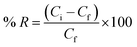Open Access Article
Open Access ArticleSelective preconcentration separation of Hg(II) and Cd(II) from water, fish muscles, and cucumber samples using recycled aluminum adsorbents†
A. B. Abdallah *a,
Ehab A. Abdelrahmanb,
Adel M. Youins
*a,
Ehab A. Abdelrahmanb,
Adel M. Youins a,
Wesam A. Ibrahima and
Magdi E. Khalifaa
a,
Wesam A. Ibrahima and
Magdi E. Khalifaa
aDepartment of Chemistry, Faculty of Science, Mansoura University, El-Gomhoria Street, Mansoura 35516, Egypt. E-mail: ahmed.bahgat@mans.edu.eg
bChemistry Department, Faculty of Science, Benha University, Benha 13518, Egypt
First published on 10th March 2022
Abstract
Modified aluminum scrap waste was used in the selective extraction of Hg(II), and Cd(II) ions. The aluminum scraps were modified with dibenzoylmethane, or isatoic anhydride, or 5-(2-chloroacetamide)-2-hydroxybenzoic acid. The modified aluminum sorbents were characterized by FT-IR, SEM, XRD, XPS, TGA, and elemental analysis. Modes of chelation between adsorbents and target metal ions were deduced via DFT. The highest adsorption capacity was observed for benzo-amino aluminum (BAA) toward Hg(II), which reached 234.56 mg g−1, while other modified sorbents ranged from 135.28 mg g−1 to 229.3 mg g−1. Under the optimized conditions, the BAA adsorbent showed a lower limit of detection (1.1 mg L−1) and limit of quantification (3.66 mg L−1) for mercury ions than other sorbents. The prepared aluminum adsorbents also exhibited significant selectivities for Hg(II) and Cd(II) ions in the presence of competing metal ions.
1. Introduction
In the last few decades, the global demand for Al (e.g. food plates, gutters, foil, soft drink cans, etc.) has seen significant growth due to its good formability, high strength and corrosion resistance.1–3 Therefore, for environmental and economic benefits, the recycling of aluminum scrap is considered as a waste management process and has also produced several useful commercial products. The recycled aluminum, especially from aluminum cans are fit for further applications including the manufacturing of paperclips and ship hulls, and extraction of various pollutants.2,3 For the adsorption of heavy metals from aqueous systems, the chemical and/or physical modification of aluminum scrap using certain organic materials including functional groups (e.g., carboxylic group, amine group, hydroxyl group, etc.) is expected to enhance their sorption process.4–6 The binding of an organic coating material to the surface of the recycled aluminum occurs by mixing the organic solution with the target adsorbent for a specific time. The solvent was allowed to evaporate, followed by air-drying to obtain the resultant adsorbent. Depending on their adsorbent modification, the removal mechanism of different metals is suggested. The target metal ions are not only extracted by adsorption on the modified alumina surface but also by surface attraction, in which chemical-bonding interactions occur between the newly added chemicals and the target heavy metal ions.With the fast development of industry, heavy metal ions (e.g., Hg(II), Cd(II), Pb(II), etc.) have been considered as a substantial issue due to their collection and expansion in various biological chains.7,8 Although these metals are essential for different living organisms, they may cause harmful health problems at certain threshold concentrations. Thus, there is a critical need for a development method for the accurate determination of trace amounts of these heavy metals. Atomic absorption spectrophotometry, capillary electrophoresis, inductively coupled plasma, flame atomic fluorescence spectrometry, and electrochemical techniques are the most frequently used methods for the determination of these metals in different real samples.9–12 Unfortunately, the accurate determination of these metal ions in environmental samples is still a difficult task owing to their low concentrations and the dominant effect of matrix components from the diverse ions. To overcome these limitations, preliminary separation and preconcentration processes become urgent steps that are needed before the direct measurement, especially in the presence of low concentrations of target ions. To date, numerous methods including solid phase extraction (SPE),13–18 cloud point extraction (CPE),19 flotation,20 and liquid–liquid extraction (LLE)21 have been designed for the separation and/or preconcentration of the heavy metal ions. Due to the high extraction efficiency, and rapid phase separation, the SPE has been widely applied as the preconcentration technique.22–24 Based on these approaches, we report a simple and efficient synthetic method for recycling aluminum scrap, especially from aluminum cans. These adsorbents showed enhanced performance for the separation and preconcentration of the target heavy metal ions (Hg(II), and Cd(II)) by modification with the promising chelating agents including dibenzoylmethane, isatoic anhydride and 5-(2-chloroacetamide)-2-hydroxybenzoic acid. The modified aluminum adsorbents were characterized by using Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), thermal gravimetric analysis (TGA), and elemental analysis. The selective extraction of Hg(II), and Cd(II) was optimized in detail prior to its determination by flame atomic absorption spectrometry (FAAS) and inductively coupled plasma optical emission spectroscopy (ICP-OES). The developed technique was validated for the studied metal ions and found to be robust and highly sensitive to low limits of detection for these heavy metal ions. Finally, these aluminum adsorbents were applied in the determination of the target ions in different real samples (e.g., water samples, fish samples, and vegetable samples).
2. Experimental section
2.1. Chemicals and reagents
The aluminum cans were obtained from commercial shops (Mansoura, Egypt). Isatoic anhydride, dibenzoylmethane, 4-amino-2-hydroxy benzoic acid, toluene, cadmium(II) chloride, chloroacetyl chloride, nickel(II) chloride hexahydrate, ethylenediaminetetraacetic acid tetrasodium salt dihydrate, zinc(II) chloride, aluminum(III) chloride hexahydrate, iron(III) chloride hexahydrate, and lead(II) chloride were obtained from Sigma-Aldrich. Hydrochloric acid, potassium chloride, sodium hydroxide, mercury(II) chloride, and copper(II) chloride dihydrate were purchased from Merck (Germany). The other chemicals were of analytical grade, obtained from Fluka (Switzerland) and were used without further purification unless otherwise stated. In addition, all aqueous solutions in this work were prepared by using doubly distilled water (resistance: 18.2 MΩ at 25 °C). It is worth mentioning that all glassware was rinsed in concentrated nitric acid solution (1% (v/v)) and washed with doubly distilled water before use. For analytical applications, the cucumber, lettuce leaves, and wastewater samples were collected from different agricultural locations in Samanoud (El-Gharbia).2.2. Instruments
The determination of metal contents was carried out using Agilent's 5100 ICP-OES (Agilent Technologies. Melbourne, Australia), and FAAS (GBC Scientific Equipment USA LLC, Hampshire, IL). A digital pH meter (HI 8519; Hanna Instruments, Italy) equipped with a combined Ag/AgCl glass electrode was used for adjusting the concentration of hydrogen ions during the extraction processes. FT-IR spectra of the modified aluminum scrap were obtained using an infrared spectrometer (Nicolet iS 10, Nicolet Instrument Co., Madison, WI, USA) over the wavenumber range from 400 cm−1 to 4000 cm−1 using pressed KBr pellets. The morphologies and compositions of the constructed sorbents were determined via scanning electron microscopy (JEOL JEM-2100 lv (HRTEM), JEOL Ltd., Tokyo, Japan), and energy-dispersive X-ray spectroscopy (X-Max 20, Oxford Instruments, UK), using an accelerating voltage of 15 kV. The percentage of the elements (C, H, N, S and O) in the ESI† was detected using a PerkinElmer 2400 CHN Elemental Analyzer (USA). The XPS spectra of the prepared sorbent before and after the adsorption of metal ions were obtained using a K-ALPHA instrument (Thermo Fisher Scientific, USA) with monochromatic X-ray Al K-alpha radiation −10 to 1350 eV and spot size of 400 μm at a pressure of 10−9 mbar. The TGA-DTG analysis was conducted for the modified sorbents at a heating rate of 10 °C min−1 in a N2 atmosphere to examine the decomposition steps and thermal stabilities of the BAA, CAA and IAA ligands after the adsorption of Hg2+ and Cd2+ ions. The crystallinity of the solid particles was examined using a Bruker AXS diffractometer (Karlsruhe, Germany), 1.54056 Å wavelength, and Cu Kα X-ray source.2.3. Preparation of aluminum scrap
Aluminum scrap was fabricated by a hydrothermal technique. Typically, 2 g of aluminum cans were dissolved in 50 mL of sodium hydroxide solution (2.5 M) after washing with boiled distilled water. Subsequently, 50 mL of sodium metasilicate pentahydrate solution, dissolved in sodium hydroxide solution, was added to the above mixture drop by drop with continuous stirring for 1 h at 850 rpm until homogenized. The produced gel was transferred into a stainless-steel autoclave and heated at 150 °C for 6 h. After that, the produced precipitate was washed several times with double distilled water and dried under vacuum for 12 h at 80 °C. Two grams of the aluminum powder precipitation was refluxed with 2.2 mL of (3-aminopropyl)trimethoxysilane in 30 mL toluene at 120–140 °C for 24 h. The resulting material was washed thoroughly with ethanol, dried at 50 °C for 24 h, ground and sieved to obtain the amino-derivative of the aluminum particles. In the subsequent reaction, the resultant amino aluminum powder (2 g) was refluxed again, for 24 h with 25 mL of ethanolic solution of dibenzoylmethane (0.36 M) in the presence of a few drops of sulfuric acid. After that, the product (benzo-amino aluminum (BAA)) was filtered, washed with ethanol, and dried at 60 °C for 12 h. Similarly, the derivative amino aluminum powder was reacted with isatoic anhydride reagent to produce the isa-amino aluminum sorbent (IAA). To produce the chloro-amino aluminum adsorbent (CAA), 0.8 g of 5-(2-chloroacetamide)-2-hydroxybenzoic acid was transferred into a round bottom flask containing 30 mL of toluene and then interacted with 1 g of amino aluminum powder at 130 °C for 24 h. The obtained CAA was filtered, washed several times with ethanol and dried at 50 °C for 6 h.2.4. Theoretical computation for geometry and binding affinity
The structures of dibenzoylmethane, isatoic anhydride and 5-(2-chloroacetamide)-2-hydroxybenzoic acid were optimized using DFT to the minimum energy with the GGA/RPBE with DNP basis set.25 All calculations were performed using the Material Studio platform via the DMOL3 tool.26,27 The chemical softness (S) and hardness (η) reactivity indices were calculated using the energies of the HOMO and LUMO MO's using the following equations:282.5. Extraction procedure
Different binding experiments were carried out to evaluate the binding ability of the constructed modified sorbents. All sorption experiments were performed depending on batch technique. At different pH values, a fixed dose of modified aluminum scrap (BAA or IAA or CAA) was suspended in 30 mL of various concentrations of target heavy metal ion (Hg(II) or Cd(II)) ranging from 50 mg L−1 to 300 mg L−1. The mixtures were then kept shaking for different interval times (2–30 min) in a thermostatic shaker bath till an equilibrium was reached at 25 °C. After that, the supernatant was filtered with a porous membrane (pore size = 0.45 μm), and the equilibrium concentrations of the metal ions were detected by ICP-OES and FAAS. The percentage of heavy metal extract was calculated using the following equation;where; Ci and Cf refer to the initial and final concentrations of the residual chemical ions (mg L−1).
2.6. Application
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), which was immediately prepared. The mixture was settled for 15 min at room temperature, then heated to near dryness and another amount of acid mixture was added. This step was repeated until the reddish-brown vapors stopped and a clear solution was obtained. The remaining sample was made up to 10 mL with DDW in a measuring flask, the pH was adjusted, and the total concentration of metal ions was determined according to the given extraction procedure.
1 v/v), which was immediately prepared. The mixture was settled for 15 min at room temperature, then heated to near dryness and another amount of acid mixture was added. This step was repeated until the reddish-brown vapors stopped and a clear solution was obtained. The remaining sample was made up to 10 mL with DDW in a measuring flask, the pH was adjusted, and the total concentration of metal ions was determined according to the given extraction procedure.3. Results and discussion
3.1. FT-IR
FT-IR spectra (Fig. 1) were utilized to clarify the modification of the aluminum scrap with different organic ligands. The aluminum spectrum is characterized by the presence of intense bands in the range 664–667 and 735–737 cm−1, which are attributed to the symmetric and asymmetric stretching vibrations of Al–O–Al groups, respectively.24 The bending vibrations of Al–O–Al groups were confirmed via the presence of a band in the range from 461 cm−1 to 465 cm−1.30 The bands that appeared at 1632 and 3424 cm−1 were assigned to the bending and stretching vibrations of OH groups, respectively.29 New absorption bands were noticed for the BAA sorbent at 1641, 3000, and 3035 cm−1, which refer to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching, and C–H stretching vibrations of aliphatic and aromatic structures, respectively. The presence of multiple bands in the region of 1449–1596 cm−1 confirmed the presence of aromatic C
N stretching, and C–H stretching vibrations of aliphatic and aromatic structures, respectively. The presence of multiple bands in the region of 1449–1596 cm−1 confirmed the presence of aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations. The presence of multiple bands in the 646–758 cm−1 region is due to aromatic CH out-of-plane bending vibrations. Moreover, the peak observed at 1346 cm−1 was due to the CH bending vibration. These results indicated the successful anchoring of dibenzoylmethane on the surface of the aluminum scrap. In the case of isatoic modification, FT-IR spectra showed a typical absorption peak at 1624 cm−1, which was assigned to the C
C stretching vibrations. The presence of multiple bands in the 646–758 cm−1 region is due to aromatic CH out-of-plane bending vibrations. Moreover, the peak observed at 1346 cm−1 was due to the CH bending vibration. These results indicated the successful anchoring of dibenzoylmethane on the surface of the aluminum scrap. In the case of isatoic modification, FT-IR spectra showed a typical absorption peak at 1624 cm−1, which was assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration of the imine group. A novel band was shown in the region of 1442–1515 cm−1 that may be due to the existence of aromatic C
N stretching vibration of the imine group. A novel band was shown in the region of 1442–1515 cm−1 that may be due to the existence of aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations.31 A sharp and strong peak was also seen at 1730 cm−1, which may be due to the C
C stretching vibrations.31 A sharp and strong peak was also seen at 1730 cm−1, which may be due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of the carbonyl group of the isatoic reagent. On the other hand, the spectra of CAA showed a sharp band at 3360 cm−1 that corresponds to the combination of N–H and –OH groups.32 Moreover, the bands that existed at 1750 cm−1, 1539 cm−1, and 782 cm−1 may be due to the stretching of the carboxyl group, C– H stretching of the aromatic backbone vibration, and aromatic C–H out-of-plane deformation of ortho disubstituted benzene, respectively.33 Additionally, the peak at 1651 cm−1 was attributed to the absorbance of the symmetric stretching vibration of COO− of the new modifier, which confirmed the presence of 5-(2-chloroacetamide)-2-hydroxybenzoic acid at the surface of the modified sorbent.
O stretching vibrations of the carbonyl group of the isatoic reagent. On the other hand, the spectra of CAA showed a sharp band at 3360 cm−1 that corresponds to the combination of N–H and –OH groups.32 Moreover, the bands that existed at 1750 cm−1, 1539 cm−1, and 782 cm−1 may be due to the stretching of the carboxyl group, C– H stretching of the aromatic backbone vibration, and aromatic C–H out-of-plane deformation of ortho disubstituted benzene, respectively.33 Additionally, the peak at 1651 cm−1 was attributed to the absorbance of the symmetric stretching vibration of COO− of the new modifier, which confirmed the presence of 5-(2-chloroacetamide)-2-hydroxybenzoic acid at the surface of the modified sorbent.
3.2. SEM and XRD
The surface morphology and particle size of the modified sorbents were characterized by scanning electron microscopy, and the SEM patterns are shown in Fig. 2. The regular crystalline structure of mesoporous aluminum overlapped or combined over an amorphous background, relatively narrow size distribution, and good dispersibility were observed. Their particle sizes were nearly less than 100 nm and hence gave an expected high surface area. To confirm the crystallinity of BAA, IAA, and CAA, X-ray diffraction patterns of these sorbents are given in Fig. S1 (ESI).† The XRD spectra of mesoporous BAA showed a wide peak in the range of 2θ = 15°–40°. A broad peak centered at 2θ of 22.9° appeared with a wider d-spacing for aluminum scrap, which was modified with isatoic anhydride reagent. The crystallite size of the modified sample was calculated using the Debye–Scherrer equation and it was found to be 16.35 nm. On the other hand, the principal points of CAA were observed in the range 2θ = 15°–40°and the crystallite size was reduced to 13.2 nm. Therefore, it is important to report that the various chelating agents did not influence the crystal structure and morphology of the aluminum scrap.3.3. Elemental analysis
The chemical composition of the constructed sorbents was elucidated by elemental analysis, and the obtained data are listed in Table 1. The appearance of nitrogen element in amino-aluminum scrap is a good indicator of the insertion of 3-aminopropyl trimethoxysilane during the chemical process. The enrichment in the percentages of carbon, hydrogen, and nitrogen after the modification with the different chelating ligands confirmed the completion of the modification processes.| Sample | C% | H% | N% |
|---|---|---|---|
| Amino-Al | 8.68 | 1.98 | 1.62 |
| BAA | 13.25 | 3.22 | 2.78 |
| IAA | 17.34 | 2.54 | 3.66 |
| CAA | 18.12 | 4.13 | 4.23 |
3.4. Thermal gravimetric analysis (TGA)
As shown in the thermograms of the complexes in Fig. S2 (ESI),† the first degradation step occurred in the temperature range of 210–280 °C with small weight loss corresponding to the loss of water molecules. From the second to the last steps of degradation in the range of 280–800 °C were attributed to the decay of organic moieties of the coordinated ligand. The final remaining weights of metal complexes were 18.29%, 16.87%, 15.23%, 24.27%, 15.42% and 25.84% for Cd–BAA, Hg–BAA, Cd–CAA, Hg–CAA, Cd–IAA and Hg–IAA complexes, respectively. The high value of the remaining weight after the adsorption of the heavy metals indicates their higher thermal stabilities.3.5. XPS analysis
XPS measurements were executed to elaborate on the chemical nature of adsorption of mercury and lead on the BAA surface. The XPS spectra before and after the adsorption of heavy metal ions at the surface of the sorbent are exhibited in Fig. S3 (ESI).† The main elements that were noted at the surface of BAA are Al, C, N, O, and Si, which demonstrated the successful preparation of the target sorbent. Furthermore, the appearance of cadmium and mercury peaks at 416.03 eV and 103.45 eV, respectively, emphasized the insertion of the target metal on the BAA surface. Also, the significant differences in intensities and peak positions of O 1s and N 1s before (532.45, and 400.45 eV for O 1s and N 1s) and after the adsorption of Hg(II) (534.6, and 400.6 eV for O 1s and N 1s) and Cd(II) (535.1, and 400.69 eV for O 1s and N 1s) confirmed the participation of nitrogen and oxygen-containing functional groups in the adsorption of metal ions.3.6. Theoretical studies for the proposed chelating ligands
To verify the reactivity of complexation between the chelating ligands and the proposed heavy metals Hg(II), and Cd(II), the MEP maps (molecular electrostatic potential maps), frontier molecular orbitals (FMOs) and the reactivity indices were evaluated. Molecular orbitals (MOs) are significant in the qualitative analysis, in which different positioning is expected for the possibility of a reaction.The concept of FMOs is introduced when studying applications such as organometallic complexes, pericyclic reactions, and acidic–basic behavior.Table 2 shows the EHOMO, ELUMO and reactivity indices (hardness ‘η’ and softness ‘S’), for the proposed ligands. The soft species (acid or base) are ones with high polarizability, while the harder ones are less polarizable.29 Based on this, it is concluded that the adsorption matrices for the different chelating ligands are soft ligands (gives values close to softness), being that the dibenzoylmethane is relatively softer than isatoic anhydride, which is softer than 5-(2-chloroacetamide)-2-hydroxybenzoic acid, as shown in Table 2. This is due to the existence of many regions that achieve resonance in ligands, such as the aromatic rings, along with the imine and carboxyl groups; this led to greater dispersion of the electrons on the ligand structures, producing an enhancement in the smoothness.
| Compound | EHOMO | ELUMO | Hardness (η) | Softness (S) |
|---|---|---|---|---|
| Dibenzoylmethane | −5.367542523 | −2.164573934 | 1.601484 | 0.800742 |
| Isatoic | −5.047153145 | −1.977711876 | 1.534721 | 0.76736 |
| Chloroacetamide | −5.275785008 | −2.425886928 | 1.424949 | 0.712475 |
For the metal ions, the Hg2+ ions show the greatest softness while the Cd2+ ions give the highest hardness.35 By considering Pearson's theory, which states that “the softer cations prefer interactions with the softer ligand while harder cations interact with harder ligands”,34 we conclude that Hg2+ ions have a greater tendency to interact with the ligands. On the other hand, the Cd2+ ions show the lowest ability for adsorption but since various ligands have relatively similar softness and hardness values, it can be deduced that mercury is the better metal for adsorption in these three ligands. Accordingly, dibenzoylmethane is the best adsorbent for Hg2+ adsorption.
The study of MEP maps is used in literature to locate the sites of interaction.36 The FMOs and MEPs are represented in Fig. S4 (ESI).† For the MEP maps, the regions of intense blue color are the positive areas (E+ attack), and the regions of orange-red color are of more negative charge ( attack). From the MPEs, as seen in Fig. S4 (ESI),† it is possible to indicate that the metallic ions (positive ions) will interact with the oxygen and nitrogen atoms of the dibenzoylmethane ligand, which has more negative charges in the areas where the oxygen and nitrogen exist. In the case of isatoic and 5-(2-chloroacetamide)-2-hydroxybenzoic acid ligands, the MEP maps indicate the involvement of the oxygen atoms of both ligands in the interaction with metal ions. From the position of the FMOs (frontier molecular orbitals) for the dibenzoylmethane, as shown in Fig. 3, the position of HOMO orbitals indicates the coordination of the dibenzoylmethane ligand with the metal cations via the nitrogen atom of the (C
attack). From the MPEs, as seen in Fig. S4 (ESI),† it is possible to indicate that the metallic ions (positive ions) will interact with the oxygen and nitrogen atoms of the dibenzoylmethane ligand, which has more negative charges in the areas where the oxygen and nitrogen exist. In the case of isatoic and 5-(2-chloroacetamide)-2-hydroxybenzoic acid ligands, the MEP maps indicate the involvement of the oxygen atoms of both ligands in the interaction with metal ions. From the position of the FMOs (frontier molecular orbitals) for the dibenzoylmethane, as shown in Fig. 3, the position of HOMO orbitals indicates the coordination of the dibenzoylmethane ligand with the metal cations via the nitrogen atom of the (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) group, as the nitrogen atom grants π-orbitals that can interact with the metal cations, while in the case of isatoic and 5-(2-chloroacetamide)-2-hydroxybenzoic acid ligands, very distinguishing π-orbitals are located over the oxygen atom of the (C
N) group, as the nitrogen atom grants π-orbitals that can interact with the metal cations, while in the case of isatoic and 5-(2-chloroacetamide)-2-hydroxybenzoic acid ligands, very distinguishing π-orbitals are located over the oxygen atom of the (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups so this would be the site of interaction for these ligands.
O) groups so this would be the site of interaction for these ligands.
The chelating ligands use their HOMO level, which contains electrons, to interact with the empty LUMO levels of the electropositive Hg2+ and Cd2+ and species that have type-d orbitals.
3.7. Optimization of extraction conditions
ln(Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − K1t Qe − K1t
| (1) |
 | (2) |
| Sample | Amount added | Found | Recovery (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BAA | IAA | CAA | BAA | IAA | CAA | ||||||||
| Cd | Hg | Cd | Hg | Cd | Hg | Cd | Hg | Cd | Hg | Cd | Hg | ||
| River Nile water (μg L−1) | — | 0.12 | 0.35 | 0.1 | 0.33 | 0.11 | 0.31 | — | — | — | — | — | — |
| 5.0 | 5.08 | 5.27 | 5.09 | 5.27 | 5.09 | 5.29 | 99.2 | 98.5 | 99.8 | 98.8 | 99.6 | 99.6 | |
| 10.0 | 10.1 | 10.31 | 10.08 | 10.31 | 10.12 | 10.18 | 99.8 | 99.6 | 99.8 | 99.8 | 100 | 98.7 | |
| Tap water (μg L−1) | — | 0.27 | 0.22 | 0.28 | 0.22 | 0.27 | 0.2 | — | — | — | — | — | — |
| 5.0 | 5.15 | 5.13 | 5.17 | 5.2 | 5.24 | 5.22 | 97.7 | 98.2 | 97.9 | 99.6 | 99.4 | 100 | |
| 10.0 | 10.17 | 10.1 | 10.29 | 10.1 | 10.21 | 10.15 | 99 | 98.8 | 100 | 98.8 | 99.4 | 99.5 | |
| Seawater (μg L−1) | — | 0.22 | 0.29 | 0.21 | 0.27 | 0.22 | 0.26 | — | — | — | — | — | — |
| 5.0 | 5.22 | 5.17 | 5.19 | 5.26 | 5.19 | 5.21 | 100 | 97.7 | 99.6 | 99.8 | 99.4 | 99 | |
| 10.0 | 10.21 | 10.11 | 10.1 | 10.27 | 10.1 | 10.1 | 99.9 | 98.2 | 98.9 | 100 | 98.8 | 98.4 | |
| Wastewater (μg L−1) | — | 0.56 | 4.12 | 0.55 | 4.1 | 0.57 | 4.2 | — | — | — | — | — | — |
| 5.0 | 5.33 | 9.09 | 5.44 | 9.09 | 5.42 | 9.15 | 95.8 | 99.6 | 98 | 99.8 | 97.3 | 99.4 | |
| 10.0 | 10.5 | 14.1 | 10.42 | 14.1 | 10.36 | 14.2 | 99.4 | 99.8 | 98.7 | 100 | 98 | 100 | |
| Fish muscles (μg L−1) | — | 0.18 | 2.65 | 0.19 | 2.61 | 0.17 | 2.59 | — | — | — | — | — | — |
| 5.0 | 5.07 | 7.51 | 5.18 | 7.49 | 5.16 | 7.48 | 97.8 | 98.1 | 99.8 | 98.4 | 99.8 | 98.5 | |
| 10.0 | 10.15 | 12.59 | 10.06 | 12.39 | 10.17 | 12.47 | 99.7 | 99.5 | 98.7 | 98.2 | 100 | 99 | |
| Cucumber (μg L−1) | — | 0.07 | 0.09 | 0.08 | 0.09 | 0.08 | 0.08 | — | — | — | — | — | — |
| 5.0 | 5.03 | 5.01 | 5.06 | 5.09 | 5.0 | 4.97 | 99.2 | 98.4 | 99.6 | 100 | 98.4 | 97.8 | |
| 10.0 | 10.0 | 10.01 | 10.07 | 9.98 | 9.94 | 10.07 | 99.3 | 99.2 | 99.9 | 98.9 | 98.6 | 99.9 | |
4. Conclusion
Newly selective and sensitive sorbents were successfully synthesized by the modification of aluminum scrap with the different promising chelating agents including dibenzoylmethane, isatoic anhydride, and 5-(2-chloroacetamide)-2-hydroxybenzoic acid. The fabricated sorbents were characterized by using IR spectroscopy, XRD, SEM, and elemental analysis. The theoretical and experimental adsorption studies revealed that the aluminum scrap modified with BAA is incredibly more selective for Hg(II) than Cd(II). Under the optimal conditions, the adsorption capacity of fabricated sorbents ranged from 135.28 mg g−1 to 229.3 mg g−1, while reaching 234.56 mg g−1 in the case of the BAA ligand. In general, the proposed sorbents showed good chemical and physical stability over a wide pH range and can be utilized repetitively in more than six cycles without significant changes in properties. This extraction procedure offers acceptable precision, high accuracy, and a relatively low detection limit and can also be applied to detect low levels of Hg(II), and Cd(II) in different real samples.Conflicts of interest
There are no conflicts to declare.References
- E. Sevigné-Itoiz, C. M. Gasol, J. Rieradevall and X. Gabarrell, Resour., Conserv. Recycl., 2014, 89, 94–103 CrossRef.
- E. van der Harst, J. Potting and C. Kroeze, Waste Manage., 2016, 48, 565–583 CrossRef CAS PubMed.
- M. E. Grigore, Recycling, 2017, 2(4), 24 CrossRef.
- Z. Xin, Colloids Surf., 2019, 567, 205–212 CrossRef.
- R. Sun, J. Zhao, Z. Li, J. Mo, Y. Pan and D. Luo, Prog. Org. Coat., 2019, 133, 77–84 CrossRef CAS.
- R. Sun, Z. Li, J. Zhao, J. Mo, Y. Pan and D. Luo, Nano, 2021, 16, 2150133 CrossRef CAS.
- G. Guzzi, A. Ronchi and P. Pigatto, Chemosphere, 2021, 263, 127990 CrossRef CAS PubMed.
- Z. Fu and S. Xi, Toxicol. Mech. Methods, 2020, 3, 167–176 CrossRef PubMed.
- V. Zarezade, M. Behbahani, F. Omidi, H. S. Abandansari and G. Hesam, RSC Adv., 2016, 6, 103499–103507 RSC.
- S. Himeno, E. Kitano and K. Morishita, Anal. Sci., 2007, 23, 959–962 CrossRef CAS PubMed.
- J. Jiang, Z. Li, Y. Wang, X. Zhang, K. Yu, H. Zhang, J. Zhang, J. Gao, X. Liu, H. Zhang, W. Wu and N. Li, Food Chem., 2020, 310, 125824 CrossRef CAS PubMed.
- R. Sun, G. Ma, X. Duan and J. Sun, Spectrochim. Acta, Part B, 2018, 141, 22–27 CrossRef CAS.
- M. Karve and R. V. Rajgor, J. Hazard. Mater., 2007, 141, 607–613 CrossRef CAS PubMed.
- Z. Zhu, S. Sun and X. Jing, Chem. Pap., 2022, 76, 401–408 CrossRef CAS.
- G. Yanyu, K. Jing, Y. Chenggen, Y. Qian and Z. Xu, Chin. J. Org. Chem., 2020, 40, 1760–1765 CrossRef.
- Z. Zhu, W. Wang, F. Zhang and J. Liu, Mater. Lett., 2020, 261, 127011 CrossRef CAS.
- J. Zhang, K. Cao, X. Zhang and Q. Zhang, Appl. Organomet. Chem., 2020, 34, e5377 CAS.
- X. Jing, C. Chen, X. Deng, X. Zhang, D. Wei and L. Yu, Appl. Organomet. Chem., 2018, 32, e4332 CrossRef.
- E. L. Silva and P. d. S. Roldan, J. Hazard. Mater., 2009, 161, 142–147 CrossRef CAS PubMed.
- L. Wang, Y. Liu, Y. Liu, Y. Mao, J. Han, W. Li and Y. Wang, Sep. Purif. Technol., 2021, 274, 118917 CrossRef CAS.
- M. Shamsipur, M. Ramezani and M. Sadeghi, Microchim. Acta, 2009, 166, 235–242 CrossRef CAS.
- M. Sajid, M. K. Nazal and I. Ihsanullah, Anal. Chim. Acta, 2021, 1141, 246–262 CrossRef CAS PubMed.
- W. Aiming, Crit. Rev. Environ. Sci. Technol., 2021, 51, 44–112 CrossRef.
- H.-L. Jiang, Q.-B. Fu, M.-L. Wang, J.-M. Lin and R.-S. Zhao, Food Chem., 2021, 345, 128841 CrossRef CAS PubMed.
- B. Hammer, L. B. Hansen and J. K. Nørskov, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 7413 CrossRef.
- B. Delley, J. Chem. Phys., 2000, 113, 7756–7764 CrossRef CAS.
- A. M. Younis, T. H. Rakha, M. M. El-Gamil and G. M. A. El-Reash, J. Inorg. Organomet. Polym. Mater., 2022, 32, 895–911 CrossRef CAS.
- R. G. Parr, in Horizons of quantum chemistry, Springer, 1980, pp. 5–15 Search PubMed.
- M. M. Hassanien, W. I. Mortada, I. M. Kenawy and H. El-Daly, Appl. Spectrosc., 2017, 71, 288–299 CrossRef CAS PubMed.
- X. Dai, F. Qiu, X. Zhou, Y. Long, W. Li and Y. Tu, Electrochim. Acta, 2014, 144, 161–167 CrossRef CAS.
- Y. Wang, E. Wang, Z. Wu, H. Li, Z. Zhu, X. Zhu and Y. Dong, Carbohydr. Polym., 2014, 101, 517–523 CrossRef CAS PubMed.
- S. Deng, G. zhang, S. Chen, Y. Xue, Z. Du and P. Wang, J. Mater. Chem. A, 2016, 4, 15851–15860 RSC.
- E. Pretsch, P. Bühlmann, C. Affolter, E. Pretsch, P. Bhuhlmann and C. Affolter, Structure determination of organic compounds, Springer, 2000 Search PubMed.
- R. G. Pearson, J. Am. Chem. Soc., 2002, 85, 3533–3539 CrossRef.
- I. H. S. Ribeiro, D. T. Reis and D. H. Pereira, J. Mol. Model., 2019, 25, 267 CrossRef PubMed.
- A. M. Younis, T. H. Rakha, M. M. El-Gamil and G. M. A. El-Reash, J. Mol. Struct., 2021, 1245, 131110 CrossRef CAS.
- M. E. Khalifa, I. M. M. Kenawy, Y. G. Abou El-Reash and A. B. Abdallah, J. Environ. Chem. Eng., 2017, 5, 3447–3454 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d2ra00028h |
| This journal is © The Royal Society of Chemistry 2022 |