Open Access Article
Open Access ArticleIn vitro digestion of designed emulsions based on milk protein and guar gum systems†
Wentao
Liu
 *a,
Mita
Lad
b and
Tim
Foster
a
*a,
Mita
Lad
b and
Tim
Foster
a
aDivision of Food, Nutrition and Dietetics, School of Biosciences, University of Nottingham, Sutton Bonington campus, LE12 5RD, UK. E-mail: wentaoliu1@hotmail.com; Tel: +44 (0)7729 243211
bJubilee Conference Centre, Jubilee Campus, University of Nottingham, NG8 1BB, UK
First published on 21st April 2022
Abstract
There is a growing interest in designing novel food microstructures that can control nutrient digestion and provide satiety for tackling obesity. In this study, phase separated microstructures of skimmed milk powder (SMP) and guar gum (GG) were the main focus, and these can be considered as water-in-water (W/W) emulsions. Through the incorporation of oil into these systems, it was possible to form model systems of SMP-GG-OIL, showing the lipid phase within the protein phase within the polysaccharide phase. The in vitro digestibility of such phase separated model systems of SMP-GG-OIL with different microstructures was investigated using a pH stat method. Confocal laser scanning microscopy also revealed structural changes that occurred to the emulsified lipid droplets as they passed through a gastrointestinal (GI) model. The microstructures were created based on the tie-lines on a previously established phase diagram of SMP-GG, and shown to be able to control lipid digestion. For a selected tie-line, the lipolysis follows the order: protein continuous > bi-continuous > polysaccharide continuous system, at a certain level of oil addition. The mechanism involved in the lipolysis of the designed formulations/microstructures was dependent upon the protein, rather than GG, and was driven by the protein concentration. These findings provide insights for potential applications in functional food designing in the food industry.
1. Introduction
To combat the obesity epidemic issues, especially in the western world, new food products/microstructures having an effect on satiety, so as to reduce the consumption of excessive calories, are considered by the food industry. Several mechanisms are known to be able to control the appetite/satiation and subsequently energy intake, such as stomach physical distension, inhibition of gastric emptying (duodenal/jejunal brake), inhibition of intestinal motility (ileal brake) and triggering of neural and hormonal pathways as a consequence of the interaction of nutrients with intestinal receptors.1–3 Other mechanisms from a sensory perspective may also play a role in satiation and energy regulation.4 However, clinical studies suggest that an intestinal mechanism (e.g. ileal brake mechanism) is more potent, although both gastric and intestinal signal interactions can modulate energy intake. The ileal brake mechanism5,6 has become of growing interest when considering the design of novel food emulsion microstructures to control (i.e. delay) lipid digestion.Until recently, many studies have focused on the investigations of controlling in vitro lipid digestion in emulsion based systems through interfacial design, gel/matrix structure design or controlling the transportation of lipase,3,7–16 and mechanisms for lipolysis have been reviewed by Wilde and Chu.17 However, the technique used to modify the interfacial structure may have some limitations, for example, the layer-by-layer (LBL) electrostatic deposition technique (building multiple layers around the droplets using different oppositely charged emulsifiers) may cause the occurrence of aggregation/flocculation of droplets during emulsion preparation and an increase of the total product cost. Some of the emulsion gel-based systems can be complex to prepare for real food application, specifically at the commercial scale. Thus, in order to avoid such problems, an attempt to develop other alternative microstructure approaches that are easy to form has become rather important.
Currently, model phase separated systems containing protein, polysaccharides and oil have shown that the oil phase in such systems remains in the protein phase,18,19 and the fat distribution in the microstructure can be controlled not only by the amount used but also by the phase where it is dispersed.20 These studies demonstrate the potential of using a mixed protein–polysaccharide system as a structuring agent of fat distribution in the system. The concept of phase separation could be used for a novel microstructure design, although recently there is a trend wherein studies are also using hydrocolloid based oleogels for oil-structuring.21 However, to date, there is limited knowledge on in vitro lipid digestion based on such model phase separated systems (containing protein, polysaccharides and oil). Although mixed biopolymer solutions (e.g. protein–polysaccharide mixtures) and their phase separation phenomena depending on type, concentration and mixing ratio have been extensively investigated,22–25 the breakdown properties of phase separated protein–polysaccharide mixtures and modelled protein–polysaccharide–oil systems in the gastro-intestinal (GI) tract are still less understood.
There has been a number of recent studies on developing a better understanding of the behaviour of milk protein stabilized emulsions during in vitro gastrointestinal digestion.12,16,26–30 However, most of these studies have been carried out using relatively simple, well-defined systems and focus on a fundamental level of understanding on the digestibility of proteins and lipids. For example, the emulsion prepared usually contains a low concentration of protein as an emulsifier (typically 1 wt% protein in a system). Therefore, due to the very limited studies on the investigation of in vitro digestion when an emulsion system containing usual product formulations is used, in this study it has been attempted to investigate how a formulation of SMP-GG-OIL with excessive protein content present in the bulk phase is digested in vitro.
In this study, focus is given to a dairy based food matrix to investigate a model system containing milk protein, guar gum and oil. Guar gum with low and high molecular weights has been chosen due to its relatively high availability and low cost and its potential appetite control effect as dietary fibre.31 Skimmed milk powder is also used, because it not only provides milk proteins, but it also allows maintaining the original ionic environment of milk (e.g. minerals and lactose), which is more consistent with the real dairy products.
Thus, the objective of this study is to investigate how phase separated mixtures of SMP-GG (W/W emulsions) are digested (measured in vitro). After the addition of oil into these mixtures to form modelled formulations of SMP-GG-OIL (O/W/W emulsions), the aim was to further investigate whether or not a phase separated microstructure (phase continuity) can control lipid digestion, and the digestive effect of GG with different molecular weights, to understand their structural changes during simulated gastric and small intestinal digestion. The hypothesis is that the phase separated structure could control (likely delay) the oil digestion due to the potential phase continuity (e.g. protein phase and/or GG phase) protection effect.
2. Materials and methods
2.1. Materials
Skimmed milk powder (SMP) used was provided by Dairy Crest (UK) and protein content was calculated to be approximately 26% (w/w). Guar gum (GG) with high molecular weight (type 400, molecular weight of 2660 kDa) and low molecular weight (LMw) (type 30, molecular weight of 420 kDa) were provided by Danisco – DuPont (Denmark). Sunflower oil was used as received from a local supermarket (Sainsbury's, UK). Nile Red, rhodamine B and Fast Green FCF dyes were obtained from Sigma-Aldrich (UK) for confocal microscopy. All other chemicals, such as salts, alkaline and acids used for the formulation of digestive fluids, were of analytical grade obtained from either Sigma (Gillingham, UK) or Fisher Scientific (UK) unless stated otherwise. The type of enzymatic materials used are given in the Methods section.2.2. Methods
| Formulations | Structures | Phase volume ratio (SMP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GG) GG) |
Starting mixing concentration (SMP + GG; wt%) | Composition (SMP + GG; wt%) |
|---|---|---|---|---|
| Ps = polysaccharide; Pr = protein. The mixing volume ratio of the starting mixing concentrations is the same as the phase volume ratio. | ||||
| TL1 (HMw GG) | ||||
| A1 | Ps-continuous | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
44% + 0.7% | 11% + 0.53% |
| B1 | Bi-continuous | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
41% + 0.72% | 20.5% + 0.36% |
| C1 | Pr-continuous | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
40% + 0.76% | 30% + 0.19% |
| TL2 (HMw GG) | ||||
| A2 | Ps-continuous | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
36% + 0.46% | 9% + 0.35% |
| B2 | Bi-continuous | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
34% + 0.46% | 17% + 0.23% |
| C2 | Pr-continuous | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
33% + 0.45% | 25% + 0.11% |
| TL2 (LMw GG) | ||||
| a | Ps-continuous | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
48% + 0.73% | 12% + 0.55% |
| b | Bi-continuous | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
44% + 0.76% | 22% + 0.38% |
| c | Pr-continuous | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
42% + 0.8% | 31.5% + 0.21% |
These mixtures, on their respective tie-lines, correspond to a polysaccharide continuous microstructure (SMP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GG in a phase ratio of 25
GG in a phase ratio of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 for A2/A1), a bi-continuous microstructure (50
75 for A2/A1), a bi-continuous microstructure (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 for B2/B1) or a protein continuous microstructure (75
50 for B2/B1) or a protein continuous microstructure (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
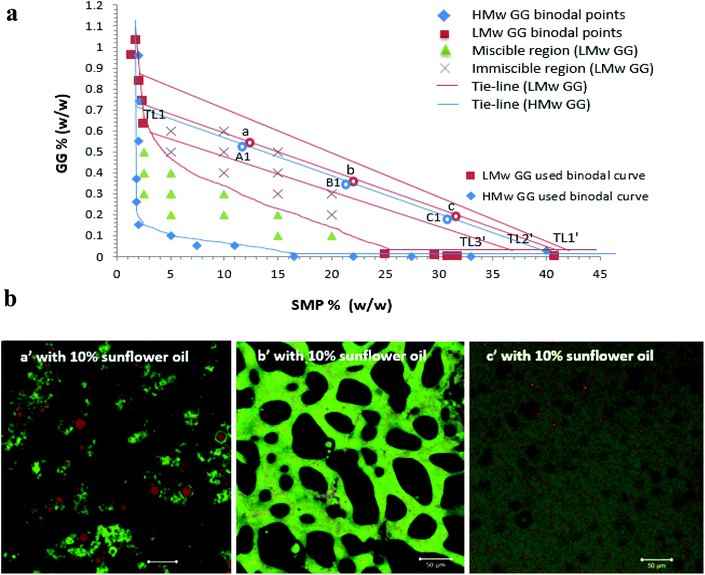
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 for C2/C1) (Table 1). Similarly, for the low molecular weight GG phase diagram, three formulations (a, b and c) on the tie-line 2 (TL2′) of the phase diagram of the SMP-LMw GG system were prepared, as shown in Table 1. These samples were prepared by pipetting certain volumes and starting mixing concentrations of SMP and GG (as shown in Table 1), followed by mixing using a Stuart roller mixer (SRT9) at 100 rpm for 20 min at room temperature before use.
25 for C2/C1) (Table 1). Similarly, for the low molecular weight GG phase diagram, three formulations (a, b and c) on the tie-line 2 (TL2′) of the phase diagram of the SMP-LMw GG system were prepared, as shown in Table 1. These samples were prepared by pipetting certain volumes and starting mixing concentrations of SMP and GG (as shown in Table 1), followed by mixing using a Stuart roller mixer (SRT9) at 100 rpm for 20 min at room temperature before use.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20; (B1′ or B2′) 40
20; (B1′ or B2′) 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20; and (C1′ or C2′) 60
20; and (C1′ or C2′) 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (SMP
20 (SMP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GG
GG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Oil), respectively. The same procedures were applied for 10% oil addition to form the emulsions, giving the ‘final’ phase volume ratios of: (A1′ or A2′) 22.5
Oil), respectively. The same procedures were applied for 10% oil addition to form the emulsions, giving the ‘final’ phase volume ratios of: (A1′ or A2′) 22.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67.5
67.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10; (B1′ or B2′) 45
10; (B1′ or B2′) 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10; and (C1′ or C2′) 67.5
10; and (C1′ or C2′) 67.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22.5
22.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (SMP
10 (SMP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GG
GG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Oil), respectively. These mixtures were subsequently homogenised using a high-speed overhead mixer at 25
Oil), respectively. These mixtures were subsequently homogenised using a high-speed overhead mixer at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 min (Ultra-Turrax, IKA T18 basic, USA) to form fine emulsions. The polysaccharide continuous and bi-continuous systems/structures refer to the Matryoshka-like structures, which can be viewed as ‘o/w/w’ emulsions; the protein continuous systems were termed ‘oil-filled w/w emulsions’, as published in our previous work.32
000 rpm for 2 min (Ultra-Turrax, IKA T18 basic, USA) to form fine emulsions. The polysaccharide continuous and bi-continuous systems/structures refer to the Matryoshka-like structures, which can be viewed as ‘o/w/w’ emulsions; the protein continuous systems were termed ‘oil-filled w/w emulsions’, as published in our previous work.32
2.2.3.1. Simulated in vitro gastric digestion. 10 mL of SMP–GG mixtures (formulations A1, B1 and C1 without oil addition) or SMP-GG-Oil emulsions (A1′, B1′ and C1′, with oil addition) were mixed with 8 mL of pre-prepared SGF stock solution, followed by adding a total of 1 mL of HCl solution (1 M and/or 5 M, variable volumes) and CaCl2 solution (0.3 M, 0.5 μL) to adjust the pH down to 3. Then, the simulated gastric digestion was started by adding 1 mL of porcine pepsin solution (11.24 mg mL−1, pepsin (P6887-1G) from porcine gastric mucosa with an activity of 3200–4500 units per mg of protein, Sigma) into the acidified mixtures. This gave a final volume of 20 mL digestion mixture with a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixing ratio of food sample to simulated gastric juice. Digestion was carried out for 2 hours (refers to averaged half-emptying timeframe) in an incubator at 37 °C with constant stirring at 200 rpm. Samples were taken periodically (0, 30, 60 and 120 min) as well as at pH 3 using a pipette from the middle of the mixing digesta for further analysis. Control experiments to investigate the impact of the presence of guar gum on in vitro digestion were conducted under the same conditions, but by using RO water instead of guar gum.
1 mixing ratio of food sample to simulated gastric juice. Digestion was carried out for 2 hours (refers to averaged half-emptying timeframe) in an incubator at 37 °C with constant stirring at 200 rpm. Samples were taken periodically (0, 30, 60 and 120 min) as well as at pH 3 using a pipette from the middle of the mixing digesta for further analysis. Control experiments to investigate the impact of the presence of guar gum on in vitro digestion were conducted under the same conditions, but by using RO water instead of guar gum.
2.2.3.2. Simulated in vitro small intestinal digestion. After 2 hours of simulated stomach digestion, only sample mixtures with oil were taken to the next stage of digestion (small intestinal digestion). Here, 20 mL mixtures from the stomach phase (gastric chyme) were adjusted to pH 7, aiming to stop gastric digestion using a total of 1.46 mL of NaOH (1 M, variable volumes) and distilled water (necessary volumes). Then, 11 mL of pre-prepared SIF, 2.5 mL of bile salt solution (porcine bile extract, B8631-100G, Sigma) and 40 μL of CaCl2 (0.3 M) were also added into the gastric phase sample. Subsequently, after a final pH check (pH = 7), the intestinal digestion was started by adding 5 mL of freshly prepared porcine pancreatin solution (pancreatin from porcine pancrease, P7545-25G, Sigma). This eventually resulted in a final mixing ratio of gastric chyme sample (20 mL) to simulated duodenum juice (20 mL) of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Digestion was conducted at 37 °C for 2 hours in a temperature controlled water bath with constant stirring and automatic pH-stat titration control. Samples were periodically (0, 30, 60, 90 and 120 min as well as at pH = 3) taken and/or immediately frozen by placing in a −80 °C freezer and stored for further analysis.
1. Digestion was conducted at 37 °C for 2 hours in a temperature controlled water bath with constant stirring and automatic pH-stat titration control. Samples were periodically (0, 30, 60, 90 and 120 min as well as at pH = 3) taken and/or immediately frozen by placing in a −80 °C freezer and stored for further analysis.
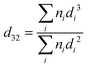 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GG
GG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Oil mixture + simulated intestinal fluids (SIF) + bile salts and pancreatin) to maintain the pH at 7 over 2 hours of digestion. The volume of NaOH that was titrated to the emulsion sample was recorded every 2 or 5 min.
Oil mixture + simulated intestinal fluids (SIF) + bile salts and pancreatin) to maintain the pH at 7 over 2 hours of digestion. The volume of NaOH that was titrated to the emulsion sample was recorded every 2 or 5 min.
The total quantity of FFA released from intestinal digestion was determined by the following equation (eqn (2)):
| FFA (mmol) = VNaOH × mNaOH, | (2) |
3. Results and discussion
3.1. Digestion of O/W/W emulsion (formulation containing SMP-GG-OIL)
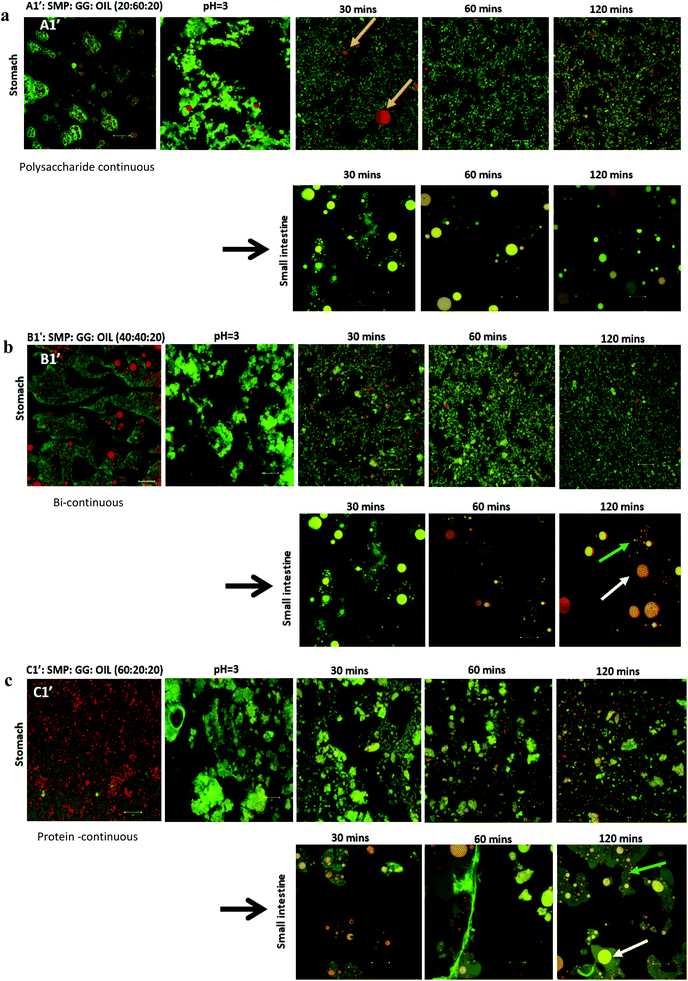
In this study, emulsions on TL1 (A1′, B1′ and C1′) and TL2 (A2′, B2′ and C2′) have been formed (‘o/w/w’ emulsions with Matryoshka-like structures (e.g. A1′ and B1′) and ‘oil-filled w/w emulsions’, e.g. C1′).32 These emulsions were selected and subjected to simulated gastric and duodenal digestion conditions; the resulting changes in the emulsion structure were recorded using confocal microscopy. The impact on lipolysis was determined by measuring free fatty acid production via the pH-stat method.Under simulated stomach digestion, the protein aggregates (entrapping oil droplets) are expected to break down into small protein fragments, such as polypeptides and peptides (see Fig. 1 for gastric digestion between 30 and 120 min), which is attributed to the protein digestion by the action of pepsin. As a result, some isolated oil droplets start to appear from 30 min in gastric digestion for all three samples; however, the large oil droplet observed in emulsion A1 (at 30 min) suggests that flocculation may occur, which promotes coalescence. Indeed, Sarkar et al.37 reported that the hydrolysis of the protein layer adsorbed at the O/W interface by pepsin would lead to the occurrence of flocculation and coalescence of droplets. Li et al.38 further studied the gastric digestive reaction of sodium caseinate-stabilized emulsions and found that digestion in simulated gastric fluid containing pepsin accelerated coalescence of the emulsion droplets. This was due to the changes of interfacial coverage and viscoelasticity as indicated by Maldonado-Valderrama and co-workers.29
In the simulated duodenal phase, triacylglycerides (TAGs) in the oil get broken down into mono and diglycerides and free fatty acids (FFAs), and then any undigested and/or partially digested protein is further hydrolysed by the action of trypsin and α-chemotrypsin. Fig. 1 shows that the oil droplets have undergone coalescence, as the droplets appear larger in size, which is associated with the loss of protein network protection due to proteolysis. This implies that oil is digested by pancreatin, generating FFA and micelles.16,39,40 Moreover, the phases (with pale yellow/orange colour) present in the duodenal phase of the three samples were found. This is in agreement with a recently published study.41 Although they did not explain what those phases are, it may indicate some of the undigested proteins, or may be the mixture of peptides, oil, digestion products and bio-surfactants (e.g. bile salt). This is clearly an area for further study, as indicated by Foster and Norton,42 who highlighted the complexity of the fat digestion, especially in the presence of released peptides, digestive products and the body of bile salts, as such mixed materials could be the sources of self-assembling structures that would complicate the system further. In these phases, apart from the large droplets (indicated by white arrows) induced from coalescence during digestion, a considerable number of small particles/droplets were also generated in the duodenal phase after long digestion time, particularly for the formulations B1′ and C1′ at 120 min (Fig. 1b and c, as indicated by green arrows). This can be attributed to the break-up of these large coalesced droplets during digestion, and these small droplets released from the large droplets may be different from the original emulsion droplets. They were probably mixed with digestion products, proteins/peptides and bile salts, where droplets were stabilized by high surface active bile salts and some lipolytic products may also have been solubilized in bile salts and may have existed as mixed micelles.
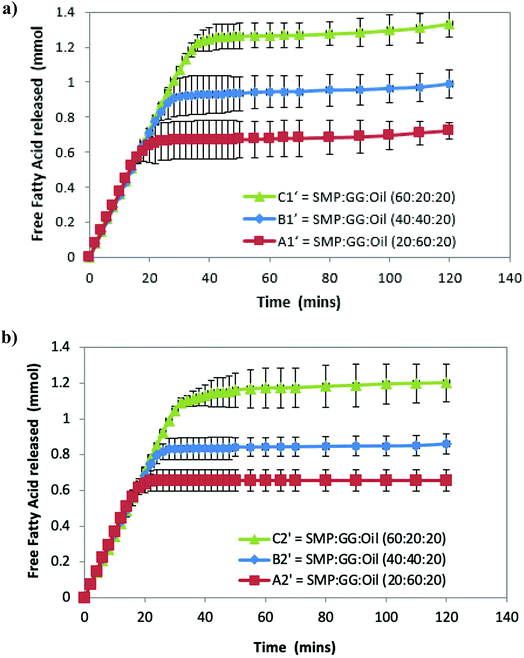
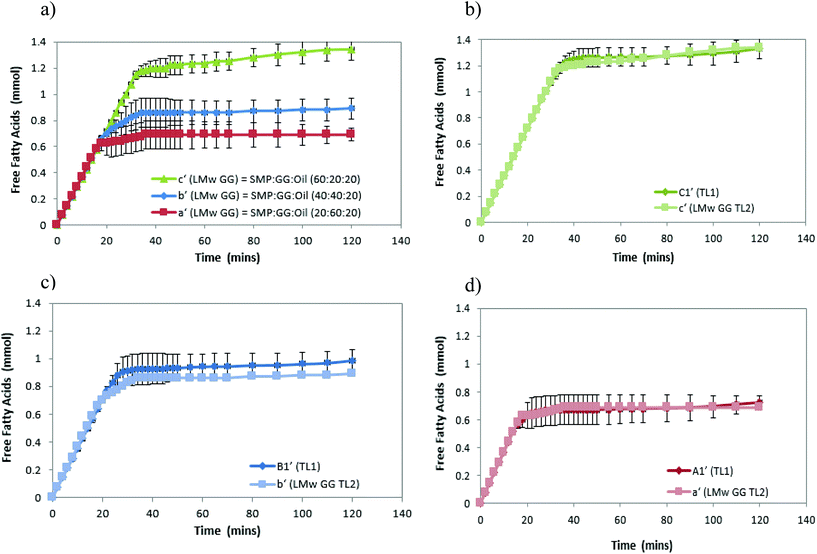
Fig. 2a shows that the amount of FFA released during lipolysis increased steadily in the first 20 min. The lipolysis of the three samples then begins to show the effect of microstructures, with the FFA production of all samples plateauing within 40 min. However, it should be noted that during the first 15–20 min, a drop in pH from 7 observed (because of the pancreatin digestive action) in emulsion C1′ was larger than that observed in B1′ and A1′, suggesting that the rate of FFA production in emulsion C1′ was faster than other two samples (followed by C1′ > B1′ > A1′), although Fig. 2 shows a same rate of lipolysis among samples during the first 20 min. This is owing to the program setting limitation of the pH stat instrument used. The limitation of the pH stat unit used was that the program could only be set at the same rate of titration of NaOH solution rather than at a dynamic titration mode; therefore, the drop in the pH (especially in the initial digestion stage) could not be immediately compensated by the titration with NaOH. This resulted in the same slope of the digestion curves (unseparated) in the initial digestion stage. Nevertheless, the extent of the lipolysis of the measured systems is well described in Fig. 2. Moreover, as a comparison, control samples that are prepared under the same treatment, but with the absence of pancreatin, have not shown a decrease in pH (pH = 7) over time, suggesting that there are no FFAs being released (data not shown).
Although all the samples reached a plateau at a long digestion time, it does not mean that lipid hydrolysis proceeded to completion. The lipolysis leveling off suggests that the O/W interface was no longer available for the enzymatic action, i.e. the reaction was inhibited by the accumulation of the lipolytic products (i.e. FFAs and MAGs) at the droplet surface in the simulated duodenal phase. However, it would be also useful to conduct a back titration at the completion of each pH stat experiment as future work, because the pH stat method has limitations that may underestimate the lipolysis due to possible incomplete titration of fatty acids (e.g. titration near or below pKa values). It is noteworthy that after the initial rapid digestion (Fig. 2), a slowdown of lipolysis rather than an immediate cessation of lipolysis (plateaus) of the samples for a short period of time (i.e. between 30 and 45 min for sample C2′, Fig. 2b) was observed. This was mainly because when the drop in pH of all samples in the initial stage was compensated by the titration of NaOH (pH reached at 7), the pH-stat was regaining control.
Table 2 shows that the total amount of FFA released in emulsion A1′ is approximately half that of emulsion C1′; while the total FFA produced in emulsion B1′ is greater than emulsion A1′, although it is less than emulsion C1′ (Table 2). The lipolysis of emulsions A2′, B2′ and C2′ with lower compositions (TL2) shows similar trends as in the case of emulsions A1′, B1′ and C1′ (Fig. 2a and b), and their total FFAs released from lipolysis also are recorded in Table 2.
| Formulation | Structure | Phase volume ratio | Total FFA/mmoL |
|---|---|---|---|
SMP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GG GG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OIL OIL |
Mean ± SDEV | ||
| Ps = polysaccharide; Pr = protein. HMw GG was used. | |||
| TL1 | |||
| A1′ | Ps-continuous | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
0.711 ± 0.048 |
| B1′ | Bi-continuous | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
0.988 ± 0.076 |
| C1′ | Pr-continuous | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
1.332 ± 0.079 |
| TL2 | |||
| A2′ | Ps-continuous | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
0.675 ± 0.058 |
| B2′ | Bi-continuous | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
0.860 ± 0.053 |
| C2′ | Pr-continuous | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
1.202 ± 0.105 |
The comparison of the formulations on different tie-lines (TLs) is setting up a way to study the influence of formulation composition on the digestibility of two samples but retaining the same structures (i.e. comparing protein continuous microstructures, C1′ and C′). We found that when changing the composition of a selected formulation without changing its structure, the formulation with a higher biopolymer concentration (TL1) had greater lipid digestibility. Moreover, when changing the amount of oil, for instance, at 10% oil, in comparison with 20%, it was also found that the lipolysis of samples still had the order C1′ > B1′ > A1′ and C2′ > B2′ > A2′ (data not shown).
These results suggest that lipolysis increases with increasing protein phase volume, which also equates to an increase in the protein concentration within the emulsion, while the protein phase composition remain the same. In other words, for a given tie-line, when there is a greater concentration of GG in the emulsion, lipolysis is reduced. From this point of view, it can be concluded that different microstructures affect lipolysis.
3.2. Digestion of W/W emulsion (mixture of SMP-GG)
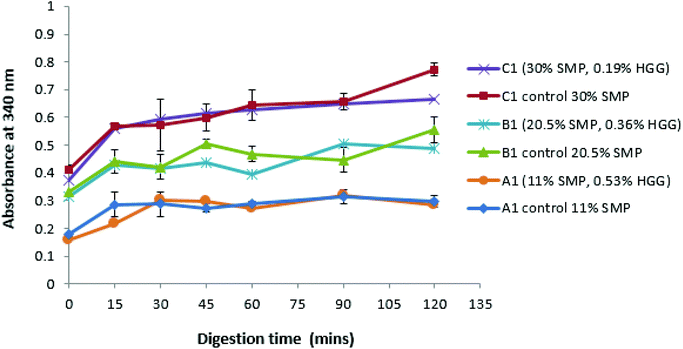
Fig. 3 shows that, with or without GG addition, there is more amino acid release as the total protein concentration increases. For example, the sample A1 and control A1 show the same amino acid release behaviour, which disproves the assumption that the sample in the absence of GG would be digested more quickly due to a lower viscosity in the system. However, it is observed that the protein digestion increases with increasing protein concentration (C1 > B1 > A1), confirmed from the control group samples. Once more, a similar effect was also found in samples from TL2 (data not shown). Nevertheless, it was surprising that an absorbance value was recorded even before the protein digestion began (at zero min), and this is probably due to the initial proteins (before digestion) having exposed reduced sulfhydryl groups, NH2 groups of primary amines which can react with o-phthaldialdehyde to form a fluorescent molecule.34
These results suggest that GG does not protect against proteolysis, or at least that the viscosity of the samples prepared in this study is not sufficient to inhibit the protein digestion. However, an extremely high viscosity in the system would have a ‘slow down’ effect on the proteolysis/digestion process.43 Amyoony et al.44 have studied the effect of the GG concentration on lipolysis and found that a 4% GG solution showed a reduction in lipolysis. They suggested that the high viscosity imparted by the high concentration of GG was the main reason due to the reduced diffusion of enzymes to the substrates. However, in this study, such high viscosity samples/formulations were not included.
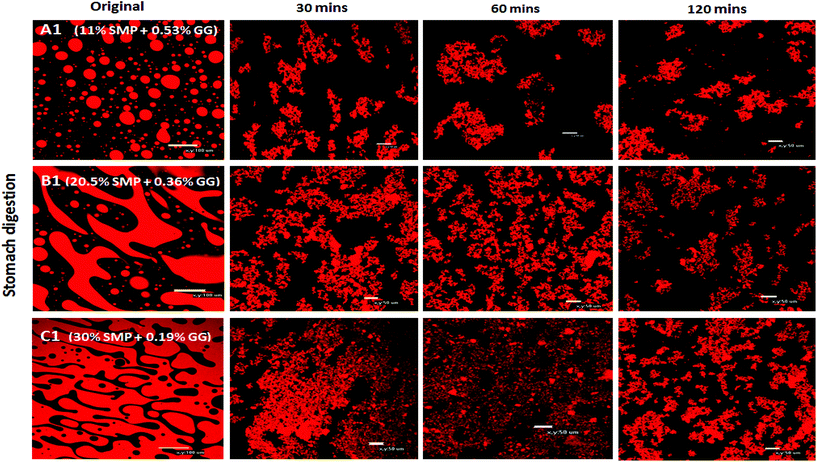
With regard to the extent of proteolysis, we found that protein digestion increased with increasing protein content, and this is also evident from the structural changes during simulated stomach digestion, as shown in the confocal images (Fig. 4), especially at the digestion end-point at 120 min, where the mixture C1 appears to have smaller protein fragments, compared to the mixture A1 that reveals more intact protein fragments/aggregates, suggesting that proteins in a system with protein continuity seem to be more easily digested than those in a system with polysaccharide continuity. In fact, it has been reported that at a constant enzyme concentration condition, the rate of protein digestion increased with increasing protein/substrate concentration until reaching a critical level;45 therefore, in this study, the enzyme concentration is not a limiting factor in milk protein digestion.
3.3. Effect of guar gum on the lipolysis of O/W/W emulsions
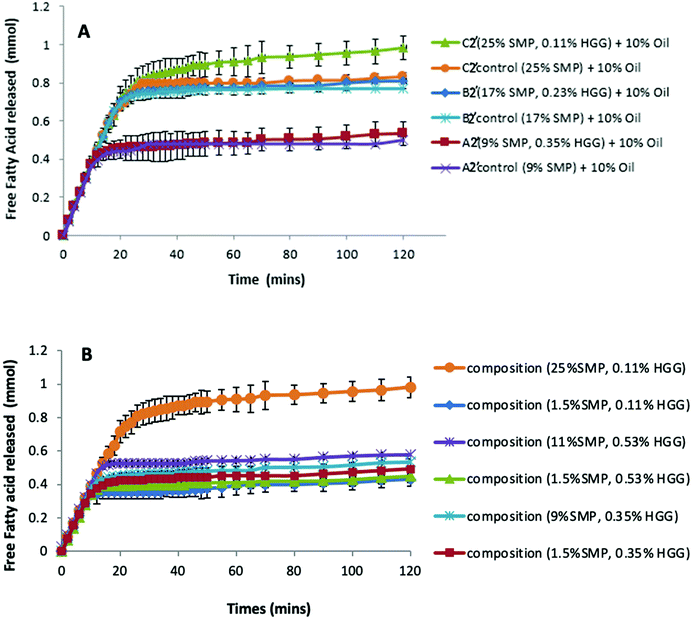
Previous studies have reported that some polysaccharides show an ability to bind with bile salts; for example, electrostatic interactions between chitosan and bile salts,46 dynamic molecular contact of beta-glucan with bile salts and entrapment of bile micelles by an arabinoxylan matrix without direct molecular interaction.47 Torcello-Gomez and Foster48 also reported the interactions between non-ionic cellulose ethers (e.g. MC, HPMC and HPC) and bile salts in the control of lipid digestion of O/W emulsions. Macierzanka et al.49 explored the interactions between bile salts with various dietary fibres in the bulk and the interfaces for understanding the digestive process.To investigate this, we therefore have compared the lipolysis of formulations/emulsions A2′, B2′ and C2′ (TL2) at 10% sunflower oil in both the presence and absence of GG (Fig. 5A.) It can be seen that in both GG-continuous and bi-continuous formulations, a similar extent of lipolysis is measured when compared to the control samples, respectively. The presence of GG in protein continuous formulations even slightly enhances lipolysis, disproving the assumption that GG would bind bile salts or interact with enzymes to reduce the accessibility of lipase to the oil/water interface, thereby delaying lipolysis. Again it is apparent that from the control samples without GG addition (Fig. 5A), the formulations with higher protein content have a greater lipolysis, corresponding to the findings on proteolysis (Fig. 3 and 4). This indicates that GG might also not be the factor to determine lipolysis during simulated small intestinal digestion.
However, when fixing the GG concentration in the formulations, but changing the protein content, we found that the sample with a higher protein concentration shows greater lipid digestion (Fig. 5B); moreover, the larger the difference of protein concentration between the two samples at the same level of GG, the greater the difference in FFA release, which suggests that the lipolysis in the formulations is protein dependent and driven by the protein concentrations.
3.4. Effect of the molecular weight of GG on the lipolysis of O/W/W emulsions
The lipolysis of two sets of formulations (a′, b′ and c′ on the TL2′ of the LMw GG phase diagram, and A1′, B1′ and C1′ on the TL1 of the HMw GG phase diagram, Fig. 6a), at 20% sunflower oil is compared in Fig. 7 (i.e. comparing a′ and A1′ and c′ and C1′). These phase diagrams were established and explained in our previous study.32 These two tie-lines have similar slopes; therefore, at a fixed phase volume, the only variable changing was the viscosity of the GG phase, due to the decrease in GG molecular weight. The compositions for these selected formulations are shown in Table 1.The phase volume ratios of SMP-GG in mixtures a (25/75), b (50/50) and c (75/25) on TL2′(LMw GG used) are the same as those in A1, B1 and C1 on TL1 (HMw GG used), respectively, which results in similar and comparable microstructures and compositions for these formulations when incorporating a certain amount of oil. For example, when adding 10% sunflower oil into the mixture a, b and c to form emulsions, the microstructures containing LMw GG shown in Fig. 6b were similar to those with HMw GG (A1′, B1′ and C1′ in Fig. 1).
As anticipated, Fig. 7b–d show that there is no significant difference (P > 0.05) in lipolysis when comparing these formulations containing LMw GG to those containing HMw GG at 20% oil, although a slight difference was observed in bi-continuous formulations (b′ and B1′), which is probably attributed to the small difference in protein concentrations. This further confirms the previous findings that lipid digestion in the formulation is very likely to be dependent upon the protein concentration, rather than the GG; therefore, changing the molecular weight of GG (viscosity) would not make any difference in lipolysis. Recently, Grundy et al.50 have investigated the impact of viscosity of oat components (guar gum as the control) on lipid digestion, and suggested that there did not seem to be a strong correlation between the biopolymer viscosity and the inhibition of lipolysis; however, they highlighted the importance of considering polysaccharide molecular properties and not just their impact on solution viscosity.
Moreover, the lipolysis in the protein continuous formulation is also shown to be greater than that in bi-continuous and GG continuous formulations (i.e. c′ > b′ > a′, Fig. 7a), confirming the previous results shown in Fig. 2. The different microstructures were a consequence of the concentration of biopolymers, but the phase continuity determining the extent of lipolysis was due to the amounts of proteins in the formulations.
It is well known that there are many factors affecting lipid digestion by different means, for example, oil droplet size/size distribution (equivalent to changes in the O/W interfacial area); physicochemical conditions (pH, ionic strength and temperature); O/W interfacial compositions (emulsifier/surfactant types, oil types and interfacial layer/film structure) and others such as enzyme activity and bio-surfactant activity (bile salts and phospholipids). In this study, all of the digestibility measurements were conducted under the same physicochemical conditions, as they all followed the same digestion model where the same concentration and activity of enzymes was used throughout the work. Thus, findings suggest that the protein content controlled lipid digestion is very likely to be due to either changing the oil droplet size or the O/W interfacial composition during the digestive processes.
In protein-stabilized emulsions, generally not all of the protein is present at the O/W interface (in the adsorbed state), especially when excessive proteins are present in the system. This means that there will be a considerable proportion of proteins residing in the aqueous phase. It has been reported that the adsorbed and unadsorbed proteins are likely to exist in different conformational states; therefore, their susceptibility to pepsin could be different.51 For instance, under a given set of simulated gastric conditions, many workers reported that protein (such as β-lactoglobulin and β-casein) in the native state was largely resistant to pepsin digestion in the aqueous phase of an emulsion, even more difficult to be digested in a solution (not in an emulsion), whereas interfacially adsorbed proteins were more susceptible to pepsin hydrolysis. This has been attributed to a possible change in the conformation of the adsorbed protein, exposing the peptic cleavage sites for proteolysis with the folded structure of unadsorbed protein in the bulk phase burying their peptic cleavage sites into the core of the structure to prevent pepsin hydrolysis.52,53
In fact, in the current experimental design, there were large amounts of proteins present in the formulations. The formulations A1′, B1′ and C1′ (SMP-GG-OIL) contained 11% SMP (∼3 wt% proteins), 20.5% SMP (5 wt% proteins) and 30% SMP (8 wt% milk proteins), respectively, corresponding to the protein concentrations in the mixtures A1, B1 and C1 (SMP-GG), respectively, as illustrated in Table 1. It has been shown that 1 wt% of milk protein is sufficient to form a fine emulsion containing 20% sunflower or soya oil,12,30 therefore, it is very likely that there were considerable amounts of unabsorbed proteins remaining in the bulk phase of the prepared formulations, which may have made a difference in simulated in vitro digestion.
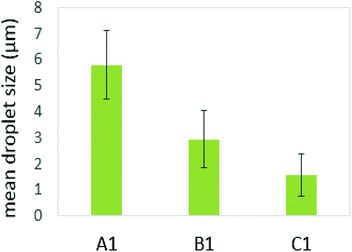
Several studies on model emulsions have shown that the net surface charge on the O/W interface decreases when emulsions undergo gastric conditions because of the hydrolysis of interfacial protein by pepsin.30,37,54 In emulsions stabilized by milk proteins, such as whey protein isolate, sodium caseinate, β-lactoglobulin or β-casein, this hydrolysis can lead to flocculation and further coalescence of the droplets, resulting from insufficient electrostatic repulsions.51 In this study, the formulation with a protein continuous structure (emulsion C1′) had a much higher protein content than those with bi-continuous (emulsion B1′) and polysaccharide continuous (emulsion A1′) structures. When these emulsions pass through the simulated gastric environment, hydrolysis of the adsorbed protein at the interface by pepsin will weaken the protein network and form peptides on the droplet surface. The reduced steric barriers and electrostatic repulsion of peptides remaining at the interface would, consequently, induce flocculation and promote coalescence of the droplets. This would result in an increase in the oil droplet size, but a decrease in the surface area where the bile salts and lipase can access in order to hydrolyse the lipid phase during the later duodenal digestion stage. However, the stability of the droplets depends on the initial amount of unadsorbed protein present in the system. According to Singh and Ye,51 in the presence of excess unadsorbed protein, the stability of the oil droplets during gastric digestion is significantly improved. Because exchanges may occure at the interface between adsorbed protein on the oil surface and non-adsorbed protein in the bulk phase, and hydrolysis of protein at the interface may induce further adsorption and hydrolysis of the protein from bulk phase to stabilise the oil droplets. This has been evident in the study of Amir et al.55 who suggested that changes in the size distribution of the droplets during pepsin hydrolysis mainly depend on the original protein concentration. A higher protein content in an emulsion during gastric digestion would generate more stable droplets; therefore, during gastric digestion, the droplets in the formulation with a protein continuous structure would tend to be more stable than those with bi-continuous and GG continuous structures. This has been supported by the droplet size data plotted in Fig. 8, based on the confocal images obtained at 60 min during simulated stomach digestion (Fig. 1).
Fig. 8 shows that the droplet size decreased with increasing the protein content in the formulations (following: C1′ < B1′ < A1′) after 60 min gastric digestion. In addition, it should be noted that the droplet sizes in the initial emulsions C1′ (before in vitro digestion) were also shown to be smaller than those in the polysaccharide continuous emulsion formulation A1′ (Fig. 1); the same observation in the case of initial emulsions A2′, B2′ and C2′ (TL2) was also recorded (data not shown). The decrease in droplet size in the emulsion C1′ or C2′ is attributed to the increase in protein concentration, which aids emulsification, generating smaller droplets. The larger surface area created from both initial small droplets in the emulsion with a higher protein content, and more stable droplets during gastric digestion would facilitate lipid digestion. This explains why the order of the lipolysis of emulsions is: C1′ > B1′ > A1′ and C2′ > B2′ > A2′.
Interestingly, Li et al.38 have reported that the changes in the droplet size and the proteolysis of the interfacial proteins of the emulsions under gastric conditions had no influence on the rate and the extent of lipid digestion in the subsequent intestinal environment, which was because bile salts, as strong bio-surfactants, would displace interfacial materials (either proteins or peptides) from O/W interfaces, which eliminated the difference in the lipolysis induced by the changes in the droplet size. However, Singh and co-workers56 and Acevedo-Fani and Singh16 suggested that the nature of the adsorbed layer of the droplets and the droplet size played a major role in controlling the action of lipase and lipid digestion. Nevertheless, it is worth pointing out that in their study, only a very low concentration of proteins was used, (typically 1 wt% protein in emulsions); therefore, the effect of the excessive unadsorbed proteins in the bulk phase on proteolysis and subsequent lipolysis has not been taken into account. In contrast, in our study, the considerable amount of proteins present in the bulk phase not only creates smaller oil droplets in the initial formulations, but also aids to stabilise the oil droplets during proteolysis under gastric conditions; therefore, this may involve different mechanisms by which it affects lipolysis, compared with those cases only containing small protein concentrations.
Based on these findings, it can therefore be argued that from the formulation point of view, the excessive protein content present in the formulations/emulsions may play an important role in controlling gastric proteolysis and subsequent lipolysis by not only creating smaller oil droplets in the initial emulsion, but also by enhancing the stability of oil droplets in the gastric digestion environment.
4. Conclusions
In the present study, it has been shown that phase separated microstructures/emulsions based on a model system (containing protein, polysaccharides and oil) were able to control lipid digestion (measured in vitro), and the extent of lipid digestibility of formulations with different phase continuities from a selected tie-line on the phase diagram was: protein continuous > bi-continuous > polysaccharide continuous systems.The investigations of the effects of polysaccharides (guar gum) on both proteolysis and lipolysis in the presence or absence of guar gum suggested that guar gum has no influence on digestion, which was further confirmed by changing the molecular weight of GG (although some other polysaccharides have been reported to have this potential). However, lipid digestibility is indeed protein concentration dependent. These findings indicated that protein content in emulsion formulation, plays an important role in controlling both gastric proteolysis and subsequent lipolysis. This is because of the mechanism that, from a formulation point of view, the excessive protein content present in the initial emulsion, would not only create smaller oil droplets, but also aid to enhance the stability of oil droplets during proteolysis under gastric digestion conditions. This corresponds to an increased interfacial area for the adsorption of pancreatic lipase-co-lipase in the duodenal phase, promoting lipolysis. These findings on protein digestibility in formulation would bring insights into the research and applications of real commercial dairy products (with designed structures), especially when there is a fortified protein strategy (high protein content in formulation) that is used, to ensure controlled nutrient release and absorption during digestion, and open a new way for potential assistance in tackling obesity issues.
Author contributions
The authors Wentao Liu and Tim Foster contributed to this whole work equally. Wentao Liu: investigation, methodology, validation, formal analysis, visualization, writing – original draft, and writing – review and editing. Tim Foster: methodology, supervision, and writing – review and editing. Mita Lad also contributed to the methodology, concept, and discussion of this publication.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank Mr Ian Ward for the confocal microscopy training support and image analysis, and also thank Dr Mohamed Gedi for the support in the pH-Stat instrument application.References
- C. Feinle, M. Christen, D. Grundy, H. Faas, O. Meier and B. Otto, Neurogastroenterol. Motil., 2002, 14, 205–213 CrossRef CAS PubMed.
- R. D. Mattes, Can. J. Diet. Pract. Res., 2007, 68(2), S1–S3 Search PubMed.
- M. Golding, T. J. Wooster, L. Day, M. Xu, L. Lundin, J. Keogh and P. Clifton, Soft Matter, 2011, 7, 3513–3523 RSC.
- D. J. McClements and E. A. Decker, Technology and Food Nutrition, New York, USA, 2009 Search PubMed.
- D. E. Cummings and J. Overduin, J. Clin. Investig., 2007, 117(1), 13–23 CrossRef CAS PubMed.
- P. W. J. Maljaars, H. P. Peters, D. J. Mela and A. A. Masclee, Physiol. Behav., 2008, 95(3), 271–281 CrossRef CAS PubMed.
- S. Mun, E. A. Decker, Y. Park, J. Weiss and F. J. McClements, Food Biophys., 2006, 1(1), 21–29 CrossRef.
- S. Mun, E. A. Decker and D. J. McClements, Food Res. Int., 2007, 40(6), 770–781 CrossRef CAS.
- D. J. McClements and Y. Li, Adv. Colloid Interface Sci., 2010, 159(2), 213–228 CrossRef CAS PubMed.
- G. A. van Aken, E. Bomhof, F. D. Zoet, M. Verbeek and A. Oosterveld, Food Hydrocolloids, 2011, 25(4), 781–788 CrossRef CAS.
- A. Sarkar, J.-M. Juan, E. JKolodziejczyk, S. Acquistapace, L. Donato-Capel and T. J. Wooster, J. Agric. Food Chem., 2015, 63, 8829–8837 CrossRef CAS PubMed.
- A. Sarkar, B. Murray, M. Holmes, A. Ettelaie, A. Abdalla and X. Y. Yang, Soft Matter, 2016, 12, 3558 RSC.
- Q. Guo, A. Q. Ye, N. Bellissimo, H. Singh and D. Rousseau, Prog. Lipid Res., 2017, 68, 109–118 CrossRef CAS PubMed.
- C. B. Dias, X. Q. Zhu, A. K. Thompson, H. Singh and M. L. Garg, Food Funct., 2019, 10(1), 112–124 RSC.
- N. Luo, A. Q. Ye, F. M. Wolber and H. Singh, Molecules, 2021, 26, 1379 CrossRef CAS PubMed.
- A. Acevedo-Fani and H. Singh, Prog. Lipid Res., 2022, 85, 101129 CrossRef CAS PubMed.
- P. J. Wilde and B. S. Chu, Adv. Colloid Interface Sci., 2011, 165, 14–22 CrossRef CAS PubMed.
- T. Moschakis, B. S. Murray and E. Dickinson, J. Colloid Interface Sci., 2005, 284(2), 714–728 CrossRef CAS PubMed.
- T. Moschakis, B. S. Murray and E. Dickinson, Langmuir, 2006, 22(10), 4710–4719 CrossRef CAS PubMed.
- H. Firoozmand and D. Rousseau, Food Hydrocolloids, 2013, 30(1), 333–342 CrossRef CAS.
- S. Bascuas, P. Morell, I. Hernando and A. Quiles, Food Hydrocolloids, 2021, 118(2), 106612 CrossRef CAS.
- T. J. Foster and I. T. Norton, Self-assembling structures in the Gastrointestinal Tract, in Designing Functional Foods; Measuring and controlling food Structure breakdown and nutrient absorption, ed. D. J. McClements and E. A. Decker, Woodhead Publishing Ltd, 2009, pp. 601–622 Search PubMed.
- C. Schorsch, A. K. Clark, M. G. Jones and I. T. Norton, Colloids Surf., B, 1999, 12, 317–329 CrossRef CAS.
- P. W. De Bont, G. M. P. van Kempen and P. Vreeker, Food Hydrocolloids, 2002, 16, 127–138 CrossRef CAS.
- V. B. Tolstoguzov, Food Polysaccharides and their Application, CRC Press, 2006, pp. 589–627 Search PubMed.
- H. Singh, A. Ye and D. Horne, Prog. Lipid Res., 2009, 48, 92–100 CrossRef CAS PubMed.
- D. J. McClements, E. A. Decker and Y. Park, Crit. Rev. Food Sci. Nutr., 2009, 49(81), 48–67 Search PubMed.
- M. Golding and J. Wooster, Curr. Opin. Colloid Interface Sci., 2010, 15, 90–101 CrossRef CAS.
- J. Maldonado-Valderrama, P. Wilde, A. Macierzanka and A. Mackie, Adv. Colloid Interface Sci., 2011, 165, 36–46 CrossRef CAS PubMed.
- J. Li, A. Ye, S. J. Lee and H. Singh, Food Funct., 2011, 3, 320 RSC.
- J. Slavin and H. Green, Nutr. Bull., 2007, 32(Suppl. 1), 32–42 CrossRef.
- W. Liu and T. J. Foster, Food Hydrocolloids Health, 2021, 100048 CAS.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carrière, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. Mackie, S. Marze, D. J. McClements, O. Ménard, I. Recio, C. N. Santos, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and A. Brodkorb, Food Funct., 2014, 5, 1113–1124 RSC.
- F. C. Church, H. E. Swaisgood, D. H. Porter and G. L. Catignani, J. Dairy Sci., 1983, 66(6), 1219–1227 CrossRef CAS.
- M. Heider, G. Hause and K. Mäder, Eur. J. Pharm. Biopharm., 2016, 109, 194–205 CrossRef CAS PubMed.
- D. J. McClements and Y. Li, Food Funct., 2010, 1, 32–59 RSC.
- A. Sarkar, K. K. T. Goh, R. P. Singh and H. Singh, Food Hydrocolloids, 2009, 23, 1563–1569 CrossRef CAS.
- J. Li, A. Ye, S. J. Lee and H. Singh, Food Funct., 2011, 3, 320 RSC.
- S. J. Hur, E. A. Decker and D. J. McClements, Food Chem., 2009, 114(1), 253–262 CrossRef CAS.
- A. Sarkar, S. Zhang and M. Holmes, Adv. Colloid Interface Sci., 2019, 263, 195–211 CrossRef CAS PubMed.
- L. Cheng, A. Ye, Y. Hemar and H. Signgh, J. Colloid Interface Sci., 2022, 608, 1286–1296 CrossRef CAS PubMed.
- T. J. Foster and I. T. Norton, Self-assembling structures in the Gastrointestinal Tract, in Designing Functional Foods; Measuring and controlling food Structure breakdown and nutrient absorption, ed. D. J. McClements and E. A. Decker, Woodhead Publishing Ltd, 2009, pp. 601–622 Search PubMed.
- M. Chen, L. Guo, J. Nsor-atindana, H. D. Goff, W. Zhang, J. Mao and F. Zhong, J. Food Hydrocolloids, 2020, 107, 105971 CrossRef CAS.
- J. Amyoony, X. Liu and A. J. Wright, Bioact. Carbohydr. Diet. Fibre, 2017, 9, 21–27 CrossRef CAS.
- H. J. Northrop, J. Gen. Physiol., 1920, 2(6), 595–611 CrossRef PubMed.
- M. Thongngam and D. J. McClements, Food Hydrocolloids, 2005, 19(5), 813–819 CrossRef CAS.
- P. Gunness, B. M. Flanagan and M. J. Gidley, J. Cereal Sci., 2010, 52(3), 444–449 CrossRef CAS.
- A. Torcello-Gómez and T. J. Foster, Carbohydr. Polym., 2014, 113, 53–61 CrossRef PubMed.
- A. Macierzanka, A. Torcello-Gómez, C. Jungnickel and J. Maldonado-Valderrama, Adv. Colloid Interface Sci., 2019, 274, 102045 CrossRef CAS PubMed.
- M. L. Grundy, D. J. McClements, S. Balance and P. J. Wilde, Food Hydrocolloids, 2018, 83, 253–264 CrossRef CAS PubMed.
- H. Singh and A. Ye, Colloid Interface Sci., 2013, 18, 360–370 CAS.
- A. Macierzanka, A. I. Sancho, E. N. C. Mills, N. M. Rigby and A. R. Mackie, Soft Matter, 2009, 5, 538–550 RSC.
- A. Sarkar, K. K. T. Goh, R. P. Singh and H. Singh, Food Hydrocolloids, 2009, 23, 1563–1569 CrossRef CAS.
- A. M. Nik, A. J. Wright and M. Corredig, Food Funct., 2010, 1, 141–148 RSC.
- M. N. Amir, A. J. Wright and M. Corredig, J. Colloid Interface Sci., 2010, 344, 372–381 CrossRef PubMed.
- H. Singh, A. Ye and D. Horne, Prog. Lipid Res., 2009, 48, 92–100 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo00592a |
| This journal is © The Royal Society of Chemistry 2022 |