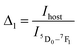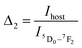Open Access Article
Open Access ArticleSynthesis and optical spectroscopy of Na3Y(VO4)2:Eu3+ phosphors for thermometry and display applications†
Ikhlas Kachoua,
Kamel Saidi a,
Rached Salhibc and
Mohamed Dammak
a,
Rached Salhibc and
Mohamed Dammak *a
*a
aLaboratoire de Physique Appliquée, Groupe de Physique des Matériaux Luminescents, Faculté des Sciences de Sfax, Département de Physique, Université de Sfax, BP 1171, 3018, Sfax, Tunisia. E-mail: madidammak@yahoo.fr; mohamed.dammak@fss.usf.tn
bLaboratory of Advanced Materials, National School of Engineers of Sfax, Sfax University, 3018 Sfax, Tunisia
cUniv Grenoble Alpes, CNRS, Grenoble INP, LMGP, Grenoble France Institute of Engineering Univ, Grenoble, 38000, France
First published on 8th March 2022
Abstract
A new Na3Y(VO4)2:Eu3+ (NYVO:Eu3+) phosphor was prepared using the sol–gel method. X-ray diffraction (XRD) and scanning electron microscopy (SEM) were used to evaluate phase purity and particle size, respectively. The optical properties were investigated by UV-visible absorption, PL, and PLE spectroscopies. The absorption measurements show the formation of the vanadate host by the presence of its characteristic band in the visible region related to VO43− groups. The experimental results show that the NYVO:Eu3+ phosphors exhibit high-brightness and thermally stable emission. Under near-ultraviolet (UV) excitation, both the broadband emission from VO43− groups and the sharp peak emissions from Eu3+ ions are observed. The highest luminescence intensity was achieved for an optimal europium concentration of 15 mol%. The study of the chromaticity parameters of these compounds gives a thermally stable hot emission in the red domain, with a color purity of about 85%, which qualifies the NYVO:Eu3+ compound as a potential phosphor for light-emitting diode (LED) applications. Thermal sensing using NYVO:Eu3+ phosphors are based on monitoring the luminescence intensity ratio between the NYVO host emission and Eu3+ luminescence lines. Notably, the optical thermometry of NYVO:Eu3+ was characterized based on the fluorescence intensity ratio of VO43− and Eu3+ emissions in the 298–440 K range, with maximum absolute and relative sensitivities of 3.4% K−1 and 0.0032 K−1 respectively and a temperature uncertainty of 0.01. NYVO:Eu3+ can then be considered as a potential red phosphor for application in ultraviolet-pumped white light-emitting diodes and as a potential optical thermometer. It provides new possibilities for the design of multifunctional materials for red light-emitting diodes and for non-contact thermometry.
1. Introduction
Rare earth doped phosphors have been widely used in recent years in a variety of applications, ranging from light sources and displays to catalysts and biomarkers.1–5 Contactless optical thermometry has recently received a lot of interest as a novel approach with great detection sensitivity, spatial resolution, and a short acquisition time.6–10 Contactless optical thermometry has recently received a lot of interest as a novel approach with great detection sensitivity, spatial resolution, and a short acquisition time. Hence, diverse optical materials have been used in contactless optical thermometry ranging from MOFs, glass, polycrystals, and their composites. Among them, phosphors are of great interest for this purpose, especially considering their low cost, easy preparation and morphology ranging from nano-to micro range.11,12 Highly sensitive thermometers are in great demand in a range of sectors such as nanomedicine, integrated photonic devices, microfluidics, and nanoelectronics.7,13–15 The luminescence intensity ratio (LIR) temperature measuring strategy is to calibrate the temperature using the relative emission peaks ratio.16 The fluorescence intensities ratio of multiple thermal coupling energy levels in a single light-emitting center is the standard LIR temperature measuring method. This temperature monitoring technique is harmful to getting increased relative and absolute sensitivity at the same time, and it has an impact on signal resolution. This restriction can be surmounted using the LIR between emissions bands associated with distinct active centers. Currently, phosphors doped with lanthanides are widely used in LIR temperature measurement systems. Optical thermometric characterization using the LIR technique with codoped or doped with rare earth and transition metal ions has been reported in several reports: K3Gd(VO4)2:Tb3+/Sm3+17 NaGd(MoO4)2:Tb3+/Pr3+,18 Y3Al5O12:RE/TM (TM = Mn4+, Cr3+, RE = Eu3+, Tb3+, Dy3+),19 Na4Mg(WO4)3:Tb3+/Mn4+,20 and Na3Y(PO4)3:Dy.21 In the LIR technique, the matrix material is usually crucial in determining the temperature-sensing performance. Based on the above assumption, selecting the right luminous host material, especially one with low phonon energies, is essential for ensuring that RE ions have good luminescent characteristics and may be used in a variety of applications.Vanadates are regarded as an excellent luminescent host materials for RE ions that have been widely investigated for solid-state lighting, solar cells, and optical displays, because of their strong absorption in the near-ultraviolet (NUV) region and good intrinsic emissions originating from the electronic transitions of 3TJ − 1A1 (J = 1, 2) in the VO43− group.22 Recently, several researches focused on the double vanadate doped with various lanthanides ions, such as Er3+, Na3Gd(VO4)2:Ho3+/Yb3+,23 Na3Y(VO4)2:Sm3+,24 Na3Y(VO4)2:Tm3+,25 K3Y(VO4)2:Ln (Ln = Eu, Er, Sm, Ho, Tm).26
In this work, Eu3+-doped Na3Y(VO4)2 phosphors are synthesized via a sol gel method. The crystal structure is examined by X-ray diffraction (XRD). The room temperature photoluminescence properties for varied concentrations, the luminescence properties and the chromaticity parameters are investigated. Ultimately, by evaluating the varied temperature responses of the VO43− group and Eu3+ ions, the temperature sensing behaviors of the NYVO phosphors were investigated and their performances are evaluated via the Sa and Sr parameters.
2. Experimental methods
2.1 Synthesis of the phosphor
A series of europium doped NYVO nanocrystals (Na3Y1−xEux(VO4)2) (x = 0, 0.05, 0.1, 0.15 and 0.20) were synthesized by combustion route method-based citrate via low temperature. Analytical grade ammonium vanadate (NH4VO3), sodium hydroxide (NaOH), citric acid monohydrate (C6H8O7) and high purity yttrium nitrate hexahydrate (Y(NO3)3·6H2O (99.9%)) and europium trinitrate pentahydrate (Eu(NO3)3·5H2O (99.9%)) were used as the starting materials. Firstly, all the precursors are placed in an Erlenmeyer and dissolved in ionized water under magnetic stirring at 70 °C. Next, citric acid is added and the color of the solution turns from green to blue. The solution was continued swirling and heated at 80 °C until it hydrolyzed into a sol and then a gel. Finally, the gel will be heated for calcination by annealing at 500 °C. The final products were reground for further characterization.2.2 Characterization
The powder X-ray diffraction (PXRD) patterns of the sample were performed at room temperature using a powder X-ray D8-Advance Bruker diffractometer, with monochromatic copper radiation CuKα1 (λ = 1.5406 Å) a step size of 0.015° from 10° to 75°, running at 45 kV and 40 mA. The phase purity is cheeked using the Fullprof program. The morphologies of the samples were investigated using a Zeiss Supra55VP FEG-SEM scanning electron microscopy (SEM) with an accelerating voltage of 2–8 kV and a Bruker XFlash 5030. UV-vis-NIR absorption was determined using a UV-vis-NIR spectrometer (PerkinElmer Lambda 950). The photoluminescence excitation (PLE) and photoluminescence (PL) spectrum was measured using a Jobin Yvon Fluoromax-4 Spectrofluorometer (Horiba, USA) with a 150 W Xe lamp as a source of excitation. An Easy Life-Horiba instrument was employed to measure the emission lifetime. The light source was a 375 nm diode laser.3. Results and discussion
3.1 XRD analysis
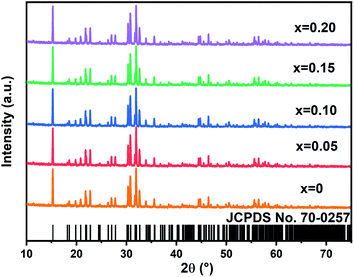
The crystal structure and phase purity of the samples were analyzed using XRD patterns. Fig. 1 shows the X-ray diffraction (XRD) patterns recorded in 10°–75° 2θ range of the nanocrystals NYVO:xEu3+ with x = 0., 0.05, 0.10, 0.15, and 0.20. The samples are shown to have a monoclinic structure based on the NYVO XRD patterns (JCPDS no. 70-0257), with P21/n space group no 14 (Z = 2) and which is isomorphous with Na3Er(VO4)2.27 The X-ray diffraction peaks were quite similar for the undoped and doped NYVO, showing that the phase of the sample remained unchanged after Eu3+ doping. In the NYVO crystal, the Eu3+ ions (r = 0.947 nm) replace the crystallographic position of the Y3+ ions (r = 0.9 nm). The sites of the Y3+ and Eu3+ ions are of C2v symmetry. The point symmetry of the (VO)43− anions is C1. The X-ray diffraction patterns were extremely similar, confirming that the phase prototype remained relatively stable after doping. The comparison with the experimental pattern and the simulated one from the X-ray data confirmed the purity of the synthesized powder. Moreover, the powder X-ray pattern was fitted by Le Bail refinement method. The refinement resulted in an excellent match between the experimental and estimated patterns (Fig. S1(a–e)†), indicating that the produced powder was pure. The obtained unit cell parameters are closer to those obtained from single crystal data. Le Bail refinement parameters of all phosphors are given in Table S1.†3.2 Morphology characterization
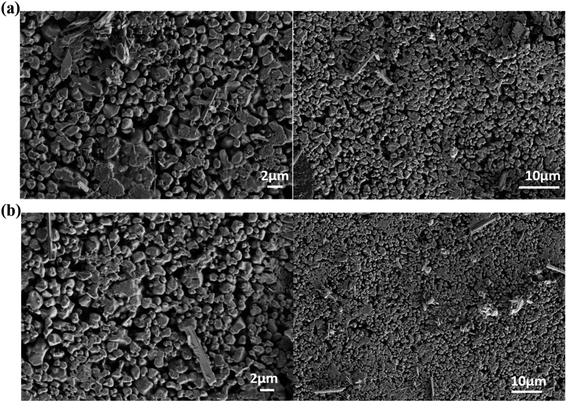
Fig. 2 shows a SEM image of NYVO:xEu3+ with x = 0 and 0.15 phosphor produced at 500 °C. The agglomerated and irregularly shaped particles are seen in the micrographs. The average diameter of the grain size is in the region of 500–800 nm. According to published research, phosphor powder with agglomeration and morphology can reduce the effective light-emitting area and packing density.284. Optical characterizations
4.1 UV-vis absorption
The diffuse reflectance spectrum (Fig. 3a) of NYVO:xEu3+ (x = 0.05, 0.1, 0.15 and 0.2) phosphors in the 200–800 nm range. The large band in 240–370 nm range is attributed to the charge transfer from the 2p oxygen orbital to 3d vanadium orbital of the (VO4)3− group.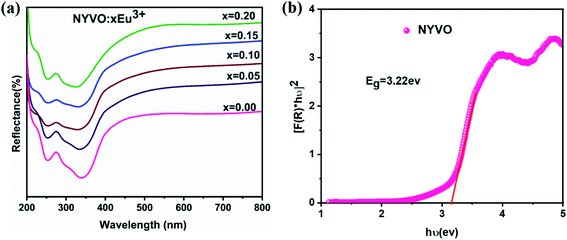 | ||
| Fig. 3 (a) Diffuse reflectance spectra of NYVO:xEu3+(x = 0.00, 0.0 5, 0.10, 0.15 and 0.20) (b) the plot of [F(R∞)hν]2 versus hν of NYVO. | ||
The Kubelka–Munk (KM) theory was used to calculate the band gap of NYVO phosphor using diffuse reflectance spectra. The relation between the diffuse reflectance of the sample (R∞), absorption coefficient (K) and scattering coefficient (S) are related by the KM remission function F(R∞):6,29
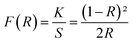 | (1) |
In the parabolic band structure, the band gap Eg and absorption coefficient α of a direct band gap semiconductor are related through the well-known Tauc equation:6
| ((F(R)hν)n) = B (hv − Eg) | (2) |
The plot of [F(R∞)hν]2 versus hν (Fig. 3b) exhibits nonlinear and linear portions, which is the characteristic of direct allowed transition. The nonlinear portion corresponds to a residual absorption involving impurity states and the linear portion characterizes the fundamental absorption. The value of Eg of NYVO was obtained (3.22 eV) by extrapolating the straight line to [F(R∞)hν]2 = 0. The energy gap values hν of the NYVO:xEu3+ (Fig. S2†) were calculated to be: 3.19, 3.18, 3.17 and 3.15 eV for x = 0.5, 0.10, 0.15, and 0.2, respectively. The energy of the gap decreases from 3.22 to 3.15 eV as the doping rates increase. These results also indicate that there is no significant change in the energy of the band gap when the europium ions are incorporated into the NYVO host matrix.
4.2 Photoluminescence properties
To gain a better understanding of the potential of the produced powder as a novel phosphor, a complete and systematic research of the photoluminescence excitation and emission of Eu3+ doped NYVO was carried out.Fig. 4a shows the excitation spectrum of the luminescence (PLE) of NYVO:0.15Eu3+ monitored at λem = 615 nm. The PLE spectrum displays a wide band, in the 250–380 nm range, which can be attributed to the V5+ → O2− charge transfer (CT) transitions of the (VO4)3− group. Other excitation peaks between 350 nm and 550 nm correspond to the intra-configuration 4f–4f electronic transition of Eu3+ ions. These peaks are attributed to the transitions from the 7F0 fundamental level to the 5L7 (382 nm), 5L6 (395 nm), 5D3 (417 nm), 5D2 (465 nm) and 5D1 (525 nm) excited levels.30 The emission spectra of NYVO:0.15Eu3+ (Fig. 4b) upon 395 nm excitation exhibit the typical emission peaks of the Eu3+ ion corresponding to the 5D0 → 7F0, 5D0 → 7F1, 5D0 → 7F2, 5D0 → 7F3, and 5D0 → 7F4 transitions situated at about 579, 593, 615, 648, and 697 nm, respectively.31 The 5D0–7FJ emission lines of Eu3+ ions have the advantage of being able to investigate the local site symmetry of optically active centers.32 The 5D0 → 7F0 transition is an electric-dipole transition, which strictly prohibited by the selection rules. It can only be observed in a Cn, Cnν or Cs type distorted environment. Thus, the presence of this transition on the emission spectrum provides information on the number of non-centrosymmetric sites in which the Eu3+ ion is found.33 The 5D0 → 7F1 transition is a magnetic-dipole transition with an intensity that is generally independent of the Eu3+ ion's surrounding chemical environment. It is dominant if the Eu3+ ions are localized in a centrosymmetric site. While the electric dipole transition 5D0 → 7F2 is hypersensitive to the environment. It will become the dominant transition if the Eu3+ ions maintain a site without a center of inversion. From the emission spectrum (Fig. 4b), it is clear that the 5D0 → 7F2 transition is the strongest emission, indicating that the Eu3+ ions occupy an asymmetric environment in NYVO host.
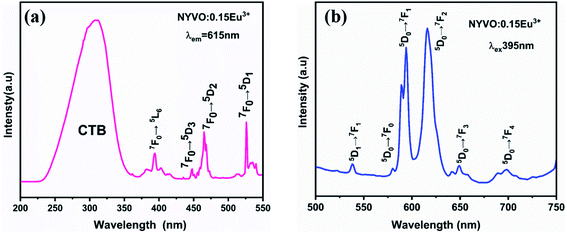 | ||
| Fig. 4 (a) Excitation spectra monitored at λem = 615 nm (b) emission spectra measured at λex = 395 nm excitation of NYVO:0.15Eu3+. | ||
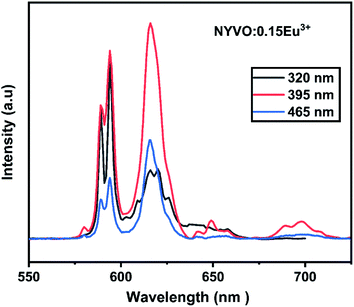
Fig. 5 depicts the emission spectra of NYVO:0.15Eu3+ at various excitation wavelengths (320 nm, 395 nm, and 465 nm). For various excitations, the emission peak positions stay unchanged. From the PL spectra, we observe that the highest intensity of transition 5D0 → 7F2 is obtained upon 395 nm excitation. But when excited at 320 nm, we observe that the 5D0 → 7F1 emission intensity dominates the 5D0 → 7F2 emission which could be explained by the energy transfer between the (VO4)3− vanadate entities and the Eu3+ ions.
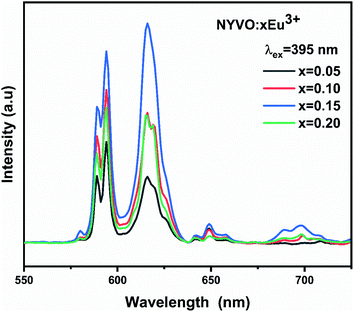
Fig. 6 shows the photoluminescence spectra of the NYVO:xEu3 phosphors with varying Eu3+ doping concentrations. For 0.15 mol Eu3+ dopage concentration, the PL intensity increased to its maximum value and then decreased for higher Eu3+ concentrations, indicating that x = 0.15 is the optimum concentration quenching in the NYVO phosphor.
For 5% and 10% Eu3+ concentrations the 5D0 → 7F1 magnetic-dipole transition dominates the emission spectra of the phosphor under 395 nm excitation which indicates that in this case the Eu3+ are located at non inversion symmetry site. However, for 15% Eu3+ concentration the 5D0 → 7F2 electric-dipole transition dominates the emission spectrum. For higher (20%) Eu3+ concentrations, the two emission intensity peaks decrease due to quenching phenomena obtained by Eu3+–Eu3+ energy transfer.
To explore the reason for concentration quenching, the critical distance (Rc) among doped Eu3+ ions are calculated using the equation presented by Blass.34,35
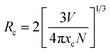 | (3) |
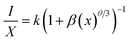 | (4) |
| (I/X) = −(θ/3)L(X) + A | (5) |
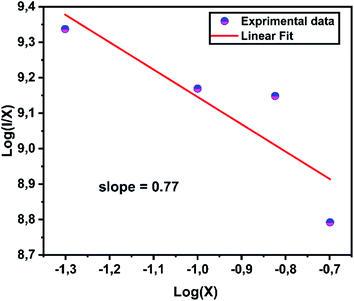
Fig. 7 exhibits the concentration relationship curve (log(I/x) − log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x), with Eu3+ concentrations. We thus obtain an affine line with a slope equal to 0.77 and the value of θ equal to 2.4, which corresponds approximately to the value 3. This indicates that the energy transfer is caused by the dipole–dipole interaction type (D–D).
x), with Eu3+ concentrations. We thus obtain an affine line with a slope equal to 0.77 and the value of θ equal to 2.4, which corresponds approximately to the value 3. This indicates that the energy transfer is caused by the dipole–dipole interaction type (D–D).
The lanthanides-activated phosphors' emission decay curves were also studied, and the correlation of activator concentration on luminescence characteristics and decay duration might strongly support concentration quenching in the energy migration process. Fig. 8 shows the logarithmic intensity emission decay curves of red emissions at 612 nm in NYVO:xEu3+ (x = 0.5, 0.10, 0.15, and 0.2) phosphors under 375 nm excitation. The monitored curves for NYVO:xEu3+ can be well fitted by a single exponential expression, as described below:
| I(t) = A + I0e(−t/τ) | (6) |
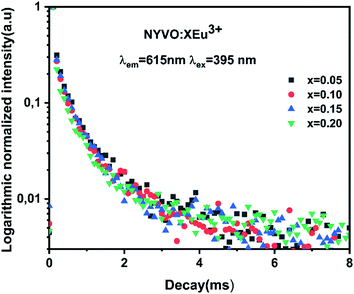 | ||
| Fig. 8 Room temperature fluorescence decay curves of NYVO doped with 0.05, 0.1, 0.15 and 0.2 of Eu3+. | ||
The single decay time suggested the uniform chemical environment of Eu3+ ions in the NYVO host, which was also consistent with the structure analysis that there is one sort of Eu3+ emission center in the phosphor. It was confirmed that the lifetime values increased monotonically and reached a maximum at x = 0.15. The concentration quenching effect is demonstrated in this study with Eu3+-dopant concentrations surpassing 15 mol%. As a result, the decay times of as-synthesized phosphors remain practically unaltered, owing to the minimal energy immigration between Eu3+ ions.
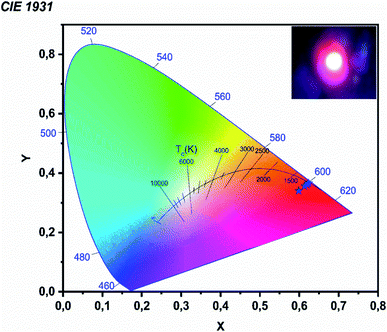
Color coordinates are used to compare phosphors. The chromaticity coordinates of NYVO:xEu3+ phosphors are estimated from the emission spectra and are displayed in Fig. 9. The color purity is an important parameter, which could be calculated by using the following equation, according to the literature.37,38
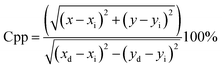 | (7) |
The obtained color purity for various Eu3+ concentrations has been reported in Table 1, with values in the range from 79% to 86%. Then the variation of the concentration causes a change in the chromaticity coordinate. This is mainly due to the variation in the ratios of the emission intensities due to the concentration extinction phenomenon. We compared the chromaticity coordinate and color purity of the ideal sample and the commercial Y2O3:Eu3+ phosphor to assess the quality of the NYVO:xEu3+ phosphor. The calculated values are near to those of the commercial sample Y2O2S:Eu3+ (x = 0.622, y = 0.351).39 The correlated color temperature (CCT) used to define the color temperature of a light source is given by:40
| CCT = −449n3 + 3525n2 − 6823n + 5520.33 | (8) |
| Doping concentration (x) | x | y | CCT | Color purity (%) |
|---|---|---|---|---|
| 0.05 | 0.5985 | 0.3402 | 2156 | 79 |
| 0.10 | 0.6137 | 0.3563 | 2040 | 84 |
| 0.15 | 0.6201 | 0.3607 | 2033 | 86 |
| 0.20 | 0.6237 | 0.3656 | 1998 | 85 |
With n = (x − xe)/(y − ye) represents the epicenter of chromaticity and xe = 0.3320 and ye = 0.1858.
It should be noted that phosphors with CCT values lower than 3200 K are generally considered as hot light sources, while those with higher TCC values at 4000 K are considered to be cold in appearance. In the present work, the TCC values obtained are all less than 3200 K, which indicates that the NYVO:xEu3+ phosphor could be used for the diodes emitting warm white light (LED).
4.3 Thermal stability
Thermal stability is an important feature for lighting applications. A phosphor with high thermal stability is then highly demanded in the application of w-LED.41 It should be noted that high-power LED chips can reach temperatures of 420 K. However, the luminescence intensity reduces significantly at this temperature. Fig. 10a displayed the PL spectra under 395 nm excitation of NYVO:0.15Eu3+ measured at various temperatures so as to determine the thermal stability of the sample. As can be seen, the profile of the PL emissions remain constant as the temperature rises, whereas the intensity of the transition gradually decreases as the temperature rises from 280 K to 440 K, due to thermal quenching. In addition, the response to temperature is different for the (VO4)3− and Eu3+ bands. The (VO4)3− band undergoes a sharp decline in intensity, whereas a slow reduction in intensity is noted for Eu3+ bands, as indicated in the histogram shown in Fig. S3.† At 440 K, the luminescence intensity remains at 77% compared to that obtained at T = 300 K this value is in the same order than that measured for the red commercial phosphorus Y2O3:Eu3+ (80%).42 It can be concluded that our phosphors have good thermal stability.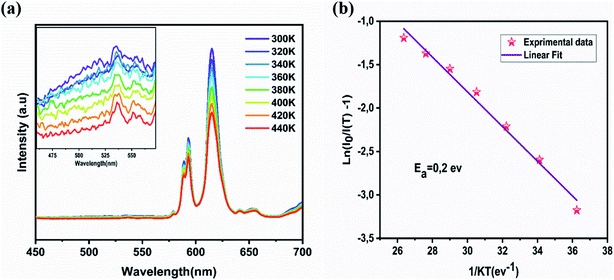 | ||
| Fig. 10 (a) The temperature-dependent PL spectra (b) variations of ln((I0/I) − 1) with 1/T in NYVO:xEu3+. | ||
Nonradiative relaxation causes thermal quenching of luminescence in most cases. The number of excited electrons increases as the temperature rises. The thermal activation process may be shown using the Arrhenius equation.
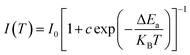 | (9) |
5. Temperature-sensing properties of NYVO:Eu3+
Fig. 11 illustrates the PL spectra of NYVO:0.15Eu3+ at various temperatures under 375 nm excitation. It's clear that rising temperatures resulted in different behavior of Eu3+ and host emission intensities. This kind of variation in the intensity of these optical centers can be exploited for optical thermometry applications based on thermometric parameters Δ. For this, we have used the TeSen calculator which is a numerical program to calculate thermometric parameters.47 Taking this into consideration, we advocated using Δ between the host band and the transition of Eu3+ as a temperature-dependent factor. The Δ approach removes temperature readout errors caused by changes in excitation intensity or detecting system, as well as nonhomogeneous distribution of the probe over the measuring surface, because the intensity ratio between two emission bands is derived from the same measurements.48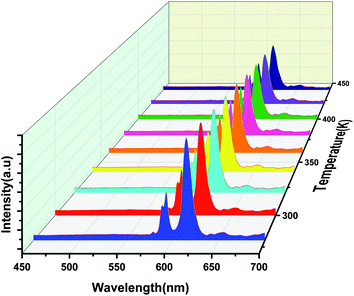 | ||
| Fig. 11 Emission spectra of the NYVO:0.15Eu3+ phosphor for different temperatures under 375 nm excitation. | ||
The LIRs between the vanadate band and the two most intense Eu3+ emission lines: LIR1 (Ihost/I(5D0–7F1)) and LIR2 (Ihost/I(5D0–7F2)) were calculated using TeSen 30 calculator (Fig. 12), developed by Kaczmarek et al..47 For the NYVO:0.15Eu3+ sample, the LIRs ratios showed a monotonic rise along with the temperature decreasing. Here, we examined the LIRs of VO43− and Eu3+ emissions over the 280 K to 440 K and found that it can be approximated by the following equation:
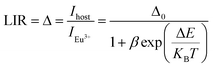 | (10) |
Sensitivity was an important and prominent characteristic for quantitatively determining the suitability of materials as an optical sensor in practical applications. Furthermore, the absolute sensitivity (Sa) and relative sensitivity (Sr) may be calculated using the formula below.15,49,50
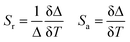 | (11) |
Fig. 13a and b show the variations of absolute sensitivities with temperature. It indicates that Sa increases with temperature up to 400 K and after that decrease. The maximum absolute sensitivity was 0.0033 K−1 for Δ1 (Ihost/I(5D0–7F1)) and 0.00105 K−1 for Δ2 at 400 K respectively.
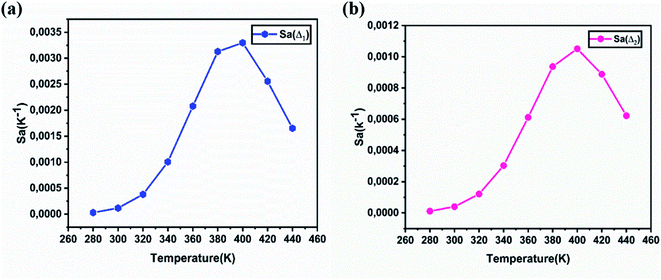 | ||
| Fig. 13 The absolute sensitivity Sa calculated for NYVO:Eu3+ as a function of the temperature, corresponding to Δ1 (a) and to Δ2 (b). | ||
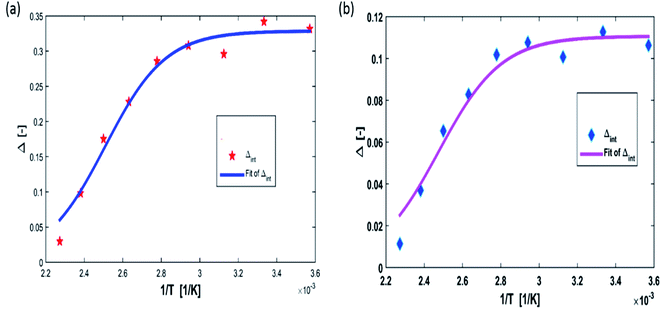
Fig. 14 show the relative sensitivity of NYVO:Eu3+ calculated for Δ1 and Δ2 ratios. As can be observed, increasing the temperature causes an increase in the Sr values. The maximum relative sensitivity is determined to be 2.7% K−1 at 440 K for Δ1 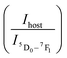 and 2.42% K−1 for Δ2
and 2.42% K−1 for Δ2 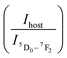 .
.
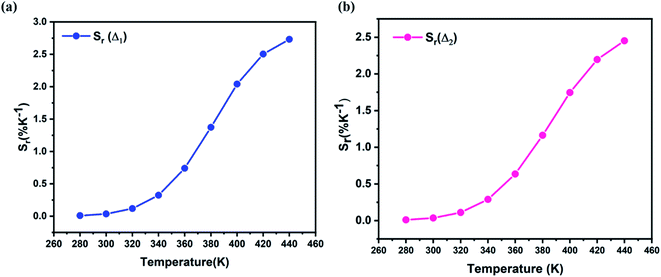 | ||
| Fig. 14 The relative sensitivity Sr values corresponding to (a) Δ1 (b) Δ2 ratios at different temperatures. | ||
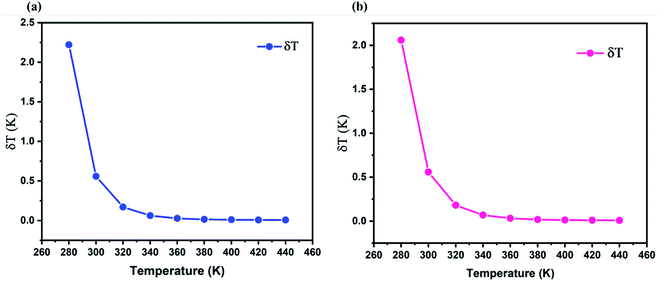
The temperature uncertainty (temperature resolution) (δT) defines the minimal change in temperature that represents a high difference in the examined parameter, which is an essential measure of the quality for a thermometer's performance.51,52 δT was estimated using the first approach using the following formula:53
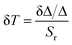 | (12) |
The relative and absolute sensitivities calculated for NYVO:0.15Eu3+ phosphor is compared with those calculated in other hosts (Table 2). NYVO:0.15Eu3+ phosphors can then be employed for optical thermometry applications.
| Compounds | T [K] | λex [nm] | Sr [% K−1] | Sa [K−1] | Ref. |
|---|---|---|---|---|---|
| Ca2NaMg2V3O12:Eu3+ | 303–503![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
335![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
1.686 | 0.0156 | 54 |
| Ba3La(VO4)3:Eu3+ | 298–573 | 320 | 1.77 | 0.0515 | 8 |
| YVO4:Eu3+ 10 at% | 298–473 | 618 | 1.08 | 0.00039 | 55 |
| Ca5Mg4V6O24:Eu3+ | 300–480 | 310 | 1.83 | — | 56 |
| CaEu2(WO4)4:Eu3+ | 300–500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
395![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
1.4 | — | 57 |
| Na3Sc2P3O12:Eu2+, Mn2+ | 293–473 | 340 | 1.556 | — | 58 |
| NaEuF4:Eu3+ | 298–523![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
394 | 0.43 | — | 59 |
| LuAG:Eu3+/Mn4+ | 303–358![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
393 | 0.7 | 0.07 | 60 |
| SnO2:Eu3+ | 298–623 | 250 | 1.83 | 0.0159 | 61 |
| Na3Y(VO4)2:Eu3+ | 298–440 | 2.7 | 0.0033 | This work | |
| 2.4 | 0.0010 | This work |
6. Conclusion
A new NYVO:Eu3+ phosphor was prepared using the sol–gel method. The NYVO mico powder crystallizes in a monoclinic system with particle size around 0.8 μm. The absorption measurements show the formation of the vanadate host by the presence of its characteristic band in the visible region related to VO43− groups, and the energy gap was estimated to be around 3.15 eV. Under near-ultraviolet (UV) excitation, both the broadband emission from VO43− groups and the sharp peak emissions from Eu3+ ions are observed. The highest luminescence intensity was achieved for an optimal europium concentration of 15% mol. The study of the chromaticity parameters gives a thermally stable hot emission in the red domain, with a color purity of about 85%. Eu3+-doped NYVO phosphors were also tested as noncontact ratiometric luminescence thermometers. Notably, the optical thermometry of NYVO:Eu3+ was characterized based on the fluorescence intensity ratio of VO43− and Eu3+ emissions in the 298–440 K range. The best room-temperature thermometric performance with a maximum absolute and relative sensitivities of 3.4% K−1 and 0.0032 K−1 respectively and a temperature uncertainty of 0.01 for the case of LIR2 (Ihost/I(5D0–7F2)). The obtained results make Eu3+-doped NYVO micro crystals as promising candidates for accurate contactless ratiometric temperature sensing and as a potential phosphor for light-emitting diodes (LED) applications.Conflicts of interest
There are no conflicts to declare.References
- S. V. Eliseeva and J.-C. G. Bünzli, Chem. Soc. Rev., 2010, 39, 189–227 RSC.
- K. Binnemans, Chem. Rev., 2009, 109, 4283–4374 CrossRef CAS PubMed.
- J.-C. G. Bünzli and C. Piguet, Chem. Soc. Rev., 2005, 34, 1048 RSC.
- M. K. Hossain, S. Hossain, M. H. Ahmed, M. I. Khan, N. Haque and G. A. Raihan, ACS Appl. Electron. Mater., 2021, 3, 3715–3746 CrossRef CAS.
- K. Saidi and M. Dammak, RSC Adv., 2020, 10, 21867–21875 RSC.
- K. Saidi and M. Dammak, J. Solid State Chem., 2021, 300, 122214 CrossRef CAS.
- E. Céspedes, J. M. Byrne, N. Farrow, S. Moise, V. S. Coker, M. Bencsik, J. R. Lloyd and N. D. Telling, Nanoscale, 2014, 6, 12958–12970 RSC.
- P. Yang, L. Li, Y. Deng, Y. Wang, S. Jiang, X. Luo, G. Xiang, Y. Lu and X. Zhou, Dalton Trans., 2019, 48, 10824–10833 RSC.
- C. D. S. Brites, A. Millán and L. D. Carlos, in Handbook on the Physics and Chemistry of Rare Earths, Elsevier, 2016, vol. 49, pp. 339–427 Search PubMed.
- C. D. S. Brites, P. P. Lima, N. J. O. Silva, A. Millán, V. S. Amaral, F. Palacio and L. D. Carlos, Nanoscale, 2012, 4, 4799 RSC.
- J. Cao, D. Xu, F. Hu, X. Li, W. Chen, L. Chen and H. Guo, J. Eur. Ceram. Soc., 2018, 38, 2753–2758 CrossRef CAS.
- J. Cao, X. Li, Z. Wang, Y. Wei, L. Chen and H. Guo, Sens. Actuators, B, 2016, 224, 507–513 CrossRef CAS.
- D. K. Chatterjee and Z. Yong, Nanomedicine, 2008, 3, 73–82 CrossRef CAS PubMed.
- C. Shen, T. K. Ng, J. T. Leonard, A. Pourhashemi, H. M. Oubei, M. S. Alias, S. Nakamura, S. P. DenBaars, J. S. Speck, A. Y. Alyamani, M. M. Eldesouki and B. S. Ooi, ACS Photonics, 2016, 3, 262–268 CrossRef CAS.
- K. Soler-Carracedo, I. R. Martín, F. Lahoz, H. C. Vasconcelos, A. D. Lozano-Gorrín, L. L. Martín and F. Paz-Buclatin, J. Alloys Compd., 2020, 847, 156541 CrossRef CAS.
- N. M. Bhiri, M. Dammak, M. Aguiló, F. Díaz, J. J. Carvajal and M. C. Pujol, J. Alloys Compd., 2020, 814, 152197 CrossRef CAS.
- Y. Hua and J. S. Yu, J. Mater. Sci. Technol., 2021, 91, 148–159 CrossRef.
- Y. Gao, F. Huang, H. Lin, J. Zhou, J. Xu and Y. Wang, Adv. Funct. Mater., 2016, 26, 3139–3145 CrossRef CAS.
- D. Chen, S. Liu, Y. Zhou, Z. Wan, P. Huang and Z. Ji, J. Mater. Chem. C, 2016, 4, 9044–9051 RSC.
- D. K. Amarasinghe and F. A. Rabuffetti, Inorg. Chem., 2021, 60, 3165–3171 CrossRef CAS PubMed.
- N. Krutyak, D. Spassky, V. Nagirnyi, A. V. Antropov and D. V. Deyneko, Opt. Mater., 2021, 122, 111738 CrossRef.
- F. Ayachi, K. Saidi, W. Chaabani and M. Dammak, J. Lumin., 2021, 240, 118451 CrossRef CAS.
- K. Saidi, M. Dammak, K. Soler-Carracedo and I. R. Martín, J. Alloys Compd., 2022, 891, 161993 CrossRef CAS.
- L. A. Jacob, S. Sisira, K. Thomas, D. Alexander, P. R. Biju, N. V. Unnikrishnan and C. Joseph, J. Solid State Chem., 2019, 280, 120998 CrossRef CAS.
- L. A. Jacob, S. Sisira, K. P. Mani, K. Thomas, D. Alexander, P. R. Biju, N. V. Unnikrishnan and C. Joseph, J. Lumin., 2020, 223, 117169 CrossRef CAS.
- M. M. Kimani, C. D. McMillen and J. W. Kolis, J. Solid State Chem., 2015, 226, 320–325 CrossRef CAS.
- R. Salmon, C. Parent, G. Le Flem and M. Vlasse, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1976, 32, 2799–2802 CrossRef.
- Sk. K. Hussain, H. S. Go, J. J. Han, H. Patnam, G. Nagaraju and J. S. Yu, J. Alloys Compd., 2019, 805, 1271–1281 CrossRef CAS.
- K. Saidi, W. Chaabani and M. Dammak, RSC Adv., 2021, 11, 30926–30936 RSC.
- I. E. Kolesnikov, A. V. Povolotskiy, D. V. Tolstikova, A. A. Manshina and M. D. Mikhailov, J. Phys. D: Appl. Phys., 2015, 48, 075401 CrossRef CAS.
- I. E. Kolesnikov, D. V. Mamonova, E. Lähderanta, A. V. Kurochkin and M. D. Mikhailov, J. Lumin., 2017, 187, 26–32 CrossRef CAS.
- K. Binnemans, Coord. Chem. Rev., 2015, 295, 1–45 CrossRef CAS.
- H. Peng, S. Huang, L. Sun and C. Yan, Phys. Lett. A, 2007, 367, 211–214 CrossRef CAS.
- Q. Liu, M. Zhang, Z. Ye, X. Wang, Q. Zhang and B. Wei, Ceram. Int., 2019, 45, 7661–7666 CrossRef CAS.
- M. Fhoula and M. Dammak, J. Lumin., 2019, 210, 1–6 CrossRef CAS.
- Z. Yahiaoui, M. A. Hassairi, M. Dammak and E. Cavalli, J. Alloys Compd., 2018, 763, 56–61 CrossRef CAS.
- V. Mahalingam and J. Thirumalai, RSC Adv., 2016, 6, 80390–80397 RSC.
- M. Fhoula, T. Koubaa and M. Dammak, Opt. Laser Technol., 2020, 130, 106352 CrossRef CAS.
- Y. Hua, Sk. K. Hussain and J. S. Yu, Ceram. Int., 2019, 45, 18604–18613 CrossRef CAS.
- C. S. McCamy, Color Res. Appl., 1992, 17, 142–144 CrossRef.
- J. Qin, C. Hu, B. Lei, J. Li, Y. Liu, S. Ye and M. Pan, J. Mater. Sci. Technol., 2014, 30, 290–294 CrossRef CAS.
- S. Som, S. Das, S. Dutta, H. G. Visser, M. K. Pandey, P. Kumar, R. K. Dubey and S. K. Sharma, RSC Adv., 2015, 5, 70887–70898 RSC.
- Q. Zhang, X. Wang, X. Ding and Y. Wang, Inorg. Chem., 2017, 56, 6990–6998 CrossRef CAS PubMed.
- S. C. Lal, J. Isuhak Naseemabeevi and S. Ganesanpotti, Mater. Adv., 2021, 2, 1328–1342 RSC.
- J. Liang, S. Zhao, X. Yuan and Z. Li, Opt. Laser Technol., 2018, 101, 451–456 CrossRef CAS.
- J. He, Z. Gao, S. Liu, J. H. Jeong, R. Yu and B. Deng, J. Lumin., 2018, 202, 7–12 CrossRef CAS.
- A. M. Kaczmarek, R. Van Deun and M. K. Kaczmarek, Sens. Actuators, B, 2018, 273, 696–702 CrossRef CAS.
- M. Sekulić, V. Đorđević, Z. Ristić, M. Medić and M. D. Dramićanin, Adv. Opt. Mater., 2018, 6, 1800552 CrossRef.
- S. A. Wade, S. F. Collins and G. W. Baxter, J. Appl. Phys., 2003, 94, 4743–4756 CrossRef CAS.
- K. Maciejewska, A. Bednarkiewicz, A. Meijerink and L. Marciniak, J. Phys. Chem. C, 2021, 125, 2659–2665 CrossRef CAS PubMed.
- J.-C. G. Bünzli, in Handbook on the Physics and Chemistry of Rare Earths, Elsevier, 2016, vol. 50, pp. 141–176 Search PubMed.
- C. D. S. Brites, A. Millán and L. D. Carlos, in Handbook on the Physics and Chemistry of Rare Earths, Elsevier, 2016, vol. 49, pp. 339–427 Search PubMed.
- S. N. Baker, T. M. McCleskey and G. A. Baker, in Ionic Liquids IIIB: Fundamentals, Progress, Challenges, and Opportunities, American Chemical Society, 2005, vol. 902, pp. 171–181 Search PubMed.
- H. Zhou, N. Guo, X. Lü, Y. Ding, L. Wang, R. Ouyang and B. Shao, J. Lumin., 2020, 217, 116758 CrossRef CAS.
- I. E. Kolesnikov, A. A. Kalinichev, M. A. Kurochkin, D. V. Mamonova, E. Yu. Kolesnikov and E. Lähderanta, J. Phys. Chem. C, 2019, 123, 5136–5143 CrossRef CAS.
- N. Zhang, J. Li, J. Wang, R. Shi, L. Chen, A. Zhang and P. Yang, RSC Adv., 2019, 9, 30045–30051 RSC.
- K. W. Meert, V. A. Morozov, A. M. Abakumov, J. Hadermann, D. Poelman and P. F. Smet, Opt Express, 2014, 22, A961–A972 CrossRef CAS PubMed.
- X. Zhang, Z. Zhu, Z. Guo, Z. Sun and Y. Chen, Chem. Eng. J., 2019, 356, 413–422 CrossRef CAS.
- Y. Tian, B. Tian, C. Cui, P. Huang, L. Wang and B. Chen, Opt Express, 2014, 39, 4164–4167 CAS.
- B. Yan, Y. Wei, W. Wang, M. Fu and G. Li, Inorg. Chem. Front., 2021, 8, 746–757 RSC.
- S. Das, S. Som, C.-Y. Yang and C.-H. Lu, Mater. Res. Bull., 2018, 97, 101–108 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d2ra00539e |
| This journal is © The Royal Society of Chemistry 2022 |