Soy isoflavone ameliorated the alterations in circulating adipokines and microRNAs of mice fed a high-fat diet†
Hyo Bin
Lee
a,
Ah Young
Lee
a,
Yumi
Jang
a and
Young Hye
Kwon
 *ab
*ab
aDepartment of Food and Nutrition, Seoul National University, Seoul, Korea. E-mail: hye0414@snu.ac.kr; Fax: +82-2-884-0305; Tel: +82-2-880-6833
bResearch Institute of Human Ecology, Seoul National University, Seoul, Korea
First published on 8th November 2022
Abstract
Soy protein, containing isoflavones and bioactive peptides, is shown to have anti-obesity effects, but the main contributor and underlying mechanisms remain unclear. Recent studies have demonstrated that circulating microRNAs (miRNAs) act as important mediators in obesity and metabolic processes. In this study, we investigated whether soy protein components have distinctive effects on adiposity and circulating miRNA profiles in obese mice. C57BL/6J mice were divided into 4 groups, and each group was fed with a control, high-fat (HF), HF with low-isoflavone soy protein (HF/S), or HF with high-isoflavone soy protein (HF/SI) diet for 16 weeks. In the HF/SI group, changes in the serum adipokine levels, adipocyte diameter, and the number of crown-like structures (CLS) were alleviated compared to those of the HF group. In the HF/S group, the number of CLS was reduced. Decreases in body and adipose tissue weights were not observed in both HF/S and HF/SI groups. Through microarray analysis of serum miRNAs, we identified 23 differentially expressed miRNAs (DEMs) among the groups. The levels of most circulating DEMs were correlated with body weight, serum biochemical parameters, and adipose tissue histology. Functional analysis of predicted target genes of DEMs from both HF vs. CON and HF/S vs. CON comparisons revealed several cancer-related pathways. Only 2 DEMs were identified in the HF/SI vs. CON comparison. In conclusion, the present study confirmed that soy isoflavones are the main contributor to the health-beneficial effects of soy protein in diet-induced obesity. Notably, the extent of serum miRNA dysregulation coincided with obesity and altered the circulating adipokine levels. These findings provide additional insights into the role of soy protein in the regulation of circulating miRNAs in diet-induced obesity. Further work is required to validate the proposed functions of miRNAs in target tissues.
1. Introduction
Obesity is characterized by excessive fat accumulation and chronic inflammation and is frequently associated with the development of various metabolic diseases, including type 2 diabetes, nonalcoholic fatty liver disease, and cancer.1 It has become evident that adipose tissue is an endocrine organ capable of secreting a number of obesity-related cell signaling adipokines into the bloodstream.2 Obesity and its related complications are commonly associated with changes in the circulating levels of adipokines, such as reduced levels of adiponectin and increased levels of leptin.3Recent advances in food and nutrition research have been dedicated to identifying food components that exert inhibitory effects on obesity-linked complications. Soybean has attracted much interest, as it is a good source of protein with a well-balanced amino acid profile. Furthermore, it contains various bioactive components, such as isoflavones and bioactive peptides.4 Several studies have shown that the intake of soybean is associated with the prevention of hypercholesterolemia, dyslipidemia, hyperglycemia, hepatic steatosis, and hypertension.5 In addition, the intake of soy products effectively reduced obesity in both humans6,7 and animals.8,9 The most beneficial health efficacy of soybean is based on the consumption of isoflavones, mainly genistein and daidzein as aglycone forms and genistin and daidzin as their respective glycosides.5 Soy isoflavones have been shown to exhibit various biological properties, such as antioxidant, anti-inflammatory, anti-microbial, and anti-cancer activities. Genistein is also known to play an important role in the differentiation of adipocytes by regulating the transcriptional activity of various nuclear receptors, including estrogen receptors10 and peroxisome proliferator-activated receptor gamma.11
In addition to isoflavones, the health beneficial effects of soy have been attributed to bioactive peptides that are produced as a result of fermentation and processing of soybeans, or protein digestion in the body.12 Various bioactive peptides are identified from soy protein, which is mainly composed of two proteins, glycinin (11S) and β-conglycinin (7S). Soy peptides are usually composed of 2 to 20 amino acids, and some of them including soymorphins are shown to play a role in the prevention of multiple chronic diseases.12 Although consumption of soy β-conglycinin was shown to reduce visceral fat mass in hyperlipidemic humans6 and high-fat diet-fed mice,13 most of the studies on soy protein and its bioactive peptides are focused on other bioactivities, such as hypolipidemic, anti-hypertensive, anti-cancer, and anti-inflammatory activities.12,14,15
MicroRNA (miRNA), a type of non-coding small RNA, regulates the expression of genes at the posttranscriptional level by translational repression or mRNA degradation in the cytoplasm.16 Moreover, miRNAs are released from many types of cells such as adipocytes, lymphocytes, macrophages, platelets, endothelial cells, and epithelial cells, into the circulation. Secreted miRNAs are mainly contained in extracellular vesicles to be protected from degradation, suggesting the possible role of circulating miRNAs as a novel cell-to-cell communicator.17 Adipose tissue is thought to be a major source of circulating miRNAs and several miRNAs in the adipose tissue have been reported to be associated with the development of obesity, suggesting the potential role of circulating miRNA profiles as a biomarker of obesity and metabolic disorders in humans.18 However, the effects of the soy protein diet on the levels and major source(s) of circulating miRNAs have not yet been studied.
Therefore, in the present study, we compared the effects of low- and high-isoflavone soy protein on obesity to investigate the distinct role of soy protein components, especially bioactive peptides and isoflavones in mice fed a high-fat diet. Also, we not only investigated the anthropometric and serum biochemical changes associated with obesity and adipose tissue inflammation, but also investigated the serum miRNA expression profile to understand the possible role of soy protein components in intercellular communication in a diet-induced obese animal model.
2. Materials and methods
2.1. Animal and diets
Five-week-old male C57BL/6J mice were obtained from Daehan Bio Link Co. (Korea). After 7 days of acclimation period with a chow diet, mice were randomly divided into four groups and each group was fed with a control diet (CON: 19.5% casein, 12.7% of total calorie from fat; n = 11), a high-fat diet (HF: 19.5% casein, 44.4% of total calorie from fat; n = 12), a high-fat/low-isoflavone soy protein diet (HF/S: 19.5% soy protein isolate, 44.4% of total calorie from fat, and 35 mg of isoflavone as aglycone equivalents per kg diet; n = 12), or a high-fat/high-isoflavone soy protein diet (HF/SI: 19.5% soy protein isolate, 44.4% of total calorie from fat, and 405 mg of isoflavone as aglycone equivalents per kg diet; n = 11) for 16 weeks (Fig. S1†). The soy protein isolate used in this study contained 0.037 mg of genistein, 0.147 mg of genistin, 0.014 mg of daidzein, and 0.064 mg of daidzin per g based on a previous study.19 The soy isoflavone concentrate contained 2.3 mg of genistein, 219 mg of genistin, 3.5 mg of daidzein, and 170 mg of daidzin per g based on a certificate of analysis provided by a supplier. Both the casein and soy protein isolate-based diets were formulated to contain equal amounts of methionine and cystine. The composition of the diets is shown in Table S1.† The animals were maintained in a temperature (22 ± 2 °C) and humidity (50 ± 5%)-controlled room with a 12 h dark/light cycle.At the end of the experiments, all animals were fasted overnight and sacrificed by CO2 gas inhalation, followed by cardiac blood collection. The blood samples were collected in serum separating tubes (BD Biosciences, USA), left at room temperature for 1 h and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 4 °C for 2 min to obtain serum. Epididymal, perirenal, and retroperitoneal adipose tissues were dissected and weighed; epididymal adipose tissues were fixed in 10% neutral-buffered formalin. Serum and adipose tissues were stored at −80 °C until use. All experimental procedures were approved by the Seoul National University Institutional Animal Care and Use Committee (SNU-200819-3-2).
000g, 4 °C for 2 min to obtain serum. Epididymal, perirenal, and retroperitoneal adipose tissues were dissected and weighed; epididymal adipose tissues were fixed in 10% neutral-buffered formalin. Serum and adipose tissues were stored at −80 °C until use. All experimental procedures were approved by the Seoul National University Institutional Animal Care and Use Committee (SNU-200819-3-2).
2.2. Serum biochemical analysis
The serum glucose, triglyceride, and total cholesterol (TC) levels were determined using commercial colorimetric kits (Asan Pharmaceutical Co., Korea). The monocyte chemoattractant protein 1 (MCP-1), leptin, and adiponectin levels were determined using commercial enzyme-linked immunosorbent assay kits (R&D Systems, USA) according to the manufacturer's protocol.2.3. Adipose tissue histology analysis
Epididymal adipose tissue fixed using formalin was cut into 4 μm thick paraffin sections and stained with hematoxylin and eosin (H&E) for histological evaluation. The morphology was observed under an ECLIPSE Ni-U microscope with a DS-Ri2 camera (Nikon, Japan). The diameter of adipocytes was measured in each 200× image and the number of crown-like structures (CLS) was counted in each 100× image. All stained analysis was blindly determined by an experienced pathologist.2.4. Microarray hybridization analysis and microRNA target gene prediction
Serum miRNA was extracted using a miRNeasy serum/plasma kit (Qiagen, USA), and the miRNA expression profiles were analyzed using GeneChip miRNA 4.0 (Affymetrix, USA) (Fig. S2†). The comparative analysis between the test and control samples was carried out using one-way analysis of variance (ANOVA) with post-hoc analysis (|fold change| ≥ 1.5 with p < 0.05). Hierarchical cluster analysis was performed using complete linkage and Euclidean distance as a measurement of similarity and visualized as a heatmap using Morpheus software (https://software.broadinstitute.org/morpheus). Serum miRNA extraction and microarray hybridization analysis was conducted blindly by Macrogen (Korea). To predict the target genes of each differentially expressed miRNA (DEM), miRWalk 3.0,20 TargetScanMouse 7.2,21 and miRDB 6.022 were used, and the target genes that are predicted by all the three algorithms were selected. Also, miRTarbase 9.023 was used to obtain the experimentally validated target genes. Only when the genes with ‘strong evidence’ existed, they were additionally added to the list. All predictions were conducted under default criteria. The target gene list was used for the Kyoto Encyclopedia of Gene and Genomes (KEGG) pathway analysis conducted by the DAVID program (https://david.ncifcrf.gov).2.5. Statistical analyses
Data were analyzed by one-way ANOVA using SPSS software (ver. 26.0; SPSS Inc., USA). Where appropriate, post hoc comparisons were made using Duncan's multiple-range test. Prior to conducting the ANOVA, the assumption of normality was evaluated by the Shapiro–Wilk test. When the normality was not satisfied (body weight, adipose tissue weight, and serum TC), a Kruskal–Wallis test and Dunn's multiple comparison test were conducted. The data are reported as the mean ± SEM and the differences were considered significant at p < 0.05. The association between factors was examined by the calculation of Pearson's correlation coefficient.3. Results
3.1. The effects of soy isoflavone on obesity, serum adipokine levels, and adipose tissue inflammation in mice fed a high-fat diet
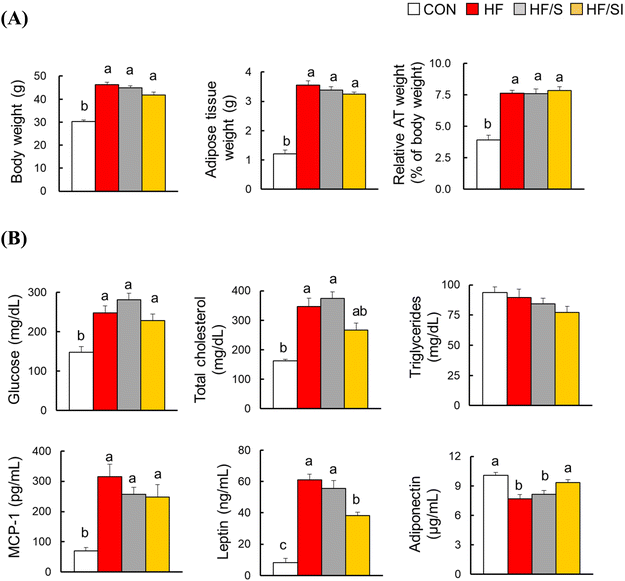
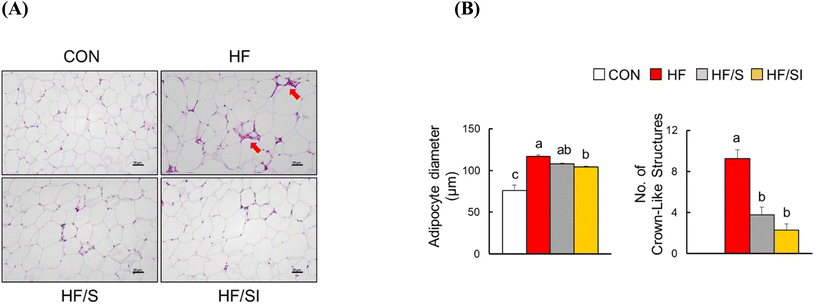
The body and adipose tissue weights were significantly increased in the HF group compared to those of the CON group (Fig. 1A). Among the HF, HF/S, and HF/SI groups, no significant differences in the body and adipose tissue weights were observed. In the HF group, serum glucose, TC, MCP-1, and leptin levels were significantly increased, and serum adiponectin levels were significantly decreased compared to those of the CON group (Fig. 1B). The changes in the serum leptin and adiponectin levels driven by a high-fat diet were significantly restored by the consumption of high-isoflavone soy protein. The serum TC and MCP-1 levels were similar among the three high-fat groups. Meanwhile, there was no significant difference in serum triglyceride levels among the groups.H&E analysis of epididymal adipose tissue revealed significant increases in adipocyte size and the number of CLS, a well-known marker of adipocyte inflammation, in the HF group. These parameters were significantly reduced in response to isoflavone supplementation (Fig. 2A & B). In the HF/S group, only the number of CLS was significantly lower compared to that of the HF group. These biochemical and histological data implicate that lipid accumulation and inflammation in adipose tissue were alleviated in the HF/SI group compared to the HF group.
3.2. The effects of soy isoflavone on the levels of circulating microRNAs in mice fed a high-fat diet
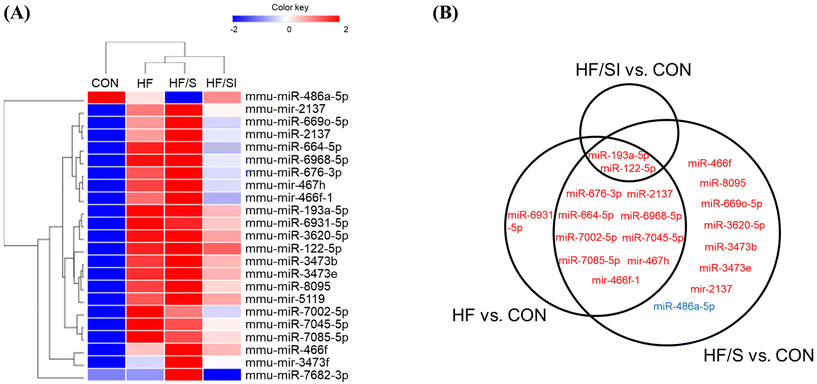
The levels of circulating miRNAs of each experimental group were investigated using microarray hybridization analysis. Through one-way ANOVA, we identified 23 DEMs (18 mature and 5 immature) among the groups. Hierarchical clustering analysis of DEMs showed that the expression profile of the CON group was most similar to that of the SPI/SI group (Fig. 3A).Compared to the CON group, the circulating levels of 12, 19, and 2 miRNAs were significantly different in the HF, HF/S, and HF/SI groups, respectively (|fold change| ≥ 1.5, p < 0.05) (Fig. 3B). Results showed significant increases in circulating miR-193a-5p and miR-122-5p levels in all 3 comparisons. Between the HF/SI and the HF/S groups, the circulating levels of miR-193a-5p, miR-486a-5p, and miR-7682-3p were significantly different. While the circulating miR-486a-5p levels were upregulated, those of miR-193a-5p and miR-7682-3p were downregulated in the HF/SI group compared to those of the HF/S group.
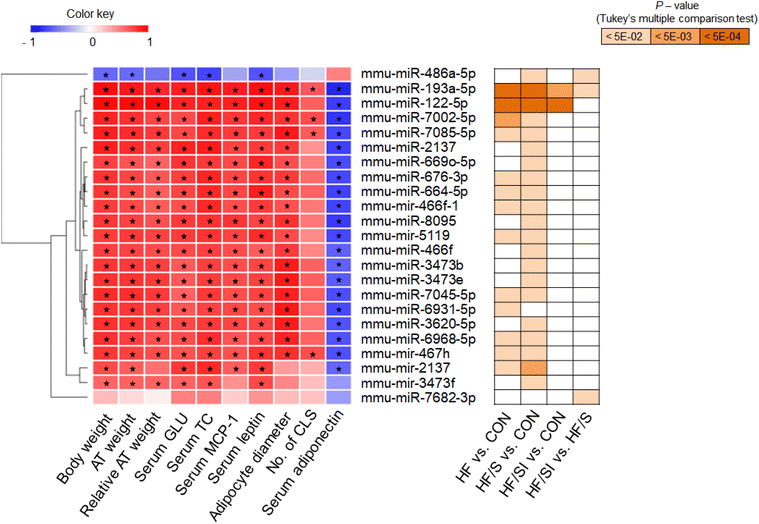
To investigate the possible role of adiposity and adipose tissue inflammation in the expression profile of miRNA, correlation analyses between anthropometric, biochemical and histological parameters, and the levels of circulating DEMs were conducted (Fig. 4). All parameters were closely correlated with the circulating levels of most DEMs. In the case of CLS incidence, only miR-193a-5p, miR-7002-5p, miR-7085-5p, and mir-467h were significantly associated. Interestingly, 2 DEMs in the HF/SI vs. HF/S comparison, miR-486a-5p and miR-7682-3p, showed different correlation aspects. The circulating miR-486a-5p levels showed an inverse correlation with the parameters; meanwhile, none of the parameters were significantly associated with the circulating miR-7682-3p levels.
3.3. The effects of soy isoflavone on functional enrichment analysis of predicted target genes of circulating miRNAs
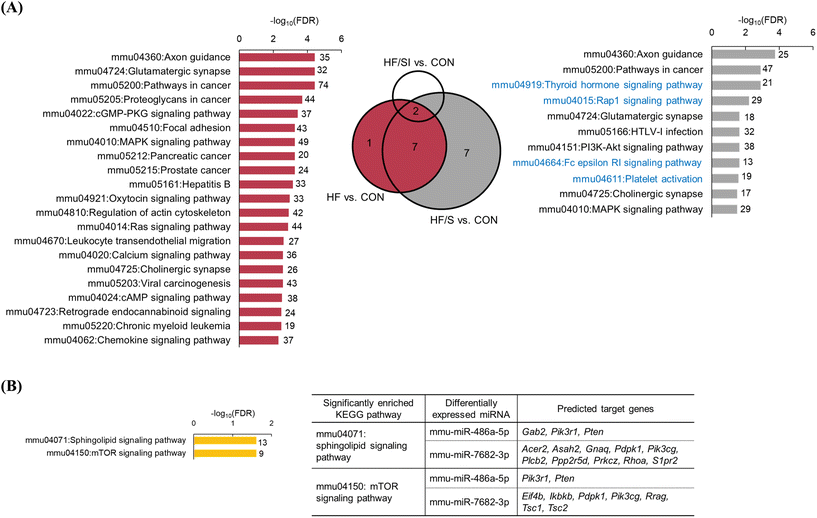
We conducted the KEGG pathway analysis of predicted target genes of 10 mature DEMs derived from the HF vs. CON comparison (Fig. 5A). The analysis results showed that the predicted target genes were significantly enriched in a total of 51 pathways. Among the top 20 significant pathways, we observed several cancer-related pathways, including ‘Pathways in cancer’ (FDR = 3.49 × 10−5), ‘Proteoglycans in cancer’ (FDR = 1.95 × 10−4), and ‘Focal adhesion’ (FDR = 5.21 × 10−4).To find out the potential effect of low-isoflavone soy protein on the replacement of casein, KEGG pathway analysis of the predicted target genes of 7 mature DEMs that were unique to the HF/S vs. CON comparison was conducted (Fig. 5A). As a result, the predicted target genes were significantly enriched in 11 KEGG pathways, including ‘Pathways in cancer (FDR = 1.27 × 10−3). Four KEGG pathways, the ‘Thyroid hormone signaling pathway’ (FDR = 1.27 × 10−3), ‘Rap1 signaling pathway’ (FDR = 6.05 × 10−3), ‘Fc epsilon RI signaling pathway’ (FDR = 2.26 × 10−2), and ‘Platelet activation’ (FDR = 2.60 × 10−2), were identified in the HF/S vs. CON comparison, but not in the HF vs. CON comparison.
Functional enrichment analysis of the predicted target genes of 3 mature DEMs (miR-193a-5p, miR-486a-5p, and miR-7682-3p) derived from the HF/SI vs. HF/S comparison revealed that target genes of miR-486a-5p and miR-7682-3p were significantly enriched in the ‘Sphingolipid signaling pathway’ (FDR = 2.48 × 10−2) and the ‘mTOR signaling pathway’ (FDR = 2.48 × 10−2) (Fig. 5B). There were no DEMs in the HF/S vs. HF comparison; meanwhile, 2 down-regulated DEMs, miR-193a-5p and miR-7002-5p, were derived from the HF/SI vs. HF comparison. However, the functional enrichment analysis of the predicted target genes of these 2 mature DEMs did not reveal any enriched KEGG pathways.
4. Discussion
This study aimed to investigate the effects of low- and high-isoflavone soy protein on obesity and miRNA profiles in diet-induced obese mice. A few studies reported the alleviating effects of a high-isoflavone soy protein diet on adiposity and circulating adipokine concentrations in rodents fed a high-fat diet.24,25 Since these studies compared soy protein isolate per se with casein, the specific bioactive component(s) were not identified. In the present study, high-isoflavone soy protein reduced the enlargement and inflammation of adipocytes and ameliorated the dysregulated secretion of adipokines. Meanwhile, decreases in adipose tissue inflammation were observed in mice fed with a low-isoflavone soy protein diet. Furthermore, the circulating miRNA profiles were apparently different in the HF/SI group compared to those of the HF group. In particular, the levels of circulating miRNAs showed significant associations with adiposity and serum levels of adipokines, including leptin and adiponectin.Various physiological and pathological states, including obesity-related metabolic disorders, are shown to regulate circulating miRNA levels.18 In the present study, we observed that circulating levels of 12 miRNAs (miR-193a-5p, miR-122-5p, miR-664-5p, miR-676-3p, miR-2137, miR-6931-5p, miR-6968-5p, miR-7002-5p, miR-7045-5p, miR-7085-5p, mir-466f-1, and mir-467h) were significantly changed in mice fed a high-fat diet compared to mice fed with a control diet. Among these DEMs, miR-122-5p and miR-193a-5p were previously identified in serum from subjects with overweight or obesity, and their levels were reduced in response to diet-induced weight loss.26 The levels of these two circulating miRNAs were also reduced in morbidly obese subjects after surgery-induced weight loss.27 Moreover, inhibition of miR-122 by miR-122 antisense oligonucleotide injection reduced hepatic steatosis and plasma cholesterol levels in a diet-induced obese mouse model.28 In accordance with our KEGG analysis data, previous studies have reported several DEMs in the HF vs. CON comparison to be associated with cancer development. The levels of miR-122-5p and miR-193a-5p, 2 DEMs identified in the intersection of three comparison sets, were upregulated in the plasma of hepatocellular carcinoma patients compared to normal individuals.29 In the case of miR-664-5p, its higher expression levels in tumor tissues were associated with poor prognosis and survival of liver cancer patients.30 Although reports on other DEMs are lacking, we observed that the circulating levels of these novel miRNAs were significantly correlated with the extent of obesity.
DEMs that are unique to the HF/S vs. CON comparison are miR-486a-5p, miR-466f, miR-3473b, miR-3473e, miR-669o-5p, miR-3620-5p, and mir-2137. Previous studies have reported that miR-486a-5p suppresses hepatocellular carcinoma31 and non-small cell lung cancer32 by targeting insulin-like growth factor 1 receptor and its downstream pathways. Except for miR-486a-5p, other DEMs were not studied extensively. Functional analysis of predicted target genes revealed that several KEGG pathways, including the ‘thyroid hormone signaling pathway’, are exclusively identified in the HF/S vs. CON comparison, but not in the HF vs. CON comparison. From this result, it could be inferred that a low-isoflavone soy protein diet exerted distinct effects on high-fat diet induced obesity, through regulating rather novel miRNAs. Among the 3 DEMs from the HF/SI vs. HF/S comparison, the circulating levels of miR-486a-5p and miR-7682-3p have distinct correlation profiles with obesity-associated parameters. In addition, the putative target genes of these DEMs are associated with the ‘sphingolipid signaling pathway’ and the ‘mTOR signaling pathway’ that are known to be involved in the regulation of homeostasis of cell cycle and metabolism.33,34 Previous studies reported that isoflavones regulate the expression of several genes associated with the biosynthesis and degradation of sphingolipids in human fibroblasts35 and reduce ceramide levels by inhibiting its biosynthesis in the heart of rats fed a high-fat diet.36
Here, we observed that consumption of isoflavone at a dose contained in commercial soy protein isolate products reduced obesity-associated complications in high-fat-fed mice. The total isoflavone content of a commercial soy protein isolate ranges from 0.26 to 2.28 mg per g of soy protein.37 The data could be converted to about 50 to 450 mg of isoflavone per kg diet under the assumption that soy protein isolate accounts for 19.5% of the total diet as in the present study. Considering the content of isoflavones in a high-isoflavone diet (405 mg as aglycone equivalents per kg), the daily consumption of isoflavones is ∼1.1 μg day−1 in mice, which is converted to ∼130 mg day−1 in humans based on the body surface area. In a human intervention study, ∼100 mg of isoflavone as aglycone equivalents per day was used to determine the effects of soy protein isolate on circulating hormone profiles in men having a higher risk of advanced prostate cancer.38 Furthermore, the bioavailability of isoflavone was investigated in American39 or Japanese women40 after the consumption of 250 mg day−1 or 130 mg day−1, respectively. According to a population-based prospective study, the daily estimated mean intake of total isoflavone by US adults was reported to be 2.35 mg.41 Meanwhile, daily consumption of isoflavone (median value of Q1 to Q5) ranged from 14.0 to 73.1 mg day−1 and from 14.5 to 72.8 mg day−1 in Japanese men and women, respectively.42 The isoflavone contents in soy-based foods that are typically consumed by Eastern Asian counties were in the range of ∼20 to 80 mg per serving.43
In previous studies, consuming similar (∼200 to 500 mg kg−1) or higher amounts (1 to 4 g kg−1) of pure isoflavone, either genistein or daidzein, during various periods (12 to 24 weeks) reduced body weight in diet-induced obese rodent models.8,9,44,45 However, we did not observe any significant decreases in body and adipose tissue weights in the HF/SI group. A variety of factors may contribute to the differential results, including the use of soy isoflavone concentrates instead of pure isoflavones. The proportion of aglycone form, genistein and daidzein, accounts for only 4.6% of total aglycone equivalents in the HF/SI diet. Although still controversial, the bioavailability of isoflavones in aglycone form was shown to be higher than those in glucoside form.40 In addition, the weight-lowering effects of the high-isoflavone soy protein diet were observed in rodents fed a high-fat diet for either too short (5 weeks to 40 days)46,47 or too long periods (160 to 180 days)24,25 compared to ours (16 weeks). The relatively low impacts of low-isoflavone soy protein on diet-induced obesity may be caused by the low amounts of bioactive peptides from soy protein since we did not conduct additional food processing like enzymatic treatments or microbial fermentation to derive more bioactive peptides from soy protein.
One of the limitations of the present study is the lack of a quantitative PCR assay to validate the levels of circulating DEMs. To replicate and extend the current observations, future research with an appropriate model to get larger blood volumes appears warranted. In addition, the secretory and target tissue(s) of circulating miRNAs were not defined. Further studies are needed to clarify whether the observed alterations in the miRNA profiles are due to the dysregulated adipose tissue or the obesity-associated changes in other tissues. Profiling of miRNAs isolated from the explant culture medium of candidate tissues as well as the candidate tissue per se would provide important information regarding the biogenesis and secretion of circulating miRNAs. Moreover, the proposed functions of DEMs in their target tissues need to be identified to provide the causal relationships between circulating miRNAs and obesity-related diseases.
In this study, a high-isoflavone soy protein diet suppressed obesity-mediated changes in circulating adipokine levels as well as adipocyte size, suggesting that soy isoflavone could possibly be the main contributor to the health-beneficial effects of soy protein. To the best of our knowledge, this is the first study that investigated the effects of a soy protein diet on circulating miRNA alterations. Overall, these findings provide additional insights into the effects of soy protein on diet-induced obesity and circulating miRNA levels. Since increasing evidence has shown the role of circulating miRNAs as the important mediator between obesity and cancer, the regulatory effects of putative miRNAs on the progression of cancer development are interesting and worthy of further study.
Author contributions
Hyo Bin Lee: methodology, validation, investigation, writing – original draft, review & editing, and visualization. Ah Young Lee: investigation and writing –review & editing. Yumi Jang: writing – original draft and review & editing. Young Hye Kwon: conceptualization, methodology, validation, writing – original draft, review & editing, supervision, and funding acquisition.Conflicts of interest
The authors declare that there are no conflicts of interest.Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (no. NRF- 2020R1A2C2010574).References
- A. L. Ghaben and P. E. Scherer, Adipogenesis and metabolic health, Nat. Rev. Mol. Cell Biol., 2019, 20, 242–258 CrossRef CAS PubMed.
- M. Fasshauer and M. Bluher, Adipokines in health and disease, Trends Pharmacol. Sci., 2015, 36, 461–470 CrossRef CAS PubMed.
- L. Forny-Germano, F. G. De Felice and M. Vieira, The role of leptin and adiponectin in obesity-associated cognitive decline and Alzheimer's disease, Front. Neurosci., 2018, 12, 1027 Search PubMed.
- M. Messina, Soy and health update: Evaluation of the clinical and epidemiologic literature, Nutrients, 2016, 8, 754 CrossRef PubMed.
- K. Yamagata and Y. Yamori, Potential effects of soy isoflavones on the prevention of metabolic syndrome, Molecules, 2021, 26, 5863 CrossRef CAS PubMed.
- M. Kohno, M. Hirotsuka, M. Kito and Y. Matsuzawa, Decreases in serum triacylglycerol and visceral fat mediated by dietary soybean beta-conglycinin, J. Atheroscler. Thromb., 2006, 13, 247–255 CrossRef CAS PubMed.
- D. B. Allison, G. Gadbury, L. G. Schwartz, R. Murugesan, J. L. Kraker, S. Heshka, K. R. Fontaine and S. B. Heymsfield, A novel soy-based meal replacement formula for weight loss among obese individuals: a randomized controlled clinical trial, Eur. J. Clin. Nutr., 2003, 57, 514–522 Search PubMed.
- S. Jeon, Y. J. Park and Y. H. Kwon, Genistein alleviates the development of nonalcoholic steatohepatitis in ApoE(-/-) mice fed a high-fat diet, Mol. Nutr. Food Res., 2014, 58, 830–841 CrossRef CAS PubMed.
- M. Gan, L. Shen, S. Wang, Z. Guo, T. Zheng, Y. Tan, Y. Fan, L. Liu, L. Chen, A. Jiang, X. Li, S. Zhang and L. Zhu, Genistein inhibits high fat diet-induced obesity through miR-222 by targeting BTG2 and adipor1, Food Funct., 2020, 11, 2418–2426 RSC.
- I. Zanella, E. Marrazzo, G. Biasiotto, M. Penza, A. Romani, P. Vignolini, L. Caimi and D. Di Lorenzo, Soy and the soy isoflavone genistein promote adipose tissue development in male mice on a low-fat diet, Eur. J. Nutr., 2015, 54, 1095–1107 CrossRef CAS.
- S. Jeong and M. Yoon, 17beta-Estradiol inhibition of PPARgamma-induced adipogenesis and adipocyte-specific gene expression, Acta Pharmacol. Sin., 2011, 32, 230–238 CrossRef CAS PubMed.
- C. Chatterjee, S. Gleddie and C. W. Xiao, Soybean bioactive peptides and their functional properties, Nutrients, 2018, 10, 1211 CrossRef PubMed.
- T. Hashidume, A. Kato, T. Tanaka, S. Miyoshi, N. Itoh, R. Nakata, H. Inoue, A. Oikawa, Y. Nakai, M. Shimizu, J. Inoue and R. Sato, Single ingestion of soy beta-conglycinin induces increased postprandial circulating FGF21 levels exerting beneficial health effects, Sci. Rep., 2016, 6, 28183 CrossRef CAS PubMed.
- I. S. Kim, W. S. Yang and C. H. Kim, Beneficial effects of soybean-derived bioactive peptides, Int. J. Mol. Sci., 2021, 22, 8570 Search PubMed.
- Q. Sun, R. Cong, H. Yan, H. Gu, Y. Zeng, N. Liu, J. Chen and B. Wang, Genistein inhibits growth of human uveal melanoma cells and affects microRNA-27a and target gene expression, Oncol. Rep., 2009, 22, 563–567 Search PubMed.
- K. Saliminejad, H. R. Khorram Khorshid, S. Soleymani Fard and S. H. Ghaffari, An overview of microRNAs: Biology, functions, therapeutics, and analysis methods, J. Cell Physiol., 2019, 234, 5451–5465 CrossRef CAS PubMed.
- C. Li, Y. Q. Ni, H. Xu, Q. Y. Xiang, Y. Zhao, J. K. Zhan, J. Y. He, S. Li and Y. S. Liu, Roles and mechanisms of exosomal non-coding RNAs in human health and diseases, Signal Transduction Targeted Ther., 2021, 6, 383 CrossRef CAS PubMed.
- C. Ji and X. Guo, The clinical potential of circulating microRNAs in obesity, Nat. Rev. Endocrinol., 2019, 15, 731–743 CrossRef CAS.
- S. B. Won, A. Han and Y. H. Kwon, Maternal consumption of low-isoflavone soy protein isolate alters hepatic gene expression and liver development in rat offspring, J. Nutr. Biochem., 2017, 42, 51–61 Search PubMed.
- C. Sticht, C. De La Torre, A. Parveen and N. Gretz, miRWalk: An online resource for prediction of microRNA binding sites, PLoS One, 2018, 13, e0206239 CrossRef.
- V. Agarwal, G. W. Bell, J. W. Nam and D. P. Bartel, Predicting effective microRNA target sites in mammalian mRNAs, eLife, 2015, 4, e05005 CrossRef PubMed.
- Y. Chen and X. Wang, miRDB: an online database for prediction of functional microRNA targets, Nucleic Acids Res., 2020, 48, D127–D131 CrossRef CAS PubMed.
- H. Y. Huang, Y. C. Lin, J. Li, K. Y. Huang, S. Shrestha, H. C. Hong, Y. Tang, Y. G. Chen, C. N. Jin, Y. Yu, J. T. Xu, Y. M. Li, X. X. Cai, Z. Y. Zhou, X. H. Chen, Y. Y. Pei, L. Hu, J. J. Su, S. D. Cui, F. Wang, Y. Y. Xie, S. Y. Ding, M. F. Luo, C. H. Chou, N. W. Chang, K. W. Chen, Y. H. Cheng, X. H. Wan, W. L. Hsu, T. Y. Lee, F. X. Wei and H. D. Huang, miRTarBase 2020: updates to the experimentally validated microRNA-target interaction database, Nucleic Acids Res., 2020, 48, D148–D154 CAS.
- I. Torre-Villalvazo, A. R. Tovar, V. E. Ramos-Barragan, M. A. Cerbon-Cervantes and N. Torres, Soy protein ameliorates metabolic abnormalities in liver and adipose tissue of rats fed a high fat diet, J. Nutr., 2008, 138, 462–468 CrossRef CAS PubMed.
- M. E. Frigolet, N. Torres, L. Uribe-Figueroa, C. Rangel, G. Jimenez-Sanchez and A. R. Tovar, White adipose tissue genome wide-expression profiling and adipocyte metabolic functions after soy protein consumption in rats, J. Nutr. Biochem., 2011, 22, 118–129 Search PubMed.
- A. L. Hess, L. H. Larsen, P. B. Udesen, Y. Sanz, T. M. Larsen and L. T. Dalgaard, Levels of circulating miR-122 are associated with weight loss and metabolic syndrome, Obesity, 2020, 28, 493–501 CrossRef CAS PubMed.
- F. J. Ortega, J. M. Mercader, V. Catalan, J. M. Moreno-Navarrete, N. Pueyo, M. Sabater, J. Gomez-Ambrosi, R. Anglada, J. A. Fernandez-Formoso, W. Ricart, G. Fruhbeck and J. M. Fernandez-Real, Targeting the circulating microRNA signature of obesity, Clin. Chem., 2013, 59, 781–792 Search PubMed.
- C. Esau, S. Davis, S. F. Murray, X. X. Yu, S. K. Pandey, M. Pear, L. Watts, S. L. Booten, M. Graham, R. McKay, A. Subramaniam, S. Propp, B. A. Lollo, S. Freier, C. F. Bennett, S. Bhanot and B. P. Monia, miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting, Cell Metab., 2006, 3, 87–98 CrossRef CAS PubMed.
- Y. Jin, Y. S. Wong, B. K. P. Goh, C. Y. Chan, P. C. Cheow, P. K. H. Chow, T. K. H. Lim, G. B. B. Goh, T. L. Krishnamoorthy, R. Kumar, T. P. Ng, S. S. Chong, H. H. Tan, A. Y. F. Chung, L. Ooi, J. P. E. Chang, C. K. Tan and C. G. L. Lee, Circulating microRNAs as potential diagnostic and prognostic biomarkers in hepatocellular carcinoma, Sci. Rep., 2019, 9, 10464 CrossRef PubMed.
- X. Wang, Z. Zhou, T. Zhang, M. Wang, R. Xu, S. Qin and S. Zhang, Overexpression of miR-664 is associated with poor overall survival and accelerates cell proliferation, migration and invasion in hepatocellular carcinoma, OncoTargets Ther., 2019, 12, 2373–2381 CrossRef CAS PubMed.
- R. A. Youness, H. M. El-Tayebi, R. A. Assal, K. Hosny, G. Esmat and A. I. Abdelaziz, MicroRNA-486-5p enhances hepatocellular carcinoma tumor suppression through repression of IGF-1R and its downstream mTOR, STAT3 and c-Myc, Oncol. Lett., 2016, 12, 2567–2573 Search PubMed.
- F. Tian, J. Wang, T. Ouyang, N. Lu, J. Lu, Y. Shen, Y. Bai, X. Xie and Q. Ge, MiR-486-5p serves as a good biomarker in nonsmall cell lung cancer and suppresses cell growth with the involvement of a target PIK3R1, Front. Genet., 2019, 10, 688 Search PubMed.
- J. Simon, A. Ouro, L. Ala-Ibanibo, N. Presa, T. C. Delgado and M. L. Martinez-Chantar, Sphingolipids in non-alcoholic fatty liver disease and hepatocellular carcinoma: Ceramide turnover, Int. J. Mol. Sci., 2019, 21, 40 Search PubMed.
- M. Laplante and D. M. Sabatini, mTOR signaling in growth control and disease, Cell, 2012, 149, 274–293 CrossRef CAS PubMed.
- M. Moskot, J. Jakobkiewicz-Banecka, E. Smolinska, B. Banecki, G. Wegrzyn and M. Gabig-Ciminska, Activities of genes controlling sphingolipid metabolism in human fibroblasts treated with flavonoids, Metab. Brain Dis., 2015, 30, 1257–1267 Search PubMed.
- I. Torre-Villalvazo, F. Gonzalez, C. A. Aguilar-Salinas, A. R. Tovar and N. Torres, Dietary soy protein reduces cardiac lipid accumulation and the ceramide concentration in high-fat diet-fed rats and ob/ob mice, J. Nutr., 2009, 139, 2237–2243 Search PubMed.
- J. E. Andrade, N. C. Twaddle, W. G. Helferich and D. R. Doerge, Absolute bioavailability of isoflavones from soy protein isolate-containing food in female BALB/c mice, J. Agric. Food Chem., 2010, 58, 4529–4536 CrossRef CAS PubMed.
- J. M. Hamilton-Reeves, S. A. Rebello, W. Thomas, J. W. Slaton and M. S. Kurzer, Isoflavone-rich soy protein isolate suppresses androgen receptor expression without altering estrogen receptor-beta expression or serum hormonal profiles in men at high risk of prostate cancer, J. Nutr., 2007, 137, 1769–1775 Search PubMed.
- L. Zubik and M. Meydani, Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women, Am. J. Clin. Nutr., 2003, 77, 1459–1465 CrossRef CAS PubMed.
- T. Izumi, M. K. Piskula, S. Osawa, A. Obata, K. Tobe, M. Saito, S. Kataoka, Y. Kubota and M. Kikuchi, Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans, J. Nutr., 2000, 130, 1695–1699 Search PubMed.
- W. Bai, C. Wang and C. Ren, Intakes of total and individual flavonoids by US adults, Int. J. Food Sci. Nutr., 2014, 65, 9–20 CrossRef CAS PubMed.
- U. Murai, N. Sawada, H. Charvat, M. Inoue, N. Yasuda, K. Yamagishi, S. Tsugane and J. S. Group, Soy product intake and risk of incident disabling dementia: the JPHC Disabling Dementia Study, Eur. J. Nutr., 2022, 61, 4045–4057 CrossRef CAS PubMed.
- L. Jian, Soy, isoflavones, and prostate cancer, Mol. Nutr. Food Res., 2009, 53, 217–226 CrossRef CAS PubMed.
- Y. M. Lee, J. S. Choi, M. H. Kim, M. H. Jung, Y. S. Lee and J. Song, Effects of dietary genistein on hepatic lipid metabolism and mitochondrial function in mice fed high-fat diets, Nutrition, 2006, 22, 956–964 Search PubMed.
- Y. Sakamoto, A. Naka, N. Ohara, K. Kondo and K. Iida, Daidzein regulates proinflammatory adipokines thereby improving obesity-related inflammation through PPARgamma, Mol. Nutr. Food Res., 2014, 58, 718–726 CrossRef CAS PubMed.
- M. J. Ronis, Y. Chen, J. Badeaux and T. M. Badger, Dietary soy protein isolate attenuates metabolic syndrome in rats via effects on PPAR, LXR, and SREBP signaling, J. Nutr., 2009, 139, 1431–1438 CrossRef CAS PubMed.
- A. Al-Dwairi, J. M. Pabona, R. C. Simmen and F. A. Simmen, Cytosolic malic enzyme 1 (ME1) mediates high fat diet-induced adiposity, endocrine profile, and gastrointestinal tract proliferation-associated biomarkers in male mice, PLoS One, 2012, 7, e46716 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo02106d |
| This journal is © The Royal Society of Chemistry 2022 |