Open Access Article
Open Access ArticleNovel hydrolytically stable Lewis acidic ionic liquid catalyst system for polybutylene succinate (PBS) synthesis†
K. S.
Savitha
b,
M.
Senthil Kumar
 a and
R. L.
Jagadish
*b
a and
R. L.
Jagadish
*b
aAlumnus, Department of Chemistry, Indian Institute of Technology Madras, Chennai, India. E-mail: senthiliit@gmail.com
bDepartment of Polymer Science Sir M. Visvesvaraya Postgraduate Centre Tubinakere, Mandya, India. E-mail: rljagadish@yahoo.com
First published on 26th August 2022
Abstract
Poly(butylene succinate) (PBS) is considered to be a potential bio-alternative for petroleum-based polymers due to its environmentally benign nature and biodegradability. Despite its various advantages, its low thermal stability and lack of high molecular weight required for industrial applications restrict its implementation on a commercial scale. The synthesis of high molecular weight PBS remains a challenging task due to the hydrolytic instability of the catalyst as well as the thermal sensitivity of PBS. Herein, we report a Lewis acidic ionic liquid (LAIL) as a novel, mild, efficient and hydrolytically stable catalyst system for the synthesis of high molecular weight PBS. Further, the thermal stability of the PBS synthesized using the novel catalyst is found to be higher than the corresponding polymer synthesized using a conventional catalyst.
Introduction
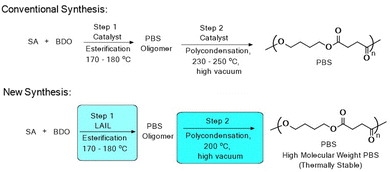
The petrochemical revolution in the last decade led to the development of various commercially important polymers such as polyethylene, polystyrene, polypropylene and poly(vinyl chloride) from fossil fuels.1 Moreover, their low cost and excellent mechanical properties have championed these polymers as the material of choice for various applications such as electrical and electronics, automobiles, food packaging, pipe and fittings, personal hygiene, cosmetics, building and construction, and pharmaceuticals.2 However, the durability and non-biodegradability of fossil fuel-based polymers has led to increased plastic pollution in the environment.3 In recent years, the design and synthesis of environmentally friendly polymers as an alternative to petroleum-based polymers have gained considerable interest among scientists in both academia and the industry.4 As a result, various bio-based polymers have been developed and have found commercial applications in replacing petroleum-based polymers.5 Among the various bio-based polymers, polybutylene succinate (PBS) has gained considerable interest as a potential bio-alternative due to its comparable physical and mechanical properties as those of polyolefins.6However, its high cost coupled with a lack of high molecular weight required for industrial application restricts its commercialization.7 Extensive research is being carried out to broaden the application of PBS by improving its mechanical and thermal properties via chemical and physical methods.8 Generally, the synthesis of PBS involves two steps: esterification or trans-esterification followed by polycondensation reactions, which are carried out at high temperatures, usually 230–250 °C under vacuum (Scheme 1).9 Significant efforts have already been made towards the development of mild and efficient catalysts in synthesizing high molecular weight PBS. As a result, various catalyst systems have been reported in the literature for the synthesis of PBS.8
However, among the various catalysts explored, titanium-based catalysts exhibit better catalytic activity than other Lewis acid catalysts. Thus, titanium alkoxides (titanium tetrabutoxide (TBT) and titanium tetraisopropoxide (TTIP)) are the most widely used catalyst for the synthesis of PBS as well as its copolymers, and the order of efficiency is found to be Ti » Zr ∼ Sn > Hf > Sb > Bi.9 However, titanium-based catalysts are more sensitive to moisture/water, and hence the addition of catalyst during the polycondensation step exhibited higher activity than adding the catalyst during the esterification step. This clearly indicates the sensitivity of titanium-based catalysts towards water, the by-product formed during the reaction.9a
Moreover, PBS is thermally sensitive and the high temperature required for the polycondensation reaction restricts the synthesis of high molecular weight PBS, as it undergoes various side reactions including degradation.10 Thus, the synthesis of high molecular weight PBS from dicarboxylic acids and diols remains a challenging task due to the thermal sensitivity of PBS and hydrolytic instability of the catalyst. Thus, the development of efficient and hydrolytically stable catalysts for the synthesis of high molecular weight PBS is much sought after. Herein, we report a novel mild, efficient and hydrolytically stable (Lewis acidic ionic liquid) catalyst system for the synthesis of high molecular weight PBS.
Results and discussion
In recent years, ionic liquids have gained considerable attention in catalysis. Various ionic liquids (LAIL) have been developed and proven to be more efficient than conventional catalysts in various organic transformations.11 To the best of our knowledge, the use of ionic liquids has not yet been explored for the synthesis of PBS. Thus, it is in our interest to explore a few Lewis acidic ionic liquids for the synthesis of PBS. Recently, a hydrolytically stable Lewis acidic ionic liquid was developed using choline chloride and the metal salts of Sn and Zn, especially for the Diels Alder reaction.12 It is in our interest to explore this catalyst system for the synthesis of PBS.Thus, ionic liquids based on Sn and Zn were prepared using choline chloride and BMIMCl (1-Butyl-3-methylimidazolium chloride) by following a reported procedure.12 The synthesized catalyst was examined for its catalytic efficiency with respect to conventional catalyst such as titanium tetrabutoxide, titanium tetraisopropoxide, stannous chloride etc., and the results are summarized in Table 1 (entry 1–12). The reported method was optimized and using the optimized synthetic method, PBS with Mn, Mw and PDI values of about 1.84 × 104 g mol−1, 4.68 × 104 g mol−1 and 2.53, respectively, were synthesized.9a Further, the optimized reaction condition was used to screen the efficiency of other catalyst systems (using 0.02 mol%) for the synthesis of PBS (Table 1).13
| S. No. | Catalysta | Temp. (°C) (Time) | M n (g mol−1) | M w (g mol−1) | PDI | T m (°C) | T c (°C) | T d (°C) |
|---|---|---|---|---|---|---|---|---|
| a 0.02 mol% catalyst was used for the reaction; Td: onset degradation temperature. | ||||||||
| 1 | Ti(OBu)4 | 230 (6.5 h) | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 536 536 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 870 870 |
2.53 | 77 | 112 | 368 |
| 2 | Ti(OiPr)4 | 230 (6.5 h) | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 855 855 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 354 354 |
2.78 | 73 | 110 | 365 |
| 3 | SnCl2 | 230 (6 h) | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 427 427 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 868 868 |
2.76 | 77 | 114 | 370 |
| 4 | ZnCl2 | 230 (8 h) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 226 226 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 226 226 |
3.15 | 70 | 112 | 360 |
| 5 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnCl2 (1 SnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
230 (7 h) | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 874 874 |
57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 957 957 |
2.65 | 78 | 115 | 379 |
| 6 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 (1 ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
230 (7 h) | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 289 289 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 483 483 |
2.15 | 74.8 | 112.7 | 358 |
| 7 | BMIM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnCl2 (1 SnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
230 (7 h) | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 923 923 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 160 |
2.41 | 72.6 | 112.6 | 362 |
| 8 | BMIM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 (1 ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
230 (7 h) | 9654 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 058 058 |
2.81 | 71.4 | 110.81 | 354 |
| 9 | BMIMCl | 230 (7 h) | 2286 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 740 |
4.70 | — | — | — |
| 10 | ChCl | 230 (7 h) | 7758 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 278 278 |
2.36 | — | — | — |
| 11 | [BMIM][HSO4] | 230 (7 h) | 3065 | 7271 | 2.37 | — | — | — |
| 12 | PMA | 230 (7 h) | 1113 | 1884 | 1.69 | — | — | — |
Interestingly, the ionic liquid prepared using choline chloride and SnCl2 exhibited better catalytic activity than the conventional catalysts (Table 1, entry 5). However, the ionic liquid prepared using zinc chloride exhibited moderate activity (Table 1, entry 6 and 8). From the experimental results, it is very clear that choline chloride-based ionic liquids are found to be more efficient in producing relatively high molecular weight PBS than the corresponding BMIMCl-based ionic liquids (Table 1, entry 5–8). Further, the reaction of succinic acid and butanediol in the presence of BMIMCl, ChCl, [BMIM][HSO4] and phosphomolybdic acid (PMA) resulted in the isolation of polymers with low molecular weight (Table 1, entry 9–12).
Furthermore, the influence of Lewis acid concentration in the ionic liquids was investigated and the results are summarized in Table 2. Since ChCl-based Lewis acidic ionic liquids exhibited better catalytic activity than the corresponding BMIMCl-based ionic liquids, a systematic investigation was carried out to understand the influence of temperature and the concentration of metal halides in ChCl-based Lewis acidic ionic liquids. An increase in the concentration of metal halides in the ChCl-based ionic liquids resulted in increased catalytic activity by reducing the reaction time required for the reaction (Table 2, entry 3); however, further increase in the concentration of Lewis acid in the catalyst system resulted in the lowering of the thermal stability of the polymer (Table 2, entry 9). Interestingly, the choline chloride-based Lewis acidic ionic liquid catalyst system was found to produce high molecular weight PBS even at low temperatures (200 °C) (Table 2, entry 6).
| S. No. | Catalysta | Temp. (°C) (Time) | M n (g mol−1) | M w (g mol−1) | PDI | T m (°C) | T c (°C) | T d (°C) |
|---|---|---|---|---|---|---|---|---|
| a 0.02 mol% catalyst was used for the reaction. | ||||||||
| 1 | BMIM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnCl2 (1 SnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
230 (7 h) | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 057 057 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 907 907 |
2.38 | 75 | 112 | 368 |
| 2 | BMIM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 (1 ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
230 (7 h) | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365 365 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 623 623 |
2.56 | 74.6 | 111 | 362 |
| 3 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnCl2 (1 SnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
230 (6 h) | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 988 988 |
72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 624 624 |
2.27 | 77 | 115 | 383 |
| 4 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 (1 ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
230 (7 h) | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902 902 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 472 472 |
2.78 | 75 | 113 | 364 |
| 5 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnCl2 (1 SnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
200 (8 h) | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 840 |
56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 826 826 |
2.60 | 77 | 114 | 378 |
| 6 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnCl2 (1 SnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
200 (7 h) | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 609 609 |
73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 031 031 |
3.38 | 76 | 115 | 389 |
| 7 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 (1 ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
200 (8.5 h) | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365 365 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 623 623 |
2.56 | 75 | 112 | 354 |
| 8 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 (1 ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
200 (7.5 h) | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823 823 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960 960 |
2.39 | 75 | 111 | 358 |
| 9 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnCl2 (1 SnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
230 (5 h) | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902 902 |
72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 990 990 |
3.33 | 77 | 114 | 368 |
To understand the hydrolytic stability of the ChCl-based Lewis acidic ionic liquid catalyst system, PBS synthesis was carried out by adding the catalyst before and after the esterification reaction (Table 3, entry 1–6). Interestingly, no significant changes in catalytic activity were observed for the newly synthesized ChCl:SnCl2 catalyst system, which clearly indicates the hydrolytic stability of the catalyst. Whereas, conventional titanium based catalyst exhibited reduced catalytic activity by adding the catalyst before esterification reaction, which clearly elucidates the hydrolytic instability of the catalyst system during PBS synthesis (Table 3, entry 1 & 4). Further, thermal stability of the PBS produced using stannous chloride-based ionic liquids was found to be higher than that of the corresponding polymer produced using the zinc-based ionic liquids and titanium-based catalysts (Tables 1 and 2).
| S. No. | Catalyste | Time | M n (g mol−1) | M w (g mol−1) | PDI | T m (°C) | T c (°C) | T d (°C) |
|---|---|---|---|---|---|---|---|---|
| a Catalyst added during the esterification step.13a b Catalyst added during the polycondensation step.13b c Temperature of the polycondensation reaction: 230 °C. d Temperature of the polycondensation reaction: 200 °C. e 0.02 mol% ppm catalyst used for the reaction. | ||||||||
| 1a | Ti(OBu)4 | 7c | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 536 536 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 870 870 |
2.53 | 77 | 112 | 362 |
| 2a | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnCl2 (1 SnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
7c | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 874 874 |
57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 957 957 |
2.65 | 77.6 | 115 | 379 |
| 3a | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnCl2 (1 SnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
6d | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 988 988 |
72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 624 624 |
2.27 | 77 | 115 | 389 |
| 4b | Ti(OBu)4 | 5c | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 074 074 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 271 271 |
2.64 | 81 | 114 | 366 |
| 5b | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnCl2 (1 SnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
7c | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 732 732 |
57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 272 272 |
2.64 | 78 | 115 | 382 |
| 6b | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnCl2 (1 SnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
6d | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 448 448 |
73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 104 104 |
2.32 | 78 | 115 | 394 |
It is reported in the literature, that the residual catalyst present in the final polymer results in the degradation of the corresponding polymer at higher temperatures.9a Trace metal analysis clearly indicates that the metal catalyst present in the final polymer (PBS) produced using Lewis acidic ionic liquids is very low when compared to the polymer produced using the corresponding metal chloride catalysts (Table 4). The removal of catalyst is found to be more effective in the case of polymerizations carried out using Lewis acidic ionic liquids compared to those with conventional metal chloride catalysts. Moreover, this resulted in the increased thermal stability of the final polymer produced using Lewis acidic ionic liquids (Table 4).
| S. No | Catalyst used for polymerization | Metal content in the final polymer (ppm) |
|---|---|---|
| 1 | Ti(OBu)4 | 225 |
| 2 | SnCl2 | 212 |
| 3 | ZnCl2 | 230 |
| 4 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnCl2 (1 SnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
96 |
| 5 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 (1 ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
108 |
Finally, from the thermal studies, it is evident that all the synthesized ionic liquid catalysts are more stable at 250 °C (temperature required for polycondensation reaction) (Table 5, entry 1–6). Even choline chloride is found to be stable at 250 °C (Table 5, entry 1). However, the stability of BMIMCl was found to be very low (61%) at 250 °C (Table 5, entry 2). Interestingly, the corresponding ionic liquids synthesized using BMIMCl are found to be more stable at 250 °C (Table 5, entry 5 and 6). This increased stability of the BMIMCl-based Lewis acidic ionic liquids is believed to be due to the self-association of quaternary ammonium salts (QASs) (hydrogen bond acceptor) and metal halides (hydrogen bond donor) to form complexes, which leads to an increase in the thermal and chemical stability of the ionic liquids.
Conclusion
A novel Lewis acidic ionic liquid catalyst has been developed for the synthesis of high molecular weight PBS. The newly developed catalyst is found to be mild and more efficient than the corresponding conventional titanium-based catalysts. Moreover, the catalyst is found to be hydrolytically stable and the polymer (PBS) produced by the catalyst exhibits higher thermal stability. In addition, choline chloride-based ionic liquids are more efficient than the corresponding imidazolium-based ionic liquids towards PBS synthesis. Furthermore, removal of the catalyst from the final polymer is found to be relatively easy using the Lewis acidic ionic liquid-based catalyst system for PBS synthesis, which in turn led to increase in thermal stability of the final polymer. We strongly believe that this development of a hydrolytically stable Lewis acidic ionic liquid-based catalyst system will find wider application in the synthesis and commercialization of bio-based polyesters, especially PBS.Author contributions
Conceptualization and investigation – K. S. Savitha Gowda; methodology and consultation – Dr M. Senthil Kumar; project administration and supervision – Prof. Dr R. L. Jagadish; writing – original draft – K. S. Savitha Gowda; review and editing – Dr M. Senthil Kumar and Prof. Dr R. L. Jagadish.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Miss. K. S. Savitha Gowda thanks Sir M. Visvesvaraya Postgraduate Centre, Mandya, Karnataka, India, for providing the infrastructure for carry out this research.References
- (a) A. Gandini, in Biocatalysis in Polymer Chemistry, ed. K. Loos, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2011, pp. 1–33 Search PubMed; (b) O. Platnieks, S. Gaidukovs, V. K. Thakur, A. Barkane and S. Beluns, Eur. Polym. J., 2021, 161, 110855 CrossRef CAS; (c) S. A. Rafiqah, A. Khalina, A. S. Harmaen, I. A. Tawakkal, K. Zaman, M. Asim, M. N. Nurrazi and C. H. Lee, Polymers, 2021, 13, 1436 CrossRef CAS PubMed; (d) M. J. Mochane, S. L. Magagula, J. S. Sefadi and T. C. Mokhena, Polymers, 2021, 13, 1200 CrossRef CAS PubMed; (e) F. Xiao, G. Fontaine and S. Bourbigot, Polym. Degrad. Stab., 2021, 183, 109466 CrossRef CAS; (f) G.-Z. Yin and X.-M. Yang, J. Polym. Res., 2020, 27, 38 CrossRef CAS; (g) Bioplastics market development update 2021, https://www.european-bioplastics.org/news/publications/.
- (a) M. K. Huda and I. Widiastuti, J. Phys.: Conf. Ser., 2021, 1808, 012015 CrossRef CAS; (b) A. L. Andrady and M. A. Neal, Philos. Trans. R. Soc., B, 2009, 364, 1977–1984 CrossRef CAS PubMed.
- I. Vroman and L. Tighzert, Materials, 2009, 2, 307–344 CrossRef CAS.
- (a) S. Spierling, E. Knupffer, H. Behnsen, M. Mudersbach, H. Krieg, S. Springer, S. Albrecht, C. Herrmann and H. J. Endres, J. Cleaner Prod., 2018, 185, 476–491 CrossRef; (b) Q. Zhang, M. Song, Y. Xu, W. Wang, Z. Wang and L. Zhang, Prog. Polym. Sci., 2021, 120, 101430 CrossRef CAS; (c) S. Rameshkumar, P. Shaiju, K. E. O’Connor and R. B. Padamati, Curr. Opin. Green Sustainable Chem., 2020, 21, 75–81 CrossRef; (d) W. Sikorska, M. Musioł, B. Zawidlak-Węgrzyńska and J. Rydz, Adv. Polym. Technol., 2021, 6695140 CAS.
- (a) J. Xu and B. H. Guo, Biotechnol. J., 2010, 5, 1149–1163 CrossRef CAS PubMed; (b) G. Guidotti, M. Soccio, V. Siracusa, M. Gazzano, E. Salatelli, A. Munari and N. Lotti, Polymers, 2017, 9, 724 CrossRef PubMed; (c) F. De Santis, V. Volpe and R. Pantani, Polym. Eng. Sci., 2016, 57, 306–311 CrossRef; (d) P. Scarfato, L. D. Maio, M. R. Milana, S. Giamberardini, M. Denaro and L. Incarnato, Food Addit. Contam., Part A: Chem., Anal., Control, Exposure Risk Assess., 2017, 34, 1730–1742 CrossRef CAS PubMed; (e) V. V. Jost, eXPRESS Polym. Lett., 2018, 12, 429–435 CrossRef CAS; (f) A. Z. Naser, I. Deiab and B. M. Darras, RSC Adv., 2021, 11, 17151 RSC; (g) C. Vilela, A. F. Sousa, A. Fonseca, A. Serra, J. Cohello, C. Freire and A. Silvestre, Polym. Chem., 2014, 5, 3119–3141 RSC; (h) European Bioplastics conference, bioplastics market development update 2021, https://docs.europeanbioplastics.org/publications/market_data/Report_Bioplastics_Market_Data_2021_short_version.pdf.
- (a) E. Pollet and L. Averous, Production, chemistry and properties of polyhydroxyalkanoates, in Biopolymers – New Materials for Sustainable Films and Coatings, ed. D. Plackett, John Wiley & Sons, Ltd, Chichester, 2011, pp. 65–86. (accessed February 16, 2016) Search PubMed; (b) L. Av_erous, J. Macromol. Sci., Part C: Polym. Rev., 2004, 44, 231–274 CrossRef; (c) M. Gigli, M. Fabbri, N. Lotti, R. Gamberini, B. Rimini and A. Munari, Eur. Polym. J., 2016, 75, 431–460 CrossRef CAS.
- L. Aliotta, M. Seggiani, A. Lazzer, V. Gigante and P. Cinelli, Polymers, 2022, 14, 844 CrossRef CAS PubMed.
- (a) K. S. Savitha, B. R. Paghadar, M. Senthil Kumar and R. L. Jagadish, Polym. Chem., 2022, 13, 3562–3612 RSC; (b) S. Y. Hwang, E. S. Yoo and S. S. Im, Polym. J., 2021, 44, 1179–1190 CrossRef; (c) Y.-R. Tang, Y. Zhang, Y. Liu, B.-H. Guo and J. Xu, J. Compos. Biodegrad. Polym., 2020, 8, 45–60 CAS.
- (a) N. Jacquel, F. Freyermouth, F. Fenouillot, A. Rousseau, J. P. Pascault, P. Fuertes and R. Saint-Loup, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 5301–5312 CrossRef CAS; (b) X. Kong, H. Qi and J. M. Curtis, J. Appl. Polym. Sci., 2014, 131, 40579 Search PubMed; (c) D. N. Bikiaris and D. Achilias, Polymer, 2006, 47, 4851–4860 CrossRef CAS; (d) C. Zhu, Z. Zhang, Q. Liu, Z. Wang and J. Jin, J. Appl. Polym. Sci., 2003, 90, 982–990 CrossRef CAS; (e) K. Y. Choi and K. B. McAuley, in Step-growth polymerization in Polymer Reaction Engineering, ed. J. M. Asua, Blackwell Publishing, Oxford, UK, 2007, ch. 7, pp. 273–314 Search PubMed.
- S.-F. Lu, M. Chen and C. H. Chen, J. Appl. Polym. Sci., 2012, 123, 3610–3619 CrossRef CAS.
- (a) A. S. Amarasekara, Chem. Rev., 2016, 116, 6133–6183 CrossRef CAS PubMed; (b) R. Ratti, Adv. Chem., 2014, 729842 Search PubMed.
- A. P. Abbott, G. Capper, D. L. Davies, H. L. Munro, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2001, 2010–2011 RSC.
-
Literature method was standardized by repeating the PBS synthesis in triplicates. Concordant values of Mn, Mw and PDI clearly indicates the repeatability and reproducibility of the synthetic procedure, which was further used to evaluate the efficiency of Lewis acidic ionic liquid catalyst system towards the synthesis of PBS.
(a) General method for the synthesis of PBS (catalyst addition during esterification reaction):
In a clean three neck RB flask fitted with distillation assembly, succinic acid (20 g, 0.17 mol) and butane diol (16 g, 0.18 mol) was taken and stirred for 10 mins. Into this catalyst (0.02 mol%) was added and the resultant mixture was heated to 175 °C till the completion of the esterification reaction (water collected approximately 5.6 mL). After completion of the esterification reaction, polycondensation was carried out at 230 °C under vacuum (10 mmHg) to give the corresponding polymer. After completion of the polycondensation reaction, reaction mixture was cooled (80 °C) and then poured into water. The solid thus obtained was filtered, washed with acetone and dried under nitrogen to give the corresponding PBS in good yield (88%). IR (neat):1714, 1157 cm−1; 1H NMR (400 MHz, CDCl3): δ 1.71 (m, 4H), 2.63 (m, 4H), 4.24 (m, 4H); 13C NMR (100 MHz, CDCl3): δ 172.3, 64.2, 29.0, 25.2.
(b) General method for the synthesis of PBS (catalyst addition during polycondensation reaction):
In a clean three neck RB flask fitted with distillation assembly, succinic acid (20 g, 0.17 mol) and butane diol (16 g, 0.18 mol) was taken and heated to 175 °C till the completion of the esterification reaction (water collected approximately 5.6 mL). After completion of the esterification reaction, catalyst (0.02 mol%) was added and the polycondensation reaction was carried out at 230 °C under vacuum (10 mmHg) to give the corresponding polymer. After completion of the polycondensation reaction, reaction mixture was cooled (80 °C) and then poured into water. The solid thus obtained was filtered, washed with acetone and dried under nitrogen to give the corresponding PBS in good yield (92%)
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma00757f |
| This journal is © The Royal Society of Chemistry 2022 |