Open Access Article
Open Access ArticleCrystallized glass tailored by controlled heat treatment for carbon dioxide capture under mild conditions†
Hyung-Ju
Kim
 *a,
Hee-Chul
Yang
*a,
Hee-Chul
Yang
 a,
Keunyoung
Lee
a,
Keunyoung
Lee
 a and
Richard I.
Foster
a and
Richard I.
Foster
 *ab
*ab
aDecommissioning Technology Research Division, Korea Atomic Energy Research Institute, 989-111 Daedeok-daero, Yuseong-gu, Daejeon 34057, Republic of Korea. E-mail: hyungjukim@kaeri.re.kr
bNuclear Research Institute for Future Technology and Policy, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 08826, Republic of Korea. E-mail: rifoster@snu.ac.kr
First published on 25th October 2022
Abstract
Described is the formation of crystallized alkaline earth oxide-containing glass adsorbents for radioactive carbon dioxide (14CO2) sequestering and mineralization under mild operating conditions; thus enabling the long-term geological disposal of hazardous 14C. The best performing crystallized glass adsorbents recorded a max CO2 capacity of 4.54 mmol g−1 and a carbonation reaction rate of 8.04 mmol g−1 h−1.
Radioactive carbon dioxide (14CO2) capture using innovative materials is desirable due to associated radiological hazards,1 and growing climate change.2,3 In recent years, modified activated carbons,4 metal–organic frameworks,5,6 modified 3D graphene,7 covalent organic frameworks,8 and natural minerals9 have been considered as adsorbents for CO2. Mineral carbonation technology (MCT) is amenable to irreversibly capture CO2, for example in the form of natural minerals,9,10 or their mimics.11,12 Typically, MCT is attractive because capturing carbon through the chemical reaction between alkaline earth metal ions and CO2 forms insoluble and significantly stable carbonates.13 However, most applications of MCT have an intrinsic restriction regarding their operational conditions since no forward reaction occurs within realistic time scales.14 Thereby, the CO2 capture performance, such as CO2 capacity and carbonation reaction rate, of MCTs and their applications are severely restricted by the difficulty of operation under mild conditions. For example, natural minerals require aggressive carbonation reaction conditions e.g. high pressure (≥20 bar), high temperature (>373 K), and pH-adjusted carrier solutions.15 To overcome such obstacles, the fabrication of alkaline earth oxides impregnated into an amorphous glass structure have been recently developed.16 They show enhanced rates of dissolution of alkaline earth metal ions and carbonation reaction due to the loosely packed glass structure and the generation of a surface coating silica gel, consequently facilitating CO2 capture under mild conditions.16
Here, we report the synthesis and application of a crystallized glass tailored by controlled heat treatment for CO2 capture under mild conditions. The controlled heat treatment of an alkaline earth oxide-containing glass gives rise to a structural transformation from amorphous to crystalline. The structural characterizations and CO2 capture performance, including CO2 capacity, carbonation reaction rate, and the dissolution rate of alkaline earth metal ion, were analyzed to reveal the impact of controlled heat treatment and phase transformation.
The materials used, fabrication procedures for amorphous glass, its crystallization, characterization, and CO2 capacity measurement are described in detail in the ESI† (I. Materials and methods). An amorphous glass can be converted into the crystalline phase when heat-treated above its crystallization temperature, as demonstrated in Fig. S1 (ESI†). This crystallized glass is often referred to as glass-ceramic.17 As shown in Fig. 1, the X-ray diffraction (XRD) pattern of the crystallized Sr-glass-Na2O, prior to CO2 capture, showed crystallinity including strontium oxide. These crystalline peaks were indexed as strontium metaborate (Joint Committee on Powder Diffraction Standards (JCPDS), SrB2O4: No. 15-0779). This definitive structure was previously reported as orthorhombic symmetry and Pbcn space group with a = 12.0135, b = 4.339, and c = 6.5864 Å.18,19 After CO2 capture, the structure of SrB2O4 was completely converted into the structure of SrCO3 (JCPDS, No. 05-0418), which coincides well with the pattern in Fig. 1 without any remaining peaks from SrB2O4. That is to say, the crystallized Sr-glass-Na2O reacted with CO2 in the aqueous phase to form carbonates, and SrB2O4 is fully converted to SrCO3.
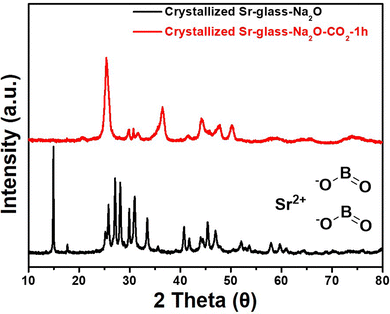 | ||
| Fig. 1 XRD patterns of the crystallized Sr-glass-Na2O (45 μm) prior and subsequent to 1 h of CO2 capture at 298 K. | ||
The Fourier transform infrared attenuated total reflectance (FT-IR/ATR) spectroscopy of the 45 μm-sized crystallized Sr-glass-Na2O prior and subsequent to 1 h of CO2 capture at 298 K was characterized as shown in Fig. 2a. The peaks near 1450 cm−1 (stretching vibration) and 850 cm−1 (bending vibration) visibly appear after CO2 capture. The presence of these peaks indicates the formation of SrCO3; an observation supporting the XRD patterns.20 The C1s peaks near 290 eV in the X-ray photoelectron spectroscopy (XPS) intensified after 1 h of CO2 exposure as shown in Fig. 2b, further confirming the capture and subsequent formation of carbonate.21 Moreover, the N2 physisorption revealed the textural properties of crystallized Sr-glass-Na2O. As shown in N2 adsorption isotherms (Fig. 3), the crystallized Sr-glass-Na2O is absolutely a nonporous material classified by International Union of Pure and Applied Chemistry Type-II adsorption isotherm.22 Subsequent to 1 h of CO2 capture at 298 K, slight porosity is tailored due to the dissolved Sr from the material and newly produced SrCO3. This is even more elucidated when the pore size distribution is analysed by Broekhoff-deBoer–Frenkel–Halsey–Hill method (Fig. S2, ESI†). Relative to the nonporous crystallized Sr-glass-Na2O, the crystallized Sr-glass-Na2O–CO2 displays higher intensity in the mesopore (2–50 nm) range. The textural properties including Brunauer–Emmett–Teller (BET) surface area and pore volume are summarized in Table S1 (ESI†). Prior and subsequent to 1 h of CO2 capture, the BET surface area increases from 1.43 to 51.13 m2 g−1 and pore volume increases from 0.014 to 0.117 cm3 g−1.
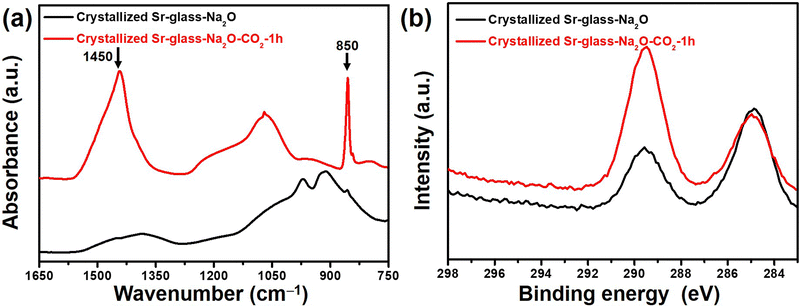 | ||
| Fig. 2 (a) FT-IR/ATR absorption spectra, and (b) XPS C1s spectra of crystallized Sr-glass-Na2O (45 μm) prior and subsequent to 1 h of CO2 capture at 298 K. | ||
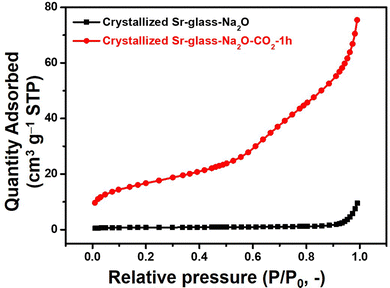 | ||
| Fig. 3 N2 adsorption isotherms at 77 K for crystallized Sr-glass-Na2O (45 μm) prior and subsequent to 1 h of CO2 capture at 298 K. | ||
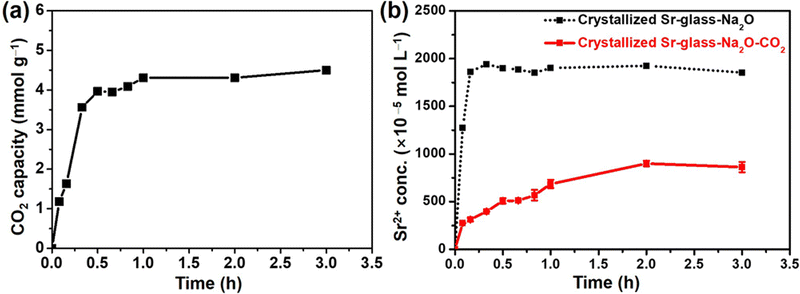
The CO2 capacities of the crystallized Sr-glass-Na2O was analyzed as a function of time. As shown in Fig. 4a, the maximum recorded CO2 capacity was 4.54 mmol g−1 for crystallized Sr-glass-Na2O after 3 h of reaction. Notably, CO2 capacity after 12 h of reaction was exactly the same as that after 3 h based on the thermogravimetric analysis curve (Fig. S3, ESI†) which demonstrates that CO2 capture was completed within 3 h, and the carbonation reaction was under equilibrium during the rest of the time. Crystallized Sr-glass-Na2O had a higher capture efficiency (90.25%) and faster carbonation reaction rate (8.04 mmol g−1 h−1 within 30 min) than our previously reported Sr-glass-Na2O (88.07% and 7.25 mmol g−1 h−1).16 Efficiency calculations were performed as a ratio of the experimental and theoretical CO2 capacities [experimental CO2 capacity/theoretical CO2 capacity × 100]. This improved performance is attributed to the enhanced dissolution of Sr2+ ion from the crystalline structure. Once the crystalline structure formed, most of the Sr2+ ions were present in the crystalline structure. Since most of the Sr2+ ions were gathered in the crystalline phase, they easily reacted on the specific sites and converted to SrCO3. Thus, CO2 capture performance was further improved comparing with amorphous Sr-glass-Na2O which had evenly distributed Sr2+ ions in the structure. To further explain the observations, the Sr2+ dissolution rate, which is a rate-limiting step, was studied by inductively coupled plasma-optical emission spectroscopy analyzing the molar concentration of Sr2+ ions released from the crystallized glass under the conditions where CO2 was either flowing or non-flowing as a function of the time (Fig. 4b). Notably, under the CO2 non-flowing condition (dotted lines), crystallized Sr-glass-Na2O possessed higher solubility than that of amorphous Sr-glass-Na2O described elsewhere,16 thereby almost 4 times higher (2000 × 10−5 mol L−1) saturated concentration of Sr2+ ion. This coincides well with the advanced CO2 capture performances (capacity, reaction rate and efficiency) of crystallized Sr-glass-Na2O. The crystallized glass also exhibited the gradual increase of ion concentrations while CO2 capture showed similar tendency as those of amorphous glass adsorbent, but the reaction rate and efficiency of crystallized Sr-glass-Na2O were much higher than other glass adsorbents because of the enhanced Sr2+ ion solubility in water.
The CO2 capture performance, max CO2 capacity in mmol g−1 and CO2 carbonation rate in mmol g−1 h−1, of newly developed crystallized Sr-glass-Na2O was compared with those of its amorphous glass adsorbent equivalent and other natural minerals under relatively mild CO2 capture conditions reported previously (Table 1). As discussed, crystallized Sr-glass-Na2O outperformed the precursor amorphous framework both in terms of CO2 capacity and CO2 carbonation rate with an almost doubling of performance. Even if natural minerals, such as serpentinite and wollastonite, containing alkaline earth metals (Mg and Ca), exhibited a superior max CO2 capacity than crystallized Sr-glass-Na2O, the aggressive CO2 capture conditions (i.e., energy intensive high temperature as well as acidic or basic reaction media) were required as shown in Table 1. Nonetheless, the crystallized glass adsorbent fabricated in this work showed an extremely high initial CO2 carbonation rate with a decent max CO2 capacity even under moderate and favourable temperature, pressure, and media, where other natural adsorbents are not able to capture CO2. For example, when the CO2 capture was performed under room temperature (298 K) and atmospheric pressure (1 bar), the acidic and basic media were inevitably required to obtain a high CO2 capacity for wollastonite,28–30 and there was no report with such mild conditions when using serpentinite to the best of the authors’ knowledge. In the crystallized Sr-glass-Na2O, alkaline earth metal ions were more easily dissolved and captured CO2 in comparison with natural minerals. This suggests that the appropriate support phase, here crystallized glass, as well as the incorporation of alkaline earth oxides make the highly efficient CO2 capture feasible under mild capture conditions, which is applicable to 14CO2 sequestration.
| Mineral carbonation material | Max CO2 capacity [mmol g−1] | CO2 carbonation rate [mmol g−1 h−1] | Temperature [K] | Media | Ref. |
|---|---|---|---|---|---|
| a CO2 carbonation rate was obtained from the initial 1 h of CO2 capture. | |||||
| Crystallized Sr-glass-Na2O | 4.54 | 8.04a | 298 | Deionized water | This work |
| Sr-glass-Na2O | 4.43 | 4.09a | 298 | Deionized water | 16 |
| Serpentinite | 7.04 | 7.04 | 343 | Orthophosphoric, oxalic, and ethylenediaminetetraacetic acid | 23,24 |
| Serpentinite | 7.58 | 2.53 | 413 | NH4HSO4/NH3 | 25,26 |
| Serpentinite | 2.53 | 0.10 | 598 | HCl/NaOH | 27 |
| Wollastonite | 7 | 2.33 | 298 | NH4OH | 28 |
| Wollastonite | 7.95 | 2.65 | 298 | NH4OH | 29 |
| Wollastonite | 7.95 | 5.30 | 298 | NH4Cl/HCl | 30 |
In conclusion, the fabrication of crystallized glass by restructuring via controlled heat treatment has been demonstrated; thereby implying better glass adsorbent for CO2 capture than other MCT materials. The newly fabricated crystallized Sr-glass-Na2O, also called SrB2O4, exhibits even higher performance in CO2 capacity, carbonation rate, and efficiency due to the enhanced dissolution of alkaline earth metal ion; in this case Sr2+. This development clearly suggests that the crystallized glass can be a technologically more desirable platform for long-term CO2 capture, especially 14CO2 disposal and storage security.
Hyung-Ju Kim: conceptualization, methodology, validation, formal analysis, investigation, writing – original draft, writing – review & editing, visualization. Hee-Chul Yang: resources. Keunyoung Lee: project administration. Richard I. Foster: writing – reviewing and editing.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. NRF-2022M2E9A2053685).References
- M. Lou Dunzik-Gougar and T. E. Smith, J. Nucl. Mater., 2014, 451, 328–335 CrossRef.
- N. Abas and N. Khan, J. CO2 Util., 2014, 8, 39–48 CrossRef CAS.
- P. Bains, P. Psarras and J. Wilcox, Prog. Energy Combust. Sci., 2017, 63, 146–172 CrossRef.
- N. Abuelnoor, A. AlHajaj, M. Khaleel, L. F. Vega and M. R. M. Abu-Zahra, Chemosphere, 2021, 282, 131111 CrossRef CAS PubMed.
- Z. Zhang, Q. Ding, S. B. Peh, D. Zhao, J. Cui, X. Cui and H. Xing, Chem. Commun., 2020, 56, 7726 RSC.
- S. Shyshkanov, T. N. Nguyen, A. Chidambaram, K. C. Stylianou and P. J. Dyson, Chem. Commun., 2019, 55, 10964 RSC.
- Y. Lin, Y. Tian, H. Sun and T. Hagio, Chemosphere, 2021, 270, 129420 CrossRef CAS PubMed.
- Y. Huang, X. Hao, S. Ma, R. Wang and Y. Wang, Chemosphere, 2022, 291, 132795 CrossRef CAS PubMed.
- S. Ó. Snæbjörnsdóttir, B. Sigfússon, C. Marieni, D. Goldberg, S. R. Gislason and E. H. Oelkers, Nat. Rev. Earth Environ., 2020, 1, 90–102 CrossRef.
- A. Sanna, M. Uibu, G. Caramanna, R. Kuusik and M. M. Maroto-Valer, Chem. Soc. Rev., 2014, 43, 8049–8080 RSC.
- I. M. Power, A. L. Harrison and G. M. Dipple, Environ. Sci. Technol., 2016, 50, 2610–2618 CrossRef CAS.
- O. Rahmani, J. CO2 Util., 2020, 35, 265 CrossRef CAS.
- A. A. Olajire, J. Pet. Sci. Eng., 2013, 109, 364–392 CrossRef CAS.
- S. P. Veetil and M. Hitch, Int. J. Environ. Sci. Technol., 2020, 17, 4359–4380 CrossRef CAS.
- S. J. Gerdemann, W. K. O’Connor, D. C. Dahlin, L. R. Penner and H. Rush, Environ. Sci. Technol., 2007, 41, 2587–2593 CrossRef CAS.
- H.-J. Kim, S.-J. Kim, H.-C. Yang, H.-C. Eun, K. Lee and J.-H. Lee, J. CO2 Util., 2022, 61, 102001 CrossRef CAS.
- R. D. Rawlings, J. P. Wu and A. R. Boccaccini, J. Mater. Sci., 2006, 41, 733–761 CrossRef CAS.
- P. D. Dernier, Acta Cryst., 1969, B25, 1001–1003 CrossRef.
- J. B. Kim, K. S. Lee, I. H. Suh, J. H. Lee, J. R. Park and Y. H. Shin, Acta Cryst., 1996, C52, 498–500 CAS.
- A. L. Vasiliu, M. V. Dinu, M. M. Zaharia, D. Peptanariu and M. Mihai, Mater. Chem. Phys., 2021, 272, 125025 CrossRef CAS.
- A. V. Shchukarev and D. V. Korolkov, Cent. Eur. J. Chem., 2004, 2, 347–362 CAS.
- K. S. W. Sing, J. Porous Mater., 1995, 2, 5–8 CrossRef CAS.
- A. H. A. Park and L. S. Fan, Chem. Eng. Sci., 2004, 59, 5241–5247 CrossRef CAS.
- A. H. A. Park, R. Jadhav and L. S. Fan, Can. J. Chem. Eng., 2003, 81, 885–890 CrossRef CAS.
- X. Wang and M. M. Maroto-Valer, ChemSusChem, 2011, 4, 1291–1300 CrossRef CAS.
- X. Wang and M. M. Maroto-Valer, Fuel, 2011, 90, 1229–1237 CrossRef CAS.
- P. C. Lin, C. W. Huang, C. T. Hsiao and H. Teng, Environ. Sci. Technol., 2008, 42, 2748–2752 CrossRef CAS PubMed.
- W. Ding, L. Fu, J. Ouyang and H. Yang, Phys. Chem. Miner., 2014, 41, 489–496 CrossRef CAS.
- W. Ding, H. Yang, J. Ouyang and H. Long, RSC Adv., 2016, 6, 78090–78099 RSC.
- H. Xie, F. Wang, Y. Wang, T. Liu, Y. Wu and B. Liang, Environ. Earth Sci., 2018, 77, 149 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, supporting figures, table. See DOI: https://doi.org/10.1039/d2ma00949h |
| This journal is © The Royal Society of Chemistry 2022 |