Open Access Article
Open Access ArticleHigh-performance delignification of invasive tree species wood with ionic liquid and deep eutectic solvent for the production of cellulose-based polyelectrolytes
Ricardo O. Almeida a,
Adriana Moreiraa,
Daniela Moreiraa,
Maria E. Pina
a,
Adriana Moreiraa,
Daniela Moreiraa,
Maria E. Pina b,
Maria G. V. S. Carvalho
b,
Maria G. V. S. Carvalho a,
Maria G. Rasteiro
a,
Maria G. Rasteiro a and
José A. F. Gamelas
a and
José A. F. Gamelas *a
*a
aUniversity of Coimbra, CIEPQPF, Department of Chemical Engineering, Rua Sílvio Lima, Pólo II, 3030-790 Coimbra, Portugal. E-mail: jafgas@eq.uc.pt
bUniversity of Coimbra, CIEPQPF, Faculty of Pharmacy, Azinhaga de Santa Comba, 3000-548 Coimbra, Portugal
First published on 1st February 2022
Abstract
An efficient and eco-friendly process for lignocellulosic biomass fractionation is essential for the production of high value-added bioproducts from biomass. The present work aimed to obtain cellulose-rich materials from the wood of an invasive tree species (Acacia dealbata) using an appropriate choice of ionic liquids (ILs) and deep eutectic solvents (DESs), and of the processing conditions, for the subsequent production of cationic wood-based polyelectrolytes. In the pretreatment step, the 1-butyl-3-methylimidazolium methyl sulfate (IL) + H2O and choline chloride + imidazole (DES) systems demonstrated a remarkable ability to remove lignin from acacia, reaching up to 92.4 and 90.2% of delignification, respectively. However, the DES pretreatment revealed to be more selective for lignin removal with lower cellulose losses (less than 15%) than the IL treatment (up to 30%) and less cellulose depolymerization. The hemicellulose was also removed but in a lesser extent with the DES treatment. Both systems could provide treated materials with a very high cellulose content (≥89%). Afterwards, cationic polyelectrolytes having a considerable content of quaternary ammonium groups (up to 3.6 mmol g−1) were obtained directly from the IL- and DES-pretreated woods. The treated woods, when used as raw materials for cationization reaction, allow to synthesize water-soluble polyelectrolytes with potential to be applied in wastewater treatment, pharmaceutical or cosmetic products.
Introduction
Lignocellulosic biomass is a low-cost, abundant and renewable feedstock that can be used, as a promising alternative to petroleum-based products, for biofuels production and high value-added chemicals.1 Lignocellulosic biomass can be found in different type of residues such as agricultural residues (rice straw, wheat straw, corncob), forest residues (Eucalyptus, acacia, pine), industrial residues (paper industry), etc. This type of biomaterial is mainly composed of cellulose (30–50%, w/w), hemicelluloses (20–35%) and lignin (15–30%) with smaller amounts of ash, extractives, pectin and proteins.2,3 The strong hydrogen and covalent bonds between carbohydrates and lignin (lignin–carbohydrate complex) results in a complex, rigid and recalcitrant structure, which renders difficult the lignin removal/isolation and the fractionation of the lignocellulosic biomass into its several components. The access of hydrolyzing enzymes to produce fermentable sugars is also hindered.4 In this sense, an efficient pretreatment is needed, in order to maximize the cellulose, hemicelluloses and lignin recovery. Several pretreatments have been applied and can be classified as physical, physicochemical, chemical and biological processes.5 The use of volatile and unsustainable organic solvents, together with the high-energy consumption and the low selectivity of conventional pretreatment processes have boosted the search for novel “green” solvents.6 In this context, ionic liquids (ILs) and deep eutectic solvents (DESs) have gained a lot of attention and have been intensively studied for the pretreatment of several sources of lignocellulosic biomass.One of the most interesting lignocellulosic biomass sources for valorization is Acacia dealbata. This is probably the most aggressive and invasive plant species in Portugal being distributed in all regions of the country.7 This invasive plant, firstly introduced as an ornamental plant, has a very fast reproduction forming dense stands and changing the chemical properties of soils, that prevent the normal growth of native vegetation.8 The high carbohydrate content allows it to be valorized in the production of a wide range of high-value products such as cellulose, xylan, furfural, hydroxymethylfurfural, bioethanol, etc. Considering this, ILs and DESs may have a very important contribution to achieve a successful valorization of A. dealbata.
ILs are organic salts composed of an organic cation and an organic or inorganic anion having a melting point lower than 100 °C. The strong electrostatic forces between the cation and the anion provide low volatility/vapor pressure and high chemical and electrochemical stability to the ILs. Besides that, ILs possess a wide liquid range, high solvating ability, high ionic conductivity, recyclability and high effectiveness in the dissolution of organic and inorganic compounds.9,10 Due to these unique properties, ILs are suitable for a wide range of applications being one of them the ability to dissolve simultaneously or selectively the main components of lignocellulosic biomass (cellulose, hemicelluloses and lignin).2 Despite these attractive properties, ILs have high viscosity, poor biodegradability and high cost of production. However, it is possible to overcome these limitations since there are innumerous combinations of cations and anions that can be used in order to tailor the physicochemical properties of ILs for a specific application.11 Swatloski et al.12 were the first to report, in 2002, that cellulose could be dissolved in an ionic liquid (1-butyl-3-methylimidazolium chloride, [BMIM]Cl), and, after that, the use of ILs was extended for lignocellulosic biomass dissolution. Over the years, imidazolium based-ILs, such as [BMIM]Cl, and 1-ethyl-3-methylimidazolium acetate, [EMIM]OAc, were widely used for dissolution/pretreatment of woody biomass, including for acacia pretreatment.1,13,14 It has been found that ILs composed of acetate and chloride ions have a high hydrogen bond basicity possessing a high cellulose dissolution capacity.9 On the other hand, it was reported, by Pu et al.,15 that kraft lignin had a high solubility in ILs with methyl sulfate anions. The authors demonstrated that for 1-butyl-3-methylimidazolium-based ILs, the order of lignin solubility for the tested anions was: [MeSO4]− > Cl− ∼ Br− ≫ [PF6]−.
More recently, the less expensive and more environmentally friendly DESs, have also been used in the lignocellulosic biomass pretreatment. DESs are prepared by the combination of at least one hydrogen bond acceptor (HBA, such as quaternary ammonium salts, typically choline chloride abbreviated as ChCl) and one hydrogen bond donor (HBD, such as amides, carboxylic acids, urea and polyols) to form eutectic mixtures.16 These eutectic mixtures possess lower melting points than the individual compounds, as a consequence of a charge delocalization that occurs through hydrogen bonding interaction between HBAs and HBDs in the DESs. In general, the higher the hydrogen bonding ability of the eutectic mixture components, the higher the decrease in the melting point. Similar to ILs, DESs are also called “Designer solvents” due to the numerous combination possibilities of HBAs and HBDs that can tune the physicochemical properties of the DESs for a specific application.17,18 DESs have comparable physicochemical properties to ILs. However, they can overcome the principal drawbacks of the ILs since DESs can be easily prepared by simply mixing the components without purification steps having lower costs of production, higher biocompatibility and higher biodegradability than ILs.19 For instance, it was reported that choline chloride-based DESs could be used for lignocellulosic biomass delignification based on mechanism of a selective cleavage of the ether bonds between phenylpropane units of lignin without affecting C–C linkages.20,21 To our knowledge, there are no studies in the literature about A. dealbata pretreatment using DESs, although several studies for the delignification of other hardwood species (such as poplar or Eucalyptus) using DESs have been recently reported.22,23
The aim of the present work was to obtain cellulose-rich materials from Acacia dealbata wood wastes for the further production of cationic polyelectrolytes. For this, conditions were optimized, namely the choice of the appropriate IL and DES, and of the processing parameters (temperature, extraction time) in order to achieve a high removal of lignin from the initial raw material with a minimal loss of cellulose and without damage of the cellulose structure. The IL, 1-butyl-3-methylimidazolium methyl sulfate ([BMIM]MeSO4) and the DES, ChCl + imidazole, were found to be the most appropriate for that purpose. After the IL and DES pretreatments, two selected the carbohydrate-rich samples, with still some amount of lignin, were cationized by a two-step reaction with sodium periodate and Girard's reagent T. The production of cationic wood-based polyelectrolytes from wood pretreated with IL and DES was never mentioned in the literature. Polyelectrolytes have potential uses in several fields including for wastewater treatment, pharmaceutics or cosmetics.24–26 In particular, because of environmental concerns, bio-based polyelectrolytes arouse an additional interest in comparison with the synthetic ones.
Materials and methods
Materials and chemicals
Acacia dealbata was the lignocellulosic biomass source used in this study. Branches of acacia were collected in the central region of Portugal and cut into smaller fractions, being ground and sieved to obtain a particle size of 0.25–0.84 mm.The chemicals used in the pretreatment of acacia were [BMIM]MeSO4 (purchased from IOLITEC), oxalic acid dihydrate (purchased from Fronteiralquimia Unipessoal, Lda), ChCl and imidazole (both purchased from Acros Organics). All the chemicals had a least 98% of purity and were used without any further purification.
For the cationization of the obtained cellulose-rich materials the following chemicals were used: lithium chloride, sodium periodate, hydroxylamine hydrochloride and Girard's reagent T (GT) (all purchased from Sigma-Aldrich). Silver nitrate and isopropanol were obtained from VWR Chemicals.
DES preparation
The two DESs used in this work (ChCl + imidazole and ChCl + oxalic acid) were prepared by mixing ChCl and the HBDs (imidazole or oxalic acid) in a 250 mL beaker placed in an ethylene glycol bath at a specific temperature for each DES. ChCl + imidazole was prepared with a molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, at 100 °C for 60 min while ChCl + oxalic acid was prepared with a molar ratio of 1
7, at 100 °C for 60 min while ChCl + oxalic acid was prepared with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, at 60 °C for 45 min.27,28 Both DESs were prepared under magnetic stirring to achieve a better homogenization of the mixtures.
1, at 60 °C for 45 min.27,28 Both DESs were prepared under magnetic stirring to achieve a better homogenization of the mixtures.
A. dealbata pretreatment with [BMIM]MeSO4 + H2O, ChCl + imidazole and ChCl + oxalic acid
Wood dissolution in [BMIM]MeSO4 + H2O. The IL pretreatment was carried out by adding 1 g (dry weight basis) of wood sawdust (0.25–0.84 mm), 2 g of distilled water and 8 g of IL to a Teflon lined stainless steel mini-reactor. Then, the closed mini-reactor was placed in an oven kept at a specific temperature. Three different temperatures (100, 120 and 140 °C) with five different reaction times for each temperature (1, 2, 4, 8 and 24 h) were evaluated. After the pretreatment, the treated sample was cooled at room temperature and 10 mL of methanol was added in order to reduce the viscosity of the mixture. After 2 h mixing, the suspension was filtered under vacuum using a glass filter crucible containing a cellulose filter paper (Whatman 541). In order to avoid losses, the content in the mini-reactor was washed with additional 10 mL of methanol. In this stage, the filtrate may be collected and stored for further recovering of lignin by precipitation. Finally, the resulting solid (cellulose-rich material, CRM) was thoroughly washed with distilled water, until the filtrate became colorless, and dried in an oven overnight at 105 °C. In order to evaluate the pretreatment reproducibility, two replicates were performed for each case. After this procedure, the pretreatment dissolution yield was calculated by eqn (1):
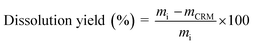 | (1) |
Wood dissolution in DESs (ChCl + imidazole and ChCl + oxalic acid). For the A. dealbata pretreatment in DESs, the wood:liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 was kept by adding 10 g of DES to 1 g (dry basis) of wood sawdust (0.25–0.84 mm) inside the mini-reactor, being then placed in an oven. The ChCl + imidazole pretreatments were performed at 120, 140, 160 and 180 °C while with ChCl + oxalic acid the pretreatments were carried out at 60 and 80 °C. In both DES systems five different reaction times were tested for each selected temperature (1, 2, 4, 8 and 24 h). After the pretreatment, the same procedure mentioned above was adopted. However, due to the lower viscosity of DESs compared to [BMIM]MeSO4 it was not necessary to wait 2 h after adding 10 mL of methanol to carry out the vacuum filtration. The dissolution yield was also calculated according to eqn (1).
10 was kept by adding 10 g of DES to 1 g (dry basis) of wood sawdust (0.25–0.84 mm) inside the mini-reactor, being then placed in an oven. The ChCl + imidazole pretreatments were performed at 120, 140, 160 and 180 °C while with ChCl + oxalic acid the pretreatments were carried out at 60 and 80 °C. In both DES systems five different reaction times were tested for each selected temperature (1, 2, 4, 8 and 24 h). After the pretreatment, the same procedure mentioned above was adopted. However, due to the lower viscosity of DESs compared to [BMIM]MeSO4 it was not necessary to wait 2 h after adding 10 mL of methanol to carry out the vacuum filtration. The dissolution yield was also calculated according to eqn (1).
Composition and characterization of treated and untreated wood
The contents in carbohydrates and lignin of treated and untreated wood samples were determined according to the analytical procedure of the National (US) Renewable Energy Laboratory, “Determination of Structural Carbohydrates and Lignin in Biomass” (NREL/TP-510-42618). The determination of klason lignin and acid-soluble lignin contents was based on a two-step hydrolysis with sulfuric acid, and then, the resulting hydrolyzed products were used for carbohydrate content determination by high-performance liquid chromatography (HPLC), using a Knauer instrument equipped with a refractive index (RI) detector and a Rezex ROA-organic acid H column from Phenomenex. After these determinations, the removals of the main wood components (cellulose, xylan and lignin) obtained after the pretreatments were calculated based on eqn (2).
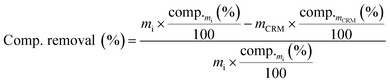 | (2) |
The composition of A. dealbata (based on three replicates) used in this work was as follows: cellulose, 47.4 ± 0.9%; xylan, 18.8 ± 0.3%; klason lignin, 17.0 ± 0.2%; acid-soluble lignin, 3.2 ± 0.1%; acetic acid (from acetyl groups), 5.8 ± 0.1%; arabinan, 1.4 ± 0.02%; glucuronic acid, 0.9 ± 0.1%; extractives, 2.4 ± 0.1%.
The initial and treated wood samples were also analyzed by Fourier transform infrared (FTIR) spectroscopy. FTIR spectra were obtained on a PerkinElmer spectrometer coupled with an attenuated total reflectance (ATR) unit, using 128 scans and a resolution of 4 cm−1, in the range of 500–4000 cm−1.
The crystallinity of the untreated and treated wood materials was also assessed using X-ray diffraction. X-ray diffraction data were collected in a Bruker D8 ADVANCE diffractometer operating in the Bragg–Brentano configuration with Cu-Kα (λ = 1.54 Å) source at a current of 40 mA and an accelerating voltage of 40 kV. Data were collected by the step counting method (step 0.01° and time 0.5 s) in the 2θ range of 4–55°. The crystallinity was determined by the empirical Segal method.29
In order to evaluate the effect of the pretreatment conditions on cellulose depolymerization, the intrinsic viscosity of the obtained cellulose-rich materials in a cupriethylenediamine solution was measured, following ISO standard 5351.
Synthesis of cationic wood-based polyelectrolytes
A two-step reaction process for the production of cationic wood-based polyelectrolytes from the obtained cellulose-rich materials was followed. The procedure was adapted, with the required changes, from other published works.30,31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8
1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.05
2.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, w/w/w/v) was placed in a 500 mL round flask covered with aluminum foil and heated under stirring in an oil bath at a given temperature. After 3 h of reaction, protected from light, the suspension was cooled and filtered under vacuum, and the solid product was thoroughly washed with distilled water. Finally, the aldehyde content of the obtained non-dried dialdehyde lignocellulose (DALC) product was determined based on the oxime reaction between aldehyde groups and NH2OH·HCl, according to a previously described procedure.32
100, w/w/w/v) was placed in a 500 mL round flask covered with aluminum foil and heated under stirring in an oil bath at a given temperature. After 3 h of reaction, protected from light, the suspension was cooled and filtered under vacuum, and the solid product was thoroughly washed with distilled water. Finally, the aldehyde content of the obtained non-dried dialdehyde lignocellulose (DALC) product was determined based on the oxime reaction between aldehyde groups and NH2OH·HCl, according to a previously described procedure.32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, w/v). Then, GT was added with two different molar ratios of GT/aldehyde (1 and 4). The pH of the reaction mixture was adjusted to 4.5 with a HCl solution and the mixture was heated at 70 °C for 1 h, under stirring. During the reaction, the solid DALC was converted to a hot water-soluble product. Afterwards, the viscous solution was cooled to room temperature and was centrifuged for 10 min at 3500 rpm, which allowed isolating a first solid fraction of the product. In order to precipitate the more soluble fraction of the product, present in the obtained supernatant, isopropanol (7.5
100, w/v). Then, GT was added with two different molar ratios of GT/aldehyde (1 and 4). The pH of the reaction mixture was adjusted to 4.5 with a HCl solution and the mixture was heated at 70 °C for 1 h, under stirring. During the reaction, the solid DALC was converted to a hot water-soluble product. Afterwards, the viscous solution was cooled to room temperature and was centrifuged for 10 min at 3500 rpm, which allowed isolating a first solid fraction of the product. In order to precipitate the more soluble fraction of the product, present in the obtained supernatant, isopropanol (7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (water), v/v) was added. The resultant mixture was then centrifuged for 30 min at 4500 rpm and the supernatant was removed. The obtained precipitate product and the precipitate of the first centrifugation (obtained without the addition of isopropanol) were washed separately with distilled isopropanol (ca. 10% of water) and centrifuged. This operation was repeated several times until the GT was not detected anymore in the supernatant. The GT presence was monitored by the addition of AgNO3 to the supernatant. When no more AgCl precipitation was observed, the washing step was completed. Finally, the cationic products were oven-dried at 60 °C and stored in a desiccator.
1 (water), v/v) was added. The resultant mixture was then centrifuged for 30 min at 4500 rpm and the supernatant was removed. The obtained precipitate product and the precipitate of the first centrifugation (obtained without the addition of isopropanol) were washed separately with distilled isopropanol (ca. 10% of water) and centrifuged. This operation was repeated several times until the GT was not detected anymore in the supernatant. The GT presence was monitored by the addition of AgNO3 to the supernatant. When no more AgCl precipitation was observed, the washing step was completed. Finally, the cationic products were oven-dried at 60 °C and stored in a desiccator.The cationic lignocellulosic products were characterized by FTIR spectroscopy and elemental analysis. FTIR-ATR spectra were obtained as aforementioned. C, H and N elemental analyses were performed using an element analyser EA 1108 CHNS-O from Fisons. 2,5-Bis(5-tert-butyl-benzoxazol-2-yl)thiophene was used as standard. From the nitrogen content, the corresponding cationicity index of the modified lignocelluloses was determined.
Results and discussion
A. dealbata dissolution in [BMIM]MeSO4 + H2O, ChCl + imidazole and ChCl + oxalic acid
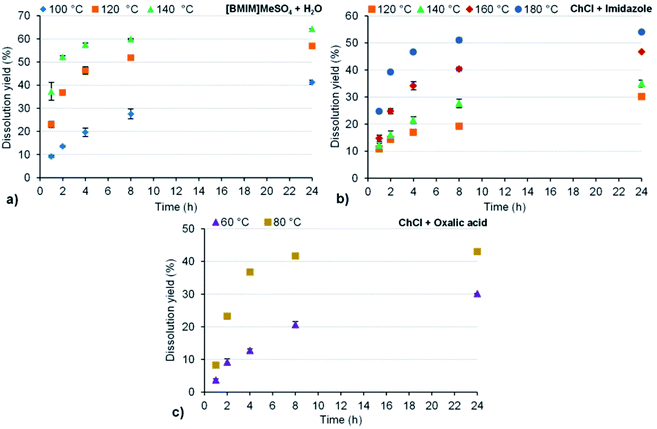
After a previous screening of different IL and DES-based solvents for lignin dissolution, A. dealbata was pretreated with [BMIM]MeSO4 + H2O (at 100, 120 and 140 °C), ChCl + imidazole (at 120, 140, 160 and 180 °C) and ChCl + oxalic acid (at 60 and 80 °C). For each temperature of treatment, the extraction time was also varied (1, 2, 4, 8 and 24 h). A range of conditions was used in order to find the best ones for an efficient and selective removal of lignin. The dissolution yield was determined based on the initial wood mass and the undissolved wood mass according to eqn (1). The results are shown in Fig. 1a, b and c, respectively. Fig. 1 shows that, regardless of the system used in the acacia pretreatment, the dissolution yield increased with the increase of temperature and extraction time. The dissolution yields obtained after 1, 2, 4 and 8 h of pretreatment with [BMIM]MeSO4 + H2O at 100 °C were very low (less than 30%), compared to the pretreatments performed at 120 and 140 °C, where the dissolution yield was already considerable after 2 h of reaction, 36.8 and 52.2%, respectively (Fig. 1a). For the same reaction time (2 h), Brandt et al.33 obtained only 10% of dissolution using [BMIM]MeSO4 + H2O (4/1) to pretreat Miscanthus at 120 °C, with the same solid/liquid ratio used in this work. However, due to the differences in the type of biomass, composition and lignin type, this comparison might not be very strict. In Fig. 1a, it can be also observed that in the first 4 h of pretreatment there was a significant increase of the dissolution yield; after that, this increase slowed down: between 4 and 24 h it was only of about 10% and 7% at 120 °C and 140 °C, respectively. With the selected IL, the maximum dissolution yield was 56.9% at 120 °C and 64.4% at 140 °C (both for 24 h of extraction time). When ChCl + imidazole was used for the pretreatment of acacia wood at these same temperatures (120 and 140 °C) (Fig. 1b), the dissolution yields were much lower than those obtained with the IL. Procentese et al.34 also used ChCl + imidazole in the corncob pretreatment with a solid/liquid ratio of 1/16 and an extraction time of 15 h. Under these conditions, they obtained a dissolution yield of 22, 42 and 42% at 80, 115 and 150 °C, respectively. Comparing to our study, if the pretreatment had been carried out for 15 h, dissolutions of about 25 and 45% would have been expected at 120 and 160 °C, respectively (based on the respective logarithmic trend lines for our data). According to these estimated values, it seems that the dissolution yields obtained with ChCl + imidazole, in this work, were more affected by the temperature increase than the results obtained by Procentese et al.34. However, once more, the distinct type of biomass does not allow a very precise comparison. Finally, the use of ChCl + oxalic acid at 60 °C led to the lowest wood dissolution and no plateau in the dissolution values was reached, while at 80 °C the dissolution yield values were much higher and after 8 h of pretreatment no significant difference was observed compared to 24 h (less than 2%) (Fig. 1c). Mamilla et al.35 used this same DES in the pretreatment of beechwood (hardwood) at 80 °C with a solid/liquid ratio of 1/10, and after 8 h the authors obtained a dissolution yield of about 30%, while in the present work it was achieved 41.7% of acacia dissolution.Overall, it can be observed that as the temperature used in the wood pretreatment is increased, the differences between the dissolution results for 8 and 24 h vanish. This means that for higher temperatures, the extraction time may be decreased saving energy in the treatment with IL or DES.
Considering that the lignin and xylan contents determined in the initial untreated wood were 20.2 and 18.8%, respectively, and even considering the content of extractives (2.4%) and acetic acid (5.8%, from acetyl groups), dissolution yields between 40 and 50%, approximately, would be the most appropriate values in order to guarantee a high removal of all these components, and at the same time, a cellulose enrichment of the treated wood. Therefore, dissolution values above 50% indicate that some amount of cellulose has probably dissolved, which was more evident when the pretreatment was performed with the IL at 120 and 140 °C (Fig. 1a). On the other hand, values below 30–35% are an indication that, probably, not too much lignin and xylan were removed during the wood pretreatment. This fact could be clearly observed for the pretreatment with the ChCl + imidazole, at 120 °C, and for the pretreatment with ChCl + oxalic acid, at 60 °C, where the dissolution values were always less than 35%.
Composition and characterization of treated wood (cellulose-rich material)
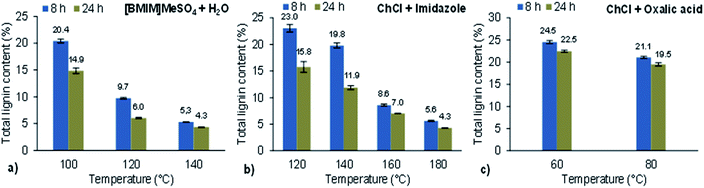
Following the acacia pretreatment, the determination of klason lignin and acid-soluble lignin was performed only on the materials obtained after 8 and 24 h of extraction time since it would be expected that, for the latter, the lignin content would be lower compared to the samples obtained after 1, 2 and 4 h. The total lignin contents of treated woods obtained after [BMIM]MeSO4 + H2O, ChCl + imidazole and ChCl + oxalic acid pretreatments, for all tested temperatures, are shown in Fig. 2a, b and c, respectively. In addition to the influence of temperature on the wood dissolution, Fig. 2 reveals that this variable also had a high impact on the lignin content present in the wood-treated materials. In other words, higher temperatures led to less total lignin content in the treated wood for both 8 and 24 h extraction times. Another trend observed in these three pretreatment systems was the larger difference in the total lignin content values obtained for lower temperatures, between the results for 8 and 24 h of pretreatment, while when the temperature was increased this difference decreased. It can also be noted that the lowest lignin contents were achieved with [BMIM]MeSO4 + H2O at 120 and 140 °C and with ChCl + imidazole at 160 and 180 °C for both 8 and 24 h treatments. In contrast, ChCl + oxalic acid demonstrated not to be effective for the lignin removal in acacia, under the tested conditions, since very high lignin contents were found in the wood treated with this DES (> 19%) even for dissolution yields higher than 40%. Comparing the two used DESs, the aromatic structure of imidazole seems to have favored the lignin dissolution, whereas the oxalic acid component is probably more suitable for cellulose and hemicellulose dissolution.Some values obtained in the treated wood are above 20.2% (the lignin content in untreated wood), namely with [BMIM]MeSO4 + H2O at 100 °C after 8 h, ChCl + imidazole at 120 °C after 8 h, ChCl + oxalic acid at 60 °C (8 and 24 h) and at 80 °C (8 h). This clearly showed that, in these cases, the lignin removal was very low and at least some portion of hemicelluloses must have been dissolved leading to an increment in the lignin concentration.
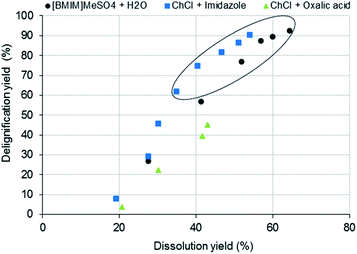
Based on the lignin contents in the treated wood samples, the delignification yield was calculated by eqn (2), and then it was related to the dissolution yield (Fig. 3).
Fig. 3 shows a trend between dissolution and delignification for all three solvent systems. In other words, the higher the wood dissolution, the higher the obtained delignification. As mentioned above, ChCl + oxalic acid pretreatment was not effective in the lignin removal, not exceeding 43% of delignification, and exhibited a lower selectivity for lignin, as shown in Fig. 3. In contrast, the highest delignification yield was obtained using [BMIM]MeSO4 + H2O (92.4%); however, at this level of delignification, the dissolution yield was also the highest which implied a higher wood loss compared to the ChCl + imidazole pretreatment. Using this DES, higher delignification yields could be achieved with less wood dissolution, which means that this DES was more selective for the lignin removal than [BMIM]MeSO4 + H2O.
The treated wood samples that showed a delignification yield higher than 60% (Fig. 3) were selected for carbohydrate content determinations, which enabled the calculation of cellulose and xylan removals by eqn (2). The detailed compositions of these treated woods and untreated wood, as well as the removals of the main wood components obtained after the pretreatments, are presented in Table 1.
| Cellulose rich-material | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| T (°C) | t (h) | Dissolution yield (%) | Lignin (%) | Cellulose (%) | Xylan (%) | Delignification yield (%) | Cellulose removal (%) | Xylan removal (%) | |
| Acacia dealbata | — | — | — | 20.2 | 47.4 | 18.8 | — | — | — |
| [BMIM]MeSO4 + H2O | 120 | 8 | 51.9 ± 0.2 | 9.7 ± 0.1 | 81.2 ± 2.8 | 7.4 ± 0.6 | 76.8 ± 0.4 | 17.7 ± 2.6 | 81.0 ± 1.4 |
| [BMIM]MeSO4 + H2O | 120 | 24 | 56.9 ± 0.8 | 6.0 ± 0.1 | 92.9 ± 1.7 | 3.7 ± 0.0 | 87.1 ± 0.5 | 15.6 ± 0.1 | 91.5 ± 0.2 |
| [BMIM]MeSO4 + H2O | 140 | 8 | 59.9 ± 0.3 | 5.3 ± 0.0 | 90.5 ± 1.0 | 3.9 ± 0.6 | 89.4 ± 0.3 | 23.2 ± 1.1 | 91.6 ± 1.4 |
| [BMIM]MeSO4 + H2O | 140 | 24 | 64.4 ± 0.0 | 4.3 ± 0.1 | 92.3 ± 1.2 | 2.3 ± 0.0 | 92.4 ± 0.2 | 30.6 ± 0.9 | 95.7 ± 0.1 |
| ChCl + imidazole | 160 | 8 | 40.4 ± 0.7 | 8.6 ± 0.2 | 72.4 ± 1.3 | 13.9 ± 0.3 | 74.6 ± 0.2 | 9.0 ± 2.8 | 55.9 ± 1.4 |
| ChCl + imidazole | 180 | 8 | 51.0 ± 0.9 | 5.6 ± 0.1 | 85.2 ± 0.5 | 9.3 ± 0.3 | 86.3 ± 0.5 | 12.0 ± 2.1 | 75.7 ± 0.3 |
| ChCl + imidazole | 140 | 24 | 35.0 ± 1.3 | 11.9 ± 0.4 | 65.0 ± 2.5 | 15.7 ± 0.6 | 61.7 ± 0.4 | 10.8 ± 1.6 | 45.8 ± 1.1 |
| ChCl + imidazole | 160 | 24 | 46.7 ± 0.3 | 7.0 ± 0.0 | 80.9 ± 1.2 | 14.7 ± 0.8 | 81.5 ± 0.0 | 9.1 ± 0.9 | 58.3 ± 1.9 |
| ChCl + imidazole | 180 | 24 | 54.0 ± 0.2 | 4.3 ± 0.1 | 89.0 ± 1.9 | 9.4 ± 0.4 | 90.2 ± 0.2 | 13.7 ± 2.3 | 76.9 ± 0.8 |
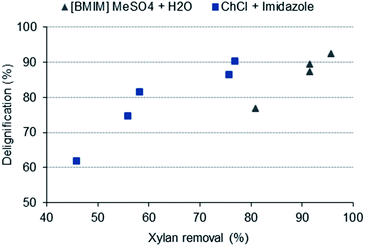
As mentioned previously, the comparatively high dissolution values obtained with the IL implied that in addition to lignin, a significant amount of, at least xylan, was also removed/dissolved. In fact, the chemical composition analysis showed very low xylan contents in the IL-treated samples corresponding to xylan removals up to ca. 95%. Additionally, as the delignification increased the xylan removal also increased, revealing a linear trend between these two variables (Fig. 4). This trend was also observed in the ChCl + imidazole pretreatment; however, the xylan removals were lower, ranging from 46 to 77% while with IL these ranged from 81 to 96% (Table 1). This simultaneous removal of lignin and hemicellulose has already been reported previously.33,34 Regarding the cellulose fraction, both extraction methods provided high cellulose enrichment in the treated wood. Due to the concomitant higher xylan removal, the cellulose contents in the IL-treated samples were the highest, ranging from 81 to 93% while the cellulose contents obtained with the ChCl + imidazole pretreatment were typically lower and ranged from 65 to 89%. Despite the fact that the IL guaranteed a higher percentage of cellulose in the treated wood, it was also the one that provided higher losses of cellulose, especially when the pretreatment was performed at 140 °C for both 8 and 24 h experiments, resulting in 23 and 31% of cellulose loss from the initial wood, respectively. On the other hand, when ChCl + imidazole was used, there was a lower removal of cellulose and the highest removal obtained was 14%, for the most drastic pretreatment conditions. Overall, these two extraction systems showed a great ability to delignify A. dealbata, reaching up to 92 and 90% of lignin removal for [BMIM]MeSO4 + H2O and ChCl + imidazole, respectively. However, the DES was more selective regarding delignification since both cellulose and xylan removals were lower than those obtained with the IL. Therefore, if the purpose is to obtain a cellulose-rich material with a higher cellulose purity, the treatment with [BMIM]MeSO4 + H2O is more appropriate. If a more selective delignification with less wood losses is desired, the treatment with ChCl + imidazole is preferable.
With the IL pretreatment, the lignin removals were substantially higher than those obtained by Yanez et al.13 and by Kim et al.,14 who were able to remove only 40 and 20% of lignin from A. dealbata and A. Auriculiformis, respectively, both with [EMIM]OAc. Despite the fact that these authors have treated the same type of wood as the one treated in this work, differences in the pretreatment conditions, in the ionic liquid structure, and in the experimental procedure used may affect the validity of these comparisons. It should be noted that the IL used in this work has never been used before for the treatment of A. dealbata; however, Doherty et al.36 pretreated Maple wood (hardwood) with [BMIM]MeSO4 at 90 °C, and after 12 h of treatment they achieved only 19% of lignin removal. When it comes to the ChCl + imidazole pretreatment, the lignin removals were in the same range of those obtained by Procentese et al.34 However, the cellulose enrichments of the pretreated wood, in the present work, were clearly superior. This proves that this DES is also capable of removing lignin from woody materials that have a more rigid and recalcitrant structure and a higher lignin content than agricultural residues such as corncob.
The FTIR spectra of untreated acacia and of some representative cellulose-rich materials obtained after the treatments with IL and DES are shown and compared in Fig. 5. The analysis of the band at 1725 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O unconjugated stretching in acetyl groups of hemicelluloses), in Fig. 5, suggested that most of the acetyl groups of hemicelluloses were removed from the initial raw material after the IL and DES treatments, which is also in agreement with the hemicellulose content reduction for the treated wood samples (as shown above for xylan). The characteristic bands of lignin at 1504 cm−1 (C
O unconjugated stretching in acetyl groups of hemicelluloses), in Fig. 5, suggested that most of the acetyl groups of hemicelluloses were removed from the initial raw material after the IL and DES treatments, which is also in agreement with the hemicellulose content reduction for the treated wood samples (as shown above for xylan). The characteristic bands of lignin at 1504 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching in aromatic structures) and 1237 cm−1 (C–O stretching in aryl ether groups) were of very low intensity (or undetected) in the cellulose-rich materials, due to their low lignin content (as shown in Table 1). The band at ca. 1595 cm−1 present in the raw material also disappeared when the treatments were run for a high delignification. The highest intensity peak associated to C–O stretching in cellulose/hemicelluloses at 1030 cm−1 apparently did not change much with the pretreatments, revealing that holocellulose was more prevalent than lignin in all the materials, which corroborate the composition data presented in Table 1.
C stretching in aromatic structures) and 1237 cm−1 (C–O stretching in aryl ether groups) were of very low intensity (or undetected) in the cellulose-rich materials, due to their low lignin content (as shown in Table 1). The band at ca. 1595 cm−1 present in the raw material also disappeared when the treatments were run for a high delignification. The highest intensity peak associated to C–O stretching in cellulose/hemicelluloses at 1030 cm−1 apparently did not change much with the pretreatments, revealing that holocellulose was more prevalent than lignin in all the materials, which corroborate the composition data presented in Table 1.
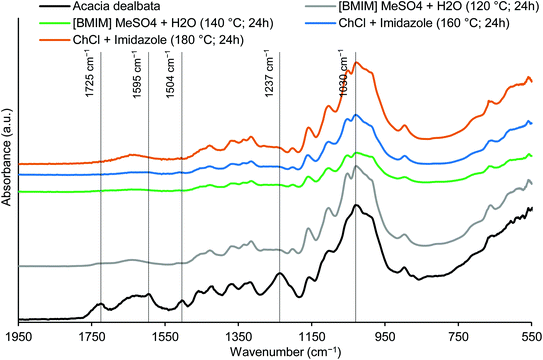 | ||
| Fig. 5 FTIR spectra of untreated A. dealbata wood and cellulose-rich materials obtained after pretreatments with [BMIM]MeSO4 + H2O and ChCl + imidazole. | ||
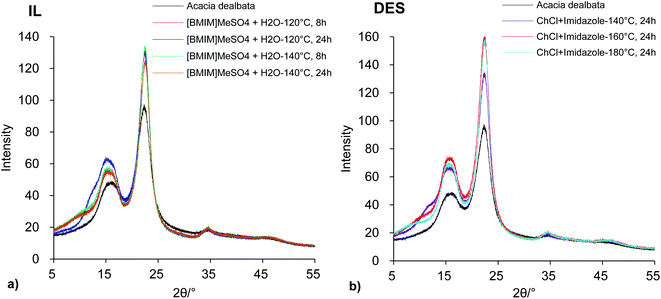
The crystallinity of the untreated wood and of some cellulose-rich materials obtained from IL and DES pretreatments was also evaluated using X-ray diffraction. The resultant diffractograms are present in Fig. 6a and b, respectively. The corresponding crystallinity index (CrI) values are presented in Table 2.
| CrI (%) | Intrinsic viscositya (cm3 g−1) | |
|---|---|---|
| a Measurements in cupriethylenediamine. | ||
| Acacia dealbata | 62.8 | — |
| [BMIM]MeSO4 + H2O – 120 °C, 8 h | 74.3 ± 0.7 | 243 ± 4 |
| [BMIM]MeSO4 + H2O – 120 °C, 24 h | 73.9 ± 0.7 | 205 ± 9 |
| [BMIM]MeSO4 + H2O – 140 °C, 8 h | 75.8 ± 0.4 | 168 ± 1 |
| [BMIM]MeSO4 + H2O – 140 °C, 24 h | 74.1 ± 0.1 | 115 ± 4 |
| ChCl + imidazole – 140 °C, 24 h | 70.5 ± 2.0 | 681 ± 10 |
| ChCl + imidazole – 160 °C, 24 h | 72.6 ± 0.3 | 591 ± 42 |
| ChCl + imidazole - 180 °C, 24 h | 75.2 ± 0.8 | 514 ± 22 |
The Acacia dealbata diffractogram (Fig. 6) showed three characteristic diffraction peaks at 2θ = 16.2, 22.5 and 34.7° corresponding to (101 + 10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ), (002) and (040) lattice planes of cellulose I polymorph. These typical peaks of cellulose I were also detected in all obtained cellulose-rich materials suggesting that the present IL and DES pretreatments did not significantly affect the cellulose structure, regardless of pretreatment conditions. On the other hand, the calculated CrI values (Table 2), revealed a significant crystallinity increase for all cellulose-rich materials compared to untreated wood. This increase was not necessarily due to an increase in cellulose crystallinity, but rather due to the removals of the amorphous components of wood (xylan and lignin) and, possibly, to the removal of the amorphous regions of cellulose. Additionally, the CrI values increased slightly with the increase of pretreatment temperature for each system: when the temperature was increased, the lignin and xylan removals were higher (as shown in Table 1), contributing to a higher crystallinity index of the undissolved solid material.
), (002) and (040) lattice planes of cellulose I polymorph. These typical peaks of cellulose I were also detected in all obtained cellulose-rich materials suggesting that the present IL and DES pretreatments did not significantly affect the cellulose structure, regardless of pretreatment conditions. On the other hand, the calculated CrI values (Table 2), revealed a significant crystallinity increase for all cellulose-rich materials compared to untreated wood. This increase was not necessarily due to an increase in cellulose crystallinity, but rather due to the removals of the amorphous components of wood (xylan and lignin) and, possibly, to the removal of the amorphous regions of cellulose. Additionally, the CrI values increased slightly with the increase of pretreatment temperature for each system: when the temperature was increased, the lignin and xylan removals were higher (as shown in Table 1), contributing to a higher crystallinity index of the undissolved solid material.
The intrinsic viscosity results (Table 2) suggested that the cellulose-rich materials obtained with IL have a considerably lower degree of polymerization (DP) when compared with those from DES. In other words, the IL pretreatment provided a higher cellulose breakdown resulting in cellulose molecules with shorter chain lengths. This fact is in accordance with the superior cellulose losses obtained with the IL treatment (Table 1). Additionally, it was also found that, regardless of the used pretreatment, the intrinsic viscosity decreased when temperature and time were increased. The intrinsic viscosity values obtained with the IL pretreatment are not far, for instance, from that obtained for a nanocellulose produced from A. dealbata by TEMPO-mediated oxidation (142 cm3 g−1).37 On the other hand, all the measured intrinsic viscosities were much lower than those of A. dealbata kraft pulps (e.g., 1241 cm3 g−1 for a pulp with kappa number of 15).38 It seems that the IL and DES pretreatments lead to a higher cellulose depolymerization than the kraft cooking, which can be an advantage depending of the subsequent application.
Synthesis and characterization of cationic wood-based lignocelluloses
Firstly, it should be noted that, to our knowledge, cellulose-rich materials obtained from wood pretreatment with ILs and DESs have never been used for the production of cationic lignocelluloses. In this work, the resulting materials obtained by [BMIM]MeSO4 + H2O (at 120 °C for 24 h) and ChCl + imidazole (at 160 °C for 24 h) pretreatments were chosen for cationic lignocellulose production. These cellulose-rich materials were selected not only based on their lignin content but also based on the obtained wood dissolution yields and considering the fact that higher pretreatment temperatures would affect more the structure and degree of polymerization of cellulose. Considering this, these specific materials were oxidized with sodium periodate in order to obtain DALC, and, afterwards, the cationic lignocellulose was produced by the reaction of DALC with GT. The reaction conditions and the results obtained in each step are listed in Table 3. The temperature for the oxidation step (50 and 60 °C for IL and DES, respectively) was optimized based on the results of several trials where temperature was changed. Higher temperatures were translated into lower yield and aldehyde group content of the products.| Treated wood | Oxidation | Cationization | |||||
|---|---|---|---|---|---|---|---|
| t (h) | T (°C) | Aldehyde group content of DALC (mmol g−1) | t (h) | T (°C) | GT/aldehyde (molar ratio) | Cationicity index (mmol g−1) | |
| a Solid fraction obtained after the first centrifugation, before adding the isopropanol. | |||||||
| [BMIM]MeSO4 + H2O (120 °C; 24 h) | 3 | 50 | 6.3 (IL-DALC) | 1 | 70 | 1 | 2.15 |
| 1.58a | |||||||
| 4 | 2.72 | ||||||
| 2.23a | |||||||
| ChCl + imidazole (160 °C; 24 h) | 3 | 60 | 8.6 (DES-DALC) | 1 | 70 | 1 | 2.59 |
| 1.97a | |||||||
| 4 | 3.59 | ||||||
| 2.74a | |||||||
The oxidation of both cellulose-rich materials yielded two DALCs with a significant difference in the aldehyde content, 6.3 and 8.6 mmol g−1 for IL-DALC and DES-DALC, respectively (Table 3). On the other hand, the obtained reaction yields were very similar, 66 and 64%, respectively. Based on the chemical composition of the initial wood-treated materials (Table 1), it could be expected that the IL-treated wood would lead to a higher reaction yield and aldehyde content since it had higher cellulose content. However, the intrinsic viscosity data (Table 2) showed that the cellulose present in this sample was much more depolymerized than in the DES-treated wood. Due to this fact, when the oxidation of the IL-treated wood was performed, some of the oxidized chains of cellulose, especially the most oxidized ones, were dissolved/lost decreasing both the reaction yield and the aldehyde group content in the isolated solid. This effect was less pronounced for the cellulose in the DES-treated wood.
After the second reaction step (cationization) at 70 °C for 1 h, with GT/aldehyde molar ratios of 1 and 4, for each DALC, two precipitate fractions were obtained: one before (first solid fraction, corresponding to 13–16%, w/w) and one after (second solid fraction, corresponding to 84–87%, w/w) the addition of isopropanol (7.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (water), v/v). As expected, when the GT/aldehyde ratio increased the cationicity index of both precipitated materials also increased (Table 3). For the same reaction conditions (GT/aldehyde ratio, temperature and reaction time), the cationicity of the materials obtained from the DES-DALC was larger than that of the materials obtained from the IL-DALC, because of the higher aldehyde group content in the initial DES-DALC, which affects the cationization efficiency. It was also found that the cationic group content of the second precipitate fraction was always higher compared to the first fraction, for each DALC and GT/aldehyde ratio, in agreement with the higher solubility in water of the second solid fraction (isopropanol was required for its precipitation). Finally, the mass ratios of final cationic product (sum of the two fractions) to initial dialdehyde varied from 1.3 (GT/aldehyde = 1) to 2.0 (GT/aldehyde = 4). The cationicity index values here obtained are in the same order of magnitude of those reported for kraft pulp cationization, especially when it was used a GT/aldehyde ratio of 4 for the cationization of DES-DALC (3.6 mmol g−1 for the main fraction). For a kraft pulp with 2.2% of lignin, Almeida et al.38 obtained 3.6 mmol g−1 of cationic group content using a similar GT/aldehyde ratio.
1 (water), v/v). As expected, when the GT/aldehyde ratio increased the cationicity index of both precipitated materials also increased (Table 3). For the same reaction conditions (GT/aldehyde ratio, temperature and reaction time), the cationicity of the materials obtained from the DES-DALC was larger than that of the materials obtained from the IL-DALC, because of the higher aldehyde group content in the initial DES-DALC, which affects the cationization efficiency. It was also found that the cationic group content of the second precipitate fraction was always higher compared to the first fraction, for each DALC and GT/aldehyde ratio, in agreement with the higher solubility in water of the second solid fraction (isopropanol was required for its precipitation). Finally, the mass ratios of final cationic product (sum of the two fractions) to initial dialdehyde varied from 1.3 (GT/aldehyde = 1) to 2.0 (GT/aldehyde = 4). The cationicity index values here obtained are in the same order of magnitude of those reported for kraft pulp cationization, especially when it was used a GT/aldehyde ratio of 4 for the cationization of DES-DALC (3.6 mmol g−1 for the main fraction). For a kraft pulp with 2.2% of lignin, Almeida et al.38 obtained 3.6 mmol g−1 of cationic group content using a similar GT/aldehyde ratio.
The FTIR spectra of the soluble fractions (Fig. 7) showed several new bands when compared to the FTIR spectra of treated and untreated wood (Fig. 5), which confirmed the functionalization of the cellulose structure: bands at 1688 and 1557 cm−1 corresponding to the carbonyl and carbon–nitrogen stretching of amide groups, respectively; bands at 1474 and 1415 cm−1 related to the asymmetric and symmetric bending vibrations of methyl groups; the asymmetric NC4 stretching of alkylammonium groups at 924 cm−1. When the band's intensity is compared, it is clearly visible the increase in the relative intensity of the characteristic band at 924 cm−1 with the cationicity index, that is, the higher the cationicity index, the higher the intensity of the band due to the presence of more alkylammonium groups.
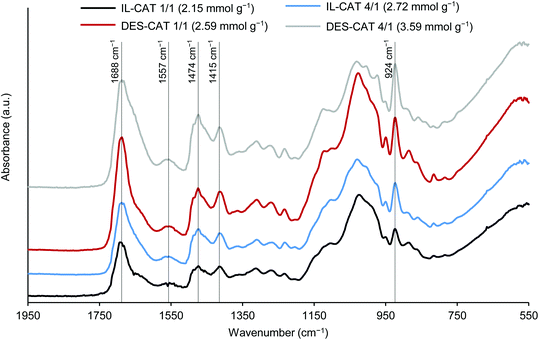 | ||
| Fig. 7 FTIR spectra of the cationic polyelectrolytes (soluble fraction). IL-CAT and DES-CAT correspond to the polyelectrolytes obtained from IL- and DES-treated wood. | ||
Bio-based cationic polyelectrolytes having a considerable content of quaternary ammonium groups can thus be obtained directly from IL- and DES-pretreated woods.
Conclusions
Under optimized conditions, the pretreatments using the [BMIM]MeSO4 (IL) + H2O system and ChCl + imidazole eutectic solvent (DES) showed both to be highly efficient for lignin removal of Acacia dealbata wood, reaching up to 92.4 and 90.2% of delignification, respectively. However, for a more selective delignification with less wood losses, the treatment with DES should be selected, since it allows almost the same level of delignification, but with lower losses of cellulose and hemicellulose.After the pretreatment step, two selected cellulose-rich materials were cationized. The selected samples were those obtained by the pretreatment with IL at 120 °C, for 24 h (lignin content of 6%; dissolution yield of 57%) and with DES at 160 °C, for 24 h (lignin content of 7.0%; dissolution yield of 47%). Despite of the amount of lignin still present in these lignocellulosic materials (6–7%), it was possible to obtain water-soluble polyelectrolytes with high alkylammonium group content (up to 3.6 mmol g−1).
The production of cationic polyelectrolytes from cellulose-rich materials extracted from wood using ionic liquids and deep eutectic solvents was successfully demonstrated for the first time, in this work. The new bio-based polyelectrolytes, produced by an environmentally friendly process from the wood wastes of an invasive plant species, may be proposed as perspective materials to be used in diverse areas such as wastewater treatment, pharmaceutics, or cosmetics.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors thank the support from project MATIS (2020 000014 MATIS 2020) funded by Agência para o Desenvolvimento e Coesão (Portugal). FCT is also acknowledged through Strategic Research Centre Project (UIDB/00102/2020).References
- Q. Hou, M. Ju, W. Li, L. Liu, Y. Chen and Q. Yang, Molecules, 2017, 22, 490 CrossRef PubMed.
- C. Wang, S. Yang, X. Song, Q. Pi, Q. Zhang, Q. Liu, Y. Xu, L. Chen and L. Ma, Adv. Sustainable Syst., 2020, 4, 2000085 CrossRef CAS.
- A. Brandt, J. Gräsvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 550–583 RSC.
- A. S. Amarasekara, Isr. J. Chem., 2018, 58, 1–15 CrossRef.
- M. Rastogi and S. Shrivastava, Renewable Sustainable Energy Rev., 2017, 80, 330–340 CrossRef.
- E. S. Morais, A. M. C. Lopes, M. G. Freire, C. S. R. Freire, J. A. P. Coutinho and A. J. D. Silvestre, Molecules, 2020, 25, 3652 CrossRef CAS PubMed.
- H. Marchante, E. Marchante and H. Freitas, Plantas invasoras em Portugal – fichas para identificação e controlo, ed. authors, Coimbra, 2005 Search PubMed.
- L. J. R. Nunes, C. I. R. Meireles, C. J. P. Gomes and N. M. C. A. Ribeiro, Forests, 2019, 10, 974 CrossRef.
- C. G. Yoo, Y. Pu and A. J. Ragauskas, Curr. Opin. Green Sustain. Chem., 2017, 5, 5–11 CrossRef.
- K. C. Badgujar and B. M. Bhanage, Bioresour. Technol., 2015, 178, 2–18 CrossRef CAS PubMed.
- S. Dutta and K. Nath, J. Water Process. Eng., 2018, 21, 163–176 CrossRef.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- R. Yáñez, B. Gómez, M. Martínez, B. Gullón and J. L. Alonso, J. Chem. Technol. Biotechnol., 2014, 89, 1337–1343 CrossRef.
- H. Kim, Y. Ahn and S. Kwak, Biomass Bioenergy, 2016, 93, 243–253 CrossRef CAS.
- Y. Pu, N. Jiang and A. J. Ragauskas, J. Wood Chem. Technol., 2007, 27, 23–33 CrossRef CAS.
- H. Xu, J. Peng, Y. Kong, Y. Liu, Z. Su, B. Li, X. Song, S. Liu and W. Tian, Bioresour. Technol., 2020, 310, 123416 CrossRef CAS PubMed.
- X. Tang, M. Zuo, Z. Li, H. Liu, C. Xiong, X. Zeng, Y. Sun, L. Hu, S. Liu, T. Lei and L. Lin, ChemSusChem, 2017, 10, 2696–2706 CrossRef CAS PubMed.
- M. Francisco, A. Bruinhorst and M. C. Kroon, Angew. Chem., Int. Ed., 2013, 52, 3074–3085 CrossRef CAS PubMed.
- L. I. N. Tomé, V. Baião, W. Silva and C. M. A. Brett, Appl. Mater. Today, 2018, 10, 30–50 CrossRef.
- Y.-L. Loow, E. K. New, G. H. Yang, L. Y. Ang, L. Y. W. Foo and T. Y. Wu, Cellulose, 2017, 24, 3591–3618 CrossRef CAS.
- C. Alvarez-Vasco, R. Ma, M. Quintero, M. Guo, S. Geleynse, K. K. Ramasamy, M. Wolcott and X. Zhang, Green Chem., 2016, 18, 5133–5141 RSC.
- Y. Chen, L. Zhang, J. Yu, Y. Lu, B. Jiang, Y. Fan and Z. Wang, R. Soc. Open Sci., 2019, 6, 181757 CrossRef CAS PubMed.
- X.-J. Shen, J.-L. Wen, Q.-Q. Mei, X. Chen, D. Sun, T.-Q. Yuan and R.-C. Sun, Green Chem., 2019, 21, 275–283 RSC.
- C. S. Lee, J. Robinson and M. F. Chong, Process Saf. Environ. Prot., 2014, 92, 489–508 CrossRef CAS.
- V. Bourganis, T. Karamanidou, O. Kammona and C. Kiparissides, Eur. J. Pharm. Biopharm., 2017, 111, 44–60 CrossRef CAS PubMed.
- A. Massironi, A. Morelli, D. Puppi and F. Chiellini, Molecules, 2020, 25, 4886 CrossRef CAS PubMed.
- Y. Hou, Y. Gu, S. Zhang, F. Yang, H. Ding and Y. Shan, J. Mol. Liq., 2008, 143, 154–159 CrossRef CAS.
- B. Socas-Rodríguez, A. Santana-Mayor, A. V. Herrera-Herrera and M. Á. Rodríguez-Delgado, in Green Sustainable Process for Chemical and Environmental Engineering and Science, ed. Inamuddin, A. M. Asiri and S. Kanchi, Elsevier, Amsterdam, 2020, ch. 5, pp.123–177 Search PubMed.
- L. Segal, J. J. Creely, A. E. Martin and C. M. Conrad, Text. Res. J., 1959, 29, 786–794 CrossRef CAS.
- J. Sirviö, A. Honka, H. Liimatainen, J. Niinimäki and O. Hormi, Carbohydr. Polym., 2011, 86, 266–270 CrossRef.
- K. Grenda, J. A. F. Gamelas, J. Arnold, O. J. Cayre and M. G. Rasteiro, RSC Adv., 2019, 9, 34814–34826 RSC.
- K. Grenda, J. Arnold, J. A. F. Gamelas and M. G. Rasteiro, Water Sci. Technol., 2017, 76, 1490–1499 CrossRef CAS PubMed.
- A. Brandt, M. J. Ray, T. Q. To, D. J. Leak, R. J. Murphy and T. Welton, Green Chem., 2011, 13, 2489–2499 RSC.
- A. Procentese, E. Johnson, V. Orr, A. G. Campanile, J. A. Wood, A. Marzocchella and L. Rehmann, Bioresour. Technol., 2015, 192, 31–36 CrossRef CAS PubMed.
- J. L. K. Mamilla, U. Novak, M. Grilc and B. Likozar, Biomass Bioenergy, 2019, 120, 417–425 CrossRef CAS.
- T. V. Doherty, M. Mora-Pale, S. E. Foley, R. J. Linhardt and J. S. Dordick, Green Chem., 2010, 12, 1967–1975 RSC.
- R. O. Almeida, A. Ramos, L. Alves, E. Potsi, P. J. T. Ferreira, M. G. V. S. Carvalho, M. G. Rasteiro and J. A. F. Gamelas, Int. J. Biol. Macromol., 2021, 188, 1003–1011 CrossRef CAS PubMed.
- R. Almeida, F. Cisneros, C. V. T. Mendes, M. G. V. S. Carvalho, M. G. Rasteiro and J. A. F. Gamelas, Ind. Crops Prod., 2021, 167, 113476 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |