Open Access Article
Open Access ArticleRecent developments of hydrogel based solar water purification technology
Shudi
Mao
 ,
Md Abu Hasan
Johir
,
Md Abu Hasan
Johir
 ,
Casey
Onggowarsito
,
Casey
Onggowarsito
 ,
An
Feng
,
An
Feng
 ,
Long D.
Nghiem
,
Long D.
Nghiem
 and
Qiang
Fu
and
Qiang
Fu
 *
*
Centre for Technology in Water and Wastewater, School of Civil and Environmental Engineering, University of Technology Sydney, NSW 2007, Australia. E-mail: qiang.fu@uts.edu.au
First published on 29th December 2021
Abstract
Water scarcity is a severe problem all over the world; however, most current large-scale water purification technologies are energy-intensive and require high capital and maintenance costs. Recently, hydrogel-based solar water purification technologies have attracted increasing attention due to their advantages, including easy preparation, less energy intensive, and highly efficient solar absorption and utilization. This review summarizes recent advances in the development of novel hydrogel materials from two perspectives: improving solar–thermal conversion efficiency, and facilitating water transport. The main strategies to achieve higher conversion efficiency include choosing good solar absorbers, constructing a surface to reduce light reflection and reducing heat loss. Adjusting wettability, tuning the physio-chemical properties of internal channels, and increasing the intermediate water content to lower the water vaporization enthalpy are the three main approaches for rapid water transport. This review also provides new insights into future directions and remaining challenges in this field.
1. Introduction
As an essential substance of life, water is one of the most essential resources for ecological well-being and economic development. Rapid population growth, economic progress, industrialisation of agriculture, dietary changes, and climate change1,2 have led to a nearly eightfold increase in fresh water demand between the 1990s and the 2010s.3 Despite recent and worldwide economic progress, by 2025, one-third of the world population will still face water scarcity. The Massachusetts Institute of Technology (MIT) Integrated Global System Model Water Resource System predicted that by 2050, about 52% of the world's projected population (9.7 billion) would live in water-stressed regions.4 Therefore, there is an ever increasing need to develop efficient, affordable and scalable purification technologies to obtain fresh water from abundant sources such as seawater, brackish water and wastewater that are independent of the hydrological cycle.Freshwater can be extracted from the ocean or brackish water sources by thermal distillation or filtration technologies.5–9 Thermal distillation processes such as multi-stage flash distillation,10 multiple effect distillation,11 and membrane distillation have been widely used for seawater desalination applications, especially in the Middle East. These technologies require significant thermal energy input, thus, many of them are co-located with and utilize waste heat from thermal power plants to lower energy consumption. Even when co-located with thermal power plants, the energy consumption of these conventional thermal distillation technologies is still quite high, in the range of 5–60 kW h m−3 of produced freshwater.7,12 Filtration-based water purification technologies, such as reverse osmosis (RO)13 and electrodialysis,14 rely mainly on the separation of salts from water via a semipermeable membrane. RO has been commercialized on a large scale and can achieve a relatively low energy consumption of ca. 3–4 kW h m−3 of produced freshwater from seawater.15–17 However, the RO membrane is susceptible to fouling during operation, and hence frequent chemical cleaning is required.18 In the electrodialysis process, cation and anion exchange membranes are used to selectively separate salts from the saline feedwater solution. Electrodialysis is only suitable for brackish water that has about one-fifth of the salinity of seawater19 and the energy consumption is also considerable.20 Hence, most of the current full-scale water purification technologies consume a significant amount of energy and are therefore costly, encouraging the researchers to develop next-generation cost-effective water purification technologies. These cost-effective water purification technologies will reduce energy consumption or will use renewable energy.
As the most abundant and renewable energy source, solar energy can be used for freshwater production.21 A solar still is a simple solar water purification technology.22Fig. 1a illustrates a conventional solar still system, consisting of a sloping glass cover, a black basin with seawater or wastewater, and a thermally insulated enclosure. Because the black basin absorbs solar energy, water evaporates and then condenses on the inner surface of the shelter. As a result, freshwater can be obtained at the lower end of the cover. Unfortunately, the solar vapor generation (SVG) efficiency of such a solar still system is too low (ca. 1–5 L m−2 d−1)23 to be widely utilized due to insufficient solar absorption and significant thermal loss. To overcome this challenge, novel hydrogel materials have been introduced and incorporated into traditional solar still systems (Fig. 1b). The thin red layer in Fig. 1b is above the water surface. It can localize heat in the evaporation interface, reduce heat loss to bulk water, and improve the solar-thermal conversion efficiency, vapor generation rate, and freshwater productivity.24–28 These materials are commonly composed of solar absorbers, which can convert solar energy into thermal energy, and porous, low-conductivity, hydrophilic materials that can reduce heat loss and transport water to the evaporation interface. Accordingly, considerable effort has been focused on improving the solar–thermal conversion efficiency, enhancing heat insulation, and speeding up water transport. In 2018, Zhao et al.25 introduced a hydrogel-based material to reduce the energy demand for evaporation and improve the solar vapor generation efficiency. Specific water states in polymer hydrogels caused by the distinct preference for water molecules of the hydrophilic polymer chains can make the water more active, thereby facilitating the evaporation process.29,30 Since then, as a landmark study, it has heightened the interest in this field, and more and more publications on hydrogel-based solar water purification have appeared recently.
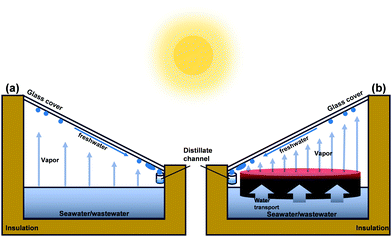 | ||
| Fig. 1 Schematic illustrations of a single slope (a) solar still and (b) hydrogel based material platform for solar water purification. | ||
Although the development of hydrogel-materials for solar water purification is still in its early stages, many research papers have been published in this field. A timely, thorough review of the recent achievements will highlight key research findings and reveal significant research trends in this field. This review summarises the design principles of hydrogel based materials for solar water purification from two primary directions: improving the solar–thermal conversion efficiency and facilitating water transport with examples from the recent three years’ representative research. We also put forward prospects on the future directions and remaining challenges in this field.
2. Solar–thermal conversion enhancement
Solar–thermal conversion is the core step of solar water purification, consisting of light-harvesting, photothermal conversion and heat insulation. The light-harvesting competence of the material platform, especially the solar absorbers, is the precondition for attaining sufficient photothermal conversion. Solar radiation might undergo reflection, transmission and absorption when it strikes the surface of solar absorbers, but only the absorbed irradiation is the net energy input for subsequent conversion. Thus, solar absorbers with high irradiation absorptivity and topographical and geometrical designs that reduce reflection and transmission are vital for achieving high conversion efficiency.31 The absorbed sunlight energy can be converted into thermal energy by the solar absorbers instead of being re-emitted. A large number of nanomaterials have been employed as solar absorbers, and various photothermal conversion mechanisms are presented based on the different interactions between electromagnetic radiation and the specific categories of solar absorbers.32,33 Finally, the generated heat will be confined in the evaporation interface layer through the heat insulation designs27,34,35 to reduce heat loss to bulk water and thereby improve the efficiency of heat utilization.Since a diverse array of factors noted above collaboratively contribute to the solar–thermal conversion competence of the entire solar water purification system, the metric energy conversion efficiency (η) has been proposed to quantitatively assess its solar–thermal conversion performance, which can be calculated according to eqn (1):36
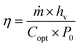 | (1) |
In order to enhance the energy conversion efficiency, considerable efforts have been devoted to choosing appropriate solar absorbers, modifying surface topography to reduce light reflection, and mitigating heat loss accordingly.
2.1 Solar absorbers
Choosing an appropriate solar absorber with full-spectrum strong solar absorption and high solar–thermal conversion capacity is one of the vital design principles. Plentiful nanomaterials have been adapted as solar absorbers in solar water purification, including inorganic semiconductors, plasmonic nanoparticles, carbon-based materials, and conjugated polymers. Different solar absorbers exhibit diverse solar–thermal conversion mechanisms and thus have different selection principles.For instance, as a widely used light-harvesting semiconductor, TiO2 has been used as a solar absorber for preparing hydrogel materials.42–45 However, under 1 sun, these hydrogels showed a low evaporation rate of <1.5 kg m−2 h−1 and a low energy conversion efficiency of <80%. This can be attributed to the wide intrinsic band-gap of TiO2 of ca. 3 eV, which corresponds to the absorption of UV-light (wavelength <400 nm).39,46,47 In contrast, Ti2O3 with an extremely narrow band-gap of ∼0.09 eV exhibits excellent energy conversion performance. Guo et al.24 incorporated Ti2O3 nanoparticles into sponge-like polyvinyl alcohol (PVA) hydrogels with interconnected pores (Fig. 2a). The SEM and the EDS mapping images (Fig. 2b–d) also confirmed that the Ti2O3 nanoparticles were uniformly distributed in the network. The optimized samples (LASG3, LASG4 and LASG5, Fig. 2e and f) showed a relatively high solar absorption efficiency of >96% and a low reflectance over a broadband of the standard solar spectrum. Correspondingly, the resultant Ti2O3/PVA hydrogels showed an extremely high evaporation rate of 3.6 kg m−2 h−1 and an excellent energy conversion efficiency of ca. 90%. In all, semiconducting solar absorbers with a narrow band-gap that can absorb more infrared energy will bring excellent solar–thermal conversion competence to the solar water purification system.
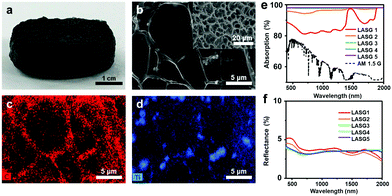 | ||
| Fig. 2 (a) Photo of the as-prepared Ti2O3–PVA sponge-like hydrogel sample. (b) SEM image of the sponge-like hydrogel and EDS mapping of (c) carbon and (d) titanium. (e) Absorption and (f) reflectance spectra in the wavelength range of 200–2000 nm. Reproduced with permission.24 Copyright 2019, American Chemical Society. | ||
As well as TiO2, MnO2,48,49 Co3O4,50 and CuO49,51,52 other transition metal oxides have been employed as solar absorbers. Furthermore, transition metal sulfides (such as MoS2,53–56 Mo2S3,57 and Bi2S3),58 nitrides (such as g-C3N4,59 TiN,42 and MoN/Mo2N),60 and other novel semiconductors (such as MXene53,61–66 and CZTSe)67,68 have also shown up in recent research progress, mostly with reasonable evaporation rates between 1.4 and 4 kg m−2 h−1 and energy conversion efficiencies of >80%. In addition, liquid metals, such as gallium and indium alloy (EGaIn) with a Ga2O3 (broad bandgap) layer on the surface to prevent the absorption of sunlight, can also be applied as solar absorbers, introducing a dipole layer to narrow the bandgap.69
Common plasmonic nanoparticles used as solar absorbers include Ag,73–76 Au,77 CuS,78–81 Cu,44,82 and so on. Recently, Wang et al.26 reported novel plasmonic Cu7S4–MoS2–Au composite nanoparticles (CMA NPs) with broad absorption and high photothermal conversion efficiency, which arises from the coupling effect among Cu7S4, MoS2 and Au, as well as the comprehensive utilization of their advantages (Fig. 3a and b). The EDS mappings (Fig. 3c) show the uniform distribution and composition of Au, S, Mo and Cu elements. It is found that the visible absorption of Au (LSPR peak at ∼520 nm) and near-infrared absorption of Cu7S4 (LSPR peak at ∼1500 nm) were successfully integrated with the LSPR absorption of CMA NPs. Compared with Cu7S4–Au, MoS2 can further increase the absorption of CMA NPs. What's more, the intense plasmon–exciton coupling between Au and MoS2 was accounted for the redshift in their absorption spectrum. Based on the above findings, it can be concluded that the synergy between these three nanoparticles successfully expands the absorption spectrum and enhances absorption in the infrared region, which matches well with the solar irradiation spectrum (Fig. 3e). When the CMA NPs are embalmed into the polydimethylsiloxane (PDMS) matrix, the entire gel material shows enhanced absorption capacity and low reflection (Fig. 3f), leading to an excellent evaporation rate (3.824 kg m−2 h−1) and energy conversion efficiency (96.6%).
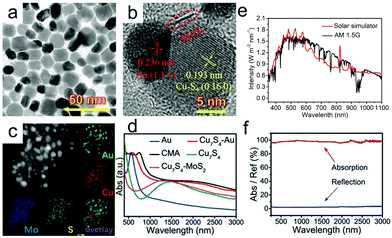 | ||
| Fig. 3 (a) TEM, (b) HRTEM and (c) HAADF-STEM images as well as corresponding elemental mapping of Cu7S4–MoS2–Au nanoparticles. (d) Absorption spectra of different nanoparticles including Au, Cu7S4, Cu7S4–Au, Cu7S4–MoS2 and CMA NPs. (e) The spectral irradiance density of the solar simulator used in the test and air mass 1.5 global (AM1.5G) tilt solar spectrum. (f) UV-Vis-NIR absorption and reflection spectra of CMA/PDMS hydrogel measured with an integrated sphere. Reproduced with permission.26 Copyright 2020, John Wiley and Sons. | ||
As for amorphous carbon, the broad light absorption ability comes from the continuous energy levels emanating from the hybrid bonds.131 To address the high reflective energy loss and thus improve their light-harvesting efficiency,39 considerable efforts have been made in tailoring surface topography,83 constructing internal channels,84,86 and introducing a second phase.27,34 Among these amorphous carbons, activated carbon and carbon black are low cost, readily available materials, and can be easily mass-produced. It is reported that carbon black can be treated with a concentrated HNO3 solution to improve its hydrophilicity and dispersibility in water.96 Considering the simple processing method and low cost of HNO3, carbon black can be arguably considered a low-cost material. Combining with a reasonable light path and design, the evaporation rate of these materials can reach 3.86 kg m−2 h−1, and the energy conversion efficiency is as high as 92%.84 Using biochar or polymer as the precursor can reduce fabrication costs91 or make the derived carbide more design-oriented.35
Different from amorphous carbon, the photothermal conversion effect of crystalline carbon (graphene and highly graphitized carbon materials) arises from the conjugated π bonds. The large number of conjugated π bonds allows electrons to be excited at almost every wavelength of the solar spectrum, resulting in various π–π* transitions. The excited electrons relax through electron–phonon coupling, so energy is transferred from the excited electrons to the vibration mode of the entire atomic lattice, which causes the macroscopic temperature of the material to increase.33 It is noted that a solar water purification system containing reduced graphene oxide (rGO) generally exhibits a relatively high evaporation rate (2.33–2.72 kg m−2 h−1) and higher energy conversion efficiency (>90%) compared to the systems based on graphene and graphene oxide (GO).59,63,74,77,102–119 This result may be attributed to the residual functional groups (i.e. epoxy, hydroxyl) on the surface of rGO, which can be further investigated.
As the youngest carbon nanomaterials, carbon dots (CDs)132–134 have attracted attention due to their broad light absorption spectrum (200–800 nm) and high photothermal conversion efficiency (>90%), so that CDs can meet the requirements of solar absorbers.135 To further improve the performance of CDs for solar water purification, future development directions include structure adjustment and surface group modification without lowering crystallinity or wetting properties.
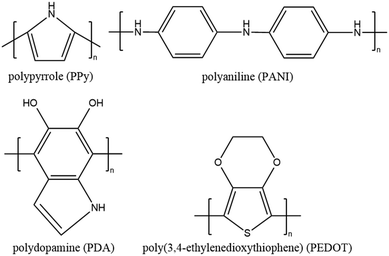 | ||
| Fig. 4 Chemical structures of PPy, PANI, PDA and PEDOT with π-conjugated backbones of sp2-hybridized carbon. | ||
A representative polymer solar absorber is polypyrrole (PPy), which has been reported in recent studies.25,75,130,137–148 For instance, Zhao et al.25 introduced PPy into a PVA-based hydrogel network and achieved a high evaporation rate of 3.2 kg m−2 h−1 with an excellent energy conversion efficiency of 94%. In the next year, the same group reported a PPy/chitosan/PVA hydrogel platform that exhibited an enhanced solar vapor generation capacity of 3.6 kg m−2 h−1.137 Recent research on conjugated polymer solar absorbers has been extended to polyaniline (PANI),149,150 poly(3,4-ethylene dioxythiophene) (PEDOT),151,152 polydopamine (PDA),66,153–156 perovskite hole layer material DPP-2T,157 and covalent organic frameworks (COFs).122,158 Furthermore, conjugated copolymer materials such as poly(aniline-co-pyrrole)34 have also shown up in recent research, proving a novel approach for solar water purification by combining the advantages of two polymers.
2.2 Surface topography modification
The most effective strategy to enhance the light-harvesting capacity of the hydrogel platforms is to tailor surface topography to reduce light reflection.69,78,83,149,159,160 Some materials78,149 are porous, so rough surfaces contribute to higher light-harvesting ability. Recently, researchers have proposed novel design methods to tailor undulating surfaces, including mold embossing,160in situ template-assisted fabrication,83 and external magnetic field-assisted methods.159Mold embossing is an ordinary method to produce hydrogels with corresponding patterns, but the cost of processing the mold is high and it is troublesome to make modifications to the mold. As a result, it is more suitable for subsequent confirmed mass production. Guo et al. first reported the in situ template-assisted fabrication method.83 They used air, glass and a specific solvent (pentanol) as templates to produce a grooved surface hydrogel (G-SH), a flat surface hydrogel (F-SH) and a sharply dimpled surface hydrogel (D-SH), respectively. The commercially available AC paper was used as the substrate and solar absorber, and a transparent PVA hydrogel layer was then prepared on the substrate to prepare a composite solar steam generator. As seen from the SEM images, G-SH has a rough surface with shallow holes (Fig. 5A and B), but a higher magnification image (Fig. 5C) reveals a relatively smooth sheet structure; F-SH exhibits a reasonably smooth outermost surface that is decorated with very shallow holes (Fig. 5D) and shows a similar sheet-like structure at higher magnification (Fig. 5E and F). For the D-SH sample, distinct “canyons” could be seen, resulting in a strongly dimpled surface topography (Fig. 5G). The porous structures are strewn through the canyon clusters (Fig. 5H). Furthermore, D-SH has nanoscale pores nested in its microsized porous structure (Fig. 5I), which neither G-SH nor F-SH has. D-SH has the highest RMS roughness with a surface area about 5 times that of its shadow area (Fig. 5K). As a result, D-SH also shows super-oleophobicity (Fig. 5J, OCA ≥ 150°), indicating that the super-wettability of PVA can be further tweaked by increasing the surface roughness. Due to the roughest surface, the most significant evaporation interface and the highest hydrophilicity, D-SH displays the best SVG performance (Fig. 5L and M) with a water evaporation rate of 2.6 kg m−2 h−1 and an energy conversion efficiency of ca. 91%.
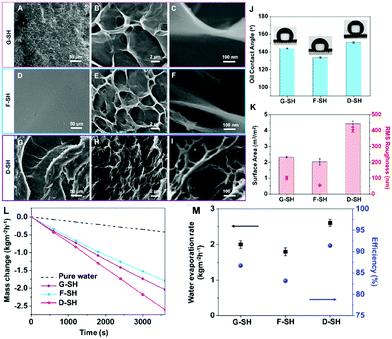 | ||
| Fig. 5 SEM images of different surface topography: (A–C) G-SH, (D–F) F-SH and (G–I) D-SH. (J) The underwater oil (1,2-dichloroethane) contact angle of each modified surface. (K) The optical profilometer test results revealed the surface area and root-mean-square (RMS) roughness of each sample. (L) The water mass changes, (M) water evaporation rates and energy conversion efficiencies of different samples under 1 sun. Reproduced with permission.83 Copyright 2019, American Chemical Society. | ||
The other novel surface modification method is only effective for solar absorber particles with magnetism, such as the Ni@RF@SiO2 core–shell nanoparticles in Yang et al.'s work.159 The nickel in the center of the core–shell structure is the source of magnetism (Fig. 6g). The method primarily involves applying an external magnetic field to the magnetic solar absorber in order to adjust its distribution and convexity on the polymer surface, thus altering the surface morphology. In Fig. 6, M-20, M-30 and M-40 represent composite membranes with particle concentrations of 20, 30, and 40 wt%, respectively. From the SEM images (Fig. 6a–d and h–k), the number and height of surface microstructures increase in proportion to the strength of the magnetic field and the population of magnetic particles. As a result, M-40 has the roughest surface due to the highest external magnetic field and highest solar absorber concentration, showing the best solar-driven steam generation performance of 2.25 kg m−2 h−1 at 95% energy conversion efficiency (Fig. 6e and f).
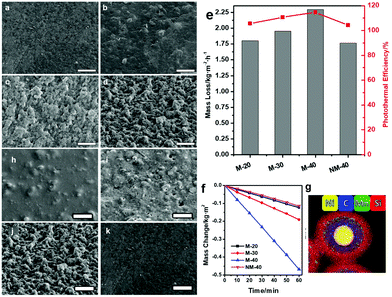 | ||
| Fig. 6 SEM images of the Ni40C20/PVA composite film, with (a) 0 mT, (b) 100 mT, (c) 200 mT and (d) 400 mT magnetic field applied during the gelation process and the SEM images of (h) M-20, (i) M-30, (j) M-40 and (k) NM-40 (scale bar = 50 μm). (e) The evaporation rates and energy conversion efficiencies of different films. (f) Water mass changes over time without light illumination at 20 °C on various films. (g) EDX mapping image of Ni@RF@SiO2 core–shell nanoparticles. Reproduced with permission.159 Copyright 2021, John Wiley and Sons. | ||
Increasing the surface roughness of the hydrogel can simultaneously improve the light harvesting competence and the effective evaporation surface area, which is beneficial to increase the production and energy conversion efficiency of the interfacial evaporator device. In contrast, the template-assisted surface construction method is more general, and suitable templates can be used for various polymers for further development, while the method based on an external magnetic field is only ideal for magnetic solar absorbing particles, and has certain limitations.
In addition, 3D printing is a promising technology to construct surface topography. Yuan et al.161 firstly reported a 3D hierarchically porous cellulose/alginate/carbon black hydrogel via direct ink writing (DIW) 3D printing. Although the evaporation performance in this work is not impressive, it proves that 3D printing technique can be used to produce hydrogels with certain surface patterns for solar water purification.
2.3 Reducing heat loss
Aside from selecting the right solar absorber and tailoring surface topography, minimizing heat loss to the atmosphere and/or the bulk water is another effective way to boost solar–thermal conversion efficiency and the overall water purification output. In principle, heat loss occurs in three ways,159 including the thermal radiation of the platform, convection between the platform and the ambient air, and heat conduction from the surface to bulk water, which are all directly linked to the temperature of the platform or bulk water. Therefore, the surface or bulk water temperature is a critical reference value for measuring the heat loss of the platform. Current methods to reduce heat loss primarily rely on changing the distribution of solar absorbers,27,35,73,75,83,100,112,141 and incorporating heat-insulating materials.As for the distribution of solar absorbers, uniform distribution of solar absorbers in the hydrogel matrix can reduce the heat flow inside the entire platform, thereby reducing heat loss compared to an unevenly dispersed system. For instance, the carboxylated CNTs can be uniformly distributed in the PAM matrix, improving energy conversion efficiency.27 However, the even distribution of solar absorbers cannot mitigate heat dissipation to the surrounding environment. Therefore, researchers have designed multi-layered structures with all solar absorbers in a thin top layer to confine the energy around the evaporation interface.73,75,83,100,112,141 On this basis, Guo et al.35 have developed a ‘top-thin-layer’ with magnetic field-driven solar absorbers to further reduce the interface contact heat loss between different layers. In this work, they use a magnet to carry the iron-based metal–organic framework (Fe-MOF) derived solar absorber particles to one side of the PVA/konjac glucomannan (KGM, which also improves the heat insulation capacity) hydrogel to form a layered hybrid hydrogel evaporator (HHE, Fig. 7f). HHE 3 with a KGM/PVA weight ratio of 0.16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 shows the highest evaporation rate (3.2 kg m−2 h−1) at the highest energy conversion efficiency (90%). As seen from Fig. 7a–c, the black solar absorbers are located at the top of HHE 3, which is also confirmed by EDS mapping (Fig. 7d and e). The surface temperature and bulk water temperature reach equilibrium temperatures of 31 and 22 °C, as shown in Fig. 7g. According to the COMSOL simulation results (Fig. 7h), HHE 3 can keep heat close to the evaporation surface to reduce heat loss. This study thus proved its higher energy conversion efficiency compared with the evaporator with evenly distributed solar absorbers. In addition, there are some other methods to confine the solar absorbers on the surface layer, such as using the difference in surface tension between ethanol and water,134 concentrating them on one end of the hydrogel under gravity,82 carbonizing the upper side of the hydrogel,90,162 and so on.
10 shows the highest evaporation rate (3.2 kg m−2 h−1) at the highest energy conversion efficiency (90%). As seen from Fig. 7a–c, the black solar absorbers are located at the top of HHE 3, which is also confirmed by EDS mapping (Fig. 7d and e). The surface temperature and bulk water temperature reach equilibrium temperatures of 31 and 22 °C, as shown in Fig. 7g. According to the COMSOL simulation results (Fig. 7h), HHE 3 can keep heat close to the evaporation surface to reduce heat loss. This study thus proved its higher energy conversion efficiency compared with the evaporator with evenly distributed solar absorbers. In addition, there are some other methods to confine the solar absorbers on the surface layer, such as using the difference in surface tension between ethanol and water,134 concentrating them on one end of the hydrogel under gravity,82 carbonizing the upper side of the hydrogel,90,162 and so on.
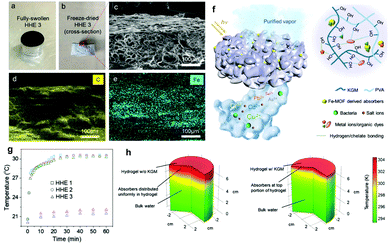 | ||
| Fig. 7 Spatial distribution of absorbers in HHE 3. Photographs of (a) fully swollen HHE 3; (b) cross-section of freeze-dried HHE 3; (c) SEM image of the top portion of HHE 3; the corresponding EDS mappings of (d) C and (e) iron elements. (f) Schematic illustration of solar water purification using HHEs. (g) Under 1 sun irradiation, the temperatures at both the hydrogel evaporator surface and in the bulk water. (h) COMSOL simulation results of the temperature distribution of a control sample (pure PVA hydrogel with uniformly distributed absorbers) and HHE 3. Reproduced with permission.35 Copyright 2020, John Wiley and Sons. | ||
For heat management, in addition to using solar absorbers as the heat-generating phase, the other phase in the platform usually exists as heat insulating substances. Hydrogels with high water content have inherent heat insulation ability. To further enhance the overall heat insulation, most studies add a heat insulation layer, such as PSS,103 PS,87,139 PE,78,79 EPE,92 TE125 foam and so on, at the bottom of the evaporation flatform. Yet, recent research incorporated heat confinement components such as specific aerogel spheres34 and hydrophobic associations27 into the hydrogels to lift their overall heat insulation performance. Tan et al.34 controlled the gelation conditions and prepared poly(aniline-co-pyrrole) hollow spheres/PVA hybrid hydrogel with gradient distribution of silica aerogel particles (Fig. 8c). The resultant hydrogel has a hydrophobic rough upper surface and a smooth hydrophilic bottom surface (Fig. 8a and b). The EDS mapping result confirmed the gradient distribution (Fig. 8g–i) and the element's contents analysis in different parts (Fig. 8j). As a result, the upper part of the hydrogel has a lower thermal conduction (0.2 W m−1 K) than the bottom part (0.54 W m−1 K), resulting in higher temperature variations in the top surface than bulk water (Fig. 8k). Zhang et al.27 used hydrophobic lauryl methyl acrylate (LMA) micelles as cross-linkers to construct hydrophobic associations in a PAM hydrogel. As shown in Fig. 8l, the hydrophobic and hydrophilic phases are coexisting in the hydrogel, resulting in isolated micropores. If the temperature of the hydrogel increases, the interaction between molecular chains and water molecules weakens, resulting in a decrease in the water content of hydrogel. Due to the higher temperature at the top of the hydrogel, there is less water around the evaporation interface (Fig. 8m), reducing heat loss from the top surface caused by excess water. A control hydrogel sample was prepared to demonstrate this speciality by omitting the hydrophobic association (C-CAH). This sample showed slower shrinkage and a larger evaporation surface area than CAH (Fig. 8n). As a result, with less heat loss, the evaporation rate of hydrophobic association hydrogel is higher than that of C-CAHs (Fig. 8o). More recently, Wu et al.163 reported a multi-layer spherical evaporator composed of a PS core and multi-layer outer coatings (PS–cellulose–PDA–PPy–PDA). This design reduces its density and restricts water transport only through the outer hydrophilic layers. Only a thin polymer coating can come in contact with the water, thereby limiting heat loss from the evaporation surface to the bulk water. In all, hydrophobic ingredients have been proven to be thermal confinement components to reduce heat loss.
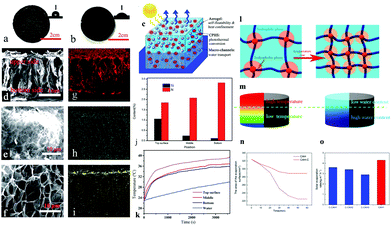 | ||
| Fig. 8 Photos of the (a) upper and (b) bottom surface of the CPHS/aerogel functionalized hydrogel; inset is the contact angle image. (c) Solar steam generation schematic based on functional hybrid hydrogels. SEM images of (d) cross-section of the hydrogel, (e) upper and (f) bottom surface of the hydrogel; and EDS mapping of (g) C, (h) N and (i) Si. (j) Si and N contents of different parts from EDS. (k) Under 1 sun, temperature–time plots of various sections of the hydrogel and bulk water. Reproduced with permission.34 Copyright 2019, Royal Society of Chemistry. Schematic illustration of (l) the composition of a hydrophobic association hydrogel's microphase separation and the structural change caused by temperature; and (m) the correlation between the temperature distribution and the water content in the hydrophobic association hydrogel. (n) Changes in the CAH's and C-CAH's evaporation surface area. (o) C-CAHs’ evaporation rates. Reproduced with permission.27 Copyright 2020, American Chemical Society. | ||
3. Enhancing water transport/activation capacity
Water management, including water transport and water activation, is another critical process that determines the overall performance of solar water purification. Fast water transport speed can ensure continuous water supply to the evaporation interface. Adjusting the wettability and tuning the internal water transport channels are the two main design principles to enhance water transport. In addition, the capacity of the platform to promote water activation is essential to decrease the water vaporization enthalpy to improve the energy conversion efficiency and the evaporation rate (see eqn (1) in Section 2).3.1 Adjusting the wettability
A practical method is to reduce the surface wettability to improve the water transport competence. Examples include using hydrophobic polydimethylsiloxane (PDMS) as the top layer,123 adding hydrophobic Si aerogels mainly in the upper part,34,104,164 selectively decorating the upper surface with PFOTS162 and reducing hydrophilic GO into hydrophobic rGO at the top side via laser.165 Furthermore, Guo et al.36 have reported a patchy surface with hydrophobic and hydrophilic parts for higher water transport speed and excellent SVG performance (4 kg m−2 h−1 with 93% energy conversion efficiency Fig. 9). The hydrogel has a partial trichloro(octadecyl)silane (OTS) modified surface, with the island-shaped patches being hydrophobic and the rest being hydrophilic (Fig. 9a). The thickness of the water film increased as a large portion of the water is confined in the hydrophilic region; thus, the effect of hydrogel surface on the outmost water molecules is decreased, resulting in faster evaporation (Fig. 9b). The optical images and increasing water contact angles confirm the successful OTS modification of the surface of the hydrogels (Fig. 9d–k). Moreover, the introduction of hydrophobic components into the hydrogels can lead to the formation of internal gaps, thus ensuring rapid water replenishment.27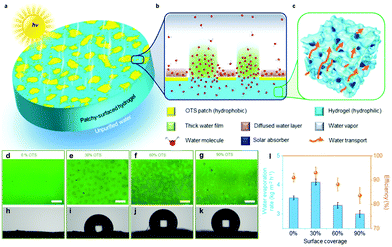 | ||
| Fig. 9 (a–c) Schematic illustration of patchy-surface hydrogels (PSHs). (d–k) Optical images and contact angles of PSHs with (d and h) 0%, (e and i) 30%, (f and j) 60%, and (g and k) 90% OTS covered surfaces. The scale bars are all 20 mm. (l) The evaporation rates and corresponding energy conversion efficiencies of PSH under 1 sun. Reproduced with permission.36 Copyright 2020, Royal Society of Chemistry. | ||
3.2 Tuning internal water channels
The internal water channel is a vital channel for rapid water transport that can be constructed from three perspectives: size,53,86,116 direction,48,64,99,124,166 and structure167 to enhance the pumping force and/or capillary effect.To tune the size of the water channel, Liang et al. have put forward a simple and fast construction method via fermentation. However, the evaporation rate is not high enough (1.611 kg m−2 h−1) due to the large pores formed (900–2540 μm).86 Li et al.53 used the ice-template method to narrow the size of the inner channel to the submicrometer range, inducing a strong capillary force to achieve rapid water transport over a long distance. The pore structures and size distribution are shown in Fig. 10a–e, and the highest evaporation rate is 6.35 kg m−2 h−1 over a long transport distance of 8 cm (Fig. 10f). In addition to the uniform size channel, Meng et al.116 proposed an N-doped rGO aerogel with ring-like gradient vertically aligned microchannels as shown in Fig. 10g.
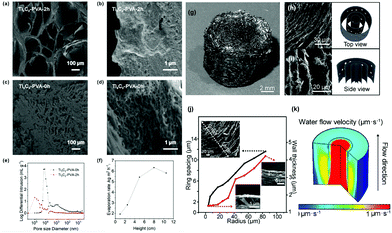 | ||
| Fig. 10 (a–d) SEM images of Ti3C2–PVA-2 h and Ti3C2–PVA-0 h. (e) Pore size distribution. (f) The evaporation rates and the corresponding maximum water transport height. Reproduced with permission.53 Copyright 2020, American Chemical Society. (g) Photo of the N-RGO aerogel with a ring-like architecture. The SEM images and corresponding schemes from (h) the top and (i) side views. (j) From the middle to the edge, the wall thickness and spacing increase. (k) Simulation of the ring-like aerogel's water flow velocity with gradient microchannels, suggesting concentrated pumping in the middle. Reproduced with permission.116 Copyright 2020, American Chemical Society. | ||
From Fig. 10h–j, it is found that the spacing and wall thickness increase from the center to the edge, and a specific channel size distribution is realized by introducing a concentration gradient of NH4OH (as antifreeze) before freeze-drying. The gradient channel size distribution can ensure concentrated pumping in the center, as reflected by the simulation results in Fig. 10k, leading to a high evaporation rate of 2.53 kg m−2 h−1.
Inspired by the water transport in trees in nature, vertically aligned internal channels have the potential to quickly transport water from the bottom of the evaporation generator to the top and release vapor quickly (Fig. 11a). The direction freezing method64,99 is usually used to construct vertically aligned channels. For example, Yu et al.64 prepared a PVA/MXene hydrogel with wood-like vertically aligned channels using the above method (Fig. 11b). The hydrogel with vertically aligned channels (TIH3) represents a higher water mass change than that of bulk water, MXene membrane (MM) and non-tree-inspired hydrogel (n-TIH3) (Fig. 11c). As a result, TIH3 shows a high evaporation rate of 2.71 kg m−2 h−1 at 90.7% energy conversion efficiency as a vapor generator in solar water purification. Besides, the tortuosity of the internal channels can be adjusted by introducing chitosan (CTS) content in the hydrogels.48,166 With a higher content of CTS, lower-tortuosity channels can be achieved to speed the water transport as well as increase the evaporation rate and energy conversion efficiency (Fig. 11d). Xu et al. have proposed a radial ice-template method (Fig. 11g) to achieve radial vertically-aligned channels, which are more similar to the internal channels of a tree (Fig. 11e).124 The aerogel with radial vertically-aligned channels shows stronger water transport capacity over a longer distance than others (Fig. 11h), but it displays a medium SVG performance with an evaporation rate of 2 kg m−2 h−1 at 85.7% energy conversion efficiency (Fig. 11i). This is relatively lower than that of the hydrogels with vertically aligned channels.
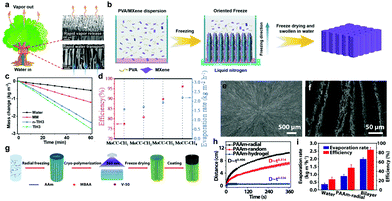 | ||
| Fig. 11 (a) Schematic illustration of the water transport in tree from root to the top with the SEM images of the top and bottom of wood. (b) Schematic illustration of the direction freezing method. (c) Water mass loss for pure water, MM, n-TIH3, and TIH3 under 1 sun. Reproduced with permission.64 Copyright 2020, John Wiley and Sons. (d) Evaporation rates and energy conversion efficiencies of MoCC-CHs. Reproduced with permission.166 Copyright 2020, American Chemical Society. (e and f) SEM images of radially aligned channels and micropores. (g) Schematic illustration of the radial freezing method. (h) Water transport distance over time. (i) Water evaporation rate and energy conversion efficiency of PAAm-based aerogels with different channel directions. Reproduced with permission.124 Copyright 2019, American Chemical Society. | ||
Gradient capillarity has been proved to facilitate strong direction-limited water transport (Fig. 12b) in hydrogels. Liang et al.167 developed an effective fabrication method based on the G-T template to fabricate gradient structure hydrogels (Fig. 12a), in which G represents glass while T represents polytetrafluoroethylene (PTFE). From Fig. 12b, it is found that between the top and bottom of the gradient-structured hydrogel, the sizes of the channel is changing continuously. The improved capillary pumping through the channels from the bottom in water to the surface in air results in rapid, one-way water replenishment, allowing for a high evaporation rate (1.684 kg m−2 h−1 at 93.4% energy conversion efficiency) and less heat loss. The optical photos and SEM images of the G side and T side reveal a considerable difference in the size of the different side channels (Fig. 12c–e).
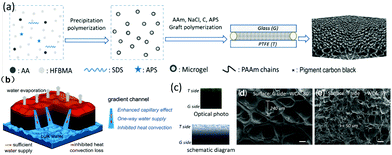 | ||
| Fig. 12 (a) Fabrication process of a gradient structured hydrogel via the G–T template method. (b) Schematic illustration of gradient structured hydrogel-based solar vapor generation. (c) Optical photo and schematic diagram of gradient structure. SEM images of the (d) G and (e) T sides. Reproduced with permission.167 Copyright 2020, Elsevier. | ||
3.3 Water activation
Specific water states in polymer hydrogels can make the water more active, thereby facilitating the evaporation process. Three forms of water have been identified in the hydrated polymer network, including bond water (BW, dark blue color area in Fig. 13a and b) with water–polymer bonding, free water (FW, light blue color area) with water–water bonding, and intermediate water (IW, yellow color area) with weakened water–polymer and water–water bonding between BW and FW. IW is the key water state which requires less energy to escape from the adjacent molecules to decrease the water evaporation enthalpy, realizing higher energy conversion efficiency (according to eqn (1)). Therefore, in order to reduce the energy demand for water evaporation from the hydrogel, it is desirable to tune the building blocks to achieve a higher amount of IW within the hydrogels.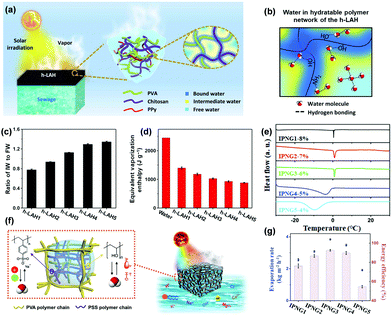 | ||
| Fig. 13 (a) Schematic illustration of solar vapor generation based on h-LAH. (b) Water state in h-LAH. (c) The IW/FW ratio in h-LAHs. (d) The equivalent water vaporization enthalpy of bulk water and the water in h-LAHs. Reproduced with permission.137 Copyright 2019, American Association for the Advancement of Science. (e) The melting activity of IW in various frozen IPNGs as depicted by DSC curves. (f) Solar water purification with controlled hydration based on IPNG. (g) IPNGs’ solar evaporation rate and energy conversion efficiency under 1 sun. Reproduced with permission.84 Copyright 2020, John Wiley and Sons. | ||
Considerable efforts have been devoted to tuning the IW content in hydrogel networks, including the adjustment of polymer concentration, the introduction of hydrophilic functional groups (–OH, –COOH, –SO3H, –NH2 and –CONH–, to form noncovalent interaction), the change of cross-linking density, and the construction of porous structures.168 For example, Zhou et al.137 tuned the PVA/CTS ratios from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 1
0 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 to form hydrogels, namely h-LAH1-5. Their IW/FW ratios increased, and the equivalent vaporization enthalpy decreased correspondingly, as shown in Fig. 13c and d. However, the evaporation enthalpy is not the only factor that can influence the water evaporation performance. h-LAH5, which has the lowest vaporization enthalpy, still has a high water content, which hinders effective energy utilization and restricts evaporation rate. After balancing these two factors, h-LAH4 with a relatively higher water vaporization enthalpy and relatively lower water content shows the highest evaporation rate of 3.6 kg m−2 h−1 and the best energy conversion efficiency of 92%.
0.25 to form hydrogels, namely h-LAH1-5. Their IW/FW ratios increased, and the equivalent vaporization enthalpy decreased correspondingly, as shown in Fig. 13c and d. However, the evaporation enthalpy is not the only factor that can influence the water evaporation performance. h-LAH5, which has the lowest vaporization enthalpy, still has a high water content, which hinders effective energy utilization and restricts evaporation rate. After balancing these two factors, h-LAH4 with a relatively higher water vaporization enthalpy and relatively lower water content shows the highest evaporation rate of 3.6 kg m−2 h−1 and the best energy conversion efficiency of 92%.
In addition, ionic polymers have electrostatic interaction with water molecules, stronger than hydrogen bonding between polymer chains and water molecules. Zhou et al.84 incorporated polystyrene sulfonate (PSS), which can be hydrated with water via electrostatic interaction and hydrogen bonding, into the PVA networks to form a semi-interpenetrating polymer network gel (IPNG, Fig. 13f). IPNG1-5 have different PSS/PVA ratios of 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1.5
1, 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, and their IW/FW ratios can be calculated from the differential scanning calorimetry (DSC) curves (Fig. 13e). It is found that with the increase of the PSS content, the ratio of IW/FW increases in response, mainly due to the increase of IW. However, when the IW content reaches the maximum limit, the ratio will drop simply because FW content will continue to increase. As a result, IPNG3 with a PSS/PVA ratio of 1.5
0, and their IW/FW ratios can be calculated from the differential scanning calorimetry (DSC) curves (Fig. 13e). It is found that with the increase of the PSS content, the ratio of IW/FW increases in response, mainly due to the increase of IW. However, when the IW content reaches the maximum limit, the ratio will drop simply because FW content will continue to increase. As a result, IPNG3 with a PSS/PVA ratio of 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 shows the highest IW/FW ratio and the highest evaporation rate (3.86 kg m−2 h−1) with an energy conversion efficiency of 92% (Fig. 13g).
1 shows the highest IW/FW ratio and the highest evaporation rate (3.86 kg m−2 h−1) with an energy conversion efficiency of 92% (Fig. 13g).
In addition to the aforementioned methods, some recent research studies also use polyelectrolyte-based hydrogels as well as some other strategies to implement strong ionic pumping,85 construct an overall water management system56,153,169 and stimulate water transport and evaporation.
4. Future research roadmap
Based on these main design principles, the solar water purification system still has many areas that can be further improved and strengthened to cope with practical water purification issues.First of all, as shown in Fig. 14 and Table 1, it is still a challenge to achieve a reasonably high evaporation rate and energy conversion efficiency at the same time (for fair comparison, studies with energy conversion efficiency >100% are not included). Taking solar water purification with CNTs as solar absorbers as an example, point B126 represents the two-layered biomimetic CNTs/bacterial cellulose hydrogel reported by Guan et al. It shows a high evaporation rate close to 3 kg m−2 h−1, but the energy conversion efficiency is only about 80%. In contrast, Zhou et al. (point C)127 proposed a 3D pillared CNTs/PAM hydrogel with selected hydrophobic modification, which has a relatively high energy conversion efficiency (96%), yet a low evaporation rate (1.42 kg m−2 h−1). In a separate design strategy, most materials only have good performance in limited aspects, but the coordinated design in multiple aspects can bring better and more comprehensive performance. For instance, in a recent study of carboxyl CNTs/PAM composite hydrogels reported by Zhang et al.27 (point A), the resulting system exhibited the highest evaporation rate (5.1 kg m−2 h−1) as well as excellent energy conversion efficiency (97%). This result can be attributed to the use of hydrophobic association in the skeleton and double-layer structure of carbon nanotubes only in the top layer to achieve lower energy loss and ultra-fast water replenishment.
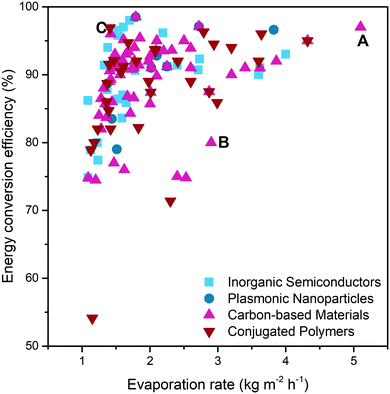 | ||
| Fig. 14 The diagram summarises the solar water purification performance of different systems. Data acquired from ref. 24–27, 34–36, 42–45, 48–69, 73–130, 132–134, 137–153, 155–160, 162–167 and 169–184 (all the studies in this figure are under the solar intensity of 1 sun; studies with energy conversion efficiency >100% are not included for fair comparison). | ||
| Design strategies | Material | Solar intensity (sun) | Evaporation rate (kg m−2 h−1) | Energy conversion efficiency | Ref. | |
|---|---|---|---|---|---|---|
| Solar–thermal conversion enhancement | Surface topography modification | 2-Layered hydrogel with PVA surface modified hydrogel/AC paper | 1 | 2.6 | 91.00% | 83 |
| CuS/Macroporous PAM | 1 | 1.46 | 92% (87.5% after 50 cycles) | 78 | ||
| Ni@C@SiO2 core–shell NPs/PVA hydrogel composite film | 1 | 2.25 | 91.20% | 159 | ||
| STA–EGaIn/lignin–CNC aerogel (SLC aerogel) with inverted pyramid topography | 1 | 1.38 | ∼94% | 69 | ||
| Flexible carbon cloth nanocomposite with a biomimetic Pelargonium hortorum-petal-like surface | 1 | 1.484 | 93.00% | 89 | ||
| Ti3C2Tx MXene/rGO-embedded PVA hydrogel | 1 | 3.62 | 91% | 160 | ||
| Reducing heat loss | Macroporous PEGDA-PANi double-network hydrogel | 1 | 1.4 | 91.50% | 149 | |
| Highly vertically ordered pillar array of graphene-assembled framework | 1 | 2.1 | 95.00% | 103 | ||
| Carbonized wood/EPE foam/hydrophilic airlaid paper | 1 | 1.45 | 91.30% | 92 | ||
| Melamine-derived carbon sponges | 1 | 1.98 | 92.00% | 87 | ||
| Mesoporous cellulose/TiO2/SiO2/TiN hydrogel | 1 | 1.235 | 77.39% | 42 | ||
| Cu/C/SiO2/TiO2 hollow microparticles with a thin shell | 1 | 1.5 | 92.20% | 44 | ||
| CNT/cellulose nanocrystal nanocomposite and PDMS sponge | 1 | 2.01 | 87.40% | 125 | ||
| KGM/Fe-MOFdAPs/PVA hybrid hydrogel | 1 | 3.2 | 90.00% | 35 | ||
| 2-Layered CNTs/PAM hydrogel | 1 | 5.1 | 97.00% | 27 | ||
| PIC/PANI hierarchically porous hydrogel | 1 | 2.79 | 96.30% | 150 | ||
| CNTs/BC hydrogel with glass bubbles/BC hydrogel/wood substrate | 1 | 2.9 | 80.00% | 126 | ||
| Carbonized loofah sponge and alkalized loofah sponge | 1 | 1.36 | 83.70% | 93 | ||
| Multilayered PDMS/CuO/Cu foil/PAM hydrogel | 1 | 1.33 | 91.40% | 51 | ||
| CuS/Bacterial cellulose (BC) hybrid gel membranes | 1 | 1.79 | 98.50% | 79 | ||
| BTA-Doped PPy hydrogel | 1 | 1.98 | 89.00% | 139 | ||
| PS–Cellulose–PDA–PPy–PDA sphere | 1 | 2.6 | 163 | |||
| Assembling CDs on vertically aligned acetate fibers | 1 | 2.6 | 93.90% | 134 | ||
| Cu/Carbon cell/PVA hydrogel | 1 | 2.08 | 93.43% | 82 | ||
| Layered graphene/polymethylmethacrylate composited membrane by electrospinning | 1 | 1.25 | ∼84% | 121 | ||
| PP–PDA/KH550/TiO2 membrane with PPy on the outer surface | 1 | 3.65 | 229.13% | 148 | ||
| Enhancing water transport/activation capacity | Adjusting the wettability | Poly(anilineco-pyrrole) hollow spheres/Si aerogel microparticles/PVA | 1 | 1.83 | 82.20% | 34 |
| GO/Si aerogel/PAM/PVA composite hydrogel | 2 | 2.696 | 104 | |||
| PVA/OTS/Ti2O3 patchy-surface hydrogel | 1 | 4 | 93.00% | 36 | ||
| rGO/Ag/sodium alginate (SA) hydrogel supported by a PU sponge | 1 | 2.02 | 91.00% | 74 | ||
| 2-Layered CNTs/PAM hydrogel | 1 | 5.1 | 97.00% | 27 | ||
| Squid ink NPs/SA/PVA/PAM floatable composite hydrogel | 4 | 2.3 | 71.38% | 164 | ||
| 2-Layered hydrogel with MWCNTs-COOH/PVA hydrogel as the bottom layer and hydrophobic PDMS as the top layer | 1 | 1.34 | 85.71% | 123 | ||
| BTA-Doped PPy hydrogel | 1 | 1.98 | 89.00% | 139 | ||
| Suedette sponge/filter paper/polydopamine | 1 | 1.8 | 92.00% | 153 | ||
| Janus monolithic chitosan scaffold (CS) aerogel with carbonized surface | 1 | 1.76 | 91% | 90 | ||
| PVA/rGO hydrogel, MFfoam and β-chitin | 1 | 1.6 | 113 | |||
| PPy/PVA-F Janus aerogel with a rough hydrophobic upper layer and a smooth hydrophilic bottom layer | 1 | 1.68 | 94.7% | 162 | ||
| Tuning internal water channels | MWCNTs/PAAm aerogel | 1 | 2 | 85.70% | 124 | |
| Ti3C2 or MoS2/PVA | 1 | 6.35 | 53 | |||
| Vertically aligned rGO/Ti3C2Tx MXene hybrid hydrogel | 1 | 2.09 | 93.50% | 63 | ||
| AC/Fermentation porous PVA hydrogel | 1 | 1.611 | 95.15% | 86 | ||
| Gradient structured C/polyacrylamide (C/PAAm) composite hydrogel | 1 | 1.684 | 93.40% | 167 | ||
| Molybdenum carbide/carbon-based chitosan hydrogel | 1 | 2.19 | 96.15% | 166 | ||
| Ti3C2Tx/PVA tree-inspired hydrogel | 1 | 2.71 | 90.70% | 64 | ||
| Porous N-doped rGO with gradient microchannel 3D aerogel | 1 | 2.53 | 74.80% | 116 | ||
| Porous Au–N-doped rGO with gradient microchannel 3D aerogel | 1 | 2.72 | 97.10% | 77 | ||
| ACET doped PAM/SA-LN(25) dual-crosslinked hydrogel | 1 | 1.64483 | 93.00% | 99 | ||
| MnO2 nanowires/chitosan hydrogel | 1 | 1.78 | 90.60% | 48 | ||
| Water activation | PVA/PPy hierarchically nanostructured hydrogel | 1 | 3.2 | 94.00% | 25 | |
| PVA/rGO hybrid hydrophilic hydrogel | 1 | 2.5 | 95.00% | 102 | ||
| PPy/PVA/CTS hydratable hydrogel | 1 | 3.6 | 92.00% | 137 | ||
| AC foam/P(SA) polyelectrolyte foam hydrogel | 1 | 1.3 | 85 | |||
| AC/PVA/PSS interpenetrating hydrogel | 1 | 3.86 | 92.00% | 84 | ||
| Sodium alginate/PEDOT/PSS 3D hydrophilic network | 1 | 1.23 | 82.00% | 151 | ||
| Nanofibrous CA/PMAA hydrogel-rGO membrane | 1 | 1.85 | 95.40% | 120 | ||
| 3D printed CB NPs/cellulose matrix | 1 | 3.01 | 170 | |||
| Polyurethane (PU) sponge with black hydrogel skin | 1 | 2.8 | 94.00% | 179 | ||
| Others | PVA/Ti2O3 sponge-like hydrogel | 1 | 3.6 | 90.00% | 24 | |
| C dots/CTS/carboxymethyl cellulose hydrogel | 1 | 1.4 | 89.00% | 133 | ||
| Protonated g-C3N4/graphene hybrid hydrogel | 1 | 1.09 | 74.8% | 59 | ||
| Double-layer Ag–PSS–agarose gel/agarose gel | 1 | 2.1 | 92.80% | 73 | ||
| Mo2C + PVA hydrogel | 1 | 1.59 | 83.60% | 61 | ||
| PVA or PU or melamine and CB NPs | 1 | 2.15 for PVA based | 98 | |||
| rGO/PAM hybrid mushroom-like cryogel | 1 | 1.76 | 86.60% | 110 | ||
| N-Doped graphene/carbon hybrid aerogels | 1 | 1.558 | 90.00% | 111 | ||
| PPy coating pre-pressed MF and pre-pressed MF 2 layered hydrogel | 1 | 1.574 | 90.40% | 141 | ||
| Flame-treated melamine foam (F-MF) assembled with expanding polyethylene (EPE) foam | 1 | 1.18 | 80.00% | 171 | ||
| Large-area polypyrrole chemically functionalized cellulose paper (PPyP) | 1 | 2.99 | 85.89% | 142 | ||
| Ni foam loading Co3O4 with nanoscale superstructures | 1 | 1.226 | 80.00% | 50 | ||
| PPy/FexOy/CTS nanostructured gel membrane | 1 | 1.93 | 143 | |||
| 2-Layered hydrogel with GO aerogel and chitosan/ZnO composite layer | 1 | 1.725 | 90.80% | 112 | ||
| Copper–zinc–tin–selenide nanocarambola assembled membrane | 1 | 1.528 | 86.40% | 67 | ||
| Ethanol-treated carrot biochar | 1 | 2.04 | 127.80% | 94 | ||
| HxMoO3/PNIPAM hydrogel | 1 | 1.65 | 85.87% | 62 | ||
| Hydrophilic acrylamide polymer (resilience)/biochar particles/SDS sponge-like hydrogel | 1 | 1.77 ± 0.05 | 94.00% | 91 | ||
| 2-Layered cellulose-based bottom hydrogel/cellulose-based CB hydrogel | 1 | 1.582 | 91.40% | 100 | ||
| Graphite nanosheets/SiO2/PVA/Fe3O4 floating magnetic hydrogel | 1.196 | 105 | ||||
| 2-Layered hydrogel with PPy/Ag/PMBA-BrIL hydrogel as the top layer and PMBA-BrIL hydrogel as the bottom layer | 1 | 1.37 | 88.70% | 75 | ||
| Squid ink/starch hydrogel | 1 | 2.07 | 93.7% | 173 | ||
| rGO hydrogel membrane | 1 | 2.33 | 106 | |||
| Fe-Mo2S3/sodium alginate starch hydrogel | 1 | 2.4 | 91.40% | 57 | ||
| Modified graphene/coal oxide aerogels | 1 | 1.62 | 76.00% | 114 | ||
| N-Doped maize straw/GO aerogel | 1 | 3.22 | 115 | |||
| G/rGO nanosheets/PVA hydrogel | 1 | 1.44 | 86.00% | 117 | ||
| Plasmonic Cu7S4-MoS2-Au NPs/PDMS | 1 | 3.824 | 96.60% | 26 | ||
| MOS2/PU/hydrophilic PDA | 1 | 1.087 | 86.20% | 54 | ||
| Hollow C nanospheres/porous mixed cellulose ester membrane | 1 | 2 | 92.70% | 174 | ||
| Rgo/KGM 3D porous sponge | 1 | 1.6 | 92.00% | 118 | ||
| Mesoporous CuO | 1 | 83.66% | 52 | |||
| CNTs/PAM hydrogel | 1 | 1.42 | 96.00% | 127 | ||
| Self-floating H1.68MoO3/airlaid paper photothermal film | 1 | 1.37 | 87.80% | 175 | ||
| A spring wrapped with Rgo/SA@cotton towel | 1 | 7.6 | 178.60% | 107 | ||
| Graphene/PS/PVA | 1 | 1.77 | 92.00% | 108 | ||
| rGO/CA hydrogel | 1 | 1.47 | 77.00% | 109 | ||
| Ppy/polyvalent cation crosslinked alginate hydrogel with styrofoam for floating | 1 | 1.15 | 54.12% | 138 | ||
| PPy/PAAM/chitosan gradient structured hydrogel | 1 | 2.41 | 92.00% | 140 | ||
| Bacterial cellulose-based cellulose nanofibril hydrogels + aerogels | 1 | 1.82 (4.32 under light air conditions 4 m s−1) | 95.00% | 97 | ||
| Bi2S3/Pd PAAH (polyacrylamide/polyacrylic acid hydrogel) | 1 | 1.61 | 97.00% | 58 | ||
| GO/PEI/PEG | 1 | 1.32 | 88.00% | 119 | ||
| CB/PVA hydrogel | 1 | 2.4 | 75.00% | 96 | ||
| BiVO4–rGO hydrogel | 1 | 1.6 | 87.00% | 172 | ||
| Ag@carbonized melamin foam/MF | 1 | 2.39 | 119.46% | 76 | ||
| Chitosan/gelatin-based IPN sponge incorporated with melanin-coated titania hollow nanospheres | 1 | 1.13 | 78.90% | 43 | ||
| PPy coating on the spike of Setaria viridis | 1 | 3.72 | 144 | |||
| CuS/BC and BC biofoam | 1 | 1.44 | 83.50% | 80 | ||
| GO–rGO janus membrane | 1 | 1.836 | 91.40% | 165 | ||
| CDs@Cotton fibers | 1 | 2.32 | 93.60% | 132 | ||
| COF/PDMS sponge | 1 | 1.39 | 84.70% | 158 | ||
| Cellulose-reinforced tannin-functionalized aerogel coated with CNTs | 1 | 1.2 | 74.46% | 128 | ||
| Ti3C2Tx (MXene)/La0.5Sr0.5CoO3 (LSC) nanohetero-structures integrated with PVA/CTS hydrogel | 1 | 2.73 | 92.30% | 65 | ||
| Poly(sodium acrylate) (PSA)/PPy cryogels | 1 | 1.41 | 96.90% | 145 | ||
| PVA–PDA network | 1 | 2.94 | 94.50% | 155 | ||
| PVA/PPy hydrogel membrane with micro-tree array | 1 | 3.64 | 96.00% | 146 | ||
| Boron nanosheets modified with MoS2 in a konjac glucomannan (KGM) sponge | 1 | 1.538 | 96.50% | 55 | ||
| Composite hydrogel MoS2@Graphene | 0.9 | 3.2 | 56 | |||
| Nanofibrous PVA based membrane (NPM) with PPy NPs and GO | 1 | 2.87 | 87.50% | 147 | ||
| Chitin membrane with MnO2 or CuO | 1 | 1.526 or 1.531 | 95.77% or 96.08% | 49 | ||
| Polyetherimide (PEI) modified cation exchange resin (CER) and PEDOT based conductive ink (EL-P 3040) | 1 | 1.42 | ∼82% | 152 | ||
| Carbonized aerogels | 1 | 2.1 | 88 | |||
| Black titania/bacterial nanocellulose | 1 | 1.71 ± 0.07 | 84.30 ± 3.40% | 45 | ||
| PDA/Ti3C2Tx MXene in a cellulose network skeleton of delignified wood (DW) | 1 | 2.08 | 93.60% | 66 | ||
| Carbonized corn straw | 1 | 1.422 | 89.30% | 95 | ||
| Copper–zinc–tin–selenide (CZTSe) nanocarambolas deposited on a hydrophilic filter membrane | 1 | 1.528 | 86.40% | 68 | ||
| Ni/C/Si core–shell sphere (SSA-Ni) film | 1 | 1.52 | ∼91% | 176 | ||
| Multi-functional carbon nanotube paper | 1 | 1.28 | ∼82% | 129 | ||
| Versatile carbon hybrid aerogel | 1 | 2.1 | 89.80% | 177 | ||
| Mussel-inspired PDA-filled cellulose aerogel | 1 | 1.36 | 86% | 156 | ||
| A cellulose paper directly with a pencil tracing and coating on surface | 1 | 1.28 | 86.48% | 178 | ||
| Polyacrylamide (PAAm)/carboxymethyl cellulose (CMC)/CuS membrane | 1 | 1.613 | 79% | 81 | ||
| Red mud/PVA/CTS gel | 1 | 2.185 | 90.74% | 180 | ||
| rGO based COF hydrogel | 1 | 1.47 | 92.07% | 122 | ||
| A 3D asymmetric evaporator by coating two CB/PVA functional films with different channel structures and water uptake at two opposite sides | 1 | 1.93 | 101 | |||
| Nanocarbon/PVA | 1 | 1.67 | 86.8% | 181 | ||
| Co–Sn alloy@PTFE film | 1 | 0.76 | 89% | 182 | ||
| Catechol-functionalized chitosan with quinone-anchored AC | 1 | 3.4 | ∼91% | 184 | ||
| CNTs/PPy/PS foam with fluorinated hydrophobic coating on the surface | 1 | 1.61 | 91.20% | 130 | ||
| Semi-coke/PDA@melamine sponge | 1 | 1.41 | 90.56% | 183 | ||
| Zwitterionic hydrogel coated PU//CNTs/PS foam/cotton swabs | 1 | 2.2 | 93.50% | 169 | ||
| DPP-2T/PVA | 1 | 2.6 | 89.00% | 157 | ||
The points representing the hydrogels of carbon-based materials ( ) and conjugated polymers (
) and conjugated polymers ( ) appear in almost all ranges of Fig. 14, which means that in most cases, these two solar absorbers are faced with simultaneously achieving high evaporation rate and energy conversion efficiency. In addition, in the existing research shown in Fig. 14, the performance of carbon-based materials is slightly better than that of conjugated polymers, because most of the points representing carbon-based materials are more concentrated in the upper right corner. Most of the hydrogels that use inorganic semiconductors as solar absorbers (
) appear in almost all ranges of Fig. 14, which means that in most cases, these two solar absorbers are faced with simultaneously achieving high evaporation rate and energy conversion efficiency. In addition, in the existing research shown in Fig. 14, the performance of carbon-based materials is slightly better than that of conjugated polymers, because most of the points representing carbon-based materials are more concentrated in the upper right corner. Most of the hydrogels that use inorganic semiconductors as solar absorbers ( ) appear around point C. This reveals that some inorganic semiconductor-based SVGs have problem with high energy conversion efficiency along with low evaporation rate, which needs to be solved in future research. For plasmonic nanoparticles (
) appear around point C. This reveals that some inorganic semiconductor-based SVGs have problem with high energy conversion efficiency along with low evaporation rate, which needs to be solved in future research. For plasmonic nanoparticles ( ), the energy conversion efficiency and evaporation rate seem to have the same trend. But because there are very few research papers in this field, it is not of valuable reference.
), the energy conversion efficiency and evaporation rate seem to have the same trend. But because there are very few research papers in this field, it is not of valuable reference.
Therefore, on the basis of complying with these design principles, the entire preparation process should be coordinated with each other from the perspectives of chemistry, materials and engineering to maximize the evaporation rate and energy conversion efficiency at the same time.
Since this technique utilizes the most abundant and inexhaustible renewable energy source, which is friendly for developing countries and poverty-stricken areas, lower capital and operating costs will make it more competitive. Using biochar or biomass-derived solar absorbers93–95,173 is a regular cost-reducing strategy because the raw materials can be obtained from nature. A research group even coated PPy on the real Setaria viridis spike to use the plant's own water transport and evaporation-promoting properties to maximize its potential.144 Other studies have reported the use of specific solid waste as solar absorbers, including red mud (residue of the aluminum mining industry),180 semi-coke,183 and pencil graphite.178 Besides, reducing the use of solar absorbers by distributing them only around the evaporation interface35,148 and constructing multilayer-structure with only one layer of them73,75,83,96,100,141 is also effective. Moreover, designing a recyclable solar water purification platform27,105 is another proven solution.
In practical application, better anti-fouling performance can guarantee long-term operation, divided into anti-biofouling and crystalline anti-fouling. The anti-bacterial performance can be achieved by adding CTS.43,112 Guo et al.184 recently attached catechol groups to the CTS hydrogel for hydrogen peroxide (a broad-acting antibacterial agent) generation, combining with the synergistic effect of quinone-modified AC, to enhance the anti-fouling properties and guarantee 3 months of performance maintenance. There are numerous methods to improve the anti-crystalline fouling ability, including the construction of the Janus structure130,165 and the umbrella-shaped platform,91 the use of ions rejection polymer,85,152,169 the introduction of bubbles in the middle section,140 the weight imbalance sensitive spherical platform capable of rotating and renewing the evaporation surface,163 the millineedle array on the surface for site-specific salt crystallization without salt clogging155 and so on. In addition, the self-floating hydrogel platform is out of the external support substrate, which is not conducive to water transport, usually by adding low-density materials (i.e. Si aerogels)34 or preparing a partially hydrophobic bottom layer.140
Drawing inspiration from existing organisms on the planet is another effective strategy to improve solar water purification performance. The most common is to imitate the water absorption of the roots of plants and the water evaporation of the leaves to enhance the water transport process.64,99 Inspired from the porifera in the sea, Wang et al.179 fabricated a hydrogel platform with the sponge as the skeleton and the black hydrogel as the skin, which could be arbitrarily compacted, folded, and twisted as well as remaining stable for more than 6 months in different environments. What's more, imitating the water absorption and release cycle of pufferfish, the inherent lower critical temperature of poly(N-isopropyl acrylamide) (PNIPAm) hydrogel was used for hydrophilic (in dark conditions) and hydrophobic (under sunlight) conversion.154 Additionally, surface biomimetic morphology is also common in studies to enhance light acquisition,69,89 anti-fouling,155 and additional water collection capacity.146
In addition to seawater desalination and wastewater purification, there are still some other particular application scenarios for solar water purification. The recent news that Japan intends to dump nuclear wastewater into the ocean has sparked international concern. Some research groups proposed using solar water purification to purify radioactive wastewater118 and recover radioactive elements, e.g. uranium.122,128,158 Besides, the application of this technology to purify specific wastewater containing nanoparticles,139 acidic,174 alkaline,94 and low-boiling-point contaminants,177 and organic dyes144,156 is also under development. Furthermore, additional functions, such as electricity generation,51,88,101,125,127,129,138,185 fog collection,146,186,187 oil/water separation,147 water disinfection,184 sensing,58 and photo degradability,65,80,188 have also been integrated into solar water purification.
5. Conclusions
In conclusion, recent progress in solar water purification shows that a higher evaporation rate and energy conversion efficiency can be achieved by designing an entire system from two key aspects of heat and water management. As for the heat management, tailoring surface topography to enhance light harvesting, selecting adequate solar absorbers with stronger full solar spectrum absorption (especially the infrared band) and higher photothermal conversion ability, and minimizing heat loss to the atmosphere and bulk water by managing the distribution of solar absorbers and heat insulation components proved to be effective strategies for harvesting sunlight. Concerning water management, adjusting wettability to ensure rapid water replenishment, tuning the size, direction and structure of the internal channel to enhance the channel pumping, and increasing the IW content in hydrogels to lower water vaporization enthalpy are the three main approaches that have been successfully implemented. However, it is still a challenge to maximize the evaporation rate and energy conversion efficiency concurrently, requiring coordination from chemistry, materials, engineering and other aspects. Additionally, lower capital and operating costs, better anti-fouling performance, self-floating capability, biomimetic design and additional integrated functions are the possible future development directions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Qiang Fu acknowledges the Australian Research Council under the Future Fellowship (FT180100312). Shudi Mao acknowledges support from the China Scholarship Council (CSC) Scholarship (202006140015).Notes and references
- D. N. Chakkaravarthy, Int. J. Agric. Environ. Biotechnol., 2019, 12, 187–193 Search PubMed.
- W. W. Immerzeel, L. P. Van Beek and M. F. Bierkens, Science, 2010, 328, 1382–1385 CrossRef CAS PubMed.
- Y. Wada, M. Flörke, N. Hanasaki, S. Eisner, G. Fischer, S. Tramberend, Y. Satoh, M. Van Vliet, P. Yillia and C. Ringler, Geosci. Model Dev., 2016, 9, 175–222 CrossRef.
- C. A. Schlosser, K. Strzepek, X. Gao, C. Fant, É. Blanc, S. Paltsev, H. Jacoby, J. Reilly and A. Gueneau, Earth's Future, 2014, 2, 341–361 CrossRef.
- P. Goh, A. Ismail and N. Hilal, Oxford Research Encyclopedia of Environmental Science, 2019 Search PubMed.
- A. Panagopoulos, K.-J. Haralambous and M. Loizidou, Sci. Total Environ., 2019, 693, 133545 CrossRef CAS PubMed.
- R. Semiat, Environ. Sci. Technol., 2008, 42, 8193–8201 CrossRef CAS PubMed.
- A. D. Khawaji, I. K. Kutubkhanah and J.-M. Wie, Desalination, 2008, 221, 47–69 CrossRef CAS.
- F. A. AlMarzooqi, A. A. Al Ghaferi, I. Saadat and N. Hilal, Desalination, 2014, 342, 3–15 CrossRef CAS.
- R. M. Morris, Desalination, 1993, 93, 57–68 CrossRef CAS.
- H. Bamufleh, F. Abdelhady, H. M. Baaqeel and M. M. El-Halwagi, Desalination, 2017, 408, 110–118 CrossRef CAS.
- A. Al-Karaghouli and L. L. Kazmerski, Renewable Sustainable Energy Rev., 2013, 24, 343–356 CrossRef CAS.
- M. Qasim, M. Badrelzaman, N. N. Darwish, N. A. Darwish and N. Hilal, Desalination, 2019, 459, 59–104 CrossRef CAS.
- H. Strathmann, Desalination, 2010, 264, 268–288 CrossRef CAS.
- J. R. Werber, A. Deshmukh and M. Elimelech, Environ. Sci. Technol. Lett., 2016, 3, 112–120 CrossRef CAS.
- R. Zhao, S. Porada, P. Biesheuvel and A. Van der Wal, Desalination, 2013, 330, 35–41 CrossRef CAS.
- N. Ghaffour, T. M. Missimer and G. L. Amy, Desalination, 2013, 309, 197–207 CrossRef CAS.
- C. Y. Tang, Z. Yang, H. Guo, J. J. Wen, L. D. Nghiem and E. Cornelissen, Environ. Sci. Technol., 2018, 52, 10215–10223 CrossRef CAS PubMed.
- N. A. A. Qasem, S. M. Zubair, B. A. Qureshi and M. M. Generous, Energy Convers. Manage., 2020, 205, 112448 CrossRef CAS.
- S. Al-Amshawee, M. Y. B. M. Yunus, A. A. M. Azoddein, D. G. Hassell, I. H. Dakhil and H. A. Hasan, Chem. – Eng. J., 2020, 380, 122231 CrossRef CAS.
- H. C. Duong, L. Xia, Z. Ma, P. Cooper, W. Ela and L. D. Nghiem, J. Membr. Sci., 2017, 542, 133–142 CrossRef CAS.
- S. Sharshir, G. Peng, L. Wu, F. Essa, A. Kabeel and N. Yang, Appl. Energy, 2017, 191, 358–366 CrossRef CAS.
- Y. Zhang, M. Sivakumar, S. Yang, K. Enever and M. Ramezanianpour, Desalination, 2018, 428, 116–145 CrossRef CAS.
- Y. Guo, X. Zhou, F. Zhao, J. Bae, B. Rosenberger and G. Yu, ACS Nano, 2019, 13, 7913–7919 CrossRef CAS PubMed.
- F. Zhao, X. Zhou, Y. Shi, X. Qian, M. Alexander, X. Zhao, S. Mendez, R. Yang, L. Qu and G. Yu, Nat. Nanotechnol., 2018, 13, 489–495 CrossRef CAS PubMed.
- H. Wang, R. Zhang, D. Yuan, S. Xu and L. Wang, Adv. Funct. Mater., 2020, 30, 2003995 CrossRef CAS.
- X. Zhang, Y. Peng, L. Shi and R. Ran, ACS Sustainable Chem. Eng., 2020, 8, 18114–18125 CrossRef CAS.
- D. Lapotko, Opt. Express, 2009, 17, 2538–2556 CrossRef CAS PubMed.
- Y. Guo, J. Bae, Z. Fang, P. Li, F. Zhao and G. Yu, Chem. Rev., 2020, 120, 7642–7707 CrossRef CAS PubMed.
- X. Zhou, Y. Guo, F. Zhao and G. Yu, Acc. Chem. Res., 2019, 52, 3244–3253 CrossRef CAS PubMed.
- Z. Xu, Z. Li, Y. Jiang, G. Xu, M. Zhu, W.-C. Law, K.-T. Yong, Y. Wang, C. Yang, B. Dong and F. Xing, J. Mater. Chem. A, 2020, 8, 25571 RSC.
- M. Gao, L. Zhu, C. K. Peh and G. W. Ho, Energy Environ. Sci., 2019, 12, 841–864 RSC.
- L. Zhu, M. Gao, C. K. N. Peh and G. W. Ho, Nano Energy, 2019, 57, 507–518 CrossRef CAS.
- M. Tan, J. Wang, W. Song, J. Fang and X. Zhang, J. Mater. Chem. A, 2019, 7, 1244–1251 RSC.
- Y. Guo, H. Lu, F. Zhao, X. Zhou, W. Shi and G. Yu, Adv. Mater., 2020, 32, 1907061 CrossRef CAS PubMed.
- Y. Guo, X. Zhao, F. Zhao, Z. Jiao, X. Zhou and G. Yu, Energy Environ. Sci., 2020, 13, 2087–2095 RSC.
- F. Yu, Y. Chen, X. Liang, J. Xu, C. Lee, Q. Liang, P. Tao and T. Deng, Prog. Nat. Sci.: Mater. Int., 2017, 27, 531–542 CrossRef CAS.
- Y. Zhao and C. Burda, Energy Environ. Sci., 2012, 5, 5564–5576 RSC.
- F. Zhao, Y. Guo, X. Zhou, W. Shi and G. Yu, Nat. Rev. Mater., 2020, 5, 388–401 CrossRef.
- C. M. Hessel, V. P. Pattani, M. Rasch, M. G. Panthani, B. Koo, J. W. Tunnell and B. A. Korgel, Nano Lett., 2011, 11, 2560–2566 CrossRef CAS PubMed.
- A. Standard, Ann. Book ASTM Standards, 2012, 14, 1–20 Search PubMed.
- Z. Sun, Z. Li, W. Li and F. Bian, Cellulose, 2019, 27, 481–491 CrossRef.
- X. Wang, Z. Li, Y. Wu, H. Guo, X. Zhang, Y. Yang, H. Mu and J. Duan, ACS Appl. Mater. Interfaces, 2021, 13, 10902–10915 CrossRef CAS PubMed.
- X. Zhang, X. Wang, W. D. Wu, X. D. Chen and Z. Wu, J. Mater. Chem. A, 2019, 7, 6963–6971 RSC.
- K. Nabeela, M. N. Thorat, S. N. Backer, A. M. Ramachandran, R. T. Thomas, G. Preethikumar, A. P. Mohamed, A. Asok, S. G. Dastager and S. Pillai, ACS Appl. Bio Mater., 2021, 4, 4373–4383 CrossRef CAS PubMed.
- C. Dette, M. A. Pérez-Osorio, C. S. Kley, P. Punke, C. E. Patrick, P. Jacobson, F. Giustino, S. J. Jung and K. Kern, Nano Lett., 2014, 14, 6533–6538 CrossRef CAS PubMed.
- J. Tao, T. Luttrell and M. Batzill, Nat. Chem., 2011, 3, 296–300 CrossRef CAS PubMed.
- M. S. Irshad, X. Wang, M. S. Abbasi, N. Arshad, Z. Chen, Z. Guo, L. Yu, J. Qian, J. You and T. Mei, ACS Sustainable Chem. Eng., 2021, 9, 3887–3900 CrossRef CAS.
- X. Li, J. Huang, L. Guo, X. Jin, L. Wang, Y. Deng, H. Xie and L. Ye, Inorg. Chem. Commun., 2021, 129, 108651 CrossRef CAS.
- P. Wang, Y. Gu, L. Miao, J. Zhou, H. Su, A. Wei, X. Mu, Y. Tian, J. Shi and H. Cai, Sustainable Mater. Technol., 2019, 20, e00106 CrossRef CAS.
- F. L. Meng, M. Gao, T. Ding, G. Yilmaz, W. L. Ong and G. W. Ho, Adv. Funct. Mater., 2020, 30, 2002867 CrossRef CAS.
- H. Zhang, K. Wang, L. Wang, H. Xie and W. Yu, Sol. Energy, 2020, 201, 628–637 CrossRef CAS.
- C. Li, L. Fan, R. Zhu, X. Li, P. Wen, X. Zhao, G. Wang, J. Zou and F. Kim, ACS Appl. Energy Mater., 2020, 3, 9216–9225 CrossRef CAS.
- Q. Wang, F. Jia, A. Huang, Y. Qin, S. Song, Y. Li and M. A. C. Arroyo, Desalination, 2020, 481, 114359 CrossRef CAS.
- J. Yin, X. You, Z. Zhang, Z. Guo, J. Wang and X. Wang, J. Water Process Eng., 2021, 41, 102048 CrossRef.
- Y. Li, X.-l. Shi, L.-j. Sun, M. Zhao, T. Jiang, W. Jiang, M. Deng, S. Yang and Y. Wang, Desalination, 2021, 515, 115192 CrossRef CAS.
- Z. Guo, F. Yu, Z. Chen, Z. Shi, J. Wang and X. Wang, Sol. Energy Mater. Sol. Cells, 2020, 211, 110531 CrossRef CAS.
- X. Geng, D. Zhang, Z. Zheng, G. Ye, S. Li, H. Tu, Y. Wan and P. Yang, Nano Energy, 2021, 82, 105700 CrossRef CAS.
- H. Su, J. Zhou, L. Miao, J. Shi, Y. Gu, P. Wang, Y. Tian, X. Mu, A. Wei, L. Huang, S. Chen and Z. Deng, Sustainable Mater. Technol., 2019, 20, e00095 CrossRef CAS.
- L. Zhu, L. Sun, H. Zhang, D. Yu, H. Aslan, J. Zhao, Z. Li, M. Yu, F. Besenbacher and Y. Sun, Nano Energy, 2019, 57, 842–850 CrossRef CAS.
- F. Yu, X. Ming, Y. Xu, Z. Chen, D. Meng, H. Cheng, Z. Shi, P. Shen and X. Wang, Adv. Mater. Interfaces, 2019, 6, 1901168 CrossRef CAS.
- S. Cao, J. Jiang, Q. Tian, C. Guo, X. Wang, K. Dai and Q. Xu, Green Energy Environ., 2020 DOI:10.1016/j.gee.2020.12.012.
- W. Li, X. Li, W. Chang, J. Wu, P. Liu, J. Wang, X. Yao and Z.-Z. Yu, Nano Res., 2020, 13, 3048–3056 CrossRef CAS.
- Z. Yu and P. Wu, Adv. Mater. Technol., 2020, 5, 2000065 CrossRef CAS.
- D. Fan, Y. Lu, H. Zhang, H. Xu, C. Lu, Y. Tang and X. Yang, Appl. Catal., B, 2021, 295, 120285 CrossRef CAS.
- Y. Chen, J. Yang, L. Zhu, X. Jia, S. Wang, Y. Li and H. Song, J. Mater. Chem. A, 2021, 9, 15482–15492 RSC.
- Y. Yang, W. Que, J. Zhao, Y. Han, M. Ju and X. Yin, Chem. Eng. J., 2019, 373, 955–962 CrossRef CAS.
- L. Yang, N. Li, C. Guo, J. He, S. Wang, L. Qiao, F. Li, L. Yu, M. Wang and X. Xu, Chem. – Eng. J., 2021, 417, 128051 CrossRef CAS.
- Z. Wei, C. Cai, Y. Huang, Y. Wang and Y. Fu, Nano Energy, 2021, 86, 106138 CrossRef CAS.
- Z. W. Seh, S. Liu, M. Low, S. Y. Zhang, Z. Liu, A. Mlayah and M. Y. Han, Adv. Mater., 2012, 24, 2310–2314 CrossRef CAS PubMed.
- M. Gao, P. K. N. Connor and G. W. Ho, Energy Environ. Sci., 2016, 9, 3151–3160 RSC.
- L. Zhu, M. Gao, C. K. N. Peh and G. W. Ho, Mater. Horiz., 2018, 5, 323–343 RSC.
- Z. Sun, J. Wang, Q. Wu, Z. Wang, Z. Wang, J. Sun and C. J. Liu, Adv. Funct. Mater., 2019, 29, 1901312 CrossRef.
- C. Liu, C. Cai, F. Ma, X. Zhao and H. Ahmad, J. Colloid Interface Sci., 2020, 560, 103–110 CrossRef CAS PubMed.
- C. Xiao, W. Liang, Q.-M. Hasi, L. Chen, J. He, F. Liu, C. Wang, H. Sun, Z. Zhu and A. Li, Mater. Today Energy, 2020, 16, 100417 CrossRef.
- Y. Shi, C. Zhang, Y. Wang, Y. Cui, Q. Wang, G. Liu, S. Gao and Y. Yuan, Desalination, 2021, 507, 115038 CrossRef CAS.
- X. Meng, J. Yang, S. Ramakrishna, Y. Sun and Y. Dai, J. Mater. Chem. A, 2020, 8, 16570–16581 RSC.
- Y. Sun, J. Gao, Y. Liu, H. Kang, M. Xie, F. Wu and H. Qiu, Chem. Eng. Sci., 2019, 207, 516–526 CrossRef CAS.
- D. Zhang, Y. Cai, Q. Liang, Z. Wu, N. Sheng, M. Zhang, B. Wang and S. Chen, ACS Sustainable Chem. Eng., 2020, 8, 9017–9026 CrossRef CAS.
- D. Zhang, M. H. Zhang, S. Y. Chen, Q. Q. Liang, N. Sheng, Z. L. Han, Y. X. Cai and H. P. Wang, Desalination, 2021, 500, 10 Search PubMed.
- J. Chen, D. Wang, X. Li, H. Sun, H. Zhao, Y. Li, X. Liu and G. Shi, ACS Appl. Polym. Mater., 2021, 3, 2402–2410 CrossRef CAS.
- C. Tian, C. Li, D. Chen, Y. Li, L. Xing, X. Tian, Y. Cao, W. Huang, Z. Liu and Y. Shen, J. Mater. Chem. A, 2021, 9, 15462–15471 RSC.
- Y. Guo, F. Zhao, X. Zhou, Z. Chen and G. Yu, Nano Lett., 2019, 19, 2530–2536 CrossRef CAS PubMed.
- X. Zhou, Y. Guo, F. Zhao, W. Shi and G. Yu, Adv. Mater., 2020, 32, 2007012 CrossRef PubMed.
- J. Zeng, Q. Wang, Y. Shi, P. Liu and R. Chen, Adv. Energy Mater., 2019, 9, 1900552 CrossRef CAS.
- X. Liang, X. Zhang, Q. Huang, H. Zhang, C. Liu and Y. Liu, Sol. Energy, 2020, 208, 778–786 CrossRef CAS.
- F. Gong, H. Li, W. Wang, J. Huang, D. Xia, J. Liao, M. Wu and D. V. Papavassiliou, Nano Energy, 2019, 58, 322–330 CrossRef CAS.
- J. Liu, X. Chen, H. Yang, J. Tang, R. Miao, K. Liu and Y. Fang, Mater. Chem. Front., 2021, 5, 1953–1961 RSC.
- Y. Xu, Z. Guo, J. Wang, Z. Chen, J. Yin, Z. Zhang, J. Huang, J. Qian and X. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 27129–27139 CrossRef CAS PubMed.
- Z. Liu, R.-K. Qing, A.-Q. Xie, H. Liu, L. Zhu and S. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 18829–18837 CrossRef CAS PubMed.
- X. Chen, Z. Wu, D. Lai, M. Zheng, L. Xu, J. Huo, Z. Chen, B. Yuan and M.-L. Fu, J. Mater. Chem. A, 2020, 8, 22645–22656 RSC.
- P.-F. Liu, L. Miao, Z. Deng, J. Zhou, H. Su, L. Sun, S. Tanemura, W. Cao, F. Jiang and L.-D. Zhao, Mater. Today Energy, 2018, 8, 166–173 CrossRef.
- Y. Lu, X. Wang, D. Fan, H. Yang, H. Xu, H. Min and X. Yang, Sustainable Mater. Technol., 2020, 25, e00180 CrossRef CAS.
- Y. Long, S. Huang, H. Yi, J. Chen, J. Wu, Q. Liao, H. Liang, H. Cui, S. Ruan and Y.-J. Zeng, J. Mater. Chem. A, 2019, 7, 26911–26916 RSC.
- J. Feng, B. Bai, L. Yang, N. Hu and H. Wang, Mater. Chem. Phys., 2021, 271, 124904 CrossRef CAS.
- H. Lu, W. Shi, F. Zhao, W. Zhang, P. Zhang, C. Zhao and G. Yu, Adv. Funct. Mater., 2021, 31, 2101036 CrossRef CAS.
- N. Li, L. Qiao, J. He, S. Wang, L. Yu, P. Murto, X. Li and X. Xu, Adv. Funct. Mater., 2021, 31, 2008681 CrossRef CAS.
- Z. Deng, L. Miao, P.-F. Liu, J. Zhou, P. Wang, Y. Gu, X. Wang, H. Cai, L. Sun and S. Tanemura, Nano Energy, 2019, 55, 368–376 CrossRef CAS.
- J. X. He, Y. K. Fan, C. H. Xiao, F. Liu, H. X. Sun, Z. Q. Zhu, W. D. Liang and A. Li, Compos. Sci. Technol., 2021, 204, 9 Search PubMed.
- N. Hu, Y. Xu, Z. Liu, M. Liu, X. Shao and J. Wang, Carbohydr. Polym., 2020, 243, 116480 CrossRef CAS PubMed.
- J. Liu, J. Gui, W. Zhou, X. Tian, Z. Liu, J. Wang, L. Yang, P. Zhang, W. Huang and J. Tu, Nano Energy, 2021, 86, 106112 CrossRef CAS.
- X. Zhou, F. Zhao, Y. Guo, Y. Zhang and G. Yu, Energy Environ. Sci., 2018, 11, 1985–1992 RSC.
- P. Zhang, Q. Liao, H. Yao, H. Cheng, Y. Huang, C. Yang, L. Jiang and L. Qu, J. Mater. Chem. A, 2018, 6, 15303–15309 RSC.
- L. Zhao, P. Wang, J. Tian, J. Wang, L. Li, L. Xu, Y. Wang, X. Fei and Y. Li, Sci. Total Environ., 2019, 668, 153–160 CrossRef CAS PubMed.
- Y. Liu, J. Tian, L. Xu, Y. Wang, X. Fei and Y. Li, New J. Chem., 2020, 44, 20181–20191 RSC.
- P. Zhuang, D. Li, N. Xu, X. Yu and L. Zhou, Global Challenges, 2020, 5, 2000053 CrossRef PubMed.
- T. Gao, X. Wu, Y. Wang, G. Owens and H. Xu, Sol. RRL, 2021, 5, 2100053 CrossRef CAS.
- W. Lei, S. Khan, L. Chen, N. Suzuki, C. Terashima, K. Liu, A. Fujishima and M. Liu, Nano Res., 2021, 14, 1135–1140 CrossRef CAS.
- G. Lou, Y. Wang, Y. Ma, J. Kou, F. Wu and J. Fan, Front. Mater. Sci., 2021, 15, 138–146 CrossRef.
- C.-S. Hu, H.-J. Li, J.-Y. Wang, A. Haleem, X.-C. Li, M. Siddiq and W.-D. He, ACS Appl. Energy Mater., 2019, 2, 7554–7563 CrossRef CAS.
- B. Huo, D. Jiang, X. Cao, H. Liang, Z. Liu, C. Li and J. Liu, Carbon, 2019, 142, 13–19 CrossRef CAS.
- X.-Y. Wang, J. Xue, C. Ma, T. He, H. Qian, B. Wang, J. Liu and Y. Lu, J. Mater. Chem. A, 2019, 7, 16696–16703 RSC.
- H. Geng, C. Lv, M. Wu, H. Ma, H. Cheng, C. Li, J. Yuan and L. Qu, Global Challenges, 2020, 4, 2000043 CrossRef PubMed.
- S. Hou, Y. Lv, X. Wu, J. Guo, Q. Sun, L. Wang and D. Jia, New J. Chem., 2020, 44, 2228–2235 RSC.
- Y. Kong, H. Dan, W. Kong, Y. Gao, Y. Shang, K. Ji, Q. Yue and B. Gao, J. Mater. Chem. A, 2020, 8, 24734–24742 RSC.
- X. Meng, J. Yang, S. Ramakrishna, Y. Sun and Y. Dai, ACS Sustainable Chem. Eng., 2020, 8, 4955–4965 CrossRef CAS.
- J. Tian, X. Huang and W. Wu, Ind. Eng. Chem. Res., 2020, 59, 1135–1141 CrossRef CAS.
- K. Yu, P. Shao, P. Meng, T. Chen, J. Lei, X. Yu, R. He, F. Yang, W. Zhu and T. Duan, J. Hazard. Mater., 2020, 392, 122350 CrossRef CAS PubMed.
- C. Liu, Y. Peng and X. Zhao, Desalination, 2021, 501, 114911 CrossRef CAS.
- L. Zang, L. Sun, S. Zhang, C. Finnerty, A. Kim, J. Ma and B. Mi, Chem. Eng. J., 2021, 422, 129998 CrossRef CAS.
- D. Li, X. Zhang, S. Zhang, D. Wang, Z. Wang, Y. Liu, X. Yu, Q. Zhao and B. Xing, Chemosphere, 2021, 267, 128916 CrossRef CAS PubMed.
- C.-R. Zhang, W.-R. Cui, C.-P. Niu, S.-M. Yi, R.-P. Liang, J.-X. Qi, X.-J. Chen, W. Jiang, L. Zhang and J.-D. Qiu, Chem. Eng. J., 2021, 428, 131178 CrossRef.
- H. Jian, Q. Qi, W. Wang and D. Yu, Sep. Purif. Technol., 2021, 264, 118459 CrossRef CAS.
- W. Xu, Y. Xing, J. Liu, H. Wu, Y. Cui, D. Li, D. Guo, C. Li, A. Liu and H. Bai, ACS Nano, 2019, 13, 7930–7938 CrossRef CAS PubMed.
- L. Zhu, T. Ding, M. Gao, C. K. N. Peh and G. W. Ho, Adv. Energy Mater., 2019, 9, 1900250 CrossRef.
- Q. F. Guan, Z. M. Han, Z. C. Ling, H. B. Yang and S. H. Yu, Nano Lett., 2020, 20, 5699–5704 CrossRef CAS PubMed.
- Y. Zhou, T. Ding, M. Gao, K. H. Chan, Y. Cheng, J. He and G. W. Ho, Nano Energy, 2020, 77, 105102 CrossRef CAS.
- X. Luo, J. Zhang, J. Tao, X. Wang, S. Zhao, Z. Chen, S. Liu, J. Li and S. Li, Chem. – Eng. J., 2021, 416, 129486 CrossRef CAS.
- Y. Duan, M. Weng, W. Zhang, Y. Qian, Z. Luo and L. Chen, Energy Convers. Manage., 2021, 241, 114306 CrossRef CAS.
- S. Sun, Y. Wang, B. Sun, F. Zhang, Q. Xu, H.-Y. Mi, H. Li, X. Tao, Z. Guo and C. Liu, ACS Appl. Mater. Interfaces, 2021, 13, 24945–24956 CrossRef CAS PubMed.
- Q. Zhao, T. Fan, J. Ding, D. Zhang, Q. Guo and M. Kamada, Carbon, 2011, 49, 877–883 CrossRef CAS.
- W. Zhang, Q. Chang, C. Xue, J. Yang and S. Hu, Sol. RRL, 2021, 5, 2100133 CrossRef CAS.
- S. Singh, N. Shauloff and R. Jelinek, ACS Sustainable Chem. Eng., 2019, 7, 13186–13194 CrossRef CAS.
- H. Zhou, C. Xue, Q. Chang, J. Yang and S. Hu, Chem. – Eng. J., 2021, 421, 129822 CrossRef CAS.
- Indriyati, I. Primadona, F. A. A. Permatasari, M. A. Irham, D. E. M. Nasir and F. Iskandar, Nanoscale, 2021, 13, 7523–7532 RSC.
- L. Xu, L. Cheng, C. Wang, R. Peng and Z. Liu, Polym. Chem., 2014, 5, 1573–1580 RSC.
- X. Zhou, F. Zhao, Y. Guo, B. Rosenberger and G. Yu, Sci. Adv., 2019, 5, eaaw5484 CrossRef CAS PubMed.
- S. H. Park, J. H. Park, J. Kim and S. J. Lee, Desalination, 2021, 500, 114900 CrossRef CAS.
- Y. Wu, L. Shen, C. Zhang, H. Gao, J. Chen, L. Jin, P. Lin, H. Zhang and Y. Xia, Desalination, 2021, 505, 114766 CrossRef CAS.
- T. Xu, Y. Xu, J. Wang, H. Lu, W. Liu and J. Wang, Chem. Eng. J., 2021, 415, 128893 CrossRef CAS.
- C. Li, D. Jiang, B. Huo, M. Ding, C. Huang, D. Jia, H. Li, C.-Y. Liu and J. Liu, Nano Energy, 2019, 60, 841–849 CrossRef CAS.
- F. Ni, P. Xiao, C. Zhang, Y. Liang, J. Gu, L. Zhang and T. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 15498–15506 CrossRef CAS PubMed.
- W. Wang, J. Niu, J. Guo, L. Yin and H. Huang, Sol. Energy Mater. Sol. Cells, 2019, 201, 110046 CrossRef CAS.
- Z. Xie, J. Zhu and L. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 9027–9035 CrossRef CAS PubMed.
- S.-L. Loo, L. Vásquez, M. Zahid, F. Costantino, A. Athanassiou and D. Fragouli, ACS Appl. Mater. Interfaces, 2021, 13, 30542–30555 CrossRef CAS PubMed.
- Y. Shi, O. Ilic, H. A. Atwater and J. R. Greer, Nat. Commun., 2021, 12, 1–10 CrossRef PubMed.
- S. Chen, Y. Liu, Y. Wang, K. Xu, X. Zhang, W. Zhong, G. Luo and M. Xing, Chem. – Eng. J., 2021, 411, 128042 CrossRef CAS.
- C. Wei, X. Zhang, S. Ma, C. Zhang, Y. Li, D. Chen, H. Jiang, Z. Xu and X. Huang, Chem. – Eng. J., 2021, 425, 130118 CrossRef CAS.
- X. Yin, Y. Zhang, Q. Guo, X. Cai, J. Xiao, Z. Ding and J. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 10998–11007 CrossRef CAS PubMed.
- F. Zhu, L. Wang, B. Demir, M. An, Z. L. Wu, J. Yin, R. Xiao, Q. Zheng and J. Qian, Mater. Horiz., 2020, 7, 3187–3195 RSC.
- X. Zhao and C. Liu, Desalination, 2020, 482, 114385 CrossRef CAS.
- C. Liu, Y. Peng, C. Cai, J. Zhang and X. Zhao, J. Environ. Chem. Eng., 2021, 9, 105272 CrossRef CAS.
- Y. Sun, X. Zong, D. Qu, G. Chen, L. An, X. Wang and Z. Sun, J. Mater. Chem. A, 2021, 9, 7122–7128 RSC.
- X. Xu, S. Ozden, N. Bizmark, C. B. Arnold, S. S. Datta and R. D. Priestley, Adv. Mater., 2021, 33, 2007833 Search PubMed.
- Z. Huang, J. Wei, Y. Wan, P. Li, J. Yu, J. Dong, S. Wang, S. Li and C. S. Lee, Small, 2021, 2101487, DOI:10.1002/smll.202101487.
- Y. Zou, J. Zhao, J. Zhu, X. Guo, P. Chen, G. Duan, X. Liu and Y. Li, ACS Appl. Mater. Interfaces, 2021, 13, 7617–7624 CrossRef CAS PubMed.
- Q. Zhao, Z. Huang, Y. Wan, J. Tan, C. Cao, S. Li and C.-S. Lee, J. Mater. Chem. A, 2021, 9, 2104–2110 RSC.
- W. R. Cui, C. R. Zhang, R. P. Liang, J. Liu and J. D. Qiu, ACS Appl. Mater. Interfaces, 2021, 13, 31561–31568 CrossRef CAS PubMed.
- F. Yang, J. X. Chen, Z. Y. Ye, D. W. Ding, N. V. Myung and Y. D. Yin, Adv. Funct. Mater., 2021, 31, 9 Search PubMed.
- Y. Lu, D. Fan, Y. Wang, H. Xu, C. Lu and X. Yang, ACS Nano, 2021, 15, 10366–10376 CrossRef PubMed.
- J. Yuan, X. Lei, C. Yi, H. Jiang, F. Liu and G. J. Cheng, Chem. – Eng. J., 2021, 132765, DOI:10.1016/j.cej.2021.132765.
- B. Wen, X. Zhang, Y. Yan, Y. Huang, S. Lin, Y. Zhu, Z. Wang, B. Zhou, S. Yang and J. Liu, Desalination, 2021, 516, 115228 CrossRef CAS.
- X. Wu, Y. Wang, P. Wu, J. Zhao, Y. Lu, X. Yang and H. Xu, Adv. Funct. Mater., 2021, 31, 2102618 CrossRef CAS.
- L. Zhao, J. Tian, Y. Liu, L. Xu, Y. Wang, X. Fei and Y. Li, Environ. Sci.: Water Res. Technol., 2020, 6, 221–230 RSC.
- H. Zhang, H. Xie, W. Han, X. Yan, X. Liu, L. He, P. Lin, Y. Xia, K. Zhang, J. A. Zapien and K.-B. Yoon, ACS Appl. Nano Mater., 2021, 4, 1916–1923 CrossRef CAS.
- F. Yu, Z. Chen, Z. Guo, M. S. Irshad, L. Yu, J. Qian, T. Mei and X. Wang, ACS Sustainable Chem. Eng., 2020, 8, 7139–7149 CrossRef CAS.
- X. Liang, X. Zhang, Z. Liu, Q. Huang, H. Zhang, C. Liu and Y. Liu, Sol. Energy, 2020, 201, 581–588 CrossRef CAS.
- Y. Guo and G. Yu, Acco. Mater. Res., 2021, 2, 374–384 CrossRef CAS.
- C. Wen, H. Guo, J. Yang, Q. Li, X. Zhang, X. Sui, M. Cao and L. Zhang, Chem. – Eng. J., 2021, 421, 130344 CrossRef CAS.
- J. J. Koh, G. J. H. Lim, S. Chakraborty, Y. Zhang, S. Liu, X. Zhang, S. C. Tan, Z. Lyu, J. Ding and C. He, Nano Energy, 2021, 79, 105436 CrossRef CAS.
- P.-F. Liu, L. Miao, Z. Deng, J. Zhou, Y. Gu, S. Chen, H. Cai, L. Sun and S. Tanemura, Appl. Energy, 2019, 241, 652–659 CrossRef CAS.
- L. L. Noureen, Z. J. Xie, M. Hussain, M. M. Li, Q. Q. Lyu, K. Wang, L. B. Zhang and J. T. Zhu, Sol. Energy Mater. Sol. Cells, 2021, 222, 7 CrossRef.
- Y. Xu, X. Xiao, X. Fan, Y. Yang, C. Song, Y. Fan and Y. Liu, J. Mater. Chem. A, 2020, 8, 24108–24116 RSC.
- H. Yang, G. Yang, Z. Qiao, H. Bao, S. Zhang, X. Li and Y. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 35193–35200 CrossRef CAS PubMed.
- Q. Zhu, K. Ye, W. Zhu, W. Xu, C. Zou, L. Song, E. Sharman, L. Wang, S. Jin, G. Zhang, Y. Luo and J. Jiang, J. Phys. Chem. Lett., 2020, 11, 2502–2509 CrossRef CAS PubMed.
- D. Ding, H. Wu, X. He, F. Yang, C. Gao, Y. Yin and S. Ding, J. Mater. Chem. A, 2021, 9, 11241–11247 RSC.
- Z. Huang, Y. Wan, J. Liang, Y. Xiao, X. Li, X. Cui, S. Tian, Q. Zhao, S. Li and C.-S. Lee, ACS Appl. Mater. Interfaces, 2021, 13, 31624–31634 CrossRef CAS PubMed.
- M. Z. Tariq, Z. Hanif, M. La, D. Choi and S. J. Park, Int. J. Energy Res., 2021, 45, 6395–6404 CrossRef CAS.
- Z. Wang, X. Wu, J. Dong, X. Yang, F. He, S. Peng and Y. Li, Chem. – Eng. J., 2022, 427, 130905 CrossRef CAS.
- P. Wang, X. Wang, S. Chen, J. Zhang, X. Mu, Y. Chen, Z. Sun, A. Wei, Y. Tian and J. Zhou, ACS Appl. Mater. Interfaces, 2021, 13, 30556–30564 CrossRef CAS PubMed.
- Y. Li, W. Hong, H. Li, Z. Yan, S. Wang, X. Liu, B. Li, H. Jiang and X. Niu, Desalination, 2021, 511, 115113 CrossRef CAS.
- Y. Wang, L. Zhao, F. Zhang, K. Yu, C. Yang, J. Jia, W. Guo, J. Zhao and F. Qu, ACS Appl. Mater. Interfaces, 2021, 13, 26879–26890 CrossRef CAS PubMed.
- L. Zhang, X. Xu, J. Feng, B. Bai, N. Hu and H. Wang, Sol. Energy Mater. Sol. Cells, 2021, 230, 111237 CrossRef CAS.
- Y. Guo, C. M. Dundas, X. Zhou, K. P. Johnston and G. Yu, Adv. Mater., 2021, 2102994, DOI:10.1002/adma.202102994.
- X. Zhou, F. Zhao, P. Zhang and G. Yu, ACS Mater. Lett., 2021, 1112–1129, DOI:10.1021/acsmaterialslett.1c00304.
- F. Zhao, X. Zhou, Y. Liu, Y. Shi, Y. Dai and G. Yu, Adv. Mater., 2019, 31, e1806446 CrossRef PubMed.
- X. Zhou, H. Lu, F. Zhao and G. Yu, ACS Mater. Lett., 2020, 2, 671–684 CrossRef CAS.
- Y. Guo, Z. Fang and G. Yu, Polym. Int., 2021, 70, 1425–1432 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |