Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The effects of a green Rooibos (Aspalathus linearis) extract on metabolic parameters and adipose tissue biology in rats fed different obesogenic diets†
L. M.
Kotzé-Hörstmann
 ab,
D. T.
Bedada
c,
R.
Johnson
ad,
L.
Mabasa
ad and
H.
Sadie-Van Gijsen
ab,
D. T.
Bedada
c,
R.
Johnson
ad,
L.
Mabasa
ad and
H.
Sadie-Van Gijsen
 *a
*a
aCentre for Cardio-metabolic Research in Africa (CARMA), Division of Medical Physiology, Department of Biomedical Sciences, Faculty of Medicine and Health Sciences, Stellenbosch University Tygerberg Campus, PO Box 241, Cape Town 8000, South Africa. E-mail: hsadie@sun.ac.za
bInstitute for Sport and Exercise Medicine (ISEM), Department of Sport Science, Faculty of Medicine and Health Sciences, Stellenbosch University Tygerberg Campus, PO Box 241, Cape Town 8000, South Africa
cDivision of Epidemiology and Biostatistics, Faculty of Medicine and Health Sciences, Stellenbosch University Tygerberg Campus, PO Box 241, Cape Town 8000, South Africa
dBiomedical Research and Innovation Platform (BRIP), South African Medical Research Council (SAMRC), PO Box 19070, Parow 7505, South Africa
First published on 28th November 2022
Abstract
Current pharmaceutical treatments addressing obesity are plagued by high costs, low efficacy and adverse side effects. Natural extracts are popular alternatives, but evidence for their anti-obesity properties is scant. We assessed the efficacy of a green (minimally-oxidized) Rooibos (Aspalathus linearis) extract (GRT) to ameliorate the effects of obesogenic feeding in rats, by examining body weight, metabolic measures, adipose tissue cellularity and tissue-resident adipose stem cells (ASCs). Furthermore, we performed statistical correlations to explore the relationships and interactions between metabolic and adipose tissue measures. Using an in vivo/ex vivo study design, male Wistar rats were maintained for 17 weeks on one of 3 diets: CON (laboratory chow), OB1 (high-sugar, medium fat) or OB2 (high-fat, high-cholesterol) (n = 24 each). From weeks 11–17, half of the animals in each group received oral GRT supplementation (60 mg per kg body weight daily). Blood and tissue samples were collected, and ASCs from each animal were cultured. Diets OB1 and OB2 induced divergent metabolic profiles compared to CON, but metabolic measures within dietary groups were mostly unaffected by GRT supplementation. Notably, diets OB1 and OB2 uncoupled the positive association between visceral adiposity and insulin resistance, while GRT uncoupled the positive association between elevated serum cholesterol and liver damage. Obesogenic feeding and GRT supplementation induced adipocyte enlargement in vivo, but lipid accumulation in cultured ASCs did not differ between dietary groups. Larger adipocyte size in subcutaneous fat was associated with favourable glucose metabolism measures in all GRT groups. In conclusion, GRT affected the associations between systemic, adipose tissue-level and cellular measures against the background of obesogenic diet-induced metabolic dysregulation.
1 Introduction
Globally, approximately 40% of adults are overweight (body mass index (BMI) of >25 kg m−2), while 15% of adults are obese (BMI >30 kg m−2).1 Obesity is a risk factor for a multitude of chronic metabolic diseases such as type II diabetes and cardiovascular disease, as well as certain types of cancer.2 Due to shifts in dietary preferences and increased sedentary lifestyle, the greatest number of obese individuals now resides in low- and middle-income countries,1 highlighting the need for affordable and widely available anti-obesity interventions.Given the scale of the problem, there has been disappointingly little success with adherence to healthy lifestyle changes or the development of anti-obesity drug therapies. Although several pharmaceutical products have been approved for the treatment of obesity, these drugs mostly induce mild weight-loss and often have adverse side-effects.3,4 In addition, many different botanical or herbal extracts are advertised as weight-loss products, but available evidence indicates that these products are also largely ineffective.5,6 The lack of success at addressing obesity may be a result of our poor understanding of adipose tissue biology, due to the continued use of research models that are inappropriate or limited in their scope.7 In humans, obesity is mostly studied retrospectively, and therefore the systemic and cellular mechanisms involved in the development and progression of obesity, especially diet-induced obesity (DIO) and diet-induced metabolic dysregulation (DIMD), are largely unknown. More work has been performed on the progression of DIO and DIMD in animal models, but these studies often do not explore molecular mechanisms on a cellular level, especially not in the tissue-resident adipose stem cells (ASCs), which have been shown to become dysfunctional during obesity and contribute to adipose tissue pathology.7 It is well established that ASCs retain a “memory” of their in vivo milieu before isolation, including adipose depot-specific characteristics and metabolic status, and this “memory” persists in cell culture. As a result, ASCs can offer a “window” into in vivo changes at the cellular level within adipose tissue.7 In contrast, immortalised adipocyte cell lines, although widely used, cannot recapitulate any of the systemic factors that may contribute to obesity, such as diet, disease, gender, pregnancy and age.7 Consequently, an in vivo/ex vivo study design, with in vivo dietary interventions followed by ex vivo culturing and investigations into ASC biology, presents a superior research model with which to explore the interactions between cellular and systemic metabolic mechanisms underpinning the impact of DIO/DIMD and putative weight-loss interventions on adipose tissue function.7,8
The Rooibos shrub (Aspalathus linearis (Burm.f.) R.Dahlgren) of the family Leguminosae (Fabaceae) is endemic to the Cederberg region of the Western Cape province in South Africa. The leaves are used to produce herbal tea and commercial dietary supplements.9 Rooibos enjoys geographical indication status, and therefore only Rooibos grown and harvested in demarcated areas of the Cederberg may legally be named as such.9 Rooibos contains several polyphenolic constituents, including the flavonoid aspalathin, which is uniquely found at high levels only in minimally-oxidised (unfermented) green Rooibos leaves.10 Although the available evidence on the potential of Rooibos to induce weight-loss is scant,11 numerous studies have demonstrated that both complex Rooibos extracts and pure aspalathin have beneficial metabolic effects. In diabetic mice, pure aspalathin and various green Rooibos extracts (GREs) containing 6–18% aspalathin exhibited glucose- and lipid-lowering properties,12–15 as well as anti-inflammatory actions.13 In addition, in vitro studies in a variety of cell-types including muscle, liver and fat cells have shown that aspalathin and GREs can enhance the activation of metabolic signalling pathways such as AMP-activated protein kinase (AMPK), Akt and insulin signalling, resulting in improved cellular glucose uptake.13–17 Anti-adipogenic and anti-lipogenic actions of fermented Rooibos extracts (FREs)18 and individual phenolic constituents of Rooibos such as rutin,19 vitexin,20 orientin20 and iso-orientin21 have also been demonstrated in vitro in 3T3-L1 immortalised pre-adipocytes, but similar studies have not been performed ex vivo in cultured ASCs.
More recently, the metabolic properties of a pharmaceutical-grade green Rooibos extract called GRT (containing 12% aspalathin22) have been assessed in a variety of animal models including rats with DIO/DIMD,23–25 diabetic mice26 and vervet monkeys.27 While the impact of GRT on body weight varied between these studies,23–27 beneficial metabolic effects such as lowered blood glucose,26,27 improved glucose tolerance24 and lipid profiles,27 and reduced liver steatosis23 were reported. However, none of these studies examined the effects of GRT on adipose tissue or ASCs. Indeed, to our knowledge, no Rooibos extracts or individual constituents have been assessed for their effects on adipose tissue and ASC function in vivo against the background of DIO/DIMD. Furthermore, it is well established that subcutaneous (peripheral) and visceral (central, intra-abdominal) adipose tissue depots differ fundamentally in terms of their contribution to the systemic metabolism. Enlargement of visceral fat volume is associated with increased metabolic risk,28,29 while subcutaneous fat expansion is considered to be metabolically protective.30,31 Correspondingly, ASCs from subcutaneous and visceral adipose depots are also inherently different in terms of their gene expression profile, adipogenic capacity and susceptibility to insult and dysfunction.7,31 However, research on the possible adipose depot-specific effects of Rooibos is lacking.
Given these in vivo and in vitro metabolic effects of Rooibos extracts collectively, and of GRT specifically, our primary hypothesis was that GRT supplementation may ameliorate the detrimental metabolic consequences of obesogenic feeding in rats, and that GRT may modulate adipose tissue and ASC function. We recently demonstrated, through direct comparisons and multivariate modelling techniques, that obesogenic diets with different relative proportions of fat, carbohydrates and sugars resulted in different metabolic profiles in rats.32 Of note, previous studies on GRT in rat DIO/DIMD models yielded mixed results in terms of changes in body weight and metabolic parameters,23–25 which may have been the consequence of different obesogenic diets used in each study. Therefore, our secondary hypothesis was that GRT may have different metabolic and adipose-specific effects against the background of different obesogenic diets. Thus, we investigated whether GRT supplementation could ameliorate the effects of two different obesogenic diets (a high-sugar/medium fat diet and high fat/high-cholesterol diet) on body composition, measures of glucose and lipid homeostasis, adipose tissue histology and ASC function, using an in vivo/ex vivo study design.8 In addition, we determined the associations between these parameters within each dietary group, and the impact of GRT on these associations, to develop a more advanced understanding of the contributions of various systemic metabolic and cellular factors during the progression of DIO and DIMD.
2 Materials and methods
2.1 Ethical approval
Ethical approval was obtained from the Stellenbosch University Research Ethics Committee: Animal Care and Use (ethics clearance number ACU-2018-6786). All experiments involving animals were performed according to the ARRIVE guidelines for in vivo animal research. All animals were housed at the Animal Research Facility, Tygerberg campus, Stellenbosch University.2.2 Materials
A detailed description of the composition and preparation of diets CON, OB1 and OB2 was published previously.32 Standard laboratory chow pellets (Rodent Breeder Customized Laboratory Animal Diet) were purchased from LabChef Research Nutrition, Stellenbosch, South Africa. Condensed milk, Holsum cooking fat (which consists mostly of solidified palm oil) and sucrose were purchased from retail supermarkets. Fructose (#F0127), cholesterol (#C8503) and casein (#C7078) were purchased from Sigma-Aldrich (Germany). Sodium pentobarbitone (Eutha-naze) was from Bayer South Africa. Vacuette 5 mL serum separator tubes (SSTs) were from Greiner Bio-one (Austria). The Milliplex MAP rat cytokine/chemokine magnetic bead panel (RECYTMAG-65K, five-plex: leptin, tumour necrosis factor-alpha (TNF-α), interleukin (IL)-18, IL-6 and IL-1α), rat/mouse insulin ELISA kits (#EZRMI-13K) and rat adiponectin ELISA kits (#EZRADP-62K) were purchased from Merck South Africa.For cell culture experiments, the following consumables were purchased from Sigma-Aldrich: human insulin (#I9278), dexamethasone (#D4902), indomethacin (#I7378), isobutyl-3-methylxanthine (#I5879) and Oil Red O (#O0625). The following cell culture consumables were purchased from Lonza, through their South African distributors, Whitehead Scientific: DMEM (high-glucose) (#BE12-604F) and DMEM (high-glucose) with UltraGlutamine (#BE12-604F/U1), penicillin–streptomycin (#17-602), trypsin (#17-161) and Hanks’ Buffered Saline Solution (#BE10-527F). Fetal bovine serum (FBS: #SV30160.03) and bovine serum albumin (BSA) were purchased from Hyclone, through their South African distributors, Separations.
2.3 Rooibos (Aspalathus linearis) extract
Aspalathus linearis (Burm.f.) R.Dahlgren (confirmed on https://www.theplantlist.org) is polymorphic in the wild,33 but only the Nortier variety of the red Rocklands type of A. linearis is cultivated for commercial use.33,34 The process for the production of GRT from green minimally-oxidized Rooibos leaves was developed and standardized by the South African Agricultural Research Council (ARC) and the South African Medical Research Council (SA-MRC). The GRT used for this study (batch number 730330) was generously donated by the SA-MRC. This extract was previously characterised by high-performance liquid chromatography (HPLC)22 and was shown to contain a variety of flavonoids, including aspalathin (∼12% m/m), nothofagin, orientin, iso-orientin (all at >1% m/m) and rutin (∼0.5% m/m).222.4 Dietary intervention study
A detailed description of the dietary intervention study, including food and energy intake data as well as comprehensive metabolic characterisation of the outcomes of diets CON, OB1 and OB2, was published previously.32 Briefly, diet CON (standard laboratory chow pellets) contained 17%E from fat, 43%E from carbohydrates (8%E from sugars) and 40%E from protein. This diet contained sufficient protein and lipids to support the growth of young male rats.35 Diet OB1 was a high sucrose/medium fat diet containing 34%E from fat, 49%E from carbohydrates (36%E from sugars) and 17%E from protein, and was prepared by mixing CON pellets with sweetened condensed milk, sucrose and Holsum. Diet OB2 was a high fat/fructose/cholesterol diet containing 68%E from fat, 15%E from carbohydrates (9%E from sugars) and 17%E from protein, and was prepared by mixing CON pellets with Holsum, fructose, cholesterol and casein.24 ESI Table 1† provides detailed information on the macronutrient composition of diets CON, OB1 and OB2. Although the protein content of diets OB1 and OB2 was lower than CON, it was similar between diets OB1 and OB2 and above the minimum protein requirements for growing male rats.32,35 For the present study, weanling male Wistar rats (6 weeks of age) were randomly assigned into one of the three dietary groups (n = 24 in each dietary group). The rats were housed in groups of four per cage, maintained at 22 °C under a 12 h/12 h day/night cycle, and had ad libitum access to food and water. Body weights of the rats were recorded weekly.The rats were introduced into the study following a staggered approach (4 rats per week over 18 weeks, for a total of 72 animals), as described previously.8 This approach was followed due to the logistical constraints relating to the primary ASC cultures, which could not be accommodated for all 72 animals simultaneously.8 All animals were the same age and approximately the same body weight (170–200 g) upon entering the study. After 10 weeks on their respective diets, half of the rats in each dietary group (n = 12 each) were randomly selected to start receiving oral GRT supplementation at a daily dose of 60 mg per kg body weight for seven weeks (dietary groups CON-GRT, OB1-GRT, OB2-GRT). The GRT dosage was identical to that used in other rat studies23–25 and comparable to that used in mice26 and vervet monkeys.27 The extract was administered in the form of strawberry-flavoured jelly blocks to encourage consumption, as described previously.8 For daily GRT administration, each rat was removed from the communal cage, briefly (<30 minutes) isolated in a smaller cage and observed until the jelly block had been wholly consumed. Rats in the non-GRT groups (CON, OB1, OB2) were administered jelly blocks without GRT, utilising the same temporary isolation protocol. All animals remained in their original dietary groups for a total of 17 weeks.
Between weeks 6 and 10, before GRT supplementation commenced, there was no significant difference in the kilojoule (kJ) consumption per cage between the different dietary groups, as reported previously.32 Once GRT supplementation commenced, GRT-fed and non-GRT-fed rats cohabitated in the same cages, and therefore the impact of GRT on food consumption could not be determined. However, it has been noted in several other published studies that GRT supplementation did not affect kJ intake.24,25,27
2.5 Sample collection
After 16 weeks of dietary intervention (6 weeks of GRT supplementation), rats were fasted for 16 hours overnight and at 09:00 in the morning, an oral glucose tolerance test (OGTT) was performed as described previously.32 Blood glucose levels were measured via tail prick at 3, 5, 10, 15, 20, 25, 30, 45, 60, 90 and 120 min.After 17 weeks of dietary intervention (7 weeks of GRT supplementation), the rats were weighed and euthanized in the non-fasted state by intraperitoneal injection with an overdose (160 mg per kg body weight) of sodium pentobarbitone, and subsequent exsanguination. All euthanizations were performed between 09:00 and 11:00, in order to minimise the impact of variations in circadian cycles on metabolic variables. Blood was collected into SSTs and internal organs were harvested and weighed. The perirenal visceral (pv) fat from one flank was dissected as described previously8 and the weight was recorded to serve as a surrogate marker for total visceral fat.32 The livers were also dissected and weighed, and the liver index was calculated as liver weight/body weight × 100.
Inguinal subcutaneous (sc) and pv fat depots from the right-hand flank were excised for ASC isolation, as described previously.8 Contralateral sc and pv fat pads, as well as epididymal (epi) fat, were collected for additional analyses as shown in ESI Fig. S1.†
2.6 ASC isolations and adipocyte differentiation
ASCs were isolated from sc and pv fat pads as described in more detail previously.8,36,37 Briefly, excised fat pads (0.5–1.5 g) were minced with a scalpel and digested with 0.075% collagenase type I (Worthington #4196) in HBSS containing 1.5% BSA for 30 minutes at 37 °C. The stromal-vascular fraction (SVF) was collected by centrifugation and seeded in a 100 mm culture dish with DMEM supplemented with 20% FBS and 1% PenStrep. After 24 hours, the media was replaced with standard growth media (SGM), which consisted of DMEM and DMEM/U1 in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, supplemented with 10% FBS and 1% PenStrep. All cultures were maintained at 37 °C with 5% CO2, and media was replaced every 72 hours. All experimental procedures were performed on cells at passage 2, as it was previously shown that this allows for ASC population homogenization.37 For adipogenic induction, cells in 12-well culture plates (two days post-confluence) were treated with either control media (CM: SGM with 0.1% EtOH and 0.1% DMSO) or adipogenic media (AM: SGM with 10 μM insulin, 0.5 mM isobutyl-3-methylxanthine, 56 μM indomethacin and 1 μM dexamethasone).36 Each treatment was performed in triplicate wells and the media was changed every 72 hours. After 12 days of treatment, the media was aspirated and 0.7% Oil Red O solution in 70% isopropanol was added directly into the culture wells without washing. After a 30-minute incubation period, the excess stain was removed, wells were rinsed 3 times with water and colour images (one in each quadrant of each well) were captured at 10x magnification using a Euromex Oxion Inverso microscope with a mounted CMEX3 camera and ImageFocus 4 software. (For a representative image, see ESI Fig. S2†). Through this method, 12 images were captured for each treatment condition (diet × fat depot × differentiation status). The percentage stained area in each image was quantified using ImageJ software, by converting images to red/green/blue stacks and calculating the dark areas in the green channel.8 A fixed threshold was used for all images, to exclude background staining and to enable comparison between images. Staining of CM-treated cells was virtually undetectable, regardless of diet or depot (Fig. S2†), and therefore only the staining values for AM-treated cells were used for statistical analysis, and no normalization relative to CM-treated cells was performed.
1 ratio, supplemented with 10% FBS and 1% PenStrep. All cultures were maintained at 37 °C with 5% CO2, and media was replaced every 72 hours. All experimental procedures were performed on cells at passage 2, as it was previously shown that this allows for ASC population homogenization.37 For adipogenic induction, cells in 12-well culture plates (two days post-confluence) were treated with either control media (CM: SGM with 0.1% EtOH and 0.1% DMSO) or adipogenic media (AM: SGM with 10 μM insulin, 0.5 mM isobutyl-3-methylxanthine, 56 μM indomethacin and 1 μM dexamethasone).36 Each treatment was performed in triplicate wells and the media was changed every 72 hours. After 12 days of treatment, the media was aspirated and 0.7% Oil Red O solution in 70% isopropanol was added directly into the culture wells without washing. After a 30-minute incubation period, the excess stain was removed, wells were rinsed 3 times with water and colour images (one in each quadrant of each well) were captured at 10x magnification using a Euromex Oxion Inverso microscope with a mounted CMEX3 camera and ImageFocus 4 software. (For a representative image, see ESI Fig. S2†). Through this method, 12 images were captured for each treatment condition (diet × fat depot × differentiation status). The percentage stained area in each image was quantified using ImageJ software, by converting images to red/green/blue stacks and calculating the dark areas in the green channel.8 A fixed threshold was used for all images, to exclude background staining and to enable comparison between images. Staining of CM-treated cells was virtually undetectable, regardless of diet or depot (Fig. S2†), and therefore only the staining values for AM-treated cells were used for statistical analysis, and no normalization relative to CM-treated cells was performed.
2.7 Adipose tissue histology
A subset of inguinal sc and epi fat pads (n = 6 per group for each depot) were randomly selected for histological analyses. The pv fat was not available for histological analysis, as the totality of pv fat was used for ASC cultures, due to its small volume (Fig. S1†). Tissue sections were prepared and stained as described elsewhere.38 Briefly, excised fat pads were rinsed in PBS and fixed by immersion in a 10% formalin/PBS solution. After a minimum fixing period of 2 weeks, tissues were dehydrated with alcohol, cleared with xylene and embedded in paraffin wax. Two sections of 5 μm thickness were prepared from each fat pad and mounted on glass slides. Haematoxylin/eosin staining was performed to visualise individual adipocytes. Two images per section (four per fat pad) were captured using a Zeiss Axioskop microscope, at 50× magnification, and Zeiss ZEN lite v2.3 image acquisition software. A scale bar of 50 μm was included in all images. The surface area of all adipocytes visible in each field of view was calculated using ImageJ image analysis software. The straight tool was used to measure the scale bar and to set the scale at a known distance of 50 μm. Measuring of the area was subsequently done using the free-hand tool function to circle the outline of the cells and the measured area was reported. All adipocytes visible within each field of view were quantified.2.8 Blood biochemistry
Blood samples collected into SSTs were allowed to clot on ice, and the serum was separated by centrifugation at 4 °C for 10 minutes at 1800g. Serum aliquots were stored at −80 °C until analysed. Quantification of serum insulin, adiponectin, ALT (alanine transaminase), AST (aspartate transaminase) and lipid profiles were all performed on serum samples collected in the fasted state (at the onset of the OGTT, as described in section 2.5), while leptin, tumour necrosis factor-alpha (TNF-α), interleukin (IL)-18, IL-6 and IL-1α were quantified in serum collected in the non-fasted state at exsanguination. Serum insulin and adiponectin concentrations were quantified using the rat/mouse insulin ELISA kit (Merck, #EZRMI-13K) and rat adiponectin ELISA kit (Merck, #EZRADP-62K), respectively. An insulin conversion factor of 1 μU ml−1 = 0.04 ng ml−1 was used to convert the mass-based measurement of insulin to active insulin units, as per the manufacturers’ instructions. The derived insulin concentration values (μU ml−1) along with fasting blood glucose concentrations were used to calculate HOMA2-IR, HOMA2-%S (insulin sensitivity) and HOMA2-%B (pancreatic beta-cell function),39 utilising the University of Oxford Diabetes Trials Unit HOMA2 calculator software (online calculator available at https://www.dtu.ox.ac.uk/homacalculator/). Blood lipid profile analyses and quantification of serum ALT and AST were performed by Pathcare licensed pathology laboratories (Cape Town, South Africa). Serum leptin, TNF-α, IL-18, IL-6 and IL-1α were quantified with a Milliplex MAP rat cytokine/chemokine magnetic bead panel (RECYTMAG-65K), utilising a Bio-plex 200 automated immunoassay array system and Bio-Plex Manager 6.1 software (Bio-Rad Laboratories) for data acquisition.2.9 Statistical analyses
Based on previous studies conducted at our research facility, the sample size of n = 12 per group used in this study was estimated from a priori power calculations40,41 to detect group differences at 95% power and 5% error probability. Data sets were assessed for normality and equality of variance. For parametric analysis, skewed data were log transformed to meet assumptions of normality. All descriptive statistics relating to metabolic variables, adipocyte size and ASC lipid accumulation were performed by Analysis of Variance (ANOVA) while main effects of diet and GRT supplementation were determined by factorial analysis (two-way ANOVA) with Bonferroni's correction. For gamma distribution analysis of adipocyte cell size data, proportions of small and large adipocytes were defined, respectively, by the 10th and 90th percentile of the gamma probability distribution curve using shape [(mean/SD)2] and scale [mean/SD2] parameters in sc and epi fat depots of each animal, as described elsewhere.42 To assess the effect of GRT treatment on adipocyte size distribution in each dietary group, we compared proportions of small and large adipocyte areas using Student's t-test with the Bonferroni correction. Pearson's correlations (r) were used to explore the relationships between body composition variables, insulin and lipid homeostasis and adipocyte area proportions in GRT-treated and -untreated CON, OB1 and OB2 groups. P-Values <0.05 were accepted as indicative of statistical significance and used for interpretation of the results. All descriptive statistics (including Area Under the Curve (AUC) calculations for OGTT) and inferential statistics were performed in GraphPad Prism version 5 (GraphPad Software, San Diego, California, USA). Correlation analyses were performed in TIBCO STATISTICA version 13.0.5.17 and gamma distribution modelling was performed in R version 3.6.3.3 Results
3.1 The impact of GRT supplementation on systemic metabolic measures
A comprehensive analysis of the differences in the metabolic outcomes between the three dietary groups was published elsewhere.32 Briefly, diets OB1 and OB2 did not induce dramatic weight gain in the rats, but visceral adiposity and serum triacylglycerol levels were significantly higher in the OB1 and OB2 dietary groups than in CON. Furthermore, fasting insulin levels, insulin resistance, serum adiponectin and leptin levels were higher in the OB1 dietary group than in the CON and OB2 groups, while serum total cholesterol, LDL-cholesterol, IL-18, ALT and AST were higher in the OB2 dietary group than in the CON and OB1 groups.32 The effect of GRT supplementation on body composition and metabolic outcomes within each dietary group are shown in Table 1. When analysed per diet group, GRT supplementation had no effect on final body weight, visceral adiposity, liver index, fasting insulin concentrations, insulin resistance, serum lipid profiles or serum concentrations of adiponectin, leptin, TNF-α and IL-18 (Table 1). A main GRT supplementation effect was identified for fasting blood glucose and OGTT AUC, and an interaction between diet and GRT supplementation was identified for serum ALT and serum AST (Table 1). However, within-group analyses indicated that these measures were not significantly different between individual GRT-supplemented dietary groups and their corresponding non-GRT-supplemented dietary groups, except for the OGTT AUC that was significantly greater in the OB2-GRT group compared to the OB2 group (ESI Fig. S3†).| Variable | CON (GRT vs. non-GRT) | OB1 (GRT vs. non-GRT) | OB2 (GRT vs. non-GRT) | Two-way ANOVA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| End-point analysis for body composition and metabolic measures, shown as GRT vs. non-GRT per diet group (n = 11–12) (values given as mean ± SD). Data was analysed with two-way ANOVA (diet × GRT) (unweighted means analysis due to differences in sample numbers between groups). No outliers were excluded. Abbreviations: ALT = alanine aminotransferase; AST = aspartate aminotransferase; D × G = interaction between diet and GRT; HDL-c = high-density lipoprotein cholesterol; HOMA2-IR = homeostatic model assessment for insulin resistance; IL = interleukin; LDL-c = low-density lipoprotein cholesterol; n.d. = not detected; OGTT AUC = oral glucose tolerance test area under the curve; pv = perirenal visceral; TC = total cholesterol; TG = triacylglycerol, TNF-α = tumour necrosis factor-alpha. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Body weight (g) | 375.00 (42.68) vs. 380.92 (33.90) | 440.58 (48.91) vs. 415.36 (33.68) | 423.58 (43.90) vs. 394.08 (26.37) | Diet: p = 0.0002 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.086 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.247 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PV weight (one flank) (g) | 0.92 (0.36) vs. 0.86 (0.25) | 1.61 (0.5) vs. 1.50 (0.52) | 1.59 (0.42) vs. 1.33 (0.59) | Diet: p < 0.0001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.192 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.738 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Visceral adiposity index (% of body weight) | 0.49 (0.17) vs. 0.45 (0.10) | 0.72 (0.18) vs. 0.72 (0.22) | 0.74 (0.15) vs. 0.66 (0.25) | Diet: p < 0.0001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.368 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.761 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Liver index (% of body weight) | 3.11 (0.21) vs. 3.25 (0.19) | 2.80 (0.29) vs. 2.81 (0.29) | 3.88 (0.48) vs. 3.74 (0.41) | Diet: p < 0.001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.919 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.363 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fasting blood glucose (mmol L−1) | 5.74 (0.40) vs. 5.28 (0.71) | 6.14 (0.91) vs. 5.88 (0.81) | 6.14 (0.69) vs. 5.74 (0.82) | Diet: p = 0.047GRT: p = 0.036D × G: p = 0.891 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OGTT AUC | 208.05 (56.49) vs. 181.47 (51.89) | 266.80 (56.58) vs. 245.76 (73.17) | 286.43 (87.08) vs. 190.62 (84.73) | Diet: p = 0.012 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.006 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.136 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fasting Insulin (ng mL−1) | 1.98 (1.06) vs. 1.95 (1.29) | 4.08 (1.87) vs. 4.14 (2.07) | 3.30 (1.70) vs. 3.00 (2.11) | Diet: p = 0.0004 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.830 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.935 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HOMA2-IR (A.U.) | 6.76 (3.27) vs. 6.50 (3.89) | 11.47 (3.51) vs. 10.88 (3.70) | 10.01 (4.47) vs. 8.14 (3.45) | Diet: p = 0.001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.340 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.764 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HOMA2-%B | 286.12 (105.43) vs. 311.00 (118.50) | 401.21 (98.90) vs. 435.04 (142.05) | 334.54 (104.42) vs. 352.50 (182.58) | Diet: p = 0.013 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.432 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.980 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HOMA2-%S | 18.51 (9.21) vs. 23.72 (21.21) | 9.67 (3.70) vs. 10.61 (5.17) | 12.62 (7.35) vs. 16.62 (13.97) | Diet: p = 0.018 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.273 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.841 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TG (mmol L−1) | 0.78 (0.38) vs. 0.60 (0.11) | 1.11 (0.30) vs. 1.36 (0.37) | 1.21 (0.47) vs. 1.32 (0.38) | Diet: p < 0.0001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.512 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.154 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TC (mmol L−1) | 1.73 (0.23) vs. 1.92 (0.29) | 1.67 (0.16) vs. 1.67 (0.31) | 2.80 (0.63) vs. 3.08 (0.67) | Diet: p < 0.0001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.181 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.604 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HDL-c (mmol L−1) | 1.11 (0.17) vs. 1.22 (0.24) | 1.03 (0.11) vs. 1.00 (0.15) | 1.12 (0.21) vs. 1.14 (0.16) | Diet: p = 0.028 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.475 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.463 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LDL-c (mmol L−1) | 0.00 (0.00) vs. 0.03 (0.09) | 0.00 (0.00) vs. 0.06 (0.21) | 0.95 (0.35) vs. 1.18 (0.58) | Diet: p < 0.0001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.190 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.553 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ALT (IU L−1) | 41.57 (12.87) vs. 36.09 (4.41) | 44.30 (16.58) vs. 40.64 (12.30) | 107.55 (39.09) vs. 179.50 (117.82) | Diet: p < 0.0001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.150 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.050 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AST (IU L−1) | 106.43 (20.35) vs. 103.18 (17.97) | 95.50 (25.34) vs. 95.09 (11.05) | 123.64 (22.17) vs. 171.17 (70.71) | Diet: p < 0.0001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.121 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.051 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Adiponectin (ng mL−1) | 50.32 (15.00) vs. 61.03 (20.30) | 89.59 (28.66) vs. 88.18 (24.95) | 54.01 (10.45) vs. 67.04 (28.59) | Diet: p < 0.0001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.174 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.508 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Leptin (ng mL−1) | 16.83 (7.32) vs. 15.90 (3.82) | 28.84 (9.59) vs. 25.66 (9.16) | 16.50 (9.03) vs. 11.68 (4.80) | Diet: p < 0.0001 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.107 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.681 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TNF-α (pg mL−1) | 0.69 (0.27) vs. 0.70 (0.55) | 0.60 (0.57) vs. 0.42 (0.49) | 0.73 (1.15) vs. 0.20 (0.33) | Diet: p = 0.428 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.129 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.344 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IL-18 (pg mL−1) | 190.21 (61.70) vs. 157.57 (89.17) | 176.37 (47.88) vs. 196.99 (61.93) | 283.66 (110.05) vs. 301.61 (169.04) | Diet: p = 0.0002 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRT: p = 0.934 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D × G: p = 0.593 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IL-6 | n.d. | n.d. | n.d. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IL-1α | n.d. | n.d. | n.d. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
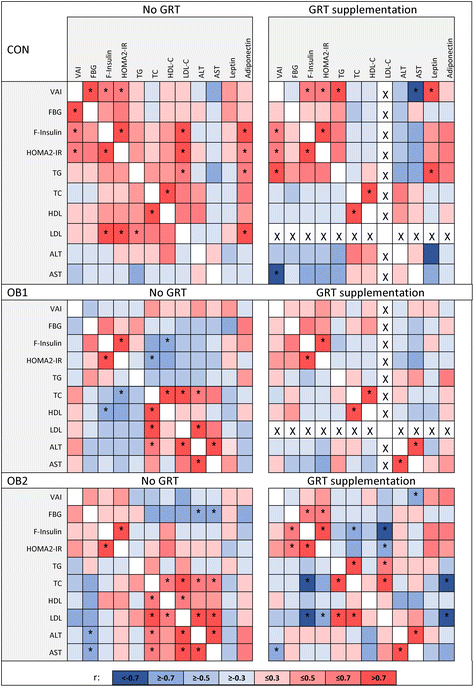
In addition, we determined the associations between body composition, insulin- and lipid homeostasis and other serum markers, and whether GRT supplementation affected these relationships similarly in each diet group. Correlation coefficients of these metabolic measures in GRT-supplemented and non-supplemented dietary groups are shown in Fig. 1, with additional data shown in ESI Data File 1.† Our previous work has already shown that diet composition has considerable impact on the associations between different metabolic measures,32 but here we demonstrate that GRT supplementation also modulated the associations between various metabolic measures within each dietary group. It is not within the scope of this manuscript to discuss all the associations identified in ESI Data File 1† and Fig. 1, but a few noteworthy observations will be mentioned here.
The visceral adiposity index (VAI) showed a significant positive association with the HOMA2-IR index of insulin resistance in the CON and CON-GRT dietary groups, but not in the other groups (Fig. 1), indicating that both diets OB1 and OB2 uncoupled the well-described43,44 association between visceral adiposity and insulin resistance. Serum concentrations of total cholesterol showed a significant positive association with serum ALT and AST in the OB1 and OB2 dietary groups, but not in the OB1-GRT and OB2-GRT groups (Fig. 1), suggesting that GRT supplementation may have ameliorated the impact of obesogenic diet-induced increases in circulating cholesterol levels on liver function and damage. Body weight at the end of the study, liver index, circulating leptin and adiponectin concentrations and fasting blood glucose concentrations did not consistently associate with any other measure included in the analysis (ESI Data File 1†).
3.2 Adipocyte size distributions in subcutaneous and epididymal adipose tissue depots
Fig. 2 shows the central tendencies of adipocyte size, including mean and median cell size, in epi fat (Fig. 2A) and sc fat (Fig. 2B) between the six dietary groups. Additional descriptive statistics (median and interquartile range) and group differences are shown in Table 2. In epi fat, the mean and median adipocyte size was significantly bigger in both the OB1 and OB2 dietary groups, compared to the CON dietary group, while the mean and median adipocyte size in sc fat was significantly bigger in dietary group OB1, compared to the CON and OB2 groups (Fig. 2 and Table 2). Furthermore, mean and median adipocyte size was significantly bigger in all GRT groups in sc and epi fat, compared to corresponding non-GRT groups (Fig. 2 and Table 2). Diet- and GRT-associated differences in adipocyte size were more pronounced in epi fat than in sc fat (Table 2). For both sc and epi fat, the relative GRT-associated difference in mean and median cell surface area was greatest in the CON dietary group (Fig. 2 and Table 2).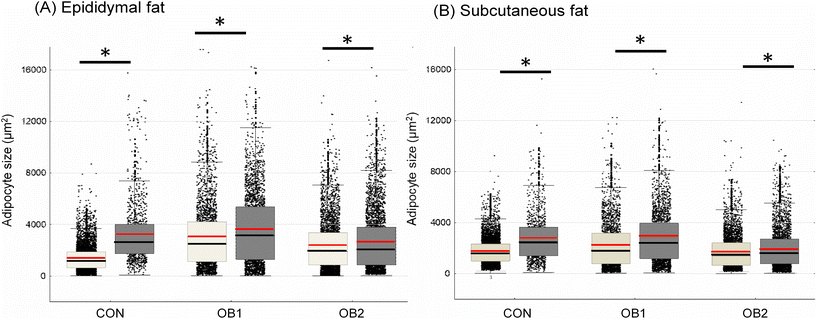 | ||
| Fig. 2 Adipocyte size central tendency in CON, OB1 and OB2 dietary groups with and without GRT supplementation size dispersion for adipocytes from epididymal fat (panel A) and subcutaneous fat (panel B) is shown, overlaid with mean (red line), median (black line), interquartile range (box) and whiskers (non-outlier range). White bars denote rats that did not receive GRT, while grey bars denote rats that received GRT. n = 800–1500 adipocytes per animal from 6 rats in each group. *p < 0.05 between GRT-supplemented and corresponding non-GRT-supplemented groups. Additional statistics and dietary group differences can be found in Table 2. | ||
| Dietary group and adipose depot | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epididymal fat | Median (μm2) and interquartile range (25th–75th percentile) | Sc fat | Median (μm2) and interquartile range (25th–75th percentile) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Descriptive statistics and group analysis of adipocyte cell size data for epididymal fat and subcutaneous fat. Cell size data (area in μm2) was obtained from histology images using ImageJ software (4 images per fat pad, n = 6 per dietary group for each depot). All visible adipocytes were counted. Data was analysed within each adipose depot using the Kruskal–Wallis test for non-parametric data, followed by Dunn's post-hoc test. *p < 0.001 vs. CON; ‡p < 0.001 vs. OB1; †p < 0.001 vs. corresponding non-GRT group; ¥p < 0.05 vs. corresponding non-GRT group. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CON | 1168 (648.1–1870) | CON | 1581 (995.8–2322) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CON-GRT | 2654 (1747–4021)* † | CON-GRT | 2382 (1420–3640)* † | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OB1 | 2465 (1126–4221)* | OB1 | 1736 (786.4–3187)* | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OB1-GRT | 3140 (1298–5389)* † | OB1-GRT | 2349 (1187–3957)* † | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OB2 | 1895 (864.1–3354)*‡ | OB2 | 1410 (662.7–2411)*‡ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OB2-GRT | 2048 (848.8–3790)* ¥ | OB2-GRT | 1579 (774–2705)* † | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
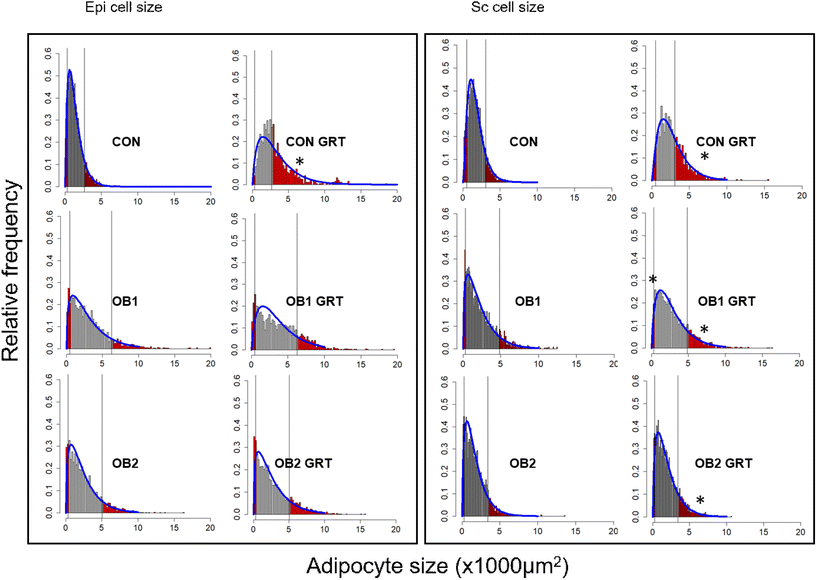
Fig. 3 shows the histogram and gamma distribution function for each dietary group. For the analysis of the small and large adipocyte size proportions, the 10th percentile was defined as the maximum size (μm2) of the smallest 10% of cells in the depot (sc-10 and epi-10, respectively), and the 90th percentile was defined as the minimum size (μm2) of the largest 10% of cells in the depot (sc-90 and epi-90, respectively) using a gamma probability distribution function.42 These cut-offs were defined separately for each of the three dietary groups (Fig. 3), and we subsequently compared GRT-supplemented to corresponding non-GRT-supplemented dietary groups.
The gamma-distribution of small and large adipocyte size proportions demonstrated that, within the adipocyte size brackets defined as sc-10 and epi-10 for each dietary group, adipose tissue samples from GRT-supplemented groups contained a significantly lower proportion of small adipocytes than tissue samples from the corresponding non-GRT-supplemented groups. Likewise, within the size brackets defined as sc-90 and epi-90 for each dietary group, there was a significantly higher proportion of large adipocytes in the GRT-supplemented groups than in the corresponding non-GRT-supplemented groups (Table 3). These findings were consistent with the results on the differences in mean and median adipocyte sizes between dietary groups (Fig. 2). Furthermore, as with the differences in mean and median adipocyte size (Fig. 2), the biggest GRT-related difference in adipocyte size proportions was observed in the CON dietary group (Table 3). Large adipocytes in subcutaneous fat (sc-90) were bigger in dietary group OB1 than in the CON and OB2 groups, while large adipocytes in epi fat (epi-90) were bigger in the OB1 and OB2 dietary groups than in the CON dietary group (Table 3).
| Epididymal fat | Subcutaneous fat | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Small adipocytes (≤322 μm2) | Large adipocytes (≥2691 μm2) | Small adipocytes (≤508 μm2) | Large adipocytes (≥3081 μm2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Percentage of small and large adipocytes were calculated form cut-off points defined, respectively, by the 10th and 90th percentile of the gamma distribution in the CON, OB1 and OB2 groups and analyzed by Student's t-test with the Bonferroni correction. * = p < 0.05 GRT-treated group vs. corresponding GRT-untreated group. n = 6 animals per group. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CON | 10% | 10% | 10% | 10% | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CON-GRT | 2% | 50.07%* | 4% | 34.71%* | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Small adipocytes (≤469 μm2) | Large adipocytes (≥6271 μm2) | Small adipocytes (≤335 μm2) | Large adipocytes (≥4812 μm2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OB1 | 10% | 10% | 10% | 10% | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OB1-GRT | 3.89% | 14.99% | 3.41%* | 15.37%* | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Small adipocytes (≤324 μm2) | Large adipocytes (≥5030 μm2) | Small adipocytes (≤278 μm2) | Large adipocytes (≥3403 μm2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OB2 | 10% | 10% | 10% | 10% | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OB2-GRT | 6.75% | 13.28% | 5.57% | 14.84%* | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3.3 Ex vivo lipid accumulation in cultured ASCs
Lipid accumulation in ex vivo-differentiated ASCs derived from rats from the six dietary groups was quantified as a measure of the adipogenic response of these cells. Group analysis indicated that the levels of intracellular lipid accumulation in differentiated scASCs (scASC-lipid, Fig. 4A) and pvASCs (pvASC-lipid, Fig. 4B) were similar between all six groups, apart from scASC-lipid in the OB2-GRT group which was significantly lower than in the CON group (Fig. 4A). However, within dietary groups, the mean lipid accumulation in ASCs derived from GRT-supplemented rats was always numerically lower than in ASCs derived from the corresponding non-GRT-supplemented rats, in both scASCs (Fig. 4A) and pvASCs (Fig. 4B), and factorial analysis demonstrated that the main suppressive effect of in vivo GRT supplementation on ex vivo lipid accumulation was significant in both scASCs (p = 0.025) and pvASCs (p = 0.004).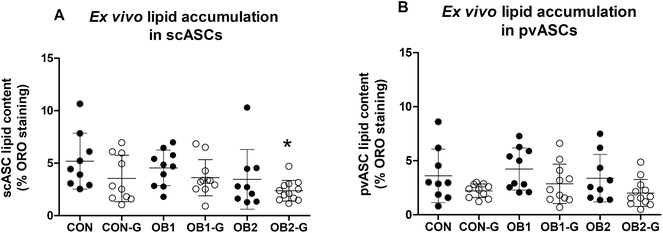 | ||
| Fig. 4 Quantification of intracellular lipid accumulation in ex vivo ASC cultures lipid accumulation in ex vivo adipocyte-differentiated subcutaneous (panel A) and perirenal visceral (panel B) ASCs (scASCs and pvASCs, respectively) is shown. Intracellular lipid droplets were stained with Oil Red O and staining was quantified with image analysis. Only adipogenic media (AM)-treated cultures were included in this analysis, as control media (CM) cultures typically contained no intracellular lipid droplets that became visible upon staining (also refer to ESI Fig. S2†). For both panels, each data point represents data for one animal (n = 9–12 per dietary group). Black circles denote animals that did not receive GRT, while open circles denote animals that received GRT. Data was analysed using one-way ANOVA and Tukey's multiple comparisons post-hoc test. *p < 0.05 relative to CON. | ||
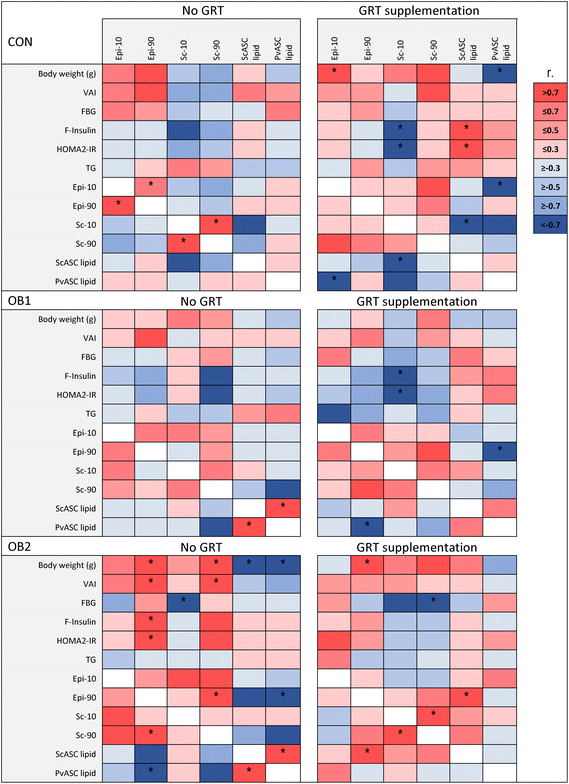
3.4 Associations between metabolic measures and adipose tissue measures
In order to explore the associations between diet- and GRT-associated differences in adipose tissue histology, ASC function and metabolic status, a Pearson's correlation analysis was conducted between these measures, as shown in Fig. 5 and ESI Data File 2.† Overall, adipose tissue measures (collectively sc-10, sc-90, epi-10, epi-90, scASC-lipid and pvASC-lipid) exhibited very few associations with concentrations of circulating factors such as leptin, adiponectin, IL-18, total cholesterol and LDL-cholesterol (ESI Data File 2†). Of note, serum triacylglycerol concentrations were not associated with any adipose tissue measure in any dietary group (Fig. 5). However, the correlation analysis clearly demonstrated how obesogenic diets and GRT supplementation affected numerous aspects of adipose tissue biology in concert. ScASC-lipid had a significant positive association with pvASC-lipid in both the OB1 and OB2 dietary groups, but not in the OB1-GRT and OB2-GRT dietary groups (Fig. 5). In addition, the larger adipocyte size in GRT-supplemented groups (Fig. 2) was associated with lower levels of ASC lipid accumulation, with significant negative associations observed between sc-10 and scASC-lipid, and epi-10 and pvASC-lipid, in the CON-GRT dietary group, as well as a significant negative association between epi-90 and pvASC-lipid in the OB1-GRT dietary group (Fig. 5). Final body weight, pv weight, VAI, fasting insulin and HOMA2-IR showed a significant positive association with sc-90 and epi-90 in the OB2 dietary group, but all of these associations, with the exception of the positive association between epi-90 and body weight, were abrogated in the OB2-GRT dietary group (Fig. 5). Sc-10 showed a significant negative association with fasting insulin concentrations and HOMA2-IR in the CON-GRT and OB1-GRT dietary groups, and sc-90 showed a significant negative association with fasting blood glucose in the OB2-GRT dietary group, but these associations were not observed in the corresponding non-GRT-supplemented groups (Fig. 5).4 Discussion
Given our expectation of weight-loss therapeutics to reduce adipocyte formation and adipose tissue volume, it is perhaps surprising that no pharmaceutical anti-obesity treatment or natural product has to date been evaluated for its effects on ASCs in vivo or in primary ex vivo cell culture.7,11,45 To our knowledge, the present study is the first of its kind, examining the effects of a putative weight-loss product on body composition, systemic markers of metabolism, adipose tissue histology and ASC biology. In this study, we examined whether supplementation with a green Rooibos extract (GRT) could ameliorate the effects of different obesogenic diets on these parameters in male rats, or alternatively, whether GRT could modulate these parameters in a diet-independent manner. While GRT supplementation had no overt effects on body composition measures or serum metabolic measures within the different dietary groups, GRT supplementation was associated with differences in the associations and correlations between these measures. In addition, GRT supplementation resulted in significantly larger adipocyte size compared to the corresponding non-GRT-supplemented dietary groups, in both epi and sc adipose depots, reduced intracellular lipid accumulation in cultured ASCs, and modulated the associations between adipose tissue measures and metabolic measures within dietary groups. Moreover, while lipid accumulation in cultured ASCs derived from these animals did not consistently associate with any systemic measures across dietary groups, the associations between ASC lipid accumulation and other measures were strongly influenced by both diet and GRT.A key feature of our model of obesogenic feeding was the isocaloric food intake between dietary groups32 before the initiation of GRT supplementation. Due to the cohabitation of GRT-supplemented and non-supplemented rats, the impact of GRT on food and energy (kJ) consumption could not be determined. This may be considered a limitation of our study, but it was previously noted in rats and vervet monkeys on control or obesogenic diets (high-fat and/or high-sugar) that supplementation with the same GRT extract, at a dose that had significant metabolic effects, did not affect energy intake.24,25,27 Correspondingly, there were also no significant group differences in body weight observed in our study. As a result, the diet- and GRT-associated differences in metabolic endpoints observed in our study could be evaluated while minimising the confounding impact of large variations in energy intake and body weight.
One of the major findings of our study was that a positive association between visceral adiposity and insulin resistance was only present in the CON and CON-GRT groups, but not in the other dietary groups. The positive relationship between visceral fat mass and insulin resistance is well documented43,44 and our findings demonstrate that this relationship exists even under non-obesogenic dietary conditions. However, we had previously reported that visceral adiposity was increased in both the OB1 and OB2 dietary groups, but that insulin resistance was increased only in the OB1 dietary group.32 Correspondingly, we demonstrate in the present study that visceral adiposity did not show an association with insulin resistance in the OB1 and OB2 dietary groups (with or without GRT supplementation), suggesting that the relationship between visceral adiposity and insulin resistance may be influenced by diet composition, or by other diet-dependent aspects of the overall metabolic status.
From the within-group analyses, it appeared as if GRT supplementation did not have any impact on body composition, glucose homeostasis, systemic lipid metabolism or liver function. However, the shifts in associations between various metabolic parameters that were observed in GRT-supplemented groups, compared to their corresponding non-GRT-supplemented dietary groups, provide evidence that GRT did have subtle metabolic effects within the context of obesogenic feeding, but most notably with regards to modulating liver function. Against the background of both obesogenic diets (diet OB1 and OB2), GRT supplementation uncoupled the association between increased serum cholesterol concentrations and liver damage. Excess dietary lipids and cholesterol accumulate in the liver and can lead to oxidative stress and endoplasmic reticulum stress, resulting in hepatocyte damage and death. It has also been proposed that cholesterol-driven oxidative stress may contribute to the conversion of relatively benign hepatic steatosis (fatty liver) to pathological steatohepatitis with fibrosis and inflammation.46 It was previously shown that GRT supplementation against the background of high-fat/high-sugar feeding improved hepatic anti-oxidant status24 and reduced hepatic steatosis in rats.23 Taken together with our results, these findings indicate that GRT may protect the liver against the consequences of obesogenic feeding, excess dietary cholesterol and elevated serum cholesterol levels, even if GRT supplementation does not lower cholesterol itself (present study and ref. 26). These findings also raise the possibility that GRT-mediated differences in measures of liver damage may have become more pronounced over a longer period of in vivo GRT supplementation. A more detailed characterisation of the effects of GRT supplementation on liver function measures such as hepatic steatosis and gene expression, against the background of diets OB1 and OB2, is currently under way (Johnson et al., manuscript in preparation).
In contrast to the lack of overt GRT effects on systemic metabolic measures, obesogenic feeding and GRT had clear effects on adipose tissue histology. In both epididymal and subcutaneous fat, adipocytes were smaller in the CON group than in all other dietary groups, with the exception of subcutaneous fat in the OB2 dietary group. This effect was more pronounced in epididymal fat than in subcutaneous fat, consistent with the findings of numerous studies in mice and humans that the relative increase in adipocyte size during obesity is greater in visceral/abdominal fat depots than in subcutaneous depots.47 Measures of in vivo adipocyte size and ex vivo lipid accumulation in cultured ASCs also exhibited numerous associations across all six dietary groups, demonstrating that both diet and GRT supplementation affected adipose tissue biology on a cellular level, impacting mature adipocytes as well as ASCs. The positive association between lipid accumulation in scASCs and pvASCs in both the OB1 and OB2 dietary groups indicate that both diets influenced the adipogenic capacity of scASCs in parallel with that of pvASCs. However, these associations were not observed in the OB1-GRT and OB2-GRT groups, suggesting that GRT counteracted obesogenic diet-induced effects on ASC biology. In the OB2 dietary group, higher body weight was associated with a higher degree of visceral adiposity and lower adipogenic capacity in both scASCs and pvASCs, consistent with the negative impact of obesity on the adipogenic capacity of ASCs (reviewed in ref. 7). In addition, in the OB2 dietary group, increased adipocyte size in both the sc and epi fat was associated with visceral adiposity and insulin resistance, consistent with the relationship between adipocyte hypertrophy and insulin resistance which is likely driven by adipose tissue inflammation.48–50 However, it is noteworthy that these associations were specific to the OB2 dietary group, but not the OB1 dietary group, providing further evidence that different obesogenic diets have different metabolic consequences, in accordance with our previous work.32 In the OB2-GRT group, cell size in epididymal fat was only associated with body weight, indicating that GRT supplementation uncoupled the relationship between epididymal adipocyte hypertrophy and insulin resistance in this dietary group.
In the CON-GRT group and OB1-GRT group, the inverse associations between ASC-lipid and adipocyte size indicate a shift away from adipogenesis and towards adipocyte enlargement (hypertrophy). Collectively, our findings suggest that GRT supplementation may predispose towards suppressed adipogenesis and concomitant adipocyte hypertrophy under certain dietary conditions. The link between adipocyte hypertrophy and loss of adipogenic capacity is well described and is thought to arise from an inability to recruit new adipocytes through adipogenesis, resulting in hypertrophic expansion of existing adipocytes.7,51,52 Notably, these associations in the CON-GRT and OB1-GRT groups were observed within the sc depot (scASC-lipid vs. sc-10) and also across two anatomically separate intra-abdominal fat depots (pvASC-lipid vs. epi-10 and epi-90), indicative of global GRT-induced adaptations in adipose tissue biology. Mildly enlarged adipocytes may indicate improved storage capacity in adipose tissue, but exaggerated adipocyte hypertrophy may result in adipose tissue inflammation and metabolic dysfunction such as insulin resistance.48–50 In the present study, increased adipocyte size in the sc fat was associated with improved insulin sensitivity in both CON-GRT and OB1-GRT groups, and with lower fasting glucose in OB2-GRT, but not in the corresponding non-GRT groups. These observations provide evidence of GRT-induced improvements in insulin signalling and homeostasis, as has been shown for other Rooibos-derived extracts and compounds in vivo17 and in vitro in cultured myotubes, hepatocytes and adipocytes,13,14,16,17 and suggest that the larger adipocytes observed in GRT-supplemented groups is metabolically favourable, at least in the short term. However, the long-term effects of possible GRT-mediated suppression of ASC adipogenic capacity warrants further attention. The suppression of adipogenesis/lipogenesis by GRT in ASCs is consistent with findings for Rooibos constituents in cultured 3T3-L1 adipocytes,18–21 and our study provides the first evidence that Rooibos could affect ASC biology in vivo. However, intracellular lipid accumulation was the only marker of ASC function included in this study, and therefore the mechanisms and pathways impacted by obesogenic diets and GRT in ASCs remain to be identified. Of note, the Rooibos constituent iso-orientin, present at 1.5% (m/m) in GRT, has been shown to reduce lipid accumulation, promote browning and improve mitochondrial energetics in cultured 3T3-L1 adipocytes.21,53 A more comprehensive characterisation of markers of adipocytic differentiation, browning, glucose handling, lipid metabolism, inflammation and the ASC secretome7 was outside the scope of the present study but is underway and will be essential to further delineate the consequences of GRT supplementation on ASCs in vivo.
The GRT used in the present study has a high (12%) aspalathin content, relative to its other constituents.22 According to our current knowledge, Rooibos is the only natural source of aspalathin, and consequently Rooibos-related research often focuses on aspalathin. However, recent work has indicated that the in vitro bio-capacity of green Rooibos extracts cannot be predicted by the content of any single constituent, including aspalathin,54 supporting the idea that complex plant-based extracts have greater value as metabolic modulators and “functional foods” than single-component products. Other green Rooibos extracts with different relative proportions of constituents may therefore be more efficacious at reversing DIO and DIMD than the GRT used in the present study. The composition of Rooibos extracts is influenced by many factors, including oxidation status of the plant material, the solvent used (methanol vs. water)55 and the temperature of the water used to prepare aqueous extracts,56 and it is therefore essential to use extracts with standardised preparation methods and known composition in studies of this kind. It is also important to consider that GRT was administered against the background of ongoing obesogenic feeding, and it may be valuable to understand whether green Rooibos extracts could contribute to enhanced recovery from obesity or reversal of “obesogenic memory” in adipose tissue57 upon cessation of obesogenic feeding. In addition, the majority of metabolic studies on Rooibos extracts and constituents have been performed either in cell culture13–21,53 or in genetic models of obesity, diabetes and hyperlipidemia.12–14,26,58 Very few studies have investigated the regulation of body weight and glucose metabolism by Rooibos extracts and single constituents in humans11 or in wild-type animal models,15,17,23–25,27 and these animal studies have all utilised different dietary conditions. Consequently, very little information is available on the interactions of Rooibos with obesogenic dietary conditions in otherwise healthy subjects in vivo. The present study therefore represents a unique attempt at mapping and comparing these diet interactions, with a particular focus on adipose tissue function. We have previously shown that obesogenic diets with different proportions of fat, carbohydrates and sugars have different metabolic consequences,32 and it is therefore reasonable to argue that anti-obesity products or drugs may have varying effects within the context of different obesogenic diets, as has already been demonstrated elsewhere for GRT in rats.23–25 This corresponds with our observations that the metabolic impact of GRT supplementation differed between the OB1 and OB2 dietary groups and may have broader implications for the efficacy of different drugs and natural products to support weight-loss and metabolic improvements in humans, against the background of a variety of dietary habits.
However, the fact that our study was only conducted in male rats presents a considerable limitation for the translatability of our data to humans. Indeed, nearly all research into the metabolic effects of Rooibos has been performed either in vitro13–21,53 or in male animals.12–15,17,23–26,58 Orlando et al.27 included female and male monkeys in their study, but the results of that study were not stratified to compare females to males. Obesity is far more prevalent in women than in men,1 and even in non-obese individuals, body fat distribution differs between men and women.59,60 ASCs from men and women are also intrinsically different.7 It is therefore clear that different metabolic mechanisms may be driving obesity in men and women. However, rat DIO/DIMD studies, including studies on the metabolic and weight-loss properties of natural products, are still mostly performed in male animals11,32 and consequently very little is known about the sex-specific effects of such products. Well-designed in vivo/ex vivo studies comparing the effects of Rooibos extracts and other natural products on systemic metabolism as well as adipose tissue and ASC function between males and females are crucial to advance our understanding of the therapeutic potential of such products to address female obesity.
To our knowledge, our in vivo/ex vivo study is the first to simultaneously assess the effects of two obesogenic diets and any potential weight-loss product on body composition, systemic metabolism, adipose tissue cellularity and ASC function, and to explore the associations between these measures. Furthermore, we applied a novel combination of statistical analysis approaches, using both univariate and gamma distribution modelling, to achieve advanced data analysis. In contrast to the univariate analyses, which yielded limited insights into diet- and GRT-associated metabolic effects, our exploration of the associations between individual measures provided a wealth of information on the effects of obesogenic diets and GRT on systemic metabolism and adipose tissue biology. In addition, our unique statistical analysis approach provide evidence of how the complex mechanistic interactions between adipose tissue and glucose homeostasis can be influenced or even uncoupled by dietary conditions and natural product intervention. As a caveat, it is important to consider that the directionality of these statistical associations cannot always be determined, and therefore causal relationships and mechanisms cannot be conclusively formulated from this data set. However, with our novel combination of biochemical and statistical tools, we have identified potential research avenues for future studies investigating the impact of Rooibos extracts and other natural medicines on adipose tissue cellularity and function.
In conclusion, our study showed that GRT supplementation induced adipocyte enlargement against the background of control and obesogenic diets, and suppressed the adipogenic differentiation of ASCs isolated from the experimental animals and cultured ex vivo. In addition, we have demonstrated that GRT supplementation may have metabolic effects specifically pertaining to preserving insulin sensitivity and liver function during DIO/DIMD. Broadly speaking, GRT affected almost all the diet-specific associations between systemic measures and adipose tissue-level measures. Our findings suggest that Rooibos extracts may impact adipose tissue function via multiple direct and indirect metabolic mechanisms.
Author contributions
L. M. Kotzé-Hörstmann: Conceptualization, software, formal analysis, investigation, data curation, writing – original draft, review and editing, visualization. D. T. Bedada: Formal analysis, data curation, manuscript – review and editing. R. Johnson: Conceptualization, investigation, resources, writing – review and editing, funding acquisition. L. Mabasa: Investigation, resources, writing – review and editing. H. Sadie-Van Gijsen: Conceptualization, methodology, formal analysis, investigation, resources, data curation, writing – original draft, review and editing, visualization, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank the following persons from the Department of Biomedical Sciences, Faculty of Medicine and Health Sciences, Stellenbosch University, for contributions to sample collection and analysis: Dr Marguerite Blignaut, Dr Bongekile Skosana, Professor Novel Chegou, Candice Snyders, Mandisa Ndebele and Reggie Williams. In addition, we wish to acknowledge Professor Birhanu Ayele (posthumous) for guidance with statistical analysis in the initial phases of the project. This work was supported by the South African Rooibos Council (SARC) (L. M. K-H., R. J. and H. S. v. G.), the South African National Research Foundation (NRF) (H. S. v. G., grant number 112254; R. J., grant number 120812) and the South African Sugar Association (SASA) (H. S. v. G., grant number 261). The authors declare that none of the funders had any influence in the study design; the collection, analysis and interpretation of data; in the writing of the report or in the decision to submit the article for publication.References
- T. Lobstein, H. Brindsen and M. Neveux, World Obesity Atlas 2022, March 2022. https://www.worldobesityday.org/assets/downloads/World_Obesity_Atlas_2022_WEB.pdf [accessed 24 May 2022].
- M. Abdelaal, C. W. le Roux and N. G. Docherty, Morbidity and mortality associated with obesity, Ann. Transl. Med., 2017, 5, 161, DOI:10.21037/atm.2017.03.107.
- Y. J. Tak and S. Y. Lee, Long-term efficacy and safety of anti-obesity treatment: Where do we stand?, Curr. Obes. Rep., 2021, 10, 14–30, DOI:10.1007/s13679-020-00422-w.
- A. K. Singh and R. Singh, Pharmacotherapy in obesity: a systematic review and meta-analysis of randomized controlled trials of anti-obesity drugs, Expert Rev. Clin. Pharmacol., 2020, 13, 53–64, DOI:10.1080/17512433.2020.1698291.
- K. Venkatakrishnan, H. F. Chiu and C. K. Wang, Extensive review of popular functional foods and nutraceuticals against obesity and its related complications with a special focus on randomized clinical trials, Food Funct., 2019, 10, 2313–2329, 10.1039/c9fo00293f.
- S. Wharton, R. Bonder, A. Jeffery and R. A. G. Christensen, The safety and effectiveness of commonly-marketed natural supplements for weight loss in populations with obesity: A critical review of the literature from 2006 to 2016, Crit. Rev. Food Sci. Nutr., 2020, 60, 1614–1630, DOI:10.1080/10408398.2019.1584873.
- H. Sadie-Van Gijsen, Adipocyte biology: It is time to upgrade to a new model, J. Cell. Physiol., 2019, 234, 2399–2425, DOI:10.1002/jcp.27266.
- H. Sadie-Van Gijsen, L. Kotzé-Hörstmann and B. Huisamen, An in vivo/ex vivo study design to investigate effects of chronic conditions and therapeutic compounds on adipose stem cells in animal models, Methods Mol. Biol., 2020, 2138, 101–118, DOI:10.1007/978-1-0716-0471-7_5.
- D. Schroeder, R. Chennells, C. Louw, L. Snyders and T. Hodges, The Rooibos benefit sharing agreement - breaking new ground with respect, honesty, fairness, and care, Cambridge Q. Healthcare Ethics, 2020, 29, 285–301, DOI:10.1017/S0963180119001075.
- L. Bramati, M. Minoggio, C. Gardana, P. Simonetti, P. Mauri and P. Pietta, Quantitative characterization of flavonoid compounds in rooibos tea (Aspalathus linearis) by LC-UV/DAD, J. Agric. Food Chem., 2002, 50, 5513–5519 CrossRef CAS PubMed.
- L.M Kotzé-Hörstmann and H. Sadie-Van Gijsen, Modulation of glucose metabolism by leaf tea constituents: a systematic review of recent clinical and pre-clinical findings, J. Agric. Food Chem., 2020, 68, 2973–3005, DOI:10.1021/acs.jafc.9b07852.
- A. Kawano, H. Nakamura, S. Hata, M. Minakawa, Y. Miura and K. Yagasaki, Hypoglycemic effect of aspalathin, a rooibos tea component from Aspalathus linearis, in type 2 diabetic model db/db mice, Phytomedicine, 2009, 16, 437–443, DOI:10.1016/j.phymed.2008.11.009.
- M. J. Son, M. Minakawa, Y. Miura and K. Yagasaki, Aspalathin improves hyperglycemia and glucose intolerance in obese diabetic ob/ob mice, Eur. J. Nutr., 2013, 52, 1607–1619, DOI:10.1007/s00394-012-0466-6.
- R. Kamakura, M. J. Son, D. de Beer, E. Joubert, Y. Miura and K. Yagasaki, Antidiabetic effect of green rooibos (Aspalathus linearis) extract in cultured cells and type 2 diabetic model KK-A(y) mice, Cytotechnology, 2015, 67, 699–710, DOI:10.1007/s10616-014-9816-y.
- C. J. Muller, E. Joubert, D. de Beer, M. Sanderson, C. J. Malherbe, S. J. Fey and J. Louw, Acute assessment of an aspalathin-enriched green rooibos (Aspalathus linearis) extract with hypoglycemic potential, Phytomedicine, 2012, 20, 32–39, DOI:10.1016/j.phymed.2012.09.010.
- S. E. Mazibuko, E. Joubert, R. Johnson, J. Louw, A. R. Opoku and C. J. Muller, Aspalathin improves glucose and lipid metabolism in 3T3-L1 adipocytes exposed to palmitate, Mol. Nutr. Food Res., 2015, 59, 2199–2208, DOI:10.1002/mnfr.201500258.
- S. E. Mazibuko-Mbeje, P. V. Dludla, C. Roux, R. Johnson, S. Ghoor and E. Joubert, et al., Aspalathin-enriched green Rooibos extract reduces hepatic insulin resistance by modulating PI3K/AKT and AMPK pathways, Int. J. Mol. Sci., 2019, 20, 633, DOI:10.3390/ijms20030633.
- M. Sanderson, S. E. Mazibuko, E. Joubert, D. de Beer, R. Johnson and C. Pheiffer, et al., Effects of fermented rooibos (Aspalathus linearis) on adipocyte differentiation, Phytomedicine, 2014, 21, 109–117, DOI:10.1016/j.phymed.2013.08.011.
- I. Choi, Y. Park, H. Choi and E. H. Lee, Anti-adipogenic activity of rutin in 3T3-L1 cells and mice fed with high-fat diet, BioFactors, 2006, 26, 273–281, DOI:10.1002/biof.5520260405.
- J. Kim, I. Lee, J. Seo, M. Jung, Y. Kim, N. Yim and K. Bae, Vitexin, orientin and other flavonoids from Spirodela polyrhiza inhibit adipogenesis in 3T3-L1 cells, Phytother. Res., 2010, 24, 1543–1548, DOI:10.1002/ptr.3186.
- K. Ziqubu, C. J. F. Muller, P. V. Dludla, S. X. H. Mthembu, N. Obonye and J. Louw, et al., Impact of isoorientin on metabolic activity and lipid accumulation in differentiated adipocytes, Molecules, 2020, 25, 1773, DOI:10.3390/molecules25081773.
- O. Patel, C. Muller, E. Joubert, J. Louw, B. Rosenkranz and C. Awortwe, Inhibitory interactions of Aspalathus linearis (Rooibos) extracts and compounds, aspalathin and Z-2-(β-d-glucopyranosyloxy)-3-phenylpropenoic acid, on cytochromes metabolizing hypoglycemic and hypolipidemic drugs, Molecules, 2016, 21, 1515, DOI:10.3390/molecules21111515.
- J. I. Layman, D. L. Pereira, N. Chellan, B. Huisamen and S. H. Kotzé, A histomorphometric study on the hepatoprotective effects of a green rooibos extract in a diet-induced obese rat model, Acta Histochem., 2019, 121, 646–656, DOI:10.1016/j.acthis.2019.05.008.
- Z. Obasa, M. A. van Vuuren, B. Huisamen and S. L. Windvogel, The modulating effects of green rooibos (Aspalathus Linearis) extract on vascular function and antioxidant status in obese Wistar rats, Cardiovasc. J. Afr., 2021, 32, 87–97, DOI:10.5830/CVJA-2020-048.
- S. E. Smit, C. Manirafasha, E. Marais, R. Johnson and B. Huisamen, Cardioprotective function of green Rooibos (Aspalathus linearis) extract supplementation in ex vivo ischemic prediabetic rat hearts, Planta Med., 2022, 88, 62–78, DOI:10.1055/a-1239-9236.
- O. Patel, C. J. F. Muller, E. Joubert, B. Rosenkranz, J. Louw and C. Awortwe, Therapeutic effects of an aspalathin-rich green rooibos extract, pioglitazone and atorvastatin combination therapy in diabetic db/db mice, PLoS One, 2021, 16, e0251069, DOI:10.1371/journal.pone.0251069.
- P. Orlando, N. Chellan, J. Louw, L. Tiano, I. Cirilli and P. Dludla, et al., Aspalathin-rich green Rooibos extract lowers LDL-cholesterol and oxidative status in high-fat diet-induced diabetic vervet monkeys, Molecules, 2019, 24, 1713, DOI:10.3390/molecules24091713.
- J.-P. Després, Is visceral obesity the cause of the metabolic syndrome?, Ann. Med., 2006, 38, 52–63, DOI:10.1080/07853890500383895.
- C. S. Fox, J. M. Massaro, U. Hoffmann, K. M. Pou, P. Maurovich-Horvat and C. Y. Liu, et al., Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study, Circulation, 2007, 116, 39–48, DOI:10.1161/CIRCULATIONAHA.106.675355.
- L. Luo and M. Liu, Adipose tissue in control of metabolism, J. Endocrinol., 2016, 231, R77–R99, DOI:10.1530/JOE-16-0211.
- H. Sadie-Van Gijsen, Is Adipose Tissue the Fountain of Youth? The Impact of Adipose Stem Cell Aging on Metabolic Homeostasis, Longevity, and Cell-Based Therapies, Adv. Exp. Med. Biol., 2021, 1286, 225–250, DOI:10.1007/978-3-030-55035-6_16.
- L. M. Kotze-Hörstmann, A. Cois, R. Johnson, L. Mabasa, S. Shabalala, P. J. van Jaarsveld and H. Sadie-Van Gijsen, Characterization and comparison of the divergent metabolic consequences of high-sugar and high-fat diets in male Wistar rats, Front. Physiol, 2022, 13, 904366, DOI:10.3389/fphys.2022.904366.
- E. Joubert, W. C. Gelderblom, A. Louw and D. de Beer, South African herbal teas: Aspalathus linearis, Cyclopia spp. and Athrixia phylicoides–a review, J. Ethnopharmacol., 2008, 119, 376–412, DOI:10.1016/j.jep.2008.06.014.
- R. Wynberg, Making sense of access and benefit sharing in the rooibos industry: Towards a holistic, just and sustainable framing, S. Afr. J. Bot., 2017, 110, 39–51, DOI:10.1016/j.sajb.2016.09.015.
- N. J. Benevenga, et al., (Subcommittee on Laboratory Animal Nutrition, Committee on Animal Nutrition, Board on Agriculture, National Research Council), Nutritional requirements of laboratory animals, National Academies Press, 4th revised edn, 1995 Search PubMed.
- H. Sadie-Van Gijsen, N. J. Crowther, F. S. Hough and W. F. Ferris, Depot-specific differences in the insulin response of adipose-derived stromal cells, Mol. Cell. Endocrinol., 2010, 328, 22–27, DOI:10.1016/j.mce.2010.06.009.
- H. Sadie-Van Gijsen, W. Smith, E. F. du Toit, J. Michie, F. S. Hough and W. F. Ferris, Depot-specific and hypercaloric diet-induced effects on the osteoblast and adipocyte differentiation potential of adipose-derived stromal cells, Mol. Cell. Endocrinol., 2012, 348, 55–66, DOI:10.1016/j.mce.2011.07.030.
- R. Berry, C. D. Church, M. T. Gericke, E. Jeffery, L. Colman and M. S. Rodeheffer, Imaging of adipose tissue, Methods Enzymol., 2014, 537, 47–73, DOI:10.1016/B978-0-12-411619-1.00004-5.
- T. M. Wallace, J. C. Levy and D. R. Matthews, Use and abuse of HOMA modelling, Diabetes Care, 2004, 27, 1487–1495, DOI:10.2337/diacare.27.6.1487.
- F. Faul, E. Erdfelder, A.-G. Lang and A. Buchner, G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences, Behav. Res. Methods, 2007, 39, 175–191, DOI:10.3758/bf03193146.
- https://www.gpower.hhu.de/ .
- C. A. Ibáñez, M. Vázquez-Martínez, J. C. León-Contreras, L. A. Reyes-Castro, G. L. Rodríguez-González and C. J. Bautista, et al., Different statistical approaches to characterization of adipocyte size in offspring of obese rats: effects of maternal or offspring exercise intervention, Front. Physiol., 2018, 9, 1571, DOI:10.3389/fphys.2018.01571.
- N. Barzilai, L. She, B. Q. Liu, P. Vuguin, P. Cohen and J. Wang, et al., Surgical removal of visceral fat reverses hepatic insulin resistance, Diabetes, 1999, 48, 94–98 CrossRef CAS PubMed.
- S. Gavi, J. J. Feiner, M. M. Melendez, D. C. Mynarcik, M. C. Gelato and M. A. McNurlan, Limb fat to trunk fat ratio in elderly persons is a strong determinant of insulin resistance and adiponectin levels, J. Gerontol., Ser. A, 2007, 62, 997–1001, DOI:10.1093/gerona/62.9.997.
- M. Khalilpourfarshbafi, K. Gholami, D. D. Murugan, M. Z. Abdul Sattar and N. A. Abdullah, Differential effects of dietary flavonoids on adipogenesis, Eur. J. Nutr., 2019, 58, 5–25, DOI:10.1007/s00394-018-1663-8.
- G. P. Püschel and J. Henkel, Dietary cholesterol does not break your heart but kills your liver, Porto Biomed. J., 2019, 3, e12, DOI:10.1016/j.pbj.0000000000000012.
- E. Börgeson, J. Boucher and C. E. Hagberg, Of mice and men: Pinpointing species differences in adipose tissue biology, Front. Cell Dev. Biol., 2022, 10, 1003118, DOI:10.3389/fcell.2022.1003118.
- H. Ruan and H. F. Lodish, Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha, Cytokine Growth Factor Rev., 2003, 14, 447–455, DOI:10.1016/s1359-6101(03)00052-2.
- P. Arner, E. Arner, A. Hammarstedt and U. Smith, Genetic predisposition for Type 2 diabetes, but not for overweight/obesity, is associated with a restricted adipogenesis, PLoS One, 2011, 6, e18284, DOI:10.1371/journal.pone.0018284.
- B. Gustafson, A. Hammarstedt, S. Hedjazifar and U. Smith, Restricted adipogenesis in hypertrophic obesity: the role of WISP2, WNT, and BMP4, Diabetes, 2013, 62, 2997–3004, DOI:10.2337/db13-0473.
- B. Gustafson, S. Gogg, S. Hedjazifar, L. Jenndahl, A. Hammarstedt and U. Smith, Inflammation and impaired adipogenesis in hypertrophic obesity in man, Am. J. Physiol. Endocrinol. Metab., 2009, 297, E999–E1003, DOI:10.1152/ajpendo.00377.2009.
- B. Gustafson, A. Nerstedt and U. Smith, Reduced subcutaneous adipogenesis in human hypertrophic obesity is linked to senescent precursor cells, Nat. Commun., 2019, 10, 2757, DOI:10.1038/s41467-019-10688-x.
- S. E. Mazibuko-Mbeje, K. Ziqubu, P. V. Dludla, L. Tiano, S. Silvestri and P. Orlando, et al., Isoorientin ameliorates lipid accumulation by regulating fat browning in palmitate-exposed 3T3-L1 adipocytes, Metab. Open, 2020, 6, 100037, DOI:10.1016/j.metop.2020.100037.
- A. Viraragavan, N. Hlengwa, D. de Beer, S. Riedel, N. Miller and S. Bowles, et al., Model development for predicting in vitro bio-capacity of green rooibos extract based on composition for application as screening tool in quality control, Food Funct., 2020, 11, 3084–3094, 10.1039/c9fo02480h.
- E. A. Hussein, C. Thron, M. Ghaziasgar, M. Vaccari, J. L. Marnewick and A. A. Hussein, Comparison of phenolic content and antioxidant activity for fermented and unfermented Rooibos samples extracted with water and methanol, Plants, 2021, 11, 16, DOI:10.3390/plants11010016.
- E. Damiani, P. Carloni, G. Rocchetti, B. Senizza, L. Tiano and E. Joubert, et al., Impact of cold versus hot brewing on the phenolic profile and antioxidant capacity of Rooibos (Aspalathus linearis) herbal tea, Antioxidants, 2019, 8, 499, DOI:10.3390/antiox8100499.
- J. Schmitz, N. Evers, M. Awazawa, H. T. Nicholls, H. S. Brönneke and A. Dietrich, et al., Obesogenic memory can confer long-term increases in adipose tissue but not liver inflammation and insulin resistance after weight loss, Mol. Metab., 2016, 5, 328–339, DOI:10.1016/j.molmet.2015.12.001.
- R. Beltrán-Debón, A. Rull, F. Rodríguez-Sanabria, I. Iswaldi, M. Herranz-López and G. Aragonès, et al., Continuous administration of polyphenols from aqueous rooibos (Aspalathus linearis) extract ameliorates dietary-induced metabolic disturbances in hyperlipidemic mice, Phytomedicine, 2011, 18, 414–424, DOI:10.1016/j.phymed.2010.11.008.
- S. Lemieux, D. Prud'homme, C. Bouchard, A. Tremblay and J. P. Després, Sex differences in the relation of visceral adipose tissue accumulation to total body fatness, Am. J. Clin. Nutr., 1993, 58, 463–467 CrossRef CAS PubMed.
- Y. D. Tchoukalova, C. Koutsari, S. B. Votruba, T. Tchkonia, N. Giorgadze and T. Thomou, et al., Sex- and depot-dependent differences in adipogenesis in normal-weight humans, Obesity, 2010, 18, 1875–1880, DOI:10.1038/oby.2010.56.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo02440c |
| This journal is © The Royal Society of Chemistry 2022 |