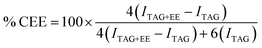Open Access Article
Open Access ArticleObtention of biodiesel through an enzymatic two-step process. Study of its performance and characteristic emissions†
Mariana Macías-Alonso ,
Rosa Hernández-Soto,
Marcelino Carrera-Rodríguez*,
Carmen Salazar-Hernández,
Juan Manuel Mendoza-Miranda,
José Francisco Villegas-Alcaraz and
Joaquín González Marrero
,
Rosa Hernández-Soto,
Marcelino Carrera-Rodríguez*,
Carmen Salazar-Hernández,
Juan Manuel Mendoza-Miranda,
José Francisco Villegas-Alcaraz and
Joaquín González Marrero *
*
Instituto Politécnico Nacional, Unidad Profesional Interdisciplinaria de Ingeniería Campus Guanajuato, Av. Mineral de Valenciana 200 Col. Fracc. Industrial Puerto Interior, Silao 36275, Guanajuato, Mexico. E-mail: jgonzalezm@ipn.mx; mcarrerar@ipn.mx
First published on 22nd August 2022
Abstract
We describe the enzymatic synthesis of biodiesel from waste cooking oil (WCO) in a two-step production process: hydrolysis of WCO, followed by acid-catalyzed esterification of free fatty acids (FFAs). Among the three commercial enzymes evaluated, the inexpensive lipase Lipex® 100L supported on Lewatit® VP OC 1600 produced the best overall biodiesel yield (96.3%). Finally, we assessed the combustion efficiency of the obtained biodiesel and its blends. All blends tested presented lower emissions of CO and HC compared to diesel. The NOx emissions were higher due to biodiesel's high volatility and viscosity. The cost of biodiesel production was calculated using the process described.
1 Introduction
Energy plays a vital role in the human economic development and welfare of societies since it is the engine of all man's activities. The global energy demand and the excessive consumption of fossil fuels are increasing rapidly, mainly in sectors such as industry and transport. For this reason, and because oil reserves are limited, alternatives are being sought to this type of fuel in order to allow future supply.1 According to the American Society of Testing and Materials (ASTM), biodiesel comprises mono-alkyl esters of long-chain fatty acids derived from vegetable oils or animal fats, designated B 100.2 Over the past few years, biodiesel has gained popularity as a suitable alternative to petroleum-based diesel fuel. However, it has some disadvantages, such as corrosion of automotive metals and degradation of elastomers softening rubber materials in the engine, which represent a loss in compression and unreliability in the engine use, leading to more spending when converting.3 An alternative to this problem is the mixture of biodiesel with petrol diesel. The existing data shows that in the case of biodiesel blends B2 (2% biodiesel, 98% petroleum diesel), these effects are practically non-existent. Some of the positive qualities of these blends are low emission of pollutants, compatibility with most internal engine accessories, the similarity in the heat of combustion and low cost of implementation because of the few modifications suffered by the engine.4Biodiesel is widely used in the European Union (EU) and the United States (US), because it can be considered as biodegradable, bio-renewable and nontoxic fuel.5 Mexico is characterized by its high biodiversity, intensive agricultural activity and residual biomass resources not used productively. Moreover, the law for the promotion and development of biofuels promotes the production and use of biofuels. Thus, Mexico has enormous potential to produce biofuels.6
Fatty acid methyl esters are produced conventionally by transesterification reaction of oil and animal fats with short chain alcohols, such as methanol and ethanol, using alkaline, acid or biocatalysis. Biotechnological tools are an alternative that solves most of the inconveniences caused by chemical catalysts. The use of lipases avoids the generation of soap, glycerol can be easily recovered, and biodiesel purification is simplified.7
Biodiesel can be synthesized from a broad spectrum of feedstock, such as edible and non-edible oils. However, the conflict regarding food vs. fuel has drawn attention towards using non-edible oils such as waste cooking oil (WCO) and animal fats.8 WCO is not easily decomposed biologically and is an environmental hazard. In most countries, WCO is discharged into drains, causing severe contamination of water and soil and health problems to society.9 Therefore, WCOs are ideal candidates to be used as feedstock in biodiesel production. They represent an environmentally friendly alternative for WCO management, as part of a circular economy, and provide renewable energy with less pollution.10 However, the high concentration of FFAs in this inexpensive waste feedstocks make necessary an additional step to decrease the FFA content below 1.0% to minimize the possibility of the alkali soap formation.11
The use of lipases is an alternative when the feedstock contains a high amount of FFAs because it allows obtaining a purer product without soap production. However, biocatalysis has limitations, mainly the high cost of enzymes and the inhibition of lipase activity by short-chain alcohols.12 Some authors have exploited several approaches to increase the enzyme yield, such as hydroesterification in two steps: hydrolysis of oils followed by esterification of the hydrolysed oils. Some advantages of hydroesterification include using feedstock with high water and FFAs contents or the prevention of the inhibition of the lipases by the organic solvents like alcohol.13 The hydrolysis of WCO with soluble lipase from Candida rugosa (CRL) followed by chemical esterification using Amberlyst 15 produced biodiesel with 99% yield.14 On the other hand, Zhou et al. used soluble CRL to obtain FFAs from unrefined Jatropha oil. The FFAs were esterified to biodiesel with an 88.6% yield, using immobilized Rhizopus oryzae IFO4697 cells as biocatalyst.15 However, these studies utilized homogeneous catalysts which often caused the recovery to be difficult and the downstream processing to be more complicated.
For all these reasons, we began a multidisciplinary project that aims to enhance biodiesel production from WCO with high oil acidity as feedstock. This started with the screening of three commercial enzymes to compare their potential as biocatalysts. Having one of them selected, we compared a transesterification reaction in a single step (Method A) vs. a two-step process (Method B), as seen in Scheme 1. In this two-step process, the selected lipase hydrolised the WCO in the presence of water as the solvent (Step B1), followed by esterification of the FFAs with ethanol (Step B2). With a selection of one of these methods, its biodiesel production was tested in a laboratory-scale diesel engine fully loaded with an increasing biodiesel ratio above 20% at different speeds. We evaluated the engine performances, emissions, and combustion characteristics for biodiesel blends compared with crude diesel to obtain the optimum blending.
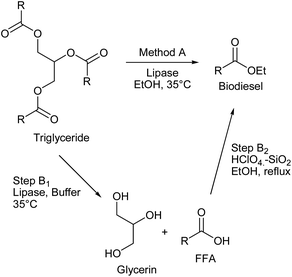 | ||
| Scheme 1 Transesterification reaction in a single step (Method A) and the two-step process (Method B: Steps B1 and B2). | ||
2 Material and methodology
2.1 General experimental procedures
All solvents and reagents used in this work were purchased from Sigma-Aldrich Co. and used without further purification. The commercial lipases used were Lipozyme TL IM, Novozym 435 and Lipex® 100L a lipase. These biocatalysts were purchased from Novozymes (Bagsvaerd, Denmark). The catalyst system HClO4–SiO2 was prepared following the procedure reported by Chakraborti et al.16 Diesel was obtained from a local PEMEX® diesel station. Analytical thin-layer chromatography (TLC) was carried out on precoated silica gel 60F254 aluminium base plates with a 230–400 mesh particle size (20 × 20 cm) (Merck). The developing mixture was 16% diethyl ether and 0.04% formic acid in 84% n-hexane. In all cases, the TLC plates were visualized by exposure to ultraviolet light. Then the spots were revealed by spraying the plates with oleum (80% acetic acid, 16% water, 4% sulphuric acid) and heating at 120 °C for 15 minutes. The NMR spectra were recorded on Bruker Avance 400 MHz spectrometers in CDCl3. Chemical shifts are given in ppm with TMS as the internal standard. IR spectra were obtained on a Bruker IFS 28/55 (FTIR) spectrometer and UV spectra on a JASCO V-560.2.2 Feedstock and pretreatment
WCO was provided by the campus cafeteria (Interdisciplinary Professional Unit of Engineering Campus Guanajuato of the National Polytechnic Institute (UPIIG-IPN), Guanajuato, Mexico). The WCO was purified, and its fatty acid composition is shown in Table 1.| Entry | Fatty acid name | Structure | wt (%) |
|---|---|---|---|
| 1 | Palmitic | C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
4.92 |
| 2 | Stearic | C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
1.78 |
| 3 | Oleic | C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (9) 1 (9) |
61.68 |
| 4 | Linoleic | C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (9, 12) 2 (9, 12) |
15.56 |
| 5 | Linolenic | C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (9, 12, 15) 3 (9, 12, 15) |
7.46 |
| 6 | Eicosenoic | C20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (11) 1 (11) |
4.03 |
2.3 Selection of enzymes
A mixture of 2 g of WCO and 50 mM phosphate buffer pH 7 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) ratio in a 15 mL Falcon plastic tube was heated at 35 °C with continuous shaking at 250 revolutions per minute (rpm) for 30 min by a shaker incubator. The reaction was initiated by adding the corresponding lipase (2%, w/w). After 24 h of incubation time, heptane (1 mL) was added to the reaction mixture and shaken for an additional 15 min. The mixture was centrifuged at 5000 rpm and 25 °C for 15 min, and the upper layer containing FFA in heptane was analysed using TLC. The reference samples were canola oil and canola oil ethyl esters in diethyl ether. TLC was performed according to a previously reported method.17 All the experiments were done in duplicate. Finally, the solvent was removed on a rotary evaporator to provide dense, yellow oil samples, which were stored at 4 °C until the analysis time.
1 (v/v) ratio in a 15 mL Falcon plastic tube was heated at 35 °C with continuous shaking at 250 revolutions per minute (rpm) for 30 min by a shaker incubator. The reaction was initiated by adding the corresponding lipase (2%, w/w). After 24 h of incubation time, heptane (1 mL) was added to the reaction mixture and shaken for an additional 15 min. The mixture was centrifuged at 5000 rpm and 25 °C for 15 min, and the upper layer containing FFA in heptane was analysed using TLC. The reference samples were canola oil and canola oil ethyl esters in diethyl ether. TLC was performed according to a previously reported method.17 All the experiments were done in duplicate. Finally, the solvent was removed on a rotary evaporator to provide dense, yellow oil samples, which were stored at 4 °C until the analysis time.
2.4 Determination of enzymatic hydrolysis efficiency
The FFA produced, were measured according to a described method.18 25 mL of isopropanol were added to 5 g of the FFA sample, and titrated against 0.2 N NaOH, previously standardized with benzoic acid, using phenolphthalein as indicator. The test was performed in triplicate for each sample. The percentage of FFA was then calculated according to the following equation: % FFA = (Vs × C ×28.2 × 100)/m, where: Vs = volume of the NaOH added to reach equivalence (mL), C = concentration of NaOH used (0.2 mol L−1), m = mass of the sample (2 g).2.5 Enzyme immobilization by entrapment
255.6 mg of Lewatit® VP OC 1600 were added to 4 mL (4.5877 g) of commercial Lipex® 100L (Protein: 25.8 mg g−1). The resulting dispersion was stirred for 24 h at room temperature. Next, the immobilized lipase was filtered off, rinsed with water, and stored at 4 °C until use. The protein concentration was measured according to the standard method described by Bradford using bovine serum albumin as the standard. The amount of immobilized enzyme was calculated by subtracting the amount of immobilized enzyme from the total lipase used for the immobilization.192.6 Two-step process
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) ratio] in an Erlenmeyer of 250 mL was heated at 35 °C at 250 rpm for 30 min by a shaker incubator (ZHCHENE, model ZHWY-200D). 546 mg of Lipex® 100L (2%, w/w), dissolved in 2.73 mL of phosphate buffer, was added to start the reaction. After 24 h, it was extracted with heptane (3 × 50 mL). The combined organic layers were washed with brine (2 × 25 mL), dried over anhydrous Na2SO4 and filtrated. The residue is used without further purification.
1 (v/v) ratio] in an Erlenmeyer of 250 mL was heated at 35 °C at 250 rpm for 30 min by a shaker incubator (ZHCHENE, model ZHWY-200D). 546 mg of Lipex® 100L (2%, w/w), dissolved in 2.73 mL of phosphate buffer, was added to start the reaction. After 24 h, it was extracted with heptane (3 × 50 mL). The combined organic layers were washed with brine (2 × 25 mL), dried over anhydrous Na2SO4 and filtrated. The residue is used without further purification.where (i) % CEE = percentage of conversion of oil into biodiesel (ii) ITAG = integration of glyceryl methylenic hydrogen of oil at 4.28–4.32 ppm; (iii) ITAG+EE = joint integration of glyceryl methylenic hydrogen of oil and ethoxy hydrogen of ester superimposed at 4.10–4.17 ppm. The fuel properties were determined as per ASTM standards (Table 2).
| Fuel properties | Diesel | Biodiesel |
|---|---|---|
| Molecular formula | C12–C25 | C12–C22 |
| Stoichiometric air/fuel ratio | 14.7 | 12.5 |
| Lower heating value (MJ kg−1) | 45.0 | 40.3 |
| Density at 40 °C (g mL−1) | 0.803 | 0.878 |
| Kinematic viscosity at 40 °C (mm2 s−1) | 2.40 | 4.29 |
| Cetane number | 52 | 54.9 |
| Oxygen content (%) | 0 | 11 |
1H NMR (CDCl3, 500 MHz) δH 0.85–0.88 (m), 1.23–1.25 (t, J = 4.0 Hz), 1.27 (m), 1.57–1.61 (m), 1.97–2.05 (m), 2.25–2.28 (t, J = 8.0 Hz), 2.29–2.33 (m), 4.08 4.12 (t, J = 7.0 Hz), 5.30–5.37 (m).
13C NMR (CDCl3, 100 MHz) δC 14.0, 14.2, 22.5, 22.6, 24.9, 25.6, 27.1, 27.2, 29.0, 29.1, 29.2, 29.3, 29.4, 29.5, 29.6, 29.7, 31.5, 31.9, 34.3, 60.1, 127.8, 128.0, 129.7, 129.9, 130.0, 131.1, 173.8.
2.7 Experimental setup
A fully functioning experiment setup includes, in addition to the CT 159 Internal Combustion Engine Basic Module, at least one engine (e.g., CT 151) and the HM 365 Universal Drive and Brake Unit (Fig. S6†). The combustion engine must then be connected to the DC motor in HM 365 that functions as a dynamometer. The engine CT 151 is an air-cooled one-cylinder 4-stroke diesel engine. Its output power has been adapted for experiments to the braking device. The main specifications of the engine are shown in Table S3.†The VEA-501 gas analyser is used for measuring carbon monoxide (CO), unburned hydrocarbons (HC) and CO2 in automotive emissions by the principle of non-dividing infrared absorption, measuring of nitrogen oxides (NOx) and O2 by the principle of electrochemical cell, calculating excessive air coefficient λ based on the composition of CO, CO2, HC and O2 measured. This instrument complies with the requirements of International Measurement Rules OIML R99/1998 (E) made by the Organization of International Measurement Law (OIML) and the National Metrological Verification Regulations # JJG 688 for class-1 instruments. It is applicable for environmental departments, vehicle inspection stations, automotive manufacturing factories and garages. Table S2† lists the technical specifications of the gas analyser.
The engine was operated at full load condition at different speeds ranging from 2500 rpm to 1000 rpm at intervals of 500 rpm. The performance parameters evaluated were brake power and brake specific fuel consumption. The concentration of carbon monoxide, unburnt hydrocarbon, and nitrogen oxides were monitored to assess the exhaust emissions. In this experimental study, the diesel fuel used is the ultra-low sulphur diesel (ULSD, or UBA in Mexico) with ten ppm sulphur content. Three blended fuels were prepared based on volumes proportion of 20%, 50% and 75% of biodiesel in the UBA, and are identified as D80B20, D50B50, and D25B75, respectively. Table S3† lists the key properties of the test fuels. The blends were obtained by mixing on a magnetic stirrer to ensure homogeneity.
3 Result and discussion
3.1 Selection of lipases
After purification of the WCO, we selected the best enzyme for biodiesel synthesis following a well-established screening procedure. The WCO was subjected to a hydrolysis process catalysed by three commercial lipases: Lipex® 100L, Lipozyme TLIM and Novozyme 435. Lipozyme TLIM and Novozyme 435 are well-known enzymes for the obtention of biodiesel, with good catalytic activity.22 On the contrary, the use of Lipex® 100L is much more limited. To our knowledge, only two previous works have used this enzyme in the transesterification reaction of vegetable oils.23,24Firstly, we selected the most effective lipase to hydrolysis 2 g of WCO using 50 mM phosphate buffer pH 7 as solvent. After stirring the reaction for 24 h, we added heptane. The organic phase was separated, and the concentration of FFAs was determined by titration of the sample with NaOH solution, using phenolphthalein as an indicator.25
The results obtained (Fig. S1†) showed that the three evaluated enzymes hydrolysed the WCO. Among them, Lipex® 100L, a commercially available enzymatic lipase preparation from T. lanuginosus, was the most effective, with a 99% yield of FFA. Moreover, according to other authors, in this case, Lipozyme TL IM is more successful in the hydrolysis of WCO than Novozyme 435.26
One of the most critical factors in the enzymatic processes for obtaining biodiesel is the amount of water present in the system.27 The presence of an appropriate oil-water interfacial area is required for the process to occur, and its size increases with the addition of water, which facilitates the process.28 The optimal water content for the enzymatic reaction is specific for each lipase. In general, if the system was free of water, no reaction occurred while the reaction rate increased with increasing water content (1–20% water weight). Contrary to this, C. antarctica (Novozym 435) shows the highest activity with little water availability.29 This fact is in accordance with the results obtained in the present study.
3.2 Biodiesel production using immobilized Lipex® 100L as biocatalyst
The principal problem for enzymatic biodiesel production is the high cost of lipases. A strategy to reduce this cost is to increase the lifetime of enzymes in the process through their immobilization in different carriers.30 As it was the most effective lipase according to our previous experiment, we selected Lipex® 100L to continue additional studies about its immobilization.The Lipex® 100L was immobilized via adsorption on Lewatit® VP OC 1600, a macroporous adsorber especially described for the immobilization of lipases.31 The enzyme was hydrophobically immobilized on this carrier by incubating for 4 h. According to the Bradford method (1976),32 the loading amount of lipase was calculated to be 60.1 mg protein per g of support, with a catalytic activity of 9 KU g−1.
The immobilized lipase was used to catalyse the hydrolysis of WCO with water to produce FFAs. Fig. S2† shows the effect of enzyme loading on FFA yield. Under the same conditions described previously, FFA yield increased from 79.3 to 95.6% when the immobilized enzyme loading ranged from 1 to 3% (w/w). Afterwards, the yield of FFAs did not show any significant enhancement when adding more biocatalyst. So, the 3% of immobilized lipase was chosen as the best dosage for economy.
The immobilization process allows an increase in the enzyme time of usage.33 Thus, although Lipex® is a relatively inexpensive enzyme (the cost of 100 mL is £19.00),34 we decided to evaluate the reuse of the immobilized lipase. After each transesterification reaction, carried out with 3% of the enzyme (grams per grams of WCO), the immobilized lipase was recovered by filtration and subsequently reused. After five cycles, the immobilized lipase maintained a relatively good activity with over a yield of 60% FFA, showing excellent reusability in the experimental conditions chosen, likely due to the induced stability caused by the enzyme interaction with the support (Fig. S2†).
Finally, after obtaining the FFA from WCO by hydrolysis with the lipase Lipex®, supported with Lewatit® VP OC 1600 and using water as the solvent, we decided to investigate the esterification of FFA to obtain biodiesel. The FFAs were dissolved in ethanol and treated with perchloric acid immobilized on silica gel as acid catalyst. After 12 h of reflux, the reaction was filtrated to obtain biodiesel in a 96.2% yield. At this point, we would like to emphasize that considering the two reaction steps, the total yield of the process is 96.3%.
When we carried out the obtention of biodiesel using the supported enzyme in a single step with ethanol as solvent, we obtained only a 70.2% yield. This result agreed with that obtained previously by Santaraite et al. In this case, the transesterification of rapeseed oil with 7% of free Lipex® 100L in the presence of ethanol produced the biodiesel in 73.4% yield.35
The physical characteristics of fuel affect their ability to form an air–fuel mixture suitable for the type of engine used.36 The results are given in Table 2. The density of biodiesel in this study is 0.878 g mL−1 at 40 °C, which is suitable for the ASTM D6751 biodiesel standard specifications (Fig. S7†). The viscosity is a critical factor for the size of liquid molecules and vapour entering the combustion chamber. For this reason, we studied the effect of temperature on the viscosity of biodiesel compared with pure diesel fuel, see Fig. S8.† The viscosity measurement at 20 °C is 6.87 mm2 s−1 for biodiesel and decreases with increasing temperature. And even though the lower heating value is roughly 10.4% lower than that of diesel, the value obtained is higher than that of other biodiesel whose average value is 38 MJ kg−1.37
The 1H NMR spectrum of the biodiesel produced (Fig. S4†) can be confirmed through the disappearance of the signal characteristic of acylglycerols and the appearance of signals at 4.10 (q, J = 7.0 Hz, 2H), 2.26 (t, J = 8.0 Hz) and 1.24 (t, J = 4.0 Hz, 3H) that together with the signal to 173.8 (s) in the 13C NMR confirm the presence of ethyl ester (Fig. S4 and S5†). The multiple peaks between 5.30 and 5.37 were assigned to olefinic hydrogens. The presence of this type of protons is confirmed by the 13C NMR olefinic carbon region (δC 127.1–131.8 ppm). In addition, the signals for the terminal methyl group (δH 0.85–0.88 ppm) and the methylenes (δH 1.23–1.61 ppm) is observed. All these data agree with the structure of biodiesel.38
3.3 Economic analysis of biodiesel production
As stated before, the catalyst's cost is the dominating factor in biodiesel's enzymatic production. Table 3 presents an estimated production cost per litre of biodiesel at a laboratory scale for each enzyme, generated based on the feedstock and reagents costs. We considered the reaction conditions described above for a global biodiesel yield of 56.8–95.3%, depending on the enzyme, for the simulation purpose. The schematic diagram of the biodiesel production process using lipase is presented in Scheme S1.† The design was based on three processing sections:| Item | Unit cost (USD)a | Quantity consumed/Cost (USD) | ||
|---|---|---|---|---|
| Lipozyme TL IM | Novozym 435 | Lipex® 100L | ||
a All-given prices are based on Mexican market data.b We calculated the quantity consumed to obtain 1 L of biodiesel based on the global yield of each enzyme: Lipozyme TL IM: 81.9%; Novozym 435![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56.8; Lipex® 100L: 96.3%.c We used a mixture of 10% new solvent and 90% recycled solvent. 56.8; Lipex® 100L: 96.3%.c We used a mixture of 10% new solvent and 90% recycled solvent. |
||||
| WCOb | 0.00/L | 1.22 L/0.0 | 1.76 L/0.0 | 1.05 L/0.0 |
| Bentonite | 0.23/kg | 0.05 kg/0.012 | 0.07 kg/0.016 | 0.04 kg/0.01 |
| Buffer | 0.81/L | 1.22 L/0.99 | 1.76 L/1.43 | 1.05 L/0.85 |
| Enzyme | 1340/kg (Lipozyme TL IM), 1280/kg (Novozym 435), 188.9/kg (Lipex® 100L) | 24.4 g/32.7 | 35.2 g/45.01 | 21 g/3.97 |
| Heptanec | 4.02/L | 0.67 L/2.7 | 0.97 L/3.9 | 0.576 L/2.32 |
| EtOHc | 2.33/L | 0.13 L/0.3 | 0.19 L/0.44 | 0.115 L/0.27 |
| Catalyst | 2.65/kg | 0.15 kg/0.40 | 0.22 kg/0.40 | 0.13 kg/0.34 |
| Total | 37.1 | 51.2 | 7.76 | |
1. The WCO was treated with bentonite in the pre-treatment process to obtain a degummed feedstock. New bentonite was used for every oil sample; no further studies on the saturation capacity of the filter were performed.
2. During the hydrolysis, the WCO is reacted with water in the presence of lipase to produce FFAs and glycerol. In the case of supported lipases, the reaction mixture is transferred into a centrifuge to separate the enzyme, which can be then recycled for the subsequent saponification reaction. The crude product containing FFAs, buffer and glycerol is transferred into a decanter and heptane is added to this mixture. The heavy-liquid phase (buffer, glycerol, and homogeneous enzyme) is removed from the bottom stream. In contrast, the FFA–heptane is removed from the light-liquid phase and sent to the heptane recovery distillation process. The glycerol obtained is stored without purification.
3. Finally, FFAs are esterified with ethanol in the presence of a solid catalyst. The reaction mixture is centrifuged to separate the solids from the crude product containing biodiesel and ethanol, which is distilled to recover ethanol. The pure biodiesel was stored at room temperature in amber glass bottles.
To calculate the cost of the production of 1 litre of biodiesel, we considered the following assumptions:
•The process is at a laboratory scale.
•Using the national market data, we considered only the costs of raw materials and catalysts (Table 3).
•We didn't include the income generated from crude glycerol.
•WCO is the most economical raw material for biodiesel production, and in this case, it was available at no cost.
The estimated biodiesel production cost in the described conditions is 7.76–51.2 USD·L−1, depending on the enzyme. Although this price is higher than those reported by other authors39 as can be observed in the table, the use of the Lipex® 100L reduces the production cost by around 80% when compared to enzymes Lipozyme TLIM and Novozym 435, all used under the same conditions. In addition, Lipex® 100L supported over Lewatit® VP OC 1600 can be recycled five times in biodiesel production. Therefore, the cost of biodiesel production could decrease to 3.51 USD·L−1.
The results are promising. Previous studies show that too small scale often makes the biodiesel production cost too high to make productions viable.40 For this reason, we think that by scaling up the process and searching for more suitable support for the enzyme, which improves its useful life, it would be possible to reduce the calculated production price and became this enzymatic biodiesel process a realistically viable industrial project.
3.4 Study of combustion performance of biodiesel
Finally, the present study investigated the engine performance and emission using the obtained biodiesel from WCO. The fuel properties of these biodiesels were determined using ASTM D6751 standards. The fuels were tested in an air-cooled one-cylinder 4-stroke diesel engine with a dynamometer and a VEA-501 exhaust gas analyser to detect the output of carbon monoxide (CO), carbon dioxide (CO2), hydrocarbons (HC), and nitrogen oxides (NOx).Brake specific fuel consumption (BSFC) is a parameter that reflects the efficiency of a combustion engine that burns fuel and produces rotational power. It is one of the most critical parameters in the technological development of machines because it is a benchmark in evaluating optimal performance, service life, and economics. The growth rate in this area is essential and necessary due to the changes in environmental policies, which demand clean technologies; this is achieved with the use of friendly to the environment alternative fuels.41 The present study's BSFC value increased 16.57% when we used biodiesel, see Fig. S10.† This result is consistent with the literature, as biodiesel has greater fuel consumption due to its lower energy content and higher density and viscosity.42,43 With this, the brake power achieved for diesel fuel was higher, about 3.71–9.06% compared to blended fuels, see Fig. S9.†
Emission analysis is an essential part of fuel testing in CI engines. Carbon monoxide (CO) occurs when there is little oxygen available for combustion and therefore the fuel does not burn completely. This phenomenon can be controlled and decreased when fuels containing oxygen in their structure and higher cetane number are used, as is the case of biodiesel compared to diesel.44 For the WCO biodiesel prepared, the trend shows a 27.13% reduction of CO emission compared to diesel, see Fig. S11.† This diminution could be attributed to biodiesel having higher oxygen content, resulting in a more complete combustion and less CO emission.45
The unburned HC emission in CI engines results from the incomplete combustion of fuel and flame quenching. For an engine in perfect condition, HC values decline as the revolutions per minute (rpm) rise, indicating that the supply system economizes appropriately, either in a carburettor or fuel injection system.46 In the present study is observed that at medium speeds (1500–2000 rpm), there is a minimum in the HC emissions with an average reduction of about 27.7% compared with diesel fuel at any speed rating, Fig. S12.† These results agree with the United States Environmental Protection Agency (US EPA), which determined that with biodiesel, the amount of hydrocarbons in the exhaust stream should decrease.47
In an engine in perfect condition, CO2 tends to rise slightly as we climb the rpm, see Fig. S13.† By comparing the concentrations for mixtures of biodiesel compared to diesel, there are factors to consider, such as: increasing of density and viscosity of the mixture difficulting the fuel injection; increasing of the BSFC due to the lower heating value in each blend and; increase in the burning time required by the mixture air–fuel entering the fuel chamber, reducing CO2 emissions.48 The CO2 reduction is achieved at all different engine speeds, where the overall reduction of CO2 reaches values between 4.02–12.64% for the blended fuels.
For all tested fuels, there is a general agreement about the effect of biodiesel on the NOx emissions with those cited in the literature, with levels up to 5%, Fig. S14†.42,48 In most tests, the NOx emissions increased with an increasing biodiesel ratio in the blended fuel and for all engine speeds in comparison with the pure diesel. In this study, he blended fuels showed an increase in NOx emissions in a range of 2.32–10.44%.
4 Conclusions
We reported a practical enzymatic synthesis of biodiesel in a two-step process. From the screening of lipases, Lipex® 100L showed the highest activity in hydrolysis of WCO to obtain FFA. This enzyme was supported on Lewatit® VP OC 1600. According to the Bradford assays, the lipase loading amount on the support was 60.1 mg protein per g of support. Under the described conditions, the yield of biodiesel obtained was 96.3% (w/w).The lower heating value and density of all blended biodiesel fuels tested have no significant differences against diesel. The brake power achieved for diesel fuel was higher, about 3.71–9.06% than the blended fuels. In general, biodiesel fuel additions to diesel fuel result in considerable reductions in HC, CO, and CO2 emissions, while there is a slight increase in NOx levels.
The results indicate that the most recommended blended fuel contains biodiesel fractions from 20% to 50% and low engine speeds. However, D50B50 provides the best performance with the following results in comparison with the corresponding values for diesel: an increase in brake power of average of 1.57%, a slight decrease in BSFC in average of 3.31%, while the related engine emissions present the following reductions, 35.55% in CO, 23.17% in HC, 0.6% in CO2, but an increase in levels of NOx, about of 2.1%.
In summary, this study documents the possibility of using Lipex® 100L for a viable industrial biodiesel production.
5 Author contributions
Conception and design of the work, M. M. A., R. H. S., M. C. R. & J. G. M.; data collection, M. M. A., C. S. H., J. M. M. M. & J. F. V. A.; analysis and interpretation of the data, M. M. A., R. H. S., M. C. R. & J. G. M.; drafting of the manuscript, M. M. A., M. C. R. & J. G. M.; critical revision of the manuscript, M. M. A., R. H. S., M. C., J. G. M, C. S. H., J. M. M. M., R. J. F. V. A. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The support by National Council on Science and Technology of Mexico (CONACYT) and Research and Postgraduate Secretary of the National Polytechnic Institute (SIP-IPN) is gratefully acknowledged. Also, acknowledge to José Adrian Corona Muñiz and Gonzalez Perez José Francisco for her technical support in this work.Notes and references
- W. V. Reid, M. K. Ali and C. B. Field, Global Change Biol., 2020, 26, 274–286 CrossRef PubMed.
- ASTM D6751-11b, Standard specification for biodiesel fuel blend stock (B100) for middle distillate fuels, West Conshohocken, ASTM Internacional, 2011 Search PubMed.
- Z. Y. Hu, J. Luo, Z. Y. Lu, Z. Wang, P. Q. Tan and D. M. Lou, ACS Omega, 2021, 6, 5046–5055 CrossRef CAS PubMed.
- R. Kamaraj, Y. K. S. S. Rao and B. Balakrishna, Environ. Sci. Pollut. Res., 2022, 29, 43770–43785 CrossRef CAS PubMed.
- R. Estevez, L. Aguado-Deblas, F. M. Bautista, D. Luna, C. Luna, J. Calero, A. Posadillo and A. A. Romero, Catalysts, 2019, 9, 1033 CrossRef CAS.
- G. S. Alemán-Nava, A. Meneses-Jácome, D. L. Cárdenas-Chávez, R. Díaz-Chavez, N. Scarlat, J. F. Dallemand, N. Ornelas-Soto, R. García-Arrazola and R. Parra, Biofuels, Bioprod. Biorefin., 2015, 9, 8–20 CrossRef.
- B. Changmai, C. Vanlalveni, A. P. Ingle, R. Bhagat and S. L. Rokhum, RSC Adv., 2020, 10, 41625–41679 RSC.
- T. A. Degfie, T. T. Mamo and Y. S. Mekonnen, Sci. Rep., 2019, 9, 18982 CrossRef CAS PubMed.
- A. Mannu, S. Garroni, J. I. Porras and A. Mele, Processes, 2020, 8, 366 CrossRef CAS.
- V. Cordero-Ravelo and J. Schallenberg-Rodriguez, J. Environ. Manage., 2018, 228, 117–129 CrossRef CAS PubMed.
- A. S. Elgharbawy, W. A. Sadik, O. M. Sadek and M. A. Kasaby, Biomass Bioenergy, 2021, 146, 105997 CrossRef CAS.
- M. Lotti, J. Pleiss, F. Valero and P. Ferrer, Biotechnol. J., 2015, 10, 22–30 CrossRef CAS PubMed.
- H. Pourzolfaghar, F. Abnisa, W. M. A. W. Daud and M. K. Aroua, Renewable Sustainable Energy Rev., 2016, 61, 245–257 CrossRef CAS.
- M. M. R. Talukder, J. C. Wu and L. P. L. Chua, Energy Fuels, 2010, 24, 2016–2019 CrossRef CAS.
- G. X. Zhou, G. Y. Chen and B. B. Yan, Biotechnol. Lett., 2015, 37, 1959–1963 CrossRef CAS PubMed.
- A. K. Chakraborti and R. Gulhane, Chem. Commun., 2003, 22, 1896–1897 RSC.
- M. Dołowy and A. Pyka, J. Chem., 2015, 2015, 120830 Search PubMed.
- S. A. Mahesar, S. T. H. Sherazi, A. R. Khaskheli, A. A. Kandhro and S. uddin, Anal. Methods, 2014, 6, 4956–4963 RSC.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- A. T. Khan, T. Parvin and L. H. Choudhury, Synthesis, 2006, 2497–2502 CrossRef CAS.
- G. F. Ghesti, J. L. Macedo, I. S. Resck, J. A. Dias and S. C. L. Dias, Energy Fuels, 2007, 21, 2475–2480 CrossRef CAS.
- G. M. Mathew, D. Raina, V. Narisetty, V. Kumar, S. Saran, A. Pugazhendi, R. Sindhu, A. Pandey and P. Binod, Sci. Total Environ., 2021, 794, 148751 CrossRef CAS PubMed.
- A. A. Mendes, H. F. de Castro and R. L. C. Giordano, Int. J. Biol. Macromol., 2014, 70, 78–85 CrossRef CAS PubMed.
- A. A. Mendes, P. C. Oliveira, A. M. Vélez, R. C. Giordano, R. L. C. Giordano and H. F. de Castro, Int. J. Biol. Macromol., 2012, 50, 503–511 CrossRef CAS PubMed.
- American Oil Chemists' Society (AOCS), Official Methods and Recommended Practices of the AOCS, AOCS Press, Denver, 2009 Search PubMed.
- M. Santaraite, E. Sendzikiene, V. Makareviciene and K. Kazancev, Energies, 2020, 13, 2588 CrossRef CAS.
- A. E. V. Petersson, P. Adlercreutz and B. Mattiasson, Biotechnol. Bioeng., 2007, 97, 235–241 CrossRef CAS PubMed.
- S. Al-Zuhair, K. V. Jayaraman, S. Krishnan and W. H. Chan, Biochem. Eng. J., 2006, 30, 212–217 CrossRef CAS.
- L. Fjerbaek, K. V. Christensen and B. Norddahl, Biotechnol. Bioeng., 2009, 102, 1298–1315 CrossRef CAS PubMed.
- X. Zhao, F. Qi, C. Yuan, W. Du and D. Liu, Renewable Sustainable Energy Rev., 2015, 44, 182–197 CrossRef CAS.
- D. F. Turati, W. G. Morais Júnior, C. R. Terrasan, S. Moreno-Perez, B. C. Pessela, G. Fernandez-Lorente, J. M. Guisan and E. C. Carmona, Molecules, 2017, 22, 339 CrossRef PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- U. Guzik, K. Hupert-Kocurek and D. Wojcieszyńska, Molecules, 2014, 19, 8995–9018 CrossRef PubMed.
- https://www.ncbe.reading.ac.uk/lipase-lipex/.
- M. Santaraite, E. Sendzikiene, V. Makareviciene and K. Kazancev, Energies, 2020, 13, 2588 CrossRef CAS.
- R. Gautam and S. Kumar, Energy Rep., 2020, 6, 2785–2793 CrossRef.
- P. S. Mehta and K. Anand, Energy Fuels, 2009, 23(8), 3893–3898 CrossRef CAS.
- B. Nieva-Echevarría, E. Goicoechea, M. J. Manzanos and M. D. Guillén, Int. Food Res. J., 2014, 66, 379–387 CrossRef.
- H. M. Khan, C. H. Ali, T. Iqbal, S. Yasin, M. Sulaiman, H. Mahmood, M. Raashid, M. Pasha and B. Mu, Chin. J. Chem. Eng., 2019, 27, 2238–2250 CrossRef CAS , and references cited there..
- E. C. Linganiso, B. Tlhaole, L. P. Magagula, S. Dziike, L. Z. Linganiso, T. E. Motaung, N. Moloto and Z. N. Tetana, Sustainability, 2022, 14, 1983 CrossRef CAS.
- I. M. Atadashi, M. K. Aroua and A. Adul Aziz, Renewable Sustainable Energy Rev., 2005, 14, 1999–2008 CrossRef.
- X. J. Man, C. S. Cheung, Z. Ning, L. Wei and Z. H. Huang, Fuel, 2016, 180, 41–49 CrossRef CAS.
- X. Wang, Y. Ge, L. Yu and X. Feng, Fuel, 2013, 107, 852–858 CrossRef CAS.
- M. M. Rahman, M. H. Hassan, M. A. Kalam, A. E. Atabani, L. A. Memon and S. M. A. Rahman, J. Cleaner Prod., 2014, 65, 304–310 CrossRef CAS.
- B. Ghobadian, H. Rahimi, A. Nikbakht, G. Najafi and T. Yusaf, Renewable Energy, 2009, 34, 976–982 CrossRef CAS.
- T. N. Nguyen, N. X. Khoa and L. A. Tuan, Energies, 2021, 14, 2986 CrossRef CAS.
- EPA 420-P-02-001, Analysis of Biodiesel Impacts on Exhaust Emissions Draft Technical Report, United States Environmental Protection Agency, Washington, DC, USA, 2002 Search PubMed.
- O. Can, Energy Convers. Manage., 2014, 87, 676–686 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra03578b |
| This journal is © The Royal Society of Chemistry 2022 |