Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Modulating the surface and mechanical properties of textile by oil-in-water emulsion design
Evangelia Argentou ab,
Carlos Amadorb,
Anju Deepali Massey Brookerb,
Serafim Bakalisac,
Peter J. Fryera and
Zhenyu Jason Zhang
ab,
Carlos Amadorb,
Anju Deepali Massey Brookerb,
Serafim Bakalisac,
Peter J. Fryera and
Zhenyu Jason Zhang *a
*a
aSchool of Chemical Engineering, University of Birmingham, Edgbaston, Birmingham, U.K. B15 2TT, UK. E-mail: z.j.zhang@bham.ac.uk
bProcter & Gamble, Newcastle Innovation Centre, Newcastle-upon-Tyne, U.K. NE12 9TS, UK
cDepartment of Food Science, University of Copenhagen, Rolighedsvej 26, Frederiksberg, DK-1958, Denmark
First published on 13th January 2022
Abstract
The synergistic effect of oil viscosity and oil droplet size on the deposition profile of oil on cotton fabric was studied using polydimethylsiloxane (PDMS) as a model oil-in-water emulsion system. Under the same preparation conditions, low viscosity PDMS produced emulsions containing small droplets, which resulted in a uniform surface deposition profile, whilst high viscosity PDMS resulted in a localised deposition profile. Interfacial phenomena such as wicking and penetration of PDMS into cotton fabrics were found to be viscosity-dependent, which agrees with the surface deposition data. Both mechanical characterisation (friction, compression, stiffness) and consumer evaluation confirm that the fabrics treated by the emulsion containing low viscosity PDMS were preferred, suggesting that a homogeneous surface deposition and an excellent penetration profile of PDMS are critical for maximising tactile sensorial benefits, which could be accomplished by optimising the emulsion formulation to contain oil of low viscosity and small PDMS droplets.
Introduction
Actives of selected molecular architecture and molar mass are commonly used to modulate the surface and tribological characteristics of materials such as textiles, resulting in an array of benefits (e.g. softness, lubricity) that are desired by consumers.1–6 To enhance product sustainability without compromising product performance, it is critical to establish a comprehensive framework correlating formulation design to comfortableness perceived by the consumer.7 The physico-chemical transformations involved in the application of softener actives include surface deposition in liquid, rearrangement during drying, and controlled surface interactions in an ambient environment, all of which are underpinned by the surface and interface phenomena.The ability to deliver and deposit actives on surfaces, alongside the knowledge that underpins the sensorial benefits of fabrics, are critical for the design of formulated products. For example, correlation between fabric friction and subjectively perceived touch properties was found for knitted, but not for woven fabrics, in a previous work.8 In the same study, relevant properties correlating friction and touch properties were identified as bending, thickness, and compressibility. Many theories have been proposed around the softening mechanism of actives such as polymers, and the most prominent one assumes the formation of a lubricating layer on the fibers upon deposition.9–15 Others have proposed that the softening benefit is due to the reduction in hydrogen bonding between water molecules and cotton fibres as a result of the deposition.16–18 Deposition of softening actives has also been explored extensively: some studies19 suggest that hydrophobic interactions due to the presence of long alkyl chains in cationic surfactants are the primary driving force for adsorption, whilst others20 have proposed that deposition is driven by electrostatic interactions. Attempts were made to correlate fabric tribology with smoothness and softness21 and others have put emphasis on studying the friction between human skin and fabrics.22 Polymers are often used in commercial fabric softeners since they can lead to improved textile properties such as wrinkle recovery, crease resistance, improved softness and better wear comfort without the disadvantages that accompany conventional softener types such as yellowing. Non-charged silicones with hydrophobic properties, such as polydimethylsiloxane (PDMS), are used extensively in the laundry industry.6,23
Surface deposition strategies of silicone have been studied extensively for the past three decades due to its ability to/cover the surface of the fibre (natural and synthetic ones), and consequently provide excellent softening properties, enhancing the sensory characteristics of fabrics.9,23,24 Deposition of PDMS on fabrics can occur in various ways, the most common being emulsions that contain PDMS/surfactant droplets. Surface deposition of actives is driven by complex physical and chemical phenomena during a wash process that are not well understood. The aqueous environment, the presence of ions, the porosity and roughness of the fabrics, the presence of surfactant monomers, the presence of shear forces as well as the hydrophobic nature of PDMS can all affect deposition.24–26
The impact of oil viscosity and emulsifiers on the resulting emulsion was investigated as early as 1990.4 It was reported that increasing oil viscosity could help to increase both the rates and extent of silicone deposition on to human hair, which was attributed to the increased attractive droplet–fibre interaction and ability to cover a large fibre surface area upon deposition.27 In a recent study, emulsions of organomodified silicones (OMS) were prepared to evaluate the effects of droplet size and charges.27 Panel tests suggested that the conventional emulsion (droplet size in the μm range) was favoured to microemulsions in which droplets were of nm size, which was attributed to the predominant surface deposition of silicone using the large emulsion. As a contrast, nano-emulsion of silicone was found to outperform micro- and macro-emulsions in terms of fabric softness, smoothness, cooler feel and thinness.28 Lastly, micro- and macro-emulsions of silicone were deposited on polyester fibres by Parvinzadeh and Hajiraissi,2 which suggested that macroemulsion was able to improve crease recovery due to the formation of silicone film that lubricates fibre–fibre friction.1 In the same study, microemulsion was found more effective in introducing crease resistance.
Upon deposition, migration of the oil droplets into fabrics is determined by wetting and wicking, both of which are multiscale phenomena that are determined by (i) the nature of the fibres such as surface charge, porosity, and hydrophobicity; (ii) characteristics of the fabrics, e.g. woven pattern and density; and (iii) surrounding environment such as pH, ionic strength. Electrokinetic properties of natural fibres have been studied extensively using techniques such as streaming potential, which has a significant impact on the deposition kinetics and characteristics of colloids.27,29,30 This equally is controlled by the oil profiles such that low viscosity and high interfacial energy would facilitate spreading.
Finally, the mechanistic relationship between amount, location, and characteristics of the deposited silicone and the sensorial benefits of fabrics remains unclear. This is partly due to the complex nature of skin tribology that is often correlated to a broad range of physical parameters. The sensory characteristics of the fabric are one of the components that determine the final product quality perception. The most important characteristics of textiles that seem to be correlated with performance attributes are appearance and “fabric handle”.31,32 The latter is defined as the perceived overall aesthetic quality that reflects the mechanical and physical properties of the fabric.33 It is commonly believed that there is a strong attraction between silicone and fabric, which enables silicone to spread and form a lubrication layer that alters the fabric tribology and therefore the “fabric handle”.34–36 It is therefore critical to consider the silicone distribution profile and its location on the fabric: should it be kept on the fabric external surface to intensify the skin-fabric lubrication or delivered into the fabrics for a homogeneous distribution.29
To design emulsion technology that contains either silicone or other alternative oils, it is therefore essential to establish a holistic approach that links oil characteristics with final sensorial perception, with a thorough consideration of the physical/chemical transformations involved. We therefore chose non-ionic silicone as a representative system for investigation in the present work. A series of emulsions of different oil droplet sizes were prepared as a function of silicone viscosity. The deposition profile of polydimethylsiloxane (PDMS) on fabrics was established using a fluorescent microscopy technique,37,38 complemented by elementary analysis and gravimetric measurements. Alteration to the physical properties of the fabrics was quantitatively investigated by mechanical testing and consumer evaluation.
Experimental
Materials
Methods
Surface morphology and elementary analysis were carried out using a tabletop SEM (Hitachi TM3030Plus), that is equipped with both Secondary Electrons (SE) and Backscattered Electron (BSE) detectors, with 5 kV and 15 kV accelerating voltage.
Coefficients of friction (COF, μs) were measured on rectangular (12 cm × 6 cm) fabric samples using a COF/Peel Tester (Thwing-Albert FP-2260). A 200 g spring sled (02250–4400), with additional load of 500 g, equivalent to 6.86 N applied load, with a geometry of 2 1/2′′ × 2 1/2′′ (63.5 mm × 63.5 mm), resulting in a contact pressure of 1701 Pa, was used to assess the COF for each fabric sample. The sliding velocity that was used was 0.8 cm s−1.
Compression measurements were conducted by measuring the force needed to compress a circular (10 cm diameter) fabric samples using an Electromechanical Testing System (MTS Insight 10). The traction capacity was at 10 kN and the speed used was 200 mm min−1. The sampling frequency was 100 Hz and the software Testworks was used to operate the machine and obtain the results. For that purpose, a round piece of the terry cotton (12 cm diameter and 5.5 mm thickness) was used.
A square piece (5 cm × 5 cm) of the fabric sample was used for stiffness measurements in ‘Taber’ stiffness units (g cm) with an industry standard 150-E Stiffness Tester (Taber Industries, New York, USA).47
Results & discussion
Size distribution of the emulsified PDMS
Previous studies26,48–51 suggest that the size of the emulsified PDMS droplets is determined by the amount of emulsifier, the emulsification time, the shear force applied, the amount and the viscosity of PDMS, of which the last two parameters were considered in the present work. Corresponding size distribution profiles of the emulsified PDMS droplets, measured by a particle size analyser, are presented in Fig. 1.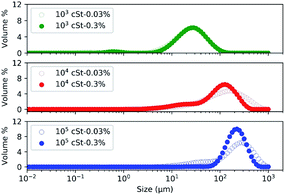 | ||
| Fig. 1 Size distribution of emulsified droplets using PDMS of different viscosity: (i) 103, (ii) 104, (iii) and 105 cSt at two concentrations: 0.03% and 0.3%. | ||
The size profiles presented in Fig. 1 confirm that the viscosity of PMDS has a significant impact on the size distribution of the resulting droplets: emulsions prepared with PDMS of low viscosity (103 cSt) contained smaller droplets in comparison to those prepared with PDMS of high viscosity (104, and 105 cSt). The median droplet sizes are 20–50 μm, 50–100 μm, and 100–500 μm from emulsions prepared by 103, 104, and 105 cSt PDMS, respectively. The use of high viscosity PDMS led to the formation of large droplets due to the high molecular weight of the PDMS molecule. Since the emulsification process, including the amount of emulsifier, shear force and time, was kept consistent, the notable variation in the size profile is likely because PDMS of high molar mass would require more energy, more time, or higher levels of surfactant molecules to emulsify and generate small droplets, as observed in a previous study.4,26 The effect of PDMS viscosity on the size distribution of emulsion would have significant implication on the industrial process whereby energy, time, and resources need to be considered.
Concentration of the emulsified droplets can be estimated from eqn (1) below
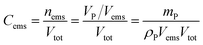 | (1) |
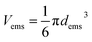 | (2) |
The diffusion coefficient of an individual emulsion droplet (Dems) can be estimated by the Stokes–Einstein equation,
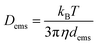 | (3) |
As for the small scale deposition, the fluid mechanics in the tergotometer was controlled under the same conditions. As such, the probability of collision (fems) between PDMS droplets and fabrics can be estimated using the following equation,52 based on the frequency of impacts of diffusive flux on a finite disc electrode:
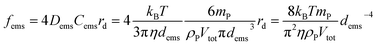 | (4) |
• Increasing droplet size would result in deposition on the surface of the fabrics due to physical entrapment, which is equally determined by the porosity of the fabrics, whilst droplets of reduced size (less than the gap between yarns, 10–40 μm) are capable of penetrating into the fabrics.
• Interfacial energy of the droplet and fibres plays a critical role. This needs to be considered under the appropriate conditions. The surface energy of PDMS is consistent, as reported previously.55
• Lastly, viscosity of the oil influences the spreading of the PDMS droplets upon contact with the fabrics.
Recent study54 suggests a quantitative correlation between droplet spreading ratio, impact velocity, and fluid properties by interpolating between the capillary regime (∝ We1/2) and viscous regime (∝ Re1/5) using a first order Padé approximation. This was further adjusted to account for low impact velocity droplet spreading, which is appropriate for the present work:
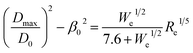 | (5) |
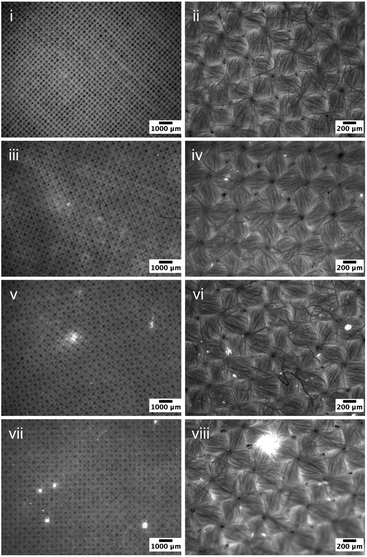
To verify the estimation above, fluorescence microscopy was used to evaluate the deposition profiles of the prepared PDMS emulsions on cotton fabrics following the small scale washing process and dried overnight (Fig. 2). Images acquired at two different magnifications are included (scale bars are 1000 and 200 μm respectively). The control sample is a standard plain weave cotton fabric that showed barely any fluorescence signal. Upon exposure to 0.03% PDMS emulsions using 103, 104, and 105 cSt PDMS, bright regions were observed on the fabrics, which indicates the presence of deposited PDMS on the fabric surfaces since there was no other fluorescence agents introduced. The fluorophore used in the present work, Pyrromethene 546, is hydrophobic and binds strongly to PDMS, and hence can provide a solid evidence concerning the location of the deposited PDMS.
Very few bright regions were observed on the samples treated by emulsions containing 103 cSt PDMS (Fig. 2iii and 2iv), indicating a limited presence of PDMS on the surface, which appears to be counter-intuitive as the probability of collision is much greater for the 103 cSt PMDS droplets that those of 104 cSt. It is very likely that the size of PDMS emulsions were significantly smaller than the size of the yarns (gaps between cotton fibre bundles), and hence were able to penetrate through the fabric into deeper regions. The presence of PDMS appeared to enhance as the viscosity of PDMS increased to 104 cSt – not only there are large patches on the fabric surface (Fig. 2v), but also significantly increased numbers of bright spots of which the size is in the region of 20–60 μm, which are the results of PDMS deposition. As for the samples exposed to the formulation prepared using 105 cSt, the number of large regions of deposition, several hundred microns in diameter, is notable (Fig. 2vii and viii). This change in the maximum spreading diameters is consistent qualitatively with the prediction based on eqn (5).
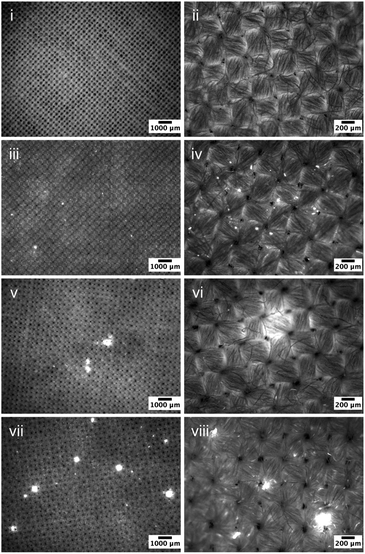
Fluorescence micrographs of the cotton samples exposed to 0.3%wt PDMS emulsions were subsequently acquired and are presented in Fig. 3. Very similar correlation can be found here: increased viscosity appears to result in an enhanced surface presence. It is worth noting that the increased concentration has a significant impact on deposition, for all formulations used, which is governed by eqn (4) whereby the collision frequency is proportional to the weight of the added PDMS, whilst the diameter of the emulsified droplets remains the same. Formulations prepared with 10 kcSt PDMS exhibited an increasingly uneven deposition profile on the fabric with the presence of large PDMS rich regions between 50-200 μm (Fig. 3v, vi). Such heterogeneity became more severe on fabrics treated by formulations using 100 kcSt PDMS: large PDMS being entrapped between the yarn intersections or deposited at the yarn surface (Fig. 3vii, viii). Their diameters are between 100-500 μm. The Fig. 3viii depicts the area occupied by the droplets of sizes 50–200 μm. Areas occupied by larger droplets can be seen in Fig. 3vii and could not be captured in higher magnification in high quality.
Observations on fabrics exposed to PDMS emulsions prepared can confirm the effects of PDMS viscosity on the surface deposition, on both emulsion droplet size and its spreadability once they are in contact with the fabric, which is consistent with previous work reported by Nazir and co-wokers.25 The PDMS emulsions of high viscosity contain large droplets (hundreds of micron) that would have: (i) strong surface adhesion to the fabric due to the increased contact area; (ii) low penetration profile due to it large size; and (iii) low flowability, which would form a localised deposition profile. As a contrast, PDMS emulsions of low viscosity resulted in droplets of low adhesion to the fabric upon deposition, capable of penetrating through the yarns, that are able to migrate with less resistance, and hence generate a more homogeneous deposition on the target fabric.
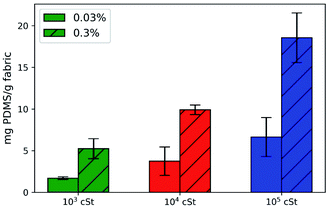
Subsequent gravimetric measurements suggest that 0.03% provides a better deposition efficacy than that of 0.3%, despite that the absolute deposition quantity is less, as presented in Fig. 4. There is a noticeable increase of amount deposited as the droplet size increases. The ratio between deposition mass is approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 between emulsions prepared by 103, 104, and 105 cSt silicone. Although the collision probability for large droplets is reduced, as rationalised earlier, the volume of each droplet is much greater (V ∼ d3), which compensates the deposition efficacy. Taking into account both collision probability and droplet size, the total volume of 20 μm droplets in the emulsion would be ten times greater than that of 200 μm droplets, and 2.5 times greater than that of 50 μm droplets, assuming that the difference in the density of PDMS is negligible. The discrepancy between our theoretical estimation and the quantities measured experimentally could be attributed primarily to the possibility that the small emulsion droplets can penetrate the gaps between cotton fibres and hence escape back into the bulk solution.
4 between emulsions prepared by 103, 104, and 105 cSt silicone. Although the collision probability for large droplets is reduced, as rationalised earlier, the volume of each droplet is much greater (V ∼ d3), which compensates the deposition efficacy. Taking into account both collision probability and droplet size, the total volume of 20 μm droplets in the emulsion would be ten times greater than that of 200 μm droplets, and 2.5 times greater than that of 50 μm droplets, assuming that the difference in the density of PDMS is negligible. The discrepancy between our theoretical estimation and the quantities measured experimentally could be attributed primarily to the possibility that the small emulsion droplets can penetrate the gaps between cotton fibres and hence escape back into the bulk solution.
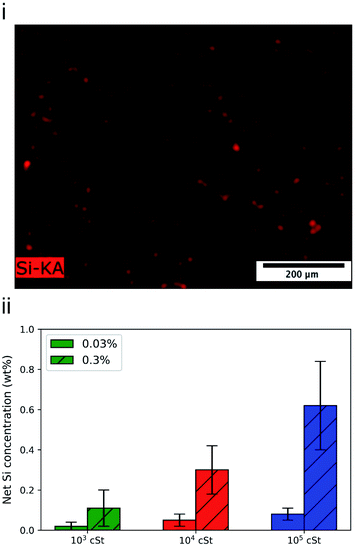
The deposition profile of PDMS seems to be homogeneous with Si being uniformly detected throughout the fabric surface (Fig. 5i). Similar micrographs were obtained for fabrics treated by the other PMDS emulsions prepared, followed by EDX analysis to quantify the deposited amount of Si (Fig. 5ii). The highest quantities of Si and thus PDMS deposited were detected for the emulsions with the 105 cSt PDMS variant (Fig. 5iii). The higher deposited quantities can be connected to the larger PDMS droplets formed and deposited on the fabric. A large portion of small PDMS droplets will be filtered through the fabric yarns resulting in less PDMS being deposited on or between the fibres. It is striking to note that the ratio between signal intensity is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, which is consistent with the gravimetric data (Fig. 4).
4, which is consistent with the gravimetric data (Fig. 4).
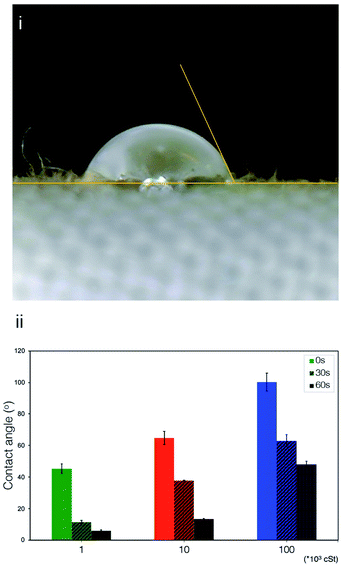
Fig. 6 shows the contact angle measurements on wet cotton fabric for all PDMS variants (103, 104, and 105 cSt), which were acquired instantaneously, and subsequently at 30 and 60 s upon deposition. PDMS sample of high viscosity (105 cSt) exhibited the greatest initial contact angle, followed by the one with medium viscosity (104 cSt), and the one with low viscosity (103 cSt) presents the lowest contact angle, which confirms the role of viscosity. As summarised by Nyoni and Brook,59 the distance of liquid travelled in a fabric is described by
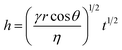 | (6) |
In recent studies,60–63 robust coating based on PDMS or other compounds was developed on textiles to render a superhydrophobic or superamphiphobic characteristics, which was evaluated by using water contact angle (WCA). These studies highlight the importance of controlling surface wettability of textile, which underpins the applications such as oil-water separation and anti-fouling. Unlike the chemical treatment routes discussed, physical deposition of emulsified PDMS was investigated in the present work. However, it is controlled by the same principles discussed early. We would, however, like to highlight that the viscosity of PDMS plays a critical role in such physical deposition process as the surface energy of all three PDMS used in the present work is the same. Another important difference to the previous work60–63 where permanent coating was developed on textiles such as cotton fabrics, PDMS layer studied in the present work is not chemically bond to the textile due to the safety regulatory requirements, and hence could be removed in the subsequent washing cycles, although there might be some build-up over the course of a §multiple washing-deposition cycle.
Mechanical properties and consumer evaluation
To assess quantitatively the effect of silicone deposition on the sensorial benefits, a range of mechanical measurements and consumer evaluation were carried out. Fig. 7 shows the normalised values of stiffness, compression and friction measurements of fabrics treated with PDMS emulsions with different PDMS variants (103, 104, and 105 cSt). Overall, it appears that the fabrics treated with PDMS emulsions exhibited better bending properties and lower stiffness values in comparison to the control sample, fabrics washed without PDMS (Fig. 7i). Samples treated with low viscosity PDMS (103 cSt) emulsions exhibited the best bending properties with 44% decrease in stiffness, while those treated with 104 cSt PDMS and 105 cSt PDMS emulsions exhibited 28% and 9% decrease in stiffness respectively. This is likely attributed to the great penetration capability of the low viscosity PDMS into cotton fabric. On the contrary, high viscosity PDMS tends to accumulate on the fabric surface and is not able to provide the same magnitude of bending benefits.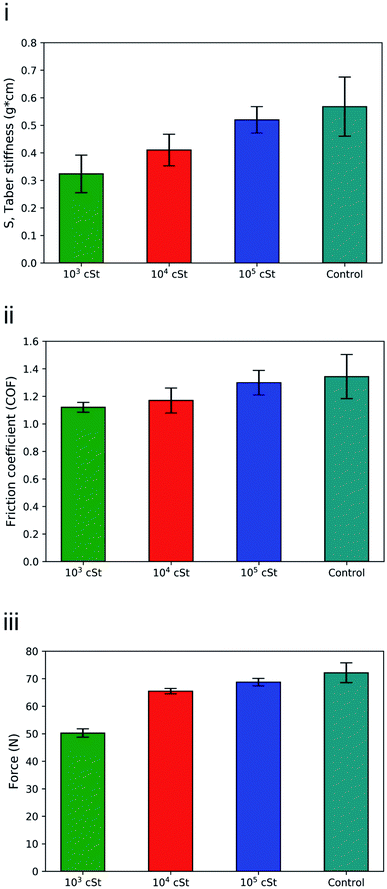 | ||
| Fig. 7 (i) stiffness; (ii) friction, and (iii) compression resistance of cotton fabrics treated by emulsion containing 1, 10, 100 kcSt PDMS. | ||
Likewise, it appears that the fabrics treated with the PDMS emulsions required less force to be compressed in comparison to the control (Fig. 7ii), which confirms the effect of PDMS in softening the fabrics. Terry cotton washed with the 103 cSt PDMS emulsion exhibited the highest compressibility, requiring 30% less force (72.16 N vs. 50.26 N) as opposed to the fabrics treated with 104 cSt PDMS and 105 cSt PDMS emulsions that required 9% and 5% less force. Both bending and compression are being viewed as critical parameters for consumer tactile benefits. Similar to the wrinkle recovery ability, for which energy dissipation plays a dominant role,64 having a thorough coverage of PDMS across the fabric appears to be very important for delivering desired performance of bending and compression properties. Surface analysis carried out by Xu et al.,65 and colleagues showed silicone adsorbed to a cellulose surface would form a smooth film that can deliver a lubrication function, which significantly reduces the energy required in bending and compressing the fabrics.
Treatment with PDMS emulsions also improved the friction properties of the terry fabrics with a reduced Coefficient of Friction (COF) after treatment (Fig. 7iii). Fabrics washed with emulsions prepared with the low viscosity PDMS (103 cSt) exhibited the lowest friction coefficient values which was 17% lower than that of the control fabrics. Fabrics treated with 104 cSt and 105 cSt PDMS emulsions exhibited a 13% and 3% decrease respectively. From the tribological perspective, our friction measurements involve a low normal load (6.86 N), slow sliding velocity (0.8 cm s−1), and small amount of deposited PDMS on the fabric surface, which imply that the friction takes place in the boundary lubrication regime whereby interfacial friction is determined by the interaction between the surfaces in contact.13,66 Although silicone of high viscosity could help to reduce interfacial friction, as previous study suggests,67 it was carried out in a scenario in which the contact interface was saturated by the silicone oil, which is a distinctively different contact mechanics to what is being discussed in the present work. Such correlation between viscosity and COF would probably be evident if the deposition profile exhibited the same uniformity for all the samples, which contradicts to our experimental observation. It is very likely that the emulsions contained large droplets of PDMS (using high viscosity PDMS) were distributed unevenly on the fabric surface, thus the total decrease in the COF value was not significant. The presence of smaller PDMS droplets in the 103 cSt PDMS emulsions, as evidenced by the size measurements and surface characterisation, resulted in a more uniform distribution on the fabric surface is linked to the decrease on the COF values, which agrees with findings reported previously.28 The result supports our explanation that smaller particles can easily penetrate into the fibres and provide coverage and thus less friction between fibres and yarns.
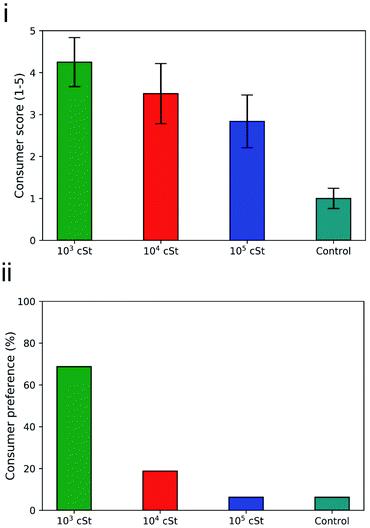 | ||
| Fig. 8 (i) Consumer grading and (ii) consumer preference on the fabrics treated by the PDMS emulsions prepared in the present work, based on an evaluation of 16 adults. | ||
Fabrics washed with 103 cSt PDMS emulsions obtained an average score of 4.25 ± 0.58 from the consumer panel with 5 being the highest score and 1 the lowest possible score. Fabrics treated with 104 cSt and 105 cSt PDMS emulsions received 3.5 ± 0.72 and 2.84 ± 0.63 respectively. The control terry fabric that did not undergo any treatment received the lowest score from the panellists 1.12 ± 0.24. Such result confirms that the surface deposited PDMS can be perceived in terms of tactile sensorial benefits, and that the PDMS of 103 cSt is the most favourable one, followed by 104 cSt and then 105 cSt. Overall consumer preference was also acquired, where the consumers were asked to choose only one of the treated fabric samples based on overall feel (Fig. 8ii). It was found that 69% (11/16) of panellists preferred the feel fabrics washed with 1 kcSt PDMS solutions, 19% (3/16) showed preference for the fabrics washed with 10 kcSt, and 6% (1/16) for the fabrics washed with 105 cSt and 6.25% (1/16). The majority of consumers preferred the fabrics washed with 103 cSt emulsions which are the samples that exhibited the lowest stiffness, lowest compression force and lowest friction coefficient values.
The results from both consumer grading and consumer preference are consistent with the results produced by the mechanical testing, which highlights, once again, the importance of not only deposition profile on the exterior surface of the fabrics but also the penetration characteristics of the oil component into the fabrics. And it is safe to conclude that controlling droplet size by changing the viscosity of a material or altering the formulation process of a product can improve the on-fabric deposition and retention improving the surface properties and leading to higher consumer acceptance.
Conclusions
In this work, we demonstrated that a coherent design framework that links formulation preparation of model silicone emulsion, their deposition profile on cotton fabrics, the resulting mechanical properties, and corresponding consumer evaluation.First of all, there is an explicit correlation between emulsion properties and PDMS deposition profile. Properties such as droplet size, viscosity, and concentration have a synergistic and significant impact on the deposition profile of PDMS on fabric. PDMS of medium and high viscosity (104 cSt, 105 cSt) lead to the formation of large PDMS droplets (100–500 μm) in the emulsion, which resulted in an increased PDMS deposition on the fabric surface. However, such large PDMS droplets with high viscosity resulted in a localised, non-even deposition profile on the fabric surface. As a contrast, emulsion prepared using PDMS of low viscosity (103 cSt) contains small droplets that were able to deposit and penetrate the fabrics, despite that the total amount of deposition is less than the counterparts of high viscosity. The subsequent mechanical properties measurements and sensorial feel evaluation confirm the superiority of low viscosity PDMS as a raw material during emulsion preparation versus the use of PDMS with higher viscosity. Formulation using 103 cSt PDMS emulsion improved bending, compression and friction properties of the cotton fabric, which was well perceived by the consumer.
To conclude, we would recommend a holistic approach for designing formulated products that contains silicone, or alternative oils, in the future by considering the synergistic effect of oil viscosity, resulting droplet size, interfacial energy on the fibre in contact under the relevant environment, in addition to the other parameters such as emulsifiers used, to accomplish the maximum consumer benefits with minimal raw materials used.
Conflicts of interest
The authors hereby confirm that there is no conflict to declare.Acknowledgements
The authors of this article would like to thank the Engineering and Physical Sciences Research Council UK (EPSRC) and Procter & Gamble UK for funding this project through the EPSRC CDT in Formulation Engineering (EP/L015153/1). ZJZ acknowledges the Industrial Fellowship with P&G sponsored by the Royal Academy of Engineering (IF2021\100).References
- M. Parvinzadeh and R. Haijraissi, Macro- and Microemulsion silicone softeners on polyester fibers: Evaluation of Different Physical Properties, J. Surfactants Deterg., 2008, 11, 269–273 CrossRef CAS.
- B. Wahle and J. Falkowski, Softeners in textile processing. Part1: an overview, Rev. Prog. Color. Relat. Top., 2002, 2, 118–124 Search PubMed.
- T. Kang and M. Kim, Effects of Silicone Treatments on the Dimensional Properties of Wool Fabric, Text. Res. J., 2001, 71, 295–300 CrossRef CAS.
- M. Berthiaume and J. Jachowicz, The effect of emulsifiers and oil viscosity on deposition of nonionic silicone oils from oil-in-water emulsions onto keratin fibres, J. Colloid Interface Sci., 1991, 141, 299–315 CrossRef CAS.
- K. Jang and K. Yeh, Effects of Silicone Softeners and Silane Coupling Agents on the Performance Properties of Cotton Fabrics, Text. Res. J., 1993, 63, 557–565 CrossRef CAS.
- F. Case, Silicones in Fabric Care, J. Surfactants Deterg., 2006, 9, 303–305 CAS.
- European Commision, Road Map Document for a Sustainable Chemical Industry, [online], 2013. Available from:https://ec.europa.eu/environment/life/project/Projects/index.cfm?fuseaction=home.showFile%26rep=file%26fil=ENERG_ICE_Road_Map.pdf Search PubMed.
- S. Mondal, V. Reddy, A. Sarkar, P. Aravindakshan and A. Ghatak, Effect of surface modification on frictional properties of polyester fabric, Tribol. Int., 2016, 97, 38 CrossRef CAS.
- A. Mohamed, M. Er-Rafik and M. Moller, Suitability of Confocal Raman microscopy for monitoring the penetration of PDMS compounds into cotton fibres, Carbohydr. Polym., 2013, 96, 305–313 CrossRef CAS PubMed.
- A. J. Miling, Surface Characterization Methods, Marcel Dekker, Inc., New York, 1st edn, 1999 Search PubMed.
- K. J. Chugg and M. M. Chaudhri, Boundary lubrication and shear properties of thin solid films of dioctadecyl dimethyl ammonium chloride (TA 100), J. Phys. D: Appl. Phys., 1993, 26, 1993–2000 CrossRef CAS.
- L. Serreau, M. Beauvais, C. Heitz and E. Barthel, Adsorption and onset of lubrication by a double-chained cationic surfactant on silica surfaces, J. Colloid Interface Sci., 2009, 33, 382–388 CrossRef PubMed.
- L. C. Gerhardt, R. Lottenbach, R. M. Rossi and S. Derler, Tribological investigation of a functional medical textile with lubricating drug-delivery finishing, Colloids Surf., B, 2013, 108, 103–109 CrossRef CAS PubMed.
- A. Vilčnik, I. Jerman, A. Šurca Vuk, M. Koželj, B. Orel, B. Tomšič, B. Simončič and J. Kovač, Structural Properties and Antibacterial Effects of Hydrophobic and Oleophobic Sol–Gel Coatings for Cotton Fabrics, Langmuir, 2009, 25, 5869–5880 CrossRef PubMed.
- J. W. Anseth, A. Bialek, R. M. Hill and G. G. Fuller, Interfacial rheology of graft-type polymeric siloxane surfactants, Langmuir, 2003, 19, 6349–6356 CrossRef CAS.
- T. Igarashi, N. Morita, Y. Okamoto and K. Nakamura, Elucidation of Softening Mechanism in Rinse Cycle Fabric Softeners. Part 1: Effect of Hydrogen Bonding, J. Surfactants Deterg., 2016, 19, 183–192 CrossRef CAS PubMed.
- T. Igarashi, K. Nakamura, M. Hoshi, T. Hara, H. Kojima, M. Itou, R. Ikeda and Y. Okamoto, Elucidation of Softening Mechanism in Rinse-Cycle Fabric Softeners. Part 2: Uneven Adsorption—The Key Phenomenon to the Effect of Fabric Softeners, J. Surfactants Deterg., 2016, 19, 759–773 CrossRef CAS PubMed.
- E. K. Oikonomou, F. Mousseau, N. Christov, G. Cristobal, A. Vacher, M. Airiau, C. Bourgaux, L. Heux and J. F. Berret, Fabric softener–cellulose nanocrystal interaction: A model for assessing surfactant deposition on cotton, J. Phys. Chem. B, 2017, 121, 2299–2307 CrossRef CAS PubMed.
- A. M. Crutzen, Study of ditallow dimethyl ammonium chloride interaction with cellulose, J. Am. Oil Chem. Soc., 1995, 72, 137–143 CrossRef CAS.
- A. Kumar, L. Gilson, F. Henrich, V. Dahl, J. Kleinen, T. Gambaryan-Roisman and J. Venzmer, Intact deposition of cationic vesicles on anionic cellulose fibers: Role of vesicle size, polydispersity, and substrate roughness studied via streaming potential measurements, J. Colloid Interface Sci., 2016, 473, 152–161 CrossRef CAS PubMed.
- X. Liao, Y. Li, J. Hu, X. Wu and Q. Li, A simultaneous measurement method to characterize touch properties of textile materials, Fibers Polym., 2014, 15, 1548–1559 CrossRef.
- L. Vilhena and A. Ramalho, Friction of Human Skin against Different Fabrics for Medical Use, Lubricants, 2016, 4, 6 CrossRef.
- P. Habereder and A. Bereck, Part 2: Silicone softeners, Rev. Prog. Color. Relat. Top., 2002, 32, 125–137 CAS.
- W. Yuan, Z. Hu, M. Liao, Y. Cai, L. Meng, Z. Liu, Y. Chen, Y. Liu and N. Lu, A novel preparation method for silicone oil nanoemulsions and its application for coating hair with silicone, Int. J. Nanomed., 2012, 5719 CrossRef PubMed.
- H. Nazir, P. Lv and L. Wang, Uniform-sized silicone oil microemulsions: Preparation, investigation of stability and deposition on hair surface, J. Colloid Interface Sci., 2011, 364, 56–64 CrossRef CAS PubMed.
- H. Nazir, W. Zhang, Y. Liu, X. Chen, L. Wang, M. Naseer and G. Ma, Silicone oil emulsions: strategies to improve their stability and applications in hair care products, Int. J. Cosmet. Sci., 2013, 36, 124–133 CrossRef PubMed.
- A. Kumar, A. Trambitas, J. Peggau, V. Dahl, J. Venzmer, T. Gambaryan-Roisman and J. Kleinen, Charge and size matters—How to formulate organomodified silicones for textile applications, Colloids Surf., A, 2019, 560, 180–188 CrossRef CAS.
- A. Jatoi, Z. Khatri, F. Ahmed and M. Hanif Memon, Effect of Silicone Nano, Nano/Micro and Nano/Macro-Emulsion Softeners on Color Yield and Physical Characteristics of Dyed Cotton Fabric, J. Surfactants Deterg., 2014, 18, 205–211 CrossRef.
- A. Kumar, J. Kleinen, T. Gambaryan-Roisman and J. Venzmer, Electrokinetic investigation of deposition of cationic fabric softener vesicles on anionic porous cotton fabrics, J. Colloid Interface Sci., 2018, 514, 132–145 CrossRef CAS PubMed.
- C. Bellmann, A. Caspari, V. Albrecht, T. T. Loan Doan, E. Mäder, T. Luxbacher and R. Kohl, Electrokinetic properties of natural fibres, Colloids Surf., A, 2005, 267, 19–23 CrossRef CAS.
- F. Chiarotti, Disegno e analisi di studi per la definizione delle caratteristiche del tessuto in relazione all'uomo e alla popolazione. Atti 5° Convegno “Tessile e Salute”, Biella, 2005 Search PubMed.
- P. Zhang, X. Liu, L. Wang and X. Wang, An experimental study on fabric softness evaluation, Int. J. Cloth. Sci. Technol., 2006, 18, 83–95 CrossRef.
- J. O. Kim and B. L. Slaten, Objective evaluation of fabric hand. Part 1: relationships of fabric hand by extraction method and related physical and surface properties, Text. Res. J., 1999, 69, 59–67 CrossRef CAS.
- P. Purohit, P. Somasundaran and R. Kulkarni, Study of properties of modified silicones at solid–liquid interface: Fabric–silicone interactions, J. Colloid Interface Sci., 2006, 298, 987–990 CrossRef CAS PubMed.
- Y. Lim, C. Park and J. Kim, Hair conditioning effect of amino silicone softeners in varied treatment conditions, Fibers Polym., 2010, 11, 507–515 CrossRef CAS.
- X. Liu, J. Song and D. Wu, Surface and Friction Behavior of a Silicone Surfactant Adsorbed on Model Textiles Substrates, Ind. Eng. Chem. Res., 2010, 49(18), 8550–8557 CrossRef CAS.
- R. Mercadé-Prieto and S. Bakalis, Methodological study on the removal of solid oil and fat stains from cotton fabrics using abrasion, Text. Res. J., 2013, 84, 52–65 CrossRef.
- R. Mercadé-Prieto, X. Pan, A. Fernández-González, Z. Zhang and S. Bakalis, Quantification of Microcapsules Deposited in Cotton Fabrics before and after Abrasion Using Fluorescence Microscopy, Ind. Eng. Chem. Res., 2012, 51, 16741–16749 CrossRef.
- D. Chou, R. Krishnamurthy, T. Randolph, J. Carpenter and M. Manning, Effects of Tween 20® and Tween 80® on the Stability of Albutropin During Agitation, J. Pharm. Sci., 2005, 94, 1368–1381 CrossRef CAS PubMed.
- Exciton.luxottica.com, 2019, available at: https://exciton.luxottica.com/.
- https://pubchem.ncbi.nlm.nih.gov/compound/Pyrromethene-546.
- F. López Arbeloa, T. López Arbeloa and I. López Arbeloa, Electronic spectroscopy of pyrromethene 546, J. Photochem. Photobiol., A, 199, 121(3), 177–182 CrossRef.
- J. C. Harris and E. L. Brown, Detergency evaluation. I. Wash test methods, J. Am. Oil Chem. Soc., 1950, 27, 564 CrossRef CAS.
- D. Kácik, P. Tatar, and I. Martinček, Measurement of PDMS refractive index by low-coherence interferometry, ELEKTRO, Rajecke Teplice, 2014, pp. 662–665 Search PubMed.
- J. Mark, Polymer data handbook, Oxford University Press, Oxford, 2009 Search PubMed.
- S. Goel, Measuring detergency of oily soils in the vicinity of phase inversion temperatures of commercial nonionic surfactants using an oil-soluble dye, J. Surfactants Deterg., 1998, 1, 221–226 CrossRef CAS.
- Taberindustries.com, 2019, Taber Stiffness Tester (Bending & Stiffness) – Taber Industries, https://www.taberindustries.com/stiffness-tester, accessed 21st April 2020.
- S. C. Mehta, P. Somasundarana and R. Kuklkarni, Variation in emulsion stabilization behavior of hybrid silicone polymers with change in molecular structure: phase diagram study, J. Colloid Interface Sci., 2009, 333, 635–640 CrossRef CAS PubMed.
- L. Fengyan, Z. Wenli, Z. Tianbo, D. Danghui and Y. Fang, Factors influencing droplet size of silicone oil emulsion with high solid content, China Pet. Process. Petrochem. Technol., 2011, 13, 21–26 Search PubMed.
- B. A. Saadevandi and J. L. Zakin, A study of silicone oil-in-water emulsions, Chem. Eng. Commun., 1997, 156, 227–246 CrossRef CAS.
- R. Rosen-Meyer, Delivery System Handbook for Personal Care and Cosmetic Products, William Andrew Inc, New York, 1st edn, 2005 Search PubMed.
- S. Sokolov, S. Eloul and E. Kätelhön, Electrode–particle impacts: a users guide, Phys. Chem. Chem. Phys., 2017, 19, 28–43 RSC.
- D. Shoup and A. Szabo, Chronoamperometric current at finite disk electrodes, J. Electroanal. Chem. Interfacial Electrochem., 1982, 140, 237–245 CrossRef CAS.
- T. De Goede, A. Moqaddam, K. Limpens, S. Kooij, D. Derome, J. Carmeliet, N. Shahidzadeh and D. Bonn, Droplet Impact of Newtonian Fluids and Blood on Simple Fabrics: Effect of Fabric Pore Size and Underlying Substrate, Phys. Fluids, 2021, 33, 033308 CrossRef CAS.
- T. Svitova, O. Theodoly and S. Christiano, Wetting Behavior of Silicone Oils on Solid Substrates Immersed in Aqueous Electrolyte Solutions, Langmuir, 2002, 18, 6821–6829 CrossRef CAS.
- A. Singh, Experimental Methodologies for the Characterization of Nanoparticles, Eng. Nanopart. Environ., 2016, 125–170 Search PubMed.
- M. Nada, Scanning Electron Microscopy, BAOJ Microbio., 2015, 1, 1–8 CrossRef.
- N. Ibrahim, B. Eid, E. El-Aziz, T. Abou Elmaaty and S. Ramadan, Multifunctional cellulose-containing fabrics using modified finishing formulations, RSC Adv.k, 2017, 7, 33219–33230 RSC.
- A. Nyoni and D. Brook, Wicking mechanisms in yarns—the key to fabric wicking performance, J. Text. Inst., 2006, 97, 119–128 CrossRef.
- X. Han and X. Gong, In Situ One-Pot Method to Prepare Robust Superamphiphobic Cotton Fabrics for High Buoyancy and Good Antifouling, ACS Appl. Mater. Interfaces, 2021, 13, 31298–31309 CrossRef CAS PubMed.
- F. Guan, Z. Song, F. Xin, H. Wang, D. Yu and G. Li, Preparation of hydrophobic transparent paper via using polydimethylsiloxane as transparent agent, J. Bioresour. Bioprod., 2020, 5, 37–43 CrossRef.
- D. Wei, H. Wei, A. Gauthier, J. Song, Y. Jin and H. Xiao, Superhydrophobic modification of cellulose and cotton textiles: Methodologies and applications, J. Bioresour. Bioprod., 2020, 5, 1–15 CrossRef CAS.
- H. Monder, L. Bielenki, H. Dodiuk, A. Dotan and S. Kenig, Poly(Dimethylsiloxane) Coating for Repellency of Polar and Non-Polar Liquids, Polymers, 2020, 12, 2423 CrossRef CAS PubMed.
- R. Zaouali, S. Msahli and F. Sakli, Energy modelling of fabrics wrinkle recovery behaviour, J. Text. Inst., 2015, 107, 1434–1441 CrossRef.
- Y. Xu, H. Yin and S. Yuan, Film morphology and orientation of amino silicone adsorbed onto cellulose substrate, Appl. Surf. Sci., 2009, 255, 8435–8442 CrossRef CAS.
- B. Jacobson, The Stribeck memorial lecture, Tribol. Int., 2003, 36, 781–789 CrossRef.
- J. Li, C. Zhang and M. Deng, Superlubricity of silicone oil achieved between two surfaces by running-in with acid solution, RSC Adv., 2015, 5, 30861–30868 RSC.
- E. Bertaux, M. Lewandowski and S. Derler, Relationship between Friction and Tactile Properties for Woven and Knitted Fabrics, Text. Res. J., 2007, 77, 387–396 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |