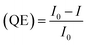Open Access Article
Open Access ArticleEfficient charge transfer from organometal lead halide perovskite nanocrystals to free base meso-tetraphenylporphyrins†
Abha
Jha
,
Hari
Shankar
,
Sandeep
Kumar
,
Muniappan
Sankar
and
Prasenjit
Kar
 *
*
Department of Chemistry, Indian Institute of Technology Roorkee, Haridwar, Uttarakhand- 247667, India. E-mail: kar.prasen@gmail.com; prasenjit.kar@cy.iitr.ac.in
First published on 15th March 2022
Abstract
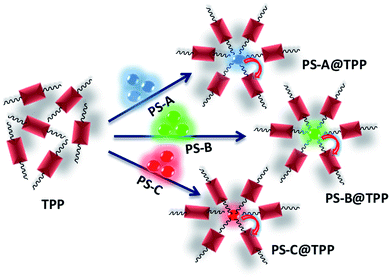
The efficient charge transfer from methylammonium lead halide, MAPbX3 (X = Br, I), perovskite nanocrystals (PNCs) to 5,10,15,20-tetraphenylporphyrin (TPP) molecules has been investigated in detail. The hydrophobically-capped MAPbX3 PNCs exhibited bright fluorescence in the solution state. However, in the presence of TPP, the fluorescence intensity was quenched, which is ascribed to the electron transfer from the PNCs to TPP. Photoluminescence (PL) spectroscopy and absolute quantum yield measurements were used to evaluate the fluorescence quenching. This efficient fluorescence quenching leads to an increase in the quenching efficiency value. The quenching of fluorescence intensity is not attributed to the change in lifetime, as evidenced by time-correlated single-photon counting (TCSPC) measurements, suggesting a static electron transfer from the PNCs to TPP molecules. Such a static fluorescence quenching corresponds to the adsorption of TPP onto the surface of hydrophobic PNCs, and has been examined via transmission electron microscopy (TEM). Cyclic voltammetry (CV) studies were used to compare the PNCs and PNCs@TPP nanocomposites, revealing that the electron transfer process takes place from the PNCs to the organic acceptor TPP molecules.
Introduction
Organic–inorganic hybrid halide perovskites have been extensively developed1,2 due to their many intriguing properties, including narrow emission bandwidth,3 size-tunable optical properties,4,5 cost effectiveness, excellent quantum yield,6 and photostability.7 The commendable properties of these materials are very important in several optoelectronic devices,8,9 such as lasers,10–12 light-emitting diodes,13,14 solar cells,15,16 photodetectors,17,18 and sensors.19 The large surface-to-volume ratio of perovskite nanocrystals (PNCs) leads to a high proportion of surface atoms with surface states and dangling bonds.20,21 Besides their ease of preparation, hybrid lead halide perovskites have sparked a lot of research interest due to their technological relevance. Additionally, extended π-conjugation, a basic N4 core, and the rigid and planar structure of porphyrin molecules play a crucial role in improving device performance due to their distinct electronic and photochemical properties.22–24 These molecules are utilized in charge generation, light harvesting, and biochemical processes.25Due to the quantum confinement effect, PNCs show intense fluorescence,26,27 which is regulated by chemical composition and bandgap engineering, and exhibit greater potential than perovskite films. Most hybrid halide perovskites are synthesized by the ligand-assisted reprecipitation (LARP) method using a capping ligand28 or on the surface of substrate.29 The tuning of the emission color of these perovskite nanocrystals from the visible to near-infrared (NIR) region is one of their fundamental advantages, which is achieved by the preparation of mixed halide perovskites through the post-synthesis halide exchange reaction and mixed halide precursors. This halide-dependent tunability of PL color results from the modification of band-edge states.30
Moreover, porphyrin-based systems have electron–transfer properties due to their exceptional photoelectric properties and broad absorption coefficient in the visible region.31,32 The main reasons for selecting TPP as an electron acceptor over other electron accepting materials are its good solubility in common solvents, high mobility, appropriate energy-level alignment and strong light harvesting potential.33 As a result, combining the properties of perovskite nanomaterials and porphyrins to create a nanocomposite will significantly improve their properties and result in further applications in optoelectronics. Currently, preparing perovskite-based nanocomposites with the electron-transfer phenomenon is one of the most prominent research areas. For example, Sundstrom et al.34 pinpointed the electron transfer study of a hybrid metal halide perovskite with an organic acceptor molecule. Sargent et al.35 reported the improved photocurrent response and stability of solar cells related to C60-layered perovskites, which corresponds to the removal of grain boundaries and surface defect passivation in perovskites by C60. Electron transport from perovskite to mesoporous titanium dioxide was studied by Gratzel et al.36 An earlier study explored the transfer of electrons from donor PNCs to classical acceptors such as TiO2,37 perylenes,38 fullerenes,39,40 benzoquinone,41 and carbon nanotubes.42 In this context, Tang et al.43 used a diammonium porphyrin to study the charge transfer dynamics in CsPbBr3 nanocrystals. Recently, Patra et al.44 demonstrated the charge transfer properties of CsPbBr3 PNCs with 5,10,15,20-tetrakis(4′-pyridyl)porphyrin.
In this work, we explicate the emission behavior of methylammonium lead halide (MAPbX3) PNCs in solutions with or without TPP. The MAPbX3 perovskite solution in the presence of TPP exhibits charge transfer and has been studied using photoluminescence (PL) spectroscopy, absolute photoluminescence quantum yield (PLQY) values and the time-correlated single-photon count (TCSPC) technique. Herein, TPP acts as an electron acceptor, and quenching of the emission of blue, green, and red luminescent PNCs has been recorded using PL spectra. A schematic representation of the electron transfer study is shown in Scheme 1. Meanwhile, the TCSPC measurement reveals the static fluorescence quenching of the PNCs. The details of the synthesis of the MAPbBr3 and MAPbI3 PNCs and TPP solutions are mentioned in the experimental section.
Characterization
The UV-vis absorption spectra were recorded using a Shimadzu UV-1800 spectrophotometer in the range of 200 to 800 nm. A Horiba Scientific Fluoromax-4C spectrophotometer was used to monitor the photoluminescence and excitation spectra. To record the absolute quantum yield values of the nanocomposites, an Edinburg FLS 980 instrument was used. A Rigaku-Smart Lab was used to record the X-ray diffraction (XRD) patterns by making a thin film with a Cu target and an angle range of 10° to 50°. TCSPC measurements were taken with a Horiba Jobin Yvon Fluorocube that was assembled with nano LEDs and spectral LEDs used as the excitation source. Fourier transform infrared (FTIR) spectroscopy was carried out using a Thermo Scientific Nicolet 6700. X-ray photoelectron (XPS) spectroscopy of the material was performed on an XPS instrument with model no. PHI 5000 Versa Probe III for the analysis of the surface. Transmission electron microscopy (TEM) images were recorded on a FEI TECHNI G2 20 S-TWIN. Electrochemical measurements were recorded with a CH instrument (CHI-620E) under argon atmosphere along with a three-electrode assembly, where Ag/AgCl was used as a reference electrode, platinum (Pt) wire as a counter electrode and 2 mm platinum button as a working electrode. 1H NMR spectra were recorded on a 500 MHz NMR spectrometer using CDCl3 solvent.Experimental details
Materials
Lead(II) bromide (99%, Aldrich), oleylamine (Aldrich), oleic acid (Aldrich), methylamine solution (33 wt% in absolute ethanol, Aldrich), chloroform (Rankem), N,N-dimethylformamide (Thomas Baker), and hydrobromic acid (48 wt% in water, SRL). All of the chemicals and solvents were acquired commercially and used as received.Methylammonium bromide (MABr) synthesis
In a 50 mL round-bottom (RB) flask, 6 ml CH3NH2 was added with 6 mL ethanol. Then, at room temperature, 5 mL 57% aqueous solution of HBr was added dropwise, followed by continuous stirring. To remove all solvents, the resulting solution was kept in a rotary evaporator at 60 °C for 1 h. To obtain the recrystallized product, the solution was washed multiple times with diethyl ether, dried and stored in a vacuum for further characterization.Methylammonium iodide (MAI) synthesis
In a 50 mL RB flask, 6 ml CH3NH2 was mixed with 6 mL ethanol. Then, HI (5 ml 57% aqueous solution) was added dropwise while stirring continuously at room temperature. The obtained solution was placed in a rotary evaporator at 60 °C for 1 h to remove all solvents. Then, to obtain the recrystallized product, the solution was washed several times with diethyl ether, dried and stored in a vacuum for further characterization.Synthesis of blue-luminescent CH3NH3PbBr3 PNCs (PS-A)
0.0112 g CH3NH3Br (0.1 mmol) and 0.0367 g PbBr2 (0.1 mmol) were dissolved in 1 mL DMF, forming a 0.1 mM solution. Next, 100 μL oleic acid and 200 μL oleylamine were added, followed by continuous stirring. Afterwards, 100 μL of this mixture was injected into 3 mL chloroform, which showed a blue color when observed under the UV chamber.Synthesis of green-luminescent CH3NH3PbBr3 PNCs (PS-B)
0.0112 g CH3NH3Br (0.1 mmol) and 0.0367 g PbBr2 (0.1 mmol) were dissolved in 1 ml DMF, forming a 0.1 mM solution. Then, 200 μL oleic acid and 38 μL oleylamine were added, followed by stirring. Afterwards, 100 μL of this mixture was injected into 3 mL chloroform, which was green when observed under the UV chamber.Synthesis of red-luminescent CH3NH3PbI3 (PS-C)
0.0112 g CH3NH3I (0.1 mmol) and 0.0461 g PbI2 (0.1 mmol) were dissolved in 1 mL DMF, forming a 0.1 mM solution. Then, 100 μL oleic acid and 200 μL oleylamine were added, followed by continuous stirring. Afterwards, 100 μL of this mixture was injected into 3 mL chloroform, showing a red color when observed under the UV chamber.Synthesis of TPP
TPP was synthesized using the Adler–Longo method (Scheme 2), as reported in the literature.45 4.41 mL benzaldehyde (43.24 mmol) and 3 mL pyrrole (43.24 mmol) were added to 180 mL hot propionic acid and refluxed for 90 min. The reaction mixture was cooled to room temperature and then filtered using a G4 sintered crucible. The purple crystalline solid was washed with methanol. The crude porphyrin was dissolved in a minimum amount of chloroform and purified by silica gel column chromatography using chloroform as the eluent, followed by recrystallization from a chloroform and methanol mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v). The purple crystalline product was dried under vacuum for 6 h. The yield was found to be 22% (1.5 g, 2.46 mmol), as shown in Scheme 2. The synthesized TPP was characterized by UV-vis and 1H NMR spectroscopy, mass spectrometry and elemental analysis. UV-vis (CH2Cl2): λmax (nm), (log
3, v/v). The purple crystalline product was dried under vacuum for 6 h. The yield was found to be 22% (1.5 g, 2.46 mmol), as shown in Scheme 2. The synthesized TPP was characterized by UV-vis and 1H NMR spectroscopy, mass spectrometry and elemental analysis. UV-vis (CH2Cl2): λmax (nm), (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 417 (5.58), 515 (4.19), 550 (3.83), 591 (3.68), and 647 (3.61). 1H NMR in CDCl3 (500 MHz, δ in ppm): 8.85 (s, 8H, β-pyrrolic H), 8.22 (d, 8H, meso-o-phenyl H), 7.79–7.74 (d, 12H, meso-m & p-phenyl H), −2.76 (d, 2H, imino-H) (Fig. S1†).
ε): 417 (5.58), 515 (4.19), 550 (3.83), 591 (3.68), and 647 (3.61). 1H NMR in CDCl3 (500 MHz, δ in ppm): 8.85 (s, 8H, β-pyrrolic H), 8.22 (d, 8H, meso-o-phenyl H), 7.79–7.74 (d, 12H, meso-m & p-phenyl H), −2.76 (d, 2H, imino-H) (Fig. S1†).
Synthesis of the CH3NH3PbX3@TPP nanocomposites
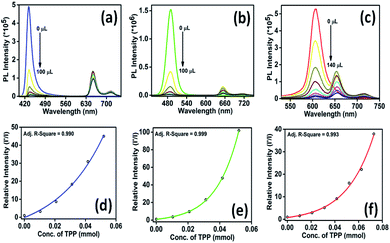
A stock solution of TPP was prepared by dissolving 0.08% w/v TPP in chloroform, following which, 50 μL synthesized perovskite solution was injected into 2.5 mL chloroform used for emission quenching. The concentration of the PNC solution employed for characterization was 0.66 × 10−7 M, while the concentration of TPP molecules required to quench the fluorescence of the PNC solution was 5 × 10−7 M.The UV-vis absorption spectra of green-luminescent (PS-B) and red-luminescent MaPbI3 perovskite nanocrystals (PS-C) with TPP were recorded at room temperature to track the optical properties of the MaPbX3@TPP nanocomposites, as shown in Fig. 1a and b. The absorption spectrum of TPP molecules dissolved in chloroform displays a characteristic Soret band (B band) at 417 nm, followed by four Q bands positions at 515, 550, 591, and 647 nm. The emission spectra of the MAPbX3@TPP nanocomposites were also recorded in chloroform at room temperature, as shown in Fig. 2. We observe an almost 100% quenching of the fluorescence intensity of PS-A, PS-B and PS-C nanocrystals with TPP (Fig. 2a–c). This indicates that efficient electron transfer occurs from the PNCs to TPP. The fluorescence quenching was monitored with the successive addition of TPP solution from 20–140 μL. An additional emission peak appeared at a higher wavelength in the range of 600–750 nm for the MAPbX3@TPP nanocomposites, which corresponds to the characteristic emission peak of the porphyrin macrocycle.46 This quenching of PL intensity is also indicated by the decrement in the quantum yield value of the nanocomposites, as mentioned in Table 1. This corroborated with the PL results, as shown in Fig. 2. Before the quenching experiment, we noticed a blue shift in the emission peak of the PNC samples as compared to a previous report47 due to the dilution effect.
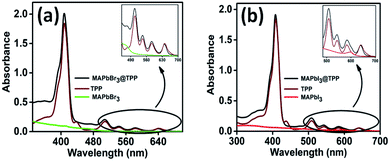 | ||
| Fig. 1 Electronic absorption spectra of (a) PS-B and (b) PS-C PNCs in the presence of TPP in CHCl3 at 298 K. | ||
| TPP volume | A 1 | A 2 | A 3 | τ 1 | τ 2 | τ 3 | τ avg (ns) | Quenching efficiency (%) | Quantum yield (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Blue-luminescent CH3NH3PbBr3 | 0 μL | 35.92 | 51.45 | 12.64 | 11.26 | 3.79 | 40.07 | 23.14 | 0 | 14 |
| 20 μL | 47.85 | 39.29 | 12.86 | 3.34 | 9.84 | 38.26 | 22.31 | 69.09 | 5 | |
| 100 μL | 40.65 | 46.34 | 13.01 | 9.33 | 3.01 | 36.88 | 21.68 | 97.77 | Less than 1 | |
| Green-luminescent CH3NH3PbBr3 | 0 μL | 74.22 | 25.78 | — | 6.65 | 20.09 | — | 13.53 | 0 | 27 |
| 20 μL | 73.14 | 26.86 | — | 6.47 | 19.31 | — | 13.18 | 72.55 | 6 | |
| 100 μL | 71.98 | 28.02 | — | 6.38 | 19.03 | — | 13.17 | 99.01 | Less than 1 | |
| Red-luminescent CH3NH3PbI3 | 0 μL | 34.09 | 55.98 | 9.93 | 6.52 | 12.55 | 44.71 | 22.00 | 0 | 62 |
| 20 μL | 33.82 | 57.50 | 8.67 | 6.35 | 12.74 | 45.40 | 21.30 | 35.40 | 25 | |
| 100 μL | 35.65 | 55.71 | 8.64 | 6.40 | 12.85 | 41.15 | 19.45 | 93.78 | 13 | |
| 140 μL | 38.20 | 53.59 | 8.22 | 6.61 | 12.95 | 40.65 | 18.93 | 98.63 | 8 |
Furthermore, to examine the effect of solvent, we recorded the emission spectrum of the PNC solution in CHCl3 in the presence and absence of TPP, as illustrated in Fig. S2, ESI†, suggesting the trivial effect of the solvent in quenching the fluorescence intensity of PNCs. This confirmed that the fluorescence quenching is caused by the adhesion of PNCs on TPP. The PL spectrum of the TPP solution displaying emission at 650 and 715 nm (at the same concentration used in the experiment) with CHCl3 is also observed and is shown in Fig. S3, ESI†. The self-excitation spectra of the PNCs, TPP as well as the PNCs@TPP nanocomposites are provided in Fig S4, ESI†. The nature of the quenching process can be understood by considering the Stern–Volmer plot. We obtained curves for the blue-, green- and red-luminescent perovskites instead of a linear line (Fig. 2d–f). The MAPbX3@TPP nanocomposites probably show a non-linear Stern–Volmer plot due to static quenching or a combination of both static and dynamic quenching.48
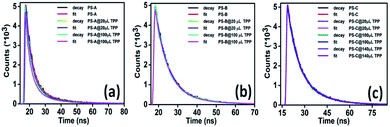
Another way to distinguish whether the quenching is static, dynamic or a combination of both is to measure the lifetime of the luminescent PNC solution in the absence and presence of TPP used as a quencher.49 The PL decay curves of the PNC solution without and with different concentrations of TPP (Fig. 3a–c) were determined by the time-correlated single-photon counting (TCSPC) technique. The fluorescence lifetimes (Table 1) of the samples remained unchanged regardless of the concentration of TPP. The constant fluorescence lifetime, along with a reduction in the number of emitted photons with an increasing amount of quencher, indicates that the excited state of the PNCs has been statically quenched by TPP.39a,40 Meanwhile, the remaining fluorescence at a specific concentration of TPP indicates that the MAPbX3 PNCs have a trace of unadsorbed TPP. In other words, it can be assumed that the continuous reduction in the quantum efficiency of the nanocomposites without any significant change in lifetime indicates the adsorption of MAPbX3 PNCs onto the organic molecules.
The surface of the PNC solution is capped with oleic acid and oleylamine (hydrophobic ligands). The presence of these hydrophobic ligands causes the PNCs to adsorb on the surface of TPP molecules. The average lifetimes of the PNCs with TPP solution are provided in Table 1. The average lifetimes were evaluated using the formula:
Quenching efficiency (QE) was calculated using the formula:
The X-ray diffraction (XRD) patterns of the nanocomposites (MAPbBr3@TPP) (Fig. S5, ESI†) have been recorded, showing the cubic phase of the perovskite nanocrystals. The XRD patterns of green-luminescent MAPbBr3 and red-luminescent MaPbI3 have also been analyzed (Fig. S6, ESI†), which confirm the phase of the PNCs, i.e. cubic and tetragonal, respectively.50 The XRD patterns of PNCs@TPP and PNCs, as illustrated in Fig. S5, ESI, and S6, ESI†, show an additional peak at 2θ = 12.95° related to unreacted PbBr2. For PNCs@TPP, the XRD pattern is the same as that of the PNCs with no shift in the peak position, indicating that there is no phase distortion after binding with TPP.
To study the surface of the nanocomposites, X-ray photoelectron spectroscopy (XPS) analysis of the PS-B (MAPbBr3) PNCs with TPP was performed (Fig. 4). The survey scan of the nanocomposites revealed the presence of Br, Pb, C and N elements. To gain further insight into the elemental configuration, the high-resolution spectra of all the elements were recorded. The narrow scan shows Pb 4f peaks at 138.36 eV and 143.16 eV, corresponding to 4f 7/2 and 4f 5/2, respectively (Fig. 4b). The peaks at 68.41 eV and 69.52 eV are ascribed to Br 3d 5/2 and Br 3d 3/2, respectively. The peaks at 284.76 eV and 401.70 eV are associated with C 1s and N 1s, respectively (Fig. 4c and d). Furthermore, to confirm the mode of interaction between TPP and the PNCs, 1H nuclear magnetic resonance (NMR) (Fig. S7, ESI†) and infrared (IR) spectroscopic studies (Fig. S8, ESI†) of TPP in the presence and absence of the PNCs have been performed. The NMR spectra show that the protons of TPP molecules slightly shift in the presence of the PNCs, indicating a weak interaction with TPP (Fig. S7, ESI†). The FT-IR spectra also corroborate this observation; the stretching vibrational frequency of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N indicates that the pyrrole ring present at 1638 cm−1 in TPP shifts by 10 cm−1 in the presence of the PNCs (Fig. S8, ESI†).
N indicates that the pyrrole ring present at 1638 cm−1 in TPP shifts by 10 cm−1 in the presence of the PNCs (Fig. S8, ESI†).
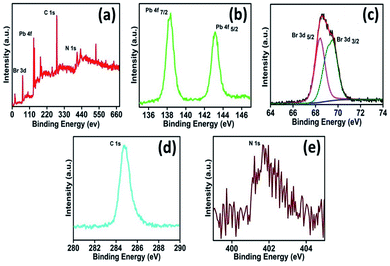 | ||
| Fig. 4 XPS spectra of (a) survey scan, (b) lead, (c) deconvoluted bromide, (d) carbon and (e) nitrogen with TPP molecules. | ||
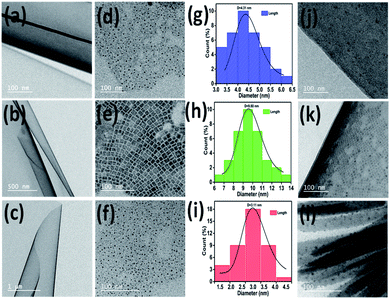
TEM images were used to study the morphology of TPP, PNCs and PNCs@TPP. The TEM images of TPP with scale bars of 100 nm (Fig. 5a), 500 nm (Fig. 5b) and 1 μm (Fig. 5c) show its sheet-like morphology. The tunability in the morphology of the perovskite nanocrystal solution (Fig. 5d–f) is evidenced by these TEM images. The TEM images of the PS-A (Fig. 5d), PS-B (Fig. 5e) and PS-C (Fig. 5f) perovskite solutions display quantum dot, nanoplate and quantum dot morphologies, respectively. The particle sizes of the blue- (Fig. 5g, 4.31 nm), green- (Fig. 5h, 9.80 nm) and red-luminescent (Fig. 5i, 3.11 nm) perovskite nanoparticles are calculated from the histogram plot. The interaction of TPP on the surface of the hydrophobically-capped PNCs is confirmed by the TEM images, as shown in Fig. 5j–l. These interactions cause electron transfer from the donor perovskite to the acceptor TPP molecules.
To measure the electrochemical properties, i.e. the reduction potential (Ered) and oxidation potential (Eox), of the PNCs and PNCs@TPP nanocomposites in solution form, cyclic voltammetry (CV) was performed at 298 K in CH2Cl2 using 0.1 M TBAPF6 as a supporting electrolyte (Fig. 6).
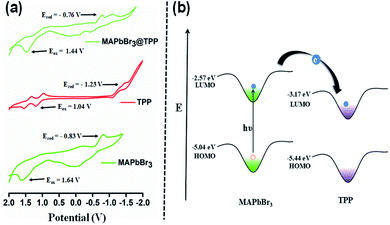 | ||
| Fig. 6 (a) Comparative CVs (vs. Ag/AgCl) of MAPbBr3, TPP and MAPbBr3@TPP solutions. (b) Schematic representation of the energy-level alignment of MAPbBr3 and TPP molecules. | ||
With the introduction of TPP solution to the perovskite nanocrystals, the reduction and oxidation potentials of the PNCs exhibited anodic and cathodic shifts as compared to the original PNCs, as shown in Fig. 6a. This indicates that TPP molecules have a significant effect on redox potential.
From the CV analysis (Fig. 6a), the bandgap values of the PNCs, PNCs@TPP and TPP solutions are calculated to be 2.47 eV, 2.20 eV and 2.27 eV, respectively. The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) are calculated as mentioned in previous reports.51
For the PNCs, the energy levels are −2.57 eV LUMO and −5.04 eV HOMO. For the PNCs@TPP nanocomposites, the energy levels are lower, with −3.64 eV LUMO and −5.84 eV HOMO, and the HOMO (−5.44 eV) and LUMO (−3.17 eV) energy levels have also been calculated for TPP. The band alignment of the HOMO and LUMO energy levels of MAPbBr3 as well as the HOMO and LUMO energy levels of TPP molecules indicate that TPP molecules withdraw electron density from the excited state of the PNC solution, and cause electron transfer from the PNCs to TPP molecules, as depicted in Fig. 6b.
Due to the spectral overlap integral and adsorption of TPP to the PNCs, fluorescence resonance energy transfer (FRET) from the PNCs to TPP was suspected to be the reason for the fluorescence quenching of the PNCs by TPP. However, the efficacy of energy transfer is highly dependent on two parameters: (i) the number of anchoring groups and (ii) the binding ability of acceptor molecules to the surface of the nanocrystals. However, TPP molecules have no valid anchor group. This leads to physical adsorption rather than anchoring of TPP molecules on the surface of the PNCs. Hence, the absence of the anchoring group and the lower emission intensity of porphyrin molecules as compared to other exciting fluorescent dyes excludes the possibility of FRET between the excited state of the PNCs and TPP.38,49
In conclusion, the charge transfer behaviour of MAPbX3 perovskite nanocrystals as electron donors and the TPP macrocycle as the acceptor was demonstrated. The addition of TPP to the PNC solution causes fluorescence quenching with no change in fluorescence lifetime. The static fluorescence quenching of the perovskite solution is attributed to the adsorption of TPP onto the surface of hydrophobically-capped PNC nanocrystals, which was also analyzed by TEM. The mode of interaction of TPP with the PNCs has been analyzed by 1H NMR and IR spectroscopy. Furthermore, by comparing the electrochemical properties of the PNCs@TPP nanocomposites with those of the PNCs, the LUMO as well as HOMO energy levels of the PNCs@TPP nanocomposites are significantly lower than those of the PNCs, indicating the effective transfer of electrons from the excited state of the PNCs to TPP molecules. These experimental findings not only open the way for understanding the electron transfer dynamics from MAPbBr3 nanocrystals to TPP molecules but also offer the opportunity for utilizing porphyrin molecules as an alternative source of the electron transporting materials for next-generation technologies, especially in optoelectronics and related applications.
Conflicts of interest
There is no conflict of interest to declare.Acknowledgements
The authors acknowledge the Institute Instrumentation Centre (IIC), IIT Roorkee, for the instrumentation facility. P. K. gratefully acknowledges the Science and Engineering Research Board (CRG/2020/000702), New Delhi, India, for their financial support. M. S. thanks the Science and Engineering Research Board (SER/CRG/2020/005958), New Delhi, for their financial support. A. J. acknowledges MHRD, India, for her doctoral fellowship. H. S. and S. K. acknowledge UGC, India, for their senior research fellowship.Notes and references
- M. D. Smith, B. A. Connor and H. I. Karunadasa, Chem. Rev., 2019, 119, 3104–3139 CrossRef CAS PubMed
.
-
(a) L. Polavarapu, B. Nickel, J. Feldmann and A. S. Urban, Adv. Energy Mater., 2017, 7, 1700267 CrossRef
; (b) A. Jha, P. Bansal, G. K. Nim and P. Kar, Opt. Mater., 2021, 111, 110660 CrossRef CAS
.
- W. Deng, H. Fang, X. Jin, X. Zhang and J. Jie, J. Mater. Chem. C, 2018, 6, 4831–4841 RSC
.
- H. Huang, L. Polavarapu, J. A. Sichert, A. S. Susha, A. S. Urban and A. L. Rogach, NPG Asia Mater., 2016, 8, e328 CrossRef CAS
.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed
.
- J.-H. Wei, X.-D. Wang, J.-F. Liao and D.-B. Kuang, ACS Appl. Electron. Mater., 2020, 2, 2707–2715 CrossRef CAS
.
-
(a) Y. Wang, H. Zhang, T. Zhang, W. Shi, M. Kan, J. Chen and Y. Zhao, Sol. RRL, 2019, 3, 1900197 CrossRef
; (b) H. Shankar, A. Jha and P. Kar, Mater. Adv., 2022, 3, 658–664 RSC
.
- S. D. Stranks and H. J. Snaith, Nat. Nanotechnol., 2015, 10, 391–402 CrossRef CAS PubMed
.
- S. T. Ha, R. Su, J. Xing, Q. Zhang and Q. H. Xiong, Chem. Sci., 2017, 8, 2522–2536 RSC
.
- Y. Fu, H. Zhu, A. W. Schrader, D. Liang, Q. Ding, P. Joshi, L. Hwang, X.-Y. Zhu and S. Jin, Nano Lett., 2016, 16, 1000–1008 CrossRef CAS PubMed
.
- G. Xing, N. Mathews, S. S. Lim, N. Yantara, X. Liu, D. Sabba, M. Gratzel, S. Mhaisalkar and T. C. Sum, Nat. Mater., 2014, 13, 476–480 CrossRef CAS PubMed
.
- S. A. Veldhuis, P. P. Boix, N. Yantara, M. Li, T. C. Sum, N. Mathews and S. G. Mhaisalkar, Adv. Mater., 2016, 28, 6804–6834 CrossRef CAS PubMed
.
- N. Wang, L. Cheng, R. Ge, S. Zhang, Y. Miao, W. Zou and W. Huang, Nat. Photonics, 2016, 10(11), 699–704 CrossRef CAS
.
- S. D. Stranks and H. J. Snaith, Nat. Nanotechnol., 2015, 10(5), 391–402 CrossRef CAS PubMed
.
- M. T. Hörantner, T. Leijtens, M. E. Ziffer, G. E. Eperon, M. G. Christoforo, M. D. McGehee and H. J. Snaith, ACS Energy Lett., 2017, 2(10), 2506–2513 CrossRef
.
- Q. Lin, A. Armin, R. C. R. Nagiri, P. L. Burn and P. Meredith, Nat. Photonics, 2015, 9(2), 106–112 CrossRef CAS
.
-
(a) P. Bansal and P. Kar, Chem. Commun., 2019, 55, 6543–6546 RSC
; (b) M. V. kovalenko, L. Protesescu and M. I. Bodnarchuk, Science, 2017, 358, 745–750 CrossRef CAS PubMed
.
- Y. Wang, Z. Lv, Q. Liao, H. Shan, J. Chen, Y. Zhou, L. Zhou, X. Chen, V. A. Roy and Z. Wang, Adv. Mater., 2018, 30, 1800327 CrossRef PubMed
.
- M.-A. Stoeckel, M. Gobbi, S. Bonacchi, F. Liscio, L. Ferlauto, E. Orgiu and P. Samorì, Adv. Mater., 2017, 29, 1702469 CrossRef PubMed
.
- D. A. Wheeler and J. Z. Zhang, Adv. Mater., 2013, 25, 2878–2896 CrossRef CAS PubMed
.
- B. Luo, Y. C. Pu, S. A. Lindley, Y. Yang, L. Lu, Y. Li, X. Li and J. Z. Zhang, Angew. Chem., Int. Ed., 2016, 55, 8864–8868 CrossRef CAS PubMed
.
- T. Higashino, Y. Fujimori, I. Nishimura and H. Imahori, J. Porphyrins Phthalocyanines, 2020, 24, 67–74 CrossRef CAS
.
- K. Kurlekar, A. Anjali, S. Sonalin, P. M. Imran and S. Nagarajan, ACS Appl. Electron. Mater., 2020, 2(10), 3402–3408 CrossRef CAS
.
- W. Li, Z. Liu, H. Wu, Y.-B. Cheng, Z. Zhao and H. He, J. Phys. Chem. C, 2015, 119(10), 5265–5273 CrossRef CAS
.
- F. Li, S. I. Yang, Y. Ciringh, J. Seth, C. H. Martin, D. L. Singh, D. Kim, R. R. Birge, D. F. Bocian, D. Holten and J. S. Lindsey, J. Am. Chem. Soc., 1998, 120, 10001–10017 CrossRef CAS
.
- Q. A. Akkerman, S. G. Motti, A. R. Srimath Kandada, E. Mosconi, V. D'Innocenzo, G. Bertoni, S. Marras, B. A. Kamino, L. Miranda, F. de Angelis, A. Petrozza, M. Prato and L. Manna, J. Am. Chem. Soc., 2016, 138, 1010–1016 CrossRef CAS PubMed
.
- G. C. Adhikari, H. Zhu, P. A. Vargas and P. Zhu, J. Phys. Chem. C, 2018, 122(26), 15041–15046 CrossRef CAS
.
- A. Jha, H. Shankar and P. Kar, New J. Chem., 2022, 46, 844–850 RSC
.
- A. Kojima, M. Ikegami, K. Teshima and T. Miyasaka, Chem. Lett., 2012, 41, 397–399 CrossRef CAS
.
- L. Chouhan, S. Ghimire, C. Subrahmanyam, T. Miyasaka and V. Biju, Chem. Soc. Rev., 2020, 49, 2869–2885 RSC
.
- K. Zeng, Z. Tong, L. Ma, W.-H. Zhu, W. Wua and Y. Xie, Energy Environ. Sci., 2020, 13, 1617–1657 RSC
.
- J. Yang, J. Jing and Y. Zhu, Adv. Mater., 2021, 33, 2101026 CrossRef CAS PubMed
.
- X. Yin, Z. Song, Z. Li and W. Tang, Energy Environ. Sci., 2020, 13, 4057–4086 RSC
.
- C. S. Ponseca Jr, E. M. Hutter, P. Piatkowski, B. Cohen, T. Pascher, A. Douhal, A. Yartsev, V. Sundstrçm and T. J. Savenije, J. Am. Chem. Soc., 2015, 137, 16043–16048 CrossRef PubMed
.
- J. Xu, A. Buin, A. H. Ip, W. Li, O. Voznyy, R. Comin, M. Yuan, S. Jeon, Z. Ning, J. J. McDowell, P. Kanjanaboos, J.-P. Sun, X. Lan, L. N. Quan, D. H. Kim, I. G. Hill, P. Maksymovych and E. H. Sargent, Nat. Commun., 2015, 6, 7081 CrossRef CAS PubMed
.
- A. Marchioro, J. Teuscher, D. Friedrich, M. Kunst, R. Krol, T. Moehl, M. Gratzel and J.-E. Moser, Nature, 2014, 508, 250–255 Search PubMed
.
- R. A. Scheidt, E. Kerns, P. V. Kamat and J. Phys, Chem. Lett., 2018, 9(20), 5962–5969 CAS
.
- R. Zhu, C. Gao, T. Sun, L. Shen, D. Sun and X. Li, Langmuir, 2016, 32, 3294–3299 CrossRef CAS PubMed
.
-
(a) V. C. Nair, C. Muthu, A. L. Rogach, R. Kohara and V. Biju, Angew. Chem., Int. Ed., 2017, 56, 1214–1218 CrossRef CAS PubMed
; (b) S. Ghimire, V. C. Nair, C. Muthu, K. Yuyama, M. Vacha and V. Biju, Nanoscale, 2019, 11, 9335–9340 RSC
.
- B. M. Sachith, T. Okamoto, S. Ghimire, T. Umeyama, Y. Takano, H. Imahori and V. Biju, J. Phys. Chem. Lett., 2021, 12, 8644–8651 CrossRef CAS PubMed
.
- K. Wu, G. Liang, Q. Shang, Y. Ren, D. Kong and T. Lian, J. Am. Chem. Soc., 2015, 137, 12792–12795 CrossRef CAS PubMed
.
- P. Bansal, X. Zhang, H. Wang, P. Kar and W. W. Yu, Nanoscale Adv., 2020, 2, 808–813 RSC
.
- X. X. Feng, X. D. Lv, Q. Liang, J. Cao and Y. Tang, ACS Appl. Mater. Interfaces, 2020, 12(14), 16236–16242 CrossRef CAS PubMed
.
- G. Ghosh, K. Marjit, S. Ghosh, A. Ghosh, R. Ahammed, A. De Sarkar and A. Patra, J. Phys. Chem. C, 2021, 125, 5859–5869 CrossRef CAS
.
-
(a) S. Kumar, N. Chaudhri, W. R. Osterloh, K. M. Kadish and M. Sankar, Dalton Trans., 2021, 50, 17086–17100 RSC
; (b) W. R. Osterloh, S. Kumar, N. Chaudhri, Y. Fang, M. Sankar and K. M. Kadish, Inorg. Chem., 2020, 59, 16737–16746 CrossRef CAS PubMed
; (c) S. Shanmugathasan, C. Edwards and R. W. Boyle, Advances in modern synthetic Porphyrin Chemistry, Tetrahedron, 2020, 56, 1025–1146 CrossRef
.
- S. M. Rojas-Montoya, M. Vonlanthen, P. Porcu, G. Flores-Rojas, A. Ruiu, D. Morales-Morales and E. Rivera, Dalton Trans., 2019, 48, 10435–10447 RSC
.
- H. S. Yang, S. H. Noh, E. H. Suh, J. Jung, J. G. Oh, K. H. Lee and J. Jang, ACS Appl. Mater. Interfaces, 2021, 13, 4374–4384 CrossRef CAS PubMed
.
- A. Pal, S. Srivastava, P. Saini, S. Raina, P. P. Ingole, R. Gupta and S. Sapra, J. Phys. Chem. C, 2015, 119(39), 22690–22699 CrossRef CAS
.
- C. Muthu, A. Vijayan and V. C. Nair, Chem. - Asian J., 2017, 12(9), 988–995 CrossRef CAS PubMed
.
- I. Levchuk, P. Herre, M. Brandl, A. Osvet, R. Hock, W. Peukert, P. Schweizer, E. Spiecker, M. Batentschuk and C. J. Brabec, Chem. Commun., 2017, 53, 244–247 RSC
.
- L. Pan, B. Hu, X. Zhu, X. Chen, J. Shang, H. Tan, W. Xue, Y. Zhu, G. Liu and R. W. Li, J. Mater. Chem. C, 2013, 1, 4556–4564 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1na00835h |
| This journal is © The Royal Society of Chemistry 2022 |