Open Access Article
Open Access ArticleStructure, preparation and properties of liquid fluoroelastomers with different end groups
Jiayu Duana,
Chen Yanga,
Hailan Kang ab,
Long Liab,
Feng Yangab,
Qinghong Fangab,
Wenchi Hanab and
Donghan Li
ab,
Long Liab,
Feng Yangab,
Qinghong Fangab,
Wenchi Hanab and
Donghan Li *ab
*ab
aCollege of Materials Science and Engineering, Shenyang University of Chemical Technology, Shenyang 110142, Liaoning, China
bLiaoning Provincial Key Laboratory of Rubber & Elastomer, Shenyang University of Chemical Technology, Shenyang 110142, Liaoning, China
First published on 25th January 2022
Abstract
In order to design and prepare liquid fluoroelastomers with different end groups, and reveal the relationship between the molecular chain structure and properties, we studied on the oxidation degradation method and functional group conversion method to prepare carboxyl-terminated and hydroxyl-terminated liquid fluoroelastomers, respectively. The reaction mechanisms were also deduced. Furthermore, the curing system was created for liquid fluoroelastomers, and systematically analyzed their properties. The sequence type and content of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– and oxygen-containing groups in the samples were measured and characterized by attenuated total reflectance/Fourier transform infrared (ATR-FTIR) spectroscopy, 1H nuclear magnetic resonance (1H-NMR), 19F-NMR spectroscopy and chemical titration, the molecular weights of liquid fluoroelastomers were measured by gel permeation chromatography (GPC). Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) were used to examine the thermal properties, while a viscometer was used to measure the dynamic viscosity of the liquid fluoroelastomers. Then the mechanical and surface properties of the cured samples were examined by universal testing machine and contact angle measurement instrument, respectively. The results show that carboxyl-terminated liquid fluoroelastomer with 2.71 wt% carboxyl terminal groups can be prepared by oxidation degradation method. When lithium aluminium hydride (LiAlH4) was used as the reducing agent, it can efficiently convert carboxyl group to hydroxyl group with a conversion rate of more than 95%. In addition, it can be seen that the dynamic viscosity of the liquid fluoroelastomers were all decreased with the increase of temperature, and it is similar to about 10 Pa s at 70 °C. Compared with carboxyl-terminated liquid fluoroelastomers, hydroxyl-terminated liquid fluoroelastomers has higher curing reactivity, higher glass transition temperature (Tg) and thermal decomposition temperature (Td), and better mechanical properties of cured samples. The two types of liquid fluoroelastomers with distinct end groups presented distinct hydrophilicity.
C– and oxygen-containing groups in the samples were measured and characterized by attenuated total reflectance/Fourier transform infrared (ATR-FTIR) spectroscopy, 1H nuclear magnetic resonance (1H-NMR), 19F-NMR spectroscopy and chemical titration, the molecular weights of liquid fluoroelastomers were measured by gel permeation chromatography (GPC). Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) were used to examine the thermal properties, while a viscometer was used to measure the dynamic viscosity of the liquid fluoroelastomers. Then the mechanical and surface properties of the cured samples were examined by universal testing machine and contact angle measurement instrument, respectively. The results show that carboxyl-terminated liquid fluoroelastomer with 2.71 wt% carboxyl terminal groups can be prepared by oxidation degradation method. When lithium aluminium hydride (LiAlH4) was used as the reducing agent, it can efficiently convert carboxyl group to hydroxyl group with a conversion rate of more than 95%. In addition, it can be seen that the dynamic viscosity of the liquid fluoroelastomers were all decreased with the increase of temperature, and it is similar to about 10 Pa s at 70 °C. Compared with carboxyl-terminated liquid fluoroelastomers, hydroxyl-terminated liquid fluoroelastomers has higher curing reactivity, higher glass transition temperature (Tg) and thermal decomposition temperature (Td), and better mechanical properties of cured samples. The two types of liquid fluoroelastomers with distinct end groups presented distinct hydrophilicity.
1. Introduction
Fluoroelastomers are synthetic polymer elastomers containing fluorine atoms on the main-chain or side-chain carbon atoms.1 They possess exceptional thermal stability, oil resistance, oxidation resistance and chemical resistance due to the high bond energy (485 kJ mol−1) of the C–F bond and the shielding effect of the fluorine atoms on the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bond in its molecular structure.2–5 However, they do not fully meet the requirements of modern fluoroelastomer transport devices because of the poor processing performance, complicated processing and complex vulcanisation procedure.6,7 As a result, low-molecular-weight fluoroelastomers with improved fluidity and plasticity, known as liquid fluoroelastomers, were developed.8,9
C– bond in its molecular structure.2–5 However, they do not fully meet the requirements of modern fluoroelastomer transport devices because of the poor processing performance, complicated processing and complex vulcanisation procedure.6,7 As a result, low-molecular-weight fluoroelastomers with improved fluidity and plasticity, known as liquid fluoroelastomers, were developed.8,9
Liquid fluoroelastomers, also known as key materials, has a wide range of potential applications in transportation, medical protection, new energy, national defence and military industries due to their unique properties. They can also be utilised to make fluorine-containing functional polymers, novel battery-sealing materials, raw materials for additive manufacturing (3D printing), high-performance adhesives, caulking agents, coatings and processing complexes.
Liquid fluoroelastomers can be prepared not only by polymerisation methods10,11 but also through oxidation degradation method.12 Because the oxidation degradation method is simpler than the polymerisation methods and produces carboxyl-terminated telechelic polymers in situ, it has attracted wide attention.13 Unfortunately, due to their low carboxyl-terminated activity and poor thermal stability, carboxyl-terminated liquid fluoroelastomers are difficult to cure at low temperatures, thus limiting their applicability in particular fields. As a result, carboxyl to hydroxyl group conversion is important for enhancing the thermal stability of liquid fluoroelastomers and for lowering the curing temperature.
In organic chemistry, carboxyl group reduction is a crucial process. Since Finholt14 discovered the preparation and application of LiAlH4 in 1946, complex metal hydrides have been used as reducing agents in organic synthesis with considerable success. They were swiftly developed and extensively employed, and the number of reagents has gradually expanded in recent years. These reductants exist in the form of complex hydrogen anion salts, which are nucleophiles that can attack the positively charged atoms in unsaturated polar bonds, resulting in a reduction process via hydrogen anion transfer.15,16 The carboxyl group can be easily converted to a hydroxyl group simultaneously.
Furthermore, liquid fluoroelastomers themselves have no strength and cannot meet the current research and application requirements, so they must be cured. Epoxy,17,18 isocyanate,19 aziridine20 and carbonised diamines are the most common curing agents used for liquid fluoroelastomers.21 Hexamethylene diisocyanate (HDI) is a particularly important aliphatic isocyanate monomer because its molecular chain contains a highly active isocyanate group (–NCO) that can react with active hydrogen groups, such as carboxyl, hydroxyl, amino, carbamate and urea, in the polymer to achieve crosslinking and obtain the desired network structure.22,23 Because HDI curing agents do not contain isocyanate groups directly attached to the carbon atoms on the benzene ring, the coatings and adhesives cured by HDI have a series of excellent properties, including weather resistance, chemical reagent resistance, yellowing resistance and powder resistance.24,25 The low surface energy of fluoroelastomers has made them popular hydrophobic materials.26,27
At present, there are few reports on the preparation methods, reaction mechanism, structure and properties of liquid fluoroelastomers with carboxyl and hydroxyl end groups. Thus, in order to design and prepare liquid fluoroelastomers with different end groups, and reveal the relationship between the molecular chain structure and properties, we studied on the oxidation degradation method and functional group conversion method to prepare carboxyl-terminated and hydroxyl-terminated liquid fluoroelastomers, respectively. The poly(VDF-co-HFP) copolymer was chosen as the raw material, the fluoroelastomer formation was confirmed based on the results of spectral analyses and chemical quantification. Furthermore, property tests and comparative analysis of the relationship between the structure and properties of the liquid fluoroelastomers with different end groups were performed.
2. Experimental details
2.1 Materials and chemical agents
The fluoroelastomer that was made of the copolymer of vinylidene fluoride and hexafluoropropene was purchased from Chenguang Research Institute of Chemical Industry. Carboxyl-terminated liquid fluoroelastomer was prepared by the oxidation degradation method of the fluoroelastomer. The hydroxyl-terminated liquid fluoroelastomer was prepared by the functional group conversion method of the carboxyl-terminated liquid fluoroelastomer. Benzyl triethyl ammonium chloride (BTEAC) and LiAlH4 were purchased from Aladdin Industrial Corporation. HDI was purchased from Yantai Wanhua Chemical Group Co., Ltd. Potassium hydroxide (KOH), hydrogen peroxide (30 wt% H2O2), hydrochloric acid (HCl), anhydrous ethanol (C2H5OH) and tetrahydrofuran (THF) were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd.2.2 Characterization
The NMR spectra were recorded on a Bruker AC 80 spectrometer (500 MHz for 1H, 470 MHz for 19F) at room temperature using acetone-d6 as the solvent and TMS (or CFCl3) as the internal standard for the 1H (or 19F) nuclei.
The carboxyl-terminated or hydroxyl-terminated liquid fluoroelastomer (m = 0.5 g) and THF (50 mL) were added into an Erlenmeyer flask. Then, phenolphthalein was added (0.01 mL), and the –COOH (ω) content was assessed by the following equation:
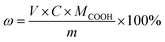 | (1) |
In eqn (1), “V” stands for the volume of the base employed for titration. Furthermore, the conversion rate of the carboxyl group was assessed by the following equation:
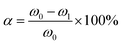 | (2) |
In eqn (2), ω0 and ω1 stand for the contents of –COOH in the carboxyl-terminated liquid fluoroelastomer and the hydroxyl-terminated liquid fluoroelastomer, respectively.
The thermal decomposition temperatures of the fluoroelastomers were estimated by thermogravimetry analysis (TGA). The TGA measurements were carried out on a NETZSCH TG209. The samples were heated from 30 °C to 600 °C at a rate of 10 °C min−1 to record the curves.
2.3 Preparation
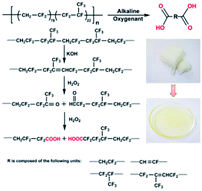
The carboxyl-terminated liquid fluoroelastomer was prepared by the oxidative degradation of the poly(VDF-co-HFP) copolymer by using an aqueous KOH solution and H2O2. The hydroxyl-terminated liquid fluoroelastomer was prepared by a reductive reaction, in which the carboxyl-terminated liquid fluoroelastomer was reacted with LiAlH4. Then, these products were cured.3. Results and discussion
3.1 Structure of the liquid fluoroelastomers
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CF3)–CH2–, –(CF3)C
C(CF3)–CH2–, –(CF3)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH– and –CH
CH– and –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CF–, respectively. This demonstrates that the fluoroelastomer underwent an oxidation degradation reaction, with the dehydrofluorination reaction producing the –C
CF–, respectively. This demonstrates that the fluoroelastomer underwent an oxidation degradation reaction, with the dehydrofluorination reaction producing the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– bond under alkaline conditions.29
C– bond under alkaline conditions.29
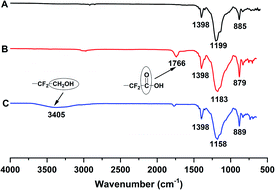
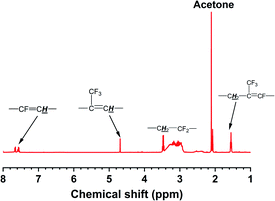
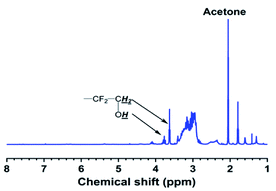
As shown in Fig. 3, compared with the carboxyl-terminated liquid fluoroelastomer, the hydroxyl-terminated liquid fluoroelastomer showed not only the characteristic structural peaks of –CH2OH at δ = 3.63 ppm and 3.75 ppm but also peaks at δ = 1.55 ppm, 4.68 ppm and 7.70–7.50 ppm. The corresponding –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– characteristic structural peaks were significantly weakened, indicating that under the action of LiAlH4, the carboxyl groups and –C
C– characteristic structural peaks were significantly weakened, indicating that under the action of LiAlH4, the carboxyl groups and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– in the carboxyl-terminated liquid fluoroelastomer were all reduced.
C– in the carboxyl-terminated liquid fluoroelastomer were all reduced.
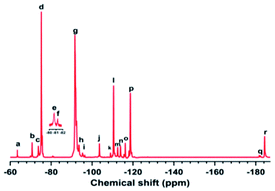
| No. | δ (ppm) | Assignment | No. | δ (ppm) | Assignment |
|---|---|---|---|---|---|
| a | −63.46 | –CF2C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) 2COOH 2COOH |
j | −103.62 | –CF2CH2C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) 2CF(CF3)CF2– 2CF(CF3)CF2– |
| b | −70.67 | –CH2CF2CF(C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) 3)CF2CH2– 3)CF2CH2– |
k | −108.96 | –CF(CF3)CH2C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) 2CF(CF3)CF2– 2CF(CF3)CF2– |
| c | −73.71 | –CF2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(C C(C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) 3)CF2– 3)CF2– |
l | −110.51 | –CF2CH2C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) 2CF2CF(CF3)– 2CF2CF(CF3)– |
| d | −75.19 | –CF2CH2CF(C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) 3)CF2CF2– 3)CF2CF2– |
m | −112.53 | –CF(CF3)CH2C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) 2CF2CH2– 2CF2CH2– |
| e | −80.66 | –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CFCF(C CFCF(C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) 3)– 3)– |
n | −113.95 | –CF2CH2C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) 2CF2CH2– 2CF2CH2– |
| f | −81.30 | –CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCF(C CHCF(C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) 3)CF2– 3)CF2– |
o | −116.24 | –CH2CH2C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) 2CF2CF(CF3)– 2CF2CF(CF3)– |
| g | −91.62 | –CF2CH2C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) 2CH2CF2– 2CH2CF2– |
p | −118.72 | –CH2CF2C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) 2CF(CF3)CH2– 2CF(CF3)CH2– |
| h | −93.56 | –CF2CH2C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) 2CH2CF(CF3)– 2CH2CF(CF3)– |
q | −181.74 | –CH2CF2C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) (CF3)CF2CH2– (CF3)CF2CH2– |
| i | −95.64 | –CH2CH2C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) 2CH2CF2– 2CH2CF2– |
r | −184.33 | –CF2CF2C![[F with combining low line]](https://www.rsc.org/images/entities/b_i_char_0046_0332.gif) (CF3)CH2CF2– (CF3)CH2CF2– |
Then, setting ∑1C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C as the integral of the –C
C as the integral of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– sequences, it was calculated as ∑1C
C– sequences, it was calculated as ∑1C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C = 2/3(I−73.71 + I−80.66 + I−81.30); ∑1(CF3 + CF2) was the integral of the other VDF and HFP sequences, ∑1(CF3 + CF2) = 2/3(I−70.67 + I−75.19) + I−63.46 + I−91.62 + I−93.56 + I−95.64 + I−103.62 + I−108.96 + I−110.51 + I−112.53 + I−113.95 + I−116.24. The molar fractions (mol%) of –C
C = 2/3(I−73.71 + I−80.66 + I−81.30); ∑1(CF3 + CF2) was the integral of the other VDF and HFP sequences, ∑1(CF3 + CF2) = 2/3(I−70.67 + I−75.19) + I−63.46 + I−91.62 + I−93.56 + I−95.64 + I−103.62 + I−108.96 + I−110.51 + I−112.53 + I−113.95 + I−116.24. The molar fractions (mol%) of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– was calculated by the following equation.34
C– was calculated by the following equation.34
mol% of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– in the copolymer:
C– in the copolymer:
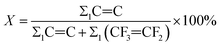 | (3) |
According to the calculations, the molar fractions of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– in the carboxyl-terminated and hydroxyl-terminated liquid fluoroelastomers were about 1.12 mol% and 0.89 mol%, respectively.
C– in the carboxyl-terminated and hydroxyl-terminated liquid fluoroelastomers were about 1.12 mol% and 0.89 mol%, respectively.
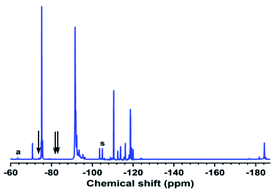
When carboxyl was converted to hydroxyl, not only did the peak assigned to –CF2COOH(a) significantly decline and the peaks assigned to –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– vanish, but the peak centred at −104.91 ppm(s) belonging to the –CF2CH2OH structure also appeared, as seen in Fig. 5.35 As a result, when carboxyl was converted to hydroxyl, the –C
C– vanish, but the peak centred at −104.91 ppm(s) belonging to the –CF2CH2OH structure also appeared, as seen in Fig. 5.35 As a result, when carboxyl was converted to hydroxyl, the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– content decreased as well.
C– content decreased as well.
Thus, the successful preparation of the carboxyl-terminated and hydroxyl-terminated liquid fluoroelastomers was confirmed, and the characterisation results matched the FTIR and 1H-NMR data.
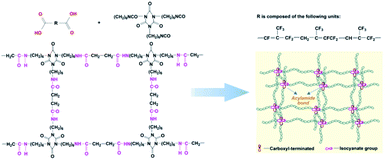
In the alkaline environment of the fluoroelastomer, the hydroxyl ion (OH−) in KOH will attack the hydrogen ion (H+) in the structure of –CF2CH2–, and a deprotonation reaction occurs, followed by a defluoridation reaction to produce –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–, as illustrated in Scheme 1. Furthermore, when there is a high concentration of alkali or a high temperature in the reaction system, oxygen in the air, the alkali and fluorine continue to interact with –C
C–, as illustrated in Scheme 1. Furthermore, when there is a high concentration of alkali or a high temperature in the reaction system, oxygen in the air, the alkali and fluorine continue to interact with –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–, resulting in an oxidation reaction. Subsequently, a hydroxyl group is introduced into the molecular chain, thus resulting in the carboxyl-terminated liquid fluoroelastomer.36,37
C–, resulting in an oxidation reaction. Subsequently, a hydroxyl group is introduced into the molecular chain, thus resulting in the carboxyl-terminated liquid fluoroelastomer.36,37
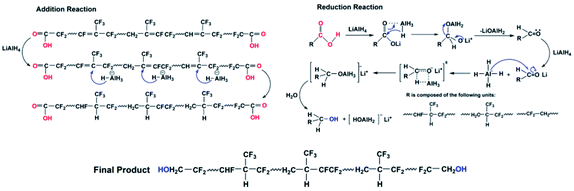
There are two stages in the reduction of the carboxyl group by LiAlH4. The carboxyl group is reduced to an aldehyde in the first stage. The carboxyl groupis first converted into a carboxylate lithium salt, and then when aluminium hydride (AlH3) is close by, it forms a complex with the carbonyl oxygen. Then, to convert LiOAlH2 to formaldehyde, the negative hydrogen from aluminium is transferred to the carbonyl carbon. The aldehyde combines with a second molecule of lithium aluminium hydride in the second step. AlH4−, being the lone offensive reagent, forms alkoxides by transferring a hydride ion to the carbon via a quaternary cyclic transition state, and these are then hydrolysed to obtain alcohols.38
The above spectral test results show that unlike hydrocarbons or polymers, when LiAlH4 was utilised as the reducing agent in a fluorine-containing system, the carboxyl group was reduced, while only a portion of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– was reduced. The reaction mechanism is shown in Scheme 2; the electron cloud density is high due to the weak polarity of –C
C– was reduced. The reaction mechanism is shown in Scheme 2; the electron cloud density is high due to the weak polarity of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–, which makes it a difficult target for attack by nucleophiles (AlH4−), and the electronegativity of the fluorine atom is relatively large, which causes the electron cloud density to shift and reduce, allowing nucleophiles to attack it. The hydride ions are then transferred to positively charged carbon atoms to form complexes, which combine with protons to complete the hydrogenation reduction process.39
C–, which makes it a difficult target for attack by nucleophiles (AlH4−), and the electronegativity of the fluorine atom is relatively large, which causes the electron cloud density to shift and reduce, allowing nucleophiles to attack it. The hydride ions are then transferred to positively charged carbon atoms to form complexes, which combine with protons to complete the hydrogenation reduction process.39
The end-group concentration in the carboxyl-terminated and hydroxyl-terminated liquid fluoroelastomers was then determined, and the conversion rate in the reduction reaction was nearly 96%. The results are shown in Table 2.
| End group | COOH (wt%) | Conversion ratio (%) |
|---|---|---|
| Carboxyl | 2.71 | — |
| Hydroxyl | 0.12 | 95.57 |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– groups in the molecular chain along with the reduction reaction. The detailed values are shown in Table 3.
C– groups in the molecular chain along with the reduction reaction. The detailed values are shown in Table 3.
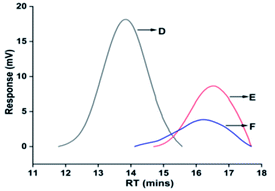 | ||
| Fig. 6 GPC spectra of the (D) poly(VDF-co-HFP) copolymer, (E) carboxyl-terminated liquid fluoroelastomer and (F) hydroxyl-terminated liquid fluoroelastomer. | ||
| Sample | Mn | Mw | PD |
|---|---|---|---|
| Poly(VDF-co-HFP) copolymer | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 724 724 |
157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 904 904 |
2.20 |
| Carboxy-terminated fluoroelastomer | 2458 | 4788 | 1.95 |
| Hydroxy-terminated fluoroelastomer | 2680 | 6904 | 2.57 |
3.2 Thermal properties of the liquid fluoroelastomers
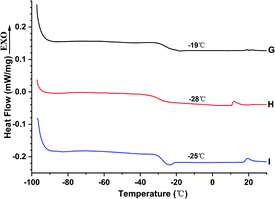
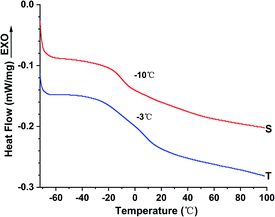
The Tg of the liquid fluoroelastomers generated from the poly(VDF-co-HFP) copolymer fell dramatically from −19 °C to −25 °C, as illustrated in Fig. 7, with a temperature difference of about −6 °C to −9 °C. Due to the presence of isolated –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– in the molecular chain, the carboxyl-terminated liquid fluoroelastomer generated by the oxidation degradation method showed increased flexibility of the molecular chain, resulting in a drop in Tg. The Tg of the hydroxyl-terminated liquid fluoroelastomer increased from −28 °C to −25 °C after the reduction reaction, and the temperature contrast was −3 °C; the –C
C– in the molecular chain, the carboxyl-terminated liquid fluoroelastomer generated by the oxidation degradation method showed increased flexibility of the molecular chain, resulting in a drop in Tg. The Tg of the hydroxyl-terminated liquid fluoroelastomer increased from −28 °C to −25 °C after the reduction reaction, and the temperature contrast was −3 °C; the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– in the molecular chain were reduced to single bonds. As a result, the number of isolated double bonds in the chain were reduced, limiting molecular chain flexibility and raising the Tg. The reason for this, based on the 19F-NMR results in Fig. 4 and 5, is the addition of hydrogen atoms to –C
C– in the molecular chain were reduced to single bonds. As a result, the number of isolated double bonds in the chain were reduced, limiting molecular chain flexibility and raising the Tg. The reason for this, based on the 19F-NMR results in Fig. 4 and 5, is the addition of hydrogen atoms to –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– during the reduction reaction, which would improve the tacticity of the molecular chains.42,43 The presence of –C
C– during the reduction reaction, which would improve the tacticity of the molecular chains.42,43 The presence of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– in the molecular chains thus had a significant impact on the low-temperature property.
C– in the molecular chains thus had a significant impact on the low-temperature property.
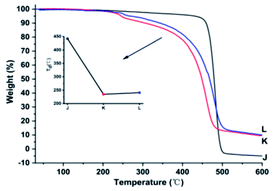
The TGA studies showed that the Td of the liquid fluoroelastomers generated using the poly(VDF-co-HFP) copolymer fell dramatically from 442 °C to 235 °C, as shown in Fig. 8. It was found that contents of carboxyl and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– were the root causes of this phenomenon. The –C
C– were the root causes of this phenomenon. The –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– in the molecular chain were reduced to single bonds, and the content of isolated double bonds in the chain decreased, thereby limiting molecular chain flexibility and increasing the Td. As a result, the Td of the hydroxyl-terminated liquid fluoroelastomer was enhanced from 235 °C to 241 °C, according to TGA results. Hence, the content of –C
C– in the molecular chain were reduced to single bonds, and the content of isolated double bonds in the chain decreased, thereby limiting molecular chain flexibility and increasing the Td. As a result, the Td of the hydroxyl-terminated liquid fluoroelastomer was enhanced from 235 °C to 241 °C, according to TGA results. Hence, the content of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– had a significant impact on the Td. Nevertheless, the TGA results showed that the material was stable and that no chemicals/residues could alter its thermal properties.
C– had a significant impact on the Td. Nevertheless, the TGA results showed that the material was stable and that no chemicals/residues could alter its thermal properties.
3.3 Dynamic viscosities of the liquid fluoroelastomers
At 30–70 °C, the dynamic viscosity of the liquid fluoroelastomers with carboxyl-terminated and hydroxyl-terminated chains was measured, and the findings are presented in Fig. 9.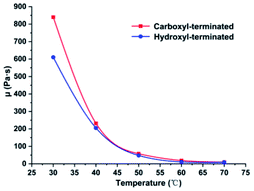 | ||
| Fig. 9 Dynamic viscosities of the carboxyl-terminated liquid fluoroelastomer and the hydroxyl-terminated liquid fluoroelastomer. | ||
The dynamic viscosity of the hydroxyl-terminated liquid fluoroelastomer was lower than that of the carboxyl-terminated liquid fluoroelastomer at 30–50 °C, as shown in Fig. 9. Because the carboxyl group has stronger polarity than the hydroxyl group, the intermolecular force of a carboxyl-terminated liquid fluoroelastomer is larger, resulting in higher dynamic viscosity. The dynamic viscosity of the hydroxyl-terminated liquid fluoroelastomer was 48 Pa s at 50 °C, which was lower than the dynamic viscosity of carboxyl-terminated liquid fluoroelastomer, which was 58 Pa s. When the test temperature exceeded 50 °C, the dynamic viscosities of them are both decreases and tends to equalise, around 10 Pa s, and the end group effect on the dynamic viscosity of the liquid fluoroelastomer disappeared.
3.4 Curing of the liquid fluoroelastomers
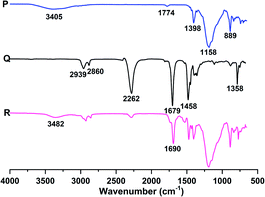
At room temperature, the liquid fluoroelastomers were viscous liquids or semi-solids with no strength. Thus, they had to be cured before use as curing might impart outstanding mechanical properties, excellent thermal stability, and chemical stability. Therefore, the liquid fluoroelastomers were cured, and the structures of the cured products, as well as the influence of heat and the amount of curing agent on the mechanical and surface properties of the cured products, were investigated and analysed.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1681 cm−1. Thus, these demonstrated that HDI could cure the carboxyl-terminated liquid fluoroelastomer.
O at 1681 cm−1. Thus, these demonstrated that HDI could cure the carboxyl-terminated liquid fluoroelastomer.
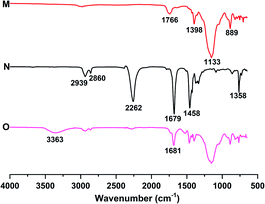 | ||
| Fig. 10 FTIR spectra of the (M) carboxyl-terminated liquid fluoroelastomer, (N) HDI trimer and (O) cured fluoroelastomer. | ||
As illustrated in Fig. 11, the characteristic peak of the hydroxyl group at 3405 cm−1 weakened significantly with the progress of the curing reaction, and the stretching vibration peaks corresponding to the N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the carbamide groups (–NHCOO–) structure appeared at 3482 cm−1 and 1690 cm−1, respectively. Thus, HDI was shown to cure the hydroxyl-terminated liquid fluoroelastomer.
O in the carbamide groups (–NHCOO–) structure appeared at 3482 cm−1 and 1690 cm−1, respectively. Thus, HDI was shown to cure the hydroxyl-terminated liquid fluoroelastomer.
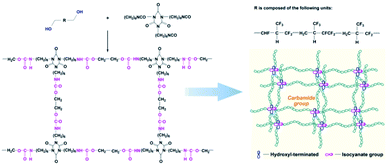
The –CH2OH of the hydroxyl-terminated liquid fluoroelastomer comprises a nucleophilic active hydrogen group containing oxygen atoms, which attacks the –NCO carbon atoms, as indicated in Scheme 4. Meanwhile, because the carbon–nitrogen double bond has low bond energy, the active hydrogen may easily interact with the nitrogen atoms in –NCO and create stable –NHCOOH–, allowing the hydroxyl-terminated liquid fluoroelastomer to be cured.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– content than the carboxyl-terminated liquid fluoroelastomer, its Tg was higher than that of the carboxyl-terminated liquid fluoroelastomer.
C– content than the carboxyl-terminated liquid fluoroelastomer, its Tg was higher than that of the carboxyl-terminated liquid fluoroelastomer.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– in the molecular chains of the liquid fluoroelastomers with different end groups, the molecular weight increased after curing, and the molecular chains formed a network structure, which reduced the flexibility of the liquid fluoroelastomer molecular chains, thus increasing their Td. Because the hydroxyl-terminated liquid fluoroelastomer had lesser –C
C– in the molecular chains of the liquid fluoroelastomers with different end groups, the molecular weight increased after curing, and the molecular chains formed a network structure, which reduced the flexibility of the liquid fluoroelastomer molecular chains, thus increasing their Td. Because the hydroxyl-terminated liquid fluoroelastomer had lesser –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– content than the carboxyl-terminated liquid fluoroelastomer, its Td was higher than that of the carboxyl-terminated liquid fluoroelastomer.
C– content than the carboxyl-terminated liquid fluoroelastomer, its Td was higher than that of the carboxyl-terminated liquid fluoroelastomer.
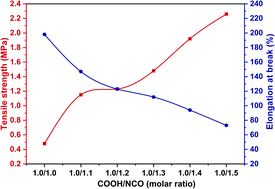 | ||
| Fig. 14 Effect of HDI content on the mechanical properties of the cured carboxyl-terminated liquid fluoroelastomer. | ||
The tensile strength of the carboxyl-terminated liquid fluoroelastomers after curing improved as the HDI proportion increased, and the elongation at break point decreased, as illustrated in Fig. 14. When the COOH/NCO molar ratio was 1.0/1.0, the tensile strength of the cured product was only 0.48 MPa, and the elongation at break was 198%; when the COOH/NCO molar ratio was 1.0/1.2, the tensile strength was 1.23 MPa, and the elongation at break was 125%; when the COOH/NCO molar ratio was further increased to 1.0/1.5, the tensile strength of the cured product increased to 2.26 MPa, and the elongation at break was the least at 73%. Because the crosslinking density in the cured product increases as the curing agents are increased, the mechanical properties of the cured product were also enhanced.
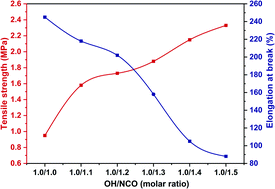
The tensile strength of the cured hydroxyl-terminated liquid fluoroelastomer increased when the amount of HDI trimer was increased, and the elongation at break decreased, as shown in Fig. 15. The compressive mechanical properties of the cured product were higher when the OH/NCO molar ratio was 1.0/1.2, with a tensile strength of 1.73 MPa and 202% elongation at break. The tensile strength of the cured product was as high as 2.33 MPa when the COOH/NCO molar ratio was 1.0/1.5, and the elongation at break was the least at 88%. The mechanical properties of the cured hydroxyl-terminated liquid fluoroelastomer product were superior to those of the HDI-cured carboxyl-terminated liquid fluoroelastomer (Fig. 14). The reaction temperature was lower and the reaction time was shorter when the hydroxyl-terminated liquid fluoroelastomer was cured with HDI, preventing thermal oxygen agentisation of the liquid fluoroelastomers and oxidation degradation of the molecular chains. Further, there was no formation of small molecule products during the reaction, and there were no internal defects and holes in the cured product, which was also one of the reasons.
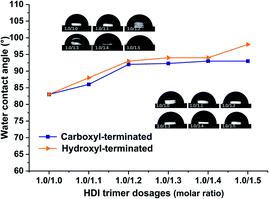 | ||
| Fig. 16 Effect of HDI content on the surface properties of the cured carboxyl-terminated and hydroxyl-terminated liquid fluoroelastomers. | ||
Furthermore, the hydroxyl-terminated liquid fluoroelastomer was more compatible with the curing agent than the carboxyl-terminated liquid fluoroelastomer. The range of surface energy loss was greater, and the samples were hydrophobic.46,47 The detailed results are shown in Table 4.
| End group | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) / /![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) /1.1 /1.1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) / /![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) / /![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) /1.4 /1.4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) /1.5 /1.5 |
|---|---|---|---|---|---|---|
| Carboxyl-terminated | 89° | 86° | 92° | 92° | 93° | 93° |
| Hydroxyl-terminated | 83° | 88° | 93° | 94° | 94° | 98° |
4. Conclusions
In summary, the molecular weight of the poly(VDF-co-HFP) copolymer reduced dramatically after the oxidation degradation reaction from 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 724 to 2458, so carboxyl-terminated liquid fluoroelastomer with 2.71 wt% carboxyl terminal groups can be prepared by oxidation degradation method, and there are about 1.12 mol% –C
724 to 2458, so carboxyl-terminated liquid fluoroelastomer with 2.71 wt% carboxyl terminal groups can be prepared by oxidation degradation method, and there are about 1.12 mol% –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– in the chains of carboxyl-terminated liquid fluoroelastomer were not completely been oxidative degraded. After reduction reaction, the concent of carboxyl group of carboxyl-terminated liquid fluoroelastomer was reduced to 0.12 wt%, with the end-group conversion rate about 96%. At the same time, the –C
C– in the chains of carboxyl-terminated liquid fluoroelastomer were not completely been oxidative degraded. After reduction reaction, the concent of carboxyl group of carboxyl-terminated liquid fluoroelastomer was reduced to 0.12 wt%, with the end-group conversion rate about 96%. At the same time, the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– content was also been reduced. The Mn of the hydroxyl-terminated liquid fluoroelastomer increased to 2680 as a result of the hydrogen-addition reaction in the molecular chain.
C– content was also been reduced. The Mn of the hydroxyl-terminated liquid fluoroelastomer increased to 2680 as a result of the hydrogen-addition reaction in the molecular chain.
Furthermore, the molecular weight and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– concentration had a direct impact on the carboxyl-terminated and hydroxyl-terminated liquid fluoroelastomers properties. The lower the molecular weight and the greater the –C
C– concentration had a direct impact on the carboxyl-terminated and hydroxyl-terminated liquid fluoroelastomers properties. The lower the molecular weight and the greater the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– concentration, the lower was the temperature resistance. Similarly, the lower the –C
C– concentration, the lower was the temperature resistance. Similarly, the lower the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– concentration and the higher the molecular weight, the better was the thermal stability. The Tg of the carboxyl-terminated liquid fluoroelastomer was −28 °C, which was 3 °C lower than that of the hydroxyl-terminated liquid fluoroelastomer, whereas the Td of the hydroxyl counterpart was higher by 6 °C at 241 °C. The dynamic viscosity of the liquid fluoroelastomers with different end groups were all decreased with the increase of temperature and similar to about 10 Pa s at 70 °C.
C– concentration and the higher the molecular weight, the better was the thermal stability. The Tg of the carboxyl-terminated liquid fluoroelastomer was −28 °C, which was 3 °C lower than that of the hydroxyl-terminated liquid fluoroelastomer, whereas the Td of the hydroxyl counterpart was higher by 6 °C at 241 °C. The dynamic viscosity of the liquid fluoroelastomers with different end groups were all decreased with the increase of temperature and similar to about 10 Pa s at 70 °C.
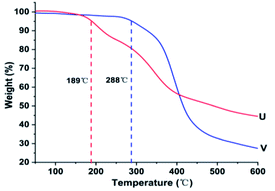
After curing, the different end groups of the liquid fluoroelastomers presented distinct curing efficiency and properties. Because the activity of the hydroxyl group is higher than that of the carboxyl group, it could be cured efficiently in a shorter time. The Td of the cured hydroxyl-terminated liquid fluoroelastomer reached 288 °C, which was about 100 °C higher than that of the cured carboxyl-terminated liquid fluoroelastomer. However, its low-temperature resistance property was poorer, with a Tg of −3 °C compared with −10 °C of its carboxyl counterpart. The tensile strength was 2.33 MPa and the elongation at break was 88%. In addition, they are both exhibited hydrophilic behaviour, the WCA of the hydroxyl-terminated liquid fluoroelastomer was 98°. And the higher the activity of the end group, the stronger was the hydrophilicity.
Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
The authors are grateful for the National Natural Science Foundation Youth Fund of China (contract grant numbers 52003165), the Doctoral Scientific Research Foundation of Liaoning Province (contract grant numbers 2019-BS-190), the Program for Young & Middle-aged Scientific and Technological Innovative Talents of Shenyang (contract grant number RC210195), the Educational Department Foundation of Liaoning Province (contract grant numbers LQ2019005), and the Program for Liaoning Innovative Talents in Universities of China (contract grant numbers LR2019053).References
- P. Ferrandez, Fluoroelastomers, FKM and FEPM, in Handbook of Specialty Elastomers, ed. C. K. Robert, Taylor & Francis Group, New York, 2008, pp. 133–154 Search PubMed.
- M. I. Abdelhamid, A. M. Aboelwafa, H. Elhadidy and A. Habib, Int. J. Polym. Mater. Polym. Biomater., 2012, 61, 505–519 CrossRef CAS.
- B. Ameduri, B. Boutevin and G. Kostov, Prog. Polym. Sci., 2001, 26, 105–187 CrossRef CAS.
- J. Li, Y. F. Lu, Y. Liu, Y. Li, X. A. Zhang and S. C. Qi, Polym.-Plast. Technol. Eng., 2014, 53, 46–53 CrossRef CAS.
- B. Ameduri, Macromolecules, 2010, 43, 10163–10184 CrossRef CAS.
- M. Destarac, K. Matyjaszewski, E. Silverman, B. Ameduri and B. Boutevin, Macromolecules, 2000, 33, 4613–4615 CrossRef CAS.
- B. Ameduri, Chem. Rev., 2019, 109, 6632–6686 CrossRef PubMed.
- R. Saint-Loup and B. Ameduri, J. Fluorine Chem., 2002, 116, 27–34 CrossRef CAS.
- S. Mitra, A. Ghanbari-Siahkali, P. Kingshott, S. Hvilsted and K. Almdal, J. Polym. Sci., Part A: Polym. Chem., 2010, 42, 6216–6229 CrossRef.
- A. Zaggia and B. Ameduri, Curr. Opin. Colloid Interface Sci., 2012, 17, 188–195 CrossRef CAS.
- M. A. Tasdelen, M. U. Kahveci and Y. Yagci, Prog. Polym. Sci., 2011, 36, 455–567 CrossRef CAS.
- Y. F. Chang, M. Y. Liao and X. Y. Li, RSC Adv., 2020, 10, 10932–10938 RSC.
- Q. Yu, Handbook of Rubber Raw Materials, Chemical Industry Press, Beijing, 2nd edn, 2007 Search PubMed.
- A. E. Finholt, A. C. jun Bond and H. I. Schlesinger, J. Am. Chem. Soc., 1947, 69, 1199–1203 CrossRef CAS.
- J. Wang, M. Y. Ju, X. H. Wang, Y. N. Ma, D. H. Wei and X. N. Chen, J. Org. Chem., 2021, 86, 5305–5316 CrossRef CAS PubMed.
- B. Thiedemann, C. M. L. Schmitz and A. Staubitz, J. Org. Chem., 2014, 79, 10284–10295 CrossRef CAS PubMed.
- L. Calabrese and A. Valenza, Eur. Polym. J., 2003, 39, 1355–1363 CrossRef CAS.
- C. W. Wise, W. D. Cook and A. A. Goodwin, Polym, 2000, 41, 4625–4633 CrossRef CAS.
- K. K. Chen, S. Yuan, X. M. Wen, C. Sang and Y. J. Luo, Propellants, Explos., Pyrotech., 2021, 46, 428–439 CrossRef CAS.
- L. Sauguet, B. Ameduri and B. Boutevin, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 4566–4578 CrossRef CAS.
- L. C. J Hesselmans, A. J. Derlken and J. A. M. van den Goorbergh, Prog. Org. Coat., 2006, 55, 142–148 CrossRef.
- Y. Ebrahimabadi, M. Mehrshad, M. Mokhtar and M. Abdollahi, J. Appl. Polym. Sci., 2021, 138, 49932. CrossRef.
- N. Wingborg, Polym. Test., 2002, 21, 283–287 CrossRef CAS.
- J. G. Kennemur and B. M. Novak, Polymer, 2011, 52, 1693–1710 CrossRef CAS.
- X. D. Zheng, S. J. Qiu, L. M. Feng, M. T. Wang, H. L. Li, X. X. Gen and Z. Z. Zhang, Polym. Bull., 2006, 56, 563–569 CrossRef CAS.
- D. Kitaga and S. Kobatake, Chem. Sci., 2012, 3, 1445–1449 RSC.
- S. A. Seyedmehdi, H. Zhang and J. Zhu, Appl. Surf. Sci., 2012, 258, 2972–2976 CrossRef CAS.
- D. Valade, F. Boschet and B. Ameduri, Macromolecules, 2009, 42, 7689–7700 CrossRef CAS.
- D. H. Li and M. Y. Liao, Mater. Rep., 2018, 32, 1730–1736 Search PubMed.
- R. Souzy, B. Ameduri, S. V. Ahsen, H. Willner and G. A. Arguello, J. Fluorine Chem., 2003, 123, 85–93 CrossRef CAS.
- A. Taguet, L. Sauguet, B. Ameduri and B. Boutevin, J. Fluorine Chem., 2007, 128, 619–630 CrossRef CAS.
- Z. Y. Yang, J. Org. Chem., 2003, 68, 5419–5421 CrossRef CAS PubMed.
- Y. Cheburkov and G. G. I. Moore, J. Fluorine Chem., 2003, 123, 227–231 CrossRef CAS.
- M. Pianca, P. Bonardelli, M. Tat, G. Cirillo and G. Moggi, Polymer, 1987, 28, 224–230 CrossRef CAS.
- M. Pianca, E. Barchiesi, G. Esposto and S. Radice, J. Fluorine Chem., 1999, 95, 71–84 CrossRef CAS.
- D. H. Li and M. Y. Liao, J. Fluorine Chem., 2017, 201, 55–67 CrossRef CAS.
- D. H. Li and M. Y. Liao, Polym. Degrad. Stab., 2018, 152, 116–125 CrossRef CAS.
- S. Lin, Z. Y. Li, G. Luo and Y. Y. Chen, Chem. Ind. Eng. Prog., 2014, 33, 1276–1284 CAS.
- A. J. Li, B. Feng and Q. C. Liu, Chin. J. Org. Chem., 2011, 31, 106–109 CAS.
- G. J. Ross, J. F. Watts, M. P. Hill and P. Morrissey, Polymer, 2001, 42, 403–413 CrossRef CAS.
- J. H. Wei, X. Zhang, J. J. Qiu and L. Brandon, J. Polym. Sci., Part B: Polym. Phys., 2015, 53, 1691–1700 CrossRef CAS.
- D. H. Li and M. Y. Liao, Key Eng. Mater., 2017, 753, 93–98 Search PubMed.
- R. Souzy, B. Boutevin and B. Ameduri, Macromolecules, 2012, 45, 3145–3160 CrossRef CAS.
- A. Taguet, B. Ameduri and B. Boutevin, J. Polym. Sci., Part A: Polym. Chem., 2010, 44, 1855–1868 CrossRef.
- W. Posthumus, A. J. Derksen, J. A. M. van den Goorbergh and L. C. j. Hesselmas, Prog. Org. Coat., 2007, 58, 231–236 CrossRef CAS.
- N. Li, F. L. Zeng, Y. Wang, D. Z. Qu, W. B. Hu, Y. G. Luan, S. Dong, J. J. Zhang and Y. P. Bai, RSC Adv., 2017, 7, 30970–30978 RSC.
- V. A. Ganesh, A. S. Nair, H. K Raut, T. T. Y. Tan, C. B. He, S. Ramakrishna and J. W. XU, J. Mater. Chem., 2012, 22, 18479–18485 RSC.
| This journal is © The Royal Society of Chemistry 2022 |