Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Molecularly imprinted polymers via reversible addition–fragmentation chain-transfer synthesis in sensing and environmental applications†
Irvin Veloz Martínez
 a,
Jackeline Iturbe Ek
a,
Jackeline Iturbe Ek
 a,
Ethan C. Ahn
a,
Ethan C. Ahn
 b and
Alan O. Sustaita
b and
Alan O. Sustaita
 *a
*a
aSchool of Engineering and Science, Tecnologico de Monterrey, Av. Eugenio Garza Sada 2501, Monterrey, N.L. 64849, Mexico. E-mail: alan.sustaita@tec.mx
bDepartment of Electrical and Computer Engineering, The University of Texas at San Antonio, San Antonio, TX 78249, USA
First published on 23rd March 2022
Abstract
Molecularly imprinted polymers (MIP) have shown their potential as artificial and selective receptors for environmental monitoring. These materials can be tailor-made to achieve a specific binding event with a template through a chosen mechanism. They are capable of emulating the recognition capacity of biological receptors with superior stability and versatility of integration in sensing platforms. Commonly, these polymers are produced by traditional free radical bulk polymerization (FRP) which may not be the most suitable for enhancing the intended properties due to the poor imprinting performance. To improve the imprinting technique and the polymer capabilities, controlled/living radical polymerization (CRP) has been used to overcome the main drawbacks of FRP. Combining CRP techniques such as RAFT (reversible addition–fragmentation chain transfer) with MIP has achieved higher selectivity, sensitivity, and sorption capacity of these polymers when implemented as the transductor element in sensors. The present work focuses on RAFT-MIP design and synthesis strategies to enhance the binding affinities and their implementation in environmental contaminant sensing applications.
1. Introduction
The increasing uncontrolled release of chemicals from primary activities and industrial processes has negatively impacted the environment and human health. For example, the constant exposure to cancer causing or promoting agents such as glyphosate is a common situation in communities near agriculture fields.1–3 The affected environmental elements also include water, soil, and atmosphere, implying a broad negative impact on human health.4 Analytical instrumentation has been implemented to address this situation by sporadically keeping track of the level of free contaminants in urban systems.5 However, this approach requires a long time between sampling and quantification, thus making it irrelevant to the real situation.6,7 The environment is dynamic and complex, and our ability to detect and neutralize pollutants must adapt.8–11Numerous analytical methods for quantification, including liquid chromatography (LC), atomic absorption spectroscopy (AAS), and gas chromatography (GC), have been reported for the determination of pollutants in different scenarios from in situ analysis stations.12–15 However, the fixed costs related to this kind of infrastructure make this solution not financially viable. In situ quantification techniques have been developed as an alternative to solve this challenge.16–18 The application of the lab-on-a-chip principle is the most promising since its objective is to achieve laboratory grade precision and accuracy with transducing devices. Although these in situ detection methods improve throughput and reduce errors associated with sample storage, processing, and complexity, low analyte concentrations and harsh environment conditions limit their accuracy and suitability.19–23 Therefore, fast, durable, and portable sensory platforms for detection and isolation of desired compounds from environmental samples are required to exhibit advantages over existing analytical techniques.24–26 These new platforms will pave the way towards an outstanding sensitive and selective performance that will undoubtedly advance future developments in food safety, environmental monitoring, and healthcare fields.2,10,27,28,146,147,152
One possible way to create such a novel sensing platform is to take advantage of biomolecules attached in electronic devices (see Fig. 1 for the schematic illustration). Its molecular recognition capability, sensitivity, and dimensions create an attractive hybrid solution that combines nature's performance and selectivity as a signal transducer with electronics99 that are used and commercialized as a sensor.30–38 However, the main challenge with this architecture is that it stills rely upon physiological conditions such as pH and temperature. A solution to overcome this challenge has been to use the molecularly imprinted polymer (MIP) technique, which mimics the key-lock principle with synthetic molecular structures.23,25,35,38–44 This technique relies upon the creation of specific size, orientation, and functional cavities for a template (Fig. 2) whose binding event can be transduced by a chemical or physicochemical event.45–47 The application of tailor-designed imprinted polymers in analytical chemistry needs to be tightly controlled since the high value applications of functional polymers in analytical science generally require well-defined interfaces, including those in precisely synthesized molecular architectures and compositions.17,38,47–49
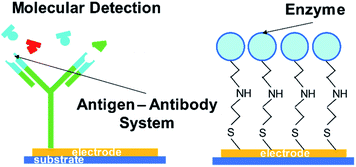 | ||
| Fig. 1 Schematic representation of hybrid devices, (left) antigen anchored to an electrode for selective sensing; (right) enzyme anchored to an electrode for specific catalysis and sensing. | ||
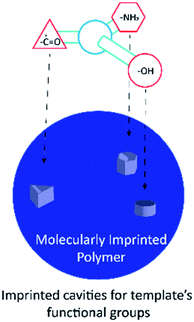 | ||
| Fig. 2 Molecularly imprinted polymer scheme, particle with imprinted sites for the template molecule. | ||
In the past few years, molecular imprinting has been considered as a promising technique that allows the creation of synthetic receptors, consisting of highly crosslinked porous rich materials with recognition properties comparable to the biological systems related to active sites or cavities with shape, size, and functional groups for a specific target molecule (also known as template).22,50,51 Another important property of these synthetic sensing materials (artificial receptors) is that they serve as one of the most attractive components of chemical biosensors because of their long term stability and low production cost compared to the synthesis and extraction of biological receptors such as antibodies and enzymes.52,53 The last mentioned was the inspiration for this technique creation as a route for polymer design and its use for compound isolation in the solid phase and electrochemical sensing applications.3,22,25,54,55
The first paper that specifically reported an “imprinted polymer” appeared in 1984, written by K. Mosbach and B. Sellergren,39 although another paper entitled “Enzyme-Analog Built Polymers” was written in 1973 by Wulff. These early researchers developed several elements of MIP.56,57 The Mosbach group focused on the development of noncovalent interactions between the functional polymer and target molecule also known as a template, while Wulff tended to research covalent binding to imprint polymers.45 The difference between these two methods is dictated by the chemistry methodology necessary to attach and remove the template from the MIP.42,45,58–61 Certainly, the covalent route is expected to produce a more homogeneous distribution of cavities and, potentially, more target specific MIP.62 As mentioned before, several authors described the development of separation and sensing materials, and Wulff and Shea extensively employed imprinted polymers in catalytic reactions, ranging from biochemical to biomedical applications.49,63,64 These applications laid the foundation for specific sensing platforms since the term “molecularly imprinted polymer sensor” started to appear by S. Piletsky in 1992 for the first time.65–68 This polymer methodology has attained great popularity due to its versatility and applicability; its applications have been found in catalysis, separation, filtration, and quantification. These can be adapted to several transducing systems, such as electrodes, transistors, and even the coupling to instrumental techniques for its specific binding capacity.53,65
This review attempts to condense the fundamental concepts, synthesis methods, and performance relating to nanostructured organic/inorganic chemical sensors based on MIP via the controlled/living radical polymerization (CRP) method of reversible addition–fragmentation chain-transfer synthesis (RAFT) and their application as selective receptors for environmental monitoring. With this specific focus, this work seeks to elucidate the potential of the polymerization technique due to their superior recognition capacity, stability and versatility. It ties the individual and segmented information of our literary research into a concise text that enlightens on the novel solution that RAFT synthesis technology for MIP offers for receptor sensing technologies. This work offers fundamental insight for the scientific community, readily capturing the advantages and characteristics of various classes of sensor nanomaterials in one place, along with their synthesis conditions, and integration towards smart synthetic materials capable of specific analyte monitoring and sensing applications.
2. Applications of imprinted polymers
Designing and constructing functional polymers is recognized as one of the most effective routes for analytical science applications such as chromatographic separation, solid-phase extraction, fluorescent or colorimetric sensing, just to list a few.23,38,69–71 The critical role of the tailor designed polymer is the capability to selectively interact with the target molecule, reducing its function as a selective adsorbent/receptor on its functional cavities.61,72,73 The capacity to design cavities with specific morphology, orientation, and functional groups has been exploited for different purposes such as extraction, synthesis, and catalysis.3,45,50,60,69,74–76 Later development and improvement of these receptor's capabilities opened up the opportunity to quantify these binding events with the combination of physicochemical methods.4,71,77 This led to the implementation of these polymers as stimulus transducing elements in sensorial platforms.78,79,99 These platforms range from biosensors for biomedical devices to food security and environmental monitoring in quality processes for water, soil, and air, toxic residual removal materials, smart/responsive structures to stimuli (thermic, magnetic and optical).29,41,65,68,80–83,143–145,149,150The aforementioned applications demand the synthesis of these polymers with controlled molecular structures and specific surface topologies to achieve the intended binding capacity.42,61 Normally, free radical bulk polymerization has been used as an effective approach for both the commercial and lab scale production of MIP because of its compatibility to a variety of monomers, mild polymerization conditions, and tolerance to many different solvents and impurities during the reaction.48,84–87 However, the main drawback is a poorly controlled polymerization process because of the fast propagation and inevitable radical termination in a variable chain length.17,88,89 Mentioning these, a series of previous works were summarized in Table S1† to identify a relationship between the research approach and the analytical application of the MIP in environmental and biological monitoring. It is found that most MIP literature studies focus on a specific application and consider an empirical approach for the design of the material.39 Also, a series of reviews highlight that using the living/controlled polymerization technique is the most accepted alternative to improve the MIP features such as selectivity, sensitivity, and maximum adsorption capacity due to the achieved molecular structure.43,69 Nonetheless, the topic is viewed from an empirical point of study since most of the works focus on the performance of the developed polymer without comparing the used polymer design fundamentals. This situation is visible in the applications column in Table S1.† Since the reported reviews analyse the MIP in a specific field of application, regarding its performance, they select those with clearly superior capabilities among the other works.19,22,24,28,30,51,90 To support this analysis, a scientific basis of polymer science could set a combination for the rational design of these polymeric receptors as the empirical performance is being compared. This situation opens the opportunity to profoundly understand how to design an MIP for each specific industry since its design depends on a series of experimental parameters, processes, and environmental conditions.25,43,73,91–93,144,151
On the other hand, there are a few works that combine computational chemistry to identify the most stable active site between the functional monomer and the template.42 This was taken as an opportunity to start analyzing the parameters to tailor design these polymeric receptors for sensing in analytical platforms, focusing on living polymerization technique effects over the imprinted cavities.
3. Molecularly imprinted polymers synthesis as a strategy for selective and durable sensing
Several methods have been established for the synthesis of MIP; Ndunda et al. stated in 2020 that these can be roughly divided into covalent, noncovalent, and semi covalent imprintings.47 On the other hand, there are several production methods concerning different host–guest binding properties.30,94 These methods rely on the presence of the template during synthesis which can follow several strategies for later remove exceeding monomer and solvent from the reaction mixture such as phase inversion using polymer precipitation by addition of an incompatible solvent or by evaporating the solvent from a networked solution of polymer plus template, and soft lithography or surface stamping.29,148 “Table 1 summarizes a series of applications for these production methods, showing that the synthesis method does not dictates the application but the template/monomer interactions. The production method relies upon the available infrastructure and material's expected binding capacity and performance since each methodology results in a different imprinting effect.” The present review focuses on this synthesis methodology since it shows advantages on energy consumption and requires simple laboratory equipment in comparison with other reported methods.| MIP production method | Applications | Reference |
|---|---|---|
| Phase inversion | Food security | 30 |
| Essential oils extraction | 14 | |
| Bio catalysis | 44 | |
| Ground bulk | Biomarker's monitoring | 15 and 43 |
| Filtration membrane | 47–49 | |
| Soil quality sensing | 29, 45 and 46 | |
| Soft lithography | Wastewater monitoring | 43 and 50 |
| Biosensors | 51 | |
| Biofluid analysis | 52 |
MIP synthesis includes several polymerization methods such as ground bulk which generates irregular size and shape particles; precipitation improves this situation with no control over molecular weight distribution; emulsion increases monodispersity, iniferter for a fine-tuned reaction advancement, and core–shell for smart/responsive particles.44,87,95,147,148,152
Unfortunately, the MIP beads/particles enlisted by the above methods exhibit limitations when prepared through a free radical polymerization, including the fact that active recognition sites embed deeply in the particle require long diffusion time, poor mass transfer, complex and non-scalable manufacturing, and bad regeneration performance, greatly reducing their application options.3,66,96–98 A comparison between these imprinting methods is discussed in Table 2.3,96
| Imprinting method | Advantage | Disadvantage |
|---|---|---|
| Bulk | Simple and fast preparation; no instrumentation required; high purity polymer | Sieving required to achieve small particle size; irregular particle shape and size |
| Suspension | One step polymerization process; spherical particles | Big particle size (hundred micrometres); poor recognition activity |
| Emulsion | High yield; water soluble | Remnants of surfactants; low imprinting |
| Seed | Spherical particles; high monodispersity | Time consuming process |
| Precipitation | One step preparation; uniform, and spherical particles | Excess of template; high dilution factor |
In the other hand, it is important to consider that this absorption property can be used as a key element when we are talking about pollutant removal from environment, the works from Foroughirad, et al. are a clear example of an holistic approach when talking about environmental applications since these materials can be designed to sense and remove pollutants from environment.141–143
The synthesis procedure of MIP generally includes three steps shown in Fig. 3. As mentioned above, a template-monomer complex is first formed via noncovalent or covalent interactions between the template molecules and functional monomers containing vinyl groups which are crucial for radical polymerization.93 The interaction between these two depends on the selected monomer and solvent nature. Although there are reported tables for the selection of these compounds depending on the intended interface with the template, the literature has shown that the imprinting method plays a key role in the performance of the material.26,38,51,65,89,100,101 An analysis of the advantages and disadvantages of free radical polymerization is shown in Table 2.38
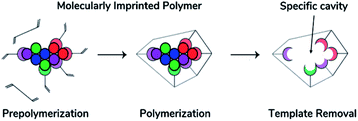 | ||
| Fig. 3 General imprinting technique process (left to right): prepolymerization, polymerization and template detachment. Modified from Belbruno, J., J. Chem. Rev., 119, 94–119 (2019). Copyright 2017 American Chemical Society.29 | ||
The second step in MIP synthesis requires the presence of the template-monomer complex which was previously prepared. If necessary, it can be added an assistant material to achieve a porous rich polymer and enhance mass transfer, and consequently, a crosslinker agent is incorporated in the reaction mixture to create a network of these complexes. Afterward, an initiator starts the polymerization reaction, and the receptor is obtained via free radical propagation which is mostly induced by heating or UV-light initiation.30,42,44,46,61,73,103,104
Finally, the template molecules are removed from the polymer via chemical etching, leaving the imprinted cavities as binding sites in the particle, which fit the template in dimension, morphology, and functional groups,51,66,68,104,105 resulting in highly selective receptors for the template or its analogues. The achieved binding selectivity is comparable to a natural antibody or enzyme, resulting in the combination of nature's sensitivity and analytical performance at a low cost and ease of preparation with a polymer's high thermal and chemical stability, and excellent reusability.30,44,61,97,103,106,107
The development of a biomimetic artificial biosensor can still be improved by engineering processes enhancing the driving forces of molecular recognition by the integration of complementary steps to control the reaction progress and tailor-design the polymer architecture and molecular weight. These properties can be tuned by the used initiation method, and the most reported technique to regulate free radical polymerization is the implementation of a controlled/living radical polymerization technique, which regulates the termination step and limits the side reactions until polymerization is achieved.
4. Controlled radical polymerization application in analytical polymeric interfaces
Controlled/living radical polymerization (CRP) has revolutionized the field of polymer science since the 1990s. A great focus has been put on its industrial origins, leaving aside the fundamentals, and achieving highly specialized properties instead of being a commodity nowadays.46,69,102,109,110 This polymerization method shows great performance in terms of polymerization degree and control over the polymer architecture. It can considerably improve the homogeneity of the crosslinked structure of MIP particles and elucidate their structure property relationship.11,28,69 Therefore, its versatility has made the CRP based techniques the most popular in the preparation of various advanced functional polymers.89 For analytical chemistry, diverse polymers with controlled architectures including homopolymers, block copolymers, molecularly imprinted copolymers, and grafted copolymers were synthesized by CRPs for target isolation or sensing.46,111 In this review, we present an overview of the recently developed advanced functional interfaces by a specific CRP approach in analytical science applications.45,1104.1. Types of controlled/living radical polymerization
The definition of controlled/living polymerization is a polymerization reaction, where the polymerization takes place in a living way. Here in this process, the side reactions (such as termination and transfer reactions) are negligibly small, and the polymerization degrees of the resulting polymers increase linearly with the monomer conversions.23,43,89 In recent years, the most extensively investigated CRPs included atom transfer radical polymerization (ATRP), reversible addition–fragmentation chain transfer (RAFT) polymerization, and iniferter induced “living” radical polymerization.23,43,69,71,77,111–115All these CRP techniques rely on the concept that the reactive growing radical species is transiently and reversely converted into the dormant state via the formation of the covalent terminal.88 This ability to activate and deactivate the radical state lets the reaction advance at a controlled rate, so most of the monomers react and the generated chains present a similar length, which is verified by analyzing the molecular weight of the synthesized polymer (Fig. 4).69,79 This is verified in the reported literature, which also evidences that ATRP is more suitable for films with a specific thickness, and it is not suitable for industrial production because it depends on a metallic catalyst and the related and indirect costs rise.69,111,116 On the other hand, RAFT polymerization shows an advantage for large scale production and offers a simple methodology. It does not require a catalyst or specific ambient conditions in comparison with ATRP which is highly sensitive to the presence of oxygen in the reaction mixture.108,116
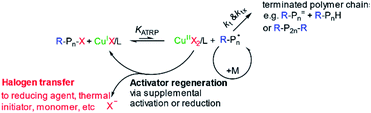 | ||
| Fig. 5 Mechanism of ATRP copper-mediated polymerization. Pn: polymer; M: metal catalyst; X: halogen, R: radical. Reprinted from Wang et al., 2016, Journal of Applied Materials and Interfaces.69 | ||
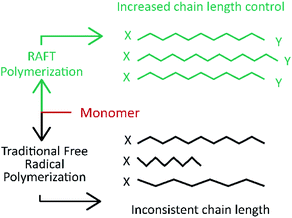 | ||
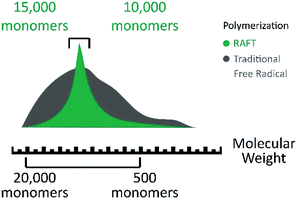
| Fig. 6 Polymer chain length comparison between RAFT polymerization (green) and traditional free radical polymerization (gray). Modified from Sigma Aldrich Copyright, RAFT polymerization products. | ||
In addition to this, RAFT polymerization has shown to be one of the most functional and adjustable methods for bestowing living attributes to radical polymerization. It provides reversible deactivation of propagating radicals by degenerate chain transfer as shown in Fig. 7. The chain transfer step has been designated as degenerate since the process involves an exchange of functionality between the chemical species.97,108,118
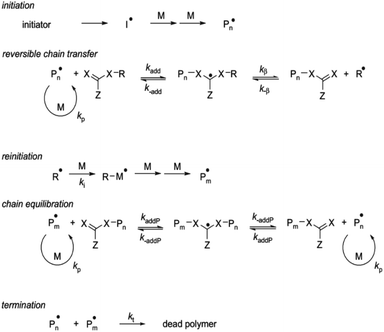 | ||
| Fig. 7 Mechanism of RAFT polymerization. Reproduced with permission of the American Chemical Society, reprinted from Wang et al., 2016, Journal of Applied Materials and Interfaces.69 | ||
The mechanism consists of an activation/deactivation that degenerates a chain transfer as shown in Fig. S1.† Primary radical binds with the CTA and creates polymeric CTAs via consecutive addition of monomers to its thiocarbonyl double bond. Polymeric CTA is converted into this state by intermediate reaction reversibly. This reaction is based on a chain equilibrium in which free radicals are not formed or destroyed. The reaction is regulated so that the concentration of initiator derived chains is negligible and only primary radicals subsist in previous steps of the reaction.118,119
Different authors have reported the use of the RAFT technique as a route to improve MIP active sites (Table S2†) in terms of homogeneity and sorption efficiency.120,121,144 This sets the path toward a study to determine the variables that can be tuned to design this functional polymer. As mentioned in the previous section, the selection of an imprinting technique is a setpoint for artificial receptor design since this implies a series of properties, needs, and performance. The selected imprinting method for this research focuses on radical polymerization in consequence of its promptitude and no requirement for sophisticated instrumentation.122,123 However, the process design does not end at this point; the selection of a specific monomer consists of identifying compatibility with the CRP type and the intended application as shown in Table S3† where a series of works in the use of RAFT-MIP were summarized. This analysis aids in the selection of the CRP technique depending on the template-monomer generated complex conceived as the active site for the MIP.
5. Reversible addition–fragmentation chain transfer polymerization used in the design of molecularly imprinted polymers
The RAFT mechanism precisely controls molecular weight, dispersity, end groups, and architecture of synthesized polymers. Regarding the architecture, preestablished structures and topologies are available such as stars, combs, and particles; this variety leads us to design the polymer particle structure just by selecting the RAFT agent.58,96,113,119 The versatility of this polymerization technique relies upon the use of “living” core polymer microspheres and surface mediated polymerization for the controlled growth of uniform MIP layers with adjustable thickness.43,102,122 The control over these properties provides the opportunity to design different advanced polymers with high efficiency and several applications. The resulting materials have superior properties compared to a traditional free radical polymerization. An example of this is the presence of homogeneous structures which produce higher affinity and an efficient mass transfer phenomenon while achieving a water compatible surface. Additionally, the RAFT mechanism can adjust the effect of decreasing binding ability produced from the reduction of apparent crosslinking degree. The higher improvement in morphology via RAFT polymerization is originated from more homogeneous distribution of crosslink points in the MIP structure.124 This broadens the application scope and performance for MIP since water compatibility is necessary for environmental monitoring. Otherwise, the material won't interact with the analytes and its performance will be affected.114,122 The following section enlists several characterization techniques for the evaluation of the polymer performance.6. Determination of RAFT-MIP materials performance
The generation of predetermined molecular weight and narrow molecular weight distributions, as mentioned before, results in more homogeneous polymer structures, hence, improving the imprinting effect and availability of the specific cavities for the template.45,47,61 Reactive terminal groups can be purposely manipulated to build additional functionality in the polymer backbone architecture including graft, star, and gradient structures.69,114 Achieving an optimal control in RAFT polymerization requires choosing an appropriate RAFT agent for the monomer to be polymerized and the reaction conditions. As shown in Fig. 8, the Z and R groups both play critical roles in determining the outcome of polymerization by participating in the chain transfer equilibrium. Its role is to determine the rate of addition and fragmentation, and they control the efficiency of chain transfer and the likelihood of retardation or inhibition of the radical state.77 Once this reaction design is made to achieve a specific molecular architecture and terminal functional group via a specific RAFT chain-transfer equilibrium, the resulting polymer needs to be evaluated so the efficiency and accuracy of the tailor made material are evaluated.125 It is important to evaluate the following properties because they are expected to be improved since RAFT was added in the reaction design despite the characterization technique is the same for both free radical and controlled radical polymerizations.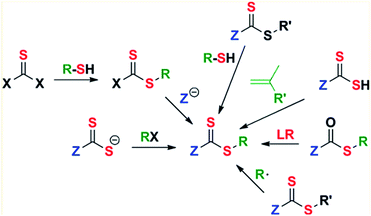 | ||
| Fig. 8 Examples of RAFT chain-transfer equilibrium possibilities. Modified from Zhang, H. et al., Eur. Polym. J., 49, 579–600 (2013).122 | ||
6.1. Dispersity and polymerization degree
The predicted dependence of the degree of polymerization and dispersity of the polymer formed on monomer conversion and the transfer coefficient (Ctr = C−tr) for an ideal polymerization (no termination) with reversible chain transfer is shown in Fig. 9. The predicted degree of polymerization is simply the ratio of [monomer consumed]: [RAFT agent consumed]. The characteristics often associated with living polymerization, namely, the straight-line dependence of molar mass on conversion (and a low dispersity, Đ < 1.2), require a Ctr of at least 10. The most effective RAFT agents have a Ctr > 100.79,119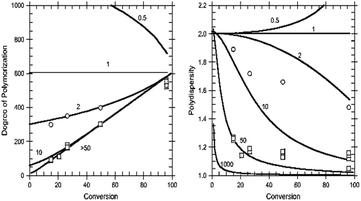 | ||
| Fig. 9 Degree of polymerization characterization of RAFT-MIP. (left) Degree of polymerization in relation to the conversion of monomer: RAFT agent; (right) disparity of the resulting polymer and its Đ value. Reprinted from Gody, G., et al., Macromolecules, 47, 639–649 (2014).126 | ||
The degree of polymerization is determined by the ratio of the concentration of consuming monomer to the sum of the concentrations of consuming transfer agent and decomposed initiator.113,119 Dispersity depends on the ratio of the rate constants of exchange reaction propagation. If the number of new chains formed by the decomposition of the initiator is much lower than that produced from the transfer agent, the rate of transfer is fast, and RAFT can control polymer chain growth by controlling the overall reaction rate.118,120 The RAFT agents should be carefully chosen for a specific polymerization system. Various dithioesters, dithiocarbamates, trithiocarbonates, and xanthates have been effectively used as CTAs to control the polymerization process.126 To efficiently evaluate this parameter, the Gel permeation chromatography (GPC), also known as size exclusion chromatography (SEC), is used for Molecular weight (Mw) determination of polymers, which is based on size exclusion phenomena.23 This technique provides the Mw and the molecular number, requiring calibration with polymers of known Mw, such as polyester standards. An example of this validation is the work done by Cai et al. (2019); they combined RAFT-MIP initiated by photo-induced electron transfer method to develop an electrochemiluminescence sensing platform for melamine quantification.109
6.2. Polymerization evaluation with Fourier transform infrared spectroscopy
Fourier transform infrared spectroscopy (FTIR) analysis aims to distinguish and compare functional groups of (1) the monomer, (2) imprinted polymer, and (3) non imprinted polymer (NIP) through the incidence of infrared radiation on the sample and consequential analysis of the generated interferences in the spectrum. When the sample interacts with the radiation, each functional group of the sample generates specific adsorption bands in the spectrum obtained. The observation that only spectra with the bands of the template monomer and the crosslinking agent appear in both the imprinted polymer and the blank or NIP verifies that the ion does not interfere with the polymerization and that the interaction is of a non-covalent type. The work done by Alahi et al. used FTIR to evaluate the type of binding between the template and the monomer to develop an ion imprinted polymer, depicts that the achieved interaction was non covalent since the template's IR band does not appear in the MIP or the NIP.20The resulting spectra provide information about the presence or absence of a functional group and this allows monitoring of the polymerization stage and is considered as a confirmation analysis of the template binding on the imprinted sites.42,77,107,127
6.3. Adsorption analysis with UV-vis
To characterize the polymerization efficiency as imprinting technique, the polymer's capacity as sorbent, and its viability of use in several applications, it is necessary to execute an adsorption kinetics study of the MIP, the NIP, and the target by placing them in a stock solution of the template. This procedure evidences the template milligrams per MIP gram adsorbed at room temperature, thus obtaining the maximum adsorption capacity and the evaluation of the intended cavities by also evaluating the blank polymer (NIP).11,91,116,128 On this scenario by Ishak, et al. the template solution was analysed with a radiation source at a wavelength of 200–205 nm corresponding to nitrate with UV-visible spectroscopy.38 In this way the concentration changes in the stock solution over time will be obtained by the attribution of the adsorption of these ions in the polymer. It is important to consider the decrease in volume generated by the aliquots to correct the concentration of the solution as the analysis evolves.39 Previous works have shown that the adsorption capacity increases for the imprinted polymer due to the generation of specific cavities; this situation is observed where the NIP presents a lower adsorption capacity.26,30,1297. RAFT-MIP applications in analytical sensing
Chemical versatility and mild reaction conditions of the RAFT mechanism make it compatible with MIP synthesis. The combination of these two achieves a homogeneous polymer network which is traduced as more accessible cavities/receptors for the template. When implemented as a transducing element in sensors, sensitivity and selectivity are improved. The most common sensor platform used in this field of analytical science is the Interdigitated Electrode (IDE) because of its simplicity of design, fabrication, and versatility to be combined with functional materials.48,130As mentioned before, the “living” mechanism of RAFT, slow polymeric chain growth, and the negligible chain termination decrease the effects of irregular imprinting, the low binding capacity, and slow mass transfer of traditional MIP synthesis. So, the potential use of the RAFT mechanism is an improved route to tailor design the molecularly imprinting technology process. On the other hand, RAFT has shown to be a versatile polymerization technique because of the several molecular architectures that can be produced. Among the previously mentioned ones, the molecular brush is the most promising structure since the proposed methodology consists of anchoring the RAFT CTA in the surface of a particle or substrate, so the polymer branch elongates attached to the substrate which is intended to play a critical role in the design of sensor devices (Fig. 10). The critical point here is the polymer deposition or integration to the sensor because depending on the synthesis method, the produced MIP's properties would be affected during and after deposition in the device. To tackle this challenge, polymer brushes are the favourite option because the in situ polymerization solves the deposition stage, and a more efficient interface between the polymer and the sensory platform is developed. This is because the traditional method of deposition of the MIP particles in the IDE does not ensure the interaction between the polymeric and the metallic interfaces so the binding event will not be correctly transduced in an electrical signal.131,132
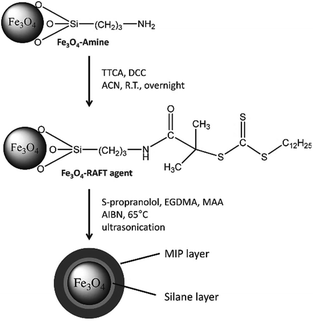 | ||
| Fig. 10 Reaction scheme of the surface modification of amino-functionalized Fe3O4 nanoparticles and the subsequent grafting of a MIP layer. Reprinted from Gonzato, C., et al., Adv. Funct. Mater., 21, 3947–3953 (2011).109 | ||
Several authors have reported the use of surface initiated RAFT-MIP synthesis to improve the interface between the MIP and the substrate. One example is a work by Gonzato et al.; they designed a core–shell particle by anchoring the RAFT agent in a Fe3O4 amine functionalized particle's surface. By doing this, the polymer chain adopted a brush like structure around the particle, so it formed a layer. The ending groups of the polymer were intended to be used as anchors to fine tune the particle's surface properties so a responsive/smart material could be developed. This property is desirable and versatile because the opportunity to grant specific properties into each layer of a particle or surface sets a new path of developing advanced and stimuli responsive materials. Its behaviour can be “programmed” by understanding and controlling the used polymerization technique.30,75,102
8. Experimental practicalities
The understanding of the polymerization technique starts by defining the monomers used in RAFT polymerization. These could be divided into two groups, namely the more active monomers (MAM) and the less active monomers (LAM).133,134 The former has a double bond conjugated with an aromatic ring such as styrene or 4-vinyl pyridine, a carbonyl group such as acrylamide, acrylic acid, or other acrylic monomers, or a nitrile such as acrylonitrile. In contrast, LAM has a double bond adjacent to an oxygen (vinyl acetate), nitrogen (N-vinylpyrrolidone), halogen (vinyl chloride), or sulphur ((4-bromophenyl) (vinyl)sulfane) lone pairs or saturated carbons.124 MAM are typically polymerized with Di thiobenzoate or trithiocarbonates RAFT agents, while less active CTAs are used for highly reactive propagating radicals provided by LAMs.135 This sets the first step to designing a MIP by implementing the RAFT mechanism because previous works by Alahi and Sellergren separately claim that the greater affinity between the monomer and the template sets the difference on the polymer's performance such as its binding site accessibility, and capacity shown. In addition to this, the result of this tailor made reaction against the FRP is that the traditional method produces polymers with a wide distribution of pores and low swelling tendency, with many poorly accessible binding sites, which results in a lower sorption capacity.30,56A series of steps are identified to synthesize a MIP by RAFT mechanism, as shown in Fig. 11. It starts by selecting the monomer–template complex with higher non covalent affinity.126 Then, the compatible RAFT agent and initiator with the intended monomer are identified, and the initiator concentration is optimized to increase the propagation rate and the fraction of living chains while achieving complete monomer conversion by selecting a monomer with high propagation rate coefficient and a RAFT agent with low retardation. The relationship of these parameters is shown in eqn (1). Next, the crosslinking agent is selected by maintaining a higher relationship with the RAFT agent concentration, so the generated material achieves a specific mechanical strength. Finally, synthesis temperature (when using heat in the propagation stage) and mixing speed are optimized according to the mixture viscosity.90,136
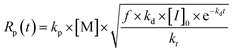 | (1) |
Polymerization rate in the RAFT process where Rp is the polymerization rate, kp the propagation rate coefficient, [M] the monomer concentration, f the initiator efficiency, kd the decomposition rate coefficient of the initiator, [I]0 the initial initiator concentration, and kt the termination rate coefficient.
9. Challenges and opportunities
Research opportunities in the field of MIP and the properties of this class of sensors include environmental safety, excellent sensitivity, selectivity, and stability.30,74 In this case there is a broad range of capabilities but the key feature of formulating MIP is the opportunity to tailor responses to a specific need.81 The relevance of this relies on the understanding of the compatibility of formulation components and the polymer's aging mechanisms. Nonetheless, many challenges affect the performance of polymers such as template leakage, low binding capacity, slow mass transfer, heterogeneity of binding sites, incompatibility with aqueous media, and hydrophilic compound imprinting and biological macromolecules.3,124 Nowadays, the main challenge of using polymers in molecular recognition is the lack of control over the polymer properties. This limitation is originated in part from the polymerization method. So, the opportunity relies upon the efficient design of the CRP for the selected need.10. Conclusion
The evolution of smart synthetic materials that mimic the natural ligand-receptor binding has experienced a quick expansion in the last years and controlled radical polymerization (CRP) has undoubtedly contributed to this result. Although free radical polymerization (FRP) is still the main approach for molecularly imprinted polymer (MIP) synthesis, the intrinsic benefits of CRP make it more attractive for imprinting. Consequently, in recent years, more and more researchers are replacing traditional FRP with CRP techniques, and this trend will likely continue in the following years, making CRP the main polymerization approach for imprinting. CRP techniques have allowed better control over the MIP architectures, obtaining more homogeneous polymer networks that present a greater homogeneity of the binding site.69,122 The materials developed under these conditions have improved kinetics, resulting in imprinted polymers with a better overall performance in terms of binding affinity and specificity. Much works still need to be done concerning ‘plastic’ or ‘synthetic’ antibodies, particularly in the context of their synthetic strategies.124 Further research still should be targeted to obtain higher imprinting yields with a more homogeneous size distribution capable of being produced on a large scale. By achieving the development of the proposed solution, a whole process would be required to expand the application of the designed polymeric films since several industries require a specific interaction for separation, extraction, and sensing.To this end, the integration of the Internet of Things (IoT) with advanced functional materials offers the great possibilities for environmental monitoring.130 Nowadays, several companies have developed technology for real time quantification and detection of specific chemical species. To create this specific interaction, they use ion selective electrodes (ISE) to selectively monitor a few chemicals such as chlorine, peroxide, and fluorine.59,137 The use of MIP as selective layers in electrodes promises to be the replacement of ISE since this material can be tailor made for each possible application for which it is intended to be applied for.138,139 The integration of these advanced sensors would create a system capable of measuring analytes concentration in real time and transfer the data to an IoT cloud server to overcome the limitations of a laboratory based sensing system and offer immediate response for emergencies, processes, and resource management by adopting an evidence based decision making protocol.64,140
11. Outlook
According to the present review, a clear trend in the field of molecularly imprinted polymers is identified. Although most previous works reported the use of FRP, more recent works focused on the polymerization design to achieve specific binding events as a tailor made artificial receptor. It is important to highlight that the combination of MIP with RAFT polymerization has taken a route to the synthesis of polymeric brushes by anchoring the CTA in the surface of particles and electrodes to simplify the fabrication process. As mentioned before, the use of electrodes is an attractive manufacturing route because of the large scale production opportunity. This sets a challenge regarding the used materials for these electrodes. This is an opportunity to achieve a market-fit solution regarding sensor fabrication and its implementation in IoT and environmental monitoring applications (Fig. 12).Funding
This research received no external funding.Author contributions
Conceptualization, I. V., A. S. and J. I.; methodology, J. I., A. S. and I. V.; validation, A. S.; formal analysis, A. S.; investigation, I. V., A. S.; resources, A. S.; data curation, J. I.; writing—original draft preparation, I. V.; writing—review and editing, E. A. and A. S.; visualization, I. V.; supervision, J. I. and A. S.; project administration, A. S.; all authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.Acknowledgements
The authors thank at Tecnológico de Monterrey, Campus Monterrey, through the Department of Chemistry and Nanotechnology, the Department of Mechanical and Advanced Materials and the Research Group of Nanotechnology for Devices Design for support on bibliographic resources. This work was supported by Laboratory of Optoelectronic Hybrid Devices (LDHO).References
- M. A. Ali, X. Wang, Y. Chen, Y. Jiao, N. K. Mahal, S. Moru, M. J. Castellano, J. C. Schnable, P. S. Schnable and L. Dong, Continuous Monitoring of Soil Nitrate Using a Miniature Sensor with Poly(3-octyl-thiophene) and Molybdenum Disulfide Nanocomposite, ACS Appl. Mater. Interfaces, 2019, 11, 29195–29206 CrossRef CAS PubMed.
- B. F. E. Matarèse, A. Kale and A. Stevenson. Enhanced ion selective membrane sensors based on a novel electroacoustic measurement approach, Proc. IEEE Conf. Nanotechnol., 2019, pp. 4–8 Search PubMed.
- M. Fizir, A. Richa, H. He, S. Touil, M. Brada and L. Fizir, A mini review on molecularly imprinted polymer based halloysite nanotubes composites: innovative materials for analytical and environmental applications, Rev. Environ. Sci. Biotechnol., 2020, 19, 241–258 CrossRef.
- J. Lehmann, D. A. Bossio, I. Kögel-Knabner and M. C. Rillig, The concept and future prospects of soil health, Nat. Rev. Earth Environ., 2020, 1–10, DOI:10.1038/s43017-020-0080-8.
- M. S. Mohsenzadeh, A. Mohammadinejad and S. A. Mohajeri, Simple and selective analysis of different antibiotics in milk using molecularly imprinted polymers: a review, Food Addit. Contam., Part A: Chem., Anal., Control, Exposure Risk Assess., 2018, 35, 1959–1974 CrossRef CAS PubMed.
- T. G. Albuquerque, M. B. P. P. Oliveira, A. Sanches-Silva and H. S. Costa, Cholesterol determination in foods: comparison between high performance and ultra-high performance liquid chromatography, Food Chem., 2016, 193, 18–25 CrossRef CAS PubMed.
- J. Fibigr, D. Šatínský and P. Solich, A UHPLC method for the rapid separation and quantification of phytosterols using tandem UV/charged aerosol detection – a comparison of both detection techniques, J. Pharm. Biomed. Anal., 2017, 140, 274–280 CrossRef CAS PubMed.
- P. Rai, S. Jung, T. Ji and V. K. Varadan, Characterization for organic ion-sensitive field effect transistor response for measurement of physiological potassium ion-concentration measurement, in Nanosensors, Biosensors, and Info-Tech Sensors and Systems 2009, ed. V. K. Varadan, SPIE, 2009, vol. 7291, p. 72910H Search PubMed.
- G. Scarpa, A. L. Idzko, A. Yadav, E. Martin and S. Thalhammer, Toward cheap disposable sensing devices for biological assays, IEEE Trans. Nanotechnol., 2010, 9, 527–532 Search PubMed.
- S. Ahmed, N. Shaikh, N. Pathak, A. Sonawane, V. Pandey and S. Maratkar, An overview of sensitivity and selectivity of biosensors for environmental applications, Tools, Tech. Protoc. Monit. Environ. Contam., 2019, 53–73, DOI:10.1016/B978-0-12-814679-8.00003-0.
- A. N. Mallya and P. C. Ramamurthy, Design and Fabrication of a Highly Stable Polymer Carbon Nanotube Nanocomposite Chemiresistive Sensor for Nitrate Ion Detection in Water, ECS J. Solid State Sci. Technol., 2018, 7, Q3054–Q3064 CrossRef CAS.
- G. A. Olah, A. Burrichter, G. Rasul, M. Hachoumy and G. K. S. Prakash, 1H, 13C, 15N NMR and Ab initio/IGLO/GIAO-MP2 study of mono-, di-, tri-, and tetraprotonated guanidine, J. Am. Chem. Soc., 1997, 119, 12929–12933 CrossRef CAS.
- S. M. Chesnut and J. J. Salisbury, The role of UHPLC in pharmaceutical development, J. Sep. Sci., 2007, 30, 1183–1190 CrossRef CAS PubMed.
- S. Fekete, J. Schappler, J. L. Veuthey and D. Guillarme, Current and future trends in UHPLC, TrAC, Trends Anal. Chem., 2014, 63, 2–13 CrossRef CAS.
- A. de Villiers, F. Lestremau, R. Szucs, S. Gélébart, F. David and P. Sandra, Evaluation of ultra performance liquid chromatography. Part I. Possibilities and limitations, J. Chromatogr. A, 2006, 1127, 60–69 CrossRef CAS PubMed.
- M. E. E. Alahi, N. Afsarimanesh, S. Mukhopadhyay, L. Burkitt and P. L. Yu, Highly selective ion imprinted polymer based interdigital sensor for nitrite detection, Proc. Int. Conf. Sens. Technol., ICST, 2016, DOI:10.1109/ICSensT.2016.7796221.
- Y. Wang, J. bo Liu, S. shan Tang, Z. giang Dai and R. fa Jin, Theoretical research on self-assembly system of molecularly imprinted polymers formed by melamine and trifluoromethacrylic acid, Struct. Chem., 2016, 27, 897–905 CrossRef CAS.
- S. Rudd, M. Dalton, P. Buss, A. Treijs, M. Portmann, N. Ktoris and D. Evans, Selective uptake and sensing of nitrate in poly(3,4-ethylenedioxythiophene), Sci. Rep., 2017, 7, 3–8 CrossRef PubMed.
- J. Zhao, H. Guo, J. Li, A. J. Bandodkar and J. A. Rogers, Body-Interfaced Chemical Sensors for Noninvasive Monitoring and Analysis of Biofluids, Trends Chem., 2019, 1, 559–571 CrossRef CAS.
- M. E. E. Alahi, S. C. Mukhopadhyay and L. Burkitt, Imprinted polymer coated impedimetric nitrate sensor for real-time water quality monitoring, Sens. Actuators, B, 2018, 259, 753–761 CrossRef CAS.
- C. Campus, Dissertation proposal for MSc Molecular Medicine TRSSA-Y on EasySpin platform: using the 2004 algorithm in combination with the popular EPR spectra simulation software to simulate all known EPR line shapes of tyrosyl radicals 1 Background, 2020, pp. 1–7 Search PubMed.
- R. Kubota, Y. Sasaki, T. Minamiki and T. Minami, Chemical Sensing Platforms Based on Organic Thin-Film Transistors Functionalized with Artificial Receptors, ACS Sens., 2019, 4, 2571–2587 CrossRef CAS PubMed.
- J. Cai, T. Chen, Y. Xu, S. Wei, W. Huang, R. Liu and J. Liu, A versatile signal-enhanced ECL sensing platform based on molecular imprinting technique via PET-RAFT cross-linking polymerization using bifunctional ruthenium complex as both catalyst and sensing probes, Biosens. Bioelectron., 2019, 124–125, 15–24 CrossRef CAS PubMed.
- A. Azizi and C. S. Bottaro, A critical review of molecularly imprinted polymers for the analysis of organic pollutants in environmental water samples, J. Chromatogr. A, 2019, 460603, DOI:10.1016/j.chroma.2019.460603.
- M. Marć and P. P. Wieczorek, Introduction to MIP synthesis, characteristics and analytical application, Compr. Anal. Chem., 2019, 86, 1–15 Search PubMed.
- F. A. C. Suquila, L. L. G. de Oliveira and C. R. T. Tarley, Restricted access copper imprinted poly(allylthiourea): the role of hydroxyethyl methacrylate (HEMA) and bovine serum albumin (BSA) on the sorptive performance of imprinted polymer, Chem. Eng. J., 2018, 350, 714–728 CrossRef CAS.
- M. A. Ali, L. Dong, J. Dhau, A. Khosla and A. Kaushik, Perspective—Electrochemical Sensors for Soil Quality Assessment, J. Electrochem. Soc., 2020, 167, 037550 CrossRef.
- D. G. Prajapati and B. Kandasubramanian, Progress in the Development of Intrinsically Conducting Polymer Composites as Biosensors, Macromol. Chem. Phys., 2019, 220, 1–26 CrossRef PubMed.
- J. J. Belbruno, Molecularly Imprinted Polymers, Chem. Rev., 2019, 119, 94–119 CrossRef CAS PubMed.
- D. Dechtrirat, B. Sookcharoenpinyo, P. Prajongtat, C. Sriprachuabwong, A. Sanguankiat, A. Tuantranontc and S. Hannongbua, An electrochemical MIP sensor for selective detection of salbutamol based on a graphene/PEDOT:PSS modified screen printed carbon electrode, Anal. Bioanal. Chem., 2019, 55, 206–212 Search PubMed.
- Z. Zeng, Y. Hoshino, A. Rodriguez, H. Yoo and K. J. Shea, Synthetic polymer nanoparticles with antibody-like affinity for a hydrophilic peptide, ACS Nano, 2010, 4, 199–204 CrossRef CAS PubMed.
- N. S. Mazlan, M. M. Ramli, M. M. A. B. Abdullah, D. S. C. Halin, S. S. M. Isa, L. F. A. Talip, N. S. Danial and S. A. Z. Murad, Interdigitated electrodes as impedance and capacitance biosensors: a review, AIP Conf. Proc., 2017, 1885 Search PubMed.
- W. Zhou, M. Vazin, T. Yu, J. Ding and J. Liu, In Vitro Selection of Chromium-Dependent DNAzymes for Sensing Chromium(III) and Chromium(VI), Chem.–Eur. J., 2016, 22, 9835–9840 CrossRef CAS PubMed.
- L. Zhang, X. Z. Cheng, L. Kuang, A. Z. Xu, R. P. Liang and J. D. Qiu, Simple and highly selective detection of arsenite based on the assembly-induced fluorescence enhancement of DNA quantum dots, Biosens. Bioelectron., 2017, 94, 701–706 CrossRef CAS PubMed.
- C. Malitesta, S. Di Masi and E. Mazzotta, From electrochemical biosensors to biomimetic sensors based on molecularly imprinted polymers in environmental determination of heavy metals, Front. Chem., 2017, 5, 1–6 Search PubMed.
- S. Shahim, R. Sukesan, I. Sarangadharan and Y. L. Wang, Multiplexed Ultra-Sensitive Detection of Cr(III) and Cr(VI) Ion by FET Sensor Array in a Liquid Medium, Sensors, 2019, 19, 5–12 CrossRef PubMed.
- A. O. Idris, E. O. Oseghe, T. A. M. Msagati, A. T. Kuvarega, U. Feleni and B. Mamba, Graphitic carbon nitride: a highly electroactive nanomaterial for environmental and clinical sensing, Sensors, 2020, 20, 1–28 CrossRef PubMed.
- N. Ishak, M. N. Ahmad, A. M. Nasir, S. F. Kamaruddin, A. K. M. S. Islam and M. M. Ariffin, Theoretical and experimental studies of ion imprinted polymer for nitrate detection, Polym. Sci., Ser. A, 2017, 59, 649–659 CrossRef CAS.
- M. A. Beluomini, J. L. Silva, A. C. de Sá, E. Buffon, T. C. Pereira and N. R. Stradiotto, Electrochemical sensors based on molecularly imprinted polymer on nanostructured carbon materials: a review, J. Electroanal. Chem., 2019, 840, 343–366 CrossRef CAS.
- B. Mattiasson, Biosensors and Molecular Imprinting. Biosensors and Molecular Imprinting, 2018, DOI:10.3390/books978-3-03842-563-2.
- L. Xie, N. Xiao, L. Li, X. Xie and Y. Li, An investigation of the intermolecular interactions and recognition properties of molecular imprinted polymers for deltamethrin through computational strategies, Polymers, 2019, 11, 2–11 Search PubMed.
- E. Abdollahi, M. Abdouss, M. Salami-Kalajahi and A. Mohammadi, Molecular Recognition Ability of Molecularly Imprinted Polymer Nano- and Micro-Particles by Reversible Addition-Fragmentation Chain Transfer Polymerization, Polym. Rev., 2016, 56, 557–583 CrossRef CAS.
- D. Liang, Y. Wang, S. Li, Y. Li, M. Zhang, Y. Li, W. Tian, J. Liu, S. Tang, B. Li and R. Jin, Study on dicyandiamide-imprinted polymers with computer-aided design, Int. J. Mol. Sci., 2016, 17, 1–11 Search PubMed.
- L. Chen, X. Wang, W. Lu, X. Wu and J. Li, Molecular imprinting: perspectives and applications, Chem. Soc. Rev., 2016, 45, 2137–2211 RSC.
- D. L. Huang, R. Z. Wang, Y. G. Liu, G. M. Zeng, C. Lai, P. Xu, B. A. Lu, J. J. Xu, C. Wang and C. Huang, Application of molecularly imprinted polymers in wastewater treatment: a review, Environ. Sci. Pollut. Res., 2014, 22, 963–977 CrossRef PubMed.
- Z. Li, M. Day, J. Ding and K. Faid, Synthesis and characterization of functional methacrylate copolymers and their application in molecular imprinting, Macromolecules, 2005, 38, 2620–2625 CrossRef CAS.
- E. N. Ndunda, Molecularly imprinted polymers—a closer look at the control polymer used in determining the imprinting effect: a mini review, J. Mol. Recognit., 2020, 1–11, DOI:10.1002/jmr.2855.
- A. Nika, P. Oikonomou, T. Manouras, P. Argitis, M. Vamvakaki, M. Sanopoulou, I. Raptis and M. Chatzichristidi, Reversible chemocapacitor system based on PDMAEMA polymers for fast sensing of VOCs mixtures, Microelectron. Eng., 2020, 227, 111304 CrossRef CAS.
- H. Zhang, Molecularly Imprinted Nanoparticles for Biomedical Applications, Adv. Mater., 2020, 32, 1–17 Search PubMed.
- R. Viveiros, S. Rebocho and T. Casimiro, Green strategies for molecularly imprinted polymer development, Polymers, 2018, 10, 2–5 CrossRef PubMed.
- F. Cui, Z. Zhou and H. S. Zhou, Molecularly imprinted polymers and surface imprinted polymers based electrochemical biosensor for infectious diseases, Sensors, 2020, 20, 2–9 Search PubMed.
- T. Minamiki, S. Tokito and T. Minami, Fabrication of a flexible biosensor based on an organic field-effect transistor for lactate detection, Anal. Sci., 2019, 35, 103–106 CrossRef CAS PubMed.
- T. Minamiki, Y. Ichikawa and R. Kurita, The power of assemblies at interfaces: nanosensor platforms based on synthetic receptor membranes, Sensors, 2020, 20, 1–22 CrossRef PubMed.
- T. Minami, Y. Sasaki, T. Minamiki, S. Wakida, R. Kurita, O. Niwa and S. Tokito, Selective nitrate detection by an enzymatic sensor based on an extended-gate type organic field-effect transistor, Biosens. Bioelectron., 2016, 81, 87–91 CrossRef CAS PubMed.
- A. J. Lang and S. Vyazovkin, Ammonium Nitrate-Polymer Glasses: A New Concept for Phase and Thermal Stabilization of Ammonium Nitrate, J. Phys. Chem. B, 2008, 112, 11236–11243 CrossRef CAS PubMed.
- C. Rossetti, O. G. Ore, B. Sellergren, T. G. Halvorsen and L. Reubsaet, Exploring the peptide retention mechanism in molecularly imprinted polymers, Anal. Bioanal. Chem., 2017, 409(24), 5631–5643 CrossRef CAS PubMed.
- H. Y. Lin, J. L. Thomas and M. H. Lee, Translational applications of molecularly imprinted polymer-based electrochemical sensors, in Handbook of Molecular Imprinting: Advanced Sensor Applications, Pan Stanford Publishing Pte. Ltd., 2012, pp. 119–139, DOI:10.1201/b13062-5.
- L. J. Schwarz, M. K. Potdar, B. Danylec, R. I. Boysen and M. T. W. Hearn, Microwave-assisted synthesis of resveratrol imprinted polymers with enhanced selectivity, Anal. Methods, 2015, 7, 150–154 RSC.
- Y. Shao, Y. Ying and J. Ping, Recent advances in solid-contact ion-selective electrodes: functional materials, transduction mechanisms, and development trends, Chem. Soc. Rev., 2020, 49, 4405–4465 RSC.
- J. B. Liu, G. Y. Wang, S. S. Tang, Q. Gao, D. D. Liang and R. F. Jin, Theoretical and experimental research on self-assembly system of molecularly imprinted polymers formed via chloramphenicol and methacrylic acid, J. Sep. Sci., 2019, 42, 769–777 CAS.
- M. S. Khan and S. Pal, Quantum mechanical studies on dioxin-imprinted polymer precursor composites: fundamental insights to enhance the binding strength and selectivity of biomarkers, J. Mol. Recognit., 2018, 31, 1–10 CrossRef PubMed.
- S. Singal, A. K. Srivastava and V. K. T. Rajesh, Electrochemical Impedance Analysis of Biofunctionalized Conducting Polymer-Modified Graphene-CNTs Nanocomposite for Protein Detection, Nano-Micro Lett., 2017, 9, 2–7 CrossRef PubMed.
- G. WULFF, Molecular Imprinting, Annals of the New York Academy of Sciences, 1984, vol. 434 Search PubMed.
- N. Afsarimanesh, M. E. E. Alahi, S. C. Mukhopadhyay and M. Kruger, Development of IoT-Based Impedometric Biosensor for Point-of-Care Monitoring of Bone Loss, IEEE J. Emerg. Sel. Top. Circuits Syst., 2018, 8, 211–220 Search PubMed.
- M. J. Whitcombe, I. Chianella, L. Larcombe, S. A. Piletsky, J. Noble, R. Porter and A. Horgan, The rational development of molecularly imprinted polymer-based sensors for protein detection, Chem. Soc. Rev., 2011, 40, 1547–1571 RSC.
- F. Canfarotta, J. Czulak, A. Guerreiro, A. Garcia Cruz, S. Piletsky, G. E. Bergdahl, M. Hedström and B. Mattiasson, A novel capacitive sensor based on molecularly imprinted nanoparticles as recognition elements, Biosens. Bioelectron., 2018, 120, 108–114 CrossRef CAS PubMed.
- T. L. Panasyuk, V. M. Mirsky, S. A. Piletsky and O. S. Wolfbeis, Electropolymerized molecularly imprinted polymers as receptor layers in capacitive chemical sensors, Anal. Chem., 1999, 71, 4609–4613 CrossRef CAS.
- O. S. Ahmad, T. S. Bedwell, C. Esen, A. Garcia-Cruz and S. A. Piletsky, Molecularly Imprinted Polymers in Electrochemical and Optical Sensors, Trends Biotechnol., 2019, 37, 294–309 CrossRef CAS PubMed.
- H. S. Wang, M. Song and T. J. Hang, Functional Interfaces Constructed by Controlled/Living Radical Polymerization for Analytical Chemistry, ACS Appl. Mater. Interfaces, 2016, 8, 2881–2898 CrossRef CAS PubMed.
- H. H. Deng, K. Y. Huang, M. J. Zhang, Z. Y. Zou, Y. Y. Xu, H. P. Peng, W. Chen and G. L. Hong, Sensitive and selective nitrite assay based on fluorescent gold nanoclusters and Fe2+/Fe3+ redox reaction, Food Chem., 2020, 317, 1–6 CrossRef PubMed.
- H. Niu, Y. Yang and H. Zhang, Efficient one-pot synthesis of hydrophilic and fluorescent molecularly imprinted polymer nanoparticles for direct drug quantification in real biological samples, Biosens. Bioelectron., 2015, 74, 440–446 CrossRef CAS PubMed.
- G. Vladislav, Computer – Aided Design of Cefotaxime-imprinted Polymer, 2019 1st Int. Conf. Control Syst. Math. Model. Autom. Energy Effic., 2019, pp. 279–282 Search PubMed.
- T. A. Sales and T. C. Ramalho, Computational design of synthetic receptors for drug detection: interaction between molecularly imprinted polymers and MDMA (3,4-methylenedioxymethamphetamine), Theor. Chem. Acc., 2020, 139, 2–10 Search PubMed.
- M. Resmini, Molecularly imprinted polymers as biomimetic catalysts, Anal. Bioanal. Chem., 2012, 402, 3021–3026 CrossRef CAS PubMed.
- L. He, B. Cui, J. Liu, Y. Song, M. Wang, D. Peng and Z. Zhang, Novel electrochemical biosensor based on core-shell nanostructured composite of hollow carbon spheres and polyaniline for sensitively detecting malathion, Sens. Actuators, B, 2018, 258, 813–821 CrossRef CAS.
- S. Science, Recent advances and applications of molecularly imprinted polymers in solid – phase extraction for real sample analysis, Anal. Bioanal. Chem., 2020, 1–117 Search PubMed.
- T. Nawaz, M. Ahmad, J. Yu, S. Wang and T. Wei, The biomimetic detection of progesterone by novel bifunctional group monomer based molecularly imprinted polymers prepared in UV light, New J. Chem., 2020, 44, 6992–7000 RSC.
- J. Hu and S. Liu, Engineering responsive polymer building blocks with host-guest molecular recognition for functional applications, Acc. Chem. Res., 2014, 47, 2084–2095 CrossRef CAS PubMed.
- L. P. Datta, D. De, U. Ghosh and T. K. Das, RAFT derived fatty acid based stimuli responsive fluorescent block copolymers as DNA sensor and cargo delivery agent, Polymer, 2018, 138, 103–112 CrossRef CAS.
- O. S. Wolfbeis, Springer Series on Chemical Sensors and Biosensors: Method and Applications, Springer, 2006, vol. 4 Search PubMed.
- R. A. Potyrailo, Polymeric sensor materials: Toward an alliance of combinatorial and rational design tools?, Angew. Chem., Int. Ed., 2006, 45, 702–723 CrossRef CAS PubMed.
- Y. H. Ngo, M. Brothers, J. A. Martin, C. C. Grigsby, K. Fullerton, R. R. Naik and S. S. Kim, Chemically Enhanced Polymer-Coated Carbon Nanotube Electronic Gas Sensor for Isopropyl Alcohol Detection, ACS Omega, 2018, 3, 6230–6236 CrossRef CAS PubMed.
- N. El Alami El Hassani, E. Llobet, L. M. Popescu, M. Ghita, B. Bouchikhi and N. El Bari, Development of a highly sensitive and selective molecularly imprinted electrochemical sensor for sulfaguanidine detection in honey samples, J. Electroanal. Chem., 2018, 823, 647–655 CrossRef CAS.
- J. Massah and K. Asefpour Vakilian, An intelligent portable biosensor for fast and accurate nitrate determination using cyclic voltammetry, Biosyst. Eng., 2019, 177, 49–58 CrossRef.
- M. S. Khan, K. Dighe, Z. Wang, I. Srivastava, E. Daza, A. S. Schwartz-Duval, J. Ghannam, S. K. Mishra and D. Pan, Detection of prostate specific antigen (PSA) in human saliva using an ultra-sensitive nanocomposite of graphene nanoplatelets with diblock-: co-polymers and Au electrodes, Analyst, 2018, 143, 1094–1103 RSC.
- G. Díaz-Díaz, D. Antuña-Jiménez, M. C. Blanco-López, J. Lobo-Castañón, A. J. Miranda-Ordieres and P. Tuñón-Blanco, New materials for analytical biomimetic assays based on affinity and catalytic receptors prepared by molecular imprinting, TrAC, Trends Anal. Chem., 2012, 33, 68–80 CrossRef.
- J. Wackerlig and R. Schirhagl, Applications of Molecularly Imprinted Polymer Nanoparticles and Their Advances toward Industrial Use: A Review, Anal. Chem., 2016, 88, 250–261 CrossRef CAS PubMed.
- M. Arvand and F. Alirezanejad, Sulfamethoxazole-Imprinted Polymeric Receptor as Ionophore for Potentiometric Transduction, Electroanalysis, 2011, 23, 1948–1957 CrossRef CAS.
- A. Kidakova, J. Reut, J. Rappich, A. Öpik and V. Syritski, Preparation of a surface-grafted protein-selective polymer film by combined use of controlled/living radical photopolymerization and microcontact imprinting, React. Funct. Polym., 2018, 125, 47–56 CrossRef CAS.
- B. Yameen and A. Farrukh, Polymer brushes: Promises and challenges, Chem.–Asian J., 2013, 8, 1736–1753 CrossRef CAS PubMed.
- H. Essousi, H. Barhoumi, M. Bibani, N. Ktari, F. Wendler, A. Al-Hamry and O. Kanoun, Ion-imprinted electrochemical sensor based on copper nanoparticles-polyaniline matrix for nitrate detection, J. Sens., 2019, 2–12 Search PubMed.
- G. Hommes, C. A. Gasser, C. B. C. Howald, R. Goers, D. Schlosser, P. Shahgaldian and P. F.-X. Corvini, Production of a robust nanobiocatalyst for municipal wastewater treatment, Bioresour. Technol., 2012, 115, 8–15 CrossRef CAS PubMed.
- G. Sharma and B. Kandasubramanian, Molecularly Imprinted Polymers for Selective Recognition and Extraction of Heavy Metal Ions and Toxic Dyes, J. Chem. Eng. Data, 2020, 65, 396–418 CrossRef CAS.
- X. Huang and S. Li, Rationally designing molecularly imprinted polymers toward a highly specific recognition by using a stoichiometric molecular self-assembly, J. Inorg. Organomet. Polym. Mater., 2008, 18, 277–283 CrossRef CAS.
- M. M. Moein, A. Abdel-Rehim and M. Abdel-Rehim, Recent applications of molecularly imprinted sol-gel methodology in sample preparation, Molecules, 2019, 24, 1–12 CrossRef PubMed.
- Y. Zheng and A. Wang, Nitrate adsorption using Poly(dimethyl diallyl ammonium chloride)/polyacrylamide hydrogel, J. Chem. Eng. Data, 2010, 55, 3494–3500 CrossRef CAS.
- Y. Liang, H. Ma, W. Zhang, P. Fu, M. Liu, X. Qiao and X. Pang, The Size Effect of Semiconductor Quantum Dots (QDs) as Photocatalysts on PET-RAFT Polymerization, Polym. Chem., 2020, 11, 4961–4967 RSC.
- Y. Liu, X. Hu, M. Meng, Z. Liu, L. Ni, X. Meng and J. Qiu, RAFT-mediated microemulsion polymerization to synthesize a novel high-performance graphene oxide3-based cadmium imprinted polymer, Chem. Eng. J., 2016, 302, 609–618 CrossRef CAS.
- C. L. Yuana, C. P. Chang and Y. Song, Hazardous industrial gases identified using a novel polymer/MWNT composite resistance sensor array, Mater. Sci. Eng., B, 2011, 176, 821–829 CrossRef CAS.
- A. H. Kamel, F. T. C. Moreira, T. S. R. Rebelo and G. F. S. Maria, Molecularly-imprinted materials for potentiometric transduction: application to the antibiotic enrofloxacin, Anal. Lett., 2011, 44, 2107–2123 CrossRef CAS.
- R. Msaadi, G. Yilmaz, A. Allushi, S. Hamadi, S. Ammar, M. M. Chehimi and Y. Yagci, Highly selective copper ion imprinted clay/polymer nanocomposites prepared by visible light initiated radical photopolymerization, Polymers, 2019, 11(286), 1–16 Search PubMed.
- J. Liu, W. Zhao, S. Tang and R. Jin, Theoretical Design and Adsorption Properties of Molecularly Imprinted Polymers Obtained from Chloramphenicol and Acrylamide, Chem. Res. Chin. Univ., 2019, 36, 915–920 CrossRef.
- T. Beduk, A. Ait Lahcen, N. Tashkandi and K. N. Salama, One-step electrosynthesized molecularly imprinted polymer on laser scribed graphene bisphenol a sensor, Sens. Actuators, B, 2020, 314, 2–8 CrossRef.
- T. Gan, J. Li, A. Zhao, J. Xu, D. Zheng, H. Wang and Y. Liu, Detection of theophylline using molecularly imprinted mesoporous silica spheres, Food Chem., 2018, 268, 1–8 CrossRef CAS PubMed.
- L. Yang, W. Wei, J. Xia and H. Tao, Artificial receptor layer for herbicide detection based on electrosynthesized molecular imprinting technique and capacitive transduction, Anal. Lett., 2004, 37, 2303–2319 CrossRef CAS.
- J. Lv, D. Chen, Y. Du, T. Wang, X. Zhang, Y. Li, L. Zhang, Y. Wang, R. Jordan and Y. Fu, Visual Detection of Thiocyanate Based on Fabry-Perot Etalons with a Responsive Polymer Brush as the Transducer, ACS Sens., 2020, 5, 303–307 CrossRef CAS PubMed.
- A. Diouf, N. El Bari and B. Bouchikhi, A novel electrochemical sensor based on ion imprinted polymer and gold nanomaterials for nitrite ion analysis in exhaled breath condensate, Talanta, 2020, 209, 120577 CrossRef CAS PubMed.
- J. Y. He and M. Lu, Photoinduced electron transfer-reversible addition-fragmentation chain transfer (PET-RAFT) polymerization of acrylonitrile in miniemulsion, J. Macromol. Sci., Part A: Pure Appl.Chem., 2019, 56, 443–449 CrossRef CAS.
- C. Gonzato, M. Courty, P. Pasetto and K. Haupt, Magnetic molecularly imprinted polymer nanocomposites via surface-initiated RAFT polymerization, Adv. Funct. Mater., 2011, 21, 3947–3953 CrossRef CAS.
- R. Yuan Song, X. Ling Hu, P. Guan, J. Li, L. Wei Qian and Q. Li Wang, Synthesis of glutathione imprinted polymer particles via controlled living radical precipitation polymerization, Chin. J. Polym. Sci., 2015, 33, 404–415 CrossRef.
- E. Elacqua, A. Croom, K. B. Manning, S. K. Pomarico, D. Lye, L. Young and M. Weck, Supramolecular Diblock Copolymers Featuring Well-defined Telechelic Building Blocks, Angew. Chem., Int. Ed., 2016, 55, 15873–15878 CrossRef CAS PubMed.
- W. F. Kuan, R. Remy, M. E. Mackay and T. H. Epps, Controlled ionic conductivity via tapered block polymer electrolytes, RSC Adv., 2015, 5, 12597–12604 RSC.
- L. Su, X. Guo and S. Han, Preparation and evaluation of vanillin molecularly imprinted polymer microspheres by reversible addition-fragmentation chain transfer precipitation polymerization, Anal. Methods, 2014, 6, 2512–2517 RSC.
- Y. Wang, S. Q. Jiao, X. L. Chen and T. X. Wei, An efficient grafting technique for producing molecularly imprinted film: via reversible addition-fragmentation chain transfer polymerization, Anal. Methods, 2017, 9, 5356–5364 RSC.
- K. P. McClelland, T. D. Clemons, S. I. Stupp and E. A. Weiss, Semiconductor Quantum Dots Are Efficient and Recyclable Photocatalysts for Aqueous PET-RAFT Polymerization, ACS Macro Lett., 2020, 9, 7–13 CrossRef CAS.
- A. Taheri Kal-Kashvandi, M. M. Heravi, S. Ahmadi and T. Hosseinnejad, Copper Nanoparticles in Polyvinyl Alcohol–Acrylic Acid Matrix: An Efficient Heterogeneous Catalyst for the Regioselective Synthesis of 1,4-Disubstituted 1,2,3-Triazoles via Click Reaction, J. Inorg. Organomet. Polym. Mater., 2018, 28, 1457–1467 CrossRef CAS.
- J. Zhang and Y. Han, Active and responsive polymer surfaces, Chem. Soc. Rev., 2010, 39, 676–693 RSC.
- Y. Zheng, H. Cao, B. Newland, Y. Dong, A. Pandit and W. Wang, 3D single cyclized polymer chain structure from controlled polymerization of multi-vinyl monomers: Beyond flory-stockmayer theory, J. Am. Chem. Soc., 2011, 133, 13130–13137 CrossRef CAS PubMed.
- S. Rahimi-Razin, V. Haddadi-Asl, M. Salami-Kalajahi, F. Behboodi-Sadabad and H. Roghani-Mamaqani, Matrix-grafted multiwalled carbon nanotubes/poly(methyl methacrylate) nanocomposites synthesized by in situ RAFT polymerization: a kinetic study, Int. J. Chem. Kinet., 2012, 44, 555–569 CrossRef CAS.
- P. Ganjeh-Anzabi, V. Hadadi-Asl, M. Salami-Kaljahi and H. Roghani-Mamaqani, A new approach for Monte Carlo simulation of RAFT polymerization, Iran. J. Chem. Chem. Eng., 2012, 31, 75–84 CAS.
- C. Boyer, J. Liu, L. Wong, M. Tippett, V. Bulmus and T. P. Davis, Stability and Utility of Pyridyl Disulfide Functionality in RAFT and Conventional Radical Polymerizations, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7207–7224 CrossRef CAS.
- H. Zhang, Controlled/“living” radical precipitation polymerization: a versatile polymerization technique for advanced functional polymers, Eur. Polym. J., 2013, 49, 579–600 CrossRef CAS.
- X. Li, M. Zhou, M. Turson, S. Lin, P. Jiang and X. Dong, Preparation of clenbuterol imprinted monolithic polymer with hydrophilic outer layers by reversible addition-fragmentation chain transfer radical polymerization and its application in the clenbuterol determination from human serum by on-line solid-phase extraction/HPLC analysis, Analyst, 2013, 138(10), 3066–3074 RSC.
- A. Gómez-Caballero, N. Unceta, M. A. Goicolea and R. J. Barrio, Plastic Receptors Developed by Imprinting Technology as Smart Polymers Imitating Natural Behavior, in Reactive and Functional Polymers, ed. T. J. Gutiérrez, Springer, Cham, 2021, vol. 3, DOI:10.1007/978-3-030-50457-1_5.
- Q. Zhu, X. Li, Y. Xiao, Y. Xiong, S. Wang, C. Xu, J. Zhang and X. Wu, Synthesis of Molecularly Imprinted Polymer via Visible Light Activated RAFT Polymerization in Aqueous Media at Room Temperature for Highly Selective Electrochemical Assay of Glucose, Macromol. Chem. Phys., 2017, 218, 1–8 CrossRef.
- G. Gody, T. Maschmeyer and P. B. Zetterlund, Polymerization for Synthesis of Polymer of High Livingness at Full, Macromolecules, 2014, 47, 639–649 CrossRef CAS.
- E. Abdollahi, A. Khalafi-Nezhad, A. Mohammadi, M. Abdouss and M. Salami-Kalajahi, Synthesis of new molecularly imprinted polymer via reversible addition fragmentation transfer polymerization as a drug delivery system, Polymer, 2018, 143, 245–257 CrossRef CAS.
- N. Abu Samah, N. A. Mat Rosli, A. H. Abdul Manap, Y. F. Abdul Aziz and M. Mohd Yusoff, Synthesis & characterization of ion imprinted polymer for arsenic removal from water: a value addition to the groundwater resources, Chem. Eng. J., 2020, 394, 2–7 CrossRef.
- Y. Rayanasukha, S. Pratontep, S. Porntheeraphat, W. Bunjongpru and J. Nukeaw, Non-enzymatic urea sensor using molecularly imprinted polymers surface modified based-on ion-sensitive field effect transistor (ISFET), Surf. Coat. Technol., 2016, 306, 147–150 CrossRef CAS.
- Z. Zhou, D. Kong, H. Zhu, N. Wang, Z. Wang, Q. Wang, W. Liu, Q. Li, W. Zhang and Z. Ren, Preparation and adsorption characteristics of an ion-imprinted polymer for fast removal of Ni(II) ions from aqueous solution, J. Hazard. Mater., 2018, 341, 355–364 CrossRef PubMed.
- E. E. Alahi and S. C. Mukhopadhyay, Smart nitrate sensor: internet of things enabled real-time water quality monitoring, 2019 Search PubMed.
- R. K. Jha, M. Wan, C. Jacob and P. K. Guha, Ammonia vapour sensing properties of in situ polymerized conducting PANI-nanofiber/WS2 nanosheet composites, New J. Chem., 2018, 42, 735–745 RSC.
- J. Lu, B. J. Park, B. Kumar, M. Castro, H. J. Choi and J. F. Feller, Polyaniline nanoparticle-carbon nanotube hybrid network vapour sensors with switchable chemo-electrical polarity, Nanotechnology, 2010, 21, 255501 CrossRef PubMed.
- W. Gao, X. Xie and E. Bakker, Direct Potentiometric Sensing of Anion Concentration (Not Activity), ACS Sens., 2020, 5, 313–318 CrossRef CAS PubMed.
- X. Hu, Y. Fan, Y. Zhang, G. Dai, Q. Cai, Y. Cao and C. Guo, Molecularly imprinted polymer coated solid-phase microextraction fiber prepared by surface reversible addition-fragmentation chain transfer polymerization for monitoring of Sudan dyes in chilli tomato sauce and chilli pepper samples, Anal. Chim. Acta, 2012, 731, 40–48 CrossRef CAS PubMed.
- M. R. Hill, R. N. Carmean and B. S. Sumerlin, Expanding the Scope of RAFT Polymerization: Recent Advances and New Horizons, Macromolecules, 2015, 48, 5459–5469 CrossRef CAS.
- E. M. Bomar, G. S. Owens and G. M. Murray, Nitrate ion selective electrode based on ion imprinted poly(N-methylpyrrole), Chemosensors, 2017, 5, 2–5 CrossRef.
- M. A. Ali, H. Jiang, N. K. Mahal, R. J. Weber, R. Kumar, M. J. Castellano and L. Dong, Microfluidic impedimetric sensor for soil nitrate detection using graphene oxide and conductive nanofibers enabled sensing interface, Sens. Actuators, B, 2017, 239, 1289–1299 CrossRef CAS.
- C. Liu, H. Tai, P. Zhang, Z. Yuan, X. Du, G. Xie and Y. Jiang, A high-performance flexible gas sensor based on self-assembled PANI-CeO2 nanocomposite thin film for trace-level NH3 detection at room temperature, Sens. Actuators, B, 2018, 261, 587–597 CrossRef CAS.
- M. E. E. Alahi and S. C. Mukhopadhyay, Detection methods of nitrate in water: a review, Sens. Actuators, A, 2018, 280, 210–221 CrossRef CAS.
- S. Foroughirad, V. Haddadi-Asl, A. Khosravi and M. Salami-Kalajahi, Mater. Today Commun., 2021, 26, 101780 CrossRef CAS.
- S. Foroughirad, V. Haddadi-Asl, A. Khosravi and M. Salami-Kalajahi, Synthesis of magnetic nanoparticles-decorated halloysite nanotubes/poly([2-(acryloyloxy)ethyl]trimethylammonium chloride) hybrid nanoparticles for removal of Sunset Yellow from water, J. Polym. Res., 2020, 27, 2–8 CrossRef.
- S. Foroughirad, V. Haddadi-Asl, A. Khosravi and M. Salami-Kalajahi, Polym. Adv. Technol., 2020, 32, 803–814 CrossRef.
- R. Wang, Y. Cui, F. Hu, W. Liu, Q. Du, Y. Zhang, J. Zha, T. Huang, M. Fizir and H. He, J. Chromatogr. A, 2019, 1591, 62–70 CrossRef CAS PubMed.
- A. Azizi, F. Shahhoseini and C. Bottaro, J. Chromatogr. A, 2020, 1610, 460534 CrossRef CAS PubMed.
- S. Xu, J. Li and L. Chen, J. Mater. Chem., 2011, 21, 4346 RSC.
- S. Xu, J. Li and L. Chen, Talanta, 2011, 85, 282–289 CrossRef CAS PubMed.
- J. Li, R. Dong, X. Wang, H. Xiong, S. Xu, D. Shen, X. Song and L. Chen, RSC Adv., 2015, 5, 10611–10618 RSC.
- X. Wu, X. Wang, W. Lu, X. Wang, J. Li, H. You, H. Xiong and L. Chen, J. Chromatogr. A, 2016, 1435, 30–38 CrossRef CAS PubMed.
- J. Li, Y. Zhou, Z. Sun, T. Cai, X. Wang, S. Zhao, H. Liu and B. Gong, J. Chromatogr. A, 2020, 1626, 461364 CrossRef CAS PubMed.
- M. Arabi, A. Ostovan, J. Li, X. Wang, Z. Zhang, J. Choo and L. Chen, Adv. Mater., 2021, 33, 2100543 CrossRef CAS PubMed.
- Z. Song, J. Li, W. Lu, B. Li, G. Yang, Y. Bi, M. Arabi, X. Wang, J. Ma and L. Chen, TrAC, Trends Anal. Chem., 2022, 146, 116504 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Table S1: RAFT-MIP works for ion detection review, Table S2: monomer and controlled radical polymerization type reported in MIP analytical applications, Table S3: MIP for ion detection review. See DOI: 10.1039/d2ra00232a |
| This journal is © The Royal Society of Chemistry 2022 |