Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A review of biodegradable thermoplastic starches, their blends and composites: recent developments and opportunities for single-use plastic packaging alternatives
Aarsha
Surendren
ab,
Amar K.
Mohanty
 *ab,
Qiang
Liu
c and
Manjusri
Misra
*ab,
Qiang
Liu
c and
Manjusri
Misra
 *ab
*ab
aSchool of Engineering, University of Guelph, Thornbrough Building, 50 Stone Road East, Guelph, Canada. E-mail: mohanty@uoguelph.ca; mmisra@uoguelph.ca
bBioproducts Discovery and Development Centre, Department of Plant Agriculture, University of Guelph, Crop Science Building, 50 Stone Road East, Guelph, Canada
cGuelph Research and Development Centre, Agriculture and Agri-Food Canada, Ontario N1G 5C9, Canada
First published on 3rd November 2022
Abstract
Single-use plastic packaging has become an inevitable part of every aspect of human life. In fact, the increase in the consumption of petroleum-based single-use plastics has resulted in the accumulation of municipal solid wastes which are a leading source of plastic pollution worldwide. Biobased materials are a virtuous replacement for single-use petroleum-based packaging products, when recycling is difficult or not practical, to fulfil environmental and economic demands. Starch is an abundant biobased polymeric material that is sustainable and biodegradable. However, the inefficiency of starch in processability due to the existence of hydrogen bonding interactions and intermolecular forces impedes its applications. An effective solution is plasticization of starch in the presence of heat and shear, and is termed plasticized starch (PS). Although different sources may refer to plasticized starch (PS) as thermoplastic starch (TPS), the plasticization process does not make the starch a thermoplastic material until it is blended with toughened polymer, making the nomenclature counterintuitive. TPS is procured through the process of plasticization of starch with water and plasticizers followed by blending with tougher polymers/biopolymers. This could enhance the flexibility and processability of the blend materials, which are an effective replacement for petroleum-based single-use plastic packaging. In this review the main focus is on analysing the effect of multiple plasticizers and compatibility enhancers such as compatibilizers, coupling agents and essential oils in TPS blends, and starch-based composites’ preparation and their effective use in single-use packaging applications. Global production and market analysis of thermoplastic starches and their challenges in real-life packaging applications are also discussed.
1. Introduction
Plastic is an inevitable and pervasive material for packaging. Global plastic production per year is estimated to be above 480 million metric tons, and the packaging sector embraces 40% of the total production.1 Packaging improves safety, health, and convenience for the manufacturer and consumer during the transportation and storage of a commodity. The diverse uses of packaging materials include consumer goods, food, beverages, cosmetics, and pharmaceuticals.2 Worldwide marketability and food production trends (fresh, half cooked, fast foods) have changed human perception of food. In their fast-paced life, insufficient time of human demanded more fast-food items, which has driven a novel evolution towards innovative and efficient packaging techniques for food packaging.3 Thus the polymer food packaging sector dominates the packaging market as it enhances food quality, safety, and shelf-life, and minimizes food waste.A predominant proportion of packaging materials is developed from fossil hydrocarbons, specifically single-use plastics that account for more than 50% of the packaging sector. Such single-use plastics have a very short life time, ranging from a few hours to weeks, thus creating an alarming environmental waste concern which appears daily in headline news. It is estimated that globally 5 trillion plastic bags are used each year, and recently the COVID-19 pandemic has impacted greatly the rise of production of single-use plastics, especially in take-away packaging from restaurants.4,5 Polyethylene, polypropylene, polyesters, polystyrene, polyvinyl chloride, and polyamides are the major fossil hydrocarbons used in the single-use plastics packaging industries, since they are cheap and accessible with good mechanical, barrier, and thermal properties.6 Most of these are non-degradable and non-recyclable, becoming a threat to the environment as they accumulate on land and in the oceans after use.6,7 This has become a great threat affecting the global ecosystem, as it disturbs the life of marine and terrestrial species and leads to the creation of microplastics (<5 mm in size), which have been found across the world's oceans and as far as Mount Everest's snow, and even in human blood. It is believed that a single plastic bag could take up to 1000 years to disintegrate completely.8 Thus, most countries have taken action against the use of single-use plastics of less than 50 microns, and a few countries have put on a high tax rate on plastic bags.9,10 Additionally, growing concern about human health and environmental aspects has driven the focus more towards eco-friendly, biodegradable, and sustainable packaging materials.11 Thus, the development of biobased or biodegradable single-use packaging materials is needed as a replacement for fossil fuel-based materials.
Globally, the production of biobased polymers is 1% of the total polymer production.12 However, the increasing need for sustainable materials has developed a dynamic rise in bioplastics production in the upcoming years. The study conducted by “European Bioplastics” in 2021 showed a significant rise in the global production capacity of bioplastics from 2021 to 2022, and this is expected to rise to around 7.59 million tonnes (MT) in 2026.13Fig. 1(a) shows the production capacity of bioplastics by region, and Fig. 1(b) represents the expected growth of bioplastics production in the period 2020–2026. Furthermore, the ability of bioplastics to replace conventional plastics has diversified the production of bioplastics such as poly(butylene adipate terephthalate) (PBAT), poly(lactic acid) (PLA), polyhydroxyalkanoates (PHA), bio polypropylene and bio polyethylene etc. Fig. 2 represents the distribution of global bioplastics production capacities from 2020 to 2026. According to the material type, PBAT is expected to have a growth of 30% and starch blends are expected to decrease by 5.2%.13 The decrease in starch blends may be due to the inability to achieve the desired material properties for the packaging sector. This indicates a requirement for further research and development of starch-based blend materials with enhanced properties for packaging and other sectors.
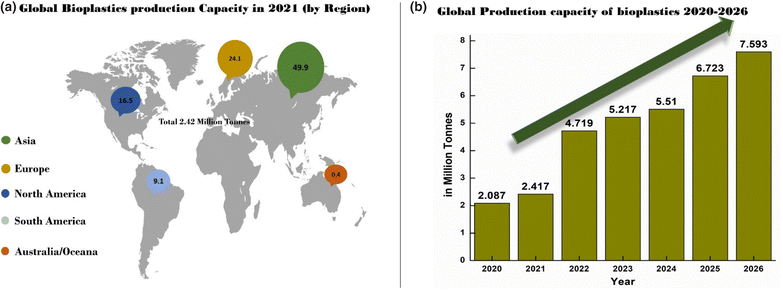 | ||
| Fig. 1 Dynamic rise in production of bioplastics from 2020 to 2026. (a) Global bioplastic production in 2021 by region, (b) global production capacity of bioplastics 2020–2026 (redrawn from data;13 accessed the website “European Bioplastics” in January 2022). | ||
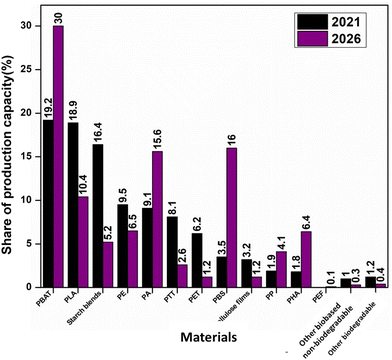 | ||
| Fig. 2 Distribution of global bioplastics production capacities in 2021 and 2026 according to the material type (redrawn from data;13 accessed the website “European Bioplastics” in January 2022). | ||
Biobased polymers are the most promising materials that are partially or completely biodegradable and synthesized from plants or microorganisms through metabolic or biochemical engineering processes.14 In addition, according to their origin, biobased polymers are classified into 3 types; these are (1) extracted from biomass: (I) Polysaccharides (starch,15 cellulose,16 chitosan,17 carrageenan,18 pectin,19 and alginate20), (II) Proteins (gelatin,21 collagen,22 zein,23 and keratin22) and (III) Lipids22; (2) synthesized from biobased monomers (PLA24 and polyesters); and (3) attained from microorganisms (PHAs25 and bacterial cellulose).26 All these materials are abundant, renewable, and ecological. Therefore, biobased packaging materials from these biopolymers can be a sustainable substitute for fossil fuel-based polymers and a solution to the associated waste disposal issues. Notably, polysaccharides are the dominant materials in the biobased packaging market.
Polysaccharides are a widely accepted biobased packaging material for films and coatings, as they provide a good barrier against penetrants like oxygen and carbon dioxide.27 These materials with varied stereochemistry exhibit a good gas barrier that improves the shelf life of the product by inhibiting the production of anaerobic regions. The film produced from these materials shows functional features; for example, it decelerates the loss of aroma compounds throughout storage time and prevents the penetration of solvent molecules into the packaged content which affect the quality of the products or may even result in toxicity, especially in food packaging.28 In addition, these films provide a better barrier against fats and oils.29
Starch and cellulose are the most abundant polysaccharides available in nature and are widely used for the production of biodegradable polymers for functional applications. They are classified as storage and structural polysaccharides according to their biological functions.30 Both polysaccharides comprise a glucopyranose unit of different glycosidic linkages, such as α-1,4-glycosidic linkages and β-1,4-glycosidic linkages for starch and cellulose, respectively.31 Furthermore, starch consists mainly of two types of bio macromolecular components, amylose and amylopectin, whereas cellulose is a linear polysaccharide that depends on the type and treatment of the raw materials, such as wood.32 Cellulosic polymeric materials are mostly available as bacterial cellulose, microcrystalline cellulose, cellulose nanocrystals or nano whiskers.33,34 They have good film-forming characteristics, as they are non-toxic and transparent film materials with exceptional mechanical, thermal and barrier properties, specifically for oxygen and oil barriers.35–37 Modified cellulose and different cellulose derivatives such as cellulose acetate and cellulose esters are predominant materials used in industry for molding, extrusion and film applications.31 However, their poor solubility and indigestibility have hampered their commercialization in the field of edible films and coatings.38 Comparing cellulose and other polysaccharides with starch, the diversity in sources of starch with different molecular weights and functional properties, such as the improved processability of starch after plasticization and miscibility with other biopolymers, is important for its market in the biodegradable polymer industries.39 However, starch has a few disadvantages such as low water resistance, weak mechanical properties and the unstable properties of starch-based plastics due to the diversity in starch molecular structure and properties.40,41 However, among polysaccharides, starch has been considered as a promising material for biobased packaging owing to its low cost, high film-forming ability, oxygen barrier properties, water solubility and thermo-plasticity.42,43
Starch is a polysaccharide obtained from plants that consists of glucan polymers such as amylose and amylopectin.44 These glucan polymers are found in granular form in different dimensions with systemized semi-crystalline and amorphous homocentric layers.40 Depending on the starch source, there are disparities in chemical composition and structure which affect the crystallinity and texture of these polymeric materials.45 However, the higher rate of crystallinity, strong intermolecular forces like hydrogen bonding, and the presence of disordered granules reduce the mechanical properties and reduce the processability of starch as a thermoplastic polymer.40 Thus, to overcome these issues, starch is plasticized with different plasticizers such as glycerol,46–49 sorbitol,50–54 formamide,55–57 urea,58,59 citric acid,60,61 glycerine,62 polyethylene glycol,63 amino acids64 and water,65 and the resultant destructurized material is termed plasticized starch (PS). Plasticization of starch disrupts the crystallinity of starch material and promotes the processability of starch in the development of thermoplastic polymer.66
There are numerous review articles that discuss different biodegradable thermoplastic starch blends and composite systems. However, there are no recent reviews that specifically focus on the effect of different plasticizers in PS and plasticized starch/biodegradable polymer-based blend systems (TPS blends). This article specifically focusses on the effects of compatibility enhancers such as co-plasticizers, compatibilizers, coupling agents, additives, surfactants and epoxidized oils in different biodegradable polymer/PS blends (TPS blends) that improve the mechanical, thermal, surface wettability and barrier properties of the blends for single-use packaging applications. Functional property enhancement of starch by reinforcement with natural fibres/fillers or organic fillers in developing starch-based bio composites and bio nanocomposites is also discussed. In addition, challenges and real-life applications of starch and the global production market of TPS are presented.
2. Starch: chemistry, structure and property co-relationship
Starch is a biodegradable polymer obtained from diverse sources of botanical species including cereal grains (corn, wheat, barley and rice), grain legumes (pea, lentil, chickpea and bean), and root tubers (cassava, taro, Canna edulis and potatoes). It is a polysaccharide polymer synthesized in plants and stored as an energy reserve.67 The purest form of starch is found in white-colored granules without any odour or taste and is unable to dissolve in cold water. The granules of starch are usually found in the diameter range of 2 to 100 μm and with a density of 1.5 g cm−3.68 Starch comprises two carbohydrate polymers units: amylose segments and amylopectin segments. These carbohydrate polymers are glucose units that are connected through glycosidic linkages. Fig. 3 depicts the chemical structural formula of (a) the amylose moiety, and (b) the amylopectin moiety.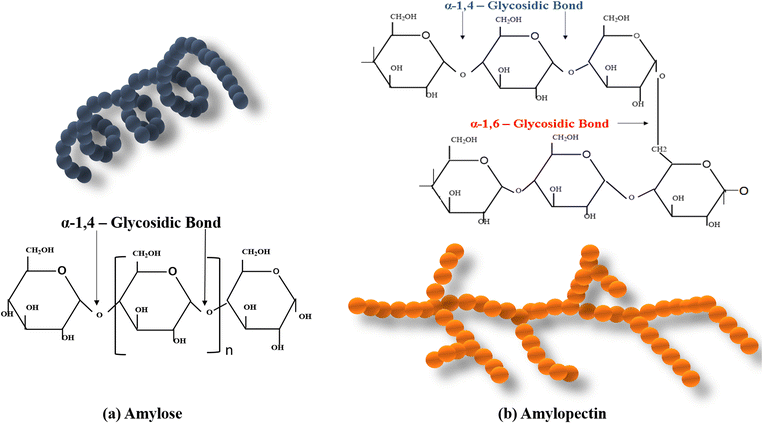 | ||
| Fig. 3 The chemical structural formula of (a) amylose moiety, and (b) amylopectin moiety (molecular structures drawn by author using ChemDraw). | ||
2.1 Biological origin of feedstock
Native starch can be acquired from various botanical sources, specifically from cereals (maize, wheat, rice, and barley) and root vegetables (cassava, tapioca, potato). Starch can also be obtained from agro-waste products and/or byproducts from food processing such as potato and pulse protein extraction. According to geographical and climatic conditions, different plant species produce starch with varying concentrations, molecular sizes and structures of amylose and amylopectin. For example, corn is cultivated in subtropical zones, rice in swamped areas, cassava in tropical regions, and wheat or sweet potatoes in moderately cold climates.69 Furthermore, the abundance in availability of starch polymer from renewable resources makes it the second most abundant biodegradable polymer in the world after cellulosic polymers. It is predicted that the mass production of starch could reach about 156.3 MT in 2025, irrespective of its use in the alimentary sector or non-alimentary sector.70 As per the global production rate of starch, corn starch is predominant with 80% of production, followed by other starch sources such as wheat, cassava, and tuber.71 Conversely, in productivity rate, cassava starch can contribute about two to four times more starch than bean, yam, taro, and around ten times more starch than that of sweet potato.72 And is estimated that the global starch market of 55.04 billion United States Dollar (USD) in 2020 is expected to rise to around 78 billion USD in the year 2026 with an annual Compound Annual Growth Rate (CAGR) of 5.63%.732.2 Structure–property relation
Native starch is a semi-crystalline granule made up of two principal types of glucan polymers: amylopectin and amylose. According to the starch source, the weight percentage of amylose components and amylopectin content varies from 20–25% and 75–80%, respectively. Amylose consists of α-(1 → 4)-D-glucopyranosyl moieties in linear or helical forms, whereas in the amylopectin macromolecule, α-(1 → 4)-D-glucopyranosyl moieties are linked with α-(1 → 6)-D-glucopyranosyl moieties at an approximate interval of 20 units, which forms a highly branched and high molecular weight macromolecule.72The higher molecular weight of starch in comparison with synthetic polymers, which affects the molecular mobility of molecules, is one of the reasons that make the processing of starch more difficult by conventional methods. Typically, the average molecular weight of amylose molecules is found in the range of 0.2–2 million Da, and amylopectin is 100–400 million Da.74 The branch chains of amylopectin components with helical structure contribute to the crystalline region of starch, and the degree of crystallinity of starch granules usually ranges from 15–45%.74 Additionally, in starch granules, amylose molecules are mostly found in the amorphous section, while individual amylose molecules are dispersed in both crystalline and amorphous phases of amylopectin clusters and in proximity with one another.75
Different studies have been conducted on starch granules to explore their molecular arrangement, internal structure, and physical and chemical properties.76,77 The chemical and physical properties of starches from different botanical sources are shown in Table 1. In the starch granule, the quantity of amylose content has a significant effect in achieving desired physical, chemical and functional properties.78 The starch source, degree of polymerization, lipids, proteins, and inorganic components are the significant aspects influencing the starch granule morphology and other functional properties.69 The complex morphological structure of starch granules can be analyzed by various characterization techniques such as scanning electron microscopy (SEM), light microscopy, atomic force microscopy (AFM) and neutron and X-ray scattering (XRS).79
| Starch source | Corn | Cassava | Wheat | Rice | Tapioca | Potato | Sago | Ref. |
|---|---|---|---|---|---|---|---|---|
| Starch granule diameter (μm) | 5.2 | 5–25 | 12.37 | <20 | 6.97 | 7.14 | 6.9 | 82 and 83 |
| Carbohydrates (%) | 99.38 | 87.8 | 99.56 | — | 99.21 | 99.39 | 99.29 | 82 |
| Amylose content (%) | 28 | 16–21 | 18.10 | 22 | 17 | 27 | 30 | 82, 84 and 85 |
| Amylopectin content (%) | 78 | 76–84 | 72 | 77 | 75 | 74 | 77 | 82, 84 and 85 |
| Protein (%) | 0.27 | 1.35 | 0.20 | 0.33 | 0.11 | 4.54 | 0.19 | 82, 84, 86 and 87 |
| Fat (%) | 0.29 | 1.0 | 0.27 | 0.34 | 0.64 | 0.16 | 0.10 | 67, 82, 87 and 88 |
| Ash content (%) | 0.06 | 0.02 | 0.17 | 0.17 | 0.044 | 0.29 | 0.06 | 82, 84, 86 and 87 |
| Density (g cm−3) | 1.4 | 1.5 | 1.11 | 1.282 | — | — | 0.76 | 67 and 89–93 |
| Moisture content (%) | 10.45 | 8–10 | 9.70 | 11.24 | 8–12 | 15.6 | 13.9 | 67, 89, 91 and 93–96 |
| Crystallinity (%) | 43–48 | 13 | 36–39 | 38 | 35–38 | 23–53 | 23.09 | 43, 97 and 98 |
| Crystalline type | A | B | A | A | C | B | C | 68 and 78 |
| Shape | Polyhedric | Semi-spherical | Polyhedric, lenticular | Polyhedric | — | Ellipsoidal | — | 99 |
Crystallization of amylopectin forms polymorphic structures and has been studied through wide-angle X-ray diffraction (WXRD). From WXRD analysis, starch granules exhibit three main types of polymorphic pattern: A-type, B-type and C-type.65 A- and B-type patterns are the common configurations found in cereals and tuber starches, respectively, while pattern C is the combination of A and B forms that is also produced naturally in pea or bean starches.76
According to the model proposed by Imberty et al.,80 A-type pattern (Fig. 4A(a)) is packed with monoclinic unit cells and four H2O molecules/unit cell, whereas in the B-type pattern (Fig. 4A(b)) water molecules are present in the center of a six double helix structure (a hexagonal unit with 36 H2O molecules/unit cell). Although amylose is not a crystalline material it can recrystallize into another pattern called V-type by complexing with guest molecules like iodine, fatty acid, emulsifiers, or butanol.81 The association of amylose with guest molecules forms a helical structure in antiparallel direction. The outer surface of the helical structure will be hydrophilic, and the inner part will be hydrophobic as it is accommodated with guest molecules, and this association is termed intrahelical association. The resultant crystal structure is termed V-hydrate (Vh) or V-anhydrous (Va), where Va is formed by shrinking Vh on losing water molecules from the unit cell. The crystalline morphologies of these starch polymorphs are usually analysed by XRD, Fourier transform infrared spectroscopy (FTIR) and electron diffraction techniques. The XDR patterns in Fig. 4B represent intensity peaks of A type starch, B-type starch and Vh and Va-type amylose.81Fig. 4C depicts the cluster model of amylopectin classified according to the branch chain type and length.
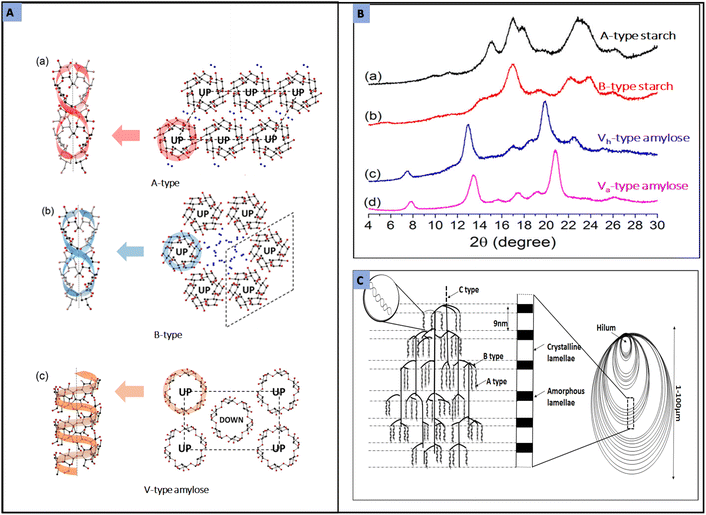 | ||
| Fig. 4 (A) Shows the helical structure and (right) unit cell projection in the c-axis of A-type, B-type and V-type amylose. The ‘UP’ and ‘DOWN’ in the unit cells represent the direction of the reducing end of the helical structure moving up and down, respectively. Blue colour dots represent the water molecules. (B) The XDR patterns in Fig. 4B have been produced by (a) A-type starch is from acid-hydrolysed and annealed pea starch, (b) B-type starch is from acid-hydrolysed and annealed Hylon starch, (c) Vh-type amylose from amylose crystallised with palmitic acid as guest molecule and (d) Va-type amylose pattern from crystallisation of amylose lacking guest. (Figure A and B reproduced from ref. 81 with permission from the American Chemical Society, Copyright 2014.) (C) Shows the cluster model of amylopectin where the terms A, B, and C are related to the chain type and length of amylopectin (drawn by author). | ||
3. Starch as a packaging material
For the past decades, starch has been gaining great prominence in the biodegradable polymer packaging industries owing to its film-forming ability, renewability, recyclability, and low cost.42,100 Furthermore, starch is valued as the best alternative for petroleum-based single-use plastic and as a blending partner for expensive commercial biopolymers for attaining better processability, production cost, heat resistance and oxygen barrier properties.101,102 Starch-based films and their coatings are optimal for food product packaging industries because of their transparency and good barrier properties (CO2 and O2 barrier).103,104 Lourdin et al.105 found that for unplasticized starch films, an increase in amylose concentration increased the elongation and tensile strength of the films. Furthermore, Forssell et al.106 analysed the effect of relative humidity on the oxygen permeability of amylose and amylopectin films prepared by the solution casting technique. The prepared film showed an oxygen barrier similar to that of commercial ethylene-vinyl alcohol (EVOH) film at ambient humidity, and on increasing the humidity above 70% RH, amylose had a better oxygen barrier than amylopectin.Corn starch that contains higher amylose content is used in the production of edible films and coatings in the food packaging sector.102 In addition, pea and rice starch with high amylose content have shown better oxygen barriers than protein-based polymers.107 However, the hydrophilic nature of starch-based films and coatings has resulted in high water solubility and poor water resistance.104 Similarly, the brittleness, retrogradation and thermal degradation of starch during melt processing can affect the mechanical integrity of the films and limits their industrial applications.101 Thus, starch modification is desirable to amplify the processability and functional properties. The physical and mechanical properties of the starch film can be improved by physical and chemical modification techniques, namely plasticization, derivatization, blending and graft polymerisation.60,108–113
4. Plasticization of starch
Native starch lacks the ability to act as a plastic owing to the presence of strong inter and intra-molecular H-bonding formed by the amylose and amylopectin of starch.55 Hence, plasticization is used to change the molecular structure of starch in the presence of plasticizer, under elevated temperature and shear.114 There is plenty of research that has been conducted on the plasticization of starch with different low molecular weight molecules (water, glycerol, sorbitol etc.) to enhance the processability of starch.47,51,54,55,115,116 The process of plasticization of starch involves different chemical and/or physical interactions such as water diffusion, and starch granules’ expansion, gelatinization, and polymer melting.114,117 Gelatinization is one of the processes that can augment starch granule disintegration and decrease the intermolecular affinity.118 However, the presence of plasticizer in the starch formulation destroys the inter and intra molecular hydrogen bonding of the starch granule, partially depolymerizing the starch backbone and further leading to the decrease in melt temperature of starch below degradation temperature.119 Thus, plasticization overcomes the brittleness of native starch by increasing the macromolecular chain mobility and enhances the processability of starch at low temperatures.To achieve efficient plasticization, the type and concentration of plasticizers play important roles.120 An efficient plasticizer would be a hydrophilic polar molecule that has high compatibility with the starch molecule, and that has a boiling point higher than that of the polymer processing temperature. Rodriguez et al.47 found that the optimum concentration of glycerol as plasticizer is 20 wt% of starch content, and further increase in plasticizer leads to phase separation and leaching out from the film.
The efficiency of the plasticizer also depends on the amylose and amylopectin content, which varies with the starch source. The study conducted by van Soest et al.121 on PS prepared by plasticizers such as glycerol and sugars showed the tendency to form retrogradation after cooling and storage, which caused brittleness to the plasticized material. Retrogradation is the process of recrystallisation of linear chains of amylose and amylopectin moieties that results in a more ordered structure during the process of storage after gelatinization.122,123 Amylose and amylopectin are the responsible components for retrogradation in starch. During cooling and storage of gelatinized starch, amylose moieties reassemble to form double helix structures and amylopectin branches form a partially ordered crystal structure.124 In this process, at the beginning of storage, amylose forms aggregates and with increasing storage time amylopectin cause crystallization that affects the deterioration of plasticized starch.
High amylose content starch forms a tough and flexible polymer because of its amorphous structure. However, on plasticization with glycerol, high amylose starch undergoes retrogradation over time and its crystallinity increases, which affects the functional properties of PS. It is important for an efficient plasticizer to have the ability to suppress retrogradation during aging, and it should enhance the flexibility. The study conducted by Ma and Yu55 found that the use of amide-based plasticizers like urea, formamide, and ethanolamine could suppress the retrogradation process and enhance the mechanical properties of PS. Urea was more effective, as the double amide group in urea could form stable hydrogen bonds with starch, resulting in an amorphous structure. This was confirmed by XRD analysis, where the crystalline peaks of starch were not visible as compared with native starch. However, urea is a small molecule with lower internal flexibility. Thus, urea-plasticized starch forms a rigid and brittle material rather than a tough thermoplastic material. Hence to rectify this issue, multiple plasticizers were introduced during plasticization of starch. The use of multiple plasticizers could enhance the plasticization efficiency and could prevent the retrogradation process.
Analysing the effect of hybrid (multiple or co-plasticization) plasticization of starch with urea and ethanolamine, Ma et al.108 demonstrated better thermal and mechanical properties as stronger bonds were formed with the hydroxyl group of starch than glycerol. In addition, this combination could effectively avoid the retrogradation process. An analysis of the mechanical properties of starch from various sources in different compositions of plasticizers is shown in Table 2. It is evident from the table that the type of starch, concentration and combination of plasticizer, relative humidity and storage time have an effect in achieving variable mechanical properties in plasticized starch. Furthermore, it shows that multiple plasticizers are depicting more percentage elongation at break (EB) than single plasticizer.
| PS formulations | Relative humidity (%) and storage time | Tensile strength (MPa) | Young's modulus (MPa) | Percentage elongation (%) | Breaking energy (N m) | Ref. |
|---|---|---|---|---|---|---|
| Corn starch plasticized with glycerol | 50, 14 days | 5.5 | 38.1 | 7 | 1.9 | 116 |
| Corn starch plasticized with ethanolamine | 50, 14 days | 6.0 | 75.3 | 14 | 2.3 | 116 |
| Corn starch plasticized with sorbitol (30%) | 7 days | 13.62 | 500 | 50 | — | 51 |
| Corn starch plasticizes with sorbitol and glycerol | 7 days | 6 | <100 | 50 | — | 51 |
Corn starch with glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) xylitol (1 xylitol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | 2.56 | — | 62.94 | — | 125 |
| Corn starch plasticized with urea (30 wt%) | 33, 7 days | 12.5 | 1664 | 5.7 | 0.32 | 108 |
| Corn starch plasticized with ethanolamine (30 wt%) | 33, 7 days | 3.1 | 61.6 | 57 | 0.75 | 108 |
| Corn starch plasticized with urea/ethanolamine (15/15 (wt%)) | 33, 7 days | 9.0 | 236 | 34.4 | 1.34 | 108 |
| Corn starch plasticized with thymol | 52, 1 day | 27.51 | 1488 | 2.22 | 126 | |
| Corn starch plasticized with glycerol and thymol | 52, 1 day | 1.52 | 73 | 66.85 | — | 126 |
| Corn starch plasticized with 1-ethyl-3-methylimidazolium acetate (1.5 wt%) and water | 50, 7 days | 4.25 | 75 | 68 | — | 127 |
| Potato starch plasticized with glycerol | 53, 7 days | 6.56 | 5.33 | 5.67 | — | 128 |
| Potato starch plasticized with sorbitol (10–50%) | 52, 4 days | 4.95–9.37 | — | 1.84–9.00 | — | 50 |
| Potato starch plasticized with 1-ethyl-3-methylimidazolium acetate (1.5 wt%) and water | 50, 7 days | 2.43 | 14.65 | 38 | — | 127 |
| Wheat starch plasticized with glycerol | 53, 7 days | 3.29 | 0.12 | 15.21 | — | 128 |
| Wheat starch plasticized with 1-ethyl-3-methylimidazolium acetate (1.5 wt%) and water | 50, 7 days | 4.99 | 87.64 | 75.59 | — | 127 |
| Rice starch plasticized with glycerol (30%) | 63, 1 day | 2.0 | — | 19.5 | — | 129 |
| Rice starch plasticized with sorbitol (40%) | 63, 1 day | 3.25 | — | 23.7 | — | 129 |
| Mango kernel starch plasticized with glycerol (40%) | 58, 2 days | 3.57 | 50.07 | 17.78 | — | 130 |
| Mango kernel starch plasticized with sorbitol (40%) | 58, 2 days | 25.06 | 959 | 4.02 | — | 130 |
Mango kernel starch plasticized with glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sorbitol (1 sorbitol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
58, 2 days | 5.73 | 96.25 | 26.13 | — | 130 |
Huneault and Li131 investigated the effect of glycerol, sorbitol, and glycerol/sorbitol mixture as a plasticizer in PS/PLA blend on achieving better morphology and mechanical strength. The processes were conducted in a series of steps such as starch plasticization, water devolatilization and PLA addition using a twin-screw extruder, where the first half was devoted to starch gelatinization followed by free water removal in the devolatilization zone, and in the second zone PLA was introduced and mixed with PS. In this study, when sorbitol was compared with glycerol, an improvement in mechanical and thermal properties was obtained for the sorbitol-plasticized starch/PLA blend because of the low rise in vapour pressure of sorbitol at higher temperature and low volatility that restricts the loss of plasticizer during melt blending. Furthermore, plasticizing starch with sorbitol could reduce the recrystallisation rate and plasticizer migration, but an increase in sorbitol content could reduce the tensile modulus (TM) of the PS/PLA blend. In contrast, the use of glycerol as a single plasticizer improves the flexibility of the starch material but decreases the thermal resistance, TM, and resistance to retrogradation. This may be due to the higher vapour pressure and weak interaction of small glycerol molecules with starch. Thus, co-plasticization is an effective method to attain a balanced thermomechanical property and retrogradation resistance in starch. This has been further proved by the study conducted by Esmaeili et al.132 on the co-plasticization effect of glycerol and sorbitol in different ratios and concentrations of plasticizers; they have shown that the mixture of plasticizer concentration raised the thermomechanical properties of the plasticized starch by providing a reasonable TS, TM, and elongation with a glass transition temperature (Tg) below room temperature (RT) (Fig. 5). Fig. 6 shows the schematic representation of hydrogen bonds formed during the plasticization of starch with glycerol, sorbitol, or glycerol/sorbitol after melting blending.132
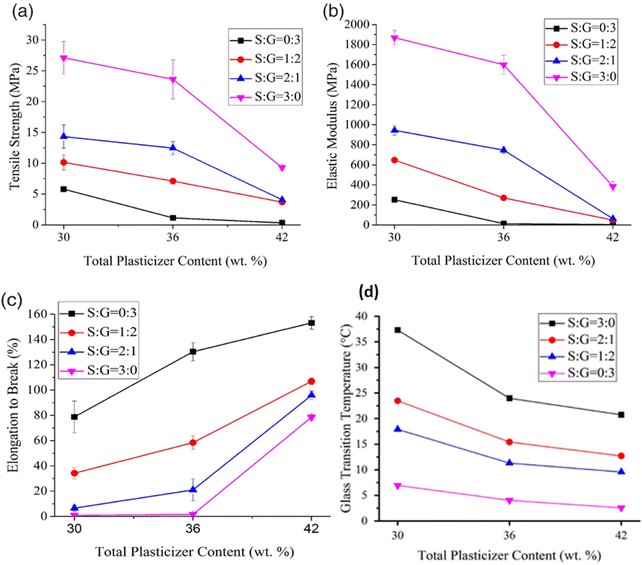 | ||
| Fig. 5 The variation in thermomechanical properties of sorbitol/glycerol co-plasticized starch at different weight ratio and total plasticizer content. (a) Tensile strength (b) elastic modulus (c) elongation at break and (d) glass transition temperature. Reproduced from ref. 132 with permission from John Wiley and Sons, copyright 2017. | ||
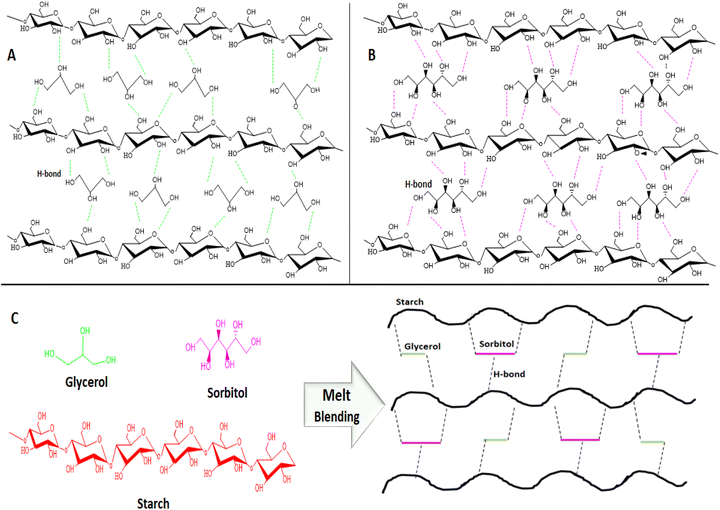 | ||
| Fig. 6 Shows the schematic representation of hydrogen bonds formed during the plasticization of starch with (A) glycerol, (B) sorbitol and (C) glycerol/sorbitol after melt blending (molecular structures drawn by author using ChemDraw).132 | ||
Recently, Kahvand and Fasihi63 investigated the effect of citric acid as co-plasticizer for starch by plasticizing starch with water/glycerol/citric acid. The terms CAPS (citric acid-plasticized starch) and PS represent water/glycerol/citric acid and water/glycerol as plasticizers, respectively. The addition of citric acid as co-plasticizer led to the development of stronger and stable hydrogen bonds with starch, and was confirmed by SEM, FTIR (Fourier transform infrared spectrometer) and XRD analysis (Fig. 7). The FTIR analysis of starch, PS, and CAPS confirmed the formation of new hydrogen bonds in starch with plasticizers by attenuating the intermolecular hydrogen bonds of starch. This was confirmed by the formation of characteristic dual peaks at 992 cm−1 and 1024 cm−1 for plasticized starch instead of a single peak of 994 cm−1 for pure starch. The intensity of these dual peaks was higher in the presence of citric acid, which represents the ability of the carboxyl group of citric acid to form stronger hydrogen bonds with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of starch. Furthermore, the formation of a characteristic peak at 1737 cm−1 in CAPS confirmed the formation of partial ester linkages in starch that enhanced the thermal resistance of starch and decreased its rate of retrogradation. Additionally, the XRD analysis of CAPS confirmed the elimination of the A-type crystal structure of starch with the formation of V-type crystal structure in CAPS, indicating the formation of a homogeneous structure without unmelted components, confirmed by SEM images.
O group of starch. Furthermore, the formation of a characteristic peak at 1737 cm−1 in CAPS confirmed the formation of partial ester linkages in starch that enhanced the thermal resistance of starch and decreased its rate of retrogradation. Additionally, the XRD analysis of CAPS confirmed the elimination of the A-type crystal structure of starch with the formation of V-type crystal structure in CAPS, indicating the formation of a homogeneous structure without unmelted components, confirmed by SEM images.
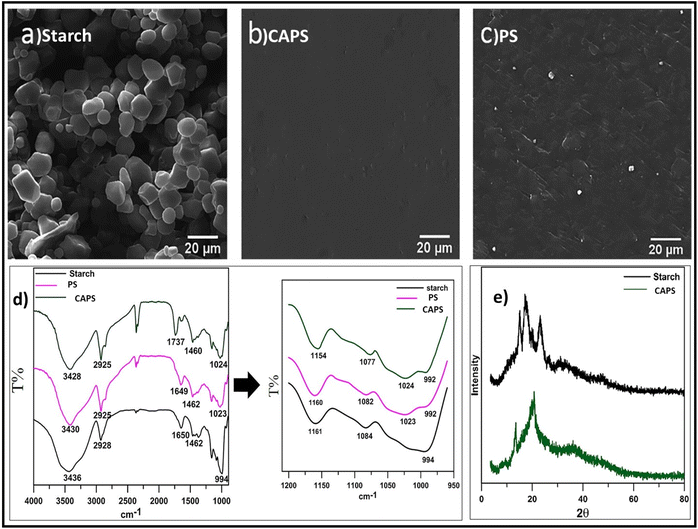 | ||
| Fig. 7 (a) SEM micrograph of starch, (b) SEM micrograph of CAPS, (c) SEM micrograph of PS, (d) FTIR spectroscopy of starch, CAPS and PS, (e) XRD of starch and CAPS. Reproduced from ref. 63 with permission from Elsevier, copyright 2019. | ||
5. Thermoplastic starch blends
Native starch is a semi-crystalline hydrophilic material with brittle characteristics which limit its application especially in the packaging sector, such as in plastic bags, mulch film, edible coatings on fruits, meats and vegetables, and food packaging. The brittleness of native starch is due to the relatively high glass transition temperature and the absence of sub Tg (β-transition).133 In addition, brittleness can increase with time through the retrogradation process.55 Furthermore, the higher melting point and lower thermal degradation temperature of starch have resulted in poor melt processability.114 However, to enhance the processability and functional properties of starch, it is converted into thermoplastic by the incorporation of one or more plasticizers followed by blending with polymer or biopolymers at elevated temperature and shear force; these are called TPS blend systems.112 In most articles the term plasticized starch refers to thermoplastic starch and is abbreviated to TPS, making the nomenclature counterintuitive. The process of plasticization of starch does not make starch a thermoplastic material until it is blended with a toughened polymer or biopolymer material. Furthermore, the lower mechanical properties and hydrophilicity of plasticized starch reduces its application in industrial use. Irrespective of the use of the term thermoplastic starch, plasticized starch cannot be remolded or reused once it is processed. Thus, for commercialization and industrial use, TPS blend systems are preferred.Thermoplastic starch-based blend systems have properties comparable with conventional polymers, and could act as an alternative for certain synthetic thermoplastic polymers. Since starch is an abundant biodegradable polymer, the TPS blend system for packaging applications will be cost-effective and environmentally friendly. There are many types of research on the development of thermoplastic starch blend systems with the incorporation of biodegradable and non-biodegradable thermoplastic polymers. However, interest in biodegradable and biobased systems has increased the development of TPS blends from biobased thermoplastic polymers and plasticized starch systems. They are prepared by thermochemical processing techniques like kneading, casting, melt blending, and compression molding.74 The processing technology, which determines the possible physical and chemical interactions, is an important aspect in achieving the blend properties.
5.1 Processing techniques
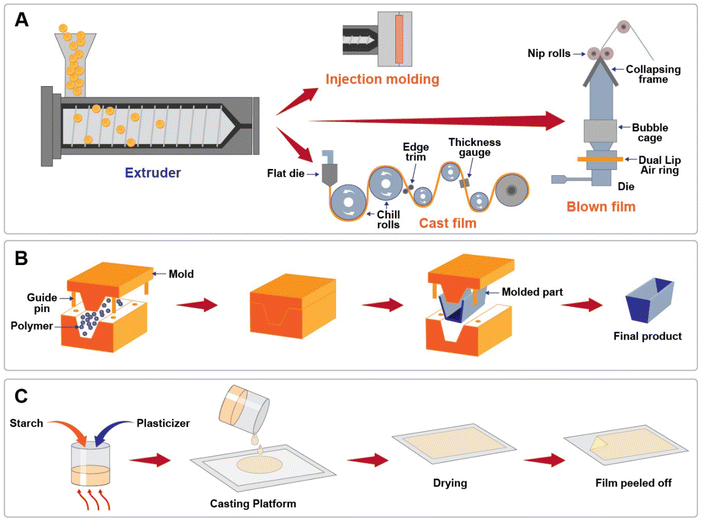
Different processing techniques have been utilized for preparation of PS and TPS blends and composite systems. In most research, the TPS blends are prepared by the solution casting technique. This is a wet process usually done at laboratory scale to analyse the plasticization, gelatinisation, and retrogradation mechanisms of starch. The ability to achieve uniform thickness distribution, dimensional stability, low haze, and optical purity are the key factors of this technology.134 However, for industrial applications extrusion techniques are preferred, as this is a reliable and continuous process. During extrusion, polymer melt is forced through a desired shaped die and formed into profiles like sheets, tubes, films etc. It is a fast and reliable technology used to manufacture thermoplastic starch-based products for commercial applications.Extrusion technology is a widely accepted thermal processing technology used to prepare PS, thermoplastic starch blends and related films and composites owing to its ability to withstand higher viscosity, and its operational flexibility in a wide range of processing conditions.43 TPS blends and composites are prepared by a two-step extrusion process such as primary and secondary extrusion. Primary extrusion is plasticization of starch to develop PS. In PS extrusion processes, the starch granules or pellets in the presence of plasticizer and/or additives are fed into a screw through the hopper and subjected to high-temperature shear mixing in different heating zones. During this process, the presence of water and plasticizer destroys the crystallites of starch which undergoes fragmentation that eases PS melting and flowability. In the secondary extrusion process, the resultant PS is blended with toughened polymer and/or reinforced with fillers or fibres to develop TPS blends and composites, and the subsequent extrudate from the die can be injection moulded into products of the desired shape, or films can be cast or blown according to the application of the final product (Fig. 8A).
Altskär et al.138 scrutinized the effect of different processing techniques such as compression molding, blown film extrusion and solution-cast film of PS obtained from glycerol and water plasticization of hydroxy propylated and oxidised potato starch (HONPS). The results showed that the composition of TPS and processing conditions affect the morphological and structural properties of the films. Furthermore, the transmission electron microscope (TEM) analyses of HONPS films from different processing techniques are shown in Fig. 9. The morphological analysis of all these processing techniques has shown phase separation according to the presence of plasticizer-rich and plasticizer-poor regions. However, the higher moisture content and molecular mobility of cast films, and recrystallisation on cooling of compression films have shown the presence of a network structure in morphology, whereas this was not found in blown film owing to its low moisture content.
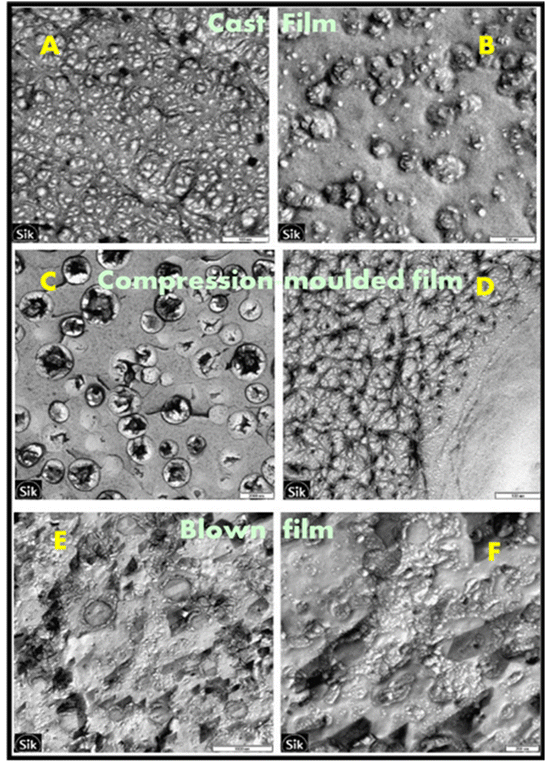 | ||
| Fig. 9 TEM micrograph of cast, compression moulded and blown HONPS films. (A) and (B) shows two different structures in cast HONPS with scale bars 100 nm, (C) and (D) shows compression moulded (HONPS) with scale bars 2000 and 100 nm, (E) and (F) shows blown HONPS films with scale bars of 1000 nm and 200 nm respectively. Reproduced from ref. 138 with permission from Elsevier, copyright 2008. | ||
In most of the studies, PS, TPS blends and composites systems were fabricated by the casting technique where the films were fabricated either from film-forming dispersion, or from an emulsion with higher percentage of water content. The process of solution casting involves mainly 3 steps: gelatinisation/plasticization or homogenisation (in the case of mixtures or emulsions), casting and drying (Fig. 8C).142 The optimization of processing temperature and gelatinisation/plasticization time depends on the starch source and plasticizer content, as the granule structure of starch varies with source. In the casting process, when starch is hydrated by being dissolved in hot water, the crystalline components of amylose and amylopectin are lost, and while forming film, the macromolecules and linear components of amylose and amylopectin rearrange and reassociate by hydrogen bonding. Thus, the resultant crystallinity of starch-based films depends on plasticizer content, and temperature and humidity conditions during drying and storage.142
6. Recent progress in TPS-based biodegradable blends
As there is much research going in the field of TPS-based biodegradable blends, the following sections separately discuss the different biodegradable polymer and starch blend systems that have been developed in the last two decades. The sections mainly focus on the different strategies that have been carried out during the processing of TPS blends to enhance the compatibility between starch and other biodegradable polymers such as PLA, PCL, poly(propylene carbonate) (PPC), PHA, poly(vinyl alcohol) (PVA), and poly(butylene succinate) (PBS). Fig. 10 shows the chemical structure of different fully biodegradable or compostable polymers.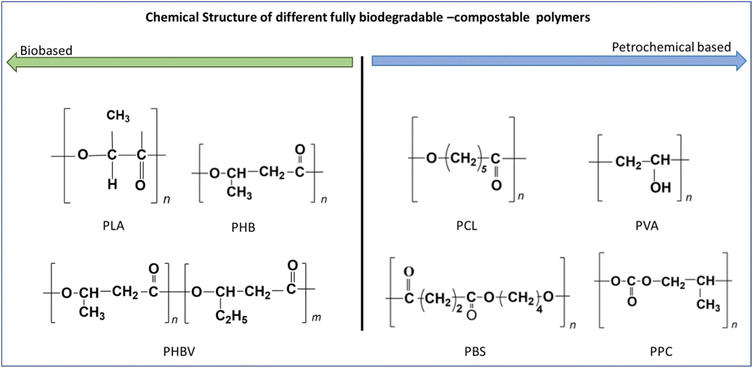 | ||
| Fig. 10 Chemical structure of different fully biodegradable and compostable polymers (drawn by author). | ||
6.1 Poly(lactic acid)/PS blends
PLA is an aliphatic polyester fabricated from renewable sources (such as starch, sugar, corn, etc.).143,144 The excellent mechanical properties and easy processability of PLA to form flexible films have shown potential applications in the field of packaging industries.145,146 However, the high cost and inherent brittleness of PLA has reduced its wide spectrum of applications.144 Thus, blending of PLA with polymers such as PCL, starch and polyethylene is cost effective and could improve the toughness of the material.147–149 Since starch is an abundant biodegradable polymer, blending of starch with PLA could result in a cost-effective blend.150 However, the lack of interfacial adhesion between hydrophilic starch and hydrophobic PLA reduces the mechanical strength of resultant blends.151 Hence, numerous studies have been performed aiming to achieve efficient compatibility in PLA/starch blend by incorporating several types and concentrations of compatibilizers, organic acids, natural oils and crosslinkers.132,152–154Initially, Zhang et al.155 studied the effect of one-step and two-step extrusion on the preparation of a PLA and starch powder (55/45) blend system with maleic anhydride (MA) as a compatibilizer and 2,5-bis(tert-butylperoxy)-2,5 dimethyl hexane (L101) as initiator. In the one-step approach, MA and L101 were added into the PLA/starch system and extruded, whereas in the two-step approach, PLA-g-MA (1% MA and 10% L101 (MA basis)) was prepared initially and blended with PLA/starch blend system. The obtained mechanical properties such as TS and EB showed that, irrespective of one-step or two-step processing, the addition of MA in the presence of initiator markedly improved the mechanical properties of the blend, and showed similar mechanical strength of that of neat PLA. Fig. 11 shows the SEM images of the PLA/starch blend system obtained through the one-step process, where PLA/starch with MA and initiator indicates better compatibility between PLA and starch.
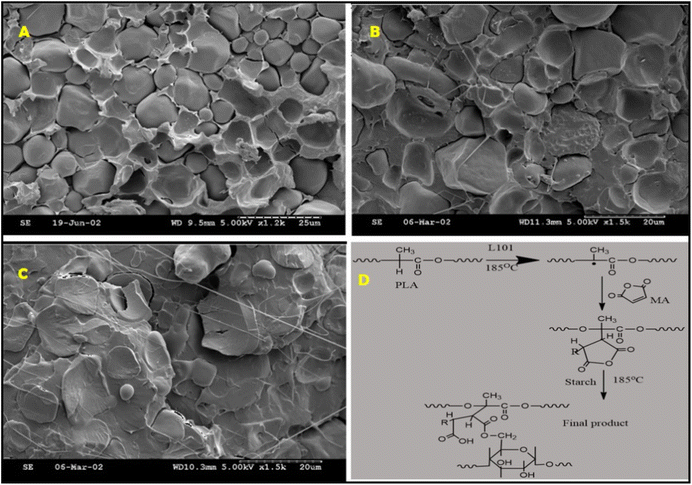 | ||
| Fig. 11 (A) Shows an immiscible PLA/starch blend without MA and initiator where PLA is the continuous phase and starch is the dispersed phase, (B) PLA/starch blend with MA and without initiator shows similar behaviour as of PLA/starch blend (without MA and initiator) with some visible cavities, (C) PLA/starch blend with MA and initiator shows uniform dispersion of starch in PLA (figure A, B, C reprinted and figure D is redrawn from ref. 155 with permission from the American Chemical Society, Copyright 2004). | ||
In later studies, utilization of PS was analysed as it enhances the properties of PLA/PS blends and additionally it could increase the starch content in blend systems, which could reduce the cost. The process of plasticization can overcome the strong intermolecular and intramolecular hydrogen bonding of starch and improve the compatibility with the blending polymer. Wang et al.156 studied the effect of different plasticizers such as glycerol and formamide in the presence and absence of water in enhancing the dispersion and interfacial adhesion of the PS/PLA blend, and the results showed that replacing glycerol with formamide in the presence of water is an efficient plasticizer PS/PLA blend system. The formamide–water plasticizer is better than the glycerol–water plasticizer system, as formamide improved the dispersion and compatibility between the polymers, evident from TGA analysis where higher Tmax (temperature at the maximum rate of weight loss) and AED (activation energy of decomposition) were obtained. Furthermore, amide group-containing plasticizers such as urea, acetamide, and formamide can reduce the retrogradation and structural changes of starch and are excellent as plasticizers, but the toxicity of these materials limits their applications in the biomedical, pharmaceutical and food packaging fields.132
On using glycerol alone as plasticizer for starch, the flexibility of the PS/PLA blend material improves, but it reduces the TM, thermal resistance and retrogradation resistance. The reduction in these properties is mainly due to the higher vapour pressure of glycerol that causes loss of plasticizer during processing, and the low molecular interaction of the small molecules of glycerol with starch molecules. Conversely, on using sorbitol as a plasticizer for starch in PS/PLA blend exhibits higher retrogradation resistance but lower flexibility. Thus, the issues of starch retrogradation, brittleness and migration of plasticizers during plasticization of starch were avoided by utilizing a mixture of plasticizers (hybrid plasticization or co-plasticization) such as formamide/urea, ethylene-bisformamide/sorbitol, sorbitol/glycerol, and glycerol/maltose.15,115,157,158 According to studies conducted by Esmaeili et al. and Huneault and Li,131,159 the combination of sorbitol and glycerol as co-plasticizers for PS/PLA blend improved the mechanical property and exhibited fine morphology compared with glycerol alone as plasticizer. This is due to the similarity in solubility parameters of PLA and plasticizers, that caused the plasticizers, specifically glycerol, to migrate to the PLA phase, decrease the storage modulus and complex viscosity of the blend systems, and thus improve the processability of the melt.
Another approach stated by Yokesahachart and Yoksan et al.160 is to use amphiphilic molecules such as Tween 60 (polyethylene glycol sorbitan monostearate or polysorbate), linoleic acid, and zein as an additive for thermoplastic starch blends prepared with glycerol as a plasticizer and followed by PS/PLA blend preparation. Amphiphilic molecules are compounds having two sets of hydrophobic hydrocarbon parts and polar hydrophilic parts like carboxylates, sulphates, sulfonates, and amine groups. The incorporation of these amphiphilic molecules resulted in a drastic improvement in elongational property and enhanced processability of plasticized starch and the consequent PS/PLA blend due to the decrease in Tg and Tm without affecting the thermal stability of the TPS blend systems.
In earlier studies, the use of reactive compatibilizers or coupling agents (such as acrylic acid, methylene-diphenyldiisocyanate (MDI), poly(hydroxyester ether) (PHEE), poly(vinyl alcohol) (PVA) and MA) was one of the methods introduced to improve the interaction between PLA and starch-based blends.71,155,161,162 Other than that, Akrami et al.152 developed a new compatibilizer of maleic anhydride-grafted polyethylene glycol-grafted starch to modify the compatibility of PLA/PS blend. This newly developed compatibilizer significantly enhanced the interfacial adhesion and ductility of the blend because of interaction between the active groups of PLAs and PS and carboxylic acid end groups of the compatibilizer. However, most of these compatibilizers are noxious and injurious for health.
To replace harmful compatibilizers and plasticizers, the use of non-toxic, renewable, and abundant epoxidized vegetable oils would be an effective solution.154,163,164 There is much research on epoxidized oil as an effective component in reducing the brittleness and enhancing the interaction of the blends. Ortega-Toro et al.165 assayed the efficiency of epoxidized sesame oil as a compatibilizing and plasticizing agent for PLA/starch blend films obtained from melt extrusion followed by compression moulding. The incorporation of epoxidized sesame oil increased the flexibility and thermal stability by providing a plasticization effect to PLA, and it acted as a coupling agent that augmented the interfacial adhesion of the blend (Fig. 12). In Fig. 12, PS is referred to as TPS.
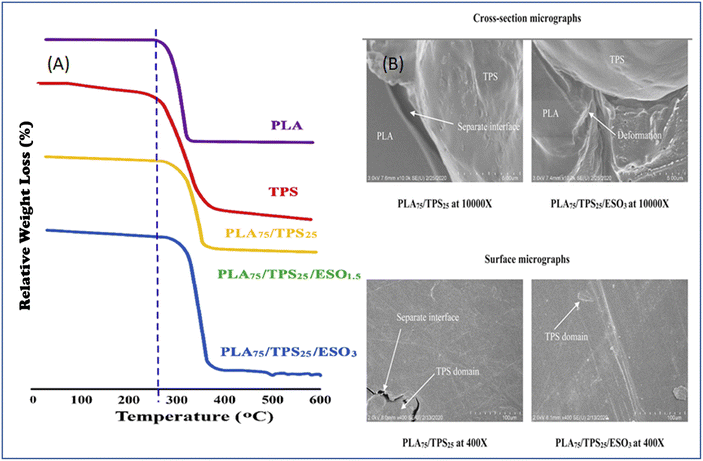 | ||
| Fig. 12 (A) Thermogravimetric analysis of PLA/starch films with or without epoxidized oil as compatibilizer/plasticizer. (B) Cross-section and surface SEM of PLA/starch film without epoxidized oil and with epoxidized. Reproduced from ref. 165 with permission from Elsevier, copyright 2021. | ||
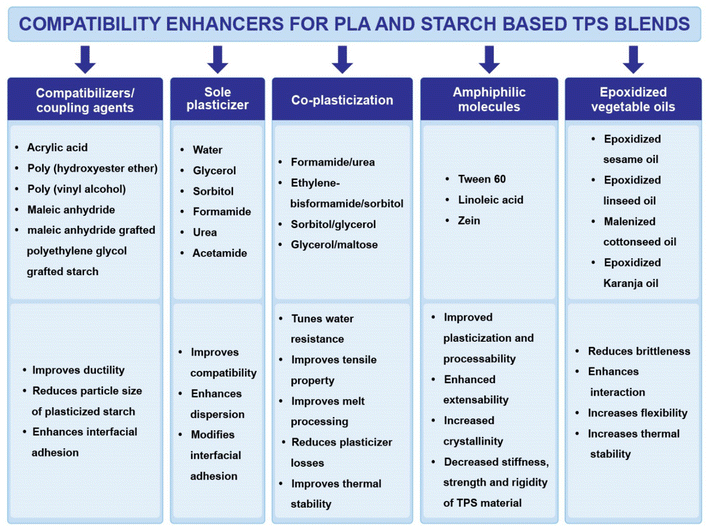
Fig. 13 shows different compatibility enhancers for PLA and starch-based blends, where co-plasticization is an effective technique to improve the thermal stability during processing and could reduce the plasticizer migration during blending of PLA and starch, whereas the addition of additives or epoxidized oils could further improve the extensivity of the blends. Replacing amide-based plasticizers such as urea and formamide with citric acid or other organic acids (acetic acid, maleic anhydride, linoleic acid and oleic acid) could be an effective option for food-contact packaging applications and prevent the retrogradation process of starch.166
6.2 Poly(ε-caprolactone)/PS blends
PCL is a commercially available biodegradable plastic that belongs to the group of linear polyesters. PCL is a tough, biodegradable, compactable and non-toxic material with high solvent, water, and oil resistance.167 It is often blended with other biopolymers such as PLA,168 PHA,169 and starch110 owing to its higher viscoelastic properties. However, the high cost of PCL restricts its broad use in applications, especially in the packaging sector.170 Thus, the incorporation of inexpensive natural polymers such as starch could reduce the cost and enhance the biodegradability of PCL. However, to overcome the poor interfacial adhesion during blending PCL and starch powder, the PCL is blended with plasticized starch. According to the study conducted by Shin et al.,110 melt blending polycaprolactone with corn-based PS as the dispersed phase, followed by compression molding to films of thickness 0.5 mm, improved the interfacial adhesion between PS/PCL blend by forming H-bonding between the ester carbonyl group of PCL and hydroxyl group of PS.In an initial study, Averous et al.167 tried to develop a cost-effective and biodegradable plasticized wheat starch/PCL blend with PS as major phase without compatibilizers. The study confirmed that the concentration of glycerol in starch and PCL have great impact on the mechanical properties of the blend, where the addition of flexible PCL into rigid PS enormously enhanced the impact strength and reduced the TS, EB and Young's modulus of the blend. This study confirmed the fact that the addition of toughened polymer could rectify the weaknesses of PS such as low resilience, high shrinkage and high moisture absorption, and the resultant blend would be an environmentally friendly material for packaging with better biodegradability than neat PCL.
For a polymer blend, control of the morphology is an essential factor in attaining effective material properties. Phase morphology of a blend depends on the viscoelastic properties of the respective components, blend composition, interfacial adhesion, and processing conditions. Different phase morphologies of polymer blends can be seen in different forms such as droplets, fibers, laminar or as co-continuous morphology. Furthermore, phase coalescence is an important process that affects the final morphology of the polymer blends and is classified as dynamic and static processes. In the dynamic process, it is a flow-dependent process, where the final morphology of the blend system depends on the balance between particle breakups and coalescence of the dispersed phase during the polymer blending,171 whereas in the static process, referred to as a quiescent process, the dispersed phase or matrix phase, or both, coarsen through coalescence over time at elevated temperature during blending. Li et al.172 analyzed the coalescence and static and dynamic mechanical properties of the PS/PCL blend, using dynamic mechanical analysis (DMA) and FTIR spectroscopy. It was confirmed that the presence of a higher concentration of plasticizers without external modifiers is more effective in achieving a dual-phase continuity region and strong hydrogen bonding between the starch and PCL in TPS blend systems. This may be because the increase in plasticizer concentration improved the chain mobility and interaction between these polymers, which resulted in enormously higher EB even in higher concentrations of PS.
Another factor to consider in PCL/PS blends is the effect of retrogradation. Recently, Hernandez et al.173 have investigated the effect of PCL in retrogradation of PS/PCL blends. It was found that irrespective of PCL concentration, the process of retrogradation of PS occurred continuously in the binary blend and changed the structural properties of the blend. XRD and FTIR analysis confirmed that the interaction between PCL and PS was negligible compared with the structural changes that were maintained within the PS phase. As a result, the mechanical properties of the blends have shown a similar trend as pure PS, where properties declined initially by plasticization through moisture absorption and then increased by retrogradation over time.
Another approach to reduce the inadequacies of individual polymers during blending is the use of a ternary blend. Bulatović et al.170 experimented with the preparation of a PLA/PCL/PS ternary blend, and showed that the addition of PS as a dispersed phase to PLA/PCL blend resulted in poor adhesion and phase separation that impaired the mechanical properties of these TPS blends, resulting in the formation of an immiscible ternary blend. However, these blend systems could be improved by adequate use of compatibilizers that could enhance the miscibility and functional properties of the blend specifically for biodegradable packaging applications.
6.3 Poly(propylene carbonate)/PS blends
PPC is an aliphatic poly(carbonic acid ester) synthesized by copolymerisation of carbon dioxide and propylene oxide in the presence of a catalyst.174–176 It is a biodegradable, biocompatible and biobased material with good transparency, gas barrier and high EB properties that could compete with and replace petroleum-based polymers in different applications like packaging, films, and solid electrolytes.177 PPC could not only minimize the consumption of petroleum resources, but also mitigate carbon dioxide emission that leads to climatic changes and global warming.178 However, the amorphous structure of PPC has low stiffness, lower Tg and poor thermal stability, limiting its industrial applications to a great extent.179 PPC is blended with several polymers, specifically with biodegradable polymers such as starch, PLA, PHB (polyhydroxyl butyrate), and chitosan to enhance the biodegradability, processability and material performance compared with individual polymers.177 Several studies have been done on preparing native corn starch/PPC blends, and the results revealed that the thermal and mechanical properties were improved due to the hydrogen bonding between the hydroxyl group of starch and the carbonyl group of PPC.180–182 However, it was found that on increasing the starch content, the mechanical properties of the blends were decreased as this weakened the interfacial bonding and compatibility of starch and PPC.180,182 Thus, plasticization of starch and blending with PPC is a great method to enhance the compatibility of the starch/PPC blend, where it disintegrates granules and overcomes the intermolecular interaction of starch.The use of reactive compatibilizers was also an effective method to improve the compatibility between starch and PPC. Ma et al.183 prepared blend compositions of PS/PPC and succinic anhydride-compatibilized PS/PPC. It was found that the incorporation of succinic anhydride as compatibilizer in the blend improved the adhesion between PPC and PS and the mechanical properties of the blends, which was attributed to the chemical interaction between the hydroxyl group of PS and the anhydride group of succinic anhydride. In addition, modification of starch through oxidation or grafting or esterification reactions was also found effective in attaining good interfacial interaction and compatibility during blending with PCL.179,184,185
Recently, much research has focused on the development of oxidised starch, where hydrophilic OH– groups of starch are converted into hydrophobic CO– groups that enhance the compatibility during blending with other degradable polymers. Fig. 14 shows the oxidation process of starch and hydrogen bonding interaction in PPC and thermoplastic oxidized starch (TPOS).179 The initial structure shows the hydrogen bonds present in native starch (in the figure shown as TPS) and is represented as hydrogen bonding 1. Blending native starch with MA-grafted PPC would usually result in intermolecular and intramolecular hydrogen bondings, represented as hydrogen bonding 1(H-bonding 1) and hydrogen bonding 2 in the figure. The presence of hydrogen bonding 1 in MA-grafted PPC/native starch blend leads to agglomeration and results in poor compatibility between the polymers in the blend. The conversion of native starch to TPOS replaces the hydroxyl group of starch with carbonyl groups and aldehyde groups; further blending TPOS with MA-grafted PPC reduces the formation of hydrogen bonding 1 and results in the formation of hydrogen bonding 2 and hydrogen bonding 3, as shown in the figure. Thus, the oxidation of starch weakens the formation of hydrogen bonding 1 and decreases agglomeration, thereby improving the interfacial interaction between PPC and starch. In the study conducted by Jiang et al.,179 TPOS and aluminic ester-grafted thermoplastic oxidized starch were melt blended with MA-grafted PPC. Aluminic ester was introduced as the coupling agent for oxidised starch to improve the interfacial adhesion between PPC and starch. The aluminic ester-grafted thermoplastic oxidized starch exhibited good dispersion of TPOS in MA-grafted PPC and showed an increase in thermal and TS of the blend.
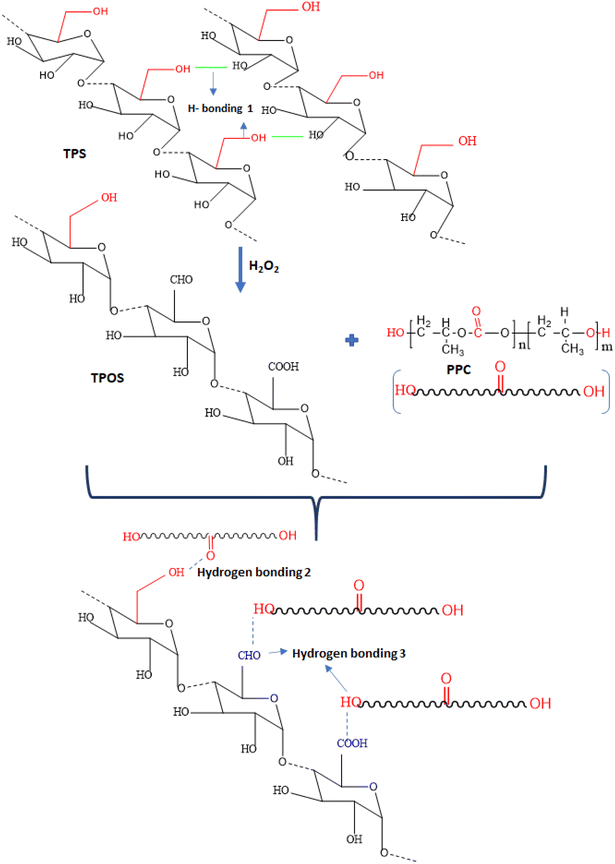 | ||
| Fig. 14 Hydrogen bonding interaction between PPC and TPOS (molecular structures have been redrawn by author using ChemDraw, from ref. 179 with permission from Taylor & Francis, copyright 2017). | ||
6.4 Polyhydroxyalkanoate/PS blends
PHAs are a group of thermoplastic polyesters derived from bacteria by the microbial fermentation process. They are crystalline, biodegradable, non-toxic, UV-resistant materials with good physical and chemical properties that are promising materials for single-use packaging applications.186 However, lower mechanical properties and poor thermal processability constrain their applications. PHB and PHBV (poly(hydroxyl butyrate-co-valerate)) are the commonly used PHA-based polymers. PHB is a homopolymer with higher degree of crystallinity (up to 70%) and efficient biodegradability in aerobic and anaerobic conditions. With higher crystallinity PHB exhibits good mechanical properties that are similar to polyethylene, and it is used for food packaging applications. In order to improve the low thermal stability, brittleness, and inadequate barrier properties, PHB is usually blended with low-melting biodegradable polymers. Thus, blending with plasticized starch is a method to improve the properties of both polymers and the resultant material would be completely biodegradable. Although several studies have been conducted on blending starch with PHA, most of them resulted in brittle materials with low elongation.187,188Lai et al.189 initially studied the possibility of PS/PHA blend and the effect of the degree of gelatinisation of starch on the mechanical properties of the blend. Different gelatinisation degrees of 25% and 33% were analysed with glycerol as plasticizer. The study revealed that lower gelatinisation degree and an increase in the concentration of PHA have a significant effect on mechanical properties such as TS and tear strength due to the dissipation of energy during the crystalline deformation of PHA and improved interaction during blending with PS.
The concentration of amylose and amylopectin content varies with starch source and determines the crystallinity of starch. High-amylose starch is more efficient for film preparation as it has a linear structure with more amorphous regions that ease gelatinisation. The study conducted by Parulekar et al.112 analysed the effect of plasticization of high-amylose starch with glycerol blended with PHA in the presence of compatibilizer. It was found that blending high-amylose PS with PHA was effective in reducing the moisture uptake and retrogradation of starch, and enhanced elongation. However, the major barrier for the PS/PHA blend is the formation of heterogeneous blends due to the higher crystallinity of PS and PHA. Thus, recently Florez et al.190 found that the active use of plasma treatment on PHB material and blending with PS matrix improved the mechanical properties of the blend, as this increases the interaction and changes the crystallinity of the blends compared with untreated PS/PHA blend.
6.5 Poly(vinyl alcohol)/PS blends
PVA is a biodegradable semicrystalline polymer consisting of 1,2-diol units or 1,3 diol units according to the degree of hydrolysis of poly(vinyl-acetate).63 The existence of a substantial number of hydroxyl groups on the surface of PVA can ease the preparation of blends and composites of PVA, which could be an ideal material for packaging applications.191 Blending of PVA with economically viable PS can reduce the cost and increase the biodegradability of the polymer. Integration of PVA into starch modifies the structure of the polymer at molecular and morphological levels to augment the mechanical and thermal properties of the starch material.63 Conventional plasticizers like glycerol and water,192 sorbitol,193 citric acid,193,194 urea195 and complex plasticizer196 are effectively employed in the preparation of PS/PVA blends.An earlier study conducted by Liu et al.192 on melt-blending starch with PVA using glycerine and water as plasticizer found that good processability, rheological and mechanical properties were attained for an optimum plasticizer composition of glycerine and water in a ratio of 50/50. Mao et al.197 studied the effect of PVA concentration on PS/PVA blend where the starch plasticized with glycerol was blended with PVA and samples were tested at 50% relative humidity. It was found that the incorporation of PVA increased the TS and EB of PS. The improvement in the mechanical properties of these blends was ascribed to the effect of intermolecular and intramolecular hydrogen bonding between PS and PVA that resulted in a more efficient compatibility among components.
Park et al.193 analysed the effect of different plasticizers, namely glycerol, sorbitol, and citric acid for PS/PVA blend for film casting. The characteristic results of these blends illustrated that the integration of citric acid provides superior properties than glycerol and sorbitol due to the formation of intermolecular and intramolecular hydrogen bonding between starch, PVA and citric acid, specifically at low-temperature drying of the film (at 5 °C). Later, in another analysis, Shi et al.194 investigated the effect of varying the concentration (5 to 30 wt%) of citric acid on the structural and physical properties of solvent-casted PS/PVA film at 140 °C. They reported that the increase in citric acid concentrations from 5 to 30 wt% decreased the water absorption from 33% to 20%. In addition, the mechanical and thermal properties were improved, where EB increased gradually from 102% to 208%. This may be due to the presence of multiple carboxylic groups in citric acid and esterification resulting in the formation of a chemically crosslinked blend with strong hydrogen bonds, which improved the thermal stability and mechanical properties of the PS/PVA blend film. Recently Kahvand and Fasihi63 investigated the effect of change in concentration and plasticizing effect of citric acid in a CAPS/PVA blend plasticized with water/glycerol/citric acid by the extrusion technique. The FTIR analysis of PVA and the CAPS/PVA blend showed a characteristic reduction in the PVA hydroxyl functional group frequency peak from 3600 cm−1 to 3400 cm−1, and formation of dual characteristic peaks at 1031 cm−1 and 995 cm−1, and the broadening of the peak in CAPS/PVA blends is attributed to the confirmation of the development of stronger hydrogen bonds between starch and PVA in the blend systems than in CAPS. Moreover, the XRD analysis of CAPS/PVA blends demonstrated the elimination of PVA crystal structural peaks in the blend with less than 50% PVA, representing the higher compatibility between PVA and starch in CAPS/PVA blend systems. The above study also states that PVA acted as a polymer-based plasticizer for starch; at a concentration above 10% and below 50% it reduced the Tg and storage modulus of the blends, whereas at below 10% PVA, it caused an anti-plasticization effect on the blend by forming a miscible structure and thereby increasing the Tg and storage modulus.
In most of the research on starch/PVA blends, the use of a complex plasticizer system has shown continuous phase morphology that resulted in good rheological and mechanical properties. Zhou et al.196 used the mixture of glycerol and urea as a complex plasticizer for a PS/PVA blend. The results revealed that the complex plasticizer can form more stable and strong hydrogen bonding with starch, PVA and water molecules than glycerol as a single plasticizer.
Another aspect to consider in blending starch with PVA is the hydrophilic nature of PS/PVA blend, which has shown susceptibility to relative humidity; on increasing the relative humidity, TS of the blend was inversely affected. To improve the mechanical and water barrier properties of the PS/PVA blend, different chemical and physical modifications during or after blending are used. Ramaraj et al. and Yoon et al.198,199 evaluated the effect of glutaraldehyde as a crosslinking agent in PS/PVA blend. Their results showed that, along with strong adhesion between PS and PVA, crosslinking at the interface of PVA and PS due to the presence of glutaraldehyde improved the mechanical properties such as TS and Young's modulus and solubility resistance.
6.6 Poly(butylene succinate)/PS blends
PBS is an aliphatic polyester derived from the polycondensation polymerisation of succinic anhydride and 1,4-butanediol.200 Lately, succinic anhydride has been derived from renewable resources that could essentially reduce the carbon footprint of PBS without loss of its overall performance.201 As a biodegradable polyester, PBS has great applications in the field of packaging films, textiles, and injection-moulded products. Besides biodegradability, these thermoplastic polymers have good melt processibility, chemical and water resistance, thermal and dimensional stability, and excellent mechanical properties.202 There is plenty of research on blending PBS with TPS to enhance the properties and processability of the polymers.Usually, the blending of hydrophilic natural polymers and hydrophobic thermoplastic polyesters would result in thermodynamically immiscible blends with poor adhesion and polymer performance. Thus, blending of these polymers was carried in the presence of suitable compatibilizers or by chemical modifications. In earlier studies, Zeng et al.202 synthesized reactive PBS having terminal NCO– group by reacting hydroxyl-terminated PBS with toluene-2,4-diisocyanate (TDI) and used this as reinforcing phase in PS/reactive PBS blend by reactive extrusion technology. This methodology has strengthened the tensile properties of the PS and resulted in low water absorption for the blend. In contrast, Li et al.203 evaluated the effect of different starch types such as waxy starch (0% amylose) and normal starch (26% amylose) on blending with PBS. This study revealed that the utilization of waxy starch eased the plasticization and processability of the blend. The presence of highly branched amylopectin in waxy starch hindered the absorption of water molecules, and further addition and increase in the concentration of PBS improved the tensile strength and water resistance properties of these blends. On comparing plasticization of starch with ionic liquid and commercial plasticizer glycerol, XRD and SEM analysis of 1-butyl-3-methylimidazolium chloride, ionic liquid plasticized starch (ILPS) showed the destruction of more crystalline phase in starch and resulted in a higher plasticization effect than glycerol.204 Thus, better dispersion of ILPS was achieved in ILPS/PBS blend, which exhibited an effective increase in TS and EB compared with the glycerol-plasticized starch/PBS blend.
7. Starch-based bio composites and bio nanocomposites
The major drawbacks of PS as a matrix material for packaging application are its high water absorption and lower mechanical properties.74 The hydrophilic nature of PS and other properties like surface tension, higher moisture absorption and thermal properties, especially Tg, further influence the applications.99 Possible approaches to enhance the properties of PS are blending with biodegradable polymers,167 incorporation of crosslinking agents199 or preparing multilayer films.205 In addition to the above techniques, the use of natural fibres/fillers or organic fillers as reinforcing material could enhance the TS, lower the density, and reduce energy consumption.206 The utilization of natural fibres over synthetic fibres improves biodegradability and minimizes health and environmental hazards. Besides, the similarity in chemical structure of plant fibres and starch offers good compatibility between matrix and reinforcing fibre. It has been found that the addition of cellulose fibres or micro fibres to PS improved the water resistance and thermal resistance of the biocomposite due to the higher crystallinity and thermal resistance of cellulose fibres compared with starch.206 The study conducted by Campos et al.207 on the effect of raw and chemically treated oil palm mesocarp fibres as reinforcement for PS proved that even with raw fibres, the composite system could achieve good intermolecular interactions and superior mechanical properties compared with treated fibres. This is because of the van der Waals force of interaction between the silica group of fibre and –OH group of starch. Lately, there have been studies on using different morphologies of PVA, such as microspheres and electrospun fibres, as a reinforcement for PS-based composites.208–210 The results showed that the addition of a lower concentration of these microspheres and fibres effectively enhanced the mechanical properties of the biocomposites.Fitch-Vargas et al.211 developed an environmentally friendly biocomposite material from acetylated sugar cane fibre-reinforced acetylated corn starch in the presence of glycerol as plasticizer. The optimum concentrations of 12.0 wt% fibre and 24 wt% glycerol content formed better interactions and shown better mechanical properties and water resistance. The fibre and matrix chemical surface modification and plasticizer content influenced the formation of more bonding between matrix and fibre than matrix–matrix interaction and enhanced the elongational properties of the composition. The researchers proposed this composition as an alternative to fossil fuel-based packaging material. Later Ferreira et al.212 developed biodegradable trays for food packaging applications from cassava starch and different agricultural residues such as sugarcane bagasse, cornhusk, malt bagasse, and orange bagasse. The resultant trays showed higher rigidity and complete biodegradation in a 60-day period compared with expanded polystyrene trays.
Interfacial tension or surface tension is a significant parameter for surface wettability. PS exhibits higher wettability that limits its packaging applications. Thus, the incorporation of fillers such as talc,213 calcium carbonate,214 clays215 and cellulose216 is widely studied. However, reinforcing micro fillers into the biopolymer matrix could reduce the mechanical properties of the biocomposites, as they act as a stress concentrator in the system. This could be minimized by reducing the particle size of fillers to the nano range. Furthermore, the integration of nano fillers such as chitin nano particles or cellulose nanocrystals could improve the surface wettability and water resistance due to the formation of a difficult tortuous path for the penetrant molecules.217 Recently, starch-based bio nanocomposite films were developed by incorporating different starch nano particles with a high surface to volume ratio; these films showed a reduction in water vapour transmission rate due to hindrance of the polymer chain moment.217 Some of the research on the preparation of starch-based bio/nano/hybrid composites developed from organic fillers and fibres with different starch sources and plasticizers is shown in Table 3.
| Starch source (plasticizer) | Polymer/reinforcement (natural fibre dimension) | Fabrication technique | Observation | Ref. |
|---|---|---|---|---|
| Corn (glycerol and natural rubber latex) | Sisal, hemp (10–15 mm) | Melt blending | Increase in fiber content improved the Tg, Young's modulus, and TS but reduced EB | 218 |
| The incorporation of rubber latex as plasticizer enhanced water resistance without affecting mechanical properties | ||||
| Corn starch (glycerol) | Poly(vinyl alcohol) fiber | Compression molding | Multilayer structure | 208 |
| Higher mechanical properties than PP and PE | ||||
| Orientation of fiber in same direction reduced stress concentration and improved mechanical properties | ||||
| Maize starch (glycerine) | Flax fiber | Extrusion | Higher impact strength and flexural strength at 20 wt% flax fiber addition | 219 |
| Fully biodegradable under aerobic condition and could be used for biogas production | ||||
| Rice (glycerol) | Cotton fiber (2.11 and 5.27 mm) | Compression molding | Improved thermal stability and Young's modulus and reduced water absorption | 220 |
| Smaller fibers with higher aspect ratio exhibit better properties | ||||
| Arrowroot starch (glycerol) | Arrowroot fiber | Solution casting | Effective stress transfer between matrix and fiber | 221 |
| Improved TS and tear strength | ||||
| Moderate resistance to water absorption on fiber loading | ||||
| Cassava (sorbitol and glycerol) | Cellulose nanocrystal from kenaf fiber (70–190 nm) | Solution casting | Crystallinity and TM of the composite have been improved | 222 |
| Good interfacial interaction between matrix and filler | ||||
| Sugar palm starch (sorbitol and glycerol) | Nanocrystalline cellulose from sugar palm | Solution casting | Improved mechanical, thermal and water barrier properties | 223 |
| Good dispersion and adhesion of filler with matrix due to H-bonding interaction between components | ||||
| Potato starch (glycerol) | Kaolin clay | Casting method | Increased water barrier, wettability and surface hardness properties on filler addition | 224 |
| Reduced mechanical properties | ||||
| Sugar palm starch (sorbitol and glycerol) | PLA/sugar palm nanocellulose | Compression molding | Increase in PS content reduced mechanical properties and water absorption | 225 |
| Low interaction between PS and PLA in absence of compatibilizer | ||||
| Pea starch (glycerol and water) | PCL/flax fiber | Compression molding | Poor compatibility and interfacial adhesion between PS and fibers | 226 |
| Presence of PCL improved TS and moisture absorption in composite system | ||||
| Cassava starch (glycerol) | PLA/PBAT/jute fiber | Extrusion | Increase in fiber content improved TM and crystallinity of the composite | 227 |
| Jute fiber acted as a nucleating agent for PLA and compatibility promoter between PS and PLA/PBAT/jute |
8. Global production and market analysis of TPS as packaging material
Thermoplastic starch-based films have been developed and marketed from the year 1999.228 The starch-based products were mainly developed by blending with polyesters such as PCL to develop biodegradable and compostable materials. Primarily, starch-based carry bags were introduced in the markets of Scandinavian and Mediterranean countries for collecting organic waste, where the waste management was well organized.229 These bags showed properties similar to the bags developed from LDPE material. However, the high cost and water permeability of biobased or biodegradable food packaging materials have limited their marketability in the food packaging sector, although the breathability of starch-based films has found applications in fruit, vegetable and bread packaging.Recently, Mordor Intelligence230 assessed the impact of COVID-19 in a growth market on the trends and forecast of thermoplastic starch manufactured by injection moulding and extrusion for bags, films, and other applications in the geographical areas of Asia-Pacific, North America, Europe, and the rest of the world in the period 2020–2025. The global production of the TPS market was about 179.58 kilo metric tons in the year 2019 and is anticipated to reach around 255.82 kilo metric tons in 2025 with a compound annual growth rate of 7.01%.230,231 The expected fastest CAGR hike rate of thermoplastic starch is due to environmental regulations and the ban of single-use plastics in the Asia-Pacific region.230 There are companies such as Novatec, Plantec, Biome, and Biotec that are focused on developing TPS for packaging applications.
Novamont is one of the largest producers of thermoplastic starch-based blends and composites, and has more than 1000 patents related to starch-based composite technology. Corn starch-based Novamont products are sold under the brand name MATER-BI®. The products developed from this material have properties similar to those of traditional polymers. They have good processability, compostability, antistatic property and mechanical properties that vary from soft to tough or rigid according to the composition.232 Biome Bioplastics (https://biomebioplastics.com/) is a branch of United Kingdom-based developer Stanelco, which develops highly functional plant-based bioplastics which are biodegradable and compostable. In collaboration with German company Biotec (https://en.biotec.de/), they develop different bioplastic resins and possess a large number of patents on development of TPS.232 Among a large range of products developed and sold through Biotec and Biome, BiomeEP1 (potato starch based) and BiomeEP2 (corn starch based) are flexible film resins without plasticizers developed by Biome that have improved adhesion and printability with good strength, flexibility, and tear strength. Similarly, Bioplast GF106/2 is a plasticizer-free potato starch-based industrial compostable resin developed by Biotec suitable for blown film extrusion processing for packaging applications. Tecnaro (https://tecnaro.de/en/) is another German company developing bioplastics resins containing more than 50% plant-based materials such as starch by grafting technology. ARBOBLEND® is a 100% biobased blend developed by Tecnaro from different biopolymers, organic additives and natural fillers, and is an efficient resin material for developing films and packaging products. Furthermore Cereplast, Inc. (https://www.cereplast.com), BIOP Biopolymer Technologies (https://www.biop.eu), and Plantic Technologies (https://www.plantic.com.au) are a few of the other companies developing starch and thermoplastic-based resins for packaging and other applications. In addition, major TPS-based companies and their products are shown in Table 4.
| Company producing TPS | Product name | Source material | Total production per year | Place/country | Applications | Ref. |
|---|---|---|---|---|---|---|
| Novamont | MASTER B | Starches, cellulose, vegetable oils | 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes 000 tonnes |
Novara/Italy | Agriculture, packaging, organic waste collection bags, food service, carrier bag | 233 |
| BioLogiQ Inc. | NuPlastiQ (Pellets) | Potato starch | 2 million tons | Hong Kong/China | Flexible bags, pouches, jugs, handle bags, trash bags, agricultural & industrial films | 234 |
| Biotec | BIOPLAST 300, BIOPLAST 400, BIOPLAST 500, BIOPLAST GF106/02 | Potato starch and other biopolymers | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons 000 metric tons |
Germany | Blown film extrusion | 235 |
| Kuraray | Plantic | Corn starch | — | Japan | Multilayer film, gas and aroma barrier film, packaging applications | 236 |
| Biome Bioplastics Limited | BiomeEP1 | Potato starch | — | UK | High-speed, full color print applications or lamination film | 237 |
| BiomeEP2 | Corn starch | |||||
| Agrana Beteiligungs | AGENACOMP, AMITROPLAST | Thermoplastic starch and biodegradable polyester | — | Austria | Carrier bags, fruit and vegetable bags, bio-waste bag, mulch film, non-woven fibers | 238 and 239 |
9. Challenges and real-life applications for starch-based materials
The dreadful effect of fossil fuel-based packaging material on the marine and terrestrial environment from depositing of waste is alarming to human and environmental health. The development of biodegradable and sustainable packaging material, especially for certain single-use plastic, would be a probable solution to reduce the rapid increase in packaging waste and its discarding.240 Starch-based blends and composites are the preferred material for certain packaging applications over synthetic and natural polymer materials, owing to their aptitude to meet commercial and ecological demands. Natural and mineral filler reinforcement is an excellent technique to improve the mechanical and water permeability of thermoplastic starch composites. Different studies have stated that the incorporation of cellulose fillers in PS not only enhanced the mechanical properties but also improved the moisture barrier properties of the starch-based composites.241,242 The decrease in moisture permeability of composite is due to the decrease in diffusion coefficient that is imposed by the cellulose crystals by forming hydrogen bonding with starch, and further due to the decrease in sorption of the penetrant.243Antimicrobial property is an integral part of packaging material in improving the shelf life of food products by preventing the growth of microbes on the surface of the food. To obtain efficient antimicrobial property biologically active agents are usually incorporated into the matrix material, such as selenium nanoparticles, essential oils etc.244 Selenium nanoparticles, derived from plant extracts or microorganisms, with properties such as antioxidant, anti-microbial, and anti-fungal characteristics and used for food packaging applications would extend the shelf-life of food products. However, there are no studies on selenium nanoparticles-incorporated starch composites for packaging applications. Essential oils are another widely used material for achieving antifungal and antimicrobial properties. Campos-Requena et al.215 used carvacrol, a natural phenolic compound, in PS/layered silicate bio nanocomposite film for an antimicrobial packaging application. The study analysed the effect of nano clay and plasticizer concentration in obtaining efficient Young's modulus and thermal resistance for packaging applications. The results showed positive change with nano clay and negative effect with plasticizer concentration that indicates the formation of intercalated or exfoliated structure in the composite with a higher concentration of silicate particles. Besides, the addition of nano clay improved the half-life of the essential oil by the optimal release of an antimicrobial agent through the difficult tortuous path. In another study, PS and PCL multilayer films were developed by Ortega-Toro et al.245 with spray coating of potassium sorbate on interlayers to plasticize and augment the adhesion between the films. This technology enhanced the mechanical, oxygen and water barrier and antimicrobial properties of the multilayer film due to the interaction between potassium sorbate with starch and PCL, and the film is an efficient material for packaging applications.
Starch foams are another material extensively used for packaging applications. Starch foams are usually prepared by softening and expanding the starch with water as blowing agent at high temperature and pressure.232 The optimum concentration of water content for foam preparation is between 16% and 18%, whereas above and below this concentration would change the foam cell structure from open to closed.246 The starch foam is an excellent replacement for polystyrene foam owing to its higher shock absorption, and antistatic, insulation and biodegradability property. However, the low mechanical strength of starch foam limits its application in food packaging. Thus, different studies have been conducted on improving the mechanical properties of starch foam by incorporating diverse agricultural residues such as malt bagasse,247 kraft fiber & chitosan,248 sesame cake,249 cotton fibre & natural rubber,250 fish scale,251 and grape stalks252 as reinforcing fillers for foam production. The integration of natural reinforcement has shown similar mechanical properties to those of commercial petroleum-based foams, and further enhances the biodegradation of the amorphous form of material. Reinforcement such as sesame cake contains a higher concentration of fibres and proteins (22.7% of fibres and 35% of proteins) that makes it ideal for food processing and in developing starch-based composites.249,253 In addition, the presence of lipids in the hydrophobic components in sesame cake or palm oil improves the moisture resistance of the starch-based composite materials.254 The above studies state that the mentioned compositions are ideal for short-term and low moisture content packaging applications such as dry foods.
Companies such as Novamont, Kuraray, Biotec etc. are leading manufacturers of thermoplastic starch-based blends and composites. These starch-based composite products have been used to develop materials such as thermoformed trays, films, foams, and for coatings. The materials developed from these manufacturers have been successfully commercialized in packaging products and are shown in Fig. 15. The commercialized products are classified here into 3 categories: films, foams and thermoformed products. The first category of commercialized starch-based film products includes food packaging films, stand-up pouches, skin packaging (a customized thin film on products, such as in dairy products, that allows product visibility, protection from the environment and minimizes tampering of products) and carry bags/garbage bags. The second category, foams, includes fruit trays, cushion foam, and packing peanuts that are used for protective packaging during shipping and transportation. The third category includes thermoform-processed products such as chocolate trays, cookie trays, coffee trays and meal/meat trays etc.
 | ||
| Fig. 15 Real-life applications of starch-based materials classified as: (i) films which includes (a) food packaging films, (b) stand-up pouches, (c) skin packaging and (d) bags/waste bags; (ii) foams which includes (e) fruit trays, (f) cushion foam, (g) packing peanuts; (iii) thermoforms which includes (h) meal/meat trays, (i) coffee trays, (j) cookies trays, (k) chocolate trays. ((a) (Reproduced from ref. 255 with permission from Elsevier, copyright 2019), (b) and (h) pixabay free images, pictures (e), (f), (g), (j), (k) have been taken from open access article under a Creative Commons licence attribution-non-commercial-no derivatives 4.0 international ref. 246, picture (i) from “Icons” of Microsoft office.) | ||
10. Conclusions
Biobased blends and composites derived from starch are an efficient replacement for petroleum-based products in single-use plastic packaging applications. The difficulty in using native starch for the development of biodegradable processed plastic products has been rectified by blending with other degradable polymers and/or by reinforcing with natural fibres/fillers or with organic fillers. The inherent hydrophilic and crystalline characteristics of starch have reduced the thermomechanical and barrier properties of the resultant starch-based blends and composites due to the inefficiency in achieving intermolecular interaction between blended polymers and reinforcements. Plasticization is an efficient methodology to enhance the thermal resistance and processability of starch by improving the intermolecular adhesion. The plasticization of starch has opened the window towards product development for the packaging industries. However, the performance limitation of starch-based blends and composites compared with the industrial standards that have been developed from petroleum-based products over the past decades is a serious challenge. Different techniques have been used to develop high-performance products from starch that are comparable with petroleum-based products. Functional techniques include the integration of compatibilizers, coupling agents, sole plasticizers, multiple plasticizers, bioactive agents and essential oils. Recently, research has proved that the presence of multiple plasticizers is an effective method to achieve the desired properties for starch-based blends and composites. Glycerol has been a widely used plasticizer for starch; however, the use of a combination of glycerol with an organic acid such as citric acid or tartaric acids could be better for minimizing the retrogradation and aging of the starch system and to reinforce plasticization efficiency. Most of the starch-based biodegradable blends have been prepared with glycerol-plasticized starch. Thus, there must be more research on using multiple plasticizers with starch-based biodegradable blends. Bioactive agents and essential oils are further found to be competent in bringing antimicrobial strength to the TPS blends. Selenium nanoparticles are bioactive agents that have been productively studied with cellulose-based blends and composite systems for packaging applications; however, studies on starch are limited. Similarly, more research must be conducted with essential oils, as these could provide better performance characteristics for starch-based blends and composites for packaging applications, and could be a replacement for harmful compatibilizers and plasticizers.Research has further proved that the presence of compatibilizers in TPS blended with fillers and fibres is effective. Maleic anhydride-grafted biobased polymers blended with PS and maleic anhydride-grafted starch blended with other biobased polymers were additionally found to be effective in developing biobased blend systems for packaging applications. Another aspect to consider is the advancement of starch-based bio nanocomposite and agro waste-based composites in single-use packaging applications. Since starch is an abundant material obtained from natural resources, deriving starch from agro-waste products and utilizing it in developing starch-based blends and composite would be cost effective and would minimise agri-food waste production.
Multilayer films are a new and innovative technology; multilayer films could be developed with minimum material and better mechanical and barrier properties. TPS blend material coated with hydrophobic polymer layers could minimise the hydrophilic characteristics of the blend and would be useful for packaging film applications such as food packaging, garbage bags, carry bags etc. More studies on developing multilayer films from thermoplastic starch formulations with better processability, wettability, and biodegradability that could meet a wide range of packaging applications are needed in the future.
Abbreviations
| AFM | Atomic force microscopy |
| CAGR | Compound annual growth rate |
| CAPS | Citric acid plasticized starch |
| DMA | Dynamic mechanical analysis |
| DP | Degree of polymerization |
| EB | Elongation at break |
| EGPS | Ethylene glycol-plasticized starch |
| AED | Activation energy of decomposition |
| EVOH | Ethylene-vinyl alcohol |
| FTIR | Fourier transform infrared spectroscopy |
| GPS | Glycerol-plasticized starch |
| HONPS | Hydroxy propylated and oxidised potato starch |
| ILPS | Ionic liquid-plasticized starch |
| LDPE | Low density polyethylene |
| MA | Maleic anhydride |
| MDI | Methylene-diphenyl diisocyanate |
| MT | Million tonnes |
| PBAT | Poly(butylene adipate terephthalate) |
| PBS | Poly(butylene succinate) |
| PCL | Polycaprolactone |
| PHA | Poly(hydroxyalkanoates) |
| PHB | Polyhydroxy butyrate |
| PHBV | Poly(hydroxyl butyrate-co-valerate) |
| PHEE | Poly(hydroxyester ether) |
| PLA | Poly(lactic acid) |
| PPC | Poly(propylene carbonate) |
| PS | Plasticized starch |
| PVA | Poly(vinyl alcohol) |
| RT | Room temperature |
| SEM | Scanning electron microscopy |
| TDI | Toluene-2,4-diisocyanate |
| TEM | Transmission electron microscope |
| T g | Glass transition temperature |
| TGA | Thermogravimetric analysis |
| T m | Melting temperature |
| TM | Tensile modulus |
| T max | Temperature at the maximum rate of weight loss |
| TPOS | Thermoplastic oxidized starch |
| TPS | Thermoplastic starch |
| TS | Tensile strength |
| USD | United States dollar |
| Va | V-anhydrous |
| Vh | V-hydrate |
| WVTR | Water vapour transmission rate |
| WXRD | Wide-angle X-ray diffraction |
| XRS | X-ray scattering |
| XRD | X-ray diffraction |
Author contributions
Project conceptualization, methodology, administration, funding acquisition and supervision, A. K. M. and M. M.; methodology, investigation, data analysis, writing—original draft preparation, A. S.; writing—review and editing, A. K. M., M. M., Q. L. and A. S.; all authors contributed to the discussion, reviews and approval of the manuscript for publication.Conflicts of interest
The authors declare that they have no known conflict of interest that could have appeared to influence the work reported in this paper.Acknowledgements
The authors would like to acknowledge the financial support of (i) the Ontario Research Fund, Research Excellence Program; Round 9 (ORF-RE09) Ontario Ministry of Economic Development, Job Creation and Trade, Canada (Project No. 053970 and 054345); (ii) the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA), Canada/University of Guelph – HQP program; (iii) OMAFRA – Bioeconomy for Industrial Uses Research Program (Project No. 030251, 030486 and 030578); (iv) OMAFRA – Ontario Agri-Food Research Initiative (Project No. 055217) (v) the Agriculture and Agri-Food Canada (AAFC), Maple Leaf Food, Canada and Bank of Montreal (BMO), Canada through Bioindustrial Innovation Canada (BIC) Bioproducts AgSci Cluster Program (Project No. 054015, 054449 and 800148); and (vi) the Natural Sciences and Engineering Research Council of Canada (NSERC), Canada Research Chair (CRC) program Project No. 460788 to carry out this study. This research also benefited from the facility funding to the Bioproducts Discovery and Development Centre (BDDC) lab by FedDev Ontario; Ontario Ministry of Agriculture, Food, and Rural affairs (OMAFRA); Canada Foundation for Innovation (CFI); Federal Post-Secondary Institutions Strategic Investment Fund (SIF); and matching funds from the province of Ontario and numerous University of Guelph Alumni.References
- J. Muncke, PLos Biol., 2021, 19, e3000961 CrossRef CAS PubMed
.
- FICCI, A report on India's plastic industry, 2016.
- J. W. Han, L. Ruiz-Garcia, J. P. Qian and X. T. Yang, Compr. Rev. Food Sci. Food Saf., 2018, 17, 860–877 CrossRef PubMed
.
- B. Misgana and G. T. Tucho, Environ. Health Insights, 2022, 16, 117863022210850 CrossRef PubMed
.
- J. Ammendolia and T. R. Walker, Sci. Total Environ., 2022, 805, 149957 CrossRef CAS PubMed
.
- V. Siracusa, P. Rocculi, S. Romani and M. D. Rosa, Trends Food Sci. Technol., 2008, 19, 634–643 CrossRef CAS
.
- E. Watt, M. Picard, B. Maldonado, M. A. Abdelwahab, D. F. Mielewski, L. T. Drzal, M. Misra and A. K. Mohanty, RSC Adv., 2021, 11, 21447–21462 RSC
.
- B. Li, J. Liu, B. Yu and X. Zheng, IOP Conf. Ser.: Earth Environ. Sci., 2022, 1011, 012050 CrossRef
.
- O. Alam, M. Billah and D. Yajie, Resour., Conserv. Recycl., 2018, 132, 121–129 CrossRef
.
-
R. M. Miller, MSc Sustainable development, Universiteit Utrecht, 2012 Search PubMed
.
- S. Arora, Int. J. Chem. Stud., 2018, 6, 2411–2418 Search PubMed
.
- The international nova biopolymer expert group, for the first time: growth rate for bio-based polymers with 8% CAGR far above overall polymer market growth, 2021.
-
European Bioplastics, Bioplastics market development update 2021, 2021, vol. 2021 Search PubMed
.
-
D. Verma and E. Fortunati, Biobased and biodegradable plastics, 2019, vol. 4 Search PubMed
.
- D. R. Lu, C. M. Xiao and S. J. Xu, eXPRESS Polym. Lett., 2009, 3, 366–375 CrossRef CAS
.
- M. F. Ismail, A. H. Jasni and D. J. Ooi, Polym. Polym. Compos., 2020, 1–13 Search PubMed
.
-
M. F. Mullah, L. Joseph, Y. A. Arfat and J. Ahmed, Glass Transition and Phase Transitions in Food and Biological Materials, 2017, pp. 281–304 Search PubMed
.
- A. Nouri, M. Tavakkoli Yaraki, M. Ghorbanpour and S. Wang, Int. J. Biol. Macromol., 2018, 115, 227–235 CrossRef CAS PubMed
.
- A. Carolina, S. de Oliveira, L. Fonseca, F. Danielly, D. O. Begali and J. Cesar, J. Polym. Environ., 2021, 29, 2546–2556 CrossRef
.
- R. Priyadarshi, H. J. Kim and J. W. Rhim, Food Hydrocolloids, 2021, 110, 106155 CrossRef CAS
.
- I. G. Ruiz-Martínez, D. Rodrigue and J. Solorza-Feria, Polym. Bull., 2021, 79, 1437–1466 CrossRef
.
- S. A. A. Mohamed, M. El-Sakhawy and M. A. M. El-Sakhawy, Carbohydr. Polym., 2020, 238, 116178 CrossRef CAS PubMed
.
- C. Xia, W. Wang, L. Wang, H. Liu and J. Xiao, Food Packag. Shelf Life, 2019, 19, 76–85 CrossRef
.
- A. Bartos, K. Nagy, J. Anggono, Antoni, H. Purwaningsih, J. Móczó and B. Pukánszky, Composites, Part A, 2021, 143, 106273 CrossRef CAS
.
- A. K. Pal, F. Wu, M. Misra and A. K. Mohanty, Composites, Part B, 2020, 198, 108141 CrossRef CAS
.
- M. Asgher, S. A. Qamar, M. Bilal and H. M. N. Iqbal, Food Res. Int., 2020, 137, 109625 CrossRef CAS PubMed
.
-
M. E. Embuscado and K. C. Huber, Edible Films and Coatings for Food Applications, Springer, New York, 2009 Search PubMed
.
- P. Cazón, G. Velazquez, J. A. Ramírez and M. Vázquez, Food Hydrocolloids, 2017, 68, 136–148 CrossRef
.
- M. G. A. Vieira, M. A. da Silva, L. O. dos Santos and M. M. Beppu, Eur. Polym. J., 2011, 47, 254–263 CrossRef CAS
.
- B. T. Wang, S. Hu, X. Y. Yu, L. Jin, Y. J. Zhu and F. J. Jin, Polymers, 2020, 12, 530–547 CrossRef CAS PubMed
.
- R. P. Babu, K. O'Connor and R. Seeram, Prog. Biomater., 2013, 2, 8 CrossRef PubMed
.
- K. J. Falua, A. Pokharel, A. Babaei-Ghazvini, Y. Ai and B. Acharya, Polymers, 2022, 14, 2215 CrossRef CAS PubMed
.
- H. Charreau, M. L. Foresti and A. Vázquez, Recent Pat. Nanotechnol., 2013, 56–80 CrossRef CAS
.
- A. K. Mohanty, S. Vivekanandhan, J.-M. Pin and M. Misra, Science, 2018, 362, 536–542 CrossRef CAS PubMed
.
- N. Noshirvani, B. Ghanbarzadeh, C. Gardrat, M. R. Rezaei, M. Hashemi, C. le Coz and V. Coma, Food Hydrocolloids, 2017, 70, 36–45 CrossRef CAS
.
- B. Fathi Achachlouei and Y. Zahedi, Carbohydr. Polym., 2018, 199, 415–425 CrossRef CAS PubMed
.
- J. Vartiainen, C. Laine, P. Willberg-Keyriläinen, M. Pitkänen and T. Ohra-aho, J. Appl. Polym. Sci., 2017, 134, 44586 CrossRef
.
- U. Amin, M. U. Khan, Y. Majeed, M. Rebezov, M. Khayrullin, E. Bobkova, M. A. Shariati, I. M. Chung and M. Thiruvengadam, Int. J. Biol. Macromol., 2021, 183, 2184–2198 CrossRef CAS PubMed
.
-
P. Mischnick and D. Momcilovic, in Chemical Structure Analysis of Starch and Cellulose Derivatives, ed. D. Horton, Academic Press, 2010, vol. 64, pp. 117–210 Search PubMed
.
- Y. I. Cornejo-Ramírez, O. Martínez-Cruz, C. L. del Toro-Sánchez, F. J. Wong-Corral, J. Borboa-Flores and F. J. Cinco-Moroyoqui, CyTA – J. Food, 2018, 16, 1003–1017 CrossRef
.
- A. K. Mohanty, F. Wu, R. Mincheva, M. Hakkarainen, J. M. Raquez, D. F. Mielewski, R. Narayan, A. N. Netravali and M. Misra, Nat. Rev. Methods Primers, 2022, 2, 1–27 CrossRef
.
- B. Hassan, S. A. S. Chatha, A. I. Hussain, K. M. Zia and N. Akhtar, Int. J. Biol. Macromol., 2018, 109, 1095–1107 CrossRef CAS PubMed
.
- Y. Zhang, C. Rempel and Q. Liu, Crit. Rev. Food Sci. Nutr., 2014, 54, 1353–1370 CrossRef CAS PubMed
.
- N. Wang, J. Yu and X. Ma, Polym. Int., 2007, 56, 1440–1447 CrossRef CAS
.
- M. Stasiak, M. Molenda, I. Opaliñski and W. Błaszczak, Czech J. Food Sci., 2013, 31, 347–354 CrossRef
.
- E. M. Teixeira, A. L. da Róz, A. J. F. Carvalho and A. A. S. Curvelo, Carbohydr. Polym., 2007, 69, 619–624 CrossRef CAS
.
- F. J. Rodriguez-Gonzalez, B. A. Ramsay and B. D. Favis, Carbohydr. Polym., 2004, 58, 139–147 CrossRef CAS
.
- P. Forssell, J. Mikkilä, T. Suortti, J. Seppälä and K. Poutanen, J. Macromol. Sci., Part A:
Pure Appl. Chem., 1996, 33, 703–715 CrossRef
.
- O. Valerio, T. Horvath, C. Pond, M. Misra and A. Mohanty, Ind. Crops Prod., 2015, 78, 141–147 CrossRef CAS
.
- L. Ballesteros-Mártinez, C. Pérez-Cervera and R. Andrade-Pizarro, NFS J., 2020, 20, 1–9 CrossRef
.
- M. D. Hazrol, S. M. Sapuan, E. S. Zainudin, M. Y. M. Zuhri and N. I. A. Wahab, Polymers, 2021, 13, 1–22 CrossRef PubMed
.
- J. Viguié, S. Molina-Boisseau and A. Dufresne, Macromol. Biosci., 2007, 7, 1206–1216 CrossRef PubMed
.
- J. h. Yang, J. g. Yu and X. f. Ma, Carbohydr. Polym., 2006, 66, 110–116 CrossRef CAS
.
- D. Thirathumthavorn and S. Charoenrein, Starch/Staerke, 2007, 59, 493–497 CrossRef CAS
.
- X. Ma and J. Yu, Carbohydr. Polym., 2004, 57, 197–203 CrossRef CAS
.
- A. Baran, P. Vrábel, M. Kovaľaková, M. Hutníková, O. Fričová and D. Olčák, J. Appl. Polym. Sci., 2020, 137, 1–9 CrossRef
.
- X. Ma and J. Yu, J. Appl. Polym. Sci., 2004, 93, 1769–1773 CrossRef CAS
.
- Y. Zuo, J. Gu, H. Tan and Y. Zhang, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2015, 30, 423–428 CrossRef CAS
.
- J. L. Wang, F. Cheng and P. X. Zhu, Carbohydr. Polym., 2014, 101, 1109–1115 CrossRef CAS PubMed
.
- R. Shi, Z. Zhang, Q. Liu, Y. Han, L. Zhang, D. Chen and W. Tian, Carbohydr. Polym., 2007, 69, 748–755 CrossRef CAS
.
- Y. Jiugao, W. Ning and M. Xiaofei, Starch/Staerke, 2005, 57, 494–504 CrossRef
.
- Z. Q. Liu, X. S. Yi and Y. Feng, J. Mater. Sci., 2001, 36, 1809–1815 CrossRef CAS
.
- F. Kahvand and M. Fasihi, Int. J. Biol. Macromol., 2019, 140, 775–781 CrossRef CAS PubMed
.
- W. J. Orts, G. A. R. Nobes, G. M. Glenn, G. M. Gray, S. Imam and B. s. Chiou, Polym. Adv. Technol., 2007, 18, 629–635 CrossRef CAS
.
- S. H. D. Hulleman, F. H. P. Janssen and H. Feil, Polymer, 1998, 39, 2043–2048 CrossRef CAS
.
- E. U. Offiong and S. L. Ayodele, Int. J. Sci. Eng. Res., 2016, 7, 438–443 Search PubMed
.
- M. K. Marichelvam, M. Jawaid and M. Asim, Fibers, 2019, 7, 32 CrossRef CAS
.
- D. le Corre, J. Bras and A. Dufresne, Biomacromolecules, 2010, 11, 1139–1153 CrossRef CAS PubMed
.
-
A. J. F. Carvalho, in Biopolymers: Biomedical and Environmental Applications, ed. L. A. Kalia Susheel, 2011, pp. 81–95 Search PubMed
.
- Reportlinker, Global Starch Industry, 2021.
- Z. N. Diyana, R. Jumaidin, M. Z. Selamat, I. Ghazali and N. Julmohammad, Polymers, 2021, 13, 1396–1320 CrossRef CAS PubMed
.
- N. W. H. Cheetham and L. Tao, Carbohydr. Polym., 1998, 36, 277–284 CrossRef CAS
.
- Maximize Market Research, Global Starch Market: Industry Analysis and Forecast (2020–2026) by Source, Type, Firm, Product, Application and Region, 2021.
- F. Xie, P. Luckman, J. Milne, L. McDonald, C. Young, C. Y. Tu, T. di Pasquale, R. Faveere and P. J. Halley, J. Renewable Mater., 2014, 2, 95–106 CrossRef
.
-
C. B. Novara, V. B. Fontaneto D’Agogna, G. D. T. Sesto Calende and R. P. Oleggio, US Pat., 5462981078, 1992 Search PubMed
.
- G. T. Oostergetel and E. F. J. van Bruggen, Carbohydr. Polym., 1993, 21, 7–12 CrossRef CAS
.
- K. Petersen, P. v. Nielsen and M. B. Olsen, Starch/Staerke, 2001, 53, 356–361 CrossRef CAS
.
- C. G. Oates, Trends Food Sci. Technol., 1997, 8, 375–382 CrossRef CAS
.
- A. Ayoub, T. Ohtani and S. Sugiyama, Starch/Staerke, 2006, 58, 475–479 CrossRef CAS
.
- A. Imberty, H. Chanzy, S. Perez, A. Buleon and V. Tran, Macromolecules, 1987, 2, 1029 Search PubMed
.
- L. Kong, C. Lee, S. H. Kim and G. R. Ziegler, J. Phys. Chem. B, 2014, 118, 1775–1783 CrossRef CAS PubMed
.
- M. D. Hossen Beg, S. b. Kormin, M. Bijarimi and H. U. Zaman, J. Polym. Eng., 2015, 35, 793–804 CrossRef CAS
.
- S. C. Alcázar-Alay and M. A. A. Meireles, Food Sci. Technol., 2015, 35, 215–236 CrossRef
.
- S. Yoo and Y. H. Chang, Prev. Nutr. Food Sci., 2018, 23, 254–259 CrossRef CAS PubMed
.
- S. Mali, L. B. Karam, L. P. Ramos, M. Victoria and E. Grossmann, J. Agric. Food Chem., 2004, 52, 7720–7725 CrossRef CAS PubMed
.
- R. Tramonte and E. R. Amante, Food Chem. Toxicol., 2007, 45(11), 2273–2278 CrossRef PubMed
.
- J. Elisa, Int. Food Res. J., 2016, 21(4), 1641–1649 Search PubMed
.
- O. O. Oladunmoye, O. C. Aworh, B. Maziya-dixon, O. L. Erukainure and G. N. Elemo, Food Sci. Nutr., 2014, 2(2), 132–138 CrossRef CAS PubMed
.
- M. I. J. Ibrahim, S. M. Sapuan, E. S. Zainudin and M. Y. M. Zuhri, BioResources, 2019, 14, 6485–6500 CAS
.
- M. I. J. Ibrahim, S. M. Sapuan, E. S. Zainudin and M. Y. M. Zuhri, Int. J. Food Prop., 2019, 22, 925–941 CrossRef CAS
.
- A. Gevaudan, G. Chuzel, S. Didider and J. Andrieu, Int. J. Food Sci. Technol., 1989, 24, 637–645 CrossRef
.
- L. Avérous and C. Fringant, Polym. Eng. Sci., 2001, 41, 727–734 CrossRef
.
- G. Satyam, S. Shivani, G. Garima, S. Nitin and P. K. Sharma, Int. J. PharmTech Res., 2010, 2, 1508–1512 Search PubMed
.
- R. F. Tester, S. J. J. Debon, H. v. Davies and M. J. Gidley, J. Sci. Food Agric., 1999, 79, 2045–2051 CrossRef CAS
.
- Y. P. Chang, P. B. Cheah and C. C. Seow, J. Food Sci., 2000, 65, 445–447 CrossRef CAS
.
- K. Jagannadham and R. Parimalavalli, Int. Food Res. J., 2015, 22, 677–683 CAS
.
- K. Dome, E. Podgorbunskikh, A. Bychkov and O. Lomovsky, Polymers, 2020, 12, 641–654 CrossRef CAS PubMed
.
- F. J. Polnaya, Haryadi, D. W. Marseno and M. N. Cahyanto, Int. Food Res. J., 2013, 20(4), 1609–1615 Search PubMed
.
-
A. J. F. Carvalho, Monomers, Polymers and Composites from Renewable Resources, 2008, pp. 321–342 Search PubMed
.
- B. R. Mose and S. M. Maranga, J. Mater. Sci. Eng. B, 2011, 1, 239–245 CAS
.
- E. Ogunsona, E. Ojogbo and T. Mekonnen, Eur. Polym. J., 2018, 108, 570–581 CrossRef CAS
.
- F. M. Fakhouri, S. M. Martelli, T. Caon, J. I. Velasco and L. H. I. Mei, Postharvest Biol. Technol., 2015, 109, 57–64 CrossRef CAS
.
- H. Neetoo, M. Ye and H. Chen, Int. J. Food Microbiol., 2010, 136, 326–331 CrossRef CAS PubMed
.
- M. Rodríguez, J. Osés, K. Ziani and J. I. Maté, Food Res. Int., 2006, 39, 840–846 CrossRef
.
- D. Lourdin, G. della Valle and P. Colonna, Carbohydr. Polym., 1995, 27, 261–270 CrossRef CAS
.
- P. Forssell, R. Lahtinen, M. Lahelin and P. Myllärinen, Carbohydr. Polym., 2002, 47, 125–129 CrossRef CAS
.
- G. F. Mehyar and J. H. Han, J. Food Sci., 2004, 69, 449–454 CrossRef
.
- X. F. Ma, J. G. Yu and J. J. Wan, Carbohydr. Polym., 2006, 64, 267–273 CrossRef CAS
.
- J. A. Ratto, P. J. Stenhouse, M. Auerbach, J. Mitchell and R. Farrell, Polymer, 1999, 40, 6777–6788 CrossRef CAS
.
- B. Y. Shin, S. i. Lee, Y. S. Shin, S. Balakrishnan and R. Narayan, Polym. Eng. Sci., 2004, 44, 1429–1438 CrossRef CAS
.
- C. Ge, B. Lansing and C. L. Lewis, Food Packag. Shelf Life, 2021, 27, 2214–2894 Search PubMed
.
- Y. Parulekar and A. K. Mohanty, Macromol. Mater. Eng., 2007, 292, 1218–1228 CrossRef CAS
.
- M. L. Sánchez, W. Patiño and J. Cárdenas, J. Build. Eng., 2020, 28, 2352–7102 Search PubMed
.
- X. L. Wang, K. K. Yang and Y. Z. Wang, J. Macromol. Sci., Polym. Rev., 2003, 43, 385–409 CrossRef
.
- X. F. Ma, J. G. Yu and Y. B. Ma, Carbohydr. Polym., 2005, 60, 111–116 CrossRef CAS
.
- M. Huang, J. Yu and X. Ma, Polym. Degrad. Stab., 2005, 90, 501–507 CrossRef CAS
.
- H. L. Abd El-Mohdy, E. A. Hegazy, E. M. El-Nesr and M. A. El-Wahab, Arabian J. Chem., 2016, 9, S1627–S1635 CrossRef CAS
.
- P. A. Perry and A. M. Donald, Biomacromolecules, 2000, 1, 424–432 CrossRef CAS PubMed
.
- R. C. R. Souza and C. T. Andrade, Adv. Polym. Technol., 2002, 21, 17–24 CrossRef CAS
.
- P. Tummala, W. Liu, L. T. Drzal, A. K. Mohanty and M. Misra, Ind. Eng. Chem. Res., 2006, 45, 7491–7496 CrossRef CAS
.
- J. J. G. van Soest, K. Benes, D. de Wit and J. F. G. Vliegenthart, Polymer, 1996, 37, 3543–3552 CrossRef CAS
.
- R. Thakur, P. Pristijono, J. B. Golding, C. E. Stathopoulos, C. Scarlett, M. Bowyer, S. P. Singh and Q. v. Vuong, Starch/Staerke, 2017, 69, 1700099 Search PubMed
.
- S. Wang, C. Li, L. Copeland, Q. Niu and S. Wang, Compr. Rev. Food Sci. Food Saf., 2015, 14, 568–585 CrossRef CAS
.
- J. Huang, Z. Wang, L. Fan and S. Ma, Int. J. Biol. Macromol., 2022, 203, 130–142 CrossRef CAS PubMed
.
- Z. Q. Fu, L. J. Wang, D. Li, Q. Wei and B. Adhikari, Carbohydr. Polym., 2011, 86, 202–207 CrossRef CAS
.
- N. Nordin, S. H. Othman, S. A. Rashid and R. K. Basha, Food Hydrocolloids, 2020, 106, 0268 CrossRef
.
- D. Domene-López, J. J. Delgado-Marín, I. Martin-Gullon, J. C. García-Quesada and M. G. Montalbán, Int. J. Biol. Macromol., 2019, 135, 845–854 CrossRef PubMed
.
- E. Basiak, A. Lenart and F. Debeaufort, Int. J. Biol. Macromol., 2017, 98, 348–356 CrossRef CAS PubMed
.
- N. Laohakunjit and A. Noomhorm, Starch/Staerke, 2004, 56, 348–356 CrossRef CAS
.
- A. Nawab, F. Alam, M. A. Haq and A. Hasnain, Starch/Staerke, 2016, 68, 919–928 CrossRef CAS
.
- M. A. Huneault and H. Li, J. Appl. Polym. Sci., 2011, 119, 2439–2448 CrossRef
.
- M. Esmaeili, G. Pircheraghi and R. Bagheri, Polym. Int., 2017, 66, 809–819 CrossRef CAS
.
- Y. Miyata, Nippon Nogeikagaku Kaishi, 1994, 68, 1318–1320 CrossRef CAS
.
- U. Siemann, Prog. Colloid Polym. Sci., 2005, 130, 1–14 CAS
.
-
T. A. Osswald, Polymer Processing Fundamentals, 1998 Search PubMed
.
- M. Thunwall, V. Kuthanová, A. Boldizar and M. Rigdahl, Carbohydr. Polym., 2008, 71, 583–590 CrossRef CAS
.
- V. M. Hernandez-Izquierdo and J. M. Krochta, J. Food Sci., 2008, 73, 30–39 CrossRef PubMed
.
- A. Altskär, R. Andersson, A. Boldizar, K. Koch, M. Stading, M. Rigdahl and M. Thunwall, Carbohydr. Polym., 2008, 71, 591–597 CrossRef
.
- J. J. G. van Soest and D. B. Borger, J. Appl. Polym. Sci., 1997, 64, 631–644 CrossRef CAS
.
- D. Lowdin, G. della Valle and P. Colonna, Carbohydr. Polym., 1995, 27, 261–270 CrossRef
.
- K. Koch, T. Gillgren, M. Stading and R. Andersson, Int. J. Biol. Macromol., 2010, 46, 13–19 CrossRef CAS PubMed
.
- A. Jiménez, M. J. Fabra, P. Talens and A. Chiralt, Food Bioprocess Technol., 2012, 5, 2058–2076 CrossRef
.
- M. S. Huda, L. T. Drzal, A. K. Mohanty and M. Misra, Compos. Sci. Technol., 2008, 68, 424–432 CrossRef CAS
.
- Z. Xiong, Y. Yang, J. Feng, X. Zhang, C. Zhang, Z. Tang and J. Zhu, Carbohydr. Polym., 2013, 92, 810–816 CrossRef CAS PubMed
.
- C. Ingrao, C. Tricase, A. Cholewa-Wójcik, A. Kawecka, R. Rana and V. Siracusa, Sci. Total Environ., 2015, 537, 385–398 CrossRef CAS PubMed
.
- I. S. M. A. Tawakkal, M. J. Cran, J. Miltz and S. W. Bigger, J. Food Sci., 2014, 79, 1477–1490 CrossRef PubMed
.
- K. S. Anderson, S. H. Lim and M. A. Hillmyer, J. Appl. Polym. Sci., 2003, 89, 3757–3768 CrossRef CAS
.
- A. Bher, R. Auras and C. E. Schvezov, J. Appl. Polym. Sci., 2018, 135, 1–15 CrossRef
.
- X. Yang, S. Liu, E. Yu and Z. Wei, ACS Omega, 2020, 5, 29284–29291 CrossRef CAS PubMed
.
- N. F. Zaaba and H. Ismail, Polym.-Plast. Technol. Mater., 2019, 58, 1945–1964 CAS
.
- T. Ke and X. S. Sun, J. Appl. Polym. Sci., 2003, 88, 2947–2955 CrossRef CAS
.
- M. Akrami, I. Ghasemi, H. Azizi, M. Karrabi and M. Seyedabadi, Carbohydr. Polym., 2016, 144, 254–262 CrossRef CAS PubMed
.
- M. Esmaeili, G. Pircheraghi, R. Bagheri and V. Altstädt, Polym. Adv. Technol., 2018, 29, 2880–2889 CrossRef CAS
.
- J. M. Ferri, D. Garcia-Garcia, L. Sánchez-Nacher, O. Fenollar and R. Balart, Carbohydr. Polym., 2016, 147, 60–68 CrossRef CAS PubMed
.
- J. F. Zhang and X. Sun, Biomacromolecules, 2004, 5, 1446–1451 CrossRef CAS PubMed
.
- N. Wang, J. Yu, P. R. Chang and X. Ma, Carbohydr. Polym., 2008, 71, 109–118 CrossRef CAS
.
- M. Kaseem, K. Hamad and F. Deri, Polym. Sci., Ser. A, 2012, 54, 165–176 CrossRef CAS
.
- K. Krogars, J. Heinämäki, M. Karjalainen, A. Niskanen, M. Leskelä and J. Yliruusi, Int. J. Pharm., 2003, 251, 205–208 CrossRef CAS PubMed
.
- M. Esmaeili, G. Pircheraghi, R. Bagheri and V. Altstädt, Polym. Adv. Technol., 2019, 30, 839–851 CrossRef CAS
.
- C. Yokesahachart and R. Yoksan, Carbohydr. Polym., 2011, 83, 22–31 CrossRef CAS
.
- H. Wang, X. Sun and P. Seib, J. Appl. Polym. Sci., 2002, 84, 1257–1262 CrossRef CAS
.
- C. S. Wu, Macromol. Biosci., 2005, 5, 352–361 CrossRef CAS PubMed
.
- J. F. Balart, V. Fombuena, O. Fenollar, T. Boronat and L. Sánchez-Nacher, Composites, Part B, 2016, 86, 168–177 CrossRef CAS
.
- A. Carbonell-Verdu, D. Garcia-Garcia, F. Dominici, L. Torre, L. Sanchez-Nacher and R. Balart, Eur. Polym. J., 2017, 91, 248–259 CrossRef CAS
.
- R. Ortega-Toro, A. López-Córdoba and F. Avalos-Belmontes, Heliyon, 2021, 7, 1–8 CrossRef PubMed
.
- C. Caicedo and H. L. C. Pulgarin, Processes, 2021, 9, 578 CrossRef CAS
.
- L. Averous, L. Moro, P. Dole and C. Fringant, Polymer, 2000, 41, 4157–4167 CrossRef CAS
.
- A. K. Matta, R. U. Rao, K. N. S. Suman and V. Rambabu, Procedia Mater. Sci., 2014, 6, 1266–1270 CrossRef CAS
.
- M. Nishida, T. Tanaka, Y. Hayakawa, T. Ogura, Y. Ito and M. Nishida, RSC Adv., 2019, 9, 1551–1561 RSC
.
- V. O. Bulatović, V. Mandić, D. Kučić Grgić and A. Ivančić, J. Polym. Environ., 2021, 29, 492–508 CrossRef
.
- Z. Yuan and B. D. Favis, AIChE J., 2005, 51, 271–280 CrossRef CAS
.
- G. Li and B. D. Favis, Macromol. Chem. Phys., 2010, 211, 321–333 CrossRef CAS
.
- J. H. Mina Hernandez, Polymers, 2021, 13, 1–19 Search PubMed
.
- H. K. T. T. Shohei Inoue, J. Polym. Sci., Part B: Polym. Lett., 1969, 7, 287–292 CrossRef
.
- S. Inoue, H. Koinuma and T. Tsuruta, Makromol. Chem., 1969, 130, 210–220 CrossRef CAS
.
- Q. Zhu, Y. Z. Meng, S. C. Tjong, X. S. Zhao and Y. L. Chen, Polym. Int., 2002, 51, 1079–1085 CrossRef CAS
.
- X. Cui, J. Jin, G. Zhao and W. Jiang, Polym. Bull., 2020, 77, 1327–1342 CrossRef CAS
.
- Q. Sun, T. Mekonnen, M. Misra and A. K. Mohanty, J. Polym. Environ., 2016, 24, 23–36 CrossRef CAS
.
- G. Jiang, J. Xu, N. Zhao, S. Zhang and H. Huang, Polym.-Plast. Technol. Eng., 2017, 56, 1084–1095 CrossRef CAS
.
- X. C. Ge, X. H. Li, Q. Zhu, L. Li and Y. Z. Meng, Polym. Eng. Sci., 2004, 44, 2134–2140 CrossRef CAS
.
- X. Ma, J. Yu and A. Zhao, Compos. Sci. Technol., 2006, 66, 2360–2366 CrossRef CAS
.
- S. Peng, X. Wang and L. Dong, Polym. Compos., 2005, 26, 37–41 CrossRef CAS
.
- X. Ma, P. R. Chang, J. Yu and N. Wang, Carbohydr. Polym., 2008, 71, 229–234 CrossRef CAS
.
- X. C. Ge, Y. Xu, Y. Z. Meng and R. K. Y. Li, Compos. Sci. Technol., 2005, 65, 2219–2225 CrossRef CAS
.
- L. X. Fu, H. Yu, H. N. Na, H. Qin, L. Li and S. X. Li, Plastics, 2008, 7, 61–63 Search PubMed
.
- E. Bugnicourt, P. Cinelli, A. Lazzeri and V. Alvarez, eXPRESS Polym. Lett., 2014, 8, 791–808 CrossRef
.
- S. H. Imam, L. Chen, S. H. Gorden, R. L. Shogren, D. Weisleder and R. v. Greene, J. Environ. Polym. Degrad., 1998, 6, 91–98 CrossRef CAS
.
- L. H. Innocentini-Mei, J. R. Bartoli and R. C. Baltieri, Macromol. Symp., 2003, 197, 77–88 CrossRef CAS
.
- S. M. Lai, T. M. Don and Y. C. Huang, J. Appl. Polym. Sci., 2006, 100, 2371–2379 CrossRef CAS
.
- J. P. Florez, M. Fazeli and R. A. Simão, Int. J. Biol. Macromol., 2019, 123, 609–621 CrossRef CAS PubMed
.
- Z. W. Abdullah, Y. Dong, I. J. Davies and S. Barbhuiya, Polym.-Plast. Technol. Eng., 2017, 56, 1307–1344 CrossRef CAS
.
- Z. Liu, Y. Feng and Y. Xiao-su, J. Appl. Polym. Sci., 1999, 74, 2667–2673 CrossRef CAS
.
- H. R. Park, S. H. Chough, Y. H. Yun and S. do Yoon, J. Polym. Environ., 2005, 13, 375–382 CrossRef CAS
.
- R. Shi, J. Bi, Z. Zhang, A. Zhu, D. Chen, X. Zhou, L. Zhang and W. Tian, Carbohydr. Polym., 2008, 74, 763–770 CrossRef CAS
.
- N. Tudorachi, C. N. Cascaval, M. Rusu and M. Pruteanu, Polym. Test., 2000, 19, 785–799 CrossRef CAS
.
- X. Y. Zhou, Y. F. Cui, D. M. Jia and D. Xie, Polym.-Plast. Technol. Eng., 2009, 48, 489–495 CrossRef CAS
.
- L. Mao, S. Imam, S. Gordon, P. Cinelli and E. Chiellini, J. Polym. Environ., 2000, 8, 205–211 CrossRef CAS
.
- B. Ramaraj, J. Appl. Polym. Sci., 2007, 103, 909–916 CrossRef CAS
.
- S. do Yoon, S. H. Chough and H. R. Park, J. Appl. Polym. Sci., 2007, 106, 2485–2493 CrossRef
.
- H. Takahashi, T. Hayakawa and M. Ueda, Chem. Lett., 2000, 684–685 CrossRef CAS
.
- R. Muthuraj, M. Misra and A. K. Mohanty, J. Polym. Environ., 2014, 22, 336–349 CrossRef CAS
.
- J. B. Zeng, L. Jiao, Y. D. Li, M. Srinivasan, T. Li and Y. Z. Wang, Carbohydr. Polym., 2011, 83, 762–768 CrossRef CAS
.
- J. Li, X. Luo, X. Lin and Y. Zhou, Starch/Staerke, 2013, 65, 831–839 CrossRef CAS
.
- D. Liu, Z. Qi, Y. Zhang, J. Xu and B. Guo, Starch/Staerke, 2015, 67, 802–809 CrossRef CAS
.
- O. Martin, E. Schwach, L. Avérous and Y. Couturier, Starch/Staerke, 2001, 53, 372–380 CrossRef CAS
.
- X. Ma, J. Yu and J. F. Kennedy, Carbohydr. Polym., 2005, 62, 19–24 CrossRef CAS
.
- A. Campos, A. R. Sena Neto, V. B. Rodrigues, B. R. Luchesi, L. H. C. Mattoso and J. M. Marconcini, Ind. Crops Prod., 2018, 124, 149–154 CrossRef CAS
.
- W. Zhou, D. Zha, X. Zhang, J. Xu, B. Guo and Y. Huang, Carbohydr. Polym., 2020, 250, 116913 CrossRef CAS PubMed
.
- B. Guo, D. Zha, B. Li, P. Yin and P. Li, Materials, 2018, 11, 640 CrossRef PubMed
.
- A. López-Córdoba, S. Estevez-Areco and S. Goyanes, Carbohydr. Polym., 2019, 215, 377–387 CrossRef PubMed
.
- P. R. Fitch-Vargas, I. L. Camacho-Hernández, F. Martínez-Bustos, A. R. Islas-Rubio, K. I. Carrillo-Cañedo, A. Calderón-Castro, N. Jacobo-Valenzuela, A. Carrillo-López, C. I. Delgado-Nieblas and E. Aguilar-Palazuelos, Carbohydr. Polym., 2019, 219, 378–386 CrossRef CAS PubMed
.
- D. C. M. Ferreira, G. Molina and F. M. Pelissari, Composites, Part B, 2020, 183, 107682 CrossRef CAS
.
- L. A. Castillo, O. v. López, M. A. García, S. E. Barbosa and M. A. Villar, Heliyon, 2019, 5, e01877 CrossRef PubMed
.
- M. Bootklad and K. Kaewtatip, Carbohydr. Polym., 2013, 97, 315–320 CrossRef CAS PubMed
.
- V. H. Campos-Requena, B. L. Rivas, M. A. Pérez, K. A. Garrido-Miranda and E. D. Pereira, Eur. Polym. J., 2018, 109, 64–71 CrossRef CAS
.
- W. Rodríguez-Castellanos, F. Martínez-Bustos, D. Rodrigue and M. Trujillo-Barragán, Eur. Polym. J., 2015, 73, 335–343 CrossRef
.
- P. Chavan, A. Sinhmar, S. Sharma, A. Dufresne, R. Thory, M. Kaur, K. S. Sandhu, M. Nehra and V. Nain, Starch/Staerke, 2022, 2100302–2100312 CrossRef CAS
.
- J. Gironès, J. P. López, P. Mutjé, A. J. F. Carvalho, A. A. S. Curvelo and F. Vilaseca, Compos. Sci. Technol., 2012, 72, 858–863 CrossRef
.
- G. Borowski, T. Klepka, M. Pawłowska, M. C. Lavagnolo, T. Oniszczuk, A. Wójtowicz and M. Combrzyński, Arch. Environ. Prot., 2020, 46, 74–82 Search PubMed
.
- J. Prachayawarakorn, P. Ruttanabus and P. Boonsom, J. Polym. Environ., 2011, 19, 274–282 CrossRef CAS
.
- J. Tarique, E. S. Zainudin, S. M. Sapuan, R. A. Ilyas and A. Khalina, Polymers, 2022, 14, 388 CrossRef CAS PubMed
.
- S. Y. Z. Zainuddin, I. Ahmad, H. Kargarzadeh, I. Abdullah and A. Dufresne, Carbohydr. Polym., 2013, 92, 2299–2305 CrossRef CAS PubMed
.
- R. A. Ilyas, S. M. Sapuan, M. R. Ishak and E. S. Zainudin, Carbohydr. Polym., 2018, 202, 186–202 CrossRef CAS PubMed
.
- A. Kwaśniewska, D. Chocyk, G. Gładyszewski, J. Borc, M. Świetlicki and B. Gładyszewska, Polymers, 2020, 12, 73 CrossRef PubMed
.
- A. Nazrin, S. M. Sapuan and M. Y. M. Zuhri, Polymers, 2020, 12, 1–18 CrossRef PubMed
.
- O. O. Fabunmi, L. G. Tabil, S. Panigrahi and P. R. Chang, J. Polym. Environ., 2011, 19, 841–848 CrossRef CAS
.
- C. Yokesahachart, R. Yoksan, N. Khanoonkon, A. K. Mohanty and M. Misra, Cellulose, 2021, 28, 5513–5530 CrossRef CAS
.
-
O. Vilpoux and L. Averous, Technology, use and potentialities of Latin American starchy tubers, 2004, pp. 521–553 Search PubMed
.
- C. Bastioli, Starch/Staerke, 2001, 53, 351–355 CrossRef CAS
.
- Mordor Intelligence, Thermoplastic starch market-growth, trends, COVID-19 impact, and forecasts (2021–2026), 2020.
- Mordor Intelligence, Global thermoplastic starch (TPS) market, 2020.
-
B. G. Laycock and P. J. Halley, Starch Polymers: From Genetic Engineering to Green Applications, Elsevier B.V., 2014, pp. 381–419 Search PubMed
.
- Novamont, Novamont increases MATER-BI® Production to 15,000 tonnes-Novamont-press, 2019.
- BioLogiQ, Starch for making NuPlastiQ, Hong Kong, 2017.
- BIOTEC, We make Bioplast, 2013.
- Kuraray, Kuraray Report 2019, 2019.
- Biome Technologies, Annual report and financial statement for the year ended 31 December 2019, 2019.
- AGRANA STÄRKE GMBH, Thermoplastic starch Made in Austria, 2004.
- Agrana starch, Bioplastic compounds, 2004.
- F. Wu, M. Misra and A. K. Mohanty, Prog. Polym. Sci., 2021, 117, 101395–101435 CrossRef CAS
.
- A. Ali, L. Yu, H. Liu, S. Khalid, L. Meng and L. Chen, J. Appl. Polym. Sci., 2017, 134, 45159 CrossRef
.
- A. Ali, S. Ali, L. Yu, H. Liu, S. Khalid, A. Hussain, M. M. N. Qayum and C. Ying, J. Appl. Polym. Sci., 2019, 136, 47978 CrossRef
.
- A. Kaushik and J. Kumra, J. Elastomers Plast., 2014, 46, 284–299 CrossRef CAS
.
- U. Shah, F. Naqash, A. Gani and F. A. Masoodi, Compr. Rev. Food Sci. Food Saf., 2016, 15, 568–580 CrossRef CAS PubMed
.
- R. Ortega-Toro, I. Morey, P. Talens and A. Chiralt, Carbohydr. Polym., 2015, 127, 282–290 CrossRef CAS PubMed
.
- T. Jiang, Q. Duan, J. Zhu, H. Liu and L. Yu, Adv. Ind. Eng. Polym. Res., 2020, 3, 8–18 Search PubMed
.
- L. R. P. F. Mello and S. Mali, Ind. Crops Prod., 2014, 55, 187–193 CrossRef CAS
.
- N. Kaisangsri, O. Kerdchoechuen and N. Laohakunjit, Ind. Crops Prod., 2012, 37, 542–546 CrossRef CAS
.
- C. M. Machado, P. Benelli and I. C. Tessaro, Ind. Crops Prod., 2017, 102, 115–121 CrossRef CAS
.
- W. Sanhawong, P. Banhalee, S. Boonsang and S. Kaewpirom, Ind. Crops Prod., 2017, 108, 756–766 CrossRef CAS
.
- C. Chiarathanakrit, S. A. Riyajan and K. Kaewtatip, Carbohydr. Polym., 2018, 188, 48–53 CrossRef CAS PubMed
.
- J. B. Engel, A. Ambrosi and I. C. Tessaro, Carbohydr. Polym., 2019, 225, 115234 CrossRef CAS PubMed
.
- E. M. da Graça Costa do Nascimento, C. W. P. Carvalho, C. Y. Takeiti, D. D. G. C. Freitas and J. L. R. Ascheri, Food Res. Int., 2012, 45, 434–443 CrossRef
.
- N. Kaisangsri, O. Kerdchoechuen and N. Laohakunjit, Carbohydr. Polym., 2014, 110, 70–77 CrossRef CAS PubMed
.
- Y. Chen, L. Yu, X. Ge, H. Liu, A. Ali, Y. Wang and L. Chen, Int. J. Biol. Macromol., 2019, 129, 944–951 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2022 |