Open Access Article
Open Access ArticlePhotoinduced degradation of thermally stable Cs2AgBiBr6 double perovskites by micro-Raman studies†
Athrey
C. Dakshinamurthy
 and
C.
Sudakar
and
C.
Sudakar
 *
*
Multifunctional Materials Laboratory, Department of Physics, Indian Institute of Technology Madras, Chennai, 600036, India. E-mail: csudakar@iitm.ac.in
First published on 14th June 2022
Abstract
The thermal stability of lead-free double perovskite Cs2AgBiBr6 for stable optoelectronic and photovoltaic devices is essential. There are contradicting reports, with some claiming stability from 300 to 400 °C based on X-ray diffraction and thermogravimetry studies and others up to 250 °C from Raman studies for Cs2AgBiBr6. We perform thermogravimetry analysis and temperature-dependent Raman studies with different laser intensities and show that Cs2AgBiBr6 is thermally stable up to ∼410 °C. A low power (3.68 mW) laser excitation source does not induce any structural changes at all temperatures. On the contrary, higher power laser light (7.15 mW) decomposes Cs2AgBiBr6 to Cs3Bi2Br9 at temperatures beyond 180 °C. Meticulous thermogravimetry, Raman, and X-ray diffraction studies confirm that Cs2AgBiBr6 is structurally stable up to 410 °C, whereas its stability decreases under light exposure beyond a certain critical intensity. This study brings out the importance of light and thermal stability of Cs2AgBiBr6, which is crucial for designing various optoelectronic devices.
1. Introduction
Halide double perovskites (HDPs) of the form A2B′B′′X6 are emerging as a promising alternative to lead-based inorganic perovskites due to their efficiency and promising applications in photovoltaics and optoelectronics.1,2 HDPs exhibit exceptional thermal stability and nontoxicity in addition to their promising optical properties.1 In HDPs, B′ and B′′ are monovalent and trivalent metal cations, thus forming a three-dimensional network of corner-connected metal-halide octahedra. Among various HDPs, Cs2AgBiBr6 is gaining significant momentum due to its reduced band gap (∼1.7–2.1 eV), long carrier lifetimes (> 1 μs), and smaller carrier effective mass, making it a promising candidate for photovoltaic and photocatalytic devices.3–6 Solar cells with Cs2AgBiBr6 as photo-absorbers have been fabricated recently, which show a promising efficiency of over 3%.7 Nevertheless, studies on the stability of double perovskites or degradation under thermal and photoinduced conditions are scarce in the literature. Burwig et al. have demonstrated through X-ray diffraction studies that the annealing temperature plays a crucial role in the phase and crystal structure evolution in Cs2AgBiBr6, with the cubic phase shown to be stable up to 300 °C.8 Beyond this temperature, it was ambiguous whether a high-temperature Cs2AgBiBr6 phase is formed, or thermal degradation begins. Contrastingly, Pistor et al. have reported that the Cs2AgBiBr6 undergoes thermal decomposition to Cs3Bi2Br9 at 250 °C.9 In fact, recent studies on solar cells fabricated using a Cs2AgBiBr6 absorber layer, reported by Ghasemi et al., demonstrated that the dual ion diffusion (Ag+ and Br−) of the material, which is an intrinsic property, affects the long term operational stability of the device.10 Thus, there is a lack of understanding of the structural stability and phase stability of Cs2AgBiBr6 double perovskites at elevated temperatures.Laser irradiation is widely used to probe various structural, vibrational, and optical properties. However, stability or degradation studies of HDPs under laser light exposure are scarce. Thermally-induced phase changes and compositional modification due to chemical effects caused by such exposure need to be understood. Various photochemical transformations such as oxidation-reduction, defect formation, phase transitions, and laser-induced degradation occur upon laser exposure.11,12 Such changes are mostly observed in lead-halide perovskites.12 Although HDPs are mostly known to be stable under ambient conditions and light exposure, it is important to explore the laser-induced effects as these compounds show applicability in high-energy X-ray and UV radiation detectors.13,14
In this communication, we demonstrate that the Cs2AgBiBr6 compound is thermally stable up to ∼410 °C, in contrast to an earlier observation9 that Cs2AgBiBr6 degrades to Cs3Bi2Br9 at 250 °C. We unequivocally show, on the other hand, that Cs2AgBiBr6 degrades to the Cs3Bi2Br9 phase even at 180 °C only upon exposure to a high intense laser power of ∼7.15 mW, revealing the instability of Cs2AgBiBr6 under intense photon interaction.
2. Experimental details
Cs2AgBiBr6 double perovskite powder is synthesized through a modified solution-based approach reported earlier.15 In a typical synthesis, stoichiometric concentrations of bismuth acetate and silver acetate are dissolved in HBr solution at 170 °C. After complete dissolution, CsBr is added to the above precursor solution. At this point, an orange-red precipitate immediately forms, indicating the crystallization and growth of Cs2AgBiBr6. The precipitate is filtered and dried at 75 °C. The crystal structure and phase purity of Cs2AgBiBr6 are analyzed by powder X-ray diffractometry (Rigaku SmartLab) with a CuKα (λ = 1.5406 Å) X-ray source. The Raman spectra are acquired using a Horiba-JobinYvon (HR 800 UV) micro-Raman spectrometer operating with a 632![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm laser excitation source. Raman spectra are also acquired at various temperatures with a Linkam stage attached to the spectrometer in a backscattered configuration with the microscope. Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) are simultaneously performed using a DSC-TGA Standard, SDT Q600 V20.9 Build 20 instrument to evaluate thermal stability and phase changes such as decomposition of Cs2AgBiBr6.
nm laser excitation source. Raman spectra are also acquired at various temperatures with a Linkam stage attached to the spectrometer in a backscattered configuration with the microscope. Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) are simultaneously performed using a DSC-TGA Standard, SDT Q600 V20.9 Build 20 instrument to evaluate thermal stability and phase changes such as decomposition of Cs2AgBiBr6.
3. Results and discussion
X-Ray diffraction (XRD) studies show that the synthesized compound exhibits phase pure composition of Cs2AgBiBr6 without the presence of any trace of other secondary phases (Fig. 1). Cs2AgBiBr6 crystallizes into a cubic elpasolite structure with the space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m symmetry. Ag+ and Bi3+ exhibit rock salt ordering in the structure, as evident from the presence of all odd Miller indices in the XRD pattern. The lattice parameter estimated using Rietveld refinement gives a = 11.2719 Å, which matches with the reported values.
m symmetry. Ag+ and Bi3+ exhibit rock salt ordering in the structure, as evident from the presence of all odd Miller indices in the XRD pattern. The lattice parameter estimated using Rietveld refinement gives a = 11.2719 Å, which matches with the reported values.
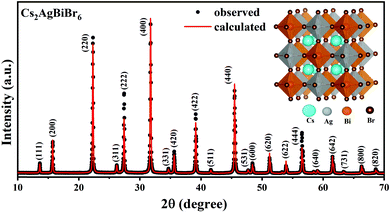 | ||
| Fig. 1 Powder XRD pattern of the synthesized Cs2AgBiBr6 along with the Rietveld refinement. Inset shows its crystal structure. | ||
Raman spectral analyses are performed on Cs2AgBiBr6 to elucidate the local structural information using octahedral vibrational modes and the stability of the compound as a function of temperature. Fig. 2 shows the Raman spectra of the Cs2AgBiBr6 compound at various temperatures in the range from 30 °C to 310 °C performed using 3.68 mW and 7.15 mW laser power of a 632 nm excitation source, which corresponds to a power density of ∼1.1 mW μm−2 and 2.2 mW μm−2, respectively. The beam size of the laser is ∼2 μm in all these measurements. Based on the Wyckoff positions of atoms and symmetry considerations, four modes viz., F2g (2 modes), Eg, and A1g are Raman active in Cs2AgBiBr6. These modes are attributed to the scissoring motion of Br atoms along with Cs motion, and asymmetric and symmetric stretching of [AgBr6]5− octahedra, respectively.16 The vibrational energies of these modes observed at 30 °C are in good agreement with the values reported in the literature.9
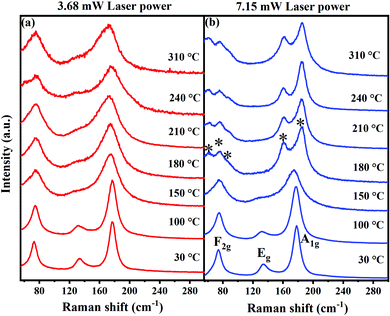 | ||
| Fig. 2 Raman spectra of Cs2AgBiBr6 acquired at various temperatures using a 632 nm He–Ne-ion laser with (a) 3.68 mW and (b) 7.15 mW laser power. | ||
The temperature stability of the Cs2AgBiBr6 and laser-induced effects on the structure can be directly inferred from the Raman spectra acquired at different temperatures using two different laser powers. When excited with lower laser power (∼3.68 mW), all the Raman modes are clearly discernible at all the temperatures suggesting the robust structural stability of Cs2AgBiBr6. The vibrational modes get substantially broadened with an increase in temperature due to the increased anharmonicities in the atomic vibrations at elevated temperatures. Most importantly, the cubic structure of Cs2AgBiBr6 is retained at high temperatures indicating that the compound Cs2AgBiBr6 is highly stable and does not undergo any thermal degradation. This agrees well with the studies reported by Burwig et al., where they demonstrated through XRD measurements that the Cs2AgBiBr6 structure is stable up to 300 °C and can be clearly assigned to the cubic polymorph.8 In their report, it is quite ambiguous, for temperatures greater than 300 °C, whether Cs2AgBiBr6 remains still in a cubic structure or undergoes phase degradation. However, a recent report by Pistor et al. showed contrastingly different results, wherein they demonstrated that the cubic structure of Cs2AgBiBr6 undergoes thermally induced phase degradation to a layered Cs3Bi2Br9 structure at 250 °C.9 This has been inferred from the changes observed in the Raman spectra acquired after annealing the samples at various temperatures. In fact, we observe a similar feature; however, when the Raman spectra are acquired with a higher laser power (7.15 mW), as shown in Fig. 2b.
Halide single perovskites are known to undergo photoinduced and laser-induced degradation resulting in phase segregations. Udalova et al. demonstrated the photochemical degradation of hybrid lead iodide perovskites upon laser irradiation, resulting in polyiodides.12 In the case of HDPs, to the best of our knowledge, such studies are scarce. However, we believe it is quite possible that the laser-induced degradation could play a significant role in converting the phase locally. To understand whether the degradation is induced by a thermal effect or due to a laser light exposure effect, Raman spectra of Cs2AgBiBr6 are acquired using a high laser power. At temperatures below 180 °C, all the Raman spectra are identical when the spectra were acquired with lower power excitation. However, at temperatures above 180 °C, we observe that the Raman modes get altered with the emergence of several new peaks (marked with the symbol *). This indicates the structural degradation of Cs2AgBiBr6 and the formation of a new phase, viz. Cs3Bi2Br9. The F2g mode at 74.8 cm−1 splits into three peaks (2Eg and 1A1g), which can be attributed to the vibrations associated with BiBr6 octahedra involving only Br atoms.17 One of the three low energy peaks (1Eg) corresponds to the octahedral vibrations around the x, y axes. The other two peaks could be due to the deformational vibrations of BiBr6 octahedra.17 This indicates a deformation of the cubic lattice. The intense A1g mode of Cs2AgBiBr6 at ∼177.5 cm−1, which is observed at all temperatures for low laser power excitation or at temperatures below 180 °C for high power laser excitation, disappears and two new peaks at ∼161 cm−1 and ∼185 cm−1 emerge at temperatures >180 °C upon excitation with a 7.15 mW, 632 nm laser source. These two modes are A1g (∼161 cm−1) and Eg (∼185 cm−1) vibrations arising from the stretching of Bi–Br bonds in the Cs3Bi2Br9 structure.17 Thus, it is evident from our Raman spectral studies that the Cs2AgBiBr6 phase degrades to Cs3Bi2Br9 only upon using a high laser power. This is in stark contrast to an earlier report by Pistor et al., in which Cs2AgBiBr6 is found to be thermally stable only up to 250 °C.9 Our study unequivocally demonstrates that (i) Cs2AgBiBr6 is stable up to high temperatures of 410 °C, as discussed in the following section, and (ii) Cs2AgBiBr6 can degrade under the influence of an intense laser source. The degradation does not happen at lower temperatures (<180 °C) as the compound is inherently stable, whereas, at higher temperatures, higher laser intensities generate a significant amount of localized heat resulting in the local degradation of Cs2AgBiBr6.
It is known that the intense laser irradiation on samples would increase the local temperature, which would cause thermal decomposition of the compounds.18 The increase in temperature upon laser exposure can be estimated19 and in the present case a rough estimate is expected to be around a few hundred Kelvin. As the sample temperature is raised using an external heater, the net temperature can go beyond the decomposition temperature locally (∼420 °C) leading to the decomposition of Cs2AgBiBr6 at 180 °C in the in situ heating experiments. This is consistent with our observation. The Raman spectra acquired at room temperature using low power laser excitation on the compound obtained by annealing it in an air ambiance up to 410 °C shows no tendency for decomposition. On the contrary, when the same study is performed under intense high power laser illumination, in in situ heating experiments, the sample decomposes at 180 °C. This clearly indicates that the intense laser photons generate localized heat in the sample. This local heat in addition to the externally applied heat (∼180 °C) can make the overall temperature reach beyond the decomposition temperature locally (>420 °C), thus resulting in decomposition to other phases.
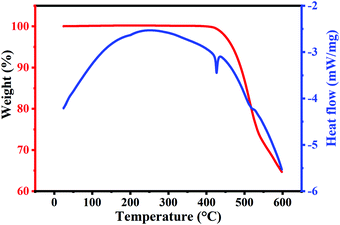
To further confirm the thermal stability of the compound, we performed both static and dynamic thermogravimetry analyses (TGAs). Simultaneous TGA with differential scanning calorimetry (DSC) studies performed on Cs2AgBiBr6 powder are shown in Fig. 3. The measurements are performed in an air ambiance from 25 °C to 600 °C with a heating rate of 5 °C min−1. The weight percentage and heat flow changes with temperature are plotted in Fig. 3. TGA studies show that the as-prepared Cs2AgBiBr6 powder does not undergo any weight loss until a temperature of ∼410 °C. DSC studies also do not show any sharp endothermic/exothermic peaks indicating that there are no phase transformations in this temperature range. This confirms the thermal stability of Cs2AgBiBr6 up to ∼410 °C. Beyond 410 °C, we observe a sharp endothermic peak at 420 °C. At this temperature, the weight loss also seems to begin. The weight loss proceeds continuously and the specimen undergoes ∼35% weight loss until 600 °C. This gradual weight loss indicates that Cs2AgBiBr6 does not undergo spontaneous decomposition. This is due to the positive values of decomposition enthalpies for its decomposition pathways.20
We also performed XRD studies on Cs2AgBiBr6 samples annealed under static conditions in open-air at 250 °C, 410 °C, and 450 °C for 30 min to understand the phase changes that would arise upon annealing at elevated temperatures (Fig. 4). It is observed that Cs2AgBiBr6 is phase-pure with the cubic structure intact up to ∼400 °C. Beyond 410 °C, Cs2AgBiBr6 decomposes, yielding other phases like Cs3Bi2Br9 and CsAgBr2. The XRD patterns for the sample annealed at 410 °C show intense cubic Cs2AgBiBr6 reflections along with minor secondary peaks, which correspond to Cs3Bi2Br9 and CsAgBr2.21,22 This indicates that Cs2AgBiBr6 starts decomposition at temperatures ∼410 °C, consistent with TGA-DSC studies, wherein no weight loss is seen until 410 °C. Upon further annealing at 450 °C, Cs2AgBiBr6 completely decomposes into binary and ternary bromide compounds and oxybromide compounds (Fig. 4 and 5). Thus, it is evident that the Cs2AgBiBr6 compound is thermally stable up to a much higher temperature, unlike the earlier report by Pistor et al.9 Although we see the similar degradation of Cs2AgBiBr6 at 180 °C through Raman spectral analyses, it is not due to the thermal degradation of the compound as reported by Pistor et al., but instead, it is laser-induced degradation of the sample at high temperatures with higher laser intensities. We have also confirmed this argument by collecting the Raman spectra of the samples annealed under static conditions at 410 °C and 450 °C (Fig. 5). The Raman spectrum of the sample annealed at 410 °C clearly shows the Raman modes of Cs2AgBiBr6 without any trace of Raman peaks from other phases. This strongly suggests that the cubic perovskite structure is intact up to 410 °C. The spectra collected for the sample annealed at 450 °C were found to be highly inhomogeneous, showing a different set of peaks at different regions. These were mostly from Cs3Bi2Br9, Cs3BiBr6, and other possible oxybromides, including BiOBr. This definitely shows the thermal degradation of the sample around 450 °C.
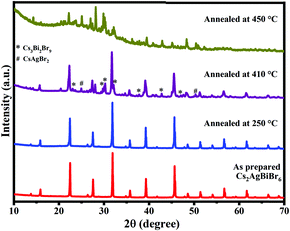 | ||
| Fig. 4 XRD patterns of Cs2AgBiBr6 samples annealed at 250 °C, 410 °C, and 450 °C, along with the as-prepared sample. | ||
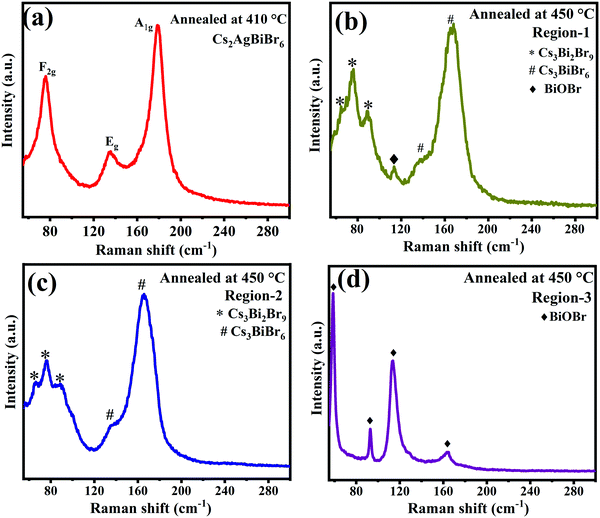 | ||
| Fig. 5 Raman spectra of Cs2AgBiBr6 annealed at (a) 410 °C, and 450 °C (b, c and d) at different regions. | ||
Furthermore, we have tried to synthesize the decomposition products like Cs3Bi2Br9, Cs3BiBr6, BiOBr and CsAgBr2 in phase-pure forms. Cs3Bi2Br9 and BiOBr compounds can be synthesized with phase purity as evident from the XRD and Raman studies (Fig. S1 and S2, ESI†). Phase-pure synthesis of Cs3BiBr6 is challenging and the solution-based synthesis of the Cs3BiBr6 compound resulted in a mixture of phases like Cs3Bi2Br9 and Cs3BiBr6 as observed from the Raman studies. Furthermore, the CsAgBr2 compound did not form when synthesized via a solution-based approach. We tried to synthesize CsAgBr2 through a solid-state reaction by thoroughly grinding equimolar concentrations of CsBr and AgBr, followed by annealing. The obtained compound shows diffraction peaks corresponding to orthorhombic CsAgBr2 along with some unidentifiable minor phases and agrees with the reported literature.23 The XRD and Raman spectra for all these compounds are acquired under the same conditions that are employed for Cs2AgBiBr6. The diffraction patterns and the spectra found in the decomposition products are in good agreement with the compounds synthesized individually (Fig. S1 and S2, ESI†). This further substantiates the origin of extra peaks and the assignment of the decomposition byproducts.
4. Conclusions
In summary, thermal stability and photostability studies are performed on the Cs2AgBiBr6 compound using temperature-dependent Raman spectra and TGA-DSC studies. Cs2AgBiBr6 is thermally stable up to ∼410 °C. However, in the presence of a high-intensity laser power source, the stability of the compound comes down to 180 °C. This could be due to the localized heating arising from intense laser light resulting in local decomposition. This study, thus, unequivocally shows the photoinduced thermal instability in an otherwise thermally stable compound. We believe such a study on this novel compound is the first of its kind and will be of great utility to effectively integrate Cs2AgBiBr6 into various optoelectronic devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
C. S. acknowledges the Ministry of Education, Government of India, for funding this work through Scheme for Transformational and Advanced Research in Sciences (STARS) vide STARS/APR2019/NS/537/FS. C. S. acknowledges the support by MHRD, India through the Institutes of Eminence (vide SP20210777DRMHRDDIRIIT) for the research initiatives on establishing the prospective Centre for Advanced Microscopy and Materials (vide SB20210844MMMHRD008277).References
- F. Igbari, Z.-K. Wang and L.-S. Liao, Adv. Energy Mater., 2019, 9, 1803150 CrossRef.
- P.-K. Kung, M.-H. Li, P.-Y. Lin, J.-Y. Jhang, M. Pantaler, D. C. Lupascu, G. Grancini and P. Chen, Sol. RRL, 2020, 4, 1900306 CrossRef CAS.
- X. Yang, W. Wang, R. Ran, W. Zhou and Z. Shao, Energy Fuels, 2020, 34, 10513–10528 CrossRef CAS.
- R. L.-Z. Hoye, L. Eyre, F. Wei, F. Brivio, A. Sadhanala, S. Sun, W. Li, K. H.-L. Zhang, J. L. MacManus-Driscoll, P. D. Bristowe, R. H. Friend, A. K. Cheetham and F. Deschler, Adv. Mater. Interfaces, 2018, 5, 1800464 CrossRef.
- D. Bartesaghi, A. H. Slavney, M. C. Gélvez-Rueda, B. A. Connor, F. C. Grozema, H. I. Karunadasa and T. J. Savenije, J. Phys. Chem. C, 2018, 122, 4809–4816 CrossRef CAS PubMed.
- F. Ji, J. Klarbring, F. Wang, W. Ning, L. Wang, C. Yin, J. S.-M. Figueroa, C. K. Christensen, M. Etter, T. Ederth, L. Sun, S. I. Simak, I. A. Abrikosov and F. Gao, Angew. Chem., Int. Ed., 2020, 59, 15191–15194 CrossRef CAS PubMed.
- B. Wang, N. Li, L. Yang, C. Dall’Agnese, A. K. Jena, S. Sasaki, T. Miyasaka, H. Tamiaki and X. Wang, J. Am. Chem. Soc., 2021, 143(5), 2207–2211 CrossRef CAS PubMed.
- T. Burwig, M. Guc, V. Izquierdo-Roca and P. Pistor, J. Phys. Chem. C, 2020, 124, 9249–9255 CrossRef CAS.
- P. Pistor, M. Meyns, M. Guc, H.-C. Wang, M. A.-L. Marques, X. Alcobé, A. Cabot and V. Izquierdo-Roca, Scr. Mater., 2020, 184, 24–29 CrossRef CAS.
- M. Ghasemi, L. Zhang, J.-H. Yun, M. Hao, D. He, P. Chen, Y. Bai, T. Lin, M. Xiao, A. Du, M. Lyu and L. Wang, Adv. Funct. Mater., 2020, 30, 2002342 CrossRef CAS.
- Y.-C. Qiao, Y.-H. Wei, Y. Pang, Y.-X. Li, D.-Y. Wang, Y.-T. Li, N.-Q. Deng, X.-F. Wang, H.-N. Zhang, Q. Wang, Z. Yang, L.-Q. Tao, H. Tian, Y. Yang and T.-L. Ren, Jpn. J. Appl. Phys., 2018, 57, 04FA01 CrossRef.
- N. N. Udalova, A. S. Tutantsev, Q. Chen, A. Kraskov, E. A. Goodilin and A. B. Tarasov, ACS Appl. Mater. Interfaces, 2020, 12, 12755–12762 CrossRef CAS PubMed.
- C. Wu, B. Du, W. Luo, Y. Liu, T. Li, D. Wang, X. Guo, H. Ting, Z. Fang, S. Wang, Z. Chen, Y. Chen and L. Xiao, Adv. Opt. Mater., 2018, 6, 1800811 CrossRef.
- W. Yuan, G. Niu, Y. Xian, H. Wu, H. Wang, H. Yin, P. Liu, W. Li and J. Fan, Adv. Funct. Mater., 2019, 29, 1900234 CrossRef.
- A. C. Dakshinamurthy and C. Sudakar, J. Phys. Chem. Lett., 2022, 13, 433–439 CrossRef CAS PubMed.
- A. C. Dakshinamurthy and C. Sudakar, Appl. Phys. Lett., 2021, 118, 131902 CrossRef.
- M. Y. Valakh, M. P. Lisitsa, E. Y. Peresh, O. V. Trylis and A. M. Yaremko, J. Mol. Struct., 1997, 436-437, 309–313 CrossRef CAS.
- L. Han, M. Zeman and A. H.-M. Smets, Nanoscale, 2015, 7, 8389–8397 RSC.
- B. H. Venkataraman, N. S. Prasad, K. B.-R. Varma, V. Rodriguez, M. Maglione, R. Vondermuhll and J. Etourneau, Appl. Phys. Lett., 2005, 87, 091113 CrossRef.
- Z. Xiao, W. Meng, J. Wang and Y. Yan, ChemSusChem, 2016, 9, 2628–2633 CrossRef CAS PubMed.
- J. Su, Y.-q Huang, H. Chen and J. Huang, Cryst. Res. Technol., 2020, 55, 1900222 CrossRef CAS.
- M. N. Tran, I. J. Cleveland and E. S. Aydil, J. Mater. Chem. C, 2020, 8, 10456–10463 RSC.
- Z. Zhang, R. Zhao, S. Teng, K. Huang, L. Zhang, D. Wang, W. Yang, R. Xie and N. Pradhan, Small, 2020, 16, 2004272 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma00179a |
| This journal is © The Royal Society of Chemistry 2022 |