Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
On the evolution of coenzyme biosynthesis†
Andreas
Kirschning

Institute of Organic Chemistry, Leibniz University Hannover, Schneiderberg 1B, D-30167 Hannover, Germany. E-mail: andreas.kirschning@oci.uni-hannover.de; Fax: +49-(0)511-762 3011; Tel: +49-(0)511-762 4614
First published on 23rd September 2022
Abstract
Covering: up to 2022
The report provides a broad approach to deciphering the evolution of coenzyme biosynthetic pathways. Here, these various pathways are analyzed with respect to the coenzymes required for this purpose. Coenzymes whose biosynthesis relies on a large number of coenzyme-mediated reactions probably appeared on the scene at a later stage of biological evolution, whereas the biosyntheses of pyridoxal phosphate (PLP) and nicotinamide (NAD+) require little additional coenzymatic support and are therefore most likely very ancient biosynthetic pathways.
1. Introduction
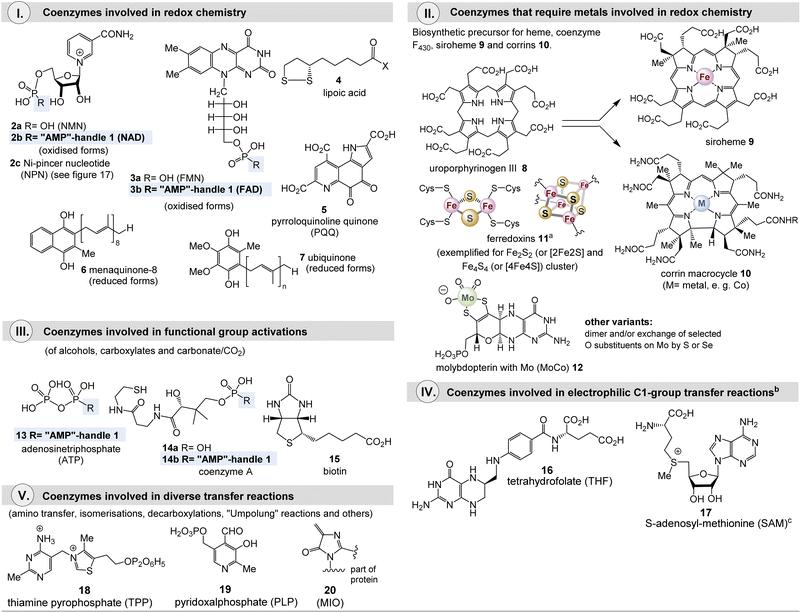
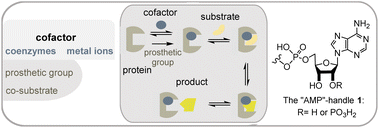
Metabolism is one of the cornerstones of life and is characterized by an elaborate network of interconnected biosynthetic pathways and their regulation.1 It is responsible for the synthesis (anabolism) and degradation (catabolism) of biomolecules. In addition to the catalytically active proteins or enzymes, other small chemical entities, called cofactors, play an important role in the catalytic abilities of enzymes. Cofactors are often divided into metal ions and small organic molecules, so-called coenzymes (Fig. 1). However, the term “cofactor” is sometimes associated exclusively with metal cations. Coenzymes can be differentiated according to the binding in the active pocket of the enzyme. The first is called a prosthetic group, which is made up of a coenzyme that is tightly, possibly covalently, and permanently bound to a protein whereas reversibly bound coenzymes are sometimes termed co-substrates. In this report, I will generally use the term coenzyme, despite the terminology just outlined.From an evolutionary point of view, cofactors and also the organic representatives must be very old and give us a clue to the origin of life, since their purpose and role have remained unchanged since the presumed starting point of life.2 It is likely that most of the examples described below already existed about 4 billion years ago.
Cofactors promote chemical reactions that the protein template cannot.3 These include (a) redox chemistry including halogenations, (b) activation of functional groups such as alcohols and carboxylates, and (c) group transfer reactions such as methylations. Coenzymes are embedded in the active site of the protein template, where they take up substrates and convert them into products (Scheme 1). Polar or ionic elements in the coenzyme, as well as functional groups of the protein, ensure the common association and alignment of the substrate in the active site. Coenzymes that mediate redox and group transfer reactions require regeneration mechanisms.
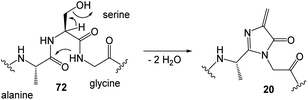
Many coenzymes are based on heterocyclic cores, often structurally modified with nucleic acid elements, as is particularly manifested in the “AMP handle” 1. In addition, elements of nucleotides are hidden in and biosynthetically derived from the heterocyclic core structures of some coenzymes, most notably guanosine triphosphate (GTP). Fig. 1 lists the major coenzymes and metal-dependent coenzymes 2–20. These are subdivided according to their chemical properties and their role in metabolism. The recently discovered nickel pincer nucleotide cofactor (NPN, 2c) is derived from nicotinamides and is a limiting case as it catalyzes racemizations of α-hydroxycarboxylates, however, this is a redox process. Since it is a metal complex, this cofactor will be discussed in section 2.2. Most of them are distributed across all phylogenetic kingdoms. 4-Methylideneimidazole-5-one (MIO, 20) is also found in the list, although it is like pyrroloquinoline quinone (PQQ, 5) a polypeptide modification.4 The common biosynthetic precursor uroporphyrinogen III 8 is shown for the macrocyclic ligands heme 9 and cobalamin 10, which are present in many metalloenzymes.
Methanogens rely on several coenzymes commonly not found in other organisms (Fig. 2).5 These prokaryotes belong to the domain of archaea and are hydrogen-dependent autotrophs that produce methane and have been proposed as good candidates for the physiological primordial state.5c As such, they are limited to carbon dioxide, formate, methanol, methylamines, and acetate as possible carbon sources.
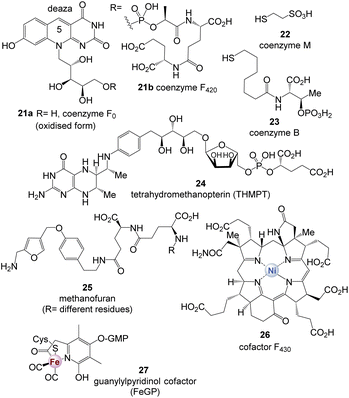 | ||
| Fig. 2 Coenzymes 21–27 found in methanogens (note: CoF420 has been found in all methanogens but also in numerous actinomycetes) (GMP = guanosine monophosphate). | ||
Such unique coenzymes are the 5-deazaflavins, coenzymes F0 and F420 (21a and 21b), which are structurally related to FMN 3a and FAD 3b, coenzyme M (22), 7-mercaptoheptanoylthreonine phosphate (coenzyme B, 23), tetrahydromethanopterin (THMPT, 24), methanofuran (25), and cofactor F430 (26).6
Coenzymes found today are formed enzymatically via biosynthetic cascades and are the result of an evolutionary process. Remarkably, in some cases nature has found distinctly different biosynthetic solutions for the same coenzyme (see below). The analysis of such different biosynthetic pathways can be seen as a way to shed light on the evolutionary development of modern coenzyme biosynthesis as was reviewed by Warren et al. before.7 Consistent with these basic considerations, this review analyzes the known biosynthetic pathways to coenzymes, specifically focusing on other coenzymes that are required for their formation. As a result, (fragmented) proposals for the evolution of coenzyme biosynthetic pathways are presented. They are not based on genetically analyzed protein-coding genes and protein clusters, but choose the perspective of a (bio)synthetic chemist and metabolism. On the basis of these evaluations, with regard to known hypothetical approaches, an additional perspective on the possible origin of an early metabolism becomes available (Table 1).2
| CO2 + 4H2 → CH4 + H2O (ΔG0′ = −131 KJ mol−1) | |
|---|---|
| Coenzyme | Role in methanogenesis |
| Coenzyme F021a | Biosynthetic precursor of coenzyme F420 |
| Coenzyme F42021b | F420H2 reduces different C1-loaded THMPT intermediates; delivers electrons to hydrogenases |
| Coenzyme M 22 | Accepts methyl from methyl-H4MPT |
| Coenzyme B 23 | Forms disulfide with S-methylated coenzyme M yielding methane |
| THMPT 24 | Binds C1-units of different oxidation states |
| Methanofuran 25 | Accepts CO2 to form N-carboxymethanofuran (1st step of methanogenesis) |
| Cofactor F43026 | Prosthetic group in methyl coenzyme M reductase: catalyses release of methane in the final step of methanogenesis. Catalysis the first step of methane oxidation |
| FeGP cofactor 27 | [Fe]-hydrogenase: reversible dehydrogenation of methylene-THMPT |
2. Biosyntheses of coenzymes
In this section, the biosyntheses of most coenzymes are summarized, and all coenzymes required for their biosynthesis in each case are listed in abbreviated form at the bottom of each graphical scheme. The classification by sections is made with reference to the chemical role of the individual coenzymes, e.g. for redox reactions. The author is aware that other classifications would have been equally possible. At the end of this review, when evolutionary considerations will be dealt with, the subdivision made will then resolve itself within the new context.2.1 Biosynthesis of redox coenzymes
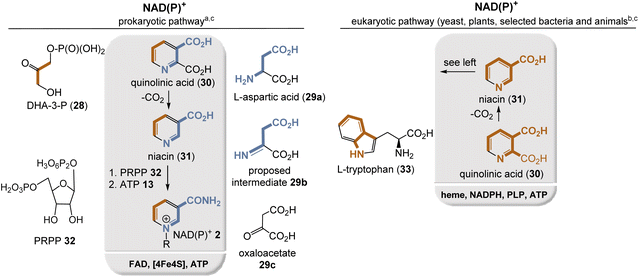
Currently, two different NAD(P)+ biosyntheses are known, with quinolinic acid (30) and niacin (31) being the key intermediates for both routes (Fig. 3). In bacteria, niacin is formed from dihydroxyacetone phosphate (DHA-3-P, 28) and L-aspartate (29a) (Fig. 3, left),9 whereas in plants, L-tryptophan (33) acts as a precursor (Fig. 3, right).10
In bacteria and archaea, aspartic acid (29a) is first oxidized by L-aspartate oxidase to the corresponding imine 29b, using FAD 3 as a coenzyme in which oxygen and hence FADHOOH serve as oxidant. Alternatively, oxaloacetate 29c, an intermediate of the TCA cycle, could also serve as a building block for intermediate 29b, so that FAD-mediated oxidation is then not required at all. Subsequently, condensation with DHA-3-P 28 takes place, which is catalyzed by quinolinate synthase, whereby, remarkably, a [4Fe–4S] cluster serves as Lewis acid and is not employed for electron transfer.11 Noteworthy, the imine 29b can also be regarded as a formal condensation adduct of oxaloacetate 29c and ammonia.12
In plants and some bacteria, L-tryptophan (33) provides all the atoms for the construction of the niacin backbone with quinolinic acid as an intermediate.12 Starting from niacin, the final steps involve the quaternization of the pyridine nitrogen atom with 5-phospho-D-ribose-1-diphosphate (PRPP, 32) as the alkylating building block, which provides NAD+ and from there ATP-mediated phosphorylation furnishes NADP+.
The aspartate pathway is clearly the simpler of the two pathways, and indeed Cleaves and Miller suggested that this pathway should in principle be feasible by chemical means under presumably prebiotic conditions.13 Another argument for why the tryptophan pathway should be more recent centers on the large number of coenzymes, including NADPH 2, required for this multistep biosynthesis and the use of O2 in association with heme. Finally, the kynurenine pathway relies on tryptophan as a starting building block, one of the rarest amino acids, which is built up via one of the most complex biosyntheses of any proteinogenic amino acid overall.12 The kynurenine pathway seems somewhat meaningless so what might its significance be? It has been shown that some organisms such as yeast (S. cerevisiae) use this pathway during aerobic growth and the de novo pathway during anaerobic growth.14
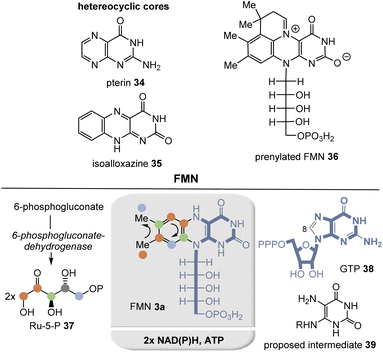
Riboflavins are biosynthesized in plants, fungi, bacteria, and archaea by a remarkable pathway (Fig. 4).15 The eastern part is derived from guanosine triphosphate (GTP, 38), losing a C atom at C8 in the form of formate, and hydrolysis yields the 5,6-diaminopyrimidine dione derivative 39. All other carbon and nitrogen atoms remain in the coenzyme. The dimethyl-substituted benzene ring is built up sequentially from two molecules of ribulose-5-phosphate (Ru-5-P, 37) by a unique mechanism.
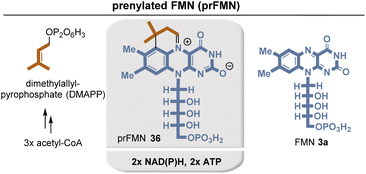
Recently, prenylated flavin (prFMN) 36 was discovered that was shown to be important in the ubiquitous microbial UbiDX system.17 UbiX acts as a flavin prenyltransferase (Fig. 5)18 while UbiD is a prFMN-dependent reversible (de)carboxylase, e.g. it promotes the decarboxylation of cinnamic acid derivatives. Although knowledge of prFMN-driven biochemistry is still in its infancy, it can be noted that it should not be able to perform classical N5-based flavin chemistry. Instead, it is capable of forming cycloadducts with dipolarophiles as well as with long-lived C4a-based radical species. In addition, photochemically driven isomerization chemistry was described.
Comparing FMN/FAD biosynthesis with that of prFMN 36, it is evident that the latter must be an evolutionary downstream metabolite that appeared on the scene only after riboflavin-based coenzyme biosynthesis was established.
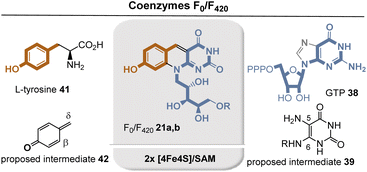
The group of 5-deazaflavins includes coenzymes F0 and F42021a and 21b, which are thought to be truly ancient redox factors.19 Functionally, 5-deazaflavin F42021b facilitates various two-electron redox reactions in methanogenic, sulfate-reducing, and probably methanotrophic archaea. In addition to its role in methanogenesis, coenzyme F420 is also involved in antibiotic biosynthesis and DNA repair.20 Both structurally and chemically, coenzymes F0 and F42021a and 21b are more closely related to nicotinamide than to flavins, which is why they have occasionally been referred to as “nicotinamide in a flavin's clothing”.21
The biosynthesis of the deazaflavin chromophore of F420 is supposed to start from tyrosine (41) and GTP 38, respectively22 and is closely related to the biosynthesis of riboflavins 3 (Fig. 6) Again, diaminopyrimidine-dione 39 serves as one key intermediate. Interestingly L-tyrosine (41) is first oxidized to the phenol radical which spontaneously fragments to p-methide quinone 42. It was suggested that Michael addition of C-5 in 39 occurs onto the δ-position in 42 and later a second Michael addition of the amino nitrogen at C-6 onto the β-position of 42 sets up the tricyclic system. In between a second H radical abstraction at the benzylic position initiates removal of the amino group at C-5 through elimination. The two radical abstraction steps are promoted by radical SAM/Fe4S4.22a
There is a direct link to thiamine pyrophosphate (TPP, 14, vide supra)23 and the [FeFe]-hydrogenase maturase HydG, the latter being responsible for the synthesis of the CO and CN–ligands present in [FeFe]-hydrogenase H cluster which is generated from tyrosine-derived dehydroglycine (NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2H) (see also section 2.2.2).24 Both enzymes yield cresol as a byproduct resulting from reduction of 42.
CHCO2H) (see also section 2.2.2).24 Both enzymes yield cresol as a byproduct resulting from reduction of 42.
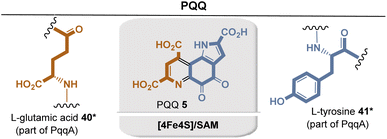 | ||
| Fig. 8 Summary of PQQ biosynthesis: building blocks are marked in sienna and blue including positions where they end up in PQQ 5. Coenzyme-dependent enzymes: Fe4S4/SAM: PqqE. | ||
SAM-mediated H-radical abstraction at the γ-position of the glutamic acid side chain by the enzyme PqqE initiates the formation of a C–C bond, with the C atom ortho to the phenol group. This step requires the presence of the chaperone PqqD, which forms a complex with PqqA prior to oxidation. Further details of PQQ biosynthesis have not yet been fully elucidated. Only the final steps promoted by the PqqC enzyme have been studied in vitro. It promotes an aza-Michael ring closure and an otherwise unknown eight-electron oxidation with O2 as oxidant.29
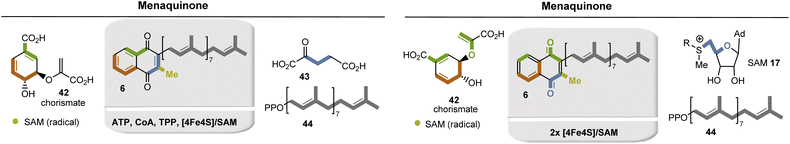
The formation of the second ring is initiated by TPP-mediated acylation with α-ketoglutarate 43 as building block and the other substituents, the methyl group and the isoprene side chains (oligoprenoid pyrophosphates 44) are finally introduced by electrophilic substitutions.
Bioinformatic analyses of whole-genome sequences have suggested that some microorganisms, such as Helicobacter pylori and Campylobacter jejuni use another route to menaquinone (Fig. 9, right).32 Both biosynthetic pathways diverge at chorismate 42 and re-converge again after the formation of naphthoquinol. Between these two points, the two pathways differ fundamentally. In contrast to the first o-succinylbenzoate pathway the futalosine pathway relies heavily on radical chemistry triggered by radical SAM.33 SAM also provides two carbons that become part of the quinone ring.
Ubiquinone (7) is an important coenzyme of electron transfer chains in proteobacteria and eukaryotes. Recent results indicate a rather large diversity of biosynthetic pathways in bacteria (Fig. 10).34 The first phase of the common pathway to ubiquinone is the shikimate pathway. Chorismate 42 is first converted to p-hydroxybenzoate, which undergoes prenylation, two monooxygenase-mediated hydroxylations, and a third after decarboxylation. In addition, two phenolic groups and the last free position on the aromatic ring are methylated. Accordingly, the coenzymes SAM 17 and FAD 3 are required repeatedly. Structurally related to ubiquinone are plastoquinone and rhodoquinone which are not covered here.34
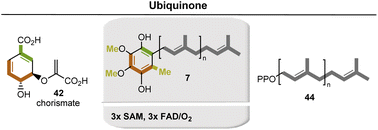 | ||
| Fig. 10 Formation of ubiquinone (7). Coenzyme-dependent enzymes: SAM: methyl transferase; FAD: monooxygenases UbiB, UbiH, and UbiF. | ||
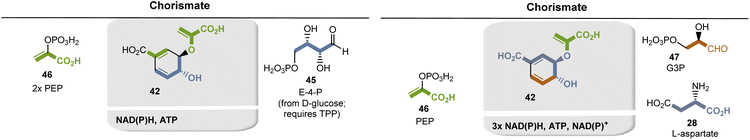
The key building block chorismate 42 is a product of the shikimate pathway. This in turn serves to provide access to aromatic amino acids and various other aromatic natural substances.35 An aldolase-mediated coupling of erythrose-4-phosphate (E4P, 45, formed from D-glucose and requires TPP) and phosphoenolpyruvate (PEP, 46), followed by an intramolecular aldol reaction producing 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP), from which shikimic acid and finally chorismate are formed (Fig. 11, left).
An alternative, TPP-free entrance into the shikimate pathway was found in some archaea such as Methanocaldococcus jannaschii (Fig. 11, right). 6-Deoxy-5-ketofructose-1-phosphate is formed from glyceraldehyde-3-phosphate (G3P, 47) and from aspartate 29a, both of which are first reduced to the corresponding aldehydes.36 Oxidative deamination leads to 4,5-dihydroxy-2,6-dioxoheptanoic acid which is converted to 3-dehydroquinic acid and hence to chorismate 42.
2.2. Biosynthesis of transition metal dependent coenzymes
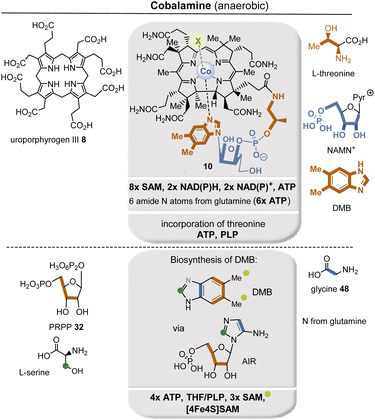
Siroheme (9) is derived from 8 and is part of the active sites of enzymes responsible for the six-electron reduction of sulfur and nitrogen. Structurally, corrin macrocycle 10 is related to uroporphyrinogen III, but the ring size is reduced by one carbon atom compared to 8. The corrin macrocycle is found in cobalamin and frequently forms complexes with cobalt(I) (see also section 2.4.1). From an evolutionary perspective, it seems reasonable to assume that transition metals such as Fe, Ni, Co, Mo, W, and others dominated redox chemistry during the transitional phase from protometabolism to the biotic world.40,41
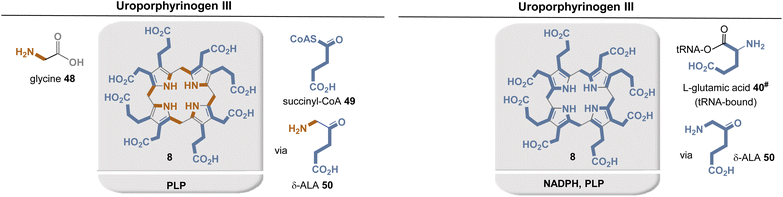
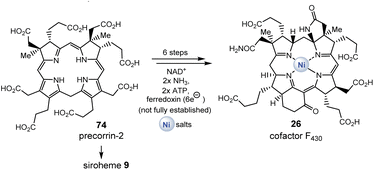
The two known biosynthetic pathways for uroporphyrinogen III (8), found in all kingdoms of life including archaea,38,42 utilize 5-aminolevulinic acid (δ-ALA, 50) as a linear precursor (Fig. 12, left). δ-ALA is biosynthesized either from glycine (48) and succinyl-CoA (49) or from glutamyl-tRNA (40#) in a two-step enzymatic process. In the first case, ALA synthase catalyzes the decarboxylative coupling of glycine to succinyl-CoA and this is catalyzed by PLP 19. In the second pathway, NADPH 2b and PLP 19 participate as coenzymes in the biosynthesis of δ-ALA 50 (Fig. 12, right). Subsequently, eight molecules of δ-ALA are condensed, which finally form macrocycle 8.
Although [Fe–S] centers in proteins can assemble spontaneously, they require iron and sulfide in concentrations that far exceed those found in cells. Such concentrations, however, would be highly toxic, and indeed a complex mechanism has evolved for their biosynthesis.44 Thus, three major pathways have been identified: the Isc system (iron–sulfur cluster), the Suf system (sulfur formation), and the Nif system (nitrogen fixation). Without going into too much detail within the scope of this report, one pathway involves the donation of sulfur catalyzed by cysteine desulfurase (IscS) in which L-cysteine (51) serves as sulfur donor (by transforming a cysteine residue in IscS into active R-S-S-H), while iron is provided by an iron chaperone (such as CyaY) or by direct iron capture at IscU (Fig. 13).45
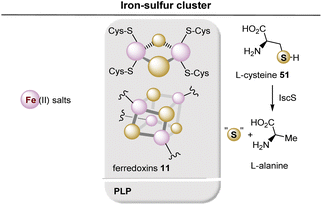 | ||
| Fig. 13 Summary of ferredoxin (iron–sulfur cluster) 11 generation. Coenzyme-dependent enzymes: PLP: cysteine desulfurase (IscS). | ||
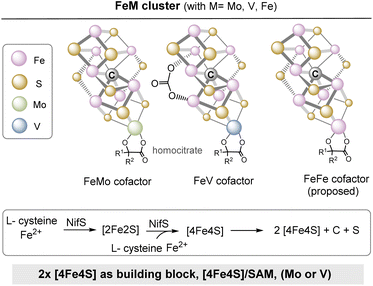
The central iron–sulfur cluster motif is replicated in several other variants that can be regarded to be “follow up” clusters. The FeMo cofactor has the stoichiometry Fe7MoS9C and is the most important cofactor of the nitrogenase.46 This is the key enzyme in nitrogen fixation, in which molecular nitrogen (N2) is reduced to ammonia (NH3).47 The cluster consists of an Fe4S3 sub-cluster and a MoFe3S3 sub-cluster and the two sub-clusters are connected by three sulfide bridges (Fig. 14). Structurally closely related are VFeco and FeFeco, in which the replacement of molybdenum by vanadium and iron, respectively, is the most important new feature.46,48 It has to be noted that the MoFe protein is an α2β2 heterotetramer, while VFe and FeFe proteins are α2β2γ2 heterohexamers.
Three proteins (NifH, NifEN, NifB) serve for the biosynthesis of the FeMo cofactor.48 NifB is responsible for the assembly of the Fe–S core, in which two [4Fe–4S] clusters are stitched together. It relies on the coenzyme SAM 17 and [4Fe–4S] to provide the carbide-like carbon atom located in the center of the iron–sulfur cluster. Thus, [4Fe–4S] has two roles here: (a) a building block and (b) cofactor to merge two [4Fe–4S] cubanes. Noteworthy, it is speculated that the FeMo cofactor, and thus the nitrogenases need the extra carbon atom as part of the metal sulfur cluster architecture to acquire catalytic activity, since the carbon atom keeps the structure rigid.
In the context of Fe- and S-containing metalloclusters, it is sensible to include hydrogenases in the discussion.
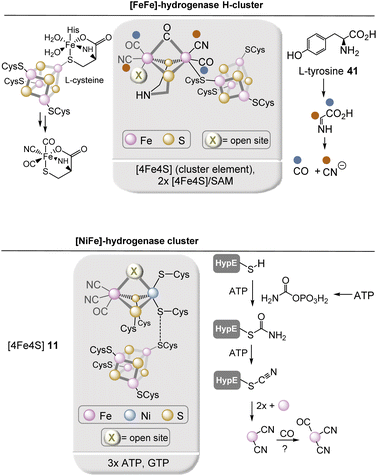
Hydrogenases are enzymes that catalyze the utilization and production of H2. They are particularly essential for the anaerobic bacteria as well as sulfate-reducing bacteria of the genus Desulfovibrio. The best known hydrogenases are [NiFe] hydrogenases found in bacteria and archaea as well as [FeFe] hydrogenases found in bacteria and eukaryotes. Key elements of these hydrogenases are [FeFe]-hydrogenase H-cluster and [NiFe]-hydrogenase cluster (Fig. 15).49 In addition to the dinuclear metal center, both hydrogenases have at least one [4Fe–4S] cluster positioned nearby. Independent of these hydrogenases, there is a third type of hydrogenase, the [Fe] hydrogenase that bears a mononuclear iron center. It will be covered in section 2.6. The biosynthesis of the [2Fe] H-subcluster50 requires several maturases that rely on or require iron–sulfur clusters. A bifunctional radical S-adenosylmethionine (SAM) enzyme (HydG) first cleaves tyrosine 41 to finally generate CO and cyanide. The resulting organometallic precursor that contains an Fe(CO)2(CN) moiety is eventually incorporated into the H-cluster. So far it is only known that these two reactions are carried out by two Fe–S clusters in HydG but many details have not been elucidated so far.
The biosynthesis of the Fe(CN)2CO complex occurs in a protein cluster composed of HypC, HypD, and HypE. Here, cyanide originates from enzyme-bound thiocarbamoylate and requires ATP for activation before cyanide is transferred to iron. Interestingly, enzyme-bound carbamothioate has been proposed as intermediate.
Other important iron–sulfur-containing clusters are associated with C1 fixation, specifically in the Wood–Ljungdahl pathway. Of particular note here are carbon monoxide dehydrogenase (CODH) and acetyl-CoA synthase (ACS), which are formed from the [4Fe–4S] cluster and are mixed with nickel atoms. However, they will not be discussed in this report.
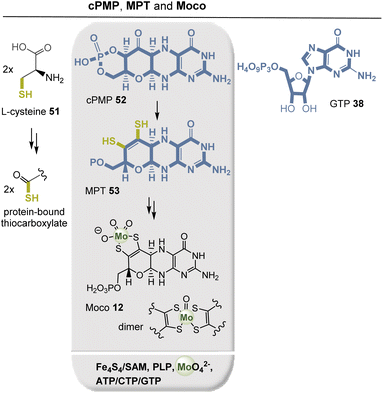
The molybdenum cofactor (Moco, 12) and its dimer (Fig. 16) are part of redox enzymes, and the pterin ligand controls the redox behavior of the metal. Moco and its dimer are capable of transferring an oxygen atom that is ultimately extracted from or incorporated into water. Typical Moco-dependent enzymes include formate dehydrogenase, sulfite oxidase, nitrate reductase, and glyceraldehyde-3-phosphate ferredoxin oxidoreductase.51,52 Moco biosynthesis is evolutionarily conserved, occurring in eukaryotes as well as eubacteria and archaea. In bacteria, the biosynthesis of cyclic pyranopterin monophosphate (cPMP, 52) and MPT 53, and thus of Moco 12, can be divided into the following main steps: (a) formation of cPMP 52, (b) formation of MPT 53, (c) insertion of molybdenum into molybdopterin to form Moco, and (d) the additional modification of Moco by addition of GMP or CMP to the phosphate group in MPT 53.53
GTP 38 serves as the starting point for the biosynthesis of all pterin-containing enzymes. Mediated by SAM, the cyclic pyranopterin monophosphate (cPMP, 52) is formed in a radical cascade. The dithiolene function in MPT is generated by PLP-mediated desulfurization of two protein-bound terminal thiocarboxylates that are generated from a persulfide made via free L-cysteine. This protein-mediated sulfur transfer is similar to the S-transfer process also found in TPP biosynthesis (see section 2.5.2). The two thiol groups in MPT 53 finally serve as ligands to trap inorganic molybdate (MoO42−). Remarkably, tungsten appears to be the physiologically active metal for MPT 53 in hyperthermophilic archaea. Tungsten plays a role in aldehyde ferredoxin oxidoreductase (AOR), formaldehyde ferredoxin oxidoreductase (FOR), and glyceraldehyde-3-phosphate ferredoxin oxidoreductase (GAPOR) in archaea.
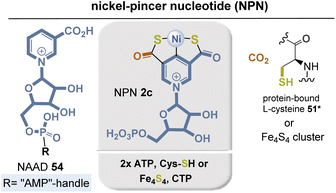
Apparently, it is recruited from NAD(P)+ biosynthesis with the intermediate nicotinic acid adenine dinucleotide (NAAD) 54 serving as precursor.55 An initial protein-bound cysteine-mediated activation of the pyridinium ring allows the introduction of the second carboxylate at C5 with CO2 as C1 source. Carboxylation is accompanied by hydrolysis of the diphosphate moiety and cleavage of AMP. Next, sulfur transfer occurs from cysteine (in L. plantarum) to form dehydroalanine or from a [4Fe–4S] cluster (in Thermotoga maritima) that repeatedly can act as a sulfur source in the presence of cysteine desulfurase (IscS) and free L-cysteine (Fig. 17). In this process, the thiocarbonyl functions are formed after ATP-mediated activation of the carboxylic acids. Finally, nickel is introduced by a CTP-dependent nickel insertase. Bioinformatics studies show that the biosynthetic genes are widely distributed in microorganisms, so the full potential of NPN has not yet been fully explored.
2.3. Coenzymes in functional group activations
The evolution of ATP synthase has been extensively studied and is thought to have begun with the merging of two functionally independent subunits, a presumably early step in evolution, since the structure and activity of ATP synthases are found in all phylogenetic trees.56
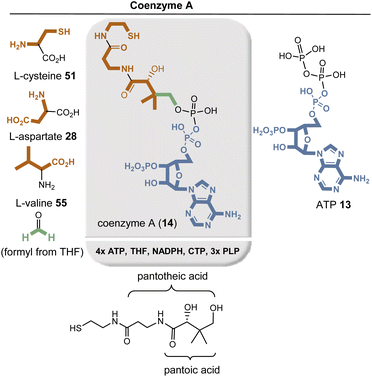
Several amino acids are required for the biosynthesis of coenzyme A (14), namely L-cysteine (51), L-aspartate (29a), and L-valine (55), as well as the nucleotide ATP 13.58 PLP-mediated decarboxylations of aspartate and cysteine, the latter already bound to pantothenic acid, give rise to the β-alanine and cysteamine units in coenzyme A (14). Biosynthesis of pantoic acid begins with L-valine (55), which is transaminated by PLP to 3-methyl-2-oxobutanoic acid and formylated with formaldehyde derived from the coenzyme THF 16. The keto group is then reduced to the secondary alcohol of pantoic acid with the assistance of NADPH, and subsequently the individual building blocks are linked together with the aid of ATP and CTP.
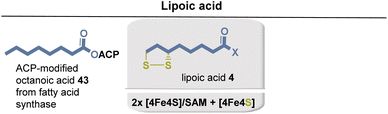
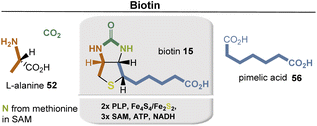
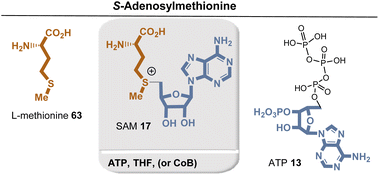
Biotin (15) is biosynthesized from L-alanine (52) and pimelic acid (56), with a variety of coenzymes involved in each enzymatic step (Fig. 19). Pimelic acid (56) is biosynthesized by a fatty acid synthase that uses malonyl-CoA methyl ester as one building block. Subsequently, several reduction steps are involved that require NADH as a coenzyme. PLP-dependent transamination, in which SAM serves as an amino donor, results in the formation of 7-keto-8-aminopelargonic acid (KAPA). This is probably the only known example of the use of SAM as a source of an amino group. The formation of the urea group from carbonate is promoted by ATP. The sulfur atom is finally inserted from an iron–sulfur cluster (Fe2S2) by a radical SAM-promoted process (Fe4S4/SAM).
2.4. Coenzymes for C1-transfer reactions
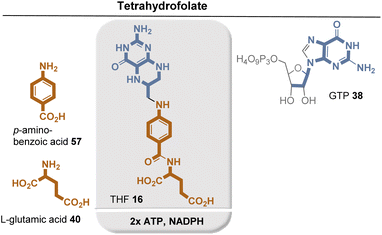
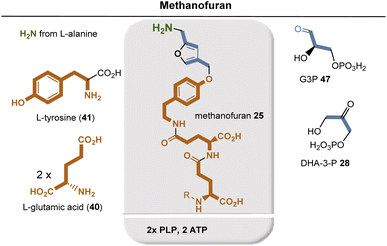
THF 16 consists of a pterin core, p-aminobenzoic acid (PABA, 49), and glutamic acid (35). It is biosynthesized in plants and fungi as well as in certain protozoa, bacteria, and archaea. Similar to the biosynthesis of riboflavins and deazaflavins and molybdenum cofactor, THF biosynthesis relies on GTP (33) as the starting point for the assembly of the pterin core.64 Zn-promoted hydrolysis of GTP by GTP cyclohydrolase I (GTPCH1h) yields the bicyclic pterin core, while formic acid is formed as a byproduct. Dihydroneopterin aldolase (DHNA) removes two carbon atoms of the original D-ribose in GTP in the form of glycoaldehyde. Only the two amide-forming steps require the assistance of the external coenzyme ATP. Finally, the resulting dihydrofolate is reduced to THF 12 by the NAD(P)H-dependent dihydrofolate reductase.
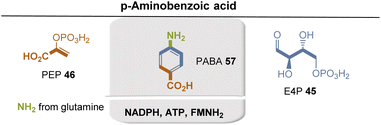
PABA 57 is formed via the shikimate metabolic pathway (see Fig. 22),35,65 which has been identified in bacteria, archaea, fungi, algae, some protozoa, and also in plants. Therefore, its biosynthesis is also analyzed in terms of the coenzymes required for its biosynthesis. PEP 46 and E4P 45 are the starting point, and three coenzyme-dependent enzymes are involved on the way to the chorismate 42, requiring NADPH, ATP, and FMNH2, respectively. Remarkably, one of these enzymes, chorismate synthase, uses FMNH2 in a redox-neutral elimination process of phosphate. Finally, the amino group is introduced at the chorismate level, with glutamine serving as the amino donor. Since chorismate 42 is an important intermediate in PABA biosynthesis, a second molecule of PEP 46 is required but removed in the final step once the aromatic system has been established.
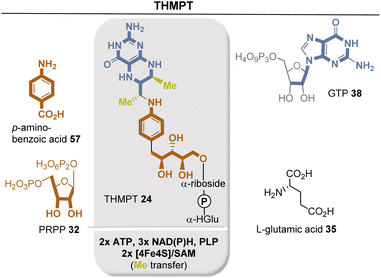
Chemically, the methanogenic coenzyme THMPT 24 and THF 16 behave similarly, as both are carbon carriers. Methanopterin is also capable of uploading oxidation states between formyl and methyl, but there are differences between the two pterin-based coenzymes. As such ATP is consumed in the entry of carbon from CO2 into the 5,6,7,8-tetrahydrofolate pathway, which is not the case for tetrahydromethanopterin.
Biosynthetically, the pterin ring is derived from GTP 38. The guanine ring of GTP is cleaved to release formic acid, followed by a recycle involving the ribose moiety (Fig. 23). Another interesting and unique transformation is the condensation of 4-aminobenzoate with the purine precursor 5-phospho-D-ribosyl diphosphate (32). This reaction is unique among known PRPP transferases in that a benzoate is decarboxylated to yield a C-riboside. Finally, SAM and a [4Fe–4S] cluster serve for the radical methylation of C7 and C9 of the pterin system.66
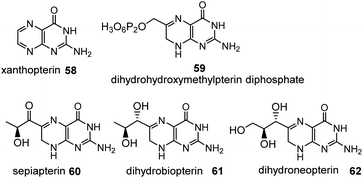
On the route to folic acid, biopterin 61 (dihydroform) and hydroxymethylpterin 59 (dehydroform) are formed, which show redox properties similar to riboflavins, with the 5,6,7,8-tetrahydro derivative being the reduced form.67 The biopteridine redox system is of particular importance in the oxidation of aromatic rings. A distinctive feature of these oxidations is that they require the presence of molecular oxygen. They occur in all kingdoms of life, including archaea.68
Xanthopterin (58) occurs as a yellow pterin pigment in butterfly wings, for example. Sepiapterin (60) is a yellow pigment found in the eyes of Drosophila and is formed from D-erythro-dihydroneopterin triphosphate.
2.5 Other coenzymes for group transfer reactions
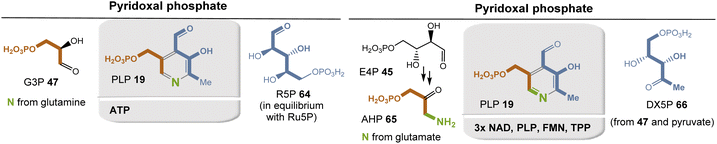
It is biosynthesized in microorganisms and plants, and to date two biosynthetic pathways have been described (Fig. 26).72 The ribose-5-phosphate-dependent (or deoxy-xylulose-phosphate-independent) pathway, found for example in Bacillus subtilis, uses glyceraldehyde-3-phosphate 47 and ribose-5-phosphate (R5P, 64 in equilibrium with ribulose-5-phosphate (RuP)) as carbon sources, while the nitrogen atom is recruited from glutamine in the form of ammonia (Fig. 26, left).73 This biosynthetic pathway has also been found to occur in archaea, fungi, and plants. PLP synthase, which carries an additional glutaminase site to provide ammonia, condenses 47 and 64 directly to PLP 19. Remarkably, the entire metabolic pathway requires no other coenzyme except ATP to regenerate glutamine from glutamate.
The second metabolic pathway (Fig. 26, right) has been studied in detail in E. coli. It utilizes 3-hydroxy-aminoacetone phosphate (AHP, 65) and 1-deoxy-xylulose 5-phosphate (DX5P, 66), which are brought together by pyridoxine 5′-phosphate synthase to form the pyridine ring. AHP 65 is formed from erythrose 4-phosphate (45), which is oxidized to 4-phosphoerythronate and further to 3-hydroxy-2-oxo-4-(phosphonatooxy)butanoate, 4-phosphohydroxy-L-threonine, and finally to AHP 65. One coenzyme is required for each of the four steps (3× NAD+ and 1× PLP). Finally, the resulting pyridoxine is oxidized to PLP 19, for which FMN serves as a redox coenzyme. The biosynthesis of DX5P 66 begins with the TPP-dependent coupling of G3P 47 with pyruvate 67. This route is clearly to be designated as a “straggler” pathway, precisely because PLP is needed here for its own formation.
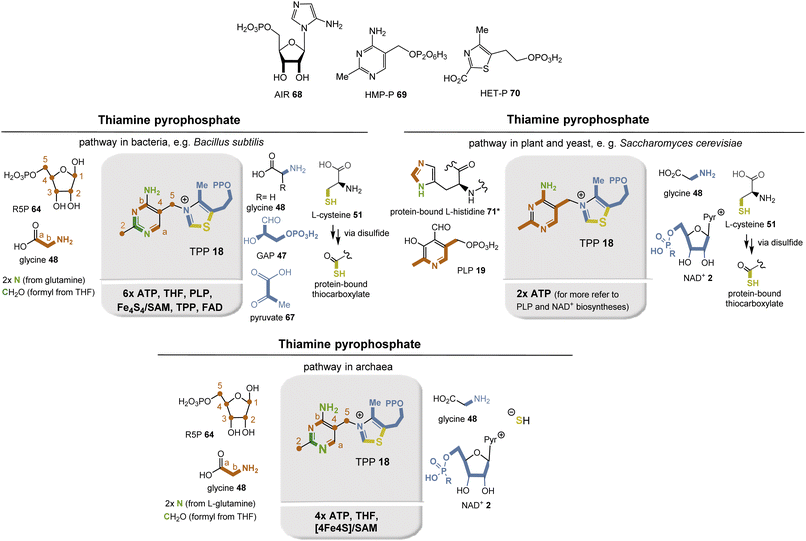
Nature has evolved three different biosynthetic pathways to TPP 18, all of which are completed by linking hydroxymethylpyrimidine phosphate (HMP-P; 69) with hydroxyethylthiazole phosphate (HET-P; 70) in a substitution reaction followed by phosphorylation to the corresponding pyrophosphate (Fig. 27).
In bacteria, plant chloroplasts, and archaea, the enzyme ThiC plays a key role. In a mechanistically highly remarkable radical SAM-mediated cascade reaction, it converts AIR 68 to HMP-P 69 (Fig. 24, top left).76 AIR 56 is an interesting intermediate in that it also serves as a general precursor for purine metabolism. The HET building block 70 is biosynthesized in most bacteria from DX5P (66) and glycine (48; in E. coli, glycine is replaced by L-tyrosine).77 The sulfur atom derives from a protein-bound thiocarboxylate that ultimately originates from the free amino acid cysteine via a persulfide intermediate. Mechanistically, this process is similar to the S-transfer in the biosynthesis of molybdopterin 54 (see above). DX5P 66 is formed from pyruvate 67 and glyceraldehyde-3-phosphate (47), and TPP 18 is required for this enzymatic step.
The second biosynthetic pathway was found in yeasts, e.g., Saccharomyces cerevisiae (Fig. 27, top right). HMP-P 69 is formed from L-histidine (71) and PLP 19, for which a remarkable mechanism has also been proposed.78 A Diels–Alder type cycloaddition is suggested, followed by radical oxidations promoted by Fe3+ and O2. It is noteworthy that coenzyme 19 acts as a building block here. HET-P 70 is formed from glycine 48, the coenzyme NAD+2, and the sulfur atom is introduced as described above. S-transfer is mediated by iron leaving a dehydroalanine residue in the protein. Remarkably, no coenzyme-dependent enzymes other than ATP-promoted phosphorylations are involved in this biosynthetic pathway. Nevertheless, the coenzymes required for the biosynthesis of the building blocks PLP 19 and NAD+2 must, of course, be included in the analysis.
Archaea harbor structural homologs of both bacterial and eukaryotic proteins for biosynthesis (Fig. 27, bottom). HMP-P 69 is essentially fed by the same building blocks as in bacteria (Fig. 27, top left), while biosynthesis of HET-P 70 follows the pathway of TPP biosynthesis in fungi (Fig. 27, top right).79 For a long time, the source of the sulfur atom remained in the dark. But thermophilic methanogens from hydrothermal vents, in which the sulfide content is high, helped to unravel this mystery. In fact, it was found that sulfide serves as a sulfur donor, with iron acting as a cofactor.
Preliminary analysis reveals that the first biosynthetic pathway found in bacteria occurred much later in evolution, requiring TPP for the synthesis of DXP 66, which is a precursor for the non-mevalonate pathway to terpenes. The second pathway uses histidine and PLP and appears to be simple in terms of the number of coenzymes required. However, the biosynthesis of histidine starts with ATP 13 and PRPP 32 and requires the coenzyme PLP 19 and 2× NAD(P)+ 2b. The third biosynthetic pathway, which occurs in archaea, is a hybrid of the other two biosynthetic routes because it feeds on their fragment biosyntheses. The recruitment of sulfur may also provide a hint on the evolution of these pathways. The protein Thi4 is required for thiazole biosynthesis, and in Methanococcus jannaschii the ortholog of Thi4 has a histidine at the site where cysteine is normally found. Consequently, sulfide had to serve as the S source. Thus, it could be argued that this mode of recruitment is the oldest, as hydrothermal vents are thought to be the habitat for early forms of life.
2.6. Coenzymes of methanogenesis
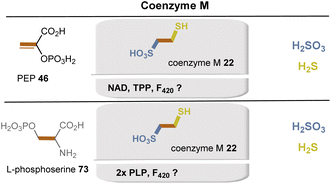
Methanogens use several unique coenzymes 21–26, which are listed in Fig. 2. The biosynthesis of coenzymes F021a and F42021b and of THMPT 24 has been described in earlier sections because of their similarities to the biosynthesis of flavins 3 and THF 16, respectively.81In methanogens, two biosynthetic pathways for coenzyme M (22) are known, with the carbon skeleton derived from either phosphoenolpyruvate 46 or L-phosphoserine 73 (Fig. 28).84 The PEP-dependent pathway begins with the Michael addition of sulfite to PEP 46, which, including an oxidation step requiring NAD+, leads to the key intermediate 2-oxo-3-sulfopropanoic acid. Decarboxylation and reductive introduction of H2S eventually forms coenzyme M (22). Details on the active electron donor for this last step have not yet been elucidated. The L-phosphoserine-dependent pathway is based on the concerted elimination of phosphate and addition of sulfite, followed by transamination. Both steps require PLP as a coenzyme. At this point, both pathways converge via the intermediate 2-oxo-3-sulfopropanoic acid.
The biosynthesis repeatedly follows a sequence of transformations encountered in the citric acid cycle (Krebs)85 and in leucine biosynthesis (Fig. 29). In the present case, it is initiated by the aldol reaction of 2-oxoglutarate and acetyl-CoA. What is remarkable about the route is the iterative nature of the 2-oxoacid elongation, which leads to a formal homologization by one carbon and the release of carbon dioxide in three rounds. The isomerization sequence, based on water elimination and regioreversed hydration, is catalyzed by an iron–sulfur cluster, which acts as a Lewis acid in the present case. Biosynthesis is completed by decarboxylation, producing an aldehyde intermediate into which H2S is reductively introduced and finally coenzyme B (23) is formed.86 Hydrogen sulfide was found to provide the sulfur atom for the formation of 7-mercaptoheptanoic acid in a reaction that has been shown to likely obtain its reducing equivalents from hydrogen via an F420-dependent hydrogenase.86
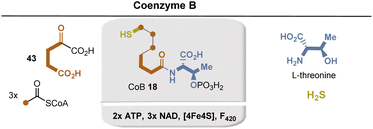 | ||
| Fig. 29 Summary of coenzyme B 23 biosyntheses: building blocks marked in sienna, blue and yellow and positions where they end up in CoB 23. Coenzyme-dependent enzymes: ATP: activation of threonine and late stage phosphorylation; enzymes not characterized yet; 3× NAD+: threo-isocitrate dehydrogenases (after each formal homologization step); Fe4S4: enzyme not characterized yet (for details see ref. 78). | ||
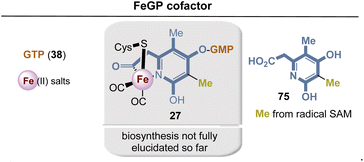 | ||
| Fig. 31 Summary of FeGP cofactor biosynthesis: building blocks are marked in blue, sienna and yellow at positions where they end up in the FeGP cofactor 27. | ||
The biosynthesis of FeGP has not yet been fully elucidated. So far, it is known that 2-(4,6-dihydroxy-3,5-dimethylpyridin-2-yl)acetic acid 75 is the biosynthetic precursor for FeGP 27, while so far only a few preliminary feeding experiments with 13C-labeled building blocks such as acetic acid and pyruvate could give some clues about the origin of the pyridine ligand.90
3. Can the analysis provide insight into coenzyme evolution?
3.1 A proposal
The compilation of biosyntheses of coenzymes presented in this article searches for metabolic relationships and dependencies of these and deliberately hides questions about bioinformatic and phylogenetic relationships of the enzymes involved in the biosyntheses. Rather, the author asks which coenzymes are required for the biosynthesis of a particular coenzyme. Biosyntheses that almost do without them would thus be older than those that rely on a large number of coenzymes. It must be emphasized, however, that this analysis in no way seeks to include the possible prebiotic existence and role of coenzymes or simpler analogs that may have served as nutrients for the first organisms. Nor does it include simplifications or truncations of coenzyme biosyntheses that might conceivably occur if some of the required building blocks, such as amino acids, were of prebiotic origin.91 Such a transitional phase on the way to life as we know it is very conceivable; it was the time before the appearance of the last unified common ancestor (LUCA).92 A recent analysis suggests that LUCA probably already had the standard repertoire of coenzymes, like the autotrophic thermophilic anaerobes today.93Obviously, the approach pursued in this account can represent only one of several possible perspectives; nevertheless, it is an attempt to address this question in a comprehensive manner. In Scheme 4, the most important coenzymes and their biosyntheses are arranged in such a way that they can be placed in a possible chronological evolutionary relationship. The scheme also contains four coenzymes that are dedicated to methanogenesis and acetogenesis. Some aspects of this sorting are highlighted and discussed below.
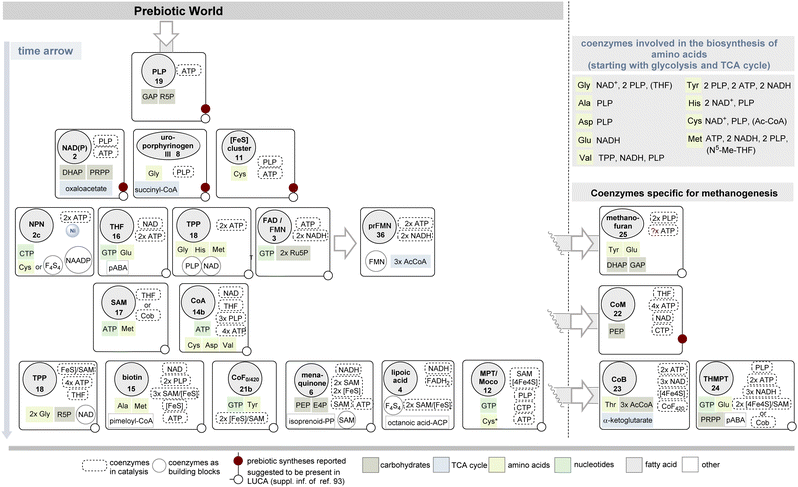 | ||
| Scheme 4 Proposed appearance in time of the biosyntheses of coenzymes under consideration of the required building blocks and coenzymes.a Their presence in LUCA according to ref. 93 as well as plausible prebiotic synthesis are listed too. NADH and FADH2 are required as coenzymes for fatty acid biosynthesis and thus for lipoic acid, as well as ATP and CoA for acyl activation; if malonyl-CoA functions as an elongation building block, biotin (15) would be required as an additional coenzyme. The evolutionary relationships of iron–sulfur cluster-derived cofactors and in the tetrapyrrole-based coenzyme family are summarized in Schemes 5 and 6. NAADP 54 is listed as a coenzyme building block, as it is part of NAD+ biosynthesis. a The FeGP 27 cofactor is not included in the list of coenzymes specific for methanogenesis, because details on the biosynthesis of the pyridinol ligand 75 are not known yet. | ||
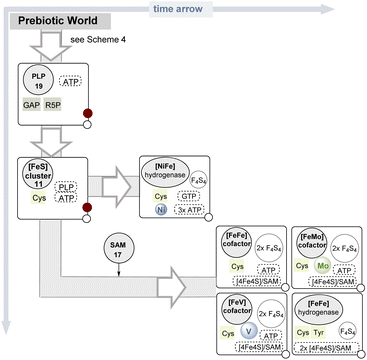 | ||
| Scheme 5 Proposed appearance in time of the biosyntheses of iron–sulfur cluster derived cofactors (symbols and color code are found in the legend in Scheme 4). | ||
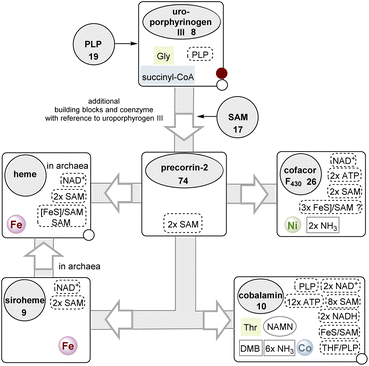 | ||
| Scheme 6 The (anaerobic) branched pathway of tetrapyrrole biosynthesis starting with uroporphyrinogen III 8 (for additional details see also Fig. 12 and 20). Fe, Ni and Co refer to metal salts actively taken from the environment. For cobalamin, the coenzymes required for the biosynthesis of DMB and the introduction of the threonine moiety are included in the list. The biosynthesis of chlorophyll is not covered here. | ||
Two pathways are known for the key biosynthetic precursor of uroporphyrinogen III 8 δ-ALA 50, which differ in the required use of coenzymes. For route A this is PLP and for route B PLP and NADH. Similarly, eukaryotic biosynthesis of nicotinamide 2 can be evolutionarily eliminated as an early development, in part because the biosynthesis of tryptophan requires NAD+ and PLP among other coenzymes.2
Metabolic evolution led to the development of three routes for TPP biosynthesis. As already indicated in section 2.5.2, the version in bacteria is the youngest, since it builds on the (MEP/DOXP) pathway for terpenes and starts with the coupling of pyruvate 67 and G3P 47, which is based on the coenzyme TPP itself. The other two biosynthetic pathways, however, are embedded in Scheme 4.
Iron–sulfur species have been regarded to be among the oldest biological coenzymes and have strictly been conserved until today.94 The in vitro chemistry of iron–sulfur clusters has been documented as several species can be generated from inorganic iron and sulfide.95 Recently, it was demonstrated that photooxidation of ferrous ions and the photolysis of organic thiols are conditions under which polynuclear iron–sulfur clusters are generated.96
The development of primitive catalysts into iron–sulfur clusters could have spontaneously occurred by assembling on polypeptide templates. In all of these complexes, cysteine also plays a central role as a proteinogenic ligand, so that in the evolution of α-amino acids it must have appeared rather earlier. In prebiotic times, other ligands, probably also based on thiols (such as methanethiol), may have first taken the later role of cysteine.
Once iron–sulfur clusters were available they could have rearranged themselves to form the different clusters that are now found in (Fe–S) proteins. These also include more complex metal clusters in which iron is exchanged for other metals. As a consequence, this evolution led to enzyme classes such as nitrogenases and hydrogenases, both key enzymes in the evolution of life. Here, based on the concept of determining the coenzymes required for their biosynthetic generation, an evolutionary relationship can be proposed (Scheme 5). Noteworthy, four of the five clusters mentioned (the [FeFe], [MoFe], and [VFe] cofactors in nitrogenases and the [FeFe] ‘H-cluster’ found in the corresponding hydrogenase) alone had to wait for SAM 17 to appear (Scheme 4) before they could step onto the stage.
From the analysis compiled in Scheme 5, it is clear that the appearance of the cofactor encountered in [NiFe]-hydrogenases should be ranked earlier. And ideas about early metabolisms suggest the same as these hydrogenases have links to ancient forms of metabolism, utilizing hydrogen as the original source of reductant on Earth.97 It has not (yet) been chemically verified whether, from the iron–sulfur clusters very likely present in the “prebiotic” world, chemical accesses to catalytically active clusters mixed with other metals did exist.
Many (Fe–S) proteins have survived up today98 but it is known that during evolution some of them have been replaced by other redox systems.99 This is exemplified for the oxidation of glucose in the Entner–Doudoroff pathway (glucose to gluconate and glyceraldehyde to glycerate). Thereby, NAD(P)+-dependent enzymes took over from the ferredoxins.100,101
Finally, an interesting finding of this analytical approach is that for these four coenzymes whose biosyntheses likely became established at an early time in evolution, chemical syntheses under plausible prebiotic conditions were also reported (Scheme 4). Or is this just a coincidence?2,91
The analysis also shows that coenzyme A 14b, a universal activator of carboxylic acids, must have arisen at a later stage of (proto)metabolism because its biosynthesis requires numerous coenzymes, including folic acid. Activation of carboxylic acids as CoA thioesters normally occurs via the mixed phosphate anhydrides with the participation of ATP 13. These, in turn, represent particularly strongly activated carboxylate derivatives, so that the development of CoA thioesters can be regarded as an evolutionary advance. This is simply because thioesters are chemically more stable than the corresponding phosphate anhydrides, i.e. more “manageable”.
Furthermore, ATP 13 is part of the nucleotide metabolism. So, if we assume that life without nucleotide biochemistry is unthinkable, also with respect to the first steps in the emergence of life from a prebiotic world, as manifested in the RNA world theory,102 then ATP 13 will be much older than CoA 14b. However, it cannot be ruled out that the reverse is true, as has been proposed for the prebiotic thioester world, but then based on chemically simpler thiols than CoA 14b.103 The (bio)molecular and physiological basis of LUCA was analyzed by genetic analyses of protein clusters from sequenced prokaryotic genomes of different phylogenetic trees.93 Its metabolism was likely dominated by FeS clusters and radical chemistry.104 Analysis suggests the presence of biosynthetic pathways for almost all coenzymes, including flavins (molybdopterin), 5-deazaflavins (coenzyme F420, 21b), S-adenosylmethionine (SAM, 17), coenzyme A (CoA, 14b), coenzyme M (CoM, 22), thiamine pyrophosphate (TPP, 18), ferredoxin (Fe–S proteins), siroheme, and corrin (Scheme 4).
Recent systems chemistry approaches have argued that the basic metabolic networks of life,106,107 such as the TCA cycle and especially its reverse counterpart, the rTCA cycle, but also others, existed long before the appearance of LUCA.108–110 These networks arose in parallel with RNA, which receives special attention in the RNA-world theory because of its catalytic and self-replicating capabilities.101
While there is hypothetical and experimental evidence for the presence of sugar-like building blocks and small carboxylic acids generated under prebiotic conditions (e.g. formose reaction,111 Sutherland's cyanosulfidic protometabolism112),91 the picture for amino acids is more complex. Thus, no plausible prebiotic approaches to methionine, tyrosine113 and histidine114 are known. In addition, the biosynthesis of methionine requires diverse coenzymes, and other considerations (e.g., evolution of the genetic code115) also suggest it to be one of the last established amino acid biosyntheses. This gives additional support for the argument that the coenzymes TPP 18, and biotin 15 are evolutionarily among the late arrivers.
Glycine is not only the structurally simplest amino acid, but is produced under almost all known plausible prebiotic conditions.91 Interestingly, it appears preferentially as a building block of the coenzyme uroporphyrinogen III (8) and cobalamin (10), which are classified as evolutionary ancient in Scheme 4.
3.2 The evolution of tetrapyrrole biosynthesis
Finally, the branched metabolic pathway responsible for the synthesis of tetrapyrrole-containing natural products will be discussed in more detail. Some aspects have already been dealt with in sections 2.2.1, 2.4.1 and Scheme 3.117Scheme 6 summarizes the evolutionary relationships between tetrapyrrole scaffold-containing coenzymes, which include cobalamin.118 Precorrin-2 (74) is the central branching site for the biosynthesis of heme,119 cofactor F43026, siroheme (9), and cobalamin (10).In the further course of the biosyntheses, starting with uroporphyrinogen III (8), SAM 17 plays a central role both as a methyl-transferring coenzyme and in radical SAM-mediated reactions120 that also allow oxidations in the absence of molecular oxygen. The analysis shows that cobalamin can by no means be an ancient coenzyme, which is also reflected in the fact that its biosynthesis is based on about 30 enzymes.121,122
This is also reflected in the fact that cobalamin was not necessarily required to be involved in the development of DNA, specifically as part of ribonucleotide reductase, since this radical deoxygenation process can instead be promoted by radical SAM (ribonucleotide reductase type III).123
4. Conclusions
The evolution of the biosynthesis of coenzymes is proposed by analyzing the individual biosynthetic pathways in terms of their demand for (other) coenzymes. Although this approach contains various imponderables, since it only chooses one particular point of view, i.e., it leaves out the topic of phylogenetic analyses of proteins and bioinformatic approaches. Indeed, it must be emphasized that the chronological-evolutionary classification of coenzymes developed here and e.g. summarised in Scheme 4 has a certain theoretical character, since it analyzes the need for coenzymes and building blocks of individual biosyntheses. This does not necessarily mean that the appearance on the scene happened at the theoretically possible moment. This shall be explained for the Ni-pincer nucleotide 2c, lipoic acid (4) and for the prFMN 36. These could have existed at a very early time based on the analysis done here. But with respect to their chemical properties, was their existence necessary in early organisms or could they have appeared much later? Martin's analysis of LUCA, compiled for coenzymes and cofactors in Scheme 4, may provide clues here. According to this, the metabolism of LUCA was not based on these coenzymes. The same is true for the FeM cofactors of the hydrogenases.Still, this report represents one of the first comprehensive attempts – another important one by Warren and coworkers7 must be mentioned here – to place coenzymes in an evolutionary context. Future “thought experiments” would need to take a more holistic approach. For example, the present report has left open the essential question of enzymatic electrophilic and radical methylations, for which nature has found various solutions, such as in the Wood–Ljungdahl C1-fixation pathway,116 methionine metabolism,124 and the methylation of uridine.125 Associated with this are the coenzymes SAM 17, THF 16, THMPT 24 and cobalamin 10. However, a deeper contemplation on this may imply the starting point for the narration of another story.126
5. Conflicts of interest
There are no conflicts to declare.6. Acknowledgements
I would like to thank Carsten Zeilinger (Center for Biomolecular Drug Research (BMWZ), Leibniz University Hannover) for the fruitful exchange of ideas. I am indebted to the reviewers for their comprehensive comments and suggestions for improvement, which were very helpful and contributed to a considerable improvement in the quality of the manuscript.7. Notes and references
- J. Nielsen, Annu. Rev. Biochem., 2017, 86, 245–275 CrossRef CAS PubMed.
- (a) A. Kirschning, Chem.–Eur. J., 2022 DOI:10.1002/chem.202201419; (b) A. Kirschning, Nat. Prod. Rep., 2021, 38, 993–1010 RSC.
- (a) T. P. Begley, Nat. Prod. Rep., 2006, 23, 15–25 RSC; (b) M. E. Webb, A. Marquet, R. R. Mendel, F. Rébeillé and A. G. Smith, Nat. Prod. Rep., 2007, 24, 988–1008 RSC; (c) T. D. Begley, A. Chatterjee, J. W. Hanes, A. Hazra and S. E. Ealick, Curr. Opin. Chem. Biol., 2008, 12, 118–125 CrossRef CAS PubMed.
- T. F. Schwede, J. Rétey and G. E. Schulz, Biochemistry, 1999, 38, 5355–5361 CrossRef CAS.
- (a) T. Sato and H. Atomi, Curr. Opin. Microbiol., 2011, 14, 307–314 CrossRef CAS PubMed; (b) A. A. DiMarco, T. A. Bobik and R. S. Wolfe, Annu. Rev. Biochem., 1990, 59, 355–394 CrossRef CAS PubMed; (c) J. M. Wolfe and G. P. Fournier, Nat. Ecol. Evol., 2018, 2, 897–903 CrossRef PubMed.
- (a) P. N. Evans, J. A. Boyd, A. O. Leu, B. J. Woodcroft, D. H. Parks, P. Hugenholtz and G. W. Tyson, Nat. Rev. Microbiol., 2019, 17, 219–232 CrossRef CAS; (b) L. G. Gorris and C. van der Drift, BioFactors, 1994, 4, 139–145 CAS.
- G. L. Holliday, J. M. Thornton, A. Marquet, A. G. Smith, F. Rebeille, R. R. Mendel, H. L. Schubert, A. D. Lawrence and M. J. Warren, Nat. Prod. Rep., 2007, 24, 972–987 RSC.
- (a) A. Mattevi, Nat. Struct. Mol. Biol., 2006, 13, 563–564 CrossRef CAS PubMed; (b) G. Noctor, G. Queval and B. Gakière, J. Exp. Bot., 2006, 57, 1603–1620 CrossRef CAS PubMed; (c) B. Gakière, J. Hao, L. de Bont, P. Pétriacq, A. Nunes-Nesi and A. R. Fernie, Crit. Rev. Plant Sci., 2018, 37, 259–307 CrossRef; (d) P. Belenky, K. L. Bogan and C. Brenner, Trends Biochem. Sci., 2007, 32, 12–19 CrossRef CAS PubMed; (e) J. C. Morales, L. Li, F. J. Fattah, Y. Dong, E. A. Bey, M. Patel, J. Gao and D. A. Boothman, Crit. Rev. Eukaryotic Gene Expression, 2014, 24, 15–28 CrossRef PubMed; (f) B. Lüscher, M. Bütepage, L. Eckei, S. Krieg, P. Verheugd and B. H. Shilto, Chem. Rev., 2018, 118, 1092–1136 CrossRef PubMed.
- S. Y. Gerdes, M. D. Scholle, M. D'Souza, A. Bernal, M. V. Baev, M. Farrell, O. V. Kurnasov, M. D. Daugherty, F. Mseeh, B. M. Polanuyer, J. W. Campbell, S. Anantha, K. Y. Shatalin, S. A. K. Chowdhury, M. Y. Fonstein and A. L. Osterman, J. Bacteriol., 2002, 184, 4555–4572 CrossRef CAS PubMed.
- G. Magni, A. Amici, M. Emanuelli, N. Raffaelli and S. Ruggieri, Adv. Enzymol. Relat. Areas Mol. Biol., 1999, 73, 135–182 CAS.
- (a) A. Volbeda, C. Darnault and J.-C. Fontecilla-Camps, J. Am. Chem. Soc., 2016, 138, 11802–11809 CrossRef CAS PubMed; (b) S. O.-de Choudens, L. Loiseau, Y. Sanakis, F. Barras and M. Fontecave, FEBS Lett., 2005, 79, 3737–3743 CrossRef; (c) T. Begley, C. Kinsland, R. Mehl, A. Osterman and P. Dorrestein, Vitam. Horm., 2001, 61, 103–119 CrossRef CAS; (d) H. Sakuraba, T. Satomura, R. Kawakami, S. Yamamoto, Y. Kawarabayasi, H. Kikuchi and T. Ohshima, Extremophiles, 2002, 6, 275–281 CrossRef CAS PubMed; (e) F. Gazzaniga, R. Stebbins, S. Z. Chang, M. A. McPeek and C. Brenner, Microbiol. Mol. Biol. Rev., 2009, 73, 529–541 CrossRef CAS PubMed.
- (a) Y. Zhang, K. L. Colabroy, T. P. Begley and S. E. Ealick, Biochemistry, 2005, 44(21), 7632–7643 CrossRef CAS PubMed; (b) W. C. Lima, A. M. Varani and C. F. Menck, Mol. Biol. Evol., 2009, 26, 399–406 CrossRef CAS PubMed; (c) For an evolutionary view on both pathways see W. C. Lima, A. M. Varani and C. F. Menck, Mol. Biol. Evol., 2009, 26, 399–406 CrossRef CAS PubMed; (d) H. B. White III, The pyridine nucleotide coenzyme, Academic Press Inc., 1982, ch. 17, pp. 225–248 Search PubMed.
- (a) H. J. Cleaves and S. L. Miller, J. Mol. Evol., 2001, 52, 73–77 CrossRef CAS PubMed; (b) H. Kim and S. A. Benner, Chem.–Eur. J., 2018, 24, 581–584 CrossRef CAS PubMed.
- (a) F. Ahmad and A. G. Moat, J. Biol. Chem., 1966, 241, 775–780 CrossRef CAS PubMed; (b) H. D. Heilmann and F. Lingens, Hoppe-Seyler's Z. Physiol. Chem., 1968, 349, 231–236 CrossRef CAS.
- (a) M. Fischer and A. Bacher, Nat. Prod. Rep., 2005, 22, 324–350 RSC; (b) A. Bacher, S. Eberhardt, M. Fischer, K. Kis and G. Richter, Annu. Rev. Nutr., 2000, 20, 153–167 CrossRef CAS PubMed; (c) M. Mack and S. Grill, Appl. Microbiol. Biotechnol., 2006, 71, 265–275 CrossRef CAS PubMed; (d) M. Fischer, A.-K. Schott, W. Römisch, A. Ramsperger, M. Augustin, A. Fidler, A. Bacher, G. Richter, R. Huber and W. Eisenreich, J. Mol. Biol., 2004, 343, 1267–1278 CrossRef PubMed; (e) M. Fischer, W. Romisch, S. Schiffmann, M. Kelly, H. Oschkinat, S. Steinbacher, R. Huber, W. Eisenreich, G. Richter and A. Bacher, J. Biol. Chem., 2002, 277, 41410–41416 CrossRef CAS PubMed; (f) S. Steinbacher, S. Schiffmann, G. Richter, R. Huber, A. Bacher and M. Fischer, J. Biol. Chem., 2003, 278, 42256–42265 CrossRef CAS PubMed.
- (a) M. Zhang, L. Wang and D. Zhong, Arch. Biochem. Biophys., 2017, 632, 158–174 CrossRef CAS PubMed; (b) A. Sancar, Biochemistry, 1994, 33, 2–9 CrossRef CAS PubMed; (c) C. A. Brautigam, B. S. Smith, Z. Ma, M. Palnitkar, D. R. Tomchick, M. Machius and J. Deisenhofer, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 12142–12147 CrossRef CAS.
- (a) D. Leys, Curr. Opin. Chem. Biol., 2018, 47, 117–125 CrossRef CAS PubMed; (b) A. Saaret, A. Balaikaite and D. Leys, Enzymes, 2020, 47, 517–549 CAS.
- P.-H. Wang, A. N. Khusnutdinova, F. Luo, J. Xiao, K. Nemr, R. Flick, G. Brown, R. Mahadevan, E. A. Edwards and F. Yakunin, Cell Chem. Biol., 2018, 25, 560–570 CrossRef CAS PubMed.
- (a) B. Reuke, S. Korn, W. Eisenreich and A. Bacher, J. Bacteriol., 1992, 174, 4042–4049 CrossRef CAS PubMed; (b) C. Greening, F. H. Ahmed, A. E. Mohamed, B. M. Lee, G. Pandey, A. C. Warden, C. Scott, J. G. Oakeshott, M. C. Taylor and C. J. Jackson, Microbiol. Mol. Biol. Rev., 2016, 80, 451–493 CrossRef CAS PubMed.
- (a) C. Walsh, Acc. Chem. Res., 1986, 19, 216–221 CrossRef CAS; (b) W. Friedrich, Vitamins, Walter de Gruyter&Co., Berlin, Germany, 1988 CrossRef.
- (a) R. Jaenchen, P. Schoenheit and R. K. Thauer, Arch. Microbiol., 1984, 137, 362–365 CrossRef CAS PubMed; (b) B. Reuke, S. Korn, W. Eisenreich and A. Bacher, J. Bacteriol., 1992, 174, 4042–4049 CrossRef CAS PubMed.
- (a) L. Decamps, B. Philmus, A. Benjdia, R. White, T. P. Begley and O. Berteau, J. Am. Chem. Soc., 2012, 134(44), 18173–18176 CrossRef CAS PubMed; (b) F. Forouhar, M. Abashidze, H. Xu, L. L. Grochowski, J. Seetharaman, M. Hussain, A. Kuzin, Y. Chen, W. Zhou, R. Xiao, T. B. Acton, G. T. Montelione, A. Galinier, R. H. White and L. Tong, J. Biol. Chem., 2008, 283, 11832–11840 CrossRef CAS PubMed.
- M. R. Challand, F. T. Martins and P. L. Roach, J. Biol. Chem., 2010, 285, 5240–5248 CrossRef CAS PubMed.
- A. Pagnier, L. Martin, L. Zeppieri, Y. Nicolet and J. C. Fontecilla-Camps, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 104–109 CrossRef CAS.
- (a) S. J. Booker, Chem. Biol., 2004, 11, 10–12 CrossRef CAS PubMed; (b) A. Marquet, B. T. S. Bui and D. Florentin, Vitam. Horm., 2001, 61, 51–101 CrossRef CAS PubMed.
- J. E. Cronan, Microbiol. Mol. Biol. Rev., 2016, 80, 429–450 CrossRef CAS PubMed.
- R. M. Cicchillo and S. J. Booker, J. Am. Chem. Soc., 2005, 127, 2860–2861 CrossRef CAS.
- (a) S. Puehringer, Mh. Metlitzky and R. Schwarzenbacher, BMC Biochem., 2008, 9, 8 CrossRef PubMed; (b) A. M. Martins, J. A. Latham, P. J. Martel, I. Barr, A. T. Iavarone and J. P. Klinman, J. Biol. Chem., 2019, 294, 15025–15036 CrossRef CAS PubMed; (c) I. Barr, J. A. Latham, A. T. Iavarone, T. Chantarojsiri, J. D. Hwang and J. P. Klinman, J. Biol. Chem., 2016, 291, 8877–8884 CrossRef CAS PubMed; (d) W. Zhu, A. M. Martins and J. P. Klinman, Methods Enzymol., 2018, 606, 389–420 CAS.
- O. T. Magnusson, H. Toyama, M. Saeki, R. Schwarzenbacher and J. P. Klinman, J. Am. Chem. Soc., 2004, 126, 5342–5343 CrossRef CAS PubMed.
- M. D. Collins and D. Jones, Microbiol. Rev., 1981, 45, 316–354 CrossRef CAS.
- R. Meganathan and O. Kwon, EcoSal Plus, 2009, 3(2) DOI:10.1128/ecosalplus.3.6.3.3.
- (a) T. Hiratsuka, K. Furihata, J. Ishikawa, H. Yamashita, N. Itoh, H. Seto and T. Dairi, Science, 2008, 321, 1670–1673 CrossRef CAS PubMed; (b) A. M. Gobe, R. Toro, X. Li, A. Ornelas, H. Fan, S. Eswaramoorthy, Y. Patskovsky, B. Hillerich, R. Seidel, A. Sali, B. K. Shoichet, S. C. Almo, S. Swaminathan, M. E. Tanner and F. M. Raushel, Biochemistry, 2013, 52, 6525–6536 CrossRef PubMed; (c) X. Li, D. Apel, E. C. Gaynot and M. E. Tanner, J. Biol. Chem., 2011, 286, 19392–19398 CrossRef CAS PubMed.
- (a) S. Joshi, D. Fedoseyenko, N. Mahanta, H. Manion, S. Naseem, T. Dairi and T. P. Begley, Curr. Opin. Chem. Biol., 2018, 47, 134–141 CrossRef CAS PubMed; (b) X.-Y. Zhi, J.-C. Yao, S.-K. Tang, Y. Huang, H.-W. Li and W.-J. Li, Genome Biol. Evol., 2014, 6, 149–160 CrossRef PubMed.
- S. S. Abby, K. Kazemzadeh, C. Vragniau, L. Pelosi and F. Pierrel, Biochim. Biophys. Acta, Bioenerg., 2020, 1861, 148259 CrossRef CAS PubMed.
- H. Maeda and N. Dudareva, Annu. Rev. Plant Biol., 2012, 63, 73–105 CrossRef CAS PubMed.
- (a) R. H. White, Biochemistry, 2004, 43, 7618–7627 CrossRef CAS PubMed; (b) R. H. White and H. Xu, Biochemistry, 2006, 45, 12366–12379 CrossRef CAS PubMed; (c) R. H. White, Biochemistry, 2008, 47, 5037–5046 CrossRef CAS PubMed; (d) D. V. Miller, M. Ruhlin, W. K. Ray, H. Xu and R. H. White, FEBS Lett., 2017, 591, 2269–2278 CrossRef CAS PubMed; (e) T. Soderberg, Archaea, 2005, 1, 347–352 CrossRef CAS.
- (a) G. Layer, J. Reichelt, D. Jahn and D. W. Heinz, Protein Sci., 2010, 19, 1137–1161 CrossRef CAS PubMed; (b) F. J. Leeper, Nat. Prod. Rep., 1989, 6, 171–203 RSC; (c) D. R. Nelson, T. Kamataki, D. J. Waxman, F. P. Guengerich, R. W. Estabrook, R. Feyereisen, F. J. Gonzalez, M. J. Coon, I. C. Gunsalus, O. Gotoh, K. Okuda and D. W. Nebert, DNA Cell Biol., 1993, 12, 1–51 CrossRef CAS PubMed.
- (a) H. A. Dailey, T. A. Dailey, S. Gerdes, D. Jahn, M. Jahn, M. R. O'Brian and M. J. Warren, Microbiol. Mol. Biol. Rev., 2017, 81, e00048-16 CrossRef PubMed; (b) T. Fan, B. Grimm and G. Layer, Adv. Bot. Res., 2019, 91, 89–131 CAS.
- (a) M. Richter, Nat. Prod. Rep., 2013, 30, 1324–1345 RSC; (b) J. D. Fischer, G. L. Holliday, S. A. Rahman and J. M. Thornton, J. Mol. Biol., 2010, 403, 803–824 CrossRef CAS PubMed; (c) T. P. Begley, Nat. Prod. Rep., 2006, 23, 15–25 RSC; (d) M. E. Webb, A. Marquet, R. R. Mendel, F. Rébeillé and A. G. Smith, Nat. Prod. Rep., 2007, 24, 988–1008 RSC; (e) M. Richter, Nat. Prod. Rep., 2013, 30, 1324–1345 RSC; (f) D. E. Scott, A. Ciulli and C. Abell, Nat. Prod. Rep., 2007, 24, 1009–1026 RSC.
- Y. Li, N. Kitadai and R. Nakamura, Life, 2018, 8, 46 CrossRef CAS PubMed.
- (a) L. Belmonte and S. S. Mansy, Elements, 2016, 12, 413–418 CrossRef CAS; (b) N. Kitadai, R. Nakamura, M. Yamamoto, K. Takai, N. Yoshida and Y. Oono, Sci. Adv., 2019, 5, eaav7848 CrossRef PubMed.
- S. Storbeck, S. Rolfes, E. Raux-Deery, M. J. Warren, D. Jahn and G. Layer, Archaea, 2010, 175050 Search PubMed.
- H. Beinert, R. H. Holm and E. Münck, Science, 1997, 277, 653–659 CrossRef CAS PubMed.
- (a) S. Bandyopadhyay, K. Chandramouli and M. K. Johnson, Biochem. Soc. Trans., 2008, 36, 1112–1119 CrossRef CAS PubMed; (b) C. Ayala-Castro, A. Saini and F. W. Outten, Microbiol. Mol. Biol. Rev., 2008, 72, 110–125 CrossRef CAS PubMed.
- (a) C. J. Schwartz, O. Djaman, J. A. Imlay and P. J. Kiley, Proc. Natl. Acad. Sci., 2000, 97, 9009–9014 CrossRef CAS PubMed; (b) D. C. Johnson, D. R. Dean, A. D. Smith and M. K. Johnson, Annu. Rev. Biochem., 2005, 74, 247–281 CrossRef CAS PubMed.
- (a) R. R. Eady, Chem. Rev., 1996, 96, 3013–3030 CrossRef CAS PubMed; (b) B. K. Burgess and D. J. Lowe, Chem. Rev., 1996, 96, 2983–3012 CrossRef CAS; (c) Y. Hu, C. C. Lee and M. W. Ribbe, Dalton Trans., 2012, 41, 1118–1127 RSC.
- D. Harris, D. Lukoyanov, S. Shaw, P. D. Compton, M. Tokmina-Lukaszewska, B. Bothner, N. L. Kelleher, D. R. Dean, B. M. Hoffman and L. C. Seefeldt, Biochemistry, 2018, 57(5), 701–710 CrossRef CAS PubMed.
- (a) J. Raymond, J. L. Siefert, C. R. Staples and R. E. Blankenship, Mol. Biol. Evol., 2004, 21, 541–554 CrossRef CAS PubMed; (b) N. Gruber and J. N. Galloway, Nature, 2008, 451, 293–296 CrossRef CAS PubMed; (c) Y. Hu and M. W. Ribbe, Coord. Chem. Rev., 2011, 255, 1218–1224 CrossRef CAS PubMed.
- (a) W. Lubitz, H. Ogata, O. Rüdiger and E. Reijerse, Chem. Rev., 2014, 114, 4081–4148 CrossRef CAS PubMed; (b) P. M. Vignais and B. Billoud, Chem. Rev., 2007, 107, 4206–4272 CrossRef CAS PubMed.
- (a) L. Tao, S. A. Pattenaude, S. Joshi, T. P. Begley, T. B. Rauchfuss and R. D. Britt, J. Am. Chem. Soc., 2020, 142, 10841–10848 CrossRef CAS PubMed; (b) G. Rao, L. Tao, D. L. M. Suess and R. D. Britt, Nat. Chem., 2018, 10, 555–560 CrossRef CAS PubMed; (c) J. B. Broderick, A. S. Byer, K. S. Duschene, B. R. Duffus, J. N. Betz, E. M. Shepard and J. W. Peters, J. Biol. Inorg Chem., 2014, 19, 747–757 CrossRef CAS PubMed; (d) D. W. Mulder, E. S. Boyd, R. Sarma, R. K. Lange, J. A. Endrizzi, J. B. Broderick and J. W. Peters, Structure, 2011, 19, 1038–1052 CrossRef CAS PubMed; (e) R. C. Driesener, M. R. Challand, S. E. McGlynn, E. M. Shepard, E. S. Boyd, J. B. Broderick, J. W. Peters and P. L. Roach, Angew. Chem., Int. Ed., 2010, 49, 1687–1690 CrossRef CAS PubMed; (f) J. M. Kuchenreuther, W. K. Myers, D. L. M. Suess, T. A. Stich, V. Pelmenschikov, S. A. Shiigi, S. P. Cramer, J. R. Swartz, R. D. Britt and S. J. George, Science, 2014, 343, 424–427 CrossRef CAS.
- R. R. Mendel, A. G. Smith, A. Marquet and M. J. Warren, Nat. Prod. Rep., 2007, 24, 963–971 RSC.
- (a) R. R. Mendel, J. Biol. Chem., 2013, 288, 13165–13172 CrossRef CAS PubMed; (b) C. Nivol and S. Leimkühler, Biochim. Biophys. Acta, Bioenerg., 2013, 1827, 1086–1101 CrossRef PubMed; (c) B. M. Hover, N. K. Tonthat, M. A. Schumacher and K. Yokoyama, Proc. Natl. Acad. Sci., 2015, 112, 6347–6352 CrossRef CAS PubMed; (d) G. Schwarz, Cell. Mol. Life Sci., 2005, 62, 2792–2810 CrossRef CAS PubMed.
- (a) G. Gutzke, B. Fischer, R. R. Mendel and G. Schwarz, J. Chem. Biol., 2001, 276, 36268–36274 CrossRef CAS PubMed; (b) M. M. Wuebbens and K. V. Rajagopalan, J. Biol. Chem., 2003, 278, 14523–14532 CrossRef CAS PubMed; (c) J. Sloan, J. R. Kinghorn and S. E. Unkles, Nucleic Acids Res., 1999, 27, 854–858 CrossRef CAS PubMed; (d) M. J. Rudolph, M. M. Wuebbens, K. V. Rajagopalan and H. Schindelin, Nat. Struct. Biol., 2001, 8, 42–46 CrossRef CAS PubMed.
- R. P. Hausinger, B. Desguin, M. Fellner, J. A. Rankin and J. Hu, Curr. Opin. Chem. Biol., 2018, 47, 18–23 CrossRef CAS PubMed.
- Review: S. Chatterjee, S. Gatreddi, S. Gupta, J. L. Nevarez, J. A. Rankin, A. Turmo, J. Hu and R. P. Hausinger, Biochem. Soc. Trans., 2022, 50, 1187–1196, DOI:10.1042/BST20220490.55.
- (a) C. Doering, B. Ermentrout and G. Oster, Biophys. J., 1995, 69, 2256–2267 CrossRef CAS; (b) K. W. Beyenbach and H. Wieczorek, J. Exp. Biol., 2006, 209, 577–589 CrossRef CAS PubMed; (c) R. L. Cross and V. Müller, FEBS Lett., 2004, 576, 1–4 CrossRef CAS PubMed.
- U. Genschel, Mol. Biol. Evol., 2004, 21, 1242–1251 CrossRef CAS PubMed.
- (a) R. Leonardi and S. Jackowski, EcoSal Plus, 2007, 2(2) DOI:10.1128/ecosalplus.3.6.3.4; (b) T. P. Begley, C. Kinsland and E. Strauss, Vitam. Horm., 2001, 61, 157–171 CrossRef CAS PubMed.
- (a) S. Lin and J. E. Cronan, Mol. Biosyst., 2011, 7, 1811–1821 RSC; (b) S. Lin, R. E. Hanson and J. E. Cronan, Nat. Chem. Biol., 2010, 6, 682–688 CrossRef CAS PubMed.
- (a) R. G. Matthews, Acc. Chem. Res., 2001, 34, 681–689 CrossRef CAS PubMed; (b) Q. Zhang, W. A. van der Donk and W. Liu, Acc. Chem. Res., 2012, 45, 555–564 CrossRef CAS PubMed; (c) A. B. Hazra, A. W. Han, A. P. Mehta, K. C. Mok, V. Osadchiy, T. P. Begley and M. E. Taga, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 10792–10797 CrossRef CAS PubMed; (d) Y. Mathur, S. Sreyas, P. M. Datar, M. B. Sathian and A. B. Hazra, J. Biol. Chem., 2020, 295, 10522–10534 CrossRef CAS PubMed.
- (a) E. Raux, H. L. Schubert and M. J. Warren, Cell. Mol. Life Sci., 2000, 57, 1880–1893 CrossRef CAS PubMed; (b) S. J. Moore, A. D. Lawrence, R. Biedendieck, E. Deery, S. Frank, M. J. Howard, S. E. J. Rigby and M. J. Warren, Proc. Natl. Acad. Sci., 2013, 110, 14906–14911 CrossRef CAS PubMed; (c) H. Fang, J. Kang and D. Zhang, Microb. Cell Fact., 2017, 16, 15 CrossRef PubMed; (d) A. B. Hazra, A. W. Han, A. P. Mehta, K. C. Mok, V. Osadchiy, T. P. Begley and M. E. Taga, Proc. Natl. Acad. Sci., 2015, 112, 10792–10797 CrossRef CAS PubMed.
- V. Gorelova, O. Bastien, O. De Clerk, S. Lespinats, F. Rébeillé and D. van Der Straeten, Sci. Rep., 2019, 9, 5731 CrossRef CAS PubMed.
- A. D. Welch, Perspect. Biol. Med., 1983, 27, 64–75 CrossRef CAS PubMed.
- A. Bermingham and J. P. Derrick, BioEssays, 2002, 24, 637–648 CrossRef CAS PubMed.
- (a) G. M. Brown, J. Biol. Chem., 1962, 237, 536–540 CrossRef CAS PubMed; (b) K. M. Herrmann, Plant Cell, 1995, 7, 907–919 CrossRef CAS; (c) A. R. Knaggs, Nat. Prod. Res., 2003, 20, 119–136 CAS.
- (a) J. McKinney and T. Tunckanat, FASEB J., 2021, 35, S1 Search PubMed; (b) K. D. Allen, H. Xu and R. H. White, J. Bacteriol., 2014, 96, 3315–3323 CrossRef PubMed.
- D. M. Howell and R. H. White, J. Bacteriol., 1997, 179, 5165–5170 CrossRef CAS PubMed.
- T. Sato and H. Atomi, Curr. Opin. Microbiol., 2011, 14, 307–314 CrossRef CAS PubMed.
- (a) S. Roje, Phytochemistry, 2006, 67, 1686–1698 CrossRef CAS PubMed; (b) A.-W. Struck, M. L. Thompson, L. S. Wong and J. Micklefield, ChemBioChem, 2012, 13, 2642–2655 CrossRef CAS PubMed; (c) K. Yokoyama and E. A. Lilla, Nat. Prod. Rep., 2018, 35, 660–694 RSC; (d) J. B. Broderick, B. R. Duffus, K. S. Duschene and E. M. Shepard, Chem. Rev., 2014, 114, 4229–4317 CrossRef CAS PubMed; (e) K. A. Shisler and J. B. Broderick, Curr. Opin. Struct. Biol., 2012, 22, 701–710 CrossRef CAS PubMed; (f) G. L. Holliday, E. Akiva, E. C.Meng, S. D. Brown, S. Calhoun, U. Pieper, A. Sali, S. J. Booker and P. C. Babbitt, Methods Enzymol., 2018, 606, 1–71 CAS; (g) A. P. Mehta, S. H. Abdelwahed, N. Mahanta, D. Fedoseyenko, B. Philmus, L. E. Cooper, Y. Liu, I. Jhulki, S. E. Ealick and T. P. Begley, J. Biol. Chem., 2015, 290, 3980–3986 CrossRef CAS.
- A. C. Eliot and J. F. Kirsch, Annu. Rev. Biochem., 2004, 73, 383–415 CrossRef CAS PubMed.
- P. A. Frey, Annu. Rev. Biochem., 2001, 70, 121–148 CrossRef CAS PubMed.
- Reviews: (a) T. B. Fitzpatrick, N. Amrhein, B. Kappes, P. Macheroux, I. Tews and T. Raschle, Biochem. J., 2007, 407, 1–13 CrossRef CAS PubMed; (b) T. Mukherjee, J. Hanes, I. Tews, S. E. Ealick and T. P. Begley, Biochim. Biophys. Acta, 2011, 1814, 1585–1596 CrossRef CAS; (c) M. Tambasco-Studart, O. Titiz, T. Raschle, G. Forster, N. Amrhein and T. B. Fitzpatrick, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 13687–13692 CrossRef CAS PubMed.
- B. Richts, J. Rosenberg and F. M. Commichau, Front. Mol. Biosci., 2019, 6, 32 CrossRef CAS PubMed.
- (a) C. T. Jurgenson, S. E. Ealick and T. P. Begley, EcoSal Plus, 2009, 3(2) DOI:10.1128/ecosalplus.3.6.3.7; (b) V. I. Bunik, A. Tylicki and N. V. Lukashev, FEBS J., 2013, 280, 6412–6442 CrossRef CAS PubMed; (c) J. A. Maupin-Furlow, B Group Vitamins: Current Uses and Perspectives, ed. J. G. LeBlanc and G. S. de Giori, 2018, ch. 2, DOI:10.5772/intechopen.77170.
- K. Zhang, J. Bian, Y. Deng, A. Smith, R. E. Nunez, M. B. Li, U. Pal, A. M. Yu, W. Qiu, S. E. Ealick and C. Li, Nat. Microbiol., 2016, 2, 16213 CrossRef CAS PubMed.
- (a) L. D. Palmer and D. M. Downs, J. Biol. Chem., 2013, 288, 30693–30699 CrossRef CAS PubMed; (b) A. Chatterjee, A. B. Hazra, S. Abdelwahed, D. G. Hilmey and T. P. Begley, Angew. Chem., Int. Ed., 2010, 49, 8653–8656 CrossRef CAS PubMed.
- T. P. Begley, D. M. Downs, S. E. Ealick, F. W. McLafferty, A. P. Van Loon, S. Taylor, N. Campobasso, H. J. Chiu, C. Kinsland, J. J. Reddick and J. Xi, Arch. Microbiol., 1999, 171, 293–300 CrossRef CAS PubMed.
- (a) P. C. Dorrestein, H. Zhai, F. W. McLafferty and T. P. Begley, Chem. Biol., 2004, 11, 1373–1381 CAS; (b) R. Y. Lai, S. Huang, M. K. Fenwick, A. Hazra, Y. Zhang, K. Rajashankar, B. Philmus, C. Kinsland, J. M. Sanders, S. E. Ealick and T. P. Begley, J. Am. Chem. Soc., 2012, 134, 9157–9159 CrossRef CAS PubMed.
- (a) S. Hwang, B. Cordova, N. Chavarria, D. Elbanna, S. McHugh, J. Rojas, F. Pfeiffer and J. A. Maupin-Furlow, BMC Microbiol., 2014, 14, 260 CrossRef PubMed; (b) J. Joshi, Q. Li, J. D. García-García, B. J. Leong, Y. Hu, S. D. Bruner and A. D. Hanson, Biochem. J., 2021, 478, 3265–3279 CrossRef CAS PubMed; (c) X. Zhang, B. E. Eser, P. K. Chanani, T. P. Begley and S. E. Ealick, Biochemistry, 2016, 55, 1826–1838 CrossRef CAS; (d) B. E. Eser, X. Zhang, P. K. Chanani, T. P. Begley and S. E. Ealick, J. Am. Chem. Soc., 2016, 138, 3639–3642 CrossRef CAS PubMed.
- H. A Cooke, C. V. Christianson and S. D. Bruner, Curr. Opin. Chem. Biol., 2009, 13, 460–468 CrossRef PubMed.
- (a) C. Welte and U. Deppenmeier, Biochim. Biophys. Acta, 2014, 1837, 1130–1147 CrossRef CAS PubMed; (b) F. Enzmann, F. Mayer, M. Rother and D. Holtmann, AMB Express, 2018, 8(1) CrossRef CAS PubMed; (c) D. E. Graham and R. H. White, Nat. Prod. Rep., 2002, 19, 133–147 RSC; (d) J. G. Ferry, FEMS Microbiol. Rev., 1999, 23, 13–38 CrossRef CAS PubMed.
- (a) R. K. Thauer, A.-K. Kaster, M. Goenrich, M. Schick, T. i. Hiromoto and S. Shima, Annu. Rev. Biochem., 2010, 79, 507–536 CrossRef CAS PubMed; (b) P. N. Evans, J. A. Boyd, A. O. Leu, B. J. Woodcroft, D. H. Parks, P. Hugenholtz and G. W. Tyson, Nat. Rev. Microbiol., 2019, 17, 219–232 CrossRef CAS PubMed.
- (a) J. R. Allen, D. D. Clark, J. G. Krum and S. A. Ensign, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 8432–8437 CrossRef CAS PubMed; (b) X. Liu and T. E. Mattes, Appl. Environ. Microbiol., 2016, 82, 3269–3279 CrossRef CAS PubMed.
- S. E. Partovi, F. Mus, A. E. Gutknech, H. A. Martinez, B. P. Tripet, B. M. Lange, J. L. DuBois and J. W. Peters, J. Biol. Chem., 2018, 293, 5236–5246 CrossRef CAS.
- D. M. Howell, M. Graupner, H. Xu and R. H. White, J. Bacteriol., 2000, 182, 5013–5016 CrossRef CAS PubMed.
- R. H. White, Biochemistry, 1989, 28, 9417–9423 CrossRef CAS.
- (a) W. Eisenreich and A. Bacher, Biol. Chem., 1992, 267, 17574–17580 CrossRef CAS; (b) D. Miller, Y. Wang, H. Xu, K. Harich and R. H. White, Biochemistry, 2014, 53, 4635–4647 CrossRef CAS PubMed.
- (a) S. W. Ragsdale, Met. Ions Life Sci., 2014, 14, 125–145 CAS; (b) R. K. Thauer, Microbiol., 1998, 144, 2377–2406 CrossRef CAS PubMed.
- S. J. Moore, S. T. Sowa, C. Schuchardt, E. Deery, A. Lawrence, J. Vazquez Ramos, S. Billig, C. Birkemeyer, P. T. Chivers, M. J. Howard, S. E. J. Rigby, G. Layer and M. J. Warren, Nature, 2017, 543, 78–82 CrossRef CAS.
- S. Schaupp, F. J. Arriaza-Gallardo, H.-j. Pan, J. Kahnt, G. Angelidou, N. Paczia, K. Costa, X. Hu and S. Shima, Angew. Chem., Int. Ed., 2022, 61, e202200994 CrossRef CAS PubMed.
- A. Kirschning, Angew. Chem., Int. Ed., 2021, 60, 6242–6269 CrossRef CAS PubMed.
- (a) W. Gilbert, Nature, 1986, 319, 618 CrossRef; (b) G. F. Joyce, Nature, 2002, 418, 214–221 CrossRef CAS PubMed; (c) M. P. Robertson and G. F. Joyce, Cold Spring Harbor Perspect. Biol., 2012, 4, a003608 Search PubMed; (d) D. Penny, Biol. Philos., 2005, 20, 633–671 CrossRef.
- (a) C. Weiss, F. Sousa, N. Mrnjavac, S. Neukirchen, M. Roettger, S. Nelson-Sathi and W. F. Martin, Nat. Microbiol., 2016, 161 Search PubMed; (b) J. L. E. Wimmer and W. F. Martin, Bunsenmagazin, 2022, 24, 135–138 Search PubMed.
- M. J. Russell and A. J. Hall, J. Geol. Soc., 1997, 154, 377–402 CrossRef CAS.
- (a) R. H. Holm, Adv. Inorg. Chem., 1992, 38, 1–71 CrossRef CAS; (b) J. M. Berg and R. H. Holm, in Iron-sulfur proteins, ed. T. G. Spiro, Wiley, New York, 1982, pp. 1–66 Search PubMed.
- (a) A. D. Tsaousis, Front. Microbiol., 2019, 10, 2478 CrossRef PubMed; (b) C. Bonfio, L. Valer, S. Scintilla, S. Shah, D. J. Evans, L. Jin, J. W. Szostak, D. D. Sasselov, J. D. Sutherland and S. S. Mansy, Nat. Chem., 2017, 9, 1229–1234 CrossRef CAS PubMed; (c) Related studies: M. Dörr, J. Käßbohrer, R. Grunert, G. Kreisel, W. A. Brand, R. A. Werner, H. Geilmann, C. Apfel, C. Robl and W. Weigand, Angew. Chem., Int. Ed., 2003, 42, 1540–1543 CrossRef PubMed.
- A. J. Finney and F. Sargent, Adv. Microb. Physiol., 2019, 74, 465–486 CrossRef.
- J. J. Braymera, S. A. Freiberta, M. Rakwalska-Bangea and R. Lilla, Biochim. Biophys. Acta, Mol. Cell Res., 2021, 1868, 118863 CrossRef.
- (a) C. H. Verhees, S. W. M. Kengen, J. E. Tuininga, G. J. Schut, M. W. W. Adams, W. M. de Vos and J. van der Oost, Biochem. J., 2004, 377, 819–822 CrossRef CAS; (b) B. Siebers, B. Tjaden, K. Michalke, C. Dörr, H. Ahmed, M. Zaparty, P. Gordon, C. W. Sensen, A. Zibat, H. Klenk, S. C. Schuster and R. Hensel, J. Bacteriol., 2004, 186, 2179–2194 CrossRef CAS; (c) H. Ahmed, T. J. G. Ettema, B. Tjaden, A. C. M. Geerling, J. van der Oost and B. Siebers, Biochem. J., 2005, 390, 529–540 CrossRef CAS.
- (a) A.-M. Sevcenco, M. W. H. Pinkse, E. Bol, G. C. Krijger, H. T. Wolterbeek, P. D. E. M. Verhaert and P.-L. Hagedoorn, Metallomics, 2009, 1, 395–402 CrossRef CAS PubMed; (b) H. Beinert, JBIC, J. Biol. Inorg. Chem., 2000, 5, 2–15 CrossRef CAS PubMed; (c) T. A. Major, H. Burd and W. B. Whitman, FEMS Microbiol. Lett., 2004, 239, 117–123 CrossRef CAS.
- R. M. Daniel and M. J. Danson, J. Mol. Evol., 1995, 40, 559–563 CrossRef CAS.
- (a) G. Joyce and L. E. Orgel, in The RNA World, ed. R. Gesteland, T. Cech and J. Atkins, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, 2nd edn, 1999, pp. 49–77 Search PubMed; (b) G. D. McDonald and M. C. Storrie-Lombardi, Astrobiology, 2010, 10, 989–1000 CrossRef CAS.
- (a) R. Krishnamurthy, Acc. Chem. Res., 2017, 50, 455–459 CrossRef CAS PubMed; (b) J. E. Goldford, H. Hartman, T. F. Smith and D. Segre, Cell, 2017, 168, 1126–1134 CrossRef CAS.
- A. Marquet, B. Tse Sum Bui, A. G. Smith and M. J. Warren, Nat. Prod. Rep., 2007, 24, 1027–1040 RSC.
- In this context, it may be of interest to note that the pair of amino acids and coenzymes is a metabolic chicken-and-egg problem (see ref. 2b).
- (a) H. J. Morowitz, Complexity, 1999, 4, 39–53 CrossRef; (b) G. Caetano-Anolles, L. S. Yafremava, H. Gee, D. Caetano-Anolles, H. S. Kim and J. E. Mittenthal, Int. J. Biochem. Cell Biol., 2009, 41, 285–297 CrossRef CAS PubMed.
- (a) H. S. Bernhardt and W. M. Patrick, J. Mol. Evol., 2014, 78, 307–309 CrossRef CAS; (b) H. S. Bernhardt and W. P. Tate, Biol. Direct, 2008, 3, 53 CrossRef PubMed; (c) K. Tamura, J. Mol. Evol., 2015, 81, 69–71 CrossRef CAS PubMed.
- (a) A. Eschenmoser, Chem. Biodiversity, 2007, 4, 554–573 CrossRef CAS; (b) S. A. Benner, H.-J. Kim and E. Biondi, Life, 2019, 9, 84 CrossRef CAS; (c) S. Harrison and N. Lane, Nat. Commun., 2018, 9, 5176 CrossRef CAS PubMed; (d) A. Wołos, R. Roszak, A. Żądło-Dobrowolska, W. Beker, B. Mikulak-Klucznik, G. Spólnik, M. Dygas, S. Szymkuć and B. A. Grzybowsk, Science, 2020, 369, eaaw1955 CrossRef PubMed.
- (a) E. Smith and H. J. Morowitz, The Origin and Nature of Life on Earth: the Emergence of the Fourth Geosphere, Cambridge University Press, New York, NY, 1st edn, 2016 CrossRef; (b) H. J. Morowitz, J. D. Kostelnik, J. Yang and G. D. Cody, Proc. Natl. Acad. Sci., 2000, 97, 7704–7708 CrossRef CAS PubMed; (c) E. Smith and H. J. Morowitz, Proc. Natl. Acad. Sci., 2004, 101, 13168–13173 CrossRef CAS PubMed; (d) R. T. Stubbs, M. Yadav, R. Krishnamurthy and G. Springsteen, Nat. Chem., 2020, 12, 1016–1022 CrossRef CAS PubMed.
- (a) M. Ralser, Biochem. J., 2018, 475, 2577–2592 CrossRef CAS; (b) K. B. Muchowska, E. Chevallot-Beroux and J. Moran, Bioorg. Med. Chem., 2019, 27, 2292–2297 CrossRef CAS PubMed.
- (a) R. Breslow, Tetrahedron Lett., 1959, 1, 22–26 CrossRef; (b) C. Appayee and R. Breslow, J. Am. Chem. Soc., 2014, 136, 3720–3723 CrossRef CAS PubMed.
- (a) J. D. Sutherland, Angew. Chem., Int. Ed., 2016, 55, 104–121 CrossRef CAS PubMed; (b) J. D. Sutherland, Angew. Chem., Int. Ed., 2016, 55, 104–12194 CrossRef CAS PubMed.
- N. Friedmann and S. L. Miller, Science, 1969, 166, 766–767 CrossRef CAS PubMed.
- (a) C. Shen, L. Yang, S. Miller and J. Oró, Origins Life Evol. Biospheres, 1987, 17, 295–305 CrossRef CAS; (b) C. Shen, L. Yang, S. L. Miller and J. Oró, J. Mol. Evol., 1990, 31, 167–174 CrossRef CAS PubMed.
- (a) M. Barbieri, Biosystems, 2019, 181, 11–19 CrossRef CAS PubMed; (b) M. Barbieri, Biosystems, 2019, 185, 104024 CrossRef CAS PubMed.
- (a) S. W. Ragsdale and E. Pierce, Biochim. Biophys. Acta, 2008, 1784, 1873–1898 CrossRef CAS PubMed; (b) S. W. Ragsdale, Ann. N. Y. Acad. Sci., 2008, 1125, 129–136 CrossRef CAS PubMed.
- M. J. Warren and A. I. Scott, Trends Biochem. Sci., 1990, 15, 486–491 CrossRef.
- G. L. Holliday, J. M. Thornton, A. Marquet, A. G. Smith, F. Rebeille, R. R. Mendel, H. L. Schubert, A. D. Lawrence and M. J. Warren, Nat. Prod. Rep., 2007, 24, 972–987 RSC.
- G. Layer, J. Reichelt, D. Jahn and D. W. Heinz, Protein Sci., 2010, 19, 1137–1161 CrossRef CAS PubMed.
- G. Layer, M. Jahn, J. Moser and D. Jahn, ACS Bio Med Chem Au, 2022, 2, 196–204, DOI:10.1021/acsbiomedchemau.1c00061.
- (a) F. Blanche, B. Cameron, J. Crouzet, L. Debussche, D. Thibaut, M. Vuilhorgne, F. J. Leeper and A. R. Battersby, Angew. Chem., Int. Ed. Engl., 1995, 34, 383 CrossRef CAS; (b) J. R. Roth, J. G. Lawrence and T. A. Bobik, Annu. Rev. Microbiol., 1996, 50, 137 CrossRef CAS.
- M. J. Warren, E. Raux, H. L. Schubert and J. C. Escalante-Semerena, Nat. Prod. Rep., 2002, 19, 390 RSC.
- S. Gambarelli, F. Luttringer, D. Padovani, E. Mulliez and M. Fontecave, ChemBioChem, 2005, 6, 1960–1962 CrossRef CAS PubMed.
- (a) M. N. Price, A. M. Deutschbauer and A. P. Arkin, PLoS Genet., 2021, 17, e100934 Search PubMed; (b) D. Sean Froese, B. Fowler and M. R. Baumgartner, J. Inherited Metab. Dis., 2019, 42, 673–685 CrossRef PubMed.
- (a) M. J. Yebra and A. S. Bhagwat, Biochemistry, 1995, 34, 14752–14757 CrossRef CAS PubMed; (b) S. Kumar, X. Cheng, S. Klimasauskas, S. Mi, J. Posfai, R. J. Roberts and G. G. Wilson, Nucleic Acids Res., 1994, 22, 1–10 CrossRef CAS PubMed; (c) T. V. Mishanina, E. M. Koehn and A. Kohen, Bioorg. Chem., 2012, 43, 37–43 CrossRef CAS PubMed.
- E. Smith and H. J. Morowitz, The Origin and Nature of Life on Earth: the Emergence of the Fourth Geosphere, Cambridge University Press, New York, NY, 1st edn, 2016 Search PubMed.
Footnote |
| † Dedicated to Heinz G. Floss (Univ. Washington, Seattle, USA). |
| This journal is © The Royal Society of Chemistry 2022 |