Open Access Article
Open Access ArticleSolution combustion synthesis of Ni-based hybrid metal oxides for oxygen evolution reaction in alkaline medium†
Aymen S. Abu Hatab ab,
Yahia H. Ahmad
ab,
Yahia H. Ahmad c,
Mohd B. Abdul Rahman
c,
Mohd B. Abdul Rahman *ab and
Siham Y. Al-Qaradawi
*ab and
Siham Y. Al-Qaradawi *c
*c
aDepartment of Chemistry, Faculty of Science, Universiti Putra Malaysia UPM, 43400 Serdang, Selangor, Malaysia. E-mail: basya@upm.edu.my
bIntegrated Chemical BioPhysics Research, Faculty of Science, Universiti Putra Malaysia UPM, 43400 Serdang, Selangor, Malaysia
cDepartment of Chemistry and Earth Sciences, College of Arts and Sciences, Qatar University, Doha 2713, Qatar. E-mail: siham@qu.edu.qa
First published on 11th January 2022
Abstract
Oxygen evolution reaction (OER) has arisen as an outstanding technology for energy generation, conversion, and storage. Herein, we investigated the synthesis of nickel-based hybrid metal oxides (NixM1−xOy) and their catalytic performance towards OER. NixM1−xOy catalysts were synthesized by solution combustion synthesis (SCS) using the metal nitrates as oxidizer and glycine as fuel. Scanning electron microscope (SEM) micrographs display a porous morphology for the hybrid binary NixM1−xOy, the common feature of combusted materials. X-ray diffraction (XRD) of NixM1−xOy depicted well-defined diffraction peaks, which confirms the crystalline nature of synthesized catalysts. The particle size of as-synthesized materials ranges between 20 and 30 nm with a mesoporous nature as revealed by N2-physisorption. The electrocatalytic performance of the as-prepared materials was evaluated towards OER in alkaline medium. Among them, NixCo1−xOy showed the best catalytic performance. For instance, it exhibited the lowest overpotential at a current density of 10 mA cm−2 (404 mV), onset potential (1.605 V), and Tafel slope (52.7 mV dec−1). The enhanced electrocatalytic performance of NixCo1−xOy was attributed to the synergism between cobalt and nickel and the alteration of the electronic structure of nickel. Also, NixCo1−xOy afforded the highest Ni3+/Ni2+ when compared to other electrocatalysts. This leads to higher oxidation states of Ni species, which promote and improve the electrocatalytic activity.
Introduction
The dramatic increase in the global energy demand, together with a growing environmental hazard resulting from fossil fuels, has triggered researchers' attention for developing new benign and clean sources of energy.1–3 The oxygen evolution reaction (OER) has grown to prominence as a critical technique in energy generation, conversion, and storage, such as hydrogen production via water electrolysis,4–6 regenerative fuel cells,7,8 and rechargeable metal-air batteries.9–11 Noble metals and their composites show enhanced catalytic performance towards OER, such as Pt, RuO2, and IrO2.12–14 However, the large-scale employment of these catalysts is hindered due to their low abundance, high cost, and/or low stability.15,16 For instance, in alkaline medium, Pt shows low activity caused by the formation of insulating platinum oxides, while RuO2 suffers from low stability at high potentials due to the generation of higher-valent ruthenium oxides.17In the last few decades, several researchers have synthesized and developed alternative electrocatalysts such as transition metal oxides and perovskites. These electrocatalysts are based on low-cost, stable, and earth-abundant nature metals such as Ni, Co, Fe, Mn, and Mo.18–21 Among them, nickel-based catalysts received considerable interest attributed to their high stability in alkaline media, low cost, and enhanced OER activity.4,7 For instance, Babar and co-workers synthesized a porous nano-wall NiO loaded directly on bare nickel foam; it exhibits remarkable OER electrocatalytic performance with an overpotential of 310 mV at a current density of 10 mA cm−2 and a Tafel slope of 54 mV dec−1, as well as enhanced OER durability and long-term stability (up to 24 hours).22
One of the reported approaches to enhance the reactivity of NiO is the mixing with other metal oxides, which is called the mixed transition metal oxides (MTMO) approach. MTMO have arisen as potential candidates for OER due to their high stability and outstanding electrochemical properties.23,24 In this regard, Ni has been coupled with several earth-abundant metals such as Co, Fe, Mn, Mo, and Cu.21,25–28 The hybrid materials show higher electrocatalytic activity and higher stability compared to pure NiO. For example, Qi et al. have reported the synthesis of a porous and high surface area Ni–Fe oxide through a coprecipitation strategy using Tween 85 as an organic surfactant. They revealed that Ni–Fe oxide is highly active for OER, showing low overpotentials of 328 mV and 420 mV at current densities of 10 mA cm−2 and 50 mA cm−2, respectively.29 Similarly, NiMoO4 has been synthesized hydrothermally and evaluated towards OER in an alkaline medium.27 It shows high electrocatalytic activity in alkaline conditions with an overpotential of 340 mV at a current density of 10 mA cm−2.27
The synthesis of hybrid Ni-based electrocatalysts for OER has been accomplished by different methods, such as solvothermal,30 hydrothermal,27,31 sol–gel combustion,32 and coprecipitation.29 Among them, solution combustion synthesis (SCS) has triggered increased interest assigned to its simplicity, quickness, repeatability, remarkable physiochemical properties, and ability for large-scale synthesis.33,34 In addition, the resulting products from SCS do not need any purification or post-synthesis treatment. Recently, two nickel-based MTMO have been synthesized through the SCS approach and evaluated as electrocatalysts towards OER. For instance, A. Kumar and co-workers synthesized NiCoO2 through a single-step SCS pathway, using the metal nitrates as oxidant (Ni and Co) and glycine as fuel. They investigated the OER performance of their as-prepared catalyst in 1.0 M KOH solution. They found that the NiCoO2 has a lower Tafel slope value (95 mV dec−1) compared to NiO (114 mV dec−1) and Co3O4 (145 mV dec−1), confirming its superior activity and performance towards OER.35 In the same context, NiMoO4 was synthesized through a low-cost and eco-friendly SCS, using ammonium molybdate tetrahydrate ((NH4)6Mo7O24·4H2O) and nickel nitrate hexahydrate (Ni(NO3)2·6H2O) as metal precursors, and agar as a fuel. The as-synthesized NiMoO4 has the lower Tafel slope value (48 mV dec−1) compared to NiO (51 mV dec−1), MoO3 (64 mV dec−1), and commercial NiMoO4 (67 mV dec−1), implying the outstanding OER performance of NiMnO4.36
Many researchers synthesized hybrid Ni-based MTMO using SCS and evaluated them as powerful electrocatalysts for OER. To our knowledge, no single study has compared the performance of different MTMO. Triggered by this, herein we reported the synthesis of Ni-based MTMO (NixM1−xOy) using the fast, reliable, and eco-friendly SCS, and we evaluated them towards OER. The as-synthesized electrocatalysts were analyzed by physico-chemical spectroscopy and morphology techniques. Their OER activity was assessed using electro-polarization methods (linear sweep voltammetry (LSV), chronoamperometry, chronopotentiometry) and electrochemical impedance spectroscopy.
Materials and method
Chemicals and materials
Nickel(II) nitrate hexahydrate (Ni(NO3)2·6H2O, ACROS Organics, ≥99%), cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O, BDH, ≥99%), iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O, Sigma Aldrich, ≥98%), manganese nitrate tetrahydrate (Mn(NO3)2·4H2O, Riedel-de Haën, ≥97%), ammonium molybdate tetrahydrate ((NH4)6Mo7O24·4H2O, BDH, ≥98%), chromium(III) nitrate nonahydrate (Cr(III)(NO3)3·9H2O, Fluka, ≥97%), copper(II) nitrate trihydrate (Cu(NO3)2·3H2O, Carl Roth, ≥98%), glycine (NH2CH2COOH, BDH, ≥99%), Nafion perfluorinated resin solution (Sigma Aldrich, 5 wt% in lower aliphatic alcohol and water), and potassium hydroxide (KOH, Carl Roth, 99.98%) were purchased and used without further purification.Synthesis of NiO by SCS
Ni(NO3)2·6H2O (872.4 mg, 3 mmol) and glycine (393.8 mg, 5.25 mmol) were weighed together and transferred to a 150 mL beaker containing 50 mL ultrapure water. The mixture was stirred continuously for 30 minutes to assure homogeneity. Afterward, the solution was heated quickly to boil until dryness. Upon dryness combustion occurs quickly with huge fire, getting a friable foam-like nanosized material. The as-synthesized product was transferred to a crucible boat and placed in a furnace oven (550 °C) overnight for calcination purposes.Synthesis of NixM1−xOy by SCS
Ni(NO3)2·6H2O (1454.0 mg, 5 mmol), metal salt source “Co, Fe, Mn, Mo, Cu, or Cr” (1 mmol), and glycine (787.5 mg, 10.5 mmol) were weighed together and transferred to 200 mL beaker containing 50 mL ultrapure water. The mixture was stirred continuously for 30 minutes to assure homogeneity. Afterward, the solution was heated quickly to boil until dryness. Upon dryness combustion occurs quickly with huge fire, getting a friable foam-like nanosized material. The as-synthesized product was transferred to a crucible boat and placed in a furnace oven (550 °C) overnight for calcination purposes.Material characterization
The size, morphology, and composition of as-synthesized materials were investigated using a field emission scanning electron microscope (FESEM, Philips XL-30, FEI Co., USA) equipped with an energy dispersive X-ray spectrometer (EDX). The powder wide-angle X-ray diffraction patterns (PXRD) were collected from an X'Pert-Pro MPD diffractometer (PANalytical Co., Netherlands) with a CuKα source of radiation (λ = 1.540598 Å). The elemental oxidation states were investigated by X-ray photoelectron spectroscopy (XPS) using a Kratos Axis Ultra XPS with a monochromatic Al-Kα radiation source (1486.6 eV). The morphology of electrocatalysts was further explored by transmission electron microscopy (TEM) using (FEI, Technai T20, Netherlands) operating at 200 kV. The specific surface area of synthesized materials was studied by from N2-physisorption experiments at the temperature of liquid nitrogen (77 K) using the BET (Brunauer–Emmett–Teller) method. The pore size and pore volume were estimated from the adsorption branch using the BJH (Barret–Joyner–Halenda) method.Electrochemical measurements
Electrochemical measurements were performed in a three-electrode system using a glassy carbon electrode (GCE, 5 mm diameter) as a working electrode, Pt wire as an auxiliary electrode, and Ag/AgCl electrode as a reference electrode. The electrolyte for the oxygen evolution reaction was 1.0 M KOH. The catalyst ink was prepared by dispersing 3 mg of each catalyst in a mixture 900 μL isopropanol and 100 μL Nafion (0.5 wt% in isopropanol). The ink solution was ultrasonicated for 1 hour. After that, 10 μL of the catalyst ink was drop-casted on the glassy carbon electrode and dried in air before electrochemical measurements. The electrochemical measurements were conducted on electrochemical analyzer (reference 3000, Gamry Co., USA).Prior to measurement, all electrodes were cleaned and activated in N2-saturated KOH solution for 200 cycles at a scan rate of 100 mV s−1. Polarization curves were recorded as linear sweep voltammetry (LSV) at a scan rate of 50 mV s−1. The electrochemical stability of NiO and NixM1−xOy were evaluated via chronopotentiometric measurements at 10 mA cm−2 for 10 h and chronoamperometric experiments at 750 mV vs. Ag/AgCl. Electrochemical impedance spectroscopy (EIS) measurements were performed at frequencies varying from 0.1 Hz to 100 kHz with an AC voltage pulse of 10 mV amplitude. The current densities (j) were normalized to the geometric surface area of the working electrode. After measurement, the structural stability of all samples was evaluated by XRD. In the same regard, all catalysts were drop-casted on a fluorine-doped tin oxide (FTO) substrate and analyzed by XRD after electrochemical measurements. The obtained potentials using Ag/AgCl electrode were converted to the reversible hydrogen electrode (RHE) by using the following equation:
| E(RHE) = E(Ag/AgCl) + 0.197 + (0.059 × pH) |
All the electrochemical data were 100% iR-corrected. The resistances (R) were obtained from the electrochemical impedance spectra.
Results and discussion
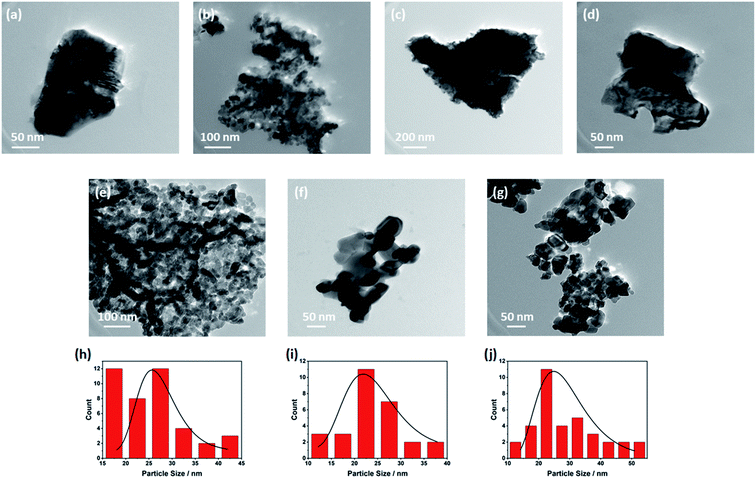
Solution combustion synthesis (SCS) is a promising approach for the preparation of nanoscale materials. It includes propagation of self-sustaining exothermic reactions in aqueous solutions. This method is considered as a powerful technique to produce a wide range of nanoscale materials, such as oxides, metals, alloys, and sulfides. SCS can be accomplished in aqueous solution of an oxidizer, usually metal nitrates (often favored because of their oxidizing property and high-water solubility) and a suitable organic fuel (contains C and H atoms) as a reducing agent (e.g., glycine, urea, sucrose, citric acid, etc.). Upon heating, the redox mixture is ignited and finally combusts in a self-sustaining rapid combustion reaction, yielding a nanocrystalline materials. Generally, the SCS method produces a substantial amount of gaseous by-products. This gasification causes a substantial expansion of the solid product and a fast drop in temperature after the reaction, resulting in a porous and finely dispersed solid product. These are regarded as the typical features in the SCS of nanoscale materials.33,34,37 Herein, we synthesized NiO and the hybrid binary NixM1−xOy materials through solution combustion synthesis pathway by mixing nickel nitrate and metal nitrate precursors such as Co, Fe, Mn, Mo, Cu, or Cr (5 mmol and 1 mmol, respectively) in the presence of glycine as a fuel. The SEM micrographs of NiO and its hybrid materials (Fig. 1) showed porous structure for all materials, which is a common feature of combusted materials. It is attributed to the evolution of a large amount of gases.34 The EDX analysis confirms the composition of NiO and binary hybrid metal oxides (Fig. S1†). The morphology and particle size of NiO and the binary oxides were further investigated by TEM analysis as displayed in Fig. 2. It shows that spherical shapes were obtained for NixCo1−xOy, NixMo1−xOy, and NixCr1−xOy (Fig. 2b, e and g, respectively) with an average particle size of 25.9 nm, 24.2 nm, and 29.0 nm, respectively. On the other hand, random agglomerates were obtained for NiO and NixFe1−xOy and NixCu1−xOy (Fig. 2a, c and f, respectively), and flakes-like structure for NixMn1−xOy (Fig. 2d).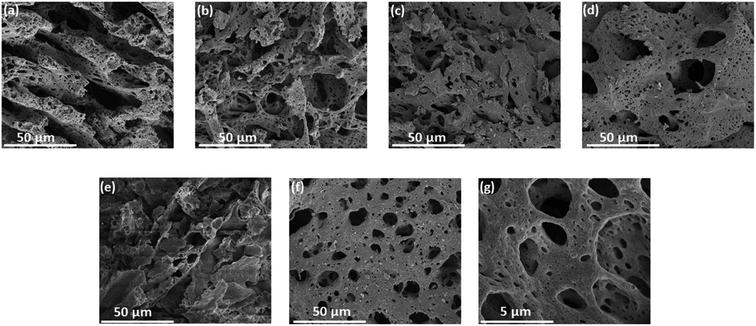 | ||
| Fig. 1 FESEM images of porous structures of (a) NiO, (b) NixCo1−xOy, (c) NixFe1−xOy, (d) NixMn1−xOy, (e) NixMo1−xOy, (f) NixCu1−xOy and (g) NixCr1−xOy. | ||
It is evident that the surface area and porosity of a catalyst are important parameters which affect the electrocatalytic performance.
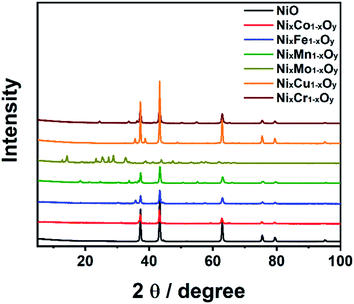
This can be attributed to the increase in the surface area that can enlarge the contact area between the electrolyte and the active sites of the catalysts, and hence improving the reaction kinetics.38–40 In this context, the textural properties of investigated catalysts were studied by N2-physisorption experiments. The N2 adsorption–desorption isotherms of NiO and the hybrid binary NixM1−xOy materials is depicted in Fig. 3. For all investigated catalysts, the isotherm was assigned to be type IV with H3 hysteresis, which is a characteristic feature for mesoporous materials.41 The surface area, pore volume and pore size for investigated electrocatalysts were calculated using Barret–Joiner–Halenda (BJH) model, and the results are presented in Table 1. The pore size distribution of all synthesized materials is presented in Fig. S2.† It was clearly found that the specific surface area of all investigated catalysts ranges between 6.8 and 29.8 m2 g−1 and pore diameter ranges between 8.5 and 12.3 nm. The pore diameter values confirm that NiO and the other hybrid binary NixM1−xOy materials are mesoporous materials. The crystalline structure and purity of the synthesized oxides were investigated using XRD measurements. Fig. 4 displays the recorded XRD patterns of NiO and NixM1−xOy's. The PXRD of NiO is consistent with the cubic NiO (JCPDS 47-1049), it shows well-defined and sharp peaks at 2θ = 37.2, 43.4, 62.9, 75.4, and 79.4, which are attributed to the crystal planes (111), (200), (220), (311) and (222), of NiO face-centered cubic (fcc, space group: Fm3hm), respectively (Fig. S3†). The XRD pattern of NixMo1−xOy can be indexed to β-NiMoO4 (JCPDS 12-0348), see Fig. S7.† On the other hand, the XRD patterns of NixCo1−xOy, NixFe1−xOy, NixMn1−xOy, NixCu1−xOy and NixCr1−xOy indicate the presence of face-centered cubic structure of NiO with the presence of weak diffraction peaks correspond to trace amount of Co3O4 (cubic, Fd3m), Fe3O4 (cubic, Fd3m), Ni6MnO8 (fcc, Fm3m), CuO (monoclinic, C2/c) and Cr2O3 (rhombohedral, R3c), respectively (Fig. S4–S6 and S8–S9†). Several reports discovered that at lower doping concentration and when the difference between the atomic radii of Mn+ (n = 2, 3 or 4) and Ni2+ is not remarkable, Ni2+ can be easily replaced with Mn+ within the same crystal lattice.42–48 It is known that the atomic radii of Ni2+, Co2+, Fe3+, Mn4+, Cr3+ and Cu2+ is 0.69 Å, 0.745 Å, 0.645 Å, 0.53 Å, 0.63 Å and 0.73 Å, respectively. This proves that at lower concentration of metal ions doping at NiO, some of the metal ions can replace Ni2+ at the rock salt of NiO with the presence of their metal oxide phase in the structure.
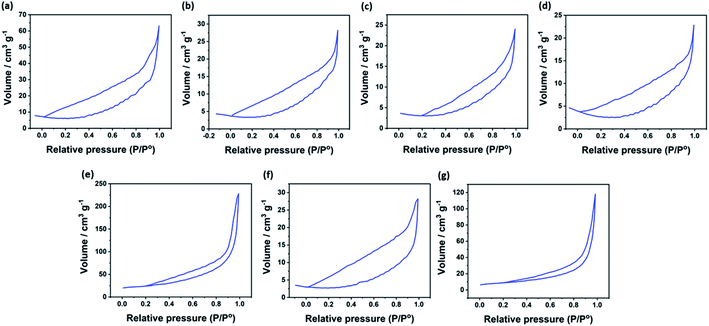 | ||
| Fig. 3 N2 adsorption–desorption isotherms of (a) NiO, (b) NixCo1−xOy, (c) NixFe1−xOy, (d) NixMn1−xOy, (e) NixMo1−xOy, (f) NixCu1−xOy and (g) NixCr1−xOy. | ||
| Catalyst | Surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore diameter (nm) |
|---|---|---|---|
| NiO | 17.3 | 0.098298 | 11.4 |
| NixCo1−xOy | 9.8 | 0.043892 | 9.0 |
| NixFe1−xOy | 8.4 | 0.037339 | 8.9 |
| NixMn1−xOy | 6.8 | 0.035503 | 10.5 |
| NixMo1−xOy | 83.4 | 0.354888 | 8.5 |
| NixCu1−xOy | 7.4 | 0.043915 | 11.9 |
| NixCr1−xOy | 29.8 | 0.183563 | 12.3 |
The chemical composition and oxidation states of elements in the hybrid binary NixM1−xOy materials were studied by XPS analysis. The survey spectra revealed the presence of Ni, O, and the corresponding element in each case, confirming their chemical composition (Fig. S10†). The emission spectrum of Ni, O, and other metals was fitted using the Gaussian fitting method. The Ni 2p spectrum is well-fitted with two spin–orbit doublets, which are characteristic of Ni2+ and Ni3+ (Fig. 5). For Ni2+, the fitting peaks were found in the range 852.7–854.1 eV and 870.3–871.8 eV for 2p3/2 and 2p1/2, respectively, whereas Ni3+ were found between 854.7–856.4 eV and 872.9–875.5 eV for 2p3/2 and 2p1/2, respectively.49–55 Two satellite peaks were also observed, which are attributed to bonding between Ni2+ and Ni3+ with oxygen.56 The O 1s spectrum is well fitted with three spin–orbit triplets, which are donated with O1, O2 and O3 (Fig. S11†). The peak of O1 is fitted located at 526.7–529.5 eV, and attributed to metal–oxygen bonding, and the peak of O2 lies between 529.4 and 531.4 eV, and correlated to the presence of defect sites related to the low oxygen coordination in small-particle size materials. Finally, the peak of O3 is located between 531.6–533.8 eV, and it can be attributed to the presence of a large number of physically and chemically bonded water molecules on or near the surface.49 The XPS of Co, Fe, Mn, Mo, Cu, and Cr in the hybrid binary NixM1−xOy materials revealed two spin–orbit doublets in all spectra. The oxidation states of all elements are as follows: first, the deconvoluted Co 2p spectrum of NixCo1−xOy showed two peaks for Co3+ and Co2+ (Fig. 6a).57 The Fe 2p spectrum for NixFe1−xOy revealed the presence of Fe2+ and Fe3+ (Fig. 6b).58 The deconvoluted Mn 2p spectrum of NixMn1−xOy revealed the formation of Mn4+ and Mn3+ oxidation states (Fig. 6c).51 The fitting of Mo 3d spectrum of NixMo1−xOy revealed two spin–orbit doublets, characteristic of Mo5+ and Mo6+ (Fig. 6d).59 The deconvoluted Cu 2p spectrum for NixCu1−xOy also shows the formation of a Cu+ and Cu2+ (Fig. 6e).53 Finally, the XPS spectrum of NixCr1−xOy revealed a pair of spin–orbit doublets for Cr3+ and Cr6+ (Fig. 6f).55
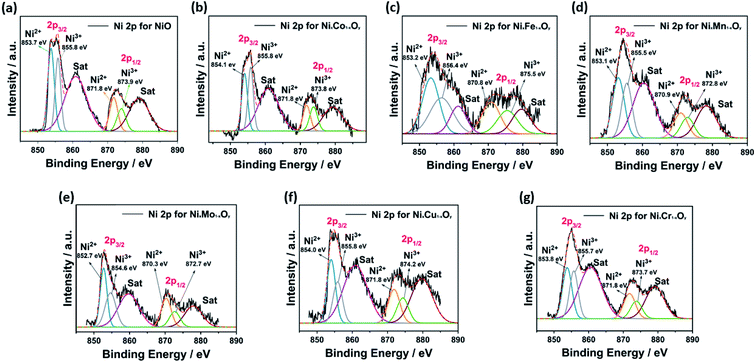 | ||
| Fig. 5 Ni 2p spectrum for (a) NiO, (b) NixCo1−xOy, (c) NixFe1−xOy, (d) NixMn1−xOy, (e) NixMo1−xOy, (f) NixCu1−xOy and (g) NixCr1−xOy. | ||
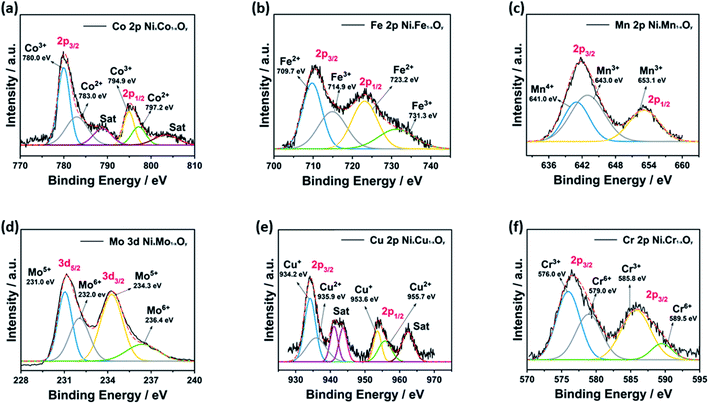 | ||
| Fig. 6 XPS analysis of (a) Co 2p of NixCo1−xOy (b) Fe 2p of NixFe1−xOy, (c) Mn 2p of NixMn1−xOy, (d) Mo 3d of NixMo1−xOy, (e) Cu 2p of NixCu1−xOy, (f) Cr 2p of NixCr1−xOy. | ||
The electrocatalytic performance of hybrid binary NixM1−xOy materials was investigated towards OER in alkaline medium. The majority of mixed metal oxides are suitable catalyst for OER in alkaline medium as their very low stability in acidic medium hindered their performance. In addition, the electrocatalysts for OER are only limited to noble metals and their oxides in acidic media.60,61 On the other hand, mixed metal oxides have their best performance in alkaline media.26 This is attributed to their high stability in alkaline medium, excellent oxygen anion mobility, and excellent oxygen exchange kinetics.26,60,61 The OER mechanism in alkaline medium can be represented by eqn (1)–(5).62
| M + OH− → MOH | (1) |
| MOH + OH− → MO + H2O(l) | (2) |
| 2MO → 2M + O2(g) | (3) |
| MO + OH− → MOOH + e− | (4) |
| MOOH + OH− → M + O2(g) + H2O(l) | (5) |
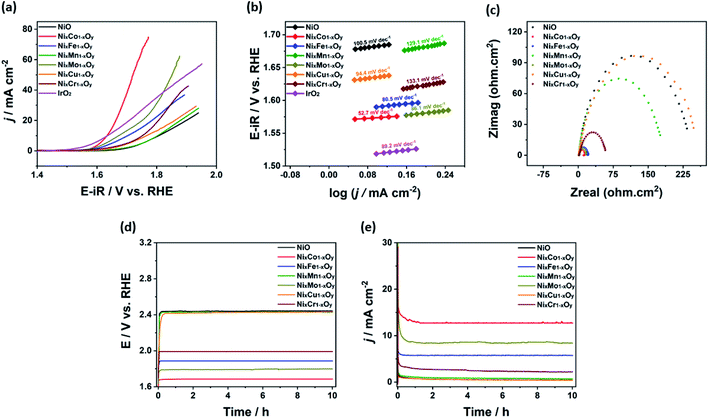
Ni-based catalysts demonstrated enhanced OER performance in alkaline medium. This was attributed to their advantageous characteristics, such as enhanced reaction kinetics and structure/performance stability.63 In this context, we studied OER performance of hybrid binary NixM1−xOy materials in 1.0 M KOH solution. Fig. 7a represents LSV of NixM1−xOy materials. The onset potential of the electrocatalysts follows the following order NixCo1−xOy < NixMo1−xOy < NixFe1−xOy < NixCr1−xOy < NixCu1−xOy < NixMn1−xOy < NiO. This confirms that the positive impact of doping Ni with other metals. OER activity was further evaluated by the value of overpotentials measured at a current density of 10 mA cm−2 (η10) and 1 mA cm−2 (η1). The calculated values for η10 were 585, 404, 473, 573, 442, 535, and 522 mV and for η1 were 443, 339, 353, 426, 344, 397, and 367 mV for NiO, NixCo1−xOy, NixFe1−xOy, NixMn1−xOy, NixMo1−xOy, NixCu1−xOy, and NixCr1−xOy, respectively. This demonstrates the preferential OER of the hybrid binary NixM1−xOy materials compared to NiO.
In addition, to obtain more insights about the OER kinetics of our electrocatalysts, the Tafel slopes were evaluated by measuring the slope of the linear part of the polarization curve (Fig. 7b). The values of Tafel slope were 100.5, 52.7, 80.5, 129.1, 86.1, 94.4, 133.1, and 89.2 mV dec−1 for NiO, NixCo1−xOy, NixFe1−xOy, NixMn1−xOy, NixMo1−xOy, NixCu1−xOy, NixCr1−xOy, and IrO2, respectively. This implies the enhanced OER performance of NixCo1−xOy electrocatalyst compared to the other hybrid binary Ni-based electrocatalysts. Table 2 summarizes the OER performance of some common electrocatalysts compared with NixCo1−xOy.
| Catalyst | Electrolyte | η1 @1 mA cm−2 (mV) | η10 @10 mA cm−2 (mV) | Tafel slope (mV dec−1) | Ref. |
|---|---|---|---|---|---|
| NixCo1−xOy | 1.0 M KOH | 339 | 404 | 52.7 | This work |
| CuO-from Cu-EA | 1.0 M KOH | 370 | 475 | 90 | 2 |
| Co3O4 | 0.1 M KOH | — | 450 | 89 | 5 |
| F-Ni-2 | 1.0 M KOH | — | 330 | 53 | 7 |
| F-NiAl LDH-NF | 1.0 M KOH | — | 250 | 77 | 7 |
| PtNi/Mn2O3–NiO | 0.1 M KOH | — | 529 | 140 | 8 |
| RuO2/Co3O4 | 1.0 M KOH | — | 305 | 69 | 14 |
| Ir/Ni oxide | 1.0 M KOH | 264 | 31 | 15 | |
| NiCo2O4 nanoflowers | 1.0 M KOH | — | 383 | 137 | 18 |
| NiCo2O4 hollow nanospheres | 1.0 M KOH | — | 428 | 141 | 18 |
| MoO3/Ni–NiO | 1.0 M KOH | 347 @100 mA cm−2 | — | 60 | 19 |
| 10% Ni-doped Mn3O4 | 1.0 M KOH | 235 @5 mA cm−2 | 283 | 165 | 20 |
NG02 (NiFe2O4/rGO 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 wt%) 5 wt%) |
1.0 M KOH | — | 302 | 63 | 21 |
| NiO/NF | 1.0 M KOH | — | 310 | 54 | 22 |
| NiCo2O4 | 1.0 M KOH | — | 292 | 62 | 23 |
| NiCo2O4/rGO (nanonet) | 0.1 M KOH | — | 450 | 53 | 25 |
| NiCo2O4/rGO (microsphere) | 0.1 M KOH | — | 510 | 62 | 25 |
| NiMoO4 nanorods | 1.0 M KOH | — | 340 | 45.6 | 27 |
| Cu2O–Cu hybrid foams | 1.0 M KOH | — | 250 | 67.52 | 28 |
| Ni–Fe oxide | 0.1 M KOH | 420 @50 mA cm−2 | 328 | 42 | 29 |
| Co0.25Ni0.75Fe2O4 | 1.0 M KOH | 380 @5 mA cm−2 | 450 | 50.5 | 32 |
| Co0.5Ni0.5Fe2O4 | 1.0 M KOH | 418 @5 mA cm−2 | 477 | 73.0 | 32 |
| Co0.75Ni0.25Fe2O4 | 1.0 M KOH | 401 @5 mA cm−2 | 460 | 52.2 | 32 |
The kinetics of the investigated electrocatalysts were studied using electrochemical impedance spectroscopy (EIS). Fig. 7c depicted the Nyquist plots of NiO and NixMo1−xOy in 1.0 M KOH solution. The plots show enhanced OER kinetics acquired by coupling Ni with other transition metals compared to NiO, Fig. 7c. It was clear that the radius of semicircle Nyquist plots decreased with increasing the performance of OER. Among the hybrid binary NixM1−xOy materials, NixCo1−xOy expressed the lowest charge transfer resistance, it has the smallest radius in semicircular Nyquist plots, suggesting faster charge transfer of charged species such as electron and OH− (electrons and OH− ions). This can be attributed to high oxidation state of Co3+ and Ni3+ existed in NixCo1−xOy, which can be confirmed from the deconvoluted fitted peaks under Ni 2p and Co 2p XPS spectra. It was clearly observed that the area ratio of Ni3+/Ni2+ for NixCo1−xOy was found to be 1.01 which is higher than other materials 0.75, 0.88, 0.77, 0.92, 0.77, and 0.85 for NiO, NixFe1−xOy, NixMn1−xOy, NixMo1−xOy, NixCu1−xOy, and NixCr1−xOy, respectively. In addition, the area ratio of Co3+/Co2+ was the highest for NixCo1−xOy (1.51) compared to the area ratio of Fe3+/Fe2+ in NixFe1−xOy (0.81), Mn4+/Mn3+ in NixMn1−xOy (0.65), Mo6+/Mo5+ (0.87), Cu2+/Cu+ (0.67) and Cr6+/Cr3+ (0.71). Several reports stated that the higher oxidation state speeds us and eases the charge transfer during the catalytic reaction in alkaline electrolytes leading to better performance on OER activity.11,64,65
The electrochemical stability of the hybrid binary NixM1−xOy materials was studied by chronoamperometric (j–t) and chronopotentiometric (E–t) measurements (Fig. 7d and e). The chronoamperometric measurement was performed at a fixed overpotential of 0.750 V in 1.0 M KOH solution versus Ag/AgCl electrode. It was clearly observed that there was no observed potential decay in curves for all prepared electrocatalysts until 10 hours. NixCo1−xOy had the highest current density at 0.750 V compared to other electrocatalysts, confirming its superior activity towards OER. In addition, the chronopotentiometric measurement was performed at a fixed current density of 10 mA cm−2 in 1.0 M KOH solution versus Ag/AgCl. It was noticeable that there was no current decay in all electrocatalysts. Similar to E–t measurements, NixCo1−xOy displayed stability at the highest overpotential when compared to other catalysts. This result is consistent with E–t measurements, LSV, and Tafel plots. To confirm the structural stability of the investigated electrocatalysts, XRD of samples after activity was performed, Fig. S12–S18.† It was found that the investigated electrocatalyst was stable and maintained crystallinity after measurement, which confirms the high stability of our electrocatalysts.
Several reports demonstrated that the presence of a high-valence state ion like Ni3+ is considered as real active sites of OER.66,67 In addition, coupling Ni to the higher oxidation states of other metal oxides i.e., Co3+, Fe3+, Mo6+, and Cr3+ enhanced the OER performance of hybrid materials.27,35,36,55,58 For example, NiCoO2 has an improved performance towards OER more than Co3O4 and NiO.35 This is due to the presence of a higher ratio of higher oxidation states species (Ni3+ and Co3+) on the surface compared to lower oxidation states species (Ni2+ and Co2+).35 This facilitates the charge carrier transfer across the catalyst/electrolyte interface, hence enhancing OER activity.35 Similarly, the presence of Fe3+ ion in the crystal lattice increases the conductivity of the catalytic sites. This suppresses the metal oxidation step and facilitates the metal reduction evolving step of O2 which results in enhancing the OER performance.68 This was proved by Xie et al. when they introduced a small amount of Fe3O4 species into a Ni/NiO. The charge transfer between Ni and Fe sites improved and provided more active sites for OER.58 In addition, it was reported that the presence of Mo6+ oxidation state as a dopant in NiFe alloy or as a part of crystal lattice in CaMoO4 boosted the OER activity.69 The presence of Mo in NiMoO4 enhanced the charge transfer process and promoted the generation of Ni3+ species, resulting in enhanced performance of OER compared to the bare MoO3 and NiO.27,36 According to earlier reports, the multivalent characteristics of Cr can also enable significant electron interactions inside the matrix of transition-metal complexes, therefore improving metal–metal synergy toward OER and hence catalytic activity.55 The electron configuration of Cr3+ is most likely t32ge0g, and this electron configuration is favorable for charge transfer and electron capture. As a result, Cr3+ can significantly enhance the formation Ni3+ species thus boost the OER activity.55 On the other hand, some reports stated that the presence of Mn and Cu with NiO might suppress the catalytic OER performance of their metals. Mn has been known to suppress the OER activity attributed to Jahn–Teller distortion, which causes suppression of OER activity. This is due to the octahedral position which is occupied by the Jahn–Teller active Mn3+, which decreases the OER activity.70 Also, thermodynamic studies revealed that Cu species do not undergo redox process in the same potential region that nickel species do. This leads to minimizing the formation of Ni3+ species during the OER process, thus suppressing the OER performance.48
According to the discussions above and to our electrocatalytic investigations, the superior OER performance of NixCo1−xOy can be assigned based on the following factors; first, the presence of Co3+ facilitates and accelerates the oxidation of Ni2+ to Ni3+ more than other metal ions. This can be clearly evidenced by deconvoluted XPS spectra of hybrid binary NixM1−xOy materials (Fig. 5, 6 and Table 1S†), where the area of deconvoluted 2p3/2 peak ratio of Ni3+/Ni2+ was obtained the highest amongst other investigated electrocatalysts. Second, from the deconvoluted XPS spectra, the ratio of the area of deconvoluted 2p3/2 peak Co3+/Co2+ was the highest when compared to other metals (see Table 1S†). As previously reported, the increase of higher oxidation states in metal oxides enhances the OER activity.11,64,65 Based on this, the activity of hybrid binary NixM1−xOy materials followed the same trend seen in the area ratio of Ni3+/Ni2+ and area ratio of Mn/Mn+m (NixCo1−xOy < NixMo1−xOy < NixFe1−xOy < NixCr1−xOy < NixCu1−xOy < NixMn1−xOy < NiO). On the other hand, NixMn1−xOy showed the lowest activity amongst all electrocatalysts. Our result was consistent with those reported for Mn-doped metal oxide, where they exhibited the lowest activities towards OER amongst other mixed metal oxides.71,72
Conclusions
In summary, this work has demonstrated the higher OER performance of the hybrid binary NixM1−xOy materials compared to bare NiO. These mesoporous nanomaterials with enhanced OER activity were synthesized by fast and economic solution combustion synthesis. They depicted porous nature with surface area ranging between 7.4 and 83.4 m2 g−1. In addition, the hybrid catalysts revealed a well crystalline structure. XPS spectra showed a mixed oxidation states for all transition metals with NixCo1−xOy revealing the highest content of high oxidation state. The hybrid binary NixM1−xOy materials showed higher OER performance than their consistent NiO in alkaline medium. NixCo1−xOy exhibited the highest performance amongst other investigated electrocatalysts. It displays an overpotential of 339 mV and 404 mV at a current density of @1 mA cm−2 and @10 mA cm−2, respectively, and a Tafel slope of 52.7 mV dec−1 in 1.0 M KOH solution. While the overpotential of other electrocatalysts ranges between 344–443 mV and 442–585 mV @1 mA cm−2 and @10 mA cm−2, respectively, and the Tafel slope ranges between 80.5-133.1 mV dec−1. The superior activity of hybrid binary NixCo1−xOy is attributed to synergistic effect between Ni and cobalt, and the amount of Ni3+ and Co3+ on surface. The research outcomes reveal a promising application of combusted nanomaterials toward OER in the future.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the technical support of Central Laboratories Unit (CLU), Center for Advanced Materials (CAM), and Gas Processing Center (GPC), Qatar University, Doha, Qatar.References
- Y. Lin, Z. Tian, L. Zhang, J. Ma, Z. Jiang, B. J. Deibert, R. Ge and L. Chen, Nat. Commun., 2019, 10, 162 CrossRef PubMed.
- X. Liu, S. Cui, M. Qian, Z. Sun and P. Du, Chem. Commun., 2016, 52, 5546–5549 RSC.
- S. Yan, Y. Xue, S. Li, G. Shao and Z. Liu, Int. J. Hydrogen Energy, 2020, 45, 1846–1856 CrossRef CAS.
- M. Favaro, W. S. Drisdell, M. A. Marcus, J. M. Gregoire, E. J. Crumlin, J. A. Haber and J. Yano, ACS Catal., 2017, 7, 1248–1258 CrossRef CAS.
- L. Li, T. Tian, J. Jiang and L. Ai, J. Power Sources, 2015, 294, 103–111 CrossRef CAS.
- S. W. Lee, C. Baik, T.-Y. Kim and C. Pak, Catal. Today, 2020, 352, 39–46 CrossRef.
- Y. Tong, H. Mao, P. Chen, Q. Sun, F. Yan and F. Xi, Chem. Commun., 2020, 56, 4196–4199 RSC.
- D. Mladenović, D. M. F. Santos, G. Bozkurt, G. S. P. Soylu, A. B. Yurtcan, Š. Miljanić and B. Šljukić, Electrochem. Commun., 2021, 124, 106963 CrossRef.
- C. Huang, Q. Ji, H. Zhang, Y. Wang, S. Wang, X. Liu, Y. Guo and C. Zhang, J. Colloid Interface Sci., 2022, 606, 654–665 CrossRef CAS PubMed.
- X. F. Lu, Y. Chen, S. Wang, S. Gao and X. W. Lou, Adv. Mater., 2019, 31, 1902339 CrossRef PubMed.
- J. Bian, Z. Li, N. Li and C. Sun, Inorg. Chem., 2019, 58, 8208–8214 CrossRef PubMed.
- Y. Wu, M. Tariq, W. Q. Zaman, W. Sun, Z. Zhou and J. Yang, ACS Appl. Energy Mater., 2019, 2, 4105–4110 CrossRef CAS.
- S. D. Ghadge, P. P. Patel, M. K. Datta, O. I. Velikokhatnyi, R. Kuruba, P. M. Shanthi and P. N. Kumta, RSC Adv., 2017, 7, 17311–17324 RSC.
- H. Liu, G. Xia, R. Zhang, P. Jiang, J. Chen and Q. Chen, RSC Adv., 2017, 7, 3686–3694 RSC.
- L. Gong, D. Ren, Y. Deng and B. S. Yeo, ACS Appl. Mater. Interfaces, 2016, 8, 15985–15990 CrossRef CAS PubMed.
- T. Gao, Z. Jin, M. Liao, J. Xiao, H. Yuan and D. Xiao, J. Mater. Chem. A, 2015, 3, 17763–17770 RSC.
- T. Maiyalagan, K. A. Jarvis, S. Therese, P. J. Ferreira and A. Manthiram, Nat. Commun., 2014, 5, 3949 CrossRef CAS PubMed.
- Z. Li, B. Li, J. Chen, Q. Pang and P. Shen, Int. J. Hydrogen Energy, 2019, 44, 16120–16131 CrossRef CAS.
- X. Li, Y. Wang, J. Wang, Y. Da, J. Zhang, L. Li, C. Zhong, Y. Deng, X. Han and W. Hu, Adv. Mater., 2020, 32, 2003414 CrossRef CAS PubMed.
- V. Maruthapandian, T. Pandiarajan, V. Saraswathy and S. Muralidharan, RSC Adv., 2016, 6, 48995–49002 RSC.
- P. Shinde, C. S. Rout, D. Late, P. K. Tyagi and M. K. Singh, Int. J. Hydrogen Energy, 2021, 46, 2617–2629 CrossRef CAS.
- P. T. Babar, A. C. Lokhande, M. G. Gang, B. S. Pawar, S. M. Pawar and J. H. Kim, J. Ind. Eng. Chem., 2018, 60, 493–497 CrossRef CAS.
- C. L. I. Flores and M. D. L. Balela, J. Solid State Electrochem., 2020, 24, 891–904 CrossRef CAS.
- M. L. Lindstrom, R. Gakhar, K. Raja and D. Chidambaram, J. Electrochem. Soc., 2020, 167, 046507 CrossRef CAS.
- E. Umeshbabu, G. Rajeshkhanna, P. Justin and G. R. Rao, J. Solid State Electrochem., 2016, 20, 2725–2736 CrossRef CAS.
- Y. H. Ahmad, K. A. Eid, S. Y. AlQaradawi and N. K. Allam, Sustainable Energy Fuels, 2017, 1, 1123–1129 RSC.
- X. Zhao, J. Meng, Z. Yan, F. Cheng and J. Chen, Chin. Chem. Lett., 2019, 30, 319–323 CrossRef CAS.
- H. Xu, J.-X. Feng, Y.-X. Tong and G.-R. Li, ACS Catal., 2017, 7, 986–991 CrossRef CAS.
- J. Qi, W. Zhang, R. Xiang, K. Liu, H.-Y. Wang, M. Chen, Y. Han and R. Cao, Adv. Sci., 2015, 2, 1500199 CrossRef PubMed.
- J. Zhang, B. Yuan, S. Cui, N. Zhang, J. Wei, X. Wang, D. Zhang, R. Zhang and Q. Huo, Dalton Trans., 2017, 46, 3295–3302 RSC.
- J. Wang, Y. Fu, Y. Xu, J. Wu, J.-H. Tian and R. Yang, Int. J. Hydrogen Energy, 2016, 41, 8847–8854 CrossRef CAS.
- V. Maruthapandian, M. Mathankumar, V. Saraswathy, B. Subramanian and S. Muralidharan, ACS Appl. Mater. Interfaces, 2017, 9, 13132–13141 CrossRef CAS PubMed.
- F.-t. Li, J. Ran, M. Jaroniec and S. Z. Qiao, Nanoscale, 2015, 7, 17590–17610 RSC.
- A. Varma, A. S. Mukasyan, A. S. Rogachev and K. V. Manukyan, Chem. Rev., 2016, 116, 14493–14586 CrossRef CAS PubMed.
- A. Ashok, A. Kumar, J. Ponraj, S. A. Mansour and F. Tarlochan, Int. J. Hydrogen Energy, 2019, 44, 16603–16614 CrossRef CAS.
- M. B. Rammal and S. Omanovic, Mater. Chem. Phys., 2020, 255, 123570 CrossRef CAS.
- S. L. González-Cortés and F. E. Imbert, Appl. Catal., A, 2013, 452, 117–131 CrossRef.
- S. Al Khateeb and T. D. Sparks, J. Mater. Res., 2019, 34, 2456–2471 CrossRef CAS.
- S. A. Khateeb, A. G. Lind, R. Santos-Ortiz, N. D. Shepherd and K. S. Jones, J. Electrochem. Soc., 2015, 162, A1667–A1674 CrossRef CAS.
- S. Al Khateeb, A. G. Lind, R. Santos-Ortiz, N. D. Shepherd and K. S. Jones, J. Mater. Sci., 2015, 50, 5174–5182 CrossRef CAS.
- F. Adam and I. A. Sugiarmawan, J. Porous Mater., 2008, 16, 321 CrossRef.
- S. Hasan, R. A. Mayanovic and M. Benamara, AIP Adv., 2018, 8, 056305 CrossRef.
- R. Sharma, A. D. Acharya, S. Moghe, S. B. Shrivastava, M. Gangrade, T. Shripathi and V. Ganesan, Mater. Sci. Semicond. Process., 2014, 23, 42–49 CrossRef CAS.
- Y. Dong, J. Yang, Y. Liu, Y. Wang, Z. Dong, M. Cui, M. Li, X. Yuan, X. Zhang and X. Dai, Dalton Trans., 2020, 49, 6355–6362 RSC.
- J. Wang, J. Cai, Y.-H. Lin and C.-W. Nan, Appl. Phys. Lett., 2005, 87, 202501 CrossRef.
- S. Hasan, R. A. Mayanovic and M. Benamara, MRS Adv., 2017, 2, 3465–3470 CrossRef CAS.
- M. Shkir, V. Ganesh, S. AlFaify, I. S. Yahia and H. Y. Zahran, J. Mater. Sci.: Mater. Electron., 2018, 29, 6446–6457 CrossRef CAS.
- A. C. Tavares, M. A. M. Cartaxo, M. I. da Silva Pereira and F. M. Costa, J. Electroanal. Chem., 1999, 464, 187–197 CrossRef CAS.
- J. Zhang, F. Liu, J. P. Cheng and X. B. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 17630–17640 CrossRef CAS PubMed.
- J. Jiang, C. Zhang and L. Ai, Electrochim. Acta, 2016, 208, 17–24 CrossRef CAS.
- X. Liu, J. Cao, C. Huang, Z. Chen, X. Fan, K. Shi, Z. Yang and W. Zhang, Mater. Res. Express, 2020, 7, 065502 CrossRef CAS.
- K. O. Egbo, S. K. Shil, C. G. Kwok, Y. Wang, C. P. Liu and K. M. Yu, J. Alloys Compd., 2021, 876, 160136 CrossRef CAS.
- Z. Fang, S. u. Rehman, M. Sun, Y. Yuan, S. Jin and H. Bi, J. Mater. Chem. A, 2018, 6, 21131–21142 RSC.
- Y. Wu, F. Li, W. Chen, Q. Xiang, Y. Ma, H. Zhu, P. Tao, C. Song, W. Shang, T. Deng and J. Wu, Adv. Mater., 2018, 30, 1803151 CrossRef PubMed.
- X. Liu and J. Wu, Electrochim. Acta, 2019, 320, 134577 CrossRef CAS.
- J. Liao, Y. Feng, W. Lin, X. Su, S. Ji, L. Li, W. Zhang, B. G. Pollet and H. Li, Int. J. Hydrogen Energy, 2020, 45, 8168–8176 CrossRef CAS.
- S. Chandra Sekhar, G. Nagaraju and J. S. Yu, Nano Energy, 2018, 48, 81–92 CrossRef CAS.
- Y. Xie, X. Wang, K. Tang, Q. Li and C. Yan, Electrochim. Acta, 2018, 264, 225–232 CrossRef CAS.
- W. Fang, P. Wang, Z. Ni, T. Sun, C. Xiang, K. Yue, R. Wang, J. Yang, Y. Zhou, C. Wang and Y. Yang, Adv. Mater. Interfaces, 2020, 7, 2000754 CrossRef CAS.
- D. Chen, C. Chen, Z. M. Baiyee, Z. Shao and F. Ciucci, Chem. Rev., 2015, 115, 9869–9921 CrossRef CAS PubMed.
- C. Hu, L. Zhang and J. Gong, Energy Environ. Sci., 2019, 12, 2620–2645 RSC.
- N. Yuan, Q. Jiang, J. Li and J. Tang, Arabian J. Chem., 2020, 13, 4294–4309 CrossRef CAS.
- Y. Chen, K. Rui, J. Zhu, S. X. Dou and W. Sun, Chem.–Eur. J., 2019, 25, 703–713 CrossRef CAS PubMed.
- C. X. Guo and C. M. Li, Chem. Commun., 2018, 54, 3262–3265 RSC.
- Y. Yan, C. Liu, H. Jian, X. Cheng, T. Hu, D. Wang, L. Shang, G. Chen, P. Schaaf, X. Wang, E. Kan and T. Zhang, Adv. Funct. Mater., 2021, 31, 2009610 CrossRef CAS.
- S. Ni, H. Qu, H. Xing, Z. Xu, X. Zhu, M. Yuan, L. Wang, J. Yu, Y. Li, L. Yang and H. Liu, ACS Appl. Mater. Interfaces, 2021, 13, 17501–17510 CrossRef CAS PubMed.
- Y. Wang, S. Tao, H. Lin, S. Han, W. Zhong, Y. Xie, J. Hu and S. Yang, RSC Adv., 2020, 10, 33475–33482 RSC.
- S. Anantharaj, S. Kundu and S. Noda, Nano Energy, 2021, 80, 105514 CrossRef CAS.
- M.-I. Jamesh and X. Sun, J. Power Sources, 2018, 400, 31–68 CrossRef CAS.
- S. Hirai, S. Yagi, A. Seno, M. Fujioka, T. Ohno and T. Matsuda, RSC Adv., 2016, 6, 2019–2023 RSC.
- B. Han, M. Risch, Y.-L. Lee, C. Ling, H. Jia and Y. Shao-Horn, Phys. Chem. Chem. Phys., 2015, 17, 22576–22580 RSC.
- M. Li, Y. Xiong, X. Liu, X. Bo, Y. Zhang, C. Han and L. Guo, Nanoscale, 2015, 7, 8920–8930 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07304d |
| This journal is © The Royal Society of Chemistry 2022 |