Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Poly(sulfur ylides): a new class of zwitterionic polymers with distinct thermal and solution behaviour†
Georgia
Poulladofonou
and
Kevin
Neumann
 *
*
Systems Chemistry Department, Institute for Molecules and Materials, Radboud University Nijmegen, Heyendaalseweg 135, 6525 AJ Nijmegen, Netherlands. E-mail: kevin.neumann@ru.nl
First published on 6th July 2022
Abstract
Zwitterionic polymers have emerged as an important class of hydrophilic polymers and found widespread applications not only in biomedical science but also in nanotechnology as antifouling coatings, drug delivery vesicles and electronic conductors. For such applications, new structural forms of zwitterionic polymers with distinct functions are in high demand. However, to this day, their design is mostly limited to polybetaine and polyampholyte scaffolds, bearing a stoichiometric amount of positive and negative charge either in a single repeating unit or across the polymer, respectively. Here, we report on the synthesis and characterization of poly(sulfur ylides), a rarely encountered class of zwitterionic polymers and demonstrate that this new form of zwitterionic polymers displays not only a remarkable stability but also distinct solution and thermal behavior. We show that the readily available poly(sulfur ylides) are a valuable addition to the chemical toolbox of polymeric materials and have the potential to become an attractive alternative to polybetaines and polyampholytes.
Introduction
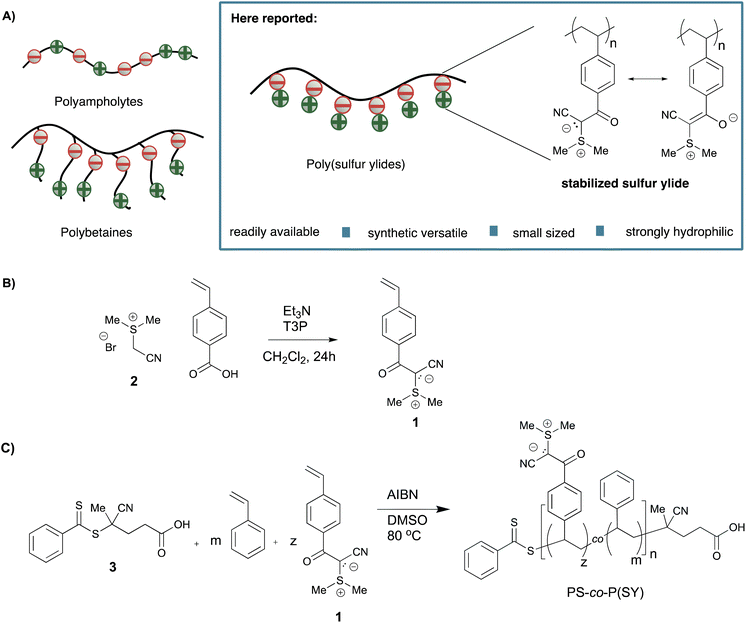
Polymers with a stoichiometric amount of positive and negative charges are called zwitterionic polymers. Such zwitterionic polymers have found widespread applications in the field of biomedical sciences because of their stimuli responsive nature.1–3 Most zwitterionic polymers can either be classified as polybetaines or polyampholytes, in which the oppositely charged moieties are either located in a single repeating unit, or statistically distributed alongside the polymer chain, respectively (Fig. 1).4,5 New applications of such zwitterionic polymers including antifouling coatings, drug delivery vesicles and phase separators make new forms of zwitterionic polymers indispensable.6–8 Recent efforts to develop new polymeric zwitterionic structures have focused predominately on developing polybetaines with new forms of cations or anions. For example, Brown et al. demonstrated that phosphonium cation bearing polybetaines are readily available forms of zwitterionic polymers displacing the frequently used ammonium cation.9While the effort to develop structurally diverse zwitterionic polymers has led to the design of several new polybetaines and polyampholytes, poly(ylides) are a rarely encountered class of zwitterionic polymers. Ylides – zwitterionic structures in which the negative charge is adjacent to the positive charge – are valuable building blocks in organic chemistry.10–12 Despite the wide use of ylides in small molecule chemistry, only very few reports of polymeric ylides exist. In these rare examples, polymeric ylides were formed as either reactive intermediates or precursors for subsequent reactions.13–15 The high reactivity of ylides attributes to the small number of reported poly(ylides), and consequently reduces their potential for applications in both the field of material science and biomedicine. Yet, we expect that poly(ylides) offer a great potential, considering their strong dipole moment, chemical versatility, and strong electrostatic interactions. Given these considerations, we sought to expand the chemical toolbox of zwitterionic polymers with a readily available and chemically diverse class of poly(ylides), namely poly(sulfur ylides). Here, we report on the synthesis and characterization of such poly(sulfur ylides) and show that their polymeric properties make them a valuable alternative to the frequently utilized polybetaines and polyampholytes (Fig. 1).
Results and discussion
We hypothesized that it is essential to chemically stabilize ylides in order to access poly(ylides) with enhanced stability and broad use for biomedical applications. To prove our hypothesis that poly(ylides) can be formed using stabilized ylide functionalities, we decided to investigate nitrile stabilized sulfur ylides, which were recently employed as remarkable stable building blocks in peptide chemistry.16,17 The reported nitrile-stabilized sulfur ylides are not only of high stability but are also characterized by high polarity, a major motivation for the design of zwitterionic polymers. At the outset of our research, we synthesized readily accessible styrenic sulfur ylide monomer 1 starting from vinyl benzoic acid and sulfonium salt 2.18,19 First, we investigated the copolymerization of bench stable monomer 1 with styrene using reversible addition−fragmentation chain transfer (RAFT) polymerization. Monitoring the progress of polymerization with 1H NMR revealed a controlled copolymerization of styrene and sulfur ylide monomer 1.Encouraged by these results, we synthesized a small library of representative copolymers with varying fractions of styrene and sulfur ylide (SY) units, ranging from FSY ≈ 0.1 to FSY ≈ 0.5 (Table 1). The polymerizations were carried out using RAFT agent 3 and AIBN at elevated temperatures. We intended to demonstrate that polymers with larger molecular weight Mn and high FSY are also accessible and synthesized PS-co-P(SY) 7 (FSY = 0.5 and Mn = 17.8 kDa). For comparison of the polymeric properties, we also synthesized one corresponding polystyrene homopolymer PS 8. Moreover, PS 8 was also utilized as a macro-RAFT agent to access block-copolymer PS-b-P(SY) 9.
| Code | X n | F SY | w SY | M n [kDa] | M n [kDa] | Đ |
|---|---|---|---|---|---|---|
| a Degree of polymerization Xn is determined by 1H NMR. b Fraction F of ylide monomer (SY) in copolymer as determined by 1H NMR. For readability, the values of FSY are rounded and the exact values are displayed in the ESI.† c Weight content w of ylide monomer (SY) in copolymer. d Molecular weight Mn determined by 1H NMR. e Molecular weight Mn determined by SEC. f Polydispersity as determined by SEC. n/a = analysis by SEC not applicable. Note: for PS-co-P(SY) with increasing amount of ylide monomer, the determined value for molecular weight Mn and Đ differs from 1H NMR because THF was no longer considered as good solvent. | ||||||
| PS-co-P(SY) 4 | 39 | 0.10 | 0.20 | 4.6 | 3.4 | 1.09 |
| PS-co-P(SY) 5 | 49 | 0.20 | 0.36 | 6.2 | 3.3 | 1.08 |
| PS-co-P(SY) 6 | 41 | 0.30 | 0.49 | 5.9 | n/a | n/a |
| PS-co-P(SY) 7 | 104 | 0.50 | 0.69 | 17.8 | n/a | n/a |
| PS 8 | 74 | 0 | 0 | 7.7 | 7.1 | 1.08 |
| PS-b-P(SY) 9 | 86 | 0.15 | 0.27 | 10.4 | 7.1 | 1.06 |
Analysis with size exclusion chromatography (SEC) revealed that the obtained copolymers displayed narrow polydispersity Đ (<1.10) and could be synthesized with high level of control. When purifying and analysing PS-co-P(SY), we readily observed that this class of poly(sulfur ylide) displayed properties distinct from polystyrene. Size exclusion chromatography in THF indicated a decreasing hydrodynamic radius for PS-co-P(SY) with increasing proportion of the ylide monomer, even by slight increase in Xn as observed for PS-co-P(SY) 4 and PS-co-P(SY) 5. This finding suggests that THF cannot be considered a good solvent anymore for copolymers bearing sulfur ylide moieties. Indeed, we observed that polymers with higher FSY (≥0.3) were insoluble in THF.
Interestingly, copolymers with high FSY become fully soluble in THF/water mixtures (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v
1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
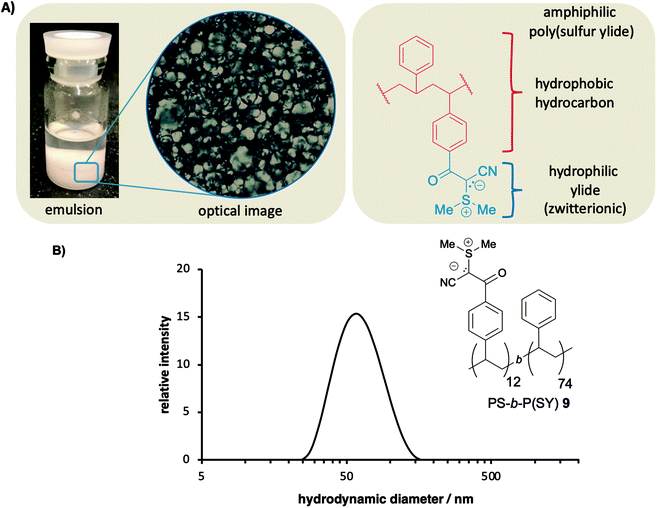
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v), which indicates a strong amphiphilic character between polymer backbone and sulfur ylide side chains. We tested the solubility in different solvent systems (ESI Table S1†) and observed that poly(sulfur ylides) indeed prefer solvent systems of large polarity. This solution behavior of poly(sulfur ylides) led us to question if this new class of zwitterionic polymers could act as emulsion stabilizer due to its strong amphiphilic behavior, a common characteristic of zwitterionic polymers.9 To test our hypothesis, we dissolved 0.5 mg mL−1 of PS-co-P(SY) 7 in a water/CHCl3 mixture (3
v), which indicates a strong amphiphilic character between polymer backbone and sulfur ylide side chains. We tested the solubility in different solvent systems (ESI Table S1†) and observed that poly(sulfur ylides) indeed prefer solvent systems of large polarity. This solution behavior of poly(sulfur ylides) led us to question if this new class of zwitterionic polymers could act as emulsion stabilizer due to its strong amphiphilic behavior, a common characteristic of zwitterionic polymers.9 To test our hypothesis, we dissolved 0.5 mg mL−1 of PS-co-P(SY) 7 in a water/CHCl3 mixture (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v
1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v). After vortexing of the mixture, an oil-in-water droplet emulsion was formed, which remained stable for several months (Fig. 2A). In contrast to PS-co-P(SY) 7, block copolymer PS-b-P(SY) 9 was utilized to stabilize water-in-oil droplets, which also remained stable for a prolonged time, thus demonstrating that already very low concentrations of sulfur ylides containing zwitterionic copolymers are sufficient for stabilizing emulsions for a prolonged time.
v). After vortexing of the mixture, an oil-in-water droplet emulsion was formed, which remained stable for several months (Fig. 2A). In contrast to PS-co-P(SY) 7, block copolymer PS-b-P(SY) 9 was utilized to stabilize water-in-oil droplets, which also remained stable for a prolonged time, thus demonstrating that already very low concentrations of sulfur ylides containing zwitterionic copolymers are sufficient for stabilizing emulsions for a prolonged time.
While defining polymeric ylides as zwitterionic polymers is discussed controversially in the field, the here reported poly(sulfur ylides) effectively prove that this class of polymers can display a key character of zwitterionic polymers, namely high hydrophilicity.20 Indeed, the high polarity observed for this class of sulfur ylide, which is stabilized by being placed in the α-position of a carbonyl moiety and conjugated to a nitrile, strongly suggests a zwitterionic structure.
One can imagine that the strong amphiphilic character of sulfur ylide containing block-copolymers can be utilized for the fabrication of polymeric nanostructures via self-assembly. Such polymeric self-assemblies including micelles and polymersomes have found wide-ranging applications, for example as tools in drug delivery or as nanoreactors.21,22 To prove this hypothesis, we investigated the behavior of block-copolymer PS-b-P(SY) 9 in aqueous solutions. Indeed, dynamic light scattering (DLS) indicates that PS-b-P(SY) 9 forms self-assemblies with size distribution of ∼67 nm in aqueous environments (Fig. 2B and ESI†). In addition, the presence of stable self-assemblies was confirmed with TEM images, which indicate a similar size distribution (ESI†). While further studies are essential to determine the exact nature of the observed self-assemblies, these results suggest that poly(sulfur ylides) hold great promises in the field of self-assembly.
In addition to the distinct solution behavior, the remarkable stability of poly(sulfur ylides) should be noted. Since stability is a crucial property for many applications of polymers, we investigated the chemical stability of such zwitterionic polymers under strongly acidic and basic aqueous environments. Finally, we tested the stability of poly(sulfur ylides) towards treatment with TFA as an organic acid under anhydrous conditions. We did not observe any degradation for PS-co-P(SY) 5 under these conditions, which demonstrates the remarkable chemical stability of poly(sulfur ylides) (ESI Fig. S2†).
Besides the solution behavior, for many applications the thermal behavior of zwitterionic polymers is crucial. For that reason, we turned our attention to the thermal behavior of sulfur ylide composing copolymers and investigated their influence on glass transition temperatures (Tg), a major characteristic of polymeric materials. For certain zwitterionic polymers higher Tg have been observed, which is attributed to restricted cooperative backbone segmental motion.23 We performed differential scanning calorimetry (DSC) analysis on copolymers 4–7 and observed a rapidly increasing Tg for copolymers with increasing FSY (Fig. 3 and ESI†). For example, PS-co-P(SY) 4 with FSY ≈ 0.1 exhibits a significantly higher Tg (117 °C) compared to unmodified PS 8 (87 °C). This trend continues also for copolymers with higher fraction of ylide moieties; for example, PS-co-P(SY) 7 with FSY ≈ 0.5 displays a Tg at 178 °C. By plotting Tg against the weight fraction wSY of the corresponding copolymers, a close alignment with the Fox equation is revealed, which indicates a truly random copolymer (Fig. 3). In addition, the good fit of the Fox-equation was confirmed by determining the Tg of polystyrene homopolymer 8 by extrapolation (88 °C), which matches the experimental value (87 °C). We also performed multiple repetitive heating/cooling cycles on these zwitterionic polymers and observed no indication of a change in the thermal behavior over time. In summary, the thermal behavior of this new class of poly(ylides) shows that sulfur ylides cannot only be used for precisely tailoring the glass transition temperature of polymers but also over a broad range of temperatures.
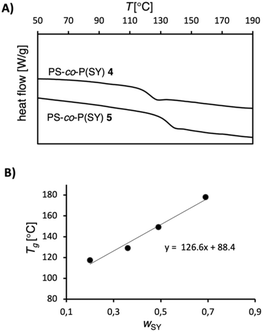 | ||
| Fig. 3 (A) Representative DSC traces of polymers 4 and 5 with a heating rate of 10 K min−1. (B) The glass transition temperatures of polymers 4–7 show a close alignment with the Fox equation. | ||
Conclusions
Poly(ylides) are a rarely encountered class of zwitterionic polymers. Here, we report on the synthesis and characterization of a new form of poly(ylides), namely poly(sulfur ylides) and their distinct solution and thermal behavior, including their strong amphiphilic character and high glass transition temperature. These distinct polymeric properties, together with their facile synthesis and remarkable chemical stability, make poly(sulfur ylides) an attractive alternative to existing zwitterionic polymers. We believe that this class of readily available and remarkably stable form of poly(ylides) will significantly extend the chemical toolbox of zwitterionic polymers, and we envision that poly(sulfur ylides) find widespread applications, not only in chemistry but also in biomedical research, nanomedicine and material science.Author contributions
G.P. carried out the experiments and collected the data. K.N. conceived, supervised and led the project. K.N. and G.P. prepared the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the startup-package of Radboud University. We would like to thank Prof. Dr Daniela A. Wilson for fruitful discussions. We thank Dr Paul White and the NMR facility of the IMM, Radboud University, for their help. We thank Helene Amatdjais-Groenen for support in SEC analysis.Notes and references
- M. Zhang, P. Yu, J. Xie and J. Li, J. Mater. Chem. B, 2022, 10, 2338–2356 RSC.
- A. Laschewsky and A. Rosenhahn, Langmuir, 2019, 35, 1056 CrossRef CAS PubMed.
- L. D. Blackman, P. A. Gunatillake, P. Cassa and K. E. S. Locock, Chem. Soc. Rev., 2019, 48, 757 RSC.
- A. B. Lowe and C. L. McCormick, Chem. Rev., 2002, 102(11), 4177 CrossRef CAS PubMed.
- K. M. Zurick and M. Bernards, J. Appl. Polym. Sci., 2014, 40069 Search PubMed.
- S. K. Lau and W. F. Yong, ACS Appl. Polym. Mater., 2021, 34390 Search PubMed.
- M. Harijan and M. Singh, J. Mol. Recognit., 2022, 1, e2944 Search PubMed.
- J. Zhao, D. Li, H. Han, J. Lin, J. Yang, Q. Wang, X. Feng, N. Yang, Y. Zhao and L. Chen, Langmuir, 2019, 35, 2630 CrossRef CAS PubMed.
- M. U. Brown, A. Triozzi and T. Emrick, J. Am. Chem. Soc., 2021, 143, 6528 CrossRef CAS PubMed.
- D. Kaiser, I. Klose, R. Oost, J. Neuhaus and N. Maulide, Chem. Rev., 2019, 119, 8701 CrossRef CAS PubMed.
- A. Sarbajna, V. S. N. Swamy and V. H. Gessner, Chem. Sci., 2021, 12, 2016 RSC.
- L. Roiser, K. Zielke and M. Waser, Asian J. Org. Chem., 2018, 7, 852 CrossRef CAS PubMed.
- D. Klinger, K. Nilles and P. Theato, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 832 CrossRef CAS.
- T. Yamamoto, B.-L. Lee, H. Hayashi, N. Saito and T. Maruyama, Polymers, 1997, 38, 4233 CrossRef CAS.
- M. Gandelman, K. M. Naing, B. Rybtchinski, E. Poverenov, Y. Ben-David, N. Ashkenazi, R. M. Gauvin and D. Milstein, J. Am. Chem. Soc., 2005, 127, 15265 CrossRef CAS PubMed.
- K. Neumann, J. Farnung, S. Baldauf and J. W. Bode, Nat. Commun., 2020, 11, 982 CrossRef CAS PubMed.
- T. Lam Pham, J. Zilke, C. C. Müller and F. Thomas, RSC Chem. Biol., 2022, 3, 426–430 RSC.
- K. W. Ratts and A. N. Yao, J. Org. Chem., 1966, 31, 1689 CrossRef CAS.
- E. Ziegler, H. Wittmann and H. Sterk, Monatsh. Chem., 1987, 118, 115 CrossRef CAS.
- A. Laschewsky, Polymers, 2014, 6, 1544 CrossRef.
- J. S. Lee and J. Feijen, J. Controlled Release, 2012, 161, 473 CrossRef CAS PubMed.
- H. Che and J. C. M. van Hest, J. Mater. Chem. B, 2016, 4, 4632 RSC.
- P. J. Scott, G. A. Spiering, Y. Wang, Z. D. Seibers, R. B. Moore, R. Kumar, B. S. Lokitz and T. E. Long, Macromolecules, 2020, 53, 11009 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2py00851c |
| This journal is © The Royal Society of Chemistry 2022 |