Open Access Article
Open Access ArticleEnhancing the quantum yield of singlet oxygen: photocatalytic degradation of mustard gas simulant 2-chloroethyl ethyl sulfide catalyzed by a hybrid of polyhydroxyl aluminum cations and porphyrin anions†
Ying Yangab,
Jianbo Yina,
Fangsheng Taoa,
Yunshan Zhou *a,
Lijuan Zhang*a,
Yuxu Zhong*b and
Yong'an Wangb
*a,
Lijuan Zhang*a,
Yuxu Zhong*b and
Yong'an Wangb
aState Key Laboratory of Chemical Resource Engineering, College of Chemistry, Beijing University of Chemical Technology, Beijing 100029, P. R. China. E-mail: zhouys@mail.buct.edu.cn
bToxicology and Medical Countermeasures, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, P. R. China
First published on 13th July 2022
Abstract
By combining the anionic salt meso-tetra(4-carboxyphenyl)porphyrin (TCPP4−) and the Keggin polyoxometalate cation cluster [Al13O4(OH)24(H2O)12]7+ via a simple ion-exchange method, a hybrid (C48H26N4O8)[Al13O4(OH)24(H2O)12]2(OH)10·18H2O (Al13–TCPP) was prepared and thoroughly characterized as a prototype of polyoxometalate–porphyrin hybrids for the photocatalytic degradation of the mustard gas simulant 2-chloroethyl ethyl sulfide (CEES). The experimental results showed that the catalytic degradation rate of CEES in the presence of Al13–TCPP reached 96.16 and 99.01% in 180 and 90 min in methanol and methanol–water solvent mixture (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), respectively. The reaction followed first-order reaction kinetics, and the half-life and kinetic constant in methanol and solvent mixture were 39.8 min, −0.017 min−1 and 14.7 min, −0.047 min−1. Mechanism analysis indicated that under visible light irradiation in air, CEES was degraded through a combination of oxidation and alcoholysis/hydrolysis in methanol and the methanol–water solvent mixture. The superoxide radical (O2˙−) and singlet molecular oxygen (1O2) generated by Al13–TCPP selectively oxidized CEES into a non-toxic sulfoxide. The singlet oxygen capture experiments showed that Al13–TCPP (Φ = 0.236) had a higher quantum yield of singlet oxygen generation than H4TCPP (Φ = 0.135) under visible light irradiation in air. The material Al13–TCPP has good reusability, and the degradation rate of CEES can still reach 98.37% after being recycled five times.
1), respectively. The reaction followed first-order reaction kinetics, and the half-life and kinetic constant in methanol and solvent mixture were 39.8 min, −0.017 min−1 and 14.7 min, −0.047 min−1. Mechanism analysis indicated that under visible light irradiation in air, CEES was degraded through a combination of oxidation and alcoholysis/hydrolysis in methanol and the methanol–water solvent mixture. The superoxide radical (O2˙−) and singlet molecular oxygen (1O2) generated by Al13–TCPP selectively oxidized CEES into a non-toxic sulfoxide. The singlet oxygen capture experiments showed that Al13–TCPP (Φ = 0.236) had a higher quantum yield of singlet oxygen generation than H4TCPP (Φ = 0.135) under visible light irradiation in air. The material Al13–TCPP has good reusability, and the degradation rate of CEES can still reach 98.37% after being recycled five times.
Introduction
Mustard gas (HD) can severely damage the respiratory tract, surface skin and other parts in humans, and thus poses a great threat to the safety of human life.1 Currently, the degradation of HD is mainly achieved through hydrolysis, elimination, oxidation and other reactions.2 The greatest disadvantage of the first two methods is the slow rate of digestion,2–6 while the oxidation method has the risk of over-oxidation to produce mustard sulfone, which has an equivalent toxicity to HD.7,8 Singlet molecular oxygen (1O2) has mild oxidizability and can selectively oxidize HD/CEES to a non-toxic sulfoxide product.8–11 Therefore, the oxidative degradation of HD by 1O2 has attracted considerable attention in recent years.Porphyrin has a large π-conjugated electronic structure, a high absorption in the visible-near infrared region, and its structure is easily adjusted. As a commonly used photosensitizer, porphyrin has the ability to convert O2 in the inert triplet ground state (3O2) into the active 1O2.10,12 Porphyrin is widely used in photodynamic therapy13 and photocatalysis.14,15 However, the ability of 1O2 production from porphyrin is limited due to the easy self-aggregation of porphyrin molecules in solvents.16,17 In order to solve this problem, some efforts have been tried, e.g., fix porphyrin molecules on the surface of a carrier through coordination bonds to prevent their self-aggregation,18 obtain porphyrin nanoparticles19 through a limited non-covalent self-assembly process, and covalently link porphyrin with (oxo)metal units to form highly periodically isolated and dispersed porphyrin–metal–organic framework compounds.9,10,20–24 These efforts lead to a greatly increased quantum yield of 1O2 by porphyrin, and further improving the performance of porphyrin-based materials in the selective oxidation of mustard gas simulant CEES to sulfoxide under visible light irradiation. In this scenario, polyoxometalates, a class of metal oxygen clusters linked by central atoms and metal atoms (such as V, W and Mo, etc.25–27) and oxygen atoms,28,29 have a wide range of practical/potential applications in various fields30–33 can also be used for CEES decontamination, such as PMo12@PDDA-rGO34 and SiW10Cu2/GO.35 Notably, the reported porphyrin–polyoxometalate hybrids show more efficient photocatalytic oxidation ability for alcohols31 and rhodamine36 than porphyrins. The combination of polyoxometalate and porphyrin has a potential to take advantage of not only both the two individual components but also their possible cooperativity/synergistic effect to increase the 1O2 and O2˙− yield of porphyrin, which can be used in the selective oxidation of HD/CEES. Due to the oleophilic nature of CEES, its low solubility in pure water limits its hydrolysis rate in water. The mixture of organic solvents and water has been proved to increase the solubility of CEES in solvents and increase the hydrolysis rate.37,38 In addition, using protic solvent can effectively improve the selectivity of CEESO formation.39–41 Therefore, methanol and methanol–water mixture were selected as solvents for research.
Based on the above analysis, the photosensitive anionic salt meso-tetra (4-carboxyphenyl) porphyrin (TCPP4−) was selected to combine with the Keggin-type polyoxometalate [Al13O4(OH)24(H2O)12]7+ cationic metal cluster to synthesize a new polyhydroxyl aluminum cationic cluster-porphyrin anion hybrid as a prototype of porphyrin–polyoxometalate hybrids for the photocatalytic degradation of HD/CEES. As expected, this material can efficiently and selectively digest CEES in methanol and methanol–water solvent mixture due to generated O2˙− and 1O2 under visible light irradiation in air.
Experimental section
Instruments and reagents
The chemicals and reagents used in this study are analytically pure and used as received without further purification. Aluminum chloride hexahydrate (AlCl3·6H2O, 99%) and sodium hydroxide (98%) were purchased from Beijing Chemical Reagent Co., Ltd (Beijing, China). Pyrrole (99%), p-formylbenzoic acid (98%), and 1-benzoquinone (98%) was purchased from Shanghai Aladdin Reagent Co., Ltd (Shanghai, China). 9,10-Diphenylanthracene (98%) and nitroblue tetrazolium (98%) were purchased from Beijing Yancheng Technology Co., Ltd (Beijing, China). Glacial acetic acid, dimethyl sulfoxide (DMSO), dichloromethane and propionic acid were purchased from Beijing Chemical Plant (Beijing, China). Methanol was obtained from Tianjin Siyou Fine Chemicals Co., Ltd (Tianjin, China), and 2-chloroethyl ethyl sulfide (95%) was purchased from Beijing Bailingwei Technology Co., Ltd (Beijing, China). Thymol phthalein (98%) was purchased from Beijing Huawei Ruike Chemical Co., Ltd (Beijing, China). The meso-tetra (4-carboxyphenyl) porphyrin (H4TCPP) was synthesized according to the literature42 and characterized by Fourier transform infrared (FTIR) spectroscopy and proton nuclear magnetic resonance (1H NMR) spectroscopy. The ε-[Al13O4(OH)24(H2O)12]7+ solution and Al13–SO4 were prepared by a previously reported method43 (see ESI†).The chemical synthesis was performed using a CEL-LAB500 Multiposition Photochemical Reaction Apparatus (Beijing Zhongjiao Jinyuan Technology Co., Ltd, Beijing, China) equipped with a PLS-LAX500 long-arc xenon lamp (Beijing Zhongjiao Jinyuan Technology Co., Ltd) as light source. The working voltage was 50 V, the working current was 10 A, and the output power was 500 W. The spectrophotometric analysis was performed on a Shimadzu UV-2550 ultraviolet-visible (UV-visible) spectrophotometer (Shimadzu Corporation, Kyoto, Japan), in the wavelength range of 300–650 nm. Solid-state 27Al NMR spectra were recorded on an Avance AV600 NMR spectrometer (Bruker AXS GmbH, Karlsruhe, Germany). Infrared spectra were collected on an ICAN9 FTIR spectrometer (Tianjin Energy Spectrum Technology Co., Ltd, Tianjin, China), using KBr tablets in the wavenumber range of 400–4000 cm−1. The thermogravimetric derivative thermogravimetric (TG-DTG) analysis were performed on a Netzsch STA449 (Netzsch Gerätebau GmbH, Selb, Germany) using the following test conditions: the air flow temperature rises from 14 °C to 1000 °C at the rate of 10 °C min−1. The elemental analysis was performed using the Elementar Vario EL CUBE Elemental Analyzer (Elementar Analysensysteme GmbH, Hanau, Germany). The chromatography-mass spectrometry (GC-MS) analysis was performed on a Thermo Scientific Trace ISQ™ GC-MS system (Thermo Fisher Scientific Inc., Waltham, MA, USA), using the following test conditions: the size of the TR-WAXMS capillary column was 30 m × 0.25 mm × 0.25 μm; the carrier gas was helium, at the flow rate of 1 mL min−1. The programmed temperature rise parameters were as follows: hold at 40 °C for 3 min, heat to 230 °C at a rate of 20 °C min−1, and hold for 3 min. The injection mode was set to split injection, the temperature of the injection port was set to 220 °C, and the split ratio was adjusted to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200. The detector was an electron ionization (EI) source, the mass spectrometer ion source temperature was 250 °C, and the transmission line temperature was 230 °C. The X-ray diffraction (XRD) analysis was performed on a Rigaku D/max 2500 X-ray diffractometer (Rigaku Corporation, Tokyo, Japan) using a scanning range from 3–90° at the scanning speed of 10° min−1. The X-ray fluorescence Spectrometer (XRF) analysis was performed on a Shimadzu XRF-1800 (Shimadzu Corporation, Kyoto, Japan).
200. The detector was an electron ionization (EI) source, the mass spectrometer ion source temperature was 250 °C, and the transmission line temperature was 230 °C. The X-ray diffraction (XRD) analysis was performed on a Rigaku D/max 2500 X-ray diffractometer (Rigaku Corporation, Tokyo, Japan) using a scanning range from 3–90° at the scanning speed of 10° min−1. The X-ray fluorescence Spectrometer (XRF) analysis was performed on a Shimadzu XRF-1800 (Shimadzu Corporation, Kyoto, Japan).
Preparation of Al13–TCPP
H4TCPP (98 mg, 0.1 mmol) was dissolved in a 0.1 mol L−1 NaOH solution of 30 mL to obtain the sodium salt. Then, the salt was slowly added to the 40 mL of the prepared Al137+ solution, and a precipitate gradually formed during the dripping process. After carefully observing the change of the color of the solution, the dripping was immediately stopped when the color changed from dark green to red. After filtering the solution, the unreacted porphyrin and Al137+ were washed with deionized water until the filtrate became colorless. Subsequently, the filter cake was placed in an 80 °C oven for 8 hours, and eventually a brown–black solid of 310 mg was obtained (yield 92%, based on H4TCPP). The CHN elemental analysis and XRF analysis values of (C48H26N4O8) [Al13O4(OH)24(H2O)12]2(OH)10·18H2O were as follows: C: 17.05%, H: 4.71%, N: 1.49%, Al: 22.12%. The theoretical value: C: 17.16%, H: 5.04%, N: 1.67%, Al: 20.90%. IR data (KBr, cm−1): 1597 (s), 1408 (s), 801 (m), 651 (m), 545 (s), 495 (w).Experimental process of the degradation of CEES
A certain quantity (particle size 500 mesh) catalyst was placed in a 50 mL quartz reaction tube, and 5 mL of methanol or 5 mL of methanol–water solvent mixture (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added as the reaction solvent, followed by the addition of 5 μL of CEES. The reaction was started by stirring under a 500 W xenon lamp light source (λ > 400 nm). At different reaction times, a 100 μL aliquot of the reaction solution was taken from the reaction tube, mixed with 200 μL of blue reagent and 100 μL of absolute ethanol and the solution was then placed in a water bath at 80 °C for 15 min. After cooling to room temperature, 5 μL of acetic acid solution (6 mol L−1) and 3 mL of 95% ethanol were added, and then the absorbance was measured at 445 nm on a UV spectrophotometer. The CEES concentration remaining after the reaction was calculated according to the standard curve (Fig. S1†), and eqn (1) was used to calculate the rate of degradation of CEES.
1) was added as the reaction solvent, followed by the addition of 5 μL of CEES. The reaction was started by stirring under a 500 W xenon lamp light source (λ > 400 nm). At different reaction times, a 100 μL aliquot of the reaction solution was taken from the reaction tube, mixed with 200 μL of blue reagent and 100 μL of absolute ethanol and the solution was then placed in a water bath at 80 °C for 15 min. After cooling to room temperature, 5 μL of acetic acid solution (6 mol L−1) and 3 mL of 95% ethanol were added, and then the absorbance was measured at 445 nm on a UV spectrophotometer. The CEES concentration remaining after the reaction was calculated according to the standard curve (Fig. S1†), and eqn (1) was used to calculate the rate of degradation of CEES.
 | (1) |
Detection of CEES degradation products
Using methanol as solvent: after placing 5 mg of Al13–TCPP (particle size 500 mesh) in a 50 mL quartz reaction tube, 5 mL of methanol and 5 μL of CEES were added to the tube. After stirring under a 500 W xenon lamp (λ > 400 nm) and reacting for 180 min, the product of the reaction product was directly analyzed by GC-MS. Using methanol–water solvent mixture (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as solvent: other steps remained the same, the solvent mixture of methanol and water (5 mL, v/v = 1
1) as solvent: other steps remained the same, the solvent mixture of methanol and water (5 mL, v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added as the reaction solvent. After 90 min of reaction, 1 mL of dichloromethane was added to extract the product, and the product was analyzed by GC-MS.
1) was added as the reaction solvent. After 90 min of reaction, 1 mL of dichloromethane was added to extract the product, and the product was analyzed by GC-MS.
Singlet oxygen and superoxide radical capture experiments
Since 9,10-diphenylanthracene (DPA) is widely used for its ability to specifically capture 1O2, we used highly reactive DPA as an 1O2 trapping agent,44–46 and H4TCPP as a reference photosensitizer.47 H4TCPP and Al13–TCPP (1 × 10−5 M) were added to 1 × 10−4 M DPA methanol solution and irradiated with the 500 W xenon lamp (λ > 400 nm). The absorbance values of DPA at the wavelength of 371 nm at different irradiation times were recorded, and the quantum yield of 1O2 was estimated by comparing the slopes of the H4TCPP and Al13–TCPP linear-square fits (eqn (2)):46–49| Φ = ΦStd × K/KStd | (2) |
The nitroblue tetrazolium (NBT) was used to detect the generation of O2˙−, because O2˙− can react with NBT to form formazan, resulting in a decrease in the characteristic absorption peak of NBT at 256 nm.50,51 Add H4TCPP and Al13–TCPP (1 × 10−5 M) to the NBT methanol solution of 5 × 10−5 M, and irradiate with the 500 W xenon lamp (λ > 400 nm). The absorbance values of NBT at the wavelength of 256 nm were recorded for different irradiation times.
Results and discussion
Characterization of Al13–TCPP
The FTIR spectra of H4TCPP, Al13–SO4 and Al13–TCPP are shown in Fig. 1. In the FTIR spectrum of H4TCPP, the band at 1683 cm−1 corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration peak of COOH, and the C
O stretching vibration peak of COOH, and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibrational peaks of the porphyrin ring at 1596 cm−1. The bands at 1410 cm−1 and 1273 cm−1 are attributed to the vibration of –OH in COOH, and the band at 961 cm−1 is ascribed to the N–H stretching vibration peak of pyrrole.52,53 In the FTIR spectrum of Al13–TCPP, the characteristic stretching vibration bands of TCPP4− appear at wavenumber 1687, 1602 and 963 cm−1, but there is a slight blue shift compared with the characteristic vibration bands of H4TCPP. The characteristic stretching vibrational bands at 1410 and 1273 cm−1 corresponding to –OH in COOH almost disappear due to the interaction between carboxyl proton and Al137+. The stretching vibrational bands of Al–OTd, Al–OH and Al–OHOh in the ε-[Al13O4(OH)24(H2O)12]7+ cluster are present at 718, 592 and 544 cm−1, respectively.54,55 Compared with the corresponding characteristic absorption bands at 721, 613 and 549 cm−1 of ε [Al13O4(OH)24(H2O)12]7+ in Al13–SO4, there is a blue shift to a certain extent, indicating that H4TCPP and ε-[Al13O4(OH)24(H2O)12]7+ form a new substance through bond forces.
N stretching vibrational peaks of the porphyrin ring at 1596 cm−1. The bands at 1410 cm−1 and 1273 cm−1 are attributed to the vibration of –OH in COOH, and the band at 961 cm−1 is ascribed to the N–H stretching vibration peak of pyrrole.52,53 In the FTIR spectrum of Al13–TCPP, the characteristic stretching vibration bands of TCPP4− appear at wavenumber 1687, 1602 and 963 cm−1, but there is a slight blue shift compared with the characteristic vibration bands of H4TCPP. The characteristic stretching vibrational bands at 1410 and 1273 cm−1 corresponding to –OH in COOH almost disappear due to the interaction between carboxyl proton and Al137+. The stretching vibrational bands of Al–OTd, Al–OH and Al–OHOh in the ε-[Al13O4(OH)24(H2O)12]7+ cluster are present at 718, 592 and 544 cm−1, respectively.54,55 Compared with the corresponding characteristic absorption bands at 721, 613 and 549 cm−1 of ε [Al13O4(OH)24(H2O)12]7+ in Al13–SO4, there is a blue shift to a certain extent, indicating that H4TCPP and ε-[Al13O4(OH)24(H2O)12]7+ form a new substance through bond forces.
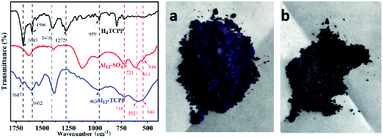 | ||
| Fig. 1 Infrared spectra of H4TCPP, Al13–SO4 and Al13–TCPP (left). Images of H4TCPP (a) and Al13–TCPP (b) samples (right). | ||
In the solid-state NMR spectrum of 27Al in Al13–TCPP (Fig. S2†), the spike at the 61.0 ppm chemical shift is ascribed to Al (AlTd) in the Al–O tetrahedron in the center of the Al137+ cationic cluster. The broad peak in the range of −90.5 to 0 ppm chemical shift corresponds to Al (AlOh) in the 12 Al–O octahedrons in the Al137+ cationic cluster structure. This result is basically consistent with the solid-state NMR shift of 27Al in the Keggin type Al137+ reported in the literature,56 indicating that the Al137+ in the Al13–TCPP material is indeed an ε-Keggin type structure.
The TG-DTG curve (Fig. S3†) reveals that the weight loss process of Al13–TCPP at room temperature to 800 °C mainly goes through three stages. In the first stage, the weight loss is 10.8% in the range of 25–120 °C, and this part loses 18 crystalline water molecules, with a theoretical value of 9.8%. In the second stage, the weight loss was 25.9% in the range of 120–440 °C, and the loss was due to the water formed by the condensation of the ligand water molecules and the Al–OH–Al group in the anion OH− and Al137+ cation cluster, with a theoretical value of 28.4%.57 In the third stage, the weight loss was 20.5% in the range of 440–650 °C, and the organic part of porphyrin TCPP was lost, with a theoretical value of 23.5%. There is no obvious weightlessness peak after 650 °C, and the main compound remaining is Al2O3, with an experimental value of 43.8% and a theoretical value of 38.3%. In general, the results of TG analysis (TGA) are basically consistent with the molecular formula obtained by elemental analysis.
The powder XRD pattern of Al13–TCPP shown in Fig. S4† reveals that Al13–TCPP has no obvious characteristic diffraction peak, suggesting that it has an amorphous structure.
Test of the degradation of CEES
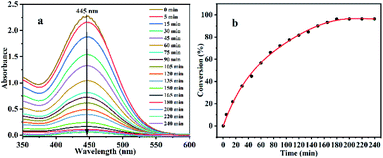
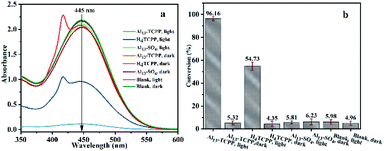
In this study, the photocatalytic degradation of the HD simulant CEES by Al13–TCPP in methanol was investigated. As shown in Fig. 2, the degradation rate of CEES increases gradually with the extension of the reaction time. When the reaction time is 180 min, the degradation rate of CEES reaches 96.16%, and with increasing reaction time, the increase of the degradation rate slows down significantly.In order to further understand the necessity of the active components and photoreaction conditions for the catalytic degradation of CEES in methanol, the degradation of CEES by Al13–TCPP, H4TCPP and Al13–SO4 in methanol with or without light was compared. As shown in Fig. 3, when the reaction time is 180 min, the best degradation rate of CEES by Al13–TCPP with light irradiation is 96.16%, but there is almost no degradation without light irradiation, indicating that light irradiation is a necessary reaction condition for catalytic oxidative degradation of CEES. H4TCPP is soluble in methanol and undergoes a homogeneous reaction (the absorption peak at the UV wavelength of 420 nm is the Soret band of H4TCPP).
With light irradiation, the degradation rate of CEES is only 54.73%, which is less than that of Al13–TCPP, indicating that the degradation rate of CEES can be enhanced by the activity of TCPP4− and Al137+ compared with that of H4TCPP. The degradation rate of CEES by Al13–SO4 with and without light irradiation is close to that of the blank group, indicating that Al13–SO4 alone has no obvious ability to degrade CEES.
HD and its simulant CEES can be hydrolyzed in water, but their lipophilic property makes their solubility in water low, which is limited by the mass transfer rate. Adding an organic solvent to water can improve the solubility of CEES in the solvent and increase its hydrolysis rate.58,59 In order to digest CEES more quickly through hydrolysis, this study further investigated the performance of Al13–TCPP in the degradation of CEES in methanol and water–methanol solvent mixtures (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
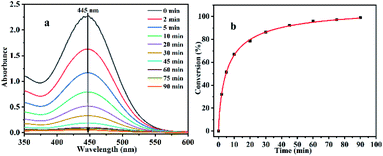
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). As shown in Fig. 4, when other reaction conditions remain unchanged, the results reveal that with the extension of the reaction time, the degradation rate of CEES by Al13–TCPP in solvent mixtures gradually increases, and when the reaction time is 90 min, the degradation rate of CEES reaches 99.01%.
1). As shown in Fig. 4, when other reaction conditions remain unchanged, the results reveal that with the extension of the reaction time, the degradation rate of CEES by Al13–TCPP in solvent mixtures gradually increases, and when the reaction time is 90 min, the degradation rate of CEES reaches 99.01%.
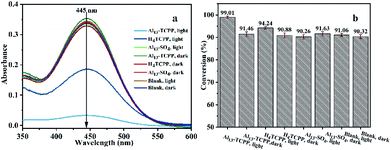
In order to further understand the active components of the materials for the catalytic degradation of CEES in methanol–water solvent mixtures and the requirement of light irradiation in the reaction, the degradation properties of Al13–TCPP, H4TCPP and Al13–SO4 in the methanol–water solvent mixtures were compared. As shown in Fig. 5, when the reaction time is 90 min, the degradation rates in methanol–water solvent mixtures in the blank control group with and without light irradiation are 91.06 and 90.32%, respectively, which are almost equal. The best degradation rate of CEES by Al13–TCPP with light irradiation is 99.01%, while the degradation performance without light irradiation (91.46%) is basically equal to that of the blank control group, indicating that light irradiation can promote the catalytic degradation of CEES by Al13–TCPP. The degradation rate of CEES by H4TCPP with light irradiation is 94.24%, and that without light irradiation is 90.88%, which is lower than that of CEES by Al13–TCPP. Al13SO4 with light irradiation is 90.26%. The degradation rate is basically equal to that of the blank control group in the methanol–water solvent mixture, indicating that Al13SO4 cannot promote the degradation of CEES.
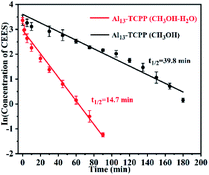
In this study, the kinetics of the catalytic degradation of CEES by Al13–TCPP in methanol and methanol–water solvent mixtures was studied. As shown in Fig. 6, the kinetic curve of the Al13–TCPP degradation of CEES is obtained by fitting the cooperative diagram of the ln (CCEES) to the reaction time, and the result is linear, which shows that the reaction follows first-order kinetics in methanol or in a methanol–water solvent mixture. The kinetic curves show that the half-life and reaction rate constant of Al13–TCPP in the degradation of CEES in methanol and methanol–water solvent mixtures are 39.8 min, −0.047 min−1 and 14.7 min, −0.017 min−1, respectively, indicating that the degradation rate of CEES by Al13–TCPP in methanol–water solvent mixtures is much higher than that in methanol.
In this study, the reusability of the catalyst (Al13–TCPP) in the photocatalytic degradation of CEES by Al13–TCPP in a methanol–water solvent mixture was investigated. After each addition of CEES, the reaction was conducted for 90 min, then CEES was added for another 90 minutes, and this process was repeated five consecutive times. As shown in Fig. S5,† the degradation rate was still 98.37% after five reaction cycles, indicating that Al13–TCPP has good reusability.
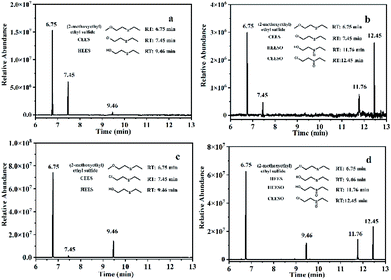
In order to study the photocatalytic degradation mechanism of CEES by Al13–TCPP in methanol solvent and methanol–water solvent mixture, the degradation products of CEES were analyzed by GC-MS (Fig. S6 and S7†). As shown in Fig. 7a and b, with light irradiation, CEES undergoes partial alcoholysis in a separate methanol solvent to form 2-methoxyethyl sulfide.60 A small amount of the hydrolysis product ethyl 2-hydroxyethyl sulfide (HEES) is generated through its hydrolysis and degradation due to the presence of a small amount of water in the solvent.55,61 When the catalyst Al13–TCPP is added to the methanol solvent, in addition to the formation of 2-methoxyethyl sulfide and a small amount of unreacted CEES, there are two oxidation products, namely ethyl hydroxyethyl sulfoxide (HEESO) and 2-chloroethyl ethyl sulfoxide (CEESO). This may be due to the production of reactive oxygen species (ROS) by Al13–TCPP in the presence of light and oxygen, which selectively oxidizes CEES and HEES into a non–toxic sulfoxide compound.10,62,63 As shown in Fig. 7c and d, methanol–water solvent mixtures mainly play the role of alcoholysis60 and hydrolysis55,61 on CEES with light irradiation, producing 2-methoxyethyl sulfide and HEES, respectively. In addition to 2-methoxyethyl sulfide and HEES, two new oxidation products, namely HEESO and CEESO, were formed after addition of the methanol–water solvent mixtures to the Al13–TCPP reaction for 90 min. The generation of CEESO is due to the direct oxidation of CEES by ROS produced by Al13–TCPP,10,64,65 and the generation of HEESO is due to the degradation of CEES by the methanol–water solvent mixture and Al13–TCPP. There are a large number of water molecules in the solvent mixtures, which may first hydrolyze CEES to HEES [48] through the mechanism of sulfur cation intermediate, and then Al13–TCPP produces ROS with light irradiation to continue to oxidize HEES to HEESO.63
In order to determine whether the ROS produced by Al13–TCPP degradation of CEES in methanol solvent are singlet oxygen or other kinds, ROS in the reaction process are identified by adding reactive oxygen scavengers. After ROS scavengers and ROS react, the degradation rate of CEES decreases. The methanol solvent itself can be used as a scavenging agent for hydroxyl radicals,66,67 so the participation of hydroxyl radicals in the degradation of CEES is basically excluded. Therefore, in this experiment O2˙− and 1O2 scavengers were added to determine the types of active oxygen, using benzoquinone as O2˙− scavenger65 and NaN3 as 1O2 scavenger.68 The experimental results listed in Table 1 show that the degradation rate of CEES is 96.16% without scavengers, 54.22 and 53.63% for CEES with benzoquinone or NaN3, respectively, and 26.53% for CEES with simultaneous addition of benzoquinone and NaN3, indicating that Al13–TCPP can degrade CEES by producing O2˙− and 1O2 in methanol solvent and with visible light irradiation. According to current reports,10,62 1O2 has mild oxidizability, and can selectively oxidize HD and its simulant CEES to non-toxic sulfoxide compounds, while O2˙− can also selectively oxidize sulfides to sulfoxide.64,65,69,70 Therefore, the hybrid used in this study accelerated the degradation of CEES by using singlet oxygen and superoxide ions produced by porphyrin as oxidants.
| Entry | Reactive oxygen scavenger | Reaction time (min) | Conversion rate (%) |
|---|---|---|---|
| a Reaction conditions: 500 W xenon lamp (λ > 400 nm), solvent volume: 5 mL methanol, catalyst dosage: 5 mg Al13–TCPP, benzoquinone (1 × 10−3 M), NaN3 (1 × 10−2 M), 5 μL CEES, reaction time: 180 min. | |||
| 1 | None | 180 | 96.16 |
| 2 | Benzoquinone | 180 | 54.22 |
| 3 | NaN3 | 180 | 53.63 |
| 4 | Benzoquinone + NaN3 | 180 | 26.53 |
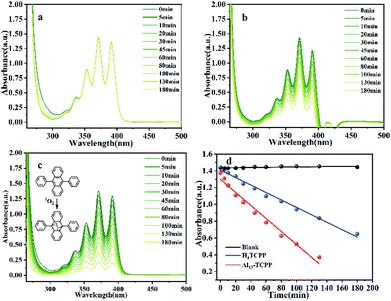
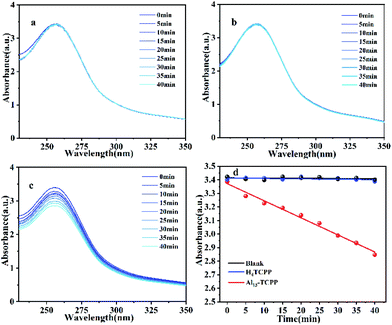
In order to determine the 1O2 and O2˙− production abilities of Al13–TCPP, DPA and NBT were used as 1O2 and O2˙− trapping agents, respectively, to capture two reactive oxygen species. As shown in Fig. 8, the absorption value of DPA at the maximum absorption wavelength of 371 nm decreases after the reaction of DPA with 1O2. The quantum yield of 1O2 can be calculated according to the change of the absorption intensity at 371 nm. The experimental results showed that the 1O2 quantum yields of Al13–TCPP and H4TCPP in methanol are Φ = 0.236 and 0.135, respectively, indicating that, compared with H4TCPP, the ionic Al13–TCPP hybrid formed by Al137+ and TCPP4− can increase the 1O2 quantum yield, and effectively overcome the problem that porphyrin is prone to self-aggregation in solvent and reduce the quantum yield of 1O2. As shown in Fig. 9, the absorbance changes of NBT in the blank control and H4TCPP are almost negligible. For the hybrid material Al13–TCPP, the absorbance of NBT at 256 nm gradually decreases with the prolongation of the illumination time, indicating that the Al13–TCPP hybrid can generate O2˙− to participate in CEES oxidative decontamination.
The above results show that CEES can spontaneously produce different degrees of alcoholysis and hydrolysis in methanol and methanol/water solvent mixtures (the rate is faster with the latter than with the former), resulting in non-toxic HEES and 2-hydroxymethyl ethyl sulfide. In the presence of Al13–TCPP and visible light irradiation, CEES and HEES produce the selective oxidation products HEESO and CEESO (Fig. S8†) caused by the 1O2 and O2˙−produced by Al13–TCPPwith light irradiation, thereby speeding up the digestion process.
Table S1† summarizes the performance of some catalysts for the photocatalytic digestion of CEES. Metal–organic frameworks (MOFs) have become the main catalysts for CEES decontamination due to their high stability and fast photocatalytic decontamination efficiency. But it is difficult to prepare in large quantities in a long time and in a short time, and most of the reaction processes need to be fed with pure O2. At present, most catalysts for degradation of CEES require a greater amount of use than the hybrid materials in this paper. In contrast, the hybrid materials synthesized in this paper have a simple preparation method and can be prepared in large quantities, in the air and visible light irradiation can generate 1O2 and O2˙− to achieve the degradation of CEES.
Conclusion
In this study, a new type of organic–inorganic ionic hybrid material, Al13–TCPP, was prepared. The photocatalytic degradation of CEES by Al13–TCPP in methanol and methanol–water solvent mixture (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was investigated. The results showed that the degradation rate of CEES was 96.16% in methanol for 180 min and 99.01% in methanol–water solvent mixture for 90 min. The degradation products of CEES were analyzed by GC-MS. The results showed that the degradation of CEES in methanol solvent is achieved through a combination of oxidation and alcoholysis mechanisms. The oxidation mechanism involves the selective oxidation of Al13–TCPP to non-toxic CEESO by using the O2˙− and 1O2 produced by CEES in air and under light irradiation, and Al13–TCPP has a higher quantum yield of 1O2 than H4TCPP. CEES is degraded through a combination of oxidation, hydrolysis and alcoholysis in the methanol–water solvent mixtures, and the final rapid degradation of CEES is the result of the interaction of Al13–TCPP material and methanol–water solvent mixtures. The material has good reusability and great potential application value in the degradation of HD. Considering the rich and diverse composition, structure, physicochemical properties and binding modes of polyoxometalate and porphyrin, this study provides a successful example for the design of porphyrin–polyoxometalate photosensitive systems with higher 1O2 quantum yield and synergistic effect between polyoxometalate and porphyrin for the efficient degradation of chemical warfare agents.
1) was investigated. The results showed that the degradation rate of CEES was 96.16% in methanol for 180 min and 99.01% in methanol–water solvent mixture for 90 min. The degradation products of CEES were analyzed by GC-MS. The results showed that the degradation of CEES in methanol solvent is achieved through a combination of oxidation and alcoholysis mechanisms. The oxidation mechanism involves the selective oxidation of Al13–TCPP to non-toxic CEESO by using the O2˙− and 1O2 produced by CEES in air and under light irradiation, and Al13–TCPP has a higher quantum yield of 1O2 than H4TCPP. CEES is degraded through a combination of oxidation, hydrolysis and alcoholysis in the methanol–water solvent mixtures, and the final rapid degradation of CEES is the result of the interaction of Al13–TCPP material and methanol–water solvent mixtures. The material has good reusability and great potential application value in the degradation of HD. Considering the rich and diverse composition, structure, physicochemical properties and binding modes of polyoxometalate and porphyrin, this study provides a successful example for the design of porphyrin–polyoxometalate photosensitive systems with higher 1O2 quantum yield and synergistic effect between polyoxometalate and porphyrin for the efficient degradation of chemical warfare agents.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The financial support from the Natural Science Foundation of China and kind support from Prof. Xue Duan of Beijing University of Chemical Technology are greatly acknowledged.Notes and references
- Y. C. Yang, J. A. Baker and J. R. Ward, Chem. Rev., 1992, 92, 1729–1743 CrossRef CAS.
- B. M. Smith, Chem. Soc. Rev., 2008, 37, 470–478 RSC.
- N. B. Munro, S. S. Talmage, G. D. Griffin, L. C. Waters, A. P. Watson, J. F. King and V. Hauschild, Environ. Health Perspect., 1999, 107, 933–974 CrossRef CAS PubMed.
- G. W. Wagner, P. W. Bartram, O. Koper and K. J. Klabunde, J. Phys. Chem. B, 1999, 103, 3225–3228 CrossRef CAS.
- G. W. Wagner, O. B. Koper, E. Lucas, S. Decker and K. J. Klabunde, J. Phys. Chem. B, 2000, 104, 5118–5123 CrossRef CAS.
- P. Horcajada, S. Surblé, C. Serre, D. Y. Hong, Y. K. Seo, J. S. Chang, J. M. Grenèche, I. Margiolaki and G. r. Férey, Chem. Commun., 2007, 2820–2822 RSC.
- M. J. Geraci, Ann. Pharmacother., 2008, 42, 237–246 CrossRef CAS PubMed.
- Y. Liu, C. T. Buru, A. J. Howarth, J. J. Mahle, J. H. Buchanan, J. B. DeCoste, J. T. Hupp and O. K. Farha, J. Mater. Chem. A, 2016, 4, 13809–13813 RSC.
- M. Cao, R. Pang, Q. Y. Wang, Z. Han, Z.-Y. Wang, X. Dong, S.-f. Li, S.-q. Zang and T. C. W. Mak, J. Am. Chem. Soc., 2019, 141, 14505–14509 CrossRef CAS PubMed.
- Y. Liu, S. Y. Moon, J. T. Hupp and O. K. Farha, ACS Nano, 2015, 9, 12358–12364 CrossRef CAS PubMed.
- Z. H. Long, D. Luo, K. Wu, Z. Y. Chen, M. M. Wu, X. P. Zhou and D. Li, ACS Appl. Mater. Interfaces, 2021, 13, 37102–37110 CrossRef CAS PubMed.
- Y. Z. Chen, Z. U. Wang, H. Wang, J. Lu, S. H. Yu and H. L. Jiang, J. Am. Chem. Soc., 2017, 139, 2035–2044 CrossRef CAS PubMed.
- M. Ethirajan, Y. Chen, P. Joshi and R. K. Pandey, Chem. Soc. Rev., 2011, 40, 340–362 RSC.
- M. Zhao, S. Ou and C. D. Wu, Acc. Chem. Res., 2014, 47, 1199–1207 CrossRef CAS PubMed.
- A. Fateeva, P. A. Chater, C. P. Ireland, A. Tahir, Y. Z. Khimyak, P. V. Wiper, J. R. Darwent and M. J. Rosseinsky, Angew. Chem., Int. Ed., 2012, 51, 7440–7444 CrossRef CAS PubMed.
- C. Tanielian, C. Wolff and M. Esch, J. Phys. Chem., 1996, 100, 6555–6560 CrossRef CAS.
- C. Tanlelian, C. Schwweitzer, R. Mechin and C. Wolff, Free Radical Biol. Med., 2001, 30, 208–212 CrossRef.
- Y. Zhang, J. Ma, D. Wang, C. Xu, S. Sheng, J. Cheng, C. Bao, Y. Li and H. Tian, Biomater. Sci., 2020, 8, 6526–6532 RSC.
- D. Wang, L. Niu, Z.-Y. Qiao, D.-B. Cheng, J. Wang, Y. Zhong, F. Bai, H. Wang and H. Fan, ACS Nano, 2018, 12, 3796–3803 CrossRef CAS PubMed.
- Y. Liu, A. J. Howarth, J. T. Hupp and O. K. Farha, Angew. Chem., Int. Ed., 2015, 54, 9001–9005 CrossRef CAS PubMed.
- C. T. Buru, M. B. Majewski, A. J. Howarth, R. H. Lavroff, C.-W. Kung, A. W. Peters, S. Goswami and O. K. Farha, ACS Appl. Mater. Interfaces, 2018, 10, 23802–23806 CrossRef CAS PubMed.
- M.-H. Xie, X.-L. Yang, C. Zou and C.-D. Wu, Inorg. Chem., 2011, 50, 5318–5320 CrossRef CAS PubMed.
- Z. H. Zhu, Y. Liu, C. Song, Y. Hu, G. Feng and B. Z. Tang, ACS Nano, 2021, 16, 1346–1357 CrossRef PubMed.
- C. F. Pereira, Y. Liu, A. Howarth, F. Figueira, J. Rocha, J. T. Hupp, O. K. Farha, J. P. C. Tomé and F. A. Almeida Paz, ACS Appl. Nano Mater., 2018, 2, 465–469 CrossRef.
- C. L. Hill, Mol. Catal., 1996, 114, 1–3 CrossRef CAS.
- C. L. Hill and C. M. McCartha, Coord. Chem. Rev., 1995, 143, 407–455 CrossRef CAS.
- J. Alcañiz-Monge, G. Trautwein, S. Parres-Esclapez and J. A. Maciá-Agulló, Microporous Mesoporous Mater., 2008, 115, 440–446 CrossRef.
- H. M. Asif, N. Qu, Y. Zhou, L. Zhang, F. K. Shehzad, Z. Shi, Y. Long and S. U. Hassan, Inorg. Chem. Front., 2017, 4, 1900–1908 RSC.
- C. L. Hill, Chem. Rev., 1998, 98, 1–2 CrossRef CAS PubMed.
- D. Schaming, C. e. Allain, R. Farha, A. Giraudeau, B. Hasenknopf, L. Ruhlmann, M. Goldmann and S. Lobstein, Langmuir, 2010, 26, 5101–5109 CrossRef CAS PubMed.
- T. Ishizuka, S. Ohkawa, H. Ochiai, M. Hashimoto, K. Ohkubo, H. Kotani, M. Sadakane, S. Fukuzumic and T. Kojima, Green Chem., 2018, 20, 1975–1980 RSC.
- G. Zhu, Y. Long, H. Ren, Y. Zhou, L. Zhang, Z. Shi, F. K. Shehzad and H. M. Asif, J. Phys. Chem. C, 2016, 120, 22549–22557 CrossRef CAS.
- S. u. Hassan, Y. Zhou, L. Zhang, Z. Shi, D. Yang, H. M. Asif and N. Qu, J. Phys. Chem. C, 2016, 120, 7757–7766 CrossRef CAS.
- Y. Wu, J. Dong, C. Liu, X. Jing, H. Liu, Y. Guo, Y. Chi and C. Hu, Dalton Trans., 2021, 50, 9796–9803 RSC.
- X. Zhang, Y. Li, Y. Li, S. Wang and X. Wang, ACS Appl. Nano Mater., 2019, 2, 6971–6981 CrossRef CAS.
- S. u. Hassan, Y. Zhou, L. Zhang, Z. Shi, D. Yang, H. M. Asif and N. Qu, Inorg. Chem., 2020, 59, 2575–2583 CrossRef PubMed.
- P. D. Bartlett and C. G. Swain, J. Am. Chem. Soc., 1949, 71, 1406–1415 CrossRef CAS PubMed.
- R. I. Tilley, Aust. J. Chem., 1993, 46, 293–300 CrossRef CAS.
- C. Ye, Y. Zhang, A. Ding, Y. Hu and H. Guo, Sci. Rep., 2018, 8, 2205–2210 CrossRef PubMed.
- F. Fringuelli, R. Pellegrino and F. Pizzo, Synth. Commun., 2006, 23, 3157–3163 CrossRef.
- X. Gu, X. Li, Y. Chai, Q. Yang, P. Li and Y. Yao, Green Chem., 2013, 15, 357–361 RSC.
- J. S. Lindsey and R. W. Wagner, J. Org. Chem., 1989, 54, 828–836 CrossRef CAS.
- M. Wang and M. Muhammed, Nanostruct. Mater., 1999, 11, 1219–1229 CrossRef CAS.
- T. Nakazono, A. R. Parent and K. Sakai, Chemistry, 2015, 21, 6723–6726 CrossRef CAS PubMed.
- C. Mongin, A. M. Ardoy, R. Mereau, D. M. Bassani and B. Bibal, Chem. Sci., 2020, 11, 1478–1484 RSC.
- N. Zheng, Z. Zhang, J. Kuang, C. Wang, Y. Zheng, Q. Lu, Y. Bai, Y. Li, A. Wang and W. Song, ACS Appl. Mater. Interfaces, 2019, 11, 18224–18232 CrossRef CAS PubMed.
- S. Liu, Y. Hua, C. Hua, Y. Xiong and M. Duan, J. Porphyrins Phthalocyanines, 2019, 23, 1084–1091 CrossRef CAS.
- J. Jin, Y. Zhu, Z. Zhang and W. Zhang, Angew. Chem., Int. Ed., 2018, 57, 16354–16358 CrossRef CAS PubMed.
- F. Nifiatis, J. C. Athas, K. D. D. Gunaratne, Y. Gurung, K. M. Monette and P. J. Shivokevich, Open Spectrosc. J., 2011, 5, 1–12 CrossRef CAS.
- Y. Zhao, X. Liang, H. Shi, Y. Wang, Y. Ren, E. Liu, X. Zhang, J. Fan and X. Hu, Chem. Eng. J., 2018, 353, 56–68 CrossRef CAS.
- H. Shi, J. Fan, Y. Zhao, X. Hu, X. Zhang and Z. Tang, J. Hazard. Mater., 2020, 381, 121006 CrossRef CAS PubMed.
- B. Zhuanga, X. Lia, R. Gea, S. Kanga, L. Qina and G. Lib, Appl. Catal., A, 2017, 533, 81–89 CrossRef.
- N. Sadeghia, S. Sharifniaa and M. S. Arabib, J. CO2 Util., 2016, 16, 450–457 CrossRef.
- C. M. Flynn Jr and T. Pope, Inorg. Chem., 1971, 10, 2524–2529 CrossRef.
- J. H. Son and Y. U. Kwon, Inorg. Chem., 2004, 43, 1929–1932 CrossRef CAS PubMed.
- J. H. Son, H. Choi, Y.-U. Kwon and O. H. Han, J. Non-Cryst. Solids, 2003, 318(1–2), 186–192 CrossRef CAS.
- J.-H. Son, H. Choi and Y.-U. Kwon, J. Am. Chem. Soc., 2000, 122(30), 7432–7433 CrossRef CAS.
- P. D. Bartlett and C. G. Swain, J. Am. Chem. Soc., 1948, 71, 1406–1415 CrossRef PubMed.
- R. I. Tilley, Aust. J. Chem., 1993, 46(3), 293–300 CrossRef CAS.
- R. M. Narske, K. J. Klabunde and S. Fultz, Langmuir, 2002, 18, 4819–4825 CrossRef CAS.
- J. T. Kloprogge, H. Ruan and R. L. Frost, J. Mater. Sci., 2001, 36, 603–607 CrossRef CAS.
- C. Changtonga, D. W. Carneya, L. Luoa, C. A. Zotoa, J. L. Lombardib and R. E. Connorsa, J. Photochem. Photobiol., A, 2013, 260, 9–13 CrossRef.
- L. J. Chen, S. Chen, Y. Qin, L. Xu, G. Q. Yin, J. Zhu, F. F. Zhu, W. Zheng, X. Li and H.-B. Yang, J. Am. Chem. Soc., 2018, 140, 5049–5052 CrossRef CAS PubMed.
- J. Jiang, R. Luo, X. Zhou, Y. Chen and H. Jia, Adv. Synth. Catal., 2018, 360, 4402–4411 CrossRef CAS.
- H. Guo, H. Xia, X. Ma, K. Chen, C. Dang, J. Zhao and B. Dick, ACS Omega, 2020, 5, 10586–10595 CrossRef CAS PubMed.
- J. S. Daniels and K. S. Gates, J. Am. Chem. Soc., 1996, 118(14), 3380–3385 CrossRef CAS.
- J. T. Schneider, D. S. Firak, R. R. Ribeiro and P. PeraltaZamora, Phys. Chem. Chem. Phys., 2020, 22, 15723–15733 RSC.
- M. Scholz, R. Dědic, T. Breitenbach and J. Hála, Photochem. Photobiol. Sci., 2013, 12, 1873–1884 CrossRef CAS PubMed.
- J. Jiang, R. Luo, X. Zhou, Y. Chen and H. Ji, Adv. Synth. Catal., 2018, 360, 4402–4411 CrossRef CAS.
- E. L. Clennan, Acc. Chem. Res., 2001, 34, 875–884 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra01821g |
| This journal is © The Royal Society of Chemistry 2022 |