Bismuth–nickel bimetal nanosheets with a porous structure for efficient hydrogen production in neutral and alkaline media†
Xueping
Yu‡
a,
Li
Qu‡
a,
Carmen
Lee
b,
Juan
Peng
 *a,
Qingyu
Yan
*a,
Qingyu
Yan
 *bc,
Hongcun
Bai
a and
Min
Yao
a
*bc,
Hongcun
Bai
a and
Min
Yao
a
aCollege of Chemistry and Chemical Engineering, State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering, Ningxia University, Yinchuan 750021, P. R. China. E-mail: pengjuan@nxu.edu.cn
bCenter for Programmable Materials, School of Materials Science and Engineering, Nanyang Technological University, Singapore 639798, Singapore. E-mail: alexyan@ntu.edu.sg
cInstitute of Materials Research and Engineering, A*STAR, 2 Fusionopolis Way, Innovis, #08-03, Singapore 138634, Singapore
First published on 14th October 2022
Abstract
Active and durable electrocatalysts are very important for efficient and economically sustainable hydrogen generation via electrocatalytic water splitting. A bismuth–nickel (Bi–Ni) bimetal nanosheet with a mesoporous structure was prepared via a self-template electrochemical in situ process. The Bi–Ni catalyst required overpotentials of 56 mV and 183 mV at 10 mA cm−2 for the hydrogen evolution reaction (HER), which were close to that of commercial Pt/C in 1.0 M KOH and 1.0 M PBS (pH 7.0), respectively. The electrocatalyst maintained a steady current density during 20 h electrolysis in 1.0 M KOH and 1.0 M PBS (pH 7.0). Density functional theory (DFT) indicated that the alloying effect could induce charge transfer from the Bi atom to Ni atom and thus modulate the d-band centre of Bi–Ni nanosheets, which could efficiently accelerate H* conversion and H2 desorption at the Ni active site. This promotes the HER kinetics. By adopting the Bi84.8Ni15.2 alloy as the cathode to establish a full-cell (IrO2∥Bi84.8Ni15.2) for water splitting in 1.0 M KOH, the required cell voltage was 1.53 V to drive 10 mA cm−2, which was lower than that of the IrO2∥Pt/C electrolyzer (1.64 V@10 mA cm−2).
1. Introduction
Due to its high energy density and environmentally friendly characteristics, hydrogen is an attractive and promising renewable energy substitute for fossil fuels.1 At present, most industrial hydrogen-generation methods are heavily reliant on fossil fuels, resulting in a large amount of CO2 emissions.2 Electrochemical water splitting stands out from the various hydrogen production strategies because it is clean, efficient, and can be coupled with renewable energy sources (solar, wind, etc.).3,4 Nowadays, electrochemical water splitting in an alkaline condition can meet the needs of industrial hydrogen production. Water electrolysis in a neutral electrolyte can potentially enable the direct usage of saltwater without the requirement for desalination.5 Additionally, the neutral hydrogen evolution reaction (HER) can also be combined with bio-processes for the production of solar fuels.6 However, the HER has sluggish reaction kinetics in alkaline and neutral circumstances, due to the high activation barrier for the dissociation of H2O molecules and the formation of H* (H2O + e− = H* + OH−).7 Therefore, it is meaningful to design HER electrocatalysts with high catalytic performance in both neutral and alkaline conditions.Bismuth nanosheets with a 2D nanostructure have been widely used in the field of electrocatalysis because of their unique physical characteristics.8 Norskov et al. predicted and verified that alloying bismuth with a noble metal (such as Pt) can effectively reduce ΔGH* and enhance the HER activity in acidic media, and that its activity is even better than that of platinum catalysts.9 However, the alloy includes a noble metal and is only used for acidic HER. Nowadays, although Pt is the most widely used catalyst in a pH-universal range, its high-cost and scarcity limit its scalable application.10,11 Therefore, it is necessary and challenging to design and develop bismuth-based non-noble metal catalysts with a wide pH range for HER. Alloying with a metal element in monophase catalysts can effectively tune the electronic structures and optimize the intermediates absorption energy of materials in HER or OER, and thus substantially improve the intrinsic catalytic properties.12–14 Notably, Ni atoms are broadly recognized as excellent water dissociation centres.15 Ni alloying/doping can play a role in strengthening the electrocatalytic activity and improving the catalytic efficiency of Ni-based catalysts by adjusting the electronic structure of the alloy's surface.15–19 Additionally, 2D porous structures feature a large surface area, abundant active sites,20,21 excellent gas-diffusion capacity and rapid electrode kinetics in the HER process.22,23 Most 2D porous electrocatalysts are prepared using porous templates24 or substrates.25,26 Nevertheless, 2D porous nanomaterials are still very rare as it is difficult to form pores directly in 2D nanostructures without a template or porous substrate.27
Herein, we demonstrate a simple self-templating strategy for the synthesis of 2D mesoporous Bi–Ni alloy via an electrochemical in situ process without using any template or substrate. Density functional theory (DFT) results suggested that the strong interaction between the surface Ni sites and OH, and between the Bi sites and H cause a breakage of the H–OH bonds (Volmer step: H2O + e− = H* + OH−) to facilitate H* formation. Subsequently, the adjacent surface Ni atoms exhibit a suitable H*-binding ability, which accelerates the H* conversion and H2 desorption (Heyrovsky step: H2O + e− + H* = H2 + OH−). This would improve the overall HER kinetics in both alkaline and neutral conditions. Furthermore, the 2D moseporous structure provided abundant active sites and accessible channels for electron transfer and mass transport. Therefore, the Bi–Ni alloy exhibited high activity and stability for HER. Here, the Bi–Ni alloy required overpotentials of 56 mV and 183 mV at 10 mA cm−2 for HER under both alkaline and neutral conditions, respectively, which were close to that of commercial Pt/C in 1.0 M KOH and 1.0 M PBS (pH 7.0). The Bi–Ni alloy was further applied as a cathode and coupled with an IrO2 anode for a full-cell in 1.0 M KOH. The required cell voltage was 1.53 V to drive 10 mA cm−2, which was lower than that of the IrO2∥Pt/C electrolyzer (1.64 V@10 mA cm−2).
2. Experimental
2.1 Chemicals and reagents
Bismuth nitrate pentahydrate (Bi(NO3)3·5H2O), disodium hydrogen phosphate dodecahydrate (Na2HPO4·12H2O), potassium hydroxide (KOH), sodium phosphate monobasic dihydrate (NaH2PO4·2H2O), and sodium hydroxide (NaOH) were acquired from Tianjin Damao Chemical Reagent Factory (China); glacial acetic acid (CH3COOH), sodium hydrogen carbonate (NaHCO3), and potassium iodide (KI) were purchased from Sinopharm (China); nickel sulfate heptahydrate (NiSO4·7H2O) was obtained from Xi'an Chemical Reagent Factory (China); Nafion solution was bought from Tianjin Incole Union Technology Co., Ltd (China); anhydrous sodium sulfate (Na2SO4) was acquired from Peking Chemical Reagent Company (China). All the reagents were used without any prior purification. DI water (18.25 MΩ cm) was used throughout the whole experiment process.2.2 Preparation of BiOI precursor
The precursor BiOI nanosheets were synthesized according to the reference.28 In short, 0.97 g of Bi(NO3)3·5H2O was added to 20 mL of glacial acetic acid aqueous solution (1.2 M) and the mixture was vigorously stirred for 20 min until completely dissolved. Then, 5 mL of KI aqueous solution (2.0 M) was added to the above mixture in a dropwise fashion. Subsequently, the pH of the mixed solution was adjusted to 6.0 using 1.0 M NaOH aqueous solution. The resulting brick-red solution was then stirred for another 30 min before transferring it into a 50 mL Teflon-lined autoclave. Finally, it was treated at 160 °C for 2 h. The final brick-red BiOI precursor was obtained by centrifugation and washed four times with DI water.2.3 Preparation of mesoporous Bi–Ni alloy nanosheets
The BiOI precursor was redispersed in 25 mL DI water. Four identical glassy carbon electrodes were prepared. Next, 5 μL of the dispersion liquid was dropped on each glassy carbon electrode (GCE), which will later be used as the working electrode. After natural drying, each working electrode was applied with 2 μL of 0.5% Nafion solution. To prepare four porous alloys samples (Bi86.5Ni13.5, Bi84.8Ni15.2, Bi80.1Ni19.9, Bi76.2Ni23.8), 15 cycles of CV in different concentrations of N2-saturated NiSO4 aqueous solution (0.01, 0.02, 0.03, and 0.04 M) were conducted within the potential window of −0.2 to −1.4 V vs. Ag/AgCl. During the reduction process, a graphite rod was used as the counter electrode. Afterwards, the working electrodes were removed and washed with DI water, followed by blow drying using nitrogen. Thus, the products of the mesoporous alloy were synthesized.2.4 Characterizations
The data for the transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM), energy dispersive X-ray spectroscopy (EDS), high angle annular dark field-scanning transmission electron microscopy (HAADF-STEM), and elemental mapping analyses for the samples were acquired using a TEM diffractometer (FEI Tecnai G2 F20) at 200 kV; scanning electron microscopy (SEM; Zeiss Sigma 300) was employed to obtain the morphologies of the catalysts; the crystal phases of the samples were obtained by X-ray diffraction (XRD; Smart Lab); the valence state of the elements on the surface of the samples was acquired by X-ray photoelectron spectrometry (XPS; Themofisher 250 XI); the atom ratio of the alloy was determined by inductively coupled plasma-optical emission spectrometry (ICP-OES; Agilent ICPOES 730); Brunauer–Emmett–Teller (BET) surface area experiments were conducted using a Micromeritics Surface Area analyzer (ASAP-2460); contact angle experiments were carried out on contact angle measuring instrument (SZ-CAMB3).2.5 Electrochemical characterizations
The electrochemical performance data of the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) were collected from a standard three-electrode set-up using a CHI 760D electrochemical analyzer (Chenhua, Shanghai). The fresh electrocatalysts, graphite rod, and saturated calomel electrode (SCE) were adopted as the working electrodes, counter electrode, and reference electrode, respectively. Polarization curves were conducted in a N2-saturated 1.0 M electrolyte (PBS or KOH) at a scan rate of 5 mV s−1 with IR compensation. All the potentials were calculated with reference to the RHE (reversible hydrogen electrode) by adopting the formula: E(V vs. RHE) = E(V vs. SCE) + 0.242 + 0.0592pH. IR correction was obtained using the formula: Ecorr = Emea − iRS (where Ecorr is the corrected potential, Emea is the measured potential, and Rs is the electrolyte resistance). The Rs value was obtained from the x-intercept of the Nyquist plots in the high-frequency region, which was then used for IR correction. Cyclic voltammetry (CV) was conducted in a 0.1 V narrow potential window at different scan rates. The current density differences of the forward and reverse scans (Janodic − Jcathodic) at 0.256 V vs. RHE (1.0 M PBS) and 0.441 V vs. RHE (1.0 M KOH) were plotted against the scan rates. The corresponding slope of the plot was two times the double-layer capacitance (Cdl). The Cdl was applied to calculate the electrochemically surface area (ECSA), while the ECSA was subsequently used to calculate the roughness factor of each sample (detailed calculation processes can be found in the ESI†). For the CV analysis, 1000 cycles of CV in the range of −0.5 to −0.8 V vs. SCE with a scan rate of 50 mV s−1 and i–t curves were used to measure the stability for hydrogen generation.The overall water splitting performance of the Bi–Ni alloy was also investigated using the polarization curve obtained from the two-electrode set-up in 1.0 M KOH electrolyte, where the carbon paper (CP) supporting the Bi–Ni alloy was adopted as the cathode and anode. Prior to electrochemical deposition, the CP substrate (1 × 2 cm2) was treated using acetone, ethanol, and deionized water followed by ultrasonic cleaning for 30 min. The Bi84.8Ni15.2 electrode was then obtained through the same electrochemical deposition method on the treated CP substrate (the coating area was 1.5 cm2, and the side without the sample was sealed using transparent tape). The stability was investigated by performing an i–t study at a fixed potential. To better compare with noble metal catalysts, 20% Pt/C and IrO2 catalysts on CP were prepared as follows: 20% Pt/C or IrO2 catalyst (8.5 mg) was dispersed in 1 mL solution comprising 750 μL DI water and 250 μL ethanol by ultrasonic treatment for about 10 min. Next, 80 μL of the ink was coated on the CP surface. After air drying, 10 μL Nafion solution (0.5 wt%) was added to the catalyst surface, thus yielding two work-electrodes with a catalyst loading of 0.45 mg cm−2 (20% Pt/C or IrO2).
2.6 DFT theoretical calculation
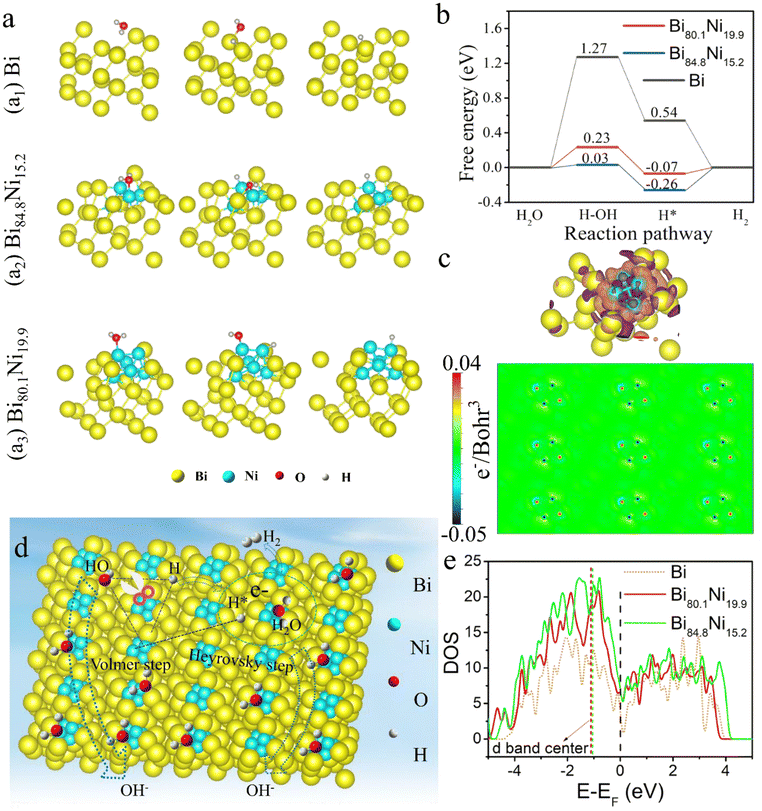
DFT was implemented in the Vienna Ab initio simulation package (VASP), which was adopted to optimize the geometric configuration for the Bi and Bi–Ni alloy.29,30 Exchange–correlation interactions were represented using the generalized gradient approximation (GGA)31 in the form of the Perdew–Burke–Ernzerhof functional (PBE).32 A 500 eV cut-off energy for plain-wave basis sets was employed. The convergence thresholds for the energy and force were 10−5 eV and 5 × 10−3 eV Å−1 respectively. Adopting the experience correction of Grimme's scheme,33 the weak interaction was expressed through DFT+D3 ways. The vacuum space setting was greater than 20 Å, which was sufficient to prevent the interaction between periodic images. For the (110) surface, a four-layer structure with 3 × 3 × 1 K-point mesh was employed during the calculation. To mimic the real experimental ratio of Ni dopants, four and six Ni cluster structures were built and put on the Bi (110) surface for the Bi80.1Ni19.9 and Bi84.8Ni15.2 models to describe the catalytic interface of the samples. Norskov et al. proposed some descriptors, which were adopted to reflect the hydrogen-generation activity on the catalyst surface. Among the descriptors, ΔGH* was a crucial parameter to determine the HER activity.34 The change in the reaction's Gibbs free energy (ΔG) of each basic step was in view of the calculated hydrogen electrode model. It can be calculated with the formula| ΔG = ΔE + ΔZPE − TΔS |
3. Results and discussion
3.1 Characterization of the mesoporous Bi–Ni alloy nanosheets
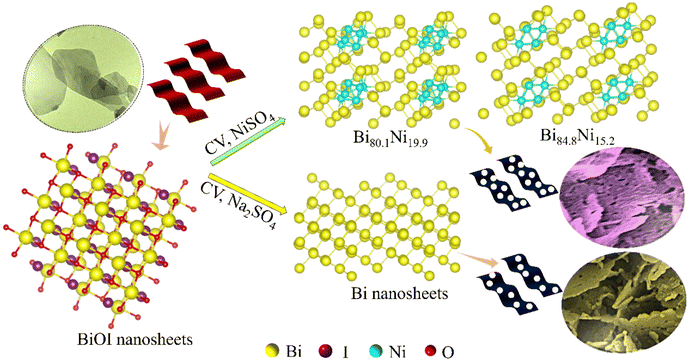
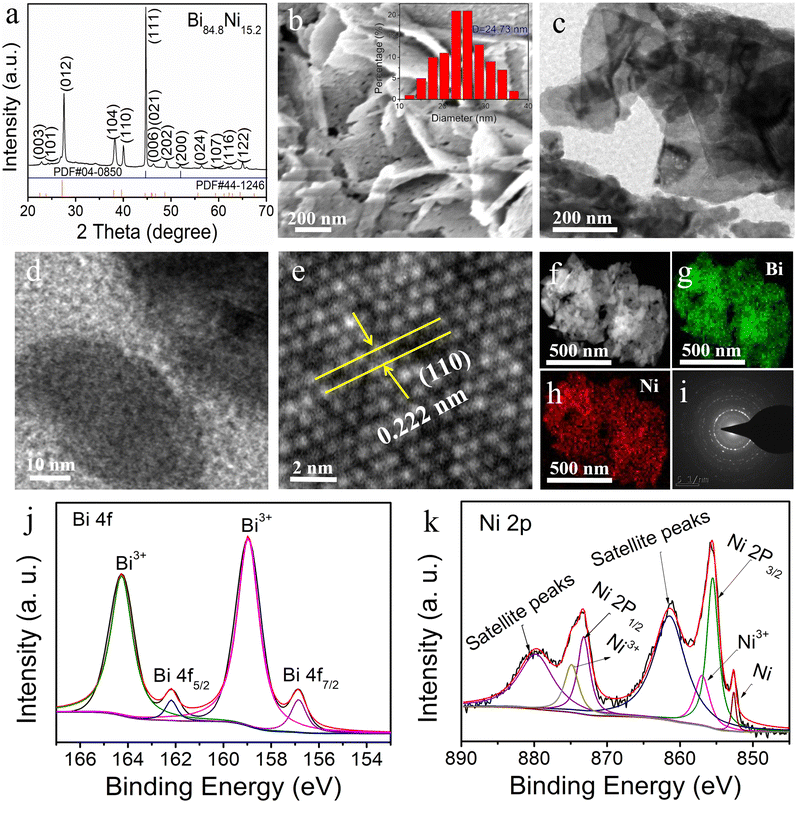
Herein, mesoporous Bi–Ni alloy and Bi nanosheets were prepared by the electrochemical reduction of BiOI precursor in N2-saturated NiSO4 and Na2SO4 electrolyte, respectively. The synthesis procedure is illustrated in Scheme 1. Inductively coupled plasma-optical emission spectrometry (ICP-OES) analysis exhibited the atomic ratio of Bi to Ni for the four alloy samples (Table S1†). Thus, these four alloy products were named Bi86.5Ni13.5, Bi84.8Ni15.2, Bi80.1Ni19.9, and Bi76.2Ni23.8, respectively. The diffraction peaks of BiOI nanosheets were consistent with the tetragonal crystalline, corresponding to the standard card (PDF#10-0445) (Fig. S1a†). After electrochemical reduction, the diffraction peaks of Bi84.8Ni15.2 were slightly shifted relative to pure Bi (PDF#44-1246) and pure Ni (PDF#04-0850) (Fig. 1a). This confirmed the successful preparation of the alloy.35,36 Furthermore, the smaller Ni atoms incorporated in the Bi lattice could induce lattice shrinkage and produce compressive lattice strain. Table S2† shows that the lattice strain of the Bi84.8Ni15.2 alloy was downregulated by 2.20% compared to pure Bi. Compressive strain in the Bi–Ni alloy could lead to a better interaction between the catalyst and the reactive intermediates of H and OH. Hence, the overall hydrogen-generation activity could be enhanced.37The SEM image displayed a uniform and smooth nanosheets structure of the BiOI sample (Fig. S1b†). On the other hand, the Bi84.8Ni15.2 alloy comprised nanosheets with abundant mesopores, which can be seen from the SEM image in Fig. 1b. The average pore size was about 24.73 nm, which corresponded to the mesopore range. Additionally, the BET surface area of the mesoporous Bi84.8Ni15.2 alloy was 43.49 m2 g−1 (Fig. S2†). The high surface area of the Bi84.8Ni15.2 alloy could be attributed to the mesopores. Such a high surface area is beneficial for the exposure of more active sites, which in turn would support a desirable electrocatalytic performance. To explore the formation mechanism of the mesopores, various electrolyte solutions were investigated in the alloy preparation process. No pore to very few pores were observed on the nanosheets when NaHCO3 or Na2SO4 aqueous solution was used as the electrolyte in the electrodeposition process (Fig. S3†). However, a large number of pores were formed when NiSO4 aqueous solution was used as the electrolyte during electrodeposition (Fig. 1b). The formation of the pores may be attributed to the Kirkendall effect, where the pores are produced due to the different diffusion rates of Bi and Ni atoms during the electrochemical reduction process.38
The TEM images of Bi84.8Ni15.2 show that the alloy was made up of 2D nanosheets (Fig. 1c and d). From the high-resolution TEM (HRTEM) images of Bi84.8Ni15.2 alloy (Fig. 1e), the lattice spacing of the (110) crystal face was approximately 0.222 nm. Compared to the (110) crystal face of Bi (0.227 nm; Fig. S4†), the lattice spacing decreased when Ni atoms were incorporated. Hence, this demonstrated the existence of compressive strain in the Bi84.8Ni15.2 alloy, which was consistent with X-ray diffractometer (XRD) result. Furthermore, both the HAADF-STEM image (Fig. 1f) and EDS mapping images (Fig. 1g and h) showed that the Bi and Ni atoms were homogeneously distributed throughout the Bi84.8Ni15.2 surface. The EDS spectrum (Fig. S5a†) showed that the Bi84.8Ni15.2 consisted of Ni and Bi elements, while the Cu peak was ascribed to the copper base. The ratio of Bi to Ni was close to the ICP-OES analysis. The selected area electron diffraction (SAED) image showed that the Bi84.8Ni15.2 alloy was polycrystalline (Fig. 1i).
The Bi 4f spectrum presented the oxidation state of Bi3+ (164.3 and 159.0 eV) and metallic state of Bi0 (156.9 and 162.2 eV).39 The oxidation state (Bi3+) may have arisen due to the exposure to air (Fig. 1j). The binding energies at 855.6 (Ni 2p3/2) and 873.2 eV (Ni 2p1/2), corresponding to satellite peaks of 861.2 and 879.7 eV in Fig. 1k, could be ascribed to the presence of Ni2+, while the peaks at 874.8 and 856.8 eV belonged to the Ni3+.40 The Ni 2p3/2 signal of porous Bi84.8Ni15.2 alloy was deconvoluted into a peak at 852.6 eV, stemming from metallic Ni0.41,42 Furthermore, XPS analysis (Fig. S5b†) showed that the Bi3+ for the Bi84.8Ni15.2 alloy and Bi80.1Ni19.9 alloy peaks were shifted to lower binding energies compared with pure Bi. This phenomenon indicated the presence of electron transfer in the Bi–Ni alloy.43 Meanwhile, the related description and characterization of the mesoporous Bi80.1Ni19.9 alloy are also provided in Fig. S6 and S7.†
3.2 Electrocatalytic HER of mesoporous Bi–Ni alloy nanosheets in alkaline electrolyte
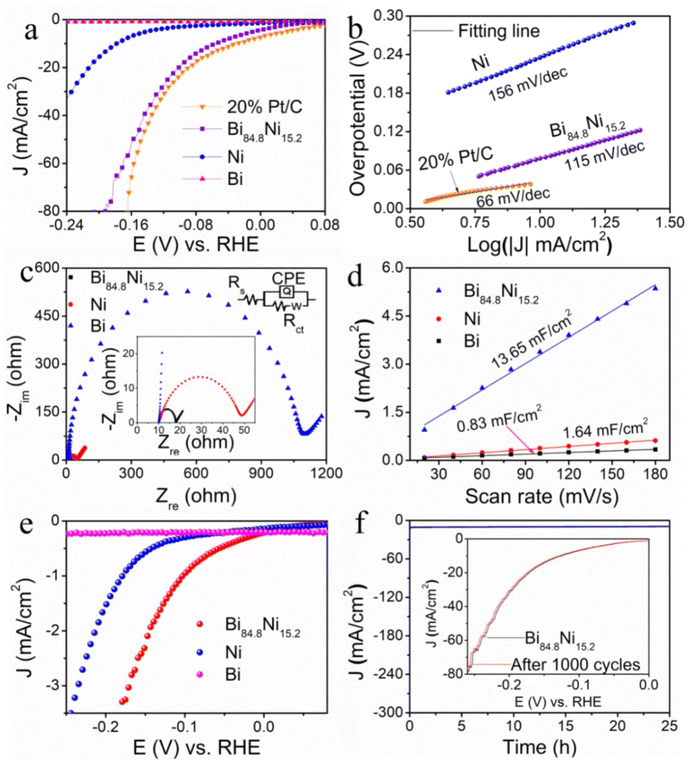
As shown in Fig. 2a, the Bi nanosheet showed almost no activity, while Ni showed relatively weak activity. On the other hand, the mesoporous Bi84.8Ni15.2 alloy exhibited prominent HER activity, in which it only required an overpotential of 56 mV to reach a current density of 10 mA cm−2 in 1.0 M KOH. Note that the activity of Bi84.8Ni15.2 alloy towards HER was comparable to the benchmark 20% Pt/C. Moreover, the mesoporous Bi84.8Ni15.2 outperformed most reported bimetallic electrocatalysts (Table S3†). The high activity of Bi84.8Ni15.2 alloy could be attributed to the formation of abundant active sites,44 numerous mesopores, and a compressive strain effect31 on the surface of the catalyst. The effects of having different Ni contents in Bi–Ni alloy on the HER activity in alkaline electrolyte were also investigated (Fig. S8†). The alloy sample had a ratio of Bi to Ni of about 84.8/15.2, which presented the best hydrogen-generation activity. Furthermore, the compressive strain of the alloy Bi84.8Ni15.2 was smaller than that of Bi80.1Ni19.9 (Bi to Ni ratio of 80.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.9) (Table S2 in the ESI†). The weak compression strain may lead to a strong interaction between the alloy electrocatalysts and the intermediate H and OH,37 thus resulting in the high activity of Bi84.8Ni15.2 in alkaline media (Fig. S8 in the ESI†). Tafel slopes were acquired using the Tafel equation: η = a + b
19.9) (Table S2 in the ESI†). The weak compression strain may lead to a strong interaction between the alloy electrocatalysts and the intermediate H and OH,37 thus resulting in the high activity of Bi84.8Ni15.2 in alkaline media (Fig. S8 in the ESI†). Tafel slopes were acquired using the Tafel equation: η = a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log|j| (where η is the applied overpotential, b is the Tafel slope, and j is the current density).45Fig. 2b shows that the Tafel slope of porous Bi84.8Ni15.2 alloy was 115 mV dec−1, which was lower than that of Ni (156 mV dec−1). Thus, this indicated the rapid HER kinetics of Bi84.8Ni15.2 alloy and that the Volmer–Heyrovsky mechanism was followed during HER.46 In Fig. 2c, the charge-transfer resistance (Rct) of porous Bi84.8Ni15.2 alloy was 7.9 Ω, which was obviously smaller than those of Bi (1090.8 Ω) and Ni (38.6 Ω), thus indicating that the charge-transfer kinetics in the HER process was much faster. In addition, the electrochemical surface area (ECSA) is employed to estimate the intrinsic activity of electrocatalysts.47 The double-layer capacitance (Cdl) values (Fig. 2d) were 13.65, 1.64, and 0.83 mF cm−2 for mesoporous Bi84.8Ni15.2 alloy, pure Ni, and pure Bi according to the above CVs (Fig. S9†). The corresponding ECSA values were 23.89, 2.87, and 1.45 cm2 and the roughness factors (RF) were 341.29, 41.00, and 20.71 (calculation process in the ESI†). These results suggest that the mesoporous Bi84.8Ni15.2 alloy had a larger electrochemical active surface area and rough surface compared to pure Bi and pure Ni. The RF of the mesoporous Bi84.8Ni15.2 alloy was about 16 times more than that of the Bi nanosheets, which was ascribed to the formation of abundant mesopores. Additionally, the catalytic efficiency of the obtained samples was evaluated by normalization of the raw current using the ECSA. As shown in Fig. 2e, the mesoporous Bi84.8Ni15.2 showed the best catalytic efficiency for HER. With the increase in overpotential, the turnover frequency (TOF) value of mesoporous Bi84.8Ni15.2 alloy was higher than that of pure Ni. This suggested a higher catalytic efficiency of mesoporous Bi84.8Ni15.2 alloy during the HER process (Fig. S10a†). Furthermore, the contact angles data in Fig. S11† present that Bi84.8Ni15.2 and Bi80.1Ni19.9 alloys and Bi nanosheets had angles of about 63°, 75.5°, and 90° after water-drop diffusion at the same time, respectively. These results demonstrate that the hydrophilicity of Bi–Ni alloy was significantly higher than pure Bi, which is conducive for the adsorption of H2O molecules. The contact angle of Bi84.8Ni15.2 alloy was smaller than that of Bi80.1Ni19.9 alloy. This phenomenon may be caused by the higher oxophilicity of Bi84.8Ni15.2 alloy than that of Bi80.1Ni19.9 alloy. The above-mentioned facts clearly indicate that the excellent catalytic activity of Bi–Ni alloys may be attributed to the mesoporous 2D nanosheet structure and the alloy effect.
log|j| (where η is the applied overpotential, b is the Tafel slope, and j is the current density).45Fig. 2b shows that the Tafel slope of porous Bi84.8Ni15.2 alloy was 115 mV dec−1, which was lower than that of Ni (156 mV dec−1). Thus, this indicated the rapid HER kinetics of Bi84.8Ni15.2 alloy and that the Volmer–Heyrovsky mechanism was followed during HER.46 In Fig. 2c, the charge-transfer resistance (Rct) of porous Bi84.8Ni15.2 alloy was 7.9 Ω, which was obviously smaller than those of Bi (1090.8 Ω) and Ni (38.6 Ω), thus indicating that the charge-transfer kinetics in the HER process was much faster. In addition, the electrochemical surface area (ECSA) is employed to estimate the intrinsic activity of electrocatalysts.47 The double-layer capacitance (Cdl) values (Fig. 2d) were 13.65, 1.64, and 0.83 mF cm−2 for mesoporous Bi84.8Ni15.2 alloy, pure Ni, and pure Bi according to the above CVs (Fig. S9†). The corresponding ECSA values were 23.89, 2.87, and 1.45 cm2 and the roughness factors (RF) were 341.29, 41.00, and 20.71 (calculation process in the ESI†). These results suggest that the mesoporous Bi84.8Ni15.2 alloy had a larger electrochemical active surface area and rough surface compared to pure Bi and pure Ni. The RF of the mesoporous Bi84.8Ni15.2 alloy was about 16 times more than that of the Bi nanosheets, which was ascribed to the formation of abundant mesopores. Additionally, the catalytic efficiency of the obtained samples was evaluated by normalization of the raw current using the ECSA. As shown in Fig. 2e, the mesoporous Bi84.8Ni15.2 showed the best catalytic efficiency for HER. With the increase in overpotential, the turnover frequency (TOF) value of mesoporous Bi84.8Ni15.2 alloy was higher than that of pure Ni. This suggested a higher catalytic efficiency of mesoporous Bi84.8Ni15.2 alloy during the HER process (Fig. S10a†). Furthermore, the contact angles data in Fig. S11† present that Bi84.8Ni15.2 and Bi80.1Ni19.9 alloys and Bi nanosheets had angles of about 63°, 75.5°, and 90° after water-drop diffusion at the same time, respectively. These results demonstrate that the hydrophilicity of Bi–Ni alloy was significantly higher than pure Bi, which is conducive for the adsorption of H2O molecules. The contact angle of Bi84.8Ni15.2 alloy was smaller than that of Bi80.1Ni19.9 alloy. This phenomenon may be caused by the higher oxophilicity of Bi84.8Ni15.2 alloy than that of Bi80.1Ni19.9 alloy. The above-mentioned facts clearly indicate that the excellent catalytic activity of Bi–Ni alloys may be attributed to the mesoporous 2D nanosheet structure and the alloy effect.
Stability is also an essential criterion for the practical application of a catalyst. In Fig. 2f, the mesoporous Bi84.8Ni15.2 alloy still maintained its initial current density for 25 h or 1000 cycles of CV electrolysis. Both the morphology and lattice spacing corresponding to the (110) crystal face of the mesoporous Bi84.8Ni15.2 alloy (Fig. S12a–c†) could still be observed after the long-term electrolysis. In Fig. S12d,† all the XRD peaks of the mesoporous Bi84.8Ni15.2 alloy remained unchanged after electrolysis, and no new peaks appeared. The high-resolution XPS analysis (Fig. S12e and f†) also revealed the chemical states of Ni and Bi did not change after continuous electrolysis. All the above results suggest that mesoporous Bi84.8Ni15.2 alloy has outstanding HER stability in alkaline electrolyte.
3.3 Electrocatalytic HER of mesoporous Bi–Ni alloy nanosheets in neutral electrolyte
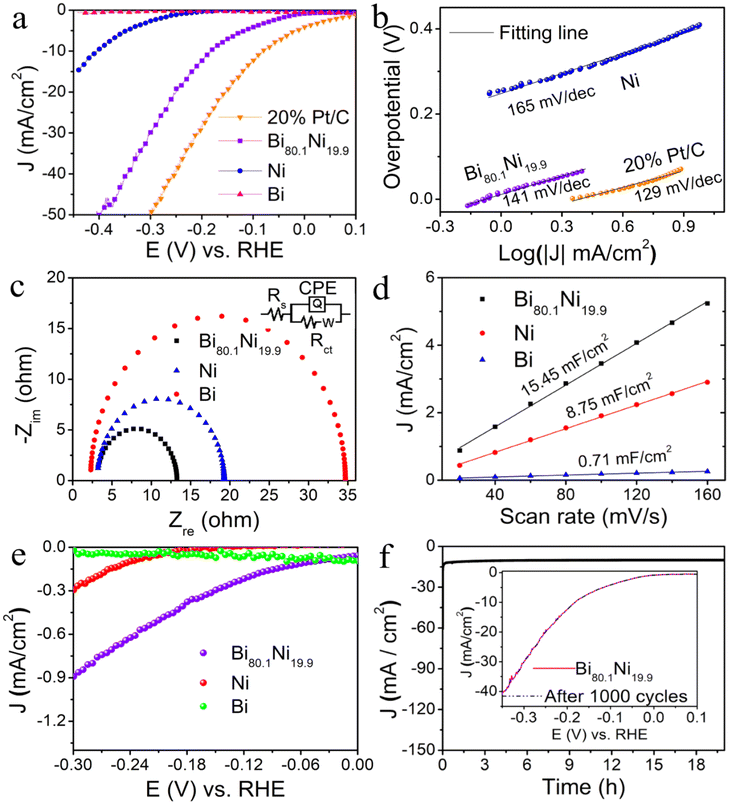
Fig. 3a clearly shows that the mesoporous Bi80.1Ni19.9 alloy needed a lower overpotential (183 mV vs. RHE@10 mA cm−2) than pure Bi and pure Ni, while the activity was inferior to mercantile 20% Pt/C. Furthermore, the HER activities of the Bi–Ni alloys with different Ni contents were also investigated in a neutral medium (Fig. S13†), in which mesoporous Bi80.1Ni19.9 alloy exhibited the optimal activity. The Tafel slope of mesoporous Bi80.1Ni19.9 alloy was 141 mV dec−1 (Fig. 3b), which indicated faster kinetic characteristics than pure Ni (165 mV dec−1).48,49 The mesoporous Bi80.1Ni19.9 alloy had the lowest Rct (Fig. 3c), and this suggested that the mesopores and the Ni incorporation into the Bi lattice are conducive for the enhancement of the charge-transfer kinetics of Bi80.1Ni19.9 alloy towards HER. The Cdl values in Fig. 3d were obtained using CVs (Fig. S14†) at variable scanning rates.50 The ECSA values of mesoporous Bi80.1Ni19.9 alloy, pure Ni, and pure Bi were calculated to be 27.04, 15.31, and 1.24 cm2, respectively, and the corresponding RF values were also calculated to be 386.29, 218.71, and 17.71 (ESI†). The high ECSA and RF of Bi80.1Ni19.9 alloy may be attributed to the abundant mesopores on the surface of the alloy, which is good for exposing more active sites, thus enhancing the HER activity. The specific activity in Fig. 3e shows that the mesoporous Bi80.1Ni19.9 alloy presented the best intrinsic catalytic activity. In Fig. S10b,† the TOF value of mesoporous Bi80.1Ni19.9 alloy was higher than that of pure Ni, which suggests that the mesoporous Bi80.1Ni19.9 alloy had higher catalytic efficiency than pure Ni in 1.0 M PBS (pH 7.0) for HER.In Fig. 3f, it can be seen that the mesoporous Bi80.1Ni19.9 catalyst could preserve its activity during the 20 h electrolysis or 1000 cycles of CV. The morphology of Bi80.1Ni19.9 alloy was also retained after long-term electrolysis, as exhibited in Fig. S15a and b.† The lattice spacing of Bi80.1Ni19.9 alloy corresponding to the (110) crystal face remained unchanged (Fig. S15c†), and the polycrystalline property of the mesoporous Bi80.1Ni19.9 alloy still existed (Fig. S15d†). Also, the phase of Bi80.1Ni19.9 alloy remained unchanged during electrolysis which was evident in the XRD patterns (Fig. S15e†). The EDS spectrum (Fig. S15f†) also showed that mesoporous Bi80.1Ni19.9 alloy still had the same elements before and after electrolysis. All the above results indicate that the mesoporous Bi80.1Ni19.9 alloy has excellent stability in neutral electrolyte for HER.
3.4 Mechanism of mesoporous Bi–Ni alloy nanosheets towards HER
To understand the mechanism of Bi–Ni alloys on the HER activity, DFT was adopted to calculate the Gibbs free energy of each step for HER in neutral and alkaline conditions. The Bi (110) and Bi–Ni alloy (110) surfaces were used for the catalysts’ theoretical models (Fig. S16†). The optimal structures at each reaction stage of Bi, Bi84.8Ni15.2, and Bi80.1Ni19.9 are shown in Fig. 4a. The Gibbs free energy of each step for HER in neutral and alkaline conditions are displayed in Fig. 4b. The dissociation energies of a water molecule on Bi80.1Ni19.9 and Bi84.8Ni15.2 surfaces were 0.23 and 0.03 eV, respectively, which were obviously smaller than that of pure Bi (1.27 eV). This shows that Ni alloying plays a crucial role in accelerating H2O dissociation and improving H* formation. Regardless of the mechanistic route, the HER always entails the formation of M–H* intermediates. Theoretically, the closer it is to zero |ΔGH*|, the higher the HER activity. A large and negative ΔGH* value represents a strong binding of H with the catalyst. The H* is firmly adsorbed on the catalyst surface. However, as H* is difficult to release from the catalyst surface, this hinders the subsequent process of the H2 generation. Conversely, a large and positive ΔGH* value indicates a weak adsorption of H on the catalyst surface, which limits the conversion rate of the H*.51,52 Therefore, ΔGH* ≈ 0 implies an optimal adsorption and desorption balance between the catalyst surface and H. The H* could be converted to H2 on the Bi80.1Ni19.9 and Bi84.8Ni15.2 alloy surfaces, with obtained ΔGH* values of only −0.07 and −0.26 eV, respectively, which were lower than for the Bi nanosheets (0.54 eV). Here, due to the unique oxophilicity of the local positive charged and unfilled d-orbital of the Ni ions produced by the oxidation of Ni, the OH from the dissociation of water molecules prefer to adsorb on the surface Ni sites.53 Meanwhile, the H prefer to adsorb on Bi sites due to the comparable electronegativity.54 Consequently, the dissociation step of water molecules is accelerated (Volmer step: H2O + e− = H* + OH−). The generated OH− desorbs from the surface Ni sites and enters the electrolyte solution. Then, one H* can recombine with one H2O molecule and one electron to produce H2 at the adjacent surface Ni atom (Heyrovsky step: H2O + e− + H* = H2 + OH−). This is because the electrons are transferred from Bi atom to Ni atom (Fig. 4c, Fig. S17a,† and Fig. 4d). It can be concluded that the alloying effect can regulate the electronic structure of the catalyst surface, thus facilitating the dissociation of a water H2O molecule and optimized ΔGH*. The ΔGH* values of Bi84.8Ni15.2 and Bi80.1Ni19.9 were different, which suggested the content of Ni has a considerable influence on the HER activity (Fig. S8 and S13†).The density of states (DOS; Fig. 4e) indicated that the d-band centre of Ni was shifted upward on Bi84.8Ni15.2 and Bi80.1Ni19.9 compared with pure Bi, suggesting Ni had a more electron-rich state. Meanwhile, in Fig. S17b–d,† compared with pure Bi, the DOS of Bi84.8Ni15.2 and Bi80.1Ni19.9 alloys orbital increased a lot at the Fermi level, resulting in more free electrons on the Ni sites. This phenomenon not only enhanced the metallic character and electrical conductivity of Bi–Ni alloy but also decreased the excessive binding effect of H on alloy surface, which boosted the HER activity.55 These results were consistent with the EIS analysis (Fig. 2c and 3c) and polarization curves (Fig. 2a and 3a).
3.5 Electrocatalytic overall water splitting performance of mesoporous Bi–Ni alloy
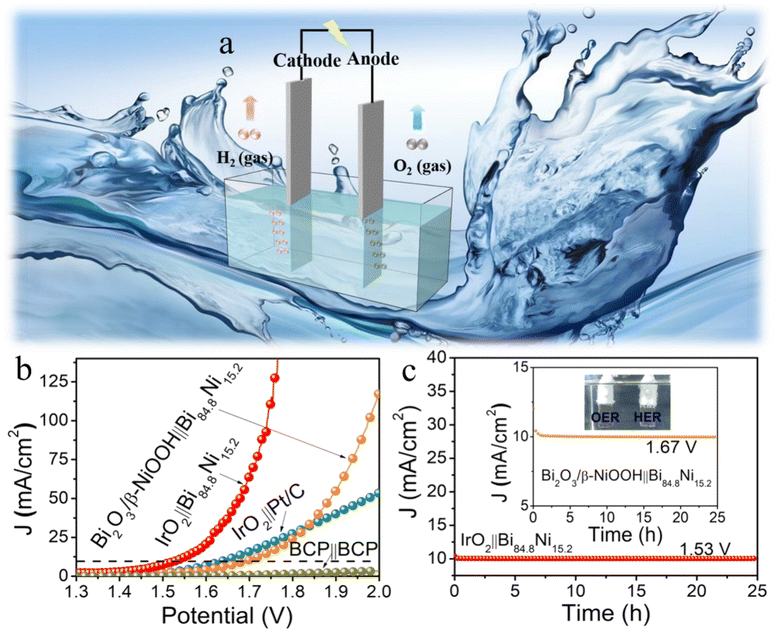
Driven by the exciting HER activity of mesoporous Bi84.8Ni15.2 alloy, a two-electrode water splitting full-cell (Fig. 5a) was constructed using Bi84.8Ni15.2 alloy as the cathode and IrO2 as the anode in 1.0 M KOH (IrO2∥Bi84.8Ni15.2). This exhibited a lower voltage (1.53 V@10 mA cm−2) than IrO2∥Pt/C (1.64 V @10 mA cm−2) (Fig. 5b) because of the rapid kinetics and bubble-diffusion procedures (inset of Fig. 5c and video S1†). The higher activity of IrO2∥Bi84.8Ni15.2 was possibly due to the stronger oxophilicity of Ni, which was caused by the local positive charged and unfilled d-orbital of the Ni ions.53,56 The OH from the dissociation of water molecules prefer to adsorb on the surface Ni sites, which makes it easy to accelerate the dissociation of water molecules in the alkaline media. Thus, the IrO2∥Bi84.8Ni15.2 system needed a low cell voltage of 1.53 V at the current density of 10 mA cm−2 in 1.0 M KOH. The oxygen evolution reaction (OER) performance of Bi84.8Ni15.2 alloy was further studied in 1.0 M solutions (PBS [pH 7.0] and KOH). The detailed information and relevant figures (Fig. S18–S22†) can be found in the ESI.† The results indicate that the real active substance Bi2O3/β-NiOOH possessed the best OER activity, with an overpotential of 479 mV@50 mA cm−2 and a low Tafel slope of 74 mV dec−1 in 1.0 M KOH (Fig. S19a and b†). The overall water splitting of Bi2O3/β-NiOOH∥Bi84.8Ni15.2 electrolyzer needed 1.67 V cell voltage to reach a current density of 10 mA cm−2 (Fig. 5b). The current density for water electrolysis using the IrO2∥Bi84.8Ni15.2 system outperformed that of Bi2O3/β-NiOOH∥Bi84.8Ni15.2 at the cell voltage of 1.5–2.0 V. This performance could be explained as follows. As shown in Fig. S19a (ESI†), the current response of Bi2O3/β-NiOOH was produced by both the water oxidation and the oxidation of Bi84.8Ni15.2 to Bi2O3/β-NiOOH at 1.6–1.8 V (vs. RHE). While using IrO2 as the anode, the current was only generated by the water oxidation. The Bi84.8Ni15.2 oxidation occurred at the cell voltage of 1.13 V in the two-electrode system (Bi2O3/β-NiOOH∥Bi84.8Ni15.2 pair) (Fig. S19e, ESI†). However, the current was provided only by the water oxidation at the cell voltage of 1.5–2.0 V. Thus, the current response of the electrolysis system (IrO2∥Bi84.8Ni15.2) was higher than that of Bi2O3/β-NiOOH∥Bi84.8Ni15.2 at the cell voltage of 1.5–2.0 V.Durability is also a key criterion for evaluating an electrolytic cell.57 The constant current response of IrO2∥Bi84.8Ni15.2 and Bi2O3/β-NiOOH∥Bi84.8Ni15.2 electrolyzers were examined by recording i–t tests for 25 h electrolysis in the same electrolyte (Fig. 5c). However, the current density of IrO2∥Pt/C declined significantly with the prolongation of time (Fig. S23†). This result is in agreement with the reported literature.58
4. Conclusions
In summary, as-fabricated 2D porous Bi–Ni alloy displayed a high intrinsic HER activity under both alkaline and neutral conditions, as well as exceptional long-time stability over 20 h of continuous electrolysis. DFT showed that the alloying effect reduced the ΔGH* value and also increased the electron density near the Fermi level of the Bi–Ni alloy. The alloying effect between Bi and Ni regulated the electronic structure, thus improving the intrinsic activity of Bi–Ni catalyst. The activity of IrO2∥Bi84.8Ni15.2 towards overall water splitting also outperformed the commercial IrO2∥Pt/C. This work shows that the incorporation of Ni element into the Bi-based catalyst could significantly boost the HER performance, which will be conducive for the development of economical and efficient electrocatalysts for energy conversion.Conflicts of interest
The authors declare that there is no competing financial interest.Acknowledgements
The authors gratefully acknowledge the National Natural Science Foundation of China (No. 22262027), and financial support from Singapore MOE Tier 1 2020-T1-001-031. This work was also supported by National First-rate Discipline Construction Project of Ningxia (NXYLXK2017A04), Ningxia Natural Science Foundation (No. 22132003), and the College Students’ Innovative and Entrepreneurship Training Program of Ningxia University, China (No. G2021107490015).References
- T. H. Wang, L. Tao, X. R. Zhu, C. Chen, W. Chen, S. Q. Du, Y. Y. Zhou, B. Zhou, D. D. Wang, C. Xie, P. Long, W. Li, Y. Y. Wang, R. Chen, Y. Q. Zou, X. Z. Fu, Y. F. Li, X. F. Duan and S. Y. Wang, Nat. Catal., 2021, 5, 66–73 CrossRef.
- R. Subbaraman, D. Tripkovic, K. C. Chang, D. Strmcnik, A. P. Paulikas, P. Hirunsit, M. Chan, J. Greeley, V. Stamenkovic and N. M. Markovic, Nat. Mater., 2012, 11, 550–557 CrossRef CAS PubMed.
- C. L. Hu, L. Zhang and J. L. Gong, Energy Environ. Sci., 2019, 12, 2620–2645 RSC.
- J. D. Holladay, J. Hu, D. L. King and Y. Wang, Catal. Today, 2009, 139, 244–260 CrossRef CAS.
- C. T. Dinh, A. Jain, F. P. G. de Arquer, P. De Luna, J. Li, N. Wang, X. L. Zheng, J. Cai, B. Z. Gregory, O. Voznyy, B. Zhang, M. Liu, D. Sinton, E. J. Crumlin and E. H. Sargent, Nat. Energy, 2019, 4, 107–114 CrossRef CAS.
- J. Staszak-Jirkovsky, C. D. Malliakas, P. P. Lopes, N. Danilovic, S. S. Kota, K. C. Chang, B. Genorio, D. Strmcnik, V. R. Stamenkovic, M. G. Kanatzidis and N. M. Markovic, Nat. Mater., 2016, 15, 197–203 CrossRef CAS PubMed.
- J. M. Wei, M. Zhou, A. C. Long, Y. M. Xue, H. B. Liao, C. Wei and Z. C. J. Xu, Nano-Micro Lett., 2018, 10, 75–89 CrossRef CAS.
- N. Han, Y. Wang, H. Yang, J. Deng, J. H. Wu, Y. F. Li and Y. G. Li, Nat. Commun., 2018, 9, 1320 CrossRef PubMed.
- J. Greeley, T. F. Jaramillo, J. Bonde, I. B. Chorkendorff and J. K. Norskov, Nat. Mater., 2006, 5, 909–913 CrossRef CAS PubMed.
- X. Q. Mu, J. N. Gu, F. Y. Feng, Z. Y. Xiao, C. Y. Chen, S. L. Liu and S. C. Mu, Adv. Sci., 2021, 8, 2002341 CrossRef CAS.
- H. J. Huang, D. S. Yu, F. Hu, S. C. Huang, J. N. Song, H. Y. Chen, L. L. Li and S. J. Peng, Angew. Chem., Int. Ed., 2022, 61, 202116068 Search PubMed.
- Y. Wang, B. Kong, D. Y. Zhao, H. T. Wang and C. Selomulya, Nano Today, 2017, 15, 26–55 CrossRef CAS.
- F. Lv, B. Huang, J. Feng, W. Zhang, K. Wang, N. Li, J. Zhou, P. Zhou, W. Yang, Y. Du, D. Su and S. Guo, Natl. Sci. Rev., 2021, 8, nwab019 CrossRef CAS PubMed.
- J. N. Song, S. Y. Qiu, F. Hu, Y. H. Ding, S. L. Han, L. L. Li, H. Y. Chen, X. P. Han, C. H. Sun and S. J. Peng, Adv. Funct. Mater., 2021, 31, 2100618 CrossRef CAS.
- J. Zhang, T. Wang, P. Liu, Z. Q. Liao, S. H. Liu, X. D. Zhuang, M. W. Chen, E. Zschech and X. L. Feng, Nat. Commun., 2017, 8, 15437 CrossRef CAS.
- P. M. Csernica, J. R. McKone, C. R. Mulzer, W. R. Dichtel, H. D. Abruna and F. J. DiSalvo, ACS Catal, 2017, 7, 3375–3383 CrossRef CAS.
- Z. X. Li, C. C. Yu, Y. Y. Wen, Y. Gao, X. F. Xing, Z. T. Wei, H. Sun, Y. W. Zhang and W. Y. Song, ACS Catal., 2019, 9, 5084–5095 CrossRef CAS.
- H. L. S. Santos, P. G. Corradini, M. Medina, J. A. Dias and L. H. Mascaro, ACS Appl. Mater. Interfaces, 2020, 12, 17492–17501 CrossRef CAS PubMed.
- Y. Ito, T. Ohto, D. Hojo, M. Wakisaka, Y. Nagata, L. H. Chen, K. L. Hu, M. Izumi, J. Fujita and T. Adschiri, ACS Catal., 2018, 8, 3579–3586 CrossRef CAS.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. B. Chorkendorff, J. K. Norskov and T. F. Jaramillo, Science, 2017, 355, 146–159 CrossRef.
- L. L. Li, D. S. Yu, P. Li, H. J. Huang, D. Y. Xie, C. C. Lin, F. Hu, H. Y. Chen and S. J. Peng, Energy Environ. Sci., 2021, 14, 6419–6427 RSC.
- H. Y. Jin, J. Wang, D. F. Su, Z. Z. Wei, Z. F. Pang and Y. Wang, J. Am. Chem. Soc., 2015, 137, 2688–2694 CrossRef CAS PubMed.
- J. Pan, S. Yu, Z. Jing, Q. Zhou, Y. Dong, X. Lou and F. Xia, Small Struct., 2021, 2100076 CrossRef CAS.
- Q. Q. Pan, C. Y. Xu, X. Li, J. F. Zhang, X. L. Hu, Y. Geng and Z. M. Su, Chem. Eng. J., 2021, 405, 126962 CrossRef CAS.
- B. N. Khiarak, M. Hasanzadeh and A. Simchi, Inorg. Chem. Commun., 2021, 127, 108525 CrossRef CAS.
- X. G. Feng, X. J. Bo and L. P. Guo, J. Power Sources, 2018, 389, 249–259 CrossRef CAS.
- K. Lan, Y. Liu, W. Zhang, Y. Liu, A. Elzatahry, R. C. Wang, Y. Y. Xia, D. Al-Dhayan, N. F. Zheng and D. Y. Zhao, J. Am. Chem. Soc., 2018, 140, 4135–4143 CrossRef CAS PubMed.
- N. Han, Y. Wang, H. Yang, J. Deng, J. H. Wu, Y. F. Li and Y. G. Li, Nat. Commun., 2018, 9, 1320–1327 CrossRef PubMed.
- G. Kresse and J. Furthmiiller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- J. P. Perdew, M. Ernzerhof and K. Burke, J. Chem. Phys., 1996, 105, 9982–9985 CrossRef CAS.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS.
- C. Wei, Y. M. Sun, G. G. Scherer, A. C. Fisher, M. Sherburne, J. W. Ager and Z. C. J. Xu, J. Am. Chem. Soc., 2020, 142, 7765–7775 CrossRef CAS PubMed.
- G. R. Chen, X. T. Yang, Z. X. Xie, F. L. Zhao, Z. Y. Zhou and Q. Yuan, J. Colloid Interface Sci., 2020, 562, 244–251 CrossRef CAS PubMed.
- F. Shen, Y. M. Wang, G. F. Qian, W. Chen, W. J. Jiang, L. Luo and S. B. Yin, Appl. Catal., B, 2020, 278, 119327 CrossRef CAS.
- X. S. Wang, Y. H. Zhu, A. Vasileff, Y. Jiao, S. M. Chen, L. Song, B. Zheng, Y. Zheng and S. Z. Qiao, ACS Energy Lett., 2018, 3, 1198–1204 CrossRef CAS.
- J. C. Li, C. Zhang, T. Zhang, Z. H. Shen, Q. W. Zhou, J. Pu, H. J. Ma, T. H. Wang, H. G. Zhang, H. M. Fan, Y. Y. Wang and H. X. Ma, Chem. Eng. J., 2020, 397, 125457 CrossRef CAS.
- Z. Khazaee, A. R. Mahjoub and A. H. C. Khavar, Appl. Catal., B, 2021, 297, 120480 CrossRef CAS.
- M. J. Qu, Y. M. Jiang, M. Yang, S. Liu, Q. F. Guo, W. Shen, M. Li and R. X. He, Appl. Catal., B, 2020, 263, 118324 CrossRef CAS.
- A. A. Dubale, Y. Y. Zheng, H. L. Wang, R. Hubner, Y. Li, J. Yang, J. W. Zhang, N. K. Sethi, L. Q. He, Z. K. Zheng and W. Liu, Angew. Chem., Int. Ed., 2020, 59, 13891–13899 CrossRef CAS PubMed.
- C. H. Wang, H. C. Yang, Y. J. Zhang and Q. B. Wang, Angew. Chem., Int. Ed., 2019, 58, 6099–6103 CrossRef CAS PubMed.
- M. F. Sanad, A. R. Puente Santiago, S. A. Tolba, M. A. Ahsan, O. Fernandez-Delgado, M. ShawkyAdly, E. M. Hashem, M. MahrousAbodouh, M. S. El-Shall, S. T. Sreenivasan, N. K. Allam and L. Echegoyen, J. Am. Chem. Soc., 2021, 143, 4064–4073 CrossRef CAS PubMed.
- F. L. Zhao, J. Y. Ye, Q. Yuan, X. T. Yang and Z. Y. Zhou, J. Mater. Chem. A, 2020, 8, 11564–11572 RSC.
- Y. G. Li, H. L. Wang, L. M. Xie, Y. Y. Liang, G. S. Hong and H. J. Dai, J. Am. Chem. Soc., 2011, 133, 7296–7299 CrossRef CAS PubMed.
- C. J. Lei, Y. Wang, Y. Hou, P. Liu, J. Yang, T. Zhang, X. D. Zhuang, M. W. Chen, B. Yang, L. C. Lei, C. Yuan, M. Qiu and X. L. Feng, Energy Environ. Sci., 2019, 12, 149–156 RSC.
- K. Zhan, C. H. Feng, X. T. Feng, D. Zhao, S. Yue, Y. J. Li, Q. Z. Jiao, H. S. Li and Y. Zhao, ACS Sustainable Chem. Eng., 2020, 8, 6273–6281 CrossRef CAS.
- M. A. Ahsan, A. R. P. Santiago, Y. Hong, N. Zhang, M. Cano, E. Rodriguez-Castellon, L. Echegoyen, S. T. Sreenivasan and J. C. Noveron, J. Am. Chem. Soc., 2020, 142, 14688–14701 CrossRef CAS.
- Q. Zhang, D. F. Yan, Z. Z. Nie, X. B. Qiu, S. Y. Wang, J. M. Yuan, D. W. Su, G. X. Wang and Z. J. Wu, ACS Appl. Energy Mater., 2018, 1, 571–579 CrossRef CAS.
- S. F. Fu, C. Z. Zhu, J. H. Song, M. H. Engelhard, X. L. Li, D. Du and Y. H. Lin, ACS Energy Lett., 2016, 1, 792–796 CrossRef CAS.
- H. Y. Li, S. M. Chen, H. F. Lin, X. B. Xu, H. Z. Yang, L. Song and X. Wang, Small, 2017, 13, 1701487 CrossRef PubMed.
- S. Shen, Z. Lin, K. Song, Z. Wang, L. Huang, L. Yan, F. Meng, Q. Zhang, L. Gu and W. Zhong, Angew. Chem., Int. Ed., 2021, 60, 12360–12365 CrossRef CAS.
- M. Gong, W. Zhou, M. C. Tsai, J. G. Zhou, M. Y. Guan, M. C. Lin, B. Zhang, Y. F. Hu, D. Y. Wang, J. Yang, S. J. Pennycook, B. J. Hwang and H. J. Dai, Nat. Commun., 2014, 5, 5695 CrossRef PubMed.
- S. B. Pillai, S. D. Dabhi and P. K. Jha, Int. J. Hydrogen Energy, 2018, 43, 21649 CrossRef CAS.
- X. Mu, J. Gu, F. Feng, Z. Xiao, C. Chen, S. Liu and S. Mu, Adv. Sci., 2021, 8, 2002341 CrossRef CAS PubMed.
- B. Ruqia and S. I. Choi, ChemSusChem, 2018, 11, 2643–2653 CrossRef CAS PubMed.
- M. S. Balogun, W. T. Qiu, H. Yang, W. J. Fan, Y. C. Huang, P. P. Fang, G. R. Li, H. B. Ji and Y. X. Tong, Energy Environ. Sci., 2016, 9, 3411–3416 RSC.
- B. H. R. Suryanto, Y. Wang, R. K. Hocking, W. Adamson and C. Zhao, Nat. Commun., 2019, 10, 2041–1723 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nr04407b |
| ‡ These authors contributed equally to the manuscript. |
| This journal is © The Royal Society of Chemistry 2022 |