in triplicate according to the method of Li et al.23 A sample volume of 1 mL was spiked with a mixture of the isotope-labeled standards (50 ng of each compound). Each sample was then filtered through a 0.45 μm PTFE syringe filter directly into a glass LC-vial. Prepared samples were stored frozen until analysis.UHPLC-Orbitrap-MS/MS analysis
Triplicate samples were analyzed with ultrahigh performance liquid chromatography coupled to a Q Exactive™ HF Hybrid Quadrupole-Orbitrap™ mass spectrometer (UHPLC-Orbitrap-MS/MS, Thermo Fisher Scientific, San Jose, USA) using electrospray ionization (ESI). The samples were injected in both ESI positive mode and ESI negative mode. The injection volume was 10 μL. Separation was achieved with a reverse-phase Hypersil GOLD™ aQ C18 polar-endcapped column (2.1 mm × 100 mm; particle size of 1.9 μm; Thermo Fisher Scientific, San Jose, USA) using a binary mobile phase gradient consisting of (A) water and (B) acetonitrile, both containing 0.1% formic acid. Details of the liquid chromatography program are provided in Table S2.† The Orbitrap-MS/MS was operated in the data-dependent acquisition (top N) mode. Mass accuracy calibration was performed regularly. Detailed information about the instrumental analysis is provided in Li et al.23HRMS data processing workflow
The data were processed using Compound Discoverer 2.1 (Thermo Fisher Scientific). The data processing workflow consisted of peak picking and integration, retention time alignment, unknown compound detection, isotope and adduct peak grouping, unknown compound grouping, blank subtraction (using blank samples prepared in Milli-Q water and methanol), and database searching (see the ESI† in Li et al.23 for relevant parameters). The workflow provided level 2 confidence in the identity of the features.26 Data acceptance criteria included detection in all of the triplicate samples and a coefficient of variation of the signal intensity <30%. The mzCloud database of accurate mass MS/MS spectra was used for annotating features. At the time of data processing (April, 2018), the database contained over 7000 compounds.Treatment efficiency was evaluated using the metric contaminant breakthrough (B), expressed in percent. B was calculated as the quotient of the contaminant concentration in effluent to that in influent. Following the method of Li et al.,23 the concentration quotient was approximated as the quotient of the peak area of the analyte in effluent and influent.
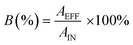 | (1) |
Chemical properties
The pKa of the identified chemicals was estimated using the Chemicalize work package.27 This pKa was used in the Henderson–Hasselbalch equation to estimate the fraction of the chemical ionized for the range of pH (6.5–7) reported in the WWTPs studied. The STPWIN model in EPI Suite™ was used to estimate the fraction of the neutral form of the chemical entering a WWTP that is removed to sludge.28Results
Quality assurance
In applying eqn (1) to calculate B, it is assumed that the response factor for the chemical is the same in influent and effluent. To test this assumption, the quotient of peak area of the labeled standards in influent and effluent was calculated. Since the same quantity of internal standard was injected in the influent and effluent samples, this quotient would equal 1 if the assumption was correct. All of the labeled standards but ibuprofen-D3 were found in both the influent and effluent samples in all 15 WWTPs, and in most cases the quotient was close to 1 (Fig. 1). Only 3 of 23 isotope-labelled standards (cotinine, fluoxetine and paracetamol) had a median quotient outside of the range 0.8–1.2. Median quotients of 0.55–0.65 for these chemicals indicated that there was more signal suppression in the influent than in the effluent. Hence, B would be overestimated by as much as a factor 2 for these 3 chemicals, while for the remaining 20 chemicals the uncertainty should be <20%. These results are consistent with our previous work on a Swedish WWTP, where 33 of 40 internal standards had quotients in the range 0.75–1.25.23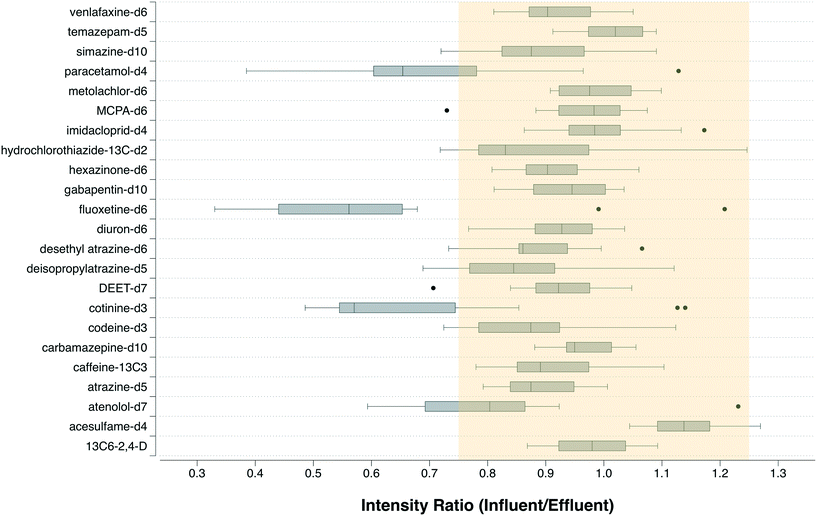 | ||
| Fig. 1 Ratio of the intensity of the signals for isotope-labeled internal standards in influent and effluent, shown as a box whisker plot of paired influent/effluent samples from 15 WWTPs. | ||
Measured contaminant breakthrough
Breakthrough was quantifiable in at least one WWTP for 361 different chemicals. In order to generate a dataset suitable for exploring the influence of WWTP technology on breakthrough, two criteria for chemical inclusion were defined. First, breakthrough data were required for at least 80% (12) of the WWTPs to reduce the likelihood of data gaps biasing the results. Second, the 75th percentile of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B had to be <2.2 (corresponding to B < 158%) to exclude chemicals that were clearly formed during treatment. A total of 293 chemicals fulfilled these criteria. Furthermore, for most of the quantitative analysis we applied a WWTP inclusion criterion of a hydraulic retention time ≤25 h. This was to exclude WWTPs for which there was high uncertainty as to whether the influent and effluent samples were matched. Two WWTPs, S66 and S67, with hydraulic retention times of 10 days and >60 days, respectively, were excluded from most of the quantitative analysis by this criterion (but included in some simpler comparisons).
B had to be <2.2 (corresponding to B < 158%) to exclude chemicals that were clearly formed during treatment. A total of 293 chemicals fulfilled these criteria. Furthermore, for most of the quantitative analysis we applied a WWTP inclusion criterion of a hydraulic retention time ≤25 h. This was to exclude WWTPs for which there was high uncertainty as to whether the influent and effluent samples were matched. Two WWTPs, S66 and S67, with hydraulic retention times of 10 days and >60 days, respectively, were excluded from most of the quantitative analysis by this criterion (but included in some simpler comparisons).
Of the 293 chemicals, 168 were estimated to be >50% ionized in the WWTP. Of the 125 chemicals that were not primarily present in the ionized form, only five were predicted by STPWIN to have a removal to sludge >5%: docosahexaenoic acid ethyl ester (22%), butylparaben (6%), ethyl palmitoleate (22%), palmiotyl ethanolamide (21%) and tributyl phosphate (11% removal to sludge). Considering all chemicals, there were weak and generally positive correlations between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B and log removal of the neutral form to sludge for the individual WWTPs (Pearson's r from −0.03 to 0.27). This suggests that the removal of the chemicals studied was controlled by processes other than sequestration to biosolids.
B and log removal of the neutral form to sludge for the individual WWTPs (Pearson's r from −0.03 to 0.27). This suggests that the removal of the chemicals studied was controlled by processes other than sequestration to biosolids.
Contaminant breakthrough versus WWTP technology
An initial indication of the influence of WWTP technology on contaminant breakthrough is provided by the summary statistics of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B for the 293 chemicals (Fig. S1†). Five WWTPs (S10, S25, S39, S53 and S66) had 75th percentiles of log
B for the 293 chemicals (Fig. S1†). Five WWTPs (S10, S25, S39, S53 and S66) had 75th percentiles of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B in excess of 1.65, i.e., at least 25% of the chemicals had a breakthrough in excess of 45%. The medians and 25th percentiles for these WWTPs were also elevated compared to the others. These WWTPs had the simplest treatment technologies of the studied plants. In order of decreasing median B, S10 had only primary treatment, S66 aerated lagoons, S39 trickling filter, S53 trickling filter and activated sludge in parallel, and S25 an activated biofilter tower followed by activated sludge. Hence, median B was inversely correlated with the sophistication of the treatment. The summary statistics showed smaller differences for the remaining 10 WWTPs (Fig. S1†).
B in excess of 1.65, i.e., at least 25% of the chemicals had a breakthrough in excess of 45%. The medians and 25th percentiles for these WWTPs were also elevated compared to the others. These WWTPs had the simplest treatment technologies of the studied plants. In order of decreasing median B, S10 had only primary treatment, S66 aerated lagoons, S39 trickling filter, S53 trickling filter and activated sludge in parallel, and S25 an activated biofilter tower followed by activated sludge. Hence, median B was inversely correlated with the sophistication of the treatment. The summary statistics showed smaller differences for the remaining 10 WWTPs (Fig. S1†).
To further explore the influence of WWTP technology, the difference between measured log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B and the mean log
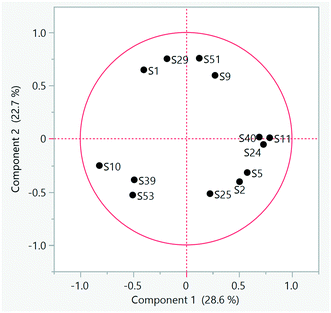
B and the mean log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B of that chemical across all WWTPs was calculated. PCA analysis was then applied to the differences (JMP 16.0.0). The 13 WWTPs separated into 3 distinct groups (Fig. 2): group 1 (S1, S9, S29 and S51) was placed in the upper portion of the loading plot, group 2 (S2, S5, S11, S24, S25, S40) in the lower right quadrant, and group 3 in the lower left quadrant (S10, S39, S53). Group 1 consisted of the 4 WWTPs with advanced treatment (S1 with ozonation, S9, S29 and S51 with filtration), group 2 included all of the WWTPs using activated sludge with nitrification/denitrification (AS-N/DN), while Group 3 WTTPs had either no secondary treatment (S10) or a trickling filter (S39 and S53). Group 3 largely coincided with the WWTPs showing high B based on summary statistics. S25 was the exception; it belonged to group 2 but was the member of that group lying closest to group 3 (Fig. 2). S25 employed an activated biofilter tower followed by an activated sludge stage.
B of that chemical across all WWTPs was calculated. PCA analysis was then applied to the differences (JMP 16.0.0). The 13 WWTPs separated into 3 distinct groups (Fig. 2): group 1 (S1, S9, S29 and S51) was placed in the upper portion of the loading plot, group 2 (S2, S5, S11, S24, S25, S40) in the lower right quadrant, and group 3 in the lower left quadrant (S10, S39, S53). Group 1 consisted of the 4 WWTPs with advanced treatment (S1 with ozonation, S9, S29 and S51 with filtration), group 2 included all of the WWTPs using activated sludge with nitrification/denitrification (AS-N/DN), while Group 3 WTTPs had either no secondary treatment (S10) or a trickling filter (S39 and S53). Group 3 largely coincided with the WWTPs showing high B based on summary statistics. S25 was the exception; it belonged to group 2 but was the member of that group lying closest to group 3 (Fig. 2). S25 employed an activated biofilter tower followed by an activated sludge stage.
The removal efficiency (= 1–B) of the 5 WWTPs using AS-N/DN (group 2 without S25) was compared with removal efficiencies for WWTPs with similar technology in the literature (Table S3†). Good agreement was found for most substances, which provides confidence in the results generated with this method. Some chemicals (e.g., caffeine, acetaminophen) were almost completely removed in all WWTPs. Others (e.g., codeine, trimethoprim) showed greater removal in WWTPs with denitrification/nitrification than in those without it. For some of the chemicals with negligible removal efficiency in this study (e.g. citalopram, venlafaxine, carbamazepine), some other studies report removal of 25% or more. However, for these same chemicals this study agreed well with the comprehensive work of Bourgin et al.11 Differences between the studies could be due to differences in sampling design (e.g., Bourgin et al. only studied removal in the biological treatment portion of the WWTP while our work studied removal across the entire WWTP), differences in sampling method, differences in the analytical procedure and related quality assurance, and differences in the actual removal efficiency in the WWTP.
In summary, there was a clear relationship between contaminant breakthrough and treatment technology. Breakthrough was reduced in the order primary treatment > trickling filter > AS-N/DN > advanced treatment (ozone or filtration).
Prediction of breakthrough with WWTP performance parameters
We tested whether it was possible to predict contaminant breakthrough from standard water quality parameters measured in the WWTP effluent, which were provided by most of the WWTPs (Table 1). For each chemical, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B was regressed against log BOD, log TSS, log P (total phosphorus), and log N (total nitrogen) for the 13 WWTPs fulfilling the inclusion criterion. Where a water quality parameter was reported as <x, x/2 was used.
B was regressed against log BOD, log TSS, log P (total phosphorus), and log N (total nitrogen) for the 13 WWTPs fulfilling the inclusion criterion. Where a water quality parameter was reported as <x, x/2 was used.
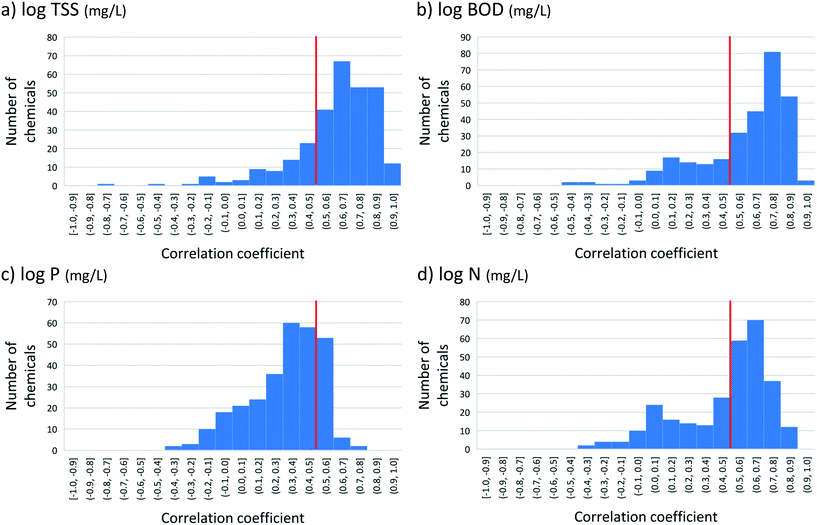
For log BOD, log TSS and log N, strong regressions were obtained for most of the 293 chemicals (Fig. 3). The best results were obtained for log TSS, which was also the variable showing the most uniform distribution across the reported range (Table 1). The correlations for 71% of the chemicals were significant at the 5% level, and 77% had correlation coefficients >0.5. This indicates that TSS was a reasonable predictor of B for the majority of the chemicals. Similar results were obtained for BOD (68% significant, 73% r > 0.5), while the results were considerably poorer for N (41%/61%). P was a poor predictor of B for most of the chemicals (21%/11%, Fig. 3). This is perhaps not surprising since the removal of BOD and to a lesser extent TSS and N reflects the quality of the biological treatment, and most of the organic contaminants here were likely removed via biotransformation. P, on the other hand, is often primarily removed via chemical treatment, and chemical treatment is likely to have little effect on the removal of most organic contaminants.
We also regressed the log of the chemical signal in the effluent against the same standard water quality parameters. Similar results were obtained to the regressions with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B (compare Fig. 3 and S2†). This can be attributed to the comparatively low inter-WWTP variability in the signal in influent compared to effluent.
B (compare Fig. 3 and S2†). This can be attributed to the comparatively low inter-WWTP variability in the signal in influent compared to effluent.
It is instructive to more closely examine these regressions. Cotinine is an example of a chemical for which log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B exhibits a strong correlation with log BOD; B ranges from 1% in WWTPs with advanced treatment (filtration) to 81% in the WWTP with only primary treatment (Fig. 4a). Other chemicals such as ethyl palmitoleate show low B (<5%) in all WWTPs but still a weak correlation with BOD (Fig. 4b). Leucylproline and dodecyl sulfate are examples of chemicals that are removed very effectively in WWTPs using AS-N/DN, but show high B in WWTPs that do not have this technology (Fig. 4c and d). Yet other chemicals such as tramadol show high B in all WWTPs except some of those with advanced treatment (Fig. 4e). Finally, there are some substances such as N,N′-diphenylguanidine (used in the vulcanization of rubber) that are not effectively removed in any of the WWTPs (Fig. 4f). This shows that B is not always a continuous function of the effluent water quality parameters; for some chemicals the relationship is better characterized by a step function where a particular technology leads to a marked reduction in breakthrough.
B exhibits a strong correlation with log BOD; B ranges from 1% in WWTPs with advanced treatment (filtration) to 81% in the WWTP with only primary treatment (Fig. 4a). Other chemicals such as ethyl palmitoleate show low B (<5%) in all WWTPs but still a weak correlation with BOD (Fig. 4b). Leucylproline and dodecyl sulfate are examples of chemicals that are removed very effectively in WWTPs using AS-N/DN, but show high B in WWTPs that do not have this technology (Fig. 4c and d). Yet other chemicals such as tramadol show high B in all WWTPs except some of those with advanced treatment (Fig. 4e). Finally, there are some substances such as N,N′-diphenylguanidine (used in the vulcanization of rubber) that are not effectively removed in any of the WWTPs (Fig. 4f). This shows that B is not always a continuous function of the effluent water quality parameters; for some chemicals the relationship is better characterized by a step function where a particular technology leads to a marked reduction in breakthrough.
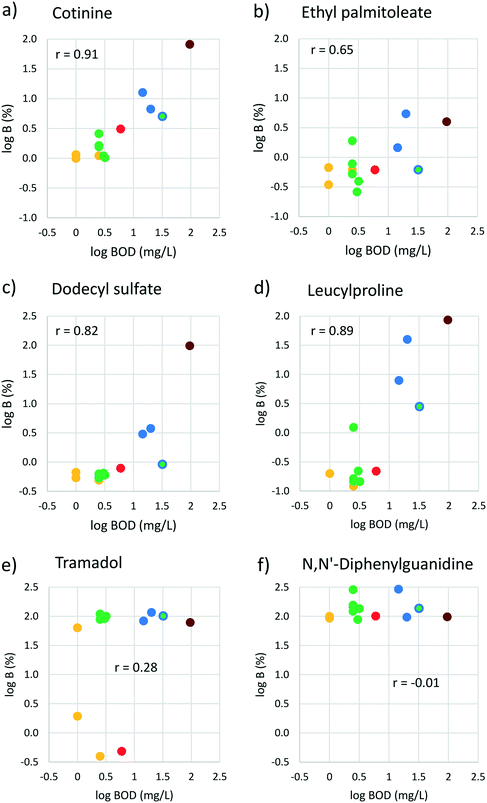 | ||
Fig. 4 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B versus log BOD plotted for 6 example chemicals. The correlation coefficient r is also shown. The colors indicate the treatment standard (see Table 1): red = ozonation; yellow = filtration; green = activated sludge with nitrification/denitrification; blue around green = biofilter/activated sludge; blue = trickling filter; brown = primary treatment. B versus log BOD plotted for 6 example chemicals. The correlation coefficient r is also shown. The colors indicate the treatment standard (see Table 1): red = ozonation; yellow = filtration; green = activated sludge with nitrification/denitrification; blue around green = biofilter/activated sludge; blue = trickling filter; brown = primary treatment. | ||
Indicator chemicals
Indicator chemicals for the effectiveness of wastewater treatment can be useful for setting treatment standards or goals and for monitoring WWTP performance, as has been done in Switzerland.29,30 We used our dataset to identify potential indicator chemicals, looking specifically for indicators of trickling filter quality treatment (compared to primary treatment only), AS-N/DN quality treatment (compared to trickling filter), advanced treatment with filtration, and advanced treatment with ozonation (each compared to AS-N/DN). For each case we extracted subsets of WWTPs that represented the two technologies to be compared (e.g., S10, S39 and S53 for comparing primary treatment only with trickling filter treatment). Within this subset of WWTPs we used simple statistics to identify chemicals that showed pronounced differences in log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B between the two treatment technologies. When a chemical showed a consistent separation in log
B between the two treatment technologies. When a chemical showed a consistent separation in log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B between the two technologies being compared, then a value of log
B between the two technologies being compared, then a value of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B between the values observed for the two technologies was selected as a breakthrough threshold.
B between the values observed for the two technologies was selected as a breakthrough threshold.
Chemicals can also be used as indicators for the breakthrough of other chemicals. This is possible when the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B of chemicals is correlated across WWTPs using a range of treatment technologies. Each of the cases introduced above represents a different pattern of breakthrough behavior among the WWTPs studied. For each of the cases we identify chemicals showing the case-specific behavior and, when possible, identify indicator chemicals that predict log
B of chemicals is correlated across WWTPs using a range of treatment technologies. Each of the cases introduced above represents a different pattern of breakthrough behavior among the WWTPs studied. For each of the cases we identify chemicals showing the case-specific behavior and, when possible, identify indicator chemicals that predict log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B of other chemicals in the group well.
B of other chemicals in the group well.
In the comparison of the two trickling filter WWTPs (S39 and S53) with the WWTP using primary treatment only (S10), many chemicals showed a pattern with close to 100% breakthrough in the primary WWTP and 2–10% breakthrough in the trickling filter WWTPs. Fig. S3† shows several of these which could be suitable indicator chemicals for trickling water treatment. They have breakthrough thresholds ranging from 10–20% (Table 2). If the breakthrough of these chemicals in a WWTP was less than the respective breakthrough threshold, this would indicate that the WWTP had (at least) trickling filter quality treatment.
| Chemical | Origin/major use | B threshold for respective treatment standard (%) | ||
|---|---|---|---|---|
| Trickling filter quality secondary treatment | AS-N/DN quality secondary treatment | Advanced treatment | ||
| 4-Pyridoxic acid | Vitamin B6 metabolite | 20 | 3 | |
| Paracetamol | Painkiller | 10 | ||
| Dodecyl sulfate | Surfactant | 10 | 1 | |
| Paraxanthine | Caffeine metabolite | 10 | 1 | |
| PEG n6 | Personal care products | 20 | ||
| Methylimidazole acetic acid | Histamine metabolite | 10 | ||
| trans-Zeatin | Plants | 30 | ||
| Acetophenone | Fragrances, food | 5 | ||
| Meprylcaine | Anesthetic | 25 | ||
| Levetiracetam | Antiepileptic | 10 | ||
| L-Phenylalanine | Essential amino acid, food supplement | 2 | ||
| Acesulfame | Sweetener | 10 | ||
| Tapentadol | Opioid analgesic | 10 | ||
| Desacetyl diltiazem | Metabolite of diltiazem | 10 | ||
Many of the chemicals displaying the above behavior also had lower, relatively uniform breakthrough in the more advanced WWTPs (Fig. S3†). The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bs of these chemicals were frequently highly correlated with each other across the different WWTPs. To identify the best indicator chemical for this kind of behavior, we searched for the chemical that was highly correlated (Pearson correlation coefficient >0.9) with the largest number of other chemicals. This was 4-pyridoxic acid, a urinary metabolite of vitamin B6, which was highly correlated with 48 other chemicals (see Fig. S3† for its log
Bs of these chemicals were frequently highly correlated with each other across the different WWTPs. To identify the best indicator chemical for this kind of behavior, we searched for the chemical that was highly correlated (Pearson correlation coefficient >0.9) with the largest number of other chemicals. This was 4-pyridoxic acid, a urinary metabolite of vitamin B6, which was highly correlated with 48 other chemicals (see Fig. S3† for its log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B in the different WWTPs, Fig. S4† for some example correlations, and Table S4† for a list of the chemicals and correlation coefficients as well as the intercepts and slopes of the linear regression against log
B in the different WWTPs, Fig. S4† for some example correlations, and Table S4† for a list of the chemicals and correlation coefficients as well as the intercepts and slopes of the linear regression against log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B of 4-pyridoxic acid). These chemicals (Table S4†), which are characterized by very good abatement in WWTPs using AS-N/DN (B < 2%), satisfactory removal in trickling filter plants (B ≈ 2–10%) and negligible removal in WWTPs with primary treatment only, include pharmaceuticals (e.g., mesalamine, paracetamol), synthetic surfactants (e.g., dodecyl sulfate), food chemicals and their metabolites (e.g., caffeine, paraxanthine, theobromine), and chemicals in personal care products (e.g., different polyethylene glycols (PEGs)), but also other natural compounds. For all of these chemicals including 4-pyridoxic acid, log
B of 4-pyridoxic acid). These chemicals (Table S4†), which are characterized by very good abatement in WWTPs using AS-N/DN (B < 2%), satisfactory removal in trickling filter plants (B ≈ 2–10%) and negligible removal in WWTPs with primary treatment only, include pharmaceuticals (e.g., mesalamine, paracetamol), synthetic surfactants (e.g., dodecyl sulfate), food chemicals and their metabolites (e.g., caffeine, paraxanthine, theobromine), and chemicals in personal care products (e.g., different polyethylene glycols (PEGs)), but also other natural compounds. For all of these chemicals including 4-pyridoxic acid, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B correlated well with log BOD (r > 0.6), the traditional indicator of biological treatment effectiveness.
B correlated well with log BOD (r > 0.6), the traditional indicator of biological treatment effectiveness.
To identify indicator chemicals and breakthrough thresholds for a treatment standard corresponding to AS-N/DN, we compared the 5 WWTPs having this treatment standard (S2, S5, S11, S24 and S40) with the 2 WWTPs with trickling filter technology (S39 and S53). The WWTP with activated biofilter/activated sludge (S25) was excluded from the comparison; for some chemicals its treatment standard was similar to trickling filter WWTPs while for others it was similar to AS-N/DN WWTPs. Mean breakthrough in the AS-N/DN WWTPs was less than mean breakthrough in the trickling filter WWTPs for almost all chemicals. This difference exceeded 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log units for 146 chemicals, and in 59 cases the difference was statistically significant at the 5% level (Table S5,† note that the statistical power in this data is low as the trickling filter group has only two WWTPs that were frequently quite different). Some of the chemicals that were good indicators for trickling filter quality treatment were also good indicators for AS-N/DN quality treatment (e.g., 4-pyridoxic acid, dodecyl sulfate and paraxanthine, Fig. S3†). For these chemicals there was a clear and consistent separation in B between both WWTPs with AS-N/DN versus trickling filter treatment as well as between WWTPs with trickling filter treatment versus primary treatment only. The breakthrough thresholds indicating AS-N/DN quality treatment were naturally lower than for trickling filter quality treatment (3%, 1% and 1%, respectively (Table 2, Fig. S3†)). There were other chemicals that were good indicators for AS-N/DN quality treatment but not for trickling filter quality treatment (Table 2, Fig. S5†). For several of these (meprylcaine, levetiracetam, trans-zeatin and acesulfame), removal efficiency with trickling filter technology was low and the breakthrough thresholds were in the 10–25% range.
log units for 146 chemicals, and in 59 cases the difference was statistically significant at the 5% level (Table S5,† note that the statistical power in this data is low as the trickling filter group has only two WWTPs that were frequently quite different). Some of the chemicals that were good indicators for trickling filter quality treatment were also good indicators for AS-N/DN quality treatment (e.g., 4-pyridoxic acid, dodecyl sulfate and paraxanthine, Fig. S3†). For these chemicals there was a clear and consistent separation in B between both WWTPs with AS-N/DN versus trickling filter treatment as well as between WWTPs with trickling filter treatment versus primary treatment only. The breakthrough thresholds indicating AS-N/DN quality treatment were naturally lower than for trickling filter quality treatment (3%, 1% and 1%, respectively (Table 2, Fig. S3†)). There were other chemicals that were good indicators for AS-N/DN quality treatment but not for trickling filter quality treatment (Table 2, Fig. S5†). For several of these (meprylcaine, levetiracetam, trans-zeatin and acesulfame), removal efficiency with trickling filter technology was low and the breakthrough thresholds were in the 10–25% range.
This last group of chemicals shows a behavior fundamentally different than for the group represented by 4-pyridoxic acid. It displays poor removal in primary treatment only and trickling filter treatment while good removal is achieved with AS-N/DN quality treatment. The clearest example of this behavior is meprylcaine (Fig. S5†). The group of chemicals for which log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B was correlated with meprylcaine (Pearson correlation coefficient >0.85 for the 2 trickling filter plants and 5 AS-N/DN plants) and log B for the trickling filter plants was low (mean log
B was correlated with meprylcaine (Pearson correlation coefficient >0.85 for the 2 trickling filter plants and 5 AS-N/DN plants) and log B for the trickling filter plants was low (mean log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B > 1.5) is shown in Table S6.† Only 8 chemicals fulfilled these criteria, indicating that there are few substances that were poorly removed by trickling filter technology but well removed by AS-N/DN technology.
B > 1.5) is shown in Table S6.† Only 8 chemicals fulfilled these criteria, indicating that there are few substances that were poorly removed by trickling filter technology but well removed by AS-N/DN technology.
Compared to the AS-N/DN WWTPs, advanced treatment with filtration (S9, S29, S51) reduced mean log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B by >1 for 17 chemicals and >0.5 for a further 30 chemicals, whereby the differences were significant at the 5% level for 11 and 15 of these, respectively. Many of these chemicals were resistant to biological treatment; mean log
B by >1 for 17 chemicals and >0.5 for a further 30 chemicals, whereby the differences were significant at the 5% level for 11 and 15 of these, respectively. Many of these chemicals were resistant to biological treatment; mean log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B for the AS-N/DN WWTPs was >1.7 (B > 50%) for 10/17 chemicals in the first group and 5/30 in the second group (see Table S7†). The WWTP with ozonation (S1) had log
B for the AS-N/DN WWTPs was >1.7 (B > 50%) for 10/17 chemicals in the first group and 5/30 in the second group (see Table S7†). The WWTP with ozonation (S1) had log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B at least 1
B at least 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log unit less than the AS-N/DN WWTPs for 39 chemicals and at least 0.5 units less for a further 49 chemicals (see Table S8†). Once again, many were resistant to biological treatment, with mean log
log unit less than the AS-N/DN WWTPs for 39 chemicals and at least 0.5 units less for a further 49 chemicals (see Table S8†). Once again, many were resistant to biological treatment, with mean log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B for the AS-N/DN WWTPs >1.7 for 23/39 chemicals in the first group. 38 of the 47 chemicals showing >0.5 unit lower log
B for the AS-N/DN WWTPs >1.7 for 23/39 chemicals in the first group. 38 of the 47 chemicals showing >0.5 unit lower log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B for filtration also showed this for ozonation. Although advanced treatment did reduce breakthrough for some compounds that had been significantly abated by AS-N/DN treatment, it was most effective for chemicals that were resistant to this treatment.
B for filtration also showed this for ozonation. Although advanced treatment did reduce breakthrough for some compounds that had been significantly abated by AS-N/DN treatment, it was most effective for chemicals that were resistant to this treatment.
There was considerable variability in the removal efficiency for the 4 WWTPs with advanced treatment, as illustrated in Fig. S6.† In some cases, B was similar for all 4 (e.g., tapentadol and desacetyl diltiazem). In other cases (e.g. sotalol, tramadol), B was similar for ozonation and two of the WWTPs with filtration, while the third, S9, had a higher B similar to the AS-N/DN WWTPs. For metoprolol and sitagliptin, S9 was again comparable to the AS-N/DN WWTPs while ozonation was clearly the most effective treatment. Irbesartan and gabapentin showed yet another pattern between the three WWTPs with filtration (Fig. S6†). These examples suggest that the effectiveness of filtration is specific to the details of the process implementation and operation, such as the kind, age and amount of filter material and the bacterial community in the filter. One explanation for the observed variability in B is significant differences in the filter material, with one WWTP (S51) having used granular activated carbon while the other two used filter coal/anthracite. No further information on filter properties was available, which made it difficult to know how representative these WWTPs are for a specific treatment standard. Consequently, they provided a poor basis for identifying indicator chemicals. However, two chemicals, tapentadol and desacetyl diltiazem, demonstrated consistently lower B at all WWTPs with advanced treatment, with a breakthrough threshold of 10% (Fig. S6,†Table 2). Finally, for 18 chemicals log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B was at least 0.5 units lower for the WWTP with ozonation (S1) than the WWTP with the next lowest log
B was at least 0.5 units lower for the WWTP with ozonation (S1) than the WWTP with the next lowest log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B. Eight of these chemicals were not well removed (log
B. Eight of these chemicals were not well removed (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B > 1) in any of the other WWTPs (Fig. S7†).
B > 1) in any of the other WWTPs (Fig. S7†).
Strengths and limitations of the methodology
A strength of the non-targeted suspect screening method employed here is that one can generate comparable data for a large number of chemicals with relatively little analytical effort. Particularly important effort-saving features are that there is no need for standards of all studied chemicals and no need for calibration curves. The quality assurance requirements (controlling instrument repeatability during the analytical run and using a collection of internal standards to assess the performance of the workflow and the similarity of response factor across samples) are also not onerous. A further advantage is that the data can be re-visited to search for new suspects once spectral information becomes available.This study resulted in an internally consistent dataset for 293 chemicals in 15 WWTPs. It provided detailed insight into the chemical specific effectiveness of different WWTP technologies and allowed indicator chemicals for different levels of wastewater treatment to be identified. Studies with a larger number of WWTPs could provide insight into the performance of a broader range of treatment technologies. They could also allow characterization of the variability of the performance of a given technology and exploration of the factors causing such variability.
This study also provided insight into similarities and differences in the behavior of different chemicals. For instance, one group of 49 chemicals was identified that was recalcitrant under primary treatment only but removed during trickling filter treatment and more strongly removed during AS-N/DN treatment. The generation of such large, internally consistent datasets of chemical breakthrough opens novel opportunities for exploring how chemical structure influences the removal efficiency of bioreactors and advanced treatment.
One limitation of the non-targeted suspect screening method is a somewhat higher uncertainty in substance identification. This method provides level 2 (probable structure) identification according to the scale of Schymanski et al.,26 which is less than the level 1 (confirmed structure) that can be achieved with target analysis. A further source of uncertainty is the possibility of different matrix effects in the analysis of influent and effluent. This difference exceeded 20–25% for ∼15% of the labeled standards used to test this, which would result in a comparable error in B. Although the B dataset showed strong overall consistency, errors of the order of a factor 2 are possible for individual chemicals. It is also important to note that even though modeling indicated that sorption was not important for the chemicals that we studied, the analytical method does not capture the sorbed fraction in the wastewater, and thus the method will not give a proper estimate of B for substances with a significant bound fraction. Another potential source of error is mismatch between the waters sampled in the influent and the effluent, which could result in a bias in the determination of B, whereby this was not a major problem in this study as indicated by B ≈ 100% for may persistent compounds (Fig. S5–S7†). One must also be aware that the interpretation of the results is more complex for compounds that are formed within the WWTP. Finally, an important limitation is that the list of potential analytes is restricted to the substances present in the available spectrum libraries (e.g., mzCloud, MassBank). For instance, at the time of this study there were comparatively few industrial chemicals in mzCloud. One way to address this is to measure spectra for chemicals of interest and upload them into an in-house data base or a publicly accessible spectrum library. As more researchers do this, the power of the method will grow.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This project was supported by the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 734522 (INTERWASTE project). The Queensland Alliance for Environmental Health Sciences, The University of Queensland, gratefully acknowledges the financial support of the Queensland Department of Health. This project was supported by an Australian Research Council Linkage Project (LP150100364). We thank C. S. McArdell (Eawag, Switzerland) for valuable input on data interpretation and N. Crosbie (Melbourne Water, Australia) for commenting on the manuscript. The authors wish to thank all of the various councils, companies and wastewater treatment plant operators who helped develop a strong network to provide samples and data associated with each sample – this project would not have been possible without your help. We also thank the QAEHS staff members who assisted in developing and conducting the wastewater sampling campaign.References
- R. Hirsch, T. Ternes, K. Haberer and K.-L. Kratz, Occurrence of antibiotics in the aquatic environment, Sci. Total Environ., 1999, 225, 109–118 CrossRef CAS PubMed.
- T. Ternes, Occurrence of drugs in German sewage treatment plants and rivers, Water Res., 1998, 32, 3245–3260 CrossRef CAS.
- BAFU Gewässerqualität: Revision der Gewässerschutzverordnung, 2015, https://www.bafu.admin.ch/bafu/de/home/themen/bildung/medienmitteilungen.msg-id-59323.html.
- J. T. Jasper and D. L. Sedlak, Phototransformation of wastewater-derived trace organic contaminants in open-water unit process treatment wetlands, Environ. Sci. Technol., 2013, 47, 10781–10790 CrossRef CAS PubMed.
- Y. Luo, W. Guo, H. H. Ngo, L. D. Nghiem, F. I. Hai, J. Zhang, S. Liang and X. C. Wang, A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment, Sci. Total Environ., 2014, 473-474, 619–641 CrossRef CAS PubMed.
- D. B. Miklos, C. Remy, M. Jekel, K. G. Linden, J. E. Drewes and U. Hübner, Evaluation of advanced oxidation processes for water and wastewater treatment – A critical review, Water Res., 2018, 139, 118–131 CrossRef CAS PubMed.
- L. Rizzo, S. Malato, D. Antakyali, V. G. Beretsou, M. B. Dolić, W. Gernjak, E. Heath, I. Ivancev-Tumbas, P. Karaolia, A. R. L. Ribeiro, G. Mascolo, C. S. McArdell, H. Schaar, A. M. T. Silva and D. Fatta-Kassinos, Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater, Sci. Total Environ., 2019, 655, 986–1008 CrossRef CAS PubMed.
- Q. Sun, M. Lv, A. Hu, X. Yang and C.-P. Yu, Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in a wastewater treatment plant in Xiamen, China, J. Hazard. Mater., 2014, 277, 69–75 CrossRef CAS PubMed.
- V. de J. Gaffney, V. V. Cardoso, E. Cardoso, A. P. Teixeira, J. Martins, M. J. Benoliel and C. M. M. Almeida, Occurrence and behaviour of pharmaceutical compounds in a Portuguese wastewater treatment plant: Removal efficiency through conventional treatment processes, Environ. Sci. Pollut. Res., 2017, 24, 14717–14734 CrossRef CAS PubMed.
- J. Hollender, S. G. Zimmermann, S. Koepke, M. Krauss, C. S. McArdell, C. Ort, H. Singer, U. von Gunten and H. Siegrist, Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration, Environ. Sci. Technol., 2009, 43, 7862–7869 CrossRef CAS PubMed.
- M. Bourgin, B. Beck, M. Boehler, E. Borowska, J. Fleiner, E. Salhi, R. Teichler, U. von Gunten, H. Siegrist and C. S. McArdell, Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products, Water Res., 2018, 129, 486–498 CrossRef CAS PubMed.
- L. F. Angeles, R. A. Mullen, I. J. Huang, C. Wilson, W. Khunjar, H. I. Sirotkin, A. E. McElroy and D. S. Aga, Assessing pharmaceutical removal and reduction in toxicity provided by advanced wastewater treatment systems, Environ. Sci.: Water Res. Technol., 2020, 6, 62 RSC.
- T. A. Ternes, C. Prasse, C. L. Eversloh, G. Knopp, P. Cornel, U. Schulte-Oehlmann, T. Schwartz, J. Alexander, W. Seitz, A. Coors and J. Oehlmann, Integrated evaluation concept to assess the efficacy of advanced wastewater treatment processes for the elimination of micropollutants and pathogens, Environ. Sci. Technol., 2017, 51, 308–319 CrossRef CAS PubMed.
- N. Collado, S. Rodriguez-Mozaz, M. Fros, A. Rubirola, D. Barceló, J. Comas, I. Rodriguez-Roda and G. Buttiglieri, Pharmaceuticals occurrence in a WWTP with significant industrial contribution and its input into the river system, Environ. Pollut., 2014, 185, 202–212 CrossRef CAS PubMed.
- O. Golovko, S. Örn, M. Sörengård, K. Frieberg, W. Nassazzi, F. Z. Lai and L. Ahrens, Occurrence and removal of chemicals of emerging concern in wastewater treatment plants and their impact on receiving water systems, Sci. Total Environ., 2021, 754, 142122 CrossRef CAS PubMed.
- M. Gros, M. Petrović, A. Ginebreda and D. Barceló, Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes, Environ. Int., 2010, 36, 15–26 CrossRef CAS PubMed.
- M. Papageorgiou, C. Kosma and D. Lambropoulou, Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece, Sci. Total Environ., 2016, 543, 547–569 CrossRef CAS PubMed.
- L. Bijlsma, E. Pitarch, E. Fonseca, M. Ibáñez, A. M. Botero, J. Claros, L. Pastor and F. Hernández, Investigation of pharmaceuticals in a conventional wastewater treatment plant: Removal efficiency, seasonal variation and impact of a nearby hospital, J. Environ. Chem. Eng., 2021, 9, 105548 CrossRef CAS.
- G. Nürenberg, U. Kunkel, A. Wick, P. Falås, A. Joss and T. A. Ternes, Nontarget analysis: A new tool for the evaluation of wastewater processes, Water Res., 2019, 163, 114842 CrossRef PubMed.
- J. E. Schollée, J. Hollender and C. S. McArdell, Characterization of advanced wastewater treatment with ozone and activated carbon using LC-HRMS based non-target screening with automated trend assignment, Water Res., 2021, 200, 117209 CrossRef PubMed.
- L. S. Lautz, J. Struijs, T. M. Nolte, A. M. Breure, E. van der Grinten, D. van de Meent and R. van Zelm, Evaluation of SimpleTreat 4.0: Simulations of pharmaceutical removal in wastewater treatment plant facilities, Chemosphere, 2017, 168, 870–876 CrossRef CAS.
- L. Wiest, A. Gosset, A. Fildier, C. Libert, M. Hervé, E. Sibeud, B. Giroud, E. Vulliet, T. Bastide, P. Polomé and Y. Perrodin, Occurrence and removal of emerging pollutants in urban sewage treatment plants using LC-QToF-MS suspect screening and quantification, Sci. Total Environ., 2021, 774, 145779 CrossRef CAS.
- Z. Li, E. Undeman, E. Papa and M. S. McLachlan, High-throughput evaluation of organic contaminant removal efficiency in a wastewater treatment plant using direct injection UHPLC-Orbitrap-MS/MS, Environ. Sci.: Processes Impacts, 2018, 20, 561–571 RSC.
- C. Ort, M. G. Lawrence, J. Rieckermann and A. Joss, Sampling for pharmaceuticals and personal care products (PPCPs) and illicit drugs in wastewater systems: Are your conclusions valid? A Critical Review, Environ. Sci. Technol., 2010, 44, 6024–6035 CrossRef CAS PubMed.
- J. W. O'Brien, S. Grant, A. P. W. Banks, R. Bruno, S. Carter, P. M. Choi, A. Covaci, N. D. Crosbie, C. Gartner, W. Hall, G. Jiang, S. Kaserzon, K. P. Kirkbride, F. Y. Lai, R. Mackie, J. Marshall, C. Ort, C. Paxman, J. Prichard, P. Thai, K. V. Thomas, B. Tscharke and J. F. Mueller, A National Wastewater Monitoring Program for a better understanding of public health: A case study using the Australian Census, Environ. Int., 2019, 122, 400–411 CrossRef PubMed.
- E. L. Schymanski, J. Jeon, R. Gulde, K. Fenner, M. Ruff, H. P. Singer and J. Hollender, Identifying small molecules via high resolution mass spectrometry: communicating confidence, Environ. Sci. Technol., 2014, 48, 2097–2098 CrossRef CAS PubMed.
- Chemicalize, https://chemicalize.com/welcome Accessed April 2020.
- US EPA, Estimation Programs Interface Suite™ for Microsoft® Windows, v 4.11, United States Environmental Protection Agency, Washington, DC, USA, Accessed April 2020 Search PubMed.
- BAFU Erläuternder Bericht zur Änderung der Gewässerschutzverordnung, Referenz/Aktenzeichen: M473–0796, 2015, https://www.newsd.admin.ch/newsd/message/attachments/41551.pdf.
- E. R. V. Dickenson, J. E. Drewes, D. L. Sedlak, E. C. Wert and S. A. Snyder, Applying surrogates and indicators to assess removal efficiency of trace organic chemicals during chemical oxidation of wastewaters, Environ. Sci. Technol., 2009, 43, 6242–6247 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ew00088a |
| This journal is © The Royal Society of Chemistry 2022 |