Iron-containing poly(ionic liquid) membranes: a heterogeneous Fenton reaction and enhanced anti-fouling ability†
Zhangbin
Guan‡
ab,
Bingyu
Wang‡
ab,
Yan
Wang
ab,
Jing
Chen
ab,
Chunyang
Bao
ab and
Qiang
Zhang
 *ab
*ab
aKey Laboratory of New Membrane Materials, Ministry of Industry and Information Technology, School of Environmental and Biological Engineering, Nanjing University of Science and Technology, Nanjing 210094, P. R. China. E-mail: zhangqiang@njust.edu.cn
bInstitute of Polymer Ecomaterials, School of Environmental and Biological Engineering, Nanjing University of Science and Technology, Nanjing 210094, P. R. China
First published on 1st December 2021
Abstract
Poly(ionic liquid)s (PILs) are widely used to improve the anti-fouling ability of membranes due to their high charge density and excellent hydrophilicity. However, it is difficult to eradicate the irreversible pollutants deposited inside the pores of membranes. In this study, an iron-containing PIL (Fe-PIL) membrane was constructed for the first time to enhance the anti-fouling property of a membrane via a heterogeneous Fenton reaction. Cu(0)-mediated reversible deactivation radical polymerization was used to synthesize polysulfone-based block copolymers with pyridine pendant groups, which were then quaternized for coordination with Fe(II) bromide to yield the targeted Fe-PILs. The presence of Fe-PILs enabled the heterogeneous Fenton reaction within the membrane pores and exhibited a wide range of pH tolerance, which could altogether remove contaminants within minutes. The membrane showed good anti-fouling performance and reusability. In addition, membrane filtration and the heterogeneous Fenton reaction exhibited a synergistic effect due to the confinement effect, improving the permeability and rejection of the membrane. This research developed a novel synthetic strategy and catalytic function of Fe-PILs for advanced membranes.
Introduction
Membrane separation technology has received more and more attention due to its advantages of simplicity, energy-saving, and high efficiency in water treatment.1–4 However, when the membrane was used for water treatment, the presence of colloids, microorganisms, and macromolecular organics could elevate fouling potentiality, causing less membrane flux and worsening the separation performance. Hence, the membrane life span was hampered by the fouling issue, which increased the economic cost.5 Therefore, the high anti-fouling performance of the membrane is expected to tackle the root of membrane fouling.6Poly(ionic liquid)s (PILs) are functional materials that combine the unique properties of ionic liquids with the functionalities of polymer materials, such as excellent stability, processability, and flexibility.7 Recently, PILs have been successfully used to prepare membranes, showing a wide range of applications in gas separation,8 fuel cells,9 seawater desalinization,10 acid recovery,11 antibacterial treatment,12 protein concentration,13 amino acid separation,14 and pigment wastewater treatment.15 PILs, as a kind of polyelectrolyte, had good hydrophilicity due to their charged structure, which made the polymer membrane less prone to fouling and easier to clean.16 It could reduce membrane fouling to a large extent; however, the irreversible pollutants that have been deposited on the surface of the polymer membrane are still difficult to remove.
A heterogeneous Fenton reaction overcame the shortcomings of the original Fenton reaction, such as being restricted by pH, producing a large amount of iron mud, etc.17–19 It has received increasing attention as a novel antifouling strategy in membrane science.20 However, when metal-based catalysts, such as Fe2O3,21 MnO2,22 TiO2,23etc., were directly loaded into the membrane, it would cause problems such as incompatibility with the polymer and higher roughness of the membrane surface. On the other hand, organometallic polymers with good solubility in the casting solution and high compatibility with other polymers could lower the surface roughness of the membrane, further reduce the degree of membrane fouling, and avoid the blockage of the membrane pores by nanoparticles.24 Wu et al. (2017) synthesized a series of polystyrene-b-PIL block copolymers to prepare a nano gold modified honeycomb membrane, which was successfully used for photocatalytic degradation of Congo red dye.25 Zheng et al. (2017) prepared an imidazolium-type iron-containing PIL (Fe-PIL) membrane to study its effect on the antibacterial activity of Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli).26 In addition, iron-containing porous polyelectrolyte membranes (PPM) can be prepared based on the ionic complex between imidazolium-based PILs and 1,1-ferrocene dicarboxylic acid.27 However, as far as we know, there are few studies on the use of Fe-PILs as catalysts for the Fenton reaction. The application of Fe-PILs to prepare membranes with enhanced anti-fouling ability has not ever been reported.
Post-polymerization modification, such as quaternization and an anion exchange reaction, effectively prepared PILs with defined structures.28 At present, living/controlled free radical polymerizations such as reversible addition fragmentation chain transfer radical polymerization (RAFT) and atom transfer radical polymerization (ATRP) are commonly used for synthesizing well-defined PILs.29,30 Among them, RAFT is well known for its versatility to a wide range of monomers and solvents.31 The use of ATRP was relatively limited, mainly due to the competition of nitrogen-containing monomers with the ATRP catalyst system.32 For example, 4-vinylpyridine could be complexed with metal ions through σ bonds and π bonds, resulting in incomplete polymerization and wide PDI, making it difficult to control the molecular weight precisely.33 The newly developed Cu(0)-mediated reversible deactivation radical polymerization (Cu(0)-RDRP) could tolerate nitrogen-containing monomers, which reacted in the aqueous system at low temperatures.34–36 We hypothesize that well-defined Fe-PILs could be synthesized by Cu(0)-RDRP following post-polymerization modification and anion exchange from pyridine-containing functional polymers.
Herein, Fe-PILs have been successfully synthesized for the fabrication of heterogeneous Fenton membranes. Functional block copolymers with pyridine pendant groups were synthesized by Cu(0)-RDRP to prepare polysulfone blend membranes. A subsequent quaternization reaction and coordination with Fe(II) bromide successfully generated Fe-PILs on the surface of the membrane. The PIL membrane showed low surface roughness, excellent anti-fouling performance, and catalytic ability to mediate heterogeneous Fenton reactions. The catalytic and self-cleaning performances of the prepared Fe-PIL membranes were evaluated using methyl blue (MB). The synergistic reaction mechanism between membrane filtration and the Fenton reaction was also discussed.
Results and discussion
Synthesis and characterization of PSF-based block copolymers with pyridine groups
The synthetic route of PSF-based block copolymers is shown in Scheme 1. PSF-OH was modified with chlorine groups via the ester reaction and the obtained PSF-Cl was used as the macroinitiator. Subsequently, the Cu(0)-RDRP of 4-VP was conducted to yield PSF-based block copolymers PSF-b-P4VP.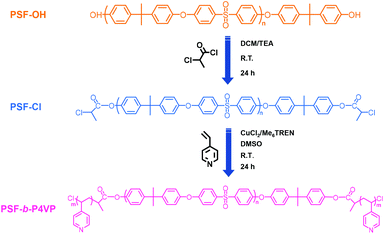 | ||
| Scheme 1 Synthesis of the poly(4-vinylpyridine)-b-polysulfone-b-poly(4-vinylpyridine) (PSF-b-P4VP) block copolymer via Cu(0)-RDRP. | ||
In this work, PSF-b-P4VP was obtained via direct Cu(0)-RDRP of 4-VP, which was conducted in a DMSO system at room temperature using chlorine terminated PSF (PSF-Cl) as the macroinitiator and Cu(0)/CuCl2/Me6TREN as the catalyst. The polymerization condition was [PSF-Cl]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuCl2]
[CuCl2]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Me6TREN]
[Me6TREN]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [4-VP] = [1]
[4-VP] = [1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [0.4]
[0.4]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [0.4]
[0.4]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [100] and the conversion could reach 93.5% after 24 hours according to the weight method. The results of FTIR, GPC, and 1H NMR were used to study the structure of PSF-based polymers.
[100] and the conversion could reach 93.5% after 24 hours according to the weight method. The results of FTIR, GPC, and 1H NMR were used to study the structure of PSF-based polymers.
The successful polymerization was directly verified using the FTIR spectra. As shown in Fig. 1A, a new peak appeared at 1760 cm−1 in the FTIR spectra of PSF-Cl compared with PSF-OH, attributed to the –COOR stretching vibration of PSF-Cl. After polymerization, new peaks at 1412 cm−1, 1557 cm−1, and 1591 cm−1 in the FTIR spectra of PSF-b-P4VP were due to the stretching vibration of the pyridine ring.37
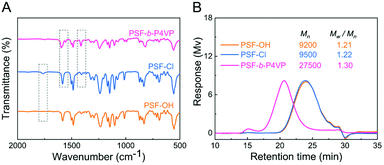 | ||
| Fig. 1 (A) Fourier transform infrared (FTIR) spectra and (B) gel permeation chromatography (GPC) spectra of PSF-based polymers. | ||
The successful synthesis of PSF-b-P4VP was also confirmed by GPC, which showed a relatively low polydispersity (Mw/Mn = 1.30, Fig. 1B) for the final polymer product PSF-b-P4VP. The Mn value of PSF-b-P4VP was significantly increased from 9500 Da (Mw/Mn = 1.22, PSF-Cl) to 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 Da (Mw/Mn = 1.30), which indicated the success of Cu(0)-RDRP. However, the Mn value of PSF-Cl showed inconspicuous change from that of PSF-OH, which showed the slight increase in the molecular weight after the modification.
500 Da (Mw/Mn = 1.30), which indicated the success of Cu(0)-RDRP. However, the Mn value of PSF-Cl showed inconspicuous change from that of PSF-OH, which showed the slight increase in the molecular weight after the modification.
The chemical composition and structure of PSF, PSF-Cl, and PSF-b-P4VP could be determined using the 1H NMR spectra, as shown in Fig. 2. The aromatic protons of the PSF-OH main chain show chemical shifts at 6–8 ppm, and a new peak of PSF-Cl at 4.1 ppm was contributed by the methine proton of CPC, indicating the successful synthesis of the macroinitiator. Meanwhile, the 1H NMR spectra of PSF-b-P4VP further indicated the presence of the C–H group of the pyridine ring in 4-vinylpridine at 8.33 ppm and 6.39 ppm.37,38
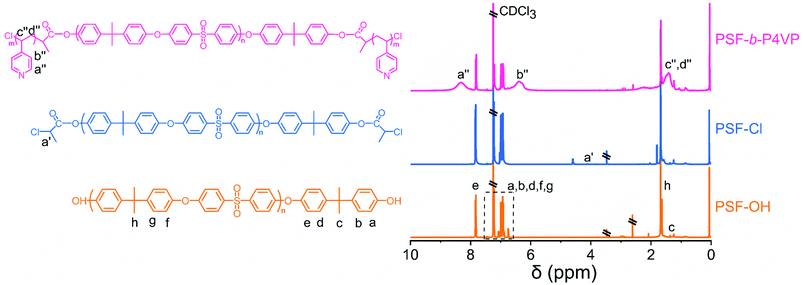 | ||
| Fig. 2 The molecular structure and 1H nuclear magnetic resonance (NMR) spectra of PSF-based polymers. | ||
Preparation and characterization of membranes
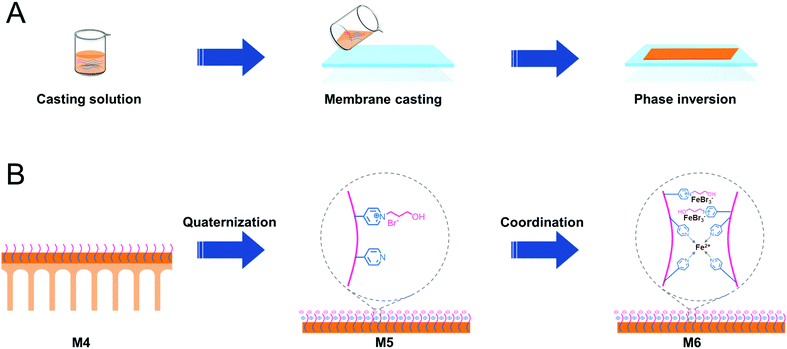
PSF-based block copolymers PSF-b-P4VP were used to prepare PSF blend membranes via the NIPS process (Scheme 2A). The pyridine groups were then quaternized with 3-bromo-1-propanol, yielding PILs on the membrane surface. Finally, the PIL-based membranes were in a saturated ethanol solution of FeBr2. FeBr2 coordinated with the pyridine ring and bromine anion of PILs, yielding the targeted Fe-PILs (Scheme 2B).According to Table S1,† the PSF-b-P4VP/PSF blend membranes (M1–M4) were prepared using various casting solutions constituted of PSF-b-P4VP and PSF in DI water via the NIPS process. Then, M4 was functionalized via quaternization with 3-bromo-1-propanol (M5) and a subsequent coordination reaction to generate Fe-PIL membranes (M6).
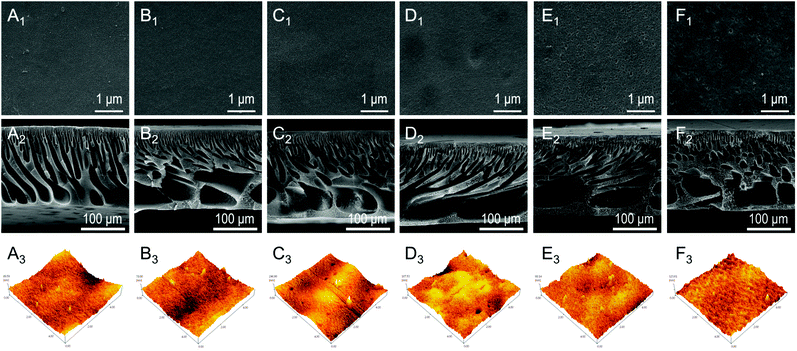
The top surface and cross-section SEM images of different membranes (M1–M6) are compared in Fig. 3. Fig. 3A1–F1 show that different membranes have a smooth surface without cracks, agglomeration, etc. A highly porous surface was observed in M5–M6 membranes (Fig. 3E1 and 3F1) compared with M1–M4 membranes (Fig. 3A1–D1). We believe that the appearance of surface pores was possibly due to the quaternization reaction in the bromo-1-propanol (10%) solution under thermal conditions. The previous study concluded that the combination of triblock copolymers and thermal and solvent annealing treatments could effectively increase the average pore size of the membrane,39 which supported this result of increasing the pore size of the M5 and M6 membranes (Table S2†). Furthermore, as shown in Fig. 3A2–F2, different cross-sectional membrane morphologies were due to various proportions casting solutions constitute. The cross-sectional image of the M1 membrane showed that the asymmetric structure of the M1 membrane was composed of a top dense skin layer, a middle finger-like structure, and a bottom cell-like structure.40 As the percentage of the polymer PSF-b-P4VP increased, the finger-like structure of the M2–M4 membranes became obvious. This can be explained as delayed mixing of the phase separation process resulting from the increasing PSF-b-P4VP content in the casting solution, leading to larger finger-shaped holes.41,42 With the introduction of PILs and Fe(II) to the membranes (M5 and M6), the larger finger-shaped structure was retained well, verifying that quaternizing the pyridine group and chelating Fe(II) on the membrane surface would not destroy the membrane structure.
The membrane surface roughness was further characterized by AFM as shown in Fig. 3A3–F3. The AFM scanning area is 5 μm × 5 μm, and the obtained roughness parameters (Ra) of all the membranes by AFM are shown in Table S2.† It can be found that as the PSF-b-P4VP content increased, the surface of M1–M4 became rougher (larger Ra value), which was attributed to the fact that self-separated hydrophilic P4VP segments on the top membrane promoted higher surface roughness.43 Interestingly, the roughness of the M5 membrane showed a downward trend from 17.01 nm to 10.02 nm after quaternization, which may be because the quaternized pyridyl group became positively charged, and the chains stretched to charge repulsion.44
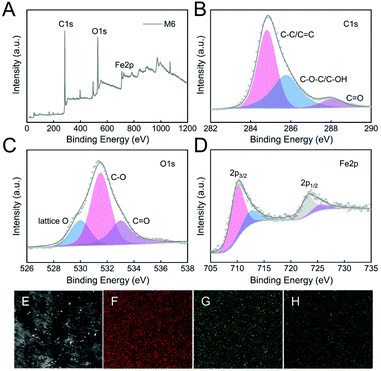
To understand the roles of different surface elements in Fenton membranes, the surface elemental content and valence state in the M6 membrane were analyzed by XPS. The results in Table S3,†Fig. 4A, and Fig. S2† show that the M6 membrane was dominated by C (70.3%), O (23.5%), and Fe (2.9%), while M1 and M4 membranes were only dominated by C (78.5–79.3%) and O (15.4–16.7%), which confirmed that the successful preparation of Fe-PIL membrane (M6).
The C 1s, O 1s, and Fe 2p deconvolution of the composite M6 membrane is further shown in Fig. 4B–D. According to the C 1s spectra deconvolution (Fig. 4B), the binding energy peaks of 284.8 eV, 285.8 eV, and 288.0 eV were assigned to C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O–C/C–OH, and C
C, C–O–C/C–OH, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the M6 membrane, respectively. The O 1s spectra deconvolution of the M6 membrane in Fig. 4C showed three binding energies at 530.0 eV, 531.5 eV, and 533.0 eV, which shows the presence of lattice oxygen, C–O, and C
O in the M6 membrane, respectively. The O 1s spectra deconvolution of the M6 membrane in Fig. 4C showed three binding energies at 530.0 eV, 531.5 eV, and 533.0 eV, which shows the presence of lattice oxygen, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the M6 membrane, respectively. The Fe 2p spectra deconvolution in Fig. 4D was at a binding energy of 710.0 eV and 712.5 eV for Fe 2p3/2, and 723.2 eV and 725.5 eV for Fe 2p1/2, which shows the presence of Fe(II) and Fe(III) in the M6 membrane.45,46 Hence, the rich hydroxyl groups content in the composite M6 membrane were attributed to the quaternization reaction. Moreover, the EDS images also showed that C, O, and Fe elements were homogeneously distributed in the surface of the M6 membrane (Fig. 4E–H), confirming that the Fe-PIL membrane had excellent dispersibility obtained by post-modification of the membrane. Besides, the excellent scalability resulting from the infinite combination of cations and anions in PILs also increased the compatibility and dispersity of C, O, and Fe elements.
O in the M6 membrane, respectively. The Fe 2p spectra deconvolution in Fig. 4D was at a binding energy of 710.0 eV and 712.5 eV for Fe 2p3/2, and 723.2 eV and 725.5 eV for Fe 2p1/2, which shows the presence of Fe(II) and Fe(III) in the M6 membrane.45,46 Hence, the rich hydroxyl groups content in the composite M6 membrane were attributed to the quaternization reaction. Moreover, the EDS images also showed that C, O, and Fe elements were homogeneously distributed in the surface of the M6 membrane (Fig. 4E–H), confirming that the Fe-PIL membrane had excellent dispersibility obtained by post-modification of the membrane. Besides, the excellent scalability resulting from the infinite combination of cations and anions in PILs also increased the compatibility and dispersity of C, O, and Fe elements.
Membrane hydrophilicity
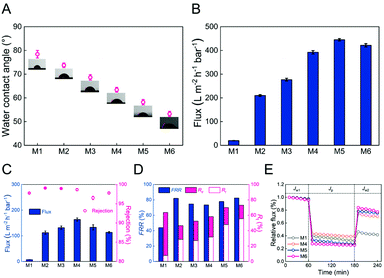
The static contact angle of the membranes (M1–M6) is shown in Fig. 5A. The M1 membrane had a contact angle of 78.4 ± 1.6°. The M2, M3, and M4 membranes had decreased contact angles (73.8 ± 1.0°, 68.7 ± 1.1°, and 63.4 ± 1.0°, respectively), presumably due to the fact that the block copolymer P4VP segment can significantly increase the membrane hydrophilicity during the NIPS process.47 In addition, the hydrophilicity of M5 membrane was further increased after the quaternization reaction (a much lower contact angle of 58.2 ± 1.1°) because of the rich hydroxyl groups content from 3-bromo-1-propanol (supported by the XPS result).28 After the coordination reaction with Fe(II), the contact area of the M6 membrane decreased due to the fact that the higher surface roughness of the membrane increased the surface tension and hydrophilicity, indicating the significance of immersed Fe(II) in increasing membrane hydrophilicity.48Separation performance and the anti-fouling ability of membranes
After pre-press at a 2 bar transmembrane pressure for 0.5 hours and controlling the pressure to 1 bar, the pure water flux of different membranes (M1–M6) is shown in Fig. 5B. It was seen that water flux of the membrane observably increased from 19.7 ± 0.2 (M1) to 392.4 ± 7.2 L m−2 h−1 bar−1 (M4) after the addition of the block copolymer PSF-b-P4VP and increased continuously to 421.7 ± 6.9 L m−2 h−1 bar−1 (M6) after being reacted with 3-bromo-1-propanol and coordinated with Fe(II). On the one hand, the presence of a P4VP or PIL segment enhanced the hydrophilicity of the membrane (static contact angle in Fig. 5A), which led to lower water diffusion resistance and thereby further promoted pure water flux. On the other hand, the average membrane pore size of M1–M6 (Table S2†) increased from 10.95 ± 0.25 to 51.91 ± 2.79 nm, which is consistent with the SEM image, that is, the main finger-like macropore membrane structure, making the pure water flux higher. Moreover, linear fitting relationships between the static contact angle or the average pore size of the membrane and the pure water flux are compared in Fig. S3.† It was found that the pure water flux of the membrane was negatively correlated with the static contact angle (p < 0.01, R2 = 0.87) and positively correlated with the pore size of the membrane in this study (p < 0.01, R2 = 0.97), which verifies that the hydrophilicity and pore size of the membrane play the most critical role in the pure water flux.In order to study the anti-fouling performance, the rejection ratio and flux of the membrane to BSA are shown in Fig. 5C. The rejection ratio of M1–M4 significantly increased (p < 0.05) from 97.8 ± 0.1% to 99.1 ± 0.1%, 98.9 ± 0.1%, and 98.7 ± 0.2%, respectively, attributed to pore size screening. Meanwhile, the BSA solution's flux to the membrane was dramatically promoted almost 22-fold from 7.2 ± 0.5 (M1) to 163.7 ± 6.0 L m−2 h−1 bar−1 (M4). It is worth noting that the M5 membrane rejection ratio (96.5 ± 0.4%) and flux (133.3 ± 10.1 L m−2 h−1 bar−1) to BSA were significantly lower (p < 0.05) than those of the M4 membrane after the quaternization reaction, which is primarily ascribed to the electrostatic attraction between the positively charged membrane and the negatively charged BSA in the PBS buffer solution (pH = 7.4), causing more severe membrane fouling.49 After coordinating with Fe(II), the electrostatic attraction between the M6 membrane and BSA was further increased, reducing the membrane flux to BSA (113.6 ± 3.1 L m−2 h−1 bar−1).
To further evaluate the antifouling ability of the membranes (M1–M6), the FRR, Rir, and Rr of the membranes are shown in Fig. 5D. Generally, 100% FRR indicates the robust antifouling ability of the membrane. The FRR of the M1 membrane was only 44.0%, and Rir was much higher than Rr, indicating that it belonged to irreversible pollutants. However, the M6 membrane had higher FRR (82.5%), and Rr was higher than Rir, which meant that compared with M1, M6 has more reversible pollutants. Fig. 5E further shows the relative flux change of different solutions (water and BSA solution) to the membrane over time. The relative flux is defined as the ratio of the real-time flux to the initial water flux. It was found that all membrane fluxes (Jw1) behave similarly with only a slight drop in 1 hour of pure water permeation. However, when pure water was converted to BSA solution, the membrane fluxes (Jp) showed a sharp drop due to a protein layer of the filter formed by deposition and adsorption to the surface of membranes. Different membranes were subjected to simple cleaning for the flux recovery test, and it was found that the M6 membrane flux recovered, which was observably higher than that of the M1 membrane. In summary, these results showed that with the addition of the PSF-b-P4VP block polymer and the quaternization/coordination reaction, the antifouling ability of the membrane was effectively improved.
Evaluation of the performance of the static heterogeneous Fenton reaction
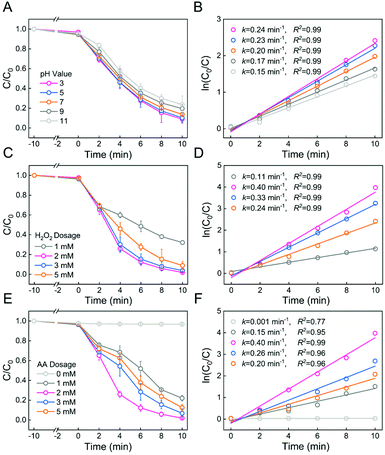
We hypothesize that Fe-PILs in the membrane could act as heterogeneous Fenton catalysts. Thus MB was first used as a model compound for the Fenton reaction in the presence of the PSF-based Fe-PIL membrane (M6). It should also be noted that the coordination of Fe(II) with pyridine-based PILs was conducted in the presence of air, which may oxidize Fe(II) into Fe(III). Thus, a widespread and friendly reducing agent, ascorbic acid (AA), was used to generate Fe(II) to accelerate the Fenton reaction. The performance of the M6 membrane to mediate the heterogeneous Fenton reaction is shown in Fig. 6, which shows the effect of the pH value, H2O2 dosage, and AA dosage on MB degradation.Fig. 6A shows the degradation kinetics of MB by the M6 membrane at different reaction pH values. It can be seen that the MB degradation rate (k) and degradation efficiency (1 − C/C0) increased with decreasing pH, and pH 3 was the optimal pH, as shown in Fig. 6A and B. The results showed that acidic conditions favored MB removal in heterogeneous Fenton reaction membrane systems, which is consistent with the previous studies.50 However, this Fenton reaction system could also work under neutral or even alkaline conditions, which was a great advantage considering that it would avoid the necessity of tuning the pH.
The effect of the H2O2 dosage on the heterogeneous Fenton membrane reaction is shown in Fig. 6C. It was observed that the MB degradation rate (k) and degradation efficiency (1 − C/C0) were initially a noticeable increase at 0–2 mM H2O2 dosage and then a mild decrease at 2–5 mM, as shown in Fig. 6C and D. It was mainly because of ˙OH scavenging at a higher H2O2 dosage.51 Therefore, an H2O2 dosage of 2 mM was selected as the best H2O2 dosage.
Moreover, the reaction efficiency of the heterogeneous Fenton system was currently limited by insufficient Fe(III)/Fe(II) cycles. The reason for this phenomenon is that the reduction rate constant of Fe(III) to Fe(II) by H2O2 was about 4 orders of magnitude weaker than that of Fe(II) oxidation by H2O2, resulting in the reaction process consuming Fe(II) rapidly.52 Thus, the Fe(III)/Fe(II) cycles were promoted by adding the reducing agent AA to increase the reaction efficiency of the heterogeneous Fenton reaction. Thus, the effect of the AA dosage on the heterogeneous Fenton membrane reaction is shown in Fig. 6E. It was observed that the MB degradation rate (k) and degradation efficiency (1 − C/C0) were originally a surging rise at 0–2 mM AA dosage and then a slight drop at 2–5 mM, as shown in Fig. 6E and F. This might be because excessive AA will react with ˙OH, which would make the degradation effect worse.53 Therefore, an AA dosage of 2 mM was selected as the best AA dosage. In summary, the heterogeneous Fenton membrane could efficiently degrade MB in minutes in a broad pH range from 3.0 to 11.0. The addition of AA could significantly accelerate the Fenton reaction.
Reusability of the Fenton membrane and reactive species during MB removal
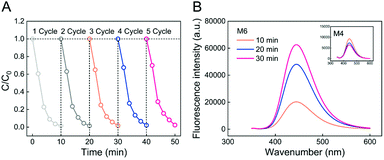
Reusability is a critical characteristic of the Fenton membrane for wastewater treatment. Thus, the reusability of the M6 membrane for MB removal in five cycles was tested as shown in Fig. 7A. After five cycles of experiments, the MB degradation by the M6 membrane was still completed within ten minutes. The removal ratio of MB in five cycles was 98.6%, 98.6%, 98.3%, 97.9%, and 97.6%, respectively, which showed that the PSF-based Fe-PIL membrane (M6) had good reliability as a heterogeneous Fenton membrane. It also showed that Fe(II) coordination with PILs is very stable, and no significant loss of Fe(II) would occur during the Fenton reaction and repeated use. This is also a significant advantage as it could avoid generating iron sludge in the traditional Fenton system.It is known that an ˙OH radical is the primary reactive species generated during Fenton catalytic degradation.50–52 To distinguish ˙OH in the Fenton membrane, the terephthalic acid (TA) scavenger was chosen in this study via fluorescent probe technology. TA (non-fluorescent) has been proposed to only react with ˙OH radicals and was converted into fluorescent 2-hydroxyterephthalic acid (HTA), which was measured using a fluorescence spectrophotometer with 315 nm excitation wavelength.54 As shown in Fig. 7B, it was found that the fluorescence intensity at 445 nm gradually increased with time, indicating that a large number of ˙OH radicals were generated during the reaction. This result proved that the generated ˙OH radicals were the primary reactive species during degradation.
Evaluation of the performance of the dynamic heterogeneous Fenton membrane reaction
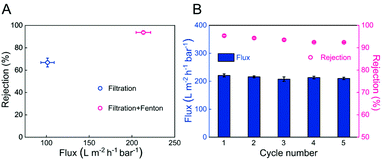
The performance of the dynamic heterogeneous Fenton reaction of the PSF-based Fe-PIL membrane (M6) was evaluated using an across-flow filtration system, that is, a single pass through the membrane. As shown in Fig. 8A, the MB rejection ratio of the M6 membrane without the addition of H2O2 and AA was only 66.9%, which was only accounted for filtration. In contrast, combining membrane filtration and the heterogeneous Fenton process, the MB rejection ratio of the M6 membrane with H2O2 and AA reached 93.6%. Meanwhile, the flux of the MB solution increased over two-fold, from 101.3 L m−2 h−1 bar−1 to 213.2 L m−2 h−1 bar−1. The lower membrane rejection ratio and flux were attributed to the membrane retention and solution concentration polarization resulting in the highest pollutant concentration near the membrane surface, which caused membrane fouling. After introducing the heterogeneous Fenton reaction, since the pore size of the membrane is 50 nm, ˙OH generated by activated H2O2 can be restricted and increased in the nano-scale domain due to the confinement effect, which improves the catalytic effect and makes the membrane possess a specific self-cleaning ability. At the same time, it prevented pollutants, whose size was larger than that of the membrane pores, from entering the membrane pores and had a specific catalytic selectivity, which could realize the separation and degradation of different pollutants.In addition, the performance of the dynamic heterogeneous Fenton membrane reaction was evaluated in five consecutive cycles to evaluate the reusability of the membrane (Fig. 8B). It was found that the flux and rejection ratio only decreased by 4.9% and 3.1% after five cycles, respectively, indicating that the PSF-based Fe-PIL membrane has good stability in the actual membrane separation process. This result indicated that the PSF-based Fe-PIL membrane had excellent self-cleaning ability and separation stability. The synergy of membrane filtration and the Fenton process could increase permeability and rejection.
Conclusions
In this study, through the combination of heterogeneous Fenton and membrane technology, a catalytic membrane based on Fe-PILs was constructed to solve the problem of membrane fouling from the source. The block copolymer PSF-b-P4VP synthesized via Cu(0)-RDRP was used to fabricate a PSF membrane via the NIPS process. The average pore size and the hydrophilicity of membranes increase with the proportion of block copolymers. By quaternizing the pyridine group on the surface of the PSF blend membrane, introducing PILs could further facilitate the hydrophilicity and anti-fouling ability of the membrane. Moreover, the membrane possessed excellent catalyst dispersibility, anti-fouling performance, and scalability due to Fe(II) coordination with PILs. The Fe-PIL membrane possessed high catalytic efficiency and excellent reusability during the heterogeneous Fenton reaction in a broad pH range from acidic to neutral and basic conditions. It was worth noting that the PSF-based Fe-PIL membrane exhibited superior synergistic performance with filtration in the dynamic heterogeneous Fenton reaction and could be maintained well after five cycles. This research provided a strategy to synthesize Fe-PILs from well-defined P4VP and a new idea to solve the problem of membrane fouling.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was financially supported by the Sichuan Science and Technology Program (2020YFSY0028).Notes and references
- D. Cohen-Tanugi, L.-C. Lin and J. C. Grossman, Nano Lett., 2016, 16, 1027–1033 CrossRef CAS PubMed.
- J. Ma, X. Tang, Y. He, Y. Fan, J. Chen and H. Yu, Desalination, 2020, 480, 114328 CrossRef CAS.
- Z. Zhu, W. Wang, D. Qi, Y. Luo, Y. Liu, Y. Xu, F. Cui, C. Wang and X. Chen, Adv. Mater., 2018, 30, 1801870 CrossRef PubMed.
- H. B. Park, J. Kamcev, L. M. Robeson, M. Elimelech and B. D. Freeman, Science, 2017, 356, 1137 CrossRef CAS PubMed.
- X. Zhao, R. Zhang, Y. Liu, M. He, Y. Su, C. Gao and Z. Jiang, J. Membr. Sci., 2018, 551, 145–171 CrossRef CAS.
- J. Chen, X. Meng, Y. Tian, X. Wang, J. Zhu, H. Zheng and L. Wang, J. Membr. Sci., 2020, 616, 118587 CrossRef CAS.
- W. Xiao, Q. Yang and S. Zhu, Sci. Rep., 2020, 10, 7825 CrossRef CAS PubMed.
- E. D. Bates, R. D. Mayton, I. Ntai and J. H. Davis, J. Am. Chem. Soc., 2002, 124, 926–927 CrossRef CAS PubMed.
- F. Liu, S. Wang, D. Wang, G. Liu, Y. Cui, D. Liang, X. Wang, Z. Yong and Z. Wang, J. Power Sources, 2021, 494, 229732 CrossRef CAS.
- X. Song, C. Wang, S. Li, H. Liu and C. Zhang, J. Membr. Sci., 2021, 632, 119360 CrossRef CAS.
- N. S. Naik, M. Padaki, S. Deon and D. H. K. Murthy, Chem. Eng. J., 2020, 401, 126148 CrossRef CAS.
- C. Fang, L. Kong, Q. Ge, W. Zhang, X. Zhou, L. Zhang and X. Wang, Polym. Chem., 2019, 10, 209–218 RSC.
- L. Qian, M. Yang, H. Chen, Y. Xu, S. Zhang, Q. Zhou, B. He, Y. Bai and W. Song, Carbohydr. Polym., 2019, 218, 154–162 CrossRef CAS PubMed.
- L. Liu, S. Xiong, L. Zeng, C. Cai, F. Li and Z. Tan, J. Colloid Interface Sci., 2021, 584, 866–874 CrossRef CAS PubMed.
- H.-F. Xiao, C.-H. Chu, W.-T. Xu, B.-Z. Chen, X.-H. Ju, W. Xing and S.-P. Sun, J. Membr. Sci., 2019, 586, 44–52 CrossRef CAS.
- E. N. Durmaz, S. Sahin, E. Virga, S. de Beer, L. C. P. M. de Smet and W. M. de Vos, ACS Appl. Polym. Mater., 2021, 3, 4347–4374 CrossRef CAS PubMed.
- J. Zhang and Y. Nosaka, J. Phys. Chem. C, 2014, 118, 10824–10832 CrossRef CAS.
- K. V. Plakas, A. Mantza, S. D. Sklari, V. T. Zaspalis and A. J. Karabelas, Chem. Eng. J., 2019, 373, 700–708 CrossRef CAS.
- J. Weiss and C. W. Humphrey, Nature, 1949, 163, 691–691 CrossRef CAS.
- S. Zhang, T. Hedtke, Q. Zhu, M. Sun, S. Weon, Y. Zhao, E. Stavitski, M. Elimelech and J.-H. Kim, Environ. Sci. Technol., 2021, 55, 9266–9275 CrossRef CAS PubMed.
- B. I. Harman, H. Koseoglu, N. O. Yigit, M. Beyhan and M. Kitis, Desalination, 2010, 261, 27–33 CrossRef CAS.
- S. Byun, S. H. Davies, A. L. Alpatova, L. M. Corneal, M. J. Baumann, V. V. Tarabara and S. J. Masten, Water Res., 2011, 45, 163–170 CrossRef CAS PubMed.
- Y. Hu, N. Milne, S. Gray, G. Morris, W. Jin, M. Duke and B. Zhu, Desalin. Water Treat., 2011, 34, 57–62 CrossRef CAS.
- Y. Wang, J. Zhang, C. Bao, X. Xu, D. Li, J. Chen, M. Hong, B. Peng and Q. Zhang, J. Membr. Sci., 2021, 624, 119121 CrossRef CAS.
- B. Wu, W. Zhang, N. Gao, M. Zhou, Y. Liang, Y. Wang, F. Li and G. Li, Sci. Rep., 2017, 7, 13973 CrossRef PubMed.
- Z. Zheng, J. Guo, H. Mao, Q. Xu, J. Qin and F. Yan, ACS Biomater. Sci. Eng., 2017, 3, 922–928 CrossRef CAS PubMed.
- A. K. Kheirabad, S. S. Garakani, L. Tan and J. Yuan, Macromol. Rapid Commun., 2021, 42, 2100077 CrossRef PubMed.
- J. Chen, D. Li, C. Bao and Q. Zhang, Chem. Commun., 2020, 56, 3665–3668 RSC.
- Q. Zhang, P. Wilson, Z. Li, R. McHale, J. Godfrey, A. Anastasaki, C. Waldron and D. M. Haddleton, J. Am. Chem. Soc., 2013, 135, 7355–7363 CrossRef CAS PubMed.
- Q. Zhang, M. Li, C. Zhu, G. Nurumbetov, Z. Li, P. Wilson, K. Kempe and D. M. Haddleton, J. Am. Chem. Soc., 2015, 137, 9344–9353 CrossRef CAS PubMed.
- M. H. Allen, S. T. Hemp, A. E. Smith and T. E. Long, Macromolecules, 2012, 45, 3669–3676 CrossRef CAS.
- N. V. Tsarevsky, W. A. Braunecker, S. J. Brooks and K. Matyjaszewski, Macromolecules, 2006, 39, 6817–6824 CrossRef CAS.
- J. Xia, X. Zhang and K. Matyjaszewski, Macromolecules, 1999, 32, 3531–3533 CrossRef CAS.
- G. R. Jones, R. Whitfield, A. Anastasaki, N. Risangud, A. Simula, D. J. Keddie and D. M. Haddleton, Polym. Chem., 2018, 9, 2382–2388 RSC.
- C. Bao, J. Chen, D. Li, A. Zhang and Q. Zhang, Polym. Chem., 2020, 11, 1386–1392 RSC.
- Z. Zheng, B. Wang, J. Chen, Y. Wang, Z. Miao, C. Shang and Q. Zhang, Eur. Polym. J., 2021, 158, 110702 CrossRef CAS.
- L. Ma, H. Kang, R. Liu and Y. Huang, Langmuir, 2010, 26, 18519–18525 CrossRef CAS PubMed.
- F. Huo, S. Li, Q. Li, Y. Qu and W. Zhang, Macromolecules, 2014, 47, 2340–2349 CrossRef CAS.
- N. Wang, T. Wang and Y. Hu, ACS Appl. Mater. Interfaces, 2017, 9, 31018–31030 CrossRef CAS PubMed.
- Y. Ma, J. Zeng, Y. Zeng, H. Zhou, G. Liu, Y. Liu, L. Zeng, J. Jian and Z. Yuan, J. Hazard. Mater., 2020, 384, 121277 CrossRef CAS PubMed.
- Y. Chen, M. Wei and Y. Wang, J. Membr. Sci., 2016, 505, 53–60 CrossRef CAS.
- L.-F. Fang, B.-K. Zhu, L.-P. Zhu, H. Matsuyama and S. Zhao, J. Membr. Sci., 2017, 524, 235–244 CrossRef CAS.
- Y. Liu, Y. Su, X. Zhao, Y. Li, R. Zhang and Z. Jiang, J. Membr. Sci., 2015, 486, 195–206 CrossRef CAS.
- S. P. Nunes, A. R. Behzad, B. Hooghan, R. Sougrat, M. Karunakaran, N. Pradeep, U. Vainio and K.-V. Peinemann, ACS Nano, 2011, 5, 3516–3522 CrossRef CAS PubMed.
- Y. Gao, S. Yan, Y. He, Y. Fan, L. Zhang, J. Ma, R. Hou, L. Chen and J. Chen, J. Membr. Sci., 2021, 626, 119192 CrossRef CAS.
- K. Jiang, Z. Liu, G. Zhang and W. Jin, J. Membr. Sci., 2020, 608, 118213 CrossRef CAS.
- J. Zeng, C. Lv, G. Liu, Z. Zhang, Z. Dong, J. Liu and Y. Wang, J. Membr. Sci., 2019, 572, 428–441 CrossRef CAS.
- S. Sarkar, T. Roy, A. Roy, S. Moitra, R. Ganguly and C. M. Megaridis, J. Colloid Interface Sci., 2021, 581, 690–697 CrossRef CAS PubMed.
- Y.-F. Zhao, L.-P. Zhu, Z. Yi, B.-K. Zhu and Y.-Y. Xu, J. Membr. Sci., 2013, 440, 40–47 CrossRef CAS.
- C. K. Duesterberg, S. E. Mylon and T. D. Waite, Environ. Sci. Technol., 2008, 42, 8522–8527 CrossRef CAS PubMed.
- W. P. Kwan and B. M. Voelker, Environ. Sci. Technol., 2002, 36, 1467–1476 CrossRef CAS PubMed.
- X. Hou, X. Huang, F. Jia, Z. Ai, J. Zhao and L. Zhang, Environ. Sci. Technol., 2017, 51, 5118–5126 CrossRef CAS PubMed.
- H. Sun, G. Xie, D. He and L. Zhang, Appl. Catal., B, 2020, 267, 118383 CrossRef CAS.
- C. Ademola Bode-Aluko, O. Pereao, H. H. Kyaw, L. Al-Naamani, M. Z. Al-Abri, M. Tay Zar Myint, A. Rossouw, O. Fatoba, L. Petrik and S. Dobretsov, Mater. Sci. Eng., B, 2021, 264, 114913 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1py01345a |
| ‡ These two authors contributed to this paper equally. |
| This journal is © The Royal Society of Chemistry 2022 |