Open Access Article
Open Access ArticleImpurities in polyvinylpyrrolidone: the key factor in the synthesis of gold nanostars†
Patricia
Taladriz-Blanco
 ac,
Miguel
Spuch-Calvar‡
a,
Anselmo del
Prado§
ac,
Miguel
Spuch-Calvar‡
a,
Anselmo del
Prado§
 a,
Christoph
Weder
a,
Christoph
Weder
 a,
Barbara
Rother-Rutishauser
a,
Barbara
Rother-Rutishauser
 a,
Alke
Petri-Fink
a,
Alke
Petri-Fink
 *ab and
Laura
Rodriguez-Lorenzo
*ab and
Laura
Rodriguez-Lorenzo
 *c
*c
aAdolphe Merkle Institute, University of Fribourg, Chemin des Verdiers 4, Fribourg, CH-1700, Switzerland. E-mail: alke.fink@unifr.ch
bChemistry Department, University of Fribourg, Chemin du Musée 9, Fribourg, CH-1700, Switzerland
cInternational Iberian Nanotechnology Laboratory (INL), Water Quality group, Av. Mestre José Veiga s/n, 4715-330 Braga, Portugal. E-mail: laura.rodriguez-lorenzo@inl.int
First published on 1st December 2021
Abstract
Control over the synthesis of anisotropic nanoparticles is crucial as slight differences in their size, shape, sharpness, or the number of tips in the case of gold nanostars, has an inordinate influence on their properties and functionality for future applications. Herein, we show that the supplier and purity of polyvinylpyrrolidone (PVP) can significantly alter the synthesis of gold nanostars, demonstrating that impurities, not PVP itself, are the main factor responsible for star-like shape formation. We demonstrate that in the presence of pure PVP and N,N-dimethylformamide, the use of hydrazine leads to the formation of branched nanoparticles. This synthetic approach opens the door to solving issues associated with the use of commercial PVP during the synthesis of gold nanostars.
Introduction
Gold (Au) nanostars (NSTs) are one of the most promising anisotropic nanoparticles (NPs) for sensing and imaging. High sensitivity detection is facilitated by concentration of the electromagnetic field at their tips, thus enhancing e.g. the Raman signal of the molecules subject to study and facilitating their detection.1,2 In this regard, Au NSTs are preferred candidates for the detection of multiple analytes by surface-enhanced Raman scattering (SERS), including bacteria,3 biomarkers,4,5 nucleic acids,6 organic and inorganic7–11 molecules.The precise control of the sharpness (i.e. length and angle) and the number of tips in nanostars allows tuning the concentration of the electromagnetic field at the tips and therefore their performance as optical nanoantennas for the induction of SERS.2,12–15
Recently, the presence of impurities in commercial chemicals has drawn scientists' attention, as it has become clear that those impurities can play an important role on the syntheses of NPs. In this context, Roger et al.16 demonstrated that the presence of impurities in polyvinylpyrrolidone (PVP) drastically influences the synthesis of silver NPs. Rioux et al.17 demonstrated that the PVP end-groups play a pivotal role in the synthesis of anisotropic NPs. The authors found that aldehyde PVP end-groups (PVP-CHO) lead to the formation of silver nanocubes, whereas PVP-OH and PVP-H yield a mixture of particles having different shapes. Warned by colleagues who observed that only one of the two PVP grades used during their experiments prevented the aggregation of Ag NPs upon dilution, Sacher et al.18 characterized the two PVPs purchased from the same supplier. The authors concluded that the commercial PVP used in the study contained 2-pyrrolidone and hydroperoxides as contaminants. In a recent editorial, Liz-Marzán et al.19 recommended considering impurities when synthesizing NPs, pointing out that the use of different commercial PVPs influences the reaction time required for the reduction of Au3+ into Au+ as well as the time needed to grow Au NSTs. In 2008, the same author published a protocol to synthesize Au NSTs20 that yields well-defined NSTs (reaction yield ∼100%) with high colloidal stability and biocompatibility using PVP. The reported synthesis is carried out at room temperature without any external stimulus (e.g. light) and involves the reduction of a chloroauric acid (HAuCl4) in the presence of N,N-dimethylformamide (DMF), PVP, and 15 nm PVP-coated gold spherical seeds. In this reaction, DMF acts as both the solvent and the reducing agent, and in the presence of metal seeds and at high concentrations of PVP (>2.5 mM) Au3+ is reduced to Au0 to grow on the surface of the metal seeds.21,22 The anisotropic growth in the [011] direction is driven by PVP, which is preferentially adsorbed on specific facets of the gold seeds and is responsible for the control of the nucleation kinetics.23 Furthermore, PVP chains provide colloidal stability to the formed stars.22
In the synthesis of Au NSTs, the role of PVP and DMF as well as temperature, size and crystallinity of the seed, or proton (H+) addition (i.e. presence of HCl) among others were already studied12,14,20,21,23,24 However, to the best of our knowledge, the impact of the presence of impurities in the PVP during the synthesis of Au NSTs has not been yet addressed. Following the protocol described by Liz-Marzán to synthesize Au NSTs with PVP,20 we demonstrate that the use of PVP from different suppliers with different purity compromises the anisotropic growth of the Au NPs. In addition, our results show that the purification of PVP drastically suppresses the formation of Au NSTs independent on its molecular weight. Finally, we explore the influence of hydrazine in the synthesis of Au NSTs using purified PVP with two different molecular weights, 10k and 40k, observing that the presence of hydrazine leads to the formation of branched NPs.
Alternatives to using PVP include the use of surfactants (such as cetyltrimethylammonium bromide25 or Triton26), buffers (e.g. HEPES27) or silver nitrate in acidic pH to induce the anisotropic growth28 among others.29
Results and discussion
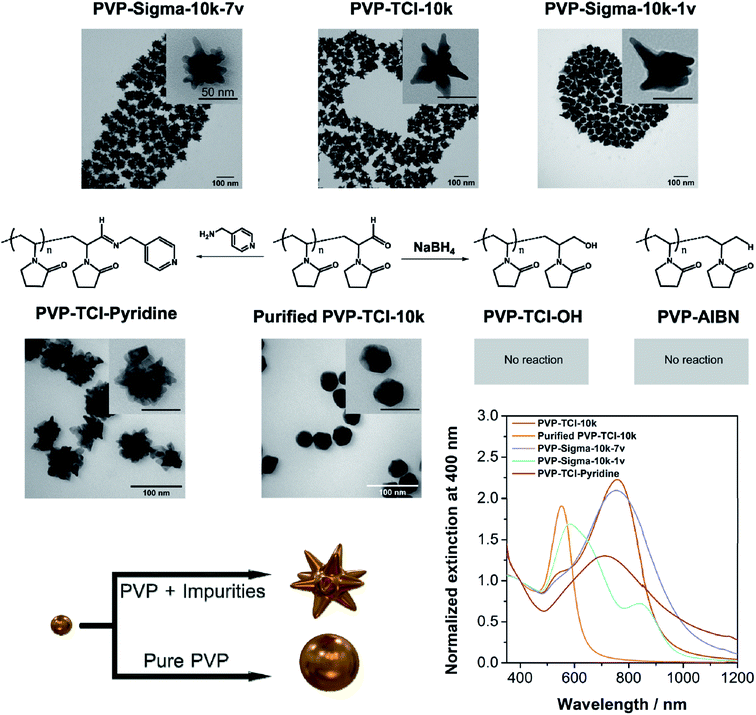
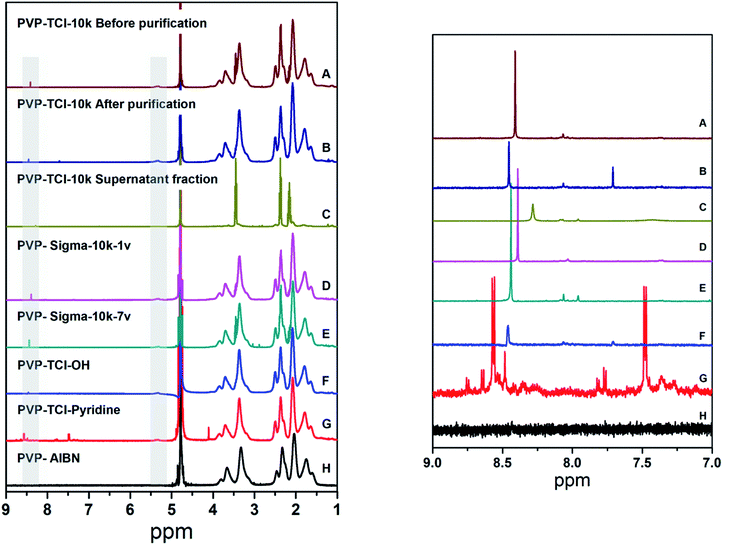
Au NSTs in the presence of three commercial PVPs (Mw = 10 kDa), PVP-TCI-10k (Lot 48NUD-DE), PVP-Sigma-10k-7v (Lot BCBM0097V), and PVP-Sigma-10k-1v (Lot BCBG5331V), respectively were prepared following the protocol described by Liz-Marzán et al.20 The results indicate that all PVPs used in our study lead to the formation of anisotropic Au NPs. Transmission electron microscopy (TEM) micrographs in Fig. 1 show that the Au NSTs prepared using PVP-TCI-10k are well-defined and exhibit large and sharp tips, while the Au NSTs prepared using PVP-Sigma-10k-7v have shorter and rounded tips. The morphology of the Au NSTs prepared with PVP-Sigma-10k-1v is ill-defined with a low number of rounded tips. The extinction spectra of the synthesized Au NSTs (Fig. 1) show that the tip-mode plasmon resonance band of PVP-Sigma-10k-7v (blue spectrum) is broader than the tip-mode band of the PVP-TCI-10k (red spectrum), which agrees with the higher number of shorter tips with a higher aperture angle in PVP-Sigma-10k-7v as visualized in the TEM micrographs. The localized surface plasmon resonance (LSPR) band corresponding to core mode resonance is predominant in the UV-Vis-NIR spectrum of PVP-Sigma-10k-1v (cyan spectrum) due to the lack of tips observed in TEM micrograph. The three PVPs were characterized by 1H-NMR spectroscopy (Fig. 2), which demonstrates the presence of impurities in PVP-TCI-10k (spectrum A) and PVP-Sigma-10k-7v (spectrum E), but not in PVP-Sigma-10k-1v (spectrum D).Based on the hypothesis that the purity of PVP strongly influences the synthesis of Au NSTs, commercial PVP-TCI-10k was purified by precipitation from chloroform into diethyl ether (see details in the ESI section Material and Methods†) which gives rise to NPs with a low degree of anisotropy (Fig. 1). The presence of impurities is drastically suppressed by this purification (Fig. 2), as shown by 1H-NMR spectra of PVP-TCI-10k before and after purification. This result was confirmed by analyzing the 1H-NMR spectrum of the fraction isolated from the supernatant after the precipitation step (Fig. 2). The broad distribution of masses detected by Matrix Assisted Laser Desorption/Ionization-Time of Flight Mass Spectrometry (MALDI-TOF) (Fig. S1†) of the supernatant fraction confirms the presence of PVP oligomers, as previously reported.30 Thus, in case of PVP-TCI-10k, PVP oligomers, which can be considered as an impurity, may serve to promote the formation of Au NSTs.
All NMR spectra show a peak at ca. 8.5 ppm and a weak band at ca. 5.2 ppm corresponding to alpha –CH– groups, suggesting the presence of aldehyde end groups. Diffusion Ordered Spectroscopy (DOSY) NMR data confirms that these residues are part of the PVP chains (Fig. S1†).
In contrast to what has been previously reported,31,32 no hydroxyl end groups were identified in the as-received and purified PVP-TCI-10k by 1H-NMR spectroscopy (Fig. 2) due to signal overlap from the PVP backbone which possesses broad signals in the region of 3–4 ppm.
As described by Straub et al.,33 the synthesis of commercial PVP often relies on hydrogen peroxide as an initiator, and this leads to the introduction of hydroxyl end groups, which may oxidize into aldehyde groups.18 In our study, the presence of aldehyde end groups, as suggested by the 1H-NMR data discussed above, was further confirmed by (1) reducing the purified PVP-TCI-10k with NaBH4 forming a hydroxyl end-terminated PVP (PVP-TCI-OH), and (2) reacting the purified PVP-TCI-10k with 4-aminopyridine (PVP-TCI-pyridine), respectively. As shown in the 1H-NMR spectra and more clearly by DOSY, the treatment of purified PVP-TCI-10k (Fig. 2 and S2†) with NaBH4 leads to the disappearance of the bands at 8.5 and 5.2 ppm, confirming the reduction of PVP-CHO to PVP-OH. The pyridine coupling via imine formation is confirmed by the appearance of two new peaks at 7.5 and 8.5 ppm corresponding to the pyridine group (Fig. 2).
Contrary to what was described by Xia et al.,32,34 no reaction was observed in attempts to produce NPs in the presence of PVP-TCI-OH (Fig. 1 and S3A†). Therefore, hydroxyl end groups in the PVP chain can neither be responsible for the reduction of the gold salt nor for the anisotropic growth. NPs synthesized in the presence of PVP-TCI-pyridine exhibit a more significant anisotropic growth than those prepared with the purified PVP-TCI-10k (Fig. 1 and S3B†). These results indicate that (1) purification of PVP drastically inhibits the formation of Au NSTs, (2) aldehydes and pyridines in the PVP chain can reduce Au3+ to Au+, (3) pyridines induce anisotropic growth, and (4) the presence of aldehydes in the PVP chain is not responsible for the formation of star-like NPs. Accordingly, no reaction was observed in attempts to produce NPs with PVP synthesized in our laboratory by conventional radical polymerization using 2,2′-azobisisobutyronitrile (AIBN) as an initiator (PVP-AIBN) (Fig. 1 and S3C†). Nevertheless, PVP-AIBN, which is –H terminated, is able to partially reduce Au3+ to Au+ (Fig. S3C†). Fig. S3D† displays the evolution of gold seeds into Au NSTs in the presence of impure PVP-TCI-10k by UV-Vis-NIR spectroscopy. After 90 minutes, the UV-Vis-NIR extinction spectra exhibit two LSPR bands centred at 546 nm (core mode) and 840 nm (tip mode), respectively. None of those bands were observed in the presence of PVP-TCI-OH, PVP-TCI-pyridine, PVP-AIBN (Fig. S3A, B and C†) or purified PVP-TCI-10k with rough spheres as the result of the synthesis. These results confirm the hypothesis that impurities in commercial PVPs are responsible for the formation of Au NSTs.
It is important to point out that these impurities are not present in the PVP we synthesized using AIBN (see 1H-NMR spectrum in Fig. 2) as no Au NSTs were formed under these conditions (Fig. 1 and S3C†). In addition, the particles obtained in the presence of pyridines suggest that the substance responsible for the anisotropic growth is a compound with reduction activity present in PVP as an impurity.
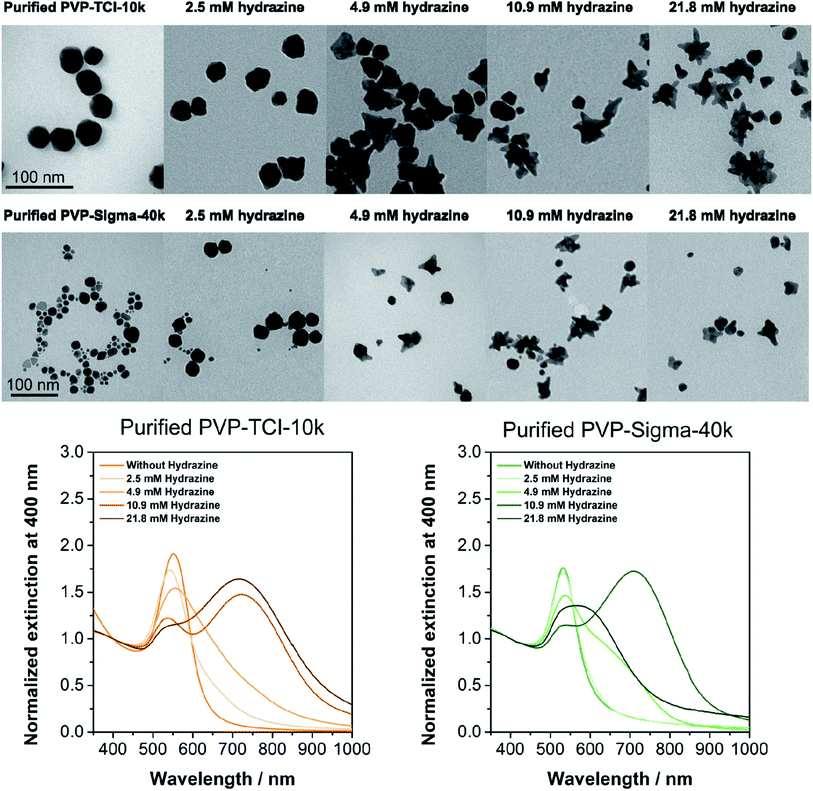
The same experimental methodology was applied using commercial PVP with higher molecular weight (PVP-Sigma-40k, Lot WXBC7054V, Mw = 40 kDa), and the results show that the formation of very well-defined stars is drastically hindered after purification of the PVP. As seen for the PVP with lower molecular weight (PVP-TCI-10k), the purified PVP-Sigma-40k lacks oligomers, supporting the hypothesis that the substance responsible for the formation of stars is present in the PVP as an impurity (Fig. S4†).
Commercial PVP is mainly produced by radical polymerization in the presence of ammonia (which is used to synthesize N-vinylpyrrolidone and remains as a residual contaminant), and hydrogen peroxide as an initiator which leads to the formation of hydrazine as a by-product.35 Meanwhile, hydrazine is commonly used as a reducing agent in the formation of spherical and anisotropic NPs, including branched NPs36–40 and it is not formed during the synthesis of PVP when using AIBN as an initiator. Speculating that hydrazine might be the impurity responsible for the formation of Au NSTs, we attempted to synthesize Au NSTs using purified PVP-TCI-10k and purified PVP-Sigma-40k in the presence of hydrazine. Different concentrations of hydrazine (2.5–21.8 mM) were used to evaluate its role in anisotropic growth. As shown in the TEM micrographs and the UV-Vis-NIR spectra (Fig. 3), an increase in hydrazine from 2.5 mM to 10.9 mM leads to the formation of branched Au NPs in the presence of both purified PVPs, PVP-TCI-10k, and PVP-Sigma-40k. In the case of purified PVP-TCI-10k, Au NST are also produced at a higher concentration of hydrazine (21.8 mM), whereas star formation appears to become stifled in the case of purified PVP-TCI-40k. This latter result and the fact that the shape and the extinction spectra of Au NST produced in the presence of “as received” and “hydrazine spiked” PVP differ somewhat suggest that the mechanism leading to the formation of Au NSTs may be complex. It is also not clear that commercial PVP grades indeed include hydrazine as an impurity. Nevertheless, our data show clearly that the addition of hydrazine to purified PVPs has a significant influence and can promote the formation of Au NSTs. However, more experiments should be carried out to elucidate the mechanism, improve the yield of the synthesis, reduce the degree of polydispersity, and the stability of the NPs.
Conclusions
In this work, we demonstrated that the chemical supplier and inherent purity of PVP play a key role in the synthesis of Au NSTs. Depending on the supplier, we observed the formation of rough or sharp stars as well as a lack of well-defined Au NSTs. We also showed that the purification of the PVP drastically suppresses the anisotropic growth and that aldehyde groups in the PVP chain are not responsible for the formation of Au NSTs. Our data also show that the addition of hydrazine to PVPs can promote the formation of Au NSTs and mitigate the absence of impurities that appear otherwise to be necessary for their formation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Swiss National Science Foundation through the National Center of Competence in Research Bio-Inspired Materials. The authors further acknowledge the support by the University of Fribourg and the Adolphe Merkle Foundation. LR-L acknowledges funding from FCT (Fundação para a Ciência e Technologia) for the Scientific Employment Stimulus Program (2020.04021.CEECIND). We thank Aaron Lee for proof-reading the manuscript, and Liliane Ackermann-Hirschi for her support in synthesis of the stars.References
- C. G. Khoury and T. Vo-Dinh, J. Phys. Chem. C, 2008, 112, 18849 CrossRef CAS PubMed.
- X.-L. Liu, J.-H. Wang, S. Liang, D.-J. Yang, F. Nan, S.-J. Ding, L. Zhou, Z.-H. Hao and Q.-Q. Wang, J. Phys. Chem. C, 2014, 118, 9659 CrossRef CAS.
- L. Rodríguez-Lorenzo, A. Garrido-Maestu, A. K. Bhunia, B. Espiña, M. Prado, L. Diéguez and S. Abalde-Cela, ACS Appl. Nano Mater., 2019, 2, 6081 CrossRef.
- R. de la Rica and M. M. Stevens, Nat. Nanotechnol., 2012, 7, 821 CrossRef CAS PubMed.
- M. Bhamidipati, H.-Y. Cho, K.-B. Lee and L. Fabris, Bioconjugate Chem., 2018, 29, 2970 CrossRef CAS PubMed.
- A. Teixeira, L. J. Paris, F. Roumani, L. Diéguez, M. Prado, B. Espiña, S. Abalde-Cela, A. Garrido-Maestu and L. Rodriguez-Lorenzo, Materials, 2020, 13, 1934 CrossRef CAS PubMed.
- A. S. D. S. Indrasekara, S. Meyers, S. Shubeita, L. C. Feldman, T. Gustafsson and L. Fabris, Nanoscale, 2014, 6, 8891 RSC.
- L. Rodríguez-Lorenzo, R. A. Álvarez-Puebla, I. Pastoriza-Santos, S. Mazzucco, O. Stéphan, M. Kociak, L. M. Liz-Marzán and F. J. García de Abajo, J. Am. Chem. Soc., 2009, 131, 4616 CrossRef PubMed.
- Y. Wang, L. Polavarapu and L. M. Liz-Marzán, ACS Appl. Mater. Interfaces, 2014, 6, 21798 CrossRef CAS PubMed.
- W. Ma, M. Sun, L. Xu, L. Wang, H. Kuang and C. Xu, Chem. Commun., 2013, 49, 4989 RSC.
- J. Qian, C. Xing, Y. Ge, R. Li, A. Li and W. Yan, Spectrochim. Acta, Part A, 2020, 232, 118146 CrossRef CAS PubMed.
- A. Kedia and P. S. Kumar, J. Mater. Chem. C, 2013, 1, 4540 RSC.
- N. Pazos-Perez, L. Guerrini and R. A. Alvarez-Puebla, ACS Omega, 2018, 3, 17173 CrossRef CAS PubMed.
- S. Barbosa, A. Agrawal, L. Rodríguez-Lorenzo, I. Pastoriza-Santos, R. A. Alvarez-Puebla, A. Kornowski, H. Weller and L. M. Liz-Marzán, Langmuir, 2010, 26, 14943 CrossRef CAS PubMed.
- S. Trigari, A. Rindi, G. Margheri, S. Sottini, G. Dellepiane and E. Giorgetti, J. Mater. Chem., 2011, 21, 6531 RSC.
- N. El Amri and K. Roger, J. Colloid Interface Sci., 2020, 576, 435 CrossRef PubMed.
- S. Jharimune, R. Pfukwa, Z. Chen, J. Anderson, B. Klumperman and R. M. Rioux, J. Am. Chem. Soc., 2021, 143, 184 CrossRef CAS PubMed.
- L. K. Mireles, M.-R. Wu, N. Saadeh, L. Yahia and E. Sacher, ACS Omega, 2020, 5, 30461 CrossRef CAS PubMed.
- L. M. Liz-Marzán, C. R. Kagan and J. E. Millstone, ACS Nano, 2020, 14, 6359 CrossRef PubMed.
- P. S. Kumar, I. Pastoriza-Santos, B. Rodríguez-González, F. J. García de Abajo and L. M. Liz-Marzán, Nanotechnol, 2008, 19, 15606 CrossRef PubMed.
- A. Kedia and P. Senthil Kumar, J. Phys. Chem. C, 2012, 116, 1679 CrossRef CAS.
- I. Pastoriza-Santos and L. M. Liz-Marzán, Adv. Funct. Mater., 2009, 19, 679 CrossRef CAS.
- K. M. Koczkur, S. Mourdikoudis, L. Polavarapu and S. E. Skrabalak, Dalton Trans., 2015, 44, 17883 RSC.
- A. Kedia and P. S. Kumar, J. Phys. Chem. C, 2012, 116, 23721 CrossRef CAS.
- L. Shao, A. S. Susha, L. S. Cheung, T. K. Sau, A. L. Rogach and J. Wang, Langmuir, 2012, 28, 8979 CrossRef CAS PubMed.
- S. Atta, M. Beetz and L. Fabris, Nanoscale, 2019, 11, 2946 RSC.
- G. Maiorano, L. Rizzello, M. A. Malvindi, S. S. Shankar, L. Martiradonna, A. Falqui, R. Cingolani and P. P. Pompa, Nanoscale, 2011, 3, 2227 RSC.
- S. Abalde-Cela, P. Taladriz-Blanco, M. G. de Oliveira and C. Abell, Sci. Rep., 2018, 8, 2440 CrossRef PubMed.
- A. Guerrero-Martínez, S. Barbosa, I. Pastoriza-Santos and L. M. Liz-Marzán, Curr. Opin. Colloid Interface Sci., 2011, 16, 118 CrossRef.
- E. Ranucci, P. Ferruti, R. Annunziata, I. Gerges and G. Spinelli, Macromol. Biosci., 2006, 6, 216 CrossRef CAS PubMed.
- K. Raith, A. V Kühn, F. Rosche, R. Wolf and R. H. H. Neubert, Pharm. Res., 2002, 19, 556 CrossRef CAS PubMed.
- Y. Xiong, I. Washio, J. Chen, H. Cai, Z.-Y. Li and Y. Xia, Langmuir, 2006, 22, 8563 CrossRef CAS PubMed.
- F. Haaf, A. Sanner and F. Straub, Polym. J., 1985, 17, 143 CrossRef CAS.
- I. Washio, Y. Xiong, Y. Yin and Y. Xia, Adv. Mater., 2006, 18, 1745 CrossRef CAS.
- Y. E. Kirsh, Water Soluble Poly-N-Vinylamides: Synthesis and Physicochemical Properties, John Wiley & Sons, Chichester, 1998 Search PubMed.
- G. H. Jeong, Y. W. Lee, M. Kim and S. W. Han, J. Colloid Interface Sci., 2009, 329, 97 CrossRef CAS PubMed.
- I. Pastoriza-Santos, A. Sánchez-Iglesias, B. Rodríguez-González and L. M. Liz-Marzán, Small, 2009, 5, 440 CrossRef CAS PubMed.
- M. Maillard, S. Giorgio and M.-P. Pileni, J. Phys. Chem. B, 2003, 107, 2466 CrossRef CAS.
- M. Sawczyk and R. Klajn, J. Am. Chem. Soc., 2017, 139, 17973 CrossRef CAS PubMed.
- A. F. Alvarez-Paneque, B. Rodríguez-González, I. Pastoriza-Santos and L. M. Liz-Marzán, J. Phys. Chem. C, 2013, 117, 2474 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1na00711d |
| ‡ Present address: Department of Applied Physics, Department of Physical Chemistry, Singular Center for Biomedical Research (CINBIO), Southern Galicia Institute of Health Research (IISGS) and Biomedical Research Networking Center for Mental Health (CIBERSAM), Universidade de Vigo, 36310 Vigo, Spain. |
| § Present address: Departamento de Química Orgánica, Facultad de Ciencias, Universidad Autónoma de Madrid, 28049 Madrid, Spain. |
| This journal is © The Royal Society of Chemistry 2022 |