Open Access Article
Open Access ArticleElectric-field-based control of molecular magnetism in TMPc/Sc2CO2 van der Waals systems
Yunlong
Wang
 ab and
Yan
Lu
*b
ab and
Yan
Lu
*b
aNational Laboratory of Solid State Microstructures, School of Physics, and Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing 210093, China
bSchool of Science, Nanchang University, Nanchang 330031, China. E-mail: ylu@ncu.edu.cn
First published on 24th November 2021
Abstract
Controlling the magnetic states of molecular structures through electric fields is a major challenge in the area of electron spin manipulation, but it can be achieved upon utilizing the sensitivity of the electron distribution at a heterostructure interface. Here, we found that the magnetic states of a transition-metal phthalocyanine (TMPc) can be effectively controlled through switching the polarization state of monolayer Sc2CO2 to achieve a transition between the nonmagnetic and magnetic states of some TMPc molecules. Moreover, the magnetic moments of some structures can be regulated through applying an external vertical electric field. These novel characteristics are caused by the effects of an intrinsic or external electric field on the d-orbital electron transfer and orbital splitting of TMPc molecules. In addition, the p–d hybrid orbital in AgPc will also be affected by the external vertical electric field, ultimately changing the magnetic moment. Therefore, we are very interested in these different magnetic effects, and the fascinating phenomena seen in this study could guide the development of ferroelectric control for use in high-performance electronic memory writing, high-density magnetic memory reading, sensors, and other spintronic devices.
Introduction
The magnetoelectric effect refers to the coupling of ferroelectricity (FE) and ferromagnetism (FM), which has great potential for use in multifunctional devices.1–3 It is an effective method for controlling the electron spin states of nanostructures using an electric field.4,5 In general, there are two degenerate polarization states (P↑ and P↓) in two-dimensional ferroelectric materials, and the out-of-plane electric field depends on the polarization states; a polarized electric field can be used to effectively control the properties of magnetic materials. However, natural multiferroic materials are really rare and the magnetoelectric coupling is usually weak; on the other hand, traditional ferroelectric and ferromagnetic materials have different d-orbital electronic requirements.6,7 Although there are materials that violate the d0 rule,8 in many cases, it is still pretty challenging to find intense coupling between FE and FM.In recent years, many two-dimensional (2D) FE materials have been widely studied, such as SnTe,9 In2Se3,10,11 Sc2CO2,12 AgBiP2Se6,13 CuInP2S6,14,15 monolayer WTe2,16 monolayer group IV chalcogenides,17 2D honeycomb binary compounds,18 vertically polarized 2D ferroelectric BN, AlN, ZnO, MoS2, GaSe,19 transition-metal dihalides,20 and CrSnTe3.21 In addition, theory predicts the existence of a variety of two-dimensional multiferrous materials with coexisting FE and FM, such as C6N8H organic networks,22 CrN,23 Hf2VC2F2,24 and monolayer CrBr3.25
van der Waals heterostructures provide multiple possibilities for the development of artificial multiferroic materials, usually accompanied by novel physical phenomena and complex coupling principles. For example, the magnetic coupling of CrI3 bilayers can be switched based on the Sc2CO2 polarization state,26via tuning the ferroelectric polarization state in In2Se3/FeI2, the FeI2 monolayer transforms from ferromagnetic to antiferromagnetic,27 and the polarization state of CuInP2S6 monolayers can be switched using an electric field.28 These reports indicate that the use of vdW heterostructures provides a method for achieving artificial multiferroicity.29
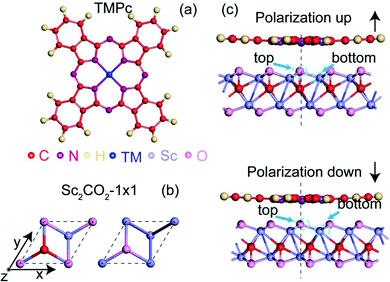
In this paper, a two-dimensional artificial multiferroic heterostructure is proposed, which is formed via vdW interactions between a transition-metal phthalocyanine molecule (TMPc) and monolayer Sc2CO2. Monolayer Sc2CO2 has out-of-plane electric polarization of 1.60 μC cm−3, and it has been studied in previous research;30 the position of the carbon atom mainly determines the polarization state, and the energy barrier between up (P↑) and down (P↓) polarization is 0.52 eV per unit.30 Besides, phthalocyanine has been widely used in chemical catalysis31,32 and has been widely studied in spintronics.33,34
We consider the case where the central atom of TMPc is a transition metal of the fourth or fifth period. Via first-principles calculations, we found that the magnetic state of TMPc can be switched through tuning the polarization state of the Sc2CO2 monolayer, the heterostructure can be switched from a magnetic state to a nonmagnetic state, and an external vertical electric field can control the magnetic moments of some heterostructures. Through further analysis, we know that the coupling variations between TMPc and Sc2CO2 are caused by a significant change in the structure arrangement and charge transfer between layers, mainly acting on the d and p orbitals. Moreover, an external electric field can further control charge transfer between layers so that the magnetic moment can be controlled. We also reveal the different origins of the magnetic variations of RuPc (P↑) and AgPc (P↑). Therefore, this research provides a novel method for designing artificial vdW multifunctional nanoelectronics and devices with strong magnetoelectric coupling.
Calculation methods
All the results are obtained based on VASP (the Vienna Ab initio Simulation Package).35 We use the projector-augmented wave (PAW) method36 to indicate the ion potential. For dealing with exchange–correlation effects between electrons, we chose the generalized gradient approximation (GGA) method established based on the Perdew–Burke–Ernzerhof (PBE) functional;37 the Hubbard U correction describes the partial filling of d orbitals, where Ueff = U − J = 3 eV (U = 3, J = 0 eV),38 and different U values do not affect the conclusions. The cutoff energy is 400 eV, and we use 25 Å vacuum space to minimize interactions between adjacent layers. In the relaxation structure, the convergence criterion is that the forces on all atoms are less than at least 0.02 eV Å−1, and that energy variations are less than at least 10−5 eV. Grimme's zero damping DFT-D3 method39 is used to describe the vdW effects of interlayers. We formed a 5 × 5 Sc2CO2 supercell to reduce the interactions of structures; the lattice parameter of Sc2CO2 is 3.37 Å, which is similar to previous studies,26 and dipole correction is considered. The differential charge distribution was drawn using Vesta software,40 and Bader41 is used for charge transfer analysis.Results and discussion
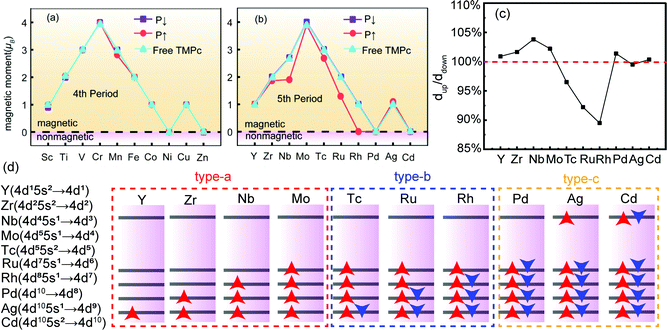
Firstly, we relax the structure to find the lowest-energy adsorption point of a TMPc molecule on Sc2CO2, as shown in Fig. 1(c). The TMPc and Sc2CO2 relative monolayer positions can be determined via referring to the oxygen atom in the nearest neighbor layer. The relative positions can be divided into top and bottom arrangements. Through rotation and translation, we find that system energy in the top position is lowest. The following research is based on this situation.As shown in Fig. 2(a and b), the transition metals of the fourth period show no evident magnetic moment variations on Sc2CO2; this may be due to the electronegativities of the fourth-period transition metals generally being weaker than those of the fifth-period transition metals. The energies of electrons occupying 4d and 5s orbitals are higher than those occupying 3d and 4s orbitals, and they can more easily participate in charge transfer processes. In terms of the D4h symmetric ligand field structure of phthalocyanine, the magnetic variations can be explained based on the “4+1” splitting phenomenon.42,43 TMPc with a central magnetic atom has four suborbitals of similar energy in its d orbital, while one has much higher energy. For example, the bonding of the central atom and ligands in VPc (3d3 4s2) causes three electrons to be in close orbitals with lower energy, so the total magnetic moment is 3 μB, and with a further increase in d-orbital-filling electrons, such as in the case of MnPc (or with Fe, Co, Tc, Ru, and Rh), the electrons adopt opposite spin states, resulting in diminishing magnetic moments.
For fifth-period transition-metal atoms, especially Nb, Ru, and Rh, Sc2CO2 (P↑) has a significant effect on the magnetic moment of the structure. In particular, the magnetic moment of the RuPc (P↑) structure is 0 μB, which means that the introduction of Sc2CO2 transforms the magnetic state of RhPc molecules. We mainly analyze the magnetic state transition of RhPc (P↑) and the magnetic moment variations of RuPc (P↑) in the following discussion. In order to explore changes in the interlayer spacing of the heterostructures after Sc2CO2 reversal, we plot the fifth-period interlayer spacing ratios in more detail, as shown in Fig. 2(c); systems that are significantly affected by the polarization of the ferroelectric layer show obvious changes in the interlayer spacing ratio, further affecting the electron density distribution and magnetic moment. We can divide the fifth-period systems into three types, the magnetic moment of a type-a material increases as the electrons occupy adjacent quadruple orbitals, while the magnetic moment of a type-b material decreases as the electrons occupy the opposite spin states of the quadruple orbitals. When the quadruple orbitals are fully occupied, the magnetic moment is 0 μB, and magnetic moment changes in type-c materials are only related to the electron occupancy of the highest energy suborbitals.
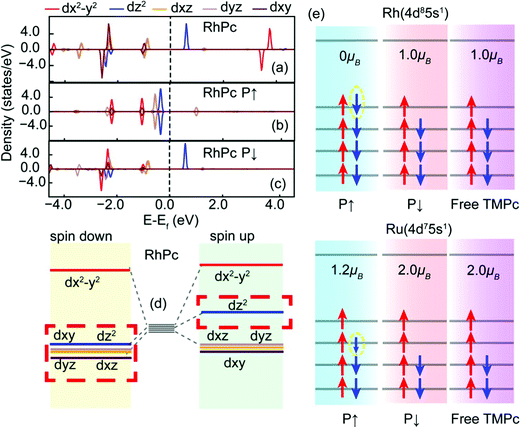
To further assess the magnetic contribution from each d-orbital sublayer in the RhPc system, we provide the following discussion. Fig. 3 illustrates the arrangements of the d-orbital sublayers. Based on similarities due to the “4+1” splitting phenomenon, the dxz and dyz orbitals of a pure RhPc molecule are degenerate. The two different spin states of the dz2 orbital are evidently split, mainly contributing to the magnetism. The energy level of the dx2−y2 orbital is significantly higher than those of dxy, dxz, dyz, and dz2 orbitals. When the RhPc molecule is adsorbed on the Sc2CO2 monolayer, due to electrons migrating to the dx2−y2 orbital, the dx2−y2 orbital moves to a lower energy level and becomes degenerate with the dxy orbital.
As shown in Fig. 3(e), firstly, the outer electron distribution of Rh is 4d8 5s1; after bonding, the d orbital loses two electrons, then RhPc (P↑) obtains one electron from the interaction between the ligand and Sc2CO2, and the total magnetic moment is 0 μB. On the other hand, Rh does not obtain electrons in the P↓ structure and there is still electron mismatch in the 4d orbital; the total magnetic moment of the structure is 1 μB. Secondly, the outer electron arrangement of Ru is 4d7 5s1, and because the electronegativity of Ru is weaker than that of Rh, the number of electrons obtained in the P↑ structure is slightly less than one, so the total magnetic moment is more than 1 μB; the P↓ structure has two electrons which are unpaired, and the total magnetic moment of the structure is 2 μB.
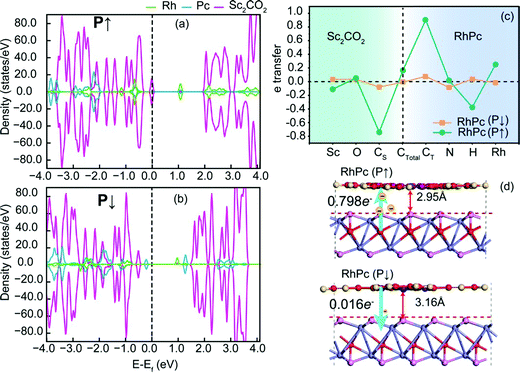
Further analysis of charge transfer in RhPc reveals the physical mechanism. This is illustrated via analyzing the density of states, charge transfer, and molecular structures of the two polarization situations. Fig. 4(a and b) shows the density distributions of electron states in RhPc (P↑) and RhPc (P↓). It can be seen that Sc2CO2 (P↑) transfers 0.798 electrons of charge to RhPc, which is much more than the 0.016 electrons of charge transferred from RhPc to Sc2CO2 (P↓); the Fermi level is lower for Sc2CO2 (P↑), showing a metallic state, which is different from the semiconductor state of Sc2CO2 (P↓). This transition differs from that of MPz/In2Se329 charge transfer, but it is essentially the transfer and redistribution of charge under the effects of a polarized electric field. Fig. 4(c) shows the internal transfer trend of electrons, where CS refers to the carbon in Sc2CO2 and CT refers to the carbon in TMPc, and CTotal = CS + CT. The number of electrons transferred has been subtracted from the intrinsic charges of the elements of TMPc and Sc2CO2; Rh gains more electrons in RhPc (P↑). According to Fig. 4(d), the interlayer distance in RhPc (P↑) is 2.95 Å, which is shorter than the distance of 3.12 Å between the layers in RhPc (P↓); this means that interactions between TMPc and Sc2CO2 are more intense in RhPc (P↑). The difference in interaction strengths leads to differences in charge transfer and orbital splitting, finally leading to the different magnetic states of TMPc in the two polarization situations.
Based on the response of TMPc to the Sc2CO2 polarized electric field, it can be predicted that the external electric field has a significant effect on the charge transfer and distribution, then affecting the total magnetic moment of the system. For the P↑ situation, the interactions between TMPc and Sc2CO2 are more intense. Therefore, the electric field controls charge transfer more significantly.
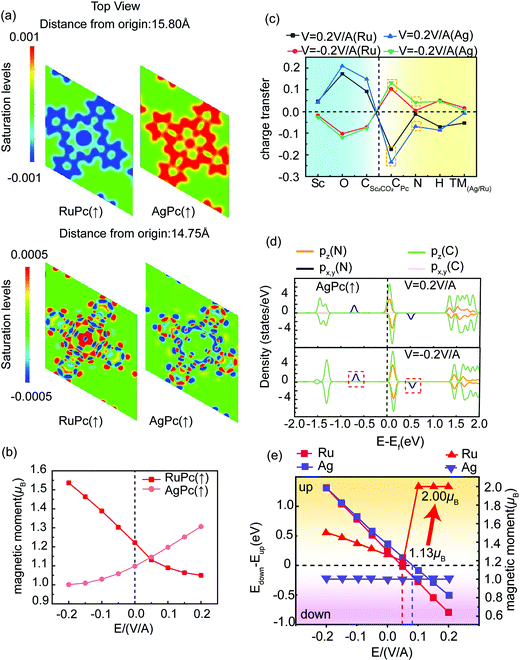
Fig. 5(a) further explains the origin of the two different magnetic moments. At a position of 15.80 Å (above the Pc structure), the covalent bond between the Ag atom and the neighboring N atom is stronger. This is different from the almost complete lack of charge interaction between the Ru atom and the N atom. A more complex bond relationship can be seen at 14.75 Å. The main reason for the increase of the RuPc (P↑) magnetic moment is that the charge of the Ru d orbital decreases, increasing the net magnetic moment, while the change of the AgPc (P↑) magnetic moment is more sophisticated.
As seen in Fig. 5(b), the magnetic moments of AgPc (P↑) and RuPc (P↑) vary with the electric field, and it can be judged that the origins of the magnetic moment variations of the two structures are quite different according to the differential charge density variations. Fig. 5(c) illustrates charge transfer under an electric field (the charge without the electric field has been subtracted). In opposite electric field directions, Ru loses different numbers of electrons, which dominates the variation of the magnetic moment, while Ag loses few electrons in the different electric fields, and the main contribution to the magnetic moment from the structure is from N and CPc, which can be explained based on the magnetic nature of carbonitride;44,45 as seen in Fig. 5(d), the px and py orbitals are degenerate, contributing part of the magnetic moment due to the asymmetry of electrons occupying spin-up and spin-down positions. AgPc (P↑) exhibits a semiconductor state in the reverse electric field direction and a half-metallic state in the forward electric field direction. The increase in magnetic moment mainly comes from the splitting of the pz orbitals of C and N near the Fermi level, which is different from the physical mechanism involved in the case of RuPc/Sc2CO2 (P↑).
In order to further explore the influence of the intensity of the interlayer exchange effect on magnetic variations, we calculated the magnetic moment of the RuPc structure while changing the interlayer spacing of the heterostructure, as shown in Fig. 6; since there is no obvious difference in magnetic moments between the RuPc/Sc2CO2 (P↓) structure and RuPc molecule, we mainly study the RuPc/Sc2CO2 (P↑) structure, where EΔ = Ed=2.2Å − Ed. d = 2.7 Å, marked in Fig. 6, is the optimized result for RuPc/Sc2CO2 (P↑) in this paper. When the interlayer spacing is too large, the result gradually approaches the behavior of two independent molecules in a vacuum. When the interlayer spacing is too small, the repulsion between electrons is strong, resulting in a drastic change in the structural distribution and the destruction of magnetism.
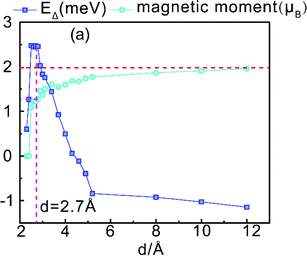 | ||
| Fig. 6 The effects of the distance d between heterostructure layers on the magnetic moment and energy of the system. | ||
Conclusions
In summary, we propose and study two-dimensional van der Waals heterostructures composed of a transition metal phthalocyanine (TMPc) molecule and the ferroelectric monolayer Sc2CO2via first-principles calculations. The magnetic state of RhPc and the magnetic moments of NbPc and RuPc can be controlled through switching the polarization of Sc2CO2 using an electric field. The physical origin of this phenomenon is that ferroelectric interfacial polarization leads to charge transfer and redistribution, which is similar to the “4+1” splitting phenomenon. The variations of the magnetic moments of AgPc (P↑) and RuPc (P↑) under an external electric field are predicted, and the different physical mechanisms are revealed. For the AgPc (P↑) structure, carbon and nitrogen contribute to the magnetism and can be further regulated by the electric field. Therefore, our research provides a theoretical method for developing high-density nonvolatile magnetic memory and it may further accelerate research in related fields.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The calculations were carried out using supercomputers at the High Performance Computing Center of Collaborative Innovation Center of Advanced Microstructures, the high-performance super-computing center of Nanjing University.References
- S. D. Bader and S. S. P. Parkin, Spintronics, Annu. Rev. Condens. Matter Phys., 2010, 1, 71–88 CrossRef CAS.
- J. T. Heron, et al., Deterministic Switching of Ferromagnetism at Room Temperature Using an Electric Field, Nature, 2014, 516, 370 CrossRef CAS PubMed.
- J. F. Scott, Data Storage – Multiferroic Memories, Nat. Mater., 2007, 6, 256–257 CrossRef CAS PubMed.
- S. E. Barnes, J. Ieda and S. Maekawa, Rashba Spin-Orbit Anisotropy and the Electric Field Control of Magnetism, Sci. Rep., 2014, 4, 4105 CrossRef PubMed.
- S. A. Wolf, D. D. Awschalom, R. A. Buhrman, J. M. Daughton, S. von Molnar, M. L. Roukes, A. Y. Chtchelkanova and D. M. Treger, Spintronics: A Spin-Based Electronics Vision for the Future, Science, 2001, 294, 1488–1495 CrossRef CAS PubMed.
- N. A. Hill and A. Filippetti, Why Are There Any Magnetic Ferroelectrics?, J. Magn. Magn. Mater., 2002, 242, 976–979 CrossRef.
- K. F. Wang, J. M. Liu and Z. F. Ren, Multiferroicity: The Coupling between Magnetic and Polarization Orders, Adv. Phys., 2009, 58, 321–448 CrossRef CAS.
- H. X. Tan, M. L. Li, H. T. Liu, Z. R. Liu, Y. C. Li and W. H. Duan, Two-Dimensional Ferromagnetic-Ferroelectric Multiferroics in Violation of the D(0)Rule, Phys. Rev. B: Condens. Matter Mater. Phys., 2019, 99, 195434 CrossRef CAS.
- K. Chang, et al., Discovery of Robust in-Plane Ferroelectricity in Atomic-Thick Snte, Science, 2016, 353, 274–278 CrossRef CAS PubMed.
- C. J. Cui, et al., Intercorrelated in-Plane and out-of-Plane Ferroelectricity in Ultrathin Two-Dimensional Layered Semiconductor In2se3, Nano Lett., 2018, 18, 1253–1258 CrossRef CAS PubMed.
- W. J. Ding, J. B. Zhu, Z. Wang, Y. F. Gao, D. Xiao, Y. Gu, Z. Y. Zhang and G. Zhu,W., Prediction of Intrinsic Two-Dimensional Ferroelectrics in In2Se3 and Other III2-VI3 van der Waals Materials, Nat. Commun., 2017, 8, 14956 CrossRef CAS PubMed.
- A. Chandrasekaran, A. Mishra and A. K. Singh, Ferroelectricity, Antiferroelectricity, and Ultrathin 2d Electron/Hole Gas in Multifunctional Monolayer Mxene, Nano Lett., 2017, 17, 3290–3296 CrossRef CAS PubMed.
- B. Xu, H. Xiang, Y. D. Xia, K. Jiang, X. G. Wan, J. He, J. Yin and Z. G. Liu, Monolayer Agbip2se6: An Atomically Thin Ferroelectric Semiconductor with out-Plane Polarization, Nanoscale, 2017, 9, 8427–8434 RSC.
- A. Belianinov, Q. He, A. Dziaugys, P. Maksymovych, E. Eliseev, A. Borisevich, A. Morozovska, J. Banys, Y. Vysochanskii and S. V. Kalinin, Cuinp2s6 Room Temperature Layered Ferroelectric, Nano Lett., 2015, 15, 3808–3814 CrossRef CAS PubMed.
- M. W. Si, et al., Room-Temperature Electrocaloric Effect in Layered FerroelectricCuinp2s6 for Solid-State Refrigeration, ACS Nano, 2019, 13, 8760–8765 CrossRef CAS PubMed.
- Z. Y. Fei, W. J. Zhao, T. A. Palomaki, B. S. Sun, M. K. Miller, Z. Y. Zhao, Q. Yan,J., X. D. Xu and D. H. Cobden, Ferroelectric Switching of a Two-Dimensional Metal, Nature, 2018, 560, 336–339 CrossRef CAS PubMed.
- R. X. Fei, W. Kang and L. Yang, Ferroelectricity and Phase Transitions in Monolayer Group-IV Monochalcogenides., Phys. Rev. Lett., 2016, 117, 097601 CrossRef PubMed.
- D. Di Sante, A. Stroppa, P. Barone, M. H. Whangbo and S. Picozzi, Emergence of Ferroelectricity and Spin-Valley Properties in Two-Dimensional Honeycomb Binary Compounds, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 161401 CrossRef.
- L. Li and M. H. Wu, Binary Compound Bilayer and Multilayer with Vertical Polarizations: Two-Dimensional Ferroelectrics, Multiferroics, and Nanogenerators, ACS Nano, 2017, 11, 6382–6388 CrossRef CAS PubMed.
- E. Bruyer, D. Di Sante, P. Barone, A. Stroppa, M. H. Whangbo and S. Picozzi, Possibility of Combining Ferroelectricity and Rashba-Like Spin Splitting in Monolayers of the 1t-Type Transition-Metal Dichalcogenides Mx2 (M = Mo, W; X = S, Se, Te), Phys. Rev. B, 2016, 94, 195402 CrossRef.
- H. L. L. Zhuang, Y. Xie, P. R. C. Kent and P. Ganesh, Computational Discovery of Ferromagnetic Semiconducting Single-Layer Crsnte3., Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 92, 035407 CrossRef.
- Z. Y. Tu, M. H. Wu and X. C. Zeng, Two-Dimensional Metal-Free Organic Multiferroic Material for Design of Multifunctional Integrated Circuits, J. Phys. Chem. Lett., 2017, 8, 1973–1978 CrossRef CAS PubMed.
- W. Luo, K. Xu and H. J. Xiang, Two-Dimensional Hyperferroelectric Metals: A Different Route to Ferromagnetic-Ferroelectric Multiferroics, Phys. Rev. B: Condens. Matter Mater. Phys., 2017, 96, 235415 CrossRef.
- J. J. Zhang, L. F. Lin, Y. Zhang, M. H. Wu, B. I. Yakobson and S. Dong, Type-Ii Multiferroic Hf2vc2f2 Mxene Monolayer with High Transition Temperature, J. Am. Chem. Soc., 2018, 140, 9768–9773 CrossRef CAS PubMed.
- C. X. Huang, Y. P. Du, H. P. Wu, H. J. Xiang, K. M. Deng and E. J. Kan, Prediction of Intrinsic Ferromagnetic Ferroelectricity in a Transition-Metal Halide Monolayer., Phys. Rev. Lett., 2018, 120 Search PubMed.
- Y. Lu, R. X. Fei, X. B. Lu, L. H. Zhu, L. Wang and L. Yang, Artificial Multiferroics and Enhanced Magnetoelectric Effect in van der Waals Heterostructures, ACS Appl. Mater. Interfaces, 2020, 12, 6243–6249 CrossRef CAS PubMed.
- W. Sun, W. Wang, D. Chen, Z. Cheng and Y. Wang, Valence Mediated Tunable Magnetism and Electronic Properties by Ferroelectric Polarization Switching in 2d Fei2/In2se3 van der Waals Heterostructures, Nanoscale, 2019, 11, 9931–9936 RSC.
- L. Z. Li and B. Z. Zhou, Theoretical Investigation of Nonvolatile Electrical Control Behavior by Ferroelectric Polarization Switching in Two-Dimensional Mncl3/Cuinp2s6 van der Waals Heterostructures, J. Mater. Chem. C, 2020, 8, 4534–4541 RSC.
- X. Tang, J. Shang, Y. D. Ma, Y. T. Gu, C. F. Chen and L. Z. Kou, Tuning Magnetism of Metal Porphyrazine Molecules by a Ferroelectric In2se3 Monolayer, ACS Appl. Mater. Interfaces, 2020, 12, 39561–39566 CrossRef CAS PubMed.
- A. Chandrasekaran, A. Mishra and A. K. Singh, Ferroelectricity, Antiferroelectricity, and Ultrathin 2d Electron/Hole Gas in Multifunctional Monolayer Mxene, Nano Lett., 2017, 17, 3290–3296 CrossRef CAS PubMed.
- N. B. McKeown, Phthalocyanine Materials: Synthesis, Structure, and Function, Cambridge University Press, Cambridge, UK, New York, 1998, vol. xvi, p. 193 Search PubMed.
- A. B. Sorokin, Phthalocyanine Metal Complexes in Catalysis, Chem. Rev., 2013, 113, 8152–8191 CrossRef CAS PubMed.
- L. Bogani and W. Wernsdorfer, Molecular Spintronics Using Single-Molecule Magnets, Nat. Mater., 2008, 7, 179–186 CrossRef CAS PubMed.
- Y. Shirota and H. Kageyama, Charge Carrier Transporting Molecular Materials and Their Applications in Devices, Chem. Rev., 2007, 107, 953–1010 CrossRef CAS PubMed.
- G. Kresse and J. Furthmuller, Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- P. E. Blochl, Projector Augmented-Wave Method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- S. L. Dudarev, G. A. Botton, S. Y. Savrasov, C. J. Humphreys and A. P. Sutton, Electron-Energy-Loss Spectra and the Structural Stability of Nickel Oxide: An Lsda+U Study, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 1505–1509 CrossRef CAS.
- S. Grimme, Semiempirical Gga-Type Density Functional Constructed with a Long- Range Dispersion Correction, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- K. Momma and F. Izumi, Vesta 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data., J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- R. F. Bader, Atoms in Molecules, Acc. Chem. Res., 1985, 18, 9–15 CrossRef CAS.
- W. J. Cho, Y. Cho, S. K. Min, W. Y. Kim and K. S. Kim, Chromium Porphyrin Arrays as Spintronic Devices, J. Am. Chem. Soc., 2011, 133, 9364–9369 CrossRef CAS PubMed.
- M. S. Liao and S. Scheiner, Electronic Structure and Bonding in Metal Porphyrins, Metal = Fe, Co, Ni, Cu, Zn, J. Chem. Phys., 2002, 117, 205–219 CrossRef CAS.
- X. Li, J. Zhou, Q. Wang, Y. Kawazoe and P. Jena, Patterning Graphitic C–N Sheets into a Kagome Lattice for Magnetic Materials, J. Phys. Chem. Lett., 2013, 4, 259–263 CrossRef CAS PubMed.
- A. Du, S. Sanvito and S. C. Smith, First-Principles Prediction of Metal-Free Magnetism and Intrinsic Half-Metallicity in Graphitic Carbon Nitride, Phys. Rev. Lett., 2012, 108, 197207 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2022 |