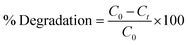Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Evaluation of bactericidal potential and catalytic dye degradation of multiple morphology based chitosan/polyvinylpyrrolidone-doped bismuth oxide nanostructures
Ahsaan
Bari
a,
Muhammad
Ikram
 *a,
Ali
Haider
b,
Anwar
Ul-Hamid
*a,
Ali
Haider
b,
Anwar
Ul-Hamid
 *c,
Junaid
Haider
d,
Iram
Shahzadi
e,
Ghazanfar
Nazir
f,
Anum
Shahzadi
g,
M.
Imran
h and
Abdul
Ghaffar
i
*c,
Junaid
Haider
d,
Iram
Shahzadi
e,
Ghazanfar
Nazir
f,
Anum
Shahzadi
g,
M.
Imran
h and
Abdul
Ghaffar
i
aSolar Cell Applications Research Lab, Department of Physics, Government College, University Lahore, Lahore, 54000, Punjab, Pakistan. E-mail: dr.muhammadikram@gcu.edu.pk
bDepartment of Clinical Sciences, Faculty of Veterinary and Animal Sciences, Muhammad Nawaz Shareef, University of Agriculture (MNSUA), 66000, Punjab, Pakistan
cCore Research Facilities, King Fahd University of Petroleum & Minerals, Dhahran 31261, Saudi Arabia. E-mail: anwar@kfupm.edu.sa
dTianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences, Tianjin 300308, China
ePunjab University College of Pharmacy, University of the Punjab, Lahore, 54000, Pakistan
fDepartment of Nanotechnology and Advanced Materials Engineering, Sejong University, Seoul 05006, Republic of Korea
gFaculty of Pharmacy, University of the Lahore, Lahore, Pakistan
hDepartment of Chemistry, Government College University Faisalabad, Pakpattan Road, Sahiwal, Punjab 57000, Pakistan
iDepartment of Physics, Government College University Lahore, 54000, Pakistan
First published on 4th May 2022
Abstract
In this study, 0.02 and 0.04 wt% of chitosan (CS) were successfully incorporated in a fixed amount of polyvinylpyrrolidone (PVP)-doped Bi2O3 nanostructures (NSs) via a co-precipitation approach. The purpose of this research was to degrade hazardous methylene blue dye and assess antimicrobial potential of the prepared CS/PVP-doped Bi2O3 nanostructures. In addition, optical characteristics, charge recombination rate, elemental composition, phase formation, surface morphology, functional groups, d-spacing, and crystallinity of the obtained nanostructures were investigated. CS/PVP-doped Bi2O3 nanostructures exhibited efficient catalytic activity (measured as 99%) in a neutral medium for dopant-free nanostructures while the inhibition zone was measured using a Vernier caliper against pathogens Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) at low and high doses to check antimicrobial activity. Strong bactericidal action was recorded against S. aureus bacteria such that a significant inhibition zone was measured at 3.09 mm.
1 Introduction
Increased economic activity and fast industrial growth have exacerbated water pollution and health-related concerns globally.1 World Health Organization (WHO) estimates that each year 2.3 million people die from water-borne (typhoid, cholera, hepatitis, and diarrhea) and carcinogenic diseases.2–4 Around 70% of water pollution is produced due to industrial waste dyes (acidic, basic, and azoic) and heavy metals (cadmium, chromium, nickel, lead, etc.). All such pollutants are highly soluble in nature and pose a serious health risk to humans and wildlife.5 Dyes are excessively used in paper coloring i.e., temporary hair colorants, cotton dyeing, and paper stock coating. Especially methylene blue (MB), a basic dye, has an aromatic molecular structure that is stable and nonbiodegradable posing strong ecological threat to aquatic life.6 According to published research, 15% of the most extensively produced dyes are released into water bodies both directly and indirectly.7 As a result, it is important to employ a method capable of degrading synthetic dye directly into non-toxic molecules such as water and carbon dioxide. Scientists use traditional approaches such as chlorination, aerobic treatment, adsorption, and ion exchange to remove organic contaminants from water. Unfortunately, these techniques have drawbacks and limitations such as high energy consumption, secondary pollution caused by inadequate removal, and transfer of dye.8,9 Catalysis in the presence of nanomaterial-based semiconductors attracted interest of researchers owing to their minimal toxicity, chemical stability, low cost and nature-friendly characteristics.10 In order to degrade synthetic dyes such as MB, this research uses a reducing agent and nano-catalyst.11–13 Mastitis has a substantial economic burden on the dairy sector. Infectious agents such as bacteria, viruses, and fungi cause mastitis. Chemical, microbial, and physical changes in milk, and clinical abnormalities in mammary gland tissues, are all associated with this disease.14Coliform, Escherichia coli and Staphylococcus aureus are the most prevalent bacterial pathogens linked to mastitis.15,16Nanomaterials have attracted researcher's attention due to their unique physiochemical properties and enhanced dye-contaminated wastewater treatment methods.17–19 Small NSs with size ranging from 1 to 100 nm have astonishing surface-to-volume ratios when compared to those of bulk chemical compositions, resulting in significant increases in chemical (biological, catalytic activity, etc.) and physical properties. Metal oxide nanomaterials have large surface area, and attractive nanostructural, optical, mechanical, and thermodynamic characteristics that are advantageous for catalysis and antibacterial activities.20 Numerous metal oxide nanomaterials (ZnO, TiO2, La2O3, CeO2, and Bi2O3) are being used in catalysis and to check antibacterial activity; particularly as an important p-type semiconductor, Bi2O3 has remarkable anode semiconductor properties including a broad band gap, low toxicity, high conductivity, antibacterial activity and degradation capability for organic dyes.21–29 Chemical (co-precipitation, sol gel, and redox reactions) and green synthesis techniques are utilized to synthesize Bi2O3 NSs.21,22,30,31 Among these, the co-precipitation method is considered as ecofriendly, inexpensive, energy-efficient and easy to use.23 A number of research studies were conducted on Bi2O3 NSs prepared through various synthesis routes to check the influence of antimicrobial activity and dye degradation.32–41 However, the obtained results were not impressive for bactericidal action and dye degradation performance. The addition of a polymer into metal oxides increases their stability and improves physiochemical properties which results in efficient dye degradation and antibacterial performance.
Polymers can interact with metal ions either through complex or ion-pair formation, which might be an attractive substitute for a stabilizer and thus can be targeted to attain specific physicochemical parameters of NSs.24 Polymeric materials have received much attention from scientists for usage in biological and environmental applications.42 Numerous types of polymers (polyvinyl alcohol, polyvinyl chloride, polyvinylpyrrolidone, and chitosan) are used for metal oxide doping to attain significant outcomes for various applications.43–45 Among them, PVP is a synthetic polymer that is considered an effective capping agent for metal oxide NSs. Its properties are attributed to the presence of both carbonyl groups and functional groups that strengthen metal oxide NSs within its composite.46,47 As it exhibits excellent physicochemical properties, it is used as an additive in different materials and to stabilize NSs.48–51 Coincidently, recent studies have shown that PVP has great water solubility, low toxicity, biocompatibility, and exhibits promising results against antimicrobial activity.52–55 Chitosan is an alkaline polymer prepared by partially hydrolyzing chitin, the primary component of crustaceans and fungus cells, and extensively used for pharmaceutical and biomedical purposes. It has superior biodegradability, biocompatibility, low toxicity, and film-forming characteristics.56 CS is mainly composed of amino and hydroxyl groups, both significant in metal ion chemical adsorption, and these groups can bind with metal ions more efficiently than any other polysaccharide, making a strong template for synthesizing metal oxide NSs.57–61
The motivation of this research is to synthesize PVP/CS-doped Bi2O3 NSs utilizing an ecologically friendly co-precipitation technique for degradation of organic dyes from contaminated water and also to assess material's bactericidal potential. Numerous characterization techniques were employed for detailed analysis of synthesized NSs. Catalytic activity (CA) tests were performed for degradation of MB dye. Furthermore, Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) pathogens were used to examine its potential for antibacterial activity.
2 Experimental section
2.1 Materials
Bismuth nitrate (Bi(NO3)3·5H2O, 98%) was acquired from BDH Laboratory Supplies. Sodium hydroxide (NaOH, 98%), polyvinylpyrrolidone (PVP) and chitosan (CS) were supplied by Sigma-Aldrich.2.2 Synthesis of polyvinylpyrrolidone/chitosan-doped bismuth oxide
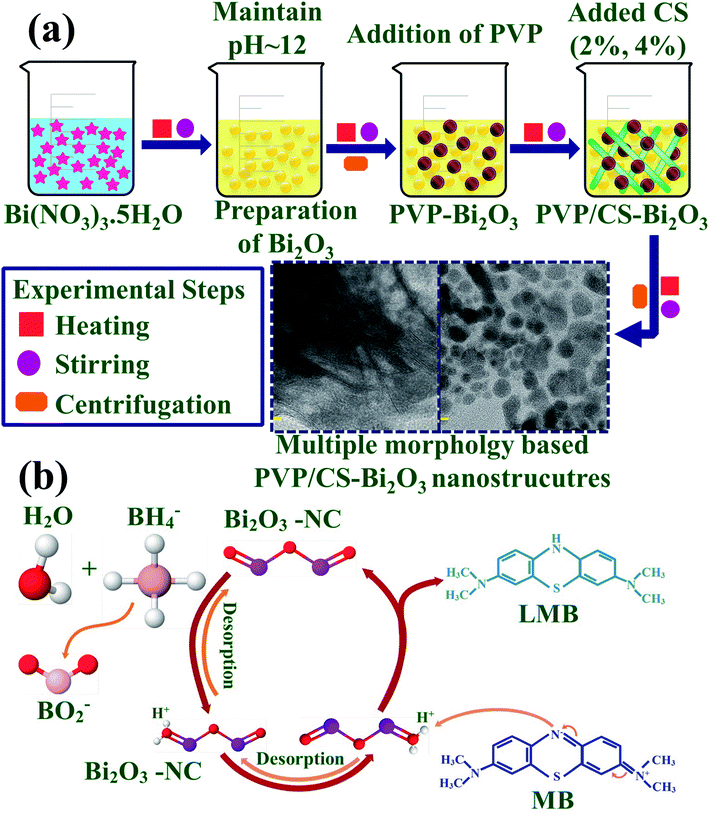
0.5 M of Bi2O3 was prepared under continuous stirring at 80 °C. NaOH was incorporated dropwise to maintain pH ≈ 12 under vigorous stirring for 1 hour. Further the colloidal solution was centrifuged at 7500 rpm repeatedly and dried for 12 hours at 150 °C. The obtained Bi2O3 NSs were crushed into fine powder. Using the above method, the desired amount of PVP was dissolved to prepare PVP-doped Bi2O3 and various concentrations of CS (2% and 4%) were added to get PVP/CS-doped Bi2O3 NSs as represented in Fig. 1(a).2.3 Catalysis
The degradation efficiency of synthetic dyes in the presence of sodium borohydride (NaBH4) and the synthesized nano-catalyst was determined through CA measurements. MB is a positively charged thiazine dye frequently used as a reductant in analytical chemistry, and is colorless in the reduced form and blue in the oxidized form.62 Using a quartz cell, 0.1 M NaBH4 solution (400 μL) was dissolved in 3 ml MB. Furthermore, 400 μL synthesized NS solution was incorporated in aqueous solution of MB. Absorption reaction progress was spectrophotometrically monitored at room temperature. In the presence of NaBH4, MB changed to leucomethylene confirming degradation of dyes. Samples without a nano-catalyst were referred to as blank. % degradation was calculated as:where C0 represents initial absorbance and Ct represents the concentration at specific time.
2.4 Isolation and identification of Staphylococcus aureus and Escherichia coli
Large quantities of dairy milk specimens were obtained from Pakistani public, private institutions and dairy farms and evaluated for surf-field mastitis. Furthermore, the acquired samples were incubated in 5% sheep blood agar. On Mannitol salt agar (MSA) and MacConkey agar (MA), colonies were formed in order to isolate Gram-positive (G +ve) S. aureus and Gram-negative (G −ve) E. coli pathogens, respectively. Pharmacological (catalase and coagulase) and morphological (gram staining) methodologies were used to identify distinctive colonies.2.5 Antimicrobial activity
Antibacterial performance of the prepared NSs was examined through the agar well diffusion approach with germ strain (G +ve and G −ve) swabbed 1.5 × 108 CFU mL−1 on MSA and MA for S. aureus and E. coli, respectively. Moreover, negative and positive controls were assigned to DIW (50 μL) and ciprofloxacin (0.005 mg/50 μL), correspondingly. Different concentrations of Bi2O3 and PVP/CS (2%, 4%)-doped Bi2O3 NSs were injected into a 6 mm well on MSA and MA plates with a micropipette and sterilized cork borer at low (0.5 mg/50 μL) and high (1.0 mg/50 μL) doses. Petri plates containing doses were incubated for 24 h at 37 °C. Furthermore, a Vernier caliper was used to assess the diameter of the inhibition zone that results in the determination of antibacterial performance. One-way variance analysis in SPSS 20 was employed to determine the bacterial efficiency by measuring the inhibitory zone.2.6 Characterization techniques
Structural and crystalline behaviors of obtained powder were determined using powder XRD ranging from 10° to 60°. FTIR spectroscopy was performed between 4000 and 400 cm−1 to identify functional groups present in PVP/CS (2%, 4%)-doped Bi2O3 NSs. The chemical composition, surface study, morphology and d-spacing of PVP/CS (2%, 4%)-doped Bi2O3 NSs were analyzed through EDS, SEM and HR-TEM respectively. Additionally, SAED analysis was performed to check crystallinity of the prepared samples. A Genesys 10S UV-vis spectrophotometer was employed to determine the optical properties while PL spectroscopy was used to investigate electron–hole recombination in the synthesized sample.3 Results and discussion
XRD identifies NSs crystallinity, crystal structure, and crystal size ranging from 2θ 10–60° (Fig. 2(a)). Diffraction peaks appearing at 2θ values 29.503° (![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) 11), 33.040° (
11), 33.040° (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 22), and 47.577° (140) revealed the monoclinic structure of Bi2O3, well matched with the XRD standard card (JCPDF 01-083-0410/00-041-1449).66 An additional broad characteristic peak observed near 13.5° corresponds to PVP and shows its amorphous nature.67 Upon doping, PVP peaks become more broadened and less intense than that of a pure nanocatalyst owing to enhancement in structural instability and the decrease in the crystallite size.68,69 Furthermore, an increasing amount of CS results in diffraction peak shift while decreased intensity is attributed to the enhanced structural disorders and significant decrease in the crystallite size. The crystallite size calculated from the most intense peak of all prepared samples using the Debye–Scherrer formula was 69.5 nm, 17.5 nm, 58.4 nm, and 26.25 nm for Bi2O3 and PVP/CS (2%, 4%)-doped Bi2O3 NSs respectively. FTIR was used to elucidate functional groups in the prepared Bi2O3 NSs (Fig. 2(b)). The Bi–O–Bi stretching vibration, C–C stretching and product vibration mode of NO3 were assigned to 540 cm−1, 1076 cm−1, 1357 cm−1 bands correspondingly.31,70,71 The manifested band at 1640 cm−1 was attributed to the bending vibration of H2O while the band at 3300–3500 cm−1 corresponded to the stretching vibration of absorbed hydroxyl function groups.72,73 Furthermore, bands at 2950 cm−1 and 1652 cm−1 in FTIR spectra of PVP were assigned to the presence of asymmetric stretching of CH2 and stretching of C–O (amide C
22), and 47.577° (140) revealed the monoclinic structure of Bi2O3, well matched with the XRD standard card (JCPDF 01-083-0410/00-041-1449).66 An additional broad characteristic peak observed near 13.5° corresponds to PVP and shows its amorphous nature.67 Upon doping, PVP peaks become more broadened and less intense than that of a pure nanocatalyst owing to enhancement in structural instability and the decrease in the crystallite size.68,69 Furthermore, an increasing amount of CS results in diffraction peak shift while decreased intensity is attributed to the enhanced structural disorders and significant decrease in the crystallite size. The crystallite size calculated from the most intense peak of all prepared samples using the Debye–Scherrer formula was 69.5 nm, 17.5 nm, 58.4 nm, and 26.25 nm for Bi2O3 and PVP/CS (2%, 4%)-doped Bi2O3 NSs respectively. FTIR was used to elucidate functional groups in the prepared Bi2O3 NSs (Fig. 2(b)). The Bi–O–Bi stretching vibration, C–C stretching and product vibration mode of NO3 were assigned to 540 cm−1, 1076 cm−1, 1357 cm−1 bands correspondingly.31,70,71 The manifested band at 1640 cm−1 was attributed to the bending vibration of H2O while the band at 3300–3500 cm−1 corresponded to the stretching vibration of absorbed hydroxyl function groups.72,73 Furthermore, bands at 2950 cm−1 and 1652 cm−1 in FTIR spectra of PVP were assigned to the presence of asymmetric stretching of CH2 and stretching of C–O (amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond), respectively.74 Bands at 1423 cm−1, 1288 cm−1, and 1652 cm−1 have been assigned to C–H bending, CH2 wagging and the –C
O bond), respectively.74 Bands at 1423 cm−1, 1288 cm−1, and 1652 cm−1 have been assigned to C–H bending, CH2 wagging and the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group respectively.74 With the addition of pure PVP in the prepared Bi2O3 NSs, the band which appeared at 1652 cm−1 shifted towards a lower wavenumber at 1640 cm−1 probably indicating that the C
O group respectively.74 With the addition of pure PVP in the prepared Bi2O3 NSs, the band which appeared at 1652 cm−1 shifted towards a lower wavenumber at 1640 cm−1 probably indicating that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond is getting weakened and there exists an interaction between metal ions and PVP through oxygen of the C
O bond is getting weakened and there exists an interaction between metal ions and PVP through oxygen of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the polymer. This distinguishing band may show the interaction of PVP with metal ions.75 Upon doping with chitosan, shift of bands toward a lower wavelength was observed, which is attributed to the –OH or NH2 functional group of CS.76–78 Strong hydrogen bonding interactions between two types of molecules form a homogeneous phase.79 Additionally, SAED analysis indicates bright circular rings of Bi2O3 and PVP/CS-Bi2O3 NSs represented in Fig. 2(c–f) suggesting highly crystalline nature of the samples. XRD measurements satisfying Bragg's diffraction conditions were well correlated with various planes of NSs.
O group of the polymer. This distinguishing band may show the interaction of PVP with metal ions.75 Upon doping with chitosan, shift of bands toward a lower wavelength was observed, which is attributed to the –OH or NH2 functional group of CS.76–78 Strong hydrogen bonding interactions between two types of molecules form a homogeneous phase.79 Additionally, SAED analysis indicates bright circular rings of Bi2O3 and PVP/CS-Bi2O3 NSs represented in Fig. 2(c–f) suggesting highly crystalline nature of the samples. XRD measurements satisfying Bragg's diffraction conditions were well correlated with various planes of NSs.
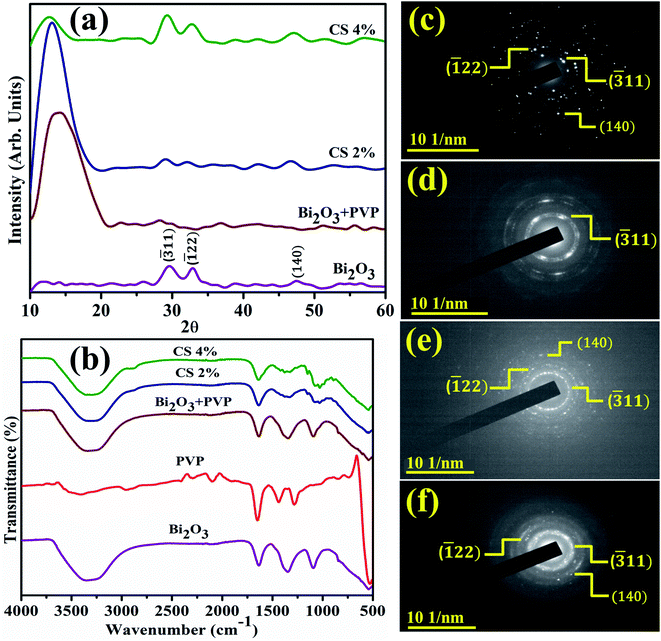
Fig. 3(a) reveals the band gap energy (Eg) and optical properties of the synthesized samples assessed with a UV-visible spectrophotometer between 250 and 500 nm. It shows a considerable absorption peak at 295 nm for Bi2O3.80 The wavelength acquired from UV-visible absorption spectra determined Eg of dopant free and PVP/CS-doped Bi2O3 NSs to be 4.18 eV, 4.27 eV, 4.09, and 4.13 eV respectively.80 Upon doping with PVP, the absorption in higher wavelength (blue shift) was observed, ascribed to an increase in Eg and decrease in the crystallite size. Furthermore, addition of CS resulted in absorption toward longer wavelength (red shift) indicating a decrease in Eg and increase in the crystallite size. Increasing amount of CS reduces the crystallite size that results in increased Eg which is well matched with the XRD results.
PL analysis elucidates the electron–hole pair recombination process in all synthesized samples as shown in Fig. 3(b). The photoluminescence signal is produced when electrons in the VB are excited to the CB at an excitation wavelength and subsequently return to the VB.81 Bi2O3 NSs emit broad emission peaks in the visible range from 520–542 nm, attributed to Bi3+ ions, when excited at 300 nm.82 The luminescence of ions in the green region is produced by the 3P1–1S0 transitions, or charge transfer between the bonding oxygen and Bi3+ ions.83–85 When PVP was incorporated, peak intensity decreased, indicating lower charge recombination while peak intensity increased upon increasing the concentration of CS, which suggests a high photo-generated charge carrier recombination tendency.86
The chemical composition of PVP/CS (2%, 4%)-doped Bi2O3 NSs determined through EDS is represented in Fig. 4(a–d). Strong peaks of Bi and O were observed that confirm the presence of Bi2O3 NSs in the synthesized samples. The carbon peak is attributed to PVP/CS used in the samples. The sodium (Na) peak was probably generated by the use of NaOH to sustain the pH of samples while Au peaks originate due to the coating sputtered upon the samples to reduce charging effects. Small peaks of Cu and Zn could be attributed to the effect of the brass sample holder utilized during EDS observation and to some contamination. Additionally, EDS mapping of the as-prepared higher doped specimen was carried out to analyze the distribution pattern of its elemental constituents in order to check additional interfacial contact as represented in (e). Five components (Bi, O, Na, Cu, and Zn) were found to spread in the higher doped samples. As already mentioned, Na, Cu, and Zn were assigned to contamination, the sample holder used for EDS analysis.
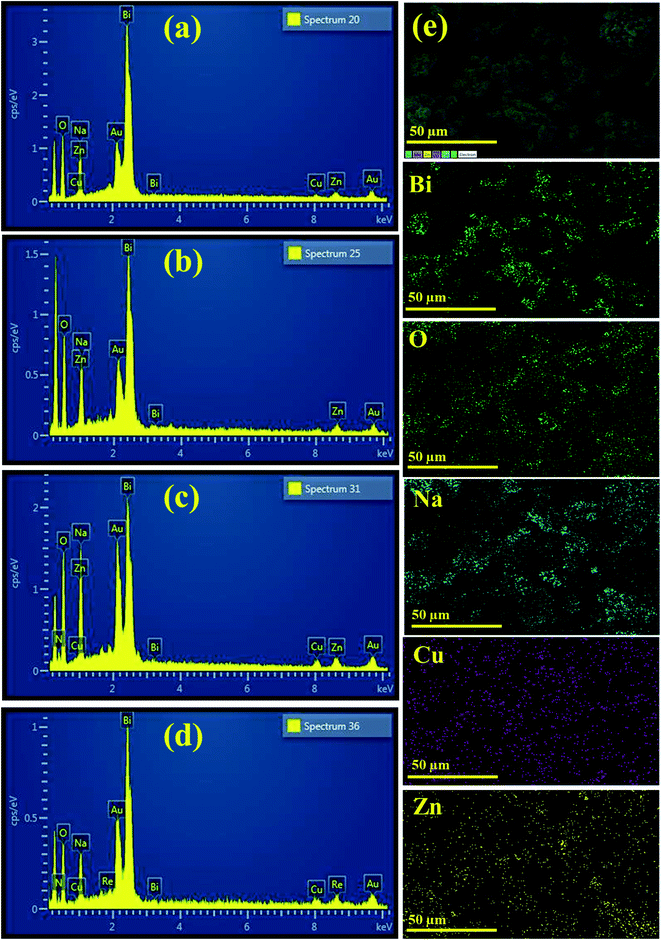 | ||
| Fig. 4 EDS image of (a) Bi2O3 (b) PVP-doped Bi2O3 (c) PVP/CS 2%-Bi2O3 (d) PVP/CS 4%-Bi2O3 and (e) mapping image of PVP/CS 4%-Bi2O3 NSs. | ||
TEM images confirmed the morphologies of Bi2O3 and doped Bi2O3 as illustrated in Fig. 5(a–d). The image of the control sample showed multiple morphologies including quantum dots while a few nanorods were also observed (Fig. 5(a)). Addition of PVP showed that quantum dots were covered with PVP (Fig. 5(b)). Addition of low concentration of CS to PVP/Bi2O3 resulted in agglomeration of nanorods and quantum dots, which led to the formation of nanoclusters (Fig. 5(c)). Upon higher amount of CS addition, agglomeration increased with the significant nanorod-type structure of CS visible (Fig. 5(d)). Additionally, interlayer d-spacing was calculated from HRTEM images using Gatan software (Fig. 5(a′–d′)). Bi2O3 and PVP/CS (2%, 4%)-doped Bi2O3 NS d-spacing values were found to be 0.271 nm, 0.311 nm, 0.193 nm, and 0.199 nm, which are well compatible with the XRD results.
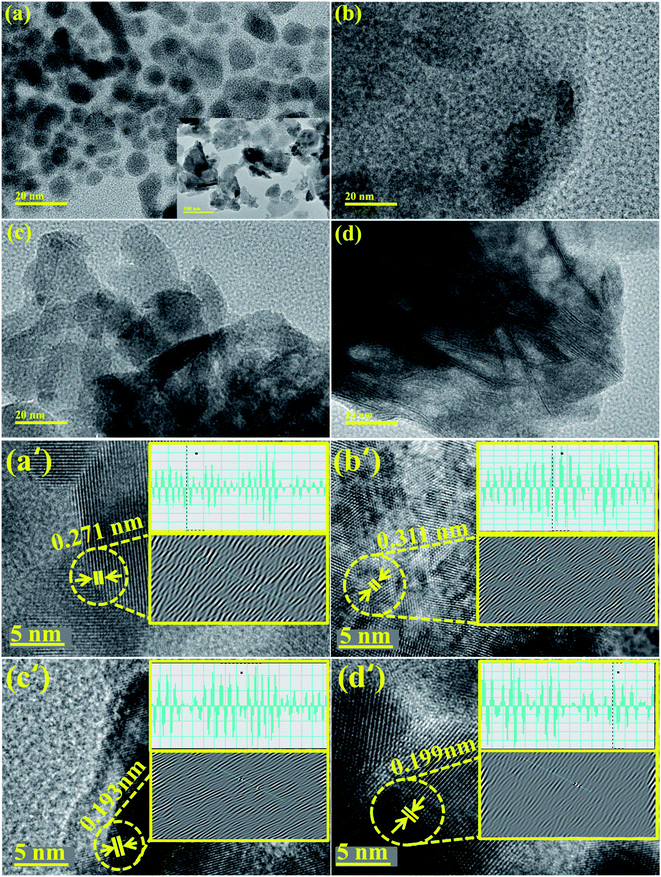 | ||
| Fig. 5 (a–d) TEM image of Bi2O3 and PVP/CS (2%, 4%)-doped Bi2O3 and (a′–d′) d spacing of Bi2O3 and PVP/CS (2%, 4%)-doped Bi2O3 NSs. | ||
Catalytic activities of pure and PVP/CS (2%, 4%)-doped Bi2O3 NSs with NaBH4 for MB degradation under acidic, neutral, and basic conditions were investigated using a UV-vis spectrophotometer. Dye sludge is frequently released at various pH levels; the rate of degradation is influenced by the pH solution and affects nano-catalysts that have been synthesized. Undoped and PVP/CS (2%, 4%)-doped Bi2O3 nanomaterials showed the maximum degradation of 99.48%, 77.90, 97.95%, and 75.54% in neutral (pH = 7), 98.35%, 98.93%, 98.27% and 96.68% in basic (pH = 12), and 89.54%, 68.07%, 94.51% and 93.84% in acidic (pH = 4) media (Fig. 6(a–c)). In all media, PVP/CS (2%)-doped Bi2O3 demonstrated the highest catalytic activity. The surface area crystallite size and shape of the nano-catalyst substantially influence CA. On doping with CS, variation in the dye degradation was observed, which is attributed to the presence of more active sites provided by catalyst's large surface area which results in high catalytic efficiency. In addition, the surface area is generally large, but the influence of the nano-catalyst is limited due to micro-porosity, which inhibits the reactants from diffusing to the catalyst surface.87 Furthermore, a slight difference between an acidic and basic medium is ascribed to increased electrostatic attraction between MB+, a positively charged dye and the catalyst which is negatively charged. The nanocatalyst surface in the basic medium tends to acquire a negative charge while absorption of cationic adsorbate species in acidic media is hindered by the catalyst's positively charged surfaces.88,89 The charge on the catalyst surface grew progressively negative as pH increased; enhancing the adsorption behavior of cationic dyes on Bi2O3 and PVP/CS (2%, 4%)-doped Bi2O3 nano-catalysts.
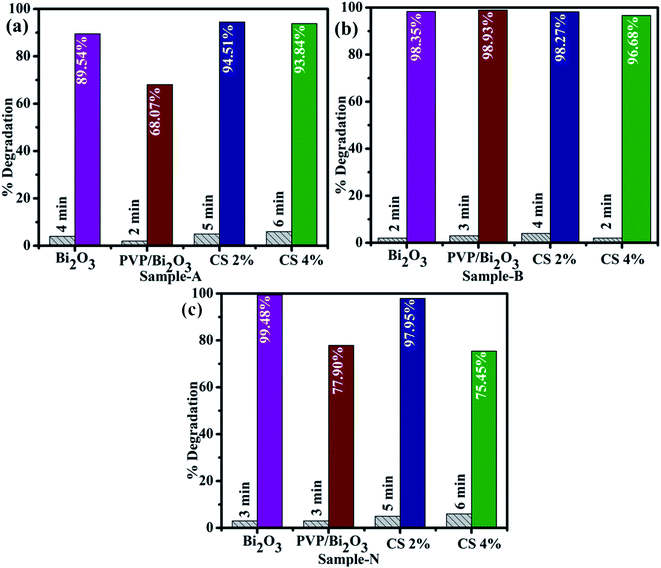 | ||
| Fig. 6 Catalytic activity of dopant free and PVP/CS (2%, 4%)-doped Bi2O3 NSs in (a) acidic medium (b) basic medium and (c) neutral medium. | ||
The large surface area of PVP/CS-doped Bi2O3 NSs resulted in enhanced catalytic activity. Consequently, the catalytic degradation of MB by PVP/CS (2%)-doped Bi2O3 NSs is significantly improved, and the dye is effectively degraded Fig. 7(a). The rate constants (k) have been calculated for catalytic degradation kinetics by measuring slopes of ln(C0/Ct) against time. Degradation rate constant k for undoped and doped Bi2O3 NSs was calculated to be 0.03095, 0.24174, 0.06604 and 0.66335 min−1, respectively Fig. 7(b).
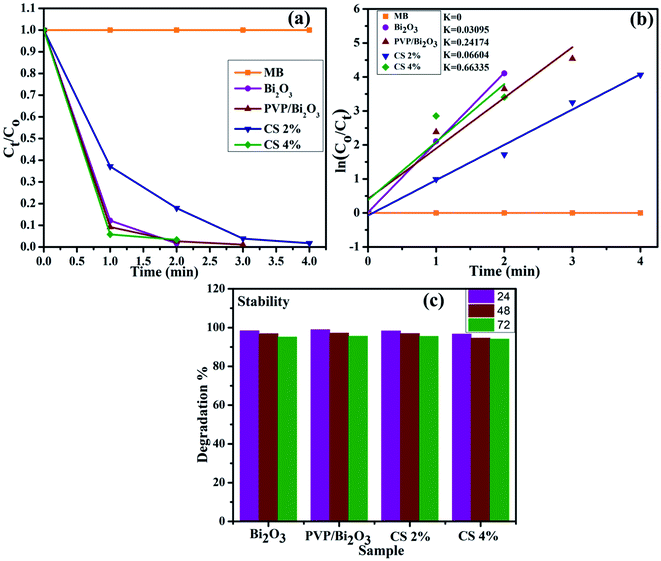 | ||
| Fig. 7 (a) Plot of the concentration ratio (Ct/C0) versus time, (b) plot of ln(C0/Ct) versus time spectra for dye reduction and (c) stability of the catalyst in the basic medium. | ||
Investigating the stability of the nano-catalyst is of economic importance. As mentioned earlier, catalytic activity in the basic medium exhibits excellent dye degradation results. Therefore, the stability of the catalyst in the basic medium was investigated by allowing the experiment to stay for at least 72 hour in order to examine whether the reduction of dye as observed in the presence of the nanocatalyst is stable or not. In this case, the degraded dye was kept in the dark and the degradation was monitored using absorption spectra obtained through a UV-vis spectrophotometer every 24 hours, as shown in Fig. 7(c). The obtained results indicate that no loss of degradation occurred under stable conditions for 72 h. Degradation was observed to be in its fairly original form which affirms the stability of the catalyst.
Fig. 8 represents bacterial activity of doped and undoped Bi2O3 NSs which is summarized in Table 1. Comparison to the E. coli results reveal that doped Bi2O3 has improved bactericidal synergism and activity against S. aureus. The inhibition zone was recorded from (0.85–1.35) to (1.25–3.09) in S. aureus at low and high doses and (0.60–1.25) in E. coli at high dose as shown in Fig. 9(a and b). All concentrations of E. coli at low dose exhibited zero efficacies as shown in Fig. 9(d). A negligible efficiency was shown by Bi2O3 for E. coli and S. aureus at low and high doses respectively Fig. 9(c and d). Furthermore, inhibition zone 4.25 mm against S. aureus and E. coli for ciprofloxacin (positive control) parallel to 0 mm DIW (negative control) was recorded. Apart from this, doped Bi2O3 NSs showed substantial (P < 0.05) antibacterial efficacy against S. aureus as compared with E. coli. In general, cell walls of Gram negative bacteria are thicker and have a more complicated structure than Gram positive bacteria. The comparison of the present work with the literature is presented in Table 2.
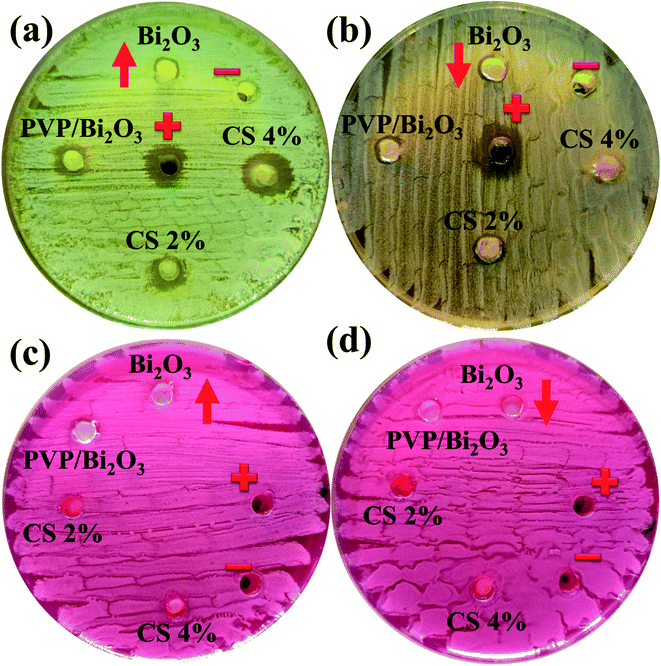 | ||
| Fig. 8 In vitro antimicrobial activity of the prepared NSs against (a) S. Aureus at high dose (b) S. Aureus at low dose (c) E. coli at high dose and (d) E. coli at low dose. | ||
| Samples | S. aureus | Inhibition zone (mm) | E. coli | Inhibition zone (mm) |
|---|---|---|---|---|
| 0.5 mg/50 μL | 1.0 mg/50 μL | 0.5 mg/50 μL | 1.0 mg/50 μL | |
| Bi2O3 | 0.85 | 1.25 | 0 | 0.60 |
| PVP doped Bi2O3 | 0.95 | 2.35 | 0 | 0.85 |
| CS 2% | 1.15 | 3.05 | 0 | 1.05 |
| CS 4% | 1.35 | 3.09 | 0 | 1.25 |
| Ciprofloxacin | 4.25 | 4.25 | 4.25 | 4.25 |
| DIW | 0 | 0 | 0 | 0 |
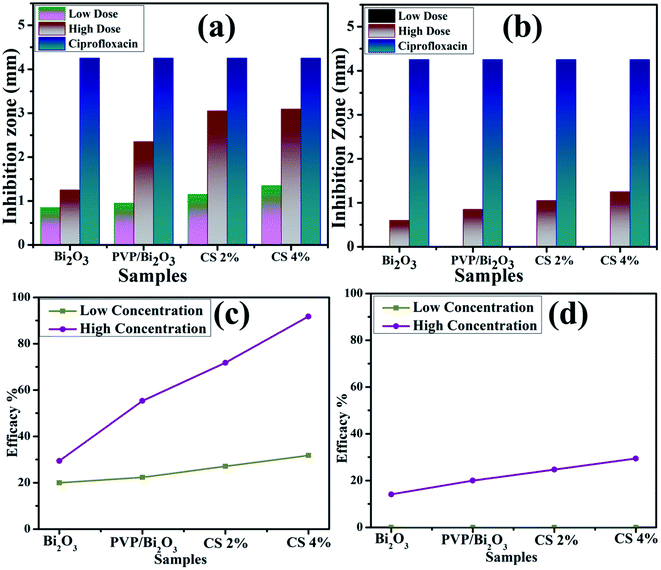 | ||
| Fig. 9 Graphical representation of antimicrobial activity of (a) S. Aureus and (b) E. coli, and efficacy of (c) S. Aureus and (b) E. coli pathogens. | ||
| Nano-catalyst | Synthesis route | Antibacterial activity | Outcome | References |
|---|---|---|---|---|
| Bi2O3 nanoparticles | Pulsed laser ablation | 0% against E. coli | No effect at different concentrations | 37 |
| Bi2O3 nanoparticles | Bacillus licheniformis on methicillin-resistant | 16% against S. aureus | — | 38 |
| Bi2O3 nanoparticles | Green synthesis | 2 mm for E. coli and 1 mm for S. aureus in 10 mg mL−1 concentration | Minor enhancement in the inhibition zone on increasing the concentration | 39 |
| RGO-Bi2O3 nanocomposite | Solvothermal method | 6.5 mm (100 g mL−1) against E. coli | Increase in concentration of RGO-Bi2O3 leads to higher toxicity | 40 |
| Chitosan biopolymer-functionalized zinc-doped bismuth oxide nano needle | Ultrasound-assisted chemical precipitation method | 14 and 15 mm against E. coli and S. aureus respectively in 200 mg mL−1 concentration | — | 41 |
| Bi2O3 nanostructures | Co-precipitation | 1.25 mm, 0.60 for S. aureus and E. coli respectively in 1.0 mg/50 μL concentration | By addition of dopants the antibacterial activity gradually increased | Present work |
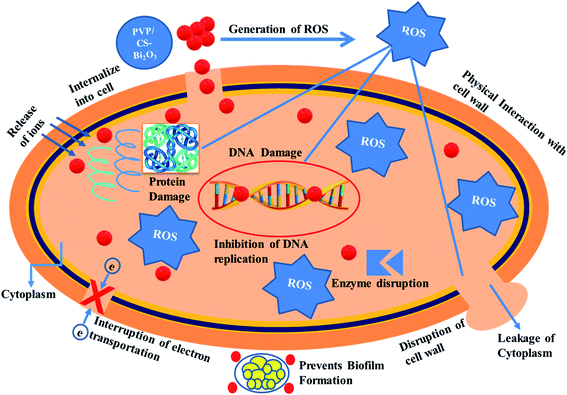
Nanomaterials produce oxidative stress that is directly proportional to their concentration, shape and size. The particle size and concentration affect antibacterial activity. The size of the material has an inverse relationship with the antimicrobial efficacy.90 Small sized particles produce more reactive oxygen species (ROS) causing cytoplasmic components to extrude and kill bacteria by harmful microorganism membrane implant.91,92 Sufficient distribution of Bi3+ inside bacterial cells increases its antimicrobial activity as it destroys bacterial membrane stability and inhibits biofilm formation as shown in Fig. 10.91
4 Conclusion
In the present work, Bi2O3 and PVP/CS-doped-Bi2O3 NSs were successfully synthesized to achieve an improved bactericidal and catalytic activity. Among all the prepared samples, CS doping in PVP-Bi2O3 with 2% and 4% concentrations showed effective catalytic and antimicrobial activities, respectively. In view of the experimental results, Bi2O3 exhibited a monoclinic structure with varying crystallite sizes (69.5 nm, 17.5 nm, 58.4 nm, and 26.25 nm) upon PVP and CS doping. The peak shifts observed towards lower wavelength revealed with FTIR confirmed the presence of dopants while a significant peak was observed at 540 cm−1 for Bi2O3. Aggregated quantum dot morphology of Bi2O3 was observed while with the addition of PVP, a layer was formed on quantum dots and a nanocluster was observed upon CS doping. Additionally, nanocluster formation was recorded with an increasing amount of CS, all of which was confirmed with TEM images. The interlayer d-spacing (0.271 nm, 0.311 nm, 0.193 nm, and 0.199 nm) was calculated with HR-TEM images showing good agreement with XRD. Optical properties and band gap (4.18 eV, 4.27 eV, 4.09, and 4.13 eV) results were obtained through a UV-vis spectrophotometer. PL spectroscopy revealed a lower peak intensity upon doping with PVP, indicating the lower charge to hole recombination rate, whereas peak intensity was increased for different concentrations of CS, showing an enhanced charge to hole recombination. In conclusion, Bi2O3 doped NSs with natural polymers were found to be ecologically friendly, low-cost and effective against pathogens and catalytic dye degradation.Abbreviations
| (Bi2O3) | Bismuth oxide |
| (PVP) | Polyvinylpyrrolidone |
| (CS) | Chitosan |
| (CA) | Catalytic activity |
| (XRD) | X-ray diffraction |
| (NSs) | Nanostructures |
| (SEM) | Scanning electron microscopy |
| (HRTEM) | High resolution transmission electron microscopy |
| (EDS) | Energy dispersive X-ray spectroscopy |
| (SAED) | Selected area electron diffraction |
| (FTIR) | Fourier transform infrared spectroscopy |
| (PL) | Photoluminescence |
| (MB) | Methylene blue |
Data availability
Data is available on suitable demand.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors are thankful to HEC, Pakistan through NRPU 20-17615.References
- M. Aqeel, M. Ikram, A. Asghar, A. Haider, A. Ul-Hamid, M. Naz, M. Imran and S. Ali, Synthesis of capped Cr-doped ZnS nanoparticles with improved bactericidal and catalytic properties to treat polluted water, Appl. Nanosci., 2020, 10, 2045–2055, DOI:10.1007/s13204-020-01268-3
.
-
J. Jalan and M. Ravallion, Does Piped Water Reduce Diarrhea for Children in Rural India?, SSRN, 2017, DOI:10.1596/1813-9450-2664
.
-
W. Bank, Pakistan Strategic Country Environmental Assessment, World Bank, Washington, DC, 2006, DOI:10.1596/33928
.
- J. Zhang, The impact of water quality on health: evidence from the drinking water infrastructure program in rural China, J. Health Econ., 2012, 31, 122–134, DOI:10.1016/j.jhealeco.2011.08.008
.
- S. Babel and T. A. Kurniawan, Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan, Chemosphere, 2004, 54, 951–967, DOI:10.1016/j.chemosphere.2003.10.001
.
- S. I. Siddiqui, O. Manzoor, M. Mohsin and S. A. Chaudhry, Nigella sativa seed based nanocomposite-MnO2/BC: an antibacterial material for photocatalytic degradation, and adsorptive removal of methylene blue from water, Environ. Res., 2019, 171, 328–340, DOI:10.1016/j.envres.2018.11.044
.
- I. A. Salem, Kinetics and mechanism of the color removal from congo red with hydrogen peroxide catalyzed by supported zirconium oxide, Transition Met. Chem., 2000, 25, 599–604, DOI:10.1023/A:1007008808372
.
- H. Eccles, Treatment of metal-contaminated wastes: why select a biological process?, Trends Biotechnol., 1999, 17, 462–465, DOI:10.1016/s0167-7799(99)01381-5
.
- S. Bhattacharya, I. Saha, A. Mukhopadhyay, D. Chattopadhyay and U. Chand, Role of nanotechnology in water treatment and purification: potential applications and implications, Int. J. Chem. Technol., 2013, 3, 59–64 Search PubMed
. https://www.urpjournals.com.
- M. Ali, S. Sharif, S. Anjum, M. Imran, M. Ikram, M. Naz and S. Ali, Preparation of Co and Ni doped ZnO nanoparticles served as encouraging nano-catalytic application, Mater. Res. Express, 2019, 6, 1250d5, DOI:10.1088/2053-1591/ab6383
.
- M. Ahmadipour, M. Arjmand, M. F. Ain, Z. A. Ahmad and S. Y. Pung, Effect of Ar:N2 flow rate on morphology, optical and electrical properties of CCTO thin films deposited by RF magnetron sputtering, Ceram. Int., 2019, 45, 15077–15081, DOI:10.1016/j.ceramint.2019.04.245
.
- M. K. Indana, B. R. Gangapuram, R. Dadigala, R. Bandi and V. Guttena, A novel green synthesis and characterization of silver nanoparticles using gum tragacanth and evaluation of their potential catalytic reduction activities with methylene blue and Congo red dyes, J. Anal. Sci. Technol., 2016, 7, 1–9, DOI:10.1186/s40543-016-0098-1
.
- O. F. S. Khasawneh, P. Palaniandy, P. Palaniandy, M. Ahmadipour, H. Mohammadi and M. R. Bin Hamdan, Removal of acetaminophen using Fe2O3-TiO2 nanocomposites by photocatalysis under simulated solar irradiation: optimization study, J. Environ. Chem. Eng., 2021, 9, 104921, DOI:10.1016/j.jece.2020.104921
.
- F. H. Dodd, Mastitis—Progress on Control, J. Dairy Sci., 1983, 66, 1773–1780, DOI:10.3168/jds.S0022-0302(83)82005-0
.
- A. Haider, M. Ijaz, S. Ali, J. Haider, M. Imran, H. Majeed, I. Shahzadi, M. M. Ali, J. A. Khan and M. Ikram, Green Synthesized Phytochemically (Zingiber officinale and Allium sativum) Reduced Nickel Oxide Nanoparticles Confirmed Bactericidal and Catalytic Potential, Nanoscale Res. Lett., 2020, 15, 1–11, DOI:10.1186/s11671-020-3283-5
.
- M. Ali, M. Ikram, M. Ijaz, A. Ul-Hamid, M. Avais and A. A. Anjum, Green synthesis and evaluation of n-type ZnO nanoparticles doped with plant extract for use as alternative antibacterials, Appl. Nanosci., 2020, 10, 3787–3803, DOI:10.1007/s13204-020-01451-6/figures/14
.
- T. Y. Shin, S. H. Yoo and S. Park, Gold nanotubes with a nanoporous wall: their ultrathin platinum coating and superior electrocatalytic activity toward methanol oxidation, Chem. Mater., 2008, 20, 5682–5686, DOI:10.1021/cm800859k
.
- Y. Xian, F. Gao and B. Cai, Synthesis of platinum nanoparticle chains based on α-chymotrypsin fibrils, Mater. Lett., 2013, 111, 39–42, DOI:10.1016/j.matlet.2013.08.051
.
- J. Ge, T. Huynh, Y. Hu and Y. Yin, Hierarchical magnetite/silica nanoassemblies as magnetically recoverable catalyst-supports, Nano Lett., 2008, 8, 931–934, DOI:10.1021/nl080020f
.
- I. M. Hamouda, Current perspectives of nanoparticles in medical and dental biomaterials, J. Biomed. Res., 2012, 26, 143–151, DOI:10.7555/jbr.26.20120027
.
-
H. Sudrajat, Low-temperature synthesis of δ-Bi2O3 hierarchical nanostructures composed of ultrathin nanosheets for efficient photocatalysis, Elsevier, 2017, https://www.sciencedirect.com/science/article/pii/S0264127517305695 Search PubMed
.
- S. S. Raut, O. Bisen and B. R. Sankapal, Synthesis of interconnected needle-like Bi2O3 using successive ionic layer adsorption and reaction towards supercapacitor application, Ionics, 2017, 23, 1831–1837, DOI:10.1007/s11581-017-1994-0
.
- X. He, W. Dong, F. Zheng, L. Fang and M. Shen, Effect of tartaric acid on the microstructure and photoluminescence of SrTiO3:Pr3+ phosphors prepared by a sol–gel method, Mater. Chem. Phys., 2010, 123, 284–288, DOI:10.1016/j.matchemphys.2010.04.012
.
- M. N. Rittner and T. Abraham, Nanostructured materials: an overview and commercial analysis, Int. J. Powder Metall., 1998, 34, 33–36 CAS
. https://search.proquest.com/openview/e8222012cb72f619f3b616ba5b9f2c53/1?pq-origsite=gscholar%26cbl=42295.
- N. R. E. Radwan, Effects of La2O3-doping on physicochemical surface and catalytic properties of nickel and manganese oxides supported on alumina, Appl. Catal., A, 2004, 257, 177–191, DOI:10.1016/j.apcata.2003.07.006
.
- Y. Huo, X. Zhang, Y. Jin, J. Zhu and H. Li, Highly active La2O3/Ti1−xBxO2 visible light photocatalysts prepared under supercritical conditions, Appl. Catal., B, 2008, 83, 78–84, DOI:10.1016/j.apcatb.2008.02.005
.
- M. Mittal, A. Gupta and O. P. Pandey, Role of oxygen vacancies in Ag/Au doped CeO2 nanoparticles for fast photocatalysis, Sol. Energy, 2018, 165, 206–216, DOI:10.1016/j.solener.2018.03.033
.
- J. Lu, I. Batjikh, J. Hurh, Y. Han, H. Ali, R. Mathiyalagan, C. Ling, J. C. Ahn and D. C. Yang, Photocatalytic degradation of methylene blue using biosynthesized zinc oxide nanoparticles from bark extract of Kalopanax septemlobus, Optik, 2019, 182, 980–985, DOI:10.1016/j.ijleo.2018.12.016
.
- V. Kumari, N. Kumar, S. Yadav, A. Mittal and S. Sharma, Novel mixed metal oxide (ZnO·La2O3·CeO2) synthesized via hydrothermal and solution combustion process – a comparative study and their photocatalytic properties, Mater. Today: Proc., 2019, 650–657, DOI:10.1016/j.matpr.2019.07.748
.
- H. Nosrati, N. Ehsani, H. Baharvandi, H. Abdizadeh and V. Mazinani, Effect of primary materials ratio and their stirring time on SiC nanoparticle production efficiency through sol gel process, Am. J. Eng. Res., 2014, 317–321 Search PubMed
, https://www.indianjournals.com/ijor.aspx?target=ijor:jmms%26volume=56%26issue=1%26article=004.
- N. Nurmalasari, Y. Yulizar and D. O. B. Apriandanu, Bi2O3 nanoparticles: synthesis, characterizations, and photocatalytic activity, IOP Conf. Ser.: Mater. Sci. Eng., 2020, 12036, DOI:10.1088/1757-899x/763/1/012036
.
- R. Sharma, M. Khanuja, S. N. Sharma and O. P. Sinha, Reduced band gap & charge recombination rate in Se doped α-Bi2O3 leads to enhanced photoelectrochemical and photocatalytic performance: theoretical & experimental insight, Int. J. Hydrogen Energy, 2017, 42, 20638–20648, DOI:10.1016/j.ijhydene.2017.07.011
.
- X. Luo, G. Zhu, J. Peng, X. Wei, M. Hojamberdiev, L. Jin and P. Liu, Enhanced photocatalytic activity of Gd-doped porous β-Bi2O3 photocatalysts under visible light irradiation, Appl. Surf. Sci., 2015, 351, 260–269, DOI:10.1016/j.apsusc.2015.05.137
.
- J. Liang, G. Zhu, P. Liu, X. Luo, C. Tan, L. Jin and J. Zhou, Synthesis and characterization of Fe-doped β-Bi2O3 porous microspheres with enhanced visible light photocatalytic activity, Superlattices Microstruct., 2014, 72, 272–282, DOI:10.1016/j.spmi.2014.05.005
.
- M. Faisal, A. A. Ibrahim, H. Bouzid, S. A. Al-Sayari, M. S. Al-Assiri and A. A. Ismail, Hydrothermal synthesis of Sr-doped α-Bi2O3 nanosheets as highly efficient photocatalysts under visible light, J. Mol. Catal. A: Chem., 2014, 387, 69–75, DOI:10.1016/j.molcata.2014.02.018
.
- J. Krishna Reddy, B. Srinivas, V. Durga Kumari and M. Subrahmanyam, Sm3+-doped Bi2O3 photocatalyst prepared by hydrothermal synthesis, ChemCatChem, 2009, 1, 492–496, DOI:10.1002/cctc.200900189
.
- J. AbdulSattar Salman, A. M. N. Jassim, S. A. Farhan, J. A. S. Salman, K. J. Khalaf and M. F. Al Marjani and M. T. Mohammed, Study the Antibacterial Effect of Bismuth Oxide and Tellurium Nanoparticles, 2015, https://www.academia.edu/download/40355314/bismoth.pdf, accessed April 5, 2022 Search PubMed.
- L. Firouzi Dalvand, F. Hosseini, S. M. Dehaghi and E. S. Torbati, Inhibitory effect of bismuth oxide nanoparticles produced by bacillus licheniformis on methicillin-resistant staphylococcus aureus strains (MRSA), Iran, J. Biotechnol., 2018, 16, 279–286, DOI:10.21859/ijb.2102
.
- N. Motakef-Kazemi and M. Yaqoubi, Green synthesis and characterization of bismuth oxide nanoparticle using mentha pulegium extract, Iran, J. Pharm. Res., 2020, 19, 70–79, DOI:10.22037/ijpr.2019.15578.13190
.
- M. Suresh and A. Sivasamy, Bismuth oxide nanoparticles decorated graphene layers for the degradation of methylene blue dye under visible light irradiations and antimicrobial activities, J. Environ. Chem. Eng., 2018, 6, 3745–3756, DOI:10.1016/j.jece.2017.01.049
.
- R. Karthik, K. Pandiselvi, K. Mariyappan, K. Park, I. Kwak and J. Sivakamavalli, Synthesis of Biogenic Chitosan Biopolymer-Functionalized Zinc-Doped Bi2O3 Nanoneedles and Its Bio-applications: In Vitro Antibacterial and Anticancer activity, Arabian J. Sci. Eng., 2021, 46, 5605–5618, DOI:10.1007/s13369-020-05099-w
.
- M. J. Tommalieh, Electrical conductivity characterization of chitosan/poly(vinyl alcohol) doped by bismuth oxide nanoparticles, Compos. Commun., 2021, 25, 100692, DOI:10.1016/j.coco.2021.100692
.
- G. Lewis, Nucleus pulposus replacement and regeneration/repair technologies: present status and future prospects, J. Biomed. Mater. Res., Part B, 2012, 100, 1702–1720, DOI:10.1002/jbm.b.32712
.
- J. Sampson and D. De Korte, DEHP-plasticised PVC: relevance to blood services, Transfusion Medicine., 2011, 21, 73–83, DOI:10.1111/j.1365-3148.2010.01056.x
.
- H. Klinkmann and J. Vienken, Membranes for dialysis, Nephrol., Dial., Transplant., 1995, 39–45, DOI:10.1093/ndt/10.supp3.39
.
- S. V. Jadhav, D. S. Nikam, V. M. Khot, N. D. Thorat, M. R. Phadatare, R. S. Ningthoujam, A. B. Salunkhe and S. H. Pawar, Studies on colloidal stability of PVP-coated LSMO nanoparticles for magnetic fluid hyperthermia, New J. Chem., 2013, 37, 3121–3130, 10.1039/c3nj00554b
.
- G. Lu, S. Li, Z. Guo, O. K. Farha, B. G. Hauser, X. Qi, Y. Wang, X. Wang, S. Han, X. Liu, J. S. Duchene, H. Zhang, Q. Zhang, X. Chen, J. Ma, S. C. J. Loo, W. D. Wei, Y. Yang, J. T. Hupp and F. Huo, Imparting functionality to a metal–organic framework material by controlled nanoparticle encapsulation, Nat. Chem., 2012, 4, 310–316, DOI:10.1038/nchem.1272
.
- M. J. Tommalieh, N. S. Awwad, H. A. Ibrahium and A. A. Menazea, Characterization and electrical enhancement of PVP/PVA matrix doped by gold nanoparticles prepared by laser ablation, Radiat. Phys. Chem., 2021, 179, 109195, DOI:10.1016/j.radphyschem.2020.109195
.
- R. Bryaskova, D. Pencheva, S. Nikolov and T. Kantardjiev, Synthesis and comparative study on the antimicrobial activity of hybrid materials based on silver nanoparticles (AgNps) stabilized by polyvinylpyrrolidone (PVP), J. Biol. Chem., 2011, 4, 185–191, DOI:10.1007/s12154-011-0063-9
.
- A. A. Menazea, One-Pot Pulsed Laser Ablation route assisted copper oxide nanoparticles doped in PEO/PVP blend for the electrical conductivity enhancement, J. Mater. Res. Technol., 2020, 9, 2412–2422, DOI:10.1016/j.jmrt.2019.12.073
.
- A. Kyrychenko, O. M. Korsun, I. I. Gubin, S. M. Kovalenko and O. N. Kalugin, Atomistic simulations of coating of silver nanoparticles with poly(vinylpyrrolidone) oligomers: effect of oligomer chain length, J. Phys. Chem. C, 2015, 119, 7888–7899, DOI:10.1021/jp510369a
.
- M. S. Al Mogbel, M. T. Elabbasy, R. S. Mohamed, A. E. Ghoniem, M. F. H. A. El-Kader and A. A. Menazea, Improvement in antibacterial activity of poly vinyl pyrrolidone/chitosan incorporated by graphene oxide NPs via laser ablation, J. Polym. Res., 2021, 28, 474, DOI:10.1007/s10965-021-02838-x
.
- M. Shahmiri, N. A. Ibrahim, F. Shayesteh, N. Asim and N. Motallebi, Preparation of PVP-coated copper oxide nanosheets as antibacterial and antifungal agents, J. Mater. Res., 2013, 28, 3109–3118, DOI:10.1557/jmr.2013.316
.
- M. Karpuraranjith and S. Thambidurai, Chitosan/zinc oxide-polyvinylpyrrolidone (CS/ZnO-PVP) nanocomposite for better thermal and antibacterial activity, Int. J. Biol. Macromol., 2017, 104, 1753–1761, DOI:10.1016/j.ijbiomac.2017.02.079
.
- M. Hu, C. Li, X. Li, M. Zhou, J. Sun, F. Sheng, S. Shi and L. Lu, Zinc oxide/silver bimetallic nanoencapsulated in PVP/PCL nanofibres for improved antibacterial activity, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 1248–1257, DOI:10.1080/21691401.2017.1366339
.
- T. S. Chung, L. Y. Jiang, Y. Li and S. Kulprathipanja, Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation, Prog. Polym. Sci., 2007, 32, 483–507, DOI:10.1016/j.progpolymsci.2007.01.008
.
- A. J. Varma, S. V. Deshpande and J. F. Kennedy, Metal complexation by chitosan and its derivatives: a review, Carbohydr. Polym., 2004, 55, 77–93, DOI:10.1016/j.carbpol.2003.08.005
.
- D. Yang, J. Li, Z. Jiang, L. Lu and X. Chen, Chitosan/TiO2 nanocomposite pervaporation membranes for ethanol dehydration, Chem. Eng. Sci., 2009, 64, 3130–3137, DOI:10.1016/j.ces.2009.03.042
.
- K. D. Khalil, E. I. Ibrahim and F. A. Al-Sagheer, A novel, efficient, and recyclable biocatalyst for Michael addition reactions and its iron(III) complex as promoter for alkyl oxidation reactions, Catal. Sci. Technol., 2016, 6, 1410–1416, 10.1039/c5cy01034a
.
- K. D. Khalil, S. M. Riyadh, S. M. Gomha and I. Ali, Synthesis, characterization and application of copper oxide chitosan nanocomposite for green regioselective synthesis of [1,2,3]triazoles, Int. J. Biol. Macromol., 2019, 130, 928–937, DOI:10.1016/j.ijbiomac.2019.03.019
.
- S. M. Riyadh, K. D. Khalil and A. Aljuhani, Chitosan-MgO Nanocomposite: One Pot Preparation and Its Utility as an Ecofriendly Biocatalyst in the Synthesis of Thiazoles and [1,3,4]thiadiazoles, Nanomater., 2018, 8, 928, DOI:10.3390/nano8110928
.
- J. M. Small and H. Hintelmann, Methylene blue derivatization then LC-MS analysis for measurement of trace levels of sulfide in aquatic samples, Anal. Bioanal. Chem., 2007, 387, 2881–2886, DOI:10.1007/s00216-007-1140-3
.
- R. Anjana and N. Geetha, Degradation of methylene blue using silver nanoparticles synthesized from cynodon dactylon (L.) pers. Leaf aqueous extract, Int. J. Life Sci. Biotechnol. Pharma Res., 2019, 8, 225–229 Search PubMed
. https://www.iopscience.iop.org/article/10.1088/1755-1315/105/1/012018/meta.
- M. Ikram, T. Inayat, A. Haider, A. Ul-Hamid, J. Haider, W. Nabgan, A. Saeed, A. Shahbaz, S. Hayat, K. Ul-Ain and A. R. Butt, Graphene Oxide-Doped MgO Nanostructures for Highly Efficient Dye Degradation and Bactericidal Action, Nanoscale Res. Lett., 2021, 16, 56, DOI:10.1186/s11671-021-03516-z
.
- A. Raza, J. Z. Hassan, M. Ikram, S. Naz, A. Haider, A. Ul-Hamid, I. Shahzadi, J. Haider, S. Goumri-Said, M. B. Kanoun and S. Ali, Molecular docking and DFT analyses of magnetic cobalt doped MoS2 and BN nanocomposites for catalytic and antimicrobial explorations, Surf. Interfaces, 2021, 27, 101571, DOI:10.1016/j.surfin.2021.101571
.
- N. Motakef-Kazemi and M. Yaqoubi, Green synthesis and characterization of bismuth oxide nanoparticle using mentha pulegium extract, Iran. J. Pharm. Res., 2020, 19, 70–79, DOI:10.22037/ijpr.2019.15578.13190
.
- X. G. Li, I. Kresse, J. Springer, J. Nissen and Y. L. Yang, Morphology and gas permselectivity of blend membranes of polyvinylpyridine with ethylcellulose, Polymer, 2001, 42, 6859–6869, DOI:10.1016/s0032-3861(01)00057-x
.
- M. P. Morales, S. Veintemillas-Verdaguer, M. I. Montero, C. J. Serna, A. Roig, L. I. Casas, B. Martínez and F. Sandiumenge, Surface and internal spin canting in γ-Fe2O3 nanoparticles, Chem. Mater., 1999, 11, 3058–3064, DOI:10.1021/cm991018f
.
- Structural, optical, dielectric and magnetic properties of PVP coated magnetite (Fe3O4) nanoparticles|Request PDF, https://www.researchgate.net/publication/343127588_Structural_optical_dielectric_and_magnetic_properties_of_PVP_coated_magnetite_Fe_3O_4_nanoparticles, accessed January 7, 2022 Search PubMed.
- Y. Wang, Y. Wen, H. Ding and Y. Shan, Improved structural stability of titanium-doped β-Bi2O3 during visible-light-activated photocatalytic processes, J. Mater. Sci., 2010, 45, 1385–1392, DOI:10.1007/s10853-009-4096-1
.
- M. Mallahi, A. Shokuhfar, M. R. Vaezi, A. Esmaeilirad and V. Mazinani, Synthesis and characterization of Bismuth oxide nanoparticles via sol-gel method, Am. J. Eng. Res., 2014, 3, 162–165 Search PubMed
. https://www.researchgate.net/profile/Ahmad-Esmaeilirad/publication/284394611_Synthesis_and_characterization_of_Bismuth_oxide_nanoparticles_via_sol-gel_method/links/591122caa6fdccbfd5b05768/Synthesis-and-characterization-of-Bismuth-oxide-nanoparticles-via-.
- W. Raza, M. M. Haque, M. Muneer, T. Harada and M. Matsumura, Synthesis, characterization and photocatalytic performance of visible light induced bismuth oxide nanoparticle, J. Alloys Compd., 2015, 648, 641–650, DOI:10.1016/j.jallcom.2015.06.245
.
- E. Bartonickova, J. Cihlar and K. Castkova, Microwave-assisted synthesis of bismuth oxide, Process. Appl. Ceram., 2007, 1, 29–33, DOI:10.2298/pac0702029b
.
- A. Rahma, M. M. Munir, Khairurrijal, A. Prasetyo, V. Suendo and H. Rachmawati, Intermolecular Interactions and the Release Pattern of Electrospun Curcumin-Polyvinyl(pyrrolidone) Fiber, Biol. Pharm. Bull., 2016, 39, 163–173, DOI:10.1248/bpb.b15-00391
.
- K. Anasuya, M. K. Veeraiah, P. Hemalatha and M. Manju, Synthesis and Characterisation of Poly (Vinylpyrrolidone) – Nickel (II) Complexes, IOSR J. Appl. Chem., 2014, 7, 61–66, DOI:10.9790/5736-07816166
.
- B. L. Wang, X. S. Liu, Y. Ji, K. F. Ren and J. Ji, Fast and long-acting antibacterial properties of chitosan-Ag/polyvinylpyrrolidone nanocomposite films, Carbohydr. Polym., 2012, 90, 8–15, DOI:10.1016/j.carbpol.2012.03.080
.
- B. L. Wang, J. L. Wang, D. D. Li, K. F. Ren and J. Ji, Chitosan/poly(vinyl pyrollidone) coatings improve the antibacterial properties of poly(ethylene terephthalate), Appl. Surf. Sci., 2012, 258, 7801–7808, DOI:10.1016/j.apsusc.2012.03.181
.
- T. Wang, X. K. Zhu, X. T. Xue and D. Y. Wu, Hydrogel sheets of chitosan, honey and gelatin as burn wound dressings, Carbohydr. Polym., 2012, 88, 75–83, DOI:10.1016/j.carbpol.2011.11.069
.
- D. Anjali Devi, B. Smitha, S. Sridhar and T. M. Aminabhavi, Novel crosslinked chitosan/poly(vinylpyrrolidone) blend membranes for dehydrating tetrahydrofuran by the pervaporation technique, J. Membr. Sci., 2006, 280, 45–53, DOI:10.1016/j.memsci.2006.01.003
.
- S. Condurache-Bota, C. Constantinescu, M. Praisler and N. Tigau, The influence of the substrate temperature on the structure and on the optical energy bandgap of bismuth oxide thin films prepared by pulsed laser deposition, Dig. J. Nanomater. Biostructures., 2015, 10, 1025–1032 Search PubMed
. https://www.researchgate.net/profile/Mirela-Praisler/publication/282002114_The_influence_of_the_substrate_temperature_on_the_structure_and_on_the_optical_energy_bandgap_of_bismuth_oxide_thin_films_prepared_by_pulsed_laser_deposition/links/5600fbd108ae0762.
- J. Tang, Z. Zou and J. Ye, Photophysical and Photocatalytic Properties of AgInW2O8, J. Phys. Chem. B, 2003, 107, 14265–14269, DOI:10.1021/jp0359891
.
- P. Hao, Z. Zhao, J. Tian, Y. Sang, G. Yu, H. Liu, S. Chen and W. Zhou, Bismuth titanate nanobelts through a low-temperature nanoscale solid-state reaction, Acta Mater., 2014, 62, 258–266, DOI:10.1016/j.actamat.2013.10.006
.
- M. Vila, C. Díaz-Guerra and J. Piqueras, Luminescence and Raman study of α-Bi2O3 ceramics, Mater. Chem. Phys., 2012, 133, 559–564, DOI:10.1016/j.matchemphys.2012.01.088
.
- P. Boutinaud, Revisiting the spectroscopy of the Bi3+ ion in oxide compounds, Inorg. Chem., 2013, 52, 6028–6038, DOI:10.1021/ic400382k
.
- L. Kumari, J. H. Lin and Y. R. Ma, Synthesis of bismuth oxide nanostructures by an oxidative metal vapour phase deposition technique, Nanotechnology, 2007, 18, 295605, DOI:10.1088/0957-4484/18/29/295605
.
- S. S. Lee, H. Bai, Z. Liu and D. D. Sun, Novel-structured electrospun TiO2/CuO composite nanofibers for high efficient photocatalytic cogeneration of clean water and energy from dye wastewater, Water Res., 2013, 47, 4059–4073, DOI:10.1016/j.watres.2012.12.044
.
- M. Ikram, S. Hayat, M. Imran, A. Haider, S. Naz, A. Ul-Hamid, I. Shahzadi, J. Haider, A. Shahzadi, W. Nabgan and S. Ali, Novel Ag/cellulose-doped CeO2 quantum dots for efficient dye degradation and bactericidal activity with molecular docking study, Carbohydr. Polym., 2021, 269, 118346, DOI:10.1016/j.carbpol.2021.118346
.
- E. Haque, J. W. Jun and S. H. Jhung, Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal–organic framework material, iron terephthalate (MOF-235), J. Hazard. Mater., 2011, 185, 507–511, DOI:10.1016/j.jhazmat.2010.09.035
.
- K. P. Singh, D. Mohan, S. Sinha, G. S. Tondon and D. Gosh, Color removal from wastewater using low-cost activated carbon derived from agricultural waste material, Ind. Eng. Chem. Res., 2003, 42, 1965–1976, DOI:10.1021/ie020800d
.
- K. Sunada, Y. Kikuchi, K. Hashimoto and A. Fujishima, Bactericidal and detoxification effects of TiO2 thin film photocatalysts, Environ. Sci. Technol., 1998, 32, 726–728, DOI:10.1021/es970860o
.
- U. Qumar, M. Ikram, M. Imran, A. Haider, A. Ul-Hamid, J. Haider, K. N. Riaz and S. Ali, Synergistic effect of Bi-doped exfoliated MoS2 nanosheets on their bactericidal and dye degradation potential, Dalt. Trans., 2020, 49, 5362–5377, 10.1039/d0dt00924e
.
- W. Fang, X. Chaofa, J. Zheng, G. Chen and K. Jiang, Fabrication of Cu-Ag bimetal nanotube-based copper silicates for enhancement of antibacterial activities, RSC Adv., 2015, 5, 39612–39619, 10.1039/c5ra06065f
.
| This journal is © The Royal Society of Chemistry 2022 |