Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Combining gellan gum with a functional low-molecular-weight gelator to assemble stiff shaped hybrid hydrogels for stem cell growth†
Carmen C.
Piras
 a,
Paul G.
Genever
b and
David K.
Smith
a,
Paul G.
Genever
b and
David K.
Smith
 *a
*a
aDepartment of Chemistry, University of York, Heslington, York, YO10 5DD, UK. E-mail: david.smith@york.ac.uk
bDepartment of Biology, University of York, Heslington, York, YO10 5NG, UK
First published on 2nd September 2022
Abstract
We report hybrid hydrogels that combine gellan gum (GG) polymer gelator (PG) with a low-molecular weight gelator (LMWG) based on 1,3:2,4-dibenzylidene sorbitol (DBS). We fabricate these gels into beads using a heat–cool cycle to set the LMWG gel and then using different calcium sources (CaCl2 and CaCO3) to subsequently crosslink the gellan gum. In the case of CaCO3, glucono-δ-lactone (GdL) is used as a slow acidification agent to slowly solubilise calcium ions and induce GG crosslinking. Alternatively the photoacid generator, diphenyliodonium nitrate (DPIN) can be used with UV irradiation to solubilise CaCO3 and induce GG gelation, in which case, a photomask applied to gels made in trays yields photopatterned gels. Combining the LMWG with gellan gum further enhances the stiffness of GG, and importantly, makes the gels significantly more resistant to shear strain. LMWG/GG hybrid gels are considerably stiffer than equivalent LMWG/alginate gels. The DBS-CONHNH2 LMWG retains its unique ability to reduce precious metal salts to nanoparticles (NPs) within the hybrid gel beads, as demonstrated by the in situ fabrication of AgNPs. The hybrid gel beads support the growth of human mesenchymal stem cells for extended periods of time. We suggest that the favourable rheological properties of these hybrid gels, combined with the ability of the LMWG to form AgNPs in situ, may enable potential future orthopedic applications.
Introduction
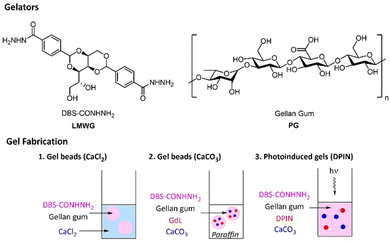
Gels are fascinating soft materials, intermediate between solids and liquids, with wide-ranging applications from food technology and personal care through to drug delivery and regenerative medicine.1 Gels can be formed by a variety of different systems – particularly important amongst these are gels formed by polymer gelators (PGs)2 and those formed by low-molecular-weight gelators (LMWGs).3 Each of these classes of gelator offers specific advantages, and in recent years, combining the two to yield hybrid hydrogels has emerged as a powerful strategy for creating addressable, tunable multi-component soft materials.4 This can provide access to shaped and patterned gel-phase materials with high added value.5 With this strategy in mind, we became interested in the use of gellan gum as the polymeric component of such hybrid gels.Gellan gum is a low-cost extracellular polysaccharide produced by Sphingomonas bacteria.6 This anionic polymer is typically composed of tetrasaccharide repeat units consisting of two D-glucose, one D-glucuronic acid and one L-rhamnose residues (i.e. [D-Glc(β1 → 4)D-GlcA(β1 → 4)D-Glc(β1 → 4)L-Rha(α1 → 3)]n, Fig. 1). Gellan gum is mainly used as a gelling agent, and is typically activated either thermally or by cross-linking with divalent metal cations. Having only been discovered in 1978, and FDA approved in 1992,7 it is a relatively ‘young’ biomaterial compared to other gelling polysaccharides, such as agarose, alginate or cellulose. However, thanks to its ease of gelation, tuneable physical properties and versatility, research on this PG has seen significant recent expansion. In the food industry, it is widely used as a thickener, stabilizer and binder, as well as a replacement for gelatine in vegan products.8 Biomedical applications of gellan gum hydrogels include, tissue engineering, repair and regeneration,9 biosensor synthesis10 and pharmaceutical formulation for oral, nasal, topical and injectable administration.11 However, gellan gum has some disadvantages in terms of its use in regenerative medicine – specifically its relatively brittle nature and a lack of attachment sites for anchorage-dependent cells.12 As such, making synthetic modifications to gellan gum and/or blending it with a different additives has become an area of significant interest.13
Research in our group has recently explored multicomponent hybrid hydrogels based on calcium alginate and the low molecular weight gelators (LMWGs) based on 1,3:2,4-dibenzylidene sorbitol (DBS), i.e. DBS-CONHNH2 and DBS-COOH (Fig. 1).14 We have demonstrated that in such hybrid gels, the LMWGs provided functionality, whilst the alginate offered mechanical support and robustness to shape and structure the LMWGs into gel beads or tubes. On starting this work with gellan gum, we wanted to explore the potential use of this PG instead of calcium alginate to develop gels with enhanced properties. Taking into account the wide range of applications of gellan gum, we reasoned that this polymer could be an interesting alternative to alginate for the formulation of LMWG/PG hybrid gels. In particular, the high stiffness of gellan gum was attractive as it may broaden the rheological range over which our LMWGs can operate, potentially opening up new applications.
We thus report the fabrication of shaped and patterned hybrid gels comprising DBS-CONHNH2 and gellan gum (GG), exploring how their rheological properties combine in a synergistic way. The LMWG retains its unique feature of inducing in situ formation of silver nanoparticles (AgNPs),15 and the resulting hybrid hydrogels are compatible with the growth of human mesenchymal stem cells. We suggest such materials may have potential future uses in regenerative medicine.
Results and discussion
Hybrid DBS-CONHNH2/GG gel beads cross-linked with CaCl2
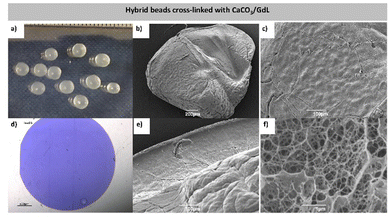
DBS-CONHNH2 was synthesized in good yield by our previously reported procedure.16 This LMWG forms hydrogels by a heat–cool cycle. Gellan gum (GG) is a commercially-available polysaccharide and was used as supplied (Alfa Aesar). GG can form stiff hydrogels when cross-linked with Ca2+ ions. Therefore, the LMWG was used as a scaffold to support the subsequent gelation of GG, induced by Ca2+.Initially DBS-CONHNH2/GG gel beads were prepared by the emulsion method previously described by us,14 with CaCl2 being used for crosslinking (Fig. 1). Briefly, DBS-CONHNH2 (0.3% wt/vol) was suspended in water (0.5 mL) and then combined with an aqueous GG solution (0.1–1.0% wt/vol, 0.5 mL). The suspension was heated until complete dissolution of the solid particles and subsequently added dropwise (20 μL per drop) to paraffin oil (40 mL). The droplets were left undisturbed for 20 min to allow assembly of the DBS-CONHNH2 network, which acts as a scaffold to support the subsequent gelation of GG. To cross-link GG, the gel beads were then transferred to a CaCl2 solution (5.0% wt/vol) and gently mixed for ca. 20 min to allow diffusion of the Ca2+ into them. After this time, to remove residual paraffin oil, the gel beads were washed with petroleum ether, then EtOH and finally water. Small beads with a 3.0–3.5 mm diameter were obtained (Fig. 2a). The bead diameter is controlled by the droplet size during fabrication – smaller gel beads could be obtained by addition of smaller volumes.14a,e
In this method, the Ca2+ ions are readily available and quickly cross-link the polymer from the outside, then penetrate inside the gel beads by diffusion. Possibly as a result of this, the surface and cross-section of the beads appear heavily wrinkled (Fig. 2b and c). Optical microscopy of the gel bead cross-section, embedded into resin and stained with toluidine blue, showed a uniform distribution of gel networks, consistent with a model in which they are woven within the gel beads (Fig. 2d). SEM microscopy of the gel beads showed a wrinkled surface and a nanofibrillar core, thus further confirming the incorporated gelators were in their self-assembled state (Fig. 2e, f and Fig. S7, ESI†), although it was not possible to distinguish the two networks using this approach. TEM images were also recorded (Fig. S4–S6, ESI†).
To confirm the two gelators were self-assembled within the gel beads, we recorded a standard 1H NMR spectrum of five gel beads in D2O, in the presence of DMSO (1.4 μL) as internal standard. The lack of any signals in the recorded spectra indicates that both gelators are completely ‘immobilised’ within the gel beads (Fig. S1, ESI†).
Another simple 1H NMR spectroscopy experiment, carried out on 10 dried beads, subsequently dissolved in DMSO-d6 in the presence of an internal standard (MeCN), allowed estimation of the amount of LMWG incorporated into each bead. By comparing the aromatic C–H integrals of DBS-CONHNH2 with the internal standard we could quantify how much LMWG had been incorporated into the beads. Given the beads were prepared using 20 μL per droplet, in theory, 1 mL can prepare 50 beads. Since an initial 0.3% wt/vol concentration of DBS-CONHNH2 was used, corresponding to 6.32 μmol, each gel bead can contain up to 0.126 μmol of LMWG. Our study indicated that each hybrid bead incorporates ca. 0.11 μmol of DBS-CONHNH2 (Fig. S2, ESI†). Therefore, the vast majority of the LMWG initially loaded is retained inside the gel beads.
The macroscopic physical properties of the gels were then studied in terms of thermal stability and rheological behaviour. Initially, the thermal stability of equivalent gels made in sample vials was evaluated using a simple tube-inversion method. The gel–sol transition temperature of DBS-CONHNH2 (0.4% wt/vol) is 86 °C. In the presence of increasing GG loadings (0.1, 0.3, 0.5 and 1.0% wt/vol), this rises to >100 °C (Table S1, ESI†). As expected therefore, the PG improves the thermal stability.
To study the thermal stability of the hybrid gel beads in more detail over time, we performed an NMR experiment at 90 °C and monitored the disassembly of the LMWG, as the LMWG becomes mobile, its 1H NMR resonances increase in intensity. After only 15 min ca. 40% of the LMWG had disassembled, increasing to a maximum of ca. 60% after 3.5 h (Table S3, ESI†). It is possible that not all LMWG is mobilised since CaCl2 cross-links GG by diffusion from the outside of the beads, a tougher GG ‘shell’ could form, somewhat protecting the LMWG network from being thermally disrupted and dissolved into the hot solvent.
Importantly, the mechanical properties of the hybrid gels were then studied by oscillatory rheology (Table 1). To record reproducible parallel plate rheometry data it was necessary to use equivalent gel discs made in sample tubes, rather than testing the beads (see ESI†). The DBS-CONHNH2 gel has an elastic modulus (G′) of ca. 800 Pa (Fig. S9, ESI†), which increases to 3980, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, 23
200, 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 and 46
500 and 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 with increasing GG loadings (0.1, 0.3, 0.5, 1.0% wt/vol) – a remarkable increase in stiffness. To some extent, this was partly expected, as GG is a stiff gel,17 however, the values obtained are even higher than those of the G′ values of the GG gels alone (3290, 4560, 10
600 with increasing GG loadings (0.1, 0.3, 0.5, 1.0% wt/vol) – a remarkable increase in stiffness. To some extent, this was partly expected, as GG is a stiff gel,17 however, the values obtained are even higher than those of the G′ values of the GG gels alone (3290, 4560, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 and 17
500 and 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 Pa; respectively at 0.4, 0.6, 0.8 and 1.3% wt/vol concentrations; Fig. S10–S13, ESI†). This suggests that the inter-penetrating PG/LMWG networks within the hybrid material significantly stiffen the gel.
300 Pa; respectively at 0.4, 0.6, 0.8 and 1.3% wt/vol concentrations; Fig. S10–S13, ESI†). This suggests that the inter-penetrating PG/LMWG networks within the hybrid material significantly stiffen the gel.
| LMWG load (%) | GG load (%) | Alginate load (%) | Total load (%) | Gel trigger | G′ (Pa) | G′/G′′ crossover (%) |
|---|---|---|---|---|---|---|
| 0.4 | — | — | 0.4 | Heat/cool | 800 | 25.1 |
| — | 0.4 | — | 0.4 | CaCl2 | 3290 | 1.0 |
| — | 0.6 | — | 0.6 | CaCl2 | 4560 | 1.1 |
| — | 0.8 | — | 0.8 | CaCl2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2.0 |
| — | 1.3 | — | 1.3 | CaCl2 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.0 |
| 0.3 | 0.1 | — | 0.4 | CaCl2 | 3980 | 6.3 |
| 0.3 | 0.3 | — | 0.6 | CaCl2 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
7.7 |
| 0.3 | 0.5 | — | 0.8 | CaCl2 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
6.9 |
| 0.3 | 1.0 | — | 1.3 | CaCl2 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
2.3 |
| — | — | 0.4 | 0.4 | CaCl2 | 490 | 6.5 |
| — | — | 0.6 | 0.6 | CaCl2 | 1420 | 19.9 |
| — | — | 0.8 | 0.8 | CaCl2 | 2500 | 2.3 |
| — | — | 1.3 | 1.3 | CaCl2 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
8.5 |
| 0.3 | — | 0.1 | 0.4 | CaCl2 | 1730 | 50.6 |
| 0.3 | — | 0.3 | 0.6 | CaCl2 | 6030 | 41.2 |
| 0.3 | — | 0.5 | 0.8 | CaCl2 | 8030 | 18.4 |
| 0.3 | — | 1.0 | 1.3 | CaCl2 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2.8 |
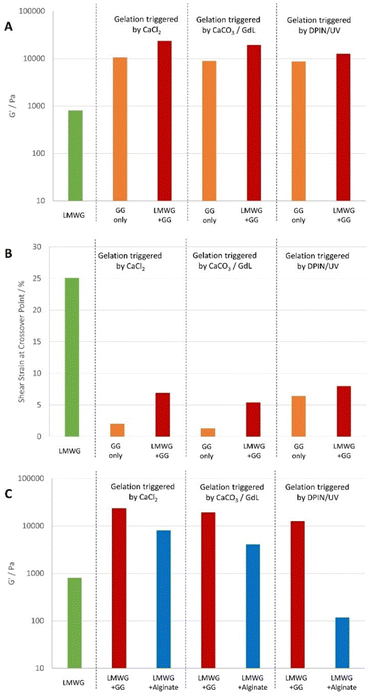
Furthermore, it is informative to consider the shear strain at which the gels break down as defined by the G′/G′′ crossover points. On its own, gellan gum, although a stiff gel, has very poor resistance to shear, and is brittle, fracturing easily. Indeed, this is a significant drawback in terms of its use in some applications.12 Indeed, we found that for GG alone, G′ = G′′ at a shear strain of only ca. 1%. However, on blending with DBS-CONHNH2, the stability to shear strain increases significantly – up to 7.7% for the sample containing 0.3% wt/vol DBS-CONHNH2 and 0.3% wt/vol GG. The high shear resistance of DBS-CONHNH2 (ca. 25% shear strain) therefore translates into these hybrid gels and very significantly improves the rheological operating window of GG.
It is worth comparing the performance of these GG/LMWG hybrid gels with the alginate/LMWG hybrid gels previously reported by us.14a As might be expected, the GG/LMWG hybrids are very significantly stiffer than those based on alginate. Indeed, with G′ values up to ca. 50 kPa, gellan gum moves these hybrids into the range of stiffnesses required for effective osteogenesis (i.e., differentiation of stem cells into bone cells).17 At the same time, the LMWG makes the GG significantly more resistant to shear strain than native GG, improving its handling. Furthermore, it adds additional function to the gels that may be useful in an osteogenesis setting (see below).
In summary therefore, GG provides DBS-CONHNH2 with stiffness, while DBS-CONHNH2 provides GG with greater shear resistance, meaning that these hybrid hydrogels may extend the potential applications of both LMWG and PG by combining the best of their individual rheological characteristics.
Hybrid DBS-CONHNH2/GG gel beads cross-linked with CaCO3/GdL
A different procedure was then applied to obtain DBS-CONHNH2/GG gel beads using CaCO3 as a cross-linker. CaCO3 is insoluble in water, and therefore cannot directly crosslink gellan gum. However it has been shown for other polysaccharides that on slow acidification, Ca2+ is released and gel crosslinking can take place.18 To achieve slow acidification, glucono-δ-lactone (GdL) is ideal – it slowly hydrolyses, and lowers pH over a period of hours.19 This approach has previously been quite extensively used to crosslink alginate hydrogels.20 GdL has also been combined with calcium carbonate to form alginate/GG hybrids, although it is not clear whether the gellan gum was actually crosslinked via the same mechanism as the alginate in this case.21 There has been some interest in incorporating CaCO3 microparticles into GG gels – they can slowly release Ca2+ even in the absence of an acid source and are also highly relevant in bone tissue engineering.22 GdL alone has also been used to trigger the assembly of emulsion gels based on GG,23 which can be achieved if the pH drops below 3.5. As far as we can tell, however, although widely used for alginate gels, it has not been clearly demonstrated that the CaCO3/GdL approach can crosslink gellan gum via Ca2+ release, and we therefore wanted to test this. We found that GG gels in vials could be produced using this approach (see ESI† and characterisation below).We therefore moved on to test this approach for making hybrid gel beads, in the same way we had previously reported for alginate/LMWG hybrids.14d After dropwise addition of the hot mixture containing the two gelators, GdL and CaCO3 into paraffin oil, the droplets were left undisturbed overnight to allow the slow release of Ca2+ ions that cross-link GG in situ (Fig. 1). After this time, small gel beads (diameter 3.0–3.5 mm) were collected from paraffin oil and washed multiple times as described above (Fig. 3a). The final pH of the gel beads is 5–6, with the precise value depending on the amount of GdL used in the formation process (Fig. S18 and S19, ESI†). As such, we are confident that the GdL is not inducing GG assembly simply by protonation of the polymer backbone itself, as that would require the pH to fall significantly lower.23
Although this method of bead formation is similar to that using CaCl2, in this case, the release of Ca2+ takes place slowly over time, throughout the bead itself, rather than rapidly from the periphery. As such, GG gelation happens in a more homogeneous, temporally-controlled manner. This leads to more uniform gel beads with smoother surfaces (Fig. 3b, c and Fig. S26, ESI†). Furthermore, because the beads are formed in paraffin, they are more uniform and spherical (Fig. 3a) than the beads crosslinked with CaCl2 (Fig. 2a). Optical microscopy of the gel bead cross-section, embedded into resin and stained with toluidine blue, showed a uniform distribution of the two gel networks, consistent with a model in which they are woven within the gel beads (Fig. 3c and Fig. S23, ESI†). SEM microscopy showed a nanofibrillar gel bead core, thus further confirming that the incorporated gelators were in their self-assembled state (Fig. 3e, f and Fig. S27, ESI†). The data were consistent with assembly of both gelators, although unfortunately did not allow us to distinguish between the self-assembled networks formed by the individual components. TEM images of the different gels were also recorded (Fig. S24 and S25, ESI†).
Once again, 1H NMR spectroscopy was used to confirm that the two gelators were self-assembled within the gel beads, with data indicating both gelators are completely ‘immobilised’ (Fig. S20, ESI†). Using NMR to estimate the amount of LMWG incorporated into each bead (see method above), we found that each CaCO3 hybrid bead incorporates ca. 0.10 μmol (Fig. S21, ESI†) of the theoretical maximum of 0.126 μmol – ca. 80%. Therefore, the majority of the LMWG is retained inside the gel beads.
Like the gels formed using CaCl2, the thermal stability of the hybrid gels triggered by CaCO3/GdL gels is >100 °C (0.15% wt/vol CaCO3/1.0% wt/vol GdL; Table S4, ESI†) as measured by tube inversion, with the PG improving the thermal stability of the gel. More detailed NMR studies indicated that when the gel beads were incubated at 90 °C, after only 15 min ca. 40% of the LMWG was disassembled (Table S3, ESI†), increasing to a maximum of ca. 71% after 5.5 h. This is broadly similar to the CaCl2 system – the slightly greater degree of LMWG disassembly may reflect the fact that for the CaCO3/GdL gel beads the exterior is less heavily crosslinked GG.
The mechanical properties of these hybrid gels were then studied by oscillatory rheology using gel discs (Table 2 and Table. S5, ESI†). As for the gels triggered by CaCl2, the presence of gellan gum increased the stiffness of the gel very significantly, with G′ values of 2580, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 and 19
300 and 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 Pa with increasing GG loadings (0.1, 0.3 and 0.5% wt/vol; Fig. S33–S35, ESI†); once again, these were higher than the G′ values of GG-only gels (1760, 4630 and 8950 Pa; Fig. S30–S32, ESI†) demonstrating the benefits of interpenetrated LMWG/PG networks. Once again, these stiffnesses are higher than those previously reported for equivalent alginate/LMWG hybrid hydrogels (data not shown).14d
300 Pa with increasing GG loadings (0.1, 0.3 and 0.5% wt/vol; Fig. S33–S35, ESI†); once again, these were higher than the G′ values of GG-only gels (1760, 4630 and 8950 Pa; Fig. S30–S32, ESI†) demonstrating the benefits of interpenetrated LMWG/PG networks. Once again, these stiffnesses are higher than those previously reported for equivalent alginate/LMWG hybrid hydrogels (data not shown).14d
| LMWG load (%) | GG load (%) | Total load (%) | Gel trigger | G′ | G′/G′′ crossover (%) |
|---|---|---|---|---|---|
| 0.4 | — | 0.4 | Heat/cool | 800 | 25.1 |
| — | 0.4 | 0.4 | CaCO3/GdL | 1760 | 0.8 |
| — | 0.6 | 0.6 | CaCO3/GdL | 4630 | 0.5 |
| — | 0.8 | 0.8 | CaCO3/GdL | 8950 | 1.3 |
| 0.3 | 0.1 | 0.4 | CaCO3/GdL | 2580 | 15.9 |
| 0.3 | 0.3 | 0.6 | CaCO3/GdL | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
7.4 |
| 0.3 | 0.5 | 0.8 | CaCO3/GdL | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
5.4 |
In terms of resistance to shear strain, the advantages of the hybrid hydrogel approach are once again evident. On its own, GG gels formed in this way are brittle, struggling to maintain integrity even at 1% shear strain. However, in the hybrid gels, this increases to at least 5%. The presence of the LMWG network significantly improves handleability of this gel, without impacting gel stiffness (indeed, stiffness is also enhanced).
To look in more detail at the effect of CaCO3 concentration on the mechanical properties of the gels, we compared the elastic moduli of hybrid gels prepared using equal amounts of the two gelators (0.3% wt/vol), a fixed GdL concentration (1.0% wt/vol) and different CaCO3 concentrations (0.075, 0.15 and 0.3% wt/vol). The G′ of the gel prepared at the lowest cross-linker concentration (0.075% wt/vol) is 5720 Pa (Fig. S36, ESI†), which increased to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 and 17
300 and 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 Pa (Fig. S37, ESI†) when the gels were prepared with 0.15% and 0.3% wt/vol of CaCO3. This is consistent with a higher availability of Ca2+ ions to cross-link GG. To explore whether GdL concentration could also affect gel stiffness, we studied the gels using a fixed CaCO3 concentration (0.15% wt/vol) and different amounts of GdL (0.8, 1.0 and 1.2% wt/vol). The gels prepared with 1.2% wt/vol GdL had the highest G′ (14
200 Pa (Fig. S37, ESI†) when the gels were prepared with 0.15% and 0.3% wt/vol of CaCO3. This is consistent with a higher availability of Ca2+ ions to cross-link GG. To explore whether GdL concentration could also affect gel stiffness, we studied the gels using a fixed CaCO3 concentration (0.15% wt/vol) and different amounts of GdL (0.8, 1.0 and 1.2% wt/vol). The gels prepared with 1.2% wt/vol GdL had the highest G′ (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 Pa; Fig. S39, ESI†) compared to the gels prepared using 0.8% wt/vol (10
300 Pa; Fig. S39, ESI†) compared to the gels prepared using 0.8% wt/vol (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 Pa; Fig. S38, ESI†). This reflects a higher percentage of CaCO3 being converted into Ca2+ and H2CO3 with higher GdL concentrations. In summary, the rheological properties can easily tuned by controlling the starting conditions – valuable for application in regenerative medicine given the impact of gel stiffness on cell fate, adhesion, migration and differentiation.24
900 Pa; Fig. S38, ESI†). This reflects a higher percentage of CaCO3 being converted into Ca2+ and H2CO3 with higher GdL concentrations. In summary, the rheological properties can easily tuned by controlling the starting conditions – valuable for application in regenerative medicine given the impact of gel stiffness on cell fate, adhesion, migration and differentiation.24
Photo-initiated and patterned gels based on DBS-CONHNH2/GG cross-linked with CaCO3/DPIN
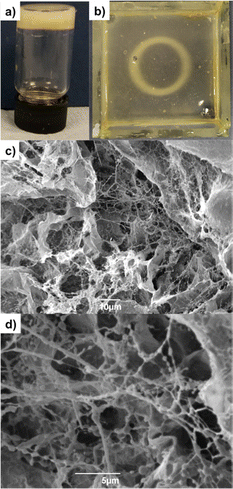
We reasoned that by using diphenyliodonium nitrate (DPIN) as a photoacid generator instead of GdL, it may be possible to induce GG crosslinking under UV light. Although this approach has been used for the photoinduced gelation and patterning of calcium alginate,25 we cannot find evidence of it having previously been applied to gellan gum.To test the method, we first combined GG (0.4, 0.6, 0.8 and 1.3% wt/vol) with CaCO3 (0.15% wt/vol) and the photo-acid generator diphenyliodonium nitrate (DPIN, 0.8% wt/vol) and exposed the mixture to a high intensity UV lamp for 2 h. After this time, self-supporting gels were obtained (Fig. S40, ESI†); thus confirming Ca2+ release and GG cross-linking could be triggered in this way. We applied this procedure to DBS-CONHNH2/GG hybrid gels. DBS-CONHNH2 (0.3% wt/vol) was dispersed in water and combined with CaCO3 (0.15% wt/vol), DPIN (0.8% wt/vol) and GG (0.5% wt/vol). The mixture was heated until dissolution of the LMWG and then exposed to the UV light for 2 h to give self-supporting UV-activated gels (Fig. 1, 4a and Fig. S40, ESI†).
Photopatterning was achieved by applying a photomask on a pre-formed DBS-CONHNH2 gel in a tray (5 cm × 5 cm), selectively triggering Ca2+ release only in the regions exposed to the UV light. This is a technique we have previously applied, and is an efficient method for photopatterning, as the pre-formed gel helps limit convection and diffusion, with the pH-responsive gelator only assembling in the regions exposed to the UV light.14d,26 DBS-CONHNH2/GG photo-patterned gels were prepared by combining DBS-CONHNH2 (0.3% wt/vol) with CaCO3 (0.15% wt/vol), DPIN (0.8% wt/vol) and GG (0.3% wt/vol). The mixture was heated until complete dissolution of the LMWG and transferred to the glass tray. The sample was left undisturbed for 15 min to allow assembly of the DBS-CONHNH2 network. A laser-printed mask was placed on top of the glass tray and the gel exposed to UV light for 2 h. To avoid disruption of the gel due to heating effects, ice was placed below the glass tray. After photo-irradiation, the desired circular pattern formed by the cross-linked polymer was clearly visible within the DBS-CONHNH2 gel (Fig. 4b).
TEM and SEM microscopy showed a self-assembled nanofibrillar network, similar to the hybrid gel prepared in sample vials using GdL (Fig. 4c, d and Fig. S41–S44, ESI†). The properties of the UV-triggered gels were studied in terms of thermal stability and rheology. Both gels had a Tgel of >100 °C, confirming that the presence of crosslinked GG improves the thermal stability of the LMWG (Table S6, ESI†). In terms of rheology, the photo-activated GG-only gel (0.6% wt/vol) has an elastic modulus of 8670 Pa (Table 3 and Table S7, Fig. S46, ESI†), higher than the G′ of the corresponding gel prepared using GdL (G′ = 4630 Pa). The hybrid gel prepared by photo-activation using an equal amount of each gelator (0.3% wt/vol) also had a slightly higher G′ (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 486 Pa; (Table 3 and Fig. S45, ESI†) than the gel prepared with GdL (11
486 Pa; (Table 3 and Fig. S45, ESI†) than the gel prepared with GdL (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 Pa). This indicates photoactivation is an effective technique for these gels. The hybrid hydrogel also shows a slightly higher resistance to shear strain than the GG-only system. These photoactivated GG-only and GG/LMWG hybrid gels are very much stiffer than the equivalent materials formed using calcium alginate (Table 3). This demonstrates that GG can yield much improved stiffness in photoactivated and photo-patterned hybrid hydrogels than alginate, which may offer significant advantages in tailored tissue engineering applications.27
300 Pa). This indicates photoactivation is an effective technique for these gels. The hybrid hydrogel also shows a slightly higher resistance to shear strain than the GG-only system. These photoactivated GG-only and GG/LMWG hybrid gels are very much stiffer than the equivalent materials formed using calcium alginate (Table 3). This demonstrates that GG can yield much improved stiffness in photoactivated and photo-patterned hybrid hydrogels than alginate, which may offer significant advantages in tailored tissue engineering applications.27
| LMWG load (%) | GG load (%) | Alginate load (%) | Total load (%) | Gelation trigger | G′ | G′/G′′ crossover (%) |
|---|---|---|---|---|---|---|
| 0.4 | — | — | 0.4 | Heat/cool | 800 | 25.1 |
| — | 0.6 | 0.6 | DPIN | 8670 | 6.4 | |
| 0.3 | 0.3 | 0.6 | DPIN | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 486 486 |
8.0 | |
| 0.6 | 0.6 | DPIN | 32.5 | 79.3 | ||
| 0.3 | 0.3 | 0.6 | DPIN | 117 | 25.1 |
Summary of rheological performance
In summary (Fig. 5), irrespective of the mode of gel triggering, the GG-only gels have high rheological stiffness, but poor stability to shear – particularly in contrast with alginate-only gels. These differences in gel mechanical properties can be attributed to intrinsic differences between the polymers (e.g. molecular weight, structure, conformation and crystallinity).28 Forming hybrid gels between GG and DBS-CONHNH2 further enhances the stiffness (Fig. 5A) because there are now two interwoven networks and also perhaps partly because GG gelation is also induced by temperature and it therefore may benefit from annealing in the two-component system. In all cases, the hybrid gels also have significantly increased resistance to shear strain (Fig. 5B), as the hybrid gels take on some of the increased resistance to shear of the LMWG system.In this way, applying the hybrid gel approach to GG significantly enhances its rheological performance. In comparison with hybrid gels based on calcium alginate (Fig. 5C), the stiffnesses are significantly higher and would be more similar to those required for bone tissue engineering.17,24 Hence there are benefits to having changed PG in terms of optimising this rheological characteristic.
In situ formation of AgNPs
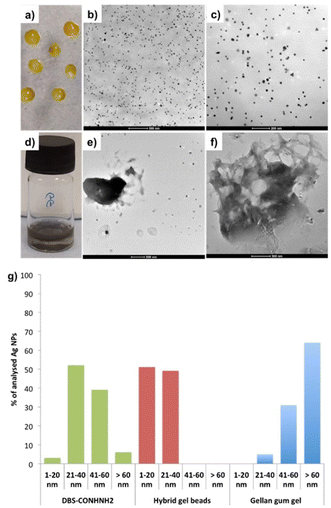
We then wanted to demonstrate that the LMWG retained its unique chemical characteristics within these hybrid gels. In particular, we decided to induce the in situ formation of AgNPs, exploiting the unique reducing power of the DBS-CONHNH2 LMWG that converts Ag(I) to Ag(0) when exposed to silver salt solutions. It is well-known that precious metal nanoparticles (NPs) can promote osteogenesis,29 and we reasoned that in the longer term, it would be useful to incorporate them in these stiff hybrid gels, which have suitable rheological properties for this type of application.17 We previously studied in situ formation of AgNPs in DBS-CONHNH2/alginate gel beads.14d,15bDBS-CONHNH2/GG gel beads were prepared by combining DBS-CONHNH2 (0.3% wt/vol) with GG (0.3% wt/vol), GdL (1.0% wt/vol) and CaCO3 (0.15% wt/vol) and compared to DBS-CONHNH2/GG gels prepared in sample vials. To remove residual ions, the beads were washed multiple times with water. AgNP formation was then induced by exposing the gels to a solution of AgNO3 (10 mM, 1 or 3 mL) for 72 h. The formation of the AgNPs was confirmed by the intense colour change of the gels (from white to orange; Fig. 6a) and by TEM, which clearly showed the presence of NPs dispersed between gel fibres with an average diameter <40 nm (Fig. 6b, c, g and Fig. S49, ESI†), similar to those formed in the DBS-CONHNH2 gel (Fig. 6g and Fig. S48, ESI†). Some sort of AgNPs were also formed in GG-only gels (Fig. 6d–g and Fig. S50, ESI†), however these gels did not undergo the intense colour change, and the NPs were not uniformly distributed, showing very variable sizes and much larger aggregates (Fig. 6g).
The maximum amount of Ag(I) incorporated into the gel beads was quantified by precipitation titration of NaCl, in the presence of K2CrO4 as an indicator. Each hybrid gel bead (20 μL volume) incorporated ca. 0.3 μmol of Ag(I), corresponding to ca. 13 μmol of Ag(I)/mL of gel (Table S8, ESI†). Each mL of gel can contain up to 6.32 μmol of gelator, clearly indicating ca. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ag
1 Ag![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DBS-CONHNH2 stoichiometry. This is consistent with a model in which the acylhydrazide groups (two per gelator) are intimately involved in reducing Ag(I) to Ag(0).15 This result is very similar to Ag(I) uptake in the DBS-CONHNH2 gels (16.7 μmol of Ag(I)/mL of gel; Table S8, ESI†), thus confirming the LMWG maintains its function within the hybrid gel beads. Ag(I) release from the gels was also investigated, with ca. 20% of the loaded Ag being rapidly released (<30 min) in the form of Ag(I) (Fig. S47, ESI†), while the remainder remains in place in the form of AgNPs. It is clear that any residual excess Ag(I) is therefore easily and rapidly removed, avoiding any problems with its potential cytotoxicity30 in our stem cell growth experiments (see below).
DBS-CONHNH2 stoichiometry. This is consistent with a model in which the acylhydrazide groups (two per gelator) are intimately involved in reducing Ag(I) to Ag(0).15 This result is very similar to Ag(I) uptake in the DBS-CONHNH2 gels (16.7 μmol of Ag(I)/mL of gel; Table S8, ESI†), thus confirming the LMWG maintains its function within the hybrid gel beads. Ag(I) release from the gels was also investigated, with ca. 20% of the loaded Ag being rapidly released (<30 min) in the form of Ag(I) (Fig. S47, ESI†), while the remainder remains in place in the form of AgNPs. It is clear that any residual excess Ag(I) is therefore easily and rapidly removed, avoiding any problems with its potential cytotoxicity30 in our stem cell growth experiments (see below).
The mechanical properties of the AgNP-loaded hybrid gels prepared in sample vials (0.3% DBS-CONHNH2, 0.3% alginate, 0.15% CaCO3 and 1.0% GdL) were also studied by oscillatory rheology. Overall, the hybrid gels loaded with AgNPs (10 μmol mL−1 of gel) had similar elastic moduli (G′ = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 Pa, Table S11 and Fig. S53, ESI†) to the unloaded gels (G′ = 11
700 Pa, Table S11 and Fig. S53, ESI†) to the unloaded gels (G′ = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 Pa). However, if the AgNP loading was increased to very high level (30 μmol mL−1 of gel), the elastic modulus fell somewhat (G′ = 4950 Pa; Table S11 and Fig. S54, ESI†). Similar trends were also observed for the corresponding DBS-CONHNH2 (Table S11 and Fig. S51–S52, ESI†) and gellan gum gels (Table S11 and Fig. S55, S56, ESI†) and it is probably due to disruption of the interactions between fibres within the gel network at very high AgNP loadings.
300 Pa). However, if the AgNP loading was increased to very high level (30 μmol mL−1 of gel), the elastic modulus fell somewhat (G′ = 4950 Pa; Table S11 and Fig. S54, ESI†). Similar trends were also observed for the corresponding DBS-CONHNH2 (Table S11 and Fig. S51–S52, ESI†) and gellan gum gels (Table S11 and Fig. S55, S56, ESI†) and it is probably due to disruption of the interactions between fibres within the gel network at very high AgNP loadings.
Stem cell growth
We then explored if the DBS-CONHNH2/GG hybrid gel beads could support stem cell growth. Preliminary cytotoxicity and viability experiments were performed on a human mesenchymal stem cell line (Y201)31 using different AgNP loadings. The gels were prepared in a 48-well plate and loaded with 0.0125 or 10.0 μmol of AgNO3/mL of gel (low loading and high loading respectively). The samples were transferred to the middle of a 6-well plate, where the cells were seeded. Due to their fragility, gels based only on DBS-CONHNH2 could not be transferred; therefore, these gels were prepared directly on the 6-well plates using bottomless vials, which did not allow loading the gels with AgNO3. After 48 h, the gels without AgNPs and those incorporating a low 0.0125 μmol loading of AgNO3/mL of gel showed normal cell growth and did not exhibit any ‘zone of inhibition’ (Fig. S57 and S58, ESI†) – as such, they were considered to be non-toxic. However, the gels incorporating a high Ag loading (10.0 μmol of AgNO3/mL of gel), showed a rather large zone of inhibition around them (2.90–3.60 mm; Fig. S58, ESI†) and must therefore be considered toxic to stem cells. This is in-line with previous studies showing high concentrations of Ag+ ions can affect mammalian cell survival.32To obtain more detailed data on biocompatibility, we performed an Alamar Blue assay on Y201 cells grown on gels with different AgNP loadings and control gels without AgNPs. DBS-CONHNH2 and GG gels were prepared directly on 96 well plates, whereas the hybrid gel beads were prepared and then transferred to the 96 well plates. To ensure the cells were adhering to the gels rather than the plate surface, we used non-adherent plates. The gels were loaded with different AgNO3 loadings (0.00625, 0.0125, 0.05, 0.1, 1.0 and 10 μmol of AgNO3/mL of gel) and cell metabolic activity was monitored over 10 days (day 0, 3, 6 and 10).
Pleasingly, the obtained results showed the cells were metabolically active in the gels without AgNPs and in those loaded with 0.00625–0.1 μmol of AgNO3/mL of gel (Fig. 7). Higher AgNO3 loadings (1.0 and 10 μmol of AgNO3/mL of gel) were, as expected, toxic across the different gel types tested (Fig. 7) in agreement with the preliminary study described above. Compared to the DBS-CONHNH2 gels, the gel beads showed higher fluorescence values over the ten days, indicative of higher cell metabolic activity, which can be related to a higher number of cells. This is probably due to the higher surface area of the gel beads available for cell anchorage and penetration inside the gels. For the GG-only gels, cell growth was initially slower than on the hybrid gel beads, which may reflect enhanced initial cell anchorage induced by the LMWG, although by day 6 the GG-only beads were equivalent to the LMWG/GG hybrids. In each case, cell growth was similar in the presence and absence of AgNPs.
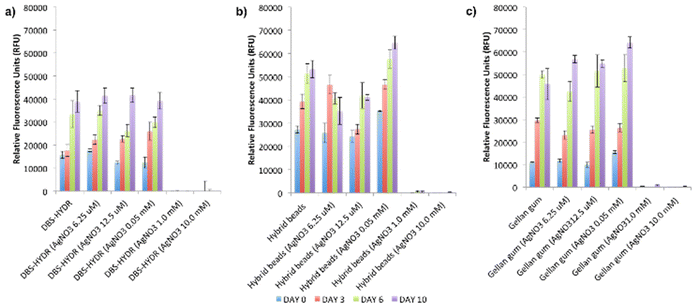 | ||
| Fig. 7 Alamar blue assay results at day 0, 3, 6 and 10 for gels loaded with different AgNO3 concentrations (N = 6, mean reported, error bars represent standard error, DBS-HYDR = DBS-CONHNH2). | ||
To verify if the gels could support cell growth over a longer period of time, a viability test was performed over 21 days (day 0, 7, 14 and 21) on the gels loaded with lower AgNO3 loadings (0.00625 and 0.0125 μmol of AgNO3/mL of gel; Fig. S59, ESI†) and control gels without AgNPs. Pleasingly, the results showed that the cells had good metabolic activity in all of the tested gels for the whole duration of the study. These preliminary biocompatibility tests demonstrate that the DBS-CONHNH2/GG hybrid gel beads (with or without AgNPs) support the growth of human mesenchymal stem cells over time and could potentially be used in tissue engineering.
Gels incorporating AgNPs have previously been demonstrated to induce osteogenesis in mesenchymal stem cells and bone tissue repair.29 Our gels could therefore be promising in this setting, particularly given the stiffness of the GG gels. Specifically, our AgNP-loaded shapeable biomaterials may be effective bone fillers to facilitate bone regeneration whilst preventing infections.33 Indeed, AgNPs are well-known to have antibacterial activity.14d,34 Future studies will explore cell function and osteogenic activity of cells grown on our AgNP gel beads as well as antimicrobial properties.
Conclusion
To conclude we have demonstrated that gellan gum can be used as a polymer gel to obtain DBS-CONHNH2/GG hybrid gels. Gel assembly can be achieved using a variety of different triggers. Exposure of pre-formed LMWG beads loaded with GG to a bath of CaCl2 induces rapid GG crosslinking from the outside inwards. Conversely, loading the beads with GG, insoluble CaCO3 and acid-generating GdL led to hybrid gel beads via an internal slow acidification gelation. By substituting GdL with the photoacid generator DPIN to induce GG crosslinking, this process could be activated by UV light exposure. Performing this process with a photo-mask made it possible to fabricate photopatterned gels with different compositions in the different domains.The gels obtained using these different triggering methods were fully characterised by a range of techniques. Importantly, in all cases, the hybrid gels were even stiffer than those formed by GG alone, as a result of the interpenetrating LMWG and PG networks. The hybrid DBS-CONHNH2/GG gels are also significantly stiffer than equivalent hybrid hydrogels previously made with calcium alginate as the PG.14a,d Furthermore, the enhanced stiffness does not come at the cost of additional sensitivity to shear strain – indeed, the hybrid gels are considerably more stable to shear strain than the GG-only gels. In summary, the presence of the LMWG enhances both the stiffness and shear strain stability of gellan gum.
To confirm the DBS-CONHNH2 kept its unique chemical function when combined with GG, we induced in situ AgNP formation and demonstrated this required the presence of the LMWG. Finally, biological tests were performed on human mesenchymal stem cells allowed us to identify non-toxic AgNP loadings and demonstrated that the cells can thrive in the hybrid gel beads for long periods of time (i.e. at least 21 days). We suggest that our shaped DBS-CONHNH2/GG gels are promising materials for orthopaedic applications. Further studies to exploit this are currently being carried out in our laboratories.
Author contributions
Funding was obtained by D. K. S. who developed the overarching LMWG shaping and patterning concept. C. C. P. conceptualised the use of gellan gum in this application and carried out all of the experimental work with supervisory guidance and input from P. G. G. (biological studies) and D. K. S. (materials chemistry). C. C. P. and D. K. S. wrote the manuscript with input from P. G. G.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank EPSRC (EP/P03361X/1) and EPSRC IAA (University of York) for funding. Karen Hodgkinson (Bioscience Technology Facility, Department of Biology, University of York) is acknowledged for optical microscopy, TEM and SEM imaging.Notes and references
- (a) A. R. Hirst, B. Escuder, J. F. Miravet and D. K. Smith, Angew. Chem., Int. Ed., 2008, 47, 8002–8018 CrossRef CAS PubMed; (b) J. Zhou, J. Li, X. Du and B. Xu, Biomaterials, 2017, 129, 1–27 CrossRef CAS PubMed; (c) J. Hoque, N. Sangaj and S. Varghese, Macromol. Biosci., 2019, 19, e1800259 CrossRef PubMed; (d) J. Mayr, C. Saldías and D. Díaz Díaz, Chem. Soc. Rev., 2018, 47, 1484–1515 RSC; (e) D. K. Smith in Molecular Gels: Structure and Dynamics, ed. R. G. Weiss, Royal Society of Chemistry, Cambridge, 2018, pp. 300–371 Search PubMed.
- (a) Polymer Gels: Fundamentals and Applications, ed. H. B. Bohidar, P. Dubin and Y. Osada, American Chemical Society, Washington DC, 2002 Search PubMed; (b) B. V. Slaughter, S. S. Khurshid, O. Z. Fisher, A. Khademhosseini and N. A. Peppas, Adv. Mater., 2009, 21, 3307–3329 CrossRef CAS PubMed; (c) J. Li and D. J. Mooney, Nat. Rev. Mater., 2016, 1, 16071 CrossRef CAS PubMed.
- (a) R. G. Weiss, J. Am. Chem. Soc., 2014, 136, 7519–7530 CrossRef CAS PubMed; (b) X. Du, J. Zhou, J. Shi and B. Xu, Chem. Rev., 2015, 115, 13165–13307 CrossRef CAS PubMed; (c) E. R. Draper and D. J. Adams, Chem, 2017, 3, 390–410 CrossRef CAS.
- For reviews see: (a) D. J. Cornwell and D. K. Smith, Mater. Horiz., 2015, 2, 279–293 RSC; (b) L. C. Li, R. Q. Sun and R. L. Zheng, Mater. Des., 2021, 197, 109209 CrossRef CAS . For selected examples: ; (c) J. Wang, Z. Wang, J. Gao, L. Wang, Z. Yang, D. Kong and Z. Yang, J. Mater. Chem., 2009, 19, 7892–7896 RSC; (d) R. Huang, W. Qi, L. Feng, R. Su and Z. He, Soft Matter, 2011, 7, 6222–6230 RSC; (e) P. Li, X.-Q. Dou, C.-L. Feng and D. Zhang, Soft Matter, 2013, 9, 3750–3757 RSC; (f) D. J. Cornwell, B. O. Okesola and D. K. Smith, Soft Matter, 2013, 9, 8730–8736 RSC; (g) J. Chen, N. Tao, S. Fang, Z. Chen, L. Liang, X. Sun, J. Li and Y.-N. Liu, New J. Chem., 2018, 42, 9651–9657 RSC; (h) B. C. Baker, I. German, G. C. Stevens, H. M. Colquhoun and W. Hayes, RSC Adv., 2018, 8, 41445 RSC; (i) B. P. Nowak and B. J. Ravoo, Soft Matter, 2020, 16, 7299–7304 RSC; (j) H. Shigemitsu, R. Kubota, K. Nakamura, T. Matsuzaki, S. Minami, T. Aoyama, K. Urayama and I. Hamachi, Nat. Commun., 2020, 11, 3859 CrossRef PubMed.
- (a) P. R. A. Chivers and D. K. Smith, Nat. Rev. Mater., 2019, 4, 463–478 CrossRef CAS; (b) G. A. Primo and A. Mata, Adv. Funct. Mater., 2021, 31, 2009574 CrossRef CAS.
- (a) I. Giavasis, L. M. Harvey and B. McNeil, Crit. Rev. Biotechnol., 2000, 20, 177–211 CrossRef CAS PubMed; (b) I. B. Bajaj, S. A. Survase, P. S. Saudagar and R. S. Singhal, Food Technol. Biotechnol., 2007, 45, 341–354 CAS; (c) A. M. Fialho, L. M. Moreira, A. T. Granja, A. O. Popescu, K. Hoffmann and I. Sa-Correia, Appl. Microbiol. Biotechnol., 2008, 79, 889–900 CrossRef CAS PubMed; (d) E. R. Morris, K. Nishinari and M. Rinaudo, Food Hydrocoll., 2012, 28, 373–411 CrossRef CAS; (e) V. D. Prajapati, G. K. Jani, B. S. Zala and T. A. Khutliwala, Carbohydr. Polym., 2013, 93, 670–678 CrossRef CAS PubMed.
- D. E. Pszczola, Food Technol., 1993, 47, 94–96 Search PubMed.
- (a) G. R. Sanderson and R. C. Clark, Food Technol., 1983, 37, 63–70 Search PubMed; (b) N. E. Wedamulla and W. A. J. P. Wijesinghe, Trends Carbohydr. Res., 2021, 13, 35–49 Search PubMed.
- L. R. Stevens, K. J. Gilmore, G. G. Wallace and M. I. H. Panhuis, Biomaterials, 2016, 4, 1276–1290 RSC.
- A. P. Chiriac, A. Ghilan, I. Neamtu, L. E. Nita, A. G. Rusu and V. M. Chiriac, Macromol. Biosci., 2019, 19, 1900187 CrossRef PubMed.
- (a) T. Osmalek, A. Froelich and S. Tasarek, Int. J. Pharm., 2014, 466, 328–340 CrossRef CAS PubMed; (b) F. S. Palumbo, S. Federico, G. Pitarresi, C. Fiorica and G. Giammona, Carbohydr. Polym., 2019, 229, 115430 CrossRef PubMed; (c) C. Villarreal-Otalvaro and J. M. Coburn, ACS Biomater. Sci. Eng., 2022 DOI:10.1021/acsbiomaterials.1c00685.
- T. Muthukumar, J. E. Song and G. Khang, Molecules, 2020, 24, 4514 CrossRef PubMed.
- (a) A. H. Bacelar, J. Silva-Correia, J. M. Oliveira and R. L. Reis, J. Mater. Chem. B, 2016, 4, 6164–6174 RSC; (b) K. M. Zia, S. Tabasum, M. F. Khan, N. Akram, N. Akhter, A. Noreen and M. Zuber, Int. J. Biol. Macromol., 2018, 109, 1068–1087 CrossRef CAS PubMed.
- (a) C. C. Piras, P. Slavik and D. K. Smith, Angew. Chem., Int. Ed., 2020, 59, 853–859 CrossRef CAS PubMed; (b) C. C. Piras, A. G. Kay, P. G. Genever and D. K. Smith, Chem. Sci., 2021, 12, 3958–3965 RSC; (c) C. C. Piras, A. K. Patterson and D. K. Smith, Chem. – Eur. J., 2021, 27, 13203–13210 CrossRef CAS PubMed; (d) C. C. Piras, C. S. Mahon, P. G. Genever and D. K. Smith, ACS Biomater. Sci Eng., 2022, 8, 1829–1840 CrossRef PubMed; (e) C. C. Piras and D. K. Smith, Chem. – Eur. J., 2021, 27, 14527–14534 CrossRef CAS PubMed.
- (a) B. O. Okesola, S. K. Suravaram, A. Parkin and D. K. Smith, Angew. Chem., Int. Ed., 2016, 55, 183–187 CrossRef CAS PubMed; (b) C. C. Piras, C. S. Mahon and D. K. Smith, Chem. – Eur. J., 2020, 26, 8452–8457 CrossRef CAS PubMed.
- (a) B. O. Okesola and D. K. Smith, Chem. Commun., 2013, 49, 11164–11166 RSC; (b) P. R. A. Chivers, J. A. Kelly, M. J. S. Hill and D. K. Smith, React. Chem. Eng., 2020, 5, 1112–1117 RSC.
- (a) Y. S. Pek, A. C. A. Wan, A. Shekaran, L. Zhuo and J. Y. Ying, Nat. Nanotechnol., 2008, 3, 671–675 CrossRef CAS PubMed; (b) R. G. M. Breuls, T. U. Jiya and T. H. Smit, Open Orthop. J., 2008, 2, 103–109 CrossRef PubMed; (c) V. Pandit, J. M. Zuidema, K. N. Venuto, J. Macione, G. Dai, R. J. Gilbert and S. P. Kotha, Tissue Eng. Part A, 2013, 19, 2452–2463 CrossRef CAS PubMed; (d) Y. Hu, W. Gao, F. Wu, H. Wu, B. He and J. He, J. Mater. Chem. B, 2016, 4, 3504–3508 RSC; (e) E. V. Alakpa, V. Jayawarna, A. Lampel, K. V. Burgess, C. C. West, S. C. J. Bakker, S. Roy, N. Javid, S. Fleming, D. A. Lamprou, J. Yang, A. Miller, A. J. Urquhart, P. W. J. M. Frederix, N. T. Hunt, B. Péault, R. V. Ulijn and M. J. Dalby, Chem, 2016, 1, 298–319 CrossRef CAS; (f) B. Yi, Q. Xu and W. Liu, Bioactive Mater., 2022, 15, 82–102 CrossRef CAS PubMed.
- P. Gurikov and I. Smirnova, Gels, 2018, 4, 14 CrossRef PubMed.
- Y. Pocker and E. Green, J. Am. Chem. Soc., 1973, 95, 113–119 CrossRef CAS PubMed.
- For selected examples see: (a) C. K. Kuo and P. X. Ma, Biomaterials, 2001, 22, 511–521 CrossRef CAS PubMed; (b) C. K. Kuo and P. X. Ma, J. Biomed. Mater., 2008, 84A, 899–907 CrossRef CAS PubMed; (c) A. Schmitt, P. Rödel, C. Anamur, C. Seeliger, A. B. Imhoff, E. Herbst, S. Vogt, M. van Griensven, G. Winter and J. Engert, PLOS One, 2015, 10, e0118937 CrossRef PubMed; (d) Z. Li, A. K. Pearce, A. P. Dove and R. K. O'Reilly, Polymers, 2021, 13, 2202 CrossRef CAS PubMed; (e) L. Sardelli, M. Tinesi, F. Briatico-Vangosa and P. Petrini, Soft Matter, 2021, 17, 8105–8117 RSC.
- A. Li, T. Gong, X. Yang and Y. R. Guo, Int. J. Biol. Macromol., 2020, 151, 257–267 CrossRef CAS PubMed.
- (a) T. E. L. Douglas, W. Piwowarczyk, E. Pamula, J. Liskova, D. Schaubroeck, S. C. G. Leeuwenburgh, G. Brackman, L. Balcauen, R. Detsch, H. Declercq, K. Cholewa-Kowalska, A. Dokupil, V. M. J. I. Cuijpers, F. Vanhaecke, R. Cornelissen, T. Coenye, A. R. Boccaccini and P. Dubruel, Biomed. Mater., 2014, 9, 045014 CrossRef CAS PubMed; (b) T. E. L. Douglas, A. Lapa, K. Reczynska, M. Krok-Borkowicz, K. Pietryga, S. K. Samal, H. A. Declercq, D. Schaubroeck, M. Boone and P. van der Voort, Biomed. Mater., 2016, 11, 065011 CrossRef PubMed.
- A. Li, T. Gong, X. Li, X. Li, X. Yang and Y. Guo, Int. J. Biol. Macromol., 2020, 156, 565–575 CrossRef CAS PubMed.
- (a) M. Ahearne, Interface Focus, 2014, 4, 20130038 CrossRef PubMed; (b) Y.-H. Tsou, J. Khoneisser, P.-C. Huang and X. Xu, Bioactive Mater., 2016, 1, 39–55 CrossRef PubMed; (c) S.-B. Han, J.-K. Kim, G. Lee and D.-H. Kim, Adv. Biosyst., 2020, 4, 2000247 CrossRef PubMed; (d) S. Yin and Y. Cao, Acta Biomater., 2021, 128, 1–20 CrossRef CAS PubMed; (e) L. Tianyi, T. Bowen, Z. Lengjing, W. Yating and L. Jinfeng, Front. Bioeng. Biotechnol., 2022, 10, 817391 CrossRef PubMed.
- (a) V. Javvaji, A. G. Baradwaj, G. F. Payne and S. R. Raghavan, Langmuir, 2011, 27, 12591–12596 CrossRef CAS PubMed; (b) L. Liu, F. Wu, X.-J. Ju, R. Xie, W. Wang, C. H. Niu and L.-Y. Chu, J. Colloid Interface Sci., 2013, 404, 85–90 CrossRef CAS PubMed; (c) A. K. Higham, C. A. Bonino, S. R. Raghavan and S. A. Khan, Soft Matter, 2014, 10, 4990–5002 RSC; (d) H. Oh, A. X. Lu, V. Javvaji, D. L. DeVoe and S. R. Raghavan, ACS Appl. Mater. Interfaces, 2016, 8, 17529–17538 CrossRef CAS PubMed; (e) T. M. Valentin, S. E. Leggett, P.-Y. Chen, J. K. Sodhi, L. H. Stephens, H. D. McClintock, J. Y. Sim and I. Y. Wong, Lab Chip, 2017, 17, 3474–3488 RSC.
- (a) D. J. Cornwell, B. O. Okesola and D. K. Smith, Angew. Chem., Int. Ed., 2014, 53, 12461–12465 CAS; (b) D. J. Cornwell, O. J. Daubney and D. K. Smith, J. Am. Chem. Soc., 2015, 137, 15486–15492 CrossRef CAS PubMed; (c) C. C. Piras and D. K. Smith, Chem. – Eur. J., 2018, 25, 11318–11326 Search PubMed; (d) D. J. Cornwell and D. K. Smith, Chem. Commun., 2020, 56, 7029–7032 RSC.
- (a) M. Guvendiren and J. A. Burdick, Curr. Opin. Biotechnol., 2013, 24, 841–846 CrossRef CAS PubMed; (b) A. Gelmi and C. E. Schutt, Adv. Healthcare Mater., 2020, 10, 2001125 CrossRef PubMed; (c) D. K. A. Kanioura, M. Chatzichristidi, K. G. Beltsios, S. E. Kakabakos and P. S. Petrou, Eur. Polym. J., 2022, 162, 110896 CrossRef.
- (a) E. Miyoshi, T. Takaya and K. Nishinari, Carbohydr. Polym., 1996, 30, 109–119 CrossRef CAS; (b) M. Jaspers, M. Dennison, M. F. J. Mabesoone, F. C. MacKintosh, A. E. Rowan and P. H. J. Kouwer, Nat. Commun., 2014, 5, 5808 CrossRef PubMed; (c) A. Li, T. Gong, X. Li, X. Li, X. Yang and Y. Guo, Int. J. Biol. Macromol., 2020, 156, 565–575 CrossRef CAS PubMed; (d) D. Ji and J. Kim, Adv. NanoBiomed. Res., 2021, 1, 2100026 CrossRef.
- (a) X. Liu, W. He, Z. Fang, A. Kienzle and Q. Feng, J. Biomed. Nanotechnol., 2014, 10, 1277–1285 CrossRef CAS PubMed; (b) H. Qin, C. Zhu, Z. An, Y. Jiang, Y. Zhao, J. Wang, X. Liu, B. Hui, X. Zhang and Y. Wang, Int. J. Nanomed., 2014, 9, 2469–2478 CrossRef PubMed; (c) A. K. Nguyen, R. Patel, J. M. Noble, J. Zheng, R. J. Narayan, G. Kumar and P. L. Goering, Appl. Vitro Toxicol., 2019, 5, 123–133 CrossRef CAS; (d) H. Xie, P. Wang and J. Wu, Artif. Cells Nanomed. Biotechnol., 2019, 47, 260–267 CrossRef CAS PubMed.
- (a) L. Kvitek, A. Panacek, R. Prucek, J. Soukupova, M. Vanickova, M. Kolar and R. Zboril, J. Phys.: Conf. Ser., 2011, 304, 012029 CrossRef; (b) C. Liao, Y. Li and S. C. Tjong, Int. J. Mol. Sci., 2019, 20, 449 CrossRef PubMed.
- S. James, J. Fox, F. Afsari, J. Lee, S. Clough, C. Knight, J. Ashmore, P. Ashton, O. Preham, M. Hoogduijn, R. D. R. Ponzoni, Y. Hancock, M. Coles and P. Genever, Stem Cell Rep., 2015, 4, 1004–1015 CrossRef CAS PubMed.
- (a) L. Kvitek, A. Panacek, R. Prucek, J. Soukupova, M. Vanickova, M. Kolar and R. Zboril, J. Phys.: Conf. Ser., 2011, 304, 012029 CrossRef; (b) C. Liao, Y. Li and S. C. Tjong, Int. J. Mol. Sci., 2019, 20, 449 CrossRef PubMed.
- (a) S. A. Brennan, C. N. Fhoghlú, B. M. DeVitt, F. J. O'Mahony, D. Brabazon and A. Walsh, Bone Joint J., 2015, 97-B, 582–589 CrossRef CAS PubMed; (b) L.-L. Li, H.-W. An, B. Peng, R. Zheng and H. Wang, Mater. Horiz., 2019, 6, 1794–1811 RSC; (c) D. Burak, B. O. Okesola, D. W. Barrett, M. D'Este, T. T. Chowdhury, D. Eglin and A. Mata, Acta Biomater., 2020, 109, 82–94 CrossRef PubMed; (d) B. O. Okesola, S. Ni, B. Derkus, C. C. Galeano, A. Hasan, Y. Wu, J. Ramis, L. Buttery, J. I. Dawson, M. D'Este, R. O. C. Oreffo, D. Eglin, H. Sun and A. Mata, Adv. Funct. Mater., 2020, 30, 1906205 CrossRef CAS; (e) B.-D. Zheng, J. Ye, Y.-C. Yang, Y.-Y. Huang and M.-T. Xiao, Carbohydr. Polym., 2021, 275, 118770 CrossRef PubMed.
- (a) N. Durán, M. Durán, M. B. de Jesus, A. B. Seabra, W. J. Fávaro and G. Nakazato, Nanomed.: Nanotechnol. Biol. Med., 2016, 12, 789–799 CrossRef PubMed; (b) M. Bilal, T. Rasheed, H. M. N. Iqbal, H. Hu and X. Zhang, Int. J. Pharmacol., 2017, 13, 832–845 CrossRef CAS; (c) A.-C. Burdusel, O. Gherasim, A. M. Grumezescu, L. Mogoanta, A. Ficai and E. Andronescu, Nanomaterials, 2018, 8, 681 CrossRef PubMed; (d) F. Paladini and M. Pollini, Materials, 2019, 12, 2540 CrossRef CAS PubMed; (e) C. Liao, Y. Li and S. C. Tjong, Int. J. Mol. Sci., 2019, 20, 449 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full details of gel fabrication methods and characterisation data, biological assay methods and additional data. See DOI: https://doi.org/10.1039/d2ma00565d |
| This journal is © The Royal Society of Chemistry 2022 |