Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structure, controlled release mechanisms and health benefits of pectins as an encapsulation material for bioactive food components
Rocío
Morales-Medina
 *a,
Stephan
Drusch
a,
Francisca
Acevedo
bc,
Alejandro
Castro-Alvarez
cd,
Astrid
Benie
e,
Denis
Poncelet
f,
Marijana M.
Dragosavac
g,
María Victoria
Defain Tesoriero
h,
Patricia
Löwenstein
h,
Verónica
Yonaha
h,
Ramiro
Iturralde
h,
Regina
Gauna Peter
h and
Paul
de Vos
i
*a,
Stephan
Drusch
a,
Francisca
Acevedo
bc,
Alejandro
Castro-Alvarez
cd,
Astrid
Benie
e,
Denis
Poncelet
f,
Marijana M.
Dragosavac
g,
María Victoria
Defain Tesoriero
h,
Patricia
Löwenstein
h,
Verónica
Yonaha
h,
Ramiro
Iturralde
h,
Regina
Gauna Peter
h and
Paul
de Vos
i
aTechnische Universität Berlin, Institute of Food Technology and Food Chemistry, Department of Food Technology and Food Material Science, Königin-Luise-Str. 22, 14195 Berlin, Germany. E-mail: morales-medina@tu-berlin.de
bDepartment of Basic Sciences, Faculty of Medicine, Universidad de La Frontera, Chile
cCenter of Excellence in Translational Medicine (CEMT), Faculty of Medicine, and Scientific and Technological Bioresource Nucleus (BIOREN), Universidad de La Frontera, Casilla 54-D, Temuco, Chile
dDepartment of Preclinical Sciences, Faculty of Medicine, Universidad de La Frontera, Casilla 54-D, Temuco, Chile
eCP Kelco ApS, Ved Banen 16, 4623 Lille Skensved, Danmark
fENCAPPROCESS, 114 allée Paul Signac, 44240 Sucé sur Erdre, France
gLoughborough University, Chemical Engineering, Leicester, Leicestershire, UK
hNational Institute of Industrial Technology (INTI), Department of New Formulation Technologies, Buenos Aires, Argentina
iImmunoendocrinology, Division of Medical Biology, Department of Pathology and Medical Biology, University of Groningen and University Medical Center Groningen, Hanzeplein 1, 9700 RB Groningen, The Netherlands
First published on 22nd September 2022
Abstract
Encapsulation of food and feed ingredients is commonly applied to avoid the loss of functionality of bioactive food ingredients. Components that are encapsulated are usually sensitive to light, pH, oxygen or highly volatile. Also, encapsulation is also applied for ingredients that might influence taste. Many polymers from natural sources have been tested for encapsulation of foods. In the past few years, pectins have been proposed as emerging broadly applicable encapsulation materials. The reasons are that pectins are versatile and inexpensive, can be tailored to meet specific demands and provide health benefits. Emerging new insight into the chemical structure and related health benefits of pectins opens new avenues to use pectins in food and feed. To provide insight into their application potential, we review the current knowledge on the structural features of different pectins, their production and tailoring process for use in microencapsulation and gelation, and the impact of the pectin structure on health benefits and release properties in the gut, as well as processing technologies for pectin-based encapsulation systems with tailor-made functionalities. This is reviewed in view of application of pectins for microencapsulation of different sensitive food components. Although some critical factors such as tuning of controlled release of cargo in the intestine and the impact of the pectin production process on the molecular structure of pectin still need more study, current insight is that pectins provide many advantages for encapsulation of bioactive food and feed ingredients and are cost-effective.
1. Introduction
With the increasing and emerging global awareness about the importance of a healthy diet and its impact on the prevention of diseases, food design and incorporation of stable functional ingredients have become the major focus of both industry and academia. Moreover, there is a growing trend of consumers who prefer foods containing natural ingredients, many of which cannot be incorporated into the food matrix without losing their health effects. Microencapsulation of these functional food components is one of the approaches to preserve the functionality of food components and facilitate the development of new and nutritionally healthy foods.The need for the market to introduce different types of ingredients into the food matrix promoted the growth of the microencapsulation field since there are currently many ingredients that cannot be directly incorporated into foods in their free form. These include flavors (e.g., citrus oil), antioxidants (e.g., β-carotene), antimicrobials (e.g., essential oils), bioactive lipids (e.g., ω-3 fatty acids), minerals (e.g., iron), vitamins (e.g., vitamin D), probiotics (e.g., lactic-acid bacteria) and enzymes (e.g., β-galactosidase).1,2
The maintenance of flavor or prevention of undesired organoleptic properties without losing functionality is one of the most mentioned reasons to encapsulate ingredients. Also, living cells such as probiotic bacteria are more often encapsulated before being applied in products.3 Microcapsules should protect these species not only from harsh conditions in the products, but also during transit through the gastrointestinal tract4,5 before reaching their site of action in the large intestine.
To maintain all the functionalities of food components, specific polymers have been applied for encapsulation. In the majority of applications, these have been natural polymers. These polymers have to meet physicochemical prerequisites as well as regulatory demands which may vary per country.6 In general, polymers for encapsulation should have adequate rheological properties at high concentrations, have the ability to disperse or emulsify the active material and stabilize the emulsion produced. It should also seal and hold the active material within its structure during processing or storage to provide maximal protection to the active ingredient against environmental conditions but release the cargo when required in its specific application. Last but not least, it should be inexpensive and food grade.7
The most commonly applied natural polymers for encapsulation of food can be categorized into four major classes: carbohydrate polymers, proteins, lipids and inorganic molecules. All these materials have their advantages and disadvantages for different applications. Some are difficult to incorporate in the food matrix, and others are for example too expensive for many applications.8,9
A special focus in recent years has been on the use of pectins in the encapsulation of food components because of some specific benefits. Pectins are anionic high-molecular-weight heteropolysaccharides that can form microcapsules around food components under relatively nonhazardous conditions.10 Pectins also have other benefits as an encapsulation polymer. Pectins not only can be used for satiety control, texture control (e.g. reduced-fat products), and targeted delivery to specific areas in the digestive tract11 but also have health benefits. The latter has been studied in great detail in the last 5 years and much new emerging scientific information is available on the benefits of pectins for health. Also, as will be outlined in this review, specific pectins can prevent inflammatory events in the gastrointestinal tract and probably even prevent the development of some tumors. Given these properties and the importance of gut health in overall health maintenance,12 pectin is a promising polymer to be used in controlled release applications. However, there are also discussions ongoing, and there are still limited data available on the effects of the use of pectins in food and feed and the type of pectin to be included in capsules. In this review a team of experts on pectin structure health benefits, gel formation for microcapsule manufacturing and pectin production have combined efforts to give a comprehensive review on the current insight into the applications of pectins in microencapsulation. The current literature has been reviewed in the light of the ability of pectin to protect specific food components and enable targeted release at specific intestinal sites.
2. Structural features of different pectins
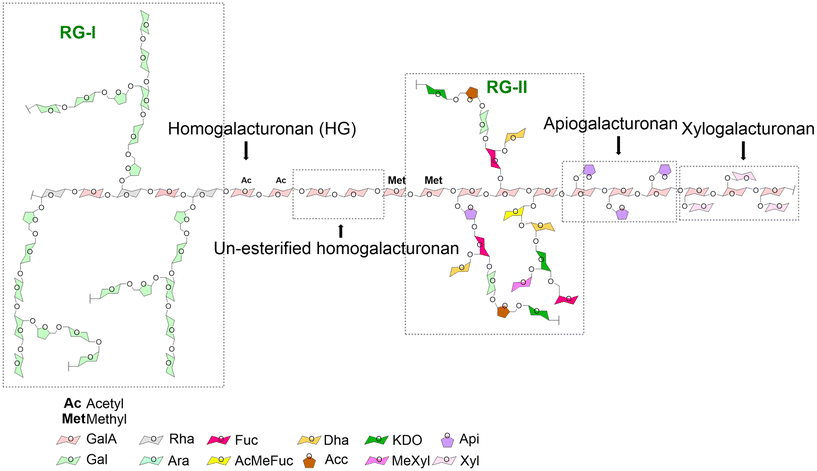
Pectins are a heterogenic and versatile family of polymers. Their structural features are determined by a variety of parameters which include, as discussed in the next section, not only the origin and method of extraction but also enzymatic modification.13 In general, pectins are hetero-polysaccharides mainly composed of α-1,4-linked galacturonic acid (GalA) residues; pectin regions where only GalA molecules can be found are called homogalacturonans. At the C6 carboxyl groups, these GalA residues can be methyl-esterified. Also, GalA in pectins can be acetylated at O-2 or O-3, which is a feature of sugar beet pectin.13 A very common feature of pectins is that they are characterized by a specific percentage of GalA residues with methyl esters. This is known as the degree of methyl-esterification (DM). Pectins can be classified as low DM pectins (DM < 50%) and high DM pectins (DM > 50%).14 These methyl-esterified GalA residues have a specific distribution in the pectin molecule. This is also called the degree of blockiness (DB) of pectins. In general, pectins with a high DB contain a more blockwise distribution of non-esterified GalA residues. In contrast, low DB pectins are characterized by a more random distribution of non-esterified GalA residues.15Pectins can also contain other structural regions in addition to homogalacturonan. These include xylogalacturonan, apiogalacturonan, rhamnogalacturonan I (RG-I) and rhamnogalacturonan II (RG-II) (Fig. 1). In xylogalacturonan containing pectins, the homogalacturonan structures are replaced by xylose molecules. In apiogalacturonan containing pectins, the homogalacturonan structures contain mono- or disaccharide apioduranosyl. In pectins containing RG-I regions, the disaccharide backbone structures consist of alternating galacturonic acid and rhamnose residues. The rhamnose residues can be branched with neutral side chains of galactose or arabinose. The GalA residues of RG-I rich pectins are in most cases non-methyl esterified. RG-II containing pectins have a different backbone from those containing RG-I. They are composed of a backbone consisting only of galacturonic acid residues. The RG-II containing pectins are probably the most complex pectin structures as they can be made up of many glycosidic linkages.16 These pectins containing regions with such a high number of complex side chains can be obtained from many fruits and vegetables including apples, sugar beet, cabbage, apricots, carrots, onions or pears.13
3. Health benefits of different pectins
Apart from tailored release properties, pectin has an additional advantage which is having health benefits as such. This is a pertinent advantage of the application of pectins in the food industry. Pectins can have such an effect by impacting gastrointestinal health at several levels. The effects are, however, dependent on the type of pectin applied and are discussed in this section to guide the user in making rational choices if they wish to favor a specific health benefit.It has been shown that pectins can beneficially influence the production of the slimy mucus layer that protects the intestinal lineage of epithelial cells from damaging agents from the gut lumen. They can do so by positively influencing mucus production by the mucus-producing goblet cells in the intestine or by their mucoadhesive effects that stimulate the strength of the mucus layer.17–19 Lower DM pectins specifically stimulate goblet cells but cannot interact with mucin due to the negative charge on the non-esterified GalA residues which interact with the negatively charged mucins.18 In contrast, higher DM pectins interact directly with mucus.20 Also, RG-I containing pectins were shown to reinforce the mucus layer in mice.19 Pectins can possibly also stimulate the barrier function of the epithelial cells that are underneath the mucus layer,21,22 but which structural features of pectins are responsible for this effect remain to be determined.23 Also, RG-I rich pectins support gut epithelial barrier function by maintaining tight-junction between cells.19
Pectins in recent years have also been recognized as important regulators of immune responses through interaction with the immune receptors galectins and Toll-like receptors.23,24 Particularly pectins rich in RG-I and RG-II and containing galactose or arabinan structures can bind to galectin-3 and support innate immune responses against e.g. tumours and pathogens.25–27 Low-DM pectins are potent modulators of Toll-like receptors and can inhibit pro-inflammatory signalling and prevent mucositis.28–30 Finally, as also outlined in the preceding section, pectins in microcapsules will be fermented and broken down by gut microbiota and contribute to better gastrointestinal health by supporting the production of e.g. short-chain fatty acids that beneficially impact health at both the metabolic and immune levels. Particularly, the gut species Bacteroides and Prevotella are pectin degraders as they possess carbohydrate-active enzymes within their polysaccharide utilization loci.31 The fermentation products of pectins can stimulate the growth of other beneficial bacteria, leading to improved gastrointestinal health. In this way, pectins used in capsules can contribute to better health. These health effects can be tailored to specific target groups by choosing specific pectin chemistries.
4. Pectin production and tailoring for microencapsulation
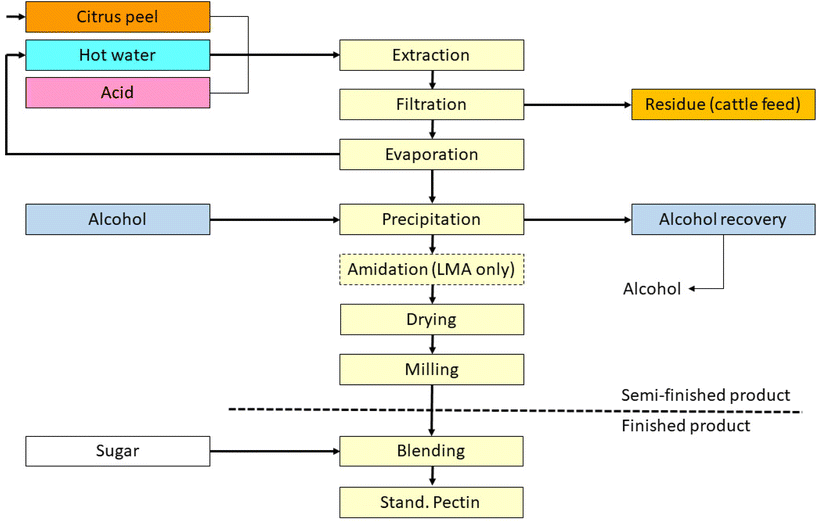
The main raw material sources for pectin production are citrus fruits including lemon, lime, orange, and grapefruit, as well as apple and sugar beet. The choice of the source determines the xylogalacturonan, apiogalacturonan, RG-I or RG-II content of the final pectin. Citrus raw materials are the most applied for pectin isolation. Sugar beet is less frequently used in industrial processes, as sugar beet pectin is less efficient as a gelling agent, which is the most common reason to include pectin in foods. The primary application of sugar beet pectin is as an emulsifier. However, sugar beet pectin has also proven benefits in terms of encapsulation properties32 and might have some specific benefits which may lead to enhanced application in the near future.Most pectins are isolated from by-products which makes them relatively inexpensive. The production process of pectins has been reviewed33 but shortly summarized here as the process determines the composition of pectin. To minimize caramelization during processing, the peels are washed in water to leach out sugars prior to drying (see Fig. 2). Later, the pretreated peels are extracted in acidified water, typically at pH 1–3 at 50–90 °C for 3–12 hours. This extraction process not only reduces the pectin polymerization degree, but also hydrolyzes ester linkages and thereby reduces the degrees of methylation and acetylation which are determinants of the health effects of pectins. The pectin yield and degrees of polymerization and esterification are determined by a fine balance between the acidity, temperature, and duration of the extraction. By tuning these parameters tailored pectins with desired properties can be produced. The extraction is then followed by filtration to separate the aqueous pectin extract from the remaining plant tissues.
Next, the extract is concentrated by evaporation, followed by alcoholic precipitation (e.g. with isopropanol), and further washing with alcohol before drying and milling. The resultant powder is ready for standardization, i.e. mixing with other pectin batches and/or sucrose to ensure a constant quality and uniformity usually defined as strength. This standardization is a mandatory step, as the properties of the botanical raw material vary due to weather and other conditions, which can significantly influence ripeness. Further variation is also introduced as the peel treatment process differs between peel manufacturers.
The manufacturing process for sugar beet pectin is essentially the same as for citrus pectin. Pectin is extracted from fresh sugar beet pieces after sugar extraction at sugar refineries. In contrast to citrus pectin, sugar beet pectin is not standardized with sugar. Normally, with the described extraction method, the DM of pectins is between 55 and 75%. For some applications, however, a lower degree of esterification is desired and created by further acidifying the extract and increasing the duration of the extraction. Furthermore, the methyl esterified carboxyl groups can be converted into primary amides by suspending precipitated pectin in a mixture of alcohol and water with ammonia. By choosing suitable conditions with respect to ammonia concentration, water activity, and temperature, pectins with various proportions of amidation, methyl esterification, blockiness of DM groups, and free carboxylate groups can be produced.
High DM pectins are often used in pH-dependent microencapsulation delivery systems where long-time release is desired, due to their pH-sensitive behavior34 and quite high hydrophobicity. Low DM pectins are of interest in many applications as they form gels with multivalent cations35 and have various health benefits. Amidated pectins have shown to have favorable characteristics for encapsulating live bacteria such as probiotic species.36 By mixing pectins with proteins, lipids35 or other polysaccharides such as chitosan37 or alginate,38 an even wider range of applications can be realized such as for optimised controlled release of pharmaceuticals in diabetes and cancer treatment or improved stability of antioxidants in the food industry. Furthermore, pectin properties can be further modified and improved by e.g. microfluidization, sonication,39 oxidation or enzymatic treatment. Due to its more complex structure, sugar beet pectin provides even more structural epitopes40 than citrus pectins and has in the past few years gained more and more attention for encapsulation.32
5. Impact of structure on the gelation of pectins
The formation of a gel is a mandatory step for microcapsule formation and is required for most applications of pectin in food. A gel is defined as a non-fluid colloidal network or polymer network that is expanded through its whole volume by a fluid.41 In the case of pectin, this network is formed through physical aggregation of the pectin molecules and intermolecular interactions resulting in the formation of so-called junction zones.42 Consequently, a prerequisite for gelation is that pectin molecules can come into close proximity with each other. The most important intermolecular interactions involved in network formation include hydrogen bonds, direct electrostatic interactions and pectin–ion interactions as well as hydrophobic interactions42 (Fig. 3).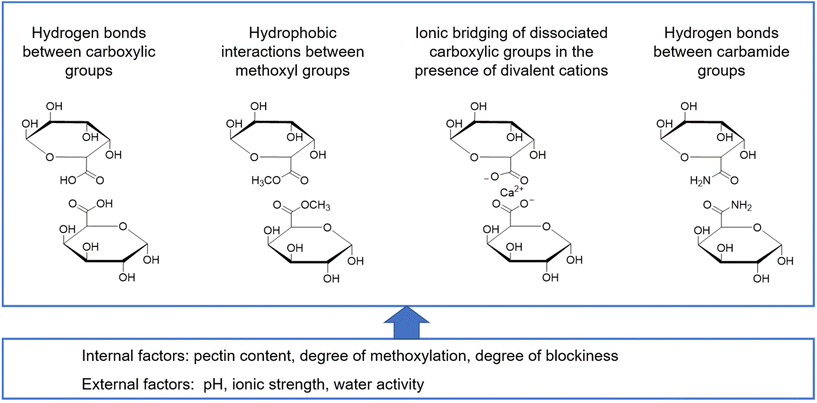 | ||
| Fig. 3 Molecular interactions between galacturonic acid derivatives and factors affecting biopolymer network formation. | ||
Based on the dominant type of interaction, one may distinguish two mechanisms of network formation: ionotropic gelation in the presence of divalent cations and cold-set gelation in an acidic environment.42 The integrity of the final gel is determined by the different types of intermolecular interactions, the nature of the junction zones and also the mechanism of gelation. As this depends on the molecular structure of pectin, the pectin composition determines the final gel properties.
Both low and high DM pectins can be applied for gel formation but by different methods. The reason is that the number of free carboxylic groups in pectin with a high DM is still sufficient to interact in junction zones. Therefore, cold-set gelation requires environmental conditions that reduce the number of dissociated groups (acidic pH of approx. 3.0–3.2) and water activity (high total solids with a high proportion of humectants, most commonly sugar). Under these conditions, repulsive interactions between similarly charged free carboxylic groups are minimized and junction zones are formed through hydrogen bonds of non-dissociated carboxylic groups and hydrophobic interactions between methoxyl groups.43 In contrast, in low DM pectins, the high number of free carboxylic groups does not allow cold-set gelation under conditions that occur in the majority of foods. However, one may take advantage of pectin–ion interactions for network formation.44 In this case, ionotropic gelation occurs, where ionic bridges between adjacent pectin molecules and divalent cations like calcium ions form and build up junction zones referred to as an “egg-box structure”.45 Electrostatic repulsion is not an issue, since these repulsive interactions and ionic bridging are similar in nature and thus have the same working range. In addition, pH is also less critical in these systems as long as a sufficient number of dissociated free carboxylic groups are available.
Apart from this overarching distinction based on the DM of pectin, in both cases, several other factors like the distribution of free carboxylic groups, the degree of acetylation, the chain length and the presence of neutral sugar side chains affect the structure formation and gel properties of pectin.46 Consequently, a given structure although classified as either low or high DM pectin may form a gel through a mechanism differing from the ones described above. Kastner et al.47 recently performed a systematic study on the structure formation and gel properties of high- and low-methoxylated pectin with either a random or block-wise distribution of the free carboxylic groups. The authors concluded that the distribution pattern significantly affects the kinetics of structure formation at a given degree of methoxylation, but not necessarily the final gel properties. When looking at the other factors in a very general way, an increase in the number of subsequent free carboxylic groups of the galacturonic acid leads to an increase in the gel strength. For low DM pectins, Fraeye et al.48 reported that a minimum of 6 to 20 subsequent free carboxylic groups are required. In contrast, reduction of the molecular weight reduces the gel strength and the presence of acetyl groups hinders ionic bridging due to steric effects. The same may hold true for neutral sugar side chains, but also a positive effect of an increased molecular entanglement by neutral sugar side chains has been reported. Zheng et al.49 recently showed that arabinan side chains stabilize cation-induced junction zones through entanglements and form dense networks in acid-induced gels through hydrogen bonds. A detailed analysis of the available literature on the influence of the pectin structure on interactions with divalent cations and its associated functionality has recently been published.50
Although much knowledge is available on the use of pectin for gel formation, little is known about the supramolecular structure of pectin in the gel state. When focusing on the major commercial sources, the pectin molecule itself is semiflexible with the maximum flexibility at a degree of methoxylation of approximately 50% as reviewed by Zdunek et al.51 The same authors showed that during calcium-induced association in a dilute regime, low-DM pectin molecules assemble in a two-fold helix. In more concentrated systems, a transition to right-handed threefold helices occurs, which is favorable for the formation of a more complex tertiary structure and which is also common for high-DM pectins.51 Based on SAXS analyses, Ventura et al.52 suggested that calcium-induced bridging of low DM pectins involves both rod-like junction zones and point-like crosslinks with their ratio varying depending on the calcium concentration. At a higher structural level, Ditta et al.53 used a rheological approach to derive fractal dimensions to describe the compactness of the resulting gel structure and observed a dependency on pectin concentration. Nanoparticle diffusometry is an upcoming technique in the field,54 which will allow one to gain a better understanding of the structural rearrangements during gel formation and the final gel structure and is thus one way to bridge the gap between molecular structure and gel functionality.
Finally, it needs to be mentioned that the gelation of a specific pectin structure also heavily depends on external factors. On one hand, undesirable side reactions like demethoxylation and molecular weight reduction may occur either during pectin extraction and modification as outlined in the preceding section47 or during processing of a pectin-based formulation. The presence of monovalent cations, which again may result from pectin modification or represents an inherent constituent of a specific formulation, alters structure formation and final gel properties due to the binding to dissociated carboxylic groups.55 Also, pH, temperature and cooling rate in the case of high DM pectins may affect the structure formation and gel properties.56 However, one may look at it as an opportunity to customize the gel structure during processing and more particularly when designing process and formulation in encapsulation as will be discussed in the section ‘Process technologies for pectin-based encapsulation systems with tailor-made functionality’.
6. Impact of pectin structure on release properties in the gut
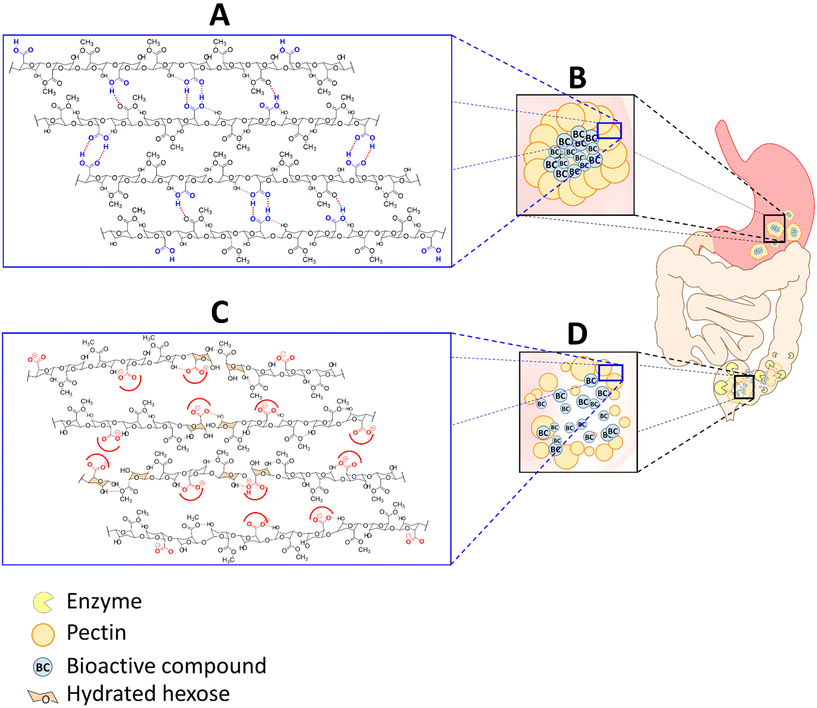
The gelling processes described in the preceding section allow the formation of microcapsules with tailored properties for several matrices for different foods.57,58,59–66,67,68 An important prerequisite is that the pectins applied in microcapsules should largely remain intact and protect the encapsulated food components when subject to the harsh circumstances in the stomach and upper gastrointestinal (GI) tract.34 Pectin is, however, ideally suitable for that. In the gel form, pectin capsules will get partly hydrated and will swell under acidic gastric conditions (pH 1.2) but will remain a stable pectin aggregate with no significant damage to the gel matrix. As observed in a study,69 in a simulated gastric fluid medium, the pectin chains are protonated and remain practically undissolved. Physiological conditions with a pH lower than pectin pKa (∼2.9–3.5) favor the protonation of the carboxyl acids of pectin. Hence, the chains are stabilized by successive hydrogen bonding between undissociated free carboxylic acids and secondary alcohol groups and by hydrophobic interactions between methyl esters.70,71 Also, most pectins are indigestible by enzymes such as proteases and amylases in the upper digestive tract in the small intestine67,72 and will remain intact until they reach the large intestine.Upon entering the cecum, the pectins will degrade which will result in the release of the cargo of the microcapsules. The mechanism of pectin degradation in microcapsules is partly swelling and erosion, but also degradation by colonic microbial-derived enzymes. It is partly pH driven as at pH 6.8 or above, the β-depolymerization and demethoxylation of pectin aggregates occur resulting in dissociation of the carboxylate (COO–) groups and destabilization of the pectin (Fig. 4). This phenomenon eventually leads to pectin chain disentanglement and the pectin network starts to dissolve supported by erosion until full dissolution.67,73–76
Additionally, the pectin polysaccharides are fermented by enzymes such as beta-D-glucosidase, beta-D-galactosidase, amylase, pectinase, xylanase, beta-D-xylosidase, and dextranase produced by numerous colonic bacteria, which expedite and facilitate the release of the bioactive cargo.72,77–80
As will be outlined in the next section, the degraded pectins will have an impact on gut microbiota and will support the health of the consumer. However, pectin also has other unique features. It can change the viscosity of the bolus in the gastrointestinal tract. A higher molecular weight of the applied pectin increases the viscosity of a pectin solution, leading to a higher interaction with the mucosal surface and hence a general tendency for adherence to mucosal surfaces.81 This mucoadhesion is due to the interaction of the carboxylic groups of pectin with functional groups on the mucosal wall such as with hydroxyl, amide, carboxyl or sulfate groups.82 These interactions may lead to hydrogen bonds and play an important role in the efficacy of the colonic delivery of pectin based microcapsules.62,63,83–86 In fact, the intimate contact between pectin and the mucus prolongs the residence time of the bioactive food components at the absorption site with enhanced release and uptake, supporting the beneficial effects of the capsule-cargo at the specific site.87,88 The bioactive release from the pectin gels occurs mainly through a diffusion mechanism89 and fits well into the Higuchi model as reported by Atyabi et al.61 and Sriamornsak et al.18
Despite the benefits of pectin-based microcapsules for colon delivery, the swelling ability of pectin hydrogels and the associated larger pore size under physiological conditions could be a limitation for application for a certain number of smaller molecular weight bioactive compounds.59,72 Another drawback of pectin-based capsules is observed when they are applied for hydrophobic molecules. The quantity and homogeneity of the bioactive food component in the gel will be limited with hydrophobic molecules as cargo due to the hydrophilic properties of the pectin gel.90 In addition, the stability and strength of the hydrogel could be reduced due to the phase separation between the hydrophobic cargo and the pectin hydrogel.91 A strategy to overcome these limitations has been the modulation of the physicochemical properties of pectin. This can be done through structural changes, functionalization, complexation with other polymers or bioactive molecules or crosslinking with di- or multi-valent cations to form a more stable structure. This usually reduces the solubility and swelling and increases the density and mechanical strength, providing controlled bioactive delivery patterns61,67,72,92–94
7. Process technologies for pectin-based encapsulation systems with tailor-made functionality
The favourable and versatile gelling properties of pectins as well as the ability to obtain pectins with different compositions and viscosities allow the use of many different cost-effective encapsulation technologies for food components which will be reviewed in this section. These can be simple dripping technologies, coacervation and emulsification.A technique that can be used is the simple extrusion of beads by means of a dripping technology. This can especially be applied for the production of pectin beads by ionic gelation of low-DM pectins. In this case, a pectin solution containing the cargo is extruded drop by drop from a needle or nozzle into a calcium bath. The droplets are subsequently gellified, leading to hydrogel beads. This process has been used for encapsulation of bacteria such as probiotics95–97 as well as for food supplements98 or intestinal drug delivery.99,100
A disadvantage of this dripping technique is that it leads to large bead sizes (2–3 mm) and low production rates (100 mL h−1), which do not meet the requirements of most of the industrial applications. There are, however, different technologies that can partly solve these limitations and reduce the size of beads and increase the productivity.101 These involve the use of e.g. nozzle resonance technologies to achieve flow rates up to liters instead of milliliters per hour and size reduction to a few hundred micrometers.101 These dripping techniques can also be employed for applications in which pectins are combined with e.g. alginate96 to enhance some application functionalities and protection of specific cargo.99 As outlined in the section on gelling high-DM pectins are gellified by cold-set gelation. Hydrogel beads may be formed by extruding a warm hydrocolloid solution in a cool water bath or by cooling in an air column.102 However, since cold-set gelation of pectin requires a low pH and reduction of water activity by e.g. sugar, this technique might only be suitable for some specific niche applications.
The second major technology is complex coacervation. Coacervation involves precipitation of a polymer or a complex of polymers by modifying the physicochemical conditions such as pH, temperature or salinity. What is usually done is that a mixture of polymers, a protein and a carbohydrate-based polymer, is lowered in pH from 6 to 8 to a pH lower than the isoelectric point of the protein (2 to 4), leading to a shift of protein charge from negative to positive. The protein then aggregates with the negatively charged carbohydrate-based biopolymer. Complex coacervation can be applied in different manners when pectins are applied. The first is by initiating coacervation under strong agitation. In this case, small polymer aggregates will be formed with some hydrophilic and hydrophobic zones. The bioactive cargo in the solution is then absorbed in one of these zones depending on its hydrophilicity. The size of the particles is generally sub-microns or even on the nanoscale (<100 nm).103 In the case of a lipophilic core material, coacervate formation occurs at the oil–water interface in an emulsion. The core material is emulsified with either a solution containing an amphiphilic biopolymer or a blend of an amphiphilic biopolymer and its counterpart for coacervate formation. In the second step, coacervate formation is induced at the oil–water interface by the addition of the second biopolymer and/or a change in the intrinsic properties of the emulsion like pH, ionic strength or temperature. This leads to directing their hydrophobic zone to the oil phase and the hydrophilic zone to the continuous aqueous phase. Depending on the size of the oil droplets, the pectin based microcapsules may vary in size from the nanoscale to hundred micrometers.104 In many cases, the resulting microcapsules are fragile and particularly do not allow drying. To improve stability they are often treated with some cross-linking agents such as glutaraldehyde or tannins.104 Complex coacervates produced with ovalbumin, tannic acid and with pectin as the encapsulation material have shown high thermal stability.105 Additionally, sugar beet pectin, bovine serum albumin and genipin as cross-linkers have been shown to efficiently encapsulate betanin and curcumin.106
An alternative technique is based on the extrusion of droplets of a charged polymer into a solution of a polymer with an opposite charge. It is sometimes referred to as interfacial coacervation or interfacial polymer complex formation. The two polymers interact subsequently at the surface of the droplet to form a membrane. The technology has been largely applied using negatively charged alginate which forms a complex with positively charged chitosan or poly-L-lysine.107 Although not broadly recognized yet, this process may also be applied with pectin as it is just like alginate negatively charged. Chitosan obtained by de-acetylation of chitin (poly-N-acetyl-D-glucosamine) has been shown to form polymer complexes with pectin and via interfacial coacervation, which is shown to be an efficacious encapsulation system.108Both techniques, gelation and complex coacervation, are sometimes discussed as potential technologies to produce nano-scale delivery systems and pectin has been used for nanoencapsulation of food components by a variety of processes.35
The processes are classified as top-down and bottom-up methods. Top-down approaches include methods based mainly on nano-emulsification,109 while bottom-up approaches involve self-assembly like nanocoacervation or nanoprecipitation.110 Several techniques classify for encapsulation of food components. This means e.g. a technique in which a composite solution of pectin and protein like gelatin is prepared at pH 7.5 and 45 °C. The pH is reduced to 3.8 and the solution is slowly brought to room temperature to promote the formation of nanocoacervates.103 Another feasible technique is to slowly add calcium to a pectin solution under high agitation, which leads to the formation of calcium pectinate nano-aggregates.111 Finally, pectin is well suitable for encapsulation by spray drying. Encapsulation involves the spraying of a pectin-containing solution or emulsion into fine droplets in a chamber supplied with warm air. The droplets are quickly dried leading to a fine powder of size between 30 and 100 μm. The cargo may be included in the initial solution as a solute, suspension or emulsion depending on its properties. Optimizing the formulation and process requires a fast drying and compact final matrix. Polymers such as maltodextrin with low viscosity at high concentration (up to 50%) are often used to provide a protective barrier against e.g. oxidation. However, they do not ensure the stability of capsules in an aqueous solution. Therefore, the release mechanism is usually a solvent-activated one and spray-dried microcapsules are incorporated in dry formulations. Due to its amphiphilic properties, sugar beet pectin or pectin with an intermediate DM and high DB may be preferred for encapsulation of lipopohilics. A composite system has been proposed by mixing e.g. maltodextrin with pectin112 but this does not improve the stability in an aqueous solution.
8. Concluding remarks and future perspectives
As outlined in the preceding sections, pectin has evolved as a promising matrix polymer for encapsulation. It has some specific features that make it particularly suitable for application in the microencapsulation of food components. It is versatile and inexpensive, can be tailored to meet specific demands and as such, depending on its structure, can provide health benefits as a molecule. The latter aspects are of utmost importance since success in encapsulation always requires maximum flexibility to match encapsulate and matrix material properties, technical requirements and process design as well as food matrix and behavior under physiological conditions. Notably, however, some challenges are also still present.Significant research efforts are required to find the optimal pectin structure for individual applications or even for encapsulation of specific food ingredients. Not all pectins that have been tested for health benefits, such as support of gastrointestinal health113–118 and lowering inflammatory processes, are suitable for microencapsulation of the wide range of food components that are currently encapsulated. It is expected that e.g. in living cells specifically lower-DM pectins have to be applied as they support cellular health,114 while for the release and support of large intestinal health, longer and higher blockiness pectins are more suitable.118 As these lower DM pectins are inexpensive and easy to process in different encapsulation settings they are applicable for a broad range of food components such as flavors, antioxidants, oils, bioactive lipids, minerals, vitamins, and enzymes.
Tuning the release of cargo is another field of research that requires some more study. Pectin capsules will generally swell under acidic gastric conditions but will stay intact due to the fact that pectin chains are protonated and remain virtually undissolved in the upper part of the gastrointestinal tract. The capsules will pass through the stomach and small intestine as most pectins are indigestible by proteolytic enzymes.67,72 In the first part of the large intestine, i.e. in the cecum, the pectins will degrade by erosion and by degradation by colonic microbial-derived enzymes. β-Depolymerization and demethoxylation of pectin aggregates will occur and result in the dissociation of the carboxylate groups. This will induce disintegration of the capsules and cargo release. However, how this release can be fine-tuned and how the composition and concentration of pectin in the microcapsules can contribute to a more specific controlled release in specific parts of the large intestine remain to be determined. Feasible approaches are lowering the pectin concentration or crosslinking degree to facilitate the release in the upper part of the gastrointestinal tract or enhancing the concentration or crosslinking of the gel to release the cargo in the more distal part of the colon.
Another field of research that needs to be carried out in order to allow broader application of pectins is to study the impact of the pectin production process, isolation and modification on the molecular structure of pectin. So far, it has been well described that the neutral sugar content and the degree of polymerization unintendedly change during processing or may actively be modified in addition to other parameters like the degree of methoxylation and the distribution of methoxylated groups. Our current understanding is limited to the impact of parameters on interactions with the encapsulate and process design of the different encapsulation techniques. Currently, pectin is applied in only a few industrially relevant microencapsulation technologies. From the present review, it becomes obvious, that this is not due to a lack of suitability, but rather due to a lack of understanding. Future research will lead to a situation, where we can customize pectin structures in a more diverse way on the industrial scale and thus take full advantage of its potential in encapsulation. The hope is that this review contributes to a wider use and application of pectin in domains other than food.
Abbreviations
| DB | Degree of blockiness |
| DM | Degree of methyl-esterification |
| GalA | α-1,4-Linked galacturonic acid |
| GI | Gastrointestinal tract |
| HM | Homogalacturonan |
| RG-I | Rhamnogalacturonan I |
| RG-II | Rhamnogalacturonan II |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 872019.References
- B. N. Estevinho and F. Rocha, Nanotechnol. Appl. Food, 2017, 1–19 CAS.
- R. Sobel, R. Versic and A. G. Gaonkar, in Microencapsulation in the Food Industry, Academic Press, 2014, pp. 3–12 Search PubMed.
- S. Palai, C. M. P. Derecho, S. S. Kesh, C. Egbuna and P. C. Onyeike, Funct. Foods, Nutraceuticals Degener. Dis. Prev., 2020, 173–196 CrossRef.
- M. P. Silva, F. L. Tulini, E. Martins, M. Penning, C. S. Fávaro-Trindade and D. Poncelet, LWT – Food Sci. Technol., 2018, 89, 392–399 CrossRef CAS.
- C. S. Favaro-Trindade, R. J. B. Heinemann and D. L. Pedroso, CAB Rev., 2011, 6, 1–8 Search PubMed.
- C. Wandrey, A. Bartkowiak and S. E. Harding, in Encapsulation Technologies for Active Food Ingredients and Food Processing, ed. N. Zuidam and V. A. Nedović, Springer, New York, 2010, pp. 31–100 Search PubMed.
- K. G. H. Desai and H. J. Park, Dry technol., 2005, 23, 1361–1394 CrossRef CAS.
- R. Domínguez, M. Pateiro, P. E. S. Munekata, D. J. McClements and J. M. Lorenzo, Molecules, 2021, 26, 3984 CrossRef PubMed.
- A. E. Quirós-Sauceda, J. F. Ayala-Zavala, G. I. Olivas and G. A. González-Aguilar, J. Food Sci. Technol., 2014, 51, 1674–1685 CrossRef PubMed.
- M. Fathi, Á. Martín and D. J. McClements, Trends Food Sci. Technol., 2014, 39, 18–39 CrossRef CAS.
- H. M. Shewan and J. R. Stokes, J. Food Eng., 2013, 119, 781–792 CrossRef CAS.
- L. Spadaro, O. Magliocco, D. Spampinato, S. Piro, C. Oliveri, C. Alagona, G. Papa, A. M. Rabuazzo and F. Purrello, Dig. Liver Dis., 2008, 40, 194–199 CrossRef CAS PubMed.
- A. G. J. Voragen, G.-J. Coenen, R. P. Verhoef and H. A. Schols, Struct. Chem., 2009, 20, 263–275 CrossRef CAS.
- B. R. Thakur, R. K. Singh, A. K. Handa and D. M. A. Rao, Crit. Rev. Food Sci. Nutr., 1997, 37, 47–73 CrossRef CAS PubMed.
- P. J. H. Daas, K. Meyer-Hansen, H. A. Schols, G. A. De Ruiter and A. G. J. Voragen, Carbohydr. Res., 1999, 318, 135–145 CrossRef CAS.
- M. A. O'Neill, T. Ishii, P. Albersheim and A. G. Darvill, Annu. Rev. Plant Biol., 2004, 55, 109–139 CrossRef PubMed.
- S. V. Popov, P. A. Markov, I. R. Nikitina, S. Petrishev, V. Smirnov and Y. S. Ovodov, World J. Gastroenterol., 2006, 12, 6646 CrossRef CAS PubMed.
- P. Sriamornsak, N. Wattanakorn and H. Takeuchi, Carbohydr. Polym., 2010, 79, 54–59 CrossRef CAS.
- D. Maria-Ferreira, A. M. Nascimento, T. R. Cipriani, A. P. Santana-Filho, P. da S. Watanabe, D. de M. G. Sant́Ana, F. B. Luciano, K. C. P. Bocate, R. M. van den Wijngaard, M. F. de P. Werner and C. H. Baggio, Sci. Rep., 2018, 8, 1–14 CAS.
- S. Hino, K. Sonoyama, H. Bito, H. Kawagishi, S. Aoe and T. Morita, J. Nutr., 2013, 143, 34–40 CrossRef CAS PubMed.
- T. Jiang, X. Gao, C. Wu, F. Tian, Q. Lei, J. Bi, B. Xie, H. Wang, S. Chen and X. Wang, Nutrients, 2016, 8, 126 CrossRef PubMed.
- E. Wilms, D. M. A. E. Jonkers, H. F. J. Savelkoul, M. Elizalde, L. Tischmann, P. de Vos, A. A. M. Masclee and F. J. Troost, Nutrients, 2019, 11, 1554 CrossRef CAS PubMed.
- L. M. Vogt, N. M. Sahasrabudhe, U. Ramasamy, D. Meyer, G. Pullens, M. M. Faas, K. Venema, H. A. Schols and P. de Vos, J. Funct. Foods, 2016, 22, 398–407 CrossRef CAS.
- X. Gao, Y. Zhi, L. Sun, X. Peng, T. Zhang, H. Xue, G. Tai and Y. Zhou, J. Biol. Chem., 2013, 288, 33953–33965 CrossRef CAS PubMed.
- V. V. Glinsky and A. Raz, Carbohydr. Res., 2009, 344, 1788–1791 CrossRef CAS PubMed.
- S. B. R. do Prado, G. F. Ferreira, Y. Harazono, T. M. Shiga, A. Raz, N. C. Carpita and J. P. Fabi, Sci. Rep., 2017, 7, 16564 CrossRef PubMed.
- L. Díaz-Alvarez and E. Ortega, Mediators Inflammation, 2017, 9247574 Search PubMed.
- N. Gasaly, P. de Vos and M. A. Hermoso, Front. Immunol., 2021, 12, 658354 CrossRef CAS PubMed.
- C. Wu, L. L. Pan, Y. Luo, W. Niu, X. Fang, W. Liang, J. Li, H. Li, X. Pan, G. Yang, W. Chen, H. Zhang, J. R. T. Lakey, B. Agerberth, P. de Vos and J. Sun, Mol. Nutr. Food Res., 2019, 1900307 CrossRef CAS PubMed.
- N. M. Sahasrabudhe, M. Beukema, L. Tian, B. Troost, J. Scholte, E. Bruininx, G. Bruggeman, M. van den Berg, A. Scheurink, H. A. Schols, M. M. Faas and P. de Vos, Front. Immunol., 2018, 9, 383 CrossRef PubMed.
- E. C. Martens, E. C. Lowe, H. Chiang, N. A. Pudlo, M. Wu, N. P. McNulty, D. W. Abbott, B. Henrissat, G. HJ, B. DN and G. JI, PLoS Biol., 2011, 9, e1001221 CrossRef CAS PubMed.
- S. Drusch, Food Hydrocolloids, 2007, 21, 1223–1228 CrossRef CAS.
- A. Zoghi, S. Vedadi, Z. H. Esfahani, H. A. Gavlighi and K. Khosravi-Darani, Biomass Convers. Biorefin., 2021, 1, 1–13 Search PubMed.
- L. S. Liu, M. L. Fishman, J. Kost and K. B. Hicks, Biomaterials, 2003, 24, 3333–3343 CrossRef CAS PubMed.
- A. Rehman, T. Ahmad, R. M. Aadil, M. J. Spotti, A. M. Bakry, I. M. Khan, L. Zhao, T. Riaz and Q. Tong, Trends Food Sci. Technol., 2019, 90, 35–46 CrossRef CAS.
- M. C. Barrera, D. Jakobs-Schoenwandt, M. I. Gómez, J. Serrato, S. Ruppel and A. V. Patel, Biotechnol. Rep., 2020, 26, e00463 CrossRef PubMed.
- E. P. Rebitski, M. Darder, R. Carraro, P. Aranda and E. Ruiz-Hitzky, New J. Chem., 2020, 44, 10102–10110 RSC.
- J. Singh, K. Kaur and P. Kumar, J. Food Sci. Technol., 2018, 55, 3625–3631 CrossRef CAS PubMed.
- W. Wang, Y. Feng, W. Chen, K. Adie, D. Liu and Y. Yin, Ultrason. Sonochem., 2021, 70, 105322 CrossRef CAS PubMed.
- T. Funami, M. Nakauma, S. Ishihara, R. Tanaka, T. Inoue and G. O. Phillips, Food Hydrocolloidsoids, 2011, 25, 221–229 CrossRef CAS.
- IUPAC, IUPAC Compend. Chem. Terminol, 2019 Search PubMed.
- S. Y. Chan, W. S. Choo, D. J. Young and X. J. Loh, Carbohydr. Polym., 2017, 161, 118–139 CrossRef CAS PubMed.
- D. G. Oakenfull, in The chemistry and technology of pectin, ed. R. H. Walter, Academic Press, San Diego, California, 1991, pp. 87–108 Search PubMed.
- M. A. V. Axelos and J.-F. Thibault, Chem. Technol. Pectin, 1991, 109–118 CAS.
- L. Cao, W. Lu, A. Mata, K. Nishinari and Y. Fang, Carbohydr. Polym., 2020, 242, 116389 CrossRef CAS PubMed.
- B. R. Thakur, R. K. Singh, A. K. Handa and M. A. Rao, Crit Rev Food Sci Nutr., 1997, 37, 47–73 CrossRef CAS PubMed.
- H. Kastner, U. Einhorn-Stoll and S. Drusch, Food Hydrocolloids, 2019, 89, 207–215 CrossRef CAS.
- I. Fraeye, E. Vandevenne, T. Duvetter, S. Van Buggenhout, P. Moldenaers, A. Van Loey and M. Hendrickx, Innovative Food Sci. Emerging Technol., 2010, 11, 401–409 CrossRef CAS.
- J. Zheng, J. Chen, H. Zhang, D. Wu, X. Ye, R. J. Linardt and S. Chen, Food Hydrocolloids, 2020, 101, 105536 CrossRef CAS.
- M. Celus, C. Kyomugasho, A. M. Van Loey, T. Grauwet and M. E. Hendrickx, Compr. Rev. Food Sci. Food Saf., 2018, 17, 1576–1594 CrossRef CAS PubMed.
- A. Zdunek, P. M. Pieczywek and J. Cybulska, Compr. Rev. Food Sci. Food Saf., 2021, 20, 1101–1117 CrossRef CAS PubMed.
- I. Ventura, J. Jammal and H. Bianco-Peled, Carbohydr. Polym., 2013, 97, 650–658 CrossRef CAS PubMed.
- L. A. Ditta, D. Bulone, P. L. San Biagio, R. Marino, D. Giacomazza and R. Lapasin, Int. J. Biol. Macromol., 2020, 158, 985–993 CrossRef CAS PubMed.
- D. W. de Kort, J. P. M. van Duynhoven, H. VanAs and F. Mariette, Trends Food Sci. Technol., 2015, 42, 13–26 CrossRef CAS.
- H. Kastner, U. Einhorn-Stoll, A. Fatouros and S. Drusch, Food Hydrocolloids, 2020, 104, 105750 CrossRef CAS.
- H. Kastner, K. Kern, R. Wilde, A. Berthold, U. Einhorn-Stoll and S. Drusch, Food Chem., 2014, 144, 44–49 CrossRef CAS PubMed.
- P. Burey, B. Bhandari, T. Howes and M. Gidley, Crit. Rev. Food Sci. Nutr., 2008, 48, 391–377 CrossRef PubMed.
- K. N. P. Humblet-Hua, G. Scheltens, E. van der Linden and L. M. C. Sagis, Food Hydrocolloids, 2011, 25, 569–576 CrossRef CAS.
- L. Liu, M. L. Fishman and K. B. Hicks, Cellulose, 2007, 14, 15–24 CrossRef CAS.
- R. N. Tharanathan, Trends Food Sci. Technol., 2003, 14, 71–78 CrossRef CAS.
- F. Atyabi, S. Majzoob, M. Iman, M. Salehi and F. Dorkoosh, Carbohydr. Polym., 2005, 61, 39–51 CrossRef CAS.
- N. Thirawong, J. Nunthanid, S. Puttipipatkhachorn and P. Sriamornsak, Eur. J. Pharm. Biopharm., 2007, 67, 132–140 CrossRef CAS PubMed.
- N. Thirawong, R. A. Kennedy and P. Sriamornsak, Carbohydr. Polym., 2008, 71, 170–179 CrossRef CAS.
- G. A. Morris, S. M. Kök, S. E. Harding and G. G. Adams, Biotechnol. Genet. Eng. Rev., 2010, 27, 257–284 CrossRef CAS PubMed.
- P. Sriamornsak, Expert Opin. Drug Delivery, 2011, 8, 1009–1023 CrossRef CAS PubMed.
- A. Butt, N. Nisar and T. Mughal, J. Pak. Med. Assoc., 2018, 68, 607–614 Search PubMed.
- M. Khotimchenko, Int. J. Biol. Macromol., 2020, 158, 1110–1124 CrossRef CAS PubMed.
- S. Y. Wang, Y. J. Meng, J. Li, J. P. Liu, Z. P. Liu and D. Q. Li, Int. J. Biol. Macromol., 2020, 157, 170–176 CrossRef CAS PubMed.
- S. Groult, S. Buwalda and T. Budtova, Eur. Polym. J., 2021, 149, 110386 CrossRef CAS.
- D. G. Oakenfull and A. Scott, J. Food Sci., 1984, 49, 1093–1098 CrossRef CAS.
- D. G. Oakenfull, in The Chemistry and Technology of Pectin, ed. R. H. Walter, Academic Press, New York, 1991, pp. 87–108 Search PubMed.
- F. Munarin, M. C. Tanzi and P. Petrini, Int. J. Biol. Macromol., 2012, 51, 681–689 CrossRef CAS PubMed.
- M. C. Ralet and J. F. Thibault, Biomacromolecules, 2002, 3, 917–925 CrossRef CAS PubMed.
- M. Ralet, E. Bonnin and J. Thibault, J. Chromatogr. B: Biomed. Sci. Appl., 2001, 753, 157–166 CrossRef CAS.
- M. Y. Khotimchenko, E. A. Kolenchenko and Y. S. Khotimchenko, J. Colloid Interface Sci., 2008, 323, 216–222 CrossRef CAS PubMed.
- L. Yang, J. S. Chu and J. A. Fix, Int. J. Pharm., 2002, 235, 1–15 CrossRef CAS PubMed.
- H. S. Sardou, A. Akhgari, H. A. Garekani and F. Sadeghi, Int. J. Pharm., 2019, 568, 118527 CrossRef PubMed.
- M. Patel, T. Shah and A. Amin, Crit. Rev. Ther. Drug Carrier Syst., 2007, 24, 147–202 CrossRef CAS PubMed.
- V. R. Sinha and R. Kumria, Int. J. Pharm., 2001, 224, 19–38 CrossRef CAS PubMed.
- Y. Cao and R. Mezzenga, Nat. Food, 2020, 1, 106–118 CrossRef.
- L. Joergensen, B. Klösgen, A. C. Simonsen, J. Borch and E. Hagesaether, Int. J. Pharm., 2011, 411, 162–168 CrossRef CAS PubMed.
- N. A. Peppas and Y. Huang, Adv. Drug Delivery Rev., 2004, 56, 1675–1687 CrossRef CAS PubMed.
- J. Schmidgall and A. Hensel, Int. J. Biol. Macromol., 2002, 30, 217–225 CrossRef CAS PubMed.
- L. Liu, M. L. Fishman, K. B. Hicks and M. Kende, Biomaterials, 2005, 26, 5907–5916 CrossRef CAS PubMed.
- P. Sriamornsak, N. Wattanakorn, J. Nunthanid and S. Puttipipatkhachorn, Carbohydr. Polym., 2008, 74, 458–467 CrossRef CAS.
- M. Singh, N. Singh, B. Chandrasekaran and P. K. Deb, in Integrative Nanomedicine for New Therapies, ed. A. Krishnan and A. Chuturgoon, Springer International Publishing, Cham, Switzerland, 2020, pp. 405–435 Search PubMed.
- B. M. Boddupalli, Z. N. K. Mohammed, R. A. Nath and D. Banji, J. Adv. Pharm. Technol. Res., 2010, 1, 381–387 CrossRef CAS PubMed.
- S. Punitha and Y. Girish, Int. J. Res. Pharm. Sci., 2010, 1, 170–186 CAS.
- A. B. Meneguin, A. L. P. Silvestre, L. Sposito, M. P. C. de Souza, R. M. Sábio, V. H. S. Araújo, B. S. F. Cury and M. Chorilli, Carbohydr. Polym., 2021, 256, 117504 CrossRef CAS PubMed.
- T. R. Hoare and D. S. Kohane, Polymer, 2008, 49, 1993–2007 CrossRef CAS.
- J. Li and D. J. Mooney, Nat. Rev. Mater., 2016, 1, 1–17 Search PubMed.
- R. Semdé, K. Amighi, M. Devleeschouwer and A. Moës, Int. J. Pharm. Pharm., 2000, 197, 181–192 Search PubMed.
- K. Ofori-Kwakye and J. T. Fell, Int. J. Pharm. Pharm., 2001, 226, 139–145 CAS.
- M. Turkoglu and T. Ugurlu, Eur. J. Pharm. Biopharm., 2002, 53, 65–73 CrossRef CAS PubMed.
- S. Zhao, Y. Zhang, Y. Liu, F. Yang, W. Yu, S. Zhang, X. Ma and G. Sun, Int. J. Biol. Macromol., 2018, 115, 29–34 CrossRef CAS PubMed.
- W. P. Voo, P. Ravindra, B. T. Tey and E. S. Chan, J. Biosci. Bioeng., 2011, 111, 294–299 CrossRef CAS PubMed.
- M. C. Tarifa, C. M. Piqueras, D. B. Genovese and L. I. Brugnoni, Int. J. Biol. Macromol., 2021, 179, 457–465 CrossRef CAS PubMed.
- A. T. B. Nguyen, P. Winckler, P. Loison, Y. Wache and O. Chambin, Colloids Surf., B, 2014, 121, 290–298 CrossRef CAS PubMed.
- J. Jung, R. D. Arnold and L. Wicker, Colloids Surf., B, 2013, 104, 116–121 CrossRef CAS PubMed.
- E. M. Jacob, A. Borah, A. Jindal, S. C. Pillai, Y. Yamamoto, T. Maekawa, D. Nair and S. Kumar, J. Mater. Res., 2020, 35, 1514–1522 CrossRef CAS.
- G. Auriemma, T. Mencherini, P. Russo, M. Stigliani, R. P. Aquino and P. Del Gaudio, Carbohydr. Polym., 2013, 92, 367–373 CrossRef CAS PubMed.
- A. Bidoret, L. Guihard, L. Cauret and D. Poncelet, Can. J. Chem. Eng., 2017, 95, 799–805 CrossRef CAS.
- M. Saravanan and K. P. Rao, Carbohydr. Polym., 2010, 80, 808–816 CrossRef CAS.
- B. Muhoza, S. Xia and X. Zhang, Food Hydrocolloids, 2019, 97, 105174 CrossRef CAS.
- B. da Silva Soares, C. W. P. de Carvalho and E. E. Garcia-Rojas, Food Bioprocess Technol., 2021, 14, 817–830 CrossRef CAS.
- X.-Y. Tang, Z.-M. Wang, H.-C. Meng, J.-W. Lin, X.-M. Guo, T. Zhang, H.-L. Chen, C.-Y. Lei and S.-J. Yu, J. Agric. Food Chem., 2021, 69, 1318–1328 CrossRef CAS PubMed.
- I. Lacík, in Fundamentals of Cell Immobilisation Biotechnology, ed. V. Nedović and R. Willaert, Springer, Dordrecht, 2004, pp. 103–120 Search PubMed.
- V. B. V. Maciel, C. M. P. Yoshida and T. T. Franco, Carbohydr. Polym., 2015, 132, 537–545 CrossRef CAS PubMed.
- C. Anandharamakrishnan, Techniques for nanoencapsulation of food ingredients, Springer, New York, 2014 Search PubMed.
- P. N. Ezhilarasi, P. Karthik, N. Chhanwal and C. Anandharamakrishnan, Food Bioprocess Technol., 2012, 6, 628–647 CrossRef.
- G. Pamunuwa, N. Anjalee, D. Kukulewa, C. Edirisinghe, F. Shakoor and D. N. Karunaratne, Carbohydr. Polym. Technol. Appl., 2020, 100008 Search PubMed.
- F. Sansone, T. Mencherini, P. Picerno, M. D'Amore, R. P. Aquino and M. R. Lauro, J. Food Eng., 2011, 105, 468–476 CrossRef CAS.
- M. Beukema, M. M. Faas and P. de Vos, Exp. Mol. Med., 2020, 52, 1364–1376 CrossRef CAS PubMed.
- S. Hu, R. Kuwabara, M. Beukema, M. Ferrari, B. J. de Haan, M. T. C. Walvoort, P. de Vos and A. M. Smink, Carbohydr. Polym., 2020, 249, 116863 CrossRef CAS PubMed.
- M. Beukema, E. Jermendi, M. A. van den Berg, M. M. Faas, H. A. Schols and P. de Vos, Carbohydr. Polym., 2021, 251, 117093 CrossRef CAS PubMed.
- M. Beukema, K. Ishisono, J. de Waard, M. M. Faas, P. de Vos and K. Kitaguchi, Food Funct., 2021, 12, 881–891 RSC.
- M. Beukema, É. Jermendi, T. Koster, K. Kitaguchi, B. J. de Haan, M. A. van den Berg, M. M. Faas, H. A. Schols and P. de Vos, Mol. Nutr. Food Res., 2021, 65, 2100222 CrossRef CAS PubMed.
- M. Beukema, R. Akkerman, É. Jermendi, T. Koster, A. Laskewitz, C. Kong, H. A. Schols, M. M. Faas and P. de Vos, Mol. Nutr. Food Res., 2021, 65, 2100346 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |