Open Access Article
Open Access ArticleCellulose based flexible and wearable sensors for health monitoring
Gargi
Dandegaonkar
a,
Abbas
Ahmed
 b,
Luyi
Sun
b,
Luyi
Sun
 bc,
Bapan
Adak
bc,
Bapan
Adak
 de and
Samrat
Mukhopadhyay
de and
Samrat
Mukhopadhyay
 *d
*d
aDepartment of Fiber and Textile Processing, Institute of Chemical Technology, Mumbai 400019, India
bPolymer Program, Institute of Materials Science, University of Connecticut, Storrs, CT 06269, USA
cDepartment of Chemical and Biomolecular Engineering, University of Connecticut, Storrs, CT 06269, USA
dDepartment of Textile and Fiber Engineering, Indian Institute of Technology, New Delhi 110016, India. E-mail: samrat@textile.iitd.ac.in
eProduct Development Department, Kusumgar Corporates Pvt Ltd, Vapi, Gujarat 396195, India
First published on 31st March 2022
Abstract
Recently, with the advancement of materials science and manufacturing methods, researchers have introduced many new and advanced materials for wearable sensors. In particular, incredible advancement has been made in flexible strain sensors for their application in wearable health monitoring, soft-robotics, human–machine interactions (HMI), and so forth. Cellulose, the most abundant natural biopolymer, is one of the most viable substrates for strain and pressure sensors, mainly due to its biocompatibility and biodegradability along with many other useful features. Cellulose is available in various forms including fibers, nanocrystals, and nanofibers, and different techniques are used to fabricate these into hydrogels, aerogels, films, paper, fabrics, and eventually into sensors. An overview of the fabrication, sensing performance, and applications of cellulose-based flexible physiological sensors has been presented in this review, along with their use in monitoring healthcare activities through different types of strain sensors, pressure sensors and HMIs. It is observed that an appropriate selection of the substrate and functional materials greatly influence the sensing characteristics of these sensors, such as sensitivity, response time, effective strain range, stability under strain/pressure, and hysteresis. The potential for further research on this subject has also been highlighted, along with the recent trends in using flexible sensors for health-monitoring.
1. Introduction
The fourth industrial revolution has brought about a radical change in society, especially in the technological context. The current scientific developments focus more on the collection, development and real-time applications of the discoveries and database that has been worked upon in the past, especially in the field of exploring new and smart materials. These smart devices find innumerable applications to obtain, change and process signals to generate useful and impactful data.1 Amongst the wide-ranging demands of the century, the one that stands out is the ongoing real-time procurement and systems administration of the physiological signals produced by human beings. This interest calls for an improvement in the available wearable electronic devices which can be put on the skin directly (for example smartwatches) using adaptable straps. However, this sometimes causes inconvenience and surprisingly restricts the checking signals, particularly when used in clinical application for monitoring human health.2–7Materials such as metal foils, silicones, and plastics are usually used to manufacture these devices. However, these materials create hurdles due to their bulkiness, lack of affinity between the material and the substrate, oxidation susceptibility, and inadequacies regarding robustness and waste disposal.8,9 Cellulose and its nano-derivatives such as cellulose nanocrystals (CNCs), cellulose nanofibrils (CNFs), and bacterial cellulose (BC) are often used as functional materials.10–12 Researchers are presently exploring the utility of cellulose and its derivatives as an advanced alternative to existing technologies due to their abundance and advantageous characteristics, such as nontoxicity, biocompatibility, strength and biodegradability13–16 The properties of cellulose such as high durability, structural strength, robustness, flexibility, and its use as a reinforcing agent make it an ideal material for manufacturing new generation flexible wearables and electronics.17–23
Wearable electronic devices can be broadly classified as (i) rigid/semi-rigid and (ii) flexible/stretchable. As indicated by the natural factors and functionalities, these sensing devices are further classified as: physical sensors that are sensitive to pressure/strain, moisture, and temperature; chemical sensors that can detect metal and non-metal ions, changes in pH, gas/vapour and toxic organic compounds, and biosensors that react to microorganisms and biomolecules.24–26
There has been enormous growth in the field of wearable sensors used for health monitoring, especially biocompatible sensors developed from cellulose materials. Several novel processes have been developed for cellulose and its use in developing functional wearable sensors, including cellulose-based textile sensors which cover areas like bio-sensing and cellulose-based composites for strain-sensors.27 However, none of the published reviews discuss the fundamental characteristics of cellulose and its green processing as a functional substrate for designing various sensors. The current review sheds light on the above-mentioned points, which may be helpful when applying cellulose-based sensors to wearable health monitoring devices.
This comprehensive review attempts to examine the latest developments in cellulose-based physiological sensors for wearable real-time health monitoring. At the onset, the structural morphology and key features of cellulose are discussed. Subsequently, several key aspects of cellulose based composite formulations have been highlighted. The next section discusses the multifaceted functional structure of cellulose composites such as hydrogels, aerogels, films and fibres for device fabrication. Additionally, several performance parameters that are crucial in the fabrication of strain and pressure sensors have been elucidated. The next section highlights the application of cellulose-based composites for wearable health monitoring in the form of strain, pressure and human–machine interfacing. The emerging applications of cellulose based flexible devices for healthcare monitoring are illustrated in Fig. 1. Overall, this review is an attempt to give insights into the persisting challenges within the field and emerging applications of sustainable, durable and multi-functional, smart sensors derived from cellulose, especially for heath monitoring.
2. Structures and features of cellulose
Cellulose is an abundantly found organic compound and a primary structural component of many green plants such as bamboo, cotton, some agricultural crops, and marine algae. It is a straight chain polymer consisting of linear β-1, 4-linked D-glucose units. Cellulose tends to form strong hydrogen bonds due to the abundant hydroxyl (–OH) active groups that are present. The D-glucose has six free hydroxyl groups that interact to form intra- and inter-chain hydrogen bonds. In plants these cellulosic molecules show van-der-Waal's forces amongst the neighbouring polymeric chains. The intra-chain hydrogen bonds stabilize the chains into a linear structure and the inter-chain hydrogen bonds facilitate parallel stacking of the adjacent chains, which results in a crystalline structure. Moreover, cellulose exhibits high tensile strength, high stability and axial stiffness.28 Some characteristics of cellulose and its derivatives like abundance of surface hydroxyl groups, large specific surface area, high aspect ratio, high crystallinity, remarkable mechanical properties and high thermal resistance make them one of the most efficient and popularly used substrates for sensing devices.29High-pressure homogenization is used to prepare a cellulose suspension to manufacture cellulose nanofibers (CNFs).30 Different techniques, for example, TEMPO-mediated oxidation31 and high-speed grinding32 are used to acquire CNFs of 5–100 nm diameter and few microns of length, with a remarkable mechanical strength.33
Plants are a major source of cellulose. These plant cells consist of multiple cellulose molecule chains which associate with each other, resulting in the formation of linear structures, known as cellulose microfibrils (Fig. 2a).34 These cellulose microfibrils have high tensile strength and consist of hemicellulose, paracrystalline cellulose and crystalline cellulose. The cellulose molecule is made of anhydrate glucose linked by beta 1–4 linkages. Strong acid hydrolysis is carried out to synthesize nanocellulose (Fig. 2b) and bacteria is used to obtain cellulose nanofibers (Fig. 2c).
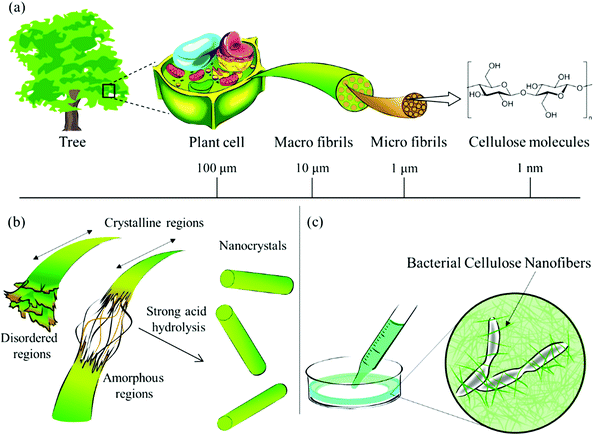 | ||
| Fig. 2 (a) Cellulose derived from plants and its structure. (b) Hydrolysis reaction between the plant cellulose carried out to acquire nanocellulose. (c) Bacterial nanocellulose obtained using cellulose-synthesizing bacteria. Reproduced with permission from ref. 34. | ||
Cellulose nanocrystals (CNCs) possess many advantages including higher dimensional stability, renewable nature, purity, non-toxicity and biocompatibility, which give them an edge over other cellulose-based nanostructured materials and other cellulose derivatives.35 CNCs have unique dimensional structures, appropriate density and size ratio, and remarkable mechanical, structural and chemical properties which help them find wide-ranging applications in the fields of materials science, electronic gadgets, biomedicine, tissue engineering and healthcare.36 There is a need for optimising the upcoming commercial production processes on the industrial scale to efficiently extract CNCs in sufficient amounts to obtain a prominent yield and high-quality smart materials. The structural, mechanical, thermal, optical, and liquid crystalline properties of CNCs have been extensively investigated to date. CNCs will thus have a large scope and a wide range of opportunities in different sectors, including healthcare. Cellulose-based nanomaterials with desired and improved characteristics can be one of the major objectives of further research. Various polymer-based nanocomposites have suffered from non-uniform dispersion due to the tendency of CNCs to agglomerate, requiring a customized chemical process for consolidating CNCs in various polymer substrates. Developments in materials science and nanotechnology will, in the near future, enable commercially feasible CNCs to also be non-toxic and environmentally safe, resulting in better long-term performance.
3. Key aspects in cellulose based composite formation
Cellulose based materials have been used for decades in many forms, mainly due to their vast availability and sustainable nature. Previously, many petrochemical based substances have been used largely in industry as the prime material for fabricating devices or they have found applications as starting materials for various quality products. But there have been concerns regarding these in recent times, mainly due to their polluting nature, hence, biomass is seen as a viable alternative to be used.37 Cellulose-based smart materials are the most sought-after materials among these biodegradable and biocompatible materials.38 Cellulose and its various derivatives like CNCs, CNF, BC, carboxymethyl cellulose (CMC), cellulose acetate (CA), methyl cellulose (MC), hydroxyethyl cellulose (HEC), and (hydroxypropyl)methyl cellulose (HPMC) find applications in different areas namely, biological sensing,39,40 water and effluent treatment,41 and as reinforcing agents42 in composites. These various forms of cellulose have different inherent properties that determine the type of substrate to be used for a specific end-use. These materials are typically strengthened by combining with certain inorganic or organic substances, thus enhancing their properties, and making them potential materials of the future. In regard to wearable sensor development, since cellulose materials are being used as base substrates, a fundamental understanding of their processing is important. For cellulose to be a source of biocompatible sensor devices, its processing medium should be free of hazardous substances. This section is devoted to discussing the concerns of cellulose processing for further development of wearable human-friendly and biocompatible sensor systems.3.1. Green solvent systems for cellulose dissolution
Cellulose has abundant hydroxyl groups and is insoluble in water and other widely used organic solvents primarily because of the inter- and intra-molecular bonds present in it.43,44 Many recent studies highlight the use of solvent systems like DMAc/LiCl,43 NaOH/Urea,44N-methylmorpholine, and N-oxide45 for cellulose dissolution. However, these solvents are hazardous to the environment and they cannot be recovered completely. This gives rise to the need for a green, environment-friendly solvent which can be used for effective cellulose dissolution and can be recovered as well. There has been interesting research on the use of ionic liquids for cellulose dissolution.46–50 Ionic liquids are comprised of an organic cation and an inorganic anion. Some ionic solvents consist of pyridinium, imidazolium cations and HCOO−, (MeO)2 PO2−, and Cl− anions. Han and team highlighted the use of PEG/NaOH as a green solvent for cellulose dissolution.51 This aqueous solution is not only environment-friendly, but is also cheaper than ionic liquids used for cellulose dissolution. The removal of residual solvent is achieved through distillation or evaporation implying that the solvents are highly volatile in nature. The leakage and evaporation of these solvents is a cause of atmospheric pollution. Green solvents overcome these issues and comply with most of the stringent environmental legislations.Nyugen et al. used a combination of a primary cellulose solvent and a natural deep eutectic solvent (NADES) to formulate a green, eco-friendly approach for preparing new cellulose solvents.52 The primary cellulose solvent and NADES both exhibit exceptional hydrogen-bonding, giving rise to a new cellulose solvent with desirable properties due to the donor–acceptor ability of both materials used. An advanced cellulose dissolvent with a lower melting point than that of a ‘pure’ solvent, was hence observed while experimenting with this novel ternary framework. For effective functioning, both primary cellulose solvent and NADES should be produced easily and have similar properties. Also, they must combine effectively in a way to create a synergistic eutectic mixture. Using this technology, innovative green solvents can be made with mild properties and cellulose-based smart materials can be tailored to specific applications. Experimentally, some advances with respect to the solubility of cellulose were observed in the NCM25% ternary solvent system. In addition, this dissolvable framework can be reused with a recycled yield of up to 95% and is capable of breaking down cellulose in aqueous media, demonstrating the substantial utility of this ternary solvent system for industrial applications. The ternary solvent, therefore, has consistent solvent properties over the temperature range of 20 to 80 °C and shows true molecular dissolution of cellulose. This novel framework, using anti-solvent precipitation, permits easy fabrication of highly anisotropic, soft cellulose particles.
3.2. Molecular modification by cross-linking
Molecular modification is carried out for different types of cellulose derivatives like nanocellulose, cellulose nanofibrils, cellulose nanocrystals, etc. Cross-linking imparts impressive properties to the materials in various areas with high aspect ratio, remarkable mechanical properties, improved aqueous, structural, and thermal stability, and tunable surface functionalization. Covalent cross-linking is carried out using a covalent cross-linking agent and it imparts some desirable mechanical properties like improved tensile strength, compression strength and Young's modulus.53,54 On the other hand, physical cross-linking is achieved by hydrophobic interactions, chain aggregation, hydrogen bonds, van der Waals forces, etc.55,56For example, Ye et al. used a chemical dual-cross-linking strategy to successfully construct robust and resilient dual-cross-linked cellulose hydrogels (DCHs). A cross-linker with a low molecular weight was used to obtain short-chain crosslinks, whereas a cross-linker with a high molecular weight was used to obtain long-chain crosslinks.57 Upon experimenting, all the short-chain cross-linking networks were found to be brittle, while the chemically cross-linked DCHs that had both long chains and short chains were found to display remarkable properties like robustness and ruggedness. The mechanical energy was generated when the bonds of the short chain crosslinks ruptured, thereby dissipating energy. The long chain crosslinks were stretchable, thereby maintaining the elasticity of the DCHs. Moreover, the high strength of DCHs could be seen because of the synergistic effect of two cross-linking points present. Values of 1.7 and 9.4 MPa were attained when these DHCs were subjected to tensile strain and compressive force, which were 26.3 and 83.9 times higher than the fracture strengths of the SCHs under similar testing conditions, when tested individually. These experiments highlighted a new methodology for making eco-friendly, resilient polymer-based hydrogels to be used as smart materials that will find potential applications of biomass in the engineering sector, with desired properties like appropriate density, biocompatibility and mechanical attributes.
Molecular modifications by cross-linking may lead to improvement in composites structural properties. Ahmed et al.58 used a simple solvent casting method to develop ultra-violet (UV) shielded cellulose/graphene oxide nanocomposite films. They used epichlorohydrin (ECH) as a crosslinker between cellulose and graphene oxide (GO). This induced remarkable interfacial adhesion and strong hydrogen bonding between GO and cellulose, resulting in a compact structure. Moreover, no micropore defects were observed in the SEM analysis. These structural characteristics were observed mainly due to the crosslinking and interaction between oxy-functionalized groups present in GO and cellulose via ECH. This resulted in enhanced mechanical and structural properties such as a higher Young's modulus (108%) and tensile strength (80%) as compared to those of the neat cellulosic (NC) film. The fabricated composite was also thermally stable when compared to the NC film.
3.3. Effect of nanomaterial dispersions
The process of de-agglomeration followed by subsequent distribution of nanomaterials within a composite matrix or a solvent is known as nanomaterial dispersion.59 Nanomaterial dispersions help in increasing the strength and thermal stability of a composite. These dispersions bring about some changes in the morphology of a composite and can be achieved using the following processes: polymer grafting,60 plasma-induced surface modification,61 ultrasonication,62etc.For instance, Khoshkava et al. used melt compounding techniques and incorporated CNC agglomerates to prepare polypropylene (PP)/(CNC) nanocomposites, using various drying techniques, namely, spray drying, freeze drying, and spray-freeze drying.63 It was observed that CNC particles and dispersions produced by spray-freeze drying (SFD) of polypropylene provided remarkable results for nanocomposite dispersion in polypropylene, as confirmed by microscopy and rheology. This was primarily due to the porous particle morphologies and web like structure that was formed. It is observed that linear viscoelasticity tests are very sensitive to filler dispersion in a polymer melt. Some rheological characteristics such as loss modulus and complex viscosity increased remarkably when spray freeze dried CNC (CNCSFD) was added. An interfacial region with an exceptional degree of scattering was observed in specimens containing CNCSFD below the rheological permeation limit of ca 2.5 wt% CNC.
3.4. Biocompatibility and non-toxicity of cellulose
A material can be regarded as a suitable biomaterial when it shows properties of biocompatibility and non-toxicity.64 Cellulose-based sensors are made from cellulose sourced from bacteria, tunicates and plants and can easily be fabricated into scaffolds.65 Cellulose-based smart materials show good adhesion properties and can be used for health monitoring in humans by attaching it to body parts. These materials do not cause any damage to the host as they exhibit the required bioactivity and biocompatibility. Moreover, it also has a versatile and customisable surface structure. It can also be removed easily from the body when used in the form of wound dressings. Cellulose-based biomaterials are also known to promote regeneration of neurons66 and mediate recovery of nerves.67 Cellulose is generally derived from natural materials. Most of these cellulose-based materials are biodegradable and do not have a negative impact on the environment.4. Cellulose based functional structures for device fabrication
Cellulose is known to have many beneficial qualities such as excellent biodegradability, cost efficiency, super flexibility, and excellent thermal and electrical properties making it a viable starting material for wearable electronic devices. Technologists have studied various methods to fabricate diverse functional materials with the requisite performance parameters and distinct morphologies, based on the remarkable developments in structural modifications and nano-morphologies, resulting in numerous fibres/films/gel-based devices along with some hybrid three-dimensional devices for human-health monitoring.68–73Table 1 summarizes the performance of various pressures sensors based on different functional structures. Some other sensors based on different functional structures are described in detail in this section.| Functional structure | Materials | Method of fabrication | Working range (%) | Sensitivity | Reference |
|---|---|---|---|---|---|
| Film | rGO/IOAC | Coating | 0.17–0.43 | 495, 0.17 < ε < 0.43% | 71 |
| Film | PDMS/GE/CNF | Mixing | 0–100 | 7.1 at 100% strain | 74 |
| Film | MWCNTs/BC | Coating | 0–0.6 | 252 | 75 |
| Paper | Au/paper | Coating | 0–0.59 | 75.8, 0 < ε < 0.59% | 76 |
| Paper | Gr/MoS2/paper | Coating | 0–2 | 60, 1.0 < ε < 1.5% | 77 |
| Fabrics | Cotton fabric | Carbonization | 0.02–140 | 64, 80 < ε < 140% | 78 |
| Fabrics | PEDOT:PSS/GNPs/cotton | Spraying | 0–47 | 4.8, e = 10% | 79 |
| Fabrics | CNT/cotton fabric | Screen printing | 0–260 | 8430, 240 < ε < 255% | 80 |
4.1. Functional hydrogels
Hydrogels are soft materials with good transparency, softness and remarkable mechanical properties and are mainly formed by cross-linking of hydrogen bonds and covalent bonds.81 According to Tong et al., chemically cross-linked cellulose ionic hydrogels (CIHs) are produced when allyl cellulose (AC) is polymerized by free radicals in the presence of NaOH/urea.82 Considering the naturally derived polymer-based hydrogels based on cellulose and its derivatives like chitin and chitosan, these hydrogels displayed remarkable properties, namely strength and high flexibility with a tensile strain of ∼126%, good transparency with a transmittance value of ∼89% at 550 nm, a superior compression strain value of ∼80% and an ionic conductivity value of ∼0.16 mS cm−1 even at −20 °C. The hydrogel and NaOH/urea aqueous system also had a difference in properties, which allowed the CIH to be incorporated as strain sensors using commercial tape. The properties can be modified by adjusting the density of chemical cross-linking.A novel biocompatible hydrogel with properties like transparency, flexibility and conductivity adapted from single-walled carbon nanotube (SWCNT) films to develop electrodes and dynamic sensors for E-skins and other wearable electronics was reported by Gilshteyn et al.83 The developed material can bear up to 100% strain when subjected to intense stretching. Because the exchange between the filter and the hydrogel surface occurs instantaneously, it eliminates the need for an intermediate layer or any other advances previously required. This is done on the surface of a surrounding aqueous hydrogel. A novel set of high-strength elements, which are flexible, were created using this methodology and found applications in sensing human motion and ECG (electrocardiography) electrodes. This strategy for the SWCNT films can be possibly utilized for different tissue designing processes or detecting applications, particularly for wearable gadgets and flexible yet durable E-skins.
Cellulose based hydrogels are commonly used as substrates for flexible strain sensors.84 These hydrogels that are conductive in nature have certain properties such as robust freezing-tolerant ability and required transparency. One such example is the organohydrogel fabricated by reinforcing CNFs and gelling of PVA and CNFs in a dimethyl sulfoxide–water (DMSO/H2O) solvent system (Fig. 3a).84 This organohydrogel showed remarkable properties such as high stretchability, high mechanical strength and robustness along with high ionic conductivity. This material also exhibited excellent freezing tolerance and maintained flexibility and conductivity even at temperatures of −70 °C. This was primarily due to the binary DMSO/H2O solvent system present (Fig. 3b). Moreover, the hydrogel displayed remarkable sensing behaviour and signal stability and was therefore used as a wearable sensor for detecting and monitoring human motions. This sensor was applied on the human wrist to monitor muscle variations and radial pulse for healthcare applications (Fig. 3c).
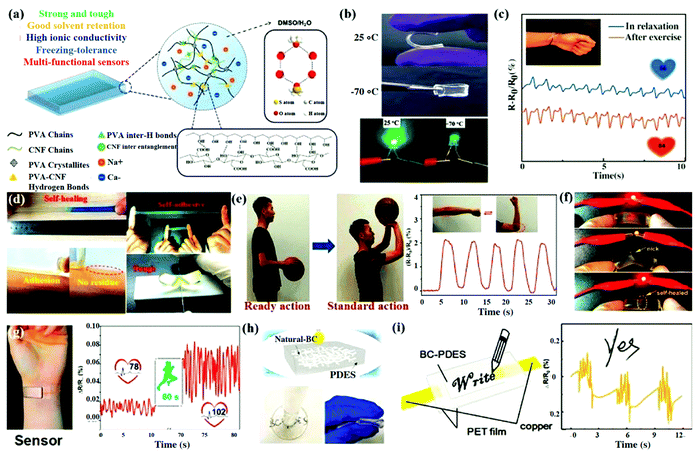 | ||
| Fig. 3 Hydrogels based on cellulose and its application as flexible sensors. (a) Fabrication of the PVA–CNF organohydrogel. (b) The optical images of the fabricated organohydrogel. (c) The PVA–CNF organohydrogel sensor used for observing movements of the wrist muscles and the radial pulse upon exercise and then relaxation. Reproduced with permission from ref. 84. (d) Cellulose nanocomposite hydrogels showing amalgamation of properties of mechanical toughness, reliable self-healing, and adhesive nature. (e) The basketball shooting action needs the wrist, elbow, and shoulder to be in an upright position. Reproduced with permission from ref. 85. (f) Ionic hydrogels based on cellulose used as conductors. (g) Cellulose-based ionic hydrogels as a wearable pulse monitor. Reproduced with permission from ref. 40. (h) The BC–PDES ionic conductor was kept in the air for 3 days and then was poked and bent. (i) BC–PDES ionic hydrogel and its variation of resistance measured at different time intervals. Reproduced with permission from ref. 86. | ||
A self-healing and self-adhesive cellulose nanocomposite hydrogel with human skin-like properties was developed. Cellulose nanocrystals coated with tannic acid (TA@CNCs) formed multiple coordination bonds with poly(acrylic acid) (PAA) chains and metal ions to impart mechanical self-healing and electrical self-healing properties to the ionic hydrogels.85 This hydrogel showed durable adhesiveness and it also detached easily, causing no damage to any tissues, when attached to human skin (Fig. 3d). Additionally, an ionic hydrogel utilised for making strain sensors was able to detect various types of human motion ranging from large scale movements such as bending of joints to small scale motion such as pulse and breathing (Fig. 3e). This data was could further be analysed on smartphones using wireless transmission. Similarly, Liu et al. developed ionic hydrogels based on a synergistic “soft and hard” hybrid network.40 These hydrogels had properties like stretchability, robustness, mechanical strength, excellent sensitivity and self-healing capability (Fig. 3f). These sensors were used to detect and differentiate human motions such as bending of fingers, breathing, pulse before and after exercise, etc, as shown in (Fig. 3g). Furthermore, an ionic hydrogel based on natural BC and PDESs (polymerizable deep eutectic solvents) was developed by Wang and team.86 This flexible, transparent and tough hydrogel was used to sense motions of the human body including coughing, breathing and writing (Fig. 3h).
Li et al. reported a mixture of polyvinyl alcohol (PVA) and polydopamine (PDA) to fabricate a strain sensor to be worn on the epidermis, that detects both super high and low signals generated by the human body.87 Incorporating self-healing, consistence, and self-bonding properties, this sensor could be utilized for attaching to the human body and monitoring human movements and epidermal distortion, which permits an interesting correlation between the epidermis and epidermal strain sensors. This resulted in perceiving the slightest signals generated from human heartbeats, changing facial movements and vibrations of the vocal cord. The results showed that even a little strain of 0.1% can be identified without a large gauge factor. Moreover, the phenomenal flexibility of this strain sensor can detect strain as high as up to 500% and exceptional self-healing properties of 250 ms at favourable temperature and stimuli help in combatting the accidental rupturing, scratching and some other mechanical inconveniences caused. This highlights the incredible capacities of the PVA/PDA hydrogel to be used as an epidermal strain sensor to screen human motion and physiological signals.
4.2. Cellulose aerogels
Cellulose aerogels are environment-friendly, biocompatible, and biodegradable. Along with this they have remarkable properties of high porosity which leads to a higher surface area and lower density.88 Despite these advantages, researchers still face some problems in the moulding and fabrication of cellulose aerogels: (i) nanocellulose and bacterial cellulose are costly, and nanocellulose tends to agglomerate during the drying process, making it difficult to recover solvents during the manufacturing process. Moreover, the solvent exchange procedure is in general a very tedious one. (ii) Additionally, modifying cellulosic aerogels with chemical coupling agents is difficult, unpredictable, and expensive. (iii) Several performance parameters such as stability, robustness, and durability do not meet the demands of practical applications, hence their value in real-time is limited. Therefore, a short, easy, and environment-friendly process using safe gel drying techniques should be developed to overcome these issues.Wang et al. successfully fabricated GO and TEMPO-oxide cellulose nanofibers TOCN carbon aerogels using the bidirectional freezing process.89 These aerogels had a regular hierarchical architecture [Fig. 4(a–e)] and remarkable properties such as improved compressibility and elasticity. The GO and TOCN interacted chemically (Fig. 4f) to give a lightweight aerogel. The improved mechanical properties, high compressibility, high electrical conductivity and high fatigue resistance were attributed to graphene used in the aerogels. Finally this shows high performance in lighting bulbs and demonstrated excellent features in various finger motion monitoring (Fig. 4(g–h)).
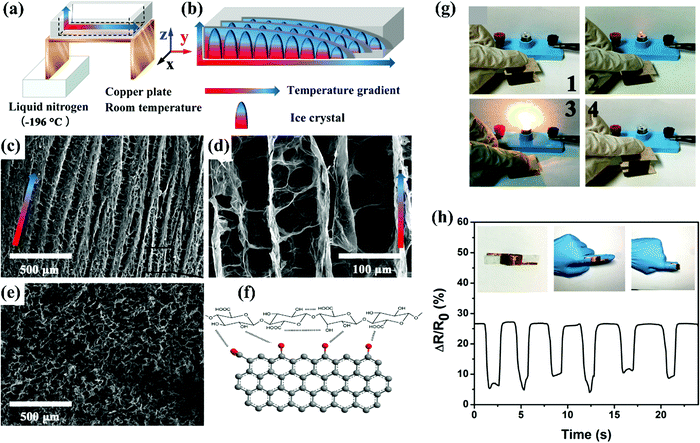 | ||
| Fig. 4 (a) Structure of cellulose. (b) Cellulose based aerogel. (c and d) G10–T10 carbon aerogel has a hierarchical architecture. The SEM images of the same aerogel observed under different magnification. (e) A direct liquid nitrogen freezing method was used to fabricate this aerogel. The SEM images of the aerogel. It exhibits porous structures which are randomly distributed. (f) The chemical reactions between GO and TOCN. (g–h) Representation of lighting bulbs and finger motion monitoring by using a cellulose based aerogel. Reproduced with permission from ref. 89. | ||
4.3. Functional films and membranes
Cellulose has an enormous potential for sensing devices and is widely used in different forms of functional structures including pure cellulosic films and membranes. BC shows better crystallinity and purity as compared to cellulose extracted from wood. Cellulose films based on wild Komagataeibacter xylinus were fabricated by Rahul et al.90 A crystallinity of 88.6–97.5% was observed for these films. The films also displayed sensitivity of around 5–20 pC/N and had remarkable linearity and stability. The fabricated films were economical and environment-friendly and can serve as potential substrates for strain sensors. Moreover, they do not consist of any other inorganic materials as well, thus eliminating a need for pure cellulose-based films.Fan et al. shed light on the fabrication process of the cellulose membranes and their recent advances in sensing applications.24 The authors of this paper highlighted the use of metal and non-metal ions and other biomolecules to create sensors that can operate under different pressures, temperatures, humidities, and pH conditions. They further discussed their functionality, as well as the environmental threats from the developed membranes. Cellulose membranes have wide application possibilities in the field of detection, which can be used by combining some other components with these cellulosic substrates. These remarkable detection properties can be attained by characterizing functional materials such as metal nanowires, metal nanoparticles, carbon dots, quantum dots, carbon nanotubes, fluorophores, organic dyes and antigen–antibodies in the cellulose membranes using numerous physio-chemical processing techniques. In spite of this, there are still some issues for the researchers to overcome when applying this technology. (i) Some parameters like sensitivity, detection limit, acute response time and reversibility in the detection should be improved. (ii) The behaviour of the cellulose membranes under extreme conditions of high acidity and alkalinity, etc. should be taken into consideration. (iii) Cellulose membrane-based sensors with multi-functional applications were rarely observed. (iv) These sensors are more or less theoretical in nature, with less understanding regarding their practical applications. There has been no industrial scale-up for most of them because they are not viable commercially. A cellulose-based detecting film has many advantages over other multidisciplinary films, such as cost-effectiveness, recyclability, biocompatibility, convenience, miniaturization, coordination, and smartness. These features have a wide range of applications in other fields and have innovative uses that can be explored in the future.
4.4. Functional fibres
Development of cellulose based functional fibres experienced unprecedented progress, and they have been used as biocompatible devices for numerous applications including wearable healthcare monitoring. Utilizing a basic filtration method Zhu et al. reported a thermoplastic polyurethane (TPU)/CNT–CNC based strain sensor.88 It was seen that the working range and the detection limits of the strain sensor can be altered by varying the mass of CNTs/CNCs proportionately with the aggregate hybrid fillers during fabrication. The presence of CNCs helped in scattering the CNT bundles and enhanced the detection limits of strain sensors when subjected to strain, by minimising the association points of the conductive framework. The fibres start elongating slowly when subjected to strain and start separating. This destruction of the conductive framework in turn increases the resistance gradually. The droplet morphology is clearly observed in the strain sensors when the corresponding volume of the filler is less than 10 ml and when the volume of these fillers arrives at 10 ml, a consistent film is shaped on the outside of the TPU membrane. As the volume of the fillers increase, a rapid increase in the resistance of the sensors is observed. This is primarily because of the widening of the cracks in some areas resulting in an extensive division of the fillers. The TPU membrane itself is exceptionally sensitive with a large gauge factor (GF) of 321 which enables the strain sensor to function in a broad sensing range of >500%, mainly because of its extensive elasticity. This strain sensor can hence be used for monitoring, sensing and detecting various human body movements.Jang and co-workers developed another fiber sensor by exploiting a dip coating method.91 They used a conductive paste and PDMS beads to manufacture a fibre-based sensor (Fig. 5(a) and (b)). The sensor had high sensitivity over a broad sensing range and excellent durability. It was regulated by using a finite element method (FEM) (Fig. 5c) and its crack structure was observed using SEM (Fig. 5d). The sensor was further used to monitor the signals generated from the human heart and resistive responses to pulse signals in various bodily conditions of hypertension, normal blood pressure, and hypotension were also observed (Fig. 5(e) and (f)). This sensor was observed to be facile, cost effective, and was fabricated without using any metallic components such as Ag or Au nanowires/nanoparticles.
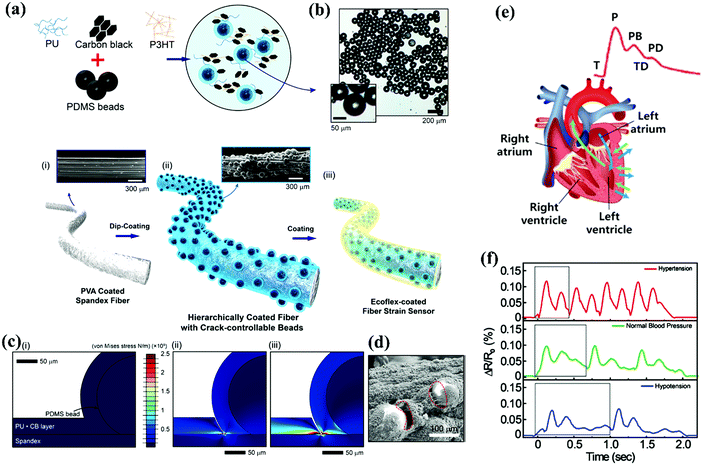 | ||
| Fig. 5 (a) Illustration of the preparation of a conductive paste for the sensor. (a, i) SEM image of the fibre sensor before coating with the conductive paste (a, ii,iii) SEM image of the fibre sensor after covering it using the conductive paste. (b) Optical microscopic image of the PDMS beads. (c, i–iii) FEM simulation to regulate the strain. (d) SEM image of the surface of the fibre when coated with beads, the red dotted line indicates the cracks upon application of strain. (e) Schematic illustration of the measurement of the signals generated from the heart in the form of waves. (f) Resistive responses to pulse signals in conditions of hypertension, normal blood pressure, and hypotension. Reproduced with permission from ref. 91. | ||
5. Perquisite performance metrics for wearable sensing
It is known that cellulose-based composites are easily made into a different variety of materials like fibres, yarns, mesh, fabrics, papers, and gels which can be then used for experimental work. These are then characterized by the sensing properties, including sensitivity, effective strain range, stability, response time, hysteresis, the linear relationship between strain and the change in relative resistance. It is widely observed that the stretchable network structure of gels and fabrics makes it functional over a wide working range, however, crack proliferation is observed in highly-sensitive papers and films. Different activities such as stretching, bending and contorting, and bowing, are distinguished using these strain sensors. Along with this, they display an excellent response and stability in pulse, motion of the vocal cords and bending of joints.92,93There are, however, many issues that need to be resolved for practical application, such as the evaluation of complex human motions, the detection of multi-dimensional structures and better fit for the human body contour.94,95 With some further improvements, cellulose-based strain sensors will have a huge impact on human health in the near future.
5.1. Stretchability and linearity
Different types of strain sensors show varying sensitivity. This behaviour is attributed to the type of the substrate and material used and the technique of fabrication. Sensors fabricated by aligning CNT on PDMS composites displayed a high stretchability of ε ≈ 280%.96 This high stretchability is attributed to the homogeneous microcrack propagation and the orientation of the aligned CNTs. Contrary to this, the strain sensors based on carbon nano papers showed a low stretchabilty value of ε ≈10%.97,98 This is mainly due to the missing of robust networks in certain nanomaterials having low aspect ratios. However, certain strain sensors fabricated using graphene-based composites showed better stretchability for example the graphene–nanocellulose nano paper composite (ε ≈ 100%) and CBs–TPE nanocomposite (ε ≈ 80%).92,99 Linearity is an important factor for strain sensors because it decides the amount of strain that can be accommodated by a particular sensor. One of the major drawbacks shown by the strain sensors of the resistive type is non-linearity.100 It was observed that strain sensors based on CNTs–polymer composites displayed a nonlinear electromechanical performance along with low sensitivity.101–103 The capacitive type strain sensors exhibited a remarkable linearity, but low sensitivity.103 Moreover, it was observed that strain sensors with high sensitivity responded with high non-linear behaviour when subjected to strain, and vice versa.5.2. Sensitivity or gauge factor
The gauge factor (GF) of the strain sensors is a measure of the slope of the relative change in the electrical signal, sometimes capacitance or resistance, against the strain applied. Zhou et al. propose a technique which includes the fabrication of sensors based on SWCNT paper and shows a high GF value of 107 at 50% applied strain.104 Altering the crack density and the thickness of these strain sensors can affect the properties and in turn the performance of the device. Moreover, the experiments suggest that we can get a set of smart materials by using different combinations of properties, namely, high sensitivity and medium flexibility or high flexibility and moderate sensitivity by using SNCWT papers of different thickness in the PDMS substrate. Altering these sensors to achieve the desired results can be done using two approaches: inter- or intra-laminar cracks, both of which yield varied results when tested for conductivity.105 Liu et al. used a basic dip coating process to manufacture a paper-based strain sensor which was economical, as well as stretchable and degradable.106 It was observed that this strain sensor had a GF of 4.3. Some other experiments show exceptionally flexible and adaptable strain sensors with a quick reaction time and remarkable sturdiness.107 Carbonized crepe paper (CCP) is obtained from cellulose, one of the most renewable, inexhaustible and biodegradable polymer resources of the planet. CCP has a corrugated surface with some carbon fibres aligned in it. The unique anisotropic arrangement of CCP allows the development of strain sensors with high GF, tensile strength, and flexibility, both parallel and perpendicular to the arranged carbon fibres. Another experiment carried out by Xia and colleagues used carbonized Chinese art paper (CCAP) as the substrate for a high-performance flexible strain sensor for monitoring human motions.108 It was observed that the fabricated CCAP strain sensor showed a high gauge factor of 68 when subjected to a strain of 100% and that of 248 under the strain in the range of 100–120%.5.3. Response time
Response time is a measure of the rate at which the strain sensors proceed towards a steady state response. A strain sensor of resistive type based on a CNT–ecoflex nanocomposite exhibited a response time of 332 ms. This large response time could possibly be a result of the ultra-soft elastomer used in fabricating the sensor.109 On the other hand, sensors of the capacitive-type based on AgNWs and CNTs showed a very quick response time of around 50–100 ms mainly due to its structural properties.110 A recovery time of 5 s was observed for the strain sensor based on aligned CNT thin films–PDMS and 100 s for the strain sensor-based CBs–elastomer composites respectively.966. Sensing approaches for wearable health monitoring
6.1. Strain sensor
Strain sensors are commonly used to detect electrical signals and to measure the strain caused by mechanical deformations. These strain sensors have a wider scope in health monitoring. At present, there are some commercially available strain sensors that have a low detection range. But there is growing interest in sensors that have suitable characteristics and can be used in various bio-medicinal applications.111,112For instance, Yan et al. fabricated a piezoresistive graphene–nanocellulose nanopaper which could be used for strain sensors, mainly due to its high stretchability.74 Vacuum filtration was used to attach cellulose fibers and crumpled graphene to a polycarbonate membrane. The stretchable nanopaper was further manufactured by embedding a flexible nanopaper in a liquid PDMS matrix (Fig. 6(a)–(c)). It was observed that the flexible nanopaper could sustain a very limited strain of 6%, whereas the fabricated stretchable nanopaper could be stretched up to 100% (Fig. 6d). The nanopaer was also used for detecting the human motions like the bending of a hand by embedding it on a glove (Fig. 6(e) and (f)).
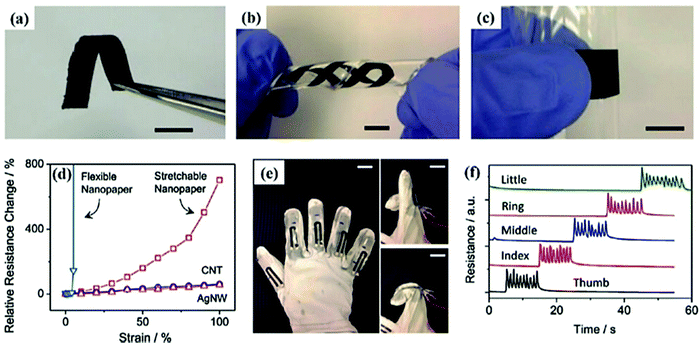 | ||
| Fig. 6 (a–c) Samples of flexible nanopaper. (d) Graph of the change of relative resistance vs. strain. A very high relative resistance change is observed at 100% strain. (e) Glove embedded with the flexible strain sensor on finger joints. (f) Relative resistance change response of a strain sensor upon bending and stretching of five finger joints. Reproduced with permission from ref. 74 | ||
Cellulose based sensors are widely exploited in several sensing platforms including healthcare monitoring. For instance, Li et al. successfully made a wearable strain/pressure sensor on the flexible conductive MXene/CNC coated TPU non-woven fabrics (NWF). These sensors displayed highly stable electrical properties and good sensing performances, owing to the hydrogen bonding of the constituents of the NWF and MXene/CNC layers which developed an effective interfacial bonding in the corresponding structure.113 As a result of the stiff conductive layer and elastic TPU matrix, a sensor with unique microcrack properties was achieved. The conductive NWF was subjected to a pre-stretching treatment. Additionally, by modifying the CNC stacking of the conductive layer, a tuneable strain detection ability was exhibited along with the construction of a modified and adjustable crack density. It was observed that the (MXene/CNC) MC0.6@p-NWF based strain sensor functioned over a large sensing range with a value of 83%, exhibited high sensitivity with a GF of ∼3405 and exhibited an ultralow detection limit with a range of 0.1%. Furthermore, these sensors were designed to provide reliable sensing across the desired frequency and response times as well as to maintain stability. It was shown that these sensors exhibited excellent properties which suggests their potential for a variety of applications, including human motion detection, signal collection, and health monitoring in stretchable E-skins and other wearable electronic devices.
Li et al. outlined a set of new, biocompatible, wearable, flexible strain sensors for monitoring human motion and health. It was found that, the major challenge in constructing a highly sensitive sensor which is also functional over a broad working range still exists, albeit the proposed different material choices and construction techniques.114 Additionally, it was observed that, when subjected to a huge strain and high pressure, these sensors lost the detection capacities as the maximum value of mechanical deformations had been reached which caused no deflections in the generated electrical signals. Additionally, these sensors, to be mounted on the human body, ought to have a versatile modulus of elasticity, similar to that of the human skin or epidermis. They should also possess enough stretchability so that they can adjust well on the human body. It is difficult to achieve compatibility between the sensing capacity and the flexibility of these devices, and striking a balance between the two is of utmost importance.
Moreover, for the sensors embedded in the body, monitoring its degradation kinetics is one of the primary concerns for its effective application as it has a direct impact on the proposed functions of the sensors.115 As of now, there have been many efforts reported in developing effective fabrication processes and in vitro experiments for biodegradable sensors, but, the limited in vivo testing restricts their function to clinical practices only. It will take a combination of several disciplines from now on, like chemistry, engineering material science, computer programming, machine-interfacing, among others, to construct the smart and adaptable sensors required for the future. The internet of things (IoT) being used for monitoring human health is yet to be developed dynamically and this Internet of Medical Things (IoMT) enables real-time communications of the patients with the doctors along with proper monitoring, detection and data collection.116 The concept is based on a portable, wireless communication system, used for a hassle-free procedure, wherein the data transfer takes place wirelessly. Several significant shifts in diagnostic methodologies from hospital-centric to patient-centric approaches are expected in the next few years. Chen and the team successfully developed a simple method to manufacture a hydrogel with anti-freezing properties, based on cellulose for wearable sensors. This material was robust, durable and showed high flexibility.117 During the preparation, first, an aqueous zinc chloride solution was used to solubilize hydroxypropyl methylcellulose in it. Then a polymerisation reaction using ceric ammonium nitrate as the initiator was carried out to graft the copolymers of acrylonitrile and acrylamide on the cellulosic chains. The results demonstrated that the hydrogel possessed properties of very high stretchability of around 1730%, remarkable tensile strength of approximately 160 kPa, good elasticity of about 90%, and toughness value of 1074.7 kJ m−3. The hydrogen bonds and dipole–dipole interactions resulted in remarkable fatigue resistance properties as well. It was observed that the introduction of zinc chloride, imparted remarkable electrical conductivity (1.54 S m−1) and effective functioning even at −33 °C. As a result, the hydrogel showed a good detection limit and sensing performances for detecting human motion.
| Materials used | Fabrication method | Elongation at break (%) | Elastic modulus | Sensitivity | Reference |
|---|---|---|---|---|---|
| MWCNTs/BSA/BC | Electrostatic self-assembly | 70 | 4.51 GPa | 1.57, 60 < ε < 70% | 119 |
| PAA–PPy/TOCNF | In situ free radical polymerization | 890 | 27.1 kPa | 7.3, 300 < ε < 800% | 120 |
| PAM/CMC | Dual-crosslinking | 1086 | 140 kPa | 1.4, 0 < ε < 30% | 121 |
| PAA/TA@CNCs | Dual-crosslinking | 2900 | 75 kPa | 4.9, 65 < ε < 75% | 85 |
| Allyl cellulose | Chemical-crosslinking | 126 | 36 kPa | 1.74, 20 < ε < 50% | 82 |
6.2. Pressure sensors
Pressure sensors are usually used in wearable devices primarily due to their excellent sensing properties. They can hence be used in pulse detection, voice recognition and motion detection.137 For example, Li et al. used a basic pyrolysis process to develop carbon cottons (CCs) which exhibited properties like low-density and electrical conductivity around 11 S m−1, using a cotton substrate.125 The composite was manufactured by vacuum aided infusing of PDMS resin in the CC structure. The CC/PDMS composite sensor exhibited a sensitivity of 6.04 kPa−1, a large working pressure up to 700 kPa, a broad response frequency from 0.01 to 5 Hz, and durability over 1000 cycles. The real time application of this sensor was realized by incorporating it into a sport shoe and a waist belt, which was used to monitor the health and sport performances of individuals. The manufacturing process of the sensor is cost effective and can be scaled-up for industrial production, mainly because cotton is used as the starting material. This sensor has a wide scope with applications in human health monitoring and wearable electronics in the form of prosthetic skins in the near future.Cellulose fibres were coated with multi-walled carbon nanotubes to develop flexible and pressure–responsive sensors.126 As shown in Fig. 7(a) and (b), the authors used cotton cellulose and multiwalled carbon nanotubes (MWCNTs) to fabricate these sensors and observed the morphologies using SEM (Fig. 7(c)–(g)). The stress–strain curves of the sensor were observed for various compositions of MWCNTs (Fig. 7h). These sensors were flexible, porous, cost-effective and a potential e-textile to be used.
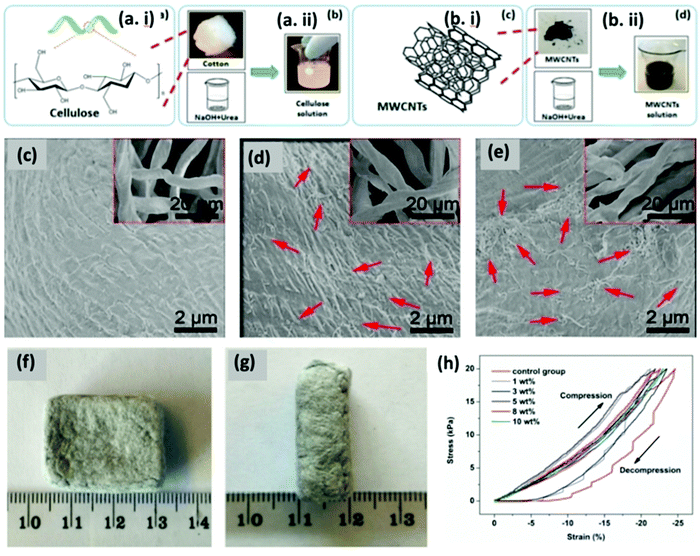 | ||
| Fig. 7 (a, i and ii) Chemical structure of the cotton cellulose and its swelling behaviour. (b, i and ii) Chemical structure of MWCNTs and its dispersion. (c) SEM image of pure cellulose substrate networks; pressure sensors with different mass fractions of MWCNTs, (d) 1 wt%, (e) 3 wt%, (f) planar view of the strain sensor, and (g) axial view of the strain sensor. (h) Stress–strain curves for the pressure sensor. Reproduced with permission from ref. 126. | ||
Li et al. fabricated conductive fibres to develop a stable textile sensor with high sensitivity and remarkable electrical properties.127 During the preparation, silver nanocomposite particles and elastic rubber was used to make these conductive fibres using coating methods with a remarkable electrical property of 0.15 Ω cm−1. Moreover, the nanoparticles' electrical networks were highly organized, showing durability and were stable to external forces for approximately 3000 bending cycles. It also exhibited high stretchability due to the presence of elastic rubber. Moreover, PDMS-coated conductive fibres were used to fabricate a textile-based capacitive pressure sensor. It showed remarkable characteristics of ultra-high sensitivity of about 0.21 kPa−1 in the low-pressure region, a quick response time interval of less than 10 ms, exceptional durability for about 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles and much less hysteresis. Furthermore, this textile sensor was woven into a fabric by integrating the fibres to develop a potential skin-like device for human machine interfacing. The sensors can also be integrated into clothes and gloves to monitor human motion wirelessly, making this a potential device for smart textile applications.
000 cycles and much less hysteresis. Furthermore, this textile sensor was woven into a fabric by integrating the fibres to develop a potential skin-like device for human machine interfacing. The sensors can also be integrated into clothes and gloves to monitor human motion wirelessly, making this a potential device for smart textile applications.
A stretchable pressure sensor was developed using cellulosic fibres by integrating swollen cellulose and MWCNTs.128 This sensor displayed intertwined, porous and selective permeable, and conductive frameworks. With 10 wt% of MWCNTs, the sensor exhibited sensitivity in the range of −0.0197 kPa−1, a reaction time of about 20 ms, a recovery time of approximately 20 ms, and a broad working pressure range from 0 to 20 kPa. Additionally, the pressure sensing devices put forth in this experiment enjoy the accompanying benefits: (i) it is sustainable and biodegradable as it has a flexible substrate made up of swollen cellulosic materials; (ii) its intertwined and porous structure facilitate air permeability paving its way for application as wearable electronic devices; (iii) it's a commercially viable process with minimal expenses and effective industrial scale-up possible.
To summarize, the above-mentioned sensors have the following merits: ultra-thin nature, flexibility, biocompatibility, broad pressure range and easy adaptability on the given substrate when compared to various other sensing devices fabricated to date. These types of sensors with much fewer morphological changes made in the functional materials could be a potential solution for the problems in the healthcare sector. Additionally, it can find a wide scope in monitoring human motions and for observing human health along with its applications in mechanical systems.
6.3. Human machine interactions
The current technological advancements focus on developing intelligent Human–Machine interfacing for various applications in the healthcare sector.95,129 There have been advancements in using cellulose-based sensors for monitoring human activities.130 The signals acquired from these sensors can be used to control smart machines and robots, allowing them to perceive and interact with the environment to provide real-time feedback, which can be used for surgical and other remote applications.131Researchers fabricated a flexible and ultra-sensitive pressure-sensing gadget with a wide detection limit using a simple, low-cost, and scalable method, based on silver nanoparticle (AgNP)–poly(3,4-ethylenedioxythiophene) (PEDOT)–paper composites.132 The intertwined fibres are covered with a layer of the conductive PEDOT and a permeable and stretchable paper is used as the supporting material. The PEDOT polymer coating imparts remarkable conductivity to the reinforcing cellulosic fibres. Additionally, it was observed that incorporating AgNPs in the PEDOT matrix increases the roughness of the polymer surface along with increasing the conductive nature of the material. Specifically, the diminished resistance and increased surface roughness of the paper composite substrate have a profound impact on the functioning of the fabricated pressure sensor. The diminished resistance reduces the energy required and the increased surface roughness extends the detection range of the sensor. The sensor endows properties like a wide distinguishing pressure range and exceptionally high sensitivity of 0.119 kPa−1 (at the 0–12 kPa range) with steady responses over 2000 cycles. These remarkable properties are coordinated to manufacture devices with sensing applications and for detecting human motions like breathing, phonation, pulses, heart beats and voice recognition and other light movements shown by the human body. Additionally, the team successfully demonstrated human–machine interaction association by voice recognition and musical translation of a piano keyboard. This paves a way for a bi-directional, twofold channel facilitating an in situ human behaviour, advanced digital signal correspondence and artificial intelligence programming.
Hang and team developed a stretchable and self-healing sensor for human motion detection using a poly(acrylamide) (PAAm) hydrogel.133 The sensor could be used in human–machine interfacing, monitoring healthcare and sports training. The sensor system converted digital signals through the analog-to-digital (ADC) convertor controlled by a microcontroller unit, MCU, as shown in Fig. 8a. The sensor was applied on a human arm to detect human motions [Fig. 8(b) and (c)] and the signals were monitored using an app on a smartphone (Fig. 8d).
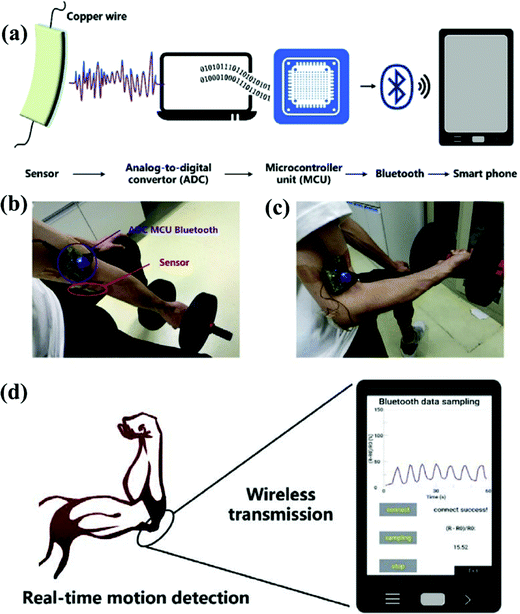 | ||
| Fig. 8 Real-time human machine interaction. (a) Schematic of a strain sensor based on a PAAm hydrogel system used to convert the digital signal through the ADC controlled by an MCU and then through wireless transfer of data to a smartphone using the bluetooth module. (b, c) The sensor mounted on an elbow to detect human motion like lifting of a dumbbell. (d) The interfacing of the app on a user's smart phone. Reproduced with permission from ref. 133. | ||
In another report, Cao et al. demonstrated a wearable and washable electronic textile that also functioned as a self-controlled motion/contact sensor for the detection and tracing of human–machine interactions.134 The screen-printed CNT used ink to make these sensors with easily engineered electrodes, along with being breathable and launderable. The E-textiles displayed tremendous adaptability and stability, and yet also provided an equivalent high conductivity of ca. 0.2 k sq−1 and air permeability of 88.2 mm s−1. Furthermore, the wearable E-textile displayed high detection limits along with a quick response to outer mechanical force to the uneven surface consisting of various microfibers based on the fabricated textile and CNTs. Moreover, this developed E-textile was found to be dependent on the single electrode mode, where the electrode array can function as a self-controlled motion sensor to examine the computer programming. The E-textile possesses numerous advantages, from wash-durability to minimal expense, and from large-scale manufacturing to biocompatibility and easy body adaptation, which make it a valuable material for wearable gadgets and human–machine interaction systems.
There have been several other advancements in the field of human–machine interfacing using textiles substrates.135,136 For instance, He et al. designed a cellulose fibre-based sensor for detecting human breathing.137 They implanted this sensor in a mask. The sensor was multifunctional, i.e. it successfully removed the PM2.5, killed bacteria and monitored breathing. Moreover, the sensors were fabricated using a low-cost method of constructing 1D CNFs onto cellulose microfibres (CM) to make a 2D nanostructure designed to synergistically remove PM2.5. Additionally, CMs were used as a template for developing Ag nanofiber membranes for wearable electronics used for monitoring breathing.
Yang and team demonstrated the use of bacterial cellulose (BC) for neural interfacing.138 They built multichannel microelectrode arrays on bacterial cellulose. BC is super-soft and has mechanical properties similar to that of the human tissue, making it an ideal material for interfacing. They used Acetobacter xylinum (ATCC53582) to produce BC by fermentation, while the electrodes were prepared using hot-pressed BC films, Au and Si3N4. These electrode arrays can find potential applications in the field of medicine, particularly in electromyography and electroencephalogy.
7. Summary and future trends
Many innovations have been made in the field of wearable electronics, but flexible strain sensors have proven to be especially important, primarily due to their adaptability. These devices find applications in many sectors and have been used for (i) E-skins; (ii) computer interfacing; (iii) monitoring human health and other aspects of the environment. The advantages of these sensors are mainly related to their easy and diverse manufacturing methods, durability, low-cost production methods, and easy scale-up for mass production. Additionally, they become biocompatible when cellulose is used as a substrate for manufacturing and display exceptional flexibility, robustness and reliable electrical sensing properties. These cellulose-based sensors can be easily moulded and provide tuneable characteristics and are widely used as biochemical and physical sensors for monitoring human health. Due to the recent advancements in research in materials science and engineering, cellulose and its derivatives have been used substantially in different forms like fibres and yarns, fabrics, membranes, paper and gels, which are moulded according to its end use. There have been remarkable efforts to transform the morphological structure of cellulose to develop smart materials with desired electrical conductivity, making it a potential smart material for wearable electronics.111,139Extensive progress has been achieved using cellulose-based smart materials for utilization in sensing devices, however, there are some major issues that need to be tackled to get productive outcomes:
(i) cellulose is a widely used biocompatible material used in different forms for making multifunctional wearable sensors. But, other components like metals, conjugated polymers and semi-conductors are also incorporated in the cellulosic substrate to increase its productivity and to give better results, which somehow restricts its use as a biocompatible wearable electronic device. Moreover, to impart elasticity, cellulose is often combined with PDMS, which is less biocompatible, making it difficult to implant these flexible epidermal sensors;
(ii) to fabricate flexible sensors that can be integrated with human organs and tissues, detailed analyses of chemical modifications, structural modifications, morphological characteristics, and conductive properties are required;
(iii) most of the available cellulose-based sensors serve only one purpose, so making multi-functional sensors is required. There are some properties of these sensors that are required to further expand their scope, such as remote and wireless sensing, self-healing, breathability and biodegradability. When a single sensor is used to detect multiple stimuli at the same time, it is difficult to differentiate between different types of stimuli due to their varying intensities. Human skin has the ability to detect multiple stimuli, effectively adapting to different temperatures and it shows self-healing properties as well. All these characteristics along with detecting various pressure and strains are essential prerequisites for making devices to monitor human health;
(iv) sensitivity and stretchability are important characteristics of a sensor along with linearity, hysteresis and response time which are some mechanical and electrical properties to realize the application of these sensors in human health monitoring. Currently, developing a broad working range for detection limits and stretchability is a challenge for cellulose-based sensors.
The above-mentioned factors are crucial for developing wearable electronics and multi-functional sensors used in human health monitoring. The following factors should be considered when developing these cellulose-based sensors in the near future: (i) constructing micro-structures with cellulose and its derivatives; (ii) making E-skins and skin-like wearable electronics with required mechanical and electrical properties; (iii) fabricating intelligent and multi-functional electronic devices using novel processing techniques for multifunctional application of storing energy, detecting pressure/strain and wireless sensing; (iv) making commercially viable and industrially scalable sensors for point-of-care (POC) devices from papers and cellulose-based fabrics with remarkable properties and its integration with other functional materials to produce a durable wearable electronic device; (v) using these devices for research can also be beneficial in other areas of biology, including drug delivery and the human body in general.112
In a nutshell, there has been continuous progress in developing cellulose and its derivatives to fabricate multifunctional sensors that give high-performance and better results. While some inspirational attempts have been made, the idea of using cellulose for a flexible sensor to monitor the health of humans is still in its infancy but has a lot of potential. Cellulose in its many forms like paper, macrofibres and cotton fabrics is used to develop cost-effective, durable, biocompatible and biodegradable sensors with exceptional stretchability and detection limits; and can be used further to get better performance. Clearly, cellulosic substrates with engineered morphologies and physicochemical properties are highly promising and have the potential to become the most sought-after material for real-time sensors.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.References
- D. dos Santos de Abreu, M. Strauss and M. Santhiago, Compr. Anal. Chem., 2020, 89, 361–395 CrossRef.
- S. Patel, H. Park, P. Bonato, L. Chan and M. Rodgers, J. Neuroeng. Rehabilitat., 2012, 9, 1–17 CrossRef PubMed.
- J. Zhong, Y. Zhang, Q. Zhong, Q. Hu, B. Hu, Z. L. Wang and J. Zhou, ACS Nano, 2014, 8, 6273–6280 CrossRef CAS PubMed.
- S. Gong, D. T. H. Lai, B. Su, K. Jye Si, Z. Ma, L. Wei Yap, P. Guo, W. Cheng, S. Gong, B. Su, K. J. Si, Z. Ma, L. W. Yap, P. Guo, W. Cheng and D. T. H. Lai, Adv. Electron. Mater., 2015, 1, 1400063 CrossRef.
- M. M. Hasan and M. M. Hossain, J. Mater. Sci., 2021, 56, 14900–14942 CrossRef CAS PubMed.
- M. O. Faruk, A. Ahmed, M. A. Jalil, M. T. Islam, A. M. Shamim, B. Adak, M. M. Hossain and S. Mukhopadhyay, Appl. Mater. Today, 2021, 23, 101025 CrossRef.
- S. Bhattacharjee, R. Joshi, M. Yasir, A. Adhikari, A. A. Chughtai, D. Heslop, R. Bull, M. Willcox and C. R. Macintyre, ACS Appl. Bio Mater., 2021, 4, 6175–6185 CrossRef CAS PubMed.
- J. Ren, C. Wang, X. Zhang, T. Carey, K. Chen, Y. Yin and F. Torrisi, Carbon, 2017, 111, 622–630 CrossRef CAS.
- G. Li, A. G. Nandgaonkar, Y. Habibi, W. E. Krause, Q. Wei and L. A. Lucia, RSC Adv., 2017, 7, 16737 RSC.
- H. Yuan, G. Yang, Q. Luo, T. Xiao, Y. Zuo, X. Guo, D. Xu and Y. Wu, Environ. Sci.: Nano, 2020, 7, 773–781 RSC.
- Q. Luo, Y. Huang, Z. Lei, J. Peng, D. Xu, X. Guo and Y. Wu, Ind. Crops Prod., 2021, 173, 114142 CrossRef CAS.
- Q. Luo, S. He, Y. Huang, Z. Lei, J. Qiao, Q. Li, D. Xu, X. Guo and Y. Wu, Ind. Crops Prod., 2022, 177, 114528 CrossRef.
- A. K. Mohanty, S. Vivekanandhan, J. M. Pin and M. Misra, Science, 2018, 362, 536–542 CrossRef CAS PubMed.
- B. Thomas, M. C. Raj, B. K. Athira, H. M. Rubiyah, J. Joy, A. Moores, G. L. Drisko and C. Sanchez, Chem. Rev., 2018, 118, 11575–11625 CrossRef CAS PubMed.
- C. Pang, C. Lee and K. Y. Suh, J. Appl. Polym. Sci., 2013, 130, 1429–1441 CrossRef CAS.
- B. K. Barman, Ø. Sele Handegård, A. Hashimoto and T. Nagao, ACS Sustainable Chem. Eng., 2021, 9, 9879–9890 CrossRef CAS.
- A. Ahmed, M. M. Hossain, B. Adak and S. Mukhopadhyay, Chem. Mater., 2020, 32, 10296–10320 CrossRef CAS.
- S. Gong, W. Schwalb, Y. Wang, Y. Chen, Y. Tang, J. Si, B. Shirinzadeh and W. Cheng, Nat. Commun., 2014, 5 DOI:10.1038/ncomms4132.
- A. Ahmed, M. A. Jalil, M. M. Hossain, M. Moniruzzaman, B. Adak, M. T. Islam, M. S. Parvez and S. Mukhopadhyay, J. Mater. Chem. C, 2020, 8, 16204–16215 RSC.
- C. L. Choong, M. B. Shim, B. S. Lee, S. Jeon, D. S. Ko, T. H. Kang, J. Bae, S. H. Lee, K. E. Byun, J. Im, Y. J. Jeong, C. E. Park, J. J. Park and U. I. Chung, Adv. Mater., 2014, 26, 3451–3458 CrossRef CAS PubMed.
- K. Takei, T. Takahashi, J. C. Ho, H. Ko, A. G. Gillies, P. W. Leu, R. S. Fearing and A. Javey, Nat. Mater., 2010, 9, 821–826 CrossRef CAS PubMed.
- S. Park, H. Kim, M. Vosgueritchian, S. Cheon, H. Kim, J. H. Koo, T. R. Kim, S. Lee, G. Schwartz, H. Chang and Z. Bao, Adv. Mater., 2014, 26, 7324–7332 CrossRef CAS PubMed.
- C. Wang, D. Hwang, Z. Yu, K. Takei, J. Park, T. Chen, B. Ma and A. Javey, Nat. Mater., 2013, 12, 899–904 CrossRef CAS PubMed.
- J. Fan, S. Zhang, F. Li, Y. Yang and M. Du, Cellulose, 2020, 27, 9157–9179 CrossRef CAS PubMed.
- P. Bingger, M. Zens and P. Woias, Biomed. Microdevices, 2012, 14, 573–581 CrossRef CAS PubMed.
- F. Lorussi, E. P. Scilingo, M. Tesconi, A. Tognetti and D. De Rossi, IEEE Trans. Inf. Technol. Biomed., 2005, 9, 372–381 CrossRef PubMed.
- A. Ahmed, S. Bain, Z. H. Prottoy, Z. Morsada, M. T. Islam, M. M. Hossain and M. Shkir, ACS Mater. Lett., 2022, 4, 68–86 CrossRef CAS.
- A. Šturcová, G. R. Davies and S. J. Eichhorn, Biomacromolecules, 2005, 6, 1055–1061 CrossRef PubMed.
- H. M. Ng, L. T. Sin, T. T. Tee, S. T. Bee, D. Hui, C. Y. Low and A. R. Rahmat, Composites, Part B, 2015, 75, 176–200 CrossRef CAS.
- D. Agarwal, W. MacNaughtan and T. J. Foster, Carbohydr. Polym., 2018, 185, 112–119 CrossRef CAS PubMed.
- P. Lu and Y. Lo Hsieh, Carbohydr. Polym., 2010, 82, 329–336 CrossRef CAS.
- S. Kalia, A. Dufresne, B. M. Cherian, B. S. Kaith, L. Avérous, J. Njuguna and E. Nassiopoulos, Int. J. Polym. Sci., 2011 DOI:10.1155/2011/837875.
- M. Jonoobi, J. Harun, A. Shakeri, M. Misra and K. Oksmand, BioResources, 2009, 4, 626–639 CAS.
- D. Miyashiro, R. Hamano and K. Umemura, Nanomaterials, 2020, 10(2), 182 CrossRef PubMed.
- J. George and S. N. Sabapathi, Nanotechnol., Sci. Appl., 2015, 8, 45–54 CrossRef CAS PubMed.
- A. Ahmed, B. Adak, Md. O. Faruk and S. Mukhopadhyay, Ind. Eng. Chem. Res., 2021, 60, 10882–10916 CrossRef CAS.
- Z.-Y. Wu, C. Li, H.-W. Liang, J.-F. Chen and S.-H. Yu, Angew. Chem., 2013, 125, 2997–3001 CrossRef.
- Q. Ding, X. Xu, Y. Yue, C. Mei, C. Huang, S. Jiang, Q. Wu and J. Han, ACS Appl. Mater. Interfaces, 2018, 10, 27987–28002 CrossRef CAS PubMed.
- C. Shao, L. Meng, M. Wang, C. Cui, B. Wang, C. R. Han, F. Xu and J. Yang, ACS Appl. Mater. Interfaces, 2019, 11(6), 5885–5895 CrossRef CAS PubMed.
- Y. J. Liu, W. T. Cao, M. G. Ma and P. Wan, ACS Appl. Mater. Interfaces, 2017, 9, 25559–25570 CrossRef CAS PubMed.
- N. Al-Dasoqi, A. Mason, R. Alkhaddar and A. Al-Shamma’A, World Environmental and Water Resources Congress 2011: Bearing Knowledge for Sustainability – Proceedings of the 2011 World Environmental and Water Resources Congress, 2011, pp. 3379–3388.
- A. Ashori and A. Nourbakhsh, Composites, Part B, 2010, 41, 578–581 CrossRef.
- A. Pinkert, K. N. Marsh and S. Pang, Ind. Eng. Chem. Res., 2010, 49, 11121–11130 CrossRef CAS.
- X. Chen, J. Chen, T. You, K. Wang and F. Xu, Carbohydr. Polym., 2015, 125, 85–91 CrossRef CAS PubMed.
- H. D. Huang, C. Y. Liu, D. Li, Y. H. Chen, G. J. Zhong and Z. M. Li, J. Mater. Chem. A, 2014, 2, 15853–15863 RSC.
- C. J. Kim, W. Khan, D. H. Kim, K. S. Cho and S. Y. Park, Carbohydr. Polym., 2011, 86, 903–909 CrossRef CAS.
- B. Adak and S. Mukhopadhyay, J. Appl. Polym. Sci., 2016, 133, 1–10 CrossRef.
- B. Adak and S. Mukhopadhyay, J. Text. Inst., 2017, 108, 1010–1017 CrossRef CAS.
- B. Adak and S. Mukhopadhyay, Single-polymer composites, CRC Press and Taylor & Francis, Boca Raton, 1st edn, 2018 Search PubMed.
- B. Adak and S. Mukhopadhyay, Cellulose, 2017, 24, 835–849 CrossRef CAS.
- D. Han and L. Yan, Carbohydr. Polym., 2010, 79, 614–619 CrossRef CAS.
- H. V. D. Nguyen, R. De Vries and S. D. Stoyanov, ACS Sustainable Chem. Eng., 2020, 8, 14166–14178 CrossRef CAS.
- X. Yang and E. D. Cranston, Chem. Mater., 2014, 26, 6016–6025 CrossRef CAS.
- W. Yang, H. Bian, L. Jiao, W. Wu, Y. Deng and H. Dai, RSC Adv., 2017, 7, 31567–31573 RSC.
- J. Leppiniemi, P. Lahtinen, A. Paajanen, R. Mahlberg, S. Metsä-Kortelainen, T. Pinomaa, H. Pajari, I. Vikholm-Lundin, P. Pursula and V. P. Hytönen, ACS Appl. Mater. Interfaces, 2017, 9, 21959–21970 CrossRef CAS PubMed.
- Q. Chen, H. Chen, L. Zhu and J. Zheng, J. Mater. Chem. B, 2015, 3, 3654–3676 RSC.
- D. Ye, C. Chang and L. Zhang, Biomacromolecules, 2019, 20, 1989–1995 CrossRef CAS PubMed.
- A. Ahmed, B. Adak, T. Bansala and S. Mukhopadhyay, ACS Appl. Mater. Interfaces, 2020, 12, 1687–1697 CrossRef CAS PubMed.
- S. Parveen, S. Rana and R. Fangueiro, J. Nanomater., 2013, 2013 Search PubMed.
- T. S. Anirudhan and S. R. Rejeena, Carbohydr. Polym., 2013, 93, 518–527 CrossRef CAS PubMed.
- J. Senthilnathan, Y. F. Liu, K. S. Rao and M. Yoshimura, Sci. Rep., 2014, 4(1), 1–7 Search PubMed.
- Q. Beuguel, J. R. Tavares, P. J. Carreau and M. C. Heuzey, J. Colloid Interface Sci., 2018, 516, 23–33 CrossRef CAS PubMed.
- V. Khoshkava and M. R. Kamal, ACS Appl. Mater. Interfaces, 2014, 6, 8146–8157 CrossRef CAS PubMed.
- R. M. A. Domingues, M. E. Gomes and R. L. Reis, Biomacromolecules, 2014, 15, 2327–2346 CrossRef CAS PubMed.
- B. V. Mohite and S. V. Patil, Biotechnol. Appl. Biochem., 2014, 61, 101–110 CrossRef CAS PubMed.
- E. C. Tsai, P. D. Dalton, M. S. Shoichet and C. H. Tator, Biomaterials, 2006, 27, 519–533 CrossRef CAS PubMed.
- M. Naseri-Nosar, M. Salehi and S. Hojjati-Emami, Int. J. Biol. Macromol., 2017, 103, 701–708 CrossRef CAS PubMed.
- D. Zhao, Y. Zhu, W. Cheng, W. Chen, Y. Wu and H. Yu, Adv. Mater., 2021, 33(28) DOI:10.1002/adma.202000619.
- S. Xu, W. Yu, M. Jing, R. Huang, Q. Zhang and Q. Fu, J. Phys. Chem. C, 2017, 121, 2108–2117 CrossRef CAS.
- M. Santhiago, C. C. Corrêa, J. S. Bernardes, M. P. Pereira, L. J. M. Oliveira, M. Strauss and C. C. B. Bufon, ACS Appl. Mater. Interfaces, 2017, 9, 24365–24372 CrossRef CAS PubMed.
- H. Xu, Y. F. Lu, J. X. Xiang, M. K. Zhang, Y. J. Zhao, Z. Y. Xie and Z. Z. Gu, Nanoscale, 2018, 10, 2090–2098 RSC.
- C. Deng, L. Pan, R. Cui, C. Li and J. Qin, J. Mater. Sci.: Mater. Electron., 2017, 28, 3535–3541 CrossRef CAS.
- H. Hosseini, M. Kokabi and S. M. Mousavi, Polymer, 2018, 137, 82–96 CrossRef CAS.
- C. Yan, J. Wang, W. Kang, M. Cui, X. Wang, C. Y. Foo, K. J. Chee and P. S. Lee, Adv. Mater., 2014, 26, 2022–2027 CrossRef CAS PubMed.
- S. Farjana, F. Toomadj, P. Lundgren, A. Sanz-Velasco, O. Naboka and P. Enoksson, J. Sens., 2013, 2013, 1–7 CrossRef.
- X. Liao, Z. Zhang, Q. Liang, Q. Liao and Y. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 4151–4158 CrossRef CAS PubMed.
- P. Sahatiya and S. Badhulika, Adv. Electron. Mater., 2018, 4(6) DOI:10.1002/aelm.201700388.
- M. Zhang, C. Wang, H. Wang, M. Jian, X. Hao and Y. Zhang, Adv. Funct. Mater., 2017, 27, 1604795 CrossRef.
- M. Zahid, E. L. Papadopoulou, A. Athanassiou and I. S. Bayer, Mater. Des., 2017, 135, 213–222 CrossRef CAS.
- M. S. Sadi, M. Yang, L. Luo, D. Cheng, G. Cai and X. Wang, Cellulose, 2019, 26, 6179–6188 CrossRef CAS.
- C. Chang and L. Zhang, Carbohydr. Polym., 2011, 84, 40–53 CrossRef CAS.
- R. Tong, G. Chen, D. Pan, H. Qi, R. Li, J. Tian, F. Lu and M. He, Biomacromolecules, 2019, 20, 2096–2104 CrossRef CAS PubMed.
- E. P. Gilshteyn, S. Lin, V. A. Kondrashov, D. S. Kopylova, A. P. Tsapenko, A. S. Anisimov, A. J. Hart, X. Zhao and A. G. Nasibulin, ACS Appl. Mater. Interfaces, 2018, 10, 28069–28075 CrossRef CAS PubMed.
- Y. Ye, Y. Zhang, Y. Chen, X. Han and F. Jiang, Adv. Funct. Mater., 2021, 403 DOI:10.1002/adfm.202003430.
- C. Shao, M. Wang, L. Meng, H. Chang, B. Wang, F. Xu, J. Yang and P. Wan, Chem. Mater., 2018, 30, 3110–3121 CrossRef CAS.
- M. Wang, R. Li, X. Feng, C. Dang, F. Dai, X. Yin, M. He, D. Liu, H. Qi and H. Qi, ACS Appl. Mater. Interfaces, 2020, 12, 27545–27554 CrossRef CAS PubMed.
- S. Liu, R. Zheng, S. Chen, Y. Wu, H. Liu, P. Wang, Z. Deng and L. Liu, J. Mater. Chem. C, 2018, 6, 4183–4190 RSC.
- D. Y. Choi, M. H. Kim, Y. S. Oh, S. H. Jung, J. H. Jung, H. J. Sung, H. W. Lee and H. M. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 1770–1780 CrossRef CAS PubMed.
- M. Wang, C. Shao, S. Zhou, J. Yang and F. Xu, Cellulose, 2018, 25, 7329–7340 CrossRef CAS.
- R. Mangayil, S. Rajala, A. Pammo, E. Sarlin, J. Luo, V. Santala, M. Karp and S. Tuukkanen, ACS Appl. Mater. Interfaces, 2017, 9, 19048–19056 CrossRef CAS PubMed.
- S. Jang, J. Kim, D. W. Kim, J. W. Kim, S. Chun, H. J. Lee, G. R. Yi and C. Pang, ACS Appl. Mater. Interfaces, 2019, 11, 15079–15087 CrossRef CAS PubMed.
- Y. R. Jeong, H. Park, S. W. Jin, S. Y. Hong, S. S. Lee and J. S. Ha, Adv. Funct. Mater., 2015, 25, 4228–4236 CrossRef CAS.
- A. Morteza, P. Aekachan, L. Sangjun, R. Seunghwa and P. Inkyu, ACS Nano, 2014, 8, 5154–5163 CrossRef PubMed.
- Alamusi, N. Hu, H. Fukunaga, S. Atobe, Y. Liu and J. Li, Sensors, 2011, 11(11), 10691–10723 CrossRef CAS PubMed.
- A. Ahmed, S. Sharma, B. Adak, M. M. Hossain, A. M. Lachance, S. Mukhopadhyay and L. Sun, InfoMat, 2022, e12295 Search PubMed.
- T. Yamada, Y. Hayamizu, Y. Yamamoto, Y. Yomogida, A. Izadi-Najafabadi, D. N. Futaba and K. Hata, Nat. Nanotechnol., 2011, 6, 296–301 CrossRef CAS PubMed.
- Y. Wang, R. Yang, Z. Shi, L. Zhang, D. Shi, E. Wang and G. Zhang, ACS Nano, 2011, 5, 3645–3650 CrossRef CAS PubMed.
- C. Mattmann, F. Clemens and G. Tröster, Sensors, 2008, 8, 3719–3732 CrossRef CAS PubMed.
- C. S. Boland, U. Khan, C. Backes, A. O’Neill, J. McCauley, S. Duane, R. Shanker, Y. Liu, I. Jurewicz, A. B. Dalton and J. N. Coleman, ACS Nano, 2014, 8, 8819–8830 CrossRef CAS PubMed.
- M. Amjadi, K. U. Kyung, I. Park and M. Sitti, Adv. Funct. Mater., 2016, 26, 1678–1698 CrossRef CAS.
- S. Tadakaluru, W. Thongsuwan and P. Singjai, Sensors, 2014, 14, 868–876 CrossRef PubMed.
- Y. Zheng, Y. Li, K. Dai, M. Liu, K. Zhou, G. Zheng, C. Liu and C. Shen, Composites, Part A, 2017, 101, 41–49 CrossRef CAS.
- D. J. Cohen, D. Mitra, K. Peterson and M. M. Maharbiz, Nano Lett., 2012, 12, 1821–1825 CrossRef CAS PubMed.
- J. Zhou, H. Yu, X. Xu, F. Han and G. Lubineau, ACS Appl. Mater. Interfaces, 2017, 9, 4835–4842 CrossRef CAS PubMed.
- I. A. Ventura, J. Zhou and G. Lubineau, Nanoscale Res. Lett., 2015, 10, 1–5 CrossRef CAS PubMed.
- H. Liu, H. Jiang, F. Du, D. Zhang, Z. Li and H. Zhou, ACS Sustainable Chem. Eng., 2017, 5, 10538–10543 CrossRef CAS.
- S. Chen, Y. Song, D. Ding, Z. Ling and F. Xu, Adv. Funct. Mater., 2018, 28(42) DOI:10.1002/adfm.201802547.
- K. Xia, X. Chen, X. Shen, S. Li, Z. Yin, M. Zhang, X. Liang and Y. Zhang, ACS Appl. Electron. Mater., 2019, 1, 2415–2421 CrossRef CAS.
- M. Amjadi, Y. J. Yoon and I. Park, Nanotechnology, 2015, 26, 375501 CrossRef PubMed.
- L. Cai, L. Song, P. Luan, Q. Zhang, N. Zhang, Q. Gao, D. Zhao, X. Zhang, M. Tu, F. Yang, W. Zhou, Q. Fan, J. Luo, W. Zhou, P. M. Ajayan and S. Xie, Sci. Rep., 2020, 3(5), 4357–4366 Search PubMed.
- M. O. Faruk, A. Ahmed, B. Adak, M. Marzana, M. M. Hossain and S. Mukhopadhyay, J. Mater. Chem. C, 2021, 9, 10193–10215 RSC.
- M. Marzana, Z. Morsada, Md. O. Faruk, A. Ahmed, Md. M. A. Khan, M. A. Jalil, Md. M. Hossain and M. M. Rahman, Chem. Rec., 2022, e202100319 Search PubMed.
- Q. Li, R. Yin, D. Zhang, H. Liu, X. Chen, Y. Zheng, Z. Guo, C. Liu and C. Shen, J. Mater. Chem. A, 2020, 8, 21131–21141 RSC.
- Y. Li, W. Chen and L. Lu, ACS Appl. Bio Mater., 2021, 4, 122–139 CrossRef CAS PubMed.
- H. Liu, Q. Li, Y. Bu, N. Zhang, C. Wang, C. Pan, L. Mi, Z. Guo, C. Liu and C. Shen, Nano Energy, 2019, 66 DOI:10.1016/j.nanoen.2019.104143.
- C. Li, C. Guo, V. Fitzpatrick, A. Ibrahim, M. J. Zwierstra, P. Hanna, A. Lechtig, A. Nazarian, S. J. Lin and D. L. Kaplan, Nat. Rev. Mater., 2020, 5, 61–81 CrossRef.
- S. B. Baker, W. Xiang and I. Atkinson, IEEE Access, 2017, 5, 26521–26544 Search PubMed.
- A. Sumboja, C. Y. Foo, X. Wang and P. S. Lee, Adv. Mater., 2013, 25, 2809–2815 CrossRef CAS PubMed.
- J. Huang, D. Li, M. Zhao, H. Ke, A. Mensah, P. Lv, X. Tian and Q. Wei, Chem. Eng. J., 2019, 373, 1357–1366 CrossRef CAS.
- Y. Chen, K. Lu, Y. Song, J. Han, Y. Yue, S. K. Biswas, Q. Wu and H. Xiao, Nanomaterials, 2019, 9(12) DOI:10.3390/nano9121737.
- Y. Cheng, X. Ren, G. Gao and L. Duan, Carbohydr. Polym., 2019, 233 DOI:10.1016/j.carbpol.2019.115051.
- R. Nur, N. Matsuhisa, Z. Jiang, M. O. G. Nayeem, T. Yokota and T. Someya, Nano Lett., 2018, 18, 5610–5617 CrossRef CAS PubMed.
- M. Marzana, Md. M. A. Khan, A. Ahmed, M. A. Jalil and Md. M. Hossain, Nanomater. Biocatal., 2022, 689–714 Search PubMed.
- H. Gullapalli, V. S. M. Vemuru, A. Kumar, A. Botello-Mendez, R. Vajtai, M. Terrones, S. Nagarajaiah and P. M. Ajayan, Small, 2010, 6, 1641–1646 CrossRef CAS PubMed.
- S. H. Park, H. B. Lee, S. M. Yeon, J. Park and N. K. Lee, ACS Appl. Mater. Interfaces, 2016, 8, 24773–24781 CrossRef CAS PubMed.
- H. Zhang, X. Sun, M. Hubbe and L. Pal, ACS Appl. Electron. Mater., 2019, 1, 1179–1188 CrossRef CAS.
- Y. Li, Y. A. Samad and K. Liao, J. Mater. Chem. A, 2015, 3, 2181–2187 RSC.
- J. Lee, H. Kwon, J. Seo, S. Shin, J. H. Koo, C. Pang, S. Son, J. H. Kim, Y. H. Jang, D. E. Kim and T. Lee, Adv. Mater., 2015, 27, 2433–2439 CrossRef CAS PubMed.
- G. Paravati and V. Gatteschi, Sensors, 2015, 15, 19487–19494 CrossRef.
- S. Jiang, L. Li, H. Xu, J. Xu, G. Gu and P. B. Shull, IEEE Trans. Ind. Electron., 2020, 67, 647–657 Search PubMed.
- G. Yang, C. Lee, J. Kim, F. Ren and S. J. Pearton, Phys. Chem. Chem. Phys., 2013, 15, 1798–1801 RSC.
- Y. J. Tsai, C. M. Wang, T. S. Chang, S. Sutradhar, C. W. Chang, C. Y. Chen, C. H. Hsieh and W. S. Liao, ACS Appl. Mater. Interfaces, 2019, 11, 10380–10388 CrossRef CAS PubMed.
- C. Z. Hang, X. F. Zhao, S. Y. Xi, Y. H. Shang, K. P. Yuan, F. Yang, Q. G. Wang, J. C. Wang, D. W. Zhang and H. L. Lu, Nano Energy, 2020, 76, 105064 CrossRef CAS.
- R. Cao, X. Pu, X. Du, W. Yang, J. Wang, H. Guo, S. Zhao, Z. Yuan, C. Zhang, C. Li and Z. L. Wang, ACS Nano, 2018, 12, 5190–5196 CrossRef CAS PubMed.
- X. Pu, S. An, Q. Tang, H. Guo and C. Hu, iScience, 2021, 24(1) DOI:10.1016/j.isci.2020.102027.
- Y. Xu, Q. Fei, M. Page, G. Zhao, Y. Ling, S. B. Stoll and Z. Yan, iScience, 2021, 24, 102736 CrossRef CAS PubMed.
- X. He, H. Zou, Z. Geng, X. Wang, W. Ding, F. Hu, Y. Zi, C. Xu, S. L. Zhang, H. Yu, M. Xu, W. Zhang, C. Lu and Z. L. Wang, Adv. Funct. Mater., 2018, 28, 1805540 CrossRef.
- J. Yang, M. Du, L. Wang, S. Li, G. Wang, X. Yang, L. Zhang, Y. Fang, W. Zheng, G. Yang and X. Jiang, ACS Appl. Mater. Interfaces, 2018, 10, 33049–33059 CrossRef CAS PubMed.
- Z. Morsada, M. M. Hossain, M. T. Islam, Md. A. Mobin and S. Saha, Appl. Mater. Today, 2021, 25, 101257 CrossRef.
| This journal is © The Royal Society of Chemistry 2022 |