A review of 3D printing technology for rapid medical diagnostic tools
Sara
Shakibania
a,
Mehrdad
Khakbiz
 *a,
Cemile Kilic
Bektas
b,
Lida
Ghazanfari
c,
Milad Tavakoli
Banizi
a and
Ki-Bum
Lee
*a,
Cemile Kilic
Bektas
b,
Lida
Ghazanfari
c,
Milad Tavakoli
Banizi
a and
Ki-Bum
Lee
 *b
*b
aDivision of Biomedical Engineering, Faculty of New Sciences and Technologies, University of Tehran, North Kargar Ave., PO Box 14395-1561, Tehran, Iran. E-mail: khakbiz@ut.ac.ir
bDepartment of Chemistry and Chemical Biology Rutgers, The State University of New Jersey, Piscataway, NJ 08854, USA. E-mail: kblee@rutgers.edu
cCenter for Nanotechnology in Drug Delivery, University of North Carolina, Chapel Hill, NC, USA
First published on 8th March 2022
Abstract
Early diagnosis of diseases leads to selecting the appropriate treatment method and prevention of problems, such as drug resistance. It also prevents the spread of diseases and pandemics in some cases and plays a crucial role in treating diseases. In some diseases, such as bacterial infections, effective diagnoses prevent antibiotic overuse and make such antibiotic treatments futile against infections. Additive manufacturing (AM) or 3D printing has received great attention in recent years and has been applied in a wide variety of biomedical applications, such as implants and diagnostic tools. The structures fabricated via this method are highly precise and economical and can have complex geometries, such as interconnecting channels, undercuts, and curvatures. Developing sensors and sensor arrays in a shorter time with high sensitivity is possible by applying AM fabrication approaches. Other fields such as dentistry also take advantage of 3D printing technology to ease the diagnosis process as it helps to fabricate complex structures with dimensions close to reality which cannot be achieved by any other method. In this review, recent advances in AM fabrication methods in producing rapid diagnosis tools have been discussed by providing a classification of advanced diagnostic tools using AM.
Design, System, ApplicationAdditive manufacturing (AM) has attracted growing interest for different industries. This article provides a review of fabrication of biosensors and diagnostic tools via additive manufacturing. This mini-review will provide an applicable classification of diagnostic tools fabricated by different AM methods such as fused deposition modeling, stereolithography, and selective laser melting. The application of bioprinting for designing sensors and different mechanisms such as extrusion, inkjet, and laser-based methods are studied. This mini review summarizes design principles and mechanisms and challenges in additive manufacturing to achieve high-performance sensors. Each of the AM methods results in specific properties and characteristics of the fabricated biosensor. Some parameters including the heat source type, build orientation, thickness of the layers, raster width and angle, air gap, and feed rate, which can affect systematically the properties and characterization of biosensors, are investigated in this paper. |
1. Introduction
Rapid diagnosis of diseases such as cancer,1 infectious diseases,2 and even dental problems plays a crucial role in choosing an appropriate treatment and will prevent serious problems. Diagnostic tests and devices based on the biosensing approach have recently received huge attention. These tools allow for economical and point-of-care testing.3 Different types of biosensors have been developed so far, such as enzyme-based, tissue-based, and immune sensors, DNA biosensors, and thermal and piezoelectric biosensors. Compared to traditional methods, good stability and sensitivity have been achieved by applying these sensors. Biosensors have various applications in the food industry, medical field, marine application, etc.4 Conventional manufacturing methods like spin coating, photolithography and screen-printing, which are mainly based on 2-dimensional fabrication processes, have several restrictions such as detrimental chemical usage, material waste, use of expensive equipment, and the impossibility of using enzymes and cells during the manufacturing process. Therefore, developing a new process to address these problems is needed.5 As a new fabrication method, additive manufacturing has started a revolution in the generation of biosensors and medical diagnostic tools in the recent years. AM, commonly known as 3D printing, allows us to control the internal shape and micro-architecture of the produced sample, which highly improves tissue regeneration and integration and the fabrication of complex structures.6 It has been considered a cost-beneficial and versatile approach to produce a complex medical application structure.7 The physical and mechanical properties of samples can be controlled by employing multiple materials, printing at changing porosities, and applying different internal designs.8 In recent years, 3D-printed electronic, force, motion, hearing, and optical devices have been widely studied. In particular, electronic and force sensing modules are highly investigated for additive manufacturing, and the rest of the sensor categories are fabricated by incorporating commercial components into 3D-printed constructions. Substrate boards, electronic ink, and printing process techniques are the main components of the 3D-printed sensors.9 In this review, advancements in the fabrication of biosensors and diagnostic tools via additive manufacturing have been discussed. This paper aims to efficiently classify diagnostic tools fabricated via fused deposition modeling, stereolithography, and selective laser melting. The outline of additive manufacturing is presented in Table 1.| Technology | Vat photopolymerization | Material extrusion | Powder bed fusion (PBF) |
|---|---|---|---|
| Method | Stereolithography | FDM/ FFF | SLS/SLM |
| Resolution (μm) | 50–200 (ref. 10) | 100–400 (ref. 11) | 50–100 (ref. 11) |
| Layer thickness (μm) | <10 μm (ref. 12) | From 100 to 250 μm (ref. 12) | From 25 to 100 μm (ref. 12) |
| Principle | Photo-polymerisation | Melt extrusion | Powder sintering |
| Materials | Photo-curable polymers13/composites/cells10 | Polymers, ceramics, metals13/composites/cells10 | Polymers, metals, ceramics13/composites10 |
| Advantages | Highly accurate | Cost-efficient14 | Highly accurate14 |
| Appropriate surface finishing14 | Facile multi-material printing14 | Acceptable strength and stiffness14 | |
| High resolution15 | |||
| Widely commercially available | |||
| Potential to process both amorphous and crystalline polymers15 | |||
| Disadvantages | Time consuming due to curing and refill intervals | Poor surface finishing14 or mechanical properties compared to injection molded parts15 | Slow building process14 |
| Poor mechanical properties15 | Limitation in size14 | ||
| Post-processing treatments needed (surface polishing, heat treatments)15 |
1.1. Why biosensing based methods
Biosensors are small devices that convert a biological reaction into measurable electrical signals proportional to the analyte's concentration.4 A biosensor typically comprises a biorecognition element, a transducer, and a signal amplifier. The biorecognition element is considered the most critical part as it determines the success of biosensing.16 It detects the analyte (e.g., nucleic acids, antibodies, ions, or enzymes17) via a reaction, specific adsorption, or other processes such as physical/chemical interactions.18 Then, the transducer converts the detected analyte to a quantifiable signal.19 So far, various types of materials have been developed for diagnostic sensors such as metallic (particularly gold) and carbon materials, carbon-based hybrids, boron-doped diamond (BDD), and paper in paper-based devices.20 Biosensors have been used in many fields, including medicine, the food industry, and marine sector, and their usage is rapidly expanding4 For example, the diagnosis of infectious diseases is one of the areas where biosensors are widely employed. Pathogens or the response of the host to pathogens plays a key role in the diagnosis of infectious diseases. Besides biosensors, the most used approaches to diagnose infectious diseases in laboratories are protein-based assays like ELISA and serology, microscopy techniques such as pathological, histological, and morphological assays, mass spectrometry, and molecular diagnostics (including quantitative (q) PCR and sequencing). Generally, these methods involve an in vitro culture and an isolation stage. For example, hepatitis diseases can be detected by a wide variety of methods such as enzyme-linked immunosorbent assay (ELISA), chemical-based methods, real-time polymerase chain reaction (PCR), etc.21 These techniques require a considerable amount of time and are highly sensitive to sample preparation. Technical restrictions due to limitations of testing a specific cell or area in a systemic sample or not having a perceptible amount of the pathogen needed for detection may result in a false answer.22 Furthermore, these techniques are both costly and complicated and entirely dependent on expert skills.21 Therefore, cost and design-efficient bio-sensing-based methods have been developed to address these limitations. Biosensors can also quantify non-polar molecules, which cannot be diagnosed via any other instrument. Relying on detection mechanisms, different biosensors such as optical, electrochemical, thermal, ion-selective, magnetic, and acoustic biosensors were developed.21 Detection of biomarkers to diagnose cardiovascular diseases is one of the examples of the medical applications of biosensors. Cardiovascular diseases have been considered the major cause of mortality in the recent years. A biosensor based on biomarker detection has been developed for this purpose. Myoglobin, interleukin-1, interleukin-6, tumor necrosis factor-alpha, low-density lipoprotein, lipoprotein-associated phospholipase, troponin I or T, C-reactive protein, and myeloperoxidase are examples of cardiac biomarkers.23 Although blood is the most widely used biological fluid for diagnostic applications, its collection and sampling is both invasive and painful.24,25 Another example of the medical application of biosensors is flexible biosensors attached to the skin (Fig. 1). These sensors are capable of quick detection of different biomarkers in body fluids such as sweat and tears, which can be an effective non-invasive alternative.26 Various types of physiological metabolites such as glucose, lactate, cortisol, and other small ions can be detected in sweat and tears.27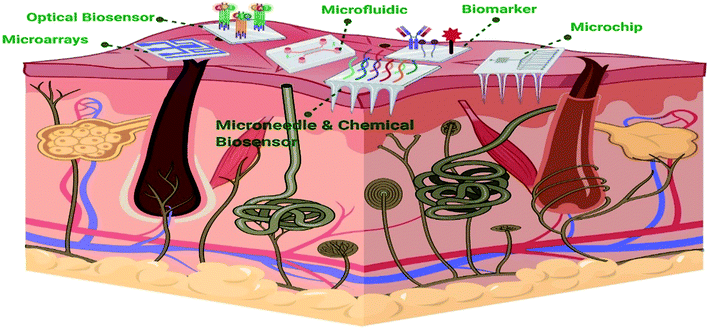 | ||
| Fig. 1 Schematic representation of different types of flexible biosensors on the skin (created with BioRender). | ||
These devices can be directly attached to the skin and report accurate and real-time measurements of biomarkers.28 These biosensors can be fabricated by different techniques such as inkjet printing, screen printing, and lift-off lithography.29
2. Additive manufacturing
Additive manufacturing (AM), namely 3D printing, is a technique to create three-dimensional (3D) objects layer by layer using computer-aided design (CAD) data. AM was developed 20 years ago for creating prototypes and models and has turned into a popular fabrication method owing to its various advantages such as availability of 3D design software, unique design freedom, ease of use, low cost, and short processing times.30The AM technique can produce structures that are either patient-specific or hard to fabricate using other methods.31 Compared to the conventional manufacturing processes such as injection molding, AM is cost-efficient as it eliminates extra tooling and re-fixturing and does not require a skilled operator, or even a long fabrication time. Complex geometric shapes can be designed and fabricated via AM technology with no additional cost, while in the conventional methods, for the more complex geometric shapes, more expensive molds are required.32 Since traditional manufacturing processes, such as injection molding, have high start-up costs, they are better suited for mass production, whereas AM is cost and time-effective for low part numbers because no startup tooling is required. Furthermore, the amount of wasted material in the AM process is remarkably low.32 As the AM manufacturing process involves a digital environment and samples are designed in digital files that can easily be shared or altered, time bottlenecks are eliminated. Another advantage of AM over other approaches is that it reduces risks associated with the workplace.14 Hence, considering the capability of fabricating samples with high geometric complexity, low wasted material, shorter time to market,14 and better efficiency of supply chains,14 AM technology is cost-efficient.32
Recent studies have concentrated on developing more cost-efficient and less time-consuming approaches with higher sensitivity. For example, in many ELISA systems, microplates of various capacities and sizes are used for antibody immobilization, necessitating a significant amount of time for incubation and washing processes. Limited surface area to immobilize antibodies is one of the challenges which restricts the use of ELISA for low-cost diagnostics.33,34 The 3D printing approach can be used to create microwells with a greater surface area, which improves the performance of microplate ELISAs. Sharafeldin et al.34 developed ELISA in 3D-printed pipet tips. The required time for the assay decreased due to the high surface area. Moreover, the roughness of the 3D-printed surface resulted in a 15–50 times higher antibody loading capacity of the surface. This increase in loading capacity lowered the required time for the assay while the sensitivity was similar to that of conventional ELISA. Moreover, 3D printing can be a cost-efficient approach for fabrication of diagnostic tools such as ELISA. Bauer et al.35 developed a 3D-printed ELISA device for detection of malaria and compared the required cost to that of different malaria-detection platforms including other 3D-printed ELISA devices, rapid diagnostic tests, and PCR. The 3D-printed ELISA device cost less than $10 depending on the reagent and printing costs while this number for rapid diagnostic tests was around $5 and, for the PCR method, could increase to $25.
2.1. Additive manufacturing techniques and process
AM comprises various approaches and technologies, including material extrusion, material jetting, powder bed fusion, directed energy deposition, binder jetting, sheet lamination, and vat photopolymerization.36,37 In all techniques, the sample is fabricated in a layer-by-layer manner.9 The selection of the AM technique depends on various factors, such as the nature of the material, chemical composition, optical character, and strength. The most common techniques applied in laboratories are particularly FDM, SLA, poly jet, and SLM printing (Fig. 2).38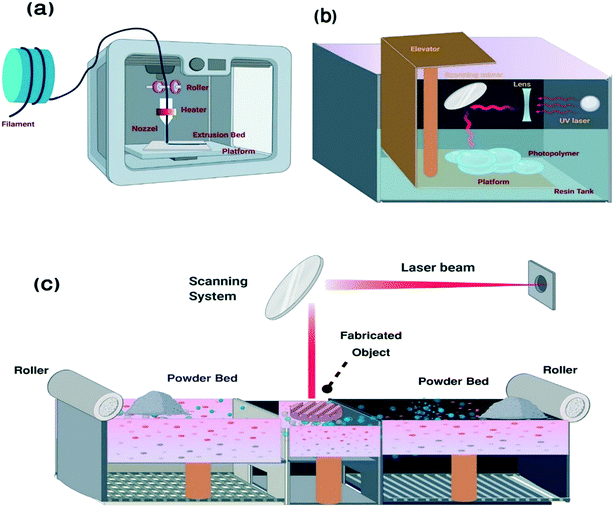 | ||
| Fig. 2 Schematic representation of AM fabrication processes: (a) fused deposition modeling, (b) stereolithography and (c) selective laser melting48 (created with BioRender). | ||
The AM procedure consists of eight general steps: 1. conceptualization and creating a CAD model. 2. Turning to STL format. 3. Conveying to the AM device and STL file manipulation. 4. Setting up the system and equipment. 5. Fabricating the sample. 6. Withdrawal and cleaning the built part. 7. Post-treatment of the fabricated samples. 8. Application.39
Sensors fabricated via AM can be highly sensitive.42 Singh et al.33 proposed a 3D printed prototype design to improve the diagnostic performance of ELISA and achieved a 2.25-fold higher sensitivity. This higher sensitivity was attributed to the larger reaction surface area in the 3D-printed samples. In another study, Guo et al.43 developed a helical structure as a multifunctional 3D liquid sensor in which the structural feature of the printed sample resulted in excellent sensitivity and selectivity as it was capable of trapping more liquid components. Petroni et al.44 fabricated an electrochemical sensor based on a graphite/acrylonitrile butadiene styrene conductive composite. The outcomes indicated better analytical performance compared to commercial carbon black/PLA conductive filaments. This is due to the production technique, which allowed for the insertion of greater amounts of conductive material in the matrix.
The performance of the sensor (such as gauge factor and linearity) can be controlled by printing parameters during the fabrication process.45 These parameters are the printing-line directions,46 needle diameter,47 ratio of components in the composites,45 and printing speed.45 Abshirini et al.47 fabricated highly flexible strain sensors by extrusion-based 3D printing. This sensor was constructed from multi-walled carbon nanotubes (MWNTs) and polydimethylsiloxane (PDMS). The influence of the needle diameter and MWNT concentrations on sensor performance was investigated. The piezoresistive sensitivity was improved when the diameter of the needle was reduced. Because the needle diameter can potentially modify the shear flow generated during the printing process, the MWNT distributions and alignment were altered as a result. Therefore the piezoresistive sensing performance of these sensors was different. Furthermore, by decreasing the amount of the MWNTs, the piezoresistive sensitivity of the printed nanocomposites was enhanced. The piezoresistive sensing mechanism depends on the MWNT network reorganization under external load and an appropriate amount of MWNTs resulted in an effective connection of MWNTs which consequently led to higher sensitivity to external loads. Vu et al.46 studied the effects of the printing-line directions (45°, 90°, 180°) on the performance of a strain sensor fabricated via the FDM method. The results showed that all three samples had acceptable performance in terms of sensitivity (GF) with the sample printed at 45° exhibiting the highest GF among the samples. The effect of other parameters such printing speed has been also studied. A change in the printing speed altered the line width of the 3D-printed sensor.45
2.1.1.1. Diagnostic tools fabricated via fused deposition modeling. Fused deposition modeling (FDM) is a 3D printing technology that uses the melt extrusion method to deposit extruded thermoplastic filaments into individual layers according to a specific pattern (Fig. 2a). FDM is a complicated process. Many parameters are involved in determining the quality of the final product and understanding how these parameters interact is typically difficult. The orientation of the structure, the layer thickness, the raster angle and width, the air gap, the infill density and design, and the feed rate are all considered critical aspects in this approach.49
In 2017, Gaal et al.50 used FDM techniques to fabricate biosensors composed of integrated, sealed and transparent polylactic acid (PLA) microchannels. The highlighted features of this construct were its appropriate transparency and reasonable price, the availability of raw material (PLA), the printing of microchannels without destroying the structures, and also the ease of combining other materials during the process. By way of illustration, pliable interdigitated electrodes were placed in a microfluidic e-tongue that could detect the basic tastes below the human threshold. Microfluidic devices is consist of polydimethylsiloxane (PDMS) because of its optical transparency, chemical inertness, non-toxicity, and gas permeability. However, microfluidic device production using PDMS has limitations, such as the cost, handling, and additional step requirements. Therefore, 3D printing to fabricate microfluidic biosensing devices enables the use of a wide range of materials and produces complex structures by avoiding multi-step processing. In 2018, Palenzuela et al.51 developed highly sensitive graphene-based electrodes for electrochemical sensing using the FDM method. They 3D-printed ring- and disc-shaped electrodes and used different redox probes (ferrocene monocarboxylic acid, K3Fe(CN)6:K4Fe(CN)6, ascorbic acid, FeCl3, and Ru(NH3)6Cl3) to study the electrochemical performance of the probes. They reported increased electroactivity by a simple activation protocol, which includes DMF-assisted limited dissolution of the insulating polymer polylactic acid. Marzo et al. (2020)52 also employed graphene and PLA to develop an enzymatic biosensor using the FDM approach in another study. The biosensors were produced by horseradish peroxidase (HRP) immobilization to create electrostatic interactions for H2O2 detection, and their results showed that the direct electron transfer of immobilized HRP was highly efficient. They further modified the biosensor by applying gold nanoparticles (AuNPs) to facilitate heterogeneous electron transfer and reported an enhanced biosensor performance. In 2020, Cardoso et al.53 developed other graphene–PLA (G–PLA) based amperometric biosensors for detecting glucose in biological fluids. The glucose level was measured using glucose oxidase and ferrocene-carboxylic acid (FCA) at a 15 μmol L−1 detection limit. They could also modify the surface of the same system (by solvent immersion and mechanical polishing) to detect nitric acid and uric acid to analyze saliva and urine. The G–PLA sensors developed via the FDM approach are flexible, biodegradable, and biocompatible. Furthermore, these types of biosensors can be fabricated on a large scale with various dimensions at a low cost. FDM techniques can also be used for disease/injury diagnosis purposes. Frizziero et al. (2019)54 reported the use of computed axial tomography (CAT) data which are converted into 3D-printed models, and these models are used to characterize the anatomical structure of fractures and lesions to provide a complete pre-surgery evaluation.
Aerosol jet printing (AJP) is a type of direct-write printing working in a contactless manner by using a directed aerosol stream where the polymer is deposited on the substrate at 1–5 mm offsets. AJP can fabricate fine features on complex substrates that generally cannot be reached by any physical nozzles and can be used in diverse applications, such as fabricating active and passive electronic components, actuators, and sensors.55 In 2016, Yang et al.56 developed silver microelectrode arrays (MEAs) using AJP techniques at a 15 μm resolution. The developed sensor was successfully applied to detecting hydrogen peroxide and glucose levels as model analytes to illustrate the system's performance. This study shows the potential of AJP as a fabrication tool for custom-shaped low-cost microelectrode arrays for a wide range of biosensor applications, including touch sensing, bio-sensing, and strain sensing. In 2018, Zachariah et al.57 reported the use of AJP to develop flexible hybrid electronics (FHE) that are wearable, comforting the human body, and light. For this purpose, they employed a silver nanoparticle (AgNP)-based ink and reported that the produced electronics could extend over 10 times their primary length without losing conductivity.58
2.1.1.2. Diagnostic tools fabricated via stereolithography. Like most 3D printing techniques, stereolithography relies on the additive fabrication process of CAD files that describe the size and geometry of the model. First, an STL file format of the model is developed and then sliced (virtually) into layers to enable layer-by-layer fabrication at high resolution (50–200 μm). The stereolithography apparatus (SLA) produces 3D objects based on the spatially controlled solidification of the liquid resin through photopolymerization.10 It is both efficient and economical in design.59Fig. 2b shows a schematic representation of stereolithography.
In 2019, Kuo et al.60 developed a microfluidic device based on a stereolithography approach using low molecular weight poly(ethylene glycol) diacrylate (MW = 258) at sub-millimeter resolution. They reported the production of complex 3D microfluidic devices such as an active micro-mixer with pneumatic micro-valves and microchannels with a high aspect ratio (37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and this resolution is not available in any other conventional rapid prototyping methods. These types of complex microfluidic devices can be applied to many different research areas, including patch-clamp chips, biosensors, organ-on-a-chip, and tumor-on-a-chip.
1), and this resolution is not available in any other conventional rapid prototyping methods. These types of complex microfluidic devices can be applied to many different research areas, including patch-clamp chips, biosensors, organ-on-a-chip, and tumor-on-a-chip.
Miller et al.61 (2011) investigated inorganic–organic hybrid microneedle-shaped materials for transdermal biosensor applications using micro-mirror device-based stereolithography instruments. The sensing mechanisms are placed in the perforation of the microneedles, and the carbon fiber electrodes are located within the hollow microneedle array created by the lithography instrument (Fig. 3). Their studies showed that the microneedles were intact after puncturing into cadaver skin. The performance of the developed ion-selective electrodes was evaluated by chemically modifying the carbon fibers to allow the detection of molecules, such as ascorbic acid and hydrogen peroxide, and measuring the current electrochemically.
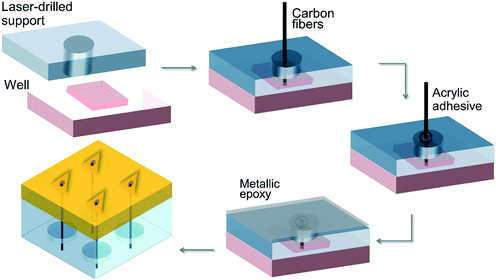 | ||
| Fig. 3 Schematic illustration of the hybrid microneedle developed by Miller et al.61 | ||
In the same year, Narayanan et al.62 fabricated a dual-mode electrochemical biosensor using the SLA technique to diagnose glucose and H2O2. The developed structure was made of tungsten coated with gold nanoparticles (AuNPs) and gold micro-wire electrodes coated with colloidal platinum (colloidal-Pt). AuNPs and colloidal-Pt acted as a support matrix to immobilize the horseradish peroxidase (HRP) and the non-enzymatic glucose biosensor, respectively. This platform was capable of identifying both glucose (with a linear range of 0.5 mM to 8 mM) and H2O2 (linearity up to 70 μM) simultaneously. This product can be considered a potential device for real-time identification of glucose and H2O2 in clinical, biological, and environmental applications.
2.1.1.3. Diagnostic tools fabricated via selective laser melting. In the selective laser melting (SLM) process or direct selective laser sintering (SLS), metallic powders are melted and fused via a high power-density laser at a high resolution (10–100 μm). The SLM process consists of the same series of steps as those in other printing techniques: obtaining CAD data, exporting the data in STL format, slicing the model into layers, and 3D printing. The 3D printing process begins with laying a thin metal powder layer on the building plate and continues with high energy63 (Fig. 2c). In the metal AM process, laser and electron beams are the most used heat sources to fuse the metal powders to the underlying layer after selectively melting in the bed. Electron beam-based approaches displayed a much higher power density and faster melting rate than laser-based sources.64
SLM is a very suitable approach in the medical and dental areas as it allows the production of complex geometries and individualized models. Moreover, multiple parts can be fabricated in a single run, enabling mass production.65 In 2007, Vandenbroucke et al.65 investigated the effect of the SLM parameters (material, surface post-treatment, the thickness of layers, the angle of slope, and the variance between the upper and lower surfaces) on two biocompatible metal alloys: Ti–6Al–4V and Co–Cr–Mo to be used as a dental prostheses. The results confirmed that optimized SLM factors resulted in achieving a part density of up to 99.98% for titanium. The printed parts were shown to have appropriate strength and stiffness, corrosion behavior, and process precision for medical or dental applications.
Kwon et al.66 used the SLS approach to fabricate copper nanoparticle thin films onto a polymer substrate and obtained a flexible, conductive, and transparent material. The method demonstrated that Cu, which normally suffers from severe oxidation, can be sintered rapidly at low annealing temperatures with significant oxidation suppression. Their results suggest that copper-based flexible electronics can be produced onto plastic substrates using the SLS technique.
Table 2 shows a summary of developed diagnosis tools via additive manufacturing.
| Year | Scientist | Material | Method | Application | Reference |
|---|---|---|---|---|---|
| 2007 | Vandenbroucke | Ti–6Al–4V/Co–Cr–Mo | SLM | Medical application | 65 |
| 2017 | Gaal | PLA | FDM | e-Tongue | 50 |
| 2017 | Arango | Metal and metal-oxide ink | — | Engineered inks for AM | 67 |
| 2018 | Zacharian | Silver-based inks | 3DP | Flexible electronic substrates | 57 |
| 2018 | Palenzuela | PLA/G | FDM | Electrochemical sensor | 51 |
| 2019 | Frizziero | — | FDM | Orthopedic device | 54 |
| 2019 | Kuo | PEG | SL | Microfluidic device | 60 |
| 2011 | Miller | Inorganic–organic hybrid materials | SL | Transdermal bio sensor | 61 |
| 2019 | Narayanan | Au NPs/W/colloidal Pt | SL | Electrochemical bio sensor | 62 |
| 2020 | Marzo | PLA/G | FDM | Enzymatic biosensor | 52 |
| 2020 | Cardosoa | PLA/G | FDM | Electrodes | 53 |
| 2021 | Kwon | Cu nanoparticles/polyethylene naphthalate | SLS | Flexible touch panel applications | 66 |
2.1.1.4. Bioprinting. The unique 3D printing fabrication approach, namely 3D bioprinting, has been recently developed in the field of tissue engineering and regenerative medicine, which is a promising substitute for scaffold-based approaches.68 3D bioprinting is a layer-by-layer fabrication process capable of precisely positioning cells, biological materials, and biochemicals.69Fig. 4 shows the steps of the bioprinting process. The shape, size, internal porosity, and interconnectivity of the fabricated samples can be controlled by the 3D bioprinting technique. Homogeneous pore size and controlled interconnectivity form ideal cell–cell and cell–matrix interaction which affects cell adhesion, proliferation, and differentiation.70 In the 3D bioprinting approach, bio-ink simulates the target tissue extracellular matrix to provide a physiologically similar environment for cell proliferation and differentiation. Extracellular matrix (ECM)-based materials mimic cellular patterns in terms of composition and structure. Decellularized ECM biomaterials are also frequently used as bio-ink to take advantage of the natural cues of the native ECM.71 The common bioprinting techniques include inkjet printing, extrusion-based printing, laser-assisted printing, and stereolithography.69 Besides, special bioprinting technologies have been also presented which are designed for fixed-point deposition of macromolecules like DNA, polycose, and cytokines.72
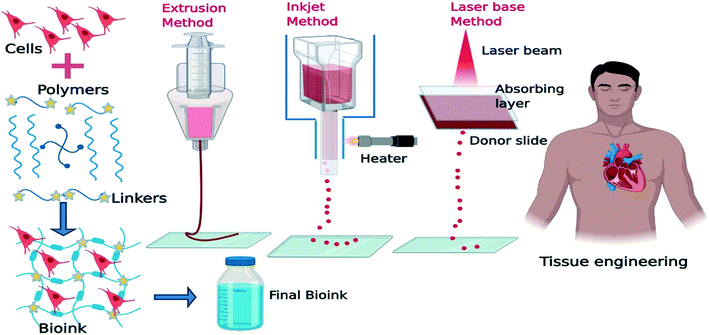 | ||
| Fig. 4 Schematic illustration of the bio-printing process in tissue engineering applications (created with BioRender). | ||
2.1.1.4.1. Bioprinting and biosensors. The basis for the development of applying living cells as bioreceptors is their capability of expressing various molecules (receptors) in various amounts. The cells can provide a quantitative response to a particular activator in a specific condition, and they can analyze over one analyte quantitatively. The development of cell-based probes provides a rapid and facile method to diagnose species that were not determined via electrochemical methods.73 Laser direct writing (LDW) and inkjet printing are promising 3D bioprinting approaches to achieve patterning of surfaces using non-contact deposition methods. This technique allows direct patterning of cells and materials without the need of specific binding chemistry. Patterning provides unique features to biosensors, such as allowing the placement of specific analytes, cells, and materials in defined areas to test specific stimuli simultaneously. 3D bioprinting, therefore, enables rapid screening of multiple analytes in a high throughput manner for diagnostic purposes.74 Some other features, such as immobilizing thin films of metal nanoparticles or nanowires on a substrate, have been achieved via some printing methods, like electrodeposition. Using the electrodeposition approach, thin films of biological materials such as bacterial cells, enzymes, proteins, polysaccharides, and nucleic acids can also be printed.74 The cell-based biosensors can investigate and track the interactions of drug–ligand complexes, environmental toxicity, bioactive agent impact, etc. A wide variety of cells can be applied in biosensor fabrication, including bacteria, yeast, fungi, algae, and eukaryotes such as fish, rat, and human cells. Microbial cells, like bacteria, fungi, yeast, and algae, have been widely applied to evaluate water quality and toxicity.75 Cui et al.76 demonstrated myotube formation of C2C12 cells when bioprinted onto micro-sized cantilevers at a 300 dpi (85 μm) resolution using a thermal inkjet printer. They reported that the printed cells fused with each other and successfully formed myotubes in 4 days compared to 14 days for randomly deposited cells. The myotubes were shown to respond to electrical stimulation. Chemical stimulation responses were also achieved upon integrating a BIO-MEMS device which demonstrates the feasibility of the developed system as a functional biosensor. In another study, Jiang et al.77 developed a biomimetic ‘intestinal microvillus’ biosensor using a stereolithography 3D bioprinting approach to detect food allergens such as wheat gliadin. For this purpose, a conductive GelMA bioink was prepared to mix with flower-like copper oxide nanoparticles and hydrazide-functionalized multi-walled carbon nanotubes. After the bioprinting process, basophilic leukemia cells were immobilized onto the structure, and wheat gliadin was sensitively detected at a 0.1–0.8 ng mL−1 linear detection range with a 0.036 ng mL−1 detection limit showing the stability and the reproducibility of the 3D bioprinting technology.
3. Conclusion
The stage of diagnosis is an essential part of treatment, and the development of diagnostic tools is highly crucial. Additive manufacturing has been recognized as an efficient approach in manufacturing diagnostic tools that are readily available, cheap, sensitive, multifunctional, and miniaturized. 3D printing technology offers many approaches, such as FDM, SLA, polyjet, and SLM printing. Each printing technique yields a unique product as the parameters such as the build orientation, thickness of the layers, raster width and angle, air gap, and feed rate change from one method to another. 3D printing enables the development of multifunctional diagnostic devices that can perform several functions simultaneously and more complex tools yet to be developed in the near future that cannot be readily produced with average bioanalytical tools without 3D printers.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge technical support and valuable assistance of the Institutes for NanoBiomedical Research, Rutgers University. We are especially grateful to anonymous reviewers for their valuable and constructive comments.References
- P. Brocken, J. B. Prins, P. R. Dekhuijzen and H. F. van der Heijden, Psycho-Oncology, 2012, 21, 1–10 CrossRef CAS PubMed.
- A. C. Ghani, D. H. Burgess, A. Reynolds and C. Rousseau, Nature, 2015, 528, S50–S52 CrossRef PubMed.
- F. S. R. R. Teles and P. D. T. Tavira, Crit. Rev. Clin. Lab. Sci., 2010, 47, 139–169 CrossRef CAS PubMed.
- P. Mehrotra, J. Oral Biol. Craniofac. Res., 2016, 6, 153–159 CrossRef PubMed.
- S. Nesaei, Y. Song, Y. Wang, X. Ruan, D. Du, A. Gozen and Y. Lin, Anal. Chim. Acta, 2018, 1043, 142–149 CrossRef CAS PubMed.
- A. A. Zadpoor and J. Malda, Ann. Biomed. Eng., 2017, 45, 1–11 CrossRef PubMed.
- C. Li, D. Pisignano, Y. Zhao and J. Xue, Engineering, 2020, 6, 1222–1231 CrossRef CAS.
- M. Padash, C. Enz and S. Carrara, Sensors, 2020, 20, 4236 CrossRef CAS PubMed.
- Y. Xu, X. Wu, X. Guo, B. Kong, M. Zhang, X. Qian, S. Mi and W. Sun, Sensors, 2017, 17, 1166 CrossRef PubMed.
- F. P. Melchels, J. Feijen and D. W. Grijpma, Biomaterials, 2010, 31, 6121–6130 CrossRef CAS PubMed.
- C. Schmidleithner and D. M. Kalaskar, Stereolithography, in 3D Printing, IntechOpen, 2018 Search PubMed.
- J. Gardan, Int. J. Prod. Res., 2016, 54, 3118–3132 CrossRef.
- M. Vaezi, S. Chianrabutra, B. Mellor and S. Yang, Virtual Phys. Prototyp., 2013, 8, 19–50 CrossRef.
- H. Choudhary, D. Vaithiyanathan and H. Kumar, MAPAN, 2021, 36, 405–422 CrossRef.
- A. Al Rashid, S. A. Khan, S. G. Al-Ghamdi and M. Koç, J. Mater. Res. Technol., 2021, 14, 910–941 CrossRef CAS.
- S. Pillai, A. Upadhyay, D. Sayson, B. H. Nguyen and S. D. Tran, Molecules, 2022, 27, 165 CrossRef CAS PubMed.
- X. Yang and H. Cheng, Micromachines, 2020, 11, 243 CrossRef PubMed.
- Y. Saylan, Ö. Erdem, S. Ünal and A. Denizli, Biosensors, 2019, 9, 65 CrossRef CAS PubMed.
- N. Verma and A. Bhardwaj, Appl. Biochem. Biotechnol., 2015, 175, 3093–3119 CrossRef CAS PubMed.
- K. Nemčeková and J. Labuda, Mater. Sci. Eng., C, 2021, 120, 111751 CrossRef PubMed.
- J. Soleymani, M. Hasanzadeh, M. H. Somi and A. Jouyban, TrAC, Trends Anal. Chem., 2018, 107, 169–180 CrossRef CAS.
- L. Tribolet, E. Kerr, C. Cowled, A. G. Bean, C. R. Stewart, M. Dearnley and R. J. Farr, Front. Microbiol., 2020, 11, 1197 CrossRef PubMed.
- N. K. Bakirhan, G. Ozcelikay and S. A. Ozkan, J. Pharm. Biomed. Anal., 2018, 159, 406–424 CrossRef CAS PubMed.
- C. Lim, Y. Lee and L. Kulinsky, Micromachines, 2018, 9, 502 CrossRef PubMed.
- Y. Yang and W. Gao, Chem. Soc. Rev., 2019, 48, 1465–1491 RSC.
- N. P. Shetti, A. Mishra, S. Basu, R. J. Mascarenhas, R. R. Kakarla and T. M. Aminabhavi, ACS Biomater. Sci. Eng., 2020, 6, 1823–1835 CrossRef CAS PubMed.
- K. Mitsubayashi, M. Suzuki, E. Tamiya and I. Karube, Anal. Chim. Acta, 1994, 289, 27–34 CrossRef CAS.
- P. Liu, Y. Zhu, S. H. Lee and M. Yun, Biomed. Microdevices, 2016, 18, 1–8 CrossRef CAS PubMed.
- M. Xu, D. Obodo and V. K. Yadavalli, Biosens. Bioelectron., 2019, 124, 96–114 CrossRef PubMed.
- D. Herzog, V. Seyda, E. Wycisk and C. Emmelmann, Acta Mater., 2016, 117, 371–392 CrossRef CAS.
- S. Bose, D. Ke, H. Sahasrabudhe and A. Bandyopadhyay, Prog. Mater. Sci., 2018, 93, 45–111 CrossRef PubMed.
- W. Gao, Y. Zhang, D. Ramanujan, K. Ramani, Y. Chen, C. B. Williams, C. C. Wang, Y. C. Shin, S. Zhang and P. D. Zavattieri, Comput. Aided Des., 2015, 69, 65–89 CrossRef.
- H. Singh, M. Shimojima, T. Shiratori, L. Van An, M. Sugamata and M. Yang, Sensors, 2015, 15, 16503–16515 CrossRef CAS PubMed.
- M. Sharafeldin, K. Kadimisetty, K. R. Bhalerao, I. Bist, A. Jones, T. Chen, N. H. Lee and J. F. Rusling, Anal. Chem., 2019, 91, 7394–7402 CrossRef PubMed.
- M. Bauer and L. Kulinsky, Micromachines, 2018, 9, 27 CrossRef PubMed.
- Standard Terminology for Additive Manufacturing Technologies, ASTM Standard, 2012.
- S. Shakibania, L. Ghazanfari, M. Raeeszadeh-Sarmazdeh and M. Khakbiz, Drug Dev. Ind. Pharm., 2021, 47, 521–534 CrossRef CAS PubMed.
- C. L. M. Palenzuela and M. Pumera, TrAC, Trends Anal. Chem., 2018, 103, 110–118 CrossRef.
- S. K. Parupelli, PhD, North Carolina Agricultural and Technical State University, 2016 Search PubMed.
- M. Sharafeldin, A. Jones and J. F. Rusling, Micromachines, 2018, 9, 394 CrossRef PubMed.
- J. Muñoz and M. Pumera, TrAC, Trends Anal. Chem., 2020, 128, 115933 CrossRef.
- Y. Ni, R. Ji, K. Long, T. Bu, K. Chen and S. Zhuang, Appl. Spectrosc. Rev., 2017, 52, 623–652 CrossRef CAS.
- S.-z. Guo, X. Yang, M.-C. Heuzey and D. Therriault, Nanoscale, 2015, 7, 6451–6456 RSC.
- J. M. Petroni, M. M. Neves, N. C. de Moraes, R. A. B. da Silva, V. S. Ferreira and B. G. Lucca, Anal. Chim. Acta, 2021, 1167, 338566 CrossRef CAS PubMed.
- M. R. Khosravani and T. Reinicke, Sens. Actuators, A, 2020, 305, 111916 CrossRef CAS.
- C. C. Vu, T. T. Nguyen, S. Kim and J. Kim, Materials, 2021, 14, 1791 CrossRef CAS PubMed.
- M. Abshirini, M. Charara, P. Marashizadeh, M. C. Saha, M. C. Altan and Y. Liu, Appl. Nanosci., 2019, 9, 2071–2083 CrossRef CAS.
- Q. Ge, A. H. Sakhaei, H. Lee, C. K. Dunn, N. X. Fang and M. L. Dunn, Sci. Rep., 2016, 6, 1–11 CrossRef PubMed.
- J. Chacón, M. A. Caminero, E. García-Plaza and P. J. Núnez, Mater. Des., 2017, 124, 143–157 CrossRef.
- G. Gaal, M. Mendes, T. P. de Almeida, M. H. Piazzetta, Â. L. Gobbi, A. Riul Jr and V. Rodrigues, Sens. Actuators, B, 2017, 242, 35–40 CrossRef CAS.
- C. L. Manzanares Palenzuela, F. Novotný, P. Krupička, Z. k. Sofer and M. Pumera, Anal. Chem., 2018, 90, 5753–5757 CrossRef CAS PubMed.
- A. M. L. Marzo, C. C. Mayorga-Martinez and M. Pumera, Biosens. Bioelectron., 2020, 151, 111980 CrossRef PubMed.
- R. M. Cardoso, P. R. Silva, A. P. Lima, D. P. Rocha, T. C. Oliveira, T. M. do Prado, E. L. Fava, O. Fatibello-Filho, E. M. Richter and R. A. Munoz, Sens. Actuators, B, 2020, 307, 127621 CrossRef.
- L. Frizziero, A. Liverani, G. Donnici, F. Osti, M. Neri, E. Maredi, G. Trisolino and S. Stilli, Symmetry, 2019, 11, 542 CrossRef.
- N. Wilkinson, M. Smith, R. Kay and R. Harris, Int. J. Adv. Manuf. Technol., 2019, 105, 4599–4619 CrossRef.
- H. Yang, M. T. Rahman, D. Du, R. Panat and Y. Lin, Sens. Actuators, B, 2016, 230, 600–606 CrossRef CAS PubMed.
- A. V. Zachariah, PhD, State University of New York at Binghamton, 2018 Search PubMed.
- R. S. Sivasubramony, N. Adams, M. Alhendi, G. S. Khinda, M. Z. Kokash, J. P. Lombardi, A. Raj, S. Thekkut, D. L. Weerawarne and M. Yadav, Isothermal Fatigue of Interconnections in Flexible Hybrid Electronics Based Human Performance Monitors, IEEE 68th Electronic Components and Technology Conference (ECTC) proceeding, San Diego, CA, USA, 2018 Search PubMed.
- I. Zein, D. W. Hutmacher, K. C. Tan and S. H. Teoh, Biomaterials, 2002, 23, 1169–1185 CrossRef CAS PubMed.
- A. P. Kuo, N. Bhattacharjee, Y. S. Lee, K. Castro, Y. T. Kim and A. Folch, Adv. Mater. Technol., 2019, 4, 1800395 CrossRef PubMed.
- P. R. Miller, S. D. Gittard, T. L. Edwards, D. M. Lopez, X. Xiao, D. R. Wheeler, N. A. Monteiro-Riviere, S. M. Brozik, R. Polsky and R. J. Narayan, Biomicrofluidics, 2011, 5, 013415 CrossRef PubMed.
- J. S. Narayanan and G. Slaughter, Bioelectrochemistry, 2019, 128, 56–65 CrossRef CAS PubMed.
- C. Y. Yap, C. K. Chua, Z. L. Dong, Z. H. Liu, D. Q. Zhang, L. E. Loh and S. L. Sing, Appl. Phys. Rev., 2015, 2, 041101 Search PubMed.
- N. Raghavan, R. Dehoff, S. Pannala, S. Simunovic, M. Kirka, J. Turner, N. Carlson and S. S. Babu, Acta Mater., 2016, 112, 303–314 CrossRef CAS.
- B. Vandenbroucke and J. P. Kruth, Rapid Prototyp. J., 2007, 13(4), 196–203 CrossRef.
- J. Kwon, H. Cho, H. Eom, H. Lee, Y. D. Suh, H. Moon, J. Shin, S. Hong and S. H. Ko, ACS Appl. Mater. Interfaces, 2016, 8, 11575–11582 CrossRef CAS PubMed.
- M. A. Torres Arango, ACS Sustainable Chem. Eng., 2017, 5(11), 10421–10429 CrossRef CAS.
- S. Y. Nam and S.-H. Park, Biomimetic Medical Materials, 2018, pp. 335–353 Search PubMed.
- S. Ostrovidov, S. Salehi, M. Costantini, K. Suthiwanich, M. Ebrahimi, R. B. Sadeghian, T. Fujie, X. Shi, S. Cannata and C. Gargioli, Small, 2019, 15, 1805530 CrossRef PubMed.
- A. F. Godier, D. Marolt, S. Gerecht, U. Tajnsek, T. P. Martens and G. Vunjak-Novakovic, Birth Defects Res. C Embryo Today, 2008, 84, 335–347 CrossRef CAS PubMed.
- S. V. Murphy and A. Atala, Nat. Biotechnol., 2014, 32, 773–785 CrossRef CAS PubMed.
- J. Li, M. Chen, X. Fan and H. Zhou, J. Transl. Med., 2016, 14, 1–15 CrossRef PubMed.
- C. Corcoran and G. Rechnitz, Trends Biotechnol., 1985, 3, 92–96 CrossRef CAS.
- A. D. Dias, D. M. Kingsley and D. T. Corr, Biosensors, 2014, 4, 111–136 CrossRef CAS PubMed.
- N. Gupta, V. Renugopalakrishnan, D. Liepmann, R. Paulmurugan and B. D. Malhotra, Biosens. Bioelectron., 2019, 141, 111435 CrossRef CAS PubMed.
- X. Cui, G. Gao and Y. Qiu, Biotechnol. Lett., 2013, 35, 315–321 CrossRef CAS PubMed.
- D. Jiang, K. Sheng, H. Jiang and L. Wang, Bioelectrochemistry, 2021, 142, 107919 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |
