Open Access Article
Open Access ArticleBioinspired carbon nanotube-based materials
Yi
Fan†
a,
Yaqi
Hou†
 ab,
Miao
Wang
ab,
Miao
Wang
 *c,
Jing
Zheng
*a and
Xu
Hou
*c,
Jing
Zheng
*a and
Xu
Hou
 *abd
*abd
aState Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China. E-mail: houx@xmu.edu.cn; zjing@xmu.edu.cn
bResearch Institute for Biomimetics and Soft Matter, Fujian Provincial Key Laboratory for Soft Functional Materials Research, Jiujiang Research Institute, College of Physical Science and Technology, Xiamen University, Xiamen 361005, China
cCollege of Materials, Xiamen University, Xiamen 361005, China. E-mail: miaowang@xmu.edu.cn
dInnovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen 361102, China
First published on 24th February 2022
Abstract
Bioinspired materials exhibit unique design strategies derived from nature, with remarkable biomimetic structures and identical biological features. Among them, bioinspired carbon nanotube (CNT)-based materials have attracted great interest because CNTs, as construction materials, have several advantages in terms of their structural, chemical, and physical properties. In this review, we present a systematic overview of the progress of CNT-based materials with design inspiration from nature in recent years. Firstly, the advantages of CNTs in the fabrication of bioinspired CNT-based materials are summarized, including their unique structure with high mechanical properties, controllable functionalized surfaces, and high conductivity. Secondly, different design strategies are categorized, where CNTs can directly act as artificial nanochannels, arrange to form CNT-assembled structures, or combine with inorganic, organic, and polymer materials to achieve CNT composites. Thirdly, their applications in reinforcement materials, energy conversion, nanopatterned surfaces, dry adhesion, and bioengineering are discussed. Finally, further challenges and perspectives are outlined, which will offer a comprehensive view and inspiration for scientists to develop bioinspired CNT-based materials with more advanced and beneficial performances.
1. Introduction
Over billions of years, biological systems have evolved into sophisticated structures with elaborate functions. Thus, ideas from the study of living organisms to create novel materials with outstanding characters (e.g., simplified devices, multifunction, superior performances, and intelligence activity) have proven to be important for solving problems in challenging situations. Bioinspired materials, products of this learning process by mankind, have been considerably utilized in aerospace, environmental treatment, energy conversion/storage, and biotechnology.1–8 Bioinspired materials are usually made of single or multi-component synthetic materials (as the construction materials). To date, numerous synthetic materials have been manufactured into bulk with particular structures, properties, and functions to construct bioinspired materials. The examples of recently developed bioinspired materials are commonly derived from the two bioinspired design strategies, i.e., mimicking structures of natural materials (e.g., chemical syntheses and biomimetic polymers toward bio-based materials, self-assembled bioinspired nanocomposites materials, and emerging artificial wood materials) and mimicking functions of living matters (e.g., gas-involved (photo) electrocatalysis with the regulation of biomimetic wetting in energy conversion, bioinspired nanofluidic iontronics, non-fouling surfaces with bioinspired dopamine chemistry and zwitterionic polymers).9–15To choose the appropriate materials to construct bioinspired materials, it is usually necessary to evaluate their application value in bioinspired design strategies. However, few materials have similar features to carbon nanotubes (CNTs), which are composed of nanoscale structures and possess outstanding chemical and physical properties.3,16–18 CNTs, discovered in 1991 by Iijima, are regarded as graphene sheets rolled up along Bravais lattice vectors to form hollow cylindrical nanostructures.19 There are mainly two types of CNTs, i.e., single-walled carbon nanotubes (SWCNTs, composed of one single graphene layer) and multi-walled carbon nanotubes (MWCNTs, formed from multiple graphene layers). The diameters of SWCNTs are generally narrower than that of MWCNTs, where the diameter of SWCNTs is in the range 0.8–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm and that of MWCNTs in the range 5–20
nm and that of MWCNTs in the range 5–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm.17,20 Different formations of SWCNTs by rolling of the graphene sheets along lattice vectors can result in three types of tubes (armchair, zigzag, and chiral tubes).20 The hollow morphology with nanoscale diameters and atomically smooth inner surfaces of CNTs make them ideal artificial materials with nanochannel geometries. Also, with the strongest carbon bonds, CNTs possess chemical stability, large surface with strong adsorption, low weight, and high mechanical strength. Together with the physical properties of electrical conductivity and possibility of versatile modifications to obtain special performances, CNTs are expected to be the most valuable materials in electronics, nanotechnology, materials science, and bioengineering.5,20–34 Nowadays, CNTs are easily obtained at a very low price due to the development of synthetic techniques. However, the demands from worldwide commercial interest will be satisfied by fabricating bioinspired CNT-based materials combined with the superior properties of CNTs, such as extremely high mechanical strength, electrical conductivity and multifunction.17Fig. 1 shows the timeline of the major advances of carbon nanotubes in their 30 year history.
nm.17,20 Different formations of SWCNTs by rolling of the graphene sheets along lattice vectors can result in three types of tubes (armchair, zigzag, and chiral tubes).20 The hollow morphology with nanoscale diameters and atomically smooth inner surfaces of CNTs make them ideal artificial materials with nanochannel geometries. Also, with the strongest carbon bonds, CNTs possess chemical stability, large surface with strong adsorption, low weight, and high mechanical strength. Together with the physical properties of electrical conductivity and possibility of versatile modifications to obtain special performances, CNTs are expected to be the most valuable materials in electronics, nanotechnology, materials science, and bioengineering.5,20–34 Nowadays, CNTs are easily obtained at a very low price due to the development of synthetic techniques. However, the demands from worldwide commercial interest will be satisfied by fabricating bioinspired CNT-based materials combined with the superior properties of CNTs, such as extremely high mechanical strength, electrical conductivity and multifunction.17Fig. 1 shows the timeline of the major advances of carbon nanotubes in their 30 year history.
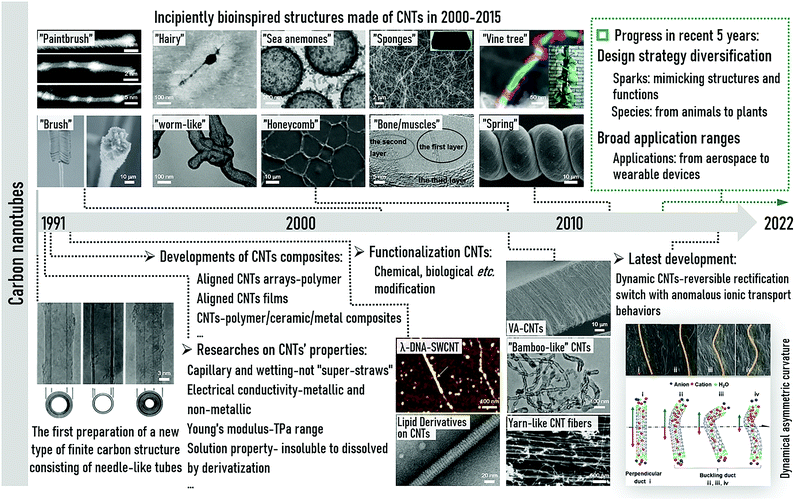 | ||
| Fig. 1 Timeline of the major advances of CNTs in their 30-year history. CNTs were firstly prepared in 1991. Reproduced with permission.19 Copyright 1991, Nature. Various studies have been conducted on their properties (e.g., capillary and wetting,35 electrical conductivity,36 Young's modulus,37 and solution properties38). In the same period, CNT composites have been developed (e.g., aligned CNT array-polymer,39 aligned CNT films,40 and CNT-polymer/ceramic/metal composites41). Also, there have been many functionalizations, normally chemical or biological modifications and CNT studies (e.g., λ-DNA-SWCNT. Reproduced with permission.42 Copyright 2003, the American Chemical Society. Lipid derivatives on CNTs. Reproduced with permission.43 Copyright 2003, Science.). Bioinspired CNT-based materials emerged in the early 20th century, normally having similar structures to natural organisms. In 2000–2015, various bioinspired structures were made by CNTs with morphologies of “brush” (Reproduced with permission.44 Copyright 2005, Nature.), “paintbrush” (Reproduced with permission.45 Copyright 2006, the American Chemical Society.), “worm-like” (Reproduced with permission.46 Copyright 2006, Wiley-VCH.), “hairy” (Reproduced with permission.47 Copyright 2007, Wiley-VCH.), “honeycomb” (Reproduced with permission.48 Copyright 2009, Wiley-VCH.), “sea anemones” (Reproduced with permission.49 Copyright 2008, Wiley-VCH.), “sponges” (Reproduced with permission.50 Copyright 2009, Wiley-VCH.), “spring” (Reproduced with permission.51 Copyright 2012, Wiley-VCH.), “vine tree” (Reproduced with permission.52 Copyright 2014, Wiley-VCH.), etc., and even adopted the “bone/muscle” strategy (Reproduced with permission.53 Copyright 2009, Wiley-VCH.), etc. New structures of CNTs, such as VA-CNTs (Reproduced with permission.54 Copyright 2004, Science.), “bamboo-like” CNTs (Reproduced with permission.55 Copyright 2005, Wiley-VCH.), and CNT yarn (Reproduced with permission.56 Copyright 2010, Wiley-VCH.), have also laid the foundation for the design of novel bioinspired CNT-based materials. In the upper right corner, the progress of bioinspired CNT-based materials in the recent 5 years is summarized. Besides, our group also has paid great attention to CNTs, including bioinspired nanoscale channels,57–60 CNT-based desalination,61–65 and CNT-based nanogenerators.66 One study is shown in the bottom right corner: dynamic CNTs. Reproduced with permission.65 Copyright 2019, Wiley-VCH. | ||
Here, we define “bioinspired CNT-based materials” as materials that are inspired by biological structures or functions and developed based on single CNT, assembled CNT or CNT composites for reinforcement, energy conversion, dry adhesion, and bioengineering applications. A systematic review on the recent progress of bioinspired CNT-based materials is provided, including superior properties of CNTs in construction, their design strategies, and applications (Fig. 2). The following content is mainly composed of three parts, as follows: (i) the superiorities of CNTs in designing bioinspired CNT-based materials; (ii) the design strategies for bioinspired CNT-based materials: CNTs directly as artificial nanochannels or as construction materials with CNT-assembled structures and CNT composites; and (iii) typical examples of bioinspired CNT-based materials in applications of reinforcement materials, energy conversion, nanopatterned surfaces, dry adhesion, and bioengineering. Finally, the current challenges and future prospects in the development of CNTs are briefly summarized. We envision that this work will inspire researchers to focus on the extensive application of bioinspired CNT-based materials and eventually realize the application of CNTs in practice.
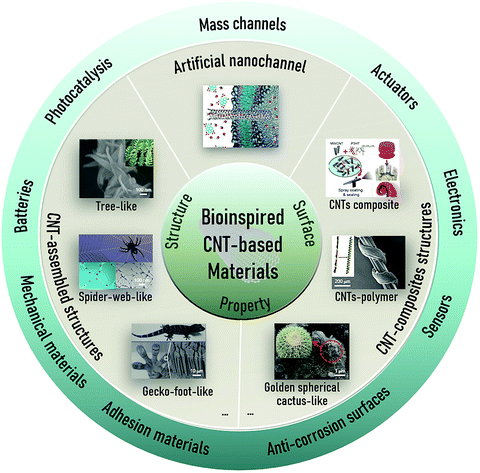 | ||
| Fig. 2 Based on the superiorities of CNTs in terms of structure, surface, and properties, diverse design strategies are used to fabricate bioinspired CNT-based materials in various fields. Artificial mass transport. Reproduced with permission.67 Copyright 2017, Science. CNT-based tree-like materials in photocatalysis. Reproduced with permission.68 Copyright 2015, Elsevier. CNT-based spider-web-like materials in designing mechanical materials. Reproduced with permission.69 Copyright 2016, the American Chemical Society. CNT-based gecko-foot-like materials in designing adhesion materials. Reproduced with permission.70,71 Copyright 2012, Wiley-VCH. Copyright 2008, Science. CNT-based golden spherical cactus-like materials in designing anti-corrosion surfaces. Reproduced with permission.72 Copyright 2018, Elsevier. CNT-polymer materials in electronics. Reproduced with permission.73 Copyright 2019, the American Chemical Society. CNT composites in actuators. Reproduced with permission.74 Copyright 2021, the American Chemical Society. | ||
2. Superiorities of CNTs in designing bioinspired CNT-based materials
2.1 Unique structure
Owing to the intrinsic morphology of hollow cylinders with nanometer diameters and allotropes of carbon made of only carbon atoms with chemical inertness through sp2 bond networks, CNTs possess a unique structure and properties. Hollow nanoscale channels have the equivalent size to that of endogenous protein channels, making CNTs ideal materials for studying the transport of ions and molecules. Resulting from their covalent sp2 bonds, CNTs are the strongest and stiffest materials reported to date with a tensile strength of up to 100 GPa and Young's modulus of 1 TPa (about 5-times higher than that of steel with a value of 210 GPa). The highest mechanical property of CNTs make them distinguished reinforcement materials in designing bioinspired CNT-based materials, mostly working as frames or substrates.16,75–78 Piling or stacking individual CNTs also results in the formation of patterned architectures in bioinspired CNT-based materials with interesting behaviors (e.g., high strength and toughness, and high dry adhesion).79 More importantly, the elaboration of other characteristics in bioinspired CNT-based materials is related to the natural structure of CNTs.2.2 Controllable surface
CNTs have nanoscale tubular structures with a surface area of up to 50–200 m2 g−1.80 Due to the presence of the strongest non-polar C–C bonds and hydrophobicity of pure sp2 hybrid carbon bonds, CNTs are naturally hydrophobic and insoluble in aqueous and organic solvents. Studies have indicated that bioinspired CNT-based materials can be obtained with rough surfaces and low surface energy by introducing CNT fragments.18,81–83 However, the hydrophobicity of CNTs restricts the applicability of bioinspired CNT-based materials. In bioengineering, the excellent along the tubular axis, which is different from graphene and the biocompatibility of bioinspired CNT-based materials with biosafety is necessary.24,84,85 Modification of the hydrophilic groups (e.g., carboxylic, carbonyl, and hydroxyl through well-established methods) on the surface of CNTs will overcome the existing bottlenecks. Due to the natural mechanical behavior and functionalized surfaces of CNTs, the bioinspired CNT-based materials will perform admirably in bioskeletons and biosubstrates. To date, several chemical modifications (e.g., minimum alteration by non-covalent functionalization and permanent modification by covalent functionalization) and doping methods have been developed for the functionalization of CNTs.86 These approaches allow the surfaces of CNTs to become controllable functionalized surfaces by integrating various functional groups, and also allow additional behaviors to be obtained through the functionalization process. The controllable functionalized surface can improve the properties of CNTs, which is another superiority to enhance the performances of bioinspired CNT-based materials.87–932.3 High conductivity
As carbon materials, CNTs are either metallic or semiconducting along their tubular axis, which is different from graphene and other carbon materials. Thus, by changing the alignment, diameter, aspect ratio, and chirality in CNTs, the electronic conductivity of bioinspired CNT-based materials can be regulated.18 Befitting from the prominent high conductivity, artificial mass transport nanochannels, and good mechanical properties of CNTs, bioinspired CNT-based materials have become popular alternative materials to solve the problems in energy conversion, for example, improving the poor electrical conductivity, quick capacity decay, and lithium anode erosion in lithium–sulfur (Li–S) batteries, solving the problems of high volume expansion or contraction in the processes of charging and discharging, poor cyclability and rate capability of oxides in lithium-ion batteries (LIBs), abating capacity fading and increasing inferior rate in sodium-ion batteries (SIBs), and even promoting photoenergy conversion in photocatalysis. Moreover, the high conductivity of bioinspired CNT-based materials make them valuable for application in biosensors and bioelectronics. In the system, once specific molecules or physical stimuli are recognized by the decorated functional groups on the surface of CNTs through specific interactions, the CNTs in the bioinspired materials can output the correlated information with electrical signals. Apparently, smart bioinspired CNT-based materials can obtain exceptional electrical responses due to the integration of CNTs.Table 1 lists the popular mimicked living organisms based on CNTs in the last 5 years, as well as the chemical components of bioinspired CNT-based materials, the functional activities of CNTs in bioinspired materials, and their potential applications. In the next section, the corresponding design strategies for bioinspired CNT-based materials are further reviewed.
| Inspiration | Material & hierarchical structure | Functional activities | Potential applications | Ref. |
|---|---|---|---|---|
| Silk fiber | CNT-SF & G–G fibers | High mechanical framework for fibers | Structural materials | 94 |
| Spider silk | BISS-SWCNTs fibrils | Stable structural substrate and conductive frameworks for electron transfer | Electro-mechanical materials | 69 |
| Muscle | CNT yarn (by mechanical training) | 95 | ||
| Mussel | Mussel-inspired chemistry: CNTs/PDA GW-hydrogel | Nanoreinforcements for increasing the conductive and mechanical properties | Self-adhesive bioelectronics | 96 |
| TA-CNTs | 97 | |||
| CNTs/PDA/PANi | One-, and two-dimensional conductivity materials | Flexible thermoelectric generators | 98 | |
| Nacre | CNT@PANI/rGO/TA | Reinforcement for high strength and conductivity | Portable and wearable electrical devices | 99 |
| Salvinia molesta leaf | E-glass/MWCNTs (by ISA-3D) | Mechanical support and surface roughness donor | 3D printed materials | 83 |
| Wood | PTCDA/NC/CNT composite | 3D conductive frameworks with micro/nanotunnels structures for ionic/electronic transport | Batteries | 100 |
| Spider web | MWCNT/γ-Fe2O3 web | Mechanical support | High-performance energy storage materials | 101 |
| Nacre | CNTs/PVP | Conductive network | Batteries | 102 |
| Stem | Sn4P3@CNT/C composite | Electron expressway and mechanical support | Batteries | 103 |
| Ear-of-wheat | MWCNTs/MONPs composite | Conducting network | Batteries | 104 |
| Trees | ciO2/CNT composites | Direct electron transport channels in photocatalysis with large specific surface and thermoelectric conductivity | Photocatalysis materials | 68 |
| Golden spherical cactus | PSU/CNTs/FEP with microsphere and nanothorns | GC-like structure donor and the network structures that conduct electrons | Superhydrophobic materials | 72 |
| Gecko feet | VA-CNT strands | Adhesion strength | Adhesion materials | 105 |
| CNTF | Adhesion to electrical and thermal management | 93 | ||
| 3DP GC | Enhancement in flexural strength | Energy conversion, energy storage, environmental or electronic systems | 106 | |
| Chameleon | HPC-PACA-CNT composite | Enhancement in conductivity and capability of reporting stimuli through resistance | Multifunctional flexible E-skins | 107 |
| Homarus americanus claws | Radial and circumferential aligned MWCNT-S | Multifunctional nanofillers with unique structures and excellent mechanical properties | Aerospace, mechanical, and tissue engineering | 108 |
| Evaporation of sweat Transpiration of plants | Porous-structured CNT films | Optical absorptivity from π-band optical transitions with a wide range of 200 nm–200 μm and fast water transport channels | Solar hygiene systems, freshwater distillation, and electrical power generation | 109 |
| Polar bear pelt | Porous Ag/cellulose/ CNT-laminated nanofiber membrane | High solar radiation absorptivity | Thermal control materials, smart garments, and wearable electronics | 110 |
| Moth eye | SWCNT coating-deposited Si | Optical properties with refraction index neff = 1.01–1.10 | Anti-reflective coatings | 111 |
3. Design strategies
Among the synthetic materials adopted for the fabrication of bioinspired materials, CNTs are regarded as unique materials. Their high availability in the formation of bioinspired CNT-based materials mainly comes from their superior chemical and physical properties with unique hollow structure, high strength, high toughness, electrical conductivity, chemical stability, etc., and their diverse construction assemblies and design strategies.67,83,98–100 In this section, we first discuss the individual CNTs with 1D natural nanochannels to mimic protein channels, and then a series of bioinspired CNT-based materials with the design strategies of CNT-assembled structures (e.g., metal oxide-anchored CNTs, CNT fibers, CNT yarns, CNT bundles, CNT web, and CNT array) and the CNT composites structures (e.g., CNT-polymer composite, CNT layer-by-layer composite, and CNT hydrogel).3.1 Directly as artificial nanochannels
Biological nanochannels, such as water, calcium (Ca2+), and sodium (Na+) protein channels, play critical roles in physiological processes, sophisticatedly regulating the release of ions and biomolecules.112,113 Inspired by these subtle nanostructures, creating artificial materials with similar morphologies by replicating biological processes has become a popular way to understand cellular events. CNTs, a promising allotrope of carbon, are composed of only carbon atoms. Their seamless cylindrical morphology with nanoscale inner diameter (for SWCNTs, their diameters are 1–6 nm, while that for MWCNTs is 2–100 nm) makes them ideal artificial nanochannel platforms for mimicking the mass transport in nano-confined space. When molecules such as water molecules pass through these nanochannels, the inner graphitic walls and confined smooth nanochannel structures of CNTs enhance the transport of water.114Computational chemistry has become a very popular and instructive method to evaluate the mass transport in CNT-based nanochannel materials. As shown in Fig. 3a, one study utilized molecular dynamics (MD) simulations to explore the ion permeation and selectivity in series computer-designed CNT-based biomimetic nanopores, in which the series models (C0, C-2, C-4 and C-1, where the C0 model nanopore has two carboxylate and two amine groups (net charge 0), C-2 model nanopore has two carboxylate groups (net charge −2), C-4 has four carboxylate groups (net charge −4), and C-1 has two carboxylate and one amine group (net charge −1)) of filters were distributed, deriving the natural Ca2+ and Na+ channels.115 These resultant nanopores had comparable ion selectivity with the structures of pivotal proteins in bacterial porins and voltage-gated channels (here, OmpE and NavAb were chosen). This study provides a novel approach to obtain advanced artificial CNT-based nanopores. Firstly, the “design principles” learned from nature are tabulated, then these principles are validated and optimized by computer simulation, and finally the principles are employed to design CNT-based materials. The size regime (nanometer-size of channels, also called nanoconfinement) is the integral parameter of CNTs, which seriously affects the transport of ions, water, and biomolecules. A smaller size has a great impact on fast, nearly friction-less flux water flow (flowing in single-file water type) and unusual ion selectivity (Fig. 3b).89,114,116 In a pore with the size of 1.5 nm under an applied electric field, two K+ cations (blue) rapidly pass through within ∼2 ns, which is accompanied by the overall flow of water molecules (red) through the CNT channel. The electrostatic effects and strong electroosmotic coupling are supposed to cause these phenomena.
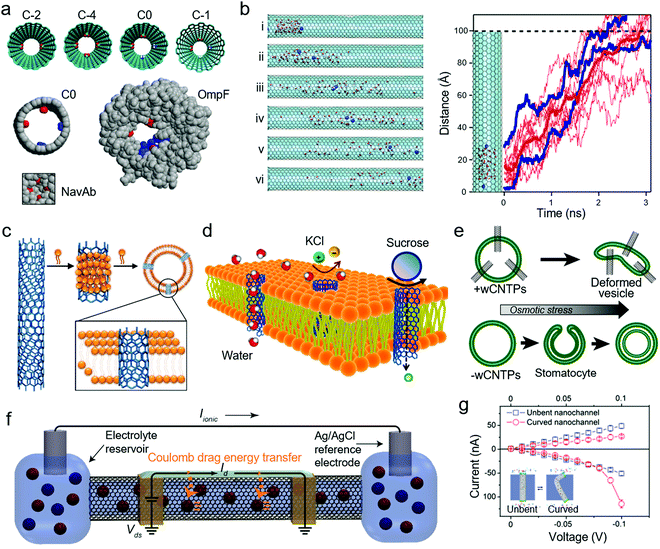 | ||
| Fig. 3 (a) Simulation of derivatized CNTs and comparison of the C0 model nanopore with two biological nanopores. Reproduced with permission.115 Copyright 2012, National Academy of Sciences. (b) MD simulations of water and ion transport in CNTPs. Reproduced with permission.116 Copyright 2019, the American Chemical Society. (c) Preparation and incorporation of CNTPs in liposomes. Reproduced with permission.117 Copyright 2014, Nature. (d) Osmotically driven transport in CNTPs. Reproduced with permission.118 Copyright 2014, the American Chemical Society. (e) Presence of CNTPs resulted in distinct polymersome responses to osmotic stress. Reproduced with permission.120 Copyright 2018, Wiley-VCH. (f) Electrically actuated, CNT-based biomimetic ion pump. Reproduced with permission.121 Copyright 2019, the American Chemical Society. (g) Dynamic curvature nanochannel-based membrane. Reproduced with permission.125 Copyright 2019, Wiley-VCH. | ||
Carbon nanotube porins (CNTPs), a system in which short pieces of CNTs self-insert into the lipid bilayer, are the representative model for bioinspired CNT-based materials to truly mimic the biological membrane channels. In 2014, Noy et al. firstly established CNTPs.117Fig. 3c shows a simple prototype for a CNT-based system as artificial nanochannels under physiological conditions. In this system, small molecules are transported but large uncharged species are rejected because of the size exclusion (Fig. 3d). These rejection characteristics were governed by the mechanisms of the electrostatic repulsion and the Donnan membrane equilibrium.118 Further studies showed that the rate of proton transport would be enhanced by an order of magnitude in the 0.8 nm-diameter CNTPs. This is quite different from that in the 1.5 nm-diameter CNTPs, where Ca2+ ions can modulate this proton conductance.119 In 0.8-nanometer-diameter CNTPs, water molecules are forced into a single-chain configuration and anion transport is blocked even in the presence of salts. The tunable ion selectivity configured the CNTPs into the switchable ionic diodes.67 It has also been reported that amphiphilic block copolymers were utilized as lipid bilayers when combining CNTs to get a fully synthetic biomimetic membrane, where they still maintained high proton and water permeability and had an additional response to osmotic stress within the robust polymersomes (Fig. 3e).120 In conclusion, these CNTPs exploit the unique structure of CNTs with suitable biomimetic nanochannels and selective mass transport performances, which brilliantly present insights to extend the applications of bioinspired CNT-based nanochannel materials in novel biomimetic systems.
To date, SWCNTs are widely understood as transporters of electronic current, electrolyte, and ions, where they act as synthetic ion channels. Integrating ion transistors and electron transistors in devices can provide SWCNT-based bionic materials with breakthrough performances in mass transport. As shown in Fig. 3f, Shepard et al. demonstrated an electrically actuated, CNT-based biomimetic ion pump with the ability to simultaneously transport electrons and nanofluids in a single SWCNT device.121 The Coulomb drag effect was engineered in this SWCNT device, which realized electrolyte transport without a potential difference and pressure gradient. In addition, chemically modified or doped CNTs have emerged, breaking the limitation that bioinspired CNT-based materials are not sufficient to satisfy diverse specific demands in real-life applications.122 Highly efficient electroosmotic flow was observed in Hinds’ lab by functionalizing a surface with a pre-microtome vertically aligned CNT-epoxy composite.123,124 One recent study in Hou's lab demonstrated another method of reversible ion transport control. The system was fabricated using a CNT array-polydimethylsiloxane composite.125 As shown in Fig. 3g, ionic rectification could be adjusted in real time by dynamically changing the channel curvature.
In conclusion, we summarized the following development characteristics of CNTs as artificial nanochannels in bioinspired CNT-based materials in the past 5 years. (1) Computational chemistry has been widely used to guide the design of new CNT-based materials for mass transport in nano-confined channels. (2) Novel systems such as the emergence of CNTPs, which have identical and even exceptional bio-molecule permeability properties. (3) Various methods, such as integrating ionic and electronic transistors with SWCNTs, chemically modifying or doping CNTs in aligned CNT-polymer composites, and controlling the curvature of CNTs, causing CNT-based material systems to have unique mass transport behaviors, which can be applied in numerous fields ranging from biosensing and nanofluidics to filtration.
3.2 As construction materials
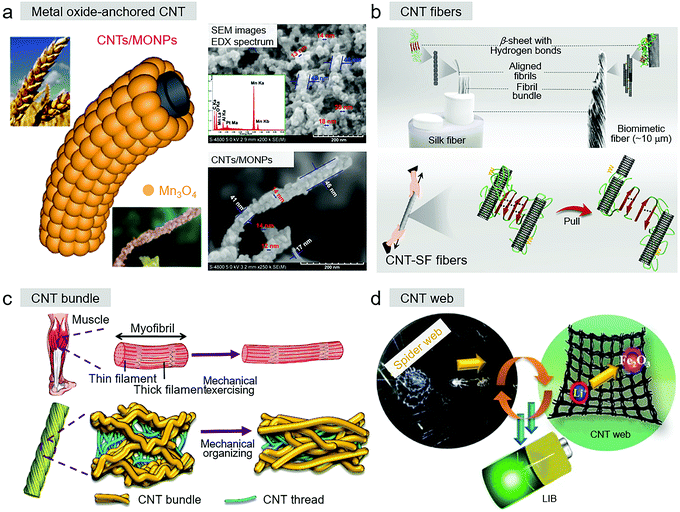 | ||
| Fig. 4 (a) Biomimetic ear-of-wheat-shaped Mn3O4 nanoparticles on CNTs. Reproduced with permission.104 Copyright 2020, Wiley-VCH. (b) Biomimetic CNT-SF fiber and its breaking process. Reproduced with permission.94 Copyright 2021, Wiley-VCH. (c) Bioinspired microstructure-reorganized behavior of CNT yarn. Reproduced with permission.95 Copyright 2020, The Royal Society of Chemistry. (d) Biomimetic spider-web-like MWCNT/γ-Fe2O3 composites. Reproduced with permission.101 Copyright 2017, Wiley-VCH. | ||
Carbon nanotube fibers (CNT fibers) and CNT yarns are almost identical assembly types of CNT-based materials in bioinspired design, which are famous for overcoming the length limitation of individual CNTs, possessing a length of up to several kilometers. However, the breaking strength of individual CNTs generally decreases when they are assembled into structures similar to CNT-based fibers or yarn materials. The weak van der Waals forces in adjacent nanotubes are the key factor in this limitation. One approach learned from nature is to enhance the mechanical strength by introducing stronger interactions between neighboring CNTs. The design inspirations come from natural masterpieces including silk and muscles, which have hierarchical structures and possess excellent mechanical properties. Learning from the biological structures of natural silk fibers, reinforcing strategies of interactions force replacement and inorganic–organic interfacial design were developed.126 Mimicking silk fibers, Zhang et al. injected silk fibroin (SF) and glycerol into spun CNT fibers and obtained composite CNT-based fibers (Fig. 4b), showing an increased breaking strength of up to 1023 MPa (+250%), improved toughness from 7.8 to 10.3 MJ m−3 (+132%), and Young's modulus of 81.3 GPa (+442%).94 The MD simulation indicated that the glycerol-rich β-sheet of SF together with abundant hydrogen bonds between SF and CNTs contributed to enhancing the mechanical properties of the CNT fibers. To construct the assembled CNT yarns with higher mechanical properties, inspired by the mechanical exercising-induced hierarchical structure of human muscles, through cyclic stretching training (cyclic stretching or cyclic loading), Xu et al. firstly created a novel microstructure-reorganized strategy to fabricate a hierarchical CNT bundle and CNT thread mechanical organizing structure (Fig. 4c).95 This structure entirely copied the structural organization behavior of human muscles and had +64% tensile strength, +148% Young's modulus, +30% conductivity, and +35% enhanced piezo-resistive sensitivity than that of the pristine CNT yarn.
The spider with a web-like shape structure is another bioinspired design strategy for bioinspired CNT-based materials with particular structures of CNT web. As shown in Fig. 4d, to improve the cell performance, a method of controlling the structure and composition of the anode materials was conducted, where a material with biomimetic spider-web-like MWCNT networks was demonstrated by Park et al., which had the structural characteristic and working patterns of sticky spider-webs.101 In this system, the MWCNT web functioned as the conductive support with 3D internetworked pathways to improve the percolated transport, and γ-Fe2O3 particles as the active sites tightly attached on the surface of the MWCNT network to make the construction more stable. Ultimately the obtained biomimetic LIB exhibited a high capacity of 822 mA h g−1 at 0.05 A g−1 with a fast rate capability of 72.3% retention at a current density in the range of 0.05 to 1 A g−1, and promising cycling stability (>88% retention after 310 cycles with >99% Coulombic efficiency).
Geckos, with extraordinary adherence and climbing ability on diverse surfaces in various atmospheres, have fascinated humans for millennia. Utilizing modern micro-nano morphological imaging techniques (SEM, TEM, and AFM), the secret of their climbing mechanism has been partly revealed. The van der Waals forces from the spatula of the geckos’ feet with a hierarchical structure are considered as the origin of their adhesion. Accordingly, inspired by the biological structure of geckos’ feet, various fabrication strategies have been developed to create synthetic dry adhesive surfaces. Among the adopted materials, the vertically aligned CNTs (VA-CNTs) are outstanding because of their remarkable mechanical properties, large aspect ratio, and exceptional alignment type by just abundant hollow individual CNTs via horizontal van der Waals interactions. Theoretical simulation has predicted that their adhesion strength is up to 500 N cm−2 on glass, which is much higher that of the natural array on geckos’ feet.
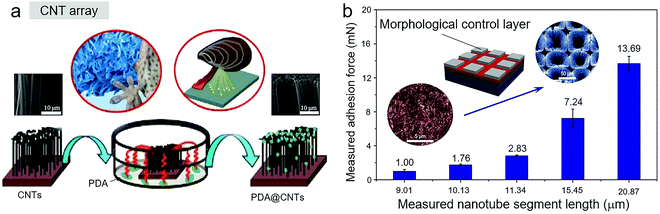
Surface modification is an efficient strategy to directly enhance the adhesion of contact surfaces. Dai et al. proved that the best adhesion strength of 29.3 N cm−2 could be achieved just by using radio-frequency tetrafluoromethane plasma.127 Also, polydopamine (PDA) in the mussel adhesive protein is considered the crucial component in simple, convenient, efficient, and environmentally friendly chemical modification, which can help improve the dry adhesion performance of traditional biomimetic CNT-based materials, even in a wet environment (Fig. 5a).105 Patterning CNTs is another useful strategy. Recently, Yao et al. reported the one-step synthesis of a CNT forest (CNTF) (Fig. 5b).128 In this system, the patterned aluminum layer controlled the morphology formation of CNTs to form CNTF, which resulted in a 5.9-times increase in adhesion.
To obtain bioinspired CNT-based materials with particular requirements such as softness, wearability, and flexibility, polymers are added. As shown in Fig. 6a, with a spring-like structure, s superelastic and electroconductive fiber was fabricated by Xu et al.73 By imitating the behavior of climbing plants, this fiber possessed biomimetic coiled tendril structures. Elastic polyester fiber (PF) was chosen as the core yarn, and flexible but conductive CNT/PDMS composite yarn (C/P CY) was then wrapped around PF to produce C/P CWY. CNTs constituted the framework and the coiled C/P CY provided abundant conductive pathways.
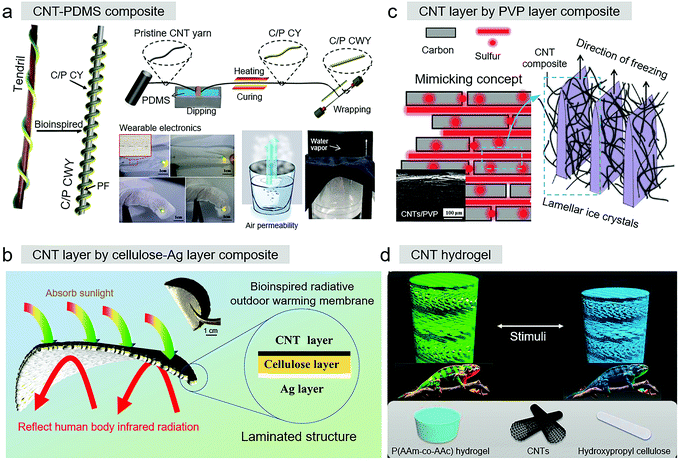 | ||
| Fig. 6 (a) Bioinspired superelastic E-fiber for wearable electronics. Reproduced with permission.73 Copyright 2019, American Chemical Society. (b) Porous Ag/cellulose/CNT-laminated nanofiber membrane. Reproduced with permission.110 Copyright 2020, American Chemical Society. (c) Nacre-like CNTs sheet. Reproduced with permission.102 Copyright 2018, Wiley-VCH. (d) Conductive CNT-based cellulose structural color hydrogel. Reproduced with permission.107 Copyright 2020, National Academy of Sciences. | ||
The polar bear pelt has an impressive radiation control strategy. Mimicking this strategy, Qiu et al. recently used a multistep to design a porous Ag/cellulose/CNT-laminated nanofiber membrane (Fig. 6b).110 This membrane had a laminated construction, where the CNT layer coating the cellulose layer was the solar radiation absorptivity layer to harvest solar thermal energy. An Ag layer was deposited via magnetron sputtering on the other side of the cellulose layer, reflecting infrared radiation from the human body. CNTs are black materials, and thus the obtained CNT layer could function as a heat collector to maximize heat input from the sun, while the Ag layer acted as the infrared reflector to minimize the human radiation heat output. Using the electrical conductivity of the additive CNTs to generate Joule heat, the membrane also had perfect thermal management.
Good conductivity is one advantage of CNTs, making bioinspired CNT-based materials excellent candidates in the field of energy conversion. Mimicking entire bulk structures to form sheet materials or composites with multidimensional pores is one design strategy. As shown in Fig. 6c, Yang et al. developed an ordered nacre-like cathode for Li–S batteries.102 A high sulfur loading was achieved with conductive polyvinylpyrrolidone (PVP) dispersed CNT-based monolith structures, in which the interval interspace was prepared via a unidirectional freeze-drying method. This nacre structure-like CNT sheet matrix resulted in cyclic stability with >99.9% coulombic efficiency and outstanding rate performance with 5 mg cm−2 sulfur loading, further increasing to 10 mg cm−2, high discharging capacity of 1236 mA h g−1 at 0.1C and 498 mA h g−1 at 2C.
Interestingly, design strategies by controlling the alignment of CNTs within polymers for the fabrication of bioinspired CNT-based materials with additional properties have also attracted attention from researchers. Through an electrically assisted 3D printing method, Chen et al. fabricated Menger structures with Bouligand-type surface-modified MECNTs (MWCNT-S) to recreate the architectures of the claws of Homarus americanus (which are mainly are made of Bouligand-type chitin-protein fibers).108 In the printing process, controlling the rotating electrical field could dynamically align the MWCNTs. A smaller rotation angle of adjacent MWCNTs led to greater energy dissipation and impact resistance. Moreover, with the requirement of multifunctional bionic electronic skins for smartly outputting multi-stimulation signals, inspired by the chameleon, Zhao et al. presented a multifunctional E-skin composited liquid-crystal hydrogel based on poly(acrylamide-co-acrylic acid) hydrogel (PACA), CNTs, and hydroxypropyl cellulose (HPC) (shown in Fig. 6d).107 With the help of additive CNTs in this system, the composite with hydrogel structure could output stimuli in electrical resistance signals. Based on a variation in color, the multifunctional bioinspired CNT-based materials had the ability to visually map the stimulating sites and quantitatively feedback external stimuli with electrical resistance changes.
Various methods, such as solvent casting, melt mixing method, and in situ polymerization have been adopted to obtain stable CNT-based composites.129 Naturally, CNTs are hydrophobic, possibly having toxicity by inducing an immune response in clinical applications.18,25 Accordingly, functionalized CNTs (e.g., carboxylic, carbonyl, and hydroxyl groups) or CNT compositions (e.g., tetrafluoromethane, polydopamine, and tannic acid) are fabricated by introducing molecules with hydrophilic, bioactive or specific groups, improving the dispersion of CNTs and the adhesion between CNTs and accompanying materials.85,97,105,127,130,131
4. Applications
The superiorities of CNTs and informed design strategies enable material engineers to fabricate bioinspired CNT-based materials, with design ideas from nature that are both ingenious and practical, which have beneficial applications. In this section, we demonstrate a few typical application examples of bioinspired CNT-based materials.4.1 Reinforcement materials
Reinforcement materials emerged in the middle of 20th century. Their promising characteristics of high stiffness, toughness with lightweight, and anticorrosion, make them applicable in aerospace, constriction, energy generators, biomedicine, etc. However, although single CNTs have the strongest intrinsic mechanical properties, the average strength of an individual CNT is only 9.6 GPa in CNT-based materials. Thus, efforts are still needed to change the weak interaction between two neighboring CNTs for the wide use of CNTs as construction materials. Bioinspired CNT-based materials, with homologous reinforcing strategies from nature, will profoundly make bioinspired CNT-based materials suitable as reinforcement materials.In Section 2, we discussed the bioinspired CNT-based reinforcement materials with enhanced mechanical properties of CNT fibers, CNT yarn, and CNT web structures. Moreover, inspired by the special hierarchical structure of natural spider silk, Pan et al. fabricated bioinspired spider silk single-walled carbon nanotubes (BISS-SWCNTs) (Fig. 7a).69 Through a multi-step fabrication procedure, the internal and external structures of these CNT-based film materials had similar appearances to that of spider silk. Specially, iron particles were embedded in BISS-SWCNT bundles as glue spots, and the highly organized SWCNTs with a skin-core structure were surrounded by an amorphous carbon layer. This bioinspired material exhibited a tensile strength of 550 MPa and Young's modulus of 6.5 GPa. Due to the above-mentioned hierarchical structure, the reinforcement was isotropic in the transformation of BISS-SWCNTs to alignment. Further equivalent superiority mechanical properties were observed in experiments on the PMMA/BISS-SWCNT/PMMA composite film, which had 300% improvement in tensile strength and 300% improvement in Young's modulus.
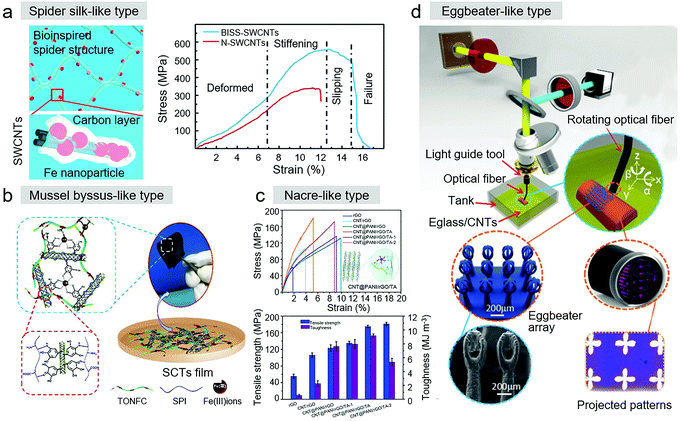 | ||
| Fig. 7 (a) With a similar spider silk-like structure, the bioinspired BISS-SWCNTs had ultrahigh mechanical properties. Reproduced with permission.69 Copyright 2016, the American Chemical Society. (b) SCT nanocomposite film. Reproduced with permission.97 Copyright 2017, Elsevier. (c) CNT@PANI/rGO/TA composite. Reproduced with permission.99 Copyright 2021, The Royal Society of Chemistry. (d) Optical system in the ISA-3D printing process for 3D-printed biomimetic super-hydrophobic structure. Reproduced with permission.83 Copyright 2017, Wiley-VCH. | ||
To obtain bio-based nanocomposites with flexible and stretchable abilities of high strength and toughness, learning from the interface engineering of mussel byssus, Li et al. described a biomimetic design strategy for the fabrication of TEMPO-oxidized nanofibrillated cellulose (TONFC)/soy protein isolate (SPI) (SPI/CT/TONFC, SCTs) nanocomposite films with strong and tough mechanical properties (Fig. 7b).97 Tannic acid-functionalized MWCNTs (PCT) were employed as cross-linkers with high functionality in the system, constructing a synergistic and sacrificial metal–ligand bonding interface between PCT and TONFC with Fe(III) mediation. The nanocomposite films exhibited a tensile strength of 11.5 MPa, toughness of 6.9 MJ m−3, elongation of 79.3%, and high electrical conductivity and good water resistance.
The direct mixture of CNTs and polymers, as one simple and cost effective method, is broadly employed to fabricate CNT-based structural materials with enhanced mechanical behavior. Nacre has extraordinary strength and toughness, relying on a brick-and-mortar architecture, also providing the possibility of creating polymer composites to obtain high-performance CNT-based reinforcement materials. Accordingly, Zhong et al. designed nacre-mimetic CNT@polyaniline/graphene oxide/biomass tannin (CNT@PANI/rGO/TA) (Fig. 7c). Biomass tannin (TA) was used as the glue, and together with “mortar” polyaniline, wrapped carbon nanotubes (CNT@PANI) to stick the reduced rGO “bricks” together.99 This bioinspired material exhibited high mechanical strength of 174.6 MPa and toughness of 9.17 MJ m−3. Similarly, individual CNTs (with the configuration of 1D fibers) were regarded as the “mortar” to link rGO to obtain CNT/rGO materials. Their tensile strength also improved to 106.2 ± 5.5 MPa and the toughness was 2.27 ± 0.4 MJ m−3.
Mimicking the Salvinia molesta superhydrophobic leaf structure via immersed surface accumulation (ISA) 3D printing, Chen et al. fabricated superhydrophobic micro-scale eggbeaters (Fig. 7d). MWCNTs, which ultimately enhanced the mechanical strength and surface roughness of the solidified microstructures, were initially added to photocurable resin.83 Consequently, stable 3D printed hairs were achieved and suspended eggbeaters, which did not collapse or adhere together, were obtained. After the addition of 0.5 wt% MWCNTs, the modulus of the structure increased from 161 to 455 MPa, while superhydrophobicity was obtained. With an increase in the MWCNT content, the surface roughness also increased.
4.2 Energy conversion
CNTs, serving as stable frameworks and substrates in bioinspired CNT-based materials, offer the feature of high electrical conductivity. Meanwhile, the interlinked micro/nano network and appropriate porosity from the compositing structure also prolong the conductive pathway of materials by increasing their ion transportation ability. These behaviors have important implications for the application of bioinspired CNT-based materials in energy conversion.Tin phosphide (Sn4P3), a metal phosphide, is a potential anode material in sodium-ion batteries (SIBs). However, in the Sn4P3 sodiation process, its low ionic and electronic conductivity lead to large volume expansion, limiting its utilization in SIBs. Recently, Knibbe et al. reported a stem-like CNT-based bioinspired material, where fructus-like Sn4P3 nanoparticles were anchored on the surface of CNTs through hydrothermal reaction followed by thermal treatment (Fig. 8a).92 With this biomimetic structure, the SIB achieved a superior electrochemical performance, with a high capacity of 742 mA h g−1 at 0.2C after 150 cycles and a superior rate of 449 mA h g−1 at 2C after 500 cycles.
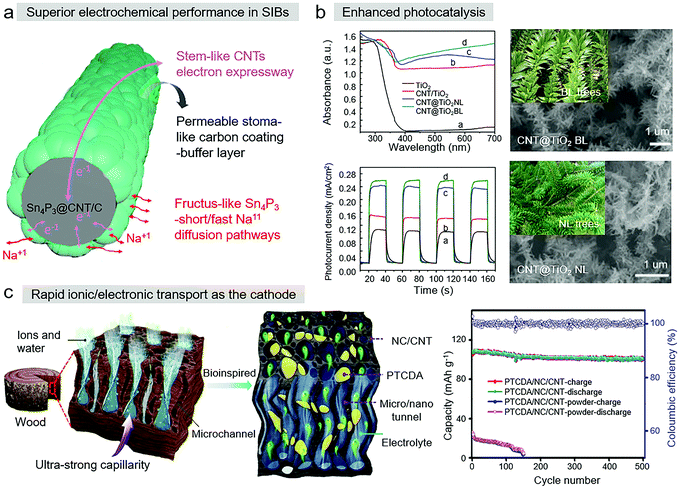 | ||
| Fig. 8 (a) Biomimetic Sn4P3 anchored on CNTs. Reproduced with permission.103 Copyright 2020, the American Chemical Society. (b) Biomimetic CNT@TiO2 composite. Reproduced with permission.68 Copyright 2015, Elsevier. (c) Design concept of the PTCDA/NC/CNT electrode. Reproduced with permission.100 Copyright 2020, Elsevier. | ||
TiO2, due to its high economic value, environment friendly nature, and chemical inertness, has been the prevailing photocatalytic material, but its application is limited because of its ineffective solar energy conversion and fast electron–hole recombination rate. Zheng et al. proved that the combination of TiO2 and CNT composites with structures inspired by trees (Fig. 8b) is an effective way to realize direct electron transport, and photocatalysis was enhanced due to the larger specific surface and thermoelectric conductivity of CNTs.57
Recently, Miao et al. was inspired by the hierarchical microchannel structure of wood to develop a CNT-participated nanofibrous organic cathode. This cathode consisted of PTCDA/nitrogen-doped carbon/carbon nanotubes (PTCDA/NC/CNTs), as shown in Fig. 8c.89 In the SIBs, this bioinspired CNT-based composite cathode had ultra-strong capillarity, highly reversible capacity, excellent rate performance, and ultra-long cyclic stability. As a conductive agent for rapid electron transfer, CNTs were employed to construct 3D interconnected conductive frameworks through electrospinning. PTCDA/NC/CNTs exhibited rapid ionic/electronic transport, high diffusion coefficients of Na+ and ultrafast reaction kinetics due to the rigid hollow nanostructure of CNTs, which formed a superb capillary. Moreover, bioinspired CNT-based nanomaterials have the advantages of good optical adsorption and free water transport, which make them promising for efficient solar steam generation. Zhu et al. fabricated a direct solar steam generation system with inspiration from the evaporation of sweat on the human skin and the transpiration in plants in biological systems.98 Utilizing these CNT films as floating photo-thermal collectors, the enhanced steam generation rate of 3.615 kg m−2 h−1 and >40% evaporation efficiency were achieved under 5 times solar intensity.
4.3 Nanopatterned surfaces
Besides their potential as reinforcing materials with high mechanical strength and chemical stability, the unique nanoscale morphology, low hydrophilicity, and optical characteristics of assembled structures of CNTs are also instructive for the application of bioinspired CNT-based materials in nanopatterned surfaces.132,133 Liz-Marzán et al. reported classical sea-anemone-like CNT-based hollow capsules with magnetic and reinforced properties. The CNT-coated Fe3O4 NP@PS spheres were used as the “hair” to provide the reinforcement.49 This outer surface structure is not only conductive to the controllable deposition of SiO2, but also stable in the washing process of the PS template. Interestingly, changing the length of CNTs would control the modification surface of the capsules, resulting in more complex structures with multi-level branching. The low hydrophilicity and chemical stability of CNTs are also beneficial in the design of surfaces with anti-fouling and anti-corrosion performances using bioinspired CNT-based materials. By incorporating the mussel-inspired material PDA, Yu et al. fabricated a PDA@CNT/PU nanofiber membrane, which exhibited superhydrophilicity and underwater superoleophobicity.134 Mimicking the micro-sphere/nano-thorn surface structure of the golden spherical cactus, Zhu et al. fabricated a superhydrophobic polysulfone (PSU)/CNT nanocomposite (Fig. 9a).72 These superhydrophobic materials could be deposited on various substrates just by electrostatic powder spraying, resulting in surfaces possessing a water contact angle greater than 164° ± 1.5°, sliding angle as low as 5° ± 0.5°, and self-cleaning ability and anti-corrosion in strong acid, alkaline and NaCl solutions. Recently, inspired by an arachnid, Li et al. developed a one-step thermal chemical vapor deposition method to fabricate a CNT stainless steel mesh (CNT@SSM) membrane (Fig. 9b). This membrane possessed excellent superhydrophilic and underwater-superoleophobic and anti-fouling properties.135 These salient surfaces in bioinspired CNT-based materials can further expand their application scope to hazardous water treatment.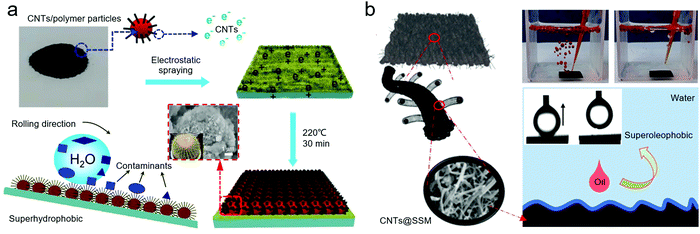 | ||
| Fig. 9 (a) Biomimetic spherical cactus superhydrophobic (PSU)/CNT coating. Reproduced with permission.72 Copyright 2018, Elsevier. (b) CNT@SSM membrane. Reproduced with permission.135 Copyright 2021, Elsevier. | ||
Additive CNTs on surfaces also have a contribution to enhanced color saturation. CNTs are ultra-black materials with a low theoretical effective index (neff = 1.01–1.10), stemming from their internal structures. Learning from the moth-eye effect, Motta et al. fabricated an optical film with SWCNT coating-deposited Si, which realized omnidirectional, broad-band, and nearly polarization-independent suppression.111 With the technology of coating optical films on arbitrary substrates, the optical absorption and emission properties of the devices were improved.
4.4 Dry adhesion
Although VA-CNTs have been proven to be suitable artificial materials for the fabrication of dry adhesive materials in theory, they still have a long way to go. To obtain the VA-CNT materials with extraordinary adhesive performances, surface modification of CNTs and patterned CNT arrays have been developed (seen in Section 3). The aim is to achieve a simple but high-efficiency procedure to obtain a perfect dry adhesive surface.Numerous bioinspired VA-CNT-based materials have been fabricated by mimicking the structures of geckos’ feet, but the design principles and synthetic mechanisms of how CNT arrays lead to dry adhesion and the key factors to enhance their performances remain unclear. In recent years, researchers have gradually focused on the influence of the key parameters in the fabrication process. Firstly, the packing density of VA-CNTs and the roughness of the surface were explored. With a high density, there was no adhesion. With an increase in roughness, the adhesion increased obviously, but the shear direction adhesion decreased (Fig. 10a).136 Gorb et al. discussed the tribological properties of densely aligned VA-CNTs with a synthetic length of up to 1.1 mm.138 A high coefficient of friction, μ, of 5–6 was showed in the initial sliding cycles, which then decreased to 2–3 in fourth and fifth cycles. The applied shear force inducing a strong contribution of adhesion was the main reason. One study showed that the incorporation of Fe and Al2O3 layers had a synergistic effect on the growth of structures and morphologies of VA-CNT materials, such as orientation, density, diameter, and growth rate (Fig. 10b).137 Conversely, the adhesion could be controlled with a change in these layers. Another study concentrated on the relation between the adhesion force of VA-CNTs and their mechanical behaviors.139 It indicated that the 3.4% higher fraction of VA-CNT arrays resulted in no adhesion effect, while at low density (less than 0.5%), the adhesion performance was strongly associated with plastic deformation. Mechanical compliance in the area near the contact interfaces may be the dominant factor.
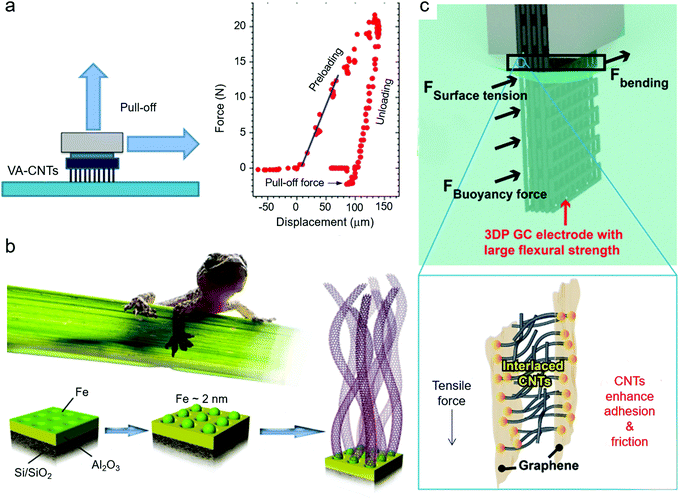 | ||
| Fig. 10 (a) Effects of packing density and surface roughness on adhesion of VA-CNTs. Reproduced with permission.136 Copyright 2015, the American Chemical Society. (b) Synergistic effects of Fe and Al2O3 layers on the growth of VA-CNTs. Reproduced with permission.137 Copyright 2018, Elsevier. (c) Gecko's feet-inspired design of 3DP GC electrode. Reproduced with permission.106 Copyright 2020, Wiley-VCH. | ||
It is worth noting that although VA-CNT materials have been utilized in bioinspired dry adhesion for no more than 20 years, their dry adhesion performances have been greatly improved. CNT-based array materials are superior to the natural geckos’ feet. However, big breakthroughs have been less frequent in recent years, and some exclusive obstacles still remain to be solved. It is necessary to move back and deeply delve into the basic principles of gecko adhesion. One reason is that geckos have a lot of natural adhesion systems that are not completely known well. Innovative applications corresponding to the dry adhesion behaviors are also urgent. One good start may be the study by Jiang et al., who reported a 3D-printed bioinspired electrode of graphene/CNTs (3DP GC) (Fig. 10c).106 Mimicking the geckos’ feet, the obtained electrode processed a hierarchical porous structure with high flexural strength of 96.2 kPa. Increasing the friction and adhesion in the neighboring 2D graphene nanosheets, the 1D CNTs played a critical role in the enhanced flexural strength.
4.5 Bioengineering
Depending on the electrical conductivity of CNTs and decorated biofunctional groups on their surface, bioinspired CNT-based materials can monitor molecular recognition and transformation processes, which broaden their applications in biosensing and biocatalysis. Swager et al. reported the fabrication of a chemiresistive glucose sensor based on poly(4-vinylpyridine) (P4VP) and SWCNT composites (Fig. 11a).140 A biomimetic surface was obtained in the system by cooperation with glucose oxidase (GOx). This biomimetic glucose sensor displayed a decrease in electrical resistance to exhibit high selectivity and instant response (within 3 s) to glucose. In another biomimetic oxidase sensor system, iron porphyrins, a type of horseradish peroxidase, were immobilized on modified MWCNTs. This sensor was proven to be a simple, economical, and efficient method to detect catechol.141 Zhang et al. firstly utilized a peptide as a DNA sensing element and built a novel CNT-DNA thin-film-transistor sensor.142 Different from the former known DNA bio-detector and NanoDrop, this bioinspired sensor had a broader sensing range of 1.6 × 10−4 to 5 × 10−6 M (the detection limit was 0.88 μg L−1). In 2015, Wang et al. firstly found that carboxyl-functionalized SWCNHs (SWCNHs-COOH) had peroxidase-like activity similar to natural peroxidase. Even under harsh reaction conditions, SWCNHs-COOH was stable and exhibited high catalytic activity, which is regarded as a potential enzymatic mimic.143 In recent years, a series of CNTs and their derivatives have emerged as highly efficient biocatalysts to mimic the function of biological enzymes, but their use is hampered by their unsatisfactory enzymatic efficiency. The accurate influence of various oxygenated groups on the surface of oxygenated group-enriched CNTs (o-CNTs) was firstly explored, including carbonyl, carboxyl, and hydroxyl groups (Fig. 11b).144 The “competitive inhibition” mechanism was proposed. When weakening the noncatalytic sites, the catalytic efficiency of o-CNTs can be greatly improved. Once the surfaces of CNTs are well functionalized, both biocatalysis and biosensing can be achieved. In the study by Tang et al., NiCoBP-MWCNT-conjugated anti-PSA antibody was fabricated.145 For glucose oxidation, the nanocomplex exhibited outstanding catalytic behavior, and for detecting PSA, it also showed excellent performance simultaneously.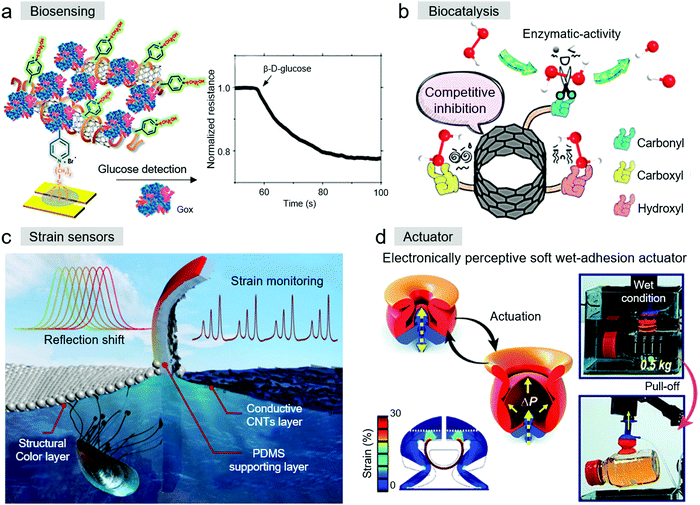 | ||
| Fig. 11 (a) P4VP-SWCNT scaffolds for a chemiresistive glucose sensor. Reproduced with permission.140 Copyright 2017, the American Chemical Society. (b) Peroxidase-like activity of o-CNTs. Reproduced with permission.144 Copyright 2018, the American Chemical Society. (c) Bioinspired Janus structural color film as visually flexible electronics. Reproduced with permission.146 Copyright 2021, Elsevier. (d) Electronic sensory soft adhesive actuator. Reproduced with permission.74 Copyright 2021, the American Chemical Society. | ||
Bioinspired CNT-based materials have been widely used in electronics and actuators, which have particular requirements such as softness, wearability, and flexibility.147–149 Mussel-inspired chemistry, which refers to the polymerization of the mussel-inspired material PDA, provides a simple but effective method for subsequent modification reactions or is utilized as a precursor for preparing functional CNT-based composite materials.150 Learning from the anti-freezing/anti-heating performances in nature, Lu et al. developed an adhesive and conductive hydrogel using mussel chemistry.96 In this system, PDA-decorated CNTs were dispersed in the hydrogel, and CNTs served as the nano reinforcements to enhance the conductive and mechanical properties. As shown in Fig. 11c, inspired by the structural color layer of mussels, a Janus structural color film was presented by Pan et al., which had a conductive CNT-based layer, a supporting polydimethylsiloxane layer, and a structural color layer formed by 2D colloidal crystals (2D-CCs).146 The experimental results indicated that these Janus structural color films exhibited stable electrical sensing and visualized color-sensing. Moreover, numerous specific sensors (e.g., mechanical sensor,151 wearable strain sensor,152 temperature sensor,153 and airflow sensor154) were developed via the methods of drop casting, air spraying, ultrasonic spraying, hybrid hydrogel, thermal mismatch design, etc. Besides, to obtain a device with the ability to catch objects with various shapes, inspired by the sensory grasping by the arm of Octopus vulgaris, Pang et al. recently fabricated an electronic sensory soft adhesive device (Fig. 11d).74 CNT-based strain sensors, which served as the artificial octopus sucker, were placed on an irregular surface via a facile 3D spray coating process to the mimic nerve-like functions of the octopus. This strain sensors identified objects through patterns of strain distribution.
5. Conclusion and perspective
In the present review, we presented the progress on bioinspired CNT-based materials in recent years. CNTs exhibit superiors properties in the design of bioinspired CNT-based materials, such as unique hollow nanochannel structures, controllable functionalized surface, and tunable high conductivity. Benefiting from these special properties of CNTs, diverse design strategies for bioinspired CNT-based materials were introduced. The natural nanochannels of CNTs are an ideal model to study the mass transport behaviors in biological systems. Besides, CNTs are construction materials used to self-assemble or composite with other materials. Mimicking the structures of natural plants, silk, muscles, spider webs, geckos’ feet, etc., bioinspired CNT-based materials have been fabricated with the CNT-assembled structures such as CNT fibers, CNT yarns, CNT web, and VA-CNT. Also, learning from tendrils, polar bears, nacre, chameleons, etc., bioinspired CNT-based materials have been designed using CNT composites such as CNT-polymer composites, CNT layer-by-layer composites and CNT hydrogels. In addition, bioinspired CNT-based materials have shown significant applications in reinforcement materials, energy conversion, nanopatterned surfaces, dry adhesion, and bioengineering.However, despite the substantial improvements, many challenges remain to be solved for bioinspired CNT-based materials. Firstly, in-depth exploration of the features and diversity of living organisms is required for achieving optimized bionic designs for CNT-based materials in both structure and function. Secondly, utilizing the unique structural, surficial, and conductive properties of CNTs as building blocks to design smart bioinspired CNT-based materials should be given exceptional attention. CNTs are easily produced, and their well-established functionalization methods can enlarge their diversity with multifunctions. Consequently, bioinspired CNT-based materials will be endowed with improved smart abilities, such as the ability to respond to heat, light, electricity, magnetism, specific molecules, etc. Thirdly, although bioinspired CNT-based materials have shown wide applications in some fields, further developments in their practical applications still have a long way to go. Overall, beyond the reported application fields, advances are anticipated in design strategies to obtain novel bioinspired CNT-based materials, feasibly leading to some burgeoning applications such as real-time virus detection, nanomaterial-based artificial synapses, and bioinspired nanofluidic iontronics.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFA0209500), the National Natural Science Foundation of China (52025132, 21975209, 21621091 and 22021001), the Fundamental Research Funds for the Central Universities (20720190037 and 20720210056), and 111 Project (B16029).References
- Q. Cheng, J. Duan, Q. Zhang and L. Jiang, ACS Nano, 2015, 9, 2231–2234 CrossRef CAS PubMed.
- N. S. Kumar, R. P. Suvarna, K. C. B. Naidu, P. Banerjee, A. Ratnamala and H. Manjunatha, Appl. Phys. A: Mater. Sci. Process., 2020, 126, 445–463 CrossRef.
- J. J. Schneider, Adv. Biosyst., 2017, 1, 1700101 CrossRef PubMed.
- D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105–1136 CrossRef CAS PubMed.
- J. K. Holt, H. G. Park, Y. M. Wang, M. Stadermann, A. B. Artyukhin, C. P. Grigoropoulos, A. Noy and O. Bakajin, Science, 2006, 312, 1034–1037 CrossRef CAS PubMed.
- J. N. Coleman, U. Khan, W. J. Blau and Y. K. Gun'ko, Carbon, 2006, 44, 1624–1652 CrossRef CAS.
- M. F. L. De Volder, S. H. Tawfick, R. H. Baughman and A. J. Hart, Science, 2013, 339, 535–539 CrossRef CAS PubMed.
- M. Moniruzzaman and K. I. Winey, Macromolecules, 2006, 39, 5194–5205 CrossRef CAS.
- L. Yan, X. Yang, Y. Zhao, Y. Wu, R. Motlhaletsi Moutloali, B. B. Mamba, P. Sorokin and L. Shao, Sep. Purif. Technol., 2022, 285, 120383 CrossRef CAS.
- M. S. Ganewatta, Z. K. Wang and C. B. Tang, Nat. Rev. Chem., 2021, 5, 753–772 CrossRef CAS.
- F. Lossada, D. Hoenders, J. Guo, D. Jiao and A. Walther, Acc. Chem. Res., 2020, 53, 2622–2635 CrossRef CAS PubMed.
- Z. L. Yu, B. Qin, Z. Y. Ma, Y. C. Gao, Q. F. Guan, H. B. Yang and S. H. Yu, Adv. Mater., 2021, 33, 2001086 CrossRef CAS PubMed.
- G. Liu, W. S. Y. Wong, M. Kraft, J. W. Ager, D. Vollmer and R. Xu, Chem. Soc. Rev., 2021, 50, 10674–10699 RSC.
- Y. Hou and X. Hou, Science, 2021, 373, 628–629 CrossRef CAS PubMed.
- A. B. Asha, Y. Chen and R. Narain, Chem. Soc. Rev., 2021, 50, 11668–11683 RSC.
- Y. Li, ACS Nano, 2021, 15, 9197–9200 CrossRef PubMed.
- M. F. De Volder, S. H. Tawfick, R. H. Baughman and A. J. Hart, Science, 2013, 339, 535–539 CrossRef CAS PubMed.
- B. Pei, W. Wang, N. Dunne and X. Li, Nanomaterials, 2019, 9, 1501 CrossRef CAS PubMed.
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS.
- N. Saifuddin, A. Z. Raziah and A. R. Junizah, J. Chem., 2013, 2013, 1–18 CrossRef.
- C. Wang, K. Xia, H. Wang, X. Liang, Z. Yin and Y. Zhang, Adv. Mater., 2019, 31, 1801072 CrossRef PubMed.
- L.-M. Peng, Z. Zhang and C. Qiu, Nat. Electron., 2019, 2, 499–505 CrossRef CAS.
- S. Mallakpour, E. Azadi and C. M. Hussain, New J. Chem., 2021, 45, 3756–3777 RSC.
- C. Xiang, Y. Zhang, W. Guo and X. J. Liang, Acta Pharm. Sin. B., 2020, 10, 239–248 CrossRef CAS PubMed.
- T. Szymanski, A. A. Mieloch, M. Richter, T. Trzeciak, E. Florek, J. D. Rybka and M. Giersig, Materials, 2020, 13, 4039 CrossRef CAS PubMed.
- Y. H. Teow and A. W. Mohammad, Desalination, 2019, 451, 2–17 CrossRef CAS.
- X. Fan, Y. Zhou, X. Jin, R. B. Song, Z. Li and Q. Zhang, Carbon Energy, 2021, 3, 449–472 CrossRef CAS.
- Y. Zhang, Q. Zhang and G. Chen, Carbon Energy, 2020, 2, 408–436 CrossRef CAS.
- W. Zhao, S. Fan, N. Xiao, D. Liu, Y. Y. Tay, C. Yu, D. Sim, H. H. Hng, Q. Zhang, F. Boey, J. Ma, X. Zhao, H. Zhang and Q. Yan, Energy Environ. Sci., 2012, 5, 5364–5369 RSC.
- A. K. K. Kyaw, H. Tantang, T. Wu, L. Ke, C. Peh, Z. H. Huang, X. T. Zeng, H. V. Demir, Q. Zhang and X. W. Sun, Appl. Phys. Lett., 2011, 99, 021107 CrossRef.
- X. Zhang, W. Lu, G. Zhou and Q. Li, Adv. Mater., 2020, 32, e1902028 CrossRef PubMed.
- L. Wang, S. Xie, Z. Wang, F. Liu, Y. Yang, C. Tang, X. Wu, P. Liu, Y. Li, H. Saiyin, S. Zheng, X. Sun, F. Xu, H. Yu and H. Peng, Nat. Biomed. Eng., 2020, 4, 159–171 CrossRef CAS PubMed.
- Y. Bai, H. Yue, J. Wang, B. Shen, S. Sun, S. Wang, H. Wang, X. Li, Z. Xu, R. Zhang and F. Wei, Science, 2020, 369, 1104–1106 CrossRef CAS PubMed.
- R. Peng, Y. Pan, B. Liu, Z. Li, P. Pan, S. Zhang, Z. Qin, A. R. Wheeler, X. S. Tang and X. Liu, Small, 2021, 17, e2100383 CrossRef PubMed.
- E. Dujardin, T. W. Ebbesen, H. Hiura and K. Tanigaki, Science, 1994, 265, 1850–1852 CrossRef CAS PubMed.
- T. W. Ebbesen, H. J. Lezec, H. Hiura, J. W. Bennett, H. F. Ghaemi and T. Thio, Nature, 1996, 382, 54–56 CrossRef CAS.
- M. M. J. Treacy, T. W. Ebbesen and J. M. Gibson, Nature, 1996, 381, 678–680 CrossRef CAS.
- J. Chen, M. A. Hamon, H. Hu, Y. S. Chen, A. M. Rao, P. C. Eklund and R. C. Haddon, Science, 1998, 282, 95–98 CrossRef CAS PubMed.
- P. M. Ajayan, O. Stephan, C. Colliex and D. Trauth, Science, 1994, 265, 1212–1214 CrossRef CAS PubMed.
- W. A. Deheer, W. S. Bacsa, A. Chatelain, T. Gerfin, R. Humphreybaker, L. Forro and D. Ugarte, Science, 1995, 268, 845–847 CrossRef CAS PubMed.
- P. J. F. Harris, Int. Mater. Rev., 2013, 49, 31–43 CrossRef.
- H. Xin and A. T. Woolley, J. Am. Chem. Soc., 2003, 125, 8710–8711 CrossRef CAS PubMed.
- C. Richard, F. Balavoine, P. Schultz, T. W. Ebbesen and C. Mioskowski, Science, 2003, 300, 775–778 CrossRef CAS PubMed.
- A. Cao, V. P. Veedu, X. Li, Z. Yao, M. N. Ghasemi-Nejhad and P. M. Ajayan, Nat. Mater., 2005, 4, 540–545 CrossRef CAS PubMed.
- D. Bonifazi, C. Nacci, R. Marega, S. Campidelli, G. Ceballos, S. Modesti, M. Meneghetti and M. Prato, Nano Lett., 2006, 6, 1408–1414 CrossRef CAS PubMed.
- M. Grzelczak, M. A. Correa-Duarte and L. M. Liz-Marzán, Small, 2006, 2, 1174–1177 CrossRef CAS PubMed.
- W. Wu, N. V. Tsarevsky, J. L. Hudson, J. M. Tour, K. Matyjaszewski and T. Kowalewski, Small, 2007, 3, 1803–1810 CrossRef CAS PubMed.
- N. Wakamatsu, H. Takamori, T. Fujigaya and N. Nakashima, Adv. Funct. Mater., 2009, 19, 311–316 CrossRef CAS.
- M. Sanles-Sobrido, V. Salgueirino-Maceira, M. A. Correa-Duarte and L. M. Liz-Marzan, Small, 2008, 4, 583–586 CrossRef CAS PubMed.
- X. Gui, J. Wei, K. Wang, A. Cao, H. Zhu, Y. Jia, Q. Shu and D. Wu, Adv. Mater., 2010, 22, 617–621 CrossRef CAS PubMed.
- Y. Shang, X. He, Y. Li, L. Zhang, Z. Li, C. Ji, E. Shi, P. Li, K. Zhu, Q. Peng, C. Wang, X. Zhang, R. Wang, J. Wei, K. Wang, H. Zhu, D. Wu and A. Cao, Adv. Mater., 2012, 24, 2896–2900 CrossRef CAS PubMed.
- M.-Q. Zhao, H.-J. Peng, G.-L. Tian, Q. Zhang, J.-Q. Huang, X.-B. Cheng, C. Tang and F. Wei, Adv. Mater., 2014, 26, 7051–7058 CrossRef CAS PubMed.
- H. Zhang, D. Wei, Y. Liu, B. Wu, L. Huang, H. Xi, J. Chen, G. Yu, H. Kajiura and Y. Li, Small, 2009, 5, 2392–2396 CrossRef CAS PubMed.
- B. J. Hinds, N. Chopra, T. Rantell, R. Andrews, V. Gavalas and L. G. Bachas, Science, 2004, 303, 62–65 CrossRef CAS PubMed.
- J. Wu, B. El Hamaoui, J. Li, L. Zhi, U. Kolb and K. Müllen, Small, 2005, 1, 210–212 CrossRef CAS PubMed.
- J. J. Vilatela and A. H. Windle, Adv. Mater., 2010, 22, 4959–4963 CrossRef CAS PubMed.
- X. Hou, Adv. Mater., 2016, 28, 7049–7064 CrossRef CAS PubMed.
- S. Chen, Y. Tang, K. Zhan, D. Sun and X. Hou, Nano Today, 2018, 20, 84–100 CrossRef CAS.
- Y. Tang, L. Cao, K. Zhan, Y. Xie, D. Sun, X. Hou and S. Chen, Sens. Actuators, B, 2019, 286, 315–320 CrossRef CAS.
- M. Wang, L. Zhou, W. Deng, Y. Hou, W. He, L. Yu, H. Sun, L. Ren and X. Hou, ACS Nano, 2022, 16, 2672–2681 CrossRef CAS PubMed.
- Y. Hou, M. Wang, X. Chen and X. Hou, Nano Res., 2020, 14, 8 Search PubMed.
- M. Wang, Y. Hou, L. Yu and X. Hou, Nano Lett., 2020, 20, 6937–6946 CrossRef CAS PubMed.
- W. He, L. Zhou, M. Wang, Y. Cao, X. Chen and X. Hou, Sci. Bull., 2021, 66, 1472–1483 CrossRef CAS.
- Y. Hou, Q. Wang, S. Wang, M. Wang, X. Chen and X. Hou, Chin. Chem. Lett., 2021 DOI:10.1016/j.cclet.2021.09.007.
- M. Wang, H. Meng, D. Wang, Y. Yin, P. Stroeve, Y. Zhang, Z. Sheng, B. Chen, K. Zhan and X. Hou, Adv. Mater., 2019, 31, 1805130 CrossRef PubMed.
- L. Yu, M. Wang and X. Hou, Huaxue Tongbao, 2020, 83, 482–487 Search PubMed.
- R. H. Tunuguntla, R. Y. Henley, Y.-C. Yao, P. Tuan Anh, M. Wanunu and A. Noy, Science, 2017, 357, 792–796 CrossRef CAS PubMed.
- J. Di, S. Li, Z. Zhao, Y. Huang, Y. Jia and H. Zheng, Chem. Eng. J., 2015, 281, 60–68 CrossRef CAS.
- C. Luo, F. Li, D. Li, Q. Fu and C. Pan, ACS Appl. Mater. Interfaces, 2016, 8, 31256–31263 CrossRef CAS PubMed.
- S. Hu and Z. Xia, Small, 2012, 8, 2464–2468 CrossRef CAS PubMed.
- L. Qu, L. Dai, M. Stone, Z. Xia and Z. Wang, Science, 2008, 322, 238–242 CrossRef CAS PubMed.
- Y. Zhu, F. Sun, H. Qian, H. Wang, L. Mu and J. Zhu, Chem. Eng. J., 2018, 338, 670–679 CrossRef CAS.
- J. Wu, Z. Wang, W. Liu, L. Wang and F. Xu, ACS Appl. Mater. Interfaces, 2019, 11, 44735–44741 CrossRef CAS PubMed.
- H. J. Lee, S. Baik, G. W. Hwang, J. H. Song, D. W. Kim, B.-Y. Park, H. Min, J. K. Kim, J.-S. Koh, T.-H. Yang and C. Pang, ACS Nano, 2021, 15, 14137–14148 CrossRef CAS PubMed.
- G. Sun, C. Zhang, Z. Dai, M. Jin, Q. Liu, J. Pan, Y. Wang, X. Gao, W. Lan, G. Sun, C. Gong, Z. Zhang, X. Pan, J. Li and J. Zhou, J. Colloid Interface Sci., 2022, 608, 459–469 CrossRef CAS PubMed.
- W. Shuangquan, D. Bo, L. Ang, W. Yanfeng, Y. Qifa and Z. Lina, Carbohydr. Polym., 2017, 174, 830–840 CrossRef PubMed.
- Q.-F. Guan, Z.-M. Han, K.-P. Yang, H.-B. Yang, Z.-C. Ling, C.-H. Yin and S.-H. Yu, Nano Lett., 2021, 21, 2532–2537 CrossRef CAS PubMed.
- L. Wang, Y. Wu, T. Hu, P. X. Ma and B. Guo, Acta Biomater., 2019, 96, 175–187 CrossRef CAS PubMed.
- M. Dasbach, M. Pyschik, V. Lehmann, K. Parey, D. Rhinow, H. M. Reinhardt and N. A. Hampp, ACS Nano, 2020, 14, 8181–8190 CrossRef CAS PubMed.
- A. Peigney, C. Laurent, E. Flahaut, R. R. Bacsa and A. Rousset, Carbon, 2001, 39, 507–514 CrossRef CAS.
- M. M. Pendergast and E. M. V. Hoek, Energy Environ. Sci., 2011, 4, 1946–1971 RSC.
- J. Lin, X. Cai, Z. Liu, N. Liu, M. Xie, B. Zhou, H. Wang and Z. Guo, Adv. Funct. Mater., 2020, 30, 2000398 CrossRef CAS.
- Y. Yang, X. J. Li, X. Zheng, Z. Y. Chen, Q. F. Zhou and Y. Chen, Adv. Mater., 2018, 30, 1704912 CrossRef PubMed.
- A. Chall, J. Stagg, A. Mixson, E. Gato, R. Quirino and V. Sittaramane, Nanotechnology, 2021, 32, 195102 CrossRef CAS PubMed.
- D. Li, S. Li, J. Liu, L. Zhan, P. Wang, H. Zhu and J. Wei, Mater. Sci. Eng., C, 2020, 112, 110887 CrossRef CAS PubMed.
- G. Hummer, J. C. Rasaiah and J. P. Noworyta, Nature, 2001, 414, 188–190 CrossRef CAS PubMed.
- O. N. Samoylova, E. I. Calixte and K. L. Shuford, Appl. Surf. Sci., 2017, 423, 154–159 CrossRef CAS.
- R. P. Singh, G. Sharma, Sonali, S. Singh, S. Bharti, B. L. Pandey, B. Koch and M. S. Muthu, Mater. Sci. Eng., C, 2017, 77, 446–458 CrossRef CAS PubMed.
- S. Xin, N. Yang, F. Gao, J. Zhao, L. Li and C. Teng, Appl. Surf. Sci., 2017, 414, 218–223 CrossRef CAS.
- H. J. Yang, J. Y. Cho, J. H. Kim, H. Y. Kim, J.-W. Lee, J. W. Wang, J. H. Kwak, S. Jung, J. H. Park, H. J. Jeong, S. Y. Jeong, S. H. Seo, G.-W. Lee and J. T. Han, Carbon, 2020, 157, 649–655 CrossRef CAS.
- L. Wang, D. Zhu, J. Chen, Y. Chen and W. Chen, Environ. Sci.: Nano, 2017, 4, 558–564 RSC.
- Y. C. Jung, H. Muramatsu, K. Fujisawa, J. H. Kim, T. Hayashi, Y. A. Kim, M. Endo, M. Terrones and M. S. Dresselhaus, Small, 2011, 7, 3292–3297 CrossRef CAS PubMed.
- J. Liu, C. Wang, X. Wang, X. Wang, L. Cheng, Y. Li and Z. Liu, Adv. Funct. Mater., 2015, 25, 384–392 CrossRef CAS.
- Z. Yin, X. Liang, K. Zhou, S. Li, H. Lu, M. Zhang, H. Wang, Z. Xu and Y. Zhang, Small, 2021, 17, 2100066 CrossRef CAS PubMed.
- Z. Wang, J. Wu, X. Wei, S. Saleemi, W. Liu, W. Li, I. Marriam and F. Xu, J. Mater. Chem. C, 2020, 8, 117–123 RSC.
- L. Han, K. Liu, M. Wang, K. Wang, L. Fang, H. Chen, J. Zhou and X. Lu, Adv. Funct. Mater., 2018, 28, 1704195 CrossRef.
- Z. Wang, S. Zhao, H. Kang, W. Zhang, S. Zhang and J. Li, Appl. Surf. Sci., 2018, 434, 1086–1100 CrossRef CAS.
- Y. Wang, S. Wu, Q. Yin, K. Du, Q. Yin, B. Jiang and S. Mo, ACS Appl. Mater. Interfaces, 2021, 13, 23970–23982 CrossRef CAS PubMed.
- D. Wu, C. Yu and W. Zhong, J. Mater. Chem. A, 2021, 9, 18356–18368 RSC.
- G. Zhou, L. Mo, C. Zhou, Y. Wu, F. Lai, Y. Lv, J. Ma, Y.-E. Miao and T. Liu, Chem. Eng. J., 2021, 420, 127597 CrossRef CAS.
- P. Bhattacharya, M. Kota, D. H. Suh, K. C. Roh and H. S. Park, Adv. Energy. Mater., 2017, 7, 1700331 CrossRef.
- Z.-Z. Pan, W. Lv, Y.-B. He, Y. Zhao, G. Zhou, L. Dong, S. Niu, C. Zhang, R. Lyu, C. Wang, H. Shi, W. Zhang, F. Kang, H. Nishihara and Q.-H. Yang, Adv. Sci., 2018, 5, 1800384 CrossRef PubMed.
- L. Ran, B. Luo, I. R. Gentle, T. Lin, Q. Sun, M. Li, M. M. Rana, L. Wang and R. Knibbe, ACS Nano, 2020, 14, 8826–8837 CrossRef CAS PubMed.
- X. Sun, M. Li, A. Ndahimana, P. Ding, Y. Xu, Q. Hu, K. Chen and T. Feng, Energy Environ. Mater., 2021, 4, 399–406 CrossRef CAS.
- W. Li, Y. Li, M. Sheng, S. Cui, Z. Wang, X. Zhang, C. Yang, Z. Yu, Y. Zhang, S. Tian, Z. Dai and Q. Xu, Langmuir, 2019, 35, 4527–4533 CrossRef CAS PubMed.
- M. Peng, D. Shi, Y. Sun, J. Cheng, B. Zhao, Y. Xie, J. Zhang, W. Guo, Z. Jia, Z. Liang and L. Jiang, Adv. Mater., 2020, 32, 1908201 CrossRef CAS PubMed.
- Z. Zhang, Z. Chen, Y. Wang and Y. Zhao, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 18310–18316 CrossRef CAS PubMed.
- Y. Yang, Z. Chen, X. Song, Z. Zhang, J. Zhang, K. K. Shung, Q. Zhou and Y. Chen, Adv. Mater., 2017, 29, 1605750 CrossRef PubMed.
- X. Wang, Y. He, X. Liu and J. Zhu, Powder Technol., 2017, 321, 276–285 CrossRef CAS.
- X. Yue, M. He, T. Zhang, D. Yang and F. Qu, ACS Appl. Mater. Interfaces, 2020, 12, 12285–12293 CrossRef CAS PubMed.
- F. De Nicola, P. Hines, M. De Crescenzi and N. Motta, Carbon, 2016, 108, 262–267 CrossRef CAS.
- J. Wu, Z. Yan, Z. Li, C. Yan, S. Lu, M. Dong and N. Yan, Science, 2015, 350, aad2395 CrossRef PubMed.
- J. Payandeh, T. Scheuer, N. Zheng and W. A. Catterall, Nature, 2011, 475, 353–359 CrossRef CAS PubMed.
- J. Koefinger, G. Hummer and C. Dellago, Phys. Chem. Chem. Phys., 2011, 13, 15403–15417 RSC.
- R. Garcia-Fandino and M. S. P. Sansom, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 6939–6944 CrossRef CAS PubMed.
- Y.-C. Yao, A. Taqieddin, M. A. Alibakhshi, M. Wanunu, N. R. Aluru and A. Noy, ACS Nano, 2019, 13, 12851–12859 CrossRef CAS PubMed.
- J. Geng, K. Kim, J. Zhang, A. Escalada, R. Tunuguntla, L. R. Comolli, F. I. Allen, A. V. Shnyrova, K. R. Cho, D. Munoz, Y. M. Wang, C. P. Grigoropoulos, C. M. Ajo-Franklin, V. A. Frolov and A. Noy, Nature, 2014, 514, 612–615 CrossRef CAS PubMed.
- K. Kim, J. Geng, R. Tunuguntla, L. R. Comolli, C. P. Grigoropoulos, C. M. Ajo-Franklin and A. Noy, Nano Lett., 2014, 14, 7051–7056 CrossRef CAS PubMed.
- R. H. Tunuguntla, F. I. Allen, K. Kim, A. Belliveau and A. Noy, Nat. Nanotechnol., 2016, 11, 639–644 CrossRef CAS PubMed.
- J. R. Sanborn, X. Chen, Y.-C. Yao, J. A. Hammons, R. H. Tunuguntla, Y. Zhang, C. C. Newcomb, J. A. Soltis, J. J. De Yoreo, A. Van Buuren, A. N. Parikh and A. Noy, Adv. Mater., 2018, 30, 1803355 CrossRef PubMed.
- J. Rabinowitz, C. Cohen and K. L. Shepard, Nano Lett., 2020, 20, 1148–1153 CrossRef CAS PubMed.
- U. Narayan-Maiti, Adv. Mater., 2014, 26, 40–67 CrossRef PubMed.
- J. Wu, K. Gerstandt, M. Majumder, X. Zhan and B. J. Hinds, Nanoscale, 2011, 3, 3321–3328 RSC.
- B. Hinds, Curr. Opin. Solid State Mater. Sci., 2012, 16, 1–9 CrossRef CAS.
- M. Wang, H. Meng, D. Wang, Y. Yin, P. Stroeve, Y. Zhang, Z. Sheng, B. Chen, K. Zhan and X. Hou, Adv. Mater., 2019, 31, 1805130 CrossRef PubMed.
- S. Dong, Z. Gan, X. Chen, Y. Pei, B. Li, J. Ren, Y. Wang, H. He and S. Ling, Mater. Chem. Front., 2021, 5, 5706–5717 RSC.
- M. Lu, Q. He, Y. Li, F. Guo and Z. Dai, Carbon, 2019, 142, 592–598 CrossRef CAS.
- K. Zhang, W. Gong, Z. Li, W. Xu and Y. Yao, Carbon, 2021, 176, 540–547 CrossRef CAS.
- V. Choudhary, B. P. Singh and R. B. Mathur, Carbon Nanotubes and Their Composites, in Syntheses and Applications of Carbon Nanotubes and Their Composites, 2013, pp. 193–222 DOI:10.5772/52897.
- F. X. Cao, P. F. Jiang, J. Z. Wang and F. Y. Yan, Polym. Adv. Technol., 2018, 29, 767–774 CrossRef CAS.
- M. S. Ata and I. Zhitomirsky, Mater. Manuf. Processes, 2016, 31, 67–73 CrossRef CAS.
- Y. Sun, Y. Wang, X. Sui, W. Liang, L. He, F. Wang and B. Yang, ACS Appl. Nano Mater., 2021, 4, 10852–10863 CrossRef CAS.
- H. Min, S. Baik, J. Kim, J. Lee, B. G. Bok, J. H. Song, M. S. Kim and C. Pang, Adv. Funct. Mater., 2021, 2107285 Search PubMed.
- M. He, H. Liu, L. Wang, X. Qin and J. Yu, Mater. Chem. Front., 2021, 5, 3673–3680 RSC.
- X. Yin, Y. He, H. Li, X. Ma, L. Zhou, T. He and S. Li, J. Colloid Interface Sci., 2021, 592, 87–94 CrossRef CAS PubMed.
- B. Chen, G. Zhong, P. G. Oppenheimer, C. Zhang, H. Tornatzky, S. Esconjauregui, S. Hofmann and J. Robertson, ACS Appl. Mater. Interfaces, 2015, 7, 3626–3632 CrossRef CAS PubMed.
- K. Ji, G. Meng, C. Yuan, E. Cui, Y. Li, J. Sun and Z. Dai, J. Manuf. Process., 2018, 33, 238–244 CrossRef.
- C. F. Schaber, T. Heinlein, G. Keeley, J. J. Schneider and S. N. Gorb, Carbon, 2015, 94, 396–404 CrossRef CAS.
- X. Yang, L. Chen, P. Zhang, H. Zhong, Y. Zhang, R. Zhang, P. Gu and Y. Zhao, Nanotechnology, 2020, 31, 295701 CrossRef CAS PubMed.
- S. Soylemez, B. Yoon, L. Toppare and T. M. Swager, ACS Sens., 2017, 2, 1123–1127 CrossRef CAS PubMed.
- B. Zou, Y. Chu and J. Xia, Bioprocess Biosyst. Eng., 2019, 42, 279–290 CrossRef PubMed.
- W. Li, Y. Gao, J. Zhang, X. Wang, F. Yin, Z. Li and M. Zhang, Nanoscale Adv., 2020, 2, 717–723 RSC.
- S. Zhu, X.-E. Zhao, J. You, G. Xu and H. Wang, Analyst, 2015, 140, 6398–6403 RSC.
- H. Wang, P. Li, D. Yu, Y. Zhang, Z. Wang, C. Liu, H. Qiu, Z. Liu, J. Ren and X. Qu, Nano Lett., 2018, 18, 3344–3351 CrossRef CAS PubMed.
- B. Zhang, Y. He, B. Liu and D. Tang, Anal. Chim. Acta, 2014, 851, 49–56 CrossRef CAS PubMed.
- D. Xu, L. Sun, Z. Zhang, Y. Wang, X. Zhang, F. Ye, Y. Zhao and J. Pan, Appl. Mater. Today, 2021, 24, 101124 CrossRef.
- Z. Yin, M. Jian, C. Wang, K. Xia, Z. Liu, Q. Wang, M. Zhang, H. Wang, X. Liang, X. Liang, Y. Long, X. Yu and Y. Zhang, Nano Lett., 2018, 18, 7085–7091 CrossRef CAS PubMed.
- M. Liu, X. Pu, C. Jiang, T. Liu, X. Huang, L. Chen, C. Du, J. Sun, W. Hu and Z. L. Wang, Adv. Mater., 2017, 29, 1703700 CrossRef PubMed.
- M. Zhang, M. Zhao, M. Jian, C. Wang, A. Yu, Z. Yin, X. Liang, H. Wang, K. Xia, X. Liang, J. Zhai and Y. Zhang, Matter, 2019, 1, 168–179 CrossRef.
- H. Huang, M. Liu, D. Xu, L. Mao, Q. Huang, F. Deng, J. Tian, Y. Wen, X. Zhang and Y. Wei, Mater. Sci. Eng., C, 2020, 106, 110157 CrossRef CAS PubMed.
- C. Jeong, H. Ko, H.-T. Kim, K. Sun, T.-H. Kwon, H. E. Jeong and Y.-B. Park, ACS Appl. Mater. Interfaces, 2020, 12, 18813–18822 CrossRef CAS PubMed.
- S. Xia, S. Song, F. Jia and G. Gao, J. Mater. Chem. B, 2019, 7, 4638–4648 RSC.
- Y. Ben-Shimon and A. Ya'akobovitz, Measurement, 2021, 172, 108889 CrossRef.
- H. Wang, S. Li, Y. Wang, H. Wang, X. Shen, M. Zhang, H. Lu, M. He and Y. Zhang, Adv. Mater., 2020, 32, 1908214 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |