 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electrocatalysis enabled transformation of earth-abundant water, nitrogen and carbon dioxide for a sustainable future
Kaili
Liu†
ab,
Pengfei
Cao†
c,
Wei
Chen†
a,
Collins I.
Ezeh
a,
Zijian
Chen
 a,
Yonglan
Luo
a,
Yonglan
Luo
 d,
Qian
Liu
e,
Haitao
Zhao
*a,
Zhenhua
Rui
f,
Shuyan
Gao
d,
Qian
Liu
e,
Haitao
Zhao
*a,
Zhenhua
Rui
f,
Shuyan
Gao
 g,
Zongyou
Yin
g,
Zongyou
Yin
 *b,
Xuping
Sun
*b,
Xuping
Sun
 *d and
Xuefeng
Yu
*d and
Xuefeng
Yu
 a
a
aMaterials Interfaces Center, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, Guangdong, China. E-mail: ht.zhao@siat.ac.cn
bResearch School of Chemistry, Australian National University, Canberra, ATC 2601, Australia. E-mail: zongyou.yin@anu.edu.au
cSchool of Chemical Engineering and Technology, Xi'an Jiaotong University, Xi'an 710049, Shanxi, China
dInstitute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu 610054, Sichuan, China. E-mail: xpsun@uestc.edu.cn
eInstitute for Advanced Study, Chengdu University, Chengdu 610106, Sichuan, China
fDepartment of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, 02139, USA
gSchool of Materials Science and Engineering, Henan Normal University, Xinxiang 453007, Henan, China
First published on 30th November 2021
Abstract
The integration of electrochemistry with catalyst systems underscores a major sustainable scheme for the production of fossil-free fuels and valuable chemicals. This undertaking necessitates the need for rational design of electrocatalysts with high catalytic activity, selectivity, and stability for electrochemical conversion. Significant progress has been made in this regard considering the importance of the products in these reaction systems. Hence, this review presents an update of both experimental and theoretical investigations that can offer insights into the design of high-performance electrocatalysts to facilitate the electrochemical conversion of H2O, N2 and CO2 into value added products. We analyse the current status of available electrocatalysts based on a standard set of figures of merit, namely yield rate, faradaic efficiency, overpotential, current density and stability. Then, we constructively compare the different electrocatalysts based on their reaction mechanisms and operation performances by evaluating the catalyst construction, electrolyte utilization and device practicality. Finally, we provide challenges and prospects from the aspects of both theoretical and experimental insights as a general guide to offer potential future directions.
1. Introduction
Fossil fuels are the primary energy source driving over 86% of the global anthropogenic effect, not to mention their limited abundance that could only sustain for the next 50 years approximately.1 Their continuous consumption will lead to an increase in the level of atmospheric CO2 with a deleterious impact on the climate. To effectively mitigate this impact for a sustainable future, it is critical to replace fossil fuels with alternative energy sources.2 Earth's atmosphere provides a universal feedstock of water, carbon dioxide, and nitrogen which can be converted into hydrogen, hydrocarbons and ammonia, respectively. This would significantly contribute to solve societal problems sustainably in terms of clean energy (hydrogen fuel), chemical sources (hydrocarbon, ammonia) and a clean environment (CO2 capturing).The development of electrochemical technology that can transform earth-abundant molecules into valuable products offers us the chance to directly address the most pressing environmental challenges and energy crisis by decreasing the utilization of fossil fuels while increasing the production of sustainable fuels and chemicals. Unfortunately, the efficiency and/or stability of such electrochemical processes are still not that satisfactory due to the sluggish kinetics of key reactions and/or the catalyst's susceptibility to the working environment. The integration of the electrochemical processes and rationally designed catalysts is essential for the transformation of these abundant molecules with efficient and versatile platforms to store and utilize green energy and/or produce valuable chemicals for other uses.3,4
An emerging sustainable scheme considers the coupling of renewable-energy plants and electrocatalytic reactors, where electrocatalysis can enable the high-performance chemical transformation with improved kinetics, efficiency, and selectivity. Electrocatalysis is an interdisciplinary area that encompasses the principles of physics, chemistry, and materials science to allow a comprehensive understanding of the reaction kinetics and stability.5 Hence, insight into electrocatalysis is an indispensable issue to ensure the scalable transformation of earth-abundant molecules for a sustainable future.6,7
The pioneering works to transform these molecules have long been recorded in history. Electrochemical water splitting was demonstrated for the production of hydrogen in 1800 by Nicholson and Carlisle.8 Following the first substantiated report on CO2 reduction reaction (CRR) via electrochemistry by Jordan and Smith in 1960,9,10 Hori and co-workers revealed the possible means of producing carbonaceous chemicals and fuels using this process in 1985.11–13 In 1990, electrocatalytic nitrogen reduction reaction to ammonia was reported by Furuya and Yoshiba.14 Because of these historical initiatives, more scientific studies adopting these chemical transformations and energy conversion processes have been conducted. Despite the recent development, these electrochemical processes are confronted with shortcomings, limiting their practical implementation. Therefore, it is of paramount importance to carry out a systematic review on the development of electrocatalysts for a sustainable future.
Nowadays, high-quality reviews on the general concept of electrocatalysis3 and electrocatalysts for energy-related reactions15 and perspectives on electrocatalytic conversion6 have been reported addressing the fundamentals and technical issues. However, there is still a paucity of information regarding the comprehensive and critical outlook on advanced electrocatalysts for the transformation of these three molecules (water, nitrogen and carbon dioxide) with a systematic and constructive survey. Moreover, the field is developing very rapidly and hence new progress is being made in understanding the role of electrocatalysts in the key reactions such as water splitting, NRR and CRR. Therefore, this field deserves a timely review encompassing the dynamic advancement of electrocatalysis in transforming earth-abundant molecules into valuable products for a sustainable future.
Hence, this study is a critical review of both experimental and theoretical investigations offering insights into catalysts for electrochemical conversion of essential molecules – water, nitrogen and carbon dioxide – and guiding the design of high-performance catalyst systems necessary to facilitate these conversions. Herein, we analyze the current status of available catalytic materials (metal and metal-free) based on a standard set of figures of merit, namely faradaic efficiency, energy efficiency, overpotential, current density, and stability. This approach enables a fair and insightful comparison among the different electrocatalysts, identifies the limiting phenomena, and forecasts the feasibility of practical applications in a much shorter timeframe.
2. Hydrogen production via water splitting
Hydrogen energy is a carbon-neutral resource for replacing fossil fuels in the future and the ‘hydrogen economy’ calls for efficient processes for the production of hydrogen from renewables.16,17 So far, there are three recognized approaches to produce hydrogen: water splitting, steam reformation of methane and coal gasification.1 Among them, water splitting is a spotless pathway and indeed represents the most sustainable strategy if backed up by renewable resources. Yet, this route accounts only for approximately 1% of the overall hydrogen production in the world.18–20 To function effectively, water splitting has been pursuing the development of catalysts that can stabilize and accelerate the reaction along a particular route. Catalysts with good charge transfer, high activity, high selectivity and extensive stability are desired.21–23 Electrocatalysts have been widely employed to enhance the conversion efficiency of water to H2 and O2.24,25 Herein, we discuss the chemistry underlying the electrosynthesis of H2 and O2 from water and thereafter, review recent advancements in the catalytic performances of electrocatalysts towards the overall water splitting.2.1. Electrochemistry of water splitting
A typical water splitting electrolytic cell was first proposed in 1789 by J. R. Deiman and Adriaan Paets van Troostwijk.26 The water splitting process involves two half-cell reactions: anodic oxidation and cathodic reduction reactions. Alternatively, these reactions are conceptualised as oxygen evolution reaction (OER) and hydrogen evolution reaction (HER), respectively.Based on the nature of the electrolytes, the specific half-cell reactions are different at the electrodes. Nevertheless, the overall reaction is the same.6,27
In an acidic electrolyte:
At the cathode:
| 2H+ + 2e− → 2H2 + O2, Ec = 0 V | (1) |
| 2H2O → O2 + 4H+ + 4e−, Ea = 1.23 V | (2) |
At the cathode:
| 2H2O + 2e− → H2 + 2OH−, Ec = −0.83 V | (3) |
| 4OH− → O2 + 2H2O + 4e−, Ea = −0.40 V | (4) |
| 2H2O → 2H2 + O2 | (5) |
| EOP = 1.23 V + ηT |
| EOP = 1.23 V + ηa + ηc + ηother | (6) |
In an acidic medium30,31 (* indicates the active site on the catalyst surface),
| H3O+ + e−+ * → H* + H2O, Volmer reaction | (7) |
| H* + H3O+ + e− → H2 + H2, Heyrovsky reaction | (8) |
| H* + H* → H2, Tafel reaction | (9) |
| H2O+ + e−+ * → H* + OH−, Volmer reaction | (10) |
| H* + H2O + e− → H2 + OH−, Heyrovsky reaction | (11) |
| H* + H* → H2, Tafel reaction | (12) |
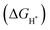 is a universally established descriptor for hydrogen-evolving materials. The
is a universally established descriptor for hydrogen-evolving materials. The 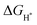 value for an optimal HER catalyst is estimated to be close to zero. The reason for this is that a large
value for an optimal HER catalyst is estimated to be close to zero. The reason for this is that a large 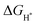 suggests the difficulty of breaking the adsorbed H* and hence, impeding H2 desorption. Moreover, a low
suggests the difficulty of breaking the adsorbed H* and hence, impeding H2 desorption. Moreover, a low 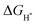 value indicates a weak H* adsorption resulting in a poor interaction between the cathode and the protons.
value indicates a weak H* adsorption resulting in a poor interaction between the cathode and the protons.
A computationally derived volcano plot of theoretical 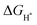 vs. the exchange current densities (log
vs. the exchange current densities (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j0) reflects the HER activities for a range of catalysts.32 This plot proposes an instinctive approach to visualize and compare the activity of a range of catalysts to enable the optimization of material design for HER. In addition, understanding the relative HER mechanism for each material is also crucial towards its design. The Tafel slope, derived from the Tafel plot and polarization curve, can be used to understand the HER mechanism.33,34 This slope is an inherent property of the catalyst, which is computed to define the rate-determining step of the HER process. In general, there are three Tafel slopes recognized to get insight into the reaction kinetics in HER, namely, 29 mV dec−1, 39 mV dec−1 and 118 mV dec−1, representing the Tafel, Heyrovsky and Volmer reactions, respectively. The rate-determining step of an electrode is defined by the proximity of its Tafel slope to that of the above-mentioned reactions. However, it is a challenge to unequivocally distinguish whether the rate-determining step is Tafel and Heyrovsky given that the values of their Tafel slopes are similar.
j0) reflects the HER activities for a range of catalysts.32 This plot proposes an instinctive approach to visualize and compare the activity of a range of catalysts to enable the optimization of material design for HER. In addition, understanding the relative HER mechanism for each material is also crucial towards its design. The Tafel slope, derived from the Tafel plot and polarization curve, can be used to understand the HER mechanism.33,34 This slope is an inherent property of the catalyst, which is computed to define the rate-determining step of the HER process. In general, there are three Tafel slopes recognized to get insight into the reaction kinetics in HER, namely, 29 mV dec−1, 39 mV dec−1 and 118 mV dec−1, representing the Tafel, Heyrovsky and Volmer reactions, respectively. The rate-determining step of an electrode is defined by the proximity of its Tafel slope to that of the above-mentioned reactions. However, it is a challenge to unequivocally distinguish whether the rate-determining step is Tafel and Heyrovsky given that the values of their Tafel slopes are similar.
For instance, Zhao and co-workers reported that the Tafel slope of a Pt electrode with the Pt(110) plane is ∼30 mV dec−1; however, the rate-determining step could be either the Tafel or Heyrovsky reaction despite the value being close to that of Tafel reaction.35 To distinguish these reactions, the rate-determining steps (Tafel or Heyrovsky) should be related to the surface coverage of H* on the electrode.35,36 The determination and interpretation of the reaction mechanisms are significant to gain theoretical insight into the elementary steps involved in HER.
These reactions occur with the involvement of adsorbed OH−, O2− and OOH− intermediates on the surface of the catalyst. Hypothetically, a generally accepted OER mechanism is illustrated as follows.38
OER in an acidic medium:
| H2O + * → HO* + H+ + e− | (13) |
| HO* → O* + H+ + e− | (14) |
| O* + H2O → HOO* + H+ + e− | (15) |
| HOO* → * + O2 + H+ + e− | (16) |
| OH− + * → HO* + e− | (17) |
| OH− + HO* → O* + H2O + e− | (18) |
| O* + OH− → HOO* + e− | (19) |
| 4HOO* → * + O2 + 2H2O + e− | (20) |
| 2H2O + * → H2O + HO* + H+ + e− | (21) |
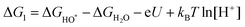 | (22) |
| H2O + HO* → H2O + O* + H+ + e− | (23) |
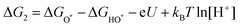 | (24) |
| O* + H2O → HOO* + H+ + e− | (25) |
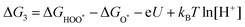 | (26) |
| HOO* → * + O2 + H+ + e− | (27) |
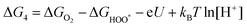 | (28) |
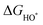 ,
, 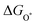 , and
, and 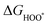 are 1.23 eV, 2.46 eV and 3.69 eV, respectively.40
are 1.23 eV, 2.46 eV and 3.69 eV, respectively.40
Under standard conditions, when the measured electrode potential is 0 versus the standard hydrogen electrode (SHE), the theoretical overpotential ηOER is defined as26
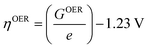 | (29) |
For minimal overpotential (ηOER = 0), ΔGOER is 1.23 eV. Given that ΔG1 = ΔG2 = ΔG3 = ΔG4 for an ideal OER catalyst under standard conditions, this suggests that the ΔG for each step at ηOER = 1.23 eV.40 Based on this hypothesis, a volcano plot analogous to that for the HER catalysts is obtained for a range of OER catalysts (Fig. 1). Here, the standard free energy 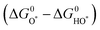 is plotted against the theoretical overpotential to reflect the OER activities for a series of catalysts.
is plotted against the theoretical overpotential to reflect the OER activities for a series of catalysts. 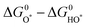 is a notable descriptor for OER activity. Deductions from the volcano plot indicate that the plot can be beneficial towards the design and optimization of highly efficient OER catalysts. Moreover, the Tafel slope, which is also correlated with the OER overpotential, can direct the understanding of the OER mechanism by providing kinetic information of OER catalysts.42
is a notable descriptor for OER activity. Deductions from the volcano plot indicate that the plot can be beneficial towards the design and optimization of highly efficient OER catalysts. Moreover, the Tafel slope, which is also correlated with the OER overpotential, can direct the understanding of the OER mechanism by providing kinetic information of OER catalysts.42
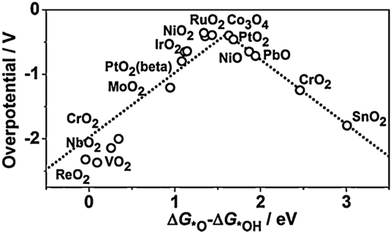 | ||
| Fig. 1 Volcano plot for OER on metal oxides. Reproduced with permission.43 Copyright 2015, Elsevier. | ||
2.2 Electrocatalysts for water splitting
To sustain an efficient and economic water splitting process, HER–OER bifunctional catalysts that can offer a minimum overpotential in aqueous alkaline or acidic medium are indispensable.44 However, the design and development of electrocatalysts are complex and challenging as several factors need to be considered in this regard.45 For instance, the electrocatalysts suitable for alkaline solutions may be unstable or inactive in acidic medium, and vice versa. Therefore, it is exceedingly attractive to develop bifunctional electrocatalysts with high activity towards both OER and HER in the same electrolyte.46,47Hitherto, both metal-based and metal-free catalyst systems have shown potential to catalyze the water splitting process. Metal-based catalyst systems including noble metals (such as iridium (Ir), palladium (Pd), platinum (Pt), ruthenium (Ru) and gold (Au)) and transition metals (TMs, such as cobalt (Co), iron (Fe), nickel (Ni), molybdenum (Mo) and copper (Cu)) and their compounds are the most widely utilized HER–OER electrocatalysts. However, owing to their structural complexities, physiochemical challenges and thermodynamic instability, these catalyst systems are constrained by their weak durability and low selectivity. This coupled with their high cost has led to research endeavor to seek for alternatives to replace metal-based electrocatalysts for water splitting.44
Following the discovery of carbon-based materials as oxygen reduction reaction (ORR) catalysts in 2009, steps were taken to design and develop metal-free electrocatalysts for water splitting. Thereafter, considerable headway has been made in this regard, particularly with respect to earth-abundant metal-free materials.44,45 Beyond their natural abundance, metal-free electrocatalysts have demonstrated good catalytic performance for water splitting due to their remarkable electrical conductivity, large surface area and high tolerance under wide operating conditions.46,48 Moreover, the flexible architecture of metal-free catalysts exposes these materials to potential heteroatom doping and structure reengineering, which can modulate the charge distribution of the carbon genomics with potential synergistic effects for water splitting.49 Notable examples of these catalysts include heteroatom-doped carbon nanotubes and graphene.50
Apart from the category, composition and structure of electrocatalysts, the properties (e.g. pH) of electrolytes play a role in the electrochemical water splitting reactions. The acidic electrolytes are beneficial for water splitting as they offer more hydroniums (H3O+) with weak covalent bonding and thereby lead to fast reaction kinetics for the reduction reaction at the cathode while the alkaline water splitting is more popular than acidic water splitting because of the availability of more choice of electrocatalysts for anodic half-cell OER reaction and the facile formation of OH* and O* species.51 Moreover, the strong acid condition requires the employment of an acid proton exchange membrane and suffers from the issue of high cost and pollution from the evaporated acid electrolyte. Comparatively, an alkaline electrolyte can relieve these problems in acid conditions for water splitting. The alkaline environment, typically realized by the adoption of 1.0 M KOH solution, has been widely employed for water splitting and has demonstrated inspiring performance over the past few decades.51 However, the drawbacks of harsh alkaline or acidic electrolytes such as the corrosion issue and the requirement of specific ion exchange membranes with good stability are inescapable when it comes to applications. Therefore, electrochemical water splitting under neutral and near-neutral conditions has gained much research interest.52,53 Different from water electrolysis under extreme acidic or alkaline conditions, water splitting under neutral or near-neutral conditions is seriously affected by reactant switching and identity and concentration of the buffered anions in similar pH values. Thus, buffer solutions, for instance phosphate buffered saline (pH 7), bicarbonate and carbonate buffer solution (pH 9.2 to 10.6), sodium sulfate solution (pH 7) and borate buffer solution (pH 8.5), are widely used in neutral electrocatalytic water splitting.54
Shu-Hong Yu and co-workers55 reported the synthesis and application of ternary Ni0.1Co0.9P porous nanosheets onto conductive carbon fiber paper which exhibited promising potential in neutral-pH water electrolysis. The ternary Ni0.1Co0.9P catalyst endows the resultant device with a voltage requirement of mere 1.81 V to reach a current density of 10 mA cm−2, showing excellent efficiency among the noble-metal-free neutral-pH electrolyzers. Jie Yu and co-workers56 reviewed the recent research on the electrocatalytic water-splitting performance of precious-metal-free catalytic materials in neutral media. Anantharaj and Aravindan54 summarized 3d transition-metal-based electrocatalysts for neutral and near-neutral water splitting from the perspective of activity, selectivity, and stability. These reviews provide a comprehensive summary of the catalytic performance of catalysts with neutral electrolytes. It is worth noting that the neutral electrolytes are good electrolytes with potential as they have more similar physical and chemical properties to seawater. However, most electrocatalysts towards overall water splitting showed better efficiency in alkaline electrolytes. It is still promising to develop efficient electrocatalysts in a neutral or near neutral pH environment.
In general, bifunctional electrocatalysts preferentially favor either the HER or OER depending on their intrinsic characteristics. Hence, to enable a high overall performance, the intrinsic activity and physiochemical properties of the catalysts should be synergistically improved.46 In this section, we critically review the recent advances in metal-based and metal-free electrocatalysts towards the overall water splitting process, and discuss the theoretical perspectives in understanding the catalytic process and recent challenges.
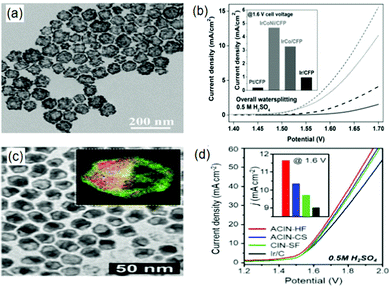 | ||
| Fig. 2 (a) TEM image of IrCoNi PHNCs and their (b) polarization curve in comparison to that of IrCo/CFP, Ir/CFP, and Pt/CFP for overall water splitting in 0.5 M H2SO4 solution at a scan rate of 5 mV s−1 (inset: with the corresponding current densities at 1.6 V). Panels (a) and (b) are reproduced with permission.61 Copyright 2017, Wiley-VCH. (c) TEM image of AuCu@IrNi and (d) polarization curves of ACIN-HF/CFP, ACIN-CS/CFP, CIN-SF/CFP and Ir/CFP for overall water splitting in 0.1 M HClO4 solution at a scan rate of 5 mV s−1 (inset: with the corresponding current densities). Panels (c) and (d) are reproduced with permission.62 Copyright 2019, Royal Society of Chemistry. | ||
Alternatively, the anchoring of noble metals on support surfaces has presented a myriad of novel heterogeneous electrocatalysts with high HER–OER activity. This approach typically augments the interaction between the metals and the support, thereby leading to an enhanced charge distribution. Specifically, exploiting surface defects on the support (particularly supports that offer enriched coordinating atoms with lone electron pairs as active centers) and fabricating porous frameworks are the most recently adopted schemes to improve the catalytic activity.60 For instance, Lee and co-workers utilized a structure-supporting hemispherical core–shell to fortify the HER–OER activity on an Ir-based multi-metallic nanoframe anchored on an Au-based core (AuCu@IrNi).62 Here, the authors demonstrated that the developed hemicore@frame AuCu@IrNi complex suitably catalyzed OER and HER in 0.1 M HClO4 with low overpotentials of 308 mV and 13.7 mV with Tafel slopes of 58 mV dec−1 and 22 mV dec−1, respectively (Fig. 2(c) and (d)).
Table 1 summarizes the catalytic activity of some of the bifunctional water splitting electrocatalysts. Generally, most noble metals are inclined to favor either the OER or HER process; therefore they are not so effective for bifunctional water splitting, particularly in the same medium. It is well documented that modulating the surface morphology, electronic structure, and element doping (bi- or multi-metallic) plays a crucial role in upgrading the activity of noble metal-based materials as OER-HER bifunctional electrocatalysts. Nonetheless, the commercial application of these catalysts is limited by their scarcity and high cost. This has driven the research interest for the design of efficient catalyst systems made from readily available and low-cost materials such as earth-abundant materials.
| Electrocatalyst | Electrolyte | Mass loading (mg cm−2) | Overpotential@j (mA cm−2) (mV) | Tafel slope (mV/dec) | Cell voltage (V)@j (mA cm−2) | Overall stability | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| HER | OER | HER | OER | ||||||
| Noble metal-based | |||||||||
| AuCu@IrNi | 0.1 M HClO4 | — | 13.7@10 | 308@10 | 22 | 58 | — | — | 62 |
| 0.5 M H2SO4 | — | — | — | — | — | 1.585@10 | 24 h | ||
| IrCo alloys | 0.5 M H2SO4 | 0.0189 (Ir) | 23.9@10 | 270@10 | 25.7 | 71.8 | 1.55@10 | 100 min | 165 |
| IrNiCo PHNC | 0.5 M H2SO4 | — | 68@10 | 309@10 | — | — | 1.52@2 | 1000 cycles | 61 |
| 0.1 M HClO4 | — | 50@17.43 | 303@10 | 31.9 | 53.8 | — | 200 min | ||
| Pt62Co23/Ir15 FBNWs/C | 0.1 M HClO4 | — | 14@10 | 308@10 | — | — | 1.53@10 | 10 h | 166 |
| Ru2Ni2 SNs | 1.0 M KOH | — | 39.3@10 | 357@10 | 25 | ∼100 | 1.58@10 | 40 h | 167 |
| Non-noble metal-based | |||||||||
| Metallic substance | |||||||||
| Cu–MOF (8%GO) | 0.5 M H2SO4 | 0.226 | 209@30 | 110@2 | 84 | 65 | — | — | 63 |
| Fe-FeOx-FeSx | 0.1 M KOH | — | 360@10 | 400@10 | — | — | 1.68@10 | 3 d | 69 |
| Ni-B/Ni foam | 1.0 M KOH | 12.3 | 125@20 | 360@100 | 93 | 76 | 1.69@15 | 10 h | 68 |
| CoSn2 | 1.0 M KOH | ∼1 | 196@10 | 299@10 | 78 | 89 | — | — | 168 |
| ∼3 | 103@10 | 230@10 | — | — | 1.55@10 | 16 h | |||
Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu (1 Cu (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) 8) |
0.1 M KOH | 1.25 | 103@10 | 313@10 | 335 ± 1.0 | 162 ± 0.7 | 1.8@10 | 10 h | 80 |
| Metal chalcogenides | |||||||||
| Ni3S2/NF | pH 14 | 1.6 | 223@10 | 260@10 | — | — | 1.76@13 | >200 h | 87 |
| NiSe/NF | 1.0 M KOH | 2.8 | 96@10 | 270@20 | 120 | 64 | 1.63@10 | 12 h | 70 |
| Zn0.1Co0.9Se2 | 1.0 M KOH | 0.285 | — | 340@10 | — | 43.2 | — | — | 169 |
| 0.5 M H2SO4 | 140@10 | — | 49.9 | — | — | — | |||
| MoS2/Ni(OH)2 | 1.0 M KOH | — | 134@10 | 233@10 | 35 | 49 | 1.46@10 | 50 h | 94 |
| MoS2/Ni3S2 | 1.0 M KOH | 7 | 110@10 | 218@10 | 83 | 88 | 1.56@10 | 10 h | 115 |
| (Ni, Fe)S2/MoS2 | 1.0 M KOH | — | 130@10 | 270@10 | 101.22 | 43.21 | 1.56@10 | 28 h | 170 |
| Ni/Ni9S8 | 1.0 M KOH | 11.04 | 230@10 | 340@30 | 123.3 | 109.8 | — | — | 139 |
| H–Fe–CoMoS | 1.0 M KOH | — | 137@10 | 282@10 | 98 | 58 | 1.60@20 | — | 171 |
| Co9S8/Ni3S2 | 1.0 M KOH | — | 128@10 | 227@10 | 97.6 | 46.5 | 1.64@10 | 12 h | 116 |
| CuCo2S4 | 1.0 M KOH | ∼2 | 158@10 | 290@20 | 113 | — | 1.66@10 | 24 h | 105 |
| Mo/Mn-NixSy/NF | 1.0 M KOH | 7.67 | 162@50 | 144@10 | 91 | 110 | 1.49@10 | 24 h | 172 |
| NCT-NiCo2S4 | 1.0 M KOH | — | 295@100 | 330@100 | 86.8 | 86.8 | 1.60@10 | 15 h | 173 |
| NiCo2S4 NA/CC | 1.0 M KOH | 4 | 263@50 | 310@50 | 141 | 89 | 1.68@10 | 12 h | 114 |
| 305@100 | 340@100 | ||||||||
| Metal phosphates and phosphides | |||||||||
| Fe2P | 0.5 M H2SO4 | — | 191@10 | — | 55 | — | — | — | 142 |
| 1.0 M KOH | — | 300@10 | 390@10 | 126 | — | — | — | ||
| FeP NTs | 0.5 M H2SO4 | 1.6 | 88@10 | — | 35.5 | — | 1.69@10 | 14 h | 141 |
| 1.0 M KOH | 120@10 | 288@10 | 59.5 | 43 | — | — | |||
| Ni-P foam | 1.0 M KOH | — | 150@10 | 350@191 | — | 179.9 | 1.44@5 | 26 h | 174 |
| Ni2P | 1.0 M KOH | 0.14 | 220@10 | 290@10 | — | 59 | 1.63@10 | 210 min | 136 |
| Ni5P4 | 0.5 M H2SO4 | 3.5 | 140@10 | — | 40 | — | 1.7@10 | 20 h | 138 |
| 1.0 M KOH | — | 150@10 | 290@10 | 53 | — | — | — | ||
| Co-P-B | 1.0 M KOH | 0.3 | 145@10 | 290@10 | 38 | 48 | 1.65@10 | 20 h | 175 |
| Ni/Ni8P3 | 1.0 M KOH | 10.58 | 130@10 | 270@30 | 58.5 | 73.2 | 1.61@10 | 24 h | 139 |
| NiFeP | 0.5 M H2SO4 | — | 143@10 | — | 67 | — | — | — | 142 |
| 1.0 M KOH | — | 255@10 | 227@10 | 83 | — | — | — | ||
| LiCoBPO | 1.0 M KOH | ∼1 | 245@10 | 293@10 | 98 | 58 | 1.94@10 | 90 h | 176 |
| ∼3 | 121@10 | 216@10 | 121 | 62 | 1.84@10 | 10 days | |||
| NaCoBPO | 1.0 M KOH | ∼1 | 298@10 | 328@10 | 124 | 60 | — | — | 176 |
| ∼3 | 207@10 | 242@10 | 128 | 99 | — | — | |||
| N-NiCoP/NCF | 1.0 M KOH | ∼2.085 | 78@10 | 225@10 | 83.17 | 66.94 | — | 100 h | 177 |
| Metal nitrides | |||||||||
| TiN@Ni3N | 1.0 M KOH | 0.6 | 21@10 | 350@10 | 42.1 | — | 1.64@10 | 16 h | 148 |
| Ni3FeN-NPs | 1.0 M KOH | 0.35 | 158@10 | 280@10 | 42 | 46 | — | 9 h | 151 |
| Ni3N-NiMoN | 1.0 M KOH | ∼1.63 | 31@10 | 277@10 | 64 | 118 | 1.54@10 | 20 h | 178 |
| Metal carbides | |||||||||
| β-Mo2C | 1.0 M KOH | 0.25 | 130@10 | 274@10 | 66.5 | 70 | 1.65@10 | 30 h | 179 |
| B, N: Mo2C/BCN | 1.0 M KOH | ∼1.0 | 100@10 | 360@100 | 62 | 61 | 1.84@100 | 20 h | 180 |
| Metal-free | |||||||||
| e-ICLDH@GDY | 1.0 M KOH | — | 43@10 | 216@10 | 98.9 | 43.6 | 1.43@10 | 60 h | 181 |
| — | 215@100 | 249@100 | — | — | 1.46@100 | — | |||
| — | 256@1000 | 278@1000 | — | — | 1.49@1000 | — | |||
| ONPPGC/OCC | 1.0 M KOH | 0.1 | 446@10 | 410@10 | 154 | 83 | 1.66@10 | 10 h | 163 |
| 0.2 M PBS | 352@1 | 420@2 | 374 | 231 | 1.71@2 | ||||
| 0.5 M H2SO4 | 386@10 | 470@10 | 109 | 200 | 1.75@5 | ||||
| PO-Ni/Ni-N-CNFs | 1.0 M KOH | 8 | 262@10 | 420@10 | 97.42 | 113.1 | 1.69@10 | 40 h | 158 |
Metallic substance. Metallic substances are well-known electrocatalysts that are commonly used in the commercial application of electrolytic water splitting.67,68 Nevertheless, this set of electrocatalysts are known to exhibit low efficiency and less stability necessitating measures to enhance their electrocatalytic activity. For instance, Martindale and Reisner reported the unstable condition of Fe due to its dissolution at the anodic and cathodic potentials under neutral and acidic media, respectively.69 Similarly, Jahan and co-workers reported the instability of Cu complexes due to their corrosion and instability in an acidic medium.63
One option is incorporating the metal based materials with non-metal or another transition metal based dopant.68,70–72 Our group reported an amorphous Ni-B nanoparticle film on Ni foam (Ni-B/Ni foam) with good water splitting potential (Fig. 3(a) and (b)). An electrolytic cell voltage of 1.69 V was required to attain 15 mA cm−2 current density in 1.0 M KOH with overpotentials of 125 mV and 360 mV at 20 mA cm−2 and 100 mA cm−2 for the HER and OER, respectively.68
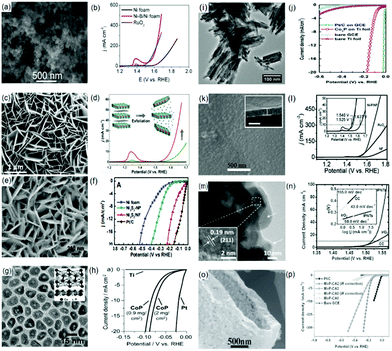 | ||
| Fig. 3 (a) SEM image of Ni-B/Ni foam. Polarization curve (in 1.0 M KOH at a scan rate of 2 mV s−1) for (b) OER on Ni foam, Ni-B/Ni foam and RuO2-Ni foam. Panels (a) and (b) are reproduced with permission.68 Copyright 2016, IOP Publishing Ltd. (c) TEM image of as-synthesized NiCo LDH nanoplates on carbon paper via HCFR. (d) Polarization curves (in O2-saturated 1 M KOH at a scan rate of 0.5 mV s−1) of NiCo LDH catalysts and carbon paper. Images (c) and (d) are reproduced with permission.113 Copyright 2015, American Chemical Society. (e) Top-view SEM image of Ni3S2/NF. (f) Steady-state current density as a function of applied voltage during HER at pH 7 over nickel foam (NF), Ni3S2-NP, Ni3S2/NF and Pt/C(20 wt%). Images (e) and (f) are reproduced with permission.87 Copyright 2015, American Chemical Society. (g) TEM image of CoP nanoparticles. (h) HER performance of the CoP/Ti electrode in 0.5 M H2SO4. Panels (g) and (h) are reproduced with permission.129 Copyright 2014, Wiley. (i) TEM image of the Co2P nanorods. (j) Polarization curves of Pt/C on GCE (loading amount: 0.285 g cm−2), Co2P on Ti foil, bare GCE, and bare Ti foil in 0.5 M H2SO4 solution (iR corrected). The bare Ti foil was subjected to the same annealing treatment as Co2P on a Ti foil sample prior to the measurement. Panels (i) and (j) are reproduced with permission.130 Copyright 2014, Elsevier. (k) SEM image of Ni-P/NF (inset: cross-sectional analysis). (l) Polarization curve (in 1.0 M KOH at a scan rate of 2 mV s−1) for OER on Ni-P/NF, NF, and Pt/C-NF. Panels (k) and (l) are reproduced with permission.85 Copyright 2015, Wiley. (m) HRTEM image of IPNTs (inset: enlarged HRTEM image). (n) iR-Corrected LSV curves measured in 1.0 M KOH (inset: the corresponding Tafel slopes). Panels (m) and (n) are reproduced with permission.141 Copyright 2015, Wiley. (o) High-magnification SEM images of the MoP-CA2 microstructure, and (p) polarization curves (in 0.5 M H2SO4 with a scan rate of 2 mV s−1) of MoP-CA2, MoP-CA0, Pt/C, and bare GCE. Reproduced with permission.64 Copyright 2014, Wiley. | ||
In the past, Co-70,73–75 and Fe-based76–78 compounds have been used as potential water splitting electrocatalysts. However, given that Fe is one of the most abundant earth metals and highly attractive for the development of low-cost catalysts, Fe-based compounds have been extensively examined as HER or OER electrocatalysts.76–78 Nonetheless, similar to Co-based metallic compounds, only a fair number of studies have reported the bifunctionally active catalysts compared to the Fe-based metallic compounds for water splitting. Among those studies, Martindale and Reisner demonstrated an Fe-only electrode to be active for catalysing both proton reduction and water oxidation in alkaline medium with superior activity than bifunctional Co and Ni electrocatalysts.69 The authors also demonstrated that the electrolyzer system with an Fe-only electrode was relatively more stable and durable. This was ascribed to the reversible interconversion of catalytically active Fe-species (an iron oxide-hydroxide (FeOx) phase under the anodic bias and Fe(0) phase under the cathodic bias).
Another example of low-cost catalytic materials is Cu and its derivatives, which are reported to be beneficial towards the activation of oxygen reduction reaction (ORR)79 and serve as HER/OER electrocatalysts.63,80 However, similar to Co-based metallic materials, the practical application of Cu-based metallic catalysts is hindered by their large overpotential and/or low stability, which requires further modifications. For instance, Jahan and co-workers fabricated a Cu-MOF composite by anchoring a Cu-centered MOF on graphene oxide (GO),63 which was employed as a tri-functional HER, OER and ORR electrocatalyst, particularly in an acidic solution. The exceptional performance of the catalyst was founded on the GO-MOF synergistic effects including the unique porous scaffold structure and improved electron transport. Likewise, anchoring Co on N-doped carbon nanostructures (Co–N–C)81,82 has been demonstrated to result in robust water splitting electrocatalysts due to the synergistic chemical coupling between the embedded Co and the N-dopant or carbon layers, leading to a better H-bonding energy necessary for HER82 and both greater mechanical and chemical rigidity.
Furthermore, the doping of these metallic catalysts with other metals or non-metals has also been shown to improve their individual electrocatalytic performance. For instance, the incorporation of Fe with Ni resulted in a Ni–Fe composite which displayed high potential as a HER–OER electrocatalyst in alkaline solutions, requiring overpotentials of only 240 and 270 mV to deliver current densities of 500 and 1000 mA cm−2, respectively. The electrode also displayed prolonged stability against bulk water electrolysis at large currents83 attributed to the optimal behaviour of this binary film due to the stabilizing effect of Fe on Ni at a higher oxidation level.84
The use of 3D catalytic substrates has been demonstrated towards improving the surface morphology, exposed active centres and electrical characteristics of the electrocatalysts.70,83,85,86 A commonly used low-cost conductive substrate is Ni foam, which when compared to other substrates such as Ni foil has shown superior activity due to its 3D macroporous structure and structure-induced electronic effect.83 In addition, Ni foam offers a much higher surface roughness than Ni foil, which tends to promote more surface activity.87,88 Other reported common conductive substrates include Ti plate27,44,89 and Cu foam.90
Metal chalcogenides. Metal chalcogenides such as sulfides70,72,85,91 and selenides44,70,89,90,92,93 have demonstrated highly efficient performance in catalyzing HER–OER. Frequently investigated metal chalcogenides for water splitting include Ni-,70,86,94 Co-,95,96 Mo- (MoS297,98 and MoSe299,100) and W-101 chalcogenides. In 2013, our group disclosed that the cobalt sulfide (Co–S) film is effective as a HER–OER electrocatalyst with a faradaic efficiency of almost 100% in 1.0 M KOH.102 The Co–S film exhibits a promising activity in the production of H2 from seawater. However, it suffers from stability issues in acidic medium (0.5 M H2SO4). Moreover, Co–S anchored on a conductive template such as Ti mesh has also revealed high catalytic activity and good stability as an OER electrocatalyst.70 Other adoptable conductive substrates include carbon-based materials such as carbon nanotubes (CNTs) and reduced graphene oxide (RGO).103,104 The interconnected CNT architecture offers improvement in porous characteristics while ensuring high electronic and mass transport. On the other hand, the RGO sheets are known to promote interfacial contact, thereby enhancing particle distribution and exposure of active sites.
Similar to the effect of bi-/multi-metallic elements on noble metal electrocatalysts, studies have shown that bi-/multi-metallic sulfides have superior activity towards HER than their single component monometallic counterparts.96,101,105 However, the nature of the involved metal atoms and their respective compositions play a paramount role in the catalytic activities. For instance, Ni0.68Co0.32S2 NWs displayed a lower HER activity than the undoped counterparts, CoS2 and NiS2.96 On the other hand Ni0.33Co0.67S2 NWs grown on Ti foil synthesized via sulfurization of the NiCo2O4 precursor displayed the best HER activity in both neutral and alkaline media as compared to the undoped CoS2 and NiS2.106 Similarly, our group demonstrated that the presence of Co (NiCo2S4 NA/CC) enhances the catalytic activity as witnessed from the observed current density of 100 mA cm−2 at an overpotential of 305 mV (for HER) and 340 mV (for OER). The outstanding performance of NiCo2S4 NA/CC was attributed to the high capacitance of the catalyst indicating a high surface roughness and surface area.
In an acidic medium, cobalt selenides are more stable electrocatalysts for water splitting than cobalt sulfides, especially when supported on carbon black,96 carbon fiber paper (CFP)107 and carbon cloth (CC).108 Attributable features for the improved HER–OER activity include the increased surface area and boosted electrical conductivity. Other notable approaches for enhancing the catalytic efficacy of cobalt selenides include grafting or anchoring other components into the structure of the electrocatalysts. In this direction, numerous composites based on CoSe2 nanobelts with tremendous water splitting capacity have been developed. These include Ni/NiO/CoSe2109 and MoS2/CoSe2110 composites for the HER process, and Mn3O4/CoSe2,111 N-doped graphene (NG)/CoSe2112 and CeO2/CoSe2106 for the OER process. The enhanced OER and HER activities of these composites are ascribed to the synergetic chemical coupling effects.
Anchoring of the catalyst system on other components has been widely adopted to boost the catalytic performance of water splitting electrocatalysts. For example, Jin and coworkers introduced a high pressure and temperature hydrothermal continuous flow reactor (HCFR) for synthesizing NiCo LDHs on carbon fiber. The HCFR enabled the stable control of the reactor pressure that assisted the better tuning of the LDH size and morphology (Fig. 3(c)), which led to a current density of 10 mA cm−2 at 367 mV (vs. RHE) (Fig. 3(d)).113 On the other hand, Liu et al. showed that the NiCo2S4 nanowire array on carbon cloth (NiCo2S4 NA/CC) showed a better HER–OER performance than NiCo2O4 NA/CC. This was because at the beginning of the OER process, NiOOH and Co(OH)2 formed at the surface of NiCo2S4 which served as the active phases for the OER.114
Furthermore, Feng and co-workers revealed that Ni3S2 nanoarrays deposited on a good conductive substrate like nickel foam (NF) are active and stable bifunctional water splitting electrocatalysts (Fig. 3(e) and (f)).87 Similarly, a NiSe nanowire film anchored on nickel foam (NiSe/NF) fabricated by means of a hydrothermal reaction of NF and NaHSe was reported to be an efficient bifunctional water splitting electrocatalyst. However, theoretical evidence reveals that the harmonious effect of the interconnected architecture of catalyst's nanoarrays and the high-index planes is responsible for its unique electrocatalytic efficacy.70
For enriching the active sites, an interfaced MoS2/Ni3S2 heterostructure engineered on NF as an advanced bifunctional electrocatalyst was developed.115 The generated abundant interfaces delivered 10 mA cm−2 current density at a very low cell voltage of 1.56 V (vs. RHE). Coupled with theoretical calculations, it was revealed that the generated interfaces synergistically facilitated the chemisorption of H- and O-bound intermediates, thus expediting the overall water splitting process. Moreover, it was also confirmed that the induced lattice defects arising from the existence of Co9S8 and Ni3S2 combined phases highly contributed to the improved chemisorption of H and O-containing intermediates.116 On these defective heterointerfaces, DFT computation revealed a lower free energy for hydrogen (ΔGH) and hydroxide (ΔGOH), indicating a conducive active site for both HER and OER processes.
Metal phosphates and phosphides. In the past few decades, a myriad of transition metal based electrocatalysts have been used as potential water splitting electrocatalysts.70,73–75,117 Among these electrocatalysts, phosphates are one of the most studied bifunctional HER–OER electrocatalysts.24,37,118–120 In 2008, Nocera and co-workers reported an in situ synthesis approach for Co2+/phosphate as OER catalysts by means of an indium tin oxide (ITO) substrate in Co2+-containing phosphate buffered saline (PBS).121,122 Nonetheless, the catalytic activity of this catalyst is pH-dependent. This is quite similar to the study of Artero and co-workers where a robust cobalt-phosphate-based electrocatalytic material (Janus H2-CoCat NP) was developed for HER in a neutral electrolyte.123 Although with a remarkable catalytic activity, these NPs have to operate under near-neutral conditions.
Cobalt phosphides are suggested to be suitable catalysts for HER–OER.124–128 In 2014, Popczun and co-workers confirmed that CoP fabricated by means of a two-step colloidal synthesis approach is highly efficient and stable for HER in an acidic medium (Fig. 3(g)). Acting as a cathode in 0.5 M H2SO4, CoP NPs (electrodeposited on Ti foil) yielded a current density of −20 mA cm−2 at 85 mV (vs. RHE) overpotential (Fig. 3(h)) with a stability of 24 h (400 cyclic voltammetric sweeps).129 Another form of cobalt phosphide that can serve as a HER electrocatalyst is Co2P (Fig. 3(i) and (j)).130 However, this material was revealed to have a larger HER overpotential than CoP. The high Co/P ratio and low Co–P character make it unlikely for Co2P NPs to enable surficial distribution of conceivable active centers on the catalyst.131
Furthermore, following the catalytic efficacy of water splitting electrocatalysts due to the presence of phosphorus, various studies have demonstrated that incorporating metal catalysts with phosphorus can generate highly efficient HER–OER activity.71,85,132–134 For instance, Wei and co-workers revealed that the catalytic activity of Ni can be tuned with the extent of P deposition with the best activity observed at 10.8 wt% P.135 Moreover, pioneering studies conducted by our group showed that nickel phosphide (Ni–P) also demonstrated high activity for OER. Here, the authors established that a Ni–P nanoparticle film electrodeposited on Ni foam (Ni–P/NF) achieved a current density of 10 mA cm−2 at 1.67 V (vs. RHE) with 80 mV and 309 mV overpotentials for HER and OER, respectively, in 1.0 M KOH (Fig. 3(k) and (l)).85 Other forms of nickel phosphides reported with high catalytic activity include Ni2P,136,137 Ni5P4138 and Ni/Ni8P3.139 Most importantly, the Ni2P nanoarray has been demonstrated to be not only a high-performance non-noble-metal 3D catalyst electrode for hydrazine oxidation reaction (HzOR), but also a bifunctional catalyst material toward more energy-efficient hydrazine-assisted electrolytic hydrogen production.137
Aside from the common colloidal synthesis method for fabricating transition metal phosphides (TMPs), a recent surfactant-free low-temperature phosphidation approach by means of topotactic conversion of the corresponding precursors is developed.140 In this route, the propagation of CoP nanostructures is achieved through the use of conductive substrates to fabricate binder-free HER cathodes. A typical example is the synthesis of porous CoP/CC with a rough surface using low-temperature phosphidation of the smooth-surfaced Co(OH)F/CC precursor. The modified roughness of the surface was attributed to the dehydration and release of gases during the annealing of the precursor. The as-synthesized CoP/CC displayed a remarkable HER performance with an overpotential of 209, 106, and 67 mV (vs. RHE) to afford a current density of 10 mA cm−2 in alkaline, neutral and acidic media, respectively. The remarkable performance can be partly related to the expedited electron transfer resulting from the improved interfacial contact between CoP and the CC conductive support, alongside the improved exposure of active centers. Similarly, our group reported the fabrication of CoP nanostructures decorated on carbon cloth (CoP/CC) via low-temperature phosphidation of the Co(OH)F/CC precursor.57 When utilized as an electrocatalyst for HER, CoP/CC also displayed a remarkable performance in alkaline and neutral media. Encouraged by the performance of Co and Ni phosphides as bifunctional electrocatalysts and the merits of 3D structured catalysts, our group synthesized Ti-supported FeP nanowire arrays (FeP NA/Ti) to catalyze overall water splitting.77 The FeP NA served as the active site while Ti acted as the current collector. In an acidic medium, the FeP NA/Ti electrode displayed a low onset overpotential of 16 mV with a Tafel slope of 38 mV dec−1 and an exchange current density of 0.42 mA cm−2. The good acidic stability of FeP NWs is depicted by the negligible changes in the overpotential after 2000 and 3000 cyclictest runs or 15 h of continuous operation. These attributes were related to the structure-induced electronic effect after phosphidation. After phosphidation, the 1D array format of FeP NWs is retained but the lengths and diameters are decreased (up to 600 nm) and increased (50–95 nm), respectively. Similarly, for exploiting the benefits of 3D architectured electrocatalysts, Wang and co-workers developed flexible 3D iron phosphide nanotubes (IPNTs) as a HER electrocatalyst.141 The prepared compound comprised of FeP coated with NiFeOx/CFP species yielded low onset overpotentials of 31 and 35 mV (vs. RHE) in alkaline and acidic solutions, respectively. Strangely, the in situ fabricated surficial iron oxide/phosphate species assisted the activation of OER with an onset overpotential of 250 mV (vs. RHE). Moreover, when the flexible 3D iron phosphide electrocatalyst was employed in a constructed alkaline electrolyzer, it displayed a good activity with a current density of 10 mA cm−2 at only 1.69 V (Fig. 3(m) and (n)).141 Another synthesis approach is the facile reaction of metal and bimetallic foils with several organophosphine sources to produce TMPs, proposed by Read and coworkers.142 The as-synthesized phosphides demonstrated outstanding OER and HER activity which compared favorably with samples prepared by means of more costly and elaborate procedures. Another TMP-based material considered as a water splitting electrocatalyst is MoP. Our group developed a closely interconnected network of MoP NPs with high specific surface area (SSA) as illustrated in Fig. 3(o). The synthesis of this microstructure was by a temperature-programmed reduction of the air-calcined precursor obtained from (NH4)6Mo7O24·4H2O, (NH4)2HPO4 and citric acid (CA) with a Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA molar ratio of 1
CA molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (MoP-CA2). The MoP-CA2 NPs displayed a current density of 0.086 mA cm−2 with an onset overpotential of 40 mV, a Tafel slope of 54 mV dec−1, and an almost 100% FE (Fig. 3(p)). In addition, a remarkable stability for 4000 cycles (at least 24 h) was maintained.64 A most compelling feature is that metal phosphides are prone to oxidation and hence their surface turns into an oxide during OER catalysis.126 This newly formed oxide surface is the one that plays the true role of a catalyst. A recent study by Shifa et al.143 revealed that Sn doped Ni5P4in situ transformed into more active SnxNiO during the OER process. The electrochemically induced oxide is catalytically more active than the pristine oxides as it is formed in the vicinity of conductive phosphide species.
2 (MoP-CA2). The MoP-CA2 NPs displayed a current density of 0.086 mA cm−2 with an onset overpotential of 40 mV, a Tafel slope of 54 mV dec−1, and an almost 100% FE (Fig. 3(p)). In addition, a remarkable stability for 4000 cycles (at least 24 h) was maintained.64 A most compelling feature is that metal phosphides are prone to oxidation and hence their surface turns into an oxide during OER catalysis.126 This newly formed oxide surface is the one that plays the true role of a catalyst. A recent study by Shifa et al.143 revealed that Sn doped Ni5P4in situ transformed into more active SnxNiO during the OER process. The electrochemically induced oxide is catalytically more active than the pristine oxides as it is formed in the vicinity of conductive phosphide species.
Metal nitrides. Metal nitrides have demonstrated superior HER–OER activities due to their good corrosion resistance and electrical conductivity.144 Comprehensive studies of this set of electrocatalysts are mostly focused on their intrinsic HER characteristics.145 Only a few studies have established the potential of metal nitrides to activate the OER.146 One among such studies is the synthesis of Ni3N nanosheets, which were reported for the first time to be a suitable OER electrocatalyst when compared to NiO nanosheets. The improved intrinsic OER activity was attributed to the enhanced electrical conductivity with metallic characteristics and atomically disordered structure.147 On this account, it was suggested that metal nitride nanosheets could serve as bifunctional HER–OER electrocatalysts, where the chemical stability of nitrides deserves special attention in addition to their efficiency.
Despite the recent developments in improving the intrinsic OER behaviour, related studies in this regard, especially for overall water splitting, are limited. This may be related to the high overpotentials resulting from the restricted charge/ion transport. To circumvent this, a rational design to enhance the morphological effect, surface electrochemical reaction and electronic conductivity was proposed by Zhang and co-workers. Here, the authors synthesized Myriophyllum-like TiN@Ni3N nanowire arrays via a chemical bath deposition approach followed by an annealing process as a bifunctional HER–OER electrocatalyst. The as-synthesized TiN@Ni3N nanowire arrays displayed good HER and OER activities, and achieved a water splitting onset of ∼1.57 V with a current retention of 63.8% after 16 h of operation.148
Compared with single-metal nitrides, specific double metal nitrides have demonstrated better electrocatalytic activity and can be easily optimized by modulating the valence and electronic states of the metal elements.149,150 Cao and co-workers demonstrated this concept by revealing the enhanced HER activity and stability of cobalt molybdenum nitride (Co0.6Mo1.4N2) with a nanoscale morphology. Synthesized via a two-step solid-state reaction, Co0.6Mo1.4N2 possessed a stacked four-layered sequence of mixed close-packed structures with alternating layers of transition metals in octahedral and trigonal prismatic coordination. Owing to this morphology, Co0.6Mo1.4N2 with a low catalyst loading of 0.24 mg cm−2 achieved a current density of 10 mA cm−2 at −0.20 V (vs. RHE) under acidic conditions.149
Similarly, Jia and co-workers synthesized Ni3FeN nanoparticles (Ni3FeN-NPs) by means of thermal ammonolysis of ultrathin NiFe-LDH nanosheets. The as-prepared NPs were highly effective for full water splitting owing to the unique electronic structure of the metallic composite, thereby facilitating charge distribution and H2O adsorption.151 Moreover, the particle size (100 nm) effect was alleged to boost the accessibility of active sites for the water splitting process.
Metal carbides. Similar to metal nitrides, metal carbides (also known as transition metal carbides (TMCs)) have a unique electronic structure, with which catalytic water splitting reactions can be accelerated through. Both theoretical and experimental verifications of improved electronic characteristics as a result of the hybridization of the d-orbitals of the metal and the sp-orbitals of carbon are provided.144,145 On this account, the d-band structure is broadened as this favors the hydrogen binding energy, thereby promoting the HER.145
Among this class of metals, molybdenum (Mo) and tungsten (W) carbides are so far the most investigated metal carbides for water splitting,152 with WC being the most stable in acidic solutions.153 On the other hand, WC, W2C, and Mo2C have similar passivation regions in alkaline/neutral medium. Overall, the stability of these compounds is influenced by the generation of surface oxide motifs in the considered pH range.152 Weigert and co-workers demonstrated the superior electrochemical stability of WC foil modified with a low coverage of Pt compared to that of pristine WC.153 The result showed that the stability of Pt-modified WC was sustained till a potential of ∼1.0 V (vs. NHE).
Among the different Mo carbides,145 Mo2C is the most reported for water splitting and was previously explored as an effective noble-metal free electrocatalyst to replace Pt owing to its similar electronic characteristics to that of Pt and optimal hydrogen-adsorption properties.100,154,155 One major setback of this compound is its large particle size which acts as a limiting factor for the exposure of its active sites. In addition, the usage of these catalyst systems is challenged by surface oxidation or corrosion.153 So far, numerous approaches are proposed to promote particle miniaturization, and among those methods, dispersion is considered to be the most effective way to increase the surface area, enrich active sites and promote electron/mass activity. Recently, an enhanced electrocatalytic performance of Mo2C was demonstrated by embedding Mo2C nanoparticles in nitrogen-doped carbon nanosheet/graphene (Mo2C@N-DC/G) aerogel films.156 The carbon nanomaterial has a high surface area which prevents the aggregation of Mo2C nanocrystals, as well as protects the metal catalysts from acid corrosion, enhances the stability in an acidic medium and simultaneously serves as “electron highways” for rapid electron transfer.157 Moreover, the heteroatom N dopant modifies the surface chemistry with different defects and alters the electronic structure of the catalysts, leading to optimized adsorption energy of the key intermediates on the surface. The synergistic effect of N-doped carbon nanomaterials and Mo2C nanoparticles enriched the electron density on the carbon surface and promoted hydrogen adsorption as well as evolution.
In summary, the above discussed studies have evidently shown that the performances of metal-based electrocatalysts (as listed in Table 1) for water splitting are dependent on several key parameters. First, their overall HER–OER activity mostly depends on the structure and specific active surface area of the catalyst system rather than the nature of the constituting metal. This deduction elaborates the importance of morphology engineering and composition towards the optimization of the density and distribution of catalytic active sites. Second, the electrolyte selection, which is also critical to efficiency and stability, is dependent on its ion species, concentration, pH values and the suitability with electrocatalysts. Since the electrolyte would directly affect the reaction kinetics and an inappropriate electrolyte would cause corrosion of the electrode, choosing a suitable electrolyte has a great influence on the performance and stability of the electrocatalysts. Third, the lifetime of a catalyst directly affects its practical application. The economic strategies for catalyst reactivation so as to further extend the shelf life of a catalyst will also play an important role in developing commercializable technologies. Additionally, the cost from material elements, catalyst preparation, electrolytes, and electrodes will decide the upscalability and hence the practicality of this technology. Although non-noble metals are preferred to minimize element cost, noble metals would be still competitive if their cost-effectiveness surpasses non-noble candidates, i.e. a tiny amount of noble metals, for example in the monodispersed single-atom catalysts, could enhance catalysis and stability greatly.
However, the investigation of the bifunctional HER and OER activity on these carbon surfaces is still limited. Therefore, exploring the application of functional carbon-based materials for overall water splitting cannot be over-exaggerated. In this regard, Lai and coworkers first conducted an extensive study on the fabrication of porous graphite nanocarbons co-doped with O, N and P heteroatoms as a self-supported 3D electrode (ONPPGC/OCC) for overall water splitting at various pH values.163 For instance, in an alkaline medium, ONPPGC/OCC electrocatalysts displayed good HER and OER activities. The electrolyzer attained a current density of 10 mA cm−2 at a cell voltage of 1.66 V with remarkable stability. The remarkable electrocatalytic performance of the porous nanocarbon was attributed to the unique 3D structure and pore distribution, highly dispersed active sites, improved transport properties, and good electrical conductivity. Qiao and co-workers presented a 3D-architectured hydrated catalyst NiCo LDH on N-doped graphene hydrogels (NG-NiCo) synthesized through ammonia-involved hydrothermal treatment of a graphene hydrogel followed by heterogeneous deposition of the obtained NiCo hydroxide on NG.164 While the presence of N-dopant reduces the catalyst's internal resistance and the graphene provides a porous 3D interconnected network, the synergistic metal–O–C and metal–N–C interactions yield interfacial active centers to activate the HER–OER. Undeniably, this study offers a unique exciting means to explore functional carbon compounds as water splitting electrocatalysts. The electrocatalytic performance of ONPPGC/OCC in other media is presented in Table 1.
Another widely used carbon-based material is graphdiyne (GDY), a novel plane carbon network consisting of sp-/sp2-co-hybridized carbon atoms.182 This porous carbon network with a unique intrinsic band gap, excellent electric conductivity, and strong stability was first synthesized by Li and co-workers in 2010.183 Given its intrinsic properties, the authors prepared a GDY anchored on CoNx nanosheets with a seamless interacting interface on Ni foam (CoNx@GDY NS/NF).182 When tested in 1.0 M KOH, CoNx@GDY NS/NF attained a current density of 10 mA cm−2 with a cell voltage of 1.48 V when employed as a bifunctional electrocatalyst. Similarly, Si and co-workers utilized GDY to develop a hierarchical heterostructure composite with NiFe LDH anchored on copper foam (GDY@NiFe-LDH/CF) to catalyze the overall water splitting.184 In its function, GDY@NiFe-LDH/CF attained a current density of 20 mA cm−2 with a cell voltage of 1.512 V. The remarkable electrochemical performance was credited to the improved interfacial chemical interaction between Fe, Ni and the triple C–C bonds in GDY. This interaction facilitated an improved electron charge distribution with a controlled diffusion rate.184 In addition, Table 1 presents a summary of other adopted metal-free electrocatalysts.
Benefitting from the synergistic effect of metallic behaviour, interconnected pores of nanowire arrays, and a distinct 3D electrode structure, Co4N porous nanowire arrays on carbon cloth attained a low overpotential of ∼0.26 V at 10 mA cm−2 and a Tafel slope of 44 mV dec−1 in an alkaline medium. Moreover, theoretical evidence shows that the metallic Co4N core with a thin cobalt oxides/hydroxides shell serves as the active centre during the OER process.146
In short, defected functional catalysts would still need the metal-based materials for the synergistic catalysis and/or as the supporting template. Besides laterally referenceable perspectives discussed above for metal-based catalysts, the synergy of interface coupling between metal-free and metallic components will play a significant role in enabling an efficient carrier (electrons in HER and holes in OER) transportation within the catalysts, thus affecting the performances.
3. Ammonia synthesis via N2 reduction reaction
Ammonia (NH3) is a commonly synthesized chemical across the world. It can function as a building block for the production of other N-based compounds and as a clean energy carrier due to its high H-content.185,186 Presently, NH3 is industrially fabricated via the Haber–Bosch process involving an exothermic interaction between N2 and H2 (N2 + 3H2 → 2NH3) in the presence of a catalyst at high pressure and high temperature.187 Theoretically, compared with Fe-based catalysts, Ru-based catalysts are more effective to operate the Haber–Bosch process under a relatively mild condition (pressure ≤ 100 bar).188 Despite this, the thermodynamics is still low (approximately 10–15%) with an energy implication estimated at 1–2% of global annual energy186,189 and the involvement of H2 in this process is undermined by the consumption of fossil fuels (with sizeable CO2 emissions).190Recently, electrocatalytic N2 reduction reaction (NRR) is viewed as an energy-saving approach for NH3 production, as the process of synthesizing NH3 is carried out under ambient temperature and pressure. Hence, electrocatalytic NRR is considered as an eco-friendly and energy-conservation approach for NH3 synthesis. However, its practical application is still constrained by the costly electrolytes, low NH3 yields and so on.188 On this account, a knowledge-driven guide towards the development of efficient electrocatalysts is a fundamental step for realizing electrocatalytic N2 fixation and accelerated NRR processes. Characteristically, NRR electrocatalysts are of three types: biocatalysts, homogeneous, and heterogeneous. The biocatalysts and homogeneous electrocatalysts contain ligand-surrounded metal centers,191 which poses a limitation due to the high cost of the ligands. Synthesis challenges as well as the low electrical properties hinder the development of these types of catalysts.192 On the other hand, heterogeneous electrocatalysts are highly durable and are more integrable with functional energy conversion devices.193 On this account, the design and development of heterogeneous electrocatalysts have been exploited for NH3 synthesis.
In this review, recent experimental and theoretical insights into the development of NRR electrocatalysts are highlighted. Particular emphasis will be devoted to the significance and implications of recent developments. First, the electrochemical NRR mechanisms are discussed, providing idealistic modalities for enhancing catalytic activity, selectivity and stability. Based on these mechanisms, various heterogeneous electrocatalysts are reviewed in terms of catalytic performance, reflecting different accumulated outcomes and mechanistic understanding of catalyst design principles. Here, electrocatalysts including metal (noble and non-noble) catalysts and metal-free catalysts are discussed.
3.1. Electrochemistry of N2 reduction reaction (NRR)
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond of the N2 molecule to form N-adatoms on the surface of the catalyst prior to the protonation process. Subsequent protonation of the adatoms on the surface results in the yield of NH3 independently. Fig. 4(a) presents a detailed schematic representation of the mechanistic pathway, with a characteristic ΔG plot to illustrate the minimum energy pathway (MEP) on the Ru NP catalyst (Fig. 4(b)).194 Deductions from the mechanistic pathway and the characteristic ΔG plot suggest that the last reduction step (*NH2 + H+ + e− → *NH3, ΔΔG = 0.32 eV uphill) is the rate-determining step.195 On the other hand, in the associative mechanism, the two N centers in N2 remain intact as a molecule while being protonated, with the release of NH3 after the severance of the N–N bond (Fig. 4(c)). This mechanism is further characterized based on the order of protonation of the two N centers (with the assumption that the N2 molecule is adsorbed on the catalyst). The protonation sequence and its associated energies will dictate the rate-determining reaction on the catalyst surface. Fig. 4(d) illustrates that the first reduction process (*N2 + H+ + e− → *N2H, ΔG = 1.03 eV uphill) is the rate-determining step of the associative reaction on the Ru NP catalyst.194
N bond of the N2 molecule to form N-adatoms on the surface of the catalyst prior to the protonation process. Subsequent protonation of the adatoms on the surface results in the yield of NH3 independently. Fig. 4(a) presents a detailed schematic representation of the mechanistic pathway, with a characteristic ΔG plot to illustrate the minimum energy pathway (MEP) on the Ru NP catalyst (Fig. 4(b)).194 Deductions from the mechanistic pathway and the characteristic ΔG plot suggest that the last reduction step (*NH2 + H+ + e− → *NH3, ΔΔG = 0.32 eV uphill) is the rate-determining step.195 On the other hand, in the associative mechanism, the two N centers in N2 remain intact as a molecule while being protonated, with the release of NH3 after the severance of the N–N bond (Fig. 4(c)). This mechanism is further characterized based on the order of protonation of the two N centers (with the assumption that the N2 molecule is adsorbed on the catalyst). The protonation sequence and its associated energies will dictate the rate-determining reaction on the catalyst surface. Fig. 4(d) illustrates that the first reduction process (*N2 + H+ + e− → *N2H, ΔG = 1.03 eV uphill) is the rate-determining step of the associative reaction on the Ru NP catalyst.194
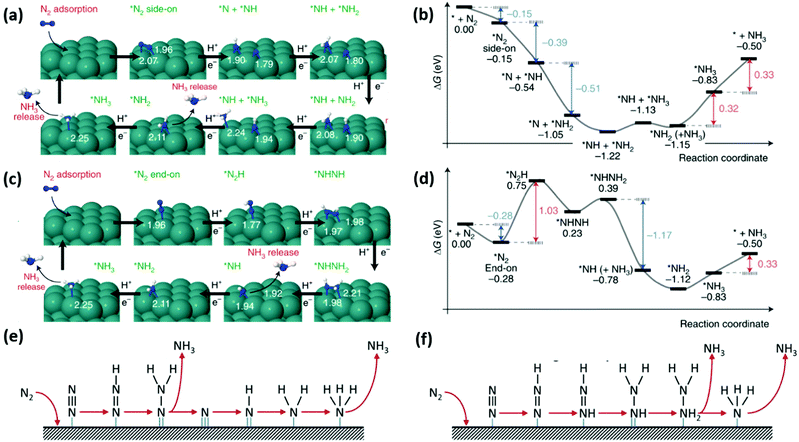 | ||
| Fig. 4 NRR mechanisms on heterogeneous catalysts. (a) Dissociative mechanism and its (b) characteristic Gibbs free energy plot illustrating the minimum energy pathway on Ru nanoparticles (Ru-NPs). (c) Associative mechanism and its (d) characteristic Gibbs free energy plot illustrating the minimum energy pathway on Ru nanoparticles (Ru-NPs). Images (a)–(d) are reproduced with permission.194 Copyright 2019, Nat. Catal. Comparison of (e) distal and (f) alternative associative mechanisms. Images (e) and (f) are reproduced with permission.196 Copyright 2017, Elsevier. | ||
Generally, protonation of the N centers occurs via two routes in the associative reaction. In the first instance, the N center farther from the catalyst surface is preferentially protonated (assuming an end-on coordination mode for the N2 molecule) resulting in the yield of NH3 and formation of a metal nitride (M–N) motif, which is later protonated to yield the second equivalent NH3. This is classified as the distal associative pathway (Fig. 4(e)). On the other hand, the alternative route relates to the protonation of each N center alternately until an N center is completely hydrogenated to NH3 and the N–N bond is severed (Fig. 4(f)).196
In both dissociative and associative mechanisms, electrochemical NRR involves a series of proton–electron transfer steps with the formation of multiple intermediates:197
| N2 + H+ + e− ↔ N2H E0 = −3.2 V vs. RHE | (31) |
| N2 + 2H+ + 2e− ↔ N2H2(g) E0 = −1.10 V vs. RHE | (32) |
| N2 + 4H+ + 4e− ↔ N2H4(g) E0 = −0.36 V vs. RHE | (33) |
| N2 + 4H2O + 6e− ↔ N2H4 + 4OH− E0 = +0.55 V vs. RHE at pH 14 | (34) |
| N2 + e− ↔ N2−(aq) E0 = −3.37 V vs. RHE at pH 14 | (35) |
Irrespective of the operating mechanism, it is evident that these enzymes are suitable catalysts for NH3 production under ambient conditions in aqueous media with an exciting energy efficiency. Consequently, electrochemistry has appeared as an attractive approach adopting the HER of the water splitting process to produce H+ and e− for the reduction of nitrogen (from air). Renewable energy sources are also suitable to power the reaction operation. Similar to the water splitting process, the NRR mechanism also depends on the nature of the electrolyte. Depending on the nature of the electrolyte, the general reactions are as follows:
In an acidic condition:
At the cathode:
| N2 + 6H+ + 6e− → 2NH3, | (36) |
| 3H2O → 3/2O2 + 6H+ + 6e− | (37) |
At the cathode:
| N2 + 6H2O + 6e− → 2NH3 + 6OH− | (38) |
| 6OH− → 3/2O2 + 3H2O + 6e− | (39) |
| N2 + 3H2O → 3/2O2 + 2NH3 | (40) |
A milestone study utilizing computational SHE via harmonic approximation and DFT calculations to investigate the reduction energetics for N2 (ad-molecules and adatoms) on different transition metal surfaces in an acidic medium was conducted by Nørskov and co-workers in 2012.201 By correlating the chemisorption energies with the reaction intermediates under the assumption that the change in free energy is proportional to the activation energy barrier in each elementary step, a volcano plot was obtained. The segmentation of the volcano plot into right and left legs indicates that the metals on each leg possess a weak and a strong N-binding energy, respectively. For instance, the metals on the left-leg (Sc, Y, Ti and Zr) have strong affinity for N-adatoms, which will facilitate significant synthesis of NH3 relative to H2, particularly at a bias of −1 V to −1.5 V (vs. SHE). At the top of the volcano plot are the most active metals for ammonia synthesis, which include Mo, Fe, Rh, and Ru. However, the faradaic efficiency (FE) of these metals is low owing to the substantial HER competition.201 Also, the authors revealed that the defect-free surfaces of TMs are catalytically more active for NH3 formation than their stepped counterparts due to the lower onset potential on the close-packed flat surfaces compared to the stepped surfaces.201
Generally, the production of NH3 is simply catalyzed by pure transition metals (TMs). Still, the N-adsorption energies (ΔE) of these metals are insufficient relative to the ideal values within the range of −0.4 eV to −1.4 eV.202 Contrarily, some early TMs (including Sc, Y, Ti and Zr) have displayed strong N-bonding within the bias range of −1 V to −1.5 V (vs. SHE). Nonetheless, these early TMs change their phase from metallic state to bulk nitrides, which hinders the potential of N2 activation due to the widened d-band centers from the Fermi level.202 For example, utilizing iron catalysts in NH3 production gives rise to the formation of a Fe4N-like structure, although it is unsure if the N-species is an ad-molecule or adatom.203
Pertaining to boosting the reduction of N2 while simultaneously suppressing the HER process, Li and co-workers proposed an electron-deficient approach to retard the HER process in an alkaline medium under ambient conditions. Here, the authors boosted the NRR performance of Cu NPs with negligible catalytic activity via a local electron depletion effect using Mott–Schottky rectifying contact with a polyimide support. The electron-depleted Cu NPs considerably enhanced N2 pre-adsorption leading to a better NH3 yield. This approach of inciting an electron deficient surface offers a novel insight into the rational design of inexpensive NRR catalyst systems with high activity and selectivity.204
Moreover, early TMs are readily oxidized to form their respective oxides in their natural state which may alter their catalytic efficacy.205 On this account, the performance of TM oxides was theoretically investigated towards electrosynthesis of NH3 under ambient conditions. Skúlason and co-workers studied the potential of TM dioxides as NRR electrocatalysts and revealed that the (110) planes of ReO2, NbO2, and TaO2 are the best suited for NH3 synthesis given their reasonably low onset potentials of −0.57 V, −1.07 V and −1.21 V (vs. SHE), respectively.206 The least overpotential was exhibited by IrO2 (−0.36 V) but this catalyst preferentially adsorbs H-adatoms which will favor the HER process.206
Lately, the possible production of NH3 using NbO2 NPs as an electrocatalyst has been experimentally proven under ambient conditions.207 This possibility is accredited to the electronic characteristics of NbO2 which are alleged to promote NH3 fixation.188 Owing to the nature of Nb4+, NbO2 NPs possess an empty d-orbital that readily accepts electrons leading to a strong surficial bonding with N2. In addition, the activation of N-admolecules/adatoms could be promoted by the back donation of the single d-electron from the Nb4+ cation. In this respect, the NbO2 NPs attained an NH3 yield rate of 11.6 μg h−1 mg−1cat. with an FE of 32% at −0.60 (vs. RHE) and −0.65 (vs. RHE), respectively, when tested in a 0.05 M H2SO4 solution.208 Similarly, other forms of niobium oxides have also shown potential applications as possible NRR electrocatalysts.209,210 For example, our group demonstrated the effectiveness of Nb2O5 as an electrocatalyst for NH3 production as both nanofibers209 and nanowires.210
Nevertheless, metals have shown good potential for N2 reduction under ambient conditions; it is worthwhile to mention that some metals and their respective oxides do not display similar catalytic efficacy particularly at room temperature. This is somewhat related to their low conductivity192 and in other cases, it is due to their strong affinity for hydrogen.202 For the latter, typical examples are the late TMs which greatly enable the HER, thereby fettering the formation of NHx intermediates.202 Unlike these metals, some metals especially the middle TMs offer moderate binding energies for N-motifs, thus making them suitable candidates for N2-to-NH3 conversion.211 Proceeding from this, it is likely that the best suited electrocatalyst can be designed by selecting the right materials with intrinsic NRR characteristics. Otherwise, it is necessary to adopt measures to promote the NRR performance of the catalyst system.
An effective scheme is to incorporate a second active center to facilitate the mechanistic spillover and hydrogenation of the activated N-motifs from the metal surface. For instance, Wang and co-workers proposed the use of LiH as a second active center to disrupt the NRR pathway on a TM or its nitride (TMN) by spilling over the activated N-motifs to its surface for direct hydrogenation. LiH is a powerful reducing agent which offers immediate H-species to bind with N-motifs to yield LiNH2. Subsequently, LiNH2 heterolytically splits to release H2 and NH3 with the regeneration of LiH. It should be emphasized that this rational scheme is an approach to improve the NRR performance of early and late 3d TMs under ambient conditions.202 However, in realistic terms, the two active centers need to be appropriately separated.
Alternatively, HER-retarding strategies are equivalently being suggested for a successful headway in the NRR process. In this regard, a common approach is the use of electrocatalysts other than pure metals. The introduction of non-metal species on metals has shown remarkable results pertaining to NH3 synthesis. A recent theoretical study involving metal nitrides as NRR electrodes suggests that VN and ZrN produced NH3 at potentials of −0.51 V (vs. NHE) and −0.76 V (vs. NHE), respectively. These results were unachievable when the nitrides were veiled with H-adatoms.205 Similarly, Abghoui and co-workers reported a parallel result for NbN and CrN.212
To date, there are no extensive computational and experimental studies investigating the efficacy of metal nitrides as catalysts for N2-to-NH3 conversion. However, Simonov and co-workers critically assessed the electrocatalytic activity of VN and Nb4N5 anchored on CC under various conditions.213 Here, the authors concluded that polycrystalline VN and Nb4N5 are electrocatalytically inactive toward NH3 synthesis regardless of the operating condition. Another related study involved the computational investigation of single TMs anchored on boron nitride (TM-BN) as a N2 fixation electrocatalyst.193 Of the investigated single TMs (Sc to Zn, Mo, Ru, Rh, Pd, and Ag), the highest electrocatalytic activity was witnessed on the defective Mo-BN nanosheet with a low overpotential of 0.19 V. The high spin-polarization, selective N2H*-motif stabilization and NH2*-motif destabilization are attributed for the high catalytic activity of the Mo-BN nanosheet for N2-to-NH3 conversion.193
Equally, it will be beneficial if similar theoretical studies are conducted on binary nitrides. For instance, Co3Mo3N is one the most active electrocatalysts for NH3 synthesis214 and was recently modeled for the conventional Haber–Bosch process.215 On a broader perspective, extensive theoretical studies are required to understand the NRR mechanism over the numerously designed electrocatalysts. Despite the countless NRR electrocatalysts proposed from laboratory tests, their current status is still unclear due to the lack of in-depth theoretical studies. These studies, although limited, are mandatory for a thorough comprehension of the reaction mechanism. Even though DFT computations may suffice as a good theoretical approximation of the catalytic activity, kinetics and evaluation of the optimization scheme, the findings may not be precise experimentally. Therefore, the need for a thorough assessment of NRR electrocatalysts, supported by both experimental and theoretical studies, is crucial.
3.2. Electrocatalysts for NRR
An important milestone in NH3 electrosynthesis was the establishment of NH3 production in aqueous solution under ambient conditions from N2 and H2via a back-to-back cell configuration by Furuya and Yoshiba in 1990.14 Of the 26 cathodes studied, ZnSe was the most suitable for the reduction of N2 with an NH3 electrode area-normalized yield rate of 0.23 mol h−1 m−2, attaining an FE of 1.3% at a potential of −1 V (vs. RHE).14 Despite the high yield rate, the FE is relatively low and was attributed to the predominant cathodic reduction of H2O at the high negative cell potential, particularly in an aqueous solution where the concentration of H2O is relatively high. For this reason, the suppression of the HER is considered by most to be the utmost challenge confronting electrochemical NRR.Moreover, the determination of N2 production in NRR is more challenging than H2 or O2 production in water splitting due to the possible contamination from the ambient environment and low production rates.216,217 Concretely, the ammonia detected may come from other routes beyond the NRR, such as ammonia contamination in the feeding gas, electrolyte and electrode surface, and decomposition or desorption from the catalyst itself, especially in the case of N-containing materials.218 Thus, it is essential to measure and prove the reliability of obtained data of NH3 amount. Suryanto, MacFarlane and co-workers194 summarized the current steps and mis-steps towards NRR in terms of experimental methodology and catalyst selection, and proposed a protocol for rigorous experimentation. They discussed the protocols of NRR experiments in detail and proposed a five-step experimental protocol including gas purification, open circuit control measurements and parallel control experiments by 15N-isotopic labelling experiments to exclude the ammonia contamination or catalyst decomposition issues for reliable proof of the occurrence of the electrochemical nitrogen reduction reaction drawing on Greenlee and co-workers.216 A similar rigorous experimental protocol consisting of standardized control experiments and quantitative isotope measurements with 15N gas is also proposed by Tang and Qiao219 based on the Nature published, Chorkendorff and colleague's landmark study about the ‘‘true or false’’ issue in the electrochemical NRR research community in 2019.220
In this regard, only with careful validation of the nitrogen source can we evaluate the NRR performance of potential catalysts. Several key parameters related to the performance of the NRR, including electrolyte, ammonia formation rate (NH3 yield), faradaic efficiency and overpotential (V) vs. RHE of reported catalysts for NRR, are listed in Table 2. It should be pointed out that since the accuracy of the determinate NRR efficiency highly relies on the meticulous measurement of NH3 amount and well controlled NRR experiments, it is necessary to refer to the original work and confirm the experimental protocols. A second party inspection of the results by using a well-accepted standard protocol will be helpful to filtrate the catalysts with reliable efficiency.
| Electrocatalysts | Electrolyte | NH3 yield | FE (%) | Overpotential (V) vs. RHE | Ref. |
|---|---|---|---|---|---|
| Noble metals | |||||
| Ag film | 0.1 M Na2SO4 | 1.27 μg h−1 cm−2 | 7.36 | −0.6 | 244 |
| Ag nanosheets | 0.1 M HCl | 2.83 μg h−1 cm−2 | 4.8 | −0.6 | 232 |
| Au flowers | 0.1 M HCl | 25.57 μg h−1 mg−1cat | 6.05 | −0.2 | 351 |
| Au/TiO2 | 0.1 M HCl | 21.4 μg h−1 mg−1cat | 8.11 | −0.2 | 352 |
| a-Au/CeOx-RGO | 0.1 M HCl | 8.3 μg h−1 mg−1cat | 10.1 | −0.2 | 353 |
| Porous Au film on Ni foam | 0.1 M Na2SO4 | 9.42 μg h−1 cm−2 | 13.36 | −0.2 | 226 |
| Pd/C | 0.1 M PBS | 4.5 μg h−1 mg−1cat | 8.2 | 0.1 | 228 |
| Rh NPs/C | 0.5 M Na2SO4 | 22.82 ± 1.49 μg h−1 mg−1cat. | ∼0.1 | −0.45 | 231 |
| Rh2Sb RNRs/C | 228.85 ± 12.96 μg h−1 mg−1cat. | ∼1.5 | |||
| Rh2Sb SNRs/C | 63.07 ± 4.45 μg h−1 mg−1cat. | ∼0.4 | |||
| Ru1 on N-doped carbon | 0.05 M H2SO4 | 120.9 μg h−1 mg−1cat | 29.6 | −0.2 | 315 |
| Non-noble metals | |||||
| Metallic substances | |||||
| Fe SA-N-C | 0.1 M KOH | 7.48 μg h−1 mg−1cat | 56.55 | 0 | 354 |
| Mo1 on N-doped porous carbon | 0.1 M KOH | 34.0 ± 3.6 μg h−1 mg−1cat. | 14.6 ± 1.6 | −0.3 | 355 |
| Metal oxides | |||||
| B-doped TiO2 | 0.1 M Na2SO4 | 14.4 μg h−1 mg−1cat. | 3.4 | −0.8 | 274 |
| C-TiO2 nanoparticles | 0.1 M Na2SO4 | 16.22 μg h−1 mg−1cat. | 1.84 | −0.7 | 273 |
| Fe2O3 | 1.0 M KOH | 0.46 μg h−1 cm−2 | 6.04 | –0.074 | 260 |
| Fe2O3 nanorods | 0.1 M Na2SO4 | 15.9 μg h−1 mg−1cat. | 0.94 | −0.8 | 265 |
| Fe2O3/TiO2 | 1.0 M KOH | 16.52 μg h−1 mg−1cat. | 0.31 | −0.577 | 356 |
| Nb2O5 nanowire array | 0.1 M Na2SO4 | 9.67 μg h−1 cm−2 | 2.26 | −0.6 | 210 |
| NbO2 nanoparticles | 0.05 M H2SO4 | 11.6 μg h−1 mg−1cat. (−0.65 V vs. RHE) | 32 (−0.6 V vs. RHE) | — | 208 |
| TiO2 nanosheet array | 0.1 M Na2SO4 | 5.62 μg h−1 cm−2 | 2.5 | −0.7 | 283 |
| TiO2-rGO | 0.1 M Na2SO4 | 15.13 μg h−1 mg−1cat. | 3.3 | −0.9 | 272 |
| Defective TiO2 on Ti mesh | 0.1 M HCl | 7.59 μg h−1 cm−2 | 9.17 | −0.15 | 284 |
| Fe/Fe3O4 | 0.1 M PBS | 0.19 μg h−1 cm−2 | 8.29 | −0.3 | 267 |
| FeOOH QDs-GS | 0.1 M LiClO4 | 27.3 μg h−1 mg−1cat. | 14.6 | −0.4 | 262 |
| Metal chalcogenides | |||||
| CoS2/NC | 0.1 M HCl | 17.45 μg h−1 mg−1cat. | 4.6 | –0.15 | 357 |
| Fe3S4 | 0.1 M HCl | 75.4 μg h−1 mg−1cat. | 6.45 | −0.4 | 358 |
| Metal carbides | |||||
| Mo2C nanorod | 0.1 M HCl | 95.1 μg h−1 mg−1cat. | 8.13 | −0.3 | 294 |
| Mo2C/C | 0.5 M LiClO4 | 11.3 μg h−1 mg−1cat. | 7.8 | −0.3 | 277 |
| Ti3C2Tx (T = F, OH) MXene nanosheets | 0.1 M HCl | 20.4 μg h−1 mg−1cat. | 9.3 | −0.4 | 270 |
| Metal nitrides | |||||
| Fe–N/C hybrid | 0.1 M KOH | 34.83 μg h−1 mg−1cat. | 9.28 | −0.2 | 359 |
| VN nanosheet array | 0.1 M HCl | 5.14 μg h−1 cm−2 | 2.25 | −0.5 | 297 |
| VN nanoparticles | 0.05 M H2SO4 | 20.2 μg h−1 cm−2 | 6 | −0.1 | 278 |
| Metal phosphides | |||||
| CoP/CNs | 0.1 M Na2SO4 | 48.9 μg h−1 mg−1cat. | 8.7 | −0.4 | 279 |
| Ni2P/N,P-C | 0.1 M KOH | 90.1 μg h−1 mg−1cat. | 19.82 | −0.2 | 280 |
| 0.1 M HCl | 34.4 μg h−1 mg−1cat. | 17.21 | — | ||
| FeP2 NP-rGO | 0.5 M LiClO4 | 35.26 μg h−1 mg−1cat. | 21.99 | −0.4 | 299 |
| Metal-free electrocatalysts | |||||
| Defect-rich carbon cloth | 0.1 M Na2SO4 | 15.9 μg h−1 cm−2 | 6.92 | −0.3 | 304 |
| 0.02 M H2SO4 | |||||
| N-Doped highly disordered carbon | 0.1 M KOH | 57.8 μg h−1 cm−2 | 10.2 | −0.3 | 310 |
| N and B co-doped carbon nanosheets | 0.1 M HCl | 7.75 μg h−1 mg−1cat. | 13.79 | −0.3 | 326 |
| Oxygen-doped hollow carbon microtubes | 0.1 M HCl | 25.12 μg h−1 mg−1cat. | 9.1 | −0.8 | 324 |
| Oxygen-doped carbon nanosheet | 0.1 M HCl | 20.15 μg h−1 mg−1cat. | 4.97 | −0.6 | 300 |
| Polymeric carbon nitride | 0.1 M HCl | 8.09 μg h−1 mg−1cat. | 11.59 | −0.2 | 311 |
| S-Doped carbon nanospheres | 0.1 M Na2SO4 | 19.07 μg h−1 mg−1cat. | 7.47 | −0.7 | 360 |
| P-Doped graphene | 0.5 M LiClO4 | 32.33 μg h−1 mg−1cat | 20.82 | −0.65 | 306 |
| S-Doped graphene | 0.1 M HCl | 27.3 μg h−1 mg−1cat. (−0.8 V vs. RHE) | 11.5 (−0.6 V vs. RHE) | — | 274 |
| Elementals and their compounds | |||||
| B-Doped graphene | 0.05 M H2SO4 | 9.8 μg h−1 cm−2 | 10.8 | −0.5 | 316 |
| B4C/CPE | 0.1 M HCl | 26.57 μg h−1 mg−1cat. | 15.95 | −0.75 | 328 |
| Boron nitride (mesoporous) | 0.1 M Na2SO4 | 18.2 μg h−1 mg−1cat. | 5.5 | −0.7 | 314 |
| Black phosphorus nanosheets | 0.01 M HCl | 31.37 μg h−1 mg−1cat. (−0.7 V vs. RHE) | 5.07 (−0.6 V vs. RHE) | — | 330 |
Furthermore, among the various research advances in electrochemical NRR, lithium-mediated nitrogen reduction has attracted much interest as it has been proven to be a good method to electrochemically synthesize ammonia in the past several years.221 The lithium-mediated nitrogen electroreduction also demonstrated good reproducibility219 but the process has so far been unstable, and the continuous deposition of lithium limits its practical applicability. The underlying mechanism of lithium-mediated NRR needs to be further investigated to ultimately contribute to green ammonia production and a sustainable society. Hence, the development of electrocatalysts for this purpose is crucial towards an effective NRR with high NH3 yield.
Au-Based electrocatalysts. Noble metal-based materials, as effective NRR electrocatalysts, have the potential for surficial interaction with N2 through electron transfer, which is required to promote N2-fixation.222 Gold (Au)-based materials, having one of the most stable metallic configurations, are targeted as NRR electrocatalysts owing to their low HER activity. Under this circumstance, electrochemical fixation of N2 is promoted,223 although theoretical studies dispute this claim.206,224 Yan and co-workers demonstrated the potential of a Au-based material (tetrahexahedral (THH) Au nanorods) as a heterogeneous NRR electrocatalyst under ambient conditions. Here, a stepped (730) facet (consisting of (210) and (310) sub-facets) of THH Au nanorods at room temperature and pressure yielded 1.648 μg h−1 cm−2 of NH3 and 0.102 μg h−1 cm−2 of N2H4·H2O with a high FE (4.02%) at −0.2 V (vs. RHE) (Fig. 5(a) and (b)).223 It was suggested that the stepped facets outperformed the flat planes due to the availability of more catalyst active sites for the adsorption and activation of N2.225
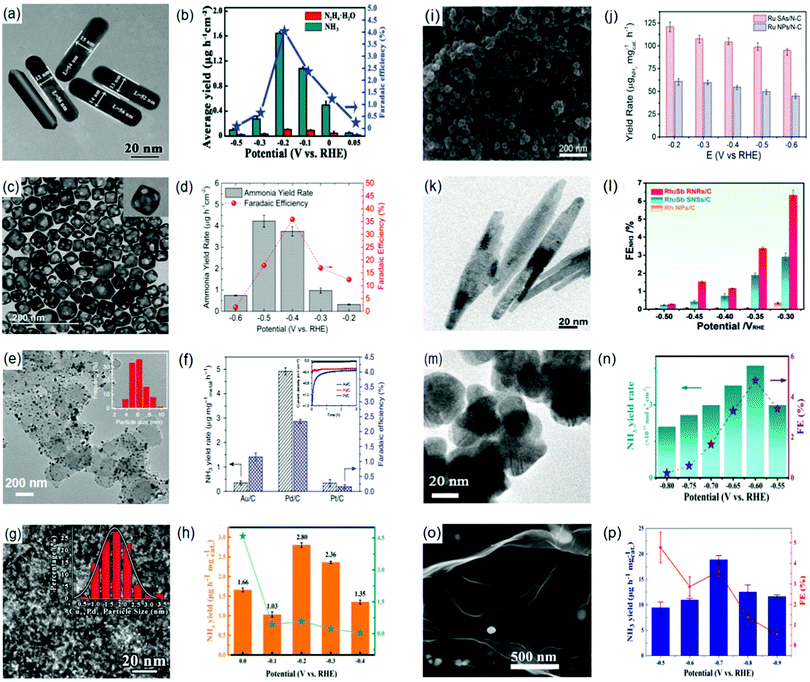 | ||
| Fig. 5 (a) TEM image of Au nanorods with aspect ratio 4 ± 0.5. (b) Yield rate of ammonia (cyan), hydrazine hydrate (red) formation, and faradaic efficiency (blue) at each given potential. Panels (a) and (b) are reproduced with permission.223 Copyright 2017, Wiley. (c) TEM image of hollow Au nanocages, and their (d) NH3 yield rate and FE at different potentials in 0.5 M LiClO4 aqueous solution. Panels (c) and (d) are reproduced with permission.227 Copyright 2018, American Chemical Society. (e) TEM image of the Pd/C catalyst (inset: particle size distribution). (f) NH3 yield rates and FE of the Pd/C catalyst in 0.05 M H2SO4, 0.1 M PBS and 0.1 M NaOH at −0.05 V (vs. RHE). Panels (e) and (f) are reproduced with permission.228 Copyright 2018, Nature Communication. (g) STEM image of Pd0.2Cu0.8/rGO (inset: particle size distribution). (h) NH3 yield rates and FE of the Pd0.2Cu0.8/rGO composite at different potentials in 0.1 M KOH. Panels (g) and (h) are reproduced with permission.229 Copyright 2018, Wiley. (i) SEM image of Ru SAs/N-C. (j) yield rate of NH3 production at different applied potentials on Ru SAs/N-C and Ru NPs/N-C. Panels (i) and (j) are reproduced with permission.230 Copyright 2018, Wiley. (k) TEM image of Rh2Sb SNRs. (l) FE in comparison to Rh2Sb RNRs/C, Rh2Sb SNRs/C, and Rh NPs/C at different potentials. Panels (k) and (l) are reproduced with permission.231 Copyright 2020, Wiley. (m) TEM image of the Ag nanosheet, and its corresponding (n) NH3 yield rate and FE at different potentials. Panels (m) and (n) are reproduced with permission.232 Copyright 2018, Royal Society of Chemistry. (o) SEM image of Ag NPs-rGO, and its corresponding (p) NH3 yield rate and FE at different potentials. Panels (o) and (p) are reproduced with permission.233 Copyright 2020, Springer Nature Switzerland AG. Part of Springer Nature. | ||
To promote access to more active sites and enhance the NH3 yield rate and selectivity, porous Au materials are suggested.226 Wang and co-workers employed a micelle-assisted electrodeposition approach to directly synthesize a porous Au film on Ni foam as an NRR electrocatalyst. In 0.1 M Na2SO4 under ambient conditions, the porous Au film presented an NH3 yield rate of 9.42 μg h−1 cm−2 and an FE of 13.36% at −0.2 V (vs. RHE).226 Nazemi and co-workers engineered hollow Au nanocages (Fig. 5(c)) of various pore size/density and Au content for the electrochemical synthesis of NH3. It was demonstrated that the 715 nm pore size (38.3 Au-wt%) displayed the optimal performance with an NH3 yield rate of 3.9 μg cm−2 h−1 and a large FE of 30.2% (Fig. 5(d)). The observed improvement in catalytic activity is attributable to the nanoscale confinement of N2 near the catalyst surface.227
Pd-Based electrocatalysts. Nowadays, neat palladium (Pd) has been argued to be not suitable as a catalyst for N2-to-NH3 conversion owing to the preferential affinity for hydrogen than nitrogen resulting in the hindrance of N2 adsorption.30 To circumvent this, strategic schemes to suppress the HER are advised. One of the strategies is adopting a hydrogen spillover phenomenon whereby the H-species are adsorbed on the catalyst and then spilled over to the adsorbed N2 for hydrogenation to occur. By doing so, the kinetics for N2-to-NH3 conversion will be accelerated. Accordingly, hydrides were suggested with the potential of boosting surface N2 hydrogenation via a hydrogen transfer mechanism.234,235 However, the formation of Pd-hydrides requires the use of highly reducing agents which could result in difficult formation of hydrides.236 In this case, studies have investigated the use of electrides as an alternative approach. Electrides are ionic compounds known for their ease of electron donation. Under this circumstance, their usage will provide electrons for the adsorbed N2 while trapping excess electrons to produce the hydride.
Xin and co-workers highlighted this effect using Pd nanoparticles on a carbon black support (Pd/C), which can generate Pd-hydrides under specific potentials (Fig. 5(e) and (f)).228 This mechanism allowed for the effective suppression of HER in 0.1 M PBS and hence facilitated the Grotthuss-like hydride transfer mechanism on α-PdH for the hydrogenation of N2. The beneficial effect of PBS in promoting N2 hydrogenation at −0.05 V (vs. RHE) (yield rate = 4.9 μg h−1 mg−1Pd) is twice the yield from 0.05 M H2SO4 (2.5 μg h−1 mg−1Pd) and 0.1 M NaOH (2.1 μg h−1 mg−1Pd). The controlled potential electrolysis on the Pd/C nanoparticles resulted in an NH3 yield rate of ∼4.5 μg h−1 mg−1Pd and a FE of 8.2% at 0.1 V (vs. RHE) (at a low overpotential of 56 mV), outperforming Au and Pt catalysts.228
Moreover, Pd catalysts can be modified for N2-to-NH3 transformation by integrating with other metals to produce alloys. For highly effective catalyst systems, Jacobsen and co-workers proposed the rational approach of forming alloys with elements from the different sides of the volcano plot. Specifically, integrating a metal with strong N2 affinity with another with weak affinity is more likely to yield optimum ammonia synthesis.237 On this account, Yan and co-workers developed an amorphous PdCu nanocluster on rGO for catalyzing NH3 synthesis229 based on the characteristics of amorphous Cu to promote the hydrogen-spillover mechanism.238 In addition to the excellent electron transport property of rGO, this support promotes the dispersion and even distribution of the alloy nanoparticles, thereby preventing particle agglomeration (Fig. 5(g)). Based upon the above principle, the optimal Pd0.2Cu0.8 alloy nanoparticles significantly outperformed the individual components and displayed an NH3 yield rate of 2.80 μg h−1 mg−1cat. with a low FE of about 0.8% at −0.2 V (vs. RHE) in 0.1 M KOH (Fig. 5(h)).229
Ru-Based electrocatalysts. Ruthenium (Ru) is an exceptional catalyst for the synthesis of NH3via the conventional Haber–Bosch process.239 Theoretically, it is revealed that Ru displays a lower N2 adsorption energy and overpotential under both associative and dissociative mechanisms than other noble metals in the electrocatalytic synthesis of NH3. Similar to other electrocatalysts, the HER impedes the NRR by decreasing the number of catalyst active sites due to the increased *H-coverage. This fact considerably affects the energy barrier for the first N2 hydrogenation and desorption of *NH2.240 One of such modalities to promote the NRR activity of Ru-based electrocatalysts is the anchoring of Ru atoms on appropriate support structures such as N-doped carbon. For instance, Geng and co-workers demonstrated that Ru atoms anchored on N-doped carbon (Ru SAs/N-C) attained a remarkably high NH3 yield rate of 120.9 μg h−1 mg−1cat. and 29.6% FE (Fig. 5(i) and (j)).230 The reason behind this is that with the aid of the porous and defective N-doped carbon, the dispersion of isolated Ru atoms throughout the Ru SAs/N-C structure promoted the adequate coordination of Ru atoms by N atoms. Similarly, atomic Ru doped in Mo2CTX MXene remarkably promoted the electrochemical N2-to-NH3 conversion.241
Rh-Based electrocatalysts. Based on several theoretical investigations, rhodium (Rh) can be considered as a promising NRR electrocatalyst as indicated by its position at the top of the volcano plot. So far, experimental studies have demonstrated various Rh nanostructures to be suitable for the electroreduction of N2 to NH3. This includes structures such as nanosheets,242 nanoparticles,231 nanowires243 and nanorods.231 For instance, Hou and co-workers recently reported that ultrathin Rh nanosheet nanoassemblies (Rh NNs) demonstrated an excellent NRR catalytic activity with a yield rate of 23.88 μg h−1 mg−1cat at −0.2 V (vs. RHE). Moreover, Rh NNs displayed good NH3 selectivity without the formation of N2H4. The remarkable activity is attributed to the unique ultrathin two-dimensional nanosheet structure (ca. 1 nm), modified electronic construction and high SSA.242
To further improve the catalytic activity, schemes to modulate the surface roughness of the catalysts were considered. Adopting a facile hydrothermal approach, Zhang and co-workers synthesized a surface-rough Rh2Sb nanorod on carbon (Rh2Sb RNRs/C) and compared its NRR performance with that of a surface-smooth Rh2Sb nanorod (Rh2Sb SNRs/C) and Rh NPs/C. The NH3 yield rates attained by these catalysts are 228.85 ± 12.96 μg h−1 mg−1Rh, 63.07 ± 4.45 μg h−1 mg−1Rh and 22.82 ± 1.49 μg h−1 mg−1Rh, respectively, at −0.45 V (vs. RHE), with 10 h stability witnessed by Rh2Sb RNRs/C (Fig. 5(k) and (l)).231 The superior catalytic activity by Rh2Sb RNRs was attributed to the high-index facets which enhanced the adsorption and activation of N2.
Ag-Based electrocatalysts. Among all the noble metals utilized as catalyst systems, silver (Ag) is the most promising candidate given that it is more abundant and offers the lowest cost relative to its catalytic activities. On this account, inquiry into its catalytic ability in the electrochemical synthesis of NH3 has gained some attention. Our group conducted a pioneering study in this regard and revealed that Ag nanosheets are an efficient electrocatalyst for N2 fixation to NH3 with remarkable stability and selectivity under ambient conditions. When tested in 0.1 M HCl, the Ag nanosheets achieved a yield rate of 4.62 × 10−11 mol s−1 cm−2 and 4.8% FE at −0.60 V (vs. RHE) with 24 h stability (Fig. 5(m) and (n)).232
To further improve the NRR efficiency, measures to retard the HER are necessary. On this account, Ji and co-workers revealed that the adsorption of halide anions on the surface of porous Ag effectively assisted the suppression of HER. In this study, the authors fabricated a nanoporous bromide-derived Ag film on Ag foil (BD-Ag/AF) with adsorbed Br- anions by means of in situ electrochemical reduction of the AgBr film on Ag foil. When tested in 0.1 M Na2SO4, BD-Ag/AF attained an improved FE efficiency of 7.36% in comparison to 0.38% of the porous Ag film alone, and a yield rate of 2.07 × 10−11 mol s−1 cm−2 at −0.6 V (vs. RHE) with 20 h stability.244
Despite the remarkable stability, the harsh self-aggregation of small-sized Ag nanoparticles affects their activity in addition to decreasing the electronic conductivity. To this effect, employing conductive substrates such as RGO to boost the catalyst's conductive features while simultaneously enabling particle dispersion has been proven to enhance the activity of the N2-to-NH3 conversion. For instance, Li and co-workers fabricated a Ag nanoparticles-reduced graphene oxide hybrid (Ag NPs-rGO) as a high-efficiency electrocatalyst for the NRR. When tested in 0.1 M Na2SO4, Ag NPs-rGO achieved an NH3 yield rate of 18.86 μg h−1 mg−1cat. and 3.60% FE at −0.7 V (vs. RHE) (Fig. 5(o) and (p)), outperforming the Ag NPs under the same conditions (yield = 9.43 μg h−1 mg−1cat. and FE = 2.25%).233
Metallic substance. Similar to noble metal based electrocatalysts, non-noble metals and their compounds have also shown good NRR activity and are considered as potential replacements for noble metal electrocatalysts. Molybdenum (Mo) is one of the many metallic elements with a strong affinity for N2 adsorption, which is a preliminary characteristic for the NRR. The (110)-oriented Mo nanofilm has been proven to be an efficient catalyst under ambient conditions. Electrochemically, the (110)-oriented Mo nanofilm yielded NH3 at a rate of 1.89 μg h−1 cm−2 and an FE of 0.72% at –0.49 V (vs. RHE) and 0.14 V (vs. RHE), respectively.245 The low NH3 yield was attributed to the strong Mo–N bonding making it difficult for the desorption of NH3. To circumvent this, it is recommended to introduce higher electronegative non-metal atoms (such as N) into the Mo lattice which could potentially assist the reduction of electron charge transfer between the Mo and N-adatoms/admolecules.205,246
Because of the unique electronic structure and sluggish HER activity, bismuth (Bi) based materials demonstrate a high NRR performance. The ammonia yield of the fragmented Bi0 nanoparticles was found to be 3.25 ± 0.08 μg cm−2 h−1 at −0.7 V vs. RHE with a faradaic efficiency of 12.11 ± 0.84% at −0.6 V vs. RHE.247 Compared with Bi0 nanoparticles, the three dimensional amorphous BiNi alloy showed an enhanced NRR activity. The NH3 yield rate of this structure was 17.5 μg h−1 mgcat−1 with a faradaic efficiency of 13.8% at −0.6 V vs. RHE. These two works indicate the significance of the electronic and geometric structure of the electrocatalysts in NRR.248
Another highly desirable metal material for the electrochemical N2-to-NH3 conversion is iron (Fe) given its important role as a catalyst system.249 A typical example is its function as an earth-abundant and low-cost catalyst for NH3 production in the industrial Haber–Bosch process.250 In terms of biological N2 fixation, Fe is also present in all three forms of nitrogenase enzymes (MoFe-, VFe-, and FeFe-nitrogenase).251 Founded on this, several investigative studies have focused on developing Fe-based catalyst systems that can support the electrosynthesis of NH3. First, theoretical evidence has revealed that Fe is one of the most promising NRR electrocatalysts among the available TMs.201 Further studies have demonstrated the associated mechanism as the NRR pathway on Fe-based electrocatalysts such as Fe2O3 with the first protonation step being the rate-determining step.252 Similar to all other metallic substances, Fe-based metal electrocatalysts display low NH3 yield resulting from passivated electrocatalytic activity from aggregated Fe-species generated during the NRR.202,203
Metal hydroxides and oxides. As previously mentioned, Fe has shown a tremendous application as a catalyst system. Likewise, iron oxide based materials such as Fe2O3 have also presented good catalytic characteristics.253,254 Experimental studies on Fe2O3 NPs over a Ni cathode have demonstrated a substantial NH3 yield rate with a FE of nearly 35%, but at a high temperature of 200 °C.253 Moreover, it was established that the yield rate of NH3 is dependent on the Fe2O3 NPs. For instance, over a 100-fold increase in yield was witnessed within the same time frame when the Fe2O3 particle size was reduced from ∼70 μm to 1–3 μm. However, for a further decrease to 10–30 nm, the NRR was too rapid and violent to be evaluated.254
In addition, the use of conductive supports has also demonstrated to improve the catalytic utilization of γ-Fe2O3 NPs. Anchoring γ-Fe2O3 NPs on porous CP resulted in an NH3 yield rate of 0.9503 μg h−1 mg−1,255 which is triple the yield from the Fe catalyst (0.3044 μg h−1 mg−1).188 Likewise with other TMs, the enhanced activity is related to the improved interface between the NPs and the carbon surface, which offers unique carbon sites for N2 fixation.256 Moreover, modulating the loading of the catalyst content on the support is suggested for optimal tuning of the NH3 formation rate.257
The nature of the electrolyte is another factor of great importance for the effective performance of NRR electrocatalysts. Generally, N2 fixation in a molten salt is associated with high temperatures requiring a high energy input. On this account, investigative studies have demonstrated the catalytic efficacy of Fe-based catalysts in different electrolytes such as N2-saturated ionic liquids ([C4mpyr], [eFAP] and [P6,6,6,14][eFAP]) (Fig. 6(a) and (b))258 or N2-saturated alkaline electrolytes (Fig. 6(c) and (d)).255 In these studies, the high solubility of N2 in the ionic liquids enabled the high NRR activity.
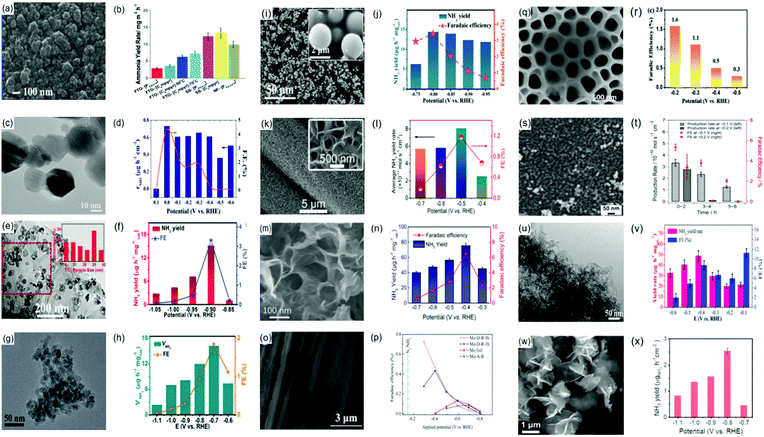 | ||
| Fig. 6 (a) SEM image of the Fe-based catalyst and the corresponding (b) NH3 yield rate on different electrodes and ionic liquids at −0.8 V (vs. NHE). Images (a) and (b) are reproduced with permission.258 Copyright 2017, Royal Society of Chemistry. (c) TEM image of γ-Fe2O3 NPs, and the corresponding (d) NH3 yield rate and FE at different potentials in N2-saturated 0.1 M KOH. Images (c) and (d) are reproduced with permission.255 Copyright 2017, American Chemical Society. (e) TEM image of TiO2-rGO (inset: the particle size distribution of TiO2) and its corresponding (f) NH3 yield rate and FE at different potentials in 0.1 M Na2SO4. Images (e) and (f) are reproduced with permission.272 Copyright 2018, Royal Society of Chemistry. (g) TEM image of C-TiO2 NP and its corresponding (h) NH3 yield rate and FE at different potentials in 0.1 M Na2SO4. Images (g) and (h) are reproduced with permission.273 Copyright 2019, Royal Society of Chemistry. (i) SEM image of B-TiO2 and its corresponding (j) NH3 yield rate and FE at different potentials in 0.1 M Na2SO4. Images (i) and (j) are reproduced with permission.274 Copyright 2019, American Chemical Society. (k) SEM image of MoS2/CC and its corresponding (l) NH3 yield rate and FEs at different potentials. Images (k) and (l) are reproduced with permission.275 Copyright 2018, Wiley. (m) SEM image of Fe3S4 nanosheets and their corresponding (n) NH3 yield rate and FEs at different potentials. Images (m) and (n) are reproduced with permission.276 Copyright 2020, Wiley. (o) SEM image of the (110)-oriented Mo nanofilm and its corresponding (p) FE at different potentials. Images (o) and (p) are reproduced with permission.245 Copyright 2017, Royal Society of Chemistry. (q) SEM image of Mo2C/C nanosheets and their corresponding (r) FE at different potentials. Images (q) and (r) are reproduced with permission.277 Copyright 2018, Wiley. (s) SEM image of VN nanoparticles. (t) Time-dependent production rate and faradaic efficiency at −0.1 V and −0.2 V for 8 h tests, respectively. Images (s) and (t) are reproduced with permission.278 Copyright 2018, American Chemical Society. (u) TEM image of CoP/CNs and their corresponding (v) NH3 yield rate and FE at different potentials. Images (u) and (v) are reproduced with permission.279 Copyright 2019, Royal Society of Chemistry. (w) SEM image and (x) NH3 formation rates at different potentials of Bi NSs. Images (w) and (x) are reproduced with permission.280 Copyright 2020, Royal Society of Chemistry. | ||
The reaction of Fe2O3 in an alkaline aqueous solution is represented by reaction (41).259 In this case, the generated Fe(OH)2 could lead to the passivation of Fe2O3, which affects the activity on the surface of Fe2O3.260 However, based on reaction (42), Fe(OH)2 can also be converted to FeOOH in an alkaline solution.
| Fe2O3 + 3H2O + 2e− → 2Fe(OH)2 + 2OH− | (41) |
| 2Fe(OH)2 + 2OH− → FeOOH + H2O + e− | (42) |
Owing to their strong affinity towards N-adatoms/admolecules than H-adatoms, Ti and its oxides are also highly considered as catalyst systems for electrochemical NH3 synthesis. However, when acting alone, Ti-based materials have low NRR activity owing to their poor electronic conductivity.271 To circumvent this, Ti-based catalysts are supported on conductive substrates such as RGO (TiO2-rGO). When tested in a neutral solution (0.1 M Na2SO4), an ammonia yield rate of 15.13 μg h−1 mg−1cat. and 3.3% FE at −0.90 V (vs. RHE) were observed over TiO2-rGO (Fig. 6(e) and (f)).272
Moreover, C-doping has shown great potential for improving the electro-conducting state of TiO2.281 When doped with carbon, C-doped TiO2 NPs displayed a high NH3 yield rate of 16.22 μg h−1 mg−1cat. and a FE of 1.84% at –0.7 V vs. RHE in 0.1 M Na2SO4 (Fig. 6(g) and (h)).273 Compared to the effect of C-dopant, B-dopants as an electron deficient atom could enrich the positively charged centers for N2 adsorption and activation (Fig. 6(i) and (j)). B-doped TiO2 produced NH3 at a rate of 14.4 μg h−1 mg−1cat. at −0.8 V vs. RHE in 0.1 M Na2SO4,274 which was slightly lower than that of C-doped TiO2 NPs.273 However, the former demonstrated a higher FE of about 3.4% when compared to the 1.84% of the latter. The variation in the activity was related to the transitioning of the semiconducting phase of TiO2 into a semi-metal state due to the appropriate B-doping resulting in the transfer of more electrons to expedite N2 activation. Also, the introduction of B-dopant resulted in the formation of O-vacant defects on the TiO2 surface which enabled the trapping of electrons at the vacant active sites for severing the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond.274
N bond.274
In view of the presence of defects, it is conceived that the occurrence of O-vacancies of NRR electrocatalysts can result in the generation of H+ defects which can trap electrons for the activation of N2.282 For instance, Zhang and co-workers highlighted the significant role of O-vacant sites in the N2-to-NH3 conversion over TiO2. Here, a yield rate of 5.62 μg h−1 cm−2 with 2.5% FE was achieved at −0.7 V vs. RHE while utilizing defective TiO2 in 0.1 M Na2SO4.283 In other studies, the presence of O-vacancies was credited for the increased activity over TiO2 to 7.59 μg h−1 cm−2 from 1.04 μg h−1 cm−2 for the perfect TiO2 in an acidic medium. Moreover, an increase in FE was also observed from 0.95% to 9.17% under ambient conditions.284
Metal chalcogenides. Following the significant role of S and Mo in nitrogenases for N2 fixation, studies have shown that the presence of S atoms can enhance the NRR activity of Mo.275 In addition to this, the preliminary study on the electroreduction of NH3 by Furuya and Yoshiba in 1990 revealed that metal chalcogenides (comprising of sulfides and selenides) displayed best NRR activity, with ZnSe being the most suitable for the reduction of N2.14 However, the FE was relatively low and was attributed to the relative competition of the HER. In this case again, suppressing the HER activity is a challenge to be confronted. Hence, the development of this set of electrocatalysts is crucial towards an effective NRR with high NH3 yield.
Recently, Sun and co-workers reported the effectiveness of MoS2 nanosheets grown on carbon cloth (Fig. 6(k)) to serve as an NRR electrocatalyst yielding NH3 at a rate of 4.945 μg h−1cm−2 with 1.17% FE at −0.5 V (vs. RHE) in 0.1 M Na2SO4 solution (Fig. 6(l)).275 Regarding the NRR performance of MoS2, Suryanto and co-workers related the suppression of HER to the enriched N2 binding sites partly resulting from the occurrence of isolated S-vacancy defects, which served as centers for hydrogenation.285
Theoretically, Abghoui and co-workers investigated the NRR potential of several TM sulphides via a DFT computational study. After structural optimization, computational results revealed that RuS2 is the most active among all examined model catalysts that could catalyze the N2-to-NH3 conversion at potentials around −0.3 V through the associative mechanism. NbS, CrS, TiS, and VS are also promising NRR catalyst systems with both associative and dissociative mechanisms at overpotentials ranging from 0.7 to 1.1 V.286 In addition to these sulfides, metal selenides have also demonstrated good NRR activity under ambient conditions. Recent development has fabricated selenium vacancy rich ReSe2@carbonized bacterial cellulose as an active electrocatalyst to attain an NH3 formation rate of 28.3 μg h−1 cm−2 with 42.5% FE at –0.25 V (vs. RHE) (Fig. 6(m) and (n)).276
Metal carbides. Another suitable candidate as an efficient NRR electrocatalyst is metal carbides (also known as TM carbides, TMCs). Apparently, the adsorption and activation of N2 depend on the electronic structure of the constituting elements of the electrocatalyst. Elements with unoccupied d-orbitals are more suited to interchange electrons with N-admolecules, which will enable N2 activation. Besides the noble metals that offer these rare abilities, TMCs are predicted to display a similar concept due to the presence of unoccupied d-orbitals.287 Coupled with this, the hybridized orbitals between the TM (sp-orbital) and the carbide (s-orbital) will facilitate more back-donation to the π orbitals of N2, thereby enhancing N2 fixation.
As mentioned earlier, a well-known metal carbide with high catalytic activity is molybdenum carbide (Mo2C) which has displayed strong affinity towards electron-rich compounds and activation of the HER.288,289 When compared to the conventional Mo electrocatalysts (Fig. 6(o) and (p)),245 Mo2C nanodots displayed a significant improvement in NH3 yield and efficiency (Fig. 6(q) and (r)). In addition to the inactivation of spilled over H-adatoms by the inlaid structure, other factors that contributed to the improved ammonia yield include enhanced N2 adsorption and activation on the enriched size-promoted active sites and the reduction of H-coverage on the catalyst surface. Despite the good NRR performance, the occurrence of a high HER activity was observed.277 To overcome this limitation, it was suggested that inducing C-vacancy defects is likely to fortify the metal–C ratio in order to retard the accumulation of H-adatoms and thereby evolution of H2.290
Recently, a new family of two-dimensional (2D) TM carbides and carbonitrides, also known as MXenes (TMn+1Xn (n = 1–3, and X = C and/or N)), have presented good catalytic activity towards the electrosynthesis of NH3.262,291 These compounds have specific structural characteristics given that their lattice TMs are exposed on both sides of the 2D layers and mostly terminated by F, O and/or OH groups with the general formula TMn+1NmXn (N = F, OH, and/or O).292 Given their nascent discovery, thorough investigative studies on their mechanism are unknown. However, it is alleged that O- and OH-terminated MXenes are the most catalytically viable given their stability and remarkable charge distribution. In addition, theoretical evidence has revealed that the O-terminated MXenes are active centers for HER which can be exploited to retard the HER process.291
Based on quantum theory, the synergistic coupling effect of TMs and the integrated C-atom resulting in the unique hybridization of their orbitals enables the TMCs to behave as catalysts with electron-enriched characteristics.287 Specifically, there is a shift in the d-band of the TM upon the integration of the TM and C atoms, which will facilitate its hybridization with the C s-orbital resulting in electron-enriched orbitals that can offer more electrons to severe the π-orbitals of N2.277 Theoretical studies to this effect have revealed the resultant low activation energy (0.32 and 0.39 eV vs. SHE) for the N2-to-NH3 conversion on V3C2 and Nb3C2, respectively.293
To gain insights into the NRR mechanistic pathway on TMCs, Shao and co-workers investigated the N2-fixation mechanism on MXenes. It was reported that the overall NRR energy is decided by electron transfer between the TMC and N2. Specifically, the donation and reception of more electrons from the TMCs are likely to indicate an exothermic and endothermic reaction, respectively. Hence, for an effective N2-to-NH3 conversion, more exothermic reaction, an extended N–N bond and substantial charge transfer are required. Based on this, mechanism-guided prediction shows that Mo2C and W2C are more suitable for electrosynthesis of NH3.222 Subsequent experimental studies validated this claim by demonstrating that Mo2C nanorods yielded NH3 at a remarkable rate of 95.1 μg h−1 mg−1cat and 8.13% FE at −0.3 V (vs. RHE) in 0.1 M HCl.294 Another reported TMC for NH3 synthesis is the F- and OH-terminated Ti3C2Nx (N = F, OH) nanosheets which displayed a yield rate of 20.4 μg h−1 mg−1cat. and 9.3% FE at –0.4 V (vs. RHE).270
Metal nitrides. Similar to TMCs, metal nitrides (also known as TMNs) are fabricated by integrating the N-atom into the skeletal structure of the TM resulting in the d-band contraction with an electronic structure similar to that of noble metals.295 Owing to the large distinction in electronegativity between the N atom and the TMs, TMNs possess enriched acid–base active centers resulting from the enhanced distribution of electron charges. The availability of the distributed active centers makes TMNs suitable candidates for NH3 electrosynthesis.
Like the TMs, TMNs can also enable the synthesis of NH3 by the direct reduction of the incorporated N atom, thereby creating a N-vacancy on the surface of the TMN catalyst which can also be repaired via N2 adsorption.215 Nonetheless, this pathway may be hindered by the competitive adsorption of other species other than N2 and consequently preventing the regeneration of the catalyst. On this account, two major conditions are necessary for the design of these catalysts. These conditions should include the repair and regeneration of the vacancies and a minimal overpotential.
In recent studies, theoretical evidence has presented the preferential repair of the vacant sites by N-atoms rather than H+, O2−,or OH− species owing to the strong energetics between these sites towards N-adatoms.296 The N-adatom adsorption energies vary with the different facets of the TMNs. Upon optimization, calculation results revealed that the rock-salt (RS) structures with (100) facets of the nitrides of V and Zr are the most favorable catalyst systems for high yield of N2 fixation at low overpotentials, while the nitrides of V and Cr are more suited for high efficiencies owing to low H2 generation.205 Besides, the (111) facet of the RS for MnN, VN and CrN also demonstrated a low overpotential towards N2-to-NH3 conversion. Nevertheless, MnN is preferentially attacked by other species other than N2 and thereby its performance as a NRR electrocatalyst is hindered.295
Founded on these theoretical calculations, several experimental studies have been conducted on VN as a suitable NRR electrocatalyst with more active centers for a high yield and conversion efficiency of N2-to-NH3. These studies include the evaluation of the VN nanosheet array on Ti mesh,297 VN NPs (Fig. 6(s) and (t))278 and VN nanowire array (on CC).298 Using the trackable 15N2 species as the feed gas, it was evident that the NRR route on VN followed the Mars–van Krevelen (MvK) mechanism with the lattice N-atom partaking in the formation of 14NH3 and 15NH3, and subsequent healing of the vacant site thereafter created.278
Metal phosphides. Among the myriads of catalyst systems for N2-fixation under ambient conditions, transition metal phosphides (TMPs) have shown good potential in this regard given their high catalytic efficacy, earth-abundance and low-cost. In addition to this, it is stipulated that the positively charged metal sites of the TMPs serve as the active centers for N2 adsorption and activation, while the adjacent negatively charged P-motifs are sites for proton anchoring via H-bonding. This electronic modulating effect of phosphorus has attracted research attention towards its adoption to boost the intrinsic characteristics of NRR electrocatalysts.
Experimentally, Zhang and co-workers investigated the potential of CoP NPs synthesized via the pyrolysis-phosphorization method as NRR electrocatalysts under ambient conditions. Here, the authors demonstrated that the as-synthesized CoP/CNs yielded NH3 at a rate of 48.9 μg h−1 mg−1cat and 8.7% FE at −0.4 V (vs. RHE) in 0.1 M Na2SO4 with an associative distal mechanism (Fig. 6(u) and (v)).279 Recently, Zhang and co-workers also revealed the significant role of support effects in the modulation of surficial electronic characteristics of Ni2P NPs.280 In this study, Ni2P NPs supported on N,P co-doped CNs (Ni2P/N,P-C) were tested in alkaline, acidic and neutral solutions under ambient conditions. The Ni2P/N,P-C catalyst displayed an excellent catalytic activity in all electrolytes with the highest performance witnessed in 0.1 M KOH with a yield rate of 90.1 μg h−1 mg−1 and 19.82% FE at −0.2 V (vs. RHE). In 0.1 M HCl, a yield rate of 34.4 μg h−1 mg−1 and 17.21% FE were witnessed (Fig. 6(w) and (x)).280 The high activity of Ni2P/N,P-C was attributed to the appropriate modulating effect of the N,P-C substrate to trap and distribute electrons. Following the establishment of the unusual role of phosphorus in modulating the NRR activity of catalyst systems and the importance of support effects, we demonstrated the high NRR performance of the FeP2 NP-RGO hybrid. When tested in 0.5 M LiClO4, an NH3 yield rate of 35.26 μg h−1 mg−1cat. and a high FE of 21.99% at −0.4 V (vs. RHE) were witnessed.299 Theoretical evidence shows that the FeP2 offers enriched active sites, higher N2 adsorption energy and a retarding effect for the HER than FeP.
Carbon-based electrocatalysts. Carbon-based electrocatalysts are one of the favorable alternatives for metal-based NRR electrocatalysts owing to their low HER selectivity,301,302 unfavorable H2 formation at the C edge site303 and high electrical conductivity and electrochemical stability.300 Also, these unique electrocatalysts easily offer to back-donate their copious π electrons to the π orbitals of N2 leading to the activation of N-admolecules. Nonetheless, pristine carbon-based materials still have a low NRR catalytic activity owing to the inert nature of the π electrons. To circumvent these shortcomings, approaches to modulate the NRR activity of carbon-based electrocatalysts could include defect introduction304 and heteroatom doping.305,306 Numerous studies have revealed that the activation of N-admolecules on a defect-rich carbon structure is easily enabled on the defective sites. To promote these defective sites on the surface of carbon-based materials, N, B, O and S-dopants are the most commonly used dopants. However, a recent study revealed a remarkably high NRR activity (NH3 yield rate = 32.33 μg h−1 mg−1cat. and FE of 20.82% at 0.65 V vs. RHE) on P-doped graphene in 0.5 M LiClO4 under ambient conditions (Fig. 7(a) and (b)). It was revealed that the P-dopant fostered graphene re-stacking which created more defect sites in the structure.306
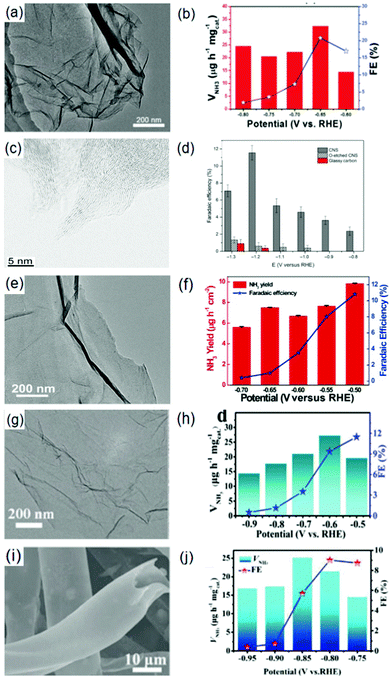 | ||
| Fig. 7 (a) TEM image of P-doped graphene and its corresponding (b) NH3 yield rate and FE at different potentials. Panels (a) and (b) are reproduced with permission.306 Copyright 2020, Royal Society of Chemistry. (c) Aberration-corrected STEM image of N-doped carbon nanospikes and their corresponding (d) FE at different potentials. Panels (c) and (d) are reproduced with permission.301 Copyright 2018, American Association for the Advancement of Science. (e) TEM image of B-doped graphene and its corresponding (f) NH3 yield rate and FE at different potentials. Panels (e) and (f) are reproduced with permission.316 Copyright 2018, Elsevier. (g) TEM image of S-doped graphene and its corresponding (h) NH3 yield rate and FE at different potentials. Panels (g) and (h) are reproduced with permission.323 Copyright 2019, Royal Society of Chemistry. (i) TEM image of O-doped hollow carbon microtubes and their corresponding (j) NH3 yield rate and FE at different potentials. Panels (i) and (j) are reproduced with permission.324 Copyright 2019, Royal Society of Chemistry. | ||
N-Dopant. To activate the π electrons of carbon-based electrocatalysts, the lone-pair electrons of the N-atom readily conjugate with π electrons, thereby making it an effective approach to enhance the NRR activity on carbon-based materials.307 In other words, N-doped carbon materials could generate additional Lewis pairs that can readily activate N2 and H2 molecules.308 Coupled with this, N-doped carbon materials are easily polarizable such that they promote adsorption of N2 and electron/mass transfer.186
Recently, experimental studies have involved incorporating N-dopant into C-based structures that exhibit remarkable NRR activity. Song and co-workers demonstrated an NH3 yield rate 97.18 ± 7.13 mg h−1 cm−2 with 11.56 ± 0.85% FE at −1.19 V (vs. RHE) over N-doped carbon nanospikes in 0.25 M LiClO4 (Fig. 7(c) and (d)).301 The sharp spike structure of the electrocatalyst provided a dense distribution of electrons at its tips, which promoted the dissolution of N2.
Liu and co-workers further revealed that the NRR performance of the N-doped carbon material can be regulated by adjusting the pyridinic and pyrrolic N content in N-doped porous carbon (NPC).186 Most importantly, the pyridinic N atom in NPC partakes in the formation of NH3 resulting in the generation of N-vacancies that can serve as active centers for further activation of N-admolecules.309
However, this is most effective in alkaline solutions as NPC undergoes severe HER in acidic electrolytes.310 Other reported N-doped carbon-based NRR catalysts include polymeric carbon nitride (PCN) with an enhanced spatial electron transfer due to the induced N-vacancies.311,312
B-Dopant. Similar to N-dopants, B-dopants have also shown great capacity to activate the π electrons of carbon-based electrocatalysts. Moreover, boron is a notable single atom catalyst for the electrochemical N2-to-NH3 transformation. In addition to their successful NRR performance,313,314 B-dopants like N-dopants have equally attracted great attention as a means to modulate the NRR activity of carbon-based electrocatalysts.315,316
Boron is an electron deficient atom with four valence electrons in the sp-orbitals, which bonds uniquely with the electronic structure of C.317 The hybridized electronic structure between these two atoms results in the generation of unoccupied orbitals that can accept lone-pair electrons from N2. Simultaneously, the occupied 2p-orbitals can back donate electrons to the π orbitals of N-admolecules. Above all, B-atoms can retard the HER by prohibiting the binding of Lewis acids in an acidic medium.316,318,319
Despite the good electronic structure, this set of electrocatalysts still suffer from high HER activity and instability of adsorbed N2, therefore requiring appropriate schemes to strengthen it.315 In this regard, optimizing the content of B-dopant has been shown to mitigate this shortcoming. For example, B-doped graphene exhibited an optimum NRR activity at a B-dopant content of 6.2% with a yield rate of 9.8 μg h−1 cm−2 and a high FE of 10.8% at −0.5 V (vs. RHE) in 0.05 M H2SO4 solution (Fig. 7(e) and (f)).316 As mentioned earlier, S plays a significant role in the biological synthesis of NH3 by means of nitrogenase enzymes.320 Nonetheless, the low electrical conductivity of S321 impedes its application in the electrocatalytic NRR. To circumvent this, the adoption of conductive supports such as RGO has been proposed as a suitable mechanism for boosting the electrical conductivity of S. For example, the S-doped dots-graphene nanohybrid demonstrated a good NH3 synthesis rate of 28.56 μg h−1 mg−1cat. and 7.07% FE at −0.85 V (vs. RHE) in 0.5 M LiClO4.322 Similar to other dopants, the S atom also possesses a modulating effect to tune the NRR activity for carbon-based materials. A typical example of this effect is portrayed in the NRR performance of graphene in 0.1 M HCl under ambient conditions. Prior to S-doping, the NRR activity on graphene resulted in an NH3 yield rate of 6.25 μg h−1 mg−1cat. and a low FE of 0.52%. However, after S-doping, a significant boost in the activity was observed with an NH3 yield rate of 27.3 μg h−1 mg−1cat. and a high FE of 11.5% under similar conditions (Fig. 7(g) and (h)).323
O-Dopant. Similar to S atoms, O atoms possess the same electronic structure and have displayed good potential to improve the electrocatalytic activity of carbon-based materials. In the same manner as S atoms, O-doping can trigger the activity of the inert π electrons of the carbon-based materials via O–C interactions. An example of this is demonstrated over an amorphous O-doped carbon nanosheet (CN) with a high NH3 yield rate of 20.15 μg h−1 mg−1cat. and a FE of 4.97% at –0.6 V (vs. RHE) in 0.1 M HCl.300 Likewise, O-doped hollow carbon microtubes revealed a superior NRR performance with a yield of 25.12 μg h−1 mg−1cat. and 9.1% FE at –0.85 V (vs. RHE) and –0.80 V (vs. RHE) in 0.1 M HCl, respectively (Fig. 7(i) and (j)).324
Elementals and their derivatives. TMs are highly recommended for NRR electrocatalysis but their selectivity towards ammonia synthesis is low. Other alternatives to replace this set of catalyst systems are the elementals and their compounds owing to their low-coordinated atoms.277 Moreover, elemental catalysts are reported to significantly retard the HER based on the structural effect as the H-motifs are preferentially adsorbed on specific sites on the catalyst.325 Supported by this ensemble effect, the suppression of the HER can be controlled.
A notable elemental catalyst for electrochemical reduction of N2 is boron (B) and its compounds particularly boron nitride (BN), which have been proven to offer good NRR activity as electrocatalysts,313,314 despite theoretical studies suggesting that B–N pairs in h-BN are inactive towards N2-to-NH3 conversion.326 For instance, Zhang and co-workers experimentally demonstrated the electrocatalytic efficacy of the B nanosheet to attain an NH3 yield rate of 13.22 μg h−1 mgcat−1 and 4.04% FE in 0.1 M Na2SO4.327 Also, at high B concentration, the boron carbide (B4C) nanosheet attained a high NH3 yield rate of 26.57 μg h−1 cm−2 and a high FE of 15.95% at −0.75 V (vs. RHE). Fortunately, the catalyst displayed remarkable stability and selectivity towards NH3 formation.328
Similarly, theoretical evidence has demonstrated the possibility of a monolayer phosphorus (P) catalyst system to catalyze N2-to-NH3 conversion.329 From an experimental perspective, few-layered black P (BP) nanosheets were used to produce NH3 with a high yield rate of 31.37 μg h−1 mg−1cat. and 5.07% FE at –0.7 V (vs. RHE) and –0.6 V (vs. RHE), respectively. In addition, the authors also revealed that the active sites for the adsorption and activation of N2 were more favorable on the zigzag and diff-zigzag edges of the BP nanosheets. On these nanosheets, computational analysis indicated that only the edges of the catalyst structure could facilitate electron donation during the NRR, which limited the performance of the catalyst.330 To circumvent this, anchoring a single-atom Fe on the monolayer P was shown to vary the charge distribution and promote electron interchangeability at the edge of the catalyst system.329
In summary, advancements in the catalyst systems to enable efficient electrochemical N2-to-NH3 conversion under ambient conditions have been substantial. An ideal catalyst system should facilitate the adsorption and activation of N2 in order to promote the NRR kinetics. Where necessary, tailoring the catalysts’ electronic structure (by defect engineering, heteroatom doping, surface functionalization and interface engineering) can enhance their intrinsic NRR characteristics. More specifically, enriching the NRR active sites/centers (size and shape modification, utilizing supports with high surface area and conductivity, and anchoring single-atoms on the catalyst system) while suppressing that of the HER is the most direct means of enhancing the N2 reduction activity.331 Generally, the HER has a lower overpotential enabling it to be preferentially selected over NH3 formation; hence, the need for HER-retarding strategies such as the use of functional composite catalysts is recommended.332,333 In addition, the electrolyte selection, stability improvement and cost-competitiveness are still the important research aspects deserving attention in order to develop competitive NRR technologies towards practicality. For a brief overview, Table 2 summarizes the recent NRR electrocatalysts and their catalytic activity, providing insights into the chemical understanding of efficient electrocatalysts for NRR.
4. Valuable fuels and chemicals via CO2 reduction reaction
Conversion of CO2 as part of carbon capture and utilization (CCU) technologies has received increased interest in the past couple of decades, especially in view of favourable prospects related to positive impact on the global climate change and renewable electricity production.334,335 Research in this field has mainly focused on fundamental and mechanistic conversion of CO2 to valuable fuels and chemicals through a variety of technologies including biochemical,336 thermochemical,337 photochemical338 and electrochemical339 reduction of CO2. Among these technologies, electrocatalytic CO2 reduction reaction (CRR) has attracted greater attention by virtue of its mild operating conditions and great potential to scale up.340–343 Although significant progress is experienced in this field, there are still major challenges, which hinder the understanding of CRR.344Pioneering studies on CRR involving different metals was initiated by Hori and co-workers more than thirty years ago.11,345 However, large-scale implementation of CRR technology is still at its infant stage because, in contrast to fundamental CRR studies, the research to understand CRR from an industrial perspective and efforts to develop an industrial/commercial CO2 electrolyzer are scarce. Due to the commercial limitations, CRR still suffers from lack of mechanistic understanding of the kinetics and thermodynamic challenges. Specifically, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond of the CO2 molecule possesses a high bonding energy (750 kJ mol−1) when compared to the binding energies of the C–C bond (336 kJ mol−1), C–H bond (411 kJ mol−1) and C–O bond (327 kJ mol−1) of conventional hydrocarbons. Hence, in the absence of an external support, it is energetically unfavourable to dissociate CO2 to generate organic compounds.346 It is therefore necessary to utilize catalyst systems to lower the energy barrier, stabilize major intermediates and facilitate reaction kinetics.
O double bond of the CO2 molecule possesses a high bonding energy (750 kJ mol−1) when compared to the binding energies of the C–C bond (336 kJ mol−1), C–H bond (411 kJ mol−1) and C–O bond (327 kJ mol−1) of conventional hydrocarbons. Hence, in the absence of an external support, it is energetically unfavourable to dissociate CO2 to generate organic compounds.346 It is therefore necessary to utilize catalyst systems to lower the energy barrier, stabilize major intermediates and facilitate reaction kinetics.
Moreover, based on the utilized catalyst system and operating condition, a wide range of reduced products can be generated from the CRR including carbon monoxide (CO), formate/formic acids (HCOO−/HCOOH), methane (CH4), methanol (CH3OH), ethane (C2H4), ethanol (C2H5OH) and so on. Principally, this is established by the reaction mechanism at a given condition and most importantly, the working electrocatalyst.347,348 In essence, it is a considerable challenge to mechanistically tune the reaction to enhance a particular product selectivity.349 Furthermore, this shortcoming is compounded by the kinetically competitive HER, as was also notable in the NRR and water splitting process. As an outcome, the rational design and development of catalyst systems for the electroreduction of CO2 are highly necessary.
4.1. Electrochemistry of CO2 reduction reaction (CRR)
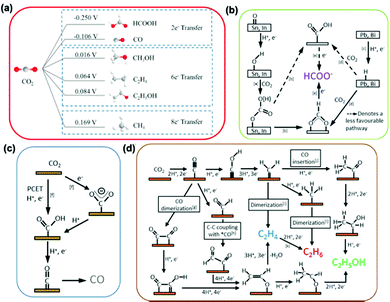 | ||
| Fig. 8 (a) CO2 reduction processes and the corresponding standard redox potentials, E0 (vs. SHE, V) for aqueous solutions. Reproduced with permission.350 Copyright 2014, Elsevier. CRR mechanisms to generate (b) formate, (c) CO, and (d) C2H4, C2H6 and C2H5OH, initiated from CO adsorption. Reproduced with permission.341 Copyright 2018, Elsevier. | ||
Conventionally, the process of heterogeneous electrocatalysis entails CO2 adsorption on the electrocatalyst surface, electron/mass transport to severe the C–O bond with the generation of C–H bonds, structural transformation and desorption of the reduced products from the electrocatalyst surface and subsequent diffusion into the electrolytic solution.363 The employed electrocatalyst and the applied electrode potential bias are one of the major factors that influence these processes and promote product selectivity.
From a thermodynamic point of view, a generally accepted CRR mechanism (pH 7 in aqueous solution (vs. SHE), 25 °C, 1 atm, and 1 M concentration of other solutes) for the primary products is illustrated below:
| CO2 + 2H+ + 2e− → CO + H2O, E0 = −0.52 V | (43) |
| CO2 + 2H+ + 2e− → HCOOH, E0 = −0.61 V | (44) |
| CO2 + 4H+ + 4e− → HCHO + H2O, E0 = −0.51 V | (45) |
| CO2 + 8H++ 8e− → CH4 + 2H2O, E0 = −0.24 V | (46) |
| CO2 + 12H+ + 12e− → C2H4 + 4H2O, E0 = −0.34 V | (47) |
| CO2 + 6H+ + 6e− → CH3OH + H2O, E0 = −0.38 V | (48) |
| 2H+ + 2e− → H2, E0 = −0.42 V | (49) |
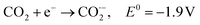 | (50) |
This reaction (reaction (50)) is the first CRR step and incites the large overpotential required to activate the reaction processes. In this step, a key intermediate CO2˙− is formed by the first electron transfer to a CO2 molecule with a large overpotential of −1.90 V (vs. SHE) due to the energy required to bend the linear CO2 molecule to a radical anion.346 Subsequently, the formed CO2˙− radical instantaneously reacts with several H+-coupled multiple-electron-transfer reactions to yield the reduced products. However, in practice, these intermediate reactions can be hindered as the OER occurs simultaneously with the CRR at the anode. To circumvent this, the cathode and anode compartments in the CO2 electrolytic cell are separated by means of an ion exchange membrane to avoid the oxidation of CRR products but promote the corresponding ion transfer.365
Pertaining to the formation of formates or formic acids, theoretical investigations into the CRR over post-transition metals have been widely conducted.367,368 With regard to formate formation, studies have revealed that the oxide layer of metal oxides plays a significant role in the formate production, as illustrated in Fig. 8(b).369 Spectroscopic analysis supported by DFT calculations suggests that the initial steps of formate production arise from the formation of surface-bound carbonate or bicarbonate intermediates from the adsorption of CO2 on the surficial O-motifs or OH-motifs, respectively.367,370 Sequentially, the formed bicarbonate species is reduced to either *COOH or *OCHO, with the latter being more thermodynamically favourable.371,372
Unlike some metals, the generation of formates does not occur via surface-bound carbonates/bicarbonates. A notable example is Pb electrodes where DFT calculations have suggested that formate formation proceeds by means of direct hydrogenation of CO2 by H-adatoms.368,373 Likewise, theoretical and experimental investigation on Bi-based electrocatalysts confirms the absence of surface-bound carbonates toward the formation of formates over Bi dendrites.374 However, both studies agree that the *OCHO intermediate is more thermodynamically viable for formate generation. Moreover, Yoo and co-workers demonstrated using DFT calculations on several modelled metal surfaces that *COOH and *H are highly correlated in their free energies. Unlike the correlation between *OCHO and *H, it is unlikely that the formation of formic acid will occur without the HER occurring along with. Based on the findings, the authors predicted that Ag and Pb are the most promising monometallic electrocatalysts with high FE for the production of HCOOH via CRR.375
Regarding the electrosynthesis of CO using CRR, several studies depict this reaction to proceed via the *COOH intermediate.340 In this case, two possible pathways are proposed. The first involves the single step proton-coupled electron transfer (PCET), while the second route is the formation of the CO2˙− radical via single electron transfer and subsequent protonation to *COOH.376Fig. 8(c) illustrates the two mechanistic routes. Irrespective of the mechanistic route, theoretical studies have shown a good correlation in the binding energies between *COOH and *CO, and *H and *CO on some metals,377 and between *COOH and *CO, and *COOH and *H over a variety of other metals.375 On this account, it is necessary that the development of electrocatalysts should consider schemes to optimize the surficial stabilization of *COOH without promoting the HER and influencing CO desorption. In this regard, further studies have revealed that the unsaturated coordinative sites such as the edges and corners are more promising sites for CO generation via CRR.377,378 Experimental validation of this concept is demonstrated in the mechanistic studies on Ag379 and Au.380 Here, the nanostructured catalyst systems possessed more active sites with unsaturated coordination than their metal foil analogues.
About the electrosynthesis of C2 products and other hydrocarbons via CRR (Fig. 8(d)), investigative studies on Cu and its compounds have been widely conducted. This has resulted in numerous proposed mechanisms, particularly for the different reduced products.381–388 Owing to this, it is unlikely to completely discuss each mechanistic insight without derailing from the scope of this study. Hence, this study will only review the theoretical insight for the electrosynthesis of C2H4. Kindly refer to the relevant literature above for further insights into other reduced products.
One of such mechanistic routes is the dimerization of *CO, which was suggested to occur over the Cu(100) electrode. In this respect, studies have demonstrated both experimentally and computationally that the formation of C2H4 occurs shortly after the rate of CO generation has peaked. In addition, it was revealed that the onset potential for the generation of C2H4 is more negative when compared to that for CO. These findings were common on three different Cu(100, 111 and 110) surfaces.389 Subsequently, computational studies have revealed that *CO dimerisation is more favourable on the Cu(100) facets despite the commonality in the reaction dynamics.390 Moreover, C2H4 formation is hindered with an increase in CO coverage, which lowers the energy barrier. Compared to the Cu(111) facet, Cu(100) exhibited a favourable potential to synthesize C2 products from CRR due to the presence of under-coordinated Cu sites, which is likely to enable the C–C coupling effect.
4.2. Electrocatalysts of CRR
It is established that the major parameters that hinder the industrial implementation of CRR are the selectivity, activity and stability,391 and each of these factors can be improved by focusing on the nature of the electrocatalyst, electrolytic solution and operating conditions.344 So far, the adopted CRR electrocatalysts include both heterogeneous and homogeneous metal based electrocatalysts consisting of earth-abundant TMs.361,362 Moreover, carbon-based electrocatalysts have also shown great potential as an efficient catalyst system for the electroreduction of CO2 to different reduced products.392Previously, research studies have demonstrated that depending on the reaction intermediates being formed and the final reduced products, crystalline bulk metals are classified into three: (i) earth-abundant TMs (such as Zn, Sn, Pb and Bi) that can generate HCOOH/HCOO−via the outer-sphere mechanism as a result of the weak binding with the CO2˙− intermediate (Fig. 8(b)); (ii) noble-metal based catalyst systems such as Au, Ag and Pd that have a strong affinity towards the *COOH intermediate resulting in its further reduction to generate the weakly bound *CO intermediate. Subsequently, CO is desorbed from the surface emerging as the main product (Fig. 8(c));361 (iii) Cu-based electrocatalysts which have demonstrated to be the only catalyst system to bind and transform the *CO intermediate into other products.393–395 In order words, Cu is the only catalyst system with the potential to facilitate CRR involving more than two electrons (2e−) transfer with significant FE.396 Specifically, at low and high overpotentials, 2e−-transfer products (such as H2, CO, HCOOH) and multi e−-transfer products (such as CH4, C2H4) are generated, respectively.
As witnessed in previous sections, suppressing the HER has been one of the focal points of most electrochemical reduction of small molecules. Similarly, attention towards retarding this reaction process is also necessary to achieve high selectivity of CRR products. On this account, schemes are employed to enhance the catalytic activity of catalyst systems, which can be reached by utilizing metal alloys, metal oxides, nanostructured metals and chalcogenides that offer enriched active centers for CO2 adsorption and activation.397–399 For instance, utilizing nanostructured Ag oxide as a CRR electrocatalyst has demonstrated a high CO selectivity of about 80% (0.49 V overpotential), which is considerably higher than the 4% selectivity of the Ag alone catalyst under similar conditions. The improved activity and selectivity were attributed to the strong *COOH stability on the active sites of the metal oxide.400
Most importantly, experimental evidence has shown that the surface nature, morphology and size of the catalyst system are influential towards the product distribution over the electrocatalysts. For instance, amorphous Cu NPs have been proven to have superior CRR activity and selectivity towards HCOOH and C2H5OH over the crystalline counterpart with 37% and 22% FE, respectively, at −1.4 V (vs. Ag/AgCl). The improved performance was ascribed to the enriched defective sites as a result of irregularity in the surface structure in the amorphous form (Fig. 9(a) and (b)).401 Sequel to this, Hwang and co-workers adopted a mix of Cu states in anodized Cu(AN-Cu) as a more stable and highly selective CRR electrocatalyst for the generation of C2H4. The improved selectivity of C2H4 over CH4 was attributed to the electrochemical reduction environment enabled by the mixed valence of the O–Cu combination catalysts (Fig. 9(c) and (d)).399 Likewise, Rosa M. et al. tuned copper's morphology and oxidation state by pulsed CO2 electrolysis and the production of C2+ products was enhanced with 76% FE at −1.0 V (vs. RHE). According to quasi in situ XPS results, they found that the improved efficiency of the Cu catalyst was due to the cooperation of Cu(I) species and continuous regeneration of defects which would promote C–C coupling pathways.402
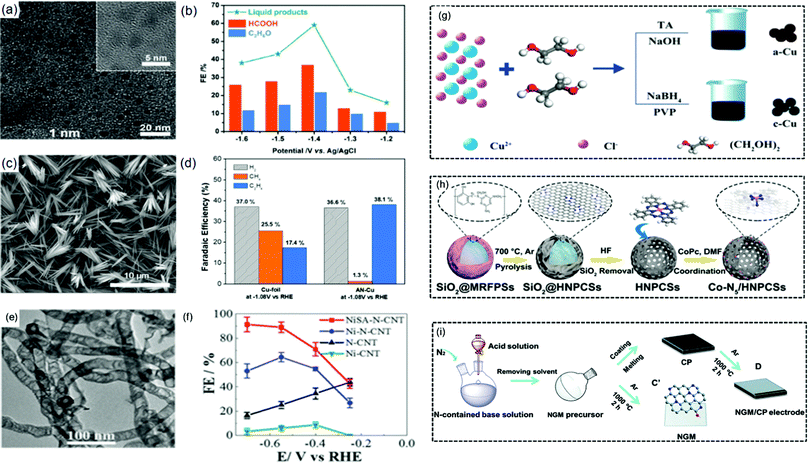 | ||
| Fig. 9 (a) TEM image of amorphous Cu NPs and their (b) FE for liquid products at each given potential for 2 h. Panels (a) and (b) are reproduced with permission.401 Copyright 2018, Wiley. (c) HRTEM image of Cu(AN-Cu) and its (d) FE for HER and hydrocarbon selectivity in comparison to Cu-foil. Panels (c) and (d) are reproduced with permission.399 Copyright 2018, American Chemical Society. (e) TEM image of NiSA-N-CNTs. (f) Faradaic efficiency of CO for NiSA-N-CNTs, Ni-N-CNTs, N-CNTs, and Ni-CNTs at −0.28, −0.40, −0.55, and −0.70 V. Panels (e) and (f) are reproduced with permission.413 Copyright 2018, Wiley. (g) Schematic illustration of the formation process of a-Cu and c-Cu.401 Copyright 2018, Wiley. (h) Schematic illustration of the formation of Co-N5/HNPCSs.412 Copyright 2018, American Chemical Society. (i) Schematic illustration of the procedures to prepare NGM and NGM/CP electrodes.348 Copyright 2016, Royal Society of Chemistry. | ||
Relative to single metals, hybrid or alloy metals have shown to offer improved CRR performance due to the potential to modulate the binding energy of specific intermediates on the catalyst surface.403,404 For instance, Hoang and co-workers demonstrated the synthesized CuAg alloy film to be a more stable and efficient CRR electrocatalyst for C2H4 and C2H5OH production with a FE reaching nearly 60% and 25%, respectively, at −0.7 V (vs. RHE).404 Elsewhere, it was elucidated that the integrating Ag and Cu atoms resulted in the occurrence of compressive strain around Cu atoms that promoted the product selectivity.405 Given the tunable effect of Cu towards the bonding of CO-motifs and selective reduction of other intermediates, several Cu-based hybrids or alloys are developed for efficient CRR electrocatalysts.405–407
Most importantly, it is worth mentioning that despite the beneficial effects of these metals on the CRR performance, the activity and selectivity can be significantly influenced by the metal content. For instance, Ma and co-workers revealed that the product selectivity during the electroreduction of CO2 using Cu-based alloys is highly affected by the Cu content.408 Therefore, an optimization of the metal content is required for an optimal performance of CO2 conversion. In addition, investigative studies towards the discovery of alloys with a suitable coupling effect in order to promote efficient and highly selective CRR performance are encouraged. To this effect, Zhang and co-workers examined the CRR activity of several Sn-based bimetallic catalysts towards the formation of HCOOH. The experimental results demonstrated that Ag–Sn and Cu–Sn are the most favourable for HCOOH production with a FE of 88.3% and 87.4%, respectively, when tested in 0.5 M NaHCO3.409
In addition to Cu, other earth-abundant TMs (such as Ni, Co, Zn, Bi and In) and their compounds have demonstrated remarkable potential as CRR electrocatalysts.71,339,410,411 For instance, Pan and co-workers demonstrated that an engineered Co-N5 catalytic site using a modified Stöber method is a highly efficient CRR electrocatalyst for CO production with FE > 99% at −0.73 and −0.79 V vs. RHE (Fig. 9(h)).412 Similarly, a carbon-anchored N-derived Zn catalyst (ZnN4/C) demonstrated high CRR selectivity and stability towards CO production with a FE of ∼95% and >75 h stability at an onset overpotential of 24 mV.413 And FeN4 sites in Fe–N–C catalysts were also obtained from the ZIF-8 which achieved 25 mA cm−2 at 0.8 V (vs. RHE) and FE was above 90% for CO in a wide potential.414 For these electrocatalysts, experimental and theoretical results reveal that the Zn–N4, Co–N5 and Fe–N4 catalytic sites facilitated the activation of CO2 and direct COOH* formation. However, the product formed via In-based catalysts is different from those of Fe, Co, and Zn based electrocatalysts which would convert CO2 to formate. Recently, Yin's group synthesized In–N–C via In-doped ZIF-8 and the atomically dispersed structure demonstrated a high CRR performance with a turnover frequency of 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 771 h−1 at −0.99 V (vs. RHE) and the maximum FE for formate was around 80%. Since In was atomically dispersed in the structure, the intermediate *OCHO was formed on isolated In sites which affected the formate formation.339
771 h−1 at −0.99 V (vs. RHE) and the maximum FE for formate was around 80%. Since In was atomically dispersed in the structure, the intermediate *OCHO was formed on isolated In sites which affected the formate formation.339
Furthermore, to enrich the distribution of active sites, enabling a large catalyst surface area can feature catalytic active centers to improve the CRR performance. On this account, a myriad of 2D materials including nanosheets and nanofilms of metals, metal oxides and chalcogenides have portrayed good CRR activity and selectivity.415–417 The versatility of 2D electrocatalysts originates from their unique electronic structure and stability. In addition, they offer the beneficial features of both heterogeneous and molecular electrocatalysts.418,419 For instance, Gao and co-workers evaluated the HCOO formation potential during CRR on two different Co-based catalytic sites. The authors revealed that surface Co atoms on the atomically thin layers displayed a higher activity and selectivity at lower overpotentials than surface Co atoms on the bulk samples. The improved activity was due to the partial oxidation of the atomic layers leading to a stable current density of 10 mA cm−2 and a FE of 90% at 0.24 V (vs. RHE) with 40 h stability.386 And very recently, Cao et al. fabricated thin bismuthene (Bi-ene) with a few layers which displayed a high selectivity with FE of nearly 100% from −0.83 V to −1.18 V (vs. RHE). Based on DFT analysis and the result of in situ ATR-IR spectra, the product formate was finally obtained from the OCHO* intermediate.417
Moreover, tailoring the intrinsic electronic structure of the electrocatalyst can benefit the CRR selectivity. Here, Xu and co-workers revealed that the partial charge delocalization in the MoSeS monolayer resulted in the tuning of the d-band electronic structure by the lengthened Mo-Se and shortened Mo-S bonds. This alteration in the electronic structure favoured the stabilization of the COOH* intermediate and facilitated the CO desorption step, which was considered as the rate-determining step. Based on this finding, the MoSeS monolayer achieved a remarkably high FE of 45.2% for the CRR towards CO formation, when compared to independent chalcogenides, MoSe2 and MoS2 monolayers, with 30.5% and 16.6%, respectively at −1.15 V vs. RHE.420
Another major disadvantage of molecular electrocatalysts is the poor recyclability and stability of the catalyst systems.424 Exploiting recent advancements in ligand mobilization, newly developed hybrid catalysts anchored on conductive supports have demonstrated more extended CRR stability.425,426 For instance, Wang and co-workers appended the CoII quaterpyridine complex [Co(qpy)]2+ on the surface of multi-walled CNTs to catalyze the electroreduction of CO2 to generate CO in water at pH 7.3. Experimental results revealed that the hybrid complex attained 100% selectivity and 100% FE with a current density of 0.94 mA cm−2 at −0.35 V (vs. RHE). Moreover, a current density of 9.3 mA cm−2 at an overpotential of only 340 mV was sustained for 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 095 catalytic cycles.426
095 catalytic cycles.426
In addition to the activity and selectivity, it is also pertinent to have an in-depth understanding of the mechanistic CRR pathway on each molecular electrocatalyst. A detailed insight into this is well documented in the literature.421 Generally, the CRR mechanism can be investigated by studying the formation of intermediates using quantum chemical simulations along with electroanalytical tools and spectroscopy (such as Fourier transform infrared spectroscopy (FTIR)-spectroelectrochemistry (SEC) and UV-vis-SEC).361,421
The electrocatalytic performance of the heteroatom-doped carbon-based catalyst systems partially depends on the electronic structure of the heteroatoms in comparison to the positively charged C atoms.
In this case, the selectivity of a specific reduced product strongly depends on the active site's affinity towards the corresponding intermediate motifs. This is quite different from the conventional pristine carbon materials. Specifically, CRR reduced products involving the 2e−-transfer (CO and HCOO−) are typically generated over the carbon-based electrocatalyst. However, other products involving multiple e−-transfer are also formed over the carbon-based electrocatalyst but with a particular composition and/or morphology.
Concerning heteroatom N-dopants, the activation of the CRR over carbon-based electrocatalysts occurs on the N-dopant.435–437 Sun and co-workers revealed that the N-motif on the N-doped carbon electrode enhanced the catalytic activity of the graphene-like carbon with high selectivity for CH4 (93.5%) at −1.4 V vs. SHE (Fig. 9(i)).348 Similarly, Duan and co-authors revealed by both theoretical and experimental studies that the pyridinic N-dopant is the most active site for the electro-reduction of CO2 to CO.392 More importantly, incorporating N-species into the carbon framework of the electrocatalyst has also been demonstrated to favour specific product selectivity. Wu and co-workers synthesized C2H4 for the first time as the major product from the CRR over a metal-free electrocatalyst. Here, 31% FE was achieved at a potential of −0.75 V (vs. RHE) over N-doped graphene quantum dots (NGQDs) in 1 M KOH.438
Another example involves the generation of C2H5OH from the electroreduction of CO2 over metal-free N-doped mesoporous carbon with a high FE of 77% at −0.56 V (vs. RHE). As mentioned earlier, the morphology of the electrocatalyst also plays a significant role in the CRR catalytic activity. In this study, Song and co-workers also demonstrated that the cylindrical structure of the N-doped mesoporous carbon enhanced the C–C coupling effect, which resulted in the high selectivity for C2H5OH while suppressing the CO formation with the potential range of −0.4 to −1.0 V. Also, experimental verification depicts that the cylindrical construct of the electrocatalyst aided the easy transport of electrons which is responsible for the enhanced C–C coupling effect.439 Besides N-dopants, other common dopants employed to improve the CRR performance of carbon-based electrocatalysts include B,430,440 P,432,433 S434 and F.403 Detailed description of the CRR activity of these catalysts and other notable CRR electrocatalysts is summarized in Table 3.
| Electrocatalyst | CRR product | Electrolyte | Faradaic efficiency (FE) | Current density at FE max (mA cm−2) | Overpotential | Ref. |
|---|---|---|---|---|---|---|
| Metal electrocatalysts | ||||||
| Ag–Sn | HCOO− | 0.5 M NaHCO3 | 88.3% | 21.3 | −0.94 V vs. RHE | 409 |
| Cu–Sn | HCOO− | 0.5 M NaHCO3 | 87.4% | 23.6 | −0.99 V vs. RHE | 409 |
| Oxidized Co4−atom-thick layer | HCOO− | 0.1 M Na2SO4 | 90% | 10 | 1.24 V vs. RHE | 386 |
| Amorphous Cu NPs | HCOOH and C2H5OH | 0.1 M KHCO3 | 37% (HCOOH), 22% (C2H5OH) | ∼4.2 | −1.4 V vs. RHE | 401 |
| Cu(I) species | C2H4, C2H5OH and n-propanol | 0.1 M KHCO3 | 76% (total) | — | −1.0 V vs. RHE | 402 |
| Co–N5 | CO | 0.2 M NaHCO3 | 99.2% | 6.2 | −0.73 V vs. RHE | 412 |
| N,P-Co-doped carbon aerogels | CO | 0.5 m [Bmim]PF6/MeCN | 99.1% | 143.6 | −2.4 V vs. Ag/AgCl | 444 |
| [Co(qpy)]2+ | CO | 0.5 M NaHCO3 | 100% | 0.94 | −0.35 V vs. RHE | 426 |
| MoSeS | CO | [Emim]BF4 (4 mol% [Emim]) | 45.2% | — | −1.15 V vs. RHE | 420 |
| MoSe2 | 30.5% | — | ||||
| MoS2 | 16.6% | — | ||||
| ZnN4/C | CO | 0.5 M KHCO3 | 95% | 4.8 | −0.43 V vs. RHE | 413 |
| FeN4/C | CO | 0.1 M KHCO3 | 90% | 25 | −0.8 V vs. RHE | 414 |
| In–N–C | Formate | 0.5 M KHCO3 | ∼80% | 24.5 | −1.1 V vs. RHE | 339 |
| AN–Cu | C2H4 | 0.1 M KHCO3 | 38.1% | 7.3 | −1.08 V vs. RHE | 399 |
| CuAg alloy | C2H4 | 1 M KOH | 60% | 300 | −0.7 V vs. RHE | 404 |
| C2H5OH | 25% | |||||
| Bi-ene | Formate | 0.5 M KHCO3 | ∼100% | 72.0 | −1.18 V vs. RHE | 417 |
| Carbon electrocatalysts | ||||||
| PEI-NCNT | HCOO− | 0.1 M KHCO3 | 85% | 7.2 | −1.8 V vs. SCE | 428 |
| PEI-NGCNT | HCOO− | 0.1 M KHCO3 | 87% | 9.5 | −1.8 V vs. SCE | 428 |
| N-Doped graphene | HCOO− | 0.5 M KHCO3 | 73% | 7.5 | −0.84 V vs. RHE | 429 |
| NCNT | HCOO− and CO | 0.1 M KHCO3 | 59% (HCOO-), 2% (CO) | 3.0 | −1.8 V vs. SCE | 428 |
| N-Doped nanodiamond | HCOO− and CH3COO− | 0.5 M NaHCO3 | 91.8% (total) | ∼1.0 | −1.0 V vs. RHE | 445 |
| N doped porous carbon | CO | 0.5 M KHCO3 | 98.4% | 3.01 | −0.55 V vs. RHE | 446 |
| B-Doped diamond | HCHO and HCOOH | CH3OH | 74% (HCHO), ∼15% (HCOOH) | — | −1.7 V vs. Ag/AgCl | 430 |
| B-Doped diamond | HCHO and HCOOH | 0.1 M NaHCO3 | 53.9% (HCHO), 26.1% (HCOOH) | — | −1.0 V vs. RHE | 440 |
| B-Doped diamond | HCOO− and CO | 0.5 M KCl | 94.7% (HCOO-), 0.6% (CO) | 2.0 | — | 431 |
| N-Doped graphene foam | CO and HCOO− | 0.1 M KHCO3 | 85% (CO), 3% (HCOO-) | 1.8 | −0.58 V vs. RHE | 437 |
| F-Doped carbon | CO | 0.1 M NaClO4 | ∼90% | ∼0.24 | −0.62 V vs. RHE | 403 |
| P-Doped onion-like carbon | CO | 0.5 M NaHCO3 | 81% | j co ∼ 4.9 | −0.90 V vs. SHE | 433 |
| S,N-Doped CNFs | CO | 0.1 M KHCO3 | 94% | ∼100 | −0.7 V vs. RHE | 434 |
| N,P-Fullerene-like carbon | CO | 0.5 M NaHCO3 | 83.30% | j co ∼ 8.52 | −0.52 V vs. RHE | 432 |
| NiSA-N-CNTs | CO | 0.5 M KHCO3 | 91.3% | 23.5 | −0.7 V vs. RHE | 413 |
| NGQDs | CO, HCOO−, CH4, C2H4, C2H5OH, CH3COO− and C3H7OH | 1 M KOH | 90% (total) | — | −0.75 V vs. RHE | 438 |
| N-Doped graphene-like carbon | CH4 and CO | [Bmim]BF4 | 93.5% (CH4), 4.2% (CO) | — | −1.4 V vs. SHE | 348 |
| N-Doped mesoporous carbon | C2H5OH | 0.1 M KHCO3 | 77% | — | −0.56 V vs. RHE | 439 |
| B,N-Doped nanodiamond | C2H5OH | 0.1 M NaHCO3 | 93.20% | — | −1.0 V vs. RHE | 440 |
Aside from the metal-free dopants, metal-doping on the carbon-based electrocatalysts has also been studied.441–443 For instance, Cheng and co-workers investigated a class of TMs that were atomically dispersed on N-doped CNTs (MSA-N-CNTs, where M = Ni, Co, NiCo, CoFe, and NiPt) using a new multistep pyrolysis approach. Among these materials, NiSA-N-CNTs demonstrated the most suitable CRR activity and selectivity towards CO production, achieving a turnover frequency (TOF) of 11.7 s−1 and an FE of 89% at −0.55 V (vs. RHE), with FE being two orders of magnitude higher than that of Ni nanoparticles supported on CNTs (Fig. 9(e) and (f)).413
Moreover, it was shown that the introduction of metal-based atoms could enhance both the efficiency and selectivity of the CRR process towards the production of C2 hydrocarbons. Jiao and co-workers proposed a molecular scaffolding approach for synthesizing a carbon-based complex with synergistic active sites to promote the CRR. It was underscored that Cu, probed on graphitic carbon nitride (g-C3N4), served as a molecular scaffold to regulate the electronic structure of Cu. Compared to the Cu(111) surface, the prepared Cu–C3N4 complex is enriched with active centers that enabled CO2 activation and further reduction to generate C2 products. Theoretical evidence relates the good catalytic performance of the complex to the strong affinity of C-bound and O-bound intermediates to Cu and g-C3N4, respectively.447
In summary, CRR reduces CO2 which is different from NRR that reduces N2, but the perspective strategies as discussed above for NRR are still laterally referenceable for CRR. Additionally, the unique characteristic for CRR lies in its product diversity, including C1, C2 and C3+ carbon based products. This proffers unlimited research space, while bringing with complex challenges at the same time. From this point, the in situ microscopy and spectroscopy techniques will offer the robust research tools for CRR to better explore the intermediates during redox reactions and hence to enable the control on customized reaction pathways for target carbon products.
5. Conclusion and outlook
In recent years, the prospective goal to develop sustainable pathways for fuels and chemicals has given rise to the advancements in electrocatalyst design and development. In this review, we extensively discussed the recent advances in electrocatalysis-enabled transformation of earth-abundant water, nitrogen and carbon dioxide with the sustainable goal of establishing environmentally sensible circulation of energy and materials.445,448,449First, the fundamental principles, mechanistic pathways, recent theoretical concepts and the energetics underlying each electrocatalytic reaction (e.g. water splitting, N2 reduction reaction and CO2 reduction reaction) were initially studied to reveal the common and distinct challenges. Second, the performances of known catalyst systems pertaining to each reaction were discussed, reflecting on the different outcomes and mechanistic understanding of catalyst design principles for developing enhanced electrocatalysts. Specifically, the current status of available metal and metal-free electrocatalysts for the reactions based on a common set of figures of merit, namely yield rate, faradaic efficiency, overpotential, current density, and stability, was explored in detail. In addition, it was found that the practical implementation of each reaction and its electrocatalysts known to date is hindered by the occurrence of different adsorbed intermediates and the accompanying energies, which alters the catalytic activity and stability. For instance, the poor performance of a myriad of NRR electrocatalysts is due to the occurrence of HER which competitively consumes abundant electrons and protons to form H2 against the formation of NH3. This circumstance intensified the challenge in the limited depth of material knowledge and scale-up dynamics of current catalyst-development strategies. Accordingly, a new strategy in the development of catalyst systems is required to counter these constraints with a special emphasis on modulating the catalytic efficiency, selectivity and stability. Moreover, another design strategy is to construct a self-supported 3D catalyst architecture that promotes larger number of active sites and improves electrical contact. This approach may include doping, chemical functionalization, alloying or defect introduction, as well as the synthesis method and conditions. Furthermore, the need to elucidate the kinetics and reaction barriers at the electrolyte–electrode interface, together with the modalities of electron/proton transfer cannot be over-exaggerated. In this regard, one of the main conclusions is that an integrated scheme is required to strengthen both experimental and theoretical insightful tools towards the design, synthesis, characterization and testing of practical catalyst systems. In spite of significant progress made as discussed above, there are still many challenges ahead in the development of electrocatalysts for overall water splitting, CO2 reduction and N2 reduction reactions and further efforts are also required to elucidate other factors that can expedite the advancements. Prospective research studies in this regard can focus on the following points.
(1) An in-depth understanding of the related mechanism for each reaction is critically sought. This would in turn provide a knowledge-driven scheme for the design and development of efficient catalysts by optimizing computational studies towards the reaction mechanism. Specifically, investigation into the mechanism can guide structural modification, electronic reconfiguration and prevention of catalyst active site degradation during cycling. In addition, theoretical calculations can discern at an atomic level the competitive interactions hindering high yield in each reaction, e.g. the competitive interaction between NRR and HER in the electrocatalytic synthesis of ammonia. Most reported computational studies are based on simplified models and hence lack accurate prediction of the actual kinetics and reaction mechanisms under given operating conditions.
(2) Morphology-engineered catalyst systems have demonstrated high performance efficacy and stability in the considered electrocatalytic processes.450,451 On this account, the catalyst with abundant active sites can be fabricated into specific configurations (such as layers, 3D, nanowires and nanotubes) in order to improve the catalyst's physiochemical properties. By doing so, the porosity and number of accessible active sites are increased, hence facilitating species adsorption, activation and electron diffusion. For instance, heteroatom doping and modulation of the composition of the catalyst alter the electronic structure of active sites so as to optimize the intrinsic activity of bifunctional HER–OER catalysts. In the case of NRR, the catalytic activity is highly dependent on the transfer of electrons, which was observed to be more effective in some special structures such as sharp spikes known to concentrate the electric field at the spikes. Hence, it is necessary to optimize the structure with respect to the morphology to end up in enhanced catalysis.
(3) Generally, extensive integration of computational and experimental studies in the design of catalyst systems is lacking, particularly in the fabrication methods for the engineered catalysts. Most reported studies in this regard are theory-based with little or no detailing of experimental schemes to effectuate the newly developed catalyst active sites. Ideally, to integrate all mechanistic information demands a rigorous standardization of experimental setups and procedures, in-depth understanding beyond surficial catalyst interactions and a multi-scale modeling involving all these aspects.
(4) Thorough knowledge-based studies on the above outlooks are essential mainly for the development of novel or improved electrocatalysts. Thereupon, the development of functional composites with better catalytic activity and stability is not far-fetched.452 For instance, various heteroatom-doped functional carbon-based materials have displayed exceptional potential towards overall water splitting due to their tunable structure, available active sites and durability in alkaline/acidic electrolytes. Another example is the adoption of the zeolitic imidazolate encapsulated catalyst, which has the potential to suppress HER in the NRR process by absorbing H-atoms. In summary, the optimization of these advanced functional materials is vital for the practical application of these processes.
(5) In addition to the optimization of composite catalysts, measures can be adopted to expedite this process via accelerating catalyst discovery. Due to advancements in machine learning and material genome databases, accelerating catalyst discovery by high-throughput assessment and non-supervised analytical techniques such as AI algorithms, aided with the identification of key synthetic parameters, is realistic.453,454 Moreover, the state-of-the-art computer-aided robotic and automated facilities enable autonomous catalyst synthesis, characterization and performance evaluation, which could significantly boost the discovery of advanced catalysts for electrochemical conversion of water, nitrogen, carbon dioxide and the other molecules.455 This critical review with more than 500 references along with groups of expertise helped to lay the foundation in this research field.
(6) Although optimizing the operating cell was not covered in detail in this study, it is worth mentioning here for future research studies. The practical application of the electrocatalytic conversion of earth-abundant molecules goes beyond the understanding of the surficial interactions on the electrocatalysts. Knowledge of the electrolytic cells and optimal operating conditions is equally vital. For instance, NH3 is theoretically reduced and detected at the cathode. However, this is not the case practically as a significant amount of NH3 is observed to crossover to the anode, which could be oxidized and subsequently reduce the yield of NH3. Therefore, the need for the design and optimization of the cell is necessary to circumvent such occurrences.
(7) In view of the technical advancement in this field, researchers underscore the importance of developing more resolute legal and techno-economic frameworks that can successfully promote sustainable solutions by internalizing environmental costs. Overall, a level of parallelism between the technological, economic and legal aspects of these technologies at the initial stage of development and coordinated efforts based on a long-term view should be established. This is valuable for the practical exploitation and commercial extension of these electrochemical conversion processes. However, despite the promising laboratory-based efficiencies, particularly for the most important reaction (HER), the processes are still long way away from practical application. Recent advancements lack feasibility tests on a pilot scale for the highly efficient electrocatalysts designed to date. In addition, it is envisaged that even with the accelerated advancement in electrocatalyst development, conventionally manufactured electrocatalysts may likely exhibit a better economy of scale, hence discouraging the implementation of sensible catalyst schemes.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52173234, 22102208), Shenzhen-Hong Kong-Macau Technology Research Programme (Type C, SGDX2020110309300301), Shenzhen Science and Technology Program (JCY20210324102008023), Shenzhen Excellent Science and Technology Innovation Talent Training Project – Outstanding Youth Project (RCJC20200714114435061), the ANU Futures Scheme (Q4601024), CCF-Tencent Open Fund and Functional Materials Interfaces Genome (FIG) project.References
- S. Chen, S. S. Thind and A. Chen, Electrochem. Commun., 2016, 63, 10–17 CrossRef CAS.
- Z. J. Schiffer and K. Manthiram, Joule, 2019, 1, 10–14 CrossRef.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Nørskov and T. F. Jaramillo, Science, 2017, 355, eaad4998 CrossRef PubMed.
- F. Naseem, P. Lu, J. Zeng, Z. Lu, Y. H. Ng, H. Zhao, Y. Du and Z. Yin, ACS Nano, 2020, 14, 7734–7759 CrossRef CAS PubMed.
- J. Hwang, R. R. Rao, L. Giordano, Y. Katayama, Y. Yu and Y. Shao-Horn, Science, 2017, 358, 751–756 CrossRef CAS PubMed.
- A. J. Martín and J. Pérez-Ramírez, Joule, 2019, 3, 2602–2621 CrossRef.
- H. Lu, J. Tournet, K. Dastafkan, Y. Liu, Y. H. Ng, S. K. Karuturi, C. Zhao and Z. Yin, Chem. Rev., 2021, 121, 10271–10366 CrossRef CAS PubMed.
- W. Kreuter and H. Hofmann, Int. J. Hydrogen Energy, 1998, 23, 661–666 CrossRef CAS.
- J. Jordan and P. T. Smith, Proc. Chem. Soc., 1960, 246–247 CAS.
- S. Meshitsuka, M. Ichikawa and K. Tamaru, J. Chem. Soc., Chem. Commun., 1974, 158–159 RSC.
- Y. Hori, A. Murata and R. Takahashi, J. Chem. Soc., Faraday Trans. 1, 1989, 85, 2309–2326 RSC.
- H. Yoshio, K. Katsuhei and S. Shin, Chem. Lett., 1985, 15, 897–898 Search PubMed.
- H. Yoshio, K. Katsuhei, M. Akira and S. Shin, Chem. Lett., 1986, 897–898 Search PubMed.
- N. Furuya and H. Yoshiba, J. Electroanal. Chem. Interfa. Electrochem., 1990, 291, 269–272 CrossRef CAS.
- J. Liu, D. Zhu, Y. Zheng, A. Vasileff and S.-Z. Qiao, ACS Catal., 2018, 8, 6707–6732 CrossRef CAS.
- M. S. Dresselhaus and I. L. Thomas, Nature, 2001, 414, 332–337 CrossRef CAS PubMed.
- J. A. Turner, Science, 2004, 305, 972 CrossRef CAS PubMed.
- Q. Liu, Z. Pu, A. M. Asiri, A. H. Qusti, A. O. Al-Youbi and X. Sun, J. Nanopart. Res., 2013, 15, 2057 CrossRef.
- J. Tian, N. Cheng, Q. Liu, W. Xing and X. Sun, Angew. Chem., Int. Ed., 2015, 54, 5493–5497 CrossRef CAS PubMed.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446–6473 CrossRef CAS PubMed.
- M. Gong, D.-Y. Wang, C.-C. Chen, B.-J. Hwang and H. Dai, Nano Res., 2016, 9, 28–46 CrossRef CAS.
- Y. Wu, M. Chen, Y. Han, H. Luo, X. Su, M.-T. Zhang, X. Lin, J. Sun, L. Wang, L. Deng, W. Zhang and R. Cao, Angew. Chem., Int. Ed., 2015, 54, 4870–4875 CrossRef CAS PubMed.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- J. Wang, W. Cui, Q. Liu, Z. Xing, A. M. Asiri and X. Sun, Adv. Mater., 2016, 28, 215–230 CrossRef CAS PubMed.
- S. Zhang, S. E. Saji, Z. Yin, H. Zhang, Y. Du and C.-H. Yan, Adv. Mater., 2021, 33, 2005988 CrossRef CAS PubMed.
- J. Wang, X. Yue, Y. Yang, S. Sirisomboonchai, P. Wang, X. Ma, A. Abudula and G. Guan, J. Alloys Compd., 2020, 819, 153346 CrossRef CAS.
- Y. Ji, L. Yang, X. Ren, G. Cui, X. Xiong and X. Sun, ACS Sustainable Chem. Eng., 2018, 6, 9555–9559 CrossRef CAS.
- B. M. Hunter, H. B. Gray and A. M. Müller, Chem. Rev., 2016, 116, 14120–14136 CrossRef CAS PubMed.
- J. Zhang, H. Zhou, X. Liu, J. Zhang, T. Peng, J. Yang, Y. Huang and S. Mu, J. Mater. Chem. A, 2016, 4, 15870–15879 RSC.
- C. G. Morales-Guio, L.-A. Stern and X. Hu, Chem. Soc. Rev., 2014, 43, 6555–6569 RSC.
- X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148–5180 RSC.
- S. Trasatti, J. Electroanal. Chem. Int. Electrochem., 1972, 39, 163–184 CrossRef CAS.
- S. Anantharaj, S. R. Ede, K. Sakthikumar, K. Karthick, S. Mishra and S. Kundu, ACS Catal., 2016, 6, 8069–8097 CrossRef CAS.
- Y. Shi and B. Zhang, Chem. Soc. Rev., 2016, 45, 1529–1541 RSC.
- X. Chang, T. Wang, Z.-J. Zhao, P. Yang, J. Greeley, R. Mu, G. Zhang, Z. Gong, Z. Luo, J. Chen, Y. Cui, G. A. Ozin and J. Gong, Angew. Chem., Int. Ed., 2018, 57, 15415–15419 CrossRef CAS PubMed.
- T. Shinagawa, A. T. Garcia-Esparza and K. Takanabe, Sci. Rep., 2015, 5, 13801 CrossRef PubMed.
- Y. Yan, B. Y. Xia, B. Zhao and X. Wang, J. Mater. Chem. A, 2016, 4, 17587–17603 RSC.
- N. T. Suen, S. F. Hung, Q. Quan, N. Zhang, Y. J. Xu and H. M. Chen, Chem. Soc. Rev., 2017, 46, 337–365 RSC.
- J. Rossmeisl, Z. W. Qu, H. Zhu, G. J. Kroes and J. K. Nørskov, J. Electroanal. Chem., 2007, 607, 83–89 CrossRef CAS.
- I. C. Man, H.-Y. Su, F. Calle-Vallejo, H. A. Hansen, J. I. Martínez, N. G. Inoglu, J. Kitchin, T. F. Jaramillo, J. K. Nørskov and J. Rossmeisl, ChemCatChem, 2011, 3, 1159–1165 CrossRef CAS.
- R. L. Doyle and M. E. G. Lyons, Photoelectrochemical Solar Fuel Production: From Basic Principles to Advanced Devices, Springer International Publishing, Cham, 2016, pp. 41–104 Search PubMed.
- Y. Matsumoto and E. Sato, Mater. Chem. Phys., 1986, 14, 397–426 CrossRef CAS.
- J. Tymoczko, V. Colic, A. Ganassin, W. Schuhmann and A. S. Bandarenka, Catal. Today, 2015, 244, 96–102 CrossRef CAS.
- X. Liu and L. Dai, Nat. Rev. Mater., 2016, 1, 16064 CrossRef CAS.
- R. Paul, L. Zhu, H. Chen, J. Qu and L. Dai, Adv. Mater., 2019, 31, 1806403 CrossRef PubMed.
- H. Wang, H.-W. Lee, Y. Deng, Z. Lu, P.-C. Hsu, Y. Liu, D. Lin and Y. Cui, Nat. Commun., 2015, 6, 7261 CrossRef CAS PubMed.
- K. Liu, F. Wang, P. He, T. A. Shifa, Z. Wang, Z. Cheng, X. Zhan and J. He, Adv. Energy Mater., 2018, 8, 1703290 CrossRef.
- Y.-N. Zhu, C.-Y. Cao, W.-J. Jiang, S.-L. Yang, J.-S. Hu, W.-G. Song and L.-J. Wan, J. Mater. Chem. A, 2016, 4, 18470–18477 RSC.
- X. Miao, D. Qu, D. Yang, B. Nie, Y. Zhao, H. Fan and Z. Sun, Adv. Mater., 2018, 30, 1704740 CrossRef PubMed.
- J. Mao, J. Iocozzia, J. Huang, K. Meng, Y. Lai and Z. Lin, Energy Environ. Sci., 2018, 11, 772–799 RSC.
- L. Li, P. Wang, Q. Shao and X. Huang, Chem. Soc. Rev., 2020, 49, 3072–3106 RSC.
- Z. Zhou, Z. Pei, L. Wei, S. Zhao, X. Jian and Y. Chen, Energy Environ. Sci., 2020, 13, 3185–3206 RSC.
- B. Kim, T. Kim, K. Lee and J. Li, ChemElectroChem, 2020, 7, 3578–3589 CrossRef CAS.
- S. Anantharaj and V. Aravindan, Adv. Energy Mater., 2020, 10, 1902666 CrossRef CAS.
- R. Wu, B. Xiao, Q. Gao, Y. R. Zheng, X. S. Zheng, J. F. Zhu, M. R. Gao and S. H. Yu, Angew. Chem., Int. Ed. Engl., 2018, 57, 15445–15449 CrossRef CAS PubMed.
- J. Yu, Y. Dai, Q. He, D. Zhao, Z. Shao and M. Ni, Mater. Rep.: Energy, 2021, 1, 100024 Search PubMed.
- J. Tian, Q. Liu, A. M. Asiri and X. Sun, J. Am. Chem. Soc., 2014, 136, 7587–7590 CrossRef CAS PubMed.
- Y. Lee, J. Suntivich, K. J. May, E. E. Perry and Y. Shao-Horn, J. Phys. Chem. Lett., 2012, 3, 399–404 CrossRef CAS PubMed.
- Y. Xu and B. Zhang, Chem. Soc. Rev., 2014, 43, 2439–2450 RSC.
- Y. Li, Y. Sun, Y. Qin, W. Zhang, L. Wang, M. Luo, H. Yang and S. Guo, Adv. Energy Mater., 2020, 10, 1903120 CrossRef CAS.
- J. Feng, F. Lv, W. Zhang, P. Li, K. Wang, C. Yang, B. Wang, Y. Yang, J. Zhou, F. Lin, G.-C. Wang and S. Guo, Adv. Mater., 2017, 29, 1703798 CrossRef PubMed.
- J. Park, S. Choi, A. Oh, H. Jin, J. Joo, H. Baik and K. Lee, Nanoscale Horiz., 2019, 4, 727–734 RSC.
- M. Jahan, Z. Liu and K. P. Loh, Adv. Funct. Mater., 2013, 23, 5363–5372 CrossRef CAS.
- Z. Xing, Q. Liu, A. M. Asiri and X. Sun, Adv. Mater., 2014, 26, 5702–5707 CrossRef CAS PubMed.
- M. S. Xie, B. Y. Xia, Y. Li, Y. Yan, Y. Yang, Q. Sun, S. H. Chan, A. Fisher and X. Wang, Energy Environ. Sci., 2016, 9, 1687–1695 RSC.
- Y. Zhu, H.-C. Chen, C.-S. Hsu, T.-S. Lin, C.-J. Chang, S.-C. Chang, L.-D. Tsai and H. M. Chen, ACS Energy Lett., 2019, 4, 987–994 CrossRef CAS.
- A. Lasia and A. Rami, J. Electroanal. Chem. Interfacial Electrochem., 1990, 294, 123–141 CrossRef CAS.
- Y. Liang, X. Sun, A. M. Asiri and Y. He, Nanotechnology, 2016, 27, 12LT01 CrossRef PubMed.
- B. C. M. Martindale and E. Reisner, Adv. Energy Mater., 2016, 6, 1502095 CrossRef.
- C. Tang, N. Cheng, Z. Pu, W. Xing and X. Sun, Angew. Chem., Int. Ed., 2015, 54, 9351–9355 CrossRef CAS PubMed.
- C. Zhao, X. Dai, T. Yao, W. Chen, X. Wang, J. Wang, J. Yang, S. Wei, Y. Wu and Y. Li, J. Am. Chem. Soc., 2017, 139, 8078–8081 CrossRef CAS PubMed.
- N. Yang, C. Tang, K. Wang, G. Du, A. M. Asiri and X. Sun, Nano Res., 2016, 9, 3346–3354 CrossRef CAS.
- L. Yang, H. Qi, C. Zhang and X. Sun, Nanotechnology, 2016, 27, 23LT01 CrossRef PubMed.
- T. Liu, L. Xie, J. Yang, R. Kong, G. Du, A. M. Asiri, X. Sun and L. Chen, ChemElectroChem, 2017, 4, 1840–1845 CrossRef CAS.
- W. Lu, T. Liu, L. Xie, C. Tang, D. Liu, S. Hao, F. Qu, G. Du, Y. Ma, A. M. Asiri and X. Sun, Small, 2017, 13, 1700805 CrossRef PubMed.
- T. Liu, Y. Liang, Q. Liu, X. Sun, Y. He and A. M. Asiri, Electrochem. Commun., 2015, 60, 92–96 CrossRef CAS.
- P. Jiang, Q. Liu, Y. Liang, J. Tian, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2014, 53, 12855–12859 CrossRef CAS PubMed.
- F. Gloaguen, J. D. Lawrence and T. B. Rauchfuss, J. Am. Chem. Soc., 2001, 123, 9476–9477 CrossRef CAS PubMed.
- S.-K. Lee, S. D. George, W. E. Antholine, B. Hedman, K. O. Hodgson and E. I. Solomon, J. Am. Chem. Soc., 2002, 124, 6180–6193 CrossRef CAS PubMed.
- D. Gao, R. Liu, J. Biskupek, U. Kaiser, Y.-F. Song and C. Streb, Angew. Chem., Int. Ed., 2019, 58, 4644–4648 CrossRef CAS PubMed.
- S. Chao, Z. Bai, Q. Cui, H. Yan, K. Wang and L. Yang, Carbon, 2015, 82, 77–86 CrossRef CAS.
- X. Zou, X. Huang, A. Goswami, R. Silva, B. R. Sathe, E. Mikmeková and T. Asefa, Angew. Chem., Int. Ed., 2014, 53, 4372–4376 CrossRef CAS PubMed.
- X. Lu and C. Zhao, Nat. Commun., 2015, 6, 6616 CrossRef CAS PubMed.
- R. D. L. Smith, M. S. Prévot, R. D. Fagan, S. Trudel and C. P. Berlinguette, J. Am. Chem. Soc., 2013, 135, 11580–11586 CrossRef CAS PubMed.
- C. Tang, A. M. Asiri, Y. Luo and X. Sun, ChemNanoMat, 2015, 1, 558–561 CrossRef CAS.
- C. Tang, Z. Pu, Q. Liu, A. M. Asiri and X. Sun, Electrochim. Acta, 2015, 153, 508–514 CrossRef CAS.
- L.-L. Feng, G. Yu, Y. Wu, G.-D. Li, H. Li, Y. Sun, T. Asefa, W. Chen and X. Zou, J. Am. Chem. Soc., 2015, 137, 14023–14026 CrossRef CAS PubMed.
- W. Zhou, X.-J. Wu, X. Cao, X. Huang, C. Tan, J. Tian, H. Liu, J. Wang and H. Zhang, Energy Environ. Sci., 2013, 6, 2921–2924 RSC.
- Z. Pu, Y. Luo, A. M. Asiri and X. Sun, ACS Appl. Mater. Interfaces, 2016, 8, 4718–4723 CrossRef CAS PubMed.
- J. Shi, J. Hu, Y. Luo, X. Sun and A. M. Asiri, Catal. Sci. Technol., 2015, 5, 4954–4958 RSC.
- C. Wang, M. Zhu, Z. Cao, P. Zhu, Y. Cao, X. Xu, C. Xu and Z. Yin, Appl. Catal., B, 2021, 291, 120071 CrossRef CAS.
- Z. Fang, L. Peng, H. Lv, Y. Zhu, C. Yan, S. Wang, P. Kalyani, X. Wu and G. Yu, ACS Nano, 2017, 11, 9550–9557 CrossRef CAS PubMed.
- J. Yang, C. Lei, H. Wang, B. Yang, Z. Li, M. Qiu, X. Zhuang, C. Yuan, L. Lei, Y. Hou and X. Feng, Nanoscale, 2019, 11, 17571–17578 RSC.
- C. Wei, C. Liu, L. Gao, Y. Sun, Q. Liu, X. Zhang and J. Guo, J. Alloys Compd., 2019, 796, 86–92 CrossRef CAS.
- W. Fang, D. Liu, Q. Lu, X. Sun and A. M. Asiri, Electrochem. Commun., 2016, 63, 60–64 CrossRef CAS.
- D. Kong, J. J. Cha, H. Wang, H. R. Lee and Y. Cui, Energy Environ. Sci., 2013, 6, 3553–3558 RSC.
- M. A. Lukowski, A. S. Daniel, F. Meng, A. Forticaux, L. Li and S. Jin, J. Am. Chem. Soc., 2013, 135, 10274–10277 CrossRef CAS PubMed.
- T. Wang, J. Zhuo, K. Du, B. Chen, Z. Zhu, Y. Shao and M. Li, Adv. Mater., 2014, 26, 3761–3766 CrossRef CAS PubMed.
- D. Kong, H. Wang, J. J. Cha, M. Pasta, K. J. Koski, J. Yao and Y. Cui, Nano Lett., 2013, 13, 1341–1347 CrossRef CAS PubMed.
- W. F. Chen, C. H. Wang, K. Sasaki, N. Marinkovic, W. Xu, J. T. Muckerman, Y. Zhu and R. R. Adzic, Energy Environ. Sci., 2013, 6, 943–951 RSC.
- T. A. Shifa, F. Wang, K. Liu, K. Xu, Z. Wang, X. Zhan, C. Jiang and J. He, Small, 2016, 12, 3802–3809 CrossRef CAS PubMed.
- Y. Sun, C. Liu, D. C. Grauer, J. Yano, J. R. Long, P. Yang and C. J. Chang, J. Am. Chem. Soc., 2013, 135, 17699–17702 CrossRef CAS PubMed.
- A. Ambrosi, C. K. Chua, A. Bonanni and M. Pumera, Chem. Rev., 2014, 114, 7150–7188 CrossRef CAS PubMed.
- S. Peng, L. Li, X. Han, W. Sun, M. Srinivasan, S. G. Mhaisalkar, F. Cheng, Q. Yan, J. Chen and S. Ramakrishna, Angew. Chem., Int. Ed., 2014, 53, 12594–12599 CAS.
- C. Zequine, S. Bhoyate, F. Wang, X. Li, K. Siam, P. K. Kahol and R. K. Gupta, J. Alloys Compd., 2019, 784, 1–7 CrossRef CAS.
- Z. Peng, D. Jia, A. M. Al-Enizi, A. A. Elzatahry and G. Zheng, Adv. Energy Mater., 2015, 5, 1402031 CrossRef.
- D. Kong, H. Wang, Z. Lu and Y. Cui, J. Am. Chem. Soc., 2014, 136, 4897–4900 CrossRef CAS PubMed.
- Q. Liu, J. Shi, J. Hu, A. M. Asiri, Y. Luo and X. Sun, ACS Appl. Mater. Interfaces, 2015, 7, 3877–3881 CrossRef CAS PubMed.
- Y.-F. Xu, M.-R. Gao, Y.-R. Zheng, J. Jiang and S.-H. Yu, Angew. Chem., Int. Ed., 2013, 52, 8546–8550 CrossRef CAS PubMed.
- M.-R. Gao, J.-X. Liang, Y.-R. Zheng, Y.-F. Xu, J. Jiang, Q. Gao, J. Li and S.-H. Yu, Nat. Commun., 2015, 6, 5982 CrossRef CAS PubMed.
- M.-R. Gao, Y.-F. Xu, J. Jiang, Y.-R. Zheng and S.-H. Yu, J. Am. Chem. Soc., 2012, 134, 2930–2933 CrossRef CAS PubMed.
- M.-R. Gao, X. Cao, Q. Gao, Y.-F. Xu, Y.-R. Zheng, J. Jiang and S.-H. Yu, ACS Nano, 2014, 8, 3970–3978 CrossRef CAS PubMed.
- H. Liang, F. Meng, M. Cabán-Acevedo, L. Li, A. Forticaux, L. Xiu, Z. Wang and S. Jin, Nano Lett., 2015, 15, 1421–1427 CrossRef CAS PubMed.
- D. Liu, Q. Lu, Y. Luo, X. Sun and A. M. Asiri, Nanoscale, 2015, 7, 15122–15126 RSC.
- J. Zhang, T. Wang, D. Pohl, B. Rellinghaus, R. Dong, S. Liu, X. Zhuang and X. Feng, Angew. Chem., Int. Ed., 2016, 55, 6702–6707 CrossRef CAS PubMed.
- F. Du, L. Shi, Y. Zhang, T. Li, J. Wang, G. Wen, A. Alsaedi, T. Hayat, Y. Zhou and Z. Zou, Appl. Catal., B, 2019, 253, 246–252 CrossRef CAS.
- Y. V. Kaneti, Y. Guo, N. L. W. Septiani, M. Iqbal, X. Jiang, T. Takei, B. Yuliarto, Z. A. Alothman, D. Golberg and Y. Yamauchi, Chem. Eng. J., 2021, 405, 126580 CrossRef CAS.
- N. L. W. Septiani, Y. V. Kaneti, K. B. Fathoni, K. Kani, A. E. Allah, B. Yuliarto, Nugraha, H. K. Dipojono, Z. A. Alothman, D. Golberg and Y. Yamauchi, Chem. Mater., 2020, 32, 7005–7018 CrossRef CAS.
- P. Bhanja, Y. Kim, B. Paul, J. Lin, S. M. Alshehri, T. Ahamad, Y. V. Kaneti, A. Bhaumik and Y. Yamauchi, ChemCatChem, 2020, 12, 2091–2096 CrossRef CAS.
- N. L. W. Septiani, Y. V. Kaneti, K. B. Fathoni, Y. Guo, Y. Ide, B. Yuliarto, X. Jiang, Nugraha, H. K. Dipojono, D. Golberg and Y. Yamauchi, J. Mater. Chem. A, 2020, 8, 3035–3047 RSC.
- M. W. Kanan and D. G. Nocera, Science, 2008, 321, 1072 CrossRef CAS PubMed.
- M. W. Kanan, Y. Surendranath and D. G. Nocera, Chem. Soc. Rev., 2009, 38, 109–114 RSC.
- S. Cobo, J. Heidkamp, P.-A. Jacques, J. Fize, V. Fourmond, L. Guetaz, B. Jousselme, V. Ivanova, H. Dau, S. Palacin, M. Fontecave and V. Artero, Nat. Mater., 2012, 11, 802–807 CrossRef CAS PubMed.
- L. Song, S. Zhang, X. Wu and K. Wang, Vacuum, 2015, 112, 12–16 CrossRef CAS.
- J. A. Cecilia, A. Infantes-Molina, E. Rodríguez-Castellón and A. Jiménez-López, Appl. Catal., B, 2009, 92, 100–113 CrossRef CAS.
- W. Li, D. Xiong, X. Gao and L. Liu, Chem. Commun., 2019, 55, 8744–8763 RSC.
- A. Dutta and N. Pradhan, J. Phys. Chem. Lett., 2017, 8, 144–152 CrossRef CAS PubMed.
- J. Xu, J. Li, D. Xiong, B. Zhang, Y. Liu, K.-H. Wu, I. Amorim, W. Li and L. Liu, Chem. Sci., 2018, 9, 3470–3476 RSC.
- E. J. Popczun, C. G. Read, C. W. Roske, N. S. Lewis and R. E. Schaak, Angew. Chem., Int. Ed., 2014, 53, 5427–5430 CrossRef CAS PubMed.
- Z. Huang, Z. Chen, Z. Chen, C. Lv, M. G. Humphrey and C. Zhang, Nano Energy, 2014, 9, 373–382 CrossRef CAS.
- J. F. Callejas, C. G. Read, E. J. Popczun, J. M. McEnaney and R. E. Schaak, Chem. Mater., 2015, 27, 3769–3774 CrossRef CAS.
- Z. Huang, Z. Chen, Z. Chen, C. Lv, H. Meng and C. Zhang, ACS Nano, 2014, 8, 8121–8129 CrossRef CAS PubMed.
- L. Feng, H. Vrubel, M. Bensimon and X. Hu, Phys. Chem. Chem. Phys., 2014, 16, 5917–5921 RSC.
- T. Liu, D. Liu, F. Qu, D. Wang, L. Zhang, R. Ge, S. Hao, Y. Ma, G. Du, A. M. Asiri, L. Chen and X. Sun, Adv. Energy Mater., 2017, 7, 1700020 CrossRef.
- Z. D. Wei, A. Z. Yan, Y. C. Feng, L. Li, C. X. Sun, Z. G. Shao and P. K. Shen, Electrochem. Commun., 2007, 9, 2709–2715 CrossRef CAS.
- L.-A. Stern, L. Feng, F. Song and X. Hu, Energy Environ. Sci., 2015, 8, 2347–2351 RSC.
- C. Tang, R. Zhang, W. Lu, Z. Wang, D. Liu, S. Hao, G. Du, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2017, 56, 842–846 CrossRef CAS PubMed.
- M. Ledendecker, S. Krick Calderón, C. Papp, H.-P. Steinrück, M. Antonietti and M. Shalom, Angew. Chem., Int. Ed., 2015, 54, 12361–12365 CrossRef CAS PubMed.
- G.-F. Chen, T. Y. Ma, Z.-Q. Liu, N. Li, Y.-Z. Su, K. Davey and S.-Z. Qiao, Adv. Funct. Mater., 2016, 26, 3314–3323 CrossRef CAS.
- Q. Liu, J. Tian, W. Cui, P. Jiang, N. Cheng, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2014, 53, 6710–6714 CrossRef CAS PubMed.
- Y. Yan, B. Y. Xia, X. Ge, Z. Liu, A. Fisher and X. Wang, Chem. – Eur. J., 2015, 21, 18062–18067 CrossRef CAS PubMed.
- C. G. Read, J. F. Callejas, C. F. Holder and R. E. Schaak, ACS Appl. Mater. Interfaces, 2016, 8, 12798–12803 CrossRef CAS PubMed.
- T. A. Shifa, K. Yusupov, G. Solomon, A. Gradone, R. Mazzaro, E. Cattaruzza and A. Vomiero, ACS Catal., 2021, 11, 4520–4529 CrossRef CAS.
- D. J. Ham and J. S. Lee, Energies, 2009, 2, 873–899 CrossRef CAS.
- W.-F. Chen, J. T. Muckerman and E. Fujita, Chem. Commun., 2013, 49, 8896–8909 RSC.
- P. Chen, K. Xu, Z. Fang, Y. Tong, J. Wu, X. Lu, X. Peng, H. Ding, C. Wu and Y. Xie, Angew. Chem., Int. Ed., 2015, 54, 14710–14714 CrossRef CAS PubMed.
- K. Xu, P. Chen, X. Li, Y. Tong, H. Ding, X. Wu, W. Chu, Z. Peng, C. Wu and Y. Xie, J. Am. Chem. Soc., 2015, 137, 4119–4125 CrossRef CAS PubMed.
- Q. Zhang, Y. Wang, Y. Wang, A. M. Al-Enizi, A. A. Elzatahry and G. Zheng, J. Mater. Chem. A, 2016, 4, 5713–5718 RSC.
- B. Cao, G. M. Veith, J. C. Neuefeind, R. R. Adzic and P. G. Khalifah, J. Am. Chem. Soc., 2013, 135, 19186–19192 CrossRef CAS PubMed.
- W.-F. Chen, K. Sasaki, C. Ma, A. I. Frenkel, N. Marinkovic, J. T. Muckerman, Y. Zhu and R. R. Adzic, Angew. Chem., Int. Ed., 2012, 51, 6131–6135 CrossRef CAS PubMed.
- X. Jia, Y. Zhao, G. Chen, L. Shang, R. Shi, X. Kang, G. I. N. Waterhouse, L.-Z. Wu, C.-H. Tung and T. Zhang, Adv. Energy Mater., 2016, 6, 1502585 CrossRef.
- M. C. Weidman, D. V. Esposito, Y.-C. Hsu and J. G. Chen, J. Power Sources, 2012, 202, 11–17 CrossRef CAS.
- E. C. Weigert, D. V. Esposito and J. G. Chen, J. Power Sources, 2009, 193, 501–506 CrossRef CAS.
- L. Liao, S. Wang, J. Xiao, X. Bian, Y. Zhang, M. D. Scanlon, X. Hu, Y. Tang, B. Liu and H. H. Girault, Energy Environ. Sci., 2014, 7, 387–392 RSC.
- H. Vrubel and X. Hu, Angew. Chem., Int. Ed., 2012, 51, 12703–12706 CrossRef CAS PubMed.
- B. Wang, X. Wu, X. Zhang, G. Pang and S. Li, New J. Chem., 2020, 44, 1147–1156 RSC.
- J.-S. Li, L.-X. Kong, Z. Wu, S. Zhang, X.-Y. Yang, J.-Q. Sha and G.-D. Liu, Carbon, 2019, 145, 694–700 CrossRef CAS.
- Z.-Y. Wu, W.-B. Ji, B.-C. Hu, H.-W. Liang, X.-X. Xu, Z.-L. Yu, B.-Y. Li and S.-H. Yu, Nano Energy, 2018, 51, 286–293 CrossRef CAS.
- C. Tang and S.-Z. Qiao, Matter, 2019, 1, 1454–1455 CrossRef.
- J. Zhang, L. Qu, G. Shi, J. Liu, J. Chen and L. Dai, Angew. Chem., Int. Ed., 2016, 55, 2230–2234 CrossRef CAS PubMed.
- Y. Zheng, Y. Jiao, Y. Zhu, L. H. Li, Y. Han, Y. Chen, A. Du, M. Jaroniec and S. Z. Qiao, Nat. Commun., 2014, 5, 3783 CrossRef PubMed.
- R. Wu, J. Zhang, Y. Shi, D. Liu and B. Zhang, J. Am. Chem. Soc., 2015, 137, 6983–6986 CrossRef CAS PubMed.
- J. Lai, S. Li, F. Wu, M. Saqib, R. Luque and G. Xu, Energy Environ. Sci., 2016, 9, 1210–1214 RSC.
- S. Chen, J. Duan, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2013, 52, 13567–13570 CrossRef CAS PubMed.
- X. Sun, F. Liu, X. Chen, C. Li, J. Yu and M. Pan, Electrochim. Acta, 2019, 307, 206–213 CrossRef CAS.
- Y. Sun, B. Huang, Y. Li, Y. Xing, M. Luo, N. Li, Z. Xia, Y. Qin, D. Su, L. Wang and S. Guo, Chem. Mater., 2019, 31, 8136–8144 CrossRef CAS.
- J. Ding, Q. Shao, Y. Feng and X. Huang, Nano Energy, 2018, 47, 1–7 CrossRef CAS.
- P. W. Menezes, C. Panda, S. Garai, C. Walter, A. Guiet and M. Driess, Angew. Chem., Int. Ed., 2018, 57, 15237–15242 CrossRef CAS PubMed.
- X. Wang, F. Li, W. Li, W. Gao, Y. Tang and R. Li, J. Mater. Chem. A, 2017, 5, 17982–17989 RSC.
- Y. Liu, S. Jiang, S. Li, L. Zhou, Z. Li, J. Li and M. Shao, Appl. Catal., B, 2019, 247, 107–114 CrossRef CAS.
- Y. Guo, X. Zhou, J. Tang, S. Tanaka, Y. V. Kaneti, J. Na, B. Jiang, Y. Yamauchi, Y. Bando and Y. Sugahara, Nano Energy, 2020, 75, 104913 CrossRef CAS.
- Y. Gong, Z. Yang, Y. Lin, T. Zhou, J. Li, F. Jiao and W. Wang, Dalton Trans., 2019, 48, 7025 RSC.
- F. Li, R. Xu, Y. Li, F. Liang, D. Zhang, W.-F. Fu and X.-J. Lv, Carbon, 2019, 145, 521–528 CrossRef CAS.
- X. Wang, W. Li, D. Xiong and L. Liu, J. Mater. Chem. A, 2016, 4, 5639–5646 RSC.
- A. Chunduri, S. Gupta, O. Bapat, A. Bhide, R. Fernandes, M. K. Patel, V. Bambole, A. Miotello and N. Patel, Appl. Catal., B, 2019, 259, 118051 CrossRef CAS.
- P. W. Menezes, A. Indra, I. Zaharieva, C. Walter, S. Loos, S. Hoffmann, R. Schlögl, H. Dau and M. Driess, Energy Environ. Sci., 2019, 12, 988–999 RSC.
- R. Zhang, J. Huang, G. Chen, W. Chen, C. Song, C. Li and K. Ostrikov, Appl. Catal., B, 2019, 254, 414–423 CrossRef CAS.
- A. Wu, Y. Xie, H. Ma, C. Tian, Y. Gu, H. Yan, X. Zhang, G. Yang and H. Fu, Nano Energy, 2018, 44, 353–363 CrossRef CAS.
- J. Xing, Y. Li, S. Guo, T. Jin, H. Li, Y. Wang and L. Jiao, Electrochim. Acta, 2019, 298, 305–312 CrossRef CAS.
- M. A. R. Anjum, M. H. Lee and J. S. Lee, ACS Catal., 2018, 8, 8296–8305 CrossRef CAS.
- L. Hui, Y. Xue, B. Huang, H. Yu, C. Zhang, D. Zhang, D. Jia, Y. Zhao, Y. Li, H. Liu and Y. Li, Nat. Commun., 2018, 9, 5309 CrossRef CAS PubMed.
- R. Matsuoka, R. Sakamoto, K. Hoshiko, S. Sasaki, H. Masunaga, K. Nagashio and H. Nishihara, J. Am. Chem. Soc., 2017, 139, 3145–3152 CrossRef CAS PubMed.
- G. Li, Y. Li, H. Liu, Y. Guo, Y. Li and D. Zhu, Chem. Commun., 2010, 46, 3256–3258 RSC.
- H.-Y. Si, Q.-X. Deng, L.-C. Chen, L. Wang, X.-Y. Liu, W.-S. Wu, Y.-H. Zhang, J.-M. Zhou and H.-L. Zhang, J. Alloys Compd., 2019, 794, 261–267 CrossRef CAS.
- R. F. Service, Science, 2014, 345, 610 CrossRef CAS PubMed.
- Y. Liu, Y. Su, X. Quan, X. Fan, S. Chen, H. Yu, H. Zhao, Y. Zhang and J. Zhao, ACS Catal., 2018, 8, 1186–1191 CrossRef CAS.
- I. Dybkjaer, Ammonia, catalysis and manufacture ed. A. Nielsen, Springer, Heidelberg, 1995, pp. 199–308 Search PubMed.
- R. Zhao, H. Xie, L. Chang, X. Zhang, X. Zhu, X. Tong, T. Wang, Y. Luo, P. Wei, Z. Wang and X. Sun, EnergyChem, 2019, 1, 100011 CrossRef.
- C. J. M. van der Ham, M. T. M. Koper and D. G. H. Hetterscheid, Chem. Soc. Rev., 2014, 43, 5183–5191 RSC.
- H. J. M. van Grinsven, L. Bouwman, K. G. Cassman, H. M. van Es, M. L. McCrackin and A. H. W. Beusen, J. Environ. Qual., 2015, 44, 356–367 CrossRef CAS PubMed.
- R. Qin, P. Liu, G. Fu and N. Zheng, Small Methods, 2018, 2, 1700286 CrossRef.
- Q. Li, L. He, C. Sun and X. Zhang, J. Phys. Chem. C, 2017, 121, 27563–27568 CrossRef CAS.
- J. Zhao and Z. Chen, J. Am. Chem. Soc., 2017, 139, 12480–12487 CrossRef CAS PubMed.
- B. H. R. Suryanto, H.-L. Du, D. Wang, J. Chen, A. N. Simonov and D. R. MacFarlane, Nat. Catal., 2019, 2, 290–296 CrossRef CAS.
- D. Wang, L. M. Azofra, M. Harb, L. Cavallo, X. Zhang, B. H. R. Suryanto and D. R. MacFarlane, ChemSusChem, 2018, 11, 3416–3422 CrossRef CAS PubMed.
- M. A. Shipman and M. D. Symes, Catal. Today, 2017, 286, 57–68 CrossRef CAS.
- X. Guo, H. Du, F. Qu and J. Li, J. Mater. Chem. A, 2019, 7, 3531–3543 RSC.
- T. Spatzal, M. Aksoyoglu, L. Zhang, S. L. A. Andrade, E. Schleicher, S. Weber, D. C. Rees and O. Einsle, Science, 2011, 334, 940 CrossRef CAS PubMed.
- L. C. Seefeldt, B. M. Hoffman and D. R. Dean, Annu. Rev. Biochem., 2009, 78, 701–722 CrossRef CAS PubMed.
- K. Kugler, B. Ohs, M. Scholz and M. Wessling, Phys. Chem. Chem. Phys., 2014, 16, 6129–6138 RSC.
- E. Skúlason, T. Bligaard, S. Gudmundsdóttir, F. Studt, J. Rossmeisl, F. Abild-Pedersen, T. Vegge, H. Jónsson and J. K. Nørskov, Phys. Chem. Chem. Phys., 2012, 14, 1235–1245 RSC.
- P. Wang, F. Chang, W. Gao, J. Guo, G. Wu, T. He and P. Chen, Nat. Chem., 2017, 9, 64–70 CrossRef CAS PubMed.
- G. Ertl, Catal. Rev., 1980, 21, 201–223 CrossRef CAS.
- D. Senthil Raja, H.-W. Lin and S.-Y. Lu, Nano Energy, 2019, 57, 1–13 CrossRef CAS.
- Y. Abghoui, A. L. Garden, V. F. Hlynsson, S. Björgvinsdóttir, H. Ólafsdóttir and E. Skúlason, Phys. Chem. Chem. Phys., 2015, 17, 4909–4918 RSC.
- Á. B. Höskuldsson, Y. Abghoui, A. B. Gunnarsdóttir and E. Skúlason, ACS Sustainable Chem. Eng., 2017, 5, 10327–10333 CrossRef.
- L. Huang, J. Wu, P. Han, A. M. Al-Enizi, T. M. Almutairi, L. Zhang and G. Zheng, Small Methods, 2019, 3, 1800386 CrossRef.
- M. Zhu, Q. Shao, Y. Qian and X. Huang, Nano Energy, 2019, 56, 330–337 CrossRef CAS.
- J. Han, Z. Liu, Y. Ma, G. Cui, F. Xie, F. Wang, Y. Wu, S. Gao, Y. Xu and X. Sun, Nano Energy, 2018, 52, 264–270 CrossRef CAS.
- W. Kong, Z. Liu, J. Han, L. Xia, Y. Wang, Q. Liu, X. Shi, Y. Wu, Y. Xu and X. Sun, Inorg. Chem. Front., 2019, 6, 423–427 RSC.
- C. Ling, Y. Ouyang, Q. Li, X. Bai, X. Mao, A. Du and J. Wang, Small Methods, 2019, 3, 1800376 CrossRef.
- Y. Abghoui, A. L. Garden, J. G. Howalt, T. Vegge and E. Skúlason, ACS Catal., 2016, 6, 635–646 CrossRef CAS.
- H.-L. Du, T. R. Gengenbach, R. Hodgetts, D. R. MacFarlane and A. N. Simonov, ACS Sustainable Chem. Eng., 2019, 7, 6839–6850 CrossRef CAS.
- C. J. H. Jacobsen, Chem. Commun., 2000, 1057–1058 RSC.
- C. D. Zeinalipour-Yazdi, J. S. J. Hargreaves and C. R. A. Catlow, J. Phys. Chem. C, 2015, 119, 28368–28376 CrossRef CAS.
- L. F. Greenlee, J. N. Renner and S. L. Foster, ACS Catal., 2018, 8, 7820–7827 CrossRef CAS.
- S. E. Saji, H. Lu, Z. Lu, A. Carroll and Z. Yin, Small Methods, 2021, 5, 2000694 CrossRef CAS PubMed.
- W. Guo, K. Zhang, Z. Liang, R. Zou and Q. Xu, Chem. Soc. Rev., 2019, 48, 5658–5716 RSC.
- C. Tang and S.-Z. Qiao, Joule, 2019, 3, 1573–1575 CrossRef.
- S. Z. Andersen, V. Čolić, S. Yang, J. A. Schwalbe, A. C. Nielander, J. M. McEnaney, K. Enemark-Rasmussen, J. G. Baker, A. R. Singh, B. A. Rohr, M. J. Statt, S. J. Blair, S. Mezzavilla, J. Kibsgaard, P. C. K. Vesborg, M. Cargnello, S. F. Bent, T. F. Jaramillo, I. E. L. Stephens, J. K. Nørskov and I. Chorkendorff, Nature, 2019, 570, 504–508 CrossRef CAS PubMed.
- S. Z. Andersen, M. J. Statt, V. J. Bukas, S. G. Shapel, J. B. Pedersen, K. Krempl, M. Saccoccio, D. Chakraborty, J. Kibsgaard, P. C. K. Vesborg, J. Nørskov and I. Chorkendorff, Energy Environ. Sci., 2020, 13, 4291–4300 RSC.
- M. Shao, Y. Shao, W. Chen, K. L. Ao, R. Tong, Q. Zhu, I. N. Chan, W. F. Ip, X. Shi and H. Pan, Phys. Chem. Chem. Phys., 2018, 20, 14504–14512 RSC.
- D. Bao, Q. Zhang, F.-L. Meng, H.-X. Zhong, M.-M. Shi, Y. Zhang, J.-M. Yan, Q. Jiang and X.-B. Zhang, Adv. Mater., 2017, 29, 1604799 CrossRef PubMed.
- J. H. Montoya, C. Tsai, A. Vojvodic and J. K. Nørskov, ChemSusChem, 2015, 8, 2180–2186 CrossRef CAS PubMed.
- Á. Logadóttir and J. K. Nørskov, J. Catal., 2003, 220, 273–279 CrossRef.
- H. Wang, H. Yu, Z. Wang, Y. Li, Y. Xu, X. Li, H. Xue and L. Wang, Small, 2019, 15, 1804769 CrossRef PubMed.
- M. Nazemi and M. A. El-Sayed, J. Phys. Chem. Lett., 2018, 9, 5160–5166 CrossRef CAS PubMed.
- J. Wang, L. Yu, L. Hu, G. Chen, H. Xin and X. Feng, Nat. Commun., 2018, 9, 1795 CrossRef PubMed.
- M.-M. Shi, D. Bao, S.-J. Li, B.-R. Wulan, J.-M. Yan and Q. Jiang, Adv. Energy Mater., 2018, 8, 1800124 CrossRef.
- Z. Geng, Y. Liu, X. Kong, P. Li, K. Li, Z. Liu, J. Du, M. Shu, R. Si and J. Zeng, Adv. Mater., 2018, 30, 1870301 CrossRef.
- N. Zhang, L. Li, J. Wang, Z. Hu, Q. Shao, X. Xiao and X. Huang, Angew. Chem., 2020, 59, 8066–8071 CrossRef CAS PubMed.
- H. Huang, L. Xia, X. Shi, A. M. Asiri and X. Sun, Chem. Commun., 2018, 54, 11427–11430 RSC.
- X. Li, H. Xie and J. Mao, J. Mater. Sci., 2020, 55, 5203–5210 CrossRef CAS.
- F. Akagi, T. Matsuo and H. Kawaguchi, Angew. Chem., Int. Ed., 2007, 46, 8778–8781 CrossRef CAS PubMed.
- T. Shima, S. Hu, G. Luo, X. Kang, Y. Luo and Z. Hou, Science, 2013, 340, 1549 CrossRef CAS PubMed.
- Y. Kobayashi, Y. Tang, T. Kageyama, H. Yamashita, N. Masuda, S. Hosokawa and H. Kageyama, J. Am. Chem. Soc., 2017, 139, 18240–18246 CrossRef CAS PubMed.
- C. J. H. Jacobsen, S. Dahl, B. S. Clausen, S. Bahn, A. Logadottir and J. K. Nørskov, J. Am. Chem. Soc., 2001, 123, 8404–8405 CrossRef CAS PubMed.
- C. I. Ezeh, X. Yang, J. He, C. Snape and X. M. Cheng, Ultrason. Sonochem., 2018, 42, 48–56 CrossRef CAS PubMed.
- R. Schlögl, Angew. Chem., Int. Ed., 2003, 42, 2004–2008 CrossRef PubMed.
- S. Back and Y. Jung, Phys. Chem. Chem. Phys., 2016, 18, 9161–9166 RSC.
- W. Peng, M. Luo, X. Xu, K. Jiang, M. Peng, D. Chen, T. S. Chan and Y. Tan, Adv. Energy Mater., 2020, 10, 2001364 CrossRef CAS.
- J. Hou, M. Yang and J. Zhang, Nanoscale, 2020, 12, 6900–6920 RSC.
- A. P. Leontiev, O. A. Brylev and K. S. Napolskii, Electrochim. Acta, 2015, 155, 466–473 CrossRef CAS.
- L. Ji, X. Shi, A. M. Asiri, B. Zheng and X. Sun, Inorg. Chem., 2018, 57, 14692–14697 CrossRef CAS PubMed.
- D. Yang, T. Chen and Z. Wang, J. Mater. Chem. A, 2017, 5, 18967–18971 RSC.
- I. Matanović, F. H. Garzon and N. J. Henson, Phys. Chem. Chem. Phys., 2014, 16, 3014–3026 RSC.
- D. Yao, C. Tang, L. Li, B. Xia, A. Vasileff, H. Jin, Y. Zhang and S. Z. Qiao, Adv. Energy Mater., 2020, 10, 2001289 CrossRef CAS.
- Z. Fang, P. Wu, Y. Qian and G. Yu, Angew. Chem., Int. Ed., 2021, 60, 4275–4281 CrossRef CAS PubMed.
- T. J. Del Castillo, N. B. Thompson and J. C. Peters, J. Am. Chem. Soc., 2016, 138, 5341–5350 CrossRef CAS PubMed.
- J. S. Anderson, J. Rittle and J. C. Peters, Nature, 2013, 501, 84–87 CrossRef CAS PubMed.
- T. M. Buscagan, P. H. Oyala and J. C. Peters, Angew. Chem., Int. Ed., 2017, 56, 6921–6926 CrossRef CAS PubMed.
- M.-T. Nguyen, N. Seriani and R. Gebauer, Phys. Chem. Chem. Phys., 2015, 17, 14317–14322 RSC.
- S. Licht, B. Cui, B. Wang, F.-F. Li, J. Lau and S. Liu, Science, 2014, 345, 637 CrossRef CAS PubMed.
- F.-F. Li and S. Licht, Inorg. Chem., 2014, 53, 10042–10044 CrossRef CAS PubMed.
- J. Kong, A. Lim, C. Yoon, J. H. Jang, H. C. Ham, J. Han, S. Nam, D. Kim, Y.-E. Sung, J. Choi and H. S. Park, ACS Sustainable Chem. Eng., 2017, 5, 10986–10995 CrossRef CAS.
- S. Chen, S. Perathoner, C. Ampelli, C. Mebrahtu, D. Su and G. Centi, Angew. Chem., Int. Ed., 2017, 56, 2699–2703 CrossRef CAS PubMed.
- S. Chen, S. Perathoner, C. Ampelli, C. Mebrahtu, D. Su and G. Centi, ACS Sustainable Chem. Eng., 2017, 5, 7393–7400 CrossRef CAS.
- F. Zhou, L. M. Azofra, M. Ali, M. Kar, A. N. Simonov, C. McDonnell-Worth, C. Sun, X. Zhang and D. R. MacFarlane, Energy Environ. Sci., 2017, 10, 2516–2520 RSC.
- B. T. Hang and D. H. Thang, J. Alloys Compd., 2016, 655, 44–49 CrossRef CAS.
- X. Cui, C. Tang, X.-M. Liu, C. Wang, W. Ma and Q. Zhang, Chem. – Eur. J., 2018, 24, 18494–18501 CrossRef CAS PubMed.
- X. Zhu, Z. Liu, Q. Liu, Y. Luo, X. Shi, A. M. Asiri, Y. Wu and X. Sun, Chem. Commun., 2018, 54, 11332–11335 RSC.
- X. Zhu, J. Zhao, L. Ji, T. Wu, T. Wang, S. Gao, A. A. Alshehri, K. A. Alzahrani, Y. Luo, Y. Xiang, B. Zheng and X. Sun, Nano Res., 2020, 13, 209–214 CrossRef CAS.
- K. Ogura, J. R. Ferrell, A. V. Cugini, E. S. Smotkin and M. D. Salazar-Villalpando, Electrochim. Acta, 2010, 56, 381–386 CrossRef CAS.
- X. Zhu, Z. Liu, H. Wang, R. Zhao, H. Chen, T. Wang, F. Wang, Y. Luo, Y. Wu and X. Sun, Chem. Commun., 2019, 55, 3987–3990 RSC.
- X. Xiang, Z. Wang, X. Shi, M. Fan and X. Sun, ChemCatChem, 2018, 10, 4530–4535 CrossRef CAS.
- X. Tang, R. Jia, T. Zhai and H. Xia, ACS Appl. Mater. Interfaces, 2015, 7, 27518–27525 CrossRef CAS PubMed.
- L. Hu, A. Khaniya, J. Wang, G. Chen, W. E. Kaden and X. Feng, ACS Catal., 2018, 8, 9312–9319 CrossRef CAS.
- X. P. Wang, Y. Hu and J. Chen, Int. Arch. Photogramm. Remote Sens. Spatial Inf. Sci., 2018, XLII-3, 1795–1798 CrossRef.
- X.-F. Li, Q.-K. Li, J. Cheng, L. Liu, Q. Yan, Y. Wu, X.-H. Zhang, Z.-Y. Wang, Q. Qiu and Y. Luo, J. Am. Chem. Soc., 2016, 138, 8706–8709 CrossRef CAS PubMed.
- J. Zhao, L. Zhang, X.-Y. Xie, X. Li, Y. Ma, Q. Liu, W.-H. Fang, X. Shi, G. Cui and X. Sun, J. Mater. Chem. A, 2018, 6, 24031–24035 RSC.
- W. Li, Y. Bai, F. Li, C. Liu, K.-Y. Chan, X. Feng and X. Lu, J. Mater. Chem., 2012, 22, 4025–4031 RSC.
- X. Zhang, Q. Liu, X. Shi, A. M. Asiri, Y. Luo, X. Sun and T. Li, J. Mater. Chem. A, 2018, 6, 17303–17306 RSC.
- K. Jia, Y. Wang, Q. Pan, B. Zhong, Y. Luo, G. Cui, X. Guo and X. Sun, Nanoscale Adv., 2019, 1, 961–964 RSC.
- Y. Wang, K. Jia, Q. Pan, Y. Xu, Q. Liu, G. Cui, X. Guo and X. Sun, ACS Sustainable Chem. Eng., 2019, 7, 117–122 CrossRef CAS.
- L. Zhang, X. Ji, X. Ren, Y. Ma, X. Shi, Z. Tian, A. M. Asiri, L. Chen, B. Tang and X. Sun, Adv. Mater., 2018, 30, 1800191 CrossRef PubMed.
- F. Lai, W. Zong, G. He, Y. Xu, H. Huang, B. Weng, D. Rao, J. A. Martens, J. Hofkens, I. P. Parkin and T. Liu, Angew. Chem., Int. Ed., 2020, 59, 13320–13327 CrossRef CAS PubMed.
- H. Cheng, L.-X. Ding, G.-F. Chen, L. Zhang, J. Xue and H. Wang, Adv. Mater., 2018, 30, 1803694 CrossRef PubMed.
- X. Yang, J. Nash, J. Anibal, M. Dunwell, S. Kattel, E. Stavitski, K. Attenkofer, J. G. Chen, Y. Yan and B. Xu, J. Am. Chem. Soc., 2018, 140, 13387–13391 CrossRef CAS PubMed.
- S. Zhang, W. Gong, Y. Lv, H. Wang, M. Han, G. Wang, T. Shi and H. Zhang, Chem. Commun., 2019, 55, 12376–12379 RSC.
- M. Yuan, H. Zhang, D. Gao, H. He, Y. Sun, P. Lu, S. Dipazir, Q. Li, L. Zhou, S. Li, Z. Liu, J. Yang, Y. Xie, H. Zhao and G. Zhang, J. Mater. Chem. A, 2020, 8, 2691–2700 RSC.
- J. Shao, W. Sheng, M. Wang, S. Li, J. Chen, Y. Zhang and S. Cao, Appl. Catal., B, 2017, 209, 311–319 CrossRef CAS.
- I. A. Amar, R. Lan, J. Humphreys and S. Tao, Catal. Today, 2017, 286, 51–56 CrossRef CAS.
- R. Zhang, X. Ren, X. Shi, F. Xie, B. Zheng, X. Guo and X. Sun, ACS Appl. Mater. Interfaces, 2018, 10, 28251–28255 CrossRef CAS PubMed.
- L. Yang, T. Wu, R. Zhang, H. Zhou, L. Xia, X. Shi, H. Zheng, Y. Zhang and X. Sun, Nanoscale, 2019, 11, 1555–1562 RSC.
- B. H. R. Suryanto, D. Wang, L. M. Azofra, M. Harb, L. Cavallo, R. Jalili, D. R. G. Mitchell, M. Chatti and D. R. MacFarlane, ACS Energy Lett., 2019, 4, 430–435 CrossRef CAS.
- Y. Abghoui, S. B. Sigtryggsson and E. Skúlason, ChemSusChem, 2019, 12, 4265–4273 CrossRef CAS PubMed.
- R. Michalsky, Y.-J. Zhang, A. J. Medford and A. A. Peterson, J. Phys. Chem. C, 2014, 118, 13026–13034 CrossRef CAS.
- X. Xu, F. Nosheen and X. Wang, Chem. Mater., 2016, 28, 6313–6320 CrossRef CAS.
- Y. Shi, Y. Yang, Y.-W. Li and H. Jiao, ACS Catal., 2016, 6, 6790–6803 CrossRef CAS.
- I. Matanovic and F. H. Garzon, Phys. Chem. Chem. Phys., 2018, 20, 14679–14687 RSC.
- G. Gao, A. P. O’Mullane and A. Du, ACS Catal., 2017, 7, 494–500 CrossRef CAS.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- L. M. Azofra, N. Li, D. R. MacFarlane and C. Sun, Energy Environ. Sci., 2016, 9, 2545–2549 RSC.
- X. Ren, J. Zhao, Q. Wei, Y. Ma, H. Guo, Q. Liu, Y. Wang, G. Cui, A. M. Asiri, B. Li, B. Tang and X. Sun, ACS Cent. Sci., 2019, 5, 116–121 CrossRef CAS PubMed.
- Y. Abghoui and E. Skúlason, Catal. Today, 2017, 286, 69–77 CrossRef CAS.
- Y. Abghoui and E. Skúlason, J. Phys. Chem. C, 2017, 121, 6141–6151 CrossRef CAS.
- R. Zhang, Y. Zhang, X. Ren, G. Cui, A. M. Asiri, B. Zheng and X. Sun, ACS Sustainable Chem. Eng., 2018, 6, 9545–9549 CrossRef CAS.
- X. Zhang, R.-M. Kong, H. Du, L. Xia and F. Qu, Chem. Commun., 2018, 54, 5323–5325 RSC.
- X. Zhu, T. Wu, L. Ji, Q. Liu, Y. Luo, G. Cui, Y. Xiang, Y. Zhang, B. Zheng and X. Sun, Chem. Commun., 2020, 56, 731–734 RSC.
- H. Huang, L. Xia, R. Cao, Z. Niu, H. Chen, Q. Liu, T. Li, X. Shi, A. M. Asiri and X. Sun, Chem. – Eur. J., 2019, 25, 1914–1917 CrossRef CAS PubMed.
- Y. Song, D. Johnson, R. Peng, D. K. Hensley, P. V. Bonnesen, L. Liang, J. Huang, F. Yang, F. Zhang, R. Qiao, A. P. Baddorf, T. J. Tschaplinski, N. L. Engle, M. C. Hatzell, Z. Wu, D. A. Cullen, H. M. Meyer, B. G. Sumpter and A. J. Rondinone, Sci. Adv., 2018, 4, e1700336 CrossRef PubMed.
- D. Yu, E. Nagelli, F. Du and L. Dai, J. Phys. Chem. Lett., 2010, 1, 2165–2173 CrossRef CAS.
- Y. Jiao, Y. Zheng, K. Davey and S.-Z. Qiao, Nat. Energy, 2016, 1, 16130 CrossRef CAS.
- W. Li, T. Wu, S. Zhang, Y. Liu, C. Zhao, G. Liu, G. Wang, H. Zhang and H. Zhao, Chem. Commun., 2018, 54, 11188–11191 RSC.
- D. S. Su, S. Perathoner and G. Centi, Chem. Rev., 2013, 113, 5782–5816 CrossRef CAS PubMed.
- T. Wu, X. Li, X. Zhu, S. Mou, Y. Luo, X. Shi, A. M. Asiri, Y. Zhang, B. Zheng, H. Zhao and X. Sun, Chem. Commun., 2020, 56, 1831–1834 RSC.
- F. Pan, H. Zhang, K. Liu, D. Cullen, K. More, M. Wang, Z. Feng, G. Wang, G. Wu and Y. Li, ACS Catal., 2018, 8, 3116–3122 CrossRef CAS.
- D. W. Stephan, Acc. Chem. Res., 2015, 48, 306–316 CrossRef CAS PubMed.
- C. Zhao, S. Zhang, M. Han, X. Zhang, Y. Liu, W. Li, C. Chen, G. Wang, H. Zhang and H. Zhao, ACS Energy Lett., 2019, 4, 377–383 CrossRef CAS.
- S. Mukherjee, D. A. Cullen, S. Karakalos, K. Liu, H. Zhang, S. Zhao, H. Xu, K. L. More, G. Wang and G. Wu, Nano Energy, 2018, 48, 217–226 CrossRef CAS.
- C. Lv, Y. Qian, C. Yan, Y. Ding, Y. Liu, G. Chen and G. Yu, Angew. Chem., Int. Ed., 2018, 57, 10246–10250 CrossRef CAS PubMed.
- G. Peng, J. Wu, M. Wang, J. Niklas, H. Zhou and C. Liu, Nano Lett., 2020, 20, 2879–2885 CrossRef CAS PubMed.
- X. Mao, S. Zhou, C. Yan, Z. Zhu and A. Du, Phys. Chem. Chem. Phys., 2019, 21, 1110–1116 RSC.
- J. Zhao, X. Ren, X. Li, D. Fan, X. Sun, H. Ma, Q. Wei and D. Wu, Nanoscale, 2019, 11, 4231–4235 RSC.
- Z. Geng, Y. Liu, X. Kong, P. Li, K. Li, Z. Liu, J. Du, M. Shu, R. Si and J. Zeng, Adv. Mater., 2018, 30, 1803498 CrossRef PubMed.
- X. Yu, P. Han, Z. Wei, L. Huang, Z. Gu, S. Peng, J. Ma and G. Zheng, Joule, 2018, 2, 1610–1622 CrossRef CAS.
- F. Ma, Y. Jiao, G. Gao, Y. Gu, A. Bilic, Z. Chen and A. Du, Nano Lett., 2016, 16, 3022–3028 CrossRef CAS PubMed.
- C. Liu, Q. Li, C. Wu, J. Zhang, Y. Jin, D. R. MacFarlane and C. Sun, J. Am. Chem. Soc., 2019, 141, 2884–2888 CrossRef CAS PubMed.
- C. Hering-Junghans, Angew. Chem., Int. Ed., 2018, 57, 6738–6740 CrossRef CAS PubMed.
- B. M. Hoffman, D. Lukoyanov, Z.-Y. Yang, D. R. Dean and L. C. Seefeldt, Chem. Rev., 2014, 114, 4041–4062 CrossRef CAS PubMed.
- Y. Fu and A. Manthiram, RSC Adv., 2012, 2, 5927–5929 RSC.
- H. Chen, X. Zhu, H. Huang, H. Wang, T. Wang, R. Zhao, H. Zheng, A. M. Asiri, Y. Luo and X. Sun, Chem. Commun., 2019, 55, 3152–3155 RSC.
- L. Xia, J. Yang, H. Wang, R. Zhao, H. Chen, W. Fang, A. M. Asiri, F. Xie, G. Cui and X. Sun, Chem. Commun., 2019, 55, 3371–3374 RSC.
- T. Wu, P. Li, H. Wang, R. Zhao, Q. Zhou, W. Kong, M. Liu, Y. Zhang, X. Sun and F. Gong, Chem. Commun., 2019, 55, 2684–2687 RSC.
- C. Choi, S. Back, N.-Y. Kim, J. Lim, Y.-H. Kim and Y. Jung, ACS Catal., 2018, 8, 7517–7525 CrossRef CAS.
- C. Chen, D. Yan, Y. Wang, Y. Zhou, Y. Zou, Y. Li and S. Wang, Small, 2019, 15, 1805029 CrossRef PubMed.
- X. Zhang, T. Wu, H. Wang, R. Zhao, H. Chen, T. Wang, P. Wei, Y. Luo, Y. Zhang and X. Sun, ACS Catal., 2019, 9, 4609–4615 CrossRef CAS.
- W. Qiu, X.-Y. Xie, J. Qiu, W.-H. Fang, R. Liang, X. Ren, X. Ji, G. Cui, A. M. Asiri, G. Cui, B. Tang and X. Sun, Nat. Commun., 2018, 9, 3485 CrossRef PubMed.
- Z. Wei, Y. Zhang, S. Wang, C. Wang and J. Ma, J. Mater. Chem. A, 2018, 6, 13790–13796 RSC.
- L. Zhang, L.-X. Ding, G.-F. Chen, X. Yang and H. Wang, Angew. Chem., 2019, 131, 2638–2642 CrossRef.
- X. Zhu, S. Mou, Q. Peng, Q. Liu, Y. Luo, G. Chen, S. Gao and X. Sun, J. Mater. Chem. A, 2020, 8, 1545–1556 RSC.
- G.-F. Chen, X. Cao, S. Wu, X. Zeng, L.-X. Ding, M. Zhu and H. Wang, J. Am. Chem. Soc., 2017, 139, 9771–9774 CrossRef CAS PubMed.
- Y. Yang, S.-Q. Wang, H. Wen, T. Ye, J. Chen, C.-P. Li and M. Du, Angew. Chem., Int. Ed., 2019, 58, 15362–15366 CrossRef CAS PubMed.
- D. T. Whipple and P. J. A. Kenis, J. Phys. Chem. Lett., 2010, 1, 3451–3458 CrossRef CAS.
- J. Artz, T. E. Müller, K. Thenert, J. Kleinekorte, R. Meys, A. Sternberg, A. Bardow and W. Leitner, Chem. Rev., 2018, 118, 434–504 CrossRef CAS PubMed.
- A. M. Appel, J. E. Bercaw, A. B. Bocarsly, H. Dobbek, D. L. DuBois, M. Dupuis, J. G. Ferry, E. Fujita, R. Hille, P. J. A. Kenis, C. A. Kerfeld, R. H. Morris, C. H. F. Peden, A. R. Portis, S. W. Ragsdale, T. B. Rauchfuss, J. N. H. Reek, L. C. Seefeldt, R. K. Thauer and G. L. Waldrop, Chem. Rev., 2013, 113, 6621–6658 CrossRef CAS PubMed.
- S. Roy, A. Cherevotan and S. C. Peter, ACS Energy Lett., 2018, 3, 1938–1966 CrossRef CAS.
- J. Wu, Y. Huang, W. Ye and Y. Li, Adv. Sci., 2017, 4, 1700194 CrossRef PubMed.
- P. Lu, X. Tan, H. Zhao, Q. Xiang, K. Liu, X. Zhao, X. Yin, X. Li, X. Hai, S. Xi, A. T. S. Wee, S. J. Pennycook, X. Yu, M. Yuan, J. Wu, G. Zhang, S. C. Smith and Z. Yin, ACS Nano, 2021, 15, 5671–5678 CrossRef CAS PubMed.
- R. Kortlever, J. Shen, K. J. P. Schouten, F. Calle-Vallejo and M. T. M. Koper, J. Phys. Chem. Lett., 2015, 6, 4073–4082 CrossRef CAS PubMed.
- L. R. L. Ting and B. S. Yeo, Curr. Opin. Electrochem., 2018, 8, 126–134 CrossRef CAS.
- L. Wang, W. Chen, D. Zhang, Y. Du, R. Amal, S. Qiao, J. Wu and Z. Yin, Chem. Soc. Rev., 2019, 48, 5310–5349 RSC.
- R. Zhao, P. Ding, P. Wei, L. Zhang, Q. Liu, Y. Luo, T. Li, S. Lu, X. Shi, S. Gao, A. M. Asiri, Z. Wang and X. Sun, Adv. Funct. Mater., 2021, 31, 2009449 CrossRef CAS.
- Y. Y. Birdja, E. Pérez-Gallent, M. C. Figueiredo, A. J. Göttle, F. Calle-Vallejo and M. T. M. Koper, Nat. Energy, 2019, 4, 732–745 CrossRef CAS.
- H. Yoshio, K. Katsuhei, M. Akira and S. Shin, Chem. Lett., 1986, 897–898 Search PubMed.
- E. Benson, C. P. Kubiak, A. J. Sathrum and J. M. Smieja, Chem. Soc. Rev., 2009, 38, 89–99 RSC.
- J. Qiao, Y. Liu, F. Hong and J. Zhang, Chem. Soc. Rev., 2014, 43, 631–675 RSC.
- X. Sun, X. Kang, Q. Zhu, J. Ma, G. Yang, Z. Liu and B. Han, Chem. Sci., 2016, 7, 2883–2887 RSC.
- O. S. Bushuyev, P. De Luna, C. T. Dinh, L. Tao, G. Saur, J. van de Lagemaat, S. O. Kelley and E. H. Sargent, Joule, 2018, 2, 825–832 CrossRef CAS.
- R. J. Lim, M. Xie, M. A. Sk, J.-M. Lee, A. Fisher, X. Wang and K. H. Lim, Catal. Today, 2014, 233, 169–180 CrossRef CAS.
- Z. Wang, Y. Li, H. Yu, Y. Xu, H. Xue, X. Li, H. Wang and L. Wang, ChemSusChem, 2018, 11, 3480–3485 CrossRef CAS PubMed.
- M.-M. Shi, D. Bao, B.-R. Wulan, Y.-H. Li, Y.-F. Zhang, J.-M. Yan and Q. Jiang, Adv. Mater., 2017, 29, 1606550 CrossRef PubMed.
- S.-J. Li, D. Bao, M.-M. Shi, B.-R. Wulan, J.-M. Yan and Q. Jiang, Adv. Mater., 2017, 29, 1700001 CrossRef PubMed.
- M. Wang, S. Liu, T. Qian, J. Liu, J. Zhou, H. Ji, J. Xiong, J. Zhong and C. Yan, Nat. Commun., 2019, 10, 341 CrossRef CAS PubMed.
- Y. Yao, Q. Feng, S. Zhu, J. Li, Y. Yao, Y. Wang, Q. Wang, M. Gu, H. Wang, H. Li, X.-Z. Yuan and M. Shao, Small Methods, 2019, 3, 1800324 CrossRef.
- R. Manjunatha, A. Karajić, V. Goldstein and A. Schechter, ACS Appl. Mater. Interfaces, 2019, 11, 7981–7989 CrossRef CAS PubMed.
- P. Wei, H. Xie, X. Zhu, R. Zhao, L. Ji, X. Tong, Y. Luo, G. Cui, Z. Wang and X. Sun, ACS Sustainable Chem. Eng., 2020, 8, 29–33 CrossRef CAS.
- X. Zhao, X. Lan, D. Yu, H. Fu, Z. Liu and T. Mu, Chem. Commun., 2018, 54, 13010–13013 RSC.
- R. Zhang, L. Ji, W. Kong, H. Wang, R. Zhao, H. Chen, T. Li, B. Li, Y. Luo and X. Sun, Chem. Commun., 2019, 55, 5263–5266 RSC.
- L. Xia, X. Wu, Y. Wang, Z. Niu, Q. Liu, T. Li, X. Shi, A. M. Asiri and X. Sun, Small Methods, 2019, 3, 1800251 CrossRef.
- R. Francke, B. Schille and M. Roemelt, Chem. Rev., 2018, 118, 4631–4701 CrossRef CAS PubMed.
- Y. Wang, J. Liu, Y. Wang, A. M. Al-Enizi and G. Zheng, Small, 2017, 13, 1701809 CrossRef PubMed.
- R. Schlögl, Angew. Chem., Int. Ed., 2015, 54, 3465–3520 CrossRef PubMed.
- J. Schneider, H. Jia, J. T. Muckerman and E. Fujita, Chem. Soc. Rev., 2012, 41, 2036–2051 RSC.
- E. V. Kondratenko, G. Mul, J. Baltrusaitis, G. O. Larrazábal and J. Pérez-Ramírez, Energy Environ. Sci., 2013, 6, 3112–3135 RSC.
- J. Albo, M. Alvarez-Guerra, P. Castaño and A. Irabien, Green Chem., 2015, 17, 2304–2324 RSC.
- M. F. Baruch, J. E. Pander, J. L. White and A. B. Bocarsly, ACS Catal., 2015, 5, 3148–3156 CrossRef CAS.
- J. E. Pander, M. F. Baruch and A. B. Bocarsly, ACS Catal., 2016, 6, 7824–7833 CrossRef CAS.
- A. Dutta, A. Kuzume, M. Rahaman, S. Vesztergom and P. Broekmann, ACS Catal., 2015, 5, 7498–7502 CrossRef CAS.
- C. Cui, J. Han, X. Zhu, X. Liu, H. Wang, D. Mei and Q. Ge, J. Catal., 2016, 343, 257–265 CrossRef CAS.
- J. T. Feaster, C. Shi, E. R. Cave, T. Hatsukade, D. N. Abram, K. P. Kuhl, C. Hahn, J. K. Nørskov and T. F. Jaramillo, ACS Catal., 2017, 7, 4822–4827 CrossRef CAS.
- J. L. White and A. B. Bocarsly, J. Electrochem. Soc., 2016, 163, H410–H416 CrossRef CAS.
- S. Back, J.-H. Kim, Y.-T. Kim and Y. Jung, Phys. Chem. Chem. Phys., 2016, 18, 9652–9657 RSC.
- J. H. Koh, D. H. Won, T. Eom, N.-K. Kim, K. D. Jung, H. Kim, Y. J. Hwang and B. K. Min, ACS Catal., 2017, 7, 5071–5077 CrossRef CAS.
- J. S. Yoo, R. Christensen, T. Vegge, J. K. Nørskov and F. Studt, ChemSusChem, 2016, 9, 358–363 CrossRef CAS PubMed.
- N. J. Firet and W. A. Smith, ACS Catal., 2017, 7, 606–612 CrossRef CAS.
- S. Back, M. S. Yeom and Y. Jung, ACS Catal., 2015, 5, 5089–5096 CrossRef CAS.
- T. Cheng, Y. Huang, H. Xiao and W. A. Goddard, J. Phys. Chem. Lett., 2017, 8, 3317–3320 CrossRef CAS PubMed.
- J. Rosen, G. S. Hutchings, Q. Lu, S. Rivera, Y. Zhou, D. G. Vlachos and F. Jiao, ACS Catal., 2015, 5, 4293–4299 CrossRef CAS.
- H.-E. Lee, K. D. Yang, S. M. Yoon, H.-Y. Ahn, Y. Y. Lee, H. Chang, D. H. Jeong, Y.-S. Lee, M. Y. Kim and K. T. Nam, ACS Nano, 2015, 9, 8384–8393 CrossRef CAS PubMed.
- A. D. Handoko, K. W. Chan and B. S. Yeo, ACS Energy Lett., 2017, 2, 2103–2109 CrossRef CAS.
- E. Pérez-Gallent, M. C. Figueiredo, F. Calle-Vallejo and M. T. M. Koper, Angew. Chem., Int. Ed., 2017, 56, 3621–3624 CrossRef PubMed.
- J. D. Goodpaster, A. T. Bell and M. Head-Gordon, J. Phys. Chem. Lett., 2016, 7, 1471–1477 CrossRef CAS PubMed.
- D. Ren, B. S.-H. Ang and B. S. Yeo, ACS Catal., 2016, 6, 8239–8247 CrossRef CAS.
- S. Lee, G. Park and J. Lee, ACS Catal., 2017, 7, 8594–8604 CrossRef CAS.
- S. Gao, Y. Lin, X. Jiao, Y. Sun, Q. Luo, W. Zhang, D. Li, J. Yang and Y. Xie, Nature, 2016, 529, 68–71 CrossRef CAS PubMed.
- S. Ma, M. Sadakiyo, R. Luo, M. Heima, M. Yamauchi and P. J. A. Kenis, J. Power Sources, 2016, 301, 219–228 CrossRef CAS.
- F. Calle-Vallejo and M. T. M. Koper, Angew. Chem., Int. Ed., 2013, 52, 7282–7285 CrossRef CAS PubMed.
- Y. Huang, A. D. Handoko, P. Hirunsit and B. S. Yeo, ACS Catal., 2017, 7, 1749–1756 CrossRef CAS.
- C. Hahn, T. Hatsukade, Y.-G. Kim, A. Vailionis, J. H. Baricuatro, D. C. Higgins, S. A. Nitopi, M. P. Soriaga and T. F. Jaramillo, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 5918 CrossRef CAS PubMed.
- H.-R. M. Jhong, S. Ma and P. J. A. Kenis, Curr. Opin. Chem. Eng., 2013, 2, 191–199 CrossRef.
- X. Duan, J. Xu, Z. Wei, J. Ma, S. Guo, S. Wang, H. Liu and S. Dou, Adv. Mater., 2017, 29, 1701784 CrossRef PubMed.
- A. Loiudice, P. Lobaccaro, E. A. Kamali, T. Thao, B. H. Huang, J. W. Ager and R. Buonsanti, Angew. Chem., Int. Ed., 2016, 55, 5789–5792 CrossRef CAS PubMed.
- M. B. Ross, C. T. Dinh, Y. Li, D. Kim, P. De Luna, E. H. Sargent and P. Yang, J. Am. Chem. Soc., 2017, 139, 9359–9363 CrossRef CAS PubMed.
- M. M. Abdelnaby, K. Liu, K. Hassanein and Z. Yin, ChemNanoMat, 2021, 7, 969–981 CrossRef CAS.
- S. Nitopi, E. Bertheussen, S. B. Scott, X. Liu and I. Chorkendorff, Chem. Rev., 2019, 119, 7610–7672 CrossRef CAS PubMed.
- B. Jiang, X.-G. Zhang, K. Jiang, D.-Y. Wu and W.-B. Cai, J. Am. Chem. Soc., 2018, 140, 2880–2889 CrossRef CAS PubMed.
- H. S. Jeon, I. Sinev, F. Scholten, N. J. Divins, I. Zegkinoglou, L. Pielsticker and B. R. Cuenya, J. Am. Chem. Soc., 2018, 140, 9383–9386 CrossRef CAS PubMed.
- S. Y. Lee, H. Jung, N.-K. Kim, H.-S. Oh, B. K. Min and Y. J. Hwang, J. Am. Chem. Soc., 2018, 140, 8681–8689 CrossRef CAS PubMed.
- M. Ma, B. J. Trześniewski, J. Xie and W. A. Smith, Angew. Chem., Int. Ed., 2016, 55, 9748–9752 CrossRef CAS PubMed.
- Y.-X. Duan, F.-L. Meng, K.-H. Liu, S.-S. Yi, S.-J. Li, J.-M. Yan and Q. Jiang, Adv. Mater., 2018, 30, 1706194 CrossRef PubMed.
- R. M. Arán-Ais, F. Scholten, S. Kunze, R. Rizo and B. Roldan Cuenya, Nat. Energy, 2020, 5, 317–325 CrossRef.
- J. Xie, X. Zhao, M. Wu, Q. Li, Y. Wang and J. Yao, Angew. Chem., 2018, 130, 9788–9792 CrossRef.
- T. T. H. Hoang, S. Verma, S. Ma, T. T. Fister, J. Timoshenko, A. I. Frenkel, P. J. A. Kenis and A. A. Gewirth, J. Am. Chem. Soc., 2018, 140, 5791–5797 CrossRef CAS PubMed.
- E. L. Clark, C. Hahn, T. F. Jaramillo and A. T. Bell, J. Am. Chem. Soc., 2017, 139, 15848–15857 CrossRef CAS PubMed.
- J. T. L. Gamler, H. M. Ashberry, S. E. Skrabalak and K. M. Koczkur, Adv. Mater., 2018, 30, 1801563 CrossRef PubMed.
- D. Kim, C. Xie, N. Becknell, Y. Yu, M. Karamad, K. Chan, E. J. Crumlin, J. K. Nørskov and P. Yang, J. Am. Chem. Soc., 2017, 139, 8329–8336 CrossRef CAS PubMed.
- S. Ma, M. Sadakiyo, M. Heima, R. Luo, R. T. Haasch, J. I. Gold, M. Yamauchi and P. J. A. Kenis, J. Am. Chem. Soc., 2017, 139, 47–50 CrossRef CAS PubMed.
- X. Zhang, F. Li, Y. Zhang, A. M. Bond and J. Zhang, J. Mater. Chem. A, 2018, 6, 7851–7858 RSC.
- H. B. Yang, S.-F. Hung, S. Liu, K. Yuan, S. Miao, L. Zhang, X. Huang, H.-Y. Wang, W. Cai, R. Chen, J. Gao, X. Yang, W. Chen, Y. Huang, H. M. Chen, C. M. Li, T. Zhang and B. Liu, Nat. Energy, 2018, 3, 140–147 CrossRef CAS.
- X. Wang, Z. Chen, X. Zhao, T. Yao, W. Chen, R. You, C. Zhao, G. Wu, J. Wang, W. Huang, J. Yang, X. Hong, S. Wei, Y. Wu and Y. Li, Angew. Chem., 2018, 130, 1962–1966 CrossRef.
- Y. Pan, R. Lin, Y. Chen, S. Liu, W. Zhu, X. Cao, W. Chen, K. Wu, W.-C. Cheong, Y. Wang, L. Zheng, J. Luo, Y. Lin, Y. Liu, C. Liu, J. Li, Q. Lu, X. Chen, D. Wang, Q. Peng, C. Chen and Y. Li, J. Am. Chem. Soc., 2018, 140, 4218–4221 CrossRef CAS PubMed.
- Y. Cheng, S. Zhao, B. Johannessen, J.-P. Veder, M. Saunders, M. R. Rowles, M. Cheng, C. Liu, M. F. Chisholm, R. De Marco, H.-M. Cheng, S.-Z. Yang and S. P. Jiang, Adv. Mater., 2018, 30, 1706287 CrossRef PubMed.
- N. M. Adli, W. T. Shan, S. Hwang, W. Samarakoon, S. Karakalos, Y. Li, D. A. Cullen, D. Su, Z. X. Feng, G. F. Wang and G. Wu, Angew. Chem., Int. Ed., 2021, 60, 1022–1032 CrossRef PubMed.
- H. Jin, C. Guo, X. Liu, J. Liu, A. Vasileff, Y. Jiao, Y. Zheng and S.-Z. Qiao, Chem. Rev., 2018, 118, 6337–6408 CrossRef CAS PubMed.
- W. Zhang, Q. Qin, L. Dai, R. Qin, X. Zhao, X. Chen, D. Ou, J. Chen, T. T. Chuong, B. Wu and N. Zheng, Angew. Chem., Int. Ed., 2018, 57, 9475–9479 CrossRef CAS PubMed.
- C. Cao, D.-D. Ma, J.-F. Gu, X. Xie, G. Zeng, X. Li, S.-G. Han, Q.-L. Zhu, X.-T. Wu and Q. Xu, Angew. Chem., Int. Ed., 2020, 59, 15014–15020 CrossRef CAS PubMed.
- F. P. García de Arquer, O. S. Bushuyev, P. De Luna, C.-T. Dinh, A. Seifitokaldani, M. I. Saidaminov, C.-S. Tan, L. N. Quan, A. Proppe, M. G. Kibria, S. O. Kelley, D. Sinton and E. H. Sargent, Adv. Mater., 2018, 30, 1802858 CrossRef PubMed.
- W. Bi, C. Wu and Y. Xie, ACS Energy Lett., 2018, 3, 624–633 CrossRef CAS.
- J. Xu, X. Li, W. Liu, Y. Sun, Z. Ju, T. Yao, C. Wang, H. Ju, J. Zhu, S. Wei and Y. Xie, Angew. Chem., Int. Ed., 2017, 56, 9121–9125 CrossRef CAS PubMed.
- N. Elgrishi, M. B. Chambers, X. Wang and M. Fontecave, Chem. Soc. Rev., 2017, 46, 761–796 RSC.
- N. Han, Y. Wang, L. Ma, J. Wen, J. Li, H. Zheng, K. Nie, X. Wang, F. Zhao, Y. Li, J. Fan, J. Zhong, T. Wu, D. J. Miller, J. Lu, S.-T. Lee and Y. Li, Chem, 2017, 3, 652–664 CAS.
- W.-H. Wang, Y. Himeda, J. T. Muckerman, G. F. Manbeck and E. Fujita, Chem. Rev., 2015, 115, 12936–12973 CrossRef CAS PubMed.
- W. Zhang, W. Lai and R. Cao, Chem. Rev., 2017, 117, 3717–3797 CrossRef CAS PubMed.
- X. Zhang, Z. Wu, X. Zhang, L. Li, Y. Li, H. Xu, X. Li, X. Yu, Z. Zhang, Y. Liang and H. Wang, Nat. Commun., 2017, 8, 14675 CrossRef PubMed.
- M. Wang, L. Chen, T.-C. Lau and M. Robert, Angew. Chem., Int. Ed., 2018, 57, 7769–7773 CrossRef CAS PubMed.
- O. G. Sánchez, Y. Y. Birdja, M. Bulut, J. Vaes, T. Breugelmans and D. Pant, Curr. Opin. Green Sust., 2019, 16, 47–56 CrossRef.
- S. Zhang, P. Kang, S. Ubnoske, M. K. Brennaman, N. Song, R. L. House, J. T. Glass and T. J. Meyer, J. Am. Chem. Soc., 2014, 136, 7845–7848 CrossRef CAS PubMed.
- H. Wang, Y. Chen, X. Hou, C. Ma and T. Tan, Green Chem., 2016, 18, 3250–3256 RSC.
- K. Nakata, T. Ozaki, C. Terashima, A. Fujishima and Y. Einaga, Angew. Chem., Int. Ed., 2014, 53, 871–874 CrossRef CAS PubMed.
- K. Natsui, H. Iwakawa, N. Ikemiya, K. Nakata and Y. Einaga, Angew. Chem., Int. Ed., 2018, 57, 2639–2643 CrossRef CAS PubMed.
- X. Xue, H. Yang, T. Yang, P. Yuan, Q. Li, S. Mu, X. Zheng, L. Chi, J. Zhu, Y. Li, J. Zhang and Q. Xu, J. Mater. Chem. A, 2019, 7, 15271–15277 RSC.
- T. Liu, S. Ali, Z. Lian, C. Si, D. S. Su and B. Li, J. Mater. Chem. A, 2018, 6, 19998–20004 RSC.
- H. Yang, Y. Wu, Q. Lin, L. Fan, X. Chai, Q. Zhang, J. Liu, C. He and Z. Lin, Angew. Chem., Int. Ed., 2018, 57, 15476–15480 CrossRef CAS PubMed.
- G.-L. Chai and Z.-X. Guo, Chem. Sci., 2016, 7, 1268–1275 RSC.
- H. Wang, J. Jia, P. Song, Q. Wang, D. Li, S. Min, C. Qian, L. Wang, Y. F. Li, C. Ma, T. Wu, J. Yuan, M. Antonietti and G. A. Ozin, Angew. Chem., Int. Ed., 2017, 56, 7847–7852 CrossRef CAS PubMed.
- J. Wu, M. Liu, P. P. Sharma, R. M. Yadav, L. Ma, Y. Yang, X. Zou, X.-D. Zhou, R. Vajtai, B. I. Yakobson, J. Lou and P. M. Ajayan, Nano Lett., 2016, 16, 466–470 CrossRef CAS PubMed.
- J. Wu, S. Ma, J. Sun, J. I. Gold, C. Tiwary, B. Kim, L. Zhu, N. Chopra, I. N. Odeh, R. Vajtai, A. Z. Yu, R. Luo, J. Lou, G. Ding, P. J. A. Kenis and P. M. Ajayan, Nat. Commun., 2016, 7, 13869 CrossRef CAS PubMed.
- Y. Song, W. Chen, C. Zhao, S. Li, W. Wei and Y. Sun, Angew. Chem., Int. Ed., 2017, 56, 10840–10844 CrossRef CAS PubMed.
- Y. Liu, Y. Zhang, K. Cheng, X. Quan, X. Fan, Y. Su, S. Chen, H. Zhao, Y. Zhang, H. Yu and M. R. Hoffmann, Angew. Chem., Int. Ed., 2017, 56, 15607–15611 CrossRef CAS PubMed.
- X.-M. Hu, H. H. Hval, E. T. Bjerglund, K. J. Dalgaard, M. R. Madsen, M.-M. Pohl, E. Welter, P. Lamagni, K. B. Buhl, M. Bremholm, M. Beller, S. U. Pedersen, T. Skrydstrup and K. Daasbjerg, ACS Catal., 2018, 8, 6255–6264 CrossRef CAS.
- X. Sun, L. Lu, Q. Zhu, C. Wu, D. Yang, C. Chen and B. Han, Angew. Chem., Int. Ed., 2018, 57, 2427–2431 CrossRef CAS PubMed.
- W. Ju, A. Bagger, G.-P. Hao, A. S. Varela, I. Sinev, V. Bon, B. Roldan Cuenya, S. Kaskel, J. Rossmeisl and P. Strasser, Nat. Commun., 2017, 8, 944 CrossRef PubMed.
- C. Chen, X. Sun, X. Yan, Y. Wu, H. Liu, Q. Zhu, B. B. A. Bediako and B. Han, Angew. Chem., Int. Ed., 2020, 59, 11123–11129 CrossRef CAS PubMed.
- Y. Liu, S. Chen, X. Quan and H. Yu, J. Am. Chem. Soc., 2015, 137, 11631–11636 CrossRef CAS PubMed.
- L. Ye, Y. Ying, D. Sun, Z. Zhang, L. Fei, Z. Wen, J. Qiao and H. Huang, Angew. Chem., Int. Ed., 2020, 59, 3244–3251 CrossRef CAS PubMed.
- Y. Jiao, Y. Zheng, P. Chen, M. Jaroniec and S.-Z. Qiao, J. Am. Chem. Soc., 2017, 139, 18093–18100 CrossRef CAS PubMed.
- J. Wu, R. M. Yadav, M. Liu, P. P. Sharma, C. S. Tiwary, L. Ma, X. Zou, X.-D. Zhou, B. I. Yakobson, J. Lou and P. M. Ajayan, ACS Nano, 2015, 9, 5364–5371 CrossRef CAS PubMed.
- B. Kumar, M. Asadi, D. Pisasale, S. Sinha-Ray, B. A. Rosen, R. Haasch, J. Abiade, A. L. Yarin and A. Salehi-Khojin, Nat. Commun., 2013, 4, 2819 CrossRef.
- H. Yin, K. Xing, Y. Zhang, D. M. A. S. Dissanayake, Z. Lu, H. Zhao, Z. Zeng, J.-H. Yun, D.-C. Qi and Z. Yin, Chem. Soc. Rev., 2021, 50, 6423–6482 RSC.
- N. Uddin, H. Zhang, Y. Du, G. Jia, S. Wang and Z. Yin, Adv. Mater., 2020, 32, 1905739 CrossRef CAS PubMed.
- Y. Yu, Y. Shi and B. Zhang, Acc. Chem. Res., 2018, 51, 1711–1721 CrossRef CAS PubMed.
- F. Lai, Z. Sun, S. E. Saji, Y. He, X. Yu, H. Zhao, H. Guo and Z. Yin, Small, 2021, 17, 2100024 CrossRef CAS PubMed.
- O. A. Moses, L. Gao, H. Zhao, Z. Wang, M. Lawan Adam, Z. Sun, K. Liu, J. Wang, Y. Lu, Z. Yin and X. Yu, Mater. Today, 2021, 50, 116–148 CrossRef CAS.
- M. Zhong, K. Tran, Y. Min, C. Wang, Z. Wang, C.-T. Dinh, P. De Luna, Z. Yu, A. S. Rasouli, P. Brodersen, S. Sun, O. Voznyy, C.-S. Tan, M. Askerka, F. Che, M. Liu, A. Seifitokaldani, Y. Pang, S.-C. Lo, A. Ip, Z. Ulissi and E. H. Sargent, Nature, 2020, 581, 178–183 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |
