DOI:
10.1039/D2RA03416F
(Paper)
RSC Adv., 2022,
12, 21066-21078
Synthesis of pyrano[3,2-c]quinolones and furo[3,2-c]quinolones via acid-catalyzed tandem reaction of 4-hydroxy-1-methylquinolin-2(1H)-one and propargylic alcohols†
Received
1st June 2022
, Accepted 17th July 2022
First published on 21st July 2022
Abstract
Two acid-catalyzed tandem reactions between 4-hydroxy-1-methylquinolin-2(1H)-one and propargylic alcohols are described. Depending mainly on the propargylic alcohol used, these tandem reactions proceed via either a Friedel–Crafts-type allenylation followed by 6-endo-dig cyclization sequence to form pyrano[3,2-c]quinolones or a Friedel–Crafts-type alkylation and 5-exo-dig ring closure sequence to afford furo[3,2-c]quinolones in moderate-to-high yields. The pyrano[3,2-c]quinolones products could be further transformed to tetracyclic 4,9-dihydro-5H-cyclopenta[lmn]phenanthridin-5-one derivatives.
Introduction
Pyrano[3,2-c]quinolone is a structural motif occurring in a number of natural products1 with a wide range of important biological activities such as anticancer,2 antibacterial,2 antimalarial,3 antiinflammatory4 and antifungal5 properties and inhibition of calcium signaling,6 TNF-α,7 platelet aggregation,8 and nitric oxide production.9 For example, alkaloids zanthosimuline and huajiaosimuline10 exhibit cytotoxicity against cancer cells, which is considered as potential anticancer agents (Scheme 1a). In addition, furo[3,2-c]quinolone derivatives such as araliopsine and almein are principally isolated from Rutaceae species11 (Scheme 1a). Furo[3,2-c]quinolone hybrids are a significant class of angularly fused tricyclic compounds among the great variety of furan derivatives, which have been shown to exhibit biological and pharmacological activity such as antimicrobial, insecticidal, antiarrhythmic, antimalarial, antiplatelet aggregation and sedative,1,12 photochemical treatment in clinic therapeutic field13 and blocking activities of the voltage-gated potassium channel Kv1.3.14 Consequently, a large number of procedures have been developed for the construction of these highly useful structures.15 However, current methods more or less suffer from limited substrate scope, complicated catalyst or noble metal catalyst systems, not easily accessible starting materials, or multistep manipulations, the development of simple methods with wide product diversity is still highly desirable. Propargylic alcohols are readily accessible synthetic building blocks in organic synthesis.16 Over the past few decades, the development of Lewis acid-catalyzed tandem annulations of propargylic alcohols has attracted interests from synthetic chemists, especially for the construction of various heterocyclic skeletons including pyrroles,17 furans,18 pyrans,19 carbazoles,20 quinolines,21 and tetrahydro-β-carbolines.22 We herein describe the development of acid-catalyzed formal [3 + 3]/[3 + 2] cascade annulation processes for the construction of pyrano[3,2-c]quinolone/furo[3,2-c]quinolone derivatives from 4-hydroxy-1-methylquinoline-2(1H)-one and propargylic alcohols (Scheme 1b).
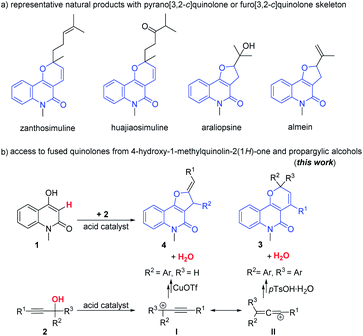 |
| | Scheme 1 Representative natural products with pyrano[3,2-c]quinolone or furo[3,2-c]quinolone skeleton. | |
Results and discussion
Our initial studies commenced with the reaction of 4-hydroxy-1-methylquinolin-2(1H)-one 1 and propargylic alcohol 2a (Table 1). No reaction occurred in the absence of an acid catalyst (Table 1, entry 1). Using 1,2-DCE (1,2-dichloroethane) as solvent, five Lewis acid catalysts and four Brønsted acid catalysts were screened and pTsOH·H2O was found to be the most efficient one for this reaction (Table 1, entries 2–10). The product 3a could be isolated in only 5% yield when the reaction was performed at 25 °C (Table 1, entry 11). Changing the solvent to THF, toluene, DMF (N,N-dimethylformamide) gave inferior results (Table 1, entries 12–14). Further screening of catalyst loading amount uncovered that 10 mol% was optimal for the reaction, while lower (5 mol%) or higher (20 mol%) loadings all led to no improvement in yields (Table 1, entries 15–16). Notably, relatively lower yields yet shorter reaction time were observed in the cases of metal Lewis acid catalysts, which might be due to faster decomposition of the propargylic alcohol 2a under these conditions as observed by thin-layer chromatography (Table 1, entries 2–10). Moreover, it is worth mentioning that the reaction is tolerant of moisture and could be performed under air.
Table 1 Screening of the reaction conditionsa,b
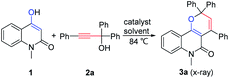
|
| Entry |
Catalyst (mol%) |
Solvent |
Time |
Yieldb (%) |
| Reaction conditions: 1 (0.5 mmol), 2a (0.5 mmol), solvent (5 mL), under air, the reaction was monitored by TLC. Yield of the isolated product. Reaction was run at 25 °C. Reaction was run at 66 °C. Reaction was run at 90 °C. |
| 1 |
No catalyst |
1,2-DCE |
24 h |
0 |
| 2 |
Yb(OTf)3 (10) |
1,2-DCE |
0.5 h |
23 |
| 3 |
Sc(OTf)3 (10) |
1,2-DCE |
0.5 h |
25 |
| 4 |
Zn(OTf)2 (10) |
1,2-DCE |
0.5 h |
23 |
| 5 |
Cu(OTf)2 (10) |
1,2-DCE |
0.5 h |
35 |
| 6 |
FeCl3·6H2O (10) |
1,2-DCE |
0.5 h |
20 |
| 7 |
pTsOH·H2O (10) |
1,2-DCE |
1 h |
70 |
| 8 |
CH3COOH (10) |
1,2-DCE |
36 h |
0 |
| 9 |
TFA (10) |
1,2-DCE |
36 h |
40 |
| 10 |
TfOH (10) |
1,2-DCE |
1 h |
57 |
| 11c |
pTsOH·H2O (10) |
1,2-DCE |
36 h |
5 |
| 12d |
pTsOH·H2O (10) |
THF |
36 h |
35 |
| 13e |
pTsOH·H2O (10) |
Toluene |
4 h |
34 |
| 14e |
pTsOH·H2O (10) |
DMF |
24 h |
0 |
| 15 |
pTsOH·H2O (5) |
1,2-DCE |
4 h |
50 |
| 16 |
pTsOH·H2O (20) |
1,2-DCE |
1 h |
65 |
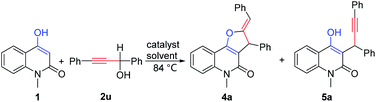
With the optimized reaction conditions (Table 1, entry 7) in hand, a series of propargylic alcohols 2 were reacted with 4-hydroxy-1-methylquinolin-2(1H)-one 1 to examine the reaction scope with regard to the formation of pyrano[3,2-c]quinolone 3. As depicted in Scheme 2, the transformation of various substituted propargylic alcohols 2 proceeded smoothly to deliver the corresponding pyrano[3,2-c]quinolone derivatives in moderate to good yields, irrespective of the electronic nature of the substituents. A variety of functional groups, including methyl, methoxyl, halogen and cyclopropyl substituents, were compatible with the reaction. Notably, cyclopropyl ring has a wide range of applications in drug molecular design23 and propargylic alcohol bearing cyclopropyl was tolerated in the reaction conditions to generate the desired product 3i in 58% yield. In addition, other alkyl groups (R1) such as methyl, n-butyl and trimethylsilyl also gave [3 + 3] products 3t–3u in 51–57% yields. Moreover, when 9-fluorenyl-substituted propargylic alcohols 2q–2s were subjected to the reaction conditions, spirocyclic products 3q–3s could be formed in the yields of 24–55% albeit with much prolonged reaction time of 10 h. It was found that product 3x was produced by hydroamination of N–H in 1 with alkyne in 2 beside normal product 3w. Then products 3y and 3z were synthesized by adjusting molar ratio of the reaction. The structure of the products 3a and 3z was additionally confirmed by X-ray crystallographic analysis (see ESI† for details).
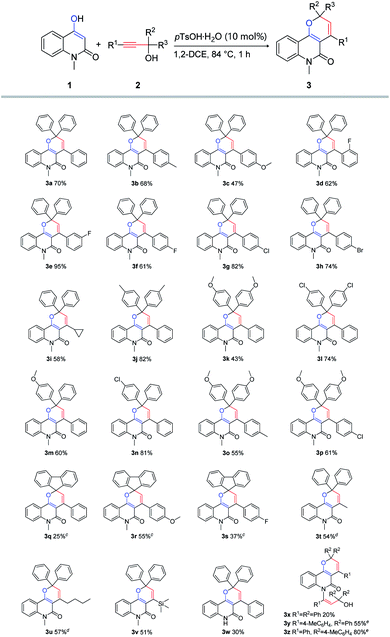 |
| | Scheme 2 Scope study with different propargylic alcohols 2a,b. aReaction conditions: 1 (0.5 mmol), 2 (0.5 mmol), pTsOH·H2O (0.05 mmol), 1,2-DCE (5 mL), 84 °C. bIsolated yield refers to pyrano[3,2-c]quinolone derivatives. cReaction time: 10 h. dReaction time: 10 min. eReaction conditions: 1 (0.5 mmol), 2 (1.0 mmol), pTsOH·H2O (0.05 mmol), 1,2-DCE (5 mL), 84 °C. | |
Based on the above experimental results, a plausible mechanism for the present cascade reactions is proposed (Scheme 3). First, in the presence of a Brønsted acid catalyst, propargylic alcohol 2a is converted to the propargylic carbocation I, which is in equilibrium with the allenic form II.24 The latter would undergo Friedel–Crafts-type reaction with 1 to form the allene intermediate III, which would be transformed to the final product 3a via 6-endo-dig cyclization.19f
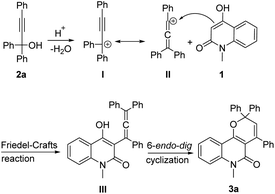 |
| | Scheme 3 A possible mechanism for the dehydrative annulation. | |
To further extend the scope of the current reaction, secondary propargylic alcohols 2u was tested as substrate. Unexpectedly, 3-(1,3-diphenylprop-2-yn-1-yl)-4-hydroxy-1-methylquinolin-2(1H)-one 5a was isolated instead of the desired pyrano[3,2-c]quinolone 3u under the above optimized reaction conditions (Scheme 4). Given the recent reports on transition-metal-catalyzed direct heterocyclization of alkynes to construct furan frameworks,25 Cu(OTf)2 was then used to catalyze the reaction and to our delight, the ring-closure compound furo[3,2-c]quinolone 4a was isolated as expected in 48% yield (Table 2, entry 2). Different Lewis acid catalysts were then screened (Table 2, entries 1–10), and CuOTf was found to be one of the best choice (Table 2, entry 3), while some catalysts could not transform 5a to 4a accordingly (Table 2, entries 1 and 4–7), indicating that the reactions were stuck at the stage of 5a. The reaction hardly occurred at room temperature (Table 2, entry 8). The use of other solvents including THF, toluene, DMF or changing the catalyst loadings made no improvement in yield (Table 2, entries 11–16).
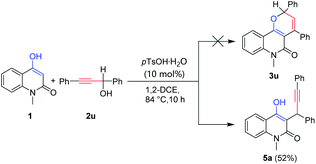 |
| | Scheme 4 Synthesis of 5a catalyzed by pTsOH·H2O. | |
Table 2 Screening of the reaction conditionsa
|
| Entry |
Catalyst (mol%) |
Solvent |
Time |
Yieldb (%) |
| 4a |
5a |
| Reaction conditions: 1 (0.5 mmol), 2u (0.5 mmol), solvent (5 mL), under air, the reaction was monitored by TLC. Yield of the isolated product. Reaction was run at 25 °C. Reaction was run at 66 °C. Reaction was run at 90 °C. |
| 1 |
pTsOH·H2O (10) |
1,2-DCE |
10 h |
0 |
52 |
| 2 |
Cu(OTf)2 (10) |
1,2-DCE |
10 h |
48 |
0 |
| 3 |
CuOTf (10) |
1,2-DCE |
10 h |
60 |
0 |
| 4 |
Yb(OTf)3 (10) |
1,2-DCE |
24 h |
0 |
51 |
| 5 |
Zn(OTf)2 (10) |
1,2-DCE |
24 h |
0 |
53 |
| 6 |
FeCl3·6H2O (10) |
1,2-DCE |
24 h |
0 |
55 |
| 7 |
HAuCl4·3H2O (10) |
1,2-DCE |
24 h |
0 |
Trace |
| 8 |
CuCl (10) |
1,2-DCE |
24 h |
0 |
0 |
| 9 |
CuBr (10) |
1,2-DCE |
24 h |
0 |
0 |
| 10 |
CuOAc (10) |
1,2-DCE |
24 h |
0 |
0 |
| 11c |
CuOTf (10) |
1,2-DCE |
24 h |
0 |
0 |
| 12d |
CuOTf (10) |
THF |
10 h |
31 |
0 |
| 13e |
CuOTf (10) |
Toluene |
10 h |
34 |
0 |
| 14e |
CuOTf (10) |
DMF |
10 h |
30 |
0 |
| 15 |
CuOTf (5) |
1,2-DCE |
10 h |
20 |
0 |
| 16 |
CuOTf (20) |
1,2-DCE |
10 h |
51 |
0 |
Next, the scope of the reaction with regard to the propargylic alcohols was investigated under the optimized reaction conditions, and the results were presented in Scheme 5. In general, the products 4 were produced in low to moderate yields (31–60%), regardless of the electronic nature and/or position of the substituents on the benzene rings (R1 or R2). It was worth noting that only stereodefined (Z)-furo[3,2-c]quinolones had been isolated. The structure of the product 4g was additionally confirmed by X-ray crystallographic analysis (see ESI† for details). The primary alcohol such as 3-phenylprop-2-yn-1-ol was tested under the same reaction conditions for 24 hours, but no new product was detected and the staring material was recovered in 95% yield. Primary alcohol may not easily form primary carbocation under this reaction conditions.
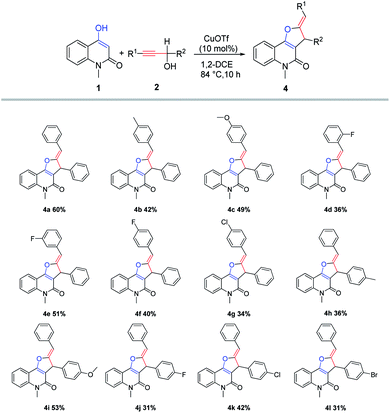 |
| | Scheme 5 Scope study with secondary propargylic alcoholsa,b. aReaction conditions: 1 (0.5 mmol), 2 (0.5 mmol), CuOTf (0.05 mmol), 1,2-DCE (5 mL), 84 °C. bIsolated yield refers to furo[3,2-c]quinolones. | |
To gain some insight into the reaction mechanism of this formal [3 + 2] annulation, some controlled experiments were conducted (Scheme 6a). Quenching the reaction between 1 and propargylic alcohols 2u at an early stage (1 hour) gave the compound 5a as the major product in 60% yield, indicating the Friedel–Crafts-type reaction was a relatively fast step in this process. Treatment of the isolated 5a under the otherwise same reaction conditions produced the final product 4a in 80% yield after 10 hours. On the basis of these results and literature reports,25b,f a mechanistic proposal for the conversion of 5a to 4a is depicted (Scheme 6b). The transformation began with the coordination of the triple bond to the copper(I) salt to facilitate the highly regioselective 5-exo-dig nucleophilic attack of the hydroxy group to form the intermediate B. Finally, protonolysis of B afforded 4a and regenerated the catalyst.
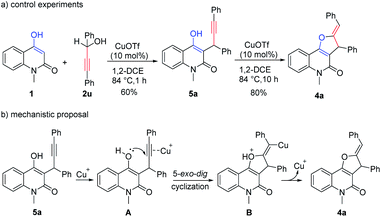 |
| | Scheme 6 Controlled experiments and mechanistic proposal for the [3 + 2]-annulation. | |
To demonstrate the practicality of this formal [3 + 3] cascade annulation, a gram-scale experiment was carried out to provide desired product 3a in 73% yield (Scheme 7a). Furthermore, novel tetracyclic compounds 6a–6e were forged from 3a and propargylic alcohols 2 in 41–60% yields, which proceeded via sequential Diels–Alder reaction of 2H-pyran with alkynes followed by retro-Diels–Alder extrusion of benzophenone under thermal reaction conditions and Friedel–Crafts-type reaction at last.26 Furo[3,2-c]quinolones 4 could be isomerized to 7 under the catalysis pTsOH·H2O with excellent yields (Scheme 7b). The structure of the product 6a was additionally confirmed by X-ray crystallographic analysis (see ESI† for details).
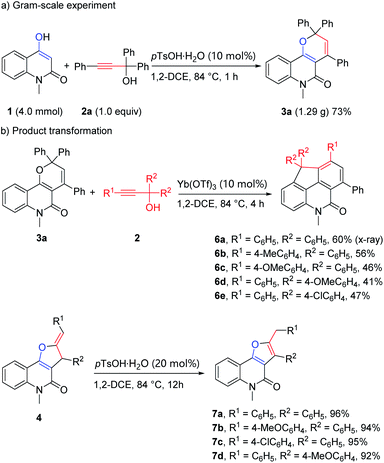 |
| | Scheme 7 Scale-up reaction and product transformations. | |
Conclusions
In conclusion, novel acid-catalyzed annulation reactions of propargylic alcohols with 4-hydroxy-1-methylquinolin-2(1H)-one were developed. This method provides a good atom- and step-economic way to useful pyrano[3,2-c]quinolone and furo[3,2-c]quinolone derivatives in moderate to good yields from readily accessible starting materials. Efforts towards the utilization of the propargylic alcohols to the synthesis of other useful cyclic compounds are underway in our laboratories.
Experimental section
General comments
Infrared spectra were obtained on a FTIR spectrometer. 1H NMR spectra were recorded on 400 MHz spectrometer in CDCl3 solution and the chemical shifts were reported relative to internal standard TMS (0 ppm). The following abbreviations are used to describe peak patterns where appropriate: br = broad, s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet. Coupling constants are reported in Hertz (Hz). 13C NMR were recorded on 100 MHz and referenced to the internal solvent signals (central peak is 77.00 ppm in CDCl3). HRMS data were obtained using ESI ionization. Melting points were measured with micro melting point apparatus.
The propargylic alcohols 2 were prepared from phenylacetylene and benzophenone according to published methods.27 Solvents were distilled prior to use. All chemicals were used as purchased unless otherwise mentioned.
General procedure for the synthesis of 3
A solution of 4-hydroxy-1-methylquinolin-2(1H)-one 1 (0.5 mmol), propargylic alcohols 2 (0.5 mmol) and pTsOH·H2O (0.05 mmol) in 1,2-DCE (5 mL) was stirred under air at 84 °C for 1 h. After being cooled down to room temperature, the solvent was evaporated and the crude product was purified by silica gel column chromatography with petroleum ether/ethyl acetate (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
General procedure for the synthesis of 4
A solution of 4-hydroxy-1-methylquinolin-2(1H)-one 1 (0.5 mmol), the secondary propargylic alcohols 2 (0.5 mmol) and CuOTf (0.05 mmol) in 1,2-DCE (5 mL) was stirred under air at 84 °C for 10 h. After being cooled down to room temperature, the solvent was evaporated and the crude product was purified by silica gel column chromatography with petroleum ether/ethyl acetate (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
Synthesis of 5a
A solution of 4-hydroxy-1-methylquinolin-2(1H)-one 1 (0.5 mmol), propargylic alcohols 2u (0.5 mmol) and CuOTf (0.05 mmol) in DCE (5 mL) was stirred under air at 84 °C for 1 h. After being cooled down to room temperature, the solvent was evaporated and the crude product was purified by silica gel column chromatography with petroleum ether/ethyl acetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
General procedure for the synthesis of 6
A solution of pyrano[3,2-c]quinolone 3a (0.5 mmol), propargylic alcohols 2 (0.6 mmol) and Yb(OTf)3 (0.05 mmol) in 1,2-DCE (5 mL) was stirred under air at 84 °C for 4 h. After being cooled down to room temperature, the solvent was evaporated and the crude product was purified by silica gel column chromatography with petroleum ether/ethyl acetate (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
General procedure for the synthesis of 7
A solution of furo[3,2-c]quinolones 4 (0.2 mmol) and pTsOH·H2O (0.04 mmol) in 1,2-DCE (3 mL) was stirred under air at 84 °C for 12 h. After being cooled down to room temperature, the solvent was evaporated and the crude product was purified by silica gel column chromatography with petroleum ether/ethyl acetate (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
Gram-scale synthesis for product 3a
A solution of 4-hydroxy-1-methylquinolin-2(1H)-one 1 (4.0 mmol), propargylic alcohols 2a (4.0 mmol) and pTsOH·H2O (0.4 mmol) in 1,2-DCE (20 mL) was stirred under air at 84 °C for 1 h. After being cooled down to room temperature, the solvent was evaporated and the crude product was purified by silica gel column chromatography with petroleum ether/ethyl acetate (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
Characterization data of products
6-Methyl-2,2,4-triphenyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3a). White solid (155 mg, 70%); mp 217–218 °C; 1H NMR (400 MHz, CDCl3) δ 8.22 (d, J = 8.2 Hz, 1H), 7.61–7.47 (m, 5H), 7.44–7.20 (m, 13H), 5.96 (s, 1H), 3.54 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.60, 156.52, 143.67, 139.88, 139.55, 135.74, 131.31, 128.17, 127.84, 127.66, 127.32, 127.24, 126.80, 126.10, 123.53, 121.75, 115.89, 113.98, 108.19, 84.31, 29.20. IR (KBr) ν 3022, 1649, 1557, 1489, 1393, 1116, 990, 756, 698 cm−1; HRMS (ESI): m/z calcd for ([C31H23NO2 + H]+): 442.1802; found: 442.1801.
6-Methyl-2,2-diphenyl-4-(p-tolyl)-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3b). White solid (155 mg, 68%); mp 192–193 °C; 1H NMR (400 MHz, CDCl3) δ 8.21 (dd, J = 8.3, 1.4 Hz, 1H), 7.65–7.43 (m, 5H), 7.41–7.21 (m, 10H), 7.16 (d, J = 7.9 Hz, 2H), 5.94 (s, 1H), 3.54 (s, 3H), 2.36 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.67, 156.52, 143.73, 139.87, 136.85, 136.62, 135.61, 131.28, 128.44, 128.16, 127.82, 127.17, 126.83, 125.73, 123.54, 121.76, 115.94, 113.99, 108.29, 84.31, 29.23, 21.28. IR (KBr) ν 3025, 2920, 1732, 1648, 1556, 1447, 1389, 1113, 988, 752, 700 cm−1; HRMS (ESI): m/z calcd for ([C32H25NO2 + H]+): 456.1958; found: 456.1960.
4-(4-Methoxyphenyl)-6-methyl-2,2-diphenyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3c). White solid (111 mg, 47%); mp 199–200 °C; 1H NMR (400 MHz, CDCl3) δ 8.21 (dd, J = 8.1, 1.5 Hz, 1H), 7.58–7.46 (m, 5H), 7.36–7.20 (m, 10H), 6.95–6.84 (m, 2H), 5.92 (s, 1H), 3.81 (s, 3H), 3.54 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.68, 158.84, 156.57, 143.75, 139.85, 135.27, 131.86, 131.29, 128.45, 128.15, 127.81, 126.81, 125.41, 123.53, 121.76, 115.94, 113.98, 113.13, 108.25, 84.30, 55.13, 29.23. IR (KBr) ν 3058, 2837, 1652, 1557, 1499, 1384, 1113, 988, 756, 700 cm−1; HRMS (ESI): m/z calcd for ([C32H25NO3 + H]+): 472.1907; found: 472.1905.
4-(2-Fluorophenyl)-6-methyl-2,2-diphenyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3d). White solid (142 mg, 62%); mp 196–197 °C; 1H NMR (400 MHz, CDCl3) δ 8.19 (d, J = 7.2 Hz, 1H), 7.57–7.49 (m, 5H), 7.42 (t, J = 7.5 Hz, 1H), 7.36–7.20 (m, 9H), 7.16 (t, J = 7.5 Hz, 1H), 7.05 (t, 1H), 6.01 (s, 1H), 3.52 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.87 (d, JC–F = 244.6 Hz), 159.86, 155.48, 143.60, 139.78, 131.31, 130.13, 129.60 (d, JC–F = 3.7 Hz), 129.01 (d, JC–F = 8.1 Hz), 128.24, 127.92, 127.74, 127.28, 126.83, 123.70 (d, JC–F = 3.3 Hz), 123.56, 121.78, 115.86, 114.73 (d, JC–F = 21.6 Hz), 114.00, 108.18, 84.05, 29.19; 19F NMR (377 MHz, CDCl3) δ −114.21 (m). IR (KBr) ν 3061, 1736, 1649, 1559, 1489, 1394, 1124, 993, 753, 699 cm−1; HRMS (ESI): m/z calcd for ([C31H22FNO2 + H]+): 460.1707; found: 460.1709.
4-(3-Fluorophenyl)-6-methyl-2,2-diphenyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3e). White solid (218 mg, 95%); mp 194–195 °C; 1H NMR (400 MHz, CDCl3) δ 8.22 (dd, J = 8.1, 1.3 Hz, 1H), 7.61–7.53 (m, 1H), 7.53–7.46 (m, 4H), 7.36–7.22 (m, 9H), 7.15 (d, J = 7.7 Hz, 1H), 7.13–7.06 (m, 1H), 7.00 (td, J = 8.4, 2.1 Hz, 1H), 5.97 (s, 1H), 3.54 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.27 (d, JC–F = 243.5 Hz), 159.50, 156.68, 143.47, 141.84 (d, JC–F = 8.0 Hz), 139.93, 134.79 (d, JC–F = 2.0 Hz), 131.54, 129.05 (d, JC–F = 8.2 Hz), 128.26, 127.97, 126.77, 126.61, 123.61, 123.18 (d, JC–F = 2.7 Hz), 121.91, 115.82, 114.50 (d, JC–F = 21.8 Hz), 114.10 (d, JC–F = 20.9 Hz), 114.09, 107.86, 84.35, 29.24; 19F NMR (377 MHz, CDCl3) δ −114.10 (m). IR (KBr) ν 3061, 2943, 1732, 1645, 1559, 1486, 1395, 1115, 756, 698 cm−1; HRMS (ESI): m/z calcd for ([C31H22FNO2 + H]+): 460.1707; found: 460.1709.
4-(4-Fluorophenyl)-6-methyl-2,2-diphenyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3f). White solid (140 mg, 61%); mp 210–211 °C; 1H NMR (400 MHz, CDCl3) δ 8.22 (d, J = 8.1 Hz, 1H), 7.60–7.45 (m, 5H), 7.39–7.21 (m, 10H), 7.04 (t, J = 8.7 Hz, 2H), 5.93 (s, 1H), 3.53 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.15 (d, JC–F = 244.1 Hz), 159.64, 156.67, 143.57, 139.88, 135.47 (d, JC–F = 3.3 Hz), 134.85, 131.48, 128.94 (d, JC–F = 8.0 Hz), 128.23, 127.93, 126.77, 126.10, 123.59, 121.89, 115.85, 114.6 (d, JC–F = 21.4 Hz), 114.06, 107.94, 84.32, 29.22; 19F NMR (377 MHz, CDCl3) δ −115.24 (s). IR (KBr) ν 3058, 2925, 1646, 1556, 1509, 1388, 1118, 988, 755, 701 cm−1; HRMS (ESI): m/z calcd for ([C31H22FNO2 + H]+): 460.1707; found: 460.1709.
4-(4-Chlorophenyl)-6-methyl-2,2-diphenyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3g). White solid (195 mg, 82%); mp 218–219 °C; 1H NMR (400 MHz, CDCl3) δ 8.21 (dd, J = 8.2, 1.1 Hz, 1H), 7.59–7.45 (m, 5H), 7.35–7.19 (m, 12H), 5.94 (s, 1H), 3.52 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.56, 156.71, 143.45, 139.86, 137.99, 134.74, 133.03, 131.52, 128.68, 128.22, 127.94, 127.85, 126.74, 126.33, 123.57, 121.91, 115.79, 114.05, 107.77, 84.32, 29.21. IR (KBr) ν 3059, 2933, 1648, 1556, 1489, 1401, 1114, 989, 755, 699 cm−1; HRMS (ESI): m/z calcd for ([C31H22ClNO2 + H]+): 476.1412; found: 476.1410.
4-(4-Bromophenyl)-6-methyl-2,2-diphenyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3h). White solid (193 mg, 74%); mp 177–178 °C; 1H NMR (400 MHz, CDCl3) δ 8.22 (dd, J = 8.1, 1.4 Hz, 1H), 7.61–7.54 (m, 1H), 7.53–7.43 (m, 6H), 7.35–7.21 (m, 10H), 5.95 (s, 1H), 3.54 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.59, 156.75, 143.46, 139.90, 138.51, 134.79, 131.56, 130.80, 129.03, 128.25, 127.97, 126.77, 126.35, 123.61, 121.94, 121.29, 115.82, 114.10, 107.73, 84.35, 29.25. IR (KBr) ν 3062, 2937, 1736, 1648, 1557, 1486, 1400, 1114, 989, 760, 700 cm−1; HRMS (ESI): m/z calcd for ([C31H22BrNO2 + H]+): 520.0907; found: 520.0904.
4-Cyclopropyl-6-methyl-2,2-diphenyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3i). White solid (118 mg, 58%); mp 213–214 °C; 1H NMR (400 MHz, CDCl3) δ 8.14 (dd, J = 7.9, 0.7 Hz, 1H), 7.50 (t, J = 7.8 Hz, 1H), 7.44–7.36 (m, 4H), 7.32–7.17 (m, 8H), 5.62 (s, 1H), 3.61 (s, 3H), 2.81–2.56 (m, 1H), 0.98–0.77 (m, 2H), 0.75–0.47 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 160.73, 155.41, 144.21, 139.56, 136.91, 131.07, 128.08, 127.64, 126.74, 123.56, 121.71, 119.57, 115.78, 113.81, 109.18, 84.04, 29.10, 13.41, 7.34. IR (KBr) ν 3056, 3000, 1643, 1554, 1497, 1399, 1108, 990, 752, 701 cm−1; HRMS (ESI): m/z calcd for ([C28H23NO2 + H]+): 406.1802; found: 406.1801.
6-Methyl-4-phenyl-2,2-di-p-tolyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3j). Blue solid (193 mg, 82%); mp 236–237 °C; 1H NMR (400 MHz, CDCl3) δ 8.19 (dd, J = 8.1, 1.5 Hz, 1H), 7.57–7.48 (m, 1H), 7.44–7.29 (m, 9H), 7.27–7.20 (m, 2H), 7.10 (d, J = 8.0 Hz, 4H), 5.92 (s, 1H), 3.52 (s, 3H), 2.29 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 159.66, 156.57, 140.90, 139.85, 139.68, 137.53, 135.42, 131.22, 128.81, 127.63, 127.32, 127.15, 126.77, 126.43, 123.58, 121.69, 116.00, 113.93, 108.12, 84.27, 29.20, 21.02. IR (KBr) ν 3032, 2932, 2838, 1733, 1649, 1555, 1461, 1385, 1113, 983, 757, 696 cm−1; HRMS (ESI): m/z calcd for ([C33H27NO2 + H]+): 470.2115; found: 470.2117.
2,2-Bis(4-methoxyphenyl)-6-methyl-4-phenyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3k). White solid (108 mg, 43%); mp 218–219 °C; 1H NMR (400 MHz, CDCl3) δ 8.17 (d, J = 7.9 Hz, 1H), 7.58–7.51 (m, 1H), 7.45–7.29 (m, 9H), 7.29–7.20 (m, 2H), 6.83 (d, J = 8.5 Hz, 4H), 5.89 (s, 1H), 3.76 (s, 6H), 3.54 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.70, 159.11, 156.56, 139.89, 139.68, 135.96, 135.42, 131.25, 128.29, 127.67, 127.33, 127.20, 126.49, 123.58, 121.71, 116.04, 113.98, 113.45, 108.12, 84.17, 55.21, 29.23. IR (KBr) ν 3056, 1652, 1556, 1489, 1393, 1116, 988, 757, 697 cm−1; HRMS (ESI): m/z calcd for ([C33H27NO4 + H]+): 502.2013; found: 502.2012.
2,2-Bis(4-chlorophenyl)-6-methyl-4-phenyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3l). White solid (189 mg, 74%); mp 264–265 °C; 1H NMR (400 MHz, CDCl3) δ 8.14 (dd, J = 7.9, 1.1 Hz, 1H), 7.62–7.52 (m, 1H), 7.48–7.39 (m, 4H), 7.39–7.32 (m, 5H), 7.31–7.22 (m, 6H), 5.85 (s, 1H), 3.55 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.44, 156.26, 141.72, 139.97, 139.06, 136.63, 134.10, 131.65, 128.52, 128.23, 127.78, 127.53, 127.25, 124.91, 123.35, 121.94, 115.62, 114.17, 108.35, 83.46, 29.29. IR (KBr) ν 3056, 1652, 1556, 1488, 1393, 1115, 988, 757, 697 cm−1; HRMS (ESI): m/z calcd for ([C31H21Cl2NO2 + H]+): 510.1022; found: 510.1024.
2-(4-Methoxyphenyl)-6-methyl-2,4-diphenyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3m). Yellow solid (141 mg, 60%); mp 253–254 °C; 1H NMR (400 MHz, CDCl3) δ 8.19 (d, J = 8.3 Hz, 1H), 7.58–7.47 (m, 3H), 7.46–7.28 (m, 9H), 7.28–7.21 (m, 3H), 6.87–6.77 (m, 2H), 5.92 (s, 1H), 3.74 (s, 3H), 3.54 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.66, 159.20, 156.54, 144.04, 139.89, 139.61, 135.58, 135.56, 131.29, 128.43, 128.14, 127.70, 127.67, 127.32, 127.23, 126.69, 126.29, 123.56, 121.74, 115.98, 113.99, 113.48, 108.15, 84.24, 55.18, 29.23. IR (KBr) ν 3049, 2931, 2840, 1648, 1558, 1498, 1387, 1116, 989, 754, 699 cm−1; HRMS (ESI): m/z calcd for ([C32H25NO3 + H]+): 472.1907; found: 472.1904.
2-(4-Chlorophenyl)-6-methyl-2,4-diphenyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3n). Yellow solid (193 mg, 81%); mp 245–246 °C; 1H NMR (400 MHz, CDCl3) δ 8.18 (d, J = 8.2 Hz, 1H), 7.60–7.53 (m, 1H), 7.52–7.42 (m, 4H), 7.41–7.21 (m, 12H), 5.91 (s, 1H), 3.54 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.54, 156.40, 143.27, 142.15, 139.94, 139.31, 136.20, 133.88, 131.50, 128.39, 128.37, 128.32, 128.04, 127.73, 127.40, 127.29, 126.69, 125.52, 123.46, 121.86, 115.78, 114.09, 108.28, 83.88, 29.27. IR (KBr) ν 3059, 2970, 1652, 1557, 1491, 1388, 1113, 986, 754, 697 cm−1; HRMS (ESI): m/z calcd for ([C31H22ClNO2 + H]+): 476.1412; found: 476.1409.
2,2-Bis(4-methoxyphenyl)-6-methyl-4-(p-tolyl)-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3o). White solid (142 mg, 55%); mp 266–267 °C; 1H NMR (400 MHz, CDCl3) δ 8.16 (dd, J = 8.0, 1.2 Hz, 1H), 7.61–7.50 (m, 1H), 7.47–7.34 (m, 4H), 7.32–7.21 (m, 4H), 7.20–7.11 (m, 2H), 6.89–6.74 (m, 4H), 5.87 (s, 1H), 3.76 (s, 6H), 3.55 (s, 3H), 2.36 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 159.72, 159.07, 156.53, 139.86, 136.76, 136.71, 136.00, 135.30, 131.18, 128.42, 128.29, 127.17, 126.09, 123.54, 121.68, 116.05, 113.95, 113.41, 108.21, 84.15, 55.19, 29.21, 21.28. IR (KBr) ν 2933, 2836, 1647, 1558, 1510, 1388, 1114, 989, 762, 717 cm−1; HRMS (ESI): m/z calcd for ([C34H29NO4 + H]+): 516.2169; found: 516.2166.
4-(4-Chlorophenyl)-2,2-bis(4-methoxyphenyl)-6-methyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3p). White solid (163 mg, 61%); mp 247–248 °C; 1H NMR (400 MHz, CDCl3) δ 8.17 (dd, J = 8.0, 1.3 Hz, 1H), 7.61–7.52 (m, 1H), 7.43–7.36 (m, 4H), 7.34–7.26 (m, 5H), 7.26–7.21 (m, 1H), 6.89–6.78 (m, 4H), 5.87 (s, 1H), 3.76 (s, 6H), 3.55 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.66, 159.17, 156.74, 139.88, 138.13, 135.74, 134.43, 132.98, 131.44, 128.69, 128.23, 127.85, 126.72, 123.61, 121.85, 115.94, 114.04, 113.51, 107.70, 84.20, 55.21, 29.22. IR (KBr) ν 2934, 2836, 1732, 1644, 1558, 1491, 1387, 1115, 991, 759, 691 cm−1; HRMS (ESI): m/z calcd for ([C33H26ClNO4 + H]+): 536.1623; found: 536.1620.
6′-Methyl-4′-phenylspiro[fluorene-9,2′-pyrano[3,2-c]quinolin]-5′(6′H)-one (3q). Yellow solid (55 mg, 25%); mp 167–168 °C; 1H NMR (400 MHz, CDCl3) δ 7.83 (dd, J = 8.0, 1.3 Hz, 1H), 7.66 (t, 4H), 7.56–7.48 (m, 1H), 7.42 (td, J = 7.5, 0.9 Hz, 2H), 7.37–7.17 (m, 8H), 7.07 (t, 1H), 5.56 (s, 1H), 3.68 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.84, 158.69, 146.49, 139.79, 139.68, 139.32, 136.35, 131.39, 130.21, 128.43, 127.57, 127.31, 127.11, 125.44, 124.09, 122.82, 121.57, 120.08, 115.80, 113.83, 106.93, 86.37, 29.39. IR (KBr) ν 3046, 1641, 1553, 1497, 1384, 1116, 986, 752, 698 cm−1; HRMS (ESI): m/z calcd for ([C31H21NO2 + H]+): 440.1645; found: 440.1648.
4′-(4-Methoxyphenyl)-6′-methylspiro[fluorene-9,2′-pyrano[3,2-c]quinolin]-5′(6′H)-one (3r). Yellow solid (129 mg, 55%); mp 130–131 °C; 1H NMR (400 MHz, CDCl3) δ 7.82 (dd, J = 8.0, 1.3 Hz, 1H), 7.65 (t, 4H), 7.55–7.46 (m, 1H), 7.41 (td, J = 7.5, 0.8 Hz, 2H), 7.31 (d, J = 8.5 Hz, 1H), 7.28–7.18 (m, 4H), 7.06 (t, J = 7.6 Hz, 1H), 6.92–6.81 (m, 2H), 5.53 (s, 1H), 3.80 (s, 3H), 3.68 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.87, 158.74, 158.72, 146.51, 139.63, 139.31, 135.91, 132.08, 131.33, 130.16, 128.42, 128.40, 125.43, 124.05, 122.07, 121.55, 120.04, 115.81, 113.79, 113.01, 107.00, 86.33, 55.12, 29.37. IR (KBr) ν 3940, 2930, 2833, 1734, 1646, 1556, 1511, 1383, 1114, 986, 754, 692 cm−1; HRMS (ESI): m/z calcd for ([C32H23NO3 + H]+): 470.1751; found: 470.1749.
4′-(4-Fluorophenyl)-6′-methylspiro[fluorene-9,2′-pyrano[3,2-c]quinolin]-5′(6′H)-one (3s). Yellow solid (85 mg, 37%); mp 134–135 °C; 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 7.9 Hz, 1H), 7.73–7.59 (m, 4H), 7.53 (t, J = 7.8 Hz, 1H), 7.42 (t, J = 7.5 Hz, 2H), 7.37–7.18 (m, 5H), 7.15–6.92 (m, 3H), 5.52 (s, 1H), 3.68 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.04 (d, JC–F = 243.8 Hz), 159.85, 158.80, 146.40, 139.67, 139.32, 135.71 (d, JC–F = 3.4 Hz), 135.42, 131.52, 130.28, 128.93 (d, JC–F = 7.9 Hz), 128.46, 125.38, 124.12, 122.88, 121.68, 120.12, 115.74, 114.47 (d, JC–F = 21.5 Hz), 113.87, 106.60, 86.36, 29.37; 19F NMR (377 MHz, CDCl3) δ −115.47 (s). IR (KBr) ν 3042, 2925, 1645, 1556, 1508, 1384, 1115, 987, 754, 692 cm−1; HRMS (ESI): m/z calcd for ([C31H20FNO2 + H]+): 458.1551; found: 458.1553.
4,6-Dimethyl-2,2-diphenyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3t). White solid (102 mg, 54%); mp 240–241 °C; 1H NMR (400 MHz, CDCl3) δ 8.15 (dd, J = 7.9, 1.1 Hz, 1H), 7.55–7.49 (m, 1H), 7.48–7.41 (m, 4H), 7.33–7.27 (m, 4H), 7.27–7.20 (m, 4H), 5.75 (s, 1H), 3.60 (s, 3H), 2.45 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 161.05, 155.52, 144.31, 139.55, 131.08, 128.11, 127.65, 126.77, 123.58, 123.09, 121.76, 115.81, 113.82, 108.87, 84.12, 29.05, 20.99. IR (KBr) ν 3052, 2920, 1648, 1627, 1606, 1558, 1450, 1389, 1316, 1201, 984, 758, 701 cm−1; HRMS (ESI): m/z calcd for ([C31H23NO2 + H]+): 380.1645; found: 380.1646.
4-Butyl-6-methyl-2,2-diphenyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3u). White solid (120 mg, 57%); mp 157–158 °C; 1H NMR (400 MHz, CDCl3) δ 8.16 (dd, J = 7.9, 1.2 Hz, 1H), 7.55–7.48 (m, 1H), 7.47–7.40 (m, 4H), 7.33–7.31 (m, 1H), 7.30–7.29 (m, 2H), 7.28–7.27 (m, 1H), 7.27–7.19 (m, 4H), 5.77 (s, 1H), 3.60 (s, 3H), 2.92 (t, 2H), 1.57–1.48 (m, 2H), 1.46–1.35 (m, 2H), 0.92 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 160.67, 155.92, 144.33, 139.54, 135.50, 131.01, 128.09, 127.63, 126.83, 123.55, 122.70, 121.70, 115.91, 113.81, 108.61, 84.02, 33.20, 31.63, 29.16, 22.59, 14.11. IR (KBr) ν 3025, 2941, 1639, 1622, 1604, 1555, 1491, 1391, 1201, 1104, 955, 750, 700 cm−1; HRMS (ESI): m/z calcd for ([C29H27NO2 + H]+): 422.2115; found: 422.2114.
6-Methyl-2,2-diphenyl-4-(trimethylsilyl)-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3v). White solid (112 mg, 51%); mp 180–181 °C; 1H NMR (400 MHz, CDCl3) δ 8.14 (d, J = 8.0 Hz, 1H), 7.50–7.40 (m, 5H), 7.33–7.26 (m, 4H), 7.26–7.17 (m, 4H), 6.29 (s, 1H), 3.58 (s, 3H), 0.32 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 161.44, 153.77, 144.05, 139.26, 135.00, 133.29, 130.76, 128.14, 127.65, 126.78, 123.25, 121.73, 116.08, 113.74, 111.17, 82.88, 29.36, 0.52. IR (KBr) ν 3058, 2952, 1643, 1618, 1591, 1492, 1384, 1126, 992, 751, 696 cm−1; HRMS (ESI): m/z calcd for ([C31H23NO2 + H]+): 438.1884; found: 438.1883.
2,2,4-Triphenyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3w). White solid (64 mg, 30%); mp 277–278 °C; 1H NMR (400 MHz, CDCl3) δ 12.29 (s, 1H), 8.07 (dd, J = 8.1, 1.0 Hz, 1H), 7.58–7.48 (m, 4H), 7.47–7.40 (m, 2H), 7.39–7.22 (m, 10H), 7.18 (t, J = 7.6 Hz, 1H), 6.67 (d, J = 8.2 Hz, 1H), 5.96 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 161.87, 158.47, 143.67, 139.42, 138.49, 135.20, 131.05, 128.21, 127.90, 127.79, 127.60, 126.87, 126.83, 125.61, 122.73, 122.04, 116.12, 114.94, 107.76, 84.55. IR (KBr) ν 3056, 2842, 1650, 1494, 1388, 1105, 950, 752, 695 cm−1; HRMS (ESI): m/z calcd for ([C30H21NO2 + H]+): 428.1645; found: 428.1647.
(Z)-6-(3-Hydroxy-1,3,3-triphenylprop-1-en-1-yl)-2,2,4-triphenyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3x). White solid (71 mg, 20%); mp 254–255 °C; 1H NMR (400 MHz, CDCl3) δ 7.92 (dd, J = 7.9, 1.2 Hz, 1H), 7.66–7.60 (m, 2H), 7.55–7.45 (m, 4H), 7.45–7.38 (m, 5H), 7.38–7.30 (m, 5H), 7.30–7.28 (m, 2H), 7.27–7.26 (m, 1H), 7.26–7.20 (m, 4H), 7.20–7.13 (m, 4H), 7.12–7.04 (m, 3H), 6.78 (d, J = 8.3 Hz, 1H), 6.33 (t, J = 7.3 Hz, 1H), 6.17 (t, J = 7.7 Hz, 2H), 5.92 (s, 1H), 5.41 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 160.60, 157.77, 148.27, 144.26, 144.03, 143.69, 139.32, 137.86, 136.49, 136.41, 135.48, 135.02, 130.49, 128.84, 128.73, 128.43, 128.39, 128.22, 128.17, 127.71, 127.67, 127.48, 127.35, 127.21, 126.85, 126.66, 126.64, 125.77, 125.70, 125.57, 125.36, 124.88, 122.86, 122.30, 117.44, 115.56, 106.85, 84.81, 75.69. IR (KBr) ν 3057, 3027, 1735, 1637, 1600, 1553, 1492, 1393, 1163, 1011, 757, 696 cm−1; HRMS (ESI): m/z calcd For ([C51H37NO3 + H]+): 712.2846; found: 712.2843.
(Z)-6-(3-Hydroxy-3,3-diphenyl-1-(p-tolyl)prop-1-en-1-yl)-2,2-diphenyl-4-(p-tolyl)-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3y). White solid (203 mg, 55%); mp 166–167 °C; 1H NMR (400 MHz, CDCl3) δ 7.91 (d, J = 7.9 Hz, 1H), 7.63 (d, J = 7.3 Hz, 2H), 7.56–7.36 (m, 7H), 7.35–7.22 (m, 8H), 7.21–7.11 (m, 4H), 7.11–6.96 (m, 7H), 6.76 (d, J = 8.4 Hz, 1H), 6.30 (t, J = 7.3 Hz, 1H), 6.13 (t, J = 7.7 Hz, 2H), 5.89 (s, 1H), 5.41 (s, 1H), 2.34 (s, 3H), 2.27 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.60, 157.70, 148.45, 144.44, 144.05, 143.79, 138.71, 137.90, 136.92, 136.42, 135.55, 135.45, 134.96, 133.59, 130.37, 129.51, 128.41, 128.36, 128.18, 128.12, 127.64, 127.31, 127.28, 126.78, 126.62, 125.81, 125.47, 125.27, 124.89, 122.78, 122.18, 117.46, 115.53, 106.95, 84.79, 75.66, 21.26, 21.11. IR (KBr) ν 3058, 3025, 2921, 1745, 1634, 1601, 1555, 1492, 1448, 1391, 1149, 755, 698 cm−1; HRMS (ESI): m/z calcd for ([C53H41NO3 + H]+): 740.3159; found: 740.3161.
(Z)-6-(3-Hydroxy-1-phenyl-3,3-di-p-tolylprop-1-en-1-yl)-4-phenyl-2,2-di-p-tolyl-2,6-dihydro-5H-pyrano[3,2-c]quinolin-5-one (3z). White solid (307 mg, 80%); mp 257–258 °C; 1H NMR (400 MHz, CDCl3) δ 7.87 (dd, J = 7.9, 1.3 Hz, 1H), 7.54 (d, J = 8.2 Hz, 2H), 7.47–7.37 (m, 5H), 7.36–7.25 (m, 7H), 7.22 (s, 5H), 7.16–7.00 (m, 6H), 6.90 (d, J = 8.1 Hz, 2H), 6.68 (d, J = 8.3 Hz, 1H), 5.92 (d, J = 8.0 Hz, 2H), 5.83 (s, 1H), 5.32 (s, 1H), 2.38 (s, 3H), 2.32 (s, 3H), 2.26 (s, 3H), 1.72 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.72, 157.73, 145.67, 141.72, 141.27, 141.02, 139.48, 138.19, 137.70, 137.31, 136.67, 136.32, 136.29, 135.61, 134.79, 134.71, 130.23, 129.20, 128.86, 128.79, 128.68, 127.60, 127.57, 127.44, 127.37, 127.24, 126.52, 125.72, 125.65, 125.34, 124.70, 122.58, 121.98, 117.38, 115.60, 106.56, 84.91, 75.29, 21.15, 21.04, 20.95, 20.50. IR (KBr) ν 3023, 2920, 1738, 1633, 1604, 1551, 1493, 1387, 1149, 925, 749, 699 cm−1; HRMS (ESI): m/z calcd for([C55H45NO3 + H]+): 768.3472; found: 768.3473.
(Z)-2-Benzylidene-5-methyl-3-phenyl-3,5-dihydrofuro[3,2-c]quinolin-4(2H)-one (4a). White solid (110 mg, 60%); mp 210–211 °C; 1H NMR (400 MHz, CDCl3) δ 8.00 (dd, J = 7.9, 1.4 Hz, 1H), 7.75–7.56 (m, 3H), 7.46–7.29 (m, 8H), 7.29–7.18 (m, 2H), 5.64 (d, J = 2.2 Hz, 1H), 5.36 (d, J = 2.2 Hz, 1H), 3.64 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.30, 159.60, 158.73, 140.91, 140.66, 134.48, 131.56, 128.73, 128.45, 128.33, 128.05, 127.35, 126.61, 123.17, 121.99, 114.73, 111.80, 111.39, 106.38, 51.20, 29.05. IR (KBr) ν 3059, 1694, 1658, 1641, 1568, 1494, 1404, 1121, 759, 700 cm−1; HRMS (ESI): m/z calcd for ([C25H19NO2 + H]+): 366.1489; found: 366.1487.
(Z)-5-Methyl-2-(4-methylbenzylidene)-3-phenyl-3,5-dihydrofuro[3,2-c]quinolin-4(2H)-one (4b). White solid (80 mg, 42%); mp 201–202 °C; 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 7.8 Hz, 1H), 7.63 (t, J = 7.9 Hz, 1H), 7.56 (d, J = 8.0 Hz, 2H), 7.45–7.28 (m, 6H), 7.28–7.13 (m, 3H), 5.61 (s, 1H), 5.34 (s, 1H), 3.63 (s, 3H), 2.36 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.33, 159.63, 158.03, 140.92, 140.83, 136.38, 131.65, 131.49, 129.15, 128.69, 128.25, 128.06, 127.28, 123.18, 121.93, 114.70, 111.82, 111.46, 106.33, 51.12, 29.03, 21.21. IR (KBr) ν 3041, 2946, 1690, 1659, 1638, 1565, 1503, 1400, 1096, 751, 702 cm−1; HRMS (ESI): m/z calcd for ([C26H21NO2 + H]+): 380.1645; found: 380.1644.
(Z)-2-(4-Methoxybenzylidene)-5-methyl-3-phenyl-3,5-dihydrofuro[3,2-c]quinolin-4(2H)-one (4c). White solid (97 mg, 49%); mp 203–204 °C; 1H NMR (400 MHz, CDCl3) δ 7.99 (d, J = 7.8 Hz, 1H), 7.71–7.54 (m, 3H), 7.45–7.29 (m, 6H), 7.28–7.19 (m, 1H), 6.92 (d, J = 8.4 Hz, 2H), 5.58 (s, 1H), 5.34 (s, 1H), 3.82 (s, 3H), 3.63 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.33, 159.66, 158.24, 157.11, 140.94, 140.92, 131.48, 129.58, 128.69, 128.05, 127.29, 127.26, 123.17, 121.91, 114.71, 113.90, 111.82, 111.48, 105.93, 55.24, 51.04, 29.03. IR (KBr) ν 3003, 2927, 2832, 2248, 1692, 1662, 1644, 1566, 1510, 1403, 1123, 757, 699 cm−1; HRMS (ESI): m/z calcd for ([C26H21NO3 + H]+): 396.1594; found: 396.1596.
(Z)-2-(2-Fluorobenzylidene)-5-methyl-3-phenyl-3,5-dihydrofuro[3,2-c]quinolin-4(2H)-one (4d). White solid (69 mg, 36%); mp 226–227 °C; 1H NMR (400 MHz, CDCl3) δ 8.19 (t, J = 6.9 Hz, 1H), 7.97 (d, J = 7.8 Hz, 1H), 7.65 (t, J = 7.9 Hz, 1H), 7.48–7.30 (m, 6H), 7.29–7.14 (m, 3H), 7.03 (t, J = 9.3 Hz, 1H), 5.93 (s, 1H), 5.38 (s, 1H), 3.65 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.17, 160.07 (d, JC–F = 2.1 Hz), 159.25 (d, JC–F = 247.7 Hz),158.01, 140.98, 140.38, 131.62, 129.56 (d, JC–F = 2.6 Hz), 128.80, 128.00 (d, JC–F = 7.8 Hz), 127.98, 127.44, 124.01 (d, JC–F = 3.6 Hz), 123.10, 122.41 (d, JC–F = 12.0 Hz), 122.00, 115.16 (d, JC–F = 22.1 Hz), 114.77, 111.94, 111.33, 97.58 (d, JC–F = 7.7 Hz), 51.41, 29.08; 19F NMR (377 MHz, CDCl3) δ −117.05 (s). IR (KBr) ν 3059, 3029, 2921, 1694, 1662, 1643, 1568, 1485, 1404, 1124, 750, 700 cm−1; HRMS (ESI): m/z calcd for ([C25H18FNO2 + H]+): 384.1394; found: 384.1397.
(Z)-2-(3-Fluorobenzylidene)-5-methyl-3-phenyl-3,5-dihydrofuro[3,2-c]quinolin-4(2H)-one (4e). Yellow solid (98 mg, 51%); mp 211–212 °C; 1H NMR (400 MHz, CDCl3) δ 7.99 (d, J = 7.8 Hz, 1H), 7.65 (t, J = 7.9 Hz, 1H), 7.51 (d, J = 10.3 Hz, 1H), 7.45–7.19 (m, 9H), 7.00–6.85 (m, 1H), 5.62 (s, 1H), 5.35 (s, 1H), 3.64 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.87 (d, JC–F = 242.6 Hz), 160.15, 159.81, 159.48, 140.96, 140.33, 136.59 (d, JC–F = 8.4 Hz), 131.67, 129.73 (d, JC–F = 8.5 Hz), 128.81, 128.02, 127.47, 124.13 (d, JC–F = 2.4 Hz), 123.13, 122.10, 114.97, 114.75, 113.41 (d, JC–F = 21.4 Hz), 111.77, 111.26, 105.41 (d, JC–F = 2.6 Hz), 51.34, 29.07; 19F NMR (377 MHz, CDCl3) δ −113.17, −113.18 (m). IR (KBr) ν 3040, 1691, 1658, 1642, 1574, 1489, 1406, 1102, 755, 702 cm−1; HRMS (ESI): m/z calcd for ([C25H18FNO2 + H]+): 384.1394; found: 384.1397.
(Z)-2-(4-Fluorobenzylidene)-5-methyl-3-phenyl-3,5-dihydrofuro[3,2-c]quinolin-4(2H)-one (4f). Yellow solid (77 mg, 40%); mp 221–222 °C; 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 7.8 Hz, 1H), 7.74–7.54 (m, 3H), 7.48–7.30 (m, 6H), 7.30–7.21 (m, 1H), 7.07 (t, J = 8.7 Hz, 2H), 5.60 (d, J = 2.1 Hz, 1H), 5.34 (d, J = 1.6 Hz, 1H), 3.64 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 161.31 (d, JC–F = 245.0 Hz), 160.20, 159.55, 158.33 (d, JC–F = 2.4 Hz), 140.90, 140.57, 131.61, 130.62 (d, JC–F = 3.2 Hz), 129.88 (d, JC–F = 7.8 Hz), 128.77, 127.99, 127.40, 123.08, 122.02, 115.33 (d, JC–F = 21.4 Hz), 114.78, 111.78, 111.31, 105.25, 51.12, 29.07; 19F NMR (377 MHz, CDCl3) δ −114.90 (m). IR (KBr) ν 3063, 2943, 1694, 1659, 1640, 1570, 1506, 1403, 1100, 753, 703 cm−1; HRMS (ESI): m/z calcd for ([C25H18FNO2 + H]+): 384.1394; found: 384.1397.
(Z)-2-(4-Chlorobenzylidene)-5-methyl-3-phenyl-3,5-dihydrofuro[3,2-c]quinolin-4(2H)-one (4g). White solid (68 mg, 34%); mp 239–240 °C; 1H NMR (400 MHz, CDCl3) δ 7.97 (dd, J = 7.9, 1.4 Hz, 1H), 7.70–7.63 (m, 1H), 7.62–7.55 (m, 2H), 7.44–7.30 (m, 8H), 7.30–7.23 (m, 1H), 5.59 (d, J = 2.2 Hz, 1H), 5.34 (d, J = 2.2 Hz, 1H), 3.65 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.15, 159.50, 159.22, 140.92, 140.39, 132.96, 132.04, 131.65, 129.50, 128.80, 128.56, 128.00, 127.45, 123.07, 122.05, 114.79, 111.77, 111.26, 105.22, 51.27, 29.08. IR (KBr) ν 3054, 2946, 1695, 1659, 1640, 1568, 1491, 1403, 1096, 753, 707 cm−1; HRMS (ESI): m/z calcd for ([C25H18ClNO2 + H]+): 400.1099; found: 400.1096.
(Z)-2-Benzylidene-5-methyl-3-(p-tolyl)-3,5-dihydrofuro[3,2-c]quinolin-4(2H)-one (4h). White solid (68 mg, 36%); mp 214–215 °C; 1H NMR (400 MHz, CDCl3) δ 7.99 (d, J = 7.8 Hz, 1H), 7.74–7.58 (m, 3H), 7.46–7.30 (m, 4H), 7.29–7.18 (m, 3H), 7.17–7.07 (m, 2H), 5.64 (d, J = 2.0 Hz, 1H), 5.32 (d, J = 1.7 Hz, 1H), 3.63 (s, 3H), 2.30 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.15, 159.60, 158.91, 140.83, 137.67, 136.92, 134.53, 131.47, 129.43, 128.41, 128.29, 127.91, 126.53, 123.12, 121.94, 114.70, 111.94, 111.39, 106.15, 50.84, 29.02, 21.10. IR (KBr) ν 3052, 3024, 1694, 1663, 1645, 1568, 1459, 1401, 1120, 752, 693 cm−1; HRMS (ESI): m/z calcd for ([C26H21NO2 + H]+): 380.1645; found: 380.1644.
(Z)-2-Benzylidene-3-(4-methoxyphenyl)-5-methyl-3,5-dihydrofuro[3,2-c]quinolin-4(2H)-one (4i). White solid (105 mg, 53%); mp 222–223 °C; 1H NMR (400 MHz, CDCl3) δ 8.00 (dd, J = 7.9, 1.4 Hz, 1H), 7.73–7.59 (m, 3H), 7.43–7.27 (m, 6H), 7.26–7.19 (m, 1H), 6.91–6.82 (m, 2H), 5.64 (d, J = 2.2 Hz, 1H), 5.32 (d, J = 2.1 Hz, 1H), 3.77 (s, 3H), 3.64 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.05, 159.64, 159.00, 158.73, 140.84, 134.52, 132.78, 131.48, 129.09, 128.43, 128.29, 126.55, 123.13, 121.95, 114.71, 114.10, 111.95, 111.40, 106.15, 55.15, 50.47, 29.02. IR (KBr) ν 3001, 2949, 2831, 1694, 1662, 1644, 1566, 1494, 1403, 1121, 753, 692 cm−1; HRMS (ESI): m/z calcd for ([C26H21NO3 + H]+): 396.1594; found: 396.1596.
(Z)-2-Benzylidene-3-(4-fluorophenyl)-5-methyl-3,5-dihydrofuro[3,2-c]quinolin-4(2H)-one (4j). White solid (59 mg, 31%); mp 216–217 °C; 1H NMR (400 MHz, CDCl3) δ 8.00 (d, J = 7.8 Hz, 1H), 7.74–7.58 (m, 3H), 7.50–7.30 (m, 6H), 7.29–7.16 (m, 1H), 7.01 (t, J = 8.6 Hz, 2H), 5.63 (s, 1H), 5.35 (s, 1H), 3.65 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.07 (d, JC–F = 244.2 Hz), 160.31, 159.60, 158.51, 140.96, 136.46 (d, JC–F = 3.3 Hz), 134.33, 131.68, 129.67 (d, JC–F = 8.0 Hz), 128.50, 128.35, 126.76, 123.21, 122.07, 115.72, 115.51, 114.78, 111.47 (d, JC–F = 24.2 Hz), 106.59, 50.47, 29.06; 19F NMR (377 MHz, CDCl3) δ −115.30 (s). IR (KBr) ν 3036, 1694, 1662, 1643, 1570, 1507, 1403, 1122, 756, 693 cm−1; HRMS (ESI): m/z calcd for ([C25H18FNO2 + H]+): 384.1394; found: 384.1397.
(Z)-2-Benzylidene-3-(4-chlorophenyl)-5-methyl-3,5-dihydrofuro[3,2-c]quinolin-4(2H)-one (4k). White solid (84 mg, 42%); mp 224–225 °C; 1H NMR (400 MHz, CDCl3) δ 8.00 (d, J = 7.7 Hz, 1H), 7.67 (d, J = 7.4 Hz, 3H), 7.52–7.13 (m, 9H), 5.62 (s, 1H), 5.35 (s, 1H), 3.65 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.44, 159.57, 158.24, 141.00, 139.19, 134.27, 133.25, 131.76, 129.49, 128.94, 128.52, 128.38, 126.82, 123.25, 122.11, 114.82, 111.42, 111.34, 106.73, 50.62, 29.08. IR (KBr) ν 3023, 1693, 1662, 1641, 1567, 1493, 1403, 1121, 754, 692 cm−1; HRMS (ESI): m/z calcd for ([C25H18ClNO2 + H]+): 400.1099; found: 400.1096.
(Z)-2-Benzylidene-3-(4-bromophenyl)-5-methyl-3,5-dihydrofuro[3,2-c]quinolin-4(2H)-one (4l). White solid (69 mg, 31%); mp 213–214 °C; 1H NMR (400 MHz, CDCl3) δ 8.01 (d, J = 7.8 Hz, 1H), 7.67 (d, J = 7.4 Hz, 3H), 7.53–7.33 (m, 6H), 7.32–7.17 (m, 3H), 5.63 (s, 1H), 5.34 (s, 1H), 3.66 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 160.48, 159.58, 158.15, 141.02, 139.72, 134.26, 131.89, 131.78, 129.87, 128.53, 128.39, 126.84, 123.26, 122.13, 121.43, 114.83, 111.35, 106.77, 50.69, 29.09. IR (KBr) ν 3021, 1694, 1662, 1641, 1567, 1505, 1402, 1120, 753, 691 cm−1; HRMS (ESI): m/z calcd for ([C25H18BrNO2 + H]+): 444.0594; found: 444.0597.
3-(1,3-Diphenylprop-2-yn-1-yl)-4-hydroxy-1-methylquinolin-2(1H)-one (5a). White solid (110 mg, 60%); mp 204–205 °C; 1H NMR (400 MHz, CDCl3) δ 8.02 (dd, J = 8.0, 1.4 Hz, 1H), 7.88 (s, 1H), 7.68–7.46 (m, 5H), 7.40–7.29 (m, 6H), 7.28–7.19 (m, 2H), 6.11 (s, 1H), 3.73 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.39, 157.59, 139.33, 139.00, 131.79, 131.15, 128.83, 128.80, 128.42, 127.32, 127.14, 123.75, 121.82, 116.21, 113.80, 110.47, 87.45, 87.02, 33.07, 29.95. IR (KBr) ν 2940, 1637, 1556, 1489, 1393, 1248, 1152, 756, 693 cm−1; HRMS (ESI): m/z calcd for ([C25H19NO2 + H]+): 366.1489; found: 366.1487.
4-Methyl-6,8,9,9-tetraphenyl-4,9-dihydro-5H-cyclopenta[lmn]phenanthridin-5-one (6a). White solid (158 mg, 60%); mp 287–288 °C; 1H NMR (400 MHz, CDCl3) δ 7.57–7.52 (m, 2H), 7.50–7.35 (m, 4H), 7.26–6.97 (m, 16H), 6.85–6.76 (m, 2H), 3.70 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.11, 152.72, 145.35, 143.39, 142.80, 142.12, 141.35, 140.25, 139.31, 135.92, 134.82, 130.76, 129.46, 129.20, 128.50, 127.85, 127.46, 127.34, 127.26, 127.23, 126.83, 123.31, 118.48, 117.99, 110.75, 69.17, 29.42. IR (KBr) ν 3024, 1648, 1591, 1490, 1268, 1123, 1012, 758, 702 cm−1; HRMS (ESI): m/z calcd for ([C39H27NO + H]+): 526.2165; found: 526.2164.
4-Methyl-6,9,9-triphenyl-8-(p-tolyl)-4,9-dihydro-5H-cyclopenta[lmn]phenanthridin-5-one (6b). White solid (151 mg, 56%); mp 264–265 °C; 1H NMR (400 MHz, CDCl3) δ 7.58–7.51 (m, 2H), 7.49–7.35 (m, 4H), 7.21–6.98 (m, 13H), 6.88 (d, J = 7.5 Hz, 2H), 6.75–6.65 (m, 2H), 3.69 (s, 3H), 2.29 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.12, 152.69, 145.39, 143.53, 142.75, 142.20, 141.35, 140.30, 136.93, 136.46, 135.89, 135.03, 130.68, 129.45, 129.09, 128.54, 128.13, 127.83, 127.31, 127.22, 126.79, 123.33, 118.35, 117.98, 110.71, 69.16, 29.39, 21.11. IR (KBr) ν 3026, 1656, 1561, 1492, 1271, 1124, 960, 756, 707 cm−1; HRMS (ESI): m/z calcd for ([C40H29NO + H]+): 540.2322; found: 540.2324.
8-(4-Methoxyphenyl)-4-methyl-6,9,9-triphenyl-4,9-dihydro-5H-cyclopenta[lmn]phenanthridin-5-one (6c). White solid (128 mg, 46%); mp 282–283 °C; 1H NMR (400 MHz, CDCl3) δ 7.58–7.51 (m, 2H), 7.49–7.34 (m, 4H), 7.21–6.99 (m, 13H), 6.78–6.69 (m, 2H), 6.66–6.57 (m, 2H), 3.75 (s, 3H), 3.69 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.11, 158.88, 152.70, 145.53, 143.19, 142.77, 142.14, 141.37, 140.30, 135.88, 135.12, 131.81, 130.68, 130.40, 129.43, 128.52, 127.84, 127.31, 127.21, 126.82, 123.33, 118.30, 117.96, 112.89, 110.70, 69.13, 55.21, 29.38. IR (KBr) ν 3032, 1650, 1514, 1490, 1269, 1181, 1037, 752, 711 cm−1; HRMS (ESI): m/z calcd for ([C40H29NO2 + H]+): 556.2271; found: 556.2269.
9,9-Bis(4-methoxyphenyl)-4-methyl-6,8-diphenyl-4,9-dihydro-5H-cyclopenta[lmn]phenanthridin-5-one (6d). White solid (120 mg, 41%); mp 265–266 °C; 1H NMR (400 MHz, CDCl3) δ 7.56–7.51 (m, 2H), 7.48–7.35 (m, 4H), 7.23–7.17 (m, 2H), 7.14–7.06 (m, 3H), 7.02 (d, J = 7.5 Hz, 1H), 6.94–6.88 (m, 4H), 6.87–6.82 (m, 2H), 6.66–6.57 (m, 4H), 3.73 (s, 6H), 3.69 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 162.13, 158.34, 153.19, 145.81, 143.21, 142.62, 141.19, 140.30, 139.42, 135.91, 134.76, 134.32, 130.71, 129.54, 129.44, 129.26, 127.46, 127.32, 127.22, 123.07, 118.42, 117.74, 113.15, 110.57, 67.90, 55.16, 29.39. IR (KBr) ν 3030, 2959, 1649, 1507, 1451, 1248, 1180, 1029, 758, 699 cm−1; HRMS (ESI): m/z calcd for ([C41H31NO3 + H]+): 586.2377; found: 586.2376.
9,9-Bis(4-chlorophenyl)-4-methyl-6,8-diphenyl-4,9-dihydro-5H-cyclopenta[lmn]phenanthridin-5-one (6e). White solid (140 mg, 47%); mp 314–315 °C; 1H NMR (400 MHz, CDCl3) δ 7.58–7.36 (m, 6H), 7.28–7.19 (m, 2H), 7.18–7.09 (m, 3H), 7.09–7.02 (m, 4H), 6.97 (d, J = 7.5 Hz, 1H), 6.93–6.85 (m, 4H), 6.84–6.77 (m, 2H), 3.70 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 161.92, 151.73, 144.39, 143.33, 143.28, 141.13, 140.40, 139.94, 138.93, 136.10, 134.80, 132.92, 130.97, 129.68, 129.40, 129.00, 128.10, 127.69, 127.57, 127.40, 127.38, 123.10, 118.63, 117.60, 111.16, 68.05, 29.45. IR (KBr) ν 3026, 1650, 1591, 1489, 1266, 1093, 1012, 753, 697 cm−1; HRMS (ESI): m/z calcd for ([C39H25Cl2NO + H]+): 594.1386; found: 594.1388.
2-Benzyl-5-methyl-3-phenylfuro[3,2-c]quinolin-4(5H)-one (7a). White solid (70 mg, 96%); mp 156–157 °C; 1H NMR (400 MHz, CDCl3) δ 8.01–7.93 (m, 1H), 7.59–7.41 (m, 5H), 7.41–7.34 (m, 2H), 7.34–7.19 (m, 6H), 4.17 (s, 2H), 3.72 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.17, 154.38, 152.22, 137.94, 137.76, 130.90, 130.21, 129.25, 128.63, 128.38, 127.95, 127.56, 126.61, 121.99, 121.83, 121.14, 114.76, 113.98, 112.83, 32.44, 29.09. IR (KBr) ν 3022, 2940, 1655, 1582, 1493, 1225, 1113, 980, 744, 701 cm−1; HRMS (ESI): m/z calcd for ([C25H19NO2 + H]+): 366.1489; found: 366.1487.
2-(4-Methoxybenzyl)-5-methyl-3-phenylfuro[3,2-c]quinolin-4(5H)-one (7b). White solid (74 mg, 94%); mp 141–142 °C; 1H NMR (400 MHz, CDCl3) δ 7.97 (dd, J = 7.9, 1.3 Hz, 1H), 7.59–7.34 (m, 7H), 7.29–7.22 (m, 1H), 7.20–7.13 (m, 2H), 6.89–6.80 (m, 2H), 4.10 (s, 2H), 3.77 (s, 3H), 3.72 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.19, 158.31, 154.32, 152.67, 137.92, 130.95, 130.22, 129.78, 129.36, 129.22, 127.93, 127.52, 121.98, 121.50, 121.14, 114.76, 114.02, 113.99, 112.86, 55.21, 31.58, 29.09. IR (KBr) ν 3052, 2991, 1658, 1512, 1251, 1179, 1111, 1039, 746, 700 cm−1; HRMS (ESI): m/z calcd for ([C26H21NO3 + H]+): 396.1594; found: 396.1596.
2-(4-Chlorobenzyl)-5-methyl-3-phenylfuro[3,2-c]quinolin-4(5H)-one (7c). White solid (76 mg, 95%); mp 151–152 °C; 1H NMR (400 MHz, CDCl3) δ 7.97 (dd, J = 7.8, 1.2 Hz, 1H), 7.57–7.49 (m, 3H), 7.48–7.35 (m, 4H), 7.31–7.23 (m, 3H), 7.20–7.13 (m, 2H), 4.13 (s, 2H), 3.73 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.13, 154.48, 151.61, 138.02, 136.19, 132.50, 130.73, 130.15, 129.72, 129.40, 128.76, 128.02, 127.69, 122.07, 121.14, 114.83, 113.98, 112.78, 31.85, 29.12. IR (KBr) ν 3035, 2895, 1653, 1581, 1489, 1308, 1220, 1111, 750, 699 cm−1; HRMS (ESI): m/z calcd for ([C25H18ClNO2 + H]+): 400.1099; found: 400.1096.
2-Benzyl-3-(4-methoxyphenyl)-5-methylfuro[3,2-c]quinolin-4(5H)-one (7d). White solid (73 mg, 92%); mp 172–173 °C; 1H NMR (400 MHz, CDCl3) δ 7.97 (dd, J = 7.9, 1.4 Hz, 1H), 7.53–7.44 (m, 3H), 7.39 (d, J = 8.5 Hz, 1H), 7.34–7.28 (m, 2H), 7.28–7.20 (m, 4H), 7.03–6.94 (m, 2H), 4.16 (s, 2H), 3.84 (s, 3H), 3.72 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 159.27, 159.08, 154.30, 151.89, 137.92, 137.88, 131.35, 129.18, 128.62, 128.36, 126.58, 123.11, 121.97, 121.46, 121.13, 114.75, 114.07, 113.50, 112.89, 55.23, 32.43, 29.07. IR (KBr) ν 3023, 2939, 1658, 1589, 1516, 1252, 1184, 1112, 752, 698 cm−1; HRMS (ESI): m/z calcd for ([C26H21NO3 + H]+): 396.1594; found: 396.1596.
Author contributions
H. Y. conducted most of the synthetic experiments. X. G., Z. F., M.-F. W., D. F. and Z. C. conducted a part of the propargylic alcohols. S. W., Y. W. and M. W. directed the projects and wrote the manuscript. All of the authors approved the final version of the manuscript.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was supported by the Excellent Young Talents Fund Program of Higher Education Institutions of Anhui Province (Grant No. gxyq2019041), the Foundation of Key Laboratory of Antiinflammatory and Immune Medicine, Ministry of Education, Anhui Medical University (KFJJ-2021-15, KFJJ-2020-10), the Natural Science Foundation of Anhui Higher Education Institutions of China (KJ2020A0619, KJ2021A0842), the National Natural Science Foundation of China (21602157), the College Students' Innovation and Entrepreneurship Program of Anhui Province (S202110368092).
Notes and references
-
(a) J. P. Michael, Quinoline, quinazoline and acridone alkaloids, Nat. Prod. Rep., 2002, 19, 742–760 RSC;
(b) J. P. Michael, Quinoline, quinazoline and acridone alkaloids, Nat. Prod. Rep., 2007, 24, 223–246 RSC.
-
(a) O. Jansen, V. Akhmedjanova, L. Angenot, G. Balansard, A. Chariot, E. Ollivier, M. Tits and M. Frédérich, Screening of 14 alkaloids isolated from Haplophyllum A. Juss. for their cytotoxic properties, J. Ethnopharmacol., 2006, 105, 241–245 CrossRef CAS PubMed;
(b) I. V. Magedov, M. Manpadi, M. A. Ogasawara, A. S. Dhawan, S. Rogelj, S. Van slambrouck, W. F. A. Steelant, N. M. Evdokimov, P. Y. Uglinskii and E. M. Elias, et al., Structural Simplification of Bioactive Natural Products with Multicomponent Synthesis. 2. Antiproliferative and Antitubulin Activities of Pyrano[3,2-c]pyridones and Pyrano[3,2-c]quinolones, J. Med. Chem., 2008, 51, 2561–2570 CrossRef CAS PubMed.
- M. Isaka, M. Tanticharoen, P. Kongsaeree and Y. Thebtaranonth, Structures of Cordypyridones A–D, Antimalarial N-Hydroxy- and N-Methoxy-2-pyridones from the Insect Pathogenic Fungus Cordyceps nipponica, J. Org. Chem., 2001, 66, 4803–4808 CrossRef CAS PubMed.
- J.-J. Chen, P.-H. Chen, C.-H. Liao, S.-Y. Huang and I.-S. Chen, New Phenylpropenoids, Bis(1-phenylethyl)phenols, Bisquinolinone Alkaloid, and Anti-inflammatory Constituents from Zanthoxylum integrifoliolum, J. Nat. Prod., 2007, 70, 1444–1448 CrossRef CAS PubMed.
-
(a) K. D. McBrien, Q. Gao, S. Huang, S. E. Klohr, R. R. Wang, D. M. Pirnik, K. M. Neddermann, I. Bursuker, K. F. Kadow and J. E. Leet, Fusaricide, a New Cytotoxic N-Hydroxypyridone from Fusarium sp., J. Nat. Prod., 1996, 59, 1151–1153 CrossRef CAS PubMed;
(b) C. L. Cantrell, K. K. Schrader, L. K. Mamonov, G. T. Sitpaeva, T. S. Kustova, C. Dunbar and D. E. Wedge, Isolation and Identification of Antifungal and Antialgal Alkaloids from Haplophyllum sieversii, J. Agric. Food Chem., 2005, 53, 7741–7748 CrossRef CAS PubMed.
- F. Koizumi, N. Fukumitsu, J. Zhao, R. Chanklan, T. Miyakawa, S. Kawahara, S. Iwamoto, M. Suzuki, S. Kakita and E. S. Rahayu, et al., YCM1008A, a Novel Ca2+-Signaling Inhibitor, Produced by Fusarium sp. YCM1008, J. Antibiot., 2007, 60, 455–458 CrossRef CAS PubMed.
- K. D. Upadhyay, N. M. Dodia, R. C. Khunt, R. S. Chaniara and A. K. Shah, Evaluation and in vivo efficacy study of pyrano[3,2-c]quinoline analogues as TNF-α inhibitors, Chem. Biol. Drug Des., 2019, 94, 1647–1655 CrossRef CAS PubMed.
- I.-S. Chen, I.-W. Tsai, C.-M. Teng, J.-J. Chen, Y.-L. Chang, F.-N. Ko, M. C. Lu and J. M. Pezzuto, Pyranoquinoline alkaloids from Zanthoxylum simulans, Phytochemistry, 1997, 46, 525–529 CrossRef CAS.
- C. Ito, M. Itoigawa, A. Furukawa, T. Hirano, T. Murata, N. Kaneda, Y. Hisada, K. Okuda and H. Furukawa, Quinolone Alkaloids with Nitric Oxide Production Inhibitory Activity from Orixa japonica, J. Nat. Prod., 2004, 67, 1800–1803 CrossRef CAS PubMed.
- I.-S. Chen, S.-J. Wu, I.-L. Tsai, T.-S. Wu, J. M. Pezzuto, M. C. Lu, H. Chai, N. Suh and C.-M. Teng, Chemical and Bioactive Constituents from Zanthoxylum simulans, J. Nat. Prod., 1994, 57, 1206–1211 CrossRef CAS PubMed.
-
(a) J. Vaquette, M. S. Hifnawy, J. L. Pousset, A. Fournet, A. Bouquet and A. Cavé, Alcaloides
d'Araliopsis soyauxii. Isolement d'un nouvel alcaloide, l'Araliopsine, Phytochemistry, 1976, 15, 743–745 CrossRef CAS;
(b) G. Bar, A. F. Parsons and C. B. Thomas, A radical approach to araliopsine and related quinoline alkaloids using manganese(III) acetate, Tetrahedron Lett, 2000, 41, 7751–7755 CrossRef CAS;
(c) Y. Tangella, K. L. Manasa, V. Laxma Nayak, M. Sathish, B. Sridhar, A. Alarifi, N. Nagesh and A. Kamal, An efficient one-pot approach for the regio- and diastereoselective synthesis of trans-dihydrofuran derivatives: cytotoxicity and DNA-binding studies, Org. Biomol. Chem., 2017, 15, 6837–6853 RSC.
- T.-S. Wu, C.-Y. Li, Y.-L. Leu and C.-Q. Hu, Limonoids and alkaloids of the root bark of Dictamnus angustifolius, Phytochemistry, 1999, 50, 509–512 CrossRef CAS.
- C. Marzano, F. Baccichetti, F. Carlassare, A. Chilin, S. Lora and F. Bordin, DNA Damage Induced by 4,6,8,9-Tetramethyl-2H-furo[2,3-h]quinolin-2-one, a New Furocoumarin Analog: Biological Consequences, Photochem. Photobiol., 2000, 71, 263–272 CrossRef CAS PubMed.
- I. Butenschön, K. Möller and W. Hänsel, Angular Methoxy-Substituted Furo- and Pyranoquinolinones as Blockers of the Voltage-Gated Potassium Channel Kv1.3, J. Med. Chem., 2001, 44, 1249–1256 CrossRef PubMed.
-
(a) R. A. Mekheimer, N. H. Mohamed and K. U. Sadek, Synthesis of Functionalized 4H-Pyrano[3,2-c]pyridines from 4-Hydroxy-6-methyl-2-pyridone and Their Reactions. Unexpected New Routes to 3,3′-Benzylidenebis[4-hydroxy-6-methyl-2(1H)-3-pyridinone]s, Bull. Chem. Soc. Jpn., 1997, 70, 1625–1630 CrossRef CAS;
(b) E. Stoyanov, I. Ivanov and D. Heber, General Method for the Preparation of Substituted 2-Amino-4H,5H-pyrano[4,3-b]pyran-5-ones and 2-Amino-4H-pyrano[3,2-c]pyridine-5-ones, Molecules, 2000, 5, 19–32 CrossRef CAS;
(c) K. Tatsuta, T. Yamaguchi, Y. Tsuda, Y. Yamaguchi, N. Hattori, H. Nagai and S. Hosokawa, The first total synthesis and structural determination of YCM1008A, Tetrahedron Lett, 2007, 48, 4187–4190 CrossRef CAS;
(d) P. Kumari, C. Narayana, S. Dubey, A. Gupta and R. Sagar, Stereoselective synthesis of natural product inspired carbohydrate fused pyrano[3,2-c]quinolones as antiproliferative agents, Org. Biomol. Chem., 2018, 16, 2049–2059 RSC;
(e) K. D. Upadhyay, N. M. Dodia, R. C. Khunt, R. S. Chaniara and A. K. Shah, Synthesis and Biological Screening of Pyrano[3,2-c]quinoline Analogues as Anti-inflammatory and Anticancer Agents, ACS Med. Chem. Lett., 2018, 9, 283–288 CrossRef CAS PubMed;
(f) G. Bar, A. F. Parsons and C. B. Thomas, Manganese(III) acetate mediated radical reactions leading to araliopsine and related quinoline alkaloids, Tetrahedron, 2001, 57, 4719–4728 CrossRef CAS;
(g) M. Ghosh and A. Hajra, DABCO-Promoted One-Pot Facile Synthesis of Angularly Fused Furoquinol-inones and Furocoumarins, Eur. J. Org. Chem., 2015, 2015, 7836–7841 CrossRef CAS;
(h) T. Katsina, E. E. Anagnostaki, F. Mitsa, V. Sarli and A. L. Zografos, Palladium-catalyzed direct alkenylation of 4-hydroxy-2-pyridones, RSC Adv, 2016, 6, 6978–6982 RSC;
(i) A. Dey and A. Hajra, FeCl3/ZnI2-Catalyzed regioselective synthesis of angularly fused furans, Org. Biomol. Chem., 2017, 15, 8084–8090 RSC;
(j) T. Guo, X.-N. Wei, H.-Y. Wang and B. Zhao, Palladium-catalyzed facile synthesis of furoquinol-inones and furopyridinones, Synth. Commun., 2018, 48, 761–767 CrossRef CAS;
(k) P. A. Sakharov, N. V. Rostovskii, A. F. Khlebnikov, T. L. Panikorovskii and M. S. Novikov, 2H-Azirines as C–C Annulation Reagents in Cu-Catalyzed Synthesis of Furo[3,2-c]quinolone Derivatives, Org. Lett., 2019, 21, 3615–3619 CrossRef CAS PubMed.
- X.-R. Song, R. Yang and Q. Xiao, Recent Advances in the Synthesis of Heterocyclics via Cascade Cyclization of Propargylic Alcohols, Adv. Synth. Catal., 2021, 363, 852–876 CrossRef CAS.
-
(a) C. R. Reddy, R. Ranjan, P. Kumaraswamy, M. D. Reddy and R. Gree, 1-Aryl Propargylic Alcohols as Handy Synthons for the Construction of Heterocycles and Carbocycles, Curr. Org. Chem., 2014, 18, 2603–2645 CrossRef CAS;
(b) S. Gandhi and B. Baire, Ag(I) Catalyzed Cascade Approach to 2-(α-Hydroxyacyl)pyrroles, ChemistrySelect, 2017, 2, 3964–3968 CrossRef CAS;
(c) X. Y. Liu, Y. L. Liu and L. Chen, Tandem Annulations of Propargylic Alcohols to Indole Derivatives, Adv. Synth. Catal., 2020, 362, 5170–5195 CrossRef CAS.
-
(a) F.-Q. Yuan and F.-S. Han, Iron-Catalyzed Direct Synthesis of Densely Substituted Benzofurans and Naphthopyrans from Phenolic Compounds and Propargylic Alcohols, Adv. Synth. Catal., 2013, 355, 537–547 CAS;
(b) W.-T. Li, W.-H. Nan and Q.-L. Luo, Metal-free sequential reaction via a propargylation, annulation and isomerization sequence for the one-pot synthesis of 2,3-disubstituted benzofurans, RSC Adv, 2014, 4, 34774–34779 RSC;
(c) P. Tharra and B. Baire, Mild Approach to 2-Acylfurans via Intercepted Meyer-Schuster Rearrangement of 6-Hydroxyhex-2-en-4-ynals, J. Org. Chem., 2015, 80, 8314–8328 CrossRef CAS PubMed;
(d) X. Cheng, Y. Yu, Z. Mao, J. Chen and X. Huang, Facile synthesis of substituted 3-aminofurans through a tandem reaction of N-sulfonyl-1,2,3-triazoles with propargyl alcohols, Org. Biomol. Chem., 2016, 14, 3878–3882 RSC;
(e) G. C. Nandi and K. Soumini, Catalyst-Controlled Straightforward Synthesis of Highly Substituted Pyrroles/Furans via Propargylation/Cycloisomerization of α-Oxoketene-N,S-acetals, J. Org. Chem., 2016, 81, 11909–11915 CrossRef CAS PubMed;
(f) A. Pareek, R. Dada, M. Rana, A. K. Sharma and S. Yaragorla, nBu4NPF6 promoted regioselective cascade synthesis of functionally embellished naphthofurans under acid, metal & solvent free conditions, RSC Adv, 2016, 6, 89732–89743 RSC;
(g) P. Tharra and B. Baire, Regioselective, cascade [3 + 2] annulation of β-naphthols (resorcinols) with Z-enoate propargylic alcohols: a novel entry for the synthesis of complex naphtho(benzo)furans, Chem. Commun., 2016, 52, 14290–14293 RSC;
(h) S. Gandhi, P. Tharra and B. Baire, Ag(I)-Catalyzed Cyclizative Hydration of Alkynes and Propargylic Alcohols. A Mild Approach to 2-Acylfuran Derivatives, Chemistryselect, 2017, 2, 1058–1062 CrossRef CAS;
(i) S. Gandhi and B. Baire, Calcium(II) Catalyzed Cycloisomerization of cis-6-Hydroxy/(Acyloxy)hex-2-en-4-ynals to 2-Acyl- and 2-(Acyloxyalkenyl)furans, Chemistryselect, 2018, 3, 4490–4494 CrossRef CAS.
-
(a) F. Bigi, S. Carloni, R. Maggi, C. Muchetti and G. Sartori, Zeolite-Induced Heterodomino Reaction. Regioselective Synthesis of 2H-1-Benzopyrans from Phenols and α-Alkynols, J. Org. Chem., 1997, 62, 7024–7027 CrossRef CAS;
(b) Y. Ishino, M. Mihara, N. Hayakawa, T. Miyata, Y. Kaneko and T. Miyata, An improved method for synthesis of 1-benzopyrans from unsaturated alcohols and phenols using a catalytic amount of acids, Synth. Commun., 2001, 31, 439–448 CrossRef CAS;
(c) Y. Nishibayashi, Y. Inada, M. Hidai and S. Uemura, Ruthenium-Catalyzed Cycloaddition of Propargylic Alcohols with Phenol Derivatives via Allenylidene Intermediates: Catalytic Use of the Allenylidene Ligand as the C3 Unit, J. Am. Chem. Soc., 2002, 124, 7900–7901 CrossRef CAS PubMed;
(d) W. Zhao and E. M. Carreira, Facile One-Pot Synthesis of Photochromic Pyrans, Org. Lett., 2003, 5, 4153–4154 CrossRef CAS PubMed;
(e) X. Xu, J. Liu, L. Liang, H. Li and Y. Li, Iron-Catalyzed Regioselective Hydroaryloxylation of C
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C Triple Bonds: An Efficient Synthesis of 2H-1-Benzopyran Derivatives, Adv. Synth. Catal., 2009, 351, 2599–2604 CrossRef CAS;
(f) Y.-P. Han, X.-S. Li, M. Li, X.-Y. Zhu and Y.-M. Liang, Lewis Acid-Catalyzed Formal [3 + 3] Annulation of Propargylic Alcohols with 4-Hydroxy-2H-chromen-2-ones, Adv. Synth. Catal., 2018, 360, 2796–2800 CrossRef CAS;
(g) H. Zhu, Q. Zhou, N. Liu, J. Xing, W. Yao and X. Dou, Relay Rhodium(I)/Acid Catalysis for Rapid Access to Benzo-2H-Pyrans and Benzofurans, Adv. Synth. Catal., 2022, 364, 1162–1167 CrossRef CAS.
C Triple Bonds: An Efficient Synthesis of 2H-1-Benzopyran Derivatives, Adv. Synth. Catal., 2009, 351, 2599–2604 CrossRef CAS;
(f) Y.-P. Han, X.-S. Li, M. Li, X.-Y. Zhu and Y.-M. Liang, Lewis Acid-Catalyzed Formal [3 + 3] Annulation of Propargylic Alcohols with 4-Hydroxy-2H-chromen-2-ones, Adv. Synth. Catal., 2018, 360, 2796–2800 CrossRef CAS;
(g) H. Zhu, Q. Zhou, N. Liu, J. Xing, W. Yao and X. Dou, Relay Rhodium(I)/Acid Catalysis for Rapid Access to Benzo-2H-Pyrans and Benzofurans, Adv. Synth. Catal., 2022, 364, 1162–1167 CrossRef CAS. -
(a) S. Wang, Z. Chai, Y. Wei, X. Zhu, S. Zhou and S. Wang, Lewis acid catalyzed cascade reaction to carbazoles and naphthalenes via dehydrative [3 + 3]-annulation, Org. Lett., 2014, 16, 3592–3595 CrossRef CAS PubMed;
(b) P. Tharra and B. Baire, Regioselective Cyclization of (Indol-3-yl)pentyn-3-ols as an Approach to (Tetrahydro)carbazoles, Org. Lett., 2018, 20, 1118–1121 CrossRef CAS PubMed.
-
(a) M. Shao, Y. Wu, Z. Feng, X. Gu and S. Wang, Synthesis of polysubstituted 1,2-dihydroquinolines and indoles via cascade reactions of arylamines and propargylic alcohols catalyzed by FeCl3·6H2O, Org. Biomol. Chem., 2016, 14, 2515–2521 RSC;
(b) Y. Wu, M. Shao, Z. Feng, X. Gu, Y. Hong, Q. Cui, L. Ren and S. Wang, Synthesis of Iodine-Substituted Quinolines, Quinolinium Salts, and Isoquinolinium Salts via a Three-Component Tandem Reaction of Aryl Azides, Propargylic Alcohols, and Iodine, Asian J. Org. Chem., 2017, 6, 76–82 CrossRef CAS.
-
(a) S. Y. Wang, Z. Chai, S. L. Zhou, S. W. Wang, X. C. Zhu and Y. Wei, A Novel Lewis Acid Catalyzed [3 + 3]-Annulation Strategy for the Syntheses of Tetrahydro-beta-Carbolines and Tetrahydroisoquinolines, Org. Lett., 2013, 15, 2628–2631 CrossRef CAS PubMed;
(b) H. Yin, Q. Ma, Y. Wang, X. Gu, Z. Feng, Y. Wu, M. Wang and S. Wang, Synthesis of tetrahydro-β-carbolines from 2-indolylmethyl azides and propargylic alcohols, RSC Adv, 2021, 11, 19639–19646 RSC.
- T. T. Talele, The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Preclinical/Clinical Drug Molecules, J. Med. Chem., 2016, 59, 8712–8756 CrossRef CAS PubMed.
-
(a) S. Swaminathan and K. V. Narayanan, Rupe and Meyer-Schuster rearrangements, Chem. Rev., 1971, 71, 429–438 CrossRef CAS;
(b) K. H. Meyer and K. Schuster, Umlagerung tertiärer Äthinyl-carbinole in ungesättigte Ketone, Chem. Ber., 1922, 55, 819–823 CrossRef;
(c) D. Roy, P. Tharra and B. Baire, Intercepted Meyer-Schuster Rearrangements in Organic Synthesis, Asian J. Org. Chem., 2018, 7, 1015–1032 CrossRef CAS.
-
(a) F. Alonso, I. P. Beletskaya and M. Yus, Transition-Metal-Catalyzed Addition of Heteroatom–Hydrogen Bonds to Alkynes, Chem. Rev., 2004, 104, 3079–3160 CrossRef CAS PubMed;
(b) Y. Liu, F. Song, Z. Song, M. Liu and B. Yan, Gold-Catalyzed Cyclization of (Z)-2-En-4-yn-1-ols: Highly Efficient Synthesis of Fully Substituted Dihydrofurans and Furans, Org. Lett., 2005, 7, 5409–5412 CrossRef CAS PubMed;
(c) B. Gabriele, R. Mancuso and G. Salerno, A Novel Synthesis of 2-Functionalized Benzofurans by Palladium-Catalyzed Cycloisomerization of 2-(1-Hydroxyprop-2-ynyl)phenols Followed by Acid-Catalyzed Allylic Isomerization or Allylic Nucleophilic Substitution, J. Org. Chem., 2008, 73, 7336–7341 CrossRef CAS PubMed;
(d) Y. Li, J. Xue, X. Li and R. Chen, A New Copper(I)-Catalyzed Cycloetherification/Acid-Catalyzed Allylic Nucleophilic Substitution for One-Pot Synthesis of 2-Substituted Benzofurans, Synlett, 2012, 23, 1043–1046 CrossRef;
(e) R. Mancuso and B. Gabriele, A Recyclable Palladium-Catalyzed Synthesis of 2-Methylene-2,3-Dihydrobenzofuran-3-ols by Cycloisomerization of 2-(1-Hydroxyprop-2-ynyl)phenols in Ionic Liquids, Molecules, 2013, 18, 10901–10911 CrossRef CAS PubMed;
(f) M. Zhang, J. Yang, Q. Xu, C. Dong, L.-B. Han and R. Shen, Copper-Catalyzed Dehydrative Cyclization of 1-(2-Hydroxyphenyl)propargyl Alcohols with P(O)H Compounds for the Synthesis of 2-Phosphorylmethylbenzofurans, Adv. Synth. Catal., 2018, 360, 334–345 CrossRef CAS.
- For selected reviews on the sequential Diels–Alder reaction
of 2-pyrones with alkenes or alkynes followed by retro-Diels–Alder extrusion of CO2 under thermal reaction conditions:
(a) K. Afarinkia, V. Vinader, T. D. Nelson and G. H. Posner, Diels–Alder cycloadditions of 2-pyrones and 2-pyridones, Tetrahedron, 1992, 48, 9111–9171 CrossRef CAS;
(b) Q. Cai, The [4 + 2] Cycloaddition of 2-Pyrone in Total Synthesis, Chin. J. Chem., 2019, 37, 946–976 CrossRef CAS;
(c) G. Huang, C. Kouklovsky and A. Torre, Inverse-Electron-Demand Diels–Alder Reactions of 2-Pyrones: Bridged Lactones and Beyond, Chem.–Eur. J., 2021, 27, 4760–4778 CrossRef CAS PubMed . For selected recent examples using this strategy:;
(d) G. L. Points III, K. T. Stout and C. M. Beaudry, Regioselective Formation of Substituted Indoles: Formal Synthesis of Lysergic Acid, Chem.–Eur. J., 2020, 26, 16655–16658 CrossRef PubMed;
(e) M.-M. Xu, X.-Y. You, Y.-Z. Zhang, Y. Lu, K. Tan, L. Yang and Q. Cai, Enantioselective Synthesis of Axially Chiral Biaryls by Diels–Alder/Retro-Diels–Alder Reaction of 2-Pyrones with Alkynes, J. Am. Chem. Soc., 2021, 143, 8993–9001 CrossRef CAS PubMed.
-
(a) D. A. Engel and G. B. Dudley, Olefination of Ketones Using a Gold(III)-Catalyzed Meyer–Schuster Rearrangement, Org. Lett., 2006, 8, 4027–4029 CrossRef CAS PubMed;
(b) H. Zhang, H. Tanimoto, T. Morimoto, Y. Nishiyama and K. Kakiuchi, Regioselective Rapid Synthesis of Fully Substituted 1,2,3-Triazoles Mediated by Propargyl Cations, Org. Lett., 2013, 15, 5222–5225 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectra for newly synthesized compounds, CIF for compounds 3a, 3z, 4g and 6a. CCDC 2160295, 2189110, 2160296 and 2168808. For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2ra03416f |
|
| This journal is © The Royal Society of Chemistry 2022 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article a,
Yunjun Wua,
Xiaoxia Gua,
Zhijun Fenga,
Meifang Wangab,
Dexiang Fenga,
Ming Wanga,
Ziyang Chenga and
Shaoyin Wang
a,
Yunjun Wua,
Xiaoxia Gua,
Zhijun Fenga,
Meifang Wangab,
Dexiang Fenga,
Ming Wanga,
Ziyang Chenga and
Shaoyin Wang *ab
*ab

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C Triple Bonds: An Efficient Synthesis of 2H-1-Benzopyran Derivatives, Adv. Synth. Catal., 2009, 351, 2599–2604 CrossRef CAS;
(f) Y.-P. Han, X.-S. Li, M. Li, X.-Y. Zhu and Y.-M. Liang, Lewis Acid-Catalyzed Formal [3 + 3] Annulation of Propargylic Alcohols with 4-Hydroxy-2H-chromen-2-ones, Adv. Synth. Catal., 2018, 360, 2796–2800 CrossRef CAS;
(g) H. Zhu, Q. Zhou, N. Liu, J. Xing, W. Yao and X. Dou, Relay Rhodium(I)/Acid Catalysis for Rapid Access to Benzo-2H-Pyrans and Benzofurans, Adv. Synth. Catal., 2022, 364, 1162–1167 CrossRef CAS.
C Triple Bonds: An Efficient Synthesis of 2H-1-Benzopyran Derivatives, Adv. Synth. Catal., 2009, 351, 2599–2604 CrossRef CAS;
(f) Y.-P. Han, X.-S. Li, M. Li, X.-Y. Zhu and Y.-M. Liang, Lewis Acid-Catalyzed Formal [3 + 3] Annulation of Propargylic Alcohols with 4-Hydroxy-2H-chromen-2-ones, Adv. Synth. Catal., 2018, 360, 2796–2800 CrossRef CAS;
(g) H. Zhu, Q. Zhou, N. Liu, J. Xing, W. Yao and X. Dou, Relay Rhodium(I)/Acid Catalysis for Rapid Access to Benzo-2H-Pyrans and Benzofurans, Adv. Synth. Catal., 2022, 364, 1162–1167 CrossRef CAS.







