Open Access Article
Open Access ArticleUnveiling the non-polar [3+2] cycloaddition reactions of cyclic nitrones with strained alkylidene cyclopropanes within a molecular electron density theory study†
Luis R. Domingo *a,
Mar Ríos-Gutiérrez
*a,
Mar Ríos-Gutiérrez a,
Rishikesh Chulanb,
M. H. H. Mahmoudc,
Mohamed M. Ibrahimc,
Salah M. El-Bahyd,
Lydia Rhyman
a,
Rishikesh Chulanb,
M. H. H. Mahmoudc,
Mohamed M. Ibrahimc,
Salah M. El-Bahyd,
Lydia Rhyman be and
Ponnadurai Ramasami
be and
Ponnadurai Ramasami *be
*be
aDepartment of Organic Chemistry, University of Valencia, Dr Moliner 50, E-46100 Burjassot, Valencia, Spain. E-mail: luisrdomingo@gmail.com
bComputational Chemistry Group, Department of Chemistry, Faculty of Science, University of Mauritius, Réduit 80837, Mauritius. E-mail: p.ramasami@uom.ac.mu
cDepartment of Chemistry, College of Science, Taif University, P.O. Box 11099, Taif 21944, Saudi Arabia
dDepartment of Chemistry, Turabah University College, Taif University, P.O. Box 11099, Taif 21944, Saudi Arabia
eCentre for Natural Product Research, Department of Chemical Sciences, University of Johannesburg, Doornfontein, Johannesburg 2028, South Africa
First published on 6th September 2022
Abstract
The role of cyclopropane substitution on the ethylene in zw-type [3+2] cycloaddition (32CA) reactions of cyclic nitrones has been studied within Molecular Electron Density Theory (MEDT) at the ωB97X-D/6-311G(d,p) computational level. Electron Localization Function (ELF) analysis of the ethylenes shows that the presence the cyclopropane only slightly increases the electron density in the C–C bonding region. Analysis of the Conceptual DFT reactivity indices indicates that the presence of the cyclopropane does not produce any remarkable change in the reactivity of these strained ethylenes. The marginal electrophilic character of ethylene makes the zw-type 32CA reactions of non-polar character. The presence of the cyclopropane in the ethylene decreases the activation enthalpy of the 32CA reactions by only 1.7 and 2.6 kcal mol−1, and also decreases the ortho regioselectivity. The loss of the strain present in the cyclopropane is responsible for the reduction of the activation enthalpy and the increase of the reaction enthalpy in these non-polar 32CA reactions. The presence of the cyclopropane does not cause any change, neither in the transition state structure (TS) geometries nor in their electronic structure. The very low global electron density transfer (GEDT) computed at the TSs confirms the non-polar character of these 32CA reactions. The ortho regioselectivity experimentally observed in these non-polar 32CA reactions is determined by the most favorable two-center interaction between the less electronegative C1 carbon of nitrone and the non-substituted methylene C5 carbon of the ethylenes.
1. Introduction
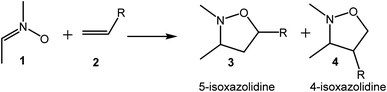
[3+2] Cycloaddition (32CA) reactions are one of the most efficient synthetic methods for the construction of five-membered heterocyclic compounds due to their ability to build organic cyclic motifs with regio- and/or stereoselectively.1–5 The 32CA reaction of nitrone 1 to an ethylene 2 is a classic synthetic strategy to generate isoxazolidines which constitute an interesting class of five-membered heterocycles which serve as valuable synthetic intermediates of fundamental significance in academia and industry.6,7 The use of non-symmetric ethylenes such as 2 can afford the formation of two regioisomeric 5- and 4-isoxazolidines 3 and 4 (see Scheme 1).The understanding of the 32CA reactions is a challenge for organic chemists as a consequence of the chameleonic electronic structures of the three-atom-components (TACs) participating in these reactions. Recent Molecular Electron Density Theory8 (MEDT) studies on 32CA reactions of a wide diversity of TACs towards ethylene allowed establishing a good correlation between the electronic structure of TACs and their reactivity in 32CA reactions.9 Nitrones are zwitterionic TACs participating in zw-type 32CA reactions with high activation energy towards ethylene 2 (R = H).10,11 Due to the nucleophilic character of nitrones, their zw-type 32CA reactions are accelerated by increasing the electrophilic character of the ethylene. However, the 32CA reactions with electron-rich ethylenes does not take place easily,11 demanding the use of Lewis acid catalysts.12
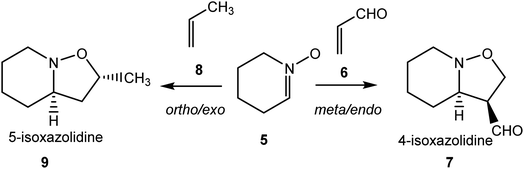
The zw-type 32CA reactions of cyclic nitrone 5 with acrolein 6 and with propene 8 have been recently studied within the MEDT (see Scheme 2).11 The reactivity and selectivity were found to be very dependent on the polar character of the reaction. Due to the strong nucleophilic character of cyclic nitrone 5, the zw-type 32CA reaction with acrolein 6 presented a very low activation energy of 1.4 kcal mol−1, the reaction being completely meta regio- and endo stereoselective yielding the 4-isoxazolidine 7, in complete agreement with the experimental outcome.13 Interestingly, the 32CA reaction with the nucleophilic propene 8 presented a higher activation energy, 7.7 kcal mol−1, and a reverse ortho regio- and exo stereoselectivity, yielding the 5-isoxazolidine 9.11
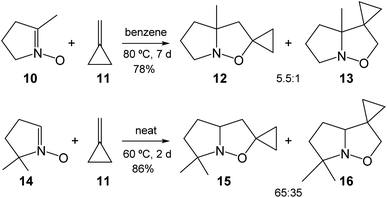
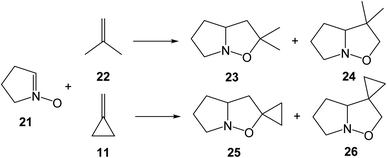
Strained compounds are an interesting topic in organic chemistry as they enable the reactions to occur under milder conditions compared to unstrained species. In addition, these reactions permit the interplay between experiment and theory. In 1986, Brandi et al. reported an experimental study on the 32CA reaction of the cyclic nitrone 10 with methylenecyclopropane (MCP) 11, a strained ethylene, yielding a mixture in the ratio of 5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the regioisomeric isoxazoline-spiro derivatives 12 and 13 (see Scheme 3).14 Likewise, the 32CA reaction of cyclic nitrone 14 with MCP 11 afforded a mixture of 15 and 16. These 32CA reactions required high temperature (above 60 °C) and long reaction time (more than 2 days). The reactions were highly regioselective yielding the 5-isoxazolidines 12 and 15 as the major products.14
1 for the regioisomeric isoxazoline-spiro derivatives 12 and 13 (see Scheme 3).14 Likewise, the 32CA reaction of cyclic nitrone 14 with MCP 11 afforded a mixture of 15 and 16. These 32CA reactions required high temperature (above 60 °C) and long reaction time (more than 2 days). The reactions were highly regioselective yielding the 5-isoxazolidines 12 and 15 as the major products.14
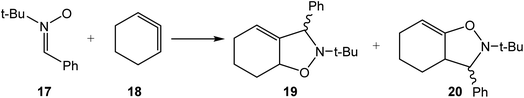
Thus, in 2017, the 32CA reaction of nitrone 17 with strained 1,2-cyclohexadiene 18 was studied within MEDT (see Scheme 4).15 This non-polar 32CA reaction showed low stereo- and regioselectivity. The strain presents in 1,2-cyclohexadiene 18 causes the easy creation of a pseudoradical center16 at the central carbon of the strained allene 18 at the beginning of the reaction with a relatively low energy cost, as it immediately promotes the formation of the first C–C single bond formation with an activation enthalpy of only 7.0 kcal mol−1.15 This scenario was completely different from that of the 32CA reaction involving the simplest allene, which presented an activation enthalpy of 27.0 kcal mol−1.15
Recently, Pagliai et al. have reported a DFT study at the PBE0-GD3/6-311G(d,p) computational level of the 32CA reactions of cyclic nitrone 21 with methylpropene (MP) 22 and with a series of alkylidene cyclopropanes, including MCP 11, in order to explain the regioselectivity in these 32CA reactions (see Scheme 5).17 These authors concluded that the electronic effect of the substituents (donor or attractor groups) at the double bond of the ethylenes determines the polarization of the double bond and the reaction mechanism, consequently determining the electrostatic interactions with nitrones and controlling the regioselectivity of these 32CA reactions. However, regioselectivity in 32CA reactions is determined by the more favorable two-center interactions taking place at the transition state structures (TSs) along the C(O)–C single bond formation.11,18
The poor reactivity of strained MCP 11 together with the loss of the complete ortho regioselectivity as observed experimentally in the 32CA reactions of MP 22 prompted us to study the 32CA reactions of cyclic nitrones 14 and 21 with MP 22, MCP 11 and dicyclopropylethylene (DCP) 27 within MEDT (see Chart 1). The main purpose of this theoretical study is to understand how the strain present in MCP 11 and DCP 27 modifies the electronic structure and reactivity of these strained ethylenes, and to explain the origin of the ortho regioselectivity in these 32CA reactions.
2. Computational details
The ωB97X-D19 functional, together with the standard 6-311G(d,p)20 basis set, which includes d-type polarization for second-row elements and p-type polarization functions for hydrogens, were used throughout this MEDT study. The suitability of this DFT computational level for the study of these non-polar zw-type 32CA reactions was asserted by performing CCSD(T)/cc-pVTZ single point energy calculations at the stationary points involved in the 32CA reaction between cyclic nitrone 17 and MCP 7 (see Table S2 in the ESI†).11 In addition, several studies of 32CA reactions emphasised that the inclusion of diffuse functions produces no notable changes in the relative energies.21 The TSs were characterized by the presence of only one imaginary frequency. The Berny method was used in the optimizations.22,23 The intrinsic reaction coordinate24 (IRC) calculations were performed to establish the unique connection given between the TSs and the corresponding minima.25,26 Solvent effects of benzene were considered by full optimization of the gas-phase structures at the same computational level using the polarizable continuum model (PCM)27,28 in the framework of the self-consistent reaction field (SCRF).29–31 Enthalpies, entropies and Gibbs free energies were calculated in benzene at 80 °C and 1 atm.20The GEDT32 values were computed using the equation GEDT(f) = Σqf, where q are the natural charges33,34 of the atoms belonging to one of the two frameworks (f) at the TS geometries. Global and local Conceptual DFT (CDFT) indices35,36 were calculated using the equations in ref. 36.
The Gaussian 16 suite of programs was used to perform the calculations.37 Electron Localization Function38 (ELF) analyses of the ωB97X-D/6-311G(d,p) monodeterminantal wavefunctions were done by using the TopMod39 package with a cubical grid of step size of 0.1 Bohr. Molecular geometries and ELF basin attractors were visualized by using the GaussView program.40
3. Results and discussion
The present MEDT study has been divided into six sections: (i) first, the ELF topological analysis at the ground state (GS) of the reactants is performed to know their electronic structures; (ii) in the second part, the chemical behaviors of the reagents is studied from the analysis of reactivity indices defined within the CDFT; (iii) in the third part, the energy profile of the 32CA reactions of cyclic nitrone 21 with MP 22, MCP 1 and DCP 27, and that of cyclic dimethyl nitrone 14 with MCP 11 is studied; (iv) in the fourth part, a BET analysis along the more favorable ortho reaction path associated with the 32CA reaction of cyclic nitrone 21 with MCP 11 is performed; (v) an ELF comparative analysis of the ortho TSs involved in the 32CA reactions of cyclic nitrone 21; and finally, (vi) the ortho regioselectivity observed in these non-polar zw-type 32CA reactions is analyzed.3.1 ELF analysis of the electronic structures of cyclic nitrones 14 and 21, and the MP 22, MCP 11 and DCP 27
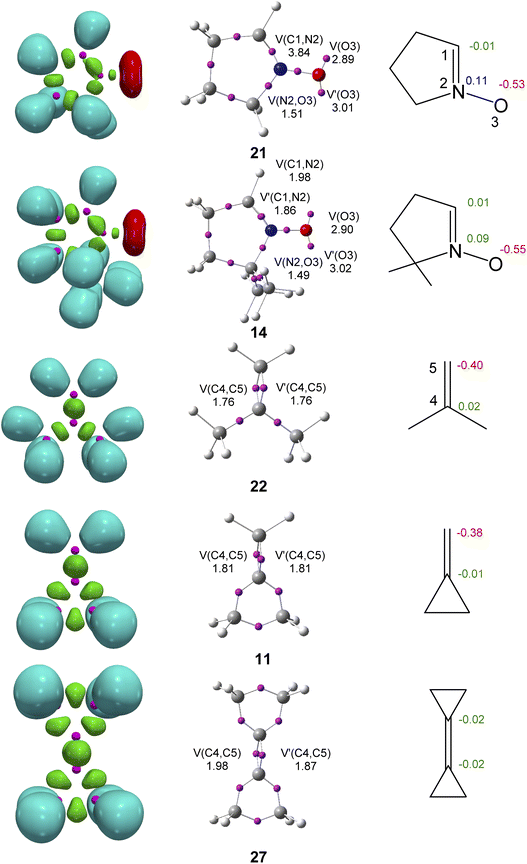
ELF38 topological analysis of the electronic structure of cyclic nitrones 14 and 21, and the MP 2, MCP 11 and DCP 27 was first performed (Fig. 1) to correlate the electronic structure with reactivity.41 Topological analysis of the ELF of cyclic nitrone 21 shows the presence of one disynaptic basin, V(C1,N2), integrating 3.84e, associated with a C1–N2 double bond, and one disynaptic basin, V(N2,O3), integrating 1.51e, associated with a depopulated N2–O3 single bond. ELF also shows the presence of two monosynaptic basins, V(O3) and V′(O3), integrating a total of 5.90e, associated with the non-bonding electron density of the O3 oxygen. ELF of the cyclic dimethyl nitrone 14 is very similar to that of nitrone 21. The only difference is the split of the V(C1,N2) disynaptic basin present in nitrone 21 into two disynaptic basins, V(C1,N2) and V′(C1,N2), integrating a total of 3.84e. Due to the absence of pseudoradical and carbenoid centres, cyclic nitrones 14 and 21 are classified as zwitterionic TACs participation in zw-type 32CA reactions.9On the other hand, ELF topological analyses of MP 22, MCP 11 and DCP 27 show the presence of two disynaptic basins V(C4,C5) and V′(C4,C5) integrating a total of 3.52e, 3.62e and 3.85e, respectively, associated with the C4–C5 double bond. The inclusion of the cyclopropane group in the ethylene increases the electron density in the C4–C5 bonding region.
The natural atomic charges of these compounds, obtained by a natural population analysis33,34 (NPA), were analyzed (see Fig. 1). At cyclic nitrones 21 and 14, while the C1 carbon is negligibly charged, the O3 oxygen in negatively charged by ca. −0.54e. The N2 nitrogen are positively charged by ca. 0.10e. On the other hand, while the C5 carbon of MP 22 and MCP 11 is negatively charged by ca. −0.39e, the C4 is negligibly charged, ca. 0.00e. The loss of the strain present at the cyclopropyl ring of MCP 11 only slightly increases the polarization of the C4–C5 double bond of MP 22. At DCP 27 the two ethylene C4 and C5 carbons are negligibly charged, −0.02e. The negative charges shown at the C5 carbons of MP 22 and MCP 11 is a consequence of the more electronegative character of the carbon atom than the hydrogen one.
3.2 Analysis of the CDFT reactivity indices of the reagents
The CDFT indices were calculated at B3LYP/6-31G(d) level of theory as it was used to define electrophilicity and nucleophilicity scales.36 The global reactivity indices, namely, the electronic chemical potential, μ, chemical hardness, η, electrophilicity, ω, and nucleophilicity, N, at the GS of cyclic nitrones 14 and 21, and the ethylenes MP 22, MCP 11 and DCP 27 are summarized in Table 1.| μ | η | ω | N | |
|---|---|---|---|---|
| Nitrone 21 | −2.90 | 5.47 | 0.77 | 3.48 |
| Dimethyl nitrone 14 | −2.83 | 5.54 | 0.72 | 3.53 |
| MCP 11 | −3.22 | 7.27 | 0.71 | 2.27 |
| DCP 27 | −3.07 | 6.87 | 0.69 | 2.62 |
| MP 22 | −2.84 | 7.37 | 0.55 | 2.60 |
The electronic chemical potentials42 of cyclic nitrones 21 and 14, μ = −2.90 and −2.83 eV, are close to those of the ethylene derivatives, μ = −3.22 (11), −3.07 (27) and −2.84 (22) eV, indicating that the corresponding 32CA reactions will have low or non-polar character.
The electrophilicity ω indices43 of cyclic nitrones 21 and 14 are 0.77 and 0.72 eV, while the nucleophilicity N indices44 are 3.48 and 3.53 eV, respectively, thus being classified as marginal electrophiles and strong nucleophiles within the electrophilicity and nucleophilicity scales.36 The presence of the two electron-releasing methyl substituents in nitrone 14 slightly increases the nucleophilcity and decreases the electrophilicity with respect to cyclic nitrone 21.
On the other hand, the electrophilicity ω and nucleophilicity N indices of MP 22 are 0.55 eV and 2.60 eV, respectively, thus being classified as a marginal electrophile and as moderate nucleophile. Thus, MP 22 will participate in polar reactions only towards strong electrophilic species. The inclusion of one or two cyclopropane rings in the ethylene increases the electrophilicity of MCP 11 and DCP 27 to 0.71 eV and 0.69 eV, respectively, while this substitution decreases the nucleophilicity N index to 2.27 eV in the case of MCP 11 but makes no changes in the N index of DCP 27, 2.62 eV. In any case, the inclusion of the cyclopropane rings does not produce any remarkable change in the reactivity of MCP 11 and DCP 27 with respect to MP 22.
Consequently, despite the strong nucleophilic character of cyclic nitrones 14 and 21, the marginal electrophilic character of MP 22, MCP 11 and DCP 27 makes that the corresponding 32CA reactions will have non-polar character, in agreement with the similar electronic chemical potentials. Consequently, these zw-type 32CA reactions will be classified as of null electron density flux (NEDF).45,46
3.3. Study of the 32CA reactions of cyclic nitrone 21 with MP 22, MCP 11 and DCP 27, and that of cyclic dimethyl nitrone 14 with MCP 11
Due to the non-symmetry of ethylenes MP 22 and MCP 11, two regioisomeric reactions paths, namely meta and ortho, are feasible for the 32CA reactions involving these species (see Scheme 6); while the meta reaction paths are associated with the formation of the C1–C5 and O3–C4 single bonds, the ortho reaction paths are associated with the formation of the O3–C5 and C1–C4 single bonds. These 32CA reactions take place through one-step mechanism involving only one TS. The relative enthalpies and Gibbs free energies, computed at 80 °C in benzene, are given in the Table 2, while the total electronic energies and thermodynamic data are given in the Tables S3 and S4 in ESI.†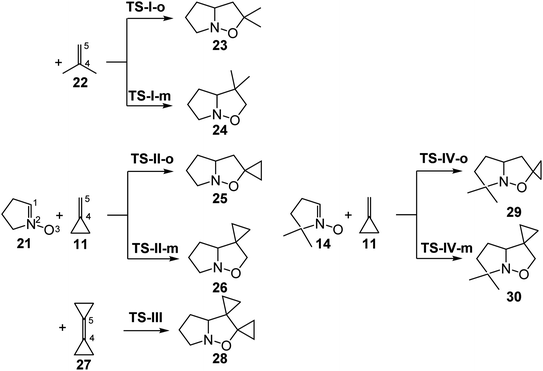 | ||
| Scheme 6 Competitive reaction paths associated with the 32CA reactions of cyclic nitrone 21 with MP 22, MCP 11 and DCP 27, and that of cyclic dimethyl nitrone 14 with MCP 11. | ||
| ΔH | ΔS | ΔG | |
|---|---|---|---|
| TS-I-o | 13.8 | −46.0 | 30.0 |
| TS-I-m | 18.3 | −47.3 | 35.0 |
| 23 | −27.8 | −49.9 | −10.1 |
| 24 | −24.3 | −51.7 | −6.0 |
| TS-II-o | 12.1 | −45.1 | 28.0 |
| TS-II-m | 13.8 | −44.7 | 29.6 |
| 25 | −36.7 | −50.1 | −19.0 |
| 26 | −34.0 | −48.3 | −17.0 |
| TS-III | 11.2 | −46.2 | 27.5 |
| 28 | −46.3 | −52.6 | −27.7 |
| TS-IV-o | 13.4 | −43.3 | 28.7 |
| TS-IV-m | 14.7 | −42.7 | 29.8 |
| 29 | −32.5 | −52.7 | −13.9 |
| 30 | −30.9 | −46.4 | −14.5 |
These zw-type 32CA reactions present activation enthalpies in the range of 11.2–13.4 kcal mol−1, the reactions being exothermic by between 27.8 – 46.3 kcal mol−1. Some appealing conclusions can be obtained from the relative enthalpies given in Table 2: (i) the activation enthalpy of the 32CA reaction of cyclic nitrone 21 with MP 22 presents a high value, 13.8 kcal mol−1. Note that the unfavorable activation enthalpy for the non-polar zw-type 32CA reaction of cyclic nitrone 21 with ethylene 31 is 13.3 kcal mol−1 (see Table S1 in ESI†); (ii) the presence of the cyclopropane in MCP 11 and DCP 27 reduces the activation enthalpy of the 32CA reactions with cyclic nitrone 21 by only 1.7 and 2.6 kcal mol−1, respectively, with respect to that with MP 11; (iii) the presence of the two methyl groups in cyclic nitrone 14 increases the activation enthalpy of the 32CA reaction with MCP 11 by 1.3 kcal mol−1 with respect to cyclic nitrones 21; (iv) the 32CA of cyclic nitrone 21 with MP 22 is completely ortho regioselective as TS-I-m is located 4.5 kcal mol−1 above TS-I-o, (v) a reduction of the regioselectivity in the 32CA reactions of nitrones 21 and 14 with MCP 11 is observed, as the meta TSs are located 1.7 and 1.3 kcal mol−1, respectively, above the ortho TSs; (vi) the strong exothermic character of these 32CA reactions make them irreversible. Consequently, the corresponding isoxazoline-5-spiro derivatives are obtained by a kinetic control; and (vii) the exothermic character of the 32CA reactions involving nitrone 21 increases in the order −27.8 (23) < −36.7 (25) < −56.3 (28) kcal mol−1. Thus, the inclusion of the cyclopropane in the ethylene framework increases the exothermic character of the 32CA reaction as a consequence of the loss of the strain present at MCP 11 and DCP 27 at the cycloadducts 25 and 28.
Inclusion of entropies and the reaction temperature to enthalpies increases the relative Gibbs free energies by between 15.1 and 18.6 kcal mol−1 due to the unfavorable activation entropies associated with these bimolecular reactions, which are found in the range −42.7 and −52.7 cal mol−1 K−1 (see in Table 2) The activation Gibbs free energies associated with these 32CA reactions via the more favorable ortho TSs rise between 27.5 and 30.0 kcal mol−1, while formation of isoxazoline-5-spiro derivatives remains exergonic by more than 10.1 kcal mol−1. Consequently, isoxazoline-5-spiro derivatives are formed by kinetic control.
Considering the Gibbs free energies associated to meta and ortho TSs given in Table 2, 4.4 (I), 1.6 (II) and 1.3 (III) kcal mol−1, and the temperature reaction, 80 °C, the following relationship between the two regioisomeric isoxazoline-5-spiro and isoxazoline-4-spiro derivatives can be estimated by using the Eyring–Polanyi relation:47 99.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 (I), 90.0
0.3 (I), 90.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.0 (II) and 86.4
10.0 (II) and 86.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.6 (III). The ortho regioselectivities found at the ωB97X-D/6-311G(d,p) computational level are in complete agreement with the experimental results in which the 32CA reactions of MP 22 are completely ortho regioselective, while the ortho regioselectivity decreases in the order I > IV.
13.6 (III). The ortho regioselectivities found at the ωB97X-D/6-311G(d,p) computational level are in complete agreement with the experimental results in which the 32CA reactions of MP 22 are completely ortho regioselective, while the ortho regioselectivity decreases in the order I > IV.
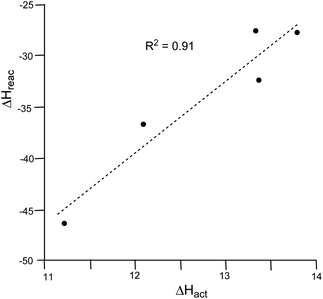
A representation of the reaction enthalpies versus the activation enthalpies for the 32CA reactions of cyclic nitrone 21 with MP 22, MCP 11, DCP 27 and ethylene 31, and that of cyclic dimethyl nitrone 14 with MCP 11, shows that there is a good linear correlation between these enthalpies (R2 = 0.91) (see Fig. 2). Thus, the loss of the strain present in the cyclopropane derivatives MCP 11 and DCP 27 along the formation of the corresponding isoxazoline-5-spiro derivatives might be responsible for both the reduction of the activation enthalpy and the increase of the reaction enthalpy in these 32CA reactions.
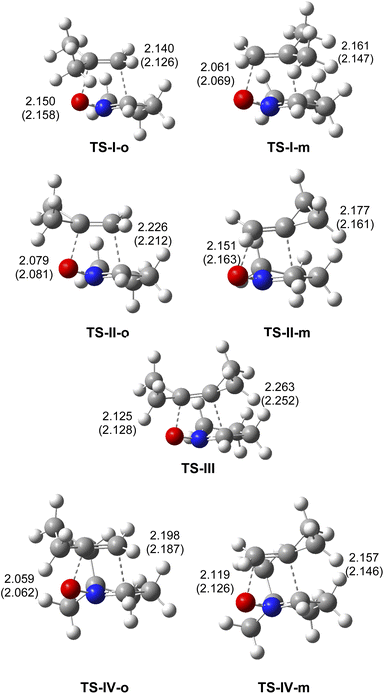
The geometries of the TSs involved in the 32CA reactions of cyclic nitrone 21 with MP 22, MCP 11 and DCP 27, and that of cyclic dimethyl nitrone 14 with MCP 11 are given in Fig. 3. In gas phase, the distances between the carbon and oxygen atoms participating in the 32CA reactions are found in the range of 2.05 and 2.26 Å, indicating a low geometrical asymmetry of both frameworks at the TSs. The C–C distances are slightly longer than that at TS-V, associated with the non-polar zw-type 32CA reaction of cyclic nitrone 21 with ethylene 31, 2.141 Å, while the C–O distances are slightly shorter, 2.111 Å (see the geometry of TS-V in ESI). Consequently, the presence of the cyclopropane in the ethylene framework does not produce remarkable changes in the geometries. Considering that the formation of the C–C and C–O single bond take place in the short range of 2.0–1.9 and 1.8–1.7 Å, respectively,9 these distances indicate the formation of any C–C and C–O single bonds have not yet begun at these TS (see later).
Inclusion of solvent effects of benzene in the geometry optimizations does not produce any remarkable change in the TS geometries (see Fig. 3). In benzene, the TSs are only slightly more delayed.
The non-polar nature of these 32CA reactions was characterized by evaluating the GEDT32 at the corresponding TSs. The very low GEDT values found at the TSs, −0.01e (TS-I-o), 0.01e (TS-I-m), 0.04e (TS-II-o), 0.06e (TS-II-m), 0.06e (TS-III), 0.02e (TS-IV-o) and 0.04e (TS-IV-m), establish the non-polar character of these 32CA reactions. The non-polar character of these 32CA reactions is a consequence of the marginal electrophilic character of these ethylene derivatives (see Table 1). Despite of this, the GEDT slightly increases in the order MP 22 > MCP 11 > DCP 27. The very low GEDT computed at the most favorable ortho TSs allows classifying these 32CA reactions as of NEDF,46 in clear agreement with the analysis of the CDFT indices.
3.4 BET analysis along the more favorable ortho reaction path associated with the 32CA reaction of cyclic nitrone 21 with MCP 11. Characterization of the zw-type mechanism
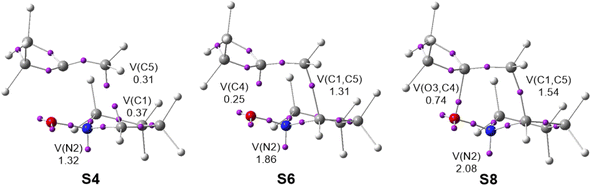
In order to characterize the C–C single bond formation along the 32CA reaction between cyclic nitrone 21 and MCP 11, and thus characterize the type of 32CA reaction, a Bonding Evolution Theory48 (BET) study along the more favorable ortho reaction path was performed. BET allows a full description of the bonding changes along a reaction path by analyzing the changes in the topology of the ELF at all the structures of the corresponding IRC path and selecting those which are chemically meaningful. Complete BET data are given in Table S5,† while ELF basin attractor positions of the more relevant structures involved in the formation of the new C1–C5 and O3–C4 single bonds are represented in Fig. 4.The most relevant mechanistic aspects obtained from the BET analysis can be summarized as follows: (i) at structure S2 a new V(N2) monosynaptic basin is created with an initial population of 0.91e. This monosynaptic basin, whose population is increased along the IRC, is associated with the non-bonding electron density present at the final isoxazoline-5-spiro 25. The electronic changes required for the formation of the V(N2) monosynaptic basin have an energy cost from structure S1 of 16.3 kcal mol−1; (ii) at structure S4, two monosynaptic basins, V(C1) and V(C5), integrating 0.37 and 0.31e, respectively, are simultaneously created (see Fig. 4). These monosynaptic basins are related to the C1 and C5 pseudoradical centres16 required for the subsequent C1–C5 single bond formation;32 (iii) at structure S5, a new V(C4) monosynaptic basin, integrating 0.18e, is created. It is required for the formation of the second O3–C4 singe bond; (iv) at structure S6, the first more relevant topological change along the IRC takes place. While the two V(C1) and V(C5) monosynaptic basins present at structure S5 have disappeared, a new V(C1,C5) disynaptic basin, with an initial population of 1.31e, is created (see Fig. 4). These topological changes indicate that the new C1–C5 single bond has been created at a C–C distance of 2.01 Å by the C-to-C coupling of the two C1 and C5 pseudoradical centers; (v) at structure S7, the two V(O3) and V′(O3) monosynaptic basins associated with the non-bonding electron density of the nitrone O3 oxygen split into three monosynaptic basins, V(O3), V′(O3) and V′(O3); and finally, (vi) at structure S8 the second more relevant topological change along the IRC takes place. While the two V′′(O3) and V(C4) monosynaptic basins present at S7 have disappeared, a new V(O3,C4) disynaptic basin, with an initial electron density of 0.72e, is created (see Fig. 4). These topological changes indicate that the new O3–C4 single bond has been created at a C–O distance of 1.76 Å by the sum of the electron density of the two V′′(O3) and V(C4) monosynaptic basins.
Some relevant conclusions can be obtained from this BET analysis: (i) the bonding changes required to reach the TS are mainly associated to the depopulation (rupture) of the C1–N2 and C4–C5 bonding regions required for the formation of the non-bonding N2 electron density and the two C1 and C5 pseudoradical centres; (ii) the energy cost associated to these changes from the first structure of the IRC, S1, rises to 17.9 kcal mol−1; (iii) these behaviors are characteristic of a non-polar zw-type 32CA reaction;11 and finally, (iv) this BET analysis clearly characterizes the asynchronous non-concerted nature of the molecular mechanism of this zw-type 32CA reactions.
3.5 ELF comparative analysis of the ortho TSs involved in the 32CA reactions of cyclic nitrone 21
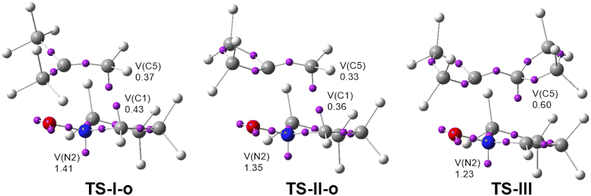
In order to analyze the role of the cyclopropane substitution in these non-polar zw-type 32CA reactions, a comparative ELF topological analysis of the electronic structure of the ortho TSs involved in the 32CA reactions of cyclic nitrone 21 with MP 22, MCP 11 and DCP 27 was performed. The ELF basin attractor positions with the most relevant valence basin populations characterizing the TSs are shown in Fig. 5.ELF of TS-II-o, involving MCP 11, is identical to that of TS-I-o, involving MP 22. It shows the presence of a V(N2) monosynaptic basin, integrating 1.35e, and two monosynaptic basins, V(C1) and V(C5), integrating 0.36 and 0.33e, respectively. These monosynaptic basins, which are not present at the reagents (see Fig. 5), are created along the reaction path (see Table S5†). While the V(N2) monosynaptic basin is related to the non-bonding electron density present at the final isoxazoline-5-spiro 25, the two V(C1) and V(C5) monosynaptic basins are related to the C1 and C5 pseudoradical centers demanded for the subsequent creation of the new C1–C5 single bond.32 The populations of these monosynaptic basins are slightly higher than those at TS-I-o as a consequence of its more advance character. ELF of TS-I-o and TS-II-o are identical to that of TS-V, associated with the 32CA reaction involving ethylene 31 (see Fig. S1 in ESI†). Consequently, the presence of the two methyl groups at MP 22 or the cyclopropane at MCP 11 does not substantially modify its reactivity with respect to that of ethylene 31. A slight difference is found at TS-II![[I with combining cedilla]](https://www.rsc.org/images/entities/char_0049_0327.gif) involving DCP 27. While the presence of the V(C1) monosynaptic basin is not observed, the population of the V(C5) monosynaptic basin is increased to 0.60e. Note that no V(C4) monosynaptic basin is observed. These topological changes with respect to the ELF of TS-II-o can by rationalized by two different behaviors: (i) TS-III is slightly more delayed than TS-II-o. Consequently, the formation of the V(C1) monosynaptic basin has not yet begun at TS-V; and (ii) the presence of the two cyclopropanes at DCP 27 makes this ethylene softer than MCP 11 (see Table 1), thus favoring the asynchronous formation of the C4 and C5 pseudoradical centers despite its symmetry.
involving DCP 27. While the presence of the V(C1) monosynaptic basin is not observed, the population of the V(C5) monosynaptic basin is increased to 0.60e. Note that no V(C4) monosynaptic basin is observed. These topological changes with respect to the ELF of TS-II-o can by rationalized by two different behaviors: (i) TS-III is slightly more delayed than TS-II-o. Consequently, the formation of the V(C1) monosynaptic basin has not yet begun at TS-V; and (ii) the presence of the two cyclopropanes at DCP 27 makes this ethylene softer than MCP 11 (see Table 1), thus favoring the asynchronous formation of the C4 and C5 pseudoradical centers despite its symmetry.
3.6 What is the origin of the ortho regioselectivity in these non-polar zw-type 32CA reactions?
The 32CA reactions of cyclic nitrone 21 with MP 22, MCP 11 or DCP 27 present a complete or high ortho regioselectivity. The presence of the strained cyclopropane at MCP 11 only slightly lowers the complete ortho regioselectivity observed in the 32CA reaction of MP 22. The well-known Parr functions49 predict regioselectivity in polar processes. However, the studied 32CA reactions are classified as NEDF reactions with non-polar character (Section 3.3). A recent MEDT study on the regioselectivity of non-polar 32CA reactions made it possible to conclude that the least electronegative C1 carbon of nitrones controls the asynchronicity in the C–X (X = C or O) single bond formation, and consequently, the regioselectivity.18 This behavior is a consequence of the fact that the non-bonding electron density donation required for the formation of the two new C–X single bonds has a lower energy demand at the least electronegative C1 carbon than at the O3 oxygen.On the other hand, the aforementioned study showed that in the non-polar 32CA reactions of nine different TACs towards MP 22, the more favorable reaction paths always involved the non-substituted methylene C5 carbon of MP 22 (see TS-I-o in Fig. 3). Note that this behavior is not modified in the 32CA reactions involving MCP 11.
NPA analysis of MP 22 and MCP 11 indicates that while the disubstituted C4 carbon is barely charged, the terminal methylene C5 carbon is considerably negatively charged as a consequence of the more electronegative character of the carbon than the hydrogen (see Fig. 1). Consequently, it is expected that along the rupture of the C4–C5 double bond, the C5 carbon will accumulate more electron density, thus favoring the earlier creation of the C5 pseudoradical center. In addition, the terminal methylene CH2 carbon is also the less hindered.
4. Conclusions
The role of the cyclopropane substitution on the ethylene in zw-type 32CA reactions of cyclic nitrones has been studied within MEDT at the ωB97X-D/6-311G(d,p) computational level.ELF topological analysis of MP 22, MCP 11 and DCP 27 shows that the presence of the cyclopropane in 11 and 27 only increases the electron density in the C4–C5 bonding region with respect to that of MP 22. Analysis of the CDFT indices indicates that the presence of the cyclopropane does not produce any remarkable change in the reactivity of MCP 11 and DCP 27 with respect to that of MP 22. Despite the strong nucleophilic character of cyclic nitrones 14 and 21, the marginal electrophilic character of ethylenes MP 22, MCP 11 and DCP 27 makes the corresponding zw-type 32CA reactions to have non-polar character.
These zw-type 32CA reactions present activation enthalpies in the Range of 11.2–13.4 kcal mol−1, the reactions being exothermic between 27.8 and 46.3 kcal mol−1. The presence of the cyclopropane in MCP 11 and DCP 27 lowers the activation enthalpies of the 32CA reaction of cyclic nitrone 21 with ethylene 31 by only 1.7 and 2.6 kcal mol−1, respectively, but also decreases the ortho regioselectivity. A linear correlation between reaction enthalpies versus the activation enthalpies is found; thus, the loss of the strain present in the cyclopropane is responsible for both the reduction of the activation enthalpies and the increase of the reaction enthalpies in these 32CA reactions.
Analysis of the geometrical parameters of the TSs indicates that the presence of the cyclopropane in the ethylene framework of MCP 11 and DCP 27 does not produce remarkable changes in the geometries. The very low GEDT computed at the more favorable ortho TSs, between 0.01e and 0.06e, permits establishing the non-polar character of these 32CA reactions, which are therefore classified as of NEDF.
ELF comparative analysis of the ortho TSs involved in the 32CA reactions of cyclic nitrone 21 shows the great similarity between them. The presence of the two methyl groups at MP 22 or the cyclopropane at MCP 11 does not substantially modify their reactivity with respect to that of ethylene 31.
The present MEDT study establishes that the ortho regioselectivity in these non-polar zw-type 32CA reactions is determined by the most favorable two-center interactions between the less electronegative C1 carbon of the nitrones and the non-substituted methylene C5 carbon of MP 22 and MCP 11.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by the Ministry of Science and Innovation (MICINN) of the Spanish Government, project PID2019-110776GB-I00 (AEI/FEDER, UE). This work has also received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 846181 (MRG). Mahmoud, Ibrahim and El-Bahy acknowledge the financial support of Taif University Researchers Supporting Project number (TURSP-2020/158), Taif University, Taif, Saudi Arabia. The authors would like to thank the anonymous reviewers for their comments to improve the manuscript.References
- W. Carruthers, in Some Modern Methods of Organic Synthesis, Cambridge University, Cambridge, UK, 1978 Search PubMed.
- A. Padwa, in 1,3-Dipolar Cycloaddition Chemistry, Wiley-Interscience, New York, NY, USA, 1984, vol. 1–2 Search PubMed.
- W. Carruthers, in Cycloaddition Reactions in Organic Synthesis, Pergamon, Oxford, UK, 1990 Search PubMed.
- A. Padwa and W. H. Pearson, in Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products, John Wiley & Sons, Inc., New York, NY, USA, 2002, vol. 59 Search PubMed.
- R. C. F. Jones and J. N. Martin, in Synthetic Application of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products, ed. A. Padwa and W. H. Pearson, Wiley, New York 2002, pp. 1–8 Search PubMed.
- K. V. Gothelf and K. A. Jorgensen, Chem. Rev., 1998, 98, 863–909 CrossRef CAS PubMed.
- K. B. G. Torssell, Nitrile Oxides, Nitrones and Nitronates in Organic Syntesis, Novel Strategies in Synthesis, ed. H. Feuer, Wiley-VCH, New York, 2008 Search PubMed.
- L. R. Domingo, Molecules, 2016, 21, 1319 CrossRef PubMed.
- M. Ríos-Gutiérrez and L. R. Domingo, Eur. J. Org. Chem., 2019, 267–282 CrossRef.
- M. Ríos-Gutiérrez, A. Daru, T. Tejero, L. R. Domingo and P. Merino, Org. Biomol. Chem., 2017, 15, 1618–1627 RSC.
- L. R. Domingo, M. Ríos-Gutiérrez and P. Pérez, J. Org. Chem., 2018, 83, 2182–2197 CrossRef CAS.
- L. R. Domingo, M. Ríos-Gutiérrez and N. Acharjee, Eur. J. Org. Chem., 2022, e202101417 CAS.
- S. A. Ali and M. I. M. Wazeer, J. Chem. Soc., Perkin Trans. 1, 1988, 597–605 RSC.
- A. Brandi, A. Guarna, A. Goti and F. De Sarlo, Tetrahedron Lett., 1986, 27, 1727–1730 CrossRef CAS.
- L. R. Domingo, M. Ríos-Gutiérrez and P. Pérez, RSC Adv., 2017, 7, 26879–26887 RSC.
- L. R. Domingo and J. A. Sáez, J. Org. Chem., 2011, 76, 373–379 CrossRef CAS PubMed.
- L. Briccolani-Bandini, M. Pagliai, F. M. Cordero, A. Brandi and G. Cardini, J. Phys. Chem. A, 2021, 125, 3892–3899 CrossRef CAS PubMed.
- L. R. Domingo, M. Ríos-Gutiérrez and S. Castellanos, Organics, 2020, 1, 19–35 CrossRef.
- J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620 RSC.
- W. J. Hehre, L. Radom, P. v. R. Schleyer and J. A. Pople, Ab initio Molecular Orbital Theory, Wiley, New York, 1986 Search PubMed.
- A. K. Nacereddine, C. Sobhi, A. Djerourou, M. Ríos-Gutiérrez and L. R. Domingo, RSC Adv., 2015, 5, 99299–99311 RSC.
- H. B. Schlegel, J. Comput. Chem., 1982, 2, 214–218 CrossRef.
- H. B. Schlegel, in Modern Electronic Structure Theory, ed. D. R. Yarkony, World Scientific Publishing, Singapore, 1994 Search PubMed.
- K. Fukui, J. Phys. Chem., 1970, 74, 4161–4163 CrossRef CAS.
- C. Gonzalez and H. B. Schlegel, J. Phys. Chem., 1990, 94, 5523–5527 CrossRef CAS.
- C. Gonzalez and H. B. Schlegel, J. Chem. Phys., 1991, 95, 5853–5860 CrossRef CAS.
- J. Tomasi and M. Persico, Chem. Rev., 1994, 94, 2027–2094 CrossRef CAS.
- B. Y. Simkin and I. Sheikhet, Quantum Chemical and Statistical Theory of Solutions-A Computational Approach, Ellis Horwood, London, 1995 Search PubMed.
- M. Cossi, V. Barone, R. Cammi and J. Tomasi, Chem. Phys. Lett., 1996, 255, 327–335 CrossRef CAS.
- E. Cances, B. Mennucci and J. Tomasi, J. Chem. Phys., 1997, 107, 3032–3041 CrossRef CAS.
- V. Barone, M. Cossi and J. Tomasi, J. Comput. Chem., 1998, 19, 404–417 CrossRef CAS.
- L. R. Domingo, RSC Adv., 2014, 4, 32415–32428 RSC.
- A. E. Reed, R. B. Weinstock and F. Weinhold, J. Chem. Phys., 1985, 83, 735–746 CrossRef CAS.
- A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899–926 CrossRef CAS.
- R. G. Parr and W. Yang, Density functional theory of atoms and molecules, Oxford University Press, New York, 1989 Search PubMed.
- L. R. Domingo, M. Ríos-Gutiérrez and P. Pérez, Molecules, 2016, 21, 748 CrossRef.
- M. J. Frisch, et al.Gaussian 16, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- A. D. Becke and K. E. Edgecombe, J. Chem. Phys., 1990, 92, 5397–5403 CrossRef CAS.
- S. Noury, X. Krokidis, F. Fuster and B. Silvi, Comput. Chem., 1999, 23, 597–604 CrossRef CAS.
- R. Dennington, T. A. Keith and J. M. Millam, GaussView, Version 6.0, Semichem Inc., Shawnee Mission, KS, 2016 Search PubMed.
- B. Silvi and A. Savin, Nature, 1994, 371, 683–686 CrossRef CAS.
- R. G. Parr and R. G. Pearson, J. Am. Chem. Soc., 1983, 105, 7512–7516 CrossRef CAS.
- R. G. Parr, L. v. Szentpaly and S. Liu, J. Am. Chem. Soc., 1999, 121, 1922–1924 CrossRef CAS.
- L. R. Domingo, E. Chamorro and P. Pérez, J. Org. Chem., 2008, 73, 4615–4624 CrossRef CAS PubMed.
- L. R. Domingo, M. Ríos-Gutiérrez and P. Pérez, RSC Adv., 2020, 10, 15394–15405 RSC.
- L. R. Domingo, K. Kula and M. Ríos-Gutiérrez, Eur. J. Org. Chem., 2020, 5938–5948 CrossRef CAS.
- M. G. Evans and M. Polanyi, Trans. Faraday Soc., 1935, 31, 875–894 RSC.
- X. Krokidis, S. Noury and B. Silvi, J. Phys. Chem. A, 1997, 101, 7277–7282 CrossRef CAS.
- L. R. Domingo, P. Pérez and J. A. Sáez, RSC Adv., 2013, 3, 1486–1494 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Non-polar zw-type 32CA reaction of cyclic nitrone 21 with ethylene 31. Comparative analyses of the MPWB1K, ωB97X-D and PBE0 functionals towards the CCSD(T)/cc-pVTZ energies in the zw-type 32CA reaction of cyclic nitrone 21 with MCP 11. Table with ωB97X-D/6-311G(d,p) total and relative energies, in gas phase and in benzene, of the stationary points associated with the 32CA reactions of cyclic nitrone 21 with MP 22, MCP 11 and DCP 27, and that of cyclic dimethyl nitrone 14 with MCP 11. Table with the ωB97X-D/6-311G(d,p) thermodynamic data for the stationary points associated with the 32CA reactions of cyclic nitrone 21 with MP 22, MCP 11 and DCP 27, and that of cyclic dimethyl nitrone 14 with MCP 11. Table with the BET analysis along the ortho reaction path of the zw-type 32CA reaction of cyclic nitrone 21 with MCP 11. Cartesian coordinates of the stationary points involved in the 32CA reactions of cyclic nitrone 21 with MP 22, MCP 11 and DCP 27, and that of cyclic dimethyl nitrone 14 with MCP 11. See https://doi.org/10.1039/d2ra03327e |
| This journal is © The Royal Society of Chemistry 2022 |