Open Access Article
Open Access ArticleSurface-initiated reversible addition fragmentation chain transfer of fluoromonomers: an efficient tool to improve interfacial adhesion in piezoelectric composites†
Vincent
Bouad
ab,
Kohji
Ohno
 c,
Ahmed
Addad
b,
Adeline
Marin
b,
Nicolas
Donzel
a,
Sophie
Barrau
c,
Ahmed
Addad
b,
Adeline
Marin
b,
Nicolas
Donzel
a,
Sophie
Barrau
 *b,
Joël
Lyskawa
*b and
Vincent
Ladmiral
*b,
Joël
Lyskawa
*b and
Vincent
Ladmiral
 *a
*a
aICGM, University of Montpellier, CNRS, ENSCM, Montpellier, France. E-mail: vincent.ladmiral@enscm.fr
bUniversité de Lille, CNRS, INRAE, Centrale Lille, UMR 8207 – UMET – Unité Matériaux et Transformations, F-59000 Lille, France. E-mail: joel.lyskawa@univ-lille.fr; sophie.barrau@univ-lille.fr
cDepartment of Materials Science, Graduate School of Engineering, Osaka Metropolitan University, Sakai, Osaka 599-8531, Japan
First published on 12th October 2022
Abstract
This article reports the surface-initiated Reversible Addition–Fragmentation chain Transfer polymerization (SI-RAFT) of trifluoroethylene (TrFE) and vinylidene fluoride (VDF) from barium titanate nanoparticles (BTO NPs) for the preparation of piezoelectric composites. A new xanthate chain transfer agent (DOPA-XA) derived from O-ethyl-S-(1-methoxy-carbonyl) ethyldithiocarbonate and integrating a catechol moiety able to efficiently bind onto the BTO NP surface was synthesized, characterized via1H and 13C NMR spectroscopy and employed to mediate SI-RAFT polymerizations. This DOPA-XA was immobilized onto BTO NPs and the grafting efficiency was evaluated by TEM images and XPS measurements while the grafting density (Γ > 0.2 chains per nm2) was calculated using TGA and BET measurements. Poly(trifluoroethylene)- (PTrFE) and poly(vinylidene fluoride-co-trifluoroethylene)- (P(VDF-co-TrFE) functionalized BTO NPs were prepared by SI-RAFT polymerization and characterized by nuclear magnetic resonance (NMR), size exclusion chromatography (SEC), thermogravimetric analysis (TGA), transmission electron macroscopy (TEM) and X-ray photoelectron microscopy (XPS). Interestingly, polymer brushes featuring relatively low dispersities (Đ < 1.5) and high grafting densities (Γ > 0.2 chains per nm2) were obtained. Piezoelectric composite films were then prepared from these fluoropolymer-decorated BTO NPs by incorporation into a P(VDF-co-TrFE) matrix using the solvent casting method. The homogeneity of the NPs dispersion in the copolymer matrix was assessed by SEM and the direct piezoelectric response of the composites was recorded after polarization and compared with composites prepared from non-modified BTO NPs. The measured piezoelectric coefficients (d33) of all the composites were of the same order of magnitude (around −9 pC N−1). Finally, the interface between the NPs and the copolymer matrix was mechanically stressed by stretching. The SEM images of the composite fracture showed, in the case of nongrafted BTO NPs, the presence of cavities close to the NPs associated with a weak interfacial adhesion while, for fluoropolymers grafted BTO NPs, the interface with the copolymer matrix was cohesive. This study leads to innovative composites with a cohesive ceramic/polymer interface for piezoelectric applications.
Introduction
Since the discovery of piezoelectricity in 1880 by Curie et al.,1 piezoelectric behaviour have been found in various materials such as natural crystals, ceramics and polymers. Piezoelectric ceramics such as Pb[ZrxTi1−x]O3 (PZT, Lead Zirconate Titanate),2 BaTiO3 (BTO, Barium Titanate)3 or Bi0.5Na0.5TiO3 (BNT, Bismuth Sodium Titanate)4 are piezoelectric materials exhibiting very high piezoelectric coefficienst (d33 up to 500 pC N−1 for PZT5). PZT, a piezoelectric ceramic with the highest piezoelectric response, has been widely used as an actuator or as an ultrasonic transducer.6,7 However, it contains harmful elements such as lead that dramatically limits its use. Therefore, BNT and BTO are progressively used to replace PZT. Nevertheless, in spite of their high piezoelectric coefficient, piezoelectric ceramics suffer from stiffness which may limit their applications in devices which require flexibility or complex shapes.The incorporation of piezoelectric nanoparticles in a polymer matrix enabled the preparation of flexible composite materials with high piezoelectric properties.8,9 The first attempts to prepare such piezoelectric composites was reported by Kitayama and Sugawara who incorporated PZT nanoparticles in a polyurethane matrix.10
In this context, since the discovery of piezoelectricity in polymers such as poly(vinylidene fluoride) (PVDF) by Kawai in 1969,11 piezoelectric composites integrating an active polymer matrix (i.e. a piezoelectric polymer matrix) have shown growing interests. Indeed, PVDF and several other fluorocopolymers are semi-crystalline and belong to the ferroelectric polymers class since they display dipoles that can be oriented by an external electric field. These fluoropolymers possess the highest piezoelectric coefficient among polymers with values around −30 pC N−1.12 In addition, P(VDF-co-TrFE) copolymer (composed of VDF (vinylidene fluoride) and TrFE (trifluoroethylene)) presents the main advantage to directly crystallize in the electroactive crystal phase13 contrary to the PVDF which requires, for instance, stretching14–16 to form the polar phase17,18 (i.e. the β-phase presenting the highest d33 compared to other crystal phases α,17δ,17 γ19 and ε20). Interestingly, P(VDF-co-TrFE) presents a d33 of −38 pC N−1,21 which is relatively close to the value of pure PVDF β-phase. Currently, the main challenge in composites integrating fluoropolymer as polymer matrix and piezoelectric ceramics is to obtain materials with enhanced piezoelectric properties resulting from the cumulative effects of both components.22,23 Because of the opposite signs of the piezoelectric coefficient d33 of the polymer (negative) and of the ceramic (positive), the main strategy to obtain cumulative effects consists in using anti-parallel poling.22–24 However the limitation of piezoelectric properties of ceramic/fluoropolymers composites may also come from the poor affinity between the ceramic NPs and the fluoropolymers matrix potentially leading to the formation of cavities at the ceramic/polymer interface,25 and ultimately to important dielectric losses which decrease the polarization of the composites.26 To solve this problem, one attractive solution consists in the grafting of a coupling agent onto the nanoparticles surface to promote interfacial compatibility and adhesion by creating physico-chemical interactions between both constituents of the composite. Such coupling agents have two major roles in piezoelectric composites: (i) firstly, they modify the surface energy of the particles thus increasing their dispersibility in the solvent during the processing step based on the solvent casting method; (ii) secondly, they improve the dispersion of the ceramic particles in the polymer matrix and reinforce the ceramic/polymer interface. This better dispersion avoids the formation of aggregates and then prevents inhomogeneities in the composites thus improving their physico-chemical properties.27–29 In this context, silanes,30,31 dopamine, dopamine derivatives32–34 and polydopamine29,35 or other molecules capable of creating strong bonds36–38 with both ceramic and polymer have been used as coupling agents.
To further enhance ceramic/polymer matrix interface, a promising approach consists in the direct grafting of polymers onto the ceramic surface. This grafting can be achieved by the functionalisation of the polymer chain with chemical groups possessing strong affinity with the ceramic surface (i.e. the “grafting to” technique) or by triggering (co)polymerization of suitable monomers directly from the ceramic surface (“grafting from” approach).39 Surface-initiated RAFT (reversible addition–fragmentation chain transfer) polymerization is an interesting tool because it leads to the formation of polymer brushes on the ceramic surface with high grafting densities.40,41 Moreover, the core–shell structure formed by these polymer-decorated particles favours and stabilizes the dispersion of the inorganic nanoparticles in the solvent during the processing route. For example, Ohno et al.42 grafted polyvinylacetate (PVAc) brushes onto silica nanoparticles, then hydrolysed the acetate groups to form polyvinylalcohol (PVA) brushes and showed that the suspension of the resulting nanoparticles in water was greatly enhanced. SI-RAFT was also used by Yang et al. to graft polystyrene (PS) brushes40 and poly(fluorinated acrylates)43 onto BTO particles. The PS@BTO nanoparticles were compression-moulded to form composites with increased dielectric constant (7.9 times higher) compared to pure PS while BTO with fluorinated shell were used to prepare by solvent casting composites using a poly(vinylidene fluoride-co-hexafluoropropene), P(VDF-co-HFP), matrix. According to Yang et al.,43 the fluorinated shell increased the dispersion of the nanoparticles in the fluoropolymer matrix, and the resulting composite displayed high energy density and low dielectric loss. The SI-RAFT approach is of particular interest for the fluoropolymers as recent work showed that VDF as well as TrFE polymerisation could be controlled using xanthate agents.44–47 Indeed, even if those monomers are prone to backwards additions, the RAFT polymerisation using O-ethyl-S-(1-methoxycarbonyl) ethyldithiocarbonate could afford an acceptable degree of control.
In the present study, piezoelectric composites integrating barium titanate (BTO) nanoparticles incorporated into a P(VDF-co-TrFE) copolymer matrix were prepared and their piezoelectric properties investigated. Barium titanate was used as an easily accessible piezoelectric ceramic featuring high piezoelectric coefficient that does not contain harmful components. The piezoelectric P(VDF-co-TrFE) copolymer was utilized as polymer matrix because it directly crystallizes in the electroactive crystal phase and it is soluble in a wide panel of organic solvents. An in situ polymerization technique was chosen to graft the fluoropolymer chains from BTO particles. A novel chain transfer agent (DOPA-XA) derived from O-ethyl-S-(1-methoxy-carbonyl) ethyldithiocarbonate, known to successfully control the RAFT polymerization of VDF48 and TrFE,45 and integrating a catechol unit was prepared and grafted onto BTO particles. The BTO particles were then decorated with PTrFE or P(VDF-co-TrFE) via surface-initiated RAFT polymerization leading to hybrid fluoropolymer/BTO particles endowed with a strong ceramic/polymer interface and able to efficiently disperse into a fluoropolymer matrix. The PTrFE@BTO and P(VDF-co-TrFE)@BTO nanoparticles were incorporated into a commercial P(VDF-co-TrFE) matrix to form composites films by solvent casting. The piezoelectric response of the resulting composites films was then examined and the cohesion of the BTO/copolymer matrix interface was investigated after mechanical solicitation. To the best of our knowledge, this work reports for the first time, the use of surface-initiated RAFT polymerization of fluoromonomers as coupling method for the preparation of piezoelectric composites.
Materials and methods
Materials
Chemical reagents were purchased from Sigma-Aldrich. O-Ethyl-S-(1-methoxycarbonyl) ethyldithiocarbonate (CTA-XA) was synthesized according to the method described by Liu et al.49 Methanol was dried by distillation on magnesium. Acetone, petroleum ether, dichloromethane, ethyl acetate, and dimethylcarbonate (DMC) were purchased from Sigma-Aldrich and used as received. BaTiO3 (or BTO) powder (99.95%, electronic grade, average particle size of 200 nm) was purchased from Inframat. P(VDF-co-TrFE) (TrFE content = 20% mol, Mn = 200–320 kDa, Đ = 2.6–2.9) was supplied from Arkema.Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500000 g mol−1). Typical sample concentration was 10 mg mL−1.
500000 g mol−1). Typical sample concentration was 10 mg mL−1.
Synthesis
1H NMR (CDCl3, ppm, Fig. S1†): 1.39–1.46 (3H, CH2CH3), 1.57–1.65 (3H, CHCH3), 4.30–4.36 (1H, CHCH3) and 4.60–4.65 (2H, CH2CH3).
1H NMR (CDCl3, ppm, Fig. S2†): 1.39–1.46 (3H, CH2CH3), 1.68–1.73 (3H, CHCH3), 2.84 (4H, CH2CH2) and 4.56–4.72 (2H, CH2CH3 and 1H, CHCH3).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binary mixture. The product was isolated as a sticky yellow oil with a 28% yield.
1 binary mixture. The product was isolated as a sticky yellow oil with a 28% yield.
1H NMR (CDCl3, ppm): 1.39–1.46 (3H, CH2CH3), 1.68–1.73 (3H, CHCH3), 2.6–2.75 (2H, NH–CH2CH2), 3.4–3.6 (2H, NH–CH2CH2), 4.15–4.35 (1H, CHCH3), 4.56–4.72 (2H, CH2CH3) and 6.5–6.9 (3H, Ph).
13C NMR (CDCl3, ppm): 13.7 (CH2CH3), 16.3 (CHCH3), 34.6 (NH–CH2CH2), 41.2 (NH–CH2CH2), 47.9 (CHCH3), 71.0 (CH2CH3) and 115.2;11.56;1;120.8 (CH, Ph), 130.6 (CH-CH2, Ph), 142.9;144.0 (CH-OH, Ph), 171.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 213.1 (C
O), 213.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S).
S).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water binary mixture (90/10) and 100 mg of DOPA-XA were added, the mixture was stirred for 48 h at room temperature (20 °C). The resulting BTO-XA particles were then washed repeatedly via cycles of centrifugation–redispersion in acetone until complete disappearance of the DOPA-XA NMR signal in the supernatant solution. The particles were subjected to TGA to estimate the amount of DOPA-XA grafted on the BTO particles.
water binary mixture (90/10) and 100 mg of DOPA-XA were added, the mixture was stirred for 48 h at room temperature (20 °C). The resulting BTO-XA particles were then washed repeatedly via cycles of centrifugation–redispersion in acetone until complete disappearance of the DOPA-XA NMR signal in the supernatant solution. The particles were subjected to TGA to estimate the amount of DOPA-XA grafted on the BTO particles.
SI-RAFT copolymerization of VDF and TrFE was carried out in thick 8 mL Carius tubes in which a solution of tert-amyl peroxy-2-ethylhexanoate (Trigonox® 121, 1.8 mg, 8.03 × 10−6 mol), CTA-XA (5.6 mg, 2.68 × 10−5 mol) and in DMC (3 mL) and 1 mL of a suspension of BTO-DOPA-XA in DMC (70 mg mL−1) was added and then degassed by performing at least three freeze–pump–thaw cycles. The gaseous monomers were introduced into the Carius tube at the liquid nitrogen temperature (TrFE, 0.66 g, 8.03 × 10−3 mol, ΔP = 0.35 bar and VDF, 1.2 g 0.019 mol, ΔP = 0.8 bar) using a custom-made manifold that enables accurate measurement of the amounts of gas (using “pressure drop vs. mass of monomer” calibration curves). The tube was then sealed under dynamic vacuum at the liquid nitrogen temperature, before being placed horizontally in a shaking water bath thermostated at 73 °C. The tubes were opened after 16 h of polymerization. The particles were washed by cycles of centrifugation–redispersion in acetone until complete disappearance of the polymer 19F NMR signal in the supernatant solution (at least 5 washing cycles). The supernatant solution was evaporated and the free polymer were analyzed by SEC and 1H and 19F NMR. The P(VDF-co-TrFE)@BTO particles were then dried overnight in a vacuum oven at 60 °C and investigated by TGA to estimate the amount of grafted polymer and the grafting density.
SI-RAFT polymerization of TrFE was carried out using the same protocol used for the copolymerization of VDF and TrFE. Briefly, a solution of tert-amyl peroxy-2-ethylhexanoate (Trigonox® 121, 1.7 mg, 7.3 × 10−6 mol), CTA-XA (5.1 mg, 2.4 × 10−5 mol) in DMC (3 mL) and 1 mL of a suspension of BTO-DOPA-XA in DMC (70 mg mL−1) was added and then degassed by performing at least three freeze–pump–thaw cycles. The gaseous monomers were introduced into the Carius tube at the liquid nitrogen temperature (TrFE, 2.0 g, 24.3 mmol, ΔP = 1.05 bar).
Results and discussion
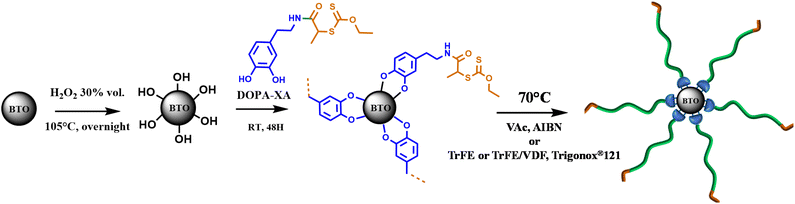
The strategy adopted to functionalize the barium titanate nanoparticles (BTO, diameter of 200 nm) is described in Scheme 1. The ceramic surface was pre-treated with hydrogen peroxide (35vol%) to increase the concentration of hydroxyl groups at the surface of the nanoparticles. Then, a new RAFT agent integrating a catechol fragment and a xanthate moiety (DOPA-XA) is grafted on the activated particles (BTO-OH) to obtain functionalized nanoparticles featuring xanthate moieties (BTO-XA). The BTO-XA surfaces were then used to mediate the SI-RAFT polymerization of TrFE and VDF from BTO nanoparticles surface.Synthesis of the DOPA-XA
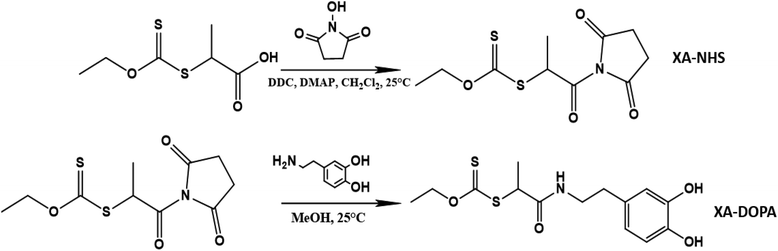
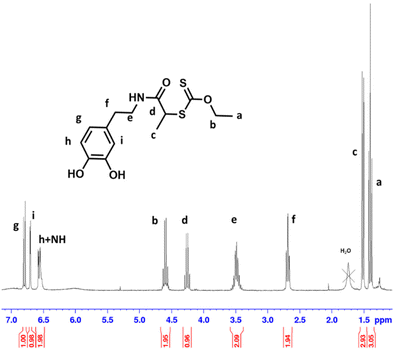
The RAFT agent DOPA-XA featuring a catechol group capable of binding onto BTO nanoparticles surface was prepared in two steps from 2-((ethoxycarbonothioyl)thio)propanoic acid and dopamine hydrochloride (Scheme 2). A particular attention was paid to introduce the catechol moiety on the R-group (re-initiating part) of the chain transfer agent to induce an efficient “grafting from” process from the BTO particles.52DOPA-XA was conveniently prepared from the coupling reaction of the N-hydroxysuccinimide activated ester of 2-((ethoxycarbonothioyl)thio)propanoic acid50 and commercially available dopamine hydrochloride. This synthesis was adapted from a previously reported procedure dealing with the preparation of a catechol-functionalised trithiocarbonate RAFT agent.53 The structure of DOPA-XA was confirmed by 1H NMR and 13C NMR (see ESI Fig. S3 and S4†). The 1H NMR spectrum of DOPA-XA (Fig. 1) revealed the presence of the characteristic signals of the catechol unit (6.5 to 7 ppm) and of the xanthate moiety at 1.3 and 4.6 ppm. Furthermore, the 13C spectrum (Fig. S1†) clearly displayed chemical shifts at 171.9 and 213.0 ppm ascribed to the amide carbonyl group and the thiocarbonyl fragment, respectively. DOPA-XA was not isolated in high yields due to significant loss during the flash chromatography purification process.
Grafting of DOPA-XA onto BTO nanoparticles
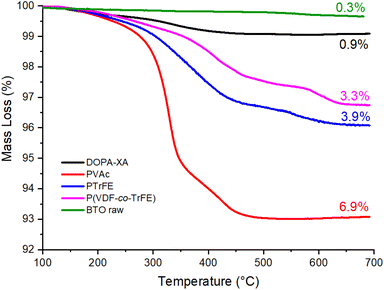
BTO nanoparticles with an average diameter of 200 nm were first activated to increase the density of hydroxyl groups on the BTO surface and allow the binding of a higher number of DOPA-XA molecules.54 Indeed, catechol groups display different interactions with surface hydroxyl groups including coordination55 (mostly with metallic materials), covalent bonding,56 and hydrogen bonding,57 the latter occurring with very hydrophilic surfaces. The activation procedure employed a solution of 35 vol% of hydrogen peroxide to generate hydroxyl groups at the surface of the BTO according to the work of Yang et al.40 and provided hydroxylated BTO surface labelled BTO-OH nanoparticles. DOPA-XA was then immobilized onto the surface of these BTO-OH nanoparticles as follows. BTO-OH particles were dispersed in a solution of DOPA-XA (10−2 M) solubilized in a protic acetone/water (90/10) binary mixture to favour the interactions between the catechol and the hydroxyl groups on the BTO-OH NPs surface.The grafting of the DOPA-XA onto the BTO NPs was investigated by TGA measurements (Fig. 2). The thermogram of the pristine BTO (raw BTO) particles showed a small mass loss of around 0.3% probably due to solvent or species poorly bonded on the BTO surface whereas the BTO-XA showed a mass loss of 0.9% in the 150 to 450 °C range corresponding to the thermal degradation of the DOPA-XA unit as depicted in Fig. S5.† The measurements of this mass loss and of the specific surface area of the raw BTO NPs by BET (ca. 4.5 m2 g−1) allowed the estimation of the DOPA-XA grafting density at 3.7 molecules per nm2 (see eqn (S1)†). This value is higher than the grafting density reported by Khani et al.58 (0.16 molecules per nm2), who modified amino-functionalized silica particles with a NHS-activated dithiobenzoate CTA but is in good agreement with previous studies by Ohno et al.42 dealing with the direct immobilisation of a silane-modified xanthate onto silica nanoparticles.
Further evidence of the grafting of DOPA-XA on the BTO NPs was obtained from XPS measurements. As depicted in Fig. 3, the XPS survey of the DOPA-XA-grafted BTO surface displayed the characteristic chemical elements of the DOPA-XA moiety. Indeed, a component at 398.4 eV was observed in the N 1s core level spectrum while two components at 151.6 and 162.7 eV were detected in the S 2p core level spectra and are attributed to nitrogen and sulfur chemical elements of DOPA-XA thus demonstrating the presence of DOPA-XA on the BTO surface. In addition, a S/N ratio of 2.38 was calculated from the XPS semi-quantitative analysis of the functionalized BTO in accordance with the theoretical ratio (S/N theoretical ratio = 2) from the DOPA-XA structure.
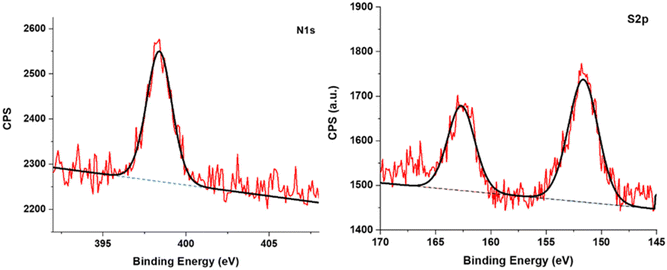 | ||
| Fig. 3 XPS spectra of the BTO nanoparticles functionalized with DOPA-XA showing the N 1s (left) and S 2p (right) core level regions. | ||
Surface-initiated RAFT polymerization from BTO
The surface-initiated RAFT polymerization was first conducted with vinyl acetate (VAc) as a test monomer to establish the suitability of the DOPA-XA@BTO NPs to mediate RAFT polymerization since VAc belongs to the Less Activated Monomers (LAMs) like VDF or TrFE and is thus controlled by xanthate RAFT agents.The conditions were adapted from the work of Ohno et al.42 who synthesized PVAc-decorated silica particles using SI-RAFT polymerization. The polymerization was carried out using O-ethyl-S-(1-methoxycarbonyl) ethyldithiocarbonate (CTA-XA) as a free xanthate agent containing the same Z group as the grafted DOPA-XA and known to control the RAFT polymerization of VDF48 and TrFE.45 According to Ohno et al., the role of this free CTA-XA in solution is to promote the exchange reactions between the free and grafted macroradicals thus affording a better control over the polymerisation. In addition, the same authors demonstrated that the free macroradicals have the same propagation rate as the grafted ones, making them remarkable tools to monitor the polymerisation.52 The reactions were conducted using 1 wt% of BTO-DOPA-XA nanoparticles without solvent. The small amount of CTA fixed on the BTO particles (4.7 mg of DOPA-XA for 1 g of BTO) was neglected as it represents only 5 mol% of the total amount of the free CTA used. The high[CTA-XA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [VAc] (1
[VAc] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) molar ratio was chosen to obtain high molar mass polymer brushes.
1000) molar ratio was chosen to obtain high molar mass polymer brushes.
After the polymerization, the free PVAc was separated from the reaction mixture by centrifugation and the conversion was analysed by NMR and the molar masses were analysed by SEC (Table 1) while the grafted NPs were analysed by TGA and TEM. 1H NMR and SEC analyses performed on the free PVAc polymer reveal a Mn of 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and a dispersity of 1.53 (entry 1, Table 1). This number average molar mass value may seem low compared to the theoretical 81
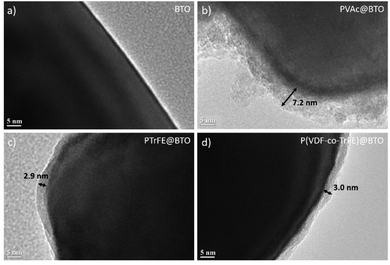
000 g mol−1 and a dispersity of 1.53 (entry 1, Table 1). This number average molar mass value may seem low compared to the theoretical 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 but could be explained by the combined effect of the PMMA calibration of the SEC instrument and the relatively high dispersity. This relatively broad molar mass distribution was expected, since VAc is prone to chain inversions which lead to a slowdown of the RAFT equilibrium and some loss of the control of the polymerization.59 The TEM images of the PVAc@BTO nanoparticles (Fig. 5b) clearly showed the PVAc polymer layer at the surface of the BTO nanoparticles with a thickness of 7.2 ± 1.5 nm. The grafting of PVAc was further demonstrated by TGA experiment which showed a mass loss of 6.9% for the PVAc@BTO NPs. Considering the average diameter of the BTO NPS, the Mn of the PVAc estimated by SEC (32
000 g mol−1 but could be explained by the combined effect of the PMMA calibration of the SEC instrument and the relatively high dispersity. This relatively broad molar mass distribution was expected, since VAc is prone to chain inversions which lead to a slowdown of the RAFT equilibrium and some loss of the control of the polymerization.59 The TEM images of the PVAc@BTO nanoparticles (Fig. 5b) clearly showed the PVAc polymer layer at the surface of the BTO nanoparticles with a thickness of 7.2 ± 1.5 nm. The grafting of PVAc was further demonstrated by TGA experiment which showed a mass loss of 6.9% for the PVAc@BTO NPs. Considering the average diameter of the BTO NPS, the Mn of the PVAc estimated by SEC (32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) and the mass loss measured by TGA, a grafting density of 0.30 chains per nm2 was calculated from eqn (S1)†. This grafting density is approximately ten times lower than that of DOPA-XA in agreement with previous reports dealing with surface initiated polymerization.60,42,52
000 g mol−1) and the mass loss measured by TGA, a grafting density of 0.30 chains per nm2 was calculated from eqn (S1)†. This grafting density is approximately ten times lower than that of DOPA-XA in agreement with previous reports dealing with surface initiated polymerization.60,42,52
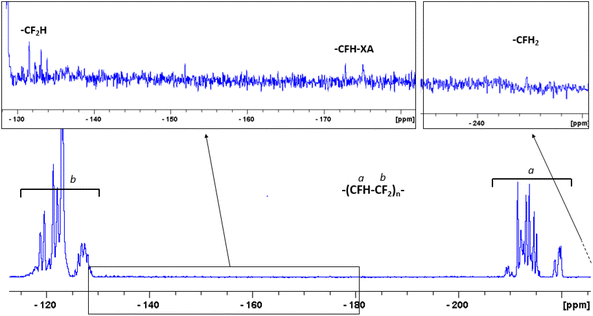 | ||
| Fig. 4 19F NMR spectrum of the free PTrFE chains recorded in CDCl3. The complexity of the backbone signal is due to tacticity and chain defects.44 | ||
| Entry | Reaction time (h) | Monomer | [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CTA] [CTA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I] d [I] d |
Conversion (%) | M nGPC (g mol−1) | Đ | Mass losse (%) | Graft density (chains per nm2) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
a Polymerization conducted in bulk at 70 °C during 5 h. I = AIBN.
b Polymerizations conducted in dimethylcarbonate, [M] = 6 mol L−1, 73 °C for 16 h. I = tert-amyl peroxy-2-ethylhexanoate.
c [VDF]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TrFE] = 7 [TrFE] = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.
d Particle concentration was around 1%wt, the amount of XA moities grafted on the particules was negleted.
e Mass loss recorded by TGA on polymer-grafted -nanoparticles. 3.
d Particle concentration was around 1%wt, the amount of XA moities grafted on the particules was negleted.
e Mass loss recorded by TGA on polymer-grafted -nanoparticles.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1a | 5 | VAc | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
94 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.53 | 6.9 | 0.30 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2b | 16 | TrFE | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3 |
–– | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.52 | 3.9 | 0.21 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3b | 16 | VDF & TrFEc | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 0.3 |
–– | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.9 | 3.3 | 0.33 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SI-RAFT polymerization was then conducted with fluoromonomers (entries 2 (TrFE) and 3 (VDF + TrFE) in Table 1). The polymerization conditions were chosen in agreement with the work of Guerre et al.47,48 and Bouad et al.44,45 The polymerization were conducted in dimethycarbonate (DMC) as this solvent has been shown to provide high polymerization rates and to be relatively less prone to transfer with the fluorinated macroradicals than other solvents.61 As for the polymerization of VAc, a high [CTA-XA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Monomer] ratio was chosen (1
[Monomer] ratio was chosen (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) to obtain high molar mass polymer brushes. The [VDF]
1000) to obtain high molar mass polymer brushes. The [VDF]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [TrFE] ratio was chosen at 7
[TrFE] ratio was chosen at 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, close to the azeotropic blend composition, to reduce drastically the compositionnal drift which would occur otherwise due to the different reactivity ratios of VDF and TrFE (rVDF−TrFE = 0.77 ± 0.04 and rTrFE−VDF = 0.32 ± 0.02).62,63 As the reaction was carried out in a sealed Carius tube, the monitoring of the reaction was impossible. At the end of the polymerization, the free polymer chains were separated from the decorated particles and subjected to 19F NMR and SEC measurements. As expected, the free PTrFE chains had high molar masses and acceptable dispersity (Mn (SEC) = 23
3, close to the azeotropic blend composition, to reduce drastically the compositionnal drift which would occur otherwise due to the different reactivity ratios of VDF and TrFE (rVDF−TrFE = 0.77 ± 0.04 and rTrFE−VDF = 0.32 ± 0.02).62,63 As the reaction was carried out in a sealed Carius tube, the monitoring of the reaction was impossible. At the end of the polymerization, the free polymer chains were separated from the decorated particles and subjected to 19F NMR and SEC measurements. As expected, the free PTrFE chains had high molar masses and acceptable dispersity (Mn (SEC) = 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1 and Đ = 1.5, entry 2 in Table 1). TrFE is even more prone to chain inversions or transfer reactions than VAc or VDF which lead to faster slowdown of the RAFT equilibrium and higher fraction of dead chains responsible for the significant increase in dispersity observed.44,45 This was confirmed by the 19F NMR investigations of the free PTrFE that showed an important loss of the chain end functionality as only 50% of these PTrFE chains were terminated by a xanthate moiety (-CFH-XA at −171 and −175 ppm, Fig. 4) at the end of the polymerization.44 The chains-ends resulting from irreversible transfer44 (i.e. terminated by a –CF2H group at −132 ppm or a –CFH2 group at −244.6 ppm, Fig. 4) constituted the other half of the PTrFE chains. The free P(VDF-co-TrFE) chains resulting from the copolymerization of VDF and TrFE mediated by the free RAFT agent had lower molar mass (13
500 g mol−1 and Đ = 1.5, entry 2 in Table 1). TrFE is even more prone to chain inversions or transfer reactions than VAc or VDF which lead to faster slowdown of the RAFT equilibrium and higher fraction of dead chains responsible for the significant increase in dispersity observed.44,45 This was confirmed by the 19F NMR investigations of the free PTrFE that showed an important loss of the chain end functionality as only 50% of these PTrFE chains were terminated by a xanthate moiety (-CFH-XA at −171 and −175 ppm, Fig. 4) at the end of the polymerization.44 The chains-ends resulting from irreversible transfer44 (i.e. terminated by a –CF2H group at −132 ppm or a –CFH2 group at −244.6 ppm, Fig. 4) constituted the other half of the PTrFE chains. The free P(VDF-co-TrFE) chains resulting from the copolymerization of VDF and TrFE mediated by the free RAFT agent had lower molar mass (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1) and higher dispersity (1.9) than the free PTrFE. Lack of knowledge on the 19F NMR signals of the chains ends of P(VDF-co-TrFE) copolymers made by RAFT polymerization prevents the estimation of the chain-end functionality. However, the relatively high dispersity observed is likely caused by the chain inversion and irreversible transfer phenomena that are known to occur for both RAFT homopolymerization of VDF and TrFE.45,47
500 g mol−1) and higher dispersity (1.9) than the free PTrFE. Lack of knowledge on the 19F NMR signals of the chains ends of P(VDF-co-TrFE) copolymers made by RAFT polymerization prevents the estimation of the chain-end functionality. However, the relatively high dispersity observed is likely caused by the chain inversion and irreversible transfer phenomena that are known to occur for both RAFT homopolymerization of VDF and TrFE.45,47
After the polymerization and several purification steps (centrifugation–redispersion), the particles were examined by TEM (Fig. 5). The TEM analyses revealed homogeneous polymer layers on the BTO nanoparticles with thicknesses of 2.9 ± 0.9 nm for the PTrFE@BTO (Fig. 5c) and 3.0 ± 1.2 nm for the P(VDF-co-TrFE)@BTO (Fig. 5d).
TGA analyses (Fig. 2) were performed to further demonstrate the grafting of the fluoropolymer layer onto the BTO nanoparticles. The PTrFE@BTO and P(VDF-co-TrFE)@BTO NPs thermograms showed mass losses of 3.9% and 3.3% respectively, leading to estimated grafting densities of 0.2 and 0.3 chains per nm2 following the calculations described in eqn (S1)†. These values are in good agreement with previous results found for the SI-RAFT of VAc.42
Finally, the presence of a fluoropolymer layer on the BTO surface was investigated by XPS experiments. The XPS survey spectra of the raw and fluoropolymer-grafted BTO surfaces are presented Fig. 6. Interestingly, a decrease of the Ba 3d and Ti 2p signals from BTO was observed on PTrFE@BTO and P(VDF-co-TrFE)@BTO survey spectra (see Table S1†) compared to the raw BTO survey spectra, suggesting the presence of a grafted layer on the surface. Moreover, the XPS surveys of the functionalized particles clearly show the presence of fluorine in the grafted film as a F 1s component at 686.9 eV was observed in both cases thus confirming the presence of fluoropolymer on the BTO surface.
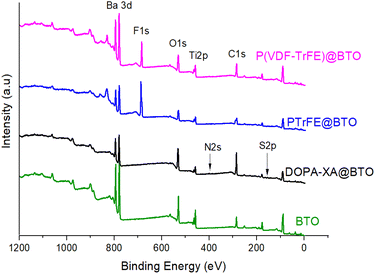 | ||
| Fig. 6 XPS survey spectra of raw BTO (green) and P(VDF-co-TrFE)@BTO (magenta), PTrFE@BTO (blue), DOPA-XA@BTO (black). Elaboration and piezoelectric properties of BTO based composites. | ||
The incorporation of nanoparticles bearing polymer brushes in a polymer matrix to promote interfacial adhesion as already been described in the literature64,65 in particular in dielectric composites.66 In this work, the same strategy was used to compatibilize the piezoelectric ceramic and the fluorocopolymer.
BTO, PTrFE@BTO and P(VDF-co-TrFE)@BTO particles were separately incorporated into a P(VDF-co-TrFE) matrix to prepare three different piezoelectric composites labelled BTO/CP, PTrFE@BTO/CP and CP@BTO/CP respectively. As expected, dispersing the PTrFE@BTO and P(VDF-co-TrFE)@BTO NPs in acetone was easier than dispersing the non-modified BTO. This observation is likely due to the presence of the polymer brushes grafted on the BTO particles which greatly stabilizes the dispersion in acetone as PTrFE and P(VDF-co-TrFE) show good solubilities in this solvent.
As the quality of the dispersion of BTO nanoparticles in the polymer matrix play an essential role on the nanocomposite physical properties, SEM images of the nanocomposites surface were recorded. As depicted in Fig. 7 the BTO/CP composite made from pristine BTO particles showed large domains of nanoparticles aggregation at a few micrometres scale with ceramic rich and polymer-rich areas. On the contrary, in the PTrFE@BTO/CP and CP@BTO/CP composites, the distribution of PTrFE@BTO and P(VDF-co-TrFE)@BTO nanoparticles appeared fairly homogeneous.
 | ||
| Fig. 7 SEM images of BTO, P(TrFE) @BTO and P(VDF-co-TrFE)@BTO nanoparticles embedded in a P(VDF-co-TrFE) matrix. The filler content was around 20 wt%. | ||
The DSC thermograms of the composites made with non-modified BTO, PTrFE@BTO and P(VDF-co-TrFE)@BTO presented in Fig. S6† did not show any difference in the Curie or melting temperatures and of the melt enthalpy. This indicates that the addition of modified BTO particles has no impact on the transition temperatures and on the crystalline properties of the polymer matrix.
In order to assess the piezoelectric responses, the three composite films were poled at 40 MV m−1 (i.e. at an electrical field lower than the breakdown strength) to allow the comparison of the piezoelectric coefficient (d33) with the same poling conditions. The measured d33 coefficient was quasi identical for the three composites with values of −9.2, −8.9 and −8.8 pC N−1 for BTO/CP, PTrFE@BTO/CP and CP@BTO/CP respectively. These results suggesting that the quality of the BTO dispersion did not have much impact on the piezoelectric response of the composites can actually be explained by the “under-solicitation” of the BTO nanoparticles during the poling step. Indeed, since the BTO particles have a much higher relative permittivity than the copolymer matrix, the effective electric field in the ceramic is greatly lower than the externally applied field.67
Finally, in order to probe the efficiency of the interphase, the composites were mechanically solicited by uniaxial stretching at 120 °C with an initial strain rate of 0.05 s−1. Fracture areas were then observed by SEM (Fig. 8).
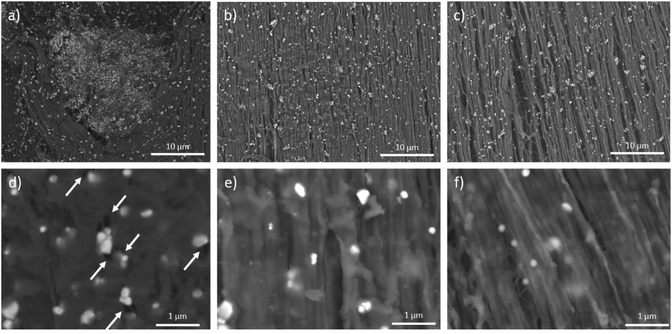 | ||
| Fig. 8 SEM images of stretched (a)–(d) BTO/CP, (b)–(e) PTrFE@BTO/CP and (c)–(f) CP@BTO/CP. Draw axis is vertical. | ||
At different microscopic scales, the SEM image of the BTO/CP composite showed a heterogeneous deformation with some stretched and unstretched areas related to the presence of BTO aggregates. In contrast, for the PTrFE@BTO/CP and CP@BTO/CP composites, fibrillar structures characteristic of the stretched copolymer68 with a fairly homogeneous BTO distribution were visible. At higher magnification, a significant number of cavities induced by the mechanical solicitation were visible close to the BTO nanoparticles in the BTO/CP composites (white arrows in Fig. 8d), while the grafting of the fluoropolymer layer onto the BTO nanoparticles was efficient enough to maintain a good interfacial adhesion with the copolymer matrix. If this strong interfacial cohesion was the desired result, it is somewhat surprising. Indeed, the relatively high grafting density of the fluoropolymer brushes (around 0.2–0.3 chains per nm2) indicates that they are in the concentrated polymer brush (CPB) regime which has been shown to hinder chain entanglement via size exclusion effect.69–71 This somewhat surprising result may be caused by the relatively high dispersity of the grafted polymers (1.5–1.9) which may prevent the size exclusion effect usually observed with polymer brushes featuring narrower molar mass distributions.
This study therefore shows the efficiency of using BTO piezoelectric nanoparticle grafted with a fluoropolymer layer such as PTrFE or P(VDF-co-TrFE) to obtain a cohesive interface with a P(VDF-co-TrFE) piezoelectric fluoropolymer matrix.
Conclusion
This work reports the preparation of Barium Titanate (BTO) – P(VDF-co-TrFE) piezoelectric composite using BTO nanoparticles decorated with fluoropolymers. A new xanthate chain transfer RAFT agent possessing a catechol unit was designed and grafted onto the surface of BTO nanoparticles with high grafting densities (>2 molecules per nm2). This grafted RAFT agent was used to mediate the SI-RAFT polymerization of vinyl acetate, and the copolymerization of trifluoroethylene and vinylidene fluoride from BTO nanoparticles surface with controlled molar mass and acceptably low molar mass distributions for these polymers. The fluoropolymer-functionalized BTO nanoparticles were incorporated into a P(VDF-co-TrFE) matrix to prepare piezoelectric composites. Interestingly, the functionalization of BTO nanoparticles by the fluoropolymers afforded better dispersions of the decorated nanoparticles in the fluoropolymer matrix. Moreover, under mechanical solicitation the interface between the functionalized BTO and the polymer matrix was shown to be more cohesive. The use of this functionalization strategy could greatly reduce the problems of compatibility between the polymer and the ceramic phases in piezoelectric composites paving the way for promising applications in piezoelectric devices. This work is aimed at getting better insights of ceramic/polymer interface in piezoelectric composites.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partly supported by the Japan Society for the Promotion of Science (JSPS) Summer program fellowship and the Kyoto University, Institute for Chemical Research International Joint Research programme 2019 (iJURC) granted to VB and VL respectively. This work was partly supported by the French National Research Agency (NanoPiC grant, ANR-16-CE08-0025). K. O. also acknowledges the funding from the Japan Society for the Promotion of Science (JSPS) KAKENHI Grants (No. 21H02000). The authors thank Arkema (Pierre-Bénite, France) for providing TrFE, VDF and P(VDF-co-TrFE). This work was carried out on the Electron Microscopy facility of the Advanced Characterization Platform of the Chevreul Institute. Technical assistance from Dr Alexandre Fadel in scanning electron microscopy experiments is gratefully acknowledged.References
- J. Curie and P. Curie, Bull. Soc. Fr. Mineral., 1880, 3, 90–93 Search PubMed
.
- W. Wersing, G. Zorn, K. Lubitz and J. Mohaupt, Jpn. J. Appl. Phys., 1985, 24, 724–726 CrossRef CAS
.
- M. Acosta, N. Novak, V. Rojas, S. Patel, R. Vaish, J. Koruza, G. A. Rossetti and J. Rödel, Appl. Phys. Rev., 2017, 4, 041305 Search PubMed
.
- A. Hamieh, F. Ponchel, S. Barrau and D. Remiens, Ferroelectrics, 2020, 556, 79–86 CrossRef CAS
.
- W. Hooker , NASA Langley Tech. Rep. Serv.
- A. Safari, J. Phys. III, 1994, 4, 1129–1149 CrossRef
.
-
B. Malič, D. Kuščer, M. Vrabelj and J. Koruza, in Magnetic, Ferroelectric, and Multiferroic Metal Oxides, Elsevier, 2018, pp. 95–120 Search PubMed
.
-
C. Ribeiro, C. M. Costa, P. Martins, V. Correia and S. Lanceros-Mendez, in Reference Module in Materials Science and Materials Engineering, Elsevier, 2018, pp. 1–11 Search PubMed
.
- M. P. Wenger, P. L. Almeida, P. Blanas, R. J. Shuford and D. K. Das-Gupta, Polym. Eng. Sci., 1999, 39, 483–492 CrossRef CAS
.
- T. Kitayama and S. Sugawara, Study Comm. Electron. Circuit Components Mater.
- H. Kawai, Jpn. J. Appl. Phys., 1969, 8, 975–976 CrossRef CAS
.
- R. G. Kepler and R. A. Anderson, Adv. Phys., 2006, 41, 1–57 CrossRef
.
- N. Jia, Q. He, J. Sun, G. Xia and R. Song, Polym. Test., 2017, 57, 302–306 CrossRef CAS
.
- P. Martins, A. C. Lopes and S. Lanceros-Mendez, Prog. Polym. Sci., 2014, 39, 683–706 CrossRef CAS
.
- A. Gebrekrstos, G. Madras and S. Bose, Cryst. Growth Des., 2019, 19, 5441–5456 CrossRef CAS
.
- J. Defebvin, S. Barrau, G. Stoclet, C. Rochas and J. M. Lefebvre, Polymer, 2016, 84, 148–157 CrossRef CAS
.
- R. Hasegawa, Y. Takahashi, Y. Chatani and H. Tadokoro, Polym. J., 1972, 3, 600–610 CrossRef CAS
.
- M. Kobayashi, K. Tashiro and H. Tadokoro, Macromolecules, 1975, 8, 158–171 CrossRef CAS
.
- S. Weinhold, M. H. Litt and J. B. Lando, Macromolecules, 1980, 13, 1178–1183 CrossRef CAS
.
- A. J. Lovinger, Macromolecules, 1982, 15, 40–44 CrossRef CAS
.
-
Y. Tajitsu, in Soft Actuators, Springer Japan, Tokyo, 2014, vol. 9784431547, pp. 203–215 Search PubMed
.
- B. Ploss, B. Ploss, F. G. Shin, H. L. W. Chan and C. L. Choy, Appl. Phys. Lett., 2000, 76, 2776–2778 CrossRef CAS
.
- S. T. Lau, K. Li and H. L. W. Chan, Ferroelectrics, 2004, 304, 19–22 CrossRef CAS
.
- L. Zhu and Q. Wang, Macromolecules, 2012, 45, 2937–2954 CrossRef CAS
.
- J. Chon, S. Ye, K. J. Cha, S. C. Lee, Y. S. Koo, J. H. Jung and Y. K. Kwon, Chem. Mater., 2010, 22, 5445–5452 CrossRef CAS
.
- F. J. Baltá Calleja, A. González Arche, T. A. Ezquerra, C. Santa Cruz, F. Batallán, B. Frick and E. López Cabarcos, Adv. Polym. Sci., 1993, 108, 3–27 CrossRef
.
- M. F. Lin, V. K. Thakur, E. J. Tan and P. S. Lee, RSC Adv., 2011, 1, 576–578 RSC
.
- K. Prabakaran, S. Mohanty and S. K. Nayak, J. Mater. Sci.: Mater. Electron., 2014, 25, 4590–4602 CrossRef CAS
.
- N. Jia, Q. Xing, G. Xia, J. Sun, R. Song and W. Huang, Mater. Lett., 2015, 139, 212–215 CrossRef CAS
.
- S. Dalle Vacche, F. Oliveira, Y. Leterrier, V. Michaud, D. Damjanovic and J. A. E. Månson, J. Mater. Sci., 2012, 47, 4763–4774 CrossRef CAS
.
- S. D. Vacche, D. Damjanovic, V. Michaud and Y. Leterrier, Materials, 2020, 13, 1–15 Search PubMed
.
- Y. Ai, J. Nie, G. Wu and D. Yang, J. Appl. Polym. Sci., 2014, 131, 41102 CrossRef
.
- A. Mayeen, M. S. Kala, S. Sunija, D. Rouxel, R. N. Bhowmik, S. Thomas and N. Kalarikkal, J. Alloys Compd., 2020, 837, 155492 CrossRef CAS
.
- J. Defebvin, S. Barrau, J. Lyskawa, P. Woisel and J. M. Lefebvre, Compos. Sci. Technol., 2017, 147, 16–21 CrossRef CAS
.
- Y. Song, Y. Shen, H. Liu, Y. Lin, M. Li and C. W. Nan, J. Mater. Chem., 2012, 22, 16491–16498 RSC
.
- Y. Zhang, J. Gao, H. Li, E. Wang, J. Zhang and L. Zhang, J. Mater. Sci.: Mater. Electron., 2016, 27, 11733–11738 CrossRef CAS
.
- Y. Niu, Y. Bai, K. Yu, Y. Wang, F. Xiang and H. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 24168–24176 CrossRef CAS PubMed
.
- Y. Niu, K. Yu, Y. Bai, F. Xiang and H. Wang, RSC Adv., 2015, 5, 64596–64603 RSC
.
- Y. Zhao and S. Perrier, Adv. Polym. Sci., 2016, 270, 77–106 CrossRef CAS
.
- K. Yang, X. Huang, L. Xie, C. Wu, P. Jiang and T. Tanaka, Macromol. Rapid Commun., 2012, 33, 1921–1926 CrossRef CAS PubMed
.
- S. Perrier, Macromolecules, 2017, 50, 7433–7447 CrossRef CAS
.
- K. Ohno, Y. Yahata, M. Sakaue and V. Ladmiral, Chem. – Eur. J., 2019, 25, 2059–2068 CrossRef CAS PubMed
.
- K. Yang, X. Huang, Y. Huang, L. Xie and P. Jiang, Chem. Mater., 2013, 25, 2327–2338 CrossRef CAS
.
- V. Bouad, M. Guerre, S. Zeliouche, B. Améduri, C. Totée, G. Silly, R. Poli and V. Ladmiral, Polym. Chem., 2021, 12, 2293–2304 RSC
.
- V. Bouad, M. Guerre, C. Totée, G. Silly, O. Gimello, B. Améduri, J.-F. Tahon, R. Poli, S. Barrau and V. Ladmiral, Polym. Chem., 2021, 12, 2271–2281 RSC
.
- M. Guerre, G. Lopez, T. Soulestin, C. Totée, B. Améduri, G. Silly and V. Ladmiral, Macromol. Chem. Phys., 2016, 217, 2275–2285 CrossRef CAS
.
- M. Guerre, S. M. W. Rahaman, B. Améduri, R. Poli and V. Ladmiral, Macromolecules, 2016, 49, 5386–5396 CrossRef CAS
.
- M. Guerre, B. Campagne, O. Gimello, K. Parra, B. Ameduri and V. Ladmiral, Macromolecules, 2015, 48, 7810–7822 CrossRef CAS
.
- X. Liu, O. Coutelier, S. Harrisson, T. Tassaing, J. D. Marty and M. Destarac, ACS Macro Lett., 2015, 4, 89–93 CrossRef CAS PubMed
.
- Y. Xiang, L. Li and S. Zheng, Polymer, 2018, 138, 113–123 CrossRef CAS
.
- F. Bargain, P. Panine, F. Domingues Dos Santos and S. Tencé-Girault, Polymer, 2016, 105, 144–156 CrossRef CAS
.
- K. Ohno, T. Akashi, Y. Huang and Y. Tsujii, Macromolecules, 2010, 43, 8805–8812 CrossRef CAS
.
- C. Zobrist, J. Sobocinski, J. Lyskawa, D. Fournier, V. Miri, M. Traisnel, M. Jimenez and P. Woisel, Macromolecules, 2011, 44, 5883–5892 CrossRef CAS
.
- J. Saiz-Poseu, J. Mancebo-Aracil, F. Nador, F. Busqué and D. Ruiz-Molina, Angew. Chem., Int. Ed., 2019, 58, 696–714 CrossRef CAS PubMed
.
- W. Laure, D. Fournier, P. Woisel and J. Lyskawa, Langmuir, 2017, 33, 3434–3443 CrossRef CAS PubMed
.
- H. Lee, N. F. Scherer and P. B. Messersmith, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12999–13003 CrossRef CAS PubMed
.
- Q. Ye, F. Zhou and W. Liu, Chem. Soc. Rev., 2011, 40, 4244 RSC
.
- M. M. Khani, D. Woo, E. L. Mumpower and B. C. Benicewicz, Polymer, 2017, 109, 339–348 CrossRef CAS
.
- M. Guerre, S. M. Wahidur Rahaman, B. Améduri, R. Poli and V. Ladmiral, Polym. Chem., 2016, 7, 6918–6933 RSC
.
- K. Ohno, T. Morinaga, K. Koh, Y. Tsujii and T. Fukuda, Macromolecules, 2005, 38, 2137–2142 CrossRef CAS
.
- A. D. Asandei, O. I. Adebolu and C. P. Simpson, J. Am. Chem. Soc., 2012, 134, 6080–6083 CrossRef CAS PubMed
.
- T. Soulestin, P. Marcelino Dos Santos Filho, V. Ladmiral, C. Totée, G. Silly, T. Lannuzel, F. Domingues Dos Santos and B. Ameduri, Macromolecules, 2017, 50, 503–514 CrossRef CAS
.
- T. Soulestin, V. Ladmiral, T. Lannuzel, F. Domingues Dos Santos and B. Améduri, Polym. Chem., 2017, 8, 735–747 RSC
.
- S. M. Maguire, N. M. Krook, A. Kulshreshtha, C. R. Bilchak, R. Brosnan, A. M. Pana, P. Rannou, M. Maréchal, K. Ohno, A. Jayaraman and R. J. Composto, Macromolecules, 2021, 54, 797–811 CrossRef CAS
.
- R. Zhang, B. Lee, M. R. Bockstaller, S. K. Kumar, C. M. Stafford, J. F. Douglas, D. Raghavan and A. Karim, Macromolecules, 2016, 49, 3965–3974 CrossRef CAS PubMed
.
- S. Virtanen, T. Krentz, J. Nelson, L. Schadler, M. Bell, B. Benicewicz, H. Hillborg and S. Zhao, IEEE Trans. Dielectr. Electr. Insul., 2014, 21, 563–570 CAS
.
- R. I. Mahdi and W. H. A. Majid, RSC Adv., 2016, 6, 81296–81309 RSC
.
- A. Ferri, S. Barrau, R. Bourez, A. Da Costa, M. H. Chambrier, A. Marin, J. Defebvin, J. M. Lefebvre and R. Desfeux, Compos. Sci. Technol., 2020, 186, 107914 CrossRef CAS
.
- C. Xu, K. Ohno, V. Ladmiral and R. J. Composto, Polymer, 2008, 49, 3568–3577 CrossRef CAS
.
- H. Chao and R. A. Riggleman, Polymer, 2013, 54, 5222–5229 CrossRef CAS
.
- S. K. Kumar, N. Jouault, B. Benicewicz and T. Neely, Macromolecules, 2013, 46, 3199–3214 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2py00825d |
| This journal is © The Royal Society of Chemistry 2022 |