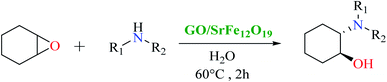Open Access Article
Open Access ArticleSynergistic effect of GO/SrFe12O19 as magnetic hybrid nanocatalyst for regioselective ring-opening of epoxides with amines under eco-friendly conditions†
Mouhsine Laayatiab,
Ayoub Abdelkader Mekkaoui ab,
Lahcen Fkharb,
Mustapha Ait Ali
ab,
Lahcen Fkharb,
Mustapha Ait Ali b,
Hafid Ananec,
Lahoucine Bahsis
b,
Hafid Ananec,
Lahoucine Bahsis cd,
Larbi El Firdoussib and
Soufiane El Houssame
cd,
Larbi El Firdoussib and
Soufiane El Houssame *a
*a
aLaboratoire des Sciences des Matériaux, Mathématiques et Environnement, Université Sultan Moulay Slimane, Faculté Polydisciplinaire de Khouribga, BP 145, Khouribga 25000, Morocco. E-mail: hous_soufiane@hotmail.com; Fax: +212 523 490354; Tel: +212 523 490359
bLaboratoire de Chimie Moléculaire, Equipe de Chimie de Coordination et de Catalyse, Département de Chimie, Faculté des Sciences Semlalia, BP 2390, Marrakech 40001, Morocco
cLaboratoire de Chimie Analytique et Moléculaire, LCAM, Faculté Polydisciplinaire de Safi, Université Cadi Ayyad, Safi 46030, Morocco
dLaboratoire de Chimie de Coordination et d'Analytique (LCCA), Département de Chimie, Faculté des Sciences d'El Jadida, Université Chouaïb Doukkali, El Jadida, Morocco
First published on 11th April 2022
Abstract
Herein, a highly efficient magnetically separable hybrid GO/SrFe12O19 nanocomposite was synthesized via dispersing M-type strontium hexaferrite (SrFe12O19) on graphene oxide (GO) sheets. First, SrFe12O19 nanoparticles (NPs) and GO sheets were prepared via chemical coprecipitation and chemical oxidation of graphite powder, respectively. Chemically reduced GO (rGO) and rGO/SrFe12O19 were also prepared for comparison purposes. Thereafter, the prepared nanostructured materials were explored by XRD, FTIR, FESEM-EDX, BET, and Zetasizer analyses. All the characterizations confirm the nanoscale and the high stability structures of the prepared materials. The prepared hybrid magnetic nanocomposite GO/SrFe12O19 exhibited a high surface area value resulting in a high catalytic activity and selectivity for the epoxide ring-opening with amines in neat water. The use of hybrid GO/SrFe12O19 compared with pure SrFe12O19 and GO sheets is of great interest for using environmentally benign heterogeneous nanocatalysts, for the synthesis of β-amino alcohols, with excellent recyclability under eco-friendly conditions. Moreover, a mechanistic study was performed through density functional theory (DFT) calculations and Parr functions to explain the observed reactivity and selectivity of SrFe-GO catalyst in the epoxide ring-opening reactions.
1. Introduction
Nanotechnology is one of the most important research fields that covers various domains such as biology, medicine, agriculture, physics, chemistry, etc.1–6 Over the last decade, this technology has been the target of fundamental and technological scientific research to develop new classes of nanostructured materials with unique properties and applications.7–12 Among the interesting nanomaterials, hexagonal ferrites represent important classes of magnetic materials in diverse areas of applications such as plastic magnets, permanent magnets, recording media, microwave components, high-frequency devices, and catalysts or photocatalysts.13–16 Since their discovery in the 1950s there has been an increasing degree of interest in the hexagonal ferrites,17 and their synthesis and study deserve a particular interest for R&D researchers.16,18–20However, magnetic nanoparticles (MNPs) have gained the attention of many scientists due to their excellent physical and chemical properties compared to traditional bulk materials, such as superparamagnetism, high surface area, high surface-to-volume ratio, simple separation under external magnetic fields, and high adsorption capacity.21–26 Nowadays, it has been found that the dispersion of magnetic NPs on 2D materials such as graphene sheets is potentially turning into a new research topic due to their improved functionalities. Therefore, their use holds considerable promise for a wide range of applications in catalysis, biomedical fields, and for the removal of contaminants from wastewater.27 Graphene, a single layer of carbon atoms packed in a two-dimensional honeycomb lattice, has a large surface area, open porous structure, flexibility, chemical stability, and very high electrical conductivity, which makes it a good candidate for the construction of graphene-based composite nanomaterials.28–33
In recent years, numerous hybrid composites have been synthesized based on strontium ferrite (SF) and reduced or non-reduced graphene oxide (rGO or GO) materials as heterogeneous catalytic systems. Recently, a nanocomposite based on polypyrrole/SrFe12O19/GO was used for the removal of tartrazine from wastewater.34 However, Aziz et al. have reported the preparation of TiO2-GO supported SrFe12O19 photocatalyst.35 Additionally, J. Luo et al. have described the preparation of RGO/SF/PANI nanocomposites in a three-step synthesis as microwave absorber materials.36 Besides, Zhao et al. produced rGO/SrFe12O19 nanocomposites by a two-step method for microwave absorption applications.37
The catalytic nucleophilic ring-opening of epoxides with amines represents one of the most important and straightforward methods for the preparation of β-amino alcohols as intermediates in the synthesis of a wide range of biologically active natural and synthetic products.38–42 Recently, Y. He et al. have used epoxy ring-opening in a two-step synthesis of dimethyl carbonate by a coupling CO2 cycloaddition and CH3OH transesterification.43,44 On the other hand, β-amino alcohols can be used as chiral ligands in various asymmetric syntheses.45,46 Thus, enantioselective hydrogenation of arylalkyl ketones is possible using Ru(II)-fi-ferrocenyl amino alcohol catalyst.47 It is noteworthy that these compounds have been widely employed for good enantioselectivity in Reformatsky reaction,48 alkynylation of aromatic aldehydes,49 or addition of divinylzinc.50 Moreover, several Lewis or Brønsted acids were used as useful activators for better regioselectivity of epoxides aminolysis.51–57 However, most of the existing methods involve high-cost processes, stoichiometric catalysts, corrosive reagents, and toxic metal ions and solvents. Therefore, it is desirable to develop new green procedures respecting the principles of green chemistry and circular economy.
Recently, we have reported M-type hexaferrite SrFe12O19 (SF) as an efficient heterogeneous bulk catalyst for amino alcohol synthesis.58 To improve the catalytic activity of SrFe12O19, we have herein selected graphene oxide as dispersion of the catalytic active sites of SF due to its intriguing characteristics including high surface area, numerous oxygen-containing functional groups, high hydrophilicity, and good dispersion in water.
In this paper, we report the synthesis of SrFe12O19 nanoparticles using the coprecipitation method, and their combination with graphene oxide is carried out to study the synergistic effect of the hybrid nanocomposite (GO/SrFe12O19) on the catalytic activity of epoxide opening ring by amine to prepare β-aminoalcohols under eco-friendly conditions. Furthermore, GO, rGO, and rGO/SrFe12O19 nanomaterials were synthesized and evaluated for the catalytic epoxide ring-opening.
2. Experimental
2.1. Materials and chemicals
All reagents and solvents were purchased from commercial sources (Sigma Aldrich) and used as received without further purification: SrCl2 (99.99%), FeCl3·6H2O (97%), NaOH (99.99%), graphite, sulfuric acid (98%), potassium permanganate (99.5%), and cetyltrimethylammonium bromide (CTAB).2.2. Catalyst characterization
The X-ray powder diffraction (XRD) analysis was conducted on a D8 Discover Bruker (AXS) using CuKα radiation (λCu = 1.5407 Å). FTIR analyses were recorded on ABB Bomem FTLA2000 in the range of 400–4000 cm−1 using KBr as a mulling agent. Raman spectra were conducted on Raman spectrometer (Confotec MR520), using a diode solid-state laser (618 nm) for irradiation. Microstructural characterizations were performed using a BRUKER (FEI, Quanta FEG 450) Scanning Electron Microscope (SEM) equipped with Energy Dispersive X-ray (EDX) detector. Zeta potential and size distribution were measured using a Malvern Panalytical Zetasizer, after dispersing 0.1 g of nanopowders in 3 mL of distilled water. The surface area of the SrFe12O19 and GO/SrFe12O19 was measured using a surface area analyzer (Micromeritics, ASAP 2010) at 77 K. Samples were degassed at 150 °C for 12 h under nitrogen flow to remove the moisture adsorbed on the solid surface. The mono-point BET method was used to evaluate the specific surface area (SBET). Aliquots samples from the reaction mixture were monitored by Shimadzu gas chromatography (GC) with a flame ionization detector using nitrogen as a carrier gas. GC parameters for capillary columns BP (25 m × 0.25 mm, SGE): injector 250 °C; detector 250 °C; oven 70 °C for 5 min then 3 °C min−1 until 250 °C for 30 min; column pressure 20 kPa; column flow 6.3 mL min−1; linear velocity 53.1 cm s−1; total flow 138 mL min−1. All obtained products were confirmed with an ISQ LT single quadrupole mass spectrometer operating in positive EI mode using a mass scan range of 50 to 400 m/z.2.3. Materials synthesis
2.4. General procedure for the catalytic epoxide ring-opening
The catalytic activity of the synthesized hybrid GO/SrFe12O19 nanocomposite was evaluated for the aminolysis of epoxides. The reaction was performed in a stoichiometric condition in presence of styrene oxide (0.66 mmol) and aniline (0.8 mmol) using GO/SrFe12O19 nanocomposite as catalyst (10 mg) and water as ecological solvent at 60 °C. The progress of the reaction was monitored using gas chromatography (GC). After the completion of the reaction, we observed the formation of foam due to the attack of amino groups at the acid functions of GO. Then, acetone was used to desorb the organic phase from the inorganic part. The catalysts were magnetically separated from the reaction mixture and were thoroughly washed with distilled water and acetone. The recovered catalysts were then dried and recycled for further use. The isolated products were analyzed using 1H and 13C NMR and GC-MS analysis.2.5. Computational details
Quantum chemical calculations were performed by using GAUSSIAN 09W software59 with the B3LYP method.60,61 The LANL2DZ basis set was applied for the iron and strontium atoms,62 and the 6-31G(d,p) for the other atoms. The global reactivity parameters such as electronic chemical potential (μ) and chemical hardness (η) are calculated using the frontier molecular orbital energies, HOMO (EH) and LUMO (EL), using the following expressions μ= (EH + EL)/2 and η= (EL − EH), respectively. At the same theoretical level, the global electrophilicity (ω) and nucleophilicity (N) indexes were calculated, and are given expressions: ω = μ2/2η; N = EH − EH (tetracyanoethylene (TCE)).63,64 The Parr functions are calculated using the Mulliken atomic spin densities.65 To get a closer understanding of the synergy effect between iron and strontium atoms for regioselective ring-opening of epoxides in the presence of amines, proposed models of the prepared catalyst were investigated using a DMol3 module66 of Material Studio 8.0. The optimization of metal clusters and adsorbing molecules were performed using the generalized gradient approximation (GGA) method67 with the Perdew–Burke–Ernzerhof (PBE) function68 and the Double Numerical plus polarization (DNP) basis sets, which is comparable to Gaussian 6-31G(d,p).693. Results and discussion
3.1. Materials characterization
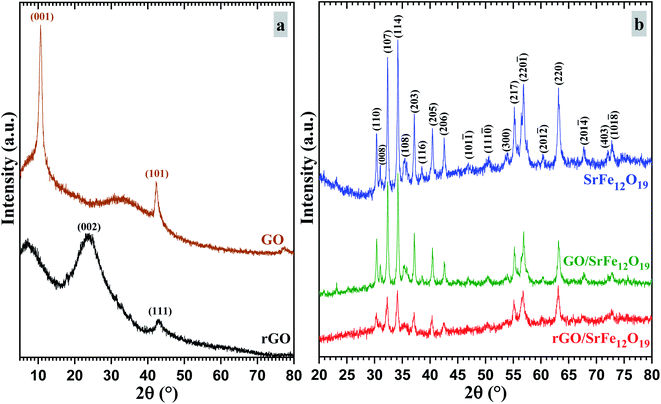
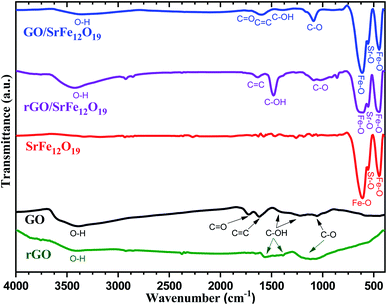
The phase purity and the stability of the synthesized materials were first investigated by XRD analyses (Fig. 1). As shown in Fig. 1a, the characteristic diffraction peaks of prepared GO corresponding to (001) and (101) planes were observed respectively at 2θ = 10.66 and 42.33°, while the prepared rGO presents both characteristic diffraction patterns (002) and (111) respectively around 2θ = 23.9 and 42.9°. In addition, the characteristic peak corresponding to (002) reflection plane of graphite was absent in both XRD spectra of GO and rGO. The XRD spectra of prepared SrFe12O19, hybrid GO/SrFe12O19, and rGO/SrFe12O19 present the same corresponding diffraction patterns of the M-type hexagonal SrFe12O19 phase and no other phases have been detected (Fig. 1b). Both prepared nanocomposites present crystalline SrFe12O19 in a hexagonal structure with a space group 194/P63 mmc, in good agreement with the standard JCPDF file (33-1340).58,70 According to the Scherrer equation,71 the crystallite size of the M-type hexagonal SrFe12O19 phase in the prepared SrFe12O19, hybrid GO/SrFe12O19, and rGO/SrFe12O19 were to be 25.098, 25.663, and 20.331 nm, respectively.The FTIR spectra were recorded for GO, rGO, pure SrFe12O19, hybrid GO/SrFe12O19, and rGO/SrFe12O19 (Fig. 2). The results confirm the oxidation of graphite and clearly show the presence of oxygen-containing functional groups in graphite oxide such as O–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–O. The spectrum of GO shows that the broad band appearing around 3395 cm−1 belonging to a strong stretching mode of OH group, the absorption band around 1723 cm−1 is attributed to C
O, and C–O. The spectrum of GO shows that the broad band appearing around 3395 cm−1 belonging to a strong stretching mode of OH group, the absorption band around 1723 cm−1 is attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode, the absorption peak around 1620 cm−1 due to C
O stretching mode, the absorption peak around 1620 cm−1 due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching mode, and the large and less intense peaks around 1395, 1170, 1120, and 1057 cm−1 were assigned to the stretching modes of C–OH and C–O, respectively. Therefore, these groups are expected to form strong surface complexes with SrFe12O19 nanoparticles. However, the absence of the C
C stretching mode, and the large and less intense peaks around 1395, 1170, 1120, and 1057 cm−1 were assigned to the stretching modes of C–OH and C–O, respectively. Therefore, these groups are expected to form strong surface complexes with SrFe12O19 nanoparticles. However, the absence of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorption band and the increase of C–OH and C–O band intensities in the FTIR spectra of rGO and rGO/SrFe12O19 confirm the reduction of GO sheets. On the other hand, the spectrum of pure SrFe12O19 NPs shows the characteristic peaks of metal–oxygen bonds at 611, 555, and 449 cm−1, respectively. These characteristic metal–oxygen peaks of SrFe12O19 arise from the vibrations of the hexaferrite structure.72 The peak observed at 449 cm−1 may be attributed to Fe–O bending vibration in the octahedral site and the higher frequency band at 611 cm−1 could be associated with Fe–O stretching vibration of the tetrahedral site.73 Moreover, the less intense peak that appeared at 555 cm−1 might be assigned to Sr–O bending vibration.73,74 Thus, the spectrum of GO/SrFe12O19 (or rGO/SrFe12O19) exhibit the presence of all characteristic peaks of GO (or rGO) and SrFe12O19 in the hybrid, with a slight difference in the intensity of O–H, C
O absorption band and the increase of C–OH and C–O band intensities in the FTIR spectra of rGO and rGO/SrFe12O19 confirm the reduction of GO sheets. On the other hand, the spectrum of pure SrFe12O19 NPs shows the characteristic peaks of metal–oxygen bonds at 611, 555, and 449 cm−1, respectively. These characteristic metal–oxygen peaks of SrFe12O19 arise from the vibrations of the hexaferrite structure.72 The peak observed at 449 cm−1 may be attributed to Fe–O bending vibration in the octahedral site and the higher frequency band at 611 cm−1 could be associated with Fe–O stretching vibration of the tetrahedral site.73 Moreover, the less intense peak that appeared at 555 cm−1 might be assigned to Sr–O bending vibration.73,74 Thus, the spectrum of GO/SrFe12O19 (or rGO/SrFe12O19) exhibit the presence of all characteristic peaks of GO (or rGO) and SrFe12O19 in the hybrid, with a slight difference in the intensity of O–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–OH, and C–O (or O–H, C–OH, and C–O) peaks due to the interaction of hexaferrite NPs with the surface of GO (or rGO) sheets.
O, C–OH, and C–O (or O–H, C–OH, and C–O) peaks due to the interaction of hexaferrite NPs with the surface of GO (or rGO) sheets.
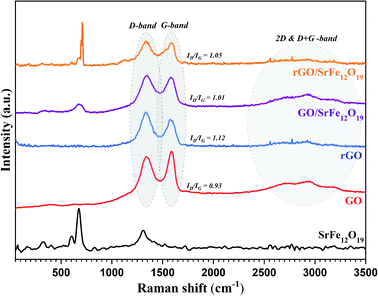
The Raman spectra were measured for all prepared materials as shown in Fig. 3. The spectrum of SrFe12O19 is in good agreement with the data reported in the literature,75,76 where the major peaks observed were around 689.48, 618.42, 535.24, 408.5 and 352.8 cm−1. The spectra of GO, rGO, GO/SrFe12O19, and rGO/SrFe12O19 present two characteristic peaks of graphene materials corresponding to D-band at 1343.62 and G-band at 1593.14 cm−1, due to the breathing mode of the k-point phonons with A1g symmetry and first order scattering E2g of the phonons from sp2 carbon atoms, respectively. Moreover, the second order of zone boundary phonons or the 2D band which is related to the nature of the graphene layer stacking was observed around 2702 cm−1 for GO. However, the ID/IG ratio increased from 0.93 in GO to 1.12 in rGO, which is due to the elimination of oxygen functionalities and the decrease in the average sp2 domain size in rGO.77 The ID/IG values of GO/SrFe12O19 about 1.01 and rGO/SrFe12O19 about 1.05 are lower than rGO value, owing to the lower degree of defects in the nanocomposites.
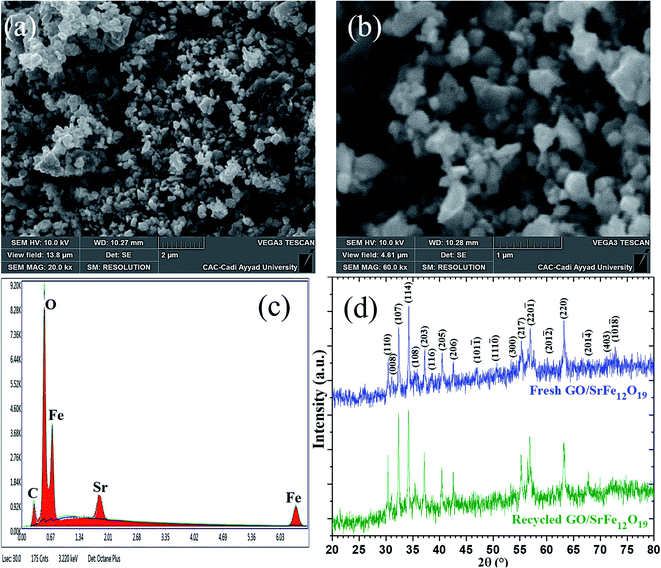
The morphology and the shape of the prepared pure SrFe12O19, GO, and rGO sheets were identified by FESEM analyses (Fig. 4). Moreover, the dispersion and the distribution of the deposited SrFe12O19 nanoparticles on GO or rGO sheets were also checked (Fig. 4). The analysis of pure SrFe12O19 FESEM images shows a nanostructured grain of the M-type hexaferrite in a regular distribution of size and morphology (Fig. 4a1 and a2). The FESEM images of GO and rGO show the preparation of microstructured sheets in a good typical morphology (Fig. 4b1, b2, d1 and d2). While the hybrid GO/SrFe12O19 and rGO/SrFe12O19 images show hexaferrite nanoparticles in good dispersion and distribution at the nanoscale range on the surface of GO and rGO sheets (Fig. 4c1, c2, e1 and e2). However, The EDX analyses of all prepared materials show good homogeneity and purity in the chemical composition (Fig. 4a3–e3).
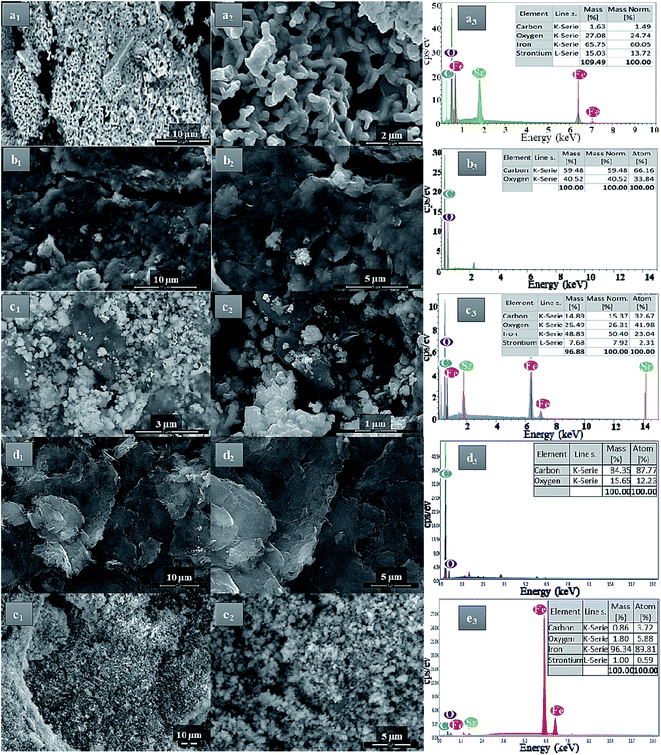 | ||
| Fig. 4 FESEM-EDX analyses of (a) SrFe12O19, (b) GO, (c) hybrid GO/SrFe12O19, (d) rGO, and (e) rGO/SrFe12O19. | ||
3.2. Catalytic epoxide ring-opening reaction
First, SrFe12O19 and the hybrid GO/SrFe12O19 nanomaterials are evaluated in the catalytic ring-opening of epoxide with various amines. In order to optimize the reaction conditions, we examined first the reaction in the presence of styrene oxide and aniline, chosen as model substrates (Scheme 1).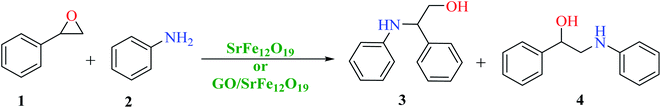 | ||
| Scheme 1 Catalytic epoxide ring-opening of styrene oxide in presence of aniline catalyzed by SrFe12O19 or GO/SrFe12O19. | ||
The catalytic performance of the prepared nanomaterials, SrFe12O19 and hybrid GO/SrFe12O19, was first evaluated in free-solvent conditions (Table 1).58 As shown in Table 1, the epoxide was converted to the resulting ring-opening products in good yields (entries 1 and 2). However, the presence of GO/SrFe12O19 improved the selectivity by reducing the reaction time and showing a complete conversion. Consequently, the hybrid GO/SrFe12O19 exhibited an excellent catalytic performance than pure SrFe12O19, owing to the outstanding improvement in the catalytic activity of SF NPs while combined with GO sheets. The improvement of the catalytic activity can be explained by the GO environment that prevents unwished aggregation of SF NPs,78 and increase the active surface area of the hybrid nanomaterial compared to pure SrFe12O19, providing a good dispersion and distribution of SF. The use of water as a solvent in the presence of hybrid GO/SrFe12O19 improves the ring-opening reaction and 100% conversion was obtained after only 2 h of reaction time (entry 3). We can assume that an interaction of GO sheets with water molecules through hydrogen bonding79 is probably involved in the reaction, proven by a lower conversion given with pure SF (entry 4). Moreover, water as a solvent promotes hydrogen bonding with the oxygen of epoxides which enhances the electrophilicity of the carbon in the alpha position.80
| Entry | Catalyst | Wt (g) | Solvent | Time (h) | Conversionb (%) | Selectivity 3c (%) | Selectivity 4c (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: styrene oxide (0.8 mmol), aniline (1 mmol), and 60 °C.b Conversion was determined by GC.c Selectivity was determined by GC. | |||||||
| 1 | SrFe12O19 | 0.01 | Neat | 17 | 95 | 74 | 26 |
| 2 | GO/SrFe12O19 | 0.01 | Neat | 5 | 100 | 92 | 8 |
| 3 | GO/SrFe12O19 | 0.01 | Water | 2 | 100 | 92 | 8 |
| 4 | SrFe12O19 | 0.01 | Water | 2 | 39 | 90 | 10 |
To shed more light on the effect of water as a solvent on the catalytic reaction and the stability of nanocomposites, zetasizer measurements of pure SrFe12O19 and hybrid GO/SrFe12O19 nanomaterials after their dispersion in water have been carried out (Table 2). The zeta potential (ZP) is applied for the determination of the surface charge of nanoparticles in colloidal solution and the ZP value can be related to the short- and long-term stability of nanoparticles in the studied colloidal solution.81 For this purpose, we have used ZP to evaluate the stability of the developed nanomaterials in water as a green and ecological solvent. The pure SrFe12O19 and hybrid GO/SrFe12O19 nanomaterials gave a ZP of −24.8 and −18 mV, respectively. It has been reported that nanoparticles having −25 > ZP > +25 mV usually present a high degree of stability,82 which means that pure SrFe12O19 is close to the threshold of agglomeration in water. Recently, we have reported the non-adaptability of water as a solvent for the ring-opening of epoxide while using SrFe12O19 as a catalyst.58 In contrast, the ZP value of hybrid GO/SrFe12O19 indicates its high stability in water, confirming the good choice of GO sheets for the dispersion of SrFe12O19 nanoparticles, which will be of great advantage for a greener catalytic application. On the other hand, the size peaks and z-average confirm the nanoscale size of hexaferrite nanoparticles and the microscale of GO sheets (confirmed by the increase in size and z-average of GO/SrFe12O19 sample). Consequently, the excellent catalytic performance of hybrid GO/SrFe12O19 in presence of water as an ecological solvent may be explained by the good dispersion of the hybrid nanomaterial in water compared to pure SF (ZP values). Moreover, hybrid GO/SrFe12O19 has a high specific surface area of (134 m2 g−1) compared to pure SF NPs (25 m2 g−1) (Table 2), which provide well dispersed and distributed active sites for adsorption of reactants.
| Nanocatalyst | Zeta potential (mV) | Size peaks | Z-Average (d nm) | BET surface area (m2 g−1) | |
|---|---|---|---|---|---|
| Size (d nm) | Intensity (%) | ||||
| SrFe12O19 | −24.8 (±5.5) | 0.651 (±0.045) | 74.1 | 11.69 | 25 |
| 2.999 (±0.358) | 25.9 | ||||
| GO/SrFe12O19 | −18.0 (±3.62) | 223.6 (±37.93) | 82.6 | 166.4 | 134 |
| 1.429 (±0.097) | 17.4 | ||||
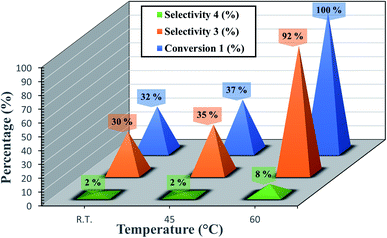
We have also checked the effect of temperature on reaction rate (Fig. 5). The best optimum was to be 60 °C. The Fig. 5 shows also that the decreasing of the temperature resulted in lower conversion.
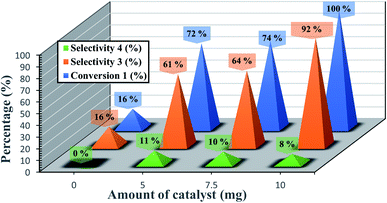
Systematic investigations on catalyst amounts were undertaken (Fig. 6). The best catalytic performance was obtained with 10 mg of catalyst. In contrast, in the absence of catalyst, only 16% of conversion and selectivity was obtained. The use of an amount of 5 or 7.5 mg of catalyst resulted in a decrease of the conversion of epoxide to 72 and 74% respectively.
A Comparison of the catalytic activity of the prepared nanomaterials was performed (Table 3). When the reaction is carried out with GO, a good conversion was obtained (entry 1). While in presence of SrFe12O19 and rGO, only 39% and 16% of conversion was observed respectively (entries 2 and 4). These results clearly demonstrate that SF NPs and acid sites of GO sheets could be both considered as active sites (entry 3). However, rGO/SrFe12O19 nanocomposite gives the same result as the pure hexaferrite NPs. Indeed, Vithalani et al. have reported that the outcome of the catalytic activity presents a mediocre performance in presence of chloro-functionalized GO compared to GO due to lesser acidic sites on the surface of the catalyst.83 Consequently, the combination of GO sheets with hexaferrite NPs effectively enhanced the catalytic activity and reusability of the developed catalyst, owing to the synergistic effect of GO and SrFe12O19 in a single magnetically separable nanostructured catalyst (hybrid GO/SrFe12O19 nanocomposite).
| Entry | Catalyst | Conversionb (%) | Selectivity 3c (%) | Selectivity 4c (%) |
|---|---|---|---|---|
| a Reaction conditions: styrene oxide (0.8 mmol), aniline (1 mmol), catalyst (10 mg), solvent (water), and 60 °C for 2 h.b Conversion was determined by GC.c Selectivity was determined by GC. | ||||
| 1 | GO | 78 | 94 | 6 |
| 2 | SrFe12O19 | 39 | 90 | 10 |
| 3 | GO/SrFe12O19 | 100 | 92 | 8 |
| 4 | rGO | 16 | 100 | 0 |
| 5 | — | 16 | 100 | 0 |
| 6 | rGO/SrFe12O19 | 37 | 100 | 0 |
The scope and limitations of the developed hybrid catalytic system GO/SrFe12O19 were investigated in the catalytic epoxide ring-opening of styrene oxide in presence of various aromatic and aliphatic amines under the optimized conditions (Scheme 2). The results obtained are collected in Table 4.
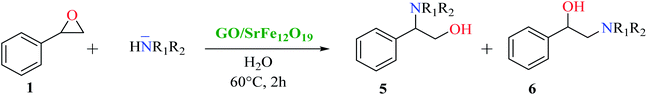 | ||
| Scheme 2 Catalytic epoxide ring-opening of styrene oxide with various amines in presence of GO/SrFe12O19. | ||
| Entry | Amine | Major product | Conversionb (%) | Selectivity 5c (%) | Selectivity 6c (%) | Isolated yieldd (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: styrene oxide (0.83 mmol), amine (1 mmol), GO/SrFe12O19 (10 mg), solvent (Water), and 60 °C for 2 h.b Conversion was determined by GC-MS.c Selectivity was determined by GC-MS.d Isolated yield of 5 and 6. | ||||||
| 1 |  |
 |
100 | 92 | 8 | 97 |
| 2 | 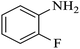 |
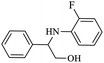 |
80 | 96 | 4 | 76 |
| 3 | 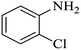 |
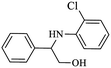 |
92 | 96 | 4 | 88 |
| 4 | 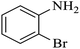 |
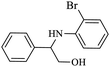 |
77 | 96 | 4 | 70 |
| 5 | 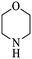 |
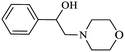 |
100 | 00 | 100 | 99 |
| 6 | 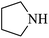 |
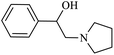 |
95 | 37 | 63 | 91 |
| 7 | 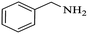 |
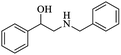 |
100 | 26 | 74 | 98 |
As shown in Table 4, with all studied aromatic and aliphatic amines, styrene oxide was converted to the corresponding β-aminoalcohol products in moderate to excellent selectivity. The reaction was faster (2 h) compared to our previous work (17 h).58 The regioselectivity of the catalytic reaction is influenced by the electronic and steric factors associated with both studied substrates epoxide and amines.84 In presence of aromatic amines, the opening reaction is mainly oriented towards amino alcohols 5 through the nucleophilic attack at the benzylic carbon atom of the epoxide ring (entries 1–4). In addition, aniline with electron-withdrawing group substituents at ortho position reduced the conversion with good regioselectivity of 96% compared to unsubstituted one (entries 1–4). On the other hand, aliphatic amines oriented the regioselectivity towards amino alcohols 6 through the nucleophilic attack at the β position of the benzylic carbon atom of the epoxide ring due to the steric effect (entries 5–7). Moreover, morpholine substrate totally oriented the regioselectivity with a complete conversion towards the corresponding amino alcohol 6 (entry 5).
The ratio of both regioisomeric products was determined by GC-MS (MS spectra in ESI†). The regioisomer of α-product (3) exhibited a molecular ion at m/z of M+ = 31 due to the CH2OH, and the regioisomer β-product (4) exhibited a molecular ion at m/z of M+ = 106 arising from the loss of PhCHO.85
To examine the extension of the reaction, we have investigated the catalytic reaction of cyclohexene oxide with various aromatic and aliphatic amines under optimized conditions (Scheme 3). With all studied amines, cyclohexene oxide was converted to the corresponding β-amino alcohols in excellent yields (Table 5). According to 1H NMR analyses (data and spectra in ESI†), exclusive trans amino alcohol derivatives were obtained in the cleavage of the epoxide ring of cyclohexene oxide, which is in good agreement with the literature.84,86 The trans stereoselectivity is confirmed by the high coupling constants (3J) between the relevant HCOH and HCNR1R2 peaks present in the region of 2.44–4.13 ppm, in contrast to the lower 3J between HOCH–CH(cis) and R1R2NCH–CH(cis).87
| Entry | Amine | Product | Conversionb (%) | Selectivityc (%) | Isolated yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: cyclohexene oxide (1.02 mmol), amine (1.1 mmol), GO/SrFe12O19 (10 mg), solvent (water), and 60 °C for 2 h.b Conversion was determined by GC-MS.c Selectivity was determined by GC-MS. | |||||
| 1 | 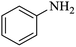 |
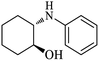 |
100 | 100 | 97 |
| 2 | 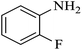 |
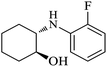 |
100 | 100 | 95 |
| 3 | 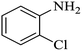 |
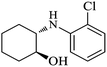 |
100 | 100 | 95 |
| 4 | 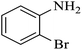 |
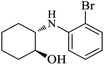 |
100 | 100 | 95 |
| 5 | 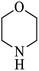 |
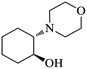 |
100 | 100 | 99 |
| 6 | 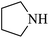 |
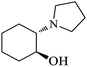 |
97 | 100 | 94 |
| 7 | 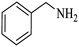 |
 |
100 | 100 | 95 |
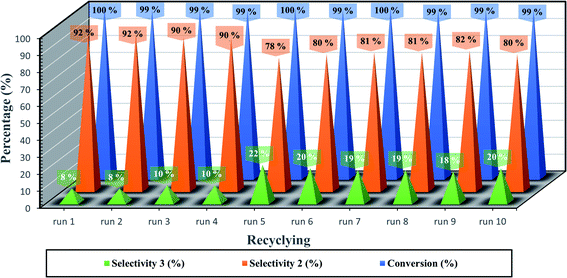
Ferrites as magnetic materials by nature point out their presence in heterogeneous catalysis as a highly recyclable catalyst. The recyclability performance of the developed catalytic system GO/SrFe12O19 as hybrid MNPs, was performed with styrene oxide and aniline under the optimized reaction conditions (Fig. 7). The catalyst was separated from the reaction mixture by applying an external magnetic field and reused for ten runs without significant loss in catalytic activity. After each run, the recycled catalyst was washed with water (3 × 5 mL) and acetone (3 × 5 mL) and dried at 100 °C for 3 h.
After the last run, the identity of the recovered hybrid nanocatalyst GO/SrFe12O19 was checked by zetasizer measurements, SEM-EDX, and XRD analyses (Table 6 and Fig. 8). The ZP of the recycled catalyst after 10 runs is close to the threshold of stability in water with an increase of z-average size, which could explain the slight loss of selectivity from 92% to 80% of amino alcohol 3 (Fig. 7). However, SEM images showed good stability in the distribution and dispersion of SrFe12O19 NPs on GO sheets (Fig. 8a and b). In addition, EDX analysis confirms the stability in the elemental composition of hybrid GO/SrFe12O19 (Fig. 8c). Moreover, XRD patterns of the recovered nanocatalyst after ten runs show good crystalline stability compared to the fresh nanocatalysts GO/SrFe12O19 (Fig. 8d).
| GO/SrFe12O19 | Zeta potential (mV) | Z-Average (d nm) | SF crystallite size (nm) |
|---|---|---|---|
| Fresh | −18.0 (±3.62) | 166.4 | 25.66 |
| Recycled | −24.6 (±3.35) | 272 | 26.01 |
3.3. Comparison of catalytic performance with other catalytic systems
A comparison of the catalytic performance of the hybrid GO/SrFe12O19 nanocatalysts with recent literature reports on styrene oxide ring-opening catalyzed by various catalytic systems is given in Table 7.| Catalyst | Reaction conditions | Conversion 1 (%) | Selectivity 3 (%) | Reusability (conversion![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) run) run) |
Ref. |
|---|---|---|---|---|---|
| Ni(S2COCH3)2 | 1 eq. amine, CH2Cl2, RT, 24 h | 98 | 83 | — | 88 |
| MS-AI | 2 eq. amine, toluene, 50 °C, 4 h | 92 | 98.5 | — | 89 |
| Zr-MOR zeolite | 1 eq. amine, free-solvent, 40 °C, 4 h | 90.2 | 93.1 | 90.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
90 |
| PTS-Im-3@GO | 10 eq. amine, free-solvent, 50 °C, 4 h | 100 | 100 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
91 |
| SrFe12O19 | 1.1 eq. amine, free-solvent, 60 °C, 17 h | 95 | 74 | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
58 |
| GO/SrFe12O19 | 1.1 eq. amine, water, 60 °C, 2 h | 100 | 92 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
This work |
3.4. Mechanistic study
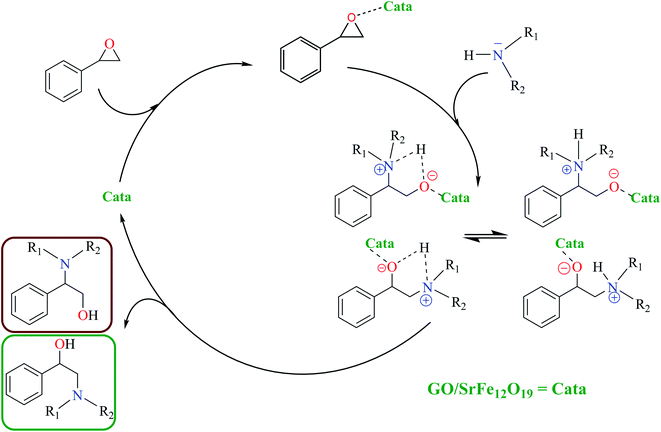
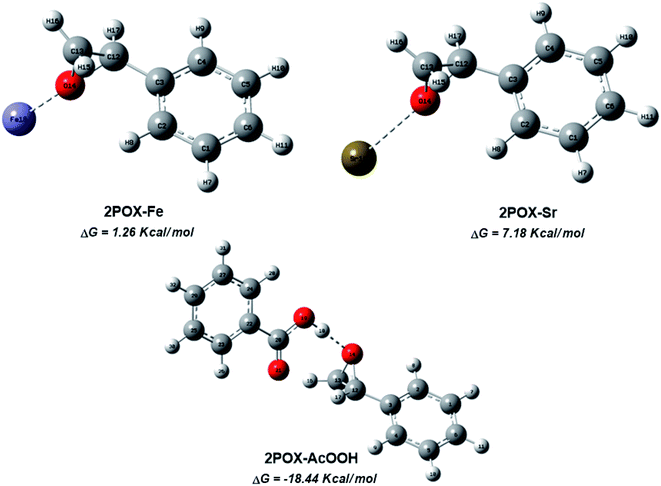
The catalytic activity of the hybrid GO/SrFe12O19 nanocomposite is mainly due to the metal ions present in the octahedral and tetrahedral sites,92 and the oxygen functionalities dispersed on GO sheets could be responsible for their catalytic activity.93 When the epoxide molecules are adsorbed on the surface of deposited hexaferrite, the acidic sites of hexaferrite and graphene oxide are activated (Scheme 4). The formation of the regioisomeric product arising from the nucleophilic attack on the electron-deficient carbon atom of the epoxide is produced. In fact, the phenyl group in styrene oxide assists in the stabilization of carbocationic character at the benzylic carbon.The epoxide ring-opening reaction in the presence of the prepared catalyst gives two possible products regarding the nature of the used amine compounds, as shown in the proposed mechanism (Scheme 4). Recently, we have used density functional theory (DFT) calculations to understand observed regio- and chemoselectivity during a studied catalytic reaction as well as the corresponding mechanistic pathway.94 To get a closer understanding of the experimental results, DFT calculations were performed using models for the prepared nanocatalyst (Scheme 5). Initially, the optimized structures of single iron and strontium atoms with 2POX, and 2POX–AcOOH complexes were performed and illustrated in Fig. 9. The coordination energy for the formation of 2POX–metal complexes is positive, which indicates that the coordination reaction between metals and 2-phenyloxirane is endothermic (Fig. 9). However, the coordination energy of the 2POX–Fe complex is lower compared to the 2POX–Sr complex, leading to the conclusion that the coordination between 2POX and Fe metal is a more stable structure of the 2POX–SrFe complex. For 2POX–AcOOH, the free energy for the formation of this complex is negative due to the hydrogen bond between AcOOH and oxygen of the epoxide ring.
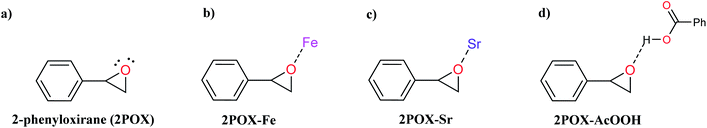 | ||
| Scheme 5 Schematic representation of the possible coordinating modes between SrFe-GO catalyst and 2-phenyloxirane (2POX). | ||
The global reactivity indexes were used as a powerful tool for the theoretical understanding which can enable to explain the regio- and chemoselectivity reactions based on the measurement of the global electron density transfer (GEDT) value.95,96 In this regard, the global properties, namely, electronic chemical potential μ, chemical hardness η, global electrophilicity ω, and global nucleophilicity N for the 2POX, aniline, morpholine and for both complexes, namely 2POX–Fe, and 2POX–Sr were calculated and reported in Table 8. From the obtained results, we can notice that in the absence of metal the electronic chemical potential of the secondary amine (morpholine), μ = −1.74 eV, is higher than that of 2POX, μ = −3.85 eV, indicating that at the TSs, the GEDT will take place from the morpholine compound towards the 2POX. In the case of primary amine (aniline), a similar chemical potential value was obtained which can explain the absence of products in this case. The coordination of 2POX with metals increases the chemical potential value that conducts to a GEDT from 2POX–metals to primary amine (aniline) and the inverse in the case of secondary amine (Table 8). The global electrophilicity and nucleophilicity indexes of reactants were calculated, see Table 8. The results indicate that the 2POX acts as a moderate electrophile (⍵ = 0.89 eV) and moderate nucleophile (N = 2.61 eV). Similar results were obtained in the case of primary amine (aniline) while morpholine acts as a stronger nucleophile compared to aniline. The coordination of 2POX with metals conducts to a strong nucleophile (N = 5.49 eV) and a moderate electrophile (⍵ = 1.90 eV), for 2POX–Fe, and a marginal electrophile (⍵ = 1.90 eV) and a strong nucleophile (N = 5.49 eV) in the case of 2POX–Sr within the electrophilicity97 and nucleophilicity scales.98 These results confirm that along with this reaction the 2POX–Fe acts as an electrophile and the amines as a nucleophile with a large polar character.
| HOMO | LUMO | μ | η | ω | N | |
|---|---|---|---|---|---|---|
| 2POX | −6.50 | −0.21 | −3.36 | 6.29 | 0.89 | 2.61 |
| Aniline | −6.54 | 0.02 | −3.26 | 6.56 | 0.81 | 2.57 |
| Morpholine | −5.71 | 2.22 | −1.74 | 7.94 | 0.19 | 3.40 |
| 2POX–Fe | −3.62 | −1.74 | −2.68 | 1.89 | 1.90 | 5.49 |
| 2POX–Sr | −3.37 | −0.75 | −2.06 | 2.62 | 0.81 | 5.74 |
| 2POX–AcOOH | −6.66 | −1.08 | −3.87 | 5.58 | 1.34 | 2.45 |
Along a polar reaction, the bond-forming process takes place at a specific position of a molecule and can explain the regio- or chemoselectivity issues of the reaction. In this regard, the epoxide ring-opening reaction in which the different approach modes of a reagent towards the other can yield two competitive isomers. The analysis of the local electrophilicity ωk at the electrophilic reagent and the local nucleophilicity Nk at the nucleophilic one derived from Parr functions allows us to explain the regioselectivity that is experimentally observed in the organic reactions with a large polar character.99 Therefore, the values of the electrophilic and nucleophilic Parr functions, the local electrophilicity, and the local nucleophilicity at the 2POX, aniline, morpholine, 2POX–Fe, 2POX–Sr, and 2POX–AcOOH are calculated and summarized in Fig. 10.
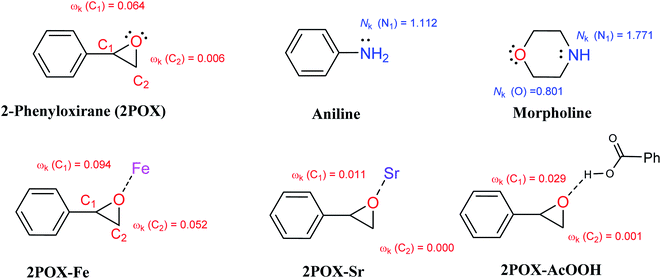 | ||
| Fig. 10 Local electrophilicity ωk (in red color) and local nucleophilicity Nk (in blue color) calculated using Parr function and all presented values are in eV. | ||
The analysis of the local electrophilicity ωk at 2POX indicates that the carbon atom C1 is the more electrophilically activated center in this compound, ωk (C1) = 0.064 eV and the analysis of the local nucleophilicity at amines indicates that the nitrogen atom is the most nucleophilic center, Nk (N1) = 1.112 and 1.771 eV for aniline and morpholine, respectively. These results confirm the selective synthesis of compound 3 in absence of catalyst (see Table 3, entry 5). The coordination of 2POX with metals indicates that the presence of iron atoms conducts to the synthesis of compound 4 while the presence of strontium atoms leads to the selective synthesis of compound 3. In the case of the coordination of 2POX with acid function, the findings show that the most electrophilic center is located in the carbon (C1) atom. Consequently, the most favorable bond formation will correspond to the N1 (amines) → C1 (2POX–AcOOH), leading to the formation of compound 3 (see Table 3, entries 1 and 4). All these results are in good agreement with the experimental observations and can explain the important role of iron, strontium atoms, and acid function in the improvement of the conversion and selectivity in the epoxide ring-opening reactions.
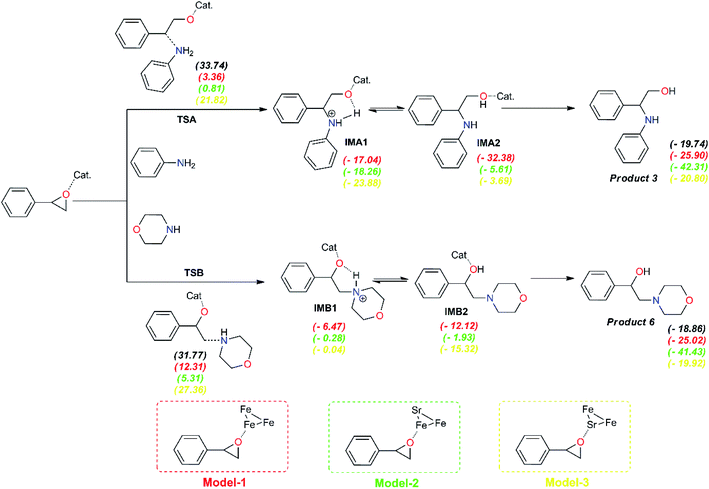
To get more light on the synergy effect between iron and strontium atoms for regioselective ring-opening of epoxides in the presence of amines, proposed models of the prepared catalyst was investigated using cluster metal atoms for the accepted mechanism to understand the selectivity obtained in the synthesis of compounds 3 and 6 using SrFe-GO catalyst (Fig. 11). Firstly, the formation of a bond between amine groups and epoxide is performed through a transition state, TSA for aniline and TSB for morpholine. The results indicate that these TSs are endothermic in both phases, in the absence and presence of catalysis. In the absence of catalyst, the computed barrier for the formation of the first N1–C1 single bond via TSA using aniline is 33.74 kcal mol−1, a value that is slightly higher than the barrier for TSB using morpholine (ca. 31.77 kcal mol−1). The epoxide ring-opening using FeSr cluster (model-3) has a higher energetic barrier in the case of aniline, ΔE(TSA) = 21.82 kcal mol−1, and also in the case of morpholine, ΔE(TSA) = 27.36 kcal mol−1. In the case of the Fe3 cluster (model-1), the energetic barrier of transition states (TSs) is reduced by ∼14 kcal mol−1 for both amines. Interestingly, the presence of the SrFe cluster (model-2) can reduce this energetic barrier in the case of aniline, ΔE(TSA) = 0.81 kcal mol−1, and also in the case of morpholine, ΔE(TSB) = 5.31 kcal mol−1. All obtained intermediates using models of SrFe catalyst are exothermic. Subsequently, the formation of products 3 and 6 are exothermic in all cases. These results allow us to explain the synergy effect between iron and strontium atoms for regioselective ring-opening of epoxides in the presence of amines and the role of metal nature especially iron in the selectivity of the corresponding products.
4. Conclusion
In a summary, we have reported a successful synthesis of magnetically separable hybrid GO/SrFe12O19 nanocomposite. First, the SrFe12O19 NPs were synthesized by a chemical coprecipitation method, while the GO sheets were prepared by the chemical oxidation of graphite powder. For comparison purposes, reduced graphene oxide (rGO) and rGO/SrFe12O19 were prepared respectively by reducing GO sheets and the prepared hybrid material. The characterization of GO/SrFe12O19, rGO/SrFe12O19, and pure SrFe12O19 by XRD presents the same corresponding diffraction patterns of M-type hexagonal ferrites structure. FTIR spectra of prepared materials exhibit the characteristic bands of GO (rGO) and SrFe12O19 in the hybrid GO/SrFe12O19 (rGO/SrFe12O19). However, FESEM-EDX analyses reveal an excellent morphology and homogeneity in composition. Moreover, the prepared nanocomposites show good dispersed and distributed SrFe12O19 NPs on the surface of GO and rGO sheets. The prepared hybrid nanocomposite GO/SrFe12O19 exhibits an excellent catalytic activity with an easy magnetic reusability and good stability for the regioselective ring-opening reaction in neat water. The marked catalytic activity of the hybrid nanocomposite compared to pure SrFe12O19 is probably due to the synergistic effect created between GO and SF NPs. The ZP values indicated a higher stability of SF NPs in the hybrid compared to the pure ones, confirming that hybrid magnetic nanocomposite is an efficient heterogeneous catalyst compared to pure ferrite NPs. In addition, a mechanistic study through DFT calculation was used to explain the synergetic role between iron and strontium atoms in the total regioselectivity of SrFe-GO catalyst in epoxide ring-opening reaction, and the obtained results are in excellent agreement with the experimental observations.Disclosure
The present research is a part of a PhD thesis work of the author Mouhsine Laayati.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
The authors are grateful to the Center for Analysis and Characterization (CAC) at Cadi Ayyad University, Marrakech, for the spectroscopic analysis.References
- S. E. McNeil, J. Leukoc. Biol., 2005, 78, 585–594 CrossRef CAS PubMed.
- A. Owen, C. Dufès, D. Moscatelli, E. Mayes, J. F. Lovell, K. V. Katti, K. Sokolov, M. Mazza, O. Fontaine, S. Rannard and V. Stone, Nanomedicine, 2014, 9, 1291–1294 CrossRef CAS PubMed.
- L. H. Reddy, J. L. Arias, J. Nicolas and P. Couvreur, Chem. Rev., 2012, 112, 5818–5878 CrossRef CAS PubMed.
- N. Dasgupta, S. Ranjan and C. Ramalingam, Environ. Chem. Lett., 2017, 15, 591–605 CrossRef CAS.
- Z. Abdin, M. A. Alim, R. Saidur, M. R. Islam, W. Rashmi, S. Mekhilef and A. Wadi, Renew. Sustain. Energy Rev., 2013, 26, 837–852 CrossRef CAS.
- W. Y. Kim, Y. C. Choi, S. K. Min, Y. Cho and K. S. Kim, Chem. Soc. Rev., 2009, 38, 2319–2333 RSC.
- F. Soofivand, F. Mohandes and M. Salavati-Niasari, Mater. Res. Bull., 2013, 48, 2084–2094 CrossRef CAS.
- F. Motahari, M. R. Mozdianfard, F. Soofivand and M. Salavati-Niasari, RSC Adv., 2014, 4, 27654–27660 RSC.
- A. R. Armstrong and P. G. Bruce, Nature, 1996, 381, 499–500 CrossRef CAS.
- M. Yousefi, F. Gholamian, D. Ghanbari and M. Salavati-Niasari, Polyhedron, 2011, 30, 1055–1060 CrossRef CAS.
- S. Ibraheem, G. Yasin, A. Kumar, M. A. Mushtaq, S. Ibrahim, R. Iqbal, M. Tabish, S. Ali and A. Saad, Appl. Catal. B Environ., 2022, 304, 120987 CrossRef CAS.
- M. Arif, G. Yasin, L. Luo, W. Ye, M. A. Mushtaq, X. Fang, X. Xiang, S. Ji and D. Yan, Appl. Catal. B Environ., 2020, 265, 118559 CrossRef CAS.
- O. Mohanta, Y. N. Singhbabu, S. K. Giri, D. Dadhich, N. N. Das and R. K. Sahu, J. Alloys Compd., 2013, 564, 78–83 CrossRef CAS.
- T. Ahmadi, G. Mohammadi Ziarani, S. M. Masoumian Hoseini, A. Badiei and M. M. Ranjbar, J. Iran. Chem. Soc., 2021, 18, 2047–2056 CrossRef CAS.
- E. Alimohammadi, S. Sheibani and A. Ataie, Mater. Chem. Phys., 2022, 275, 125312 CrossRef CAS.
- S. Chen, Y. Di, H. Li, M. Wang, B. Jia, R. Xu and X. Liu, Appl. Surf. Sci., 2021, 559, 149855 CrossRef CAS.
- R. C. Pullar, Prog. Mater. Sci., 2012, 57, 1191–1334 CrossRef CAS.
- E. Govea-Alcaide, J. Matilla-Arias, F. Guerrero, P. Mariño-Castellanos, K. Montero-Rey, F. Rosales-Saiz and I. F. Machado, J. Magn. Magn. Mater., 2021, 533, 167966 CrossRef CAS.
- X. Zhu, X. Wang, K. Liu, H. Yuan, R. Boudaghi and M. N. Akhtar, J. Alloys Compd., 2021, 873, 159818 CrossRef CAS.
- S. S. Nikitin, O. V. Merkulov, A. D. Bamburov and M. V. Patrakeev, J. Alloys Compd., 2021, 873, 159677 CrossRef CAS.
- J. L. Gong, B. Wang, G. M. Zeng, C. P. Yang, C. G. Niu, Q. Y. Niu, W. J. Zhou and Y. Liang, J. Hazard. Mater., 2009, 164, 1517–1522 CrossRef CAS PubMed.
- L. Chen, Z. Xu, H. Dai and S. Zhang, J. Alloys Compd., 2010, 497, 221–227 CrossRef CAS.
- M. D. Kaminski and L. Nuñez, J. Magn. Magn. Mater., 1999, 194, 31–36 CrossRef CAS.
- C. Chen, J. Hu, D. Shao, J. Li and X. Wang, J. Hazard. Mater., 2009, 164, 923–928 CrossRef CAS PubMed.
- S. Imine, F. Schoenstein, S. Mercone, M. Zaghrioui, N. Bettahar and N. Jouini, J. Eur. Ceram. Soc., 2011, 31, 2943–2955 CrossRef CAS.
- J. Ding, C. Zhou, Z. Wu, C. Chen, N. Feng, L. Wang, H. Wan and G. Guan, Appl. Catal. Gen., 2021, 616, 118080 CrossRef CAS.
- Y. Zhang, B. Chen, L. Zhang, J. Huang, F. Chen, Z. Yang, J. Yao and Z. Zhang, Nanoscale, 2011, 3, 1446–1450 RSC.
- J. Yan, T. Wei, W. Qiao, B. Shao, Q. Zhao, L. Zhang and Z. Fan, Electrochim. Acta, 2010, 55, 6973–6978 CrossRef CAS.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- G. Eda and M. Chhowalla, ACS Nano, 2011, 5, 4265–4268 CrossRef CAS PubMed.
- G. Yasin, M. Arif, T. Mehtab, M. Shakeel, M. A. Mushtaq, A. Kumar, T. A. Nguyen, Y. Slimani, M. T. Nazir and H. Song, Inorg. Chem. Front., 2020, 7, 402–410 RSC.
- M. A. Mushtaq, M. Arif, X. Fang, G. Yasin, W. Ye, M. Basharat, B. Zhou, S. Yang, S. Ji and D. Yan, J. Mater. Chem. A, 2021, 9, 2742–2753 RSC.
- G. Yasin, S. Ibraheem, S. Ali, M. Arif, S. Ibrahim, R. Iqbal, A. Kumar, M. Tabish, M. A. Mushtaq, A. Saad, H. Xu and W. Zhao, Mater. Today Chem., 2022, 23, 100634 CrossRef CAS.
- S. Ebrahimpoor, V. Kiarostami, M. Khosravi, M. Davallo and A. Ghaedi, Fibers Polym., 2021, 22, 159–170 CrossRef CAS.
- A. Aziz, Y. H. Yau, G. L. Puma, C. Fischer, S. Ibrahim and S. Pichiah, Chem. Eng. J., 2014, 235, 264–274 CrossRef.
- J. Luo, P. Shen, W. Yao, C. Jiang and J. Xu, Nanoscale Res. Lett., 2016, 11, 141 CrossRef PubMed.
- C. Zhao, M. Shen, Z. Li, R. Sun, A. Xia and X. Liu, J. Alloys Compd., 2016, 689, 1037–1043 CrossRef CAS.
- M. T. Barros, M. A. J. Charmier, C. D. Maycock and T. Michaud, Tetrahedron, 2005, 61, 7960–7966 CrossRef CAS.
- E. J. Corey and F. Y. Zhang, Angew. Chem. Int. Ed., 1999, 38, 1931–1934 CrossRef CAS PubMed.
- C. W. Johannes, M. S. Visser, G. S. Weatherhead and A. H. Hoveyda, J. Am. Chem. Soc., 1998, 120, 8340–8347 CrossRef CAS.
- S. Pulla, C. M. Felton, Y. Gartia, P. Ramidi and A. Ghosh, ACS Sustain. Chem. Eng., 2013, 1, 309–312 CrossRef CAS.
- D. J. Ager, I. Prakash and D. R. Schaad, Chem. Rev., 1996, 96, 835–875 CrossRef CAS PubMed.
- Y. He, H. Lu, X. Li, J. Wu, T. Pu, W. Du, H. Li, J. Ding, H. Wan and G. Guan, Green Chem., 2021, 23, 8571–8580 RSC.
- Y. He, X. Li, W. Cai, H. Lu, J. Ding, H. Li, H. Wan and G. Guan, ACS Sustain. Chem. Eng., 2021, 9, 7074–7085 CrossRef CAS.
- M. Palmer, T. Walsgrove and M. Wills, J. Org. Chem., 1997, 62, 5226–5228 CrossRef CAS.
- E. Alza, A. Bastero, S. Jansat and M. A. Pericàs, Tetrahedron: Asymmetry, 2008, 19, 374–378 CrossRef CAS.
- A. Patti and S. Pedotti, Tetrahedron: Asymmetry, 2003, 14, 597–602 CrossRef CAS.
- Y. Fujiwara, T. Katagiri and K. Uneyama, Tetrahedron Lett., 2003, 44, 6161–6163 CrossRef CAS.
- Y. F. Kang, R. Wang, L. Liu, C. S. Da, W. J. Yan and Z. Q. Xu, Tetrahedron Lett., 2005, 46, 863–865 CrossRef CAS.
- W. Oppolzer and R. N. Radinov, Tetrahedron Lett., 1988, 29, 5645–5648 CrossRef CAS.
- N. Azizi and M. R. Saidi, Tetrahedron, 2007, 63, 888–891 CrossRef CAS.
- J. Agarwal, A. Duley, R. Rani and R. Peddinti, Synthesis, 2009, 2009, 2790–2796 CrossRef.
- R. Outouch, M. Rauchdi, B. Boualy, L. El Firdoussi, A. Roucoux and M. A. Ali, Acta Chim. Slov., 2014, 61, 67–72 CAS.
- A. T. Placzek, J. L. Donelson, R. Trivedi, R. A. Gibbs and S. K. De, Tetrahedron Lett., 2005, 46, 9029–9034 CrossRef CAS.
- G. Sabitha, G. S. Kumar Reddy, K. Bhaskar Reddy and J. S. Yadav, Synthesis, 2003, 15, 2298–2300 CrossRef.
- M. C. Singh and R. K. Peddinti, Tetrahedron Lett., 2007, 48, 7354–7357 CrossRef CAS.
- J. R. Rodríguez and A. Navarro, Tetrahedron Lett., 2004, 45, 7495–7498 CrossRef.
- M. Laayati, A. Hasnaoui, N. Abdallah, S. Oubaassine, L. Fkhar, O. Mounkachi, S. El Houssame, M. Ait Ali and L. El Firdoussi, J. Chem., 2020, 2020, 1–10 CrossRef.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian Inc, Wallingford CT, 2009 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed.
- L. E. Roy, P. J. Hay and R. L. Martin, J. Chem. Theory Comput., 2008, 4, 1029–1031 CrossRef CAS PubMed.
- R. G. Parr, L. V. Szentpály and S. Liu, J. Am. Chem. Soc., 1999, 121, 1922–1924 CrossRef CAS.
- L. R. Domingo and P. Pérez, Org. Biomol. Chem., 2011, 9, 7168–7175 RSC.
- L. R. Domingo, P. Pérez and J. A. Sáez, RSC Adv., 2013, 3, 1486–1494 RSC.
- B. Delley, J. Chem. Phys., 2000, 113, 7756–7764 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- M. Ernzerhof and G. E. Scuseria, J. Chem. Phys., 1999, 110, 5029–5036 CrossRef CAS.
- Y. Inada and H. Orita, J. Comput. Chem., 2008, 29, 225–232 CrossRef CAS PubMed.
- P. Sivakumar, L. Shani, Y. Yeshurun, A. Shaulov and A. Gedanken, J. Mater. Sci. Mater. Electron., 2016, 27, 5707–5714 CrossRef CAS.
- P. Scherrer, in Kolloidchemie Ein Lehrbuch, Springer Berlin Heidelberg, Berlin, Heidelberg, 1912, pp. 387–409 Search PubMed.
- X. Meng, Y. Zhu, S. Xu and T. Liu, RSC Adv., 2016, 6, 4946–4949 RSC.
- T. Ben Ghzaiel, W. Dhaoui, A. Pasko and F. Mazaleyrat, J. Alloys Compd., 2016, 671, 245–253 CrossRef CAS.
- M. A. Almessiere, Y. Slimani, H. Güngüneş, A. Baykal, S. V. Trukhanov and A. V. Trukhanov, Nanomaterials, 2019, 9, 1–18 Search PubMed.
- L. Zhang and Z. Li, J. Alloys Compd., 2009, 469, 422–426 CrossRef CAS.
- G. P. Nethala, R. Tadi, G. R. Gajula, K. N. Chidambara Kumar and V. Veeraiah, Phys. B Condens. Matter, 2018, 550, 136–144 CrossRef CAS.
- Z. Lin, Y. Yao, Z. Li, Y. Liu, Z. Li and C. P. Wong, J. Phys. Chem. C, 2010, 114, 14819–14825 CrossRef CAS.
- Y. Yao, Z. Yang, D. Zhang, W. Peng, H. Sun and S. Wang, Ind. Eng. Chem. Res., 2012, 51, 6044–6051 CrossRef CAS.
- X. Huang, Z. Yin, S. Wu, X. Qi, Q. He, Q. Zhang, Q. Yan, F. Boey and H. Zhang, Small, 2011, 7, 1876–1902 CrossRef CAS PubMed.
- B. Mouhsine, A. Karim, C. Dumont and M. Sauthier, Green Chem., 2020, 22, 950–955 RSC.
- G. W. Lu and P. Gao, in Handbook of Non-Invasive Drug Delivery Systems, Elsevier, 2010, pp. 59–94 Search PubMed.
- A. J. Shnoudeh, I. Hamad, R. W. Abdo, L. Qadumii, A. Y. Jaber, H. S. Surchi and S. Z. Alkelany, in Biomaterials and Bionanotechnology, Elsevier, 2019, pp. 527–612 Search PubMed.
- R. S. Vithalani, C. K. Modi, V. Sharma, P. K. Jha and H. Srivastava, J. Mol. Struct., 2022, 1249, 131620 CrossRef CAS.
- B. P. Shivani and A. K. Chakraborti, J. Org. Chem., 2007, 72, 3713–3722 CrossRef CAS PubMed.
- M. Hosseini-Sarvari, Can. J. Chem., 2008, 86, 65–71 CrossRef CAS.
- N. Azizi, P. Kamrani and M. Saadat, Appl. Organomet. Chem., 2016, 30, 431–434 CrossRef CAS.
- S. Liu, J. Xie, W. Li, W. Kong, L. Wang and Q. Zhou, Org. Lett., 2009, 11, 4994–4997 CrossRef CAS PubMed.
- G. Kumar, R. Kumar, G. Ogruc-ildiz, R. Fausto and A. Husain, J. Mol. Struct., 2019, 1177, 33–46 CrossRef CAS.
- A. Iqbal, T. K. Hou, U. S. Shaari, F. Adam and N. H. H. A. Bakar, Mater. Today Proc., 2018, 5, 21584–21593 CrossRef CAS.
- B. Tang, W.-C. Song, S.-Y. Li, E.-C. Yang and X.-J. Zhao, New J. Chem., 2018, 42, 13503–13511 RSC.
- A. V Nakhate and G. D. Yadav, ChemistrySelect, 2018, 3, 4547–4556 CrossRef.
- A. Sheoran, M. Dhiman, S. Bhukal, R. Malik, J. Agarwal, B. Chudasama and S. Singhal, Mater. Chem. Phys., 2019, 222, 207–216 CrossRef CAS.
- S. Verma, H. P. Mungse, N. Kumar, S. Choudhary, S. L. Jain, B. Sain and O. P. Khatri, Chem. Commun., 2011, 47, 12673–12675 RSC.
- A. A. Mekkaoui, H. Ben El Ayouchia, H. Anane, R. Chahboun, L. El Firdoussi and S. El Houssame, J. Mol. Struct., 2021, 1235, 130221 CrossRef CAS.
- P. Geerlings, F. De Proft and W. Langenaeker, Chem. Rev., 2003, 103, 1793–1873 CrossRef CAS PubMed.
- L. Domingo, M. Ríos-Gutiérrez and P. Pérez, Molecules, 2016, 21, 748 CrossRef PubMed.
- L. R. Domingo, M. J. Aurell, P. Pérez and R. Contreras, Tetrahedron, 2002, 58, 4417–4423 CrossRef CAS.
- P. Jaramillo, L. R. Domingo, E. Chamorro and P. Pérez, J. Mol. Struct., 2008, 865, 68–72 CrossRef CAS.
- L. R. Domingo, RSC Adv., 2014, 4, 32415–32428 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra00984f |
| This journal is © The Royal Society of Chemistry 2022 |