Open Access Article
Open Access ArticleExtended air, light, and heat-resistive organolead halide perovskite single-crystalline microrods for high-performance photodetectors†
Chang-Yu
Lin
 *ab,
Rajesh Kumar
Ulaganathan
*ab,
Rajesh Kumar
Ulaganathan
 *cde,
Ambika
Subramanian
a,
Huei-Chu
Weng
a,
Yaw-Jen
Chang
a,
Raghavan Chinnambedu
Murugesan
f,
Raman
Sankar
*cde,
Ambika
Subramanian
a,
Huei-Chu
Weng
a,
Yaw-Jen
Chang
a,
Raghavan Chinnambedu
Murugesan
f,
Raman
Sankar
 c and
Alex
Rozhin
f
c and
Alex
Rozhin
f
aDepartment of Mechanical Engineering, Chung Yuan Christian University, Taoyuan-32023, Taiwan. E-mail: cylin@cycu.edu.tw
bResearch Center for Semiconductor Materials and Advanced Optics, Chung Yuan Christian University, Taoyuan-32023, Taiwan
cInstitute of Physics, Academia Sinica, Taipei-11529, Taiwan
dCenter for Condensed Matter Sciences, National Taiwan University, Taipei-10617, Taiwan
eDepartment of Electrical and Photonics Engineering, Technical University of Denmark, Roskilde-4000, Denmark. E-mail: rajul@fotonik.dtu.dk
fAston Institute of Photonic Technologies, Aston University, Birmingham-B4 7ET, UK
First published on 17th September 2022
Abstract
Two-dimensional organic–inorganic hybrid perovskites are much attracted due to promising stable optoelectronic properties with tunable quantum well structures. Herein, we report the photodetector performance of the structurally tuned FA-incorporated hybrid perovskite FA-(N-MPDA)PbBr4 (FA = formamidinium and N-MPDA = N1-methylpropane-1,3-diammonium) high-quality single-crystalline microrods obtained from over supersaturated solution by a slow evaporation at constant temperature (SECT) growth method. The single crystalline FA-(N-MPDA)PbBr4 microrod exhibits exceptional structural, thermal, and optical stabilities against ambient, light, and heat exposures, which were systematically monitored using X-ray diffraction, photoluminescence, and thermogravimetric analysis/differential scanning calorimetry techniques. The photodetector device fabricated using stable FA-(N-MPDA)PbBr4 crystalline microrod exhibits good responsivity ∼40 A W−1 with a response time of less than 50 ms by shining a 405 nm laser. In addition, the microrods exhibit high specific detectivity of 7.8 × 1010 Jones at an incident light of 53 μW cm−2. These results demonstrate the potential of organic–inorganic perovskite microrods formed with a long-chain organic diammonium spacer suitable for stable and high-performance optoelectronic devices.
1. Introduction
Three-dimensional (3D) organometal halide ABX3 perovskites, A = methyl ammonium CH3NH3+ (MA)/or formamidinium (FA) HC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH)NH3+ cations, B = Pb2+/Sn2+, and X = Cl−, Br−, and I− are widely explored for optoelectronic applications such as photodetectors,1 field-effect transistors,2 solar cells,3 and random lasers.4–6 However, this class of materials often suffers degradation due to poor stability against light, moisture, and heat atmosphere.7,8 Two-dimensional (2D) layered perovskites have recently gained much attention because of their stability and inherent quantum well (QW) structures.9–11 The 2D perovskites consist of an inorganic and organic portion, while inorganic perovskite slabs, which are sandwiched between the organic barrier layers to form a QW assembly, form the 2D conducting layers. There is a dielectric constant difference between the organic and inorganic structure, which creates the efficient confinement of electron–hole pairs in the 2D perovskite system. Consequently, this induces a stable structure in the ambient environment.12–14
NH)NH3+ cations, B = Pb2+/Sn2+, and X = Cl−, Br−, and I− are widely explored for optoelectronic applications such as photodetectors,1 field-effect transistors,2 solar cells,3 and random lasers.4–6 However, this class of materials often suffers degradation due to poor stability against light, moisture, and heat atmosphere.7,8 Two-dimensional (2D) layered perovskites have recently gained much attention because of their stability and inherent quantum well (QW) structures.9–11 The 2D perovskites consist of an inorganic and organic portion, while inorganic perovskite slabs, which are sandwiched between the organic barrier layers to form a QW assembly, form the 2D conducting layers. There is a dielectric constant difference between the organic and inorganic structure, which creates the efficient confinement of electron–hole pairs in the 2D perovskite system. Consequently, this induces a stable structure in the ambient environment.12–14
Compared to 3D hybrid halide perovskites, 2D materials offer significant advantages in tuning the physical properties by tailoring the crystal structure using different organic spacers, varying perovskite layer thickness, and halide ion modifications.15,16 Based on the crystallographic studies, layered inorganic perovskite sheets are typically formed by slicing 3D perovskites using the (001) or (110) planes. There are many reports on the 2D hybrid perovskites with different organic spacers, metal ions, and halides.17,18 Recently, alkyl diammonium cations having different carbon chain lengths, such as N1-methylethane-1,3-diammonium (NMEDA) and N1-methylpropane-1,3-diammonium (NMPDA), were used as organic spacers to form the self-assembly of (110)- and (001)-oriented (N-MEDA)PbBr4 (corrugated crystal structure) and (N-MEDA)PbBr4 perovskites, respectively. The 2D (N-MEDA)PbBr4 perovskite exhibits a strong, broadband white light emission, whereas (N-MPDA)PbBr4-based 2D hybrid perovskite exhibits a strong excitonic absorption band at 420 nm (blue region). The growth of a high-quality single crystal of this material is essential for the fundamental understanding and application points of view.
Moreover, hybrid perovskites in the single crystal form exhibit long carrier diffusion, high absorption, enhanced charge transport due to large grain boundaries, and low defect concentration.19–21 We have recently reported unprecedented random lasing from FA-incorporated FA-(NMPDA)PbBr4 single-crystalline rods grown by the SECT growth method as the optical gain media. The millimeter-sized single-crystalline FA-(NMPDA)PbBr4 microrods reveal low threshold (∼0.5 μJ cm−2) random lasing behaviors with a sharp laser having linewidth (∼0.1 nm) and a high-quality factor (∼5350) at room temperature.22 The FA was incorporated into the 2D perovskite lattice to form the quasi-2D perovskite-like structure and enhance the stability. The FA-(NMPDA)PbBr4 single-crystalline microrods showed excellent stability for more than 2 hours against laser irradiation.22
Motivated by the outstanding stability against laser irradiation of the single-crystalline FA-(NMPDA)PbBr4 microrods, in this study, we have further extended its application as a single crystalline microrod conductive channel material in the photodetector device structure. Furthermore, the single crystalline microrods were subjected to essential characterization to evaluate the quality and structure–property relations. The FA-(N-MPDA)PbBr4 microrods exhibit high photoresponsivity of ∼40 A W−1 (under 405 nm laser illumination) and high specific directivity with robust photoswitching stability under continuous laser irradiations with a fast response speed. These results demonstrate that the FA-(N-MPDA)PbBr4 microrod holds great promise for optoelectronic applications, exclusively for constructing photodetectors with great photocurrent generation, fast response, and long-term stability.
2. Results and discussion
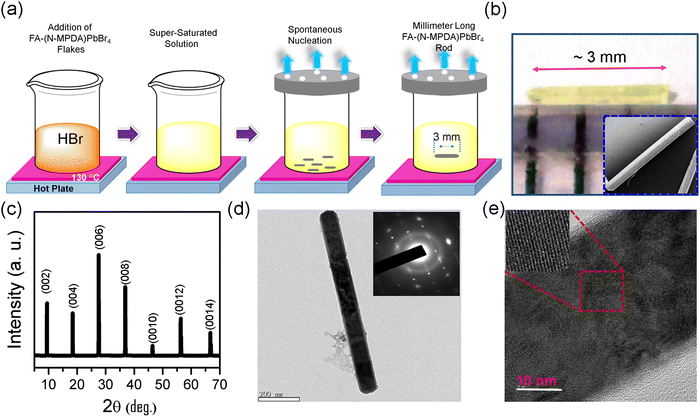
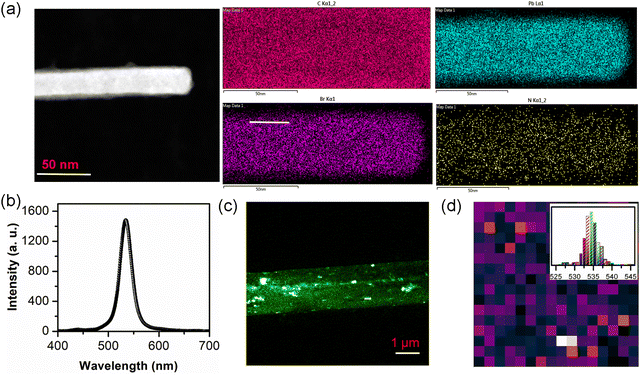
Fig. 1a illustrates the SECT process for the growth of millimeter-sized perovskite single-crystalline microrods. At first, the as-synthesized FA-(N-MPDA)PbBr4 compound (Fig. S1, ESI†) was saturated by dissolving into HBr solvent at 110 °C under continuous magnetic stirring. The solution was subjected to constant magnetic stirring to form a clear solution without undissolved debrides. The homogeneous saturated solution obtained by the above process allowed the slow evaporation at a constant temperature for supersaturation. During the nucleation, the excessive solute in the supersaturated solution crystallizes at the bottom of the beaker. Further, the solution was left for continuous slow evaporation for 2–3 days at a constant temperature without any disturbance to achieve high-quality single-crystalline, a millimeter-sized microrod. The optical image of the as-synthesized transparent yellowish single-crystalline FA-(N-MPDA)PbBr4 microrod is shown in Fig. 1b. The size of the as-grown microrod is 3 mm in length and 2 μm in diameter. The inset in Fig. 1b is the scanning electron microscopy (SEM) image of the as-grown millimeter-sized perovskite microrod having a smooth surface. The XRD pattern shown in Fig. 1c confirms the solid diffraction peaks consistent with interplanar spacing at 2θ values of 9.08°, 18.18°, 27.25°, 36.63°, and 46.20°, which correspond to the lattice planes of (002), (004), (006), (008), and (0010), respectively.22 From the single crystal XRD study, the single crystalline microrod exhibits a monoclinic crystal structure, and the growth direction is oriented along the c-axis. The lattice parameter values are identified as a = 12.83, b = 5.94, and c = 13.17 with the space group of P2/m. A transmission electron microscopy (TEM) study was carried out to reveal the single crystallinity of the FA-(N-MPDA)PbBr4 microrod, as shown in Fig. 1d. The single crystalline surface of the perovskite microrod is very smooth. It has no surface damage due to the incident electron beam. The inset Fig. 1d shows the selected area electron diffraction (SAED) pattern with linearly ordered diffraction spots, which indicates the single-crystalline nature of the FA-(N-MPDA)PbBr4 microrod. The careful observation of the SAED pattern reveals the dual/multi-sports features, indicating the stacking fault in the atomic arrangement in the perovskite lattices. The lattice fringes were observed from the high-resolution transmission electron microscopy (Fig. 1e), and the interplanar distance was found to be ∼0.25 nm. Moreover, the stacking fault and break in lattice arrangement are revealed in the high-resolution TEM pattern, which correlates well with the SAED result. The formation of stacking fault and unparalleled fringes in the SAED (inset of Fig. 1d) and HRTEM (inset of Fig. 1e) might be attributed to the FA cation in the crystal structure.To assess the chemical uniformity, the as-grown FA-(N-MPDA)PbBr4 microrod crystal was further examined by energy-dispersive X-ray (EDX) spectroscopy. EDX mapping (Fig. 2a) confirms the uniformly distributed C, N, Pb, and Br atoms in the single-crystalline microrod. The EDX spectrum of the FA-(N-MPDA)PbBr4 microrod is displayed in Fig. S2 of the ESI,† which shows the prominent individual peaks of all the atoms such as Pb, Br, N, and C. Further, to study the optical properties and spectral uniformity of the as-grown FA-(N-MPDA)PbBr4 microrod single crystals, we performed photoluminescence (PL) spectral analysis. The microrods were placed on a clean silicon substrate and excited by a 405 nm laser using a 10× objective with a light intensity of 1 mW. Fig. 2b is the PL emission spectrum of the FA-(N-MPDA)PbBr4 microrod with a narrow emission band at a wavelength of 535 nm. The PL emission for the pristine (NMPDA)PbBr4 without FA was observed at the blue band region of wavelength of about 435 nm (Fig. S3, ESI†). The PL emission at the green band region for the FA-(N-MPDA)PbBr4 is well correlated to the change in the crystal structure compared to the pristine (NMPDA)PbBr4. To ensure the spectral uniformity of the microrod, spatially-correlated PL mapping was performed. Fig. 2c shows the microrod optical mapping image (in Fig. S4, ESI†), and the distribution PL emission mapping is shown in Fig. 2d, recorded with 64 × 64 pixels. The distribution of the PL emission peaks was visualized over the entire crystal through spectral mapping, endorsing the reasonable quality of the FA-(N-MPDA)PbBr4 microrods. Finally, the histogram of the whole mapping area was calculated. The peak distribution was identified between 526 nm and 545 nm (inset Fig. 2d). The absorption spectrum of the FA-(N-MPDA)PbBr4 microrods was observed in the 400–800 nm wavelength region, which shows strong absorption (Fig. S5, ESI†).
The structural, optical, and thermal stability of the FA-(N-MPDA)PbBr4 microrod exposed under ambient atmosphere (room temperature 30 °C and humidity 70%) were systematically monitored using XRD, PL, and TGA/DSC, and the results are summarized in Fig. 3. The XRD measurement was conducted from days 1 to 7 (Fig. 3a), revealing no changes in the XRD patterns. The peak positions and intensities of the FA-(N-MPDA)PbBr4 crystalline microrod remain almost the same as it was observed freshly, and there are no additional peaks corresponding to the impurity/secondary phases, which confirms the exceptional structural stability and crystallinity against ambient degradation. Fig. 3b shows the PL emission spectrum of the FA-(N-MPDA)PbBr4 microrod under continuous laser irradiation of 405 nm. The PL intensity of the emission spectrum remains the same even after continuous laser light irradiation for about two hours. The results above prove that the FA-(N-MPDA)PbBr4 microrod is stable against light and ambient exposure. To evaluate the thermal stability, chemical decomposition, and phase transition behavior of the material, TGA/DSC measurement was performed on the FA-(N-MPDA)PbBr4 powder sample, as shown in Fig. 3c. The FA-(N-MPDA)PbBr4 exhibits stable weight until 290 °C without any loss of chemical decomposition, indicating the heat resistance of the material until 290 °C, which is 50 °C and 100 °C higher than that of the 2D(PEA)2PbBr4 and (BA)2PbI4 hybrid perovskite materials, respectively.23,24 After 290 °C, the material drops to about 70% of its original weight before reaching 350 °C due to the loss of organic moieties, such as the decomposition of FA and N-MPDA species.25 The formation of a sharp endothermic peak at 110 °C was observed from the differential scanning calorimetric analysis (Fig. 3c), indicating the structural phase transition of the material.
Moreover, the phase transition of the material occurred before the decomposition temperature, as observed in the TGA result. The superior stability of the FA-(N-MPDA)PbBr4 perovskite crystalline microrod can be reasoned to form a stable perovskite crystal structure due to the high tolerance factor of the organic species (FA and long-chain diammonium N-MPDA spacer) and Br− ion in the perovskite lattices.26,27 Moreover, the higher lattice energy was caused by a smaller Pb–Br bond length in the perovskite lattices avoiding materials decomposition, which quickly occurred in iodide-based perovskites due to low lattice energy.26 In overall consideration, the FA-(N-MPDA)PbBr4 crystalline microrod possesses excellent stability against heat, light, and moisture, which typically appears to be a critical challenge in other 3D and 2D hybrid halide perovskites for implementing this material into practical applications.
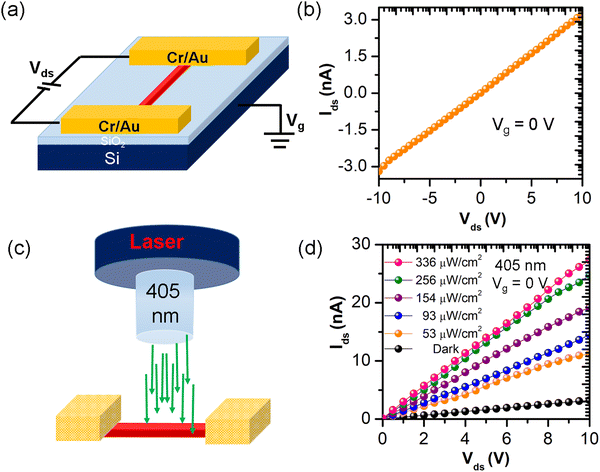
A photodetector device was made on the as-grown FA-(N-MPDA)PbBr4 microrod single crystal to evaluate the optoelectronic performances by depositing source–drain electrodes (Cr/Au). This was achieved using a copper grid as a shadow mask on both ends of the FA-(N-MPDA)PbBr4 perovskite microrod, and the entire device structure is on a silicon substrate having a 300 nm dielectric layer.28,29 The schematic view of the device structure is shown in Fig. 4a. The source–drain voltage vs. source–drain current (Ids–Vds) characteristic of the FA-(N-MPDA)PbBr4 microrod was measured under dark between −10 and 10 V at Vg = 0 V in the atmospheric environment (Fig. 4b). Fig. S6 of the ESI,† shows linear Ids–Vds behavior at a lower voltage of −0.5 to 0.5 V, confirming the Ohmic contact between the metal pad and perovskite microrod. The schematic illustration of the photoresponse of the FA-(N-MPDA)PbBr4 microrod under a 405 nm laser illumination is depicted in Fig. 4c.
Fig. 4d shows the plot of the generated photocurrent (Iph) as a function of various light intensities. The photocurrent increased with increasing laser intensity (53 to 336 μW cm−2) as well as with the bias voltage (0 to 10 V) compared to the dark measurement (Fig. 4d). The Iph value was obtained by subtracting the dark current (Idark) from the light current (Ilight). At the illumination of 336 μW cm−2 and a bias of 10 V, the device attained a high photocurrent of 24 nA (Fig. 5a). The photocurrent vs. bias voltage is shown in Fig. S7 of the ESI.† From Fig. S7, the photocurrent increases with an increase in the bias voltage. The photoresponsivity (Rλ) of the FA-(N-MPDA)PbBr4 microrod photodetector is estimated using Rλ = Iph/(P × S), where Iph is the photocurrent, P is light irradiance, and S is the active area of the device.30,31 From the above relation, the photoresponsivity of the FA-(N-MPDA)PbBr4 microrod is ∼3.8 A W−1 (Fig. 5b) under a light illumination of 53 μW cm−2 (Vg = 0 V and Vds = 10 V). Photoresponsivity is considered to be a critical figure of merit of the photodetector.32–36 The high photoresponsivity value on the perovskite microrod was attributed to the strong absorption coefficient, unidirectional charge transport, high-quality factor, quantum confinements, and low non-radiative recombination rates.
The other two key factors of photosensing are external quantum efficiency (EQE) and specific detectivity (D*). EQE defines photoexcited electrons produced per incident photons and is expressed as EQE = Rλ·[hc/(eλ)], where Rλ is the photoresponsivity, h is Planck's constant, c is the speed of light, e is the elementary charge, and λ is the incident light wavelength.37,38 From Fig. 5c, the estimated EQE for the FA-(N-MPDA)PbBr4 single-crystalline microrod is ∼103. The obtained high value of EQE can be related to the prolonged lifetime of the photoexcited charge carriers, which transit the device several times, enhance the efficiency, and recombine. The other parameter (D*) defines the photodetector sensitivity by detecting the minimum light optical signals. It is calculated by D* = (S·Δf)1/2/NEP, where S is the effective area, Δf is the electrical bandwidth, and NEP is the noise equivalent power. The above relation is rewritten as RλS1/2/(2eIdark)1/2 at a lower NEP value, where Rλ, S, e, and Idark are the photoresponsivity, effective area, elementary charge, and dark current, respectively.39,40 The calculated D* value is 7.8 × 1010 Jones (Fig. 5d) at 53 μW cm−2 (Vg = 0 V and Vds = 10 V).
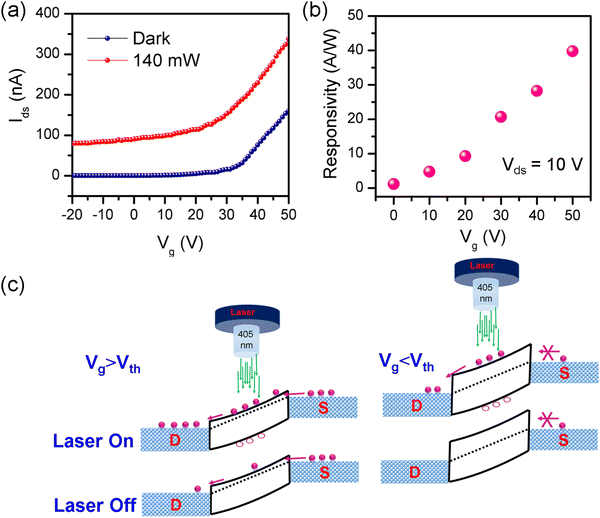
Fig. 6a illustrates the Ids curve of the FA-(N-MPDA)PbBr4 microrod at different gate voltage ranging from −20 V to 50 V under dark and laser irradiation conditions. At 140 mW of 405 nm illumination, the Ids current undoubtedly enhances than that in the dark. At positive Vg, the current increases, depicting the n-type nature of the material with more electrons as a charge carrier. The Iph is extracted and plotted against Vg from the transfer curve (Fig. 6a), as shown in the ESI,† in Fig. S8. The Rλ is calculated concerning various Vg (0 to 50 V) at 10 V of Vds shown in Fig. 6b. The Rλ increases when Vg rises and gives a maximum Rλ of 40 A W−1 at 50 V. The mechanism behind this high value of Rλ is demonstrated in Fig. 6c. In (Laser Off) condition at Vg > Vth, the Ids continually increases when the Vg is more than the threshold voltage (Vth) due to the Fermi level band shifting upward, thus allowing more charge carrier flows into the source–drain electrodes. In the case of Vg < Vth, the Ids value is much lower because the Fermi level shifts to a more downward direction, which creates a solid barrier for the circulation of charge carriers during electric field supply. During Laser On at Vg > Vth, the photons constantly produce numerous electron–hole pairs with less barrier for the circulation of carriers; both cause a much larger source–drain current, resulting in significant photocurrent enhancement, while at Vg < Vth, only a few exciting carriers will attain a chance to transit toward the electrode due to its long barrier.41,42
Typically, the presence of a long chain organic spacer in the hybrid perovskite impacts the conductivity, prohibiting the charge carrier transport process along its orientation, which might be attributed to the low conductivity of the FA-(N-MPDA)PbBr4 microrod. The less conductance leads to low current (∼3 nA) during bias between the source and drain. However, superior stability was observed from the microrod device under ambient air for several days. The long-term stability of the photodetector device was monitored on different days, as shown in the ESI,† in Fig. S9. The device exhibited good stability under ambient air for several days. Moreover, the PD device also reveals good thermal stability and increasing source–drain current until about 100 °C, as shown in Fig. S10 (ESI†). Later, the source–drain value decreased above 100 °C, indicating the degradation of organic molecules in the FA-(N-MPDA)PbBr4 at higher temperatures.
Finally, real-time measurements were performed to show the response speed and photoswitching stability of the FA-(N-MPDA)PbBr4 microrod photodetector with laser on-off illumination. Fig. 7a shows the single on-off cycle response, where a fast rise in the current (Ids) under “on” and a quick drop after switching “off” the laser illumination are observed. The photocurrent increases rapidly, then decreases rapidly until it reaches a saturation level. This slight instability might be attributed to the presence of defects and surface oxygen, resulting in the generation and recombination of charge carriers, which significantly impact the capacity of generating and storing charges during the processes of photoresponse. In addition, the microrod defects or light/bias-induced degradation could also impact this instability of the photocurve. The photogenerated signal determined that the rising time of the FA-(N-MPDA)PbBr4 microrod device is less than 50 ms. In the case of falling, there is a combination of fast decay and slow relaxation. To understand the successive photoswitching, the robustness and stability of the FA-(N-MPDA)PbBr4 microrod photodetector were further inspected by a train of pulsed illumination for a long run-up to five-minute (Fig. 7b) and with various Vds of 5 V and 10 V, where the Ids increased when Vds is higher (Fig. 7c). The reproducible on-off cycle without any degradation against laser illumination and atmospheric environment, along with maintainable rise times, reveals the device robustness, reproducible response, and switching stability of the FA-(N-MPDA)PbBr4 microrod photodetector.43,44
3. Conclusion
In summary, the millimeter-sized FA-(N-MPDA)PbBr4 perovskite single-crystalline microrod was grown by the SECT solution growth method for photodetector application. The FA-(N-MPDA)PbBr4 exhibits exceptional structural, optical, and thermal stability against ambient, light, and heat, resulting in high-performance stable photodetectors. The FA-(N-MPDA)PbBr4 single-crystalline microrod photodetector reveals stable photoresponse with a responsivity of ∼40 A W−1 and a specific detectivity of 7.8 × 1010 Jones at room temperature. Moreover, the device shows a fast response speed of less than 50 ms with robust photoswitching stability. High stability against natural degradation and good optoelectronic performance of the FA-(N-MPDA)PbBr4 microrod would be a highly competitive perovskite material for implementing practical applications.4. Experimental section
4.1. Precursor material for the single-crystal growth of (N-MPDA)PbBr4
Lead oxide (PbO), hydrobromic acid (HBr), N1-methylpropane-1,3-diammonium (CH3NH(CH2)3NH2) (N-MPDA), and formamidinium chloride (HC(NH2)2Cl) raw chemicals were purchased from Sigma Aldrich. 10 mmol PbO was dissolved in 20 mL HBr at 70 °C under constant stirring until a clear, bright yellow solution (Solution A) formed. On the other side, 3.33 mmol CH3NH(CH2)3NH2 was added dropwise into the HBr solution under ice bath conditions to prepare (N-MPDA)+Br− salt solution (Solution B). To attain the 2D (N-MPDA)PbBr4 perovskite, the cold salt solution B was added to hot solution A at 120 °C. Initially, light yellow precipitation will occur, redissolved with excessive HBr, and stirred to obtain a bright yellow solution.22 This bright yellow solution is kept constant under slow evaporation22,44,45 at a fixed temperature to obtain the (N-MPDA)PbBr4 perovskite compound.4.2. Formation of the FA-(N-MPDA)PbBr4 perovskite
Formamidinium chloride (FACl) was added into the PbO solution first, followed by the addition of (N-MPDA)+Br− with continued stirring. Following SECT growth techniques, the supersaturated solution evaporates slowly for 2–3 days while maintaining a constant temperature.44,45 In nucleation, the excess solute in the supersaturated solution crystallizes at the bottom of the solution in millimeter sizes.4.3. Characterization
The crystal structure and phase purity of the as-grown FA-(N-MPDA)PbBr4 single crystal were examined with an X'Pert PRO-PANalytical XRD using CuKα (λ = 1.5406 Å) source, step size 0.01°, scan speed 0.5 s step−1, and operation voltage 40 kV. SEM (FEI, Nova 200) investigated the morphology and elemental compositions. TEM (JEOL, JEM-2100F) was employed at 200 kV to study the microstructures and SAED patterns. TGA/DSC was performed on the powdered samples to observe the thermal stability using Mettler Toledo. The samples were taken in alumina crucibles and heated up to 450 °C at a 10 °C min−1 rate under an argon gas flow. A home-built setup was used to map under a 450 nm pulse laser (Pico Quant). The electrical and optical properties were measured at room temperature using (Lakeshore, TTPX) probe station. The system was combined with a source meter (Keithley, 2636A) with the optical system of a 405 nm laser.4.4. Device fabrication
FA-(N-MPDA)PbBr4 microrod-FETs were fabricated using a standard shadow mask technique with a copper grid. Gold/Chromium electrodes were deposited at about 100/10 nm on both ends of the microrods with the help of a thermal evaporator to attain the source and drain electrodes.28,29 The effective illuminated area of the device was about 150 μm2.Conflicts of interest
There are no conflicts to declare.Acknowledgements
C.-Y. L. acknowledges the financial support provided by the National Science and Technology Council (NSTC), Taiwan, under grant numbers NSTC-111-2112-M-033-005-, NSTC-110-2221-E-033-022, and NSTC-111-2628-E-033-002-MY3. R. S. acknowledges MOST-111-2124-M-001-009, MOST-110-2112-M-001-065-MY3, and Academia Sinica for the budget of AS-iMATE-19-112. R. C. M. and A. R. acknowledge the Marie Skłodowska-Curie Individual Fellowship (MOFUS, #795356).References
- R. Dong, Y. Fang, J. Chae, J. Dai, Z. Xiao, Q. Dong, Y. Yuan, A. Centrone, X. C. Zeng and J. Huang, Adv. Mater., 2015, 27, 1912 CrossRef CAS PubMed.
- S. Shao, W. Talsma, M. Pitaro, J. Dong, S. Kahmann, A. J. Rommens, G. Portale and M. A. Loi, Adv. Funct. Mater., 2021, 31, 2008478 CrossRef CAS.
- Y. Jiang, E. J. J.-Perez, Q. Ge, S. Wang, M. R. Leyden, L. K. Ono, S. R. Raga, J. Hu and Y. Qi, Mater. Horiz., 2016, 3, 548 RSC.
- A. Zhizhchenko, S. Syubaev, A. Berestennikov, A. V. Yulin, A. Porfirev, A. Pushkarev, I. Shishkin, K. Golokhvast, A. A. Bogdanov, A. A. Zakhidov, A. A. Kuchmizhak, Y. S. Kivshar and S. V. Makarov, ACS Nano, 2019, 13, 4140 CrossRef CAS.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050 CrossRef CAS.
- S. Huang, P. Huang, L. Wang, J. Han, Y. Chen and H. Zhong, Adv. Mater., 2019, 31, 1903830 CrossRef CAS PubMed.
- Y. Zhou and Y. Zhao, Energy Environ. Sci., 2019, 12, 1495 RSC.
- J. Bisquert and E. J. J.-Perez, J. Phys. Chem. Lett., 2019, 10, 5889 CrossRef CAS.
- F. A. Roghabadi, M. Alidaei, S. M. Mousavi, T. Ashjari, A. S. Tehrani, V. Ahmadi and S. M. Sadrameli, J. Mater. Chem. A, 2019, 7, 5898 RSC.
- M. You, H. Wang, F. Cao, C. Zhang, T. Zhang, L. Kong, L. Wang, D. Zhao, J. Zhang and X. Yang, ACS Appl. Mater. Interfaces, 2020, 12, 43018 CrossRef CAS.
- H. Yu, Y. Xie, J. Zhang, J. Duan, X. Chen, Y. Liang, K. Wang and L. Xu, Adv. Sci., 2021, 8, 2004510 CrossRef CAS.
- X. Zhang, X. Ren, B. Liu, R. Munir, X. Zhu, D. Yang, J. Li, Y. Liu, D.-M. Smilgies, R. Li, Z. Yang, T. Niu, X. Wang, A. Amassian, K. Zhao and S. Liu, Energy Environ. Sci., 2017, 10, 2095 RSC.
- Y. Chen, Y. Sun, J. Peng, W. Zhang, X. Su, K. Zheng, T. Pullerits and Z. Liang, Adv. Energy Mater., 2017, 7, 1700162 CrossRef.
- Z. Li, N. Liu, K. Meng, Z. Liu, Y. Hu, Q. Xu, X. Wang, S. Li, L. Cheng and G. Chen, Nano Lett., 2019, 19, 5237 CrossRef CAS.
- C. C. Stoumpos, D. H. Cao, D. J. Clark, J. Young, J. M. Rondinelli, J. I. Jang, J. T. Hupp and M. G. Kanatzidis, Chem. Mater., 2016, 28, 2852 CrossRef CAS.
- C. Liang, H. Gu, Y. Xia, Z. Wang, X. Liu, J. Xia, S. Zuo, Y. Hu, X. Gao, W. Hui, L. Chao, T. Niu, M. Fang, H. Lu, H. Dong, H. Yu, S. Chen, X. Ran, L. Song, B. Li, J. Zhang, Y. Peng, G. Shao, J. Wang, Y. Chen, G. Xing and W. Huang, Nat. Energy, 2021, 6, 38 CrossRef CAS.
- L. Mao, Y. Wu, C. C. Stoumpos, M. R. Wasielewski and M. G. Kanatzidis, J. Am. Chem. Soc., 2017, 139, 5210 CrossRef CAS.
- D. B. Mitzi, K. Chondroudis and C. R. Kagan, IBM J. Res. Dev., 2001, 45, 29 CAS.
- M. Seitz, A. J. Magdaleno, N. A. Cano, M. Melendez, T. J. Lubbers, S. W. Walraven, S. Pakdel, E. Prada, R. D.-Buscalioni and F. Prins, Nat. Commun., 2020, 11, 2035 CrossRef CAS.
- A. O. E.-Ballouli, O. M. Bakr and O. F. Mohammed, J. Phys. Chem. Lett., 2020, 11, 5705 CrossRef.
- M. Wang, J. Tang, H. Wang, C. Zhang, Y. S. Zhao and J. Yao, Adv. Opt. Mater., 2020, 8, 1901780 CrossRef CAS.
- P. K. Roy, R. K. Ulaganathan, C. M. Raghavan, S. M. Mhatre, H. I. Lin, W. L. Chen, Y. M. Chang, A. Rozhin, Y. T. Hsu, Y. F. Chen, R. Sankar, F. C. Chou and C. T. Liang, Nanoscale, 2020, 12, 18269 RSC.
- Y. Zhang, Y. Liu, Z. Xu, H. Ye, Q. Li, M. Hu, Z. Yang and S. Liu, J. Mater. Chem. C, 2019, 7, 1584 RSC.
- T. Li, W. A. D. Shohl, E. W. Reinheimer, P. L. Magueres and D. B. Mitzi, Chem. Sci., 2019, 10, 1168 RSC.
- T. Li, W. A. D. Shohl, Q. Han and D. B. Mitzi, Chem. Mater., 2017, 29, 6200 CrossRef CAS.
- E. S. Vasileiadou, I. Hadar, M. Kepenekian, J. Even, Q. Tu, C. D. Malliakas, D. Friedrich, I. Spanopoulos, J. M. Hoffman, V. P. Dravid and M. G. Kanatzidis, Chem. Mater., 2021, 33, 5085 CrossRef CAS.
- G. P. Nagabhushana, R. Shivaramaiah and A. Navrotsky, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 7717 CrossRef CAS.
- J. Yang, W. Kang, Z. Liu, M. Pi, L. B. Luo and C. Li, J. Phys. Chem. Lett., 2020, 11, 6880 CrossRef CAS.
- M. Shoaib, X. Zhang, X. Wang, H. Zhou, T. Xu, X. Wang, X. Hu, H. Liu, X. Fan, W. Zheng, T. Yang, S. Yang, Q. Zhang, X. Zhu, L. Sun and A. Pan, J. Am. Chem. Soc., 2017, 139, 15592 CrossRef CAS PubMed.
- J. Yao and G. Yang, Nanoscale, 2020, 12, 454 RSC.
- O. L.-Sanchez, D. Lembke, M. Kayci, A. Radenovic and A. Kis, Nat. Nanotechnol., 2013, 8, 497 CrossRef PubMed.
- S. Lim, M. Ha, Y. Lee and H. Ko, Adv. Opt. Mater., 2018, 6, 1800615 CrossRef.
- F.-X. Liang, J.-J. Jiang, Y.-Z. Zhao, Z.-X. Zhang, D. Wu, L.-H. Zeng, Y. H. Tsang and L.-B. Luo, Adv. Funct. Mater., 2020, 30, 2001033 CrossRef CAS.
- J. Zhou, Y. Chu and J. Huang, ACS Appl. Mater. Interfaces, 2016, 8, 25660 CrossRef CAS.
- R. Dong, C. Lan, X. Xu, X. Liang, X. Hu, D. Li, Z. Zhou, L. Shu, S. Yip, C. Li, S.-W. Tsang and J. C. Ho, ACS Appl. Mater. Interfaces, 2018, 10, 19019 CrossRef CAS PubMed.
- R. Dong, C. Lan, F. Li, S. Yip and J. C. Ho, Nanoscale Horiz., 2019, 4, 1342 RSC.
- W. Deng, L. Huang, X. Xu, X. Zhang, X. Jin and S.-T. Lee, Nano Lett., 2017, 17, 2482 CrossRef CAS PubMed.
- Z. Lian, Q. Yan, Q. Lv, Y. Wang, L. Liu, L. Zhang, S. Pan, Q. Li, L. Wang and J.-L. Sun, Sci. Rep., 2015, 5, 16563 CrossRef.
- M. Buscema, J. O. Island, D. J. Groenendijk, S. I. Blanter, G. A. Steele, H. S. J. van ser Zant and A. C. Gomez, Chem. Soc. Rev., 2015, 44, 3691 RSC.
- G. Su, V. G. Hadjiev, P. E. Loya, J. Zhang, S. Lei, S. Maharjan, P. Dong, P. M. Ajayan, J. Lou and H. Peng, Nano Lett., 2015, 15, 506 CrossRef CAS PubMed.
- N. Huo and G. Konstantatos, Nat. Commun., 2017, 8, 572 CrossRef PubMed.
- J. Kwon, Y. Shin, H. Kwon, J. Y. Lee, H. Park, K. Watanabe, T. Taniguchi, J. Kim, C. H. Lee, S. Im and G. H. Lee, Sci. Rep., 2019, 9, 10354 CrossRef PubMed.
- Z. Tan, Y. Wu, H. Hong, J. Yin, J. Zhang, L. Lin, M. Wang, X. Sun, L. Sun, Y. Huang, K. Liu, Z. Liu and H. Peng, J. Am. Chem. Soc., 2016, 138, 16612 CrossRef CAS PubMed.
- R. K. Ulaganathan, R. C. Murugesan, C. Y. Lin, A. Subramanian, W. L. Chen, Y. M. Chang, A. Rozhin and R. Sankar, Adv. Funct. Mater., 2022, 32, 2112277 CrossRef CAS.
- C. M. Raghavan, T. P. Chen, S. S. Li, W. L. Chen, C. Y. Lo, Y. M. Liao, G. Haider, C. C. Lin, C. C. Chen, R. Sankar, Y. M. Chang, F. G. Chou and C. W. Chen, Nano Lett., 2018, 18, 3221 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma00602b |
| This journal is © The Royal Society of Chemistry 2022 |