Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mixing and flow-induced nanoprecipitation for morphology control of silk fibroin self-assembly†
Saphia A. L. Matthewa,
Refaya Rezwan b,
Jirada Kaewchuchuenad,
Yvonne Perriea and
F. Philipp Seib
b,
Jirada Kaewchuchuenad,
Yvonne Perriea and
F. Philipp Seib *ac
*ac
aStrathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, 161 Cathedral Street, Glasgow, G4 0RE, UK. E-mail: philipp.seib@strath.ac.uk; Tel: +44 (0)141 548 2510
bDepartment of Pharmacy, ASA University Bangladesh, 23/3 Bir Uttam A. N. M. Nuruzzaman Sarak, Dhaka 1207, Bangladesh
cEPSRC Future Manufacturing Research Hub for Continuous Manufacturing and Advanced Crystallisation (CMAC), University of Strathclyde, Technology and Innovation Centre, 99 George Street, Glasgow G1 1RD, UK
dFaculty of Nursing, HRH Princess Chulabhorn College of Medical Science, Chulabhorn Royal Academy, Bangkok, Thailand
First published on 4th March 2022
Abstract
Tuning silk fibroin nanoparticle morphology using nanoprecipitation for bottom-up manufacture is an unexplored field that has the potential to improve particle performance characteristics. The aim of this work was to use both semi-batch bulk mixing and micro-mixing to modulate silk nanoparticle morphology by controlling the supersaturation and shear rate during nanoprecipitation. At flow rates where the shear rate was below the critical shear rate for silk, increasing the concentration of silk in both bulk and micro-mixing processes resulted in particle populations of increased sphericity, lower size, and lower polydispersity index. At high flow rates, where the critical shear rate was exceeded, the increased supersaturation with increasing concentration was counteracted by increased rates of shear-induced assembly. The morphology could be tuned from rod-like to spherical assemblies by increasing supersaturation of the high-shear micro-mixing process, thereby supporting a role for fast mixing in the production of narrow-polydispersity silk nanoparticles. This work provides new insight into the effects of shear during nanoprecipitation and provides a framework for scalable manufacture of spherical and rod-like silk nanoparticles.
Introduction
The control of silk fibroin multiscale structure under shear flow is important to the function of this biopolymer in the natural world and can be exploited in organic solvent-induced nanoprecipitation processes. Silk fibroin produced by the Bombyx mori silkworm is increasingly proposed for a range of drug delivery applications,1 as this biopolymer exhibits several favourable characteristics, including biocompatibility and biodegradability,2–4 and a number of products have been translated to the clinic.5 A variety of material types5 and crystallinities5–8 can also be accessed from the reverse-engineered silk solution, as the block copolymer primary structure can exist in a range of polymorphic states, notably silk I–II.9 The silk I polymorph has a high composition of β-turns, helices and random coils which bestow aqueous solubility. This metastable solution is found in the silkworm gland or is obtained by regeneration of the degummed silkworm cocoon.10The rate of structural conversion of aqueous silk I to the more thermodynamically stable and solid silk II structure can be increased by displacement of the protein hydration layer with a water-miscible organic solvent11–13 and the application of physical shear forces under flow.10 The intermolecular hydrogen-bonding ability of silk molecules under shear flow enables the spinning of liquid silk dope at ambient conditions and remarkably little work input.14 Similarly, this fundamental property determines the outcome of high shear fluid processing of the regenerated aqueous solutions into structures such as nanoparticles.
The physicochemical properties of nanoparticle drug delivery systems dictate the in vivo performance following parenteral administration, including immunogenicity, volume of distribution, controlled drug release and intracellular trafficking.15 Nanoparticles smaller than 100 nm in size can be filtered from systemic circulation by the hepatic endothelial fenestration, thereby lowering plasma half-life by accumulation in the liver.15 Conversely, the plasma half-life of nanoparticles above 200 nm in size can be reduced due to opsonisation and clearance by the mononuclear reticuloendothelial system.15 Therefore, nanoparticles of sizes between 100 and 200 nm with a low polydispersity index have shown the greatest clinical success as drug delivery systems.15 The nanoparticle morphology can also impart specific delivery and rheological properties16,17 and a change from spherical to cylindrical carriers has been shown to improve circulation in vivo.18
Silk particles of sub-micron size (25–200 nm) are suitable for drug delivery and can be manufactured by eight major methods (reviewed previously19): capillary microdot printing,20 desolvation,11,13,21,22 supercritical CO2,23 electrospraying,24 emulsification,25 ionic liquid dissolution,4 milling26 and electrogelation.27 Of these methods, desolvation is an accessible, low-energy–expenditure nanoprecipitation process which has been used to tune the key quality attributes of protein12,13,28 and polymeric29 nanoparticles. The one-step fabrication technique involves solvent shifting of aqueous, molecularly dissolved silk using at least a 200% v/v excess of a water-miscible organic solvent11,21,22 in which at least one of the blocks has a low solubility, resulting in supersaturation and spontaneous precipitation.29,30
Silk nanoformulations have many advantages, but further progress is needed for the manufacture of non-spherical particles by nanoprecipitation and for a better understanding of the silk self-assembly mechanism in semi-batch and continuous formats. Nanoprecipitation occurs due to the interdiffusion of the antisolvent and water molecules; therefore, it is highly dependent on the mixing conditions, as well as on the composition of the aqueous, solute and antisolvent components used in the mixture.29,30 This raises concerns regarding the commonly accepted lab-scale process of manual drop-by-drop silk feeding to an organic antisolvent in semi-batch format (e.g. 11 and 31), as this method results in a time-dependent change in the mixture composition. For this reason, in the last decade, microfluidics has emerged as an alternative to dropwise fabrication, as it can provide a continuous route that subjects all solute molecules to the same conditions, thereby allowing increased control over the mixture composition. Microfluidics have been used most extensively for silk water-in-oil emulsions, with narrow polydispersity particles with rod and spherical morphologies achieved by flow-focusing droplet microfluidics (145–200 μm).32,33 However, the micro-size of the emulsions obtained using microchannels32–34 limits the application of the resulting particles. The low flow rates required for the generation of nano-sized emulsions (51–1500 nm)25 can also limit the throughput speed of production on scale-up.
One solution has been the use of the staggered herringbone micromixer, which can provide high-throughput microfluidic-assisted manufacture of nanoparticles (110–310 nm) using silk nanoprecipitation in acetone and isopropanol antisolvents.12 High mixing efficiency occurs in the microchannel as the fluid interface is stretched and folded by asymmetric bas-relief herringbone structures that cause chaotic advection by creating continually switching transverse vortices.35 The staggered herringbone micromixer therefore decreases the mixing time compared to laminar flow regimes and semi-batch macromixing.35 Use of this micromixer has demonstrated a dependence of the physicochemical properties of the nanoparticles on the solvent properties, the total flow rate and the flow rate ratio of the aqueous and organic phases.12
An optimal nanoparticle batch, with a size of 110 nm, a polydispersity of 0.14, a zeta potential of −29.8 mV, a spherical morphology and aqueous stability, has been obtained in a staggered herringbone micromixer using a flow ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 isopropanol to silk and a total flow rate of 1 mL min−1.12 These conditions were used in further work to analyse the impact of the precursor molecular weight on nanoparticle properties by varying the sericin degumming time of silk cocoons.13 In both manual semi-batch and continuous formats, increasing the degumming time resulted in a greater molecular weight polydispersity of the silk stocks and reduced the nanoparticle size, polydispersity and zeta potential.13 By comparison, semi-batch production resulted in a significant decrease in surface charge,13 indicating that changes in the mixing time and the supersaturation between the two formats impacted the process of silk–silk association. However, spherical particle morphologies were consistently obtained for regenerated silk stocks.
1 isopropanol to silk and a total flow rate of 1 mL min−1.12 These conditions were used in further work to analyse the impact of the precursor molecular weight on nanoparticle properties by varying the sericin degumming time of silk cocoons.13 In both manual semi-batch and continuous formats, increasing the degumming time resulted in a greater molecular weight polydispersity of the silk stocks and reduced the nanoparticle size, polydispersity and zeta potential.13 By comparison, semi-batch production resulted in a significant decrease in surface charge,13 indicating that changes in the mixing time and the supersaturation between the two formats impacted the process of silk–silk association. However, spherical particle morphologies were consistently obtained for regenerated silk stocks.
The aim of the present study was to control the morphology and multiscale structure of silk self-assembly by varying the shear processing and supersaturation conditions under bulk and microfluidic mixing regimes. The effects of mixing on self-assembly were assessed across a range of silk precursor concentrations and shear rates using semi-batch mixing conditions of low and high mixing times as examples of bulk mixing processes for comparison with the staggered herringbone micromixer. This comparative work illustrates that control of silk nanoprecipitation across multiple length scales can be achieved under conditions of high shear and fast mixing and provides a platform for tuning high-shear, semi-batch processes.
Experimental
Materials
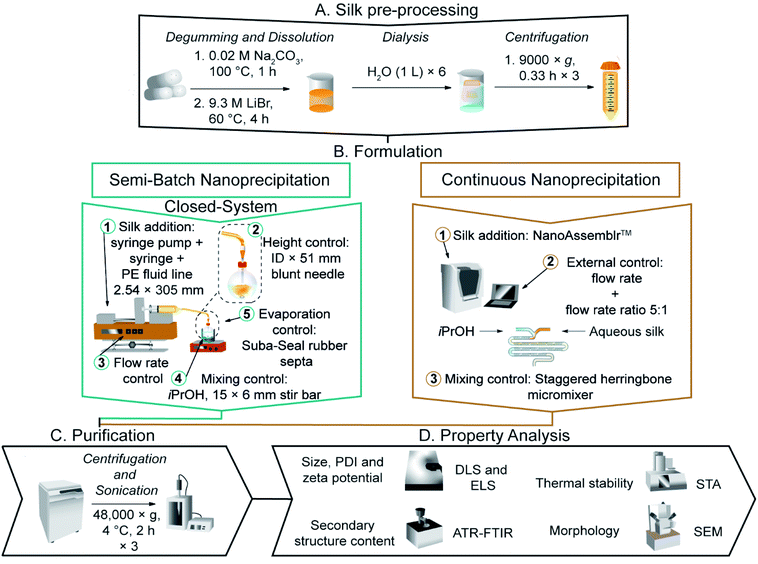
All studies were carried out at 18–22 °C, unless otherwise stated. Reagents and solvents were acquired from Acros Organics™ or Sigma Aldrich at >98% purity and used without additional purification. Runs for nanoparticle production in continuous format were defined as complete nanoparticle collection using one silk precursor solution (1 mL). Nanoparticle batches were obtained in continuous format and in semi-batch format using 0.5% w/v aqueous silk by mixing the product suspensions from three runs that used the same silk precursor solution prior to ultracentrifugation. Each independent experiment was repeated in triplicate using three different silk precursor stock solutions.Regeneration of B. mori silk
Silk fibroin from B. mori cocoons was regenerated using the 1 h sodium carbonate and 4 h lithium bromide method detailed previously,36 and described in video format elsewhere.11General drop-by-drop manufacture of silk nanoparticles in semi-batch format
Silk nanoparticles were manufactured by the addition of a 3% w/v silk solution (1 mL) to isopropanol (5 mL) in a short-neck round-bottom flask. The silk feed was supplied at room temperature using a syringe pump (Harvard Apparatus 22, Holliston, MA, USA) held at an inclination of 0–0.1° and equipped with a BD PLASTIPACK™ syringe, polyethylene Luer lock fluid line (2.54 × 305 mm) and blunt needle (Fig. 1). The flask was stoppered, and the mother liquor was incubated at room temperature for no longer than 0.5 h before dilution with ultrapure H2O in a polypropylene ultracentrifugation tube and centrifugation at 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 × g for 2 h at 4 °C (Beckmann Coulter Avanti® J-E equipped with JA-20 rotor). The supernatant was removed, the pellet was resuspended in ultrapure H2O (20 mL) and the suspension was sonicated twice for 30 seconds at 30% amplitude with a Sonoplus HD 2070 sonicator (ultrasonic homogeniser, Bandelin, Berlin, Germany). Ultrapure H2O (23 mL) was added to the suspension, and the centrifugation, washing and resuspension steps were repeated for a total of three times. The final pellet was suspended in ultrapure H2O (2–3 mL) and stored at 4 °C until use. Each experiment was repeated in triplicate using three different silk precursor stocks.
400 × g for 2 h at 4 °C (Beckmann Coulter Avanti® J-E equipped with JA-20 rotor). The supernatant was removed, the pellet was resuspended in ultrapure H2O (20 mL) and the suspension was sonicated twice for 30 seconds at 30% amplitude with a Sonoplus HD 2070 sonicator (ultrasonic homogeniser, Bandelin, Berlin, Germany). Ultrapure H2O (23 mL) was added to the suspension, and the centrifugation, washing and resuspension steps were repeated for a total of three times. The final pellet was suspended in ultrapure H2O (2–3 mL) and stored at 4 °C until use. Each experiment was repeated in triplicate using three different silk precursor stocks.
Calculations for needle residence time and shear rate were based on the literature value for dynamic viscosity (27 mPa s) of regenerated 3% aqueous silk37 and the calculated density (1.02 g mL−1) for the 3% w/v aqueous silk solution, with an assumed newtonian flow (Table 1).37 The Reynolds number was estimated based on the internal diameter of the needle.38 An upper limit of the residence time was estimated using the linear velocity and the needle length.39 The maximum shear rate was taken as the wall shear rate and, for simplicity, the shear rate calculations used the geometry of a straight cylinder. Calculations for the 3, 10 and 50 mL syringes used in the study were performed similarly, using the internal diameters stated by the manufacturer (Table S1†).
| Semi-batch format | Micro-mixer | ||||||
|---|---|---|---|---|---|---|---|
| Needle internal diameter/mm | Flow rate/mL min−1 | Residence time/ms | Maximum shear rate/s−1 | Re | Flow rate/mL min−1 | Maximum shear rate/s−1 | Re |
| a The average wall shear rate under laminar and newtonian flow is reported. The micromixer geometry was simplified to a rectangular channel by ignoring the groove depth. Residence times in the fluid line and needles were estimated using the linear velocity while the volumetric flow rate was used for the micromixer. | |||||||
| 0.33 | 0.017 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 335 335 |
80 | 0.04 | 0.001 | 80.1 | 0.04 |
| 1.000 | 261 | 4724 | 2.5 | ||||
| 3.510 | 74 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 581 581 |
8.5 | 0.059 | 4727 | 2.4 | |
| 7.000 | 37 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 068 068 |
17 | ||||
| 8.485 | 31 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 083 083 |
21 | ||||
| 16.96 | 15 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 119 119 |
41 | 0.50 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 073 073 |
20 | |
| 0.41 | 3.510 | 115 | 8646 | 6.9 | |||
| 0.6 | 3.510 | 246 | 2759 | 4.7 | |||
| 0.84 | 3.510 | 481 | 1005 | 3.4 | 1.00 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 114 114 |
40 |
| 1.19 | 3.510 | 966 | 354 | 2.4 | |||
| 1.60 | 3.510 | 1746 | 145 | 1.8 | |||
The effects of flow rate and initial addition height in closed, semi-batch format
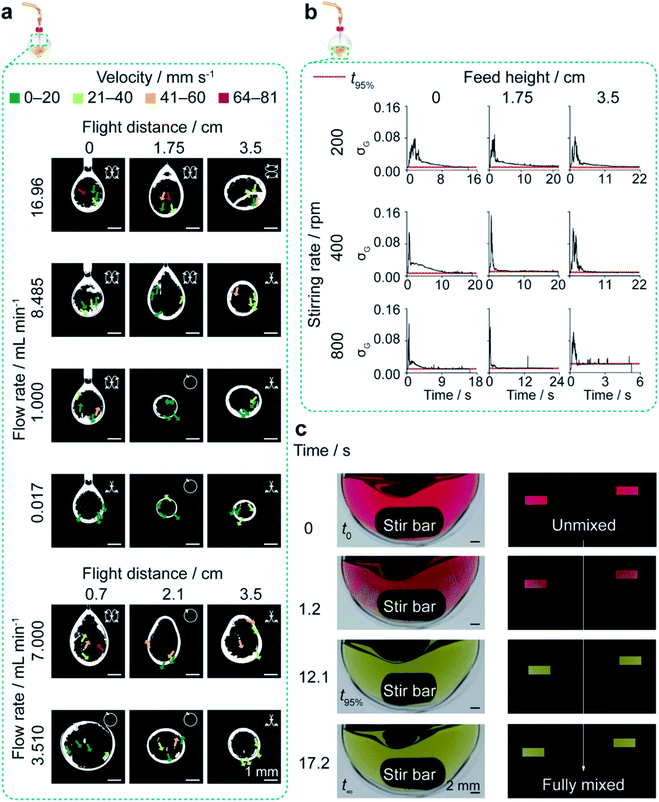
Silk nanoparticles were manufactured in 10 mL round-bottom flasks using a blunt needle (0.33 × 51 mm). At room temperature, a 3% w/v silk solution was added to isopropanol at flow rates of 0.017, 1.000, 3.510 or 7.000 mL min−1, while varying the initial addition height at 0.7, 2.1 or 3.5 cm from the isopropanol surface. The Reynolds numbers at 0.017, 1.000, 3.510 and 7.000 mL min−1 (calculated as 5.4 × 10−3, 0.32, 1.1 and 2.2 for the fluid line, and as 0.04, 2.5, 8.5 and 17 for the needle) indicated laminar flow (Tables S2† and 1).The effect of flow rate and concentration in the closed, semi-batch format
Silk nanoparticles were manufactured using a blunt needle (0.33 × 51 mm). Silk solution was added to isopropanol at rates of 0.017, 1.000, 8.485 or 16.96 mL min−1 from an addition height of 1.75 cm and the stirring rate was varied from 0 to 400 rpm using the egg-shaped stir bar. The silk concentration was 0.5, 2 or 3% w/v. The Reynolds numbers at 8.485 and 16.96 mL min−1 (calculated as 2.7 and 5.4 for the fluid line and 21 and 41 for the needle) indicated laminar flow (Table 1).The dual indicator system for mixing time described by Melton et al.40 and Weheliye et al.41,42 was used, with some adaptations. Stock solutions of thymol blue (0.095 mg mL−1) and methyl red (0.135 mg mL−1) were prepared in ethanol. The working solution was prepared by mixing and diluting the stock solutions to give 4.3 mg mL−1 thymol blue and methyl red in 70% v/v ethanol. To each 5 mL aliquot of the working solution, 0.5 M HCl was added at 0.5 mL L−1, and the system was equilibrated for at least 10 revolutions. An equivalent amount of NaOH (10.5 μL of 0.15 M NaOH) was then added to the mixture using a 20 μL Eppendorf pipette (attached to a clamp stand for control of the feed location and height). The mixing process was captured on an iPhone SE (Apple, Cupertino, CA, USA) reverse camera at a capture speed and resolution of 240 fps and 1080 p using FiLMiC Pro (FiLMiC Inc., Seattle, WA, USA). Each condition was repeated at least four times.
Images were extracted at 240 fps using FFmpeg.43 The images were processed using custom MATLAB (Mathworks, Natick, USA) scripts to apply rectangular masks of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pixels and to calculate the standard deviation of the normalised green channel intensity, as described by Rodriguez et al.44 The standard deviation of the fully mixed condition was calculated as the average of the final ten images, and the mixing time (t95%) was estimated as the time required to reach 95% of the standard deviation at the fully mixed condition (Table 2).
000 pixels and to calculate the standard deviation of the normalised green channel intensity, as described by Rodriguez et al.44 The standard deviation of the fully mixed condition was calculated as the average of the final ten images, and the mixing time (t95%) was estimated as the time required to reach 95% of the standard deviation at the fully mixed condition (Table 2).
| Semi-batch reactor | Micro-mixer | ||||
|---|---|---|---|---|---|
| Feed height/cm | Stirring rate/rpm | Mixing time/s | Flow rate/mL min−1 | Residence time/ms | Mixing time/ms |
| 0 | 200 | 16 ± 5.1 | 0.001 | 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| 400 | 15.7 ± 3.7 | ||||
| 800 | 5.3 ± 1.1 | 0.059 | 2034 | 306 | |
| 1.75 | 200 | 28.0 ± 3.7 | |||
| 400 | 8.4 ± 3.0 | 0.50 | 240 | 40 | |
| 800 | 2.0 ± 1.0 | ||||
| 3.50 | 200 | 16.9 ± 3.5 | 1.00 | 120 | 21 |
| 400 | 3.3 ± 3.0 | ||||
| 800 | 0.7 ± 0.1 | ||||
Semi-batch droplet analysis
Microfluidic-assisted manufacture of silk nanoparticles
Silk nanoparticles were manufactured using the NanoAssemblr™ benchtop instrument version 1.5 (model number NA-1.5-16; NanoAssemblr™, Precision Nano-Systems Inc. Vancouver, Canada) equipped with a cyclic olefin copolymer microfluidic cartridge (product codes: 1207 and 1151-034 BENCHTOP CARTRIDGE), as described elsewhere.12 Using the Y-junction of the two 25 mm inlet channels, the fluids were mixed in a 27 mm rectangular mixing channel (79 μm × 200 μm) having a series of raised grooves (31 μm × 50 μm) and four switchback turns.46 A 1 mL volume of aqueous silk solution (0.5–3% w/v) and isopropanol (5 mL) were injected into separate chamber inlets, and the nanoparticles formed in the staggered herringbone mixer were collected from the outlet. The total flow rate of the isopropanol and silk mixture was varied between 0.001–1.0 mL min−1, and the flow rate ratio was 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The cartridge was cleaned between runs using a water wash and a prime. The water wash consisted of a flow ratio of 1
1. The cartridge was cleaned between runs using a water wash and a prime. The water wash consisted of a flow ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ultrapure H2O/ultrapure H2O, a total volume of 2 mL and a total flow rate of 4 mL min−1; the wash was repeated in triplicate. The priming procedure consisted of a flow ratio of 5
1 ultrapure H2O/ultrapure H2O, a total volume of 2 mL and a total flow rate of 4 mL min−1; the wash was repeated in triplicate. The priming procedure consisted of a flow ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 isopropanol/ultrapure H2O, a total volume of 6 mL and a total flow rate of 1 mL min−1. The mother liquor suspension was incubated for no longer than 0.5 h before purification.
1 isopropanol/ultrapure H2O, a total volume of 6 mL and a total flow rate of 1 mL min−1. The mother liquor suspension was incubated for no longer than 0.5 h before purification.
Calculations were based on the literature viscosity value (3.14 mPa s) and density (0.837 g mL−1) values for the 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v isopropanol/water mixture measured at 20 °C,47 and newtonian flow was assumed.37 Increasing the flow rate from 0.001 to 1 mL min−1 resulted in mixing time estimates of 21 to 14
1 v/v isopropanol/water mixture measured at 20 °C,47 and newtonian flow was assumed.37 Increasing the flow rate from 0.001 to 1 mL min−1 resulted in mixing time estimates of 21 to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ms respectively, according to the manufacturer's guidelines and based on an analytical model for a similar system published elsewhere, by calculating the Peclet number to achieve a coefficient of variation of <0.1 (Table 2).48 The Peclet numbers were estimated as 4.27 × 108, 2.52 × 1010, 2.14 × 1011, and 4.27 × 1011 using the hydraulic diameter of the channel38 (142 μm) and the diffusion coefficient (3.5 × 10−10 m2 s−1) of the 5
500 ms respectively, according to the manufacturer's guidelines and based on an analytical model for a similar system published elsewhere, by calculating the Peclet number to achieve a coefficient of variation of <0.1 (Table 2).48 The Peclet numbers were estimated as 4.27 × 108, 2.52 × 1010, 2.14 × 1011, and 4.27 × 1011 using the hydraulic diameter of the channel38 (142 μm) and the diffusion coefficient (3.5 × 10−10 m2 s−1) of the 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 isopropanol/water mixture.49 The Reynolds numbers (0.04, 2.4, 20, 40) indicated laminar flow (Table 1). An upper limit of the residence time was estimated using the total fluidic volume and flow rate.39 The residence time was longer than the mixing time at all flow rates, indicating that complete mixing had occurred in the micromixer. The maximum shear rate was taken as the wall shear rate, with the assumptions that chaotic advection did not significantly affect the wall shear rate and that it created a significantly lower shear within the channel. For simplicity, the shear rate calculations used the geometry of a straight rectangular channel and did not take into account the groove depth.50
1 isopropanol/water mixture.49 The Reynolds numbers (0.04, 2.4, 20, 40) indicated laminar flow (Table 1). An upper limit of the residence time was estimated using the total fluidic volume and flow rate.39 The residence time was longer than the mixing time at all flow rates, indicating that complete mixing had occurred in the micromixer. The maximum shear rate was taken as the wall shear rate, with the assumptions that chaotic advection did not significantly affect the wall shear rate and that it created a significantly lower shear within the channel. For simplicity, the shear rate calculations used the geometry of a straight rectangular channel and did not take into account the groove depth.50
The correlation coefficients I of silk films, freeze-dried silk and nanoparticles were calculated by adaptation of a literature protocol53 using the air-dried silk film of an aqueous silk precursor batch as a reference. The second derivative of the FTIR absorption spectra was calculated and smoothed twice with a five-point Savitzky–Golay function and a polynomial order of 2. The processed silk sample was then compared with the reference over the spectral range 1600–1700 cm−1 using eqn (1).
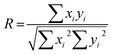 | (1) |
Results
Silk nanoparticle characterisation
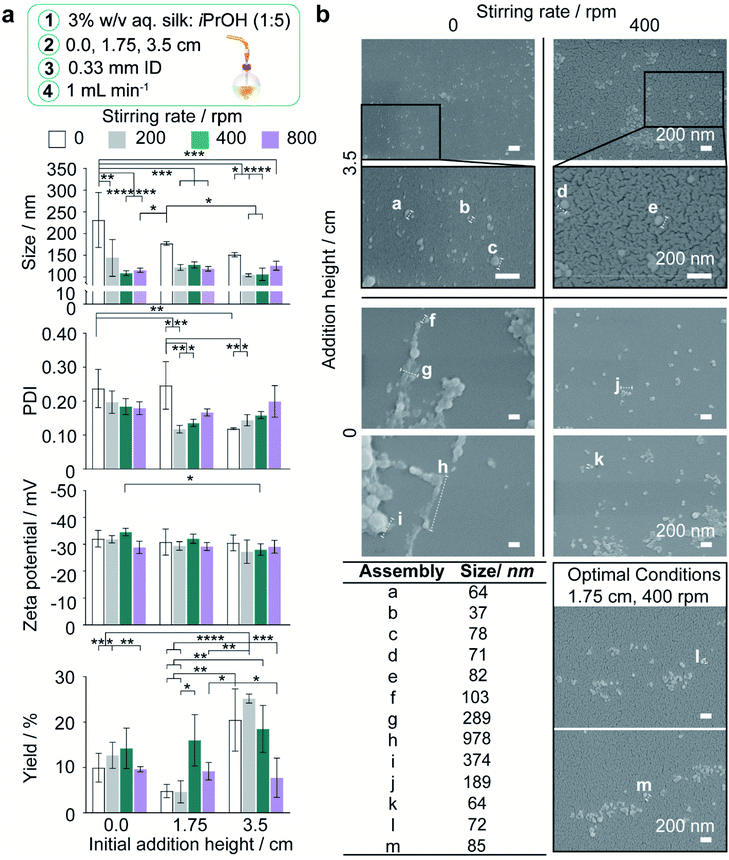
As manufacture in the closed, semi-batch system without stirring resulted in large nanoparticles with a wide size distribution, the effect of varying the time and shear stress of mixing was then determined at a 1.000 mL min−1 flow rate (shear-induced nucleation). The mixing time was reduced by increasing the stirring rate from 0 to 800 rpm and increasing the droplet velocity by raising the feed height from 0.0 cm to 3.5 cm (Tables 2, S4,† and Fig. 2). Increasing the levels of both these factors significantly reduced the nanoparticle size and polydispersity index (Fig. 3a). At 0 rpm, an increase in the feed height from 0.0 to 3.5 cm significantly decreased the nanoparticle size (from 232 to 151 nm) and the polydispersity index (from 0.24 to 0.12) (Fig. 3a). Equally, an increase in the stirring rate from 0 to 800 rpm significantly decreased the nanoparticle size to 116 nm at 0.0 cm feed height and the polydispersity index from 0.25 to 0.14 at 1.75 cm feed height. The SEM views confirmed the reduction in assembly size and the increase in nanoparticle curvature as mixing time decreased (Fig. 3b).
Generally, the nanoparticle size and size distribution were optimal at 1.75 and 3.5 cm feed heights combined with stirring rates of 200 and 400 rpm (104–128 nm; 0.12–0.16) (Fig. 3a). As feed height increased, silk nanoparticles were also produced with a higher zeta potential at 400 rpm, and in greater yield at low stirring rates, where the yield reached a maximum of 25% at 3.5 cm and 200 rpm. Further reductions in the mixing time at 3.5 cm (Table 2) obtained by increasing the stirring rate to 800 rpm caused a significant drop in nanoparticle yield to 8%. Overall, the nanoparticle size, polydispersity index and zeta potential were optimised at 400 rpm stirring rate. At 1.75 cm feed height a sufficient yield was returned while maintaining low diffusion length scales and shear exposure caused by circulatory flow during droplet flight (Fig. 2a, 3a, and Table S4†). These factor levels were set for further investigations of the effect of flow rate and silk concentration.
 | ||
| Fig. 4 The impact of increasing the flow rate and silk feed concentration in the staggered herringbone micromixer and in semi-batch systems of high and low mixing time. The hydrodynamic diameter, polydispersity index, zeta potential and yield for (a) semi-batch format and (b) microfluidic format. For the unstirred semi-batch processes, two-way ANOVA was used to compare multiple groups across concentration and flow rate, followed by Tukey's pairwise multiple comparison post hoc test for yield and Tukey's simple effect multiple comparison post hoc test for size, polydispersity index and zeta potential. In stirred semi-batch format and microfluidic format, multiple groups were evaluated by two-way ANOVA, followed by Tukey's pairwise multiple comparison post-hoc test for all properties error bars are hidden in the bars when not visible, ±SD, n = 3. Asterisks denote statistical significance determined using post hoc tests as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. For ease, all statistically significant interactions have been omitted and are shown in Fig. S3.† | ||
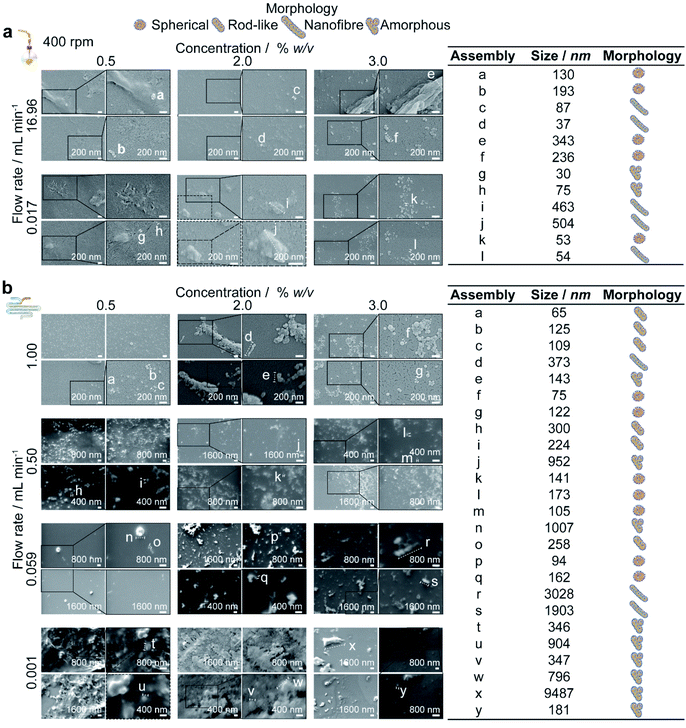
Conversely, at the flow rate of 0.001 mL min−1 in the micromixer, increases in the silk concentration from 0.5 to 2 and 3% resulted in a significant increase, followed by a decrease, in nanoparticle size (from 536 to 600 to 259 nm), whereas the polydispersity index remained high (ranging from 0.57 to 0.42) (Fig. 4b). Increasing the concentration from 0.5 to 3% did not significantly affect the zeta potential or yield, which ranged from −27 to −31 mV and from 9 to 6%, respectively. The DLS results were supported by SEM observations, which showed a mixture of spherical secondary building units and amorphous aggregates for all silk feeds (Fig. 5b).
At the 400 rpm stirring rate, moving from low to high shear by increasing the flow rate from 0.017 to 16.96 mL min−1 caused a significant increase in the nanoparticle size from 89 to 252 nm with 2% silk. The size distribution and zeta potential also significantly increased with flow rate at silk concentrations of 0.5 and 2%. SEM examinations confirmed that these results were due to a greater degree of shear-induced self-assembly for all silk feeds as the flow rate increased (Fig. 5a). For example, at 3% silk concentration, tertiary units were present as nanofibres, while at 0.5% and 2%, amorphous, lamellar aggregates also formed. As for mixing-induced nanoprecipitation, the polydispersity index decreased significantly with increasing silk concentration from 0.5 and 2% to 3% across all high-shear flow rates. The yield increased significantly with silk concentration at a flow rate of 1.000 mL min−1 and reached a maximum at 3% silk (tested from 1 to 16%).
Similarly, for the high-shear microfluidic format at flow rates between 0.059 and 1.0 mL min−1, increasing the silk concentration from 0.5% and 2% to 3% generally resulted in a significant decrease in particle size and an increase in yield (Fig. 4b). At a flow rate of 0.059 mL min−1, the size and polydispersity index significantly decreased (from 276 to 138 nm and from 0.36 to 0.19) with increased silk concentration from 0.5 to 2%. The size, polydispersity index and zeta potential magnitude subsequently decreased (from 138 to 194 nm, 0.19 to 0.39 and −36 to −26 mV) as the silk concentration increased further to 3%. The SEM observations (Fig. 5b) reinforced the DLS results and indicated that the primary assemblies formed using 2 and 3% silk stocks underwent secondary and tertiary assembly to give mixtures containing nanofibre aggregates, whereas the 0.5% silk stock formed rod-like particles in mixtures with amorphous aggregates. At 1.0 mL min−1, the opposite trend was observed with an increase in the silk concentration from 0.5 to 2%, as the size and zeta potential significantly increased (from 139 to 269 nm and −37 to −30 mV) due to the increased formation of amorphous aggregates. Subsequent increases from 2 to 3% silk resulted in a decreased nanoparticle size (to 103 nm) and an increased sphericity.
For 0.5 and 3% silk feeds, increasing the flow rate from 0.001 to 1.0 mL min−1 caused significant reductions in the assembly size (from 536 to 139 nm and from 259 to 103 nm, respectively) and polydispersity index (from 0.43 to 0.17 and from 0.42 to 0.09, respectively). This size reduction was supported by the observed reduction in aggregates as the flow rate increased for both silk feeds (Fig. 5b). Increasing the flow for 3% silk feeds significantly decreased the zeta potential, from −31 mV at 0.059 mL min−1 to −35 mV at 1.0 mL min−1. For 0.5% silk, changing the flow rate from 0.001 to 1.0 mL min−1 also significantly reduced the zeta potential (from −27 to −37 mV) while for 2% silk feedstocks, increasing the flow rate from 0.001 to 0.059 mL min−1 reduced particle size (from 600 to 138 nm), polydispersity index (from 0.57 to 0.19) and zeta potential (from −29 to −36 mV) and increased the yield (from 6 to 12%). These changes were accompanied by a morphological shift from amorphous aggregates at 0.001 mL min−1 to spherical nanoparticles and nanofibre agglomerates at 0.059 mL min−1. Raising the flow rate to 0.5 and 1.0 mL min−1 increased the particle size (to 269 nm) and zeta potential (to −26 mV) while lowering the yield (to 8%). At these flow rates, amorphous and nanofibre aggregates were produced in mixtures with spherical nanoparticles. The SEM views reinforced the DLS measurements and confirmed that, at 0.5 and 1.0 mL min−1, increasing the silk feed concentration resulted in a shift from rod-like particles to aggregates and then spherical particles (Fig. 4b).
Secondary structure measurement
The correlation of the silk nanoparticle secondary structure content with formulation conditions and the shear-induced assembly of the silk precursor by extrusion through the feed needle were evaluated by attenuated total reflectance-FTIR (ATR-FTIR) analysis and deconvolution of the amide I region (Fig. S1†). The structural changes caused by the nanoprecipitation process were also evaluated by analysing the amide I region (1600–1700 cm−1) using the spectral correlation coefficient method.53In the stirred system, the nanoparticle β-sheet content generally increased with feed height from 0.0 to 1.75 cm (Fig. S4d†). For example, at 200 rpm, increasing the feed height from 0.0 to 1.75 cm increased the β-sheet content from 51% to 56% (Fig. S4d†). Increasing the feed height also caused a main effect decrease in the nanoparticle β-turn content. As the stirring rate increased from 0 to 200 rpm and then to 800 rpm, the nanoparticle antiparallel β-sheet content showed a main effect decrease and then an increase, respectively (Fig. S4d†).
The β-sheet content of 0.5% extruded silk increased with flow rate from 19% at 0.017 mL min−1 to 31% at 8.485 mL min−1 (Fig. S4e†). The interaction between flow rate and concentration also significantly increased the silk β-sheet content, from 19% to 29% and 37%, as the flow rate was increased from 0.017 mL min−1 to 16.96 mL min−1 and 16.96 mL min−1 at silk concentrations of 0.5%, 2.0% and 3.0%, respectively (Fig. S4e†). These results were supported by the reduction in the correlation coefficient of extruded silk from 0.78 to 0.32 and 0.23 as the flow rate increased from 0.017 mL min−1 to 8.485 mL min−1 and 16.96 mL min−1, respectively, at a silk concentration of 3.0% (Fig. S4e†).
In the micromixer, the nanoparticle β-sheet content did not significantly vary with silk concentration or flow rate, and the correlation coefficients displayed no general trend (Fig. S5b†). However, the nanoparticle α-helix and random coil content significantly increased with increases in silk concentration from 0.5 and 2 to 3% at 0.059 mL min−1 (from 20 and 19 to 28%) and decreased (from 22 to 19%) at 1.0 mL min−1 flow rate (Fig. S5b†). Further, the nanoparticle α-helix and random coil content generally increased with flow rate. For example, increasing the flow rate from 0.001 to 1.0 mL min−1 caused a significant increase from 18 to 22% for 0.5% silk feeds; by contrast, for 3% silk this content increased from 20 to 28% between 0.001 to 0.059 mL min−1 and decreased to 19% at 1.0 mL min−1.
Discussion
In the current study, the optimised formulation variables11–13 for preparing silk nanoparticles were used to investigate the impact of flow and mixing on silk self-assembly. Consequently, all silk feed concentrations were below the ≈10% w/w critical micelle concentration37 of regenerated silk fibroin. We used the closed, semi-batch process for lab-scale nanoprecipitation, and the NanoAssemblr™ platform for the continuous process, with a commercially available staggered herringbone mixer Operating under conditions of laminar flow (Re < 2000).The effect of flow rate, addition height and stirring rate in closed, semi-batch format
The flow rates between 0.017 and 16.96 mL min−1 resulted in Reynolds numbers <2000 within the tubing and with all needles used; hence, laminar and newtonian flow was used to calculate the average wall shear rate.37 The average wall shear rates in the syringe and tubing (Table 1), combined with the residence times, were not expected to provide sufficient work (≈105 Pa)14 for shear-induced nucleation of the silk molecules at flow rates between 0.017 and 16.96 mL min−1. However, conditions of high shear were introduced at flow rates above 0.017 mL min−1 in needles with diameters less than 1.6 mm (Table 1).Regenerated silk fibroin can undergo a primary assembly process into silk fibroin micellar structures (secondary building units), followed by secondary and tertiary assembly of the micellar units into nanofibers and lamellar structures (Fig. 6).54 The hydrophilic blocks of the silk fibroin polymer are hydrated in aqueous solution, resulting in an extended conformation.54 The shear-induced desolvation breaks the stabilizing intermolecular bonds with water and enables hydrophobic interactions between silk molecules.54 Scanning electron microscopy showed that shear-induced nucleation resulted in secondary and tertiary assembly of silk micelles into nanofibres and lamellar structures, prior to antisolvent addition and completion of mixing (Fig. S2c, d† and 3b).55 This was reinforced by the significantly lower correlation coefficients of the silk extruded from the 0.33 mm needle at all flow rates above 0.017 mL min−1 (Fig. S4b†).
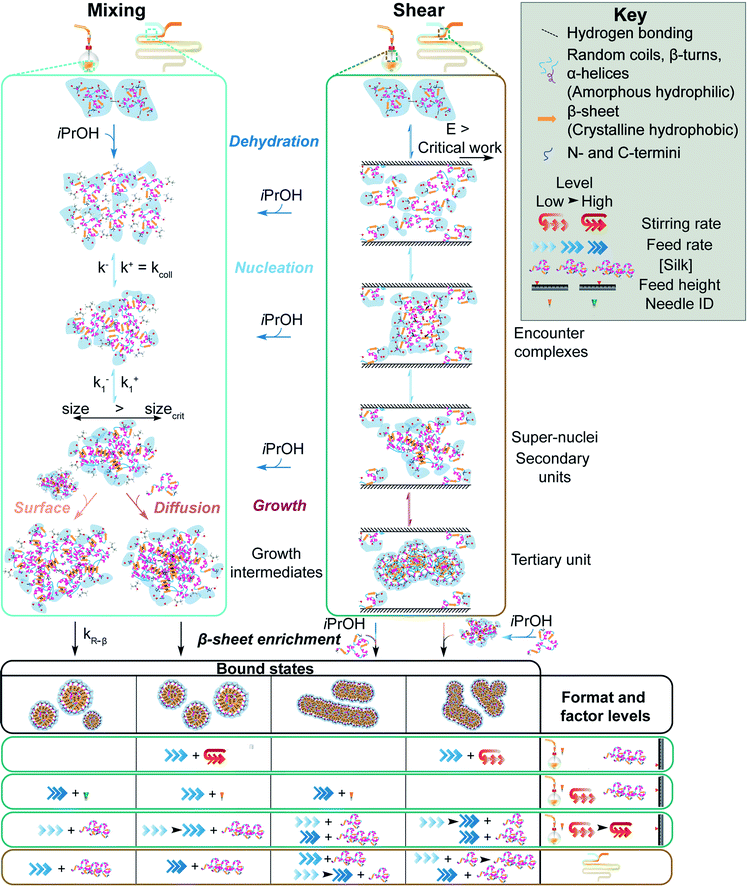 | ||
| Fig. 6 Schematic of protein–protein association and β-sheet assembly of silk fibroin via anti-solvent and shear-induced desolvation. The mechanism under shear flow is adapted from Dunderdale et al.14 and Zhang et al.54 Silk molecules, nanoparticles and stoichiometry of association are not drawn to scale. | ||
At all feed heights, mixing of silk within free falling droplets was driven by convection due to the high fluid velocities (Table S4†). Consequently, the impact of non-uniform silk concentration on solvent-antisolvent mixing efficiency can be neglected. However, for flow conditions of high shear, self-assembly within the droplet during flight could occur due to the microscale diffusion length of the silk molecules (Table S4†). The incorporation of water into, and size of, shear-induced silk assemblies would increase with feed height and time of flight. The concomitant reduction in free water concentration within the droplet could have reduced the solvent-antisolvent mixing efficiency and resulted in large, polydisperse assemblies (Fig. S2†).
Without stirring, at a 1.000 mL min−1 feed rate and 0.0 cm height, the shear-induced nucleation, and the antisolvent-induced dehydration resulted in nanoparticle formation at the phase boundary upon addition of the aqueous silk feed to the isopropanol bulk. This resulted in phase separation and low reproducibility due to the uncontrolled manual mixing of the two phases during purification. Increasing the stirring rate and feed height increased the rate and degree of antisolvent-induced nucleation by reducing the mixing time (Table 2).
Assembly growth was disfavoured under conditions of low mixing time, resulting in low nanoparticle size and polydispersity with increased yield. Additionally, the shear stress of the liquid flow arising from stirring rates and feed heights of 200 rpm and 1.75 cm could exceed the shear stress of agglomerates formed during silk extrusion from the feed needle. Breaking the intermolecular bridges holding the agglomerates together, combined with desolvation, would result in kinetic locking of the secondary building units. This was supported by the increase of nanoparticle β-sheet content with feed height (Fig. S4d†) and the low curvature morphology of tertiary assemblies formed at low heights and stirring rates (Fig. 3b).
The effect of flow rate and concentration in closed semi-batch and microfluidic formats
In the stirred semi-batch process, at feed rates of 0.017, 1.000, 8.485 and 16.96 mL min−1, the shear rates experienced by silk in the fluid line were estimated as 0.18, 10, 88 and 176 s−1, respectively, while the shear rates in the needle were estimated as 80, 4724, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 083 and 80
083 and 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 114 s−1 (Tables S2† and 1). At 1.000 mL min−1 and above, the needle shear rates lay above the critical shear rate and work required for nucleation.56 Shear-induced nucleation was reinforced by a decrease in the correlation coefficients of extruded silk as the flow rate increased across all concentrations and an increase in the β-sheet content of extruded silk as the flow rate increased at 0.5 and 2.0% (Fig. S4e†). Consequently, the following arguments assume that shear-induced nucleation, followed by isopropanol-induced desolvation, occurred above a flow rate of 0.017 mL min−1.
114 s−1 (Tables S2† and 1). At 1.000 mL min−1 and above, the needle shear rates lay above the critical shear rate and work required for nucleation.56 Shear-induced nucleation was reinforced by a decrease in the correlation coefficients of extruded silk as the flow rate increased across all concentrations and an increase in the β-sheet content of extruded silk as the flow rate increased at 0.5 and 2.0% (Fig. S4e†). Consequently, the following arguments assume that shear-induced nucleation, followed by isopropanol-induced desolvation, occurred above a flow rate of 0.017 mL min−1.
For homogenous nucleation at 0.017 mL min−1, increasing the concentration from 0.5 to 3.0% reduced the assembly size and polydispersity index due to a greater degree of supersaturation in both low and high mixing time processes (Fig. 4a). Samples prepared using 3.0% silk at flow rates of 0.017 and 1.000 mL min−1 and stirring at 400 rpm could be considered monodisperse. At 0.017 mL min−1, the reduction in the growth phase with increasing concentration significantly increased the assembly curvature (Fig. 5a) and packing due to decreases in the α-helix and random coil contents (Fig. S5a†). The reduced growth observed at higher silk concentrations in low-shear, semi-batch nanoprecipitation contrasts with results of previous work by D. Pham et al.,31 who used lower antisolvent:silk volumetric ratios.
In the unstirred system, under high-shear conditions and at all flow rates exceeding 0.017 mL min−1, increasing the silk feed concentration from 0.5 to 3.0% significantly reduced the particle size, whereas in the stirred system, the decreases in size were not significant (Fig. 4a). A mixture of low sphericity particles and aggregates were formed under high shear at 0.5 and 2.0%, while a mixture of nanofibres, lamellar structures and spherical particles were formed at 3.0% (Fig. 5a). This possibly reflected a balance between increased antisolvent-induced nucleation rate and increased rates of shear-induced self-assembly with concentration. The greater degree of shear-induced self-assembly with increasing concentration, from 0.5 to 3.0%, was reinforced by the increase in total and intermolecular β-sheet contents of the nanoparticles, the increased total β-sheet content of the extruded silk, the reduction in the nanoparticle correlation coefficients with concentration and the decrease in extruded silk correlation coefficients at 8.485 and 16.96 mL min−1 (Fig. S4e†). Consequently, as the formation of shear-induced micelles increased with feed rate, the reduced silk concentration prohibited antisolvent-induced nucleation.
The feed rate of silk also impacts mixing-induced self-assembly by altering the mixing time. For example, the droplet surface area decreased as the feed rate increased from 0.017 to 1.000 mL min−1 and then increased as the feed rate was raised to 16.96 mL min−1 (Table S4†). The reduced sphericity of the droplets produced in the jetting regime above 7.000 mL min−1 (Fig. 2a) could also cause non-uniform mixing. The effect of decreasing the mixing time as the feed rate was increased could be observed in the unstirred system at 0.5% and 2% silk feed concentrations. At these concentrations, the nanoparticle size and polydispersity index decreased with flow rate because the increase in the antisolvent-induced nucleation rates outweighed the increase in shear-induced assembly rates. Further, the feed rate controls the degree and rate of supersaturation onset of the antisolvent-induced desolvation. This means that the type and rate of antisolvent-induced nucleus growth could be impacted by the rate of silk addition. Increasing the feed rate favours mononuclear surface-controlled growth by increasing the mass transfer of silk from the solution to the assembly surface. For example, as the feed rate increased in the stirred semi-batch process for 2% silk feeds, the resulting assemblies increased in size, size distribution and zeta potential (Fig. 4a).
Continuous manufacture is a well-recognised strategy that simplifies the scale-up process of nanoformulations.57 We therefore used the NanoAssemblr™ as an example of a scale-independent process. Assuming newtonian flow, the flow rates of 0.001 mL min−1 to 1 mL min−1 resulted in Reynolds numbers of 0.04 to 40. These numbers were below the high vorticity and low transverse flow regime (Reynolds numbers > 1000). This ensured a high mixing efficiency, with the mixing time decreasing as flow rate increased from 0.001 to 1.0 mL min−1. The residence times at all flow rates were longer than the mixing times (Table 2), indicating that complete mixing occurred in the micromixer. The wall shear rates increased with flow rate, and when combined with the residence time, shear-induced nucleation of silk fibroin was likely to occur at all flow rates greater than 0.001 mL min−1, as both the critical shear rate56 and work14 were exceeded. Because the shear rates in both formats were analogous, the effects of shear and mixing in the micromixer could be compared with semi-batch processes with low and high mixing times (Fig. 6).
In the micromixer and with 0.5% silk feeds, as the shear rate and mixing efficiency increased with flow rate from 0.001 to 1.0 mL min−1, the assembly morphology shifted from amorphous aggregates to mixtures of rod-like and spherical particles. We speculate that the observation of rod-like morphologies only with 0.5% silk feeds was due to the low degrees of nucleation and long shear-induced and antisolvent-induced growth phases associated with insufficient surpassing of the nucleation energy barrier (Fig. 5b). The size reduction in the nanoassemblies with increasing flow rate indicated that the enhancement of the antisolvent-induced nucleation rates dominated over the increase in shear-induced assembly rates.
The association and assembly processes were favoured by an increase in the silk concentration from 0.5 to 2%,54 which resulted in the formation of larger nanofibres and lamellar tertiary structures with a higher polydispersity index under homogenous nucleation at 0.001 mL min−1 (Fig. 4b and 5b). This effect indicated that the increase in the silk concentration still did not greatly exceed the nucleation barrier. The opposite trend was observed for semi-batch processes at low and high mixing times due to the higher saturation induced by larger initial antisolvent-to-silk ratios.
Following the increase in flow rate from 0.001 to 0.059 mL min−1 for 2% feeds, the reduction in the mixing time dominated over the increase in shear-induced nucleation and assembly (Fig. 4b). The resulting particles showed spherical morphologies, although shear-induced assembly also resulted in the secondary assembly of nanoparticles into nanofibres. However, for 2% silk, further increases in the flow rate produced nanoparticles of increasing size due to secondary and tertiary growth processes that gave rise to amorphous and fibre-like assemblies. This trend was also observed for the low-mixing-time, semi-batch system, supporting the idea that the increase in the rates of shear-induced nucleation and growth in the microchannel dominated over the effect of reduced mixing time.
A silk concentration increase from 2 to 3%, under homogeneous nucleation at 0.001 mL min−1, significantly reduced both the nanoparticle size and the polydispersity index due to an increase in supersaturation and the antisolvent-induced nucleation rates. Increasing the silk concentration to 3% caused gradual increases in viscosity and rapid decreases in the surface tension.56,58 We speculate that these changes increased the water transport rate between the aqueous and isopropanol phases for 3% silk feeds, thereby providing less time for secondary self-assembly processes.
The extent of self-assembly for 3% silk feedstocks decreased as the flow rate increased from 0.001 to 1 mL min−1, with reductions in nanoparticle size, polydispersity index and zeta potential, but particles were produced in greater yield. The lower degree of self-assembly with increasing flow rate was confirmed by the increase in particle curvature (Fig. 5b) and the increase in α-helix and random coil content (Fig. S5b†). Increasing the silk feed concentration from 0.5 and 2% to 3% reduced the critical shear rate.56 Consequently, at flow rates above 0.001 mL min−1 in the micromixer, the shear-induced nucleation rates for 3% silk were likely greater than those for 0.5 and 2% silk feeds. As the flow rate increased, the assembly growth phase period for 3% silk was shortened due to the higher shear-induced nucleation rates, combined with the increased rates of antisolvent-induced nucleation and kinetic locking of the micellar structure. The narrower growth phases compared to the lower silk concentrations were supported by the greater nanoparticle sphericities for 3% feeds. This trend was not observed with 3% silk for either the low-mixing-time or high-mixing-time semi-batch processes.
The dependence of particle morphology and properties on the flow rate through the microchannel was supported by previous demonstrations in the staggered herringbone micromixer.12 The kinetic freezing of secondary building units observed with 3% silk at the 1 mL min−1 flow rate agreed with previous literature reports.12,13 Further, for 3% silk feeds, secondary and tertiary self-assembly were promoted by shear processing following an increase in the flow rate from 1 to 12 mL min−1 (shear rate 961![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 499 s−1), resulting in reduced particle curvature and increased overall size and polydispersity.12 Consequently, a critical flow rate exists between 1 and 12 mL min−1 for 3% silk feeds, beyond which the increase in the antisolvent-induced nucleation rate is out-competed by the increase in the rate of shear-induced assembly. This non-linear trend of self-assembly reflects the trend observed for 2% silk, but was shifted to higher flow rates due to the shortened growth phase occurring from greater supersaturation.
499 s−1), resulting in reduced particle curvature and increased overall size and polydispersity.12 Consequently, a critical flow rate exists between 1 and 12 mL min−1 for 3% silk feeds, beyond which the increase in the antisolvent-induced nucleation rate is out-competed by the increase in the rate of shear-induced assembly. This non-linear trend of self-assembly reflects the trend observed for 2% silk, but was shifted to higher flow rates due to the shortened growth phase occurring from greater supersaturation.
Conclusions
The flow properties of silk fibroin, which are fundamental to the natural role of the polymer as a polymorphic material, can be exploited in nanoprecipitation processes. Here, to control the morphology of silk nanoparticles, we utilised shear-induced nucleation of silk fibroin under flow in semi-batch and microfluidic formats. The morphology of the resulting silk assemblies varied with shear processing and silk precursor concentration in bulk mixing, although nanofibre and lamellar assemblies were formed as mixtures with spherical particles. Conversely, the high-shear, low-mixing-time conditions provided by the micromixer identifies this as a promising platform for tuning primary–tertiary silk self-assembly. Due to the sufficiently low mixing time, the silk concentration could be used as a controllable input factor for the formation of monodisperse, rod-like and spherical nanoassemblies suitable for use as nanomedicines. The information provided in this study delineates rational guidelines for the modulation of silk fibroin multiscale structures under shear and antisolvent-induced desolvation by varying the supersaturation, shear rate and mixing time.Author contributions
S. A. L. M. designed, collected, analysed, and interpreted the data and generated the manuscript draft. R. R. conducted silk concentration experiments at 1 mL min−1 using microfluidics and interpreted results. J. K. collected SEM images and interpreted results. Y. P. and F. P. S. provided training, advised on experimental design, and contributed to the interpretation of the results. All authors discussed the results and/or provided advice on the experimental analysis. F. P. S. supervised the project and content-edited the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Professor Andrea Ducci (University College London, England, UK), Professor Nigel Mottram (University of Glasgow, Scotland, UK), Dr Alice Turner, Dr Deborah Bowering and Dr Maider Olasolo (University of Strathclyde, Scotland, UK) for providing training and technical advice. The authors acknowledge that this work was carried out in part at the EPSRC Future Manufacturing Research Hub for Continuous Manufacturing and Advanced Crystallisation (CMAC) (EP/P006965/1) and was supported by a UK Research Partnership Fund award from the Higher Education Funding Council for England (Grant HH13054). The authors acknowledge that the electron scanning microscopy work was carried out at the Advanced Materials Research Laboratory, housed within the University of Strathclyde. The authors acknowledge Engineering and Physical Sciences Research Council funding EP/V034995/1 to establish the Thermal Equipment Suite, housed within the University of Strathclyde. S. A. L. M. is supported by a Medical Research Scotland PhD Studentship (PhD-1292-2018). J. K. received a fellowship support from the Development and Promotion of Science and Technology Talents Project under the Royal Government of Thailand Scholarship. J. K. is supported by Faculty of Nursing, HRH Princess Chulabhorn College of Medical Science, Chulabhorn Royal Academy, Bangkok, Thailand. All data created during this research are openly available from the University of Strathclyde-Pure, at https://10.15129/59c261cb-cdb7-4888-acfb-860e717c5149https://10.15129/59c261cb-cdb7-4888-acfb-860e717c5149https://10.15129/59c261cb-cdb7-4888-acfb-860e717c5149.Notes and references
- R. Konwarh, Bio-Des. Manuf., 2019, 2, 278–286 CrossRef.
- D. Chouhan and B. B. Mandal, Acta Biomater., 2020, 103, 24–51 CrossRef CAS PubMed.
- M. F. Maitz, C. Sperling, T. Wongpinyochit, M. Herklotz, C. Werner and F. P. Seib, Nanomedicine, 2017, 13, 2633–2642 CrossRef CAS PubMed.
- A. A. Lozano-Pérez, M. G. Montalbán, S. D. Aznar-Cervantes, F. Cragnolini, J. L. Cenis and G. Víllora, J. Appl. Polym. Sci., 2015, 132, 41702 Search PubMed.
- C. Holland, K. Numata, J. Rnjak-Kovacina and F. P. Seib, Adv. Healthcare Mater., 2019, 8, 1800465 CrossRef PubMed.
- L. Xiao, G. Lu, Q. Lu and D. L. Kaplan, ACS Biomater. Sci. Eng., 2016, 2, 2050–2057 CrossRef CAS PubMed.
- S. Mehrotra, D. Chouhan, R. Konwarh, M. Kumar, P. K. Jadi and B. B. Mandal, ACS Biomater. Sci. Eng., 2019, 5, 2054–2078 CrossRef CAS PubMed.
- E. Wenk, H. P. Merkle and L. Meinel, J. Controlled Release, 2011, 150, 128–141 CrossRef CAS PubMed.
- M. A. Tomeh, R. Hadianamrei and X. Zhao, Pharmaceutics, 2019, 11, 494 CrossRef CAS PubMed.
- H.-J. Jin and D. L. Kaplan, Nature, 2003, 424, 1057–1061 CrossRef CAS PubMed.
- T. Wongpinyochit, B. F. Johnston and F. P. Seib, J. Visualized Exp., 2016, e54669 Search PubMed.
- T. Wongpinyochit, J. D. Totten, B. F. Johnston and F. P. Seib, Nanoscale Adv., 2019, 1, 873–883 RSC.
- J. I. Solomun, J. D. Totten, T. Wongpinyochit, A. J. Florence and F. P. Seib, ACS Biomater. Sci. Eng., 2020, 6, 2796–2804 CrossRef CAS PubMed.
- G. J. Dunderdale, S. J. Davidson, A. J. Ryan and O. O. Mykhaylyk, Nat. Commun., 2020, 11, 3372 CrossRef PubMed.
- M. Wacker, Int. J. Pharm., 2013, 457, 50–62 CrossRef CAS PubMed.
- W. Richtering, I. Alberg and R. Zentel, Small, 2020, 16, 1–8 CrossRef PubMed.
- M. C. Arno, M. Inam, A. C. Weems, Z. Li, A. L. A. Binch, C. I. Platt, S. M. Richardson, J. A. Hoyland, A. P. Dove and R. K. O'Reilly, Nat. Commun., 2020, 11, 1420 CrossRef CAS PubMed.
- J. Zhao and M. H. Stenzel, Polym. Chem., 2018, 9, 259–272 RSC.
- B. Crivelli, S. Perteghella, E. Bari, M. Sorrenti, G. Tripodo, T. Chlapanidas and M. L. Torre, Soft Matter, 2018, 14, 546–557 RSC.
- V. Gupta, A. Aseh, C. N. Ríos, B. B. Aggarwal and A. B. Mathur, Int. J. Nanomed., 2009, 4, 115–122 CrossRef CAS PubMed.
- F. P. Seib, G. T. Jones, J. Rnjak-Kovacina, Y. Lin and D. L. Kaplan, Adv. Healthcare Mater., 2013, 2, 1606–1611 CrossRef CAS PubMed.
- T. Wongpinyochit, P. Uhlmann, A. J. Urquhart and F. P. Seib, Biomacromolecules, 2015, 16, 3712–3722 CrossRef CAS PubMed.
- Z. Zhao, Y. Li and M.-B. Xie, Int. J. Mol. Sci., 2015, 16, 4880–4903 CrossRef CAS PubMed.
- A. Gholami, H. Tavanai and A. R. Moradi, J. Nanopart. Res., 2011, 13, 2089–2098 CrossRef CAS.
- Z. Toprakcioglu, P. K. Challa, D. B. Morse and T. Knowles, Sci. Adv., 2020, 6, eaay7952 CrossRef CAS PubMed.
- M. Kazemimostaghim, R. Rajkhowa and X. Wang, Powder Technol., 2015, 283, 321–327 CrossRef CAS.
- Q. Lu, Y. Huang, M. Li, B. Zuo, S. Lu, J. Wang, H. Zhu and D. L. Kaplan, Acta Biomater., 2011, 7, 2394–2400 CrossRef CAS PubMed.
- M. Tarhini, H. Greige-Gerges and A. Elaissari, Int. J. Pharm., 2017, 522, 172–197 CrossRef CAS PubMed.
- E. Lepeltier, C. Bourgaux and P. Couvreur, Adv. Drug Delivery Rev., 2014, 71, 86–97 CrossRef CAS PubMed.
- R. Botet and K. Roger, Curr. Opin. Colloid Interface Sci., 2016, 22, 108–112 CrossRef CAS.
- D. T. Pham, N. Saelim and W. Tiyaboonchai, Int. J. Appl. Pharm., 2018, 10, 195–201 CrossRef CAS.
- D. N. Breslauer, S. J. Muller and L. P. Lee, Biomacromolecules, 2010, 11, 643–647 CrossRef CAS PubMed.
- U. Shimanovich, F. S. Ruggeri, E. De Genst, J. Adamcik, T. P. Barros, D. Porter, T. Müller, R. Mezzenga, C. M. Dobson, F. Vollrath, C. Holland and T. P. J. Knowles, Nat. Commun., 2017, 8, 15902 CrossRef CAS PubMed.
- Z. Toprakcioglu, A. Levin and T. P. J. Knowles, Biomacromolecules, 2017, 18, 3642–3651 CrossRef CAS PubMed.
- S. P. Kee and A. Gavriilidis, Chem. Eng. J., 2008, 142, 109–121 CrossRef CAS.
- S. A. L. Matthew, J. D. Totten, S. Phuagkhaopong, G. Egan, K. Witte, Y. Perrie and F. P. Seib, ACS Biomater. Sci. Eng., 2020, 6, 6748–6759 CrossRef CAS PubMed.
- A. Nisal, C. Kalelkar, J. Bellare and A. Lele, Rheol. Acta, 2013, 52, 833–840 CrossRef CAS.
- M. A. Ianovska, P. P. M. F. A. Mulder and E. Verpoorte, RSC Adv., 2017, 7, 9090–9099 RSC.
- T. Rode García, A. García Ac, A. Lalloz, F.-X. Lacasse, P. Hildgen, J.-M. Rabanel and X. Banquy, Langmuir, 2018, 34, 5772–5780 CrossRef PubMed.
- L. A. Melton, C. W. Lipp, R. W. Spradling and K. A. Paulson, Chem. Eng. Commun., 2002, 189, 322–338 CrossRef CAS.
- W. Weheliye, G. Rodriguez, T. Anderlei, M. Micheletti, M. Yianneskis and A. Ducci, in Proceedings of the 14th European Conference on Mixing, Warszawa, Warsaw, Poland, 2012, pp. 503–508 Search PubMed.
- G. Rodriguez, W. Weheliye, T. Anderlei, M. Micheletti, M. Yianneskis and A. Ducci, Chem. Eng. Res. Des., 2013, 91, 2084–2097 CrossRef CAS.
- S. Tomar, Linux J., 2006, 146, 10 Search PubMed.
- G. Rodriguez, T. Anderlei, M. Micheletti, M. Yianneskis and A. Ducci, Biochem. Eng. J., 2014, 82, 10–21 CrossRef CAS.
- X. Jiang, L. Zheng, H. Wu and J. Zhang, Math. Biosci. Eng., 2021, 18, 4071–4083 Search PubMed.
- Z. Xu, C. Lu, J. Riordon, D. Sinton and M. G. Moffitt, Langmuir, 2016, 32, 12781–12789 CrossRef CAS PubMed.
- F.-M. Pang, C.-E. Seng, T.-T. Teng and M. H. Ibrahim, J. Mol. Liq., 2007, 136, 71–78 CrossRef CAS.
- M. S. Williams, K. J. Longmuir and P. Yager, Lab Chip, 2008, 8, 1121–1129 RSC.
- A. Mialdun, V. Yasnou, V. Shevtsova, A. Königer, W. Köhler, D. Alonso De Mezquia and M. M. Bou-Ali, J. Chem. Phys., 2012, 136, 244512 CrossRef CAS PubMed.
- Y. Son, Polymer, 2007, 48, 632–637 CrossRef CAS.
- H. Yang, S. Yang, J. Kong, A. Dong and S. Yu, Nat. Protoc., 2015, 10, 382–396 CrossRef CAS PubMed.
- X. Hu, D. Kaplan and P. Cebe, Macromolecules, 2006, 39, 6161–6170 CrossRef CAS.
- K. Griebenow, A. M. Santos and K. G. Carrasquillo, Internet J. Vib. Spectrosc., 1999, 3, 1–2 Search PubMed.
- Y. Zhang, Y. Zuo, S. Wen, Y. Hu and Y. Min, Biomacromolecules, 2018, 19, 1223–1233 CrossRef CAS PubMed.
- Zainuddin, T. T. Le, Y. Park, T. V. Chirila, P. J. Halley and A. K. Whittaker, Biomaterials, 2008, 29, 4268–4274 CrossRef CAS PubMed.
- A. Matsumoto, A. Lindsay, B. Abedian and D. L. Kaplan, Macromol. Biosci., 2008, 8, 1006–1018 CrossRef CAS PubMed.
- C. Webb, N. Forbes, C. B. Roces, G. Anderluzzi, G. Lou, S. Abraham, L. Ingalls, K. Marshall, T. J. Leaver, J. A. Watts, J. W. Aylott and Y. Perrie, Int. J. Pharm., 2020, 582, 119266 CrossRef CAS PubMed.
- D. E. Chung and I. C. Um, Fibers Polym., 2014, 15, 153–160 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra07764c |
| This journal is © The Royal Society of Chemistry 2022 |