Open Access Article
Open Access ArticleFoldable batteries: from materials to devices
Insu
Jeong†
 a,
Dong-Yeob
Han†
a,
Jongha
Hwang†
b,
Woo-Jin
Song
*b and
Soojin
Park
a,
Dong-Yeob
Han†
a,
Jongha
Hwang†
b,
Woo-Jin
Song
*b and
Soojin
Park
 *a
*a
aDepartment of Chemistry, Pohang University of Science and Technology, Pohang 37673, South Korea. E-mail: soojin.park@postech.ac.kr
bDepartment of Organic Materials Engineering, Chungnam National University, Daejeon 34134, South Korea. E-mail: wjsong@cnu.ac.kr
First published on 3rd February 2022
Abstract
Wearable electronics is a growing field that has important applications in advanced human-integrated systems with high performance and mechanical deformability, especially foldable characteristics. Although foldable electronics such as rollable TVs (LG signature OLED R) or foldable smartphones (Samsung Galaxy Z fold/flip series) have been successfully established in the market, these devices are still powered by rigid and stiff batteries. Therefore, to realize fully wearable devices, it is necessary to develop state-of-the-art foldable batteries with high performance and safety in dynamic deformation states. In this review, we cover the recent progress in developing materials and system designs for foldable batteries. The Materials section is divided into three sections aimed at helping researchers choose suitable materials for their systems. Several foldable battery systems are discussed and the combination of innovative materials and system design that yields successful devices is considered. Furthermore, the basic analysis process of electrochemical and mechanical properties is provided as a guide for researchers interested in the evaluation of foldable battery systems. The current challenges facing the practical application of foldable batteries are briefly discussed. This review will help researchers to understand various aspects (from material preparation to battery configuration) of foldable batteries and provide a brief guideline for evaluating the performance of these batteries.
1. Introduction
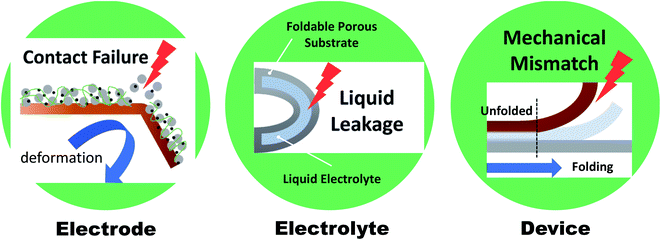
Wearable electronics have gained increasing attention over the past few years due to their potential for use in applications such as health care devices, sensors, and implantable devices.1–4 Wearable electronics should be attachable to human skin or implantable in the human body and hence should be both deformable and soft.5 Deformation commonly includes bending, twisting, folding, and stretching, but can be simplified to only folding and stretching. A wide range of “folding” processes include all deformations, except for stretching that is governed by a different mechanism and therefore requires further innovative strategies.6 Hereafter, deformability will refer mainly to foldability (rather than stretchability), and various systems will be discussed in this regard. In recent years, considerable efforts are focused on achieving foldability accompanied by comfort, light weight, and high performance. Foldable displays, represented by rollable TVs (LG signature OLED R) or foldable smartphones (Samsung Galaxy Z fold/flip series), have been developed and successfully established in the market. These displays showed that foldability can be achieved even in multilayered structures, and that the realization of next-generation wearable devices has begun. In this regard, batteries, which supply power to a device (including displays), must satisfy the current state-of-the-art foldability requirements while maintaining excellent performance. However, the development remains in an early stage. A significant breakthrough is required for foldable batteries.Normally, batteries consist of electrodes, a separator, and an electrolyte. However, direct use of the materials comprising these components in foldable batteries is limited by various factors (Fig. 1). Electrodes are commonly manufactured via slurry casting on rigid metal current collectors (copper and aluminum). The slurry is composed of active materials mixed with polymeric binders (e.g., polyvinylidene fluoride (PVDF)) and conductive additives, and can be easily delaminated from the collector when external mechanical stimuli are applied. Moreover, electrolytes consisting of organic solvents (e.g., ethylene carbon) and lithium salts (e.g., lithium hexafluorophosphate (LiPF6)) can lead to leakage from packing materials during the deformation process. Solid-state or gel-polymer electrolytes, which can serve as a separator membrane, are considered promising alternatives to their liquid counterparts as the leakage and flammability issue can be avoided. However, even when all components are successfully substituted with suitable materials for foldable batteries, the whole system may exhibit insufficient foldability due to mechanical modulus mismatch of each component under an external stress. Therefore, the entire battery system and each component of the battery must be reinvented in order to satisfy the requirements of high mechanical durability and excellent and stable electrochemical performance for practical applications.
Intrinsically foldable components have been developed for achieving excellent foldability and electrochemical properties. This is especially true for electrodes, where replacement of the alternating rigid metal current collector was considered urgent. For example, carbon materials such as carbon nanotubes, graphene, or carbon cloths can serve as an excellent backbone structure, which is highly conductive and foldable. Unlike the conventional battery systems, the reported systems may sometimes (rather than always) include components (such as binders, current collectors, or separators) for realizing high durability under repeated deformation.7 Material development and innovative system designs such as patterning,8 island structures,9 or kirigami/origami structures10,11 have been reported as strategies for achieving foldable batteries by decoupling the system into an energy-storage section and a foldable section. The reported systems exhibited high deformability and employed rigid but highly conductive components of conventional batteries.
Several reviews have discussed the materials and battery system designs associated with flexible battery systems.7,12,13 However, these reviews have focused only on lithium-ion batteries or primarily on materials, and in most cases the definition of flexibility between folding and stretching is ambiguous. A comprehensive review, which considers materials with battery system designs and a wide range of foldable batteries, is therefore still necessary. In this review, we focus mainly on the design strategies of materials and various devices for achieving foldability with practical electrochemical and mechanical performances. First, recent progress in developing materials and system designs for foldable batteries is summarized. The Materials section is further divided into three parts (based on the dimensional nanostructures) in order to help researchers choose suitable materials for their systems. Various foldable battery systems including lithium-ion batteries, air batteries (Zn–air and Li–air), and multi-valent batteries (Zn and Mg) are then introduced and the combination of innovative materials and system designs for achieving successful devices is discussed. In addition, the basic process of electrochemical and mechanical analysis is provided to guide researchers in the evaluation of foldable battery systems. At the end of this review, current challenges facing the practical applications of foldable batteries are briefly covered. This review will help researchers to understand various aspects (from material preparation to battery configuration) of foldable batteries and provide a brief guideline for evaluating the performance of such batteries.
2. Morphologies of materials
A well-designed structure of materials can maintain the original formation in the folding or bending state in foldable batteries and hence the morphologies of materials play a significant role in the flexibility of these batteries.14–16 Furthermore, the dimensional stability of electrodes is critical to mechanical properties associated with the excellent cohesion and adhesion among active materials, binders, and conducting agents.17 Even if flexible materials are included in the electrode, selecting unsuitable materials for electrode flexibility can destroy the device in the folding state.18 Therefore, we should determine the mechanical properties of each electrode component material with various dimensions in order to achieve flexibility of foldable and wearable devices. For the perfect fabrication of foldable batteries, we must consider various materials as current collectors, active materials, binders, and additives, and hence, in this work, we investigate the specific materials according to multiple dimensions for foldable batteries. These materials are mainly divided into three categories in the flexible battery system: one-dimensional (1D), two-dimensional (2D), and three-dimensional (3D).19–23 Many kinds of 1D, 2D, or 3D materials have been studied with the aim of achieving foldable batteries with high dimensional stability. These include carbon fibers (CFs),24 carbon cloths (CCs),25 carbon nanotubes (CNTs),26 graphene,27 polymeric materials,28 metal oxides,29 transition-metal carbides (TMCs),30 or metal–organic frameworks (MOFs).31 The mechanical properties, electrochemical performance, and current applicability of these materials in foldable batteries are discussed in this section.2.1 One-dimensional materials
1D materials such as CFs, CNTs, and metal wires are considered quite suitable for many applications, owing to their numerous active sites and effective channels that generate short ion diffusion pathways for foldable batteries with excellent mechanical performance.32–34 These materials can enable the entanglement of active materials, and hence mechanical stability is maintained in the folding state. Therefore, 1D materials, which can be classified as carbon-based materials and metal-based materials, are considered excellent candidates for realizing outstanding performance of foldable batteries.35,36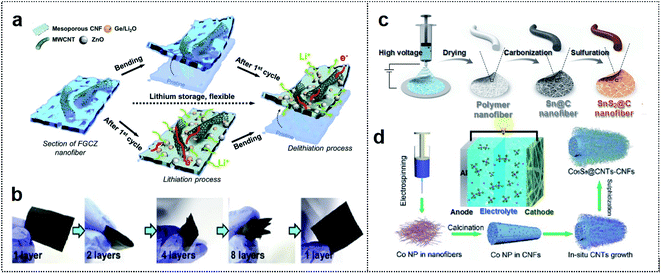 | ||
| Fig. 2 Carbon-based foldable 1D electrode. (a) Schematic illustration of FGCZ structures and the mechanism for lithium storage. (b) Digital images of the multi-folded FGCZ film and the recovery state of the FGCZ film. Reproduced from ref. 43. Copyright 2019, Elsevier. (c) Schematic illustration of SnS2@CF by the electrospinning method. Reproduced from ref. 44. Copyright 2021, Royal Society of Chemistry. (d) Schematic illustration of the binder-free and free-standing Co9S8@CNT–CNF mats via the electrospinning method. Reproduced from ref. 45. Copyright 2018, Wiley-VCH. | ||
A polymer-based electrospinning method can also be utilized for the fabrication of 1D CF scaffold. Ci and co-workers reported SnS2-based nitrogen/sulfur (N/S) co-doped CFs as flexible anode materials for foldable K-ion batteries.44 Polyacrylonitrile (PAN) fiber-derived 1D CF was utilized as the scaffold for depositing Sn particles (Fig. 2c). The carbonized PAN fiber with metal particles could provide a high capacity of 323.9 mA h g−1 after 30 cycles (80.8% capacity retention). In addition, the fiber enabled operation of 27 LEDs, which remained in operation in 1-fold (180° deformation) and 2-fold states in the SnS2@C anode-based K ion battery.
1D carbon-based materials can also be used as cathode materials in foldable batteries. Wang and co-workers used an electrospinning method to design a binder-free and free-standing CF film with cobalt sulfide encapsulated CNTs (Co9S8@CNT–CNF) as a cathode electrode in flexible Al ion batteries (Fig. 2d).45 1D CNTs and CNFs could provide entanglement with a porous network of high surface area (115.1 m2 g−1), and Co9S8 particles were well distributed among the CNTs. Consequently, the Co9S8@CNT–CNF-based Al ion battery exhibited outstanding cycle stability (87 mA h g−1 at 1 A g−1 and 90% coulombic efficiency after 6000 cycles) and powered LEDs in the deformation state. 1D carbon-based materials can provide good mechanical properties with high flexibility and good adhesion among flexible substrates, active materials, and conductive additives. However, these materials are sometimes easily aggregated, leading to self-entanglement due to strong π–π interactions, and limited flexibility of the electrodes.46,47 Therefore, the appropriate utilization and combination of 1D carbon-based materials are crucial for various foldable batteries.
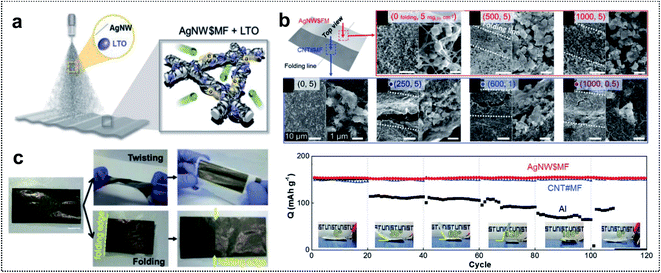 | ||
| Fig. 3 Metal-based foldable 1D electrode. (a) Schematic illustration of LTO-loaded AgNW$MF. (b) SEM images of the foldable electrode. (up) Top-view images of LTO-loaded AgNW$MF: before folding, after 500 folding cycles, and after 1000 folding cycles. (Down) Top-view images of LTO-loaded CNT#MF: in 5 mgLTO cm−2 before folding, in 5 mgLTO cm−2 after 250 folding cycles, in 1 mgLTO cm−2 after 600 folding cycles and in 0.5 mgLTO cm−2 after 1000 folding cycles. (c) Digital images of LTO-loaded AgNW$MF after twisting and folding. (d) Capacity retention of folded cells at different angles at 1C. Reproduced from ref. 55. Copyright 2018, Wiley-VCH. | ||
2.2 Two-dimensional materials
The second category of materials is 2D-based materials, composed of flexible sheet-type components such as graphene and TMC (MXene). Generally, 2D crystalline sheets are composed of atomically thin layers with intralayer covalent bonding and interlayer van der Waals bonding,56,57 which can provide outstanding mechanical properties and high electrical conductivity for foldable batteries.58–60 These materials lack dangling surface bonds and exhibit excellent electrochemical stability.61–63 Moreover, 2D sheet-type materials are suitable for flat large-area nanomanufacturing technologies that generate foldable devices.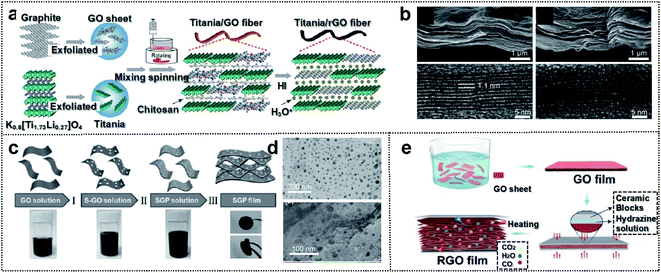 | ||
| Fig. 4 Carbon-based foldable 2D electrode. (a) Schematic illustration of the fabrication process for the hybrid fiber of titania/rGO (b) SEM (upper panel) and HRTEM (lower panel) images for (left) as-spun dry titania/GO fiber and (right) HI-reduced titania/rGO fiber. Reproduced from ref. 74. Copyright 2017, American Chemical Society. (c) Schematic illustration of the fabrication process of SGP cathodes. (d) TEM images of (up) nano-S grown on GO, and (down) SGP. Reproduced from ref. 75. Copyright 2017, Wiley-VCH. (e) Schematic illustration of the fabrication process of the ultraflexible and porous graphene film. Reproduced from ref. 76. Copyright 2018, Wiley-VCH. | ||
Yu and co-workers reported a solid-state foldable supercapacitor.76 They engineered the micro-void distribution of rGO films through a leavening-accompanied reduction treatment of GO films with the spillage of gaseous species such as water and carbon dioxide. This treatment expanded the graphene layers, thereby yielding a porous structure with micro-voids (Fig. 4e). The micro-voids could improve the foldability of the rGO foam structure because the porous films could prevent stress localization and structural breaks at the folding site (no crack formation after 2000 folding cycles). The rGO-based supercapacitor with micro-voids resulted in almost 100% capacitance retention at 5 mV s−1 after 2000 cycles of single or double folding (capacitance: 221.6 mF cm−2 and energy density: 30.8 μW h cm−2). These studies indicate that the structural engineering of 2D graphene is crucial for the design of foldable storage devices.
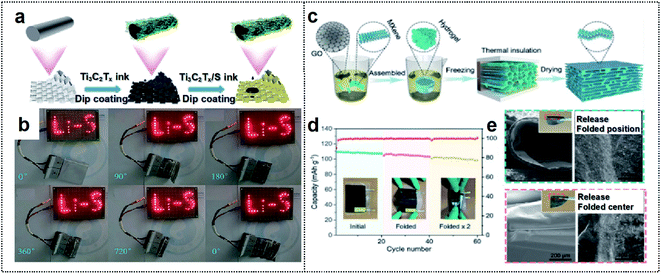 | ||
| Fig. 5 MXene-based foldable 2D electrode. (a) Schematic illustration of the fabrication process of the MF@Ti3C2Tx/S cathode. (b) A “Li–S” shaped string of light contains 30 LEDs lit by MF@Ti3C2Tx/S50 electrode soft-packaged Li–S batteries at different bending angles. Reproduced from ref. 82. Copyright 2021, Royal Society of Chemistry. (c) Schematic illustration of the fabrication process of the MGA material. (d) Cycling performance of the LMO//MGA@Zn pouch cells at various folding times. (e) SEM images of the MGA sample after folding (left) and releasing (right) once (up) and twice (down). The inset images indicate the optical pictures of the MGA sample after folding once (up) and twice (down), respectively. Reproduced from ref. 83. Copyright 2021, Wiley-VCH. | ||
MXenes can also be used to fabricate composites with other 2D materials (such as graphene) to extend structural advantages of the material. Chen and co-workers used an MXene to fabricate an aerogel with 2D graphene (MGA) for a foldable electrode (Fig. 5c).83 The resulting MXene/rGO composites provided a large surface area for the dense deposition of active materials. Their integrating strategy provided mechanical strength and a large electrode/electrolyte contact area for high flexibility. Furthermore, the fluorine termination of MXene could inhibit the dendritic growth of Zn during the charge/discharge process, thereby promoting long-term stability. Therefore, the MGA-based electrode exhibited a high coulombic efficiency of 99.67% at a high current density of 10 mA cm−2 after 600 cycles. This electrode achieved a lower overpotential (88 vs. 33 mV at 60 cycles) than that of commercial Cu foil. The flexible MGA-based Zn-ion batteries with a LiMn2O4 cathode exhibited excellent deformability without the loss of active materials during the charge/discharge process and a high capacity retention of over 91% after folding cycles (Fig. 5d and e). As a result, the construction of 2D-based materials has a high potential, owing to the excellent mechanical properties and high electrical conductivity achievable for foldable batteries.
2.3 Three-dimensional materials
Regarding foldable devices, 3D-based materials with pores can also be candidates, owing to available mechanical movement of internal space occurring in the free volume and high ion-absorption area from the electrolyte in the deformation state.84,85 Two fabrication strategies are typically used to construct 3D porous materials: (i) the utilization of 3D precursors such as carbon cloth,86 carbon aerogel,87 and metal mesh.88 (ii) The combination of 1D and 2D materials.89,90 In the case of 3D substrates, an intrinsically strong and porous network structure can provide mechanical stability to the electrodes and excellent adhesion of active materials, binders, and additives without spalling in the deformation state. Xue and co-workers reported the fabrication process of Ag nanoparticle-deposited 3D carbon cloth obtained via the inkjet printing method (Fig. 6a).91 This method is a cost-efficient process that allows direct deposition on the 3D carbon support for uniform and dense deposition of Ag nanoparticles (Fig. 6b). The results suggested that a heterometallic seed-mediated method prevented dendrite growth of Zn into a 3D substrate via the inkjet printing method, as confirmed through in situ optical microscopy during the Zn-plating process of AgNPs@CC scaffolds. The full cell with AgNPs@CC/Zn exhibited a high capacity of 282 mA h g−1 at 0.5 A g−1. Correspondingly, foldable batteries with cycling stability (1200 cycles of capacitance retention at 5.0 A g−1) and high capacity at different deformations (∼95% of capacity retention in flat, bent, fold, and flat states) were obtained.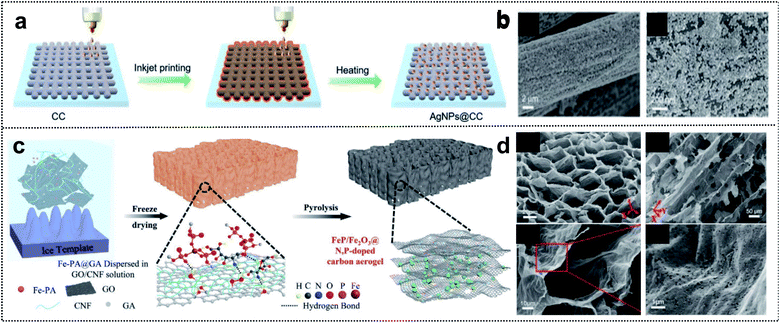 | ||
| Fig. 6 Foldable 3D electrode. (a) Schematic illustration of the fabrication process of AgNPs@CC. (b) SEM images of AgNPs@CC scaffolds. Reproduced from ref. 91. Copyright 2021, Wiley-VCH. (c) Schematic illustration of the fabrication process of FeP/Fe2O3@NPCA. (d) SEM images of FeP/Fe2O3@NPCA at different magnifications. Reproduced from ref. 92. Copyright 2020, Wiley-VCH. | ||
Compared with the utilization of 3D precursors, the construction of 1D and 2D composites includes chemical bonding such as covalent bonding among the functional groups of 1D and 2D materials. This has structural advantages for foldable devices due to good accessibility of the electrolyte and the mechanical strength of the 3D network entanglement. Lu and co-workers designed a 3D honeycomb-like carbon aerogel with an FeP/Fe2O3@N/P-doped carbon aerogel by utilizing 2D GO and 1D cellulose nanofibrils (CNFs) for foldable cathode materials (Fig. 6c).92 The strategy exploited the good dispersibility of GO and flexible CNF building blocks in water for excellent mechanical properties, and 1D CNFs prevented the stacking of reduced GO sheets, thereby resulting in a porous structure (Fig. 6d). This carbon aerogel provided outstanding mechanical stability during bending and compression and contributed to efficient electrolyte diffusion and ionic conductivity. The low energy gap of the FeP/Fe2O3@N/P-doped carbon aerogel as the electrochemical catalyst for Zn–air batteries was considerably better than those of other catalysts (0.79 vs. 0.93 V for FeNx–C) and even similar to that of commercial noble catalysts (0.73 V). Zn–air batteries with the FeP/Fe2O3@N/P-doped carbon aerogel exhibited a high capacity of 676 mA h g−1 and an energy density of 517 W h kg−1. Moreover, in the bending state, the assembled foldable battery exhibited high stability with the operation of 12 yellow LEDs. We summarize the advantages of each dimension of materials for foldable batteries. 1D materials guarantee short ion diffusion pathways and outstanding adhesion properties. 2D materials have outstanding mechanical properties and high electrical conductivity. Lastly, 3D materials have both the internal space of free volume and high ion-absorption area in the mechanical deformation state. These material strategies for 1D, 2D, and 3D composites provide inspiration for the development of foldable electrode materials and the practical application of foldable batteries.
2.4 Structural design
Structure design represents a new method for achieving system-level-integration of energy-storage-devices without changing the dimension of materials; this method is compatible with the standard manufacturing processes and is cost effective.22,93–97 The design of a foldable energy-storage device begins with decoupling of the energy-storage parts (rigid parts) from the flexibility providing parts (soft parts). Furthermore, an appropriate balance between the two sets of parts is necessary for realizing high-performance foldable batteries with stable electrochemical properties. The inspiration (such as calligraphy,98 folding beds,99 accordions,100 spines,101 and joints102) for the structural design of the foldable energy-storage-devices arises mainly from our daily lives. Recently, inspired by the structure of the human spine, Yang and co-workers fabricated a spine-like battery with a high energy density. The schematic presented in Fig. 7a shows the configuration of the battery, which consists of hard components (energy storage) and soft components (flexibility providers) corresponding to the vertebrae and marrow, respectively. The conventional anode, separator, cathode, and polyethylene supporting film were stacked and cut into a long strip with multiple branches. Afterward, the strips, which were wrapped around the backbone, formed thick stacks for high energy density, and unwound parts between the strips provided excellent flexibility to the whole device. The cycling performance was determined in both the flat state and bent state of the spine-like battery (Fig. 7b). Capacity retentions of 99.4% after 20 cycles in the flexed state and 99.4% after 10 cycles in the twisted state (95.6% capacity retention after 50 cycles in the flat state) were observed. This suggested that, owing to the unique structural design of the battery, the deformations had no effect on the cycling performance of the battery. Moreover, during a dynamic mechanical load experiment, the spine-like battery was charged/discharged during repetitive deformations (Fig. 7c). In the dynamic flexed state at 0.5C, the specific discharge capacity decreased in the initial cycles of bending (3.8 mA h g−1 from cycles 6–10), but remained steady from the 10th cycle (125.7 mA h g−1) to the 15th cycle (124.6 mA h g−1).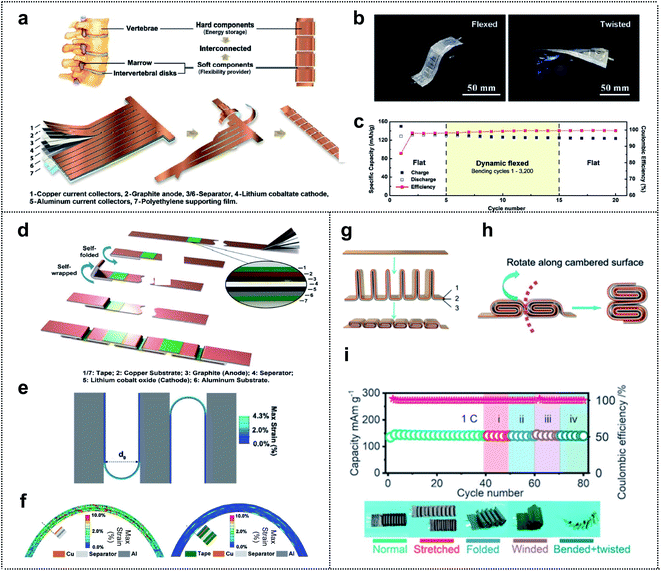 | ||
| Fig. 7 Structure design. (a) The schematic of the structure and the fabrication process of a spine-like battery. (b) Optical images of the spine battery in the flexed and twisted states. (c) The cycling performance of a spine-like battery in different configurations by a repetitive mechanical load test. Reproduced from ref. 101. Copyright 2018, Wiley-VCH. (d) Fabrication process of a zigzag-like foldable battery. (e) Folding deformation of a zigzag-like battery structure with a bending diameter of 3 mm where the tape length is half of the stack length. (f) Strain contours of electrode multilayers at folding joints without (left) and with (right) protective tapes. Reproduced from ref. 103. Copyright 2019, Wiley-VCH. (g) The schematic of the structure and fabrication process of the flexible batteries, where 1, 2 and 3 correspond to the anode, separator, and cathode, respectively. (h) The folding schematic of the battery with cubic units. (i) Charge/discharge cycling test of the cell with cylindrical units in different configurations at 1C. Reproduced from ref. 102. Copyright 2021, Royal Society of Chemistry. | ||
Further developments in structural design included a zigzag-like foldable battery that was fabricated by Yang and co-workers a year after the spine-like battery was developed.103 The zigzag battery, with excellent foldability and high energy density, was composed of a conventional graphite anode/separator/lithium cobalt oxide cathode with its metal current collectors (Cu and Al foil) and tape. As shown in Fig. 7d, the division segments that act as future folding joints (soft parts) were protected by the thin tape for future deformation. This tape occupied <4% of the area and had little effect on the energy density (<0.5%). Based on the finite element calculation results of the mechanical foldability and durability, Yang et al. optimized the structural design for excellent flexibility while maintaining the electrochemical performance of the cell. Fig. 7e shows the selected gap-less structure (zigzag) that can be folded by 180° and become self-compacted, and the smallest bending diameter (d0) for the fixed length of folding joints compared with that of other structural designs. Regarding the usefulness of thin tape, they showed the strain contours of electrode multilayers at folding joints with and without protective thin tapes in 180° folding states (Fig. 7f). The results revealed that 0.5% and 5.5% of the shear strain were borne by the metal foil in the electrode with and without the protective tape, respectively. Thus, the role of the tape, which covers both sides of the folding joints, in enhancing the mechanical durability of the foldable battery during the deformation was verified.
Zhi and co-workers, inspired by the human joints that can accommodate large deformation, fabricated a foldable lithium-ion battery that is similar to the joints that enable bending, twisting, and even folding. Fig. 7g shows the stack of an anode (1), separator (2), and cathode (3) that is divided into interconnected segments, with each segment folded in half (rigid parts). The two rigid stacks are connected by a junction (soft parts), which serves as the flexibility-providing ligament. Therefore, the designed units (here, cubic units) move along the cambered surface of the thick stack during bending and the additional space between the rigid parts is unnecessary and may be advantageous in terms of increasing energy density (Fig. 7h). They also proposed three types of units, namely triangular prism-shaped, cubic, and cylindrical units. Among batteries consisting of these units, the foldable battery with cylindrical units can bear more severe deformations due to the characteristics of a circle, as confirmed by finite element analysis. To evaluate the electrochemical performance of the designed foldable batteries, the batteries were tested at 1C with various configurations including normal, stretched, folded, wound, bent, and twisted (Fig. 7i). No significant capacity fading for the cells was observed, even under severe mechanical deformation conditions, thereby confirming the well-designed structure of the foldable lithium-ion batteries (LIBs).
3. Devices
3.1 Li-ion batteries
Lithium-ion batteries are considered one of the most promising energy-storage systems for next-generation wearable devices as they have current state-of-the art electrochemical properties such as high energy density and working voltage, and excellent cycle life.104 Foldable lithium-ion batteries (FLIBs) exhibit excellent mechanical durability and the aforementioned electrochemical characteristics even under repeated deformation. FLIBs can be realized by using foldable electrodes94,105–107 and electrolytes108–110 or introducing innovative structural designs.93Recently, Lu and co-workers obtained FLIBs by introducing carbon coated ion exchange synthesized nickel oxide nanosheet arrays into carbon cloth (C@IENiO-CC) as a foldable electrode. The electrode can be fabricated through a sequential process – carbon coating, ion exchange, and dehydration, as shown in Fig. 8a. This process allows the electrode to avoid the reduction issue of transition-metal oxides during the high-temperature formation step of carbon coating and preserve the nanostructure of the starting template, such as interwoven Mg(OH)2 nanosheets. FLIBs were assembled into a pouch cell (see Fig. 8b). The cell maintained excellent cycling performance in different bending states (0°, 90°, and 180° followed by returning to 0°) at a current density of 0.25 mA cm−2 (Fig. 8c) and successfully powered green LED bulbs in these states (Fig. 8d).
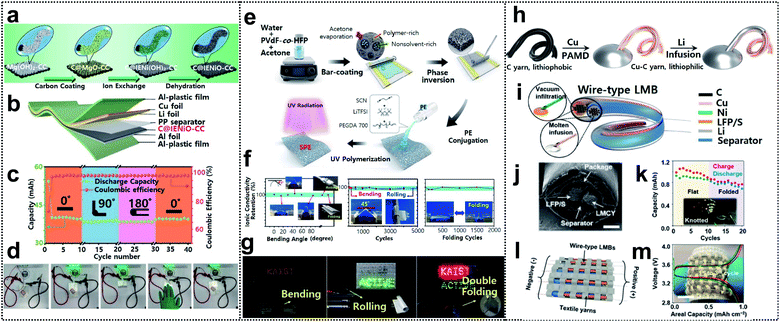 | ||
Fig. 8 Foldable lithium-ion batteries. (a) Schematic illustration of fabrication of carbon-coated IE-synthesized NiO nanosheet arrays on CC(C@IENiO-CC), (b) a pouch cell structure based on C@IENiO-CC, (c) cycling performance of the cell in bending states and (d) optical images of the cell lighting up LED bulbs in flat and bended states. Reproduced from ref. 107. Copyright 2021, Wiley-VCH. (e) Schematic procedure for preparing porous PVDF-co-HFP intertwined SPE, (f) ionic conductivity under several deformation processes and (g) optical images of LEDs lighted by LFP/40-INSPE 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/Li with bending, rolling, and double-folding. Reproduced from ref. 109. Copyright 2020, American Chemical Society. (h) Fabrication process of a lithium–metal composite yarn (LMCY), (i) structural design of the wire-type lithium–metal batteries (LMBs), (j) sectional SEM image of the as-fabricated wire-type LFP‖LMCY battery, (k) capacity retention of the battery in flat and folded states, and inset shows the optical image of powered LEDs by the knotted batteries, (l) schematic image of the battery textile and (m) voltage profiles of the battery textile, the inset shows the optical image of the as-fabricated battery textile. Reproduced from ref. 111. Copyright 2021, Wiley-VCH. 2/Li with bending, rolling, and double-folding. Reproduced from ref. 109. Copyright 2020, American Chemical Society. (h) Fabrication process of a lithium–metal composite yarn (LMCY), (i) structural design of the wire-type lithium–metal batteries (LMBs), (j) sectional SEM image of the as-fabricated wire-type LFP‖LMCY battery, (k) capacity retention of the battery in flat and folded states, and inset shows the optical image of powered LEDs by the knotted batteries, (l) schematic image of the battery textile and (m) voltage profiles of the battery textile, the inset shows the optical image of the as-fabricated battery textile. Reproduced from ref. 111. Copyright 2021, Wiley-VCH. | ||
Oh and co-workers reported an intertwined nanosponge solid-state polymer electrolyte (INSPE) for FLIBs. The INSPE was conjugated with intertwined nanosponge (IN) of poly(vinylidene fluoride-co-hexafluoro-propylene) (PVDF-co-HFP) and ion-conducting polymer electrolyte (PE) containing poly(ethylene glycol)diacrylate (PEGDA), succinonitrile (SCN) plasticizer, and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI). The fabrication process of INSPE was divided into two steps, as shown in Fig. 8e. First, the porous IN was prepared via phase separation, as acetone rapidly evaporates after bar-casting of the PVDF-co-HFP in water–acetone solution. Afterward, the PE solution was poured onto IN and solidified through the ultraviolet (UV)-polymerization of PEGDA and free-standing INSPE, which maintained the high mechanical strength of PVDF-co-HFP, and good ionic conductivity of PE was thereby obtained. The pore size and the component configuration of PE were further optimized. In addition, 40-INSPE 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (synthesized from 40 mg mL−1 of PVDF-co-HFP solution and PE including PEGDA and SCN in a ratio of 1
2 (synthesized from 40 mg mL−1 of PVDF-co-HFP solution and PE including PEGDA and SCN in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) with the highest ionic conductivity of 1.04 mS cm−1 at 27 °C, was selected. 40-INSPE 1
2) with the highest ionic conductivity of 1.04 mS cm−1 at 27 °C, was selected. 40-INSPE 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was evaluated in an LFP half-cell, which exhibited a discharge capacity of 144 mA h g−1, high coulombic efficiency of 87.3% after 200 cycles, and good rate capability at several current density values. Further evaluation of 40-INSPE 1
2 was evaluated in an LFP half-cell, which exhibited a discharge capacity of 144 mA h g−1, high coulombic efficiency of 87.3% after 200 cycles, and good rate capability at several current density values. Further evaluation of 40-INSPE 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 under deformation (see Fig. 8f) revealed excellent ionic conductivity retention after increasing the bending angle to 85° or repeated bending, rolling, and folding cycles. A pouch cell with 40-INSPE 1
2 under deformation (see Fig. 8f) revealed excellent ionic conductivity retention after increasing the bending angle to 85° or repeated bending, rolling, and folding cycles. A pouch cell with 40-INSPE 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 laminated between LFP and Li foil successfully lit up the LEDs under bending, rolling, and double folding, as shown in Fig. 8g.
2 laminated between LFP and Li foil successfully lit up the LEDs under bending, rolling, and double folding, as shown in Fig. 8g.
As another approach, Zheng and co-workers demonstrated a Li-metal composite yarn (LMCY) as a flexible anode for wire-type FLIBs. The LMCY was fabricated via infusion of molten Li into a flexible and lithiophilic Cu–C yarn as shown in Fig. 8h.111 The pristine carbon yarn was a twist of ∼200 fibers, each with a diameter of 10 μm. The C yarn was already flexible and provided enough capillarity to liquids that wet its surface, but was lithiophobic as indicated by the large contact angle (120°) with the molten Li. To obtain a lithiophilic yarn, Cu (with a contact angle of 30°) was further coated via polymer-assisted metal deposition with a uniform thickness of 200 nm onto each carbon fiber. The LMCY exhibited much lower linear resistance (∼1 ohm cm−1) than the carbonaceous materials (e.g., ∼20 ohm cm−1 for the C yarn) and similar overpotentials even for different lengths of the yarn, due to the highly conductive Cu–C yarn. The LMCY also exhibited a stable overpotential of ∼94 mV for more than 400 h, thereby outperforming the Li metal wire. Wire-type FLIBs were assembled with the LMCY as the anode and LFP or S yarn as the cathode (Fig. 8i). The cathode was fabricated by dripping the active material slurry onto the nickel-coated carbon yarn. The as-prepared electrodes were aligned in parallel, electrically isolated by a polypropylene separator, and encapsulated in a polyolefin tube, as shown in Fig. 8j. The battery delivered an initial capacity of 150 mA h g−1 and showed a high capacity retention of 76% after 400 cycles. In addition, the battery maintained its performance in the folded state and was able to power 36 LEDs even under knotted conditions (Fig. 8k). Wire-type materials could be directly woven with cotton yarns to create wearable power fabrics (see Fig. 8l). The battery textile, consisting of six LFP‖LMCY fibers connected in parallel, delivered a capacity of 1 mA h cm−2 (Fig. 8m).
3.2 Supercapacitors
In general, supercapacitors (SCs) are divided into two types: pseudocapacitors and electrochemical double layer capacitors (EDLCs). Transition-metal oxides are one of the most promising candidates, for pseudocapacitors, because they provide high energy density through the redox reactions occurring within the bulk material.19,112–119 Conductive polymers are also promising candidates.120 However, for EDLCs, the electrical energy is stored by electrostatic accumulation on the interfacial electrolyte/electrode surface, mainly for carbon-based materials with a high specific surface area. Nowadays, supercapacitors have gained increasing attention in the field of wearable devices due to their electrochemical stability, long cycle life, exceptionally high charge–discharge speed, and safety. Electrodes, as one of the components of a supercapacitor (electrodes, separator, electrolyte, current collector, and packing shell), seem to have a considerable impact on the electrochemical performance of the capacitor. Thus, various electrode materials, including activated carbon,121–124 CNTs,117,125,126 CNFs,127–130 graphene,76,131–134 and MOFs,135–138 have been developed.Xu and co-workers reported that excellent mechanical properties were obtained for conductive metal–organic framework (c-MOF) nanolayers on cellulose nanofibers (CNFs), with hierarchical micro–mesoporosity, serving as electrodes in a foldable supercapacitor.139Fig. 9a shows a schematic of the synthesis process for the CNF@c-MOF hybrid nanofibers. First, the extracted CNFs from the green algae were subjected to TEMPO oxidation (TEMPO = 2,2,6,6-tetramethylpiperidin-1-yloxyl) aimed at developing carboxyl surfaces and achieving ion exchange with Ni2+ ions. Afterward, an aqueous solution containing an organic ligand (HTTP) was added for the construction of Ni-HTTP and a homogeneous suspension of CNF@c-MOF nanofibers was obtained. The electrochemical performance of the electrodes under mechanical deformation was evaluated by obtaining cyclic voltammetry (CV) curves under different folding angles (0°, 90°, 120°, and 180°). The results revealed (Fig. 9b) that folding the device had no influence on the CV curves within the potential windows (0–0.7 V) considered (scan rate: 100 mV s−1). Fig. 9c shows a red LED that can be powered by bent or even folded devices.
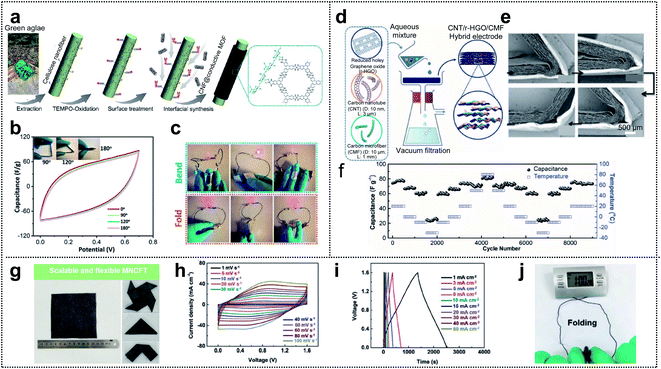 | ||
| Fig. 9 Supercapacitor. (a) Schematic of the synthesis procedure of CNF@c-MOF hybrid nanofibers. (b) Cyclic voltammetry curves (scan rate: 100 mV s−1) at different folding angles. (c) Photograph of a LED powered by the devices in series under different deformations. Reproduced from ref. 139. Copyright 2019, American Chemical Society. (d) Schematic illustration of the preparation of LPCSs. (e) A set of cross-sectional SEM images of a foldable film showing a recovery process. (f) Low-temperature foldability of SCs made with LPCSs. Reproduced from ref. 140. Copyright 2019, Wiley-VCH. (g) Optical images of large-scale MNCFTs and flexibility exhibition. (h) Cyclic voltammetry curves measured at different scan rates between 0 and 1.6 V. (i) Galvanostatic charge–discharge curves collected at different current densities between 0 and 1.6 V. (j) Images of textile-based electrochemical energy storage devices driving an electronic watch at the folding state. Reproduced from ref. 113. Copyright 2020, Wiley-VCH. | ||
As another method of fabricating foldable electrodes for supercapacitors, Zheng and co-workers fabricated new free-standing and foldable electrodes made of pure carbon and referred to as lamellar porous carbon stacks (LPCSs).140 A uniform and free-standing LPCS (hybrid electrode) was obtained through vacuum filtration of an aqueous solution including HRGO nanosheets, CNTs, and CFs (Fig. 9d). The thickness of each layer and the space between the layers in the resulting multilayer lamellar structure were ∼20 μm and 50 μm, respectively. To confirm foldability, they showed the structural change in the LPCS during a folding and unfolding cycle (see Fig. 9e). The lack of fractures or cracks (even at a small folding radius of <20 μm) may have resulted from the dissipation of inner compressive stress and outer tensile stress by the gaps between neighboring layers. Furthermore, based on the highly stable LPCS and the low-temperature-tolerant ionic liquid used as the electrolyte, they assessed the electrochemical performance of LPCS SCs over a wide range of temperatures (80 °C to −30 °C; see Fig. 9f). They performed 500 cycles at each temperature stage (capacitance values of 76.8, 67.4, 61.4, and 25.2 F g−1 obtained at 20 °C, 0 °C, −10 °C, and −30 °C, respectively). After the repeated temperature change, the specific capacitance of the LPSC SCs easily recovered its original value (73.6 F g−1), indicating excellent structural integrity and good stability of the SCs at various temperatures.
Owing to their high conductivity, good flexibility, and good mechanical properties, carbon fiber textiles (CFTs) are a promising candidate for substrates used in the loading of active materials. Yuan and co-workers fabricated tailorable, wearable, and foldable solid-state asymmetric supercapacitors (ASCs) based on CFTs.113 The suggested positive and negative electrodes of the ASCs were O/N-functionalized CFTs (ONCFTs) and N-doped CFTs with MXene ink (MNCFTs), respectively, and polyvinyl alcohol (PVA)/H2SO4 gel electrolytes were inserted between the electrodes. Fig. 9g shows a 10 × 10 cm2 region of the MNCFT electrodes with different folded shapes. The lack of fractures demonstrates the excellent flexibility and mechanical stability of these electrodes. Furthermore, they compared the operating potential windows of the MNCFT (−0.6 to 0.3 V) and ONCFT electrodes (0 to 1 V) with that of the assembled ASC, which is capable of extending the voltage window to 1.6 V. Based on the extended voltage window, ASCs exhibited undistorted CV shapes even at a high scan rate of 100 mV s−1, indicating high rate capability (Fig. 9h). Additionally, the galvanostatic charge–discharge profiles were only slightly distorted compared with the ideal symmetric triangular shape (current density: 1–50 mA cm−2) suggesting good reversibility and pseudocapacitive properties (Fig. 9i). The excellent rate capability resulted in 60% capacitance retention (548 and 330 mF cm−2) even when the scan rate was increased from 10 to 100 mV s−1. For practical applications, the textile-based electrochemical energy-storage-devices were able to power an electronic watch under repeated folding conditions, thereby demonstrating good flexibility and excellent integrity (Fig. 9j).
3.3 Air batteries
Metal–air batteries are potential candidates for foldable batteries with high energy density.141,142 For example, Li–air and Zn–air batteries are promising for next-generation energy-storage-devices (such as electric vehicle batteries) due to their excellent power storage capacity, safety, low cost, and abundant materials.143,144 Li–air batteries have a high theoretical energy density (3500 W h kg−1).145 However, these batteries are plagued by problems such as low charge/discharge efficiency, unstable electrolyte of partial discharge products, and dendrite formation of unstable anodes.146,147 Many studies have focused on overcoming these issues. For example, Feng and co-workers developed a foldable Li–air battery (LAB) using two approach strategies (Fig. 10a),148 namely the design of a stable anode and the utilization of gel-polymer electrolyte (GPE). For a stable anode, reduced graphene oxide (rGO) played a critical role in alleviating the Li dendrite formation and increasing the flexibility and mechanical strength. A GPE with 4 wt% SiO2 and LiI imparted a high ionic conductivity (1.01 mS cm−1) and reduced the air-attacking-induced Li passivation. Moreover, LiI and SiO2 assisted in the prevention of discharge products and improved the flame resistance. For a fixed capacity of 500 mA h g−1, the SiO2–LiI–GPE-based LAB exhibited a lower voltage than the SiO2–GPE-based LAB (100 cycles vs. 59 cycles for a discharge terminal voltage >2.0 V). This result indicated that LiI additives in the GPE could improve the reversibility of LABs. In addition, the SiO2–GPE-based flexible belt-shaped LAB exhibited a long cycle life of 100 cycles with a small voltage gap of ∼1.45 V and powered a red LED under twisting to 90° or 360°.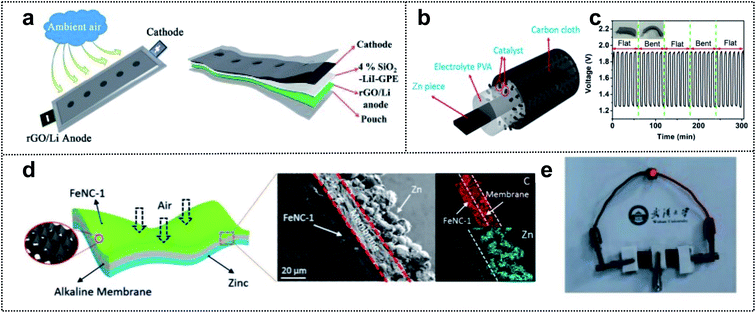 | ||
| Fig. 10 Foldable air batteries. (a) Schematic illustration of the flexible belt-shaped Li–air battery structure. Reproduced from ref. 148. Copyright 2018, Royal Society of Chemistry. (b) Schematic illustration of a solid-state Zn–air battery. (c) Stability of the solid-state rechargeable Zn–air batteries under flat and folded states. Reproduced from ref. 148. Copyright 2018, Elsevier. (d) Schematic illustration of a substrate-free flexible ZAB. (e) Digital image of the red-light LED powered by two tandem devices. Reproduced from ref. 153. Copyright 2019, Wiley-VCH. | ||
Similar to LABs, rechargeable Zn–air batteries have a high theoretical energy density (1086 W h kg−1).149 However, some catalysts with platinum (Pt) or iridium (Ir) in the air cathode are expensive, rare, and have low electrochemical stability in Zn–air batteries.150,151 The relatively low kinetics of the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) at the cathode can be overcome by developing electrochemical catalysts that will yield high-performance foldable Zn–air batteries.152 In order to enhance the catalytic effect for the ORR and OER in these batteries, Cao and co-workers designed a solid-state Zn–air battery using single-atom cobalt electrocatalysts for high performance.153 They utilized Zn, CC, and PVA as the anode, current collector, and PE to prevent leakage of liquid devices for foldable batteries (Fig. 10b). In addition, the single-atom Co–N–C composite (CoN4/NG) was used as a high-performance electrocatalyst with a low overpotential (0.74 V, EORR–EOER). The ORR onset potential of CoN4/NG (0.98 V) was considerably higher than that of Co-NPs/NG (0.92 V). The assembled foldable Zn–air battery was characterized by excellent discharging and charging properties (730 vs. 711 mA h g−1 for commercial Pt/C) with a high power density, energy density (671 W h kg−1), and long stability (6 h in flat and folded states) (Fig. 10c). Chen and co-workers reported an alkaline polymer membrane-based foldable Zn–air battery.154 In this work, two strategies (self-standing active layer and gel-electrolyte coated separators) were employed through an ink-casting/hot-pressing approach for obtaining a high-performance flexible battery (Fig. 10d). An interconnected Fe/N-doped 3D carbon-based electrocatalyst was used as an ultrathin cathode material without the backing gas-diffusion layer for outstanding catalytic properties. Moreover, a three-in-one design with Zn powder, an alkaline polymer membrane, and electrocatalysts achieved a large ion exchange capacity (1 mmol g−1), high ionic conductivity (>0.1 S cm−1), chemical stability, and outstanding mechanical properties for flexible electrodes. Consequently, the all-solid-state foldable Zn–air battery exhibited excellent flexibility and exceptional battery performance (power density of 250 mW cm−3 and a high capacity of 150.4 mA h cm−3 at a fast-discharging rate of 8.3 mA cm−3) (Fig. 10e). Therefore, the specific design of highly active electrocatalysts and a flexible polymer-based membrane, as well as the appropriate combination of current collectors and packing agents, can yield high-performance foldable air batteries.
3.4 Multi-valent batteries
Multi-valent batteries such as Zn or Al-ion batteries have attracted great interest due to low-cost, high-power density, and high capacity.155,156 Compared with Li- and Na-ion batteries, multi-valent batteries can have double or triple capacities for the same number of ions because the number of ions that react at the electrodes decreases within the electrolyte when we substitute M+ with Mn+ (n = 2 and 3) charge carriers.157 In addition, multi-valent metals such as Zn, Ca, Mg or Al are abundant in nature.158 This means that multi-valent batteries can serve as next-generation wearable and foldable energy-storage-devices that overcome issues regarding the battery cost, energy density, power density, and resources.159–161 Despite these advantages, Al ion batteries (AIBs) suffer from the kinetics limitation of Al3+, which is associated with slow ion insertion and extraction.162 The selection of electrode materials is therefore very crucial. Huang and co-workers obtained a safe flexible aqueous AIB for wearable electronic devices with long cycling stability, high capacity, and good rate capability using an AlxVOPO4 cathode, a MoO3 anode, and a gelatin–polyacrylamide hydrogel electrolyte (Fig. 11a and b).163 VOPO4 enabled fast cation intercalation, owing to the coordination of the V atom (Fig. 11c). Furthermore, MoO3 is a 2D layered structure with good electrochemical intercalation and easy coupling of multivalent-state Mo atoms. Grafted polymer electrolytes with gelatin and polyacrylamide (PAM) provided flexibility and a high ionic conductivity (20.83 vs. 0.65 mS cm−1 for PVA–H3PO4 gel electrolyte). Consequently, this foldable AIB exhibited good durability (86.2% after 2800 cycles), a high specific capacity (88 mA h g−1), and a high rate capability (6 A g−1). This indicates that flexible AIBs capable of overcoming low kinetics possess considerable potential for wearable devices used as foldable batteries.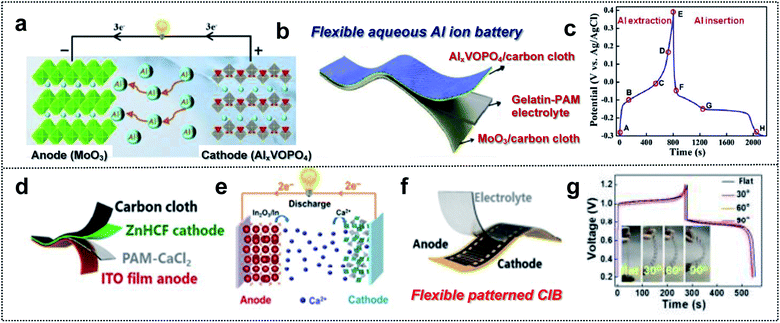 | ||
| Fig. 11 Foldable multi-valent batteries. (a) Schematic illustration of the working principle of aqueous AIBs based on the intercalation electrode. (b) Schematic illustration of a flexible aqueous AIB. (c) Charge/discharge curves of VOPO4 at a current density of 1 A g−1. Reproduced from ref. 163. Copyright 2020, Elsevier. (d) Schematic illustration of a flexible aqueous CIB, (e) working principle schematic of aqueous CIB, (f) schematic illustration of a flexible patterned CIB and (g) GCD curve of a flexible CIB at different bending angles. Reproduced from ref. 167. Copyright 2021, Springer Nature. | ||
Due to a moderate charge density and polarization strength, Ca-ion batteries (CIBs) are characterized by better kinetics than that of the batteries based on other multi-valent ions.164,165 However, the number of electrode materials capable of hosting Ca2+ cations is limited. For example, layered potassium birnessite (K0.31MnO2·0.25H2), a well-established host material, exhibits low rate capability and fast capacity drops (51%) for the initial 20 cycles, owing to poor kinetics and instability.166 Huang and co-workers used the Sn-doped In2O3 (ITO) film as the aqueous CIB anode with a zinc hexacyanoferrate (ZnHCF) cathode (Fig. 11d).167 In this system, an ITO electrode assisted the insertion/extraction reaction of Ca cations in the reduction of In3+ for charge balance. Ca2+ intercalation/deintercalation occurred during the partial reduction of Fe3+ to Fe2+ in the ZnHCF cathode and the conversion reaction at the ITO anode (Fig. 11e). For the foldable CIB, they utilized PAM/CaCl2 as the GPE and ZnHCF/CC as flexible electrodes (Fig. 11d). This well-designed flexible CIB exhibited a high specific capacity of 75.3 mA h g−1 at 0.4 A g−1 and high coulombic efficiency of 97.5%. In addition, excellent foldable properties for powering an electronic watch in a flat or bending state were realized (Fig. 11f and g). Regarding multi-valent batteries, optimized electrode materials should be considered. Moreover, the cell configuration of GPE, flexible substrates, and mechanically stable electrode materials is significant for foldable batteries.
4. Analysis
4.1 Electrochemical characterization
Electrochemical characterization of foldable batteries is the same as that of conventional batteries, except for an additional analysis confirming that the system performance is maintained even in the folded state. Previous studies have already performed the aforementioned analysis process implicitly, but the basic guideline is still necessary for researchers who will start investigations of foldable battery systems. Two examples are introduced in this section as a means of proving a common guideline for evaluating foldable electrodes or separators.Foldable electrodes usually have non-conventional electrode structures because rigid metal current collectors are unsuitable for use under deformation conditions. Therefore, the electrical conductivity of these electrodes should be comparable to that of metal current collectors in both the unfolded and folded states. Song and co-workers reported similar findings for active material loaded silver-nanowire-wound microfiber (AgNW$MF) or carbon-nanotube-webbed microfiber (CNT#MF) electrodes for FLIBs.55 The electrical conductivity of the as-fabricated electrodes was measured in the unfolded state and also tracked as a form of resistance change during repeated folding cycles, as shown in Fig. 12a. The conductivity of LTO-loaded AgNW$MF was four orders of magnitude higher than that of a practical LTO-slurry coated aluminum electrode and the resistance was consistent for 1000 folding cycles. The lithiation/delithiation electrochemistry and rate capability of the electrodes were further analyzed in a half-cell including lithium metal used for panels (Fig. 12b). Compared with LTO-loaded AgNW$MF, the foldable electrode exhibited a similar capacity retention but with a higher gravimetric capacity and good rate capability due to the low weight of the electrode and excellent electrical conductivity, respectively. The same analysis was performed for two- and four-time folded electrodes that were assembled into pouch-type half-cells, as shown in Fig. 12c. LFP-loaded CNT#MF and LTO-loaded AgNW$MF were assembled into pouch-type full cells and tested after confirming the electrochemical performance of the electrodes in the unfolded and folded states (Fig. 12d). To confirm that the electrochemical performance was maintained under deformation conditions (see Fig. 12e), the relative capacity was measured during charge/discharge cycles with intermittent 20 folding and unfolding events every 20 cycles. The cell exhibited excellent capacity retention even when four-time folded electrodes were used. Moreover, LED bulb show-up was demonstrated after folding, crumpling, and hammering (Fig. 12f).
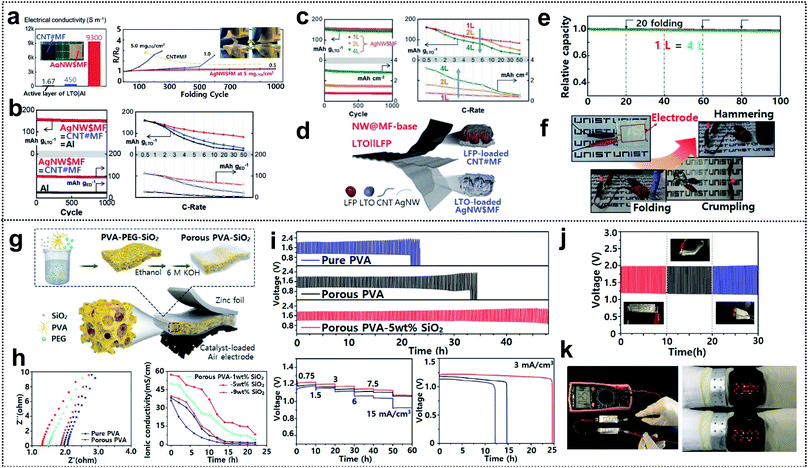 | ||
| Fig. 12 Electrochemical characterization. (a) Initial electrical conductivities of LTO containing electrodes and resistance change along with repeated folding cycles, (b) lithiation electrochemistry of LTO loaded on AgNW$MF, CNT#MF, and aluminum foil, (c) capacity retention and rate capabilities of folded AgNW$MFs (2 L and 4 L) versus the unfolded one, (d) schematic image of a flexible LTO‖LFP battery, (e) capacity retention of unfolded and folded batteries and (f) optical images of LEDs powered by a battery under folding, crumpling and hammering. Reproduced from ref. 55. Copyright 2017, Wiley VCH. (g) Schematic diagram of a foldable zinc–air battery (FZAB) and the preparation process, (h) impedance spectra and ionic conductivity of PVA-based GPEs, (i) several electrochemical characterization studies of FZABs based on various PVA-based GPEs, (j) cycle performance under different bending conditions and (k) open-circuit voltage demonstration with two FZABs in series and demonstration of a LED watch powered by bracelet-type FZABs. Reproduced from ref. 173. Copyright 2018, Elsevier. | ||
GPEs and solid-state electrolytes (SPEs) are widely used as both electrolytes and separators for foldable batteries due to their high mechanical durability and non-susceptibility to electrolyte leakage.168–171 The ionic conductivity of GPEs and SPEs directly affects the whole cell performance (the operating voltage, lifespan, and power output) and must therefore be sufficiently high.172 Hu and co-workers demonstrated porous PVA-based GPEs for foldable zinc air batteries (FZABs) and showed a detailed electrochemical analysis process from the GPE step to a full cell test.173 The GPEs were fabricated through a simple phase-inversion method and then sandwiched between zinc foil and a catalyst-loaded air electrode for FZAB assembly, as shown in Fig. 12g. The GPEs consisted of a PVA matrix immersed in a 6 M KOH solution and SiO2 was introduced on the surface for increased water retention. Initial impedance spectra were measured for GPEs with different contents of the SiO2 filler. Subsequently, the ionic conductivity was monitored over time to identify the optimum conditions that yield the highest ionic conductivity and electrolyte absorption capacity of GPEs (Fig. 12h). The resistance decreased with increasing SiO2 filler content. However, over a certain point (9 wt%), excessive SiO2 could lead to aggregation and disruption of the ion transport, thereby affecting the overall performance, as indicated by the high resistance and poor water retention properties. FZABs were assembled using optimized porous PVA-5 wt% SiO2 GPEs and were then subjected to several galvanostatic charge/discharge measurements, as shown in Fig. 12i. The electrochemical performance (lifespan, rate capability, and specific capacity (maximum value: 68.0 mA h cm−3)) of the GPEs was superior to that of pure PVA or porous PVA. The cycle performance of FZABs with the GPEs was measured at bending angles of 0°, 60°, and 180° (Fig. 12j). The FZABs exhibited excellent stability, i.e., the potential changed only modestly with the bending angle. Furthermore, two FZABs were connected in series in order to realize a high open-circuit voltage of 2.54 V, which was enough to power an LED watch even in the form of a bracelet, as shown in Fig. 12k.
4.2 Mechanical characterization
Several review papers reported significant progress in the field of foldable LIBs and supercapacitors (SCs) exhibiting high electrochemical performance and flexibility.174–176 However, compared with the evaluation of electrochemical performance, diverse methods and parameters are employed for evaluation of the flexibility, and unified assessment criteria are lacking. Recently, various efforts have focused on precise and proper mechanical characterization of foldable energy-storage-devices (ESDs).109,177–183 In this section, we discuss various measurement methods and their effectiveness in determining the mechanical properties of deformable ESDs.Zhi and co-workers investigated the validity of the widely used parameters for bending durability and assessed the softness of flexible ESDs.184 They proposed three parameters, θ (the bending angle), R (the bending radius of curvature), and L (the length of the device), for precise evaluation of the durability (see Fig. 13a). The figure shows that lowering the bending angle or bending radius increases the area of stress regions and the degree of the tensile/compressive strain on the outer/inner surface. Similarly, for a given bending angle and bending radius, they suggested that (compared with a shorter device) a longer device may be less affected by bending as the stressed region is localized. To demonstrate the effects of the parameters, they compared the dependence of capacity retention during cycling on different bending parameters (θ, R, and L). Fig. 13b shows the cycle retention of Zn/MnO2 batteries for a fixed bending radius and bending angle (1.5 cm and 90°, respectively) and different lengths. When the length of the device decreased from 12 cm to 5 cm, the capacity retention for 100 cycles decreased from ∼99% to ∼91%. This capacity decay of the cell was attributed to cracks in the electrodes and interfacial contact deterioration induced by the repeated mechanical deformation, i.e., mechanical tests affect the electrochemical performance. However, they confirmed the mechanical properties after the electrochemical tests and the stress–strain curve revealed a negligible effect on the mechanical properties of ESDs (Fig. 13c). In addition, they discussed the wearability and comfort assessment of a flexible ESD in terms of softness. They proposed softness as a parameter for evaluating the softness of such ESDs, which can be evaluated using a commercially available leather and fabric softness tester, as shown in Fig. 13d. During the evaluation, a cylindrical load of defined mass was lowered at a specified rate onto a securely clamped area of the ESD and the distension from the ESD was recorded as the softness (unit: mm). Foldable current collectors are an inevitable component of foldable ESDs with high softness. Thus, they measured the softness of flexible batteries using different current collectors such as CNT paper, CC, graphene paper, and steel foil (Fig. 13e). The highest value of softness (4.06 mm) was obtained for batteries with CNT paper (current collectors in descending order of softness: CNT paper, CC, graphene paper, and steel foil).
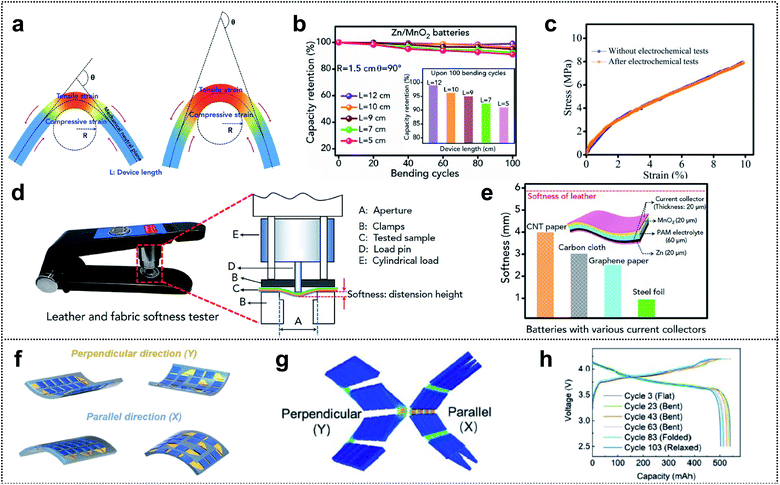 | ||
| Fig. 13 Mechanical characterization. (a) Schematics of structures and the three key parameters (L, θ, and R) that are commonly used to demonstrate the bending state of flexible and wearable energy storage devices (ESDs). (b) Zn–MnO2 batteries with different lengths at a certain bending angle (90°) and bending radius (1.5 cm). (c) Stress–strain curve of flexible Zn/MnO2 batteries before and after electrochemical tests (100 charge/discharge cycles). (d) Photograph of the softness tester and schematic illustration for measuring softness. (e) Softness of flexible Zn–MnO2 batteries constructed with different flexible current collectors (the inset shows the schematic illustration of the structure of flexible Zn–MnO2 batteries and the thickness of different components). Reproduced from ref. 184. Copyright 2019, Elsevier. (f) Schematic illustration of the different types of snake-origami batteries with bidirectional deformation. (g) Finite element calculations of snake-origami batteries in the bending state with bidirectional deformation: parallel (X) and perpendicular (Y) directions. (h) The cycle performance of snake-origami batteries in different states: flat, bent, fold, and relaxed state under a constant current density of 0.5C. Reproduced from ref. 188. Copyright 2021, Wiley-VCH. | ||
In addition to mechanical characterization through the actual deformation of a foldable ESD, mathematical analysis (computational tools) of the detailed deformation has rapidly been developed with significant evolution of computer technology. Finite element analysis (FEA), a numerical method, provides the stress distribution of a foldable ESD by formulating and combining the algebraic equations of sub-units. In FEA simulation, a continuous complex structure is discretized into finite elements and a fine element mesh is generated by connecting the fine elements involving material and structural properties. Based on the density of the mesh, the strain distribution between the different deformation states and different structures can be measured.111,185–187
Song and co-workers reported a novel bidirectional snake-origami battery with a scalable planar structure, which was obtained by separating the energy-storage and mechanical deformation segments (Fig. 13f).188 FEA of the deformation at soft connecting segments was used to elucidate the mechanical durability of the batteries. Fig. 13g shows that the batteries with 4 × 4 arrays were bent along two directions and the stress was concentrated only on the soft segments, rather than on the energy-storage segments. Moreover, via simulations, they determined the effectiveness of the battery flexibility and the relationship between the energy density and the minimum bending radius as a function of the dimensionless gap width. Based on the mechanical characterization, they evaluated the performance of the batteries in various mechanical deformation states including flat, bent, folded, and relaxed. The voltage profiles of each cycle (see Fig. 13h) revealed negligible capacity degradation (0.066% for each cycle) or overpotential, indicating the excellent flexibility of the snake-origami batteries.
5. Outlook and perspective
In this review, we briefly cover state-of-the-art and some pioneering studies on the foldable ESDs reported so far. This review highlights the morphologies of materials (1D, 2D, 3D, and structural design) for foldable electrodes and discusses the foldable ESDs (Li-ion batteries, supercapacitors, air batteries, and multi-valent batteries). Furthermore, beyond foldability, this review discusses the evaluation methods (electrochemical and mechanical analysis) for these devices. The features of various strategies for different foldable ESDs based on the system, active materials, electrolytes, capacity, active material mass loading, cycle stability, and energy density are summarized in Table 1. Although considerable progress has already been accomplished in this area, challenges (including low energy density, high cost, poor productivity, packaging materials, and safety issues) are encountered in the practical application and commercialization of these devices. Thus, in this chapter, we discuss the challenges and perspectives for the future improvement of foldable ESDs.| Systemref. | Active materials (cathode/anode) | Electrolyte | Capacity | Mass loading (mg cm−2) | Cycle stability | Energy density |
|---|---|---|---|---|---|---|
| Lithium ion-battery43 | LCO@CNT//GeOx/ZnO/C | 1 M LiPF6 in EC/DMC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v/v) 1, v/v/v) |
890 mA h g−1 | 1.2 | 464 mA h g−1 over 500 cycles at 1 A g−1 | — |
| Potassium-ion battery44 | (KFeII[(FeIII(CN)6)])//SnS2@C | 0.8 M KPF6 in EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
457.4 mA h g−1 | 1.2 | 183.1 mA h g−1 after 1000 cycles at 2 A g−1 | — |
| Aluminum-ion battery45 | Co9S8@CNT–CNF//- | AlCl3 and [EMIm]Cl | 315 mA h g−1 | 1.5 | 87 mA h g−1 after 6000 cycles at 1 A g−1 | 300 W h kg−1 |
| Lithium ion-battery55 | CNT#MF@LFP//AgNW$MF@LTO | 1 M LiPF6 in EC/DEC (3/7, w/w) | 150 mA h g−1 | 5.0 | 150 mA h g−1 after 1000 cycles at 1C | — |
| Lithium ion-battery74 | LMO//titania/rGO | 1 M LiPF6 in EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
168 mA h g−1 | 100 mA h g−1 after 100 cycles at 0.017 mA | — | |
| Lithium–sulfur battery75 | GO@sulfur@PEDOT:PSS//Li foil | 1 M Li2S6 in DOL/DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
1584 mA h g−1 | 2.0 | (80%) 806 mA h g−1 after 500 cycles at 1C | — |
| Lithium–sulfur battery82 | MF@Ti3C2Tx/S//Li foil | 0.1 M Li2S6 and 1 M LiTFSI in DOL/DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
916 mA h g−1 | 1.5 | 674 mA h g−1 after 1000 cycles at 1C | — |
| Zinc-ion battery83 | LMO//MXene/graphene@Zn | PVA@MXene | 126 mA h g−1 | 25 | 90.3% capacity retention for 60 cycles at 2C | — |
| Zinc-ion battery91 | NVO//AgNPs@CC/Zn | 1 M Zn(CF3SO3)2 aqueous solution | 321 mA h g−1 | 4 | 184 mA h g−1 after 1200 cycles at 5 A g−1 | — |
| Lithium-ion battery101 | LCO//graphite | 1 M LiPF6 in EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
151.4 mA h g−1 | 12.29 | 145.7 mA h g−1 after 20 cycles at 0.2C (flexed state) | 242 W h L−1 |
| Lithium-ion battery102 | LCO//graphite | 1 M LiPF6 in EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
132.9 mA h g−1 | — | 131.5 mA h g−1 after 50 cycles at 1C (dynamic bending and twisting) | 371.9 W h L−1 |
| Lithium-ion battery103 | LCO//graphite | 1 M LiPF6 in EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
148.6 mA h g−1 | 12.8 | 124.2 mA h g−1 after 15 cycles at 1C (dynamic folding) | 245 W h L−1 |
| Lithium-ion battery109 | LFP//lithium metal | Intertwined nanosponge solid-state polymer electrolyte | 144 mA h g−1 | 6.0 | 87.3% capacity retention after 200 cycles at 0.2C | — |
| Lithium–metal battery111 | Yarn-like LFP//Li–metal composite yarn | 1 M LiTFSI in DOL/DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) + 2 wt% LiNO3 1, v/v) + 2 wt% LiNO3 |
150 mA h g−1 | 1.2 | 88% capacity retention after 170 cycles | 292.4 W h L−1 |
| Supercapacitor113 | MNCFT//ONCFT | PVA/H2SO4 gel electrolyte | 148 F g−1 | 3 | 90% capacitance retention after 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 50 mA cm−2 000 cycles at 50 mA cm−2 |
277.3 μW h cm−2 |
| Supercapacitor139 | CNF@c-MOF//CNF@c-MOF | PVA/KCl gel electrolyte | 75 F g−1 | 0.7 | 90% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1 A g−1 000 cycles at 1 A g−1 |
— |
| Supercapacitor140 | LPCS (lamellar porous carbon stack)//LPCS | BMIMBF4 electrolyte | 65 F g−1 | 20.2 | 95% capacity retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at −30 °C 000 cycles at −30 °C |
2.1 mW h cm−2 |
| Lithium–air battery148 | Air//rGO/Li | 4% SiO2–LiI–GPE | 500 mA h g−1 | 1.0 | Average voltage gap of 1.45 V during 100 cycles | — |
| Zinc–air battery153 | Air//CoN4/NG/Zn | 6 M KOH and 0.2 M ZnO | 730 mA h g−1 | 100 hours | 671 W h kg−1 | |
| Aluminum-ion battery161 | VOPO4//MoO3 | Gelatin–polyacrylamide hydrogel electrolyte | 73 mA h g−1 | 1.5 | 86.2% capacity retention after 2800 cycles at 1 A g−1 | — |
| Calcium-ion battery165 | ZnHCF//Sn-doped ITO | Polyacrylamide hydrogel electrolyte | 71.2 mA h g−1 | 0.8 | 75.3 mA h g−1 at 0.4 A g−1 | — |
Firstly, the energy density (W h L−1 or W h g−1) is closely related to the lifetime and portability of electronic devices and is hence the most important factor for evaluating the performance of energy devices. This is especially crucial for wearable devices (such as smart watches, smart lenses, and electronic patches) where a long lifespan associated with their operating hours and portability is required. In this context, the working voltage, capacity, weight, and volume should be carefully considered in order to achieve a high energy density. Foldable energy devices, unlike conventional ESDs, need electrochemically inactive components, which provide foldability to the whole device. Unfortunately, these components usually lower the energy density of the devices and can even lead to deterioration of the electrochemical performance. Thus, the electrode of foldable ESDs should be composed of all-electrochemically active materials. The weight fractions of the electrolyte, separator, and packaging materials in these devices will probably be reduced in order to obtain a high energy density. In addition, a high mass loading of active materials is needed for realizing a high energy density and can be simply achieved by increasing the film thickness. The drawbacks resulting from thick electrodes (including low foldability and inefficient ion diffusion) can be overcome by a well-engineered porous structure containing moderate space for the mechanical strain and the electrolyte near the active materials.75,189–193
Secondly, the high cost of the materials and poor productivity of foldable ESDs should also be considered. Substrates for foldable electrodes such as graphene, CC, and CNT paper have good electrical conductivity and mechanical properties but their high cost, compared with that of the conventional substrates (e.g., copper and aluminum foil), impede the commercialization of foldable ESDs. Additionally, the large-scale production of foldable electrodes and their substrates is quite challenging, although laboratory-scale production for assembling various prototypes of foldable ESDs is quite straightforward. The current large-scale coating or deposition technologies (e.g., roll-to-roll coating) were developed for rigid substrates and not adapted to foldable substrates for loading active materials.
Thirdly, packaging materials of foldable ESDs are frequently neglected. Metal cases are required to prevent the air and moisture penetration in battery packaging. For example, during the washing of wearable electronics, water and laundry detergents, which can cause severe damage to the batteries, may penetrate the batteries as existing packaging materials and technologies are ineffective in protecting the batteries. Moreover, although the foldability of electrodes, separators, and current collectors is assured, the packaging must also provide the same level of foldability to maintain the battery system under severe deformation. The modification of packaging materials with the aim of preserving the integrity of foldable ESDs under various deformations should therefore be seriously considered as a means of releasing the strain in metal layers.
Lastly, the electrochemical performance and durability of foldable ESDs under extreme conditions should be investigated for practical applications. The previously reported studies have focused only on the performance of foldable ESDs in ambient environments. The performances under harsh conditions, such as extremely high humidity, underwater environments, high pressure, and high/low temperatures, are rarely reported.94,194–196 Moreover, under various harsh conditions, inevitable issues (including leakage, flammability, and volatility) associated with organic-based liquid electrolytes and related to the safety of devices are encountered. Non-flammable aqueous electrolytes are good candidates for overcoming these issues. However, the low operating voltage resulting from the irreversible decomposition (oxygen evolution reaction and hydrogen evolution reaction) of aqueous electrolytes hinders realization of high energy density foldable systems.83,197,198 Replacing conventional flammable liquid electrolytes with solid-state electrolytes can provide improved safety and integrity but challenges such as low ionic conductivity and side reactions between the electrode/electrolyte interfaces are encountered.20,109,112,153,199
In summary, although numerous efforts have been made to develop foldable ESDs for commercialization, many hurdles still remain to hamper the further approaches near to commercialization. Low energy density, high cost with poor productivity, ignorance of packaging materials, and insufficient evaluation conditions are included as main issues for significant improvements in this field. Among them, critical challenges in terms of durability and performance of foldable batteries are low energy density and insufficient evaluation conditions. To handle the former challenge, one possible key can be to reduce the fraction of inactive components for decreasing the weight/volume of the cells and the other key can be to use high-capacity active materials such as alloy-type (e.g., silicon, germanium, and tin) lithium metal anodes, and sulfur cathodes for increasing the energy. Also, constructing parameters and criteria for the extreme conditions (e.g., humidity, low/high temperature, and external shock) should be considered for determining precise durability, performance, and safety under the certain conditions. It is believed that the cost/productivity and packaging material issues can be resolved by fabricating appropriate flexible current collectors and flexible packaging materials with the combined efforts in materials science and electrochemistry.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by Ministry of Education (2019R1I1A1A01050857 and NRF-2021M3H4A1A02099354).References
- J. A. Rogers, Z. Bao, K. Baldwin, A. Dodabalapur, B. Crone, V. R. Raju, V. Kuck, H. Katz, K. Amundson, J. Ewing and P. Drzaic, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 4835–4840 CrossRef CAS PubMed
.
- I. You, B. Kim, J. Park, K. Koh, S. Shin, S. Jung and U. Jeong, Adv. Mater., 2016, 28, 6359–6364 CrossRef CAS PubMed
.
- W. J. Song, S. Yoo, G. Song, S. Lee, M. Kong, J. Rim, U. Jeong and S. Park, Batteries Supercaps, 2019, 2, 181–199 CrossRef
.
- W. J. Song, S. Lee, G. Song, H. B. Son, D. Y. Han, I. Jeong, Y. Bang and S. Park, Energy Storage Mater., 2020, 30, 260–286 CrossRef
.
- S. I. Park, D. S. Brenner, G. Shin, C. D. Morgan, B. A. Copits, H. U. Chung, M. Y. Pullen, K. N. Noh, S. Davidson, S. J. Oh, J. Yoon, K. I. Jang, V. K. Samineni, M. Norman, J. G. Grajales-Reyes, S. K. Vogt, S. S. Sundaram, K. M. Wilson, J. S. Ha, R. Xu, T. Pan, T. I. Kim, Y. Huang, M. C. Montana, J. P. Golden, M. R. Bruchas, R. W. t. Gereau and J. A. Rogers, Nat. Biotechnol., 2015, 33, 1280–1286 CrossRef CAS PubMed
.
- D. G. Mackanic, T. H. Chang, Z. Huang, Y. Cui and Z. Bao, Chem. Soc. Rev., 2020, 49, 4466–4495 RSC
.
- G. M. Zhou, F. Li and H. M. Cheng, Energy Environ. Sci., 2014, 7, 1307–1338 RSC
.
- T. Li, Z. G. Suo, S. P. Lacour and S. Wagner, J. Mater. Res., 2005, 20, 3274–3277 CrossRef CAS
.
- S. Wagner, S. P. Lacour, J. Jones, P. H. I. Hsu, J. C. Sturm, T. Li and Z. G. Suo, Phys. E, 2004, 25, 326–334 CrossRef
.
- Z. Song, T. Ma, R. Tang, Q. Cheng, X. Wang, D. Krishnaraju, R. Panat, C. K. Chan, H. Yu and H. Jiang, Nat. Commun., 2014, 5, 3140 CrossRef PubMed
.
- Z. Song, X. Wang, C. Lv, Y. An, M. Liang, T. Ma, D. He, Y. J. Zheng, S. Q. Huang, H. Yu and H. Jiang, Sci. Rep., 2015, 5, 10988 CrossRef CAS PubMed
.
- K. K. Fu, J. Cheng, T. Li and L. B. Hu, ACS Energy Lett., 2016, 1, 1065–1079 CrossRef CAS
.
- Z. H. Fang, J. Wang, H. C. Wu, Q. Q. Li, S. S. Fan and J. P. Wang, J. Power Sources, 2020, 454, 227932 CrossRef CAS
.
- A. Sumboja, J. Liu, W. G. Zheng, Y. Zong, H. Zhang and Z. Liu, Chem. Soc. Rev., 2018, 47, 5919–5945 RSC
.
- L. Kong, C. Tang, H. J. Peng, J. Q. Huang and Q. Zhang, SmartMat, 2020, 1, 1–35 CrossRef
.
- M. J. Park and J. S. Lee, Adv. Electron. Mater., 2019, 5, 1800411 CrossRef
.
- H. Li, Y. Ma and Y. Huang, Mater. Horiz., 2021, 8, 383–400 RSC
.
- Z. Y. Wang, W. K. Zhang, X. L. Li and L. Z. Gao, J. Mater. Res., 2016, 31, 1648–1664 CrossRef CAS
.
- L. B. Dong, C. J. Xu, Y. Li, Z. H. Huang, F. Y. Kang, Q. H. Yang and X. Zhao, J. Mater. Chem. A, 2016, 4, 4659–4685 RSC
.
- Y. Yang, Nanoscale, 2020, 12, 3560–3573 RSC
.
- J. Liu, J. Wang, C. Xu, H. Jiang, C. Li, L. Zhang, J. Lin and Z. X. Shen, Adv. Sci., 2018, 5, 1700322 CrossRef PubMed
.
- W. Liu, M. S. Song, B. Kong and Y. Cui, Adv. Mater., 2017, 29, 1603436 CrossRef PubMed
.
- R. Z. Hou, G. S. Gund, K. Qi, P. Nakhanivej, H. F. Liu, F. Li, B. Y. Xia and H. S. Park, Energy Storage Mater., 2019, 19, 212–241 CrossRef
.
- Y. Han, Y. Z. Lu, S. H. Shen, Y. Zhong, S. Liu, X. H. Xia, Y. X. Tong and X. H. Lu, Adv. Funct. Mater., 2019, 29, 1806329 CrossRef
.
- K. Wang, Y. Huang, M. Y. Wang, M. Yu, Y. D. Zhu and J. S. Wu, Carbon, 2017, 125, 375–383 CrossRef CAS
.
- Q. Yu, B. Jiang, J. Hu, C. Y. Lao, Y. Gao, P. Li, Z. Liu, G. Suo, D. He, W. A. Wang and G. Yin, Adv. Sci., 2018, 5, 1800782 CrossRef PubMed
.
- L. Manjakkal, C. G. Nunez, W. T. Dang and R. Dahiya, Nano Energy, 2018, 51, 604–612 CrossRef CAS
.
- H. Yao, F. Zhang, G. W. Zhang, H. Y. Luo, L. Liu, M. H. Shen and Y. Y. Yang, Chem. Eng. J., 2018, 334, 2547–2557 CrossRef CAS
.
- J. Lee, J. Y. Seok, S. Son, M. Yang and B. Kang, J. Mater. Chem. A, 2017, 5, 24585–24593 RSC
.
- C. Y. Wang, Z. J. Zheng, Y. Q. Feng, H. Ye, F. F. Cao and Z. P. Guo, Nano Energy, 2020, 74, 104817 CrossRef CAS
.
- C. W. Li, Q. C. Zhang, J. Sun, T. T. Li, E. Songfeng, Z. Z. Zhu, B. He, Z. Y. Zhou, Q. L. Li and Y. G. Yao, ACS Energy Lett., 2018, 3, 2761–2768 CrossRef CAS
.
- Y. H. Wang, J. R. Zeng, J. Li, X. Q. Cui, A. M. Al-Enizi, L. J. Zhang and G. F. Zheng, J. Mater. Chem. A, 2015, 3, 16382–16392 RSC
.
- B. Yuan and L. Cademartiri, J. Mater. Sci. Technol., 2015, 31, 607–615 CrossRef CAS
.
- X. Wang, Z. Li, J. Shi and Y. Yu, Chem. Rev., 2014, 114, 9346–9384 CrossRef CAS PubMed
.
- J. B. Wu, Z. W. Zhu, H. W. Zhang, H. M. Fu, H. Li, A. M. Wang and H. F. Zhang, Sci. Rep., 2016, 6, 1–7 CrossRef PubMed
.
- L. Wang, G. R. Yang, S. J. Peng, J. N. Wang, W. Yan and S. Ramakrishna, Energy Storage Mater., 2020, 25, 443–476 CrossRef
.
- J. N. Coleman, U. Khan, W. J. Blau and Y. K. Gun'ko, Carbon, 2006, 44, 1624–1652 CrossRef CAS
.
- Y. Liu and S. Kumar, ACS Appl. Mater. Interfaces, 2014, 6, 6069–6087 CrossRef CAS PubMed
.
- E. Z. Zhou, J. B. Xi, Y. Guo, Y. J. Liu, Z. Xu, L. Peng, W. W. Gao, J. Ying, Z. C. Chen and C. Gao, Carbon, 2018, 133, 316–322 CrossRef CAS
.
- B. Shi, Y. Shang, Y. Pei, S. Pei, L. Wang, D. Heider, Y. Y. Zhao, C. Zheng, B. Yang, S. Yarlagadda, T. W. Chou and K. K. Fu, Nano Lett., 2020, 20, 5504–5512 CrossRef CAS PubMed
.
- Y. Yao, R. Xu, M. L. Chen, X. L. Cheng, S. F. Zeng, D. J. Li, X. F. Zhou, X. J. Wu and Y. Yu, ACS Nano, 2019, 13, 4695–4704 CrossRef CAS PubMed
.
- D. Gueon, J. T. Hwang, S. B. Yang, E. Cho, K. Sohn, D. K. Yang and J. H. Moon, ACS Nano, 2018, 12, 226–233 CrossRef CAS PubMed
.
- X. He, Y. Hu, R. Z. Chen, Z. Shen, K. S. Wu, Z. L. Cheng and P. Pan, Chem. Eng. J., 2019, 360, 1020–1029 CrossRef CAS
.
- D. Li, L. Dai, X. Ren, F. Ji, Q. Sun, Y. Zhang and L. Ci, Energy Environ. Sci., 2021, 14, 424–436 RSC
.
- Y. X. Hu, D. L. Ye, B. Luo, H. Hu, X. B. Zhu, S. C. Wang, L. L. Li, S. J. Peng and L. Z. Wang, Adv. Mater., 2018, 30, 1703824 CrossRef PubMed
.
- H. Isobe, T. Tanaka, R. Maeda, E. Noiri, N. Solin, M. Yudasaka, S. Iijima and E. Nakamura, Angew. Chem., Int. Ed., 2006, 45, 6676–6680 CrossRef CAS PubMed
.
- B. Koh and W. Cheng, Langmuir, 2014, 30, 10899–10909 CrossRef CAS PubMed
.
- Y. H. Zhu, X. Y. Yang, T. Liu and X. B. Zhang, Adv. Mater., 2020, 32, e1901961 CrossRef PubMed
.
- L. Ye, Y. Hong, M. Liao, B. J. Wang, D. C. Wei, H. S. Peng, L. Ye, Y. Hong, M. Liao, B. Wang, D. Wei and H. Peng, Energy Storage Mater., 2020, 28, 364–374 CrossRef
.
- Y. C. Li, J. W. Zhou, T. B. Zhang, T. S. Wang, X. L. Li, Y. F. Jia, J. L. Cheng, Q. Guan, E. Z. Liu, H. S. Peng and B. Wang, Adv. Funct. Mater., 2019, 29, 1808117 CrossRef
.
- M. Tehrani, Phys. Status Solidi A, 2021, 218, 2000704 CrossRef CAS
.
- B. Xu, H. R. Wang, Q. Z. Zhu, N. Sun, B. Anasori, L. F. Hu, F. Wang, Y. B. Guan and Y. Gogotsi, Energy Storage Mater., 2018, 12, 128–136 CrossRef
.
- Y. He, W. Chen, C. Gao, J. Zhou, X. Li and E. Xie, Nanoscale, 2013, 5, 8799–8820 RSC
.
- Y. Shao, M. F. El-Kady, L. J. Wang, Q. Zhang, Y. Li, H. Wang, M. F. Mousavi and R. B. Kaner, Chem. Soc. Rev., 2015, 44, 3639–3665 RSC
.
- C. Hwang, W. J. Song, J. G. Han, S. Bae, G. Song, N. S. Choi, S. Park and H. K. Song, Adv. Mater., 2018, 30, 1705445 CrossRef PubMed
.
- D. Akinwande, N. Petrone and J. Hone, Nat. Commun., 2014, 5, 5678 CrossRef CAS PubMed
.
- X. Y. Chia and M. Pumera, Nat. Catal., 2018, 1, 909–921 CrossRef CAS
.
- P. Xiong, L. L. Peng, D. H. Chen, Y. Zhao, X. Wang and G. H. Yu, Nano Energy, 2015, 12, 816–823 CrossRef CAS
.
- P. Y. Chen, M. Liu, Z. Wang, R. H. Hurt and I. Y. Wong, Adv. Mater., 2017, 29, 1605096 CrossRef PubMed
.
- J. W. Ju, J. Ma, Y. T. Wang, Y. Y. Cui, P. X. Han and G. L. Cui, Energy Storage Mater., 2019, 20, 269–290 CrossRef
.
- L. L. Peng, Y. Zhu, D. H. Chen, R. S. Ruoff and G. H. Yu, Adv. Energy Mater., 2016, 6, 1600025 CrossRef
.
- E. Pomerantseva and Y. Gogotsi, Nat. Energy, 2017, 2, 17089 CrossRef CAS
.
- B. Mendoza-Sanchez and Y. Gogotsi, Adv. Mater., 2016, 28, 6104–6135 CrossRef CAS PubMed
.
- K. S. Novoselov, V. I. Fal'ko, L. Colombo, P. R. Gellert, M. G. Schwab and K. Kim, Nature, 2012, 490, 192–200 CrossRef CAS PubMed
.
- Z. Qin, M. Taylor, M. Hwang, K. Bertoldi and M. J. Buehler, Nano Lett., 2014, 14, 6520–6525 CrossRef CAS PubMed
.
- R. Van Noorden, Nature, 2012, 483, S32–S33 CrossRef PubMed
.
- M. Lotya, P. J. King, U. Khan, S. De and J. N. Coleman, ACS Nano, 2010, 4, 3155–3162 CrossRef CAS PubMed
.
- S. Bae, H. Kim, Y. Lee, X. Xu, J. S. Park, Y. Zheng, J. Balakrishnan, T. Lei, H. R. Kim, Y. I. Song, Y. J. Kim, K. S. Kim, B. Ozyilmaz, J. H. Ahn, B. H. Hong and S. Iijima, Nat. Nanotechnol., 2010, 5, 574–578 CrossRef CAS PubMed
.
- Y. Lee, S. Bae, H. Jang, S. Jang, S. E. Zhu, S. H. Sim, Y. I. Song, B. H. Hong and J. H. Ahn, Nano Lett., 2010, 10, 490–493 CrossRef CAS PubMed
.
- K. P. Loh, Q. Bao, G. Eda and M. Chhowalla, Nat. Chem., 2010, 2, 1015–1024 CrossRef CAS PubMed
.
- V. Chabot, D. Higgins, A. P. Yu, X. C. Xiao, Z. W. Chen and J. J. Zhang, Energy Environ. Sci., 2014, 7, 1564–1596 RSC
.
- V. Agarwal and P. B. Zetterlund, Chem. Eng. J., 2021, 405, 127018 CrossRef CAS
.
- S. Thakur and N. Karak, Carbon, 2015, 94, 224–242 CrossRef CAS
.
- T. Hoshide, Y. Zheng, J. Hou, Z. Wang, Q. Li, Z. Zhao, R. Ma, T. Sasaki and F. Geng, Nano Lett., 2017, 17, 3543–3549 CrossRef CAS PubMed
.
- P. Xiao, F. Bu, G. Yang, Y. Zhang and Y. Xu, Adv. Mater., 2017, 29, 1703324 CrossRef PubMed
.
- R. Huang, M. Huang, X. Li, F. An, N. Koratkar and Z. Z. Yu, Adv. Mater., 2018, 30, e1707025 CrossRef PubMed
.
- Z. Ling, C. E. Ren, M. Q. Zhao, J. Yang, J. M. Giammarco, J. Qiu, M. W. Barsoum and Y. Gogotsi, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 16676–16681 CrossRef CAS PubMed
.
- A. Lipatov, H. Lu, M. Alhabeb, B. Anasori, A. Gruverman, Y. Gogotsi and A. Sinitskii, Sci. Adv., 2018, 4, eaat0491 CrossRef PubMed
.
- M. Q. Zhao, C. E. Ren, Z. Ling, M. R. Lukatskaya, C. Zhang, K. L. Van Aken, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2015, 27, 339–345 CrossRef CAS PubMed
.
- Z. W. Zhang, H. N. Li, G. D. Zou, C. Fernandez, B. Z. Liu, Q. R. Zhang, J. Hu and Q. M. Peng, ACS Sustainable Chem. Eng., 2016, 4, 6763–6771 CrossRef CAS
.
- M. O. Faruk, A. Ahmed, B. Adak, M. Marzana, M. M. Hossain and S. Mukhopadhyay, J. Mater. Chem. C, 2021, 9, 10193–10215 RSC
.
- K. Liu, Y. Fan, A. Ali and P. K. Shen, Nanoscale, 2021, 13, 2963–2971 RSC
.
- J. Zhou, M. Xie, F. Wu, Y. Mei, Y. Hao, L. Li and R. Chen, Adv. Mater., 2022, 34, 2106897 CrossRef CAS PubMed
.
- H. Zhang, W. Q. Zhao, M. C. Zou, Y. S. Wang, Y. J. Chen, L. Xu, H. S. Wu and A. Y. Cao, Adv. Energy Mater., 2018, 8, 1800013 CrossRef
.
- X. Wang, K. Chen, G. Wang, X. Liu and H. Wang, ACS Nano, 2017, 11, 11602–11616 CrossRef CAS PubMed
.
- M. Govindasamy, S. Shanthi, E. Elaiyappillai, S. F. Wang, P. M. Johnson, H. Ikeda, Y. Hayakawa, S. Ponnusamy and C. Muthamizhchelvan, Electrochim. Acta, 2019, 293, 328–337 CrossRef CAS
.
- X. G. Gao, Y. Huang, Z. X. Guang and X. Li, Energy Fuels, 2020, 34, 3931–3940 CrossRef CAS
.
- Q. T. Zhou, J. N. Kim, K. W. Han, S. W. Oh, S. Umrao, E. J. Chae and I. K. Oh, Nano Energy, 2019, 59, 120–128 CrossRef CAS
.
- Z. Guo, H. Nie, Z. Yang, W. Hua, C. Ruan, D. Chan, M. Ge, X. Chen and S. Huang, Adv. Sci., 2018, 5, 1800026 CrossRef PubMed
.
- J. Ren, R. P. Ren and Y. K. Lv, Chem. Eng. J., 2018, 353, 419–424 CrossRef CAS
.
- T. Chen, Y. A. Wang, Y. Yang, F. Huang, M. K. Zhu, B. T. W. Ang and J. M. Xue, Adv. Funct. Mater., 2021, 31, 2101607 CrossRef CAS
.
- K. Wu, L. Zhang, Y. Yuan, L. Zhong, Z. Chen, X. Chi, H. Lu, Z. Chen, R. Zou, T. Li, C. Jiang, Y. Chen, X. Peng and J. Lu, Adv. Mater., 2020, 32, e2002292 CrossRef PubMed
.
- G. Y. Qian, X. B. Liao, Y. X. Zhu, F. Pan, X. Chen and Y. Yang, ACS Energy Lett., 2019, 4, 690–701 CrossRef CAS
.
- T. Liu, M. Zhang, Y. L. Wang, Q. Y. Wang, C. Lv, K. X. Liu, S. Suresh, Y. H. Yin, Y. Y. Hu, Y. S. Li, X. B. Liu, S. W. Zhong, B. Y. Xia and Z. P. Wu, Adv. Energy Mater., 2018, 8, 1802349 CrossRef
.
- F. Wu, X. Gao, X. Xu, Y. Jiang, X. Gao, R. Yin, W. Shi, W. Liu, G. Lu and X. Cao, ChemSusChem, 2020, 13, 1537–1545 CrossRef CAS PubMed
.
- T. Zhang, L. Zhang, L. Zhao, X. Huang, W. Li, T. Li, T. Shen, S. Sun and Y. Hou, Small, 2020, 16, e2005302 CrossRef PubMed
.
- C. Chen, S. Xu, Y. Kuang, W. Gan, J. Song, G. Chen, G. Pastel, B. Liu, Y. Li, H. Huang and L. Hu, Adv. Energy Mater., 2019, 9, 1802964 CrossRef
.
- J. H. Li, Y. L. Shao, Q. W. Shi, C. Y. Hou, Q. H. Zhang, Y. G. Li, R. B. Kaner and H. Z. Wang, Nano Energy, 2017, 38, 429–437 Search PubMed
.
- Z. Luo, C. Liu and S. Fan, ACS Appl. Mater. Interfaces, 2019, 11, 42172–42178 CrossRef CAS PubMed
.
- C. M. Shi, T. Y. Wang, X. B. Liao, B. Y. Qie, P. F. Yang, M. J. Chen, X. Wang, A. Srinivasan, Q. Cheng, Q. Ye, A. C. Li, X. Chen and Y. Yang, Energy Storage Mater., 2019, 17, 136–142 CrossRef
.
- G. Qian, B. Zhu, X. Liao, H. Zhai, A. Srinivasan, N. J. Fritz, Q. Cheng, M. Ning, B. Qie, Y. Li, S. Yuan, J. Zhu, X. Chen and Y. Yang, Adv. Mater., 2018, 30, e1704947 CrossRef PubMed
.
- A. Chen, X. Guo, S. Yang, G. J. Liang, Q. Li, Z. Chen, Z. D. Huang, Q. Yang, C. P. Han and C. Y. Zhi, Energy Environ. Sci., 2021, 14, 3599–3608 RSC
.
- X. B. Liao, C. M. Shi, T. Y. Wang, B. Y. Qie, Y. L. Chen, P. F. Yang, Q. Cheng, H. W. Zhai, M. J. Chen, X. Wang, X. Chen and Y. Yang, Adv. Energy Mater., 2019, 9, 1802998 CrossRef
.
- N. Nitta, F. X. Wu, J. T. Lee and G. Yushin, Mater. Today, 2015, 18, 252–264 CrossRef CAS
.
- K. Amin, Q. Meng, A. Ahmad, M. Cheng, M. Zhang, L. Mao, K. Lu and Z. Wei, Adv. Mater., 2018, 30, 1703868 CrossRef PubMed
.
- J. M. Son, S. Oh, S. H. Bae, S. Nam and I. K. Oh, Adv. Energy Mater., 2019, 9, 1900477 CrossRef
.
- S. R. Chen, R. M. Tao, J. Tu, P. M. Guo, G. Yang, W. J. Wang, J. Y. Liang and S. Y. Lu, Adv. Funct. Mater., 2021, 31, 2101199 CrossRef CAS
.
- G. H. Chen, F. Zhang, Z. M. Zhou, J. R. Li and Y. B. Tang, Adv. Energy Mater., 2018, 8, 1801219 CrossRef
.
- S. Oh, V. H. Nguyen, V. T. Bui, S. Nam, M. Mahato and I. K. Oh, ACS Appl. Mater. Interfaces, 2020, 12, 11657–11668 CrossRef CAS PubMed
.
- D. Wei, W. Shen, T. Xu, K. Li, L. Yang, Y. Zhou, M. Zhong, F. Yang, X. Xu, Y. Wang, M. Zheng, Y. Zhang, Q. Li, Z. Yong, H. Li and Q. Wang, Mater. Today Energy, 2022, 23, 100889 CrossRef CAS
.
- Y. Gao, H. Hu, J. Chang, Q. Y. Huang, Q. N. Zhuang, P. Li and Z. J. Zheng, Adv. Energy Mater., 2021, 11, 2101809 CrossRef CAS
.
- J. L. Xu, Y. H. Liu, X. Gao, S. Shen and S. D. Wang, Energy Storage Mater., 2019, 22, 402–409 CrossRef
.
- Y. Wang, X. Wang, X. Li, X. Li, Y. Liu, Y. Bai, H. Xiao and G. Yuan, Adv. Funct. Mater., 2021, 31, 2008185 CrossRef CAS
.
- L. Kumar, P. K. Boruah, M. R. Das and S. Deka, ACS Appl. Mater. Interfaces, 2019, 11, 37665–37674 CrossRef CAS PubMed
.
- N. R. Chodankar, S. Selvaraj, S. H. Ji, Y. Kwon and D. H. Kim, Small, 2019, 15, 1803716 CrossRef PubMed
.
- C. Chen, J. Cao, Q. Q. Lu, X. Y. Wang, L. Song, Z. Q. Niu and J. Chen, Adv. Funct. Mater., 2017, 27, 1604639 CrossRef
.
- H. Wu, Y. N. Zhang, W. Y. Yuan, Y. X. Zhao, S. H. Luo, X. W. Yuan, L. X. Zheng and L. F. Cheng, J. Mater. Chem. A, 2018, 6, 16617–16626 RSC
.
- Q. Qin, J. Liu, W. Mao, C. Xu, B. Lan, Y. Wang, Y. Zhang, J. Yan and Y. Wu, Nanoscale, 2018, 10, 7377–7381 RSC
.
- Y. H. Liu, J. L. Xu, X. Gao, Y. L. Sun, J. J. Lv, S. Shen, L. S. Chen and S. D. Wang, Energy Environ. Sci., 2017, 10, 2534–2543 RSC
.
- A. M. Bryan, L. M. Santino, Y. Lu, S. Acharya and J. M. D'Arcy, Chem. Mater., 2016, 28, 5989–5998 CrossRef CAS
.
- J. Gamby, P. L. Taberna, P. Simon, J. F. Fauvarque and M. Chesneau, J. Power Sources, 2001, 101, 109–116 CrossRef CAS
.
- L. B. Dong, C. J. Xu, Q. Yang, J. Fang, Y. Li and F. Y. Kang, J. Mater. Chem. A, 2015, 3, 4729–4737 RSC
.
- J. H. Jun, H. Song, C. Kim, I. S. Choi, Y. Jeong and J. H. Lee, Small, 2018, 14, 1702145 CrossRef PubMed
.
- M. C. G. Santos, D. R. da Silva, P. S. Pinto, A. S. Ferlauto, R. G. Lacerda, W. P. Jesus, T. H. R. da Cunha, P. F. R. Ortega and R. L. Lavall, Electrochim. Acta, 2020, 349, 136241 CrossRef CAS
.
- L. B. Dong, G. M. Liang, C. J. Xu, D. Y. Ren, J. J. Wang, Z. Z. Pan, B. H. Li, F. Y. Kang and Q. H. Yang, J. Mater. Chem. A, 2017, 5, 19934–19942 RSC
.
- F. H. Su and M. H. Miao, Electrochim. Acta, 2014, 127, 433–438 CrossRef CAS
.
- J. P. Li, S. Qiu, B. F. Liu, H. Y. Chen, D. S. Xiao and H. Li, J. Power Sources, 2021, 483, 229219 CrossRef CAS
.
- Q. Z. Liu, Z. H. Chen, S. S. Jing, H. Zhuo, Y. J. Hu, J. C. Liu, L. X. Zhong, X. W. Peng and C. F. Liu, J. Mater. Chem. A, 2018, 6, 20338–20346 RSC
.
- Y. J. Li, W. Ou-Yang, X. T. Xu, M. Wang, S. J. Hou, T. Lu, Y. F. Yao and L. K. Pan, Electrochim. Acta, 2018, 271, 591–598 CrossRef CAS
.
- Y. Liu, J. Zhou, L. Chen, P. Zhang, W. Fu, H. Zhao, Y. Ma, X. Pan, Z. Zhang, W. Han and E. Xie, ACS Appl. Mater. Interfaces, 2015, 7, 23515–23520 CrossRef CAS PubMed
.
- Y. Y. Shen, Z. Y. Qin, S. Hu, L. F. Yang, X. Xu, L. Ding and Y. W. Zhang, Carbon, 2020, 158, 711–718 CrossRef CAS
.
- L. Huang, D. Santiago, P. Loyselle and L. Dai, Small, 2018, 14, e1800879 CrossRef PubMed
.
- K. Lee, H. Lee, Y. Shin, Y. Yoon, D. Kim and H. Lee, Nano Energy, 2016, 26, 746–754 CrossRef CAS
.
- G. de Souza Augusto, J. Scarmínio, P. R. C. Silva, A. de Siervo, C. S. Rout, F. Rouxinol and R. V. Gelamo, Electrochim. Acta, 2018, 285, 241–253 CrossRef CAS
.
- C. J. Ye, Q. Q. Qin, J. Q. Liu, W. P. Mao, J. Yan, Y. Wang, J. W. Cui, Q. Zhang, L. P. Yang and Y. C. Wu, J. Mater. Chem. A, 2019, 7, 4998–5008 RSC
.
- F. N. Dai, X. K. Wang, S. H. Zheng, J. P. Sun, Z. D. Huang, B. Xu, L. L. Fan, R. M. Wang, D. F. Sun and Z. S. Wu, Chem. Eng. J., 2021, 413, 127520 CrossRef CAS
.
- Y. Yan, P. Gu, S. S. Zheng, M. B. Zheng, H. Pang and H. G. Xue, J. Mater. Chem. A, 2016, 4, 19078–19085 RSC
.
- W. Zhao, Y. W. Zheng, L. Cui, D. D. Jia, D. Wei, R. K. Zheng, C. Barrow, W. R. Yang and J. Q. Liu, Chem. Eng. J., 2019, 371, 461–469 CrossRef CAS
.
- S. Y. Zhou, X. Y. Kong, B. Zheng, F. W. Huo, M. Stromme and C. Xu, ACS Nano, 2019, 13, 9578–9586 CrossRef CAS PubMed
.
- Y. Yang, S. W. Ng, D. Chen, J. Chang, D. Wang, J. Shang, Q. Huang, Y. Deng and Z. Zheng, Small, 2019, 15, e1902071 CrossRef PubMed
.
- K. F. Blurton and A. F. Sammells, J. Power Sources, 1979, 4, 263–279 CrossRef CAS
.
- M. A. Rahman, X. J. Wang and C. E. Wen, J. Electrochem. Soc., 2013, 160, A1759–A1771 CrossRef CAS
.
- J. S. Lee, S. T. Kim, R. Cao, N. S. Choi, M. Liu, K. T. Lee and J. Cho, Adv. Energy Mater., 2011, 1, 34–50 CrossRef CAS
.
- Q. F. Liu, Z. F. Pan, E. D. Wang, L. An and G. Q. Sun, Energy Storage Mater., 2020, 27, 478–505 CrossRef
.
- L. Wang, J. Pan, Y. Zhang, X. Cheng, L. Liu and H. Peng, Adv. Mater., 2018, 30, 1704378 CrossRef PubMed
.
- J. Lai, Y. Xing, N. Chen, L. Li, F. Wu and R. Chen, Angew. Chem., Int. Ed., 2020, 59, 2974–2997 CrossRef CAS PubMed
.
- X. Chi, M. Li, J. Di, P. Bai, L. Song, X. Wang, F. Li, S. Liang, J. Xu and J. Yu, Nature, 2021, 592, 551–557 CrossRef CAS PubMed
.
- Z. Y. Guo, J. L. Li, Y. Xia, C. Chen, F. M. Wang, A. G. Tamirat, Y. G. Wang, Y. Y. Xia, L. Wang and S. H. Feng, J. Mater. Chem. A, 2018, 6, 6022–6032 RSC
.
- C. Tang, B. Wang, H. F. Wang and Q. Zhang, Adv. Mater., 2017, 29, 1703185 CrossRef PubMed
.
- H. F. Wang, C. Tang and Q. Zhang, Adv. Funct. Mater., 2018, 28, 1803329 CrossRef
.
- X. Y. Cai, L. F. Lai, J. Y. Lin and Z. X. Shen, Mater. Horiz., 2017, 4, 945–976 RSC
.
- Y. G. Zhang, Y. P. Deng, J. Y. Wang, Y. Jiang, G. L. Cui, L. L. Shui, A. P. Yu, X. Wang and Z. W. Chen, Energy Storage Mater., 2021, 35, 538–549 CrossRef
.
- L. Yang, L. Shi, D. Wang, Y. L. Lv and D. P. Cao, Nano Energy, 2018, 50, 691–698 CrossRef CAS
.
- W. Wang, M. Tang, Z. Zheng and S. Chen, Adv. Energy Mater., 2019, 9, 1803628 CrossRef
.
- T. Zhang, Y. Tang, S. Guo, X. Cao, A. Pan, G. Fang, J. Zhou and S. Liang, Energy Environ. Sci., 2020, 13, 4625–4665 RSC
.
- X. Shen, T. Sun, L. Yang, A. Krasnoslobodtsev, R. Sabirianov, M. Sealy, W.-N. Mei, Z. Wu and L. Tan, Nat. Commun., 2021, 12, 820 CrossRef CAS PubMed
.
- A. Ponrouch, J. Bitenc, R. Dominko, N. Lindahl, P. Johansson and M. R. Palacin, Energy Storage Mater., 2019, 20, 253–262 CrossRef
.
- Y. Liang, H. Dong, D. Aurbach and Y. Yao, Nat. Energy, 2020, 5, 646–656 CrossRef CAS
.
- P. Canepa, G. Sai Gautam, D. C. Hannah, R. Malik, M. Liu, K. G. Gallagher, K. A. Persson and G. Ceder, Chem. Rev., 2017, 117, 4287–4341 CrossRef CAS PubMed
.
- H. L. Ye and Y. G. Li, Energy Fuels, 2021, 35, 7624–7636 CrossRef CAS
.
- J. Xie and Q. Zhang, Small, 2019, 15, e1805061 CrossRef PubMed
.
- S. L. Lu, M. Y. Wang, F. Guo, J. G. Tu, A. J. Lv, Y. F. Chen and S. Q. Jiao, Chem. Eng. J., 2020, 389, 124370 CrossRef CAS
.
- P. P. Wang, Z. Chen, H. Wang, Z. Y. Ji, Y. P. Feng, J. Q. Wang, J. Liu, M. M. Hu, J. B. Fei, W. Gan and Y. Huang, Energy Storage Mater., 2020, 25, 426–435 CrossRef
.
- A. Ponrouch, C. Frontera, F. Barde and M. R. Palacin, Nat. Mater., 2016, 15, 169–172 CrossRef CAS PubMed
.
- M. Wang, C. Jiang, S. Zhang, X. Song, Y. Tang and H. M. Cheng, Nat. Chem., 2018, 10, 667–672 CrossRef CAS PubMed
.
- J. Hyoung, J. W. Heo and S. T. Hong, J. Power Sources, 2018, 390, 127–133 CrossRef CAS
.
- P. Wang, H. Wang, Z. Chen, J. Wu, J. Luo and Y. Huang, Nano Res., 2021, 15, 701–708 CrossRef
.
- X. Zou, Q. Lu, Y. Zhong, K. Liao, W. Zhou and Z. Shao, Small, 2018, 14, e1801798 CrossRef PubMed
.
- D. Xie, M. Zhang, Y. Wu, L. Xiang and Y. Tang, Adv. Funct. Mater., 2019, 30, 1906770 CrossRef
.
- Z. X. Liu, Q. Yang, D. H. Wang, G. J. Liang, Y. H. Zhu, F. N. Mo, Z. D. Huang, X. L. Li, L. T. Ma, T. C. Tang, Z. G. Lu and C. Y. Zhi, Adv. Energy Mater., 2019, 9, 1902473 CrossRef CAS
.
- H. F. Li, C. P. Han, Y. Huang, Y. Huang, M. S. Zhu, Z. X. Pei, Q. Xue, Z. F. Wang, Z. X. Liu, Z. J. Tang, Y. K. Wang, F. Y. Kang, B. H. Li and C. Y. Zhi, Energy Environ. Sci., 2018, 11, 941–951 RSC
.
- F. Baskoro, H. Q. Wong and H. J. Yen, ACS Appl. Energy Mater., 2019, 2, 3937–3971 CrossRef CAS
.
- X. Y. Fan, J. Liu, Z. S. Song, X. P. Han, Y. D. Deng, C. Zhong and W. B. Hu, Nano Energy, 2019, 56, 454–462 CrossRef CAS
.
- V. Vallem, Y. Sargolzaeiaval, M. Ozturk, Y. C. Lai and M. D. Dickey, Adv. Mater., 2021, 33, e2004832 CrossRef PubMed
.
- Z. Wu, Y. Wang, X. Liu, C. Lv, Y. Li, D. Wei and Z. Liu, Adv. Mater., 2019, 31, e1800716 CrossRef PubMed
.
- Y. Zhang, Q. Wang, S. Bi, M. Yao, F. Wan and Z. Niu, Nanoscale, 2019, 11, 17630–17636 RSC
.
- D. Lee, H. Lee, O. Gwon, O. Kwon, H. Y. Jeong, G. Kim and S. Y. Lee, J. Mater. Chem. A, 2019, 7, 24231–24238 RSC
.
- B. Wang, J. Li, C. Hou, Q. Zhang, Y. Li and H. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 46005–46014 CrossRef CAS PubMed
.
- H. Z. Liu, J. H. Li, X. N. Zhang, X. X. Liu, Y. Yan, F. J. Chen, G. H. Zhang and H. G. Duan, Adv. Funct. Mater., 2021, 31, 2106550 CrossRef CAS
.
- K. D. Harris, A. L. Elias and H. J. Chung, J. Mater. Sci., 2016, 51, 2771–2805 CrossRef CAS
.
- A. M. Gaikwad and A. C. Arias, ACS Appl. Mater. Interfaces, 2017, 9, 6390–6400 CrossRef CAS PubMed
.
- H. Wang, J. Fu, C. Wang, R. Zhang, Y. Li, Y. Yang, H. Li, Q. Sun and H. Li, InfoMat, 2021, 4, e12241 Search PubMed
.
- L. J. Mao, Q. H. Meng, A. Ahmad and Z. X. Wei, Adv. Energy Mater., 2017, 7, 1700535 CrossRef
.
- H. F. Li, Z. J. Tang, Z. X. Liu and C. Y. Zhi, Joule, 2019, 3, 613–619 CrossRef
.
- S. H. Kim, N. Y. Kim, U. J. Choe, J. M. Kim, Y. G. Lee and S. Y. Lee, Adv. Energy Mater., 2021, 11, 2100531 CrossRef CAS
.
- Y. Bao, G. Hong, Y. Chen, J. Chen, H. Chen, W. L. Song and D. Fang, ACS Appl. Mater. Interfaces, 2020, 12, 780–788 CrossRef CAS PubMed
.
- C. J. Xu, L. Weng, B. B. Chen, L. Ji, J. Q. Zhou, R. Cai and S. L. Lu, J. Power Sources, 2020, 446, 227353 CrossRef CAS
.
- N. Li, H. Chen, S. Yang, H. Yang, S. Jiao and W. L. Song, Adv. Sci., 2021, 8, e2101372 CrossRef PubMed
.
- Y. Mao, G. Li, Y. Guo, Z. Li, C. Liang, X. Peng and Z. Lin, Nat. Commun., 2017, 8, 14628 CrossRef PubMed
.
- R. Z. Chen, Y. Hu, Z. Shen, P. Pan, X. He, K. S. Wu, X. W. Zhang and Z. L. Cheng, J. Mater. Chem. A, 2017, 5, 12914–12921 RSC
.
- A. Mahmood, Z. W. Yuan, X. Sui, M. A. Riaz, Z. X. Yu, C. Liu, J. S. Chen, C. Wang, S. L. Zhao, N. Mahmood, Z. X. Pei, L. Wei and Y. Chen, Energy Storage Mater., 2021, 41, 395–403 CrossRef
.
- W. G. Chong, Y. Xiao, J. Q. Huang, S. Yao, J. Cui, L. Qin, C. Gao and J. K. Kim, Nanoscale, 2018, 10, 21132–21141 RSC
.
- Z. Wang, A. Malti, L. Ouyang, D. Tu, W. Tian, L. Wagberg and M. M. Hamedi, Small, 2018, 14, e1803313 CrossRef PubMed
.
- S. Y. Zhao, Y. Y. Zuo, T. Liu, S. Zhai, Y. W. Dai, Z. J. Guo, Y. Wang, Q. J. He, L. C. Xia, C. Y. Zhi, J. Bae, K. L. Wang and M. Ni, Adv. Energy Mater., 2021, 11, 2101749 CrossRef CAS
.
- C. Shu, J. Long, S. X. Dou and J. Wang, Small, 2019, 15, e1804701 CrossRef PubMed
.
- Y. N. Zhang, H. L. Qin, M. Alfred, H. Z. Ke, Y. B. Cai, Q. Q. Wang, F. L. Huang, B. Liu, P. F. Lv and Q. F. Wei, Energy Storage Mater., 2021, 42, 88–96 CrossRef
.
- G. Zan, T. Wu, Z. Zhang, J. Li, J. Zhou, F. Zhu, H. Chen, M. Wen, X. Yang, X. Peng, J. Chen and Q. Wu, Adv. Sci., 2022, 9, 2103714 CrossRef PubMed
.
- J. H. Park, M. J. Kwak, C. Hwang, K. N. Kang, N. Liu, J. H. Jang and B. A. Grzybowski, Adv. Mater., 2021, 33, e2101726 CrossRef PubMed
.
- A. Sumboja, M. Lubke, Y. Wang, T. An, Y. Zong and Z. L. Liu, Adv. Energy Mater., 2017, 7, 1700927 CrossRef
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |