Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemical diversity, medicinal potentialities, biosynthesis, and pharmacokinetics of anthraquinones and their congeners derived from marine fungi: a comprehensive update†
Mohamed Sebak
 a,
Fatma Molham
a,
Claudio Greco
a,
Fatma Molham
a,
Claudio Greco
 b,
Mohamed A. Tammam
b,
Mohamed A. Tammam
 c,
Mansour Sobeh
d and
Amr El-Demerdash
c,
Mansour Sobeh
d and
Amr El-Demerdash
 *ef
*ef
aMicrobiology and Immunology Department, Faculty of Pharmacy, Beni-Suef University, Beni-Suef 62514, Egypt
bMolecular Microbiology Department, The John Innes Center, Norwich Research Park, Norwich NR4 7UH, UK
cDepartment of Biochemistry, Faculty of Agriculture, Fayoum University, Fayoum 63514, Egypt
dAgroBioSciences Department, Mohammed VI Polytechnic University (UM6P), Ben Guerir, Morocco
eOrganic Chemistry Division, Department of Chemistry, Faculty of Science, Mansoura University, Mansoura 35516, Egypt. E-mail: a_eldemerdash83@mans.edu.eg; Amr.El-Demerdash@jic.ac.uk; Tel: +00447834240424
fDepartment of Metabolic Biology and Biological Chemistry, The John Innes Center, Norwich Research Park, Norwich NR4 7UH, UK
First published on 1st September 2022
Abstract
Marine fungi receive excessive attention as prolific producers of structurally unique secondary metabolites, offering promising potential as substitutes or conjugates for current therapeutics, whereas existing research has only scratched the surface in terms of secondary metabolite diversity and potential industrial applications as only a small share of bioactive natural products have been identified from marine-derived fungi thus far. Anthraquinones derived from filamentous fungi are a distinct large group of polyketides containing compounds which feature a common 9,10-dioxoanthracene core, while their derivatives are generated through enzymatic reactions such as methylation, oxidation, or dimerization to produce a large variety of anthraquinone derivatives. A considerable number of reported anthraquinones and their derivatives have shown significant biological activities as well as highly economical, commercial, and biomedical potentialities such as anticancer, antimicrobial, antioxidant, and anti-inflammatory activities. Accordingly, and in this context, this review comprehensively covers the state-of-art over 20 years of about 208 structurally diverse anthraquinones and their derivatives isolated from different species of marine-derived fungal genera along with their reported bioactivity wherever applicable. Also, in this manuscript, we will present in brief recent insights centred on their experimentally proved biosynthetic routes. Moreover, all reported compounds were extensively investigated for their in-silico drug-likeness and pharmacokinetics properties which intriguingly highlighted a list of 20 anthraquinone-containing compounds that could be considered as potential drug lead scaffolds.
1 Introduction
Throughout history, different natural sources have been used for treatment of diseases, and more recently as sources and valuable suppliers of biologically active compounds with diverse bioactivities that can be developed to be used in new drugs.1–6 Intriguingly, marine organisms and microorganisms were among the valuable sources of new natural products.2 Microbial secondary metabolites have been known for their chemical diversity and a broad range of bioactivities.6,7 Marine microorganisms are considered highly productive sources of physiologically active compounds including peptides, polyketides, terpenes, and alkaloids.8–10 Some marine-based compounds have been approved as drugs with different pharmacological uses,11,12 while several others are under different clinical trials before their approval as new drugs.11During the last few decades, numerous drug discovery programs focused on marine-derived microbial natural products due to their great potential for the production of structurally diverse biologically active secondary metabolites.13,14 Among the hot microbes responsible for the production of interesting compounds, fungi, served as the primary source for mining the first reported antibiotic, penicillin, whereas they are still one of the main sources for discovering novel bioactive compounds from different niches including the marine fungi which have high biological diversifications.15,16 Therefore, the bioactive secondary metabolites recovered from the marine-derived fungi have gained great interest as promising sources of therapeutics. Interestingly, more than a thousand compounds have been isolated from marine fungi with a wide range of bioactivities including antiviral, anticancer, and antibacterial activities.17 Even though only one bioactive compound, cyclosporine A, has been approved for clinical use in the market. This might be attributed to problems in the optimization methods or the screening approaches of natural product discovery.18
Studying the marine-derived fungi has been started around two centuries ago when the first fungal species, Sphaeria posidoniae (Halotthia posidoniae) was reported on a rhizome of the marine grass Posidonia oceanica in 1846.19 Marine fungi have been isolated from different habitats including algae, mobile, and sessile invertebrates, sediments, marine mammals, and driftwood from different marine locations.20 Despite the importance of marine fungi as a promising source for novel bioactive secondary metabolites, marine fungi are still less investigated sources for natural product discovery programmes compared to other niches of fungi.18,21 Although the estimated number of fungal species on the earth is ranging from 1.5 to 5 million species, only around 1100 species have been exclusively isolated from the marine niche.18,20
Marine-derived fungi produce various classes of different compounds with both chemical and biological diversities.22,23 For instance, they produce varieties of bioactive compounds such as terpenes, alkaloids, peptides, and polyketides.18 Polyketides have been reported in many previous studies as dominant natural products from marine filamentous fungi.24,25 They are a large group of complex chemical architectures such as anthraquinones, hydroxyanthraquinones, naphthoquinones, macrolides, flavonoids, polyenes, and tetracyclines. Around 700 anthraquinones and their derivatives have been reported from different natural sources, while anthraquinones are widely produced by marine filamentous fungi.16,26 Chemically, anthraquinones are a group of polyketides of the quinone family with a basic cyclic scaffold of three fused benzene rings including two ketone groups on the central 9, 10-carbons with a chemical formula of C14H8O2, while their derivatives are generated by the decoration of the around free protons with different functional groups16 or by enzymatic reaction of the rings or the keto groups such as reduction, oxidation, dehydration or dimerization to result in a wide range of derivatives.27 Interestingly, many reported anthraquinones and their derivatives exhibited potent biological activities including antitumor, antibacterial, antifungal, antioxidant, and immunomodulatory bioactivities.16
Drug-likeness and pharmacokinetics properties using SWISSADME online platform, which intriguingly highlighted a list of 20 anthraquinone containing compounds (ESI†) that could be considered as potential drug leads scaffolds. Such a massive connection between chemical spaces and bioactivities highlights the huge capacity of marine-derived fungi as an attractive biological source that is worth further exploitations with distinguished anticipations for the global pharmaceuticals industries.
Several interesting review articles have focused recently on the marine anthraquinones and their derivatives such as Fouillaud et al. who reported the chemical diversity, specific bioactivities, biosynthetic pathways, biological sources, and the producing fungal genera of tens of marine-derived anthraquinones and their derivatives discovered before 2016.16 Also, another review by Masi and Evidente presented a comprehensive update of the bioactive fungal anthraquinones and analogues including the marine-derived anthraquinones produced via the acetate route over the period 1966–2020 with their sources, biosynthesis, biological activities, and industrial applications.28 Whereas Greco et al. in their recent review critically described the marine-derived anthraquinones which showed anti-tumor activity as well as their mutagenic and genotoxic potentialities.29 Herein and as a part of our continuous program on pharmacologically active fungal natural products,4,30,31 we are presenting an extensive coverage over the period 2000–2020 for 208 anthraquinones and their derivatives, extensively reported from different marine-derived fungal genera such as Nigrospora, Aspergillus, Penicillium, Stemphylium, Alternaria, Eurotium, Trichoderma, Halorosellinia, and Fusarium. In addition, we reported here their different biological activities, drug-likeliness and pharmacokinetics properties wherever applicable, in addition to a general overview of their proposed biogenesis pathway. The investigation of in-silico drug-likeness and pharmacokinetics properties of the marine-derived anthraquinones and their derivatives in this review could be advantageous in predicting the possibility of anthraquinones as drug candidates.
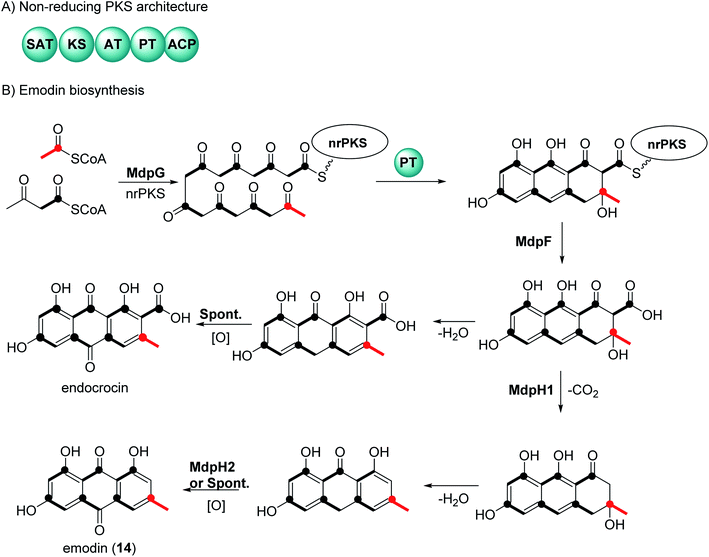
2 General biosynthetic pathway of anthraquinones
There have been extensive studies since the 1950s to determine the biosynthetic pathway of anthraquinones and the related natural products called xanthones.32,33 Feeding experiments using labelled acetates in fungi, first reported by Birch et al., showed that anthraquinone and xanthones are biosynthesized by polyketides.32,33 Genome sequencing and genetic transformation experiments have confirmed that the core structure of anthraquinones is synthesized in fungi by non-reducing polyketide synthase (nrPKS).34,35 This class of PKS share a common domain architecture which consists of an SAT (starter unit-ACP transacylase), KS (ketosynthase), AT (acyl transferase), PT (product template), and ACP (acyl carrier protein) (Fig. 1A). The biosynthesis of anthraquinones can be generalized using emodin (14) and endocrocin as examples (Fig. 1B).27,36 The nrPKS (MdpG) synthesize the polyketide, which is then cyclized with the loss of two water molecules by the PT domain. The polyketide is released by a metallo-hydrolase protein (MdpF) to obtain atrochrysone carboxylic acid, which can in most cases, undergo decarboxylation by a decarboxylase (MdpH1). This is followed by spontaneous dehydration and oxidation by an anthroneoxidase (MdpH2) to afford emodin (14).27,36 Some reports also have described that the final oxidation step could occur spontaneously.36 Further modification by tailoring proteins give rise to a huge diversity, these include methylation, dehydration, and dimerization.273 Chemistry and medicinal potentialities of anthraquinones and their congeners derived from marine-derived fungi
In this manuscript, we provide extensive insights about chemical and biological investigations centered on anthraquinones and their derivatives exclusively derived from marine fungi. For the handling of this documentation, all isolated anthraquinones are classified and tabulated according to the marine fungal genera where they have been recovered along with their recorded biological potentialities whenever applicable.3.1. Anthraquinones isolated from Nigrospora sp.
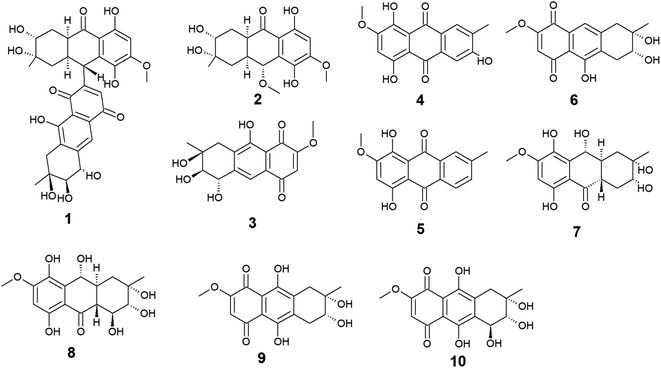
Ten anthraquinones or their derivatives 1–10 were reported from the marine-derived fungus Nigrospora sp. Nigrodiquinone A (1) was isolated for the first time as a new hydroanthraquinone dimer from the zoanthid-derived fungus Nigrospora sp.37 Another four anthraquinone derivatives namely 4a-epi-9α-methoxydihydrodeoxybostrycin (2), 10-deoxybostrycin (3), 3,5,8-trihydroxy-7-methoxy-2-methyl-anthracene-9,10-dione (4), and austrocortirubin (5) were reported from both sea anemone-derived38 and zoanthid-derived fungus Nigrospora sp.,37 while austrocortirubin (5) was also recorded from the sea fan-derived fungus Fusarium sp.,39 and the mangrove endophytic fungi Guignardia sp.40 and Halorosellinia sp.40,41 Although nigrodiquinone A (1) showed no antiviral or antibacterial activities,37 compounds 4 and 5 displayed mild antiviral activity with IC50 value of 93.7 μM against coxsackievirus and 74.0 μM against the respiratory syncytial virus (RSV), respectively.Notably, compounds 2 and 3 showed potent antibacterial activity against both the Gram-positive bacteria, Staphylococcus aureus and Micrococcus tetragenus and the Gram-negative bacteria, Escherichia coli (E. coli), Vibrio anguillarum (V. anguillarum), and V. parahemolyticus. Compound 3 displayed MIC of equal to or less than 2.5 μM against all tested bacteria, whereas compound 2 exhibited MIC of equal to or less than 2.5 μM against all tested bacteria except V. anguillarum and V. parahemolyticus against which it showed MIC value of 25.0 μM.38 In addition, compound 3 showed potent cytotoxic activity against the human lung cancer cell line, A-549 with an IC50 value of 4.56 μM,38 while austrocortirubin (5) displayed an IC50 value of 6.3 μM against the human breast adenocarcinoma cells, MCF-7 39.
Further anthraquinone derivatives 6–10 were previously isolated from the sea anemone-derived fungus Nigrospora sp.38 Also, some of these anthraquinone derivatives have been isolated from other marine fungal species such as Fusarium sp. PSU-F14 from which compounds 6–8 and 10 were recovered,39 while compounds 7, 8 and 10 were also isolated from the marine-derived fungus Aspergillus sp.42
Compounds 6–10 exhibited different interesting biological activities. For instance, nigrosporin B (6) displayed modest anti-mycobacterial activity,43 phytotoxic activity,44 and potent antibacterial and cytotoxic activity.38 Also, 4-deoxybostrycin (9) showed modest anti-mycobacterial activity,43 potent antibacterial activity,38 and moderate antitumor activity.45 Nigrosporin B (6) and 4-deoxybostrycin (9) displayed potent antibacterial activity against both the Gram-positive bacteria, Bacillus subtilis (B. subtilis), B. cereus, Staphylococcus albus (S. albus), S. aureus, and Micrococcus tetragenus and the Gram-negative bacteria E. coli, V. anguillarum, and V. parahemolyticus with MIC values equal to or less than 2.5 and 3.12 μM, respectively.38 Moreover, both compounds exhibited modest anti-mycobacterial activity against several mycobacterial species including two multidrug-resistant Mycobacterium tuberculosis (M. tuberculosis) with MIC values of less than 30.0 μg mL−1.43
An additional example of anthraquinones isolated from Nigrospora sp. with multiple bioactivities is tetrahydrobostrycin (8) which exhibited moderate to high antibacterial activity against the Gram-positive bacteria, B. subtilis and B. cereus (MIC values of 2.5 μM), S. aureus and Micrococcus luteus (MIC values of 2.5 μM), and Micrococcus tetragenus (MIC value of 1.25 μM).38 Compound 8 also displayed good antibacterial activity against the Gram-negative bacteria E. coli (MIC value of 6.25 μM), V. anguillarum (MIC value of 1.56 μM), and V. parahemolyticus (MIC value of 12.5 μM).38 Additionally, it exhibited potent activity against M. tuberculosis with a MIC value of 12.50 μg mL−1 and was also active as antimalarial agent against Plasmodium falciparum with an IC50 value of 7.94 μg mL−146 (Fig. 2).
3.2. Anthraquinones isolated from Aspergillus sp.
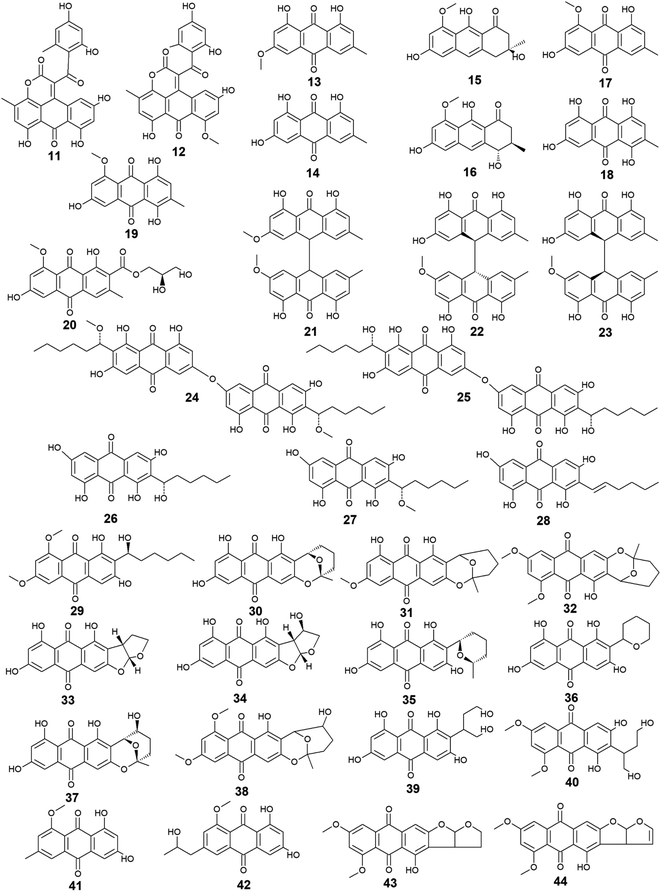
Aspergillus was the richest source of marine anthraquinones and their derivatives among all marine-derived fungi with 73 reported compounds including the previously mentioned 7, 8, and 10 as well as other seventy anthraquinones 11–80. For instance, thirteen compounds 11–23 were isolated from the marine-derived fungus Aspergillus glaucus (A. glaucus).47 Aspergiolide A (11), which features a naphtho[1,2,3-de]chromene-2,7-dione skeleton was isolated as a novel anthraquinone derivative from the marine-derived fungus A. glaucus.48 Aspergiolide B (12) was isolated from A. glaucus as a new analogue for aspergiolide A (11).47 Aspergiolides A and B (11 and 12) exhibited potent cytotoxic activities against both adenocarcinoma human alveolar basal epithelial cell line, A-549 with IC50 values of 0.13 and 0.24 μM and human leukemia cell line, HL-60 with IC50 values of 0.28 and 0.51 μM, respectively47,48 indicating that methylation of one hydroxyl group in aspergiolide A (11) to be a methoxy group in aspergiolide B (12) slightly affected the cytotoxicity of aspergiolide A.Physcion (13) was also isolated from other species of Aspergillus such as A. glaucus,47 A. wentii ,49 and the halotolerant A. variecolor50 besides the marine-derived fungus Microsporum sp.51 Physcion (13) displayed a wide array of biological activities including cytotoxic activity against human cervical carcinoma HeLa cells,51 moderate antifungal activity against Trichophyton mentagrophytes with a MIC value of 25.0 μg mL−1 and weak antifungal activity against both C. albicans and Cryptococcus neoformans with MIC value of 50.0 μg mL−1.52 It also exhibited weak free radical scavenging activity against 1,1-diphenyl-2-picrylhydrazyl (DPPH) with an IC50 value of 99.4 μg mL−1.49
Furthermore, emodin (14) which was reported from the marine-derived fungus A. glaucus, was also recovered from many other marine fungal species such as Penicillium citrinum (P. citrinum),53 Trichoderma aureoviride (T. aureoviride),54 Monodictys sp.,55 Gliocladium sp.,56 Paecilomyces sp.,57 Eurotium rubrum (Eu. rubrum)58 and A. versicolor.59 Emodin (14) showed moderate antibacterial against Pseudomonas putida with a MIC value of 25.0 μM60 and significant anti-mycobacterial activity against M. tuberculosis with a MIC value of 12.5 μg mL−1 and modest antifungal activity against Candida albicans (C. albicans) with an IC50 value of 11.0 μg mL−1.61 Noteworthy, it showed potent cytotoxic activity against both oral human epidermoid carcinoma cell line, KB and human breast cancer cell line, MCF7 with IC50 values of 0.88 and 2.8 μg mL−1, respectively.61
Further anthraquinones 17 and 18, and 20 which were isolated from both A. glaucus47 and the halotolerant A. variecolor,50 showed variable bioactivities. Questin (17) and catenarin (18) exhibited DPPH radical scavenging activity62 and potent antibacterial activity against Brevibacillus brevis with a MIC value of 1.0 μg mL−1,63 respectively, while (+)-variecolorquinone A (20) displayed positive cytotoxicity against the human hepatocellular carcinoma cell line, BEL-7402, mouse lymphoma cell line, P388, human leukemia cell line, HL-60, and adenocarcinoma human alveolar basal epithelial cells, A-549 with IC50 values of 114.0, 266.0, 309.0, and 3.0 μM, respectively.50
Notably, the known anthraquinone dimer 21, as well as two new isomers of anthraquinone dimer 22 and 23, were also isolated from A. glaucus.47 However, compound 21 was not evaluated for any relevant bioactivity, the trans isomer of emodin-physcion bianthrone (22) showed good cytotoxicity against the cell lines; A-549 and HL-60 with IC50 values of 9.2 and 7.8 μM, respectively. On the other hand, its cis isomer 23 was less active as its IC50 values were 14.2 and 44.0 μM, respectively,47 suggesting that isomerization has affected the cytotoxicity of compound 22.
Additional thirty anthraquinones 24–54 have been isolated from the marine-derived fungus A. versicolor. Two new anthraquinone dimers 24 and 25 besides three other known closely related anthraquinone derivatives 26–28 were isolated from the marine-derived fungus A. versicolor.64 Averantin (26) and its derivative 1′-O-methyl-averantin (27) were also isolated earlier from the marine-derived fungus P. purpurogenum G59 ref. 65 and Aspergillus sp. SCSIO F063 ,66 while averythrin (28) was formerly reported from the marine-derived fungus Aspergillus sp. SCSIO F063.66
Compounds 24 and 25 showed selective antibacterial activity against the Gram-positive bacterium, S. aureus using the disk diffusion method at a concentration of 30.0 μg per well,64 whereas the same study revealed that compound 24 had a selective cytotoxic activity against human CNS cancer cells, XF-498 with an IC50 value of 22.39 μg mL−1. In addition, averantin (26) and its derivative 1′-O-methyl-averantin (27) displayed a weak antitumor activity against the bone marrow cancer cell line, K562 at a concentration of 100.0 μg mL−1.65 Another study mentioned that compound 27 exhibited modest cytotoxic activity against the human glioblastoma SF-268, human breast adenocarcinoma MCF-7 and human large-cell lung carcinoma NCI–H460 cell lines with IC50 values ranging from 33.59 to 44.22 μM, whilst compounds 26 and 28 displayed weak to moderate cytotoxic activity against MCF-7 with IC50 values of 45.47 and 29.69 μM, respectively.66 Also, compounds 26 and 27 displayed potent antioxidant activity, whereas compound 28 exhibited weak antioxidant activity in terms of antioxidant capacity compared to Trolox67 suggesting that the presence of oxygen in the side chain of the anthraquinones may play role in their antioxidant activity.
Additionally, compound 26 displayed promising antibacterial activity against different strains of the Gram-positive bacteria, Streptococcus pyogenes (Str. pyogenes) and S. aureus with MIC values of equal to or less than 3.13 μg mL−1, while its 1′-O-methylated derivative, 27 showed weaker antibacterial activity as it was only active against one strain of Str. pyogenes with a MIC value of 6.25 μg mL−1 with no activity against the other strain of Str. pyogenes or any strain of S. aureus up to a concentration of 12.5 μg mL−1,68 indicating that O-methylation at position 1 greatly affected the antibacterial activity of averantin (26).
Compound 29 which is another derivative of averantin (26) was isolated from another marine-derived fungus A. versicolor EN-7.69 Compound 29 showed weak antibacterial activity against only E. coli at a concentration of 20.0 μg per disk with no activity against S. aureus,69 suggesting that the O,O′-dimethylation of averantin (26) decreased its antibacterial activity against the Gram-positive bacteria.
The aflatoxin, averufin (30) and its O-methylated derivatives 6-O-methyl-averufin (31) and 6,8-O,O′-dimethyl-averufin (32) were also isolated from different strains of the marine-derived fungus A. versicolor,68,69 whereas averufin (30) was also isolated from other species of Aspergillus such as A. niger70 and A. nidulans.71 Averufin (30) exhibited different bioactivities including potent antioxidant activity in terms of Trolox equivalent antioxidant capacity,67 weak cytotoxic activity,68 and moderate inhibitory activity against the multiplication of Tobacco Mosaic virus,70 in addition to weak antibacterial activity against the Gram-positive Str. pyogenes and S. aureus with MIC values equal to or less than 12.5 μg mL−1.68 On the other hand, neither 6-O-methyl-averufin (31) nor 6,8-O,O′-dimethyl-averufin (32) showed any antimicrobial activity69 or anti-neuroinflammatory effect,72 respectively.
Moreover, further eight bioactive compounds 33–40 were also isolated from the marine-derived fungus A. versicolor67–69,73 including versicolorin B (33), averufanin (35) nidurufin (37), and versiconol (39) as well as their derivatives 1′-hydroxyversicolorin B (34), noraverufanin (36), 6,8-O,O′-dimethyl-nidurufin (38) and 6,8-O,O′-dimethyl-versiconol (40), respectively. Both versicolorin B (33) and its hydroxyl derivative, 1′-hydroxyversicolorin B (34) showed potent antioxidant activity as they displayed antioxidant capacity approximately equivalent to Trolox,67 while an old study revealed that 1′-hydroxyversicolorin B (34) (UCT1072M1) had potent cytotoxicity against the human cervical cell adenocarcinoma, HeLa S3 and the human lung giant cell carcinoma, Lu-65 with IC50 values of 2.1 and 2.2 μM, respectively.74
Indeed, averufanin (35) displayed a good antioxidant activity in terms of antioxidant capacity to Trolox,67 and weak activity against both acyl-CoA: cholesterol acyltransferase type 1 and 2 in the cell-based assay with IC50 values of 28.0 and 12.0 μM, respectively,75 whereas noraverufanin (36) exhibited a weak HIV latency–reversal activity with reactivation of 43.3% at concentration of 10.0 μM.73 Nidurufin (37) which has been also isolated from the marine fungi A. niger70 as well as P. purpurogenum G59,65 showed weak antitumor activity against the bone marrow cancer cell line, K562 with an inhibition rate percentage of 25.5% at a concentration of 100.0 μg mL−165 and moderate antioxidant capacity with 0.62 as Trolox equivalent as antioxidant.67
Another previous study showed that nidurufin (37) had exhibited strong anticancer activity against the A-549 cells, the human ovarian cancer cells, SK-OV-3, the human skin cancer cells, SK-MEL-2, the human CNS cancer cells, XF-498, and the human colon cancer HCT-15 with IC50 values of 1.83, 3.39, 3.16, 1.78, and 2.2 μg mL−1 beside good antibacterial activity against different strains of the Gram-positive bacteria Str. pyogenes and S. aureus with MIC values of equal to or less than 3.13 μg mL−1.68
Compound 38 (6,8-O,O′-dimethyl-nidurufin), showed weak antibacterial activity against the Gram-positive S. aureus as well as the Gram-negative E. coli with inhibition zones of 7 and 6.5 mm, respectively using the disk diffusion method at a concentration of 20.0 μg per disk,69 suggesting that the new derivatization by O,O′-dimethylation in position 6 and 8 in this compound had affected the antibacterial activity of the parent metabolite, nidurufin (37) which showed better antibacterial activity when tested against the Gram-positive bacteria as discussed above.
Versiconol (39) exhibited weak anticancer activity against the A-549 cells, the SK-OV-3 cells, the SK-MEL-2 cells, the XF-498 cells, and the HCT-15 cells with IC50 values of 20.45, 15.29, 15.86, 23.73, and 19.2 μg mL−1, respectively,68 whilst its O,O′-dimethylated derivative, 6,8-O,O′-dimethyl-versiconol (40) showed selective weak antibacterial activity against S. aureus with inhibition zones of 6.5 mm using disk diffusion method at a concentration of 20.0 μg per disk when tested against both S. aureus and E. coli.69
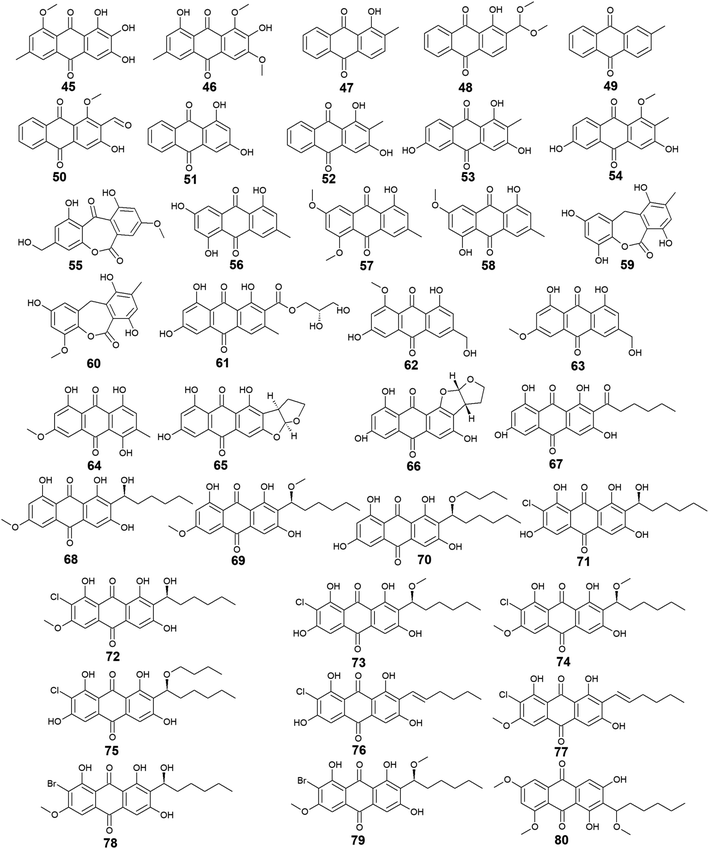
Other bioactive compounds isolated from the marine fungus A. versicolor were compounds 41 and 42, 47 and 48, and 50–54.59,69,76 1-methyl-emodin (41) which is an O-methylated derivative of emodin (14) and both were isolated from A. versicolor,59 exhibited better cytotoxic activity than emodin (14) itself against human epidermoid carcinoma cell line, KBv200 with an IC50 value of 190.81 μM,40 although 41 did not show any cytotoxicity against the human leukemia cell line, CCRF-CEM and some other solid tumors including the human lung H-125, human colon HCT-116, and human liver Hep-G2 cells.76 On the other hand, compound 41 showed less inhibitory activity against Hepatitis C virus (HCV) protease than its parent 14 with IC50 values of 40.2 and 22.5 μg mL−1, respectively.76 The same study showed that the new metabolite from A. versicolor; isorhodoptilometrin-1-methyl-ether (42) displayed moderate antibacterial activity against B. cereus, B. subtilis, and S. aureus at a concentration of 50.0 μg per disk and mild selective cytotoxicity against the Hep-G2 cell line.76
Additionally, 1-hydroxy-2-methyl-anthraquinone (47) and its novel dimethoxy derivative; 2-(dimethoxy methyl)-1-hydroxy-9,10-anthraquinone (48) were evaluated for their antibacterial activity against two strains of methicillin-resistant S. aureus (MRSA) (CGMCC 1.12409 and ATCC 43300) and three strains of Vibrio (V. rotiferianus, V. vulnificus, and V. campbellii). Noteworthy, the dimethoxy derivative 48 was highly active against the MRSA strains showing MIC values of 7.8 and 3.9 μg mL−1, respectively, and was moderately active against the Vibrio strains with MIC values ranging from 15.6 to 62.5 μg mL−1.59 The same study mentioned that a molecular docking study was conducted to explain the cause behind this antimicrobial activity revealing the least binding energy of compound 48 with both AmpC β-lactamase and topoisomerase IV (Topo IV).59 On the other hand, its parent compound 47 displayed potent larvicidal activity against the larvae of Aedes aegypti with an IC50 value of 1.8 μg mL−1.77
Moreover, another anthraquinone derivative, damnacanthal (50) which was reported from A. versicolor59 exhibited strong larvicidal activity against the larvae of Aedes aegypti with an IC50 value of 7.4 μg mL−177 and weak antibacterial activity against some strains of MRSA and Vibrio with MIC values ranging from 31.3 to 125.0 μg mL−1.59 Similarly, xanthopurpurin (51) showed weak antibacterial properties against some strains of MRSA and Vibrio with the same MIC range of damnacanthal (50).59 Also, compound 51 previously showed strong antiplatelet aggregation activity via inhibition of collagen-induced aggregation.78 In addition, a chemically related rubiadin (52) showed a strong inhibitory activity on the formation of advanced glycation end products with an IC50 value of 179.31 μM.79 Notably, its hydroxylated derivative; 6-hydroxyrubiadin (53) displayed potent inhibitory activity on phosphatase of regenerating liver-3 with an IC50 value of 1.3 μg mL−1 causing inhibition of migration of phosphatase of regenerating liver-3 expressed tumor cells with no cytotoxicity.80
Additional four derivatives 55–58 were isolated from the marine-derived fungus A. wentii.49,81 Wentiquinone C (55) showed no free radical scavenging activity up to a concentration of 1000.0 μg mL−1,49 whereas compounds 56–58 were not tested for any relevant bioactivity.81
Further derivatives including compounds 59–64 were isolated from the halotolerant fungus A. variecolor,50 while compounds 65–67 were reported from A. nidulans.71 Compounds 59 and 60 exhibited potent DPPH radical scavenging activity (antioxidant activity) with IC50 values of 6.0 and 11.0 μM, respectively50 suggesting that the O-methylation of eurotinone (59), slightly affected its antioxidant activity. Interestingly, Questinol (62) which was also isolated from the marine-derived fungi Talaromyces stipitatus KUFA 0207 82 and Eu. amstelodami,83 displayed significant anti-inflammatory activity via different mechanisms including inhibition of both nitric oxide and prostaglandin E2 production and, inhibition of the production of some inflammatory cytokines such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α. Compound 62 also showed slight inhibitory activity against cyclooxygenase-2 (COX-2) expression at a concentration of 200.0 μM.83 In addition, compound 62 exhibited potent anti-obesity activity with a 60% reduction in the stained lipids with an IC50 value of 0.95 μM, while the chemically related compound, fallacinol (63) showed no significant anti-obesity activity.82 Interestingly, versicolorin C (65) displayed selective potent antibacterial activity against both E. coli and V. parahaemolyticus with a MIC value of 1.0 μg mL−1 and, against V. anguillarum and Edwardsiella ictaluri with MIC values of 4.0 and 8.0 μg mL−1, respectively, whilst the closely related congener isoversicolorin C (66) displayed selective potent antibacterial activity against both V. alginolyticus and Edwardsiella ictaluri with MIC values of 1.0 and 4.0 μg mL−1, respectively.71 Further, twelve anthraquinones including three non-halogenated ones 68–70, seven new chlorinated anthraquinone derivatives 71–77, and two new brominated anthraquinone derivatives 78 and 79 were isolated from the marine-derived fungus Aspergillus sp. SCSIO F063,66 in addition to compound 80 which was reported from another marine-derived fungus Aspergillus sp. SF-6796.72 Compounds 68–70 are chemically related to each other and are derivatives of averantin (26) which was isolated in the same study as a metabolite from Aspergillus sp. SCSIO F063,66 while it was isolated earlier from the marine-derived fungi A. versicolor.64 Averantin-1′-butyl-ether (70) exhibited weak cytotoxicity against SF-268 and MCF-7 cell lines with IC50 values of 47.19 and 40.47 μM, respectively, revealing slightly better cytotoxicity than its parent; averantin (26) which only showed activity against the MCF-7 cell line with an IC50 value of 45.47 μM,66 suggesting that the structural modification in 70 has improved its bioactivity. By contrast, neither compound 68 nor 69 displayed any cytotoxicity against all tested human cell lines including NCI–H460, SF-268, and MCF-7 ref. 66 indicating that O-methylation of averantin (26) in compounds 68 and 69 may negatively influence their cytotoxicity. It is noteworthy that the chlorinated anthraquinone derivative, 72 exhibited potent cytotoxicity against NCI–H460, SF-268, and MCF-7 cells with IC50 values of 7.42, 7.11, and 6.64 μM, respectively. While 71 showed weak cytotoxicity against only the MCF-7 cell line with an IC50 value of 36.41 μM, 73 displayed better cytotoxic activity against the three cell lines; NCI–H460, SF-268, and MCF-7 with IC50 values of 37.19, 34.06 and 26.09 μM, respectively.66 The other chlorinated anthraquinones, 75 and 77 demonstrated weak to modest cytotoxic activity against only the MCF-7 cell line with IC50 values of 49.53 and 24.38 μM, respectively. The same study revealed that from the two isolated brominated anthraquinones, only 78 displayed modest cytotoxicity against NCI–H460, SF-268, and MCF-7 cell lines with IC50 values of 18.91, 24.69, and 25.62 μM, respectively.66 Furthermore, another bioactive derivative of averantin (26) isolated from Aspergillus sp. is 6,8,1′-O,O′,O′′-trimethyl-averantin (80) which showed an anti-neuroinflammatory effect via different mechanisms including suppression of the overproduction of many pro-inflammatory mediators including COX-2, prostaglandin E2, and nitric oxide in lipopolysaccharide-activated BV2 microglial cells72 (Fig. 3 and 4).
3.3. Anthraquinones from Penicillium sp.
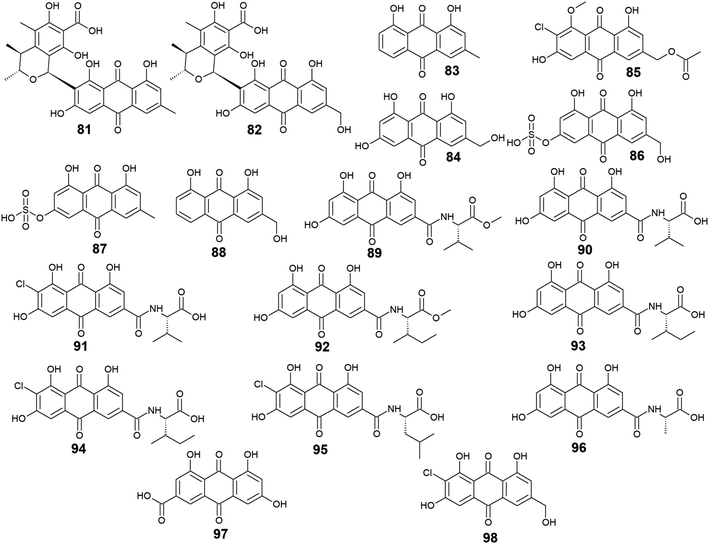
Furthermore, eighteen compounds 81–98 besides the previously reported compounds 14, 17, 26, 27, and 37 were isolated from different species of the marine-derived fungus Penicillium. Indeed, penicillanthranin A (81) and B (82) which are anthraquinone-citrinin derivatives, as well as chrysophanol (83) and ω-hydroxyemodin (84), were isolated from the marine fungus P. citrinum PSU-F51.53 Penicillanthranin A (81) and chrysophanol (83) exhibited selective antibacterial activity against the Gram-positive S. aureus ATCC25923 with MIC value of 16.0 μg mL−1 for both compounds and MRSA SK1 with MIC values of 16.0 and 64.0 μg mL−1, respectively, while compounds 82 and 84 were not screened for their antimicrobial activity in the same study.53 Interestingly, some earlier studies revealed that ω-hydroxyemodin (84) showed moderate activity against MRSA SK1 and mild activity against S. aureus ATCC 25923 with MIC values of 32.0 and 200.0 μg mL−1, respectively,54 in addition to good anti-mycobacterial activity against M. tuberculosis H37Ra with a MIC value of 12.5 μg mL−1.61 It also showed potent cytotoxicity against the human oral epidermoid carcinoma KB cells with an IC50 value of 4.5 μg mL−1, and weak cytotoxic activity against both the human breast cancer cells, MCF7 and the human lung carcinoma cells, NCI–H187 with IC50 values of 22.0 and 39.0 μg mL−1, respectively.61 In contrast, penicillanthranin A (81) showed selective cytotoxicity to the KB cell lines with an IC50 value of 30.0 μg mL−1.53Another bioactive metabolite, 2′-acetoxy-7-chlorocitreorosein (85) which was first recovered from a mangrove-derived fungus P. citrinum HL-5126 ref. 84 demonstrated moderate antibacterial activity against S. aureus and significant activity against V. parahaemolyticus with MIC values of 22.8 and 10.0 μg mL−1, respectively,84 suggesting that such modification in its structure by acetylation, chlorination, and O-methylation of ω-hydroxyemodin (84) resulted in significant improvement in its antibacterial activity. Further anthraquinone derivatives discovered from the marine fungus P. oxalicum, including citreorosein-3-O-sulphate (86), emodin-3-O-sulphate (87), and aloe-emodin (88) were not tested for any relevant activity.85 However, other previous studies revealed that aloe-emodin (88) displayed modest antimalarial activity against Plasmodium falciparum (MRC-2) with an EC50 value of 22.0 μg mL−186 and weak antimicrobial activity against the Gram-positive bacteria, S. aureus, S. epidermidis, B. cereus, B. subtilis, and Micrococcus kristinae, and the Gram-negative bacteria, E. coli, Enterobacter aerogenes, Proteus vulgaris, and Shigella sonnei with MIC values ranging from 62.5 to 250.0 μg mL−1.87
Additional ten bioactive compounds including eight newly isolated anthraquinone–amino acid conjugates, namely emodacidamide A–H (89–96) along with the previously isolated anthraquinone derivatives; emodic acid (97) and 2-chloro-1,3,8-trihydroxy-6 (hydroxymethyl)-anthracene-9,10 dione (98), were isolated from the marine fungus Penicillium sp. SCSIO sof101.88 Emodacidamides A–H (89–96) displayed immunomodulatory activity with inhibitory activity against IL-2 production from Jurkat cells.88 Intriguingly, emodacidamides A (89), C (91), and E (93) showed potent IL-2 inhibitory activity with IC50 values of 4.1, 5.1, and 5.4 μM, respectively.88 Meanwhile, emodic acid (97) showed no remarkable inhibition of IL-2 secretion at a concentration of 20.0 μM, indicating that amino acid conjugation with the anthraquinone derivatives enhanced their inhibitory effect on IL-2 secretion.88
On the other side, emodic acid (97) which was previously isolated from the marine endophytic fungus Eu. rubrum,58 evoked potent inhibition of p56lck tyrosine kinase with an IC50 value of 1.07 μg mL−1.89 In addition, compound 97 demonstrated a potent inhibitory effect on both the tyrosine kinase domain of the epidermal growth factor receptor and protein tyrosine kinase p59fyn with IC50 values of 0.078 and 0.080 μg mL−1, respectively without any noted cytotoxicity on human foreskin fibroblast89 (Fig. 5).
3.4. Anthraquinones from Stemphylium sp.
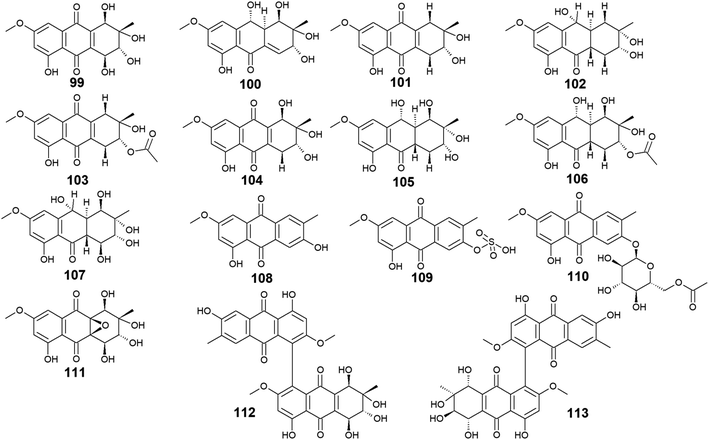
The marine-derived fungus Stemphylium is another good source of the bioactive anthraquinones with thirty-two recovered compounds 99–130. A group of twenty-five anthraquinones derivatives 99–123 were reported from a mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 ref. 90 including the bioactive altersolanol A, B, C (99, 101, 104) and L (105) as well as their derivatives dihydroaltersolanol A (100), tetrahydroaltersolanol B (102), 2-O-acetylaltersolanol B (103).
231 ref. 90 including the bioactive altersolanol A, B, C (99, 101, 104) and L (105) as well as their derivatives dihydroaltersolanol A (100), tetrahydroaltersolanol B (102), 2-O-acetylaltersolanol B (103).
Altersolanol A (99) showed selective antimicrobial activity against S. aureus, E. coli, B. subtilis, and Micrococcus tetragenus with MIC values of 2.07, 4.1, 4.1, and 8.2 μM, respectively, whereas altersolanol B (101) displayed similar antibacterial activity against S. aureus, E. coli and B. subtilis as well as the Gram-positive bacterium, Kocuria rhizophila with MIC values of 7.8 μM for all strains.90 The same study revealed that altersolanol C (104) had a narrow spectrum of activity against only B. subtilis with a MIC value of 8.8 μM, while altersolanol L (105) had no antibacterial activity against the tested strains.90 In the contrast, another recent study demonstrated that altersolanol L(105), had a modest antifungal activity against P. italicum and Rhizoctonia solani with MIC values of 35.0 and 50.0 μg mL−1, respectively.91
Additionally, a recent study showed that both altersolanol A (99) and B (101) had strong cytotoxicity against MCF-7 and HCT-116 cell lines with IC50 values of [7.21, 1.3 μM] for altersolanol A (99) and, [9.0, 3.5 μM] for altersolanol B (101), respectively.92 By contrast, dihydroaltersolanol A (100) did not show any antibacterial activity or cytotoxicity when tested against various microbes and cell lines,90,93 suggesting that the derivatization of its parent altersolanol A (99) into dihydroaltersolanol A (100) lead to a significant change in its biological activities.
Furthermore, ampelanol (107), macrosporin (108) and its sulphate derivative, macrosporin-7-O-sulphate (109), in addition to its glycosidic derivative, macrosporin 2-O-(6′-acetyl)-α-D-glucopyranoside (110), as well as auxarthrol C (111), were also recovered from the marine fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231.90 Ampelanol (107) displayed moderate cytotoxicity against the murine lymphoma cell line, L5178Y,94 whereas macrosporin (108) exhibited significant antibacterial activity against Micrococcus tetragenus, E. coli, and S. aureus with MIC values of 4.6, 4.6, and 9.2 μM, respectively.90 On the other hand, both derivatives of macrosporin (108), macrosporin-7-O-sulphate (109) and macrosporin 2-O-(6′-acetyl)-α-D-glucopyranoside (110) displayed no antibacterial activity against the same indicator strains up to a concentration of 10.0 μM,90 indicating that these modifications in the chemical structure of macrosporin (108) have greatly affected its antibacterial activity. Additionally, macrosporin (108) was shown to have potent antifungal activity against Fusarium oxysporum (F. oxysporum) with a MIC value of 3.75 μg mL−1 and modest antifungal activity against Colletotrichum musae, F. graminearum, P. italicum, and Colletotrichum gloeosporioides with MIC values ranging from 30.0 to 60.0 μg mL−1.91 Noteworthy, compound 110 demonstrated a remarkable brine shrimp lethality using Artemia salina with an LD50 value of 10.0 μM,90 while the parent compound 108, and its derivative 109 showed no lethality in the same study90 suggesting that brine shrimp lethality might be dependent on acetylation and/or glycosylation of this compound. Also, the same study revealed that auxarthrol C (111) displayed selective antibacterial activity against only the Gram-negative organism, E. coli with a MIC value of 9.8 μM with no notable cytotoxicity or brine shrimp lethal effect.90
231.90 Ampelanol (107) displayed moderate cytotoxicity against the murine lymphoma cell line, L5178Y,94 whereas macrosporin (108) exhibited significant antibacterial activity against Micrococcus tetragenus, E. coli, and S. aureus with MIC values of 4.6, 4.6, and 9.2 μM, respectively.90 On the other hand, both derivatives of macrosporin (108), macrosporin-7-O-sulphate (109) and macrosporin 2-O-(6′-acetyl)-α-D-glucopyranoside (110) displayed no antibacterial activity against the same indicator strains up to a concentration of 10.0 μM,90 indicating that these modifications in the chemical structure of macrosporin (108) have greatly affected its antibacterial activity. Additionally, macrosporin (108) was shown to have potent antifungal activity against Fusarium oxysporum (F. oxysporum) with a MIC value of 3.75 μg mL−1 and modest antifungal activity against Colletotrichum musae, F. graminearum, P. italicum, and Colletotrichum gloeosporioides with MIC values ranging from 30.0 to 60.0 μg mL−1.91 Noteworthy, compound 110 demonstrated a remarkable brine shrimp lethality using Artemia salina with an LD50 value of 10.0 μM,90 while the parent compound 108, and its derivative 109 showed no lethality in the same study90 suggesting that brine shrimp lethality might be dependent on acetylation and/or glycosylation of this compound. Also, the same study revealed that auxarthrol C (111) displayed selective antibacterial activity against only the Gram-negative organism, E. coli with a MIC value of 9.8 μM with no notable cytotoxicity or brine shrimp lethal effect.90
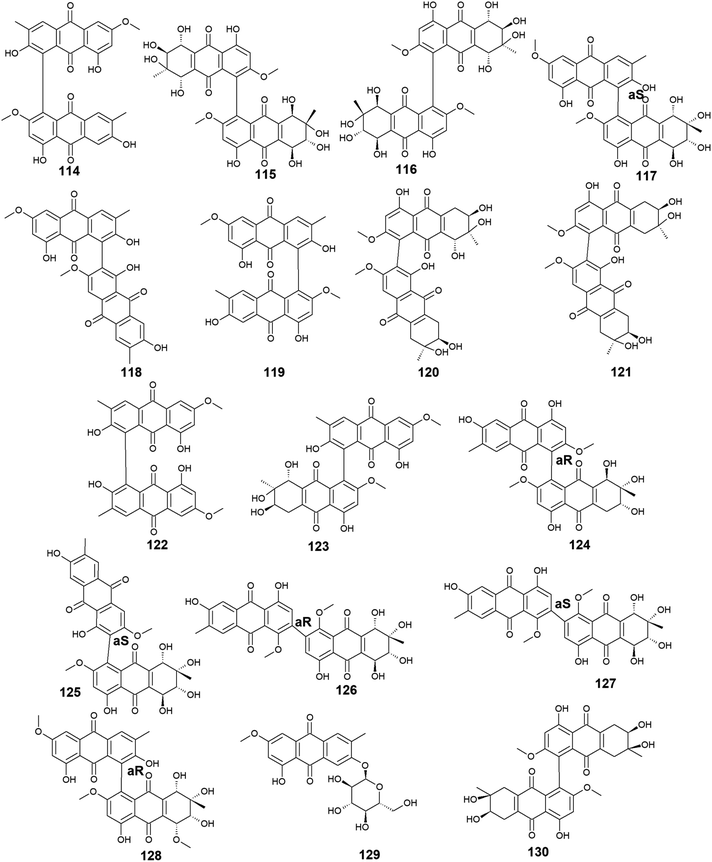
Moreover, other bioactive anthraquinone dimers including alterporriols B–E (113–116), N (117), Q (118), U (121), and V (122) were also isolated from the same fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231.90 The anthraquinone dimers, alterporriols B–E (113–116) displayed positive antibacterial activity, whereas alterporriol A (112) did not show either antibacterial or cytotoxic activity.90 Alterporriol B (113) showed a narrow spectrum of antimicrobial activity against B. cereus with a MIC value of 7.9 μM, whereas alterporriol C (114) showed selective antibacterial activity against S. albus with a MIC value of 8.9 μM. Interestingly, alterporriol D (115) exhibited notable antibacterial activity against both S. aureus and E. coli and with MIC values of 5.0 and 7.5 μM, respectively, while alterporriol E (116) displayed potent antimicrobial activity against both B. cereus and E. coli with MIC values of 2.5 and 5.0 μM, respectively.90 The same study demonstrated that alterporriol Q (118) and R (119) showed no antimicrobial activity against various tested microbes up to a concentration of 10.0 μM.90 This finding was confirmed in another study which showed that both compounds did not display any antibacterial activity against different Gram-positive bacteria as well as E. coli from the Gram-negative bacteria up to a concentration of 20.0 μM.93 However, alterporriol Q (118) exhibited strong antiviral activity against the porcine reproductive and respiratory syndrome virus with a MIC value of 22.0 μM, whereas alterporriol R (119) showed no antiviral activity.93 Also, the same study revealed that alterporriol C (114) had a modest antiviral activity with a MIC value of 39.0 μM.93 In addition, the other anthraquinone dimers, alterporriol U (121) and V (122) exhibited a narrow spectrum of antibacterial bioactivity against the Gram-positive bacterium, B. cereus with MIC values of 8.3 and 8.1 μM, respectively.90
231.90 The anthraquinone dimers, alterporriols B–E (113–116) displayed positive antibacterial activity, whereas alterporriol A (112) did not show either antibacterial or cytotoxic activity.90 Alterporriol B (113) showed a narrow spectrum of antimicrobial activity against B. cereus with a MIC value of 7.9 μM, whereas alterporriol C (114) showed selective antibacterial activity against S. albus with a MIC value of 8.9 μM. Interestingly, alterporriol D (115) exhibited notable antibacterial activity against both S. aureus and E. coli and with MIC values of 5.0 and 7.5 μM, respectively, while alterporriol E (116) displayed potent antimicrobial activity against both B. cereus and E. coli with MIC values of 2.5 and 5.0 μM, respectively.90 The same study demonstrated that alterporriol Q (118) and R (119) showed no antimicrobial activity against various tested microbes up to a concentration of 10.0 μM.90 This finding was confirmed in another study which showed that both compounds did not display any antibacterial activity against different Gram-positive bacteria as well as E. coli from the Gram-negative bacteria up to a concentration of 20.0 μM.93 However, alterporriol Q (118) exhibited strong antiviral activity against the porcine reproductive and respiratory syndrome virus with a MIC value of 22.0 μM, whereas alterporriol R (119) showed no antiviral activity.93 Also, the same study revealed that alterporriol C (114) had a modest antiviral activity with a MIC value of 39.0 μM.93 In addition, the other anthraquinone dimers, alterporriol U (121) and V (122) exhibited a narrow spectrum of antibacterial bioactivity against the Gram-positive bacterium, B. cereus with MIC values of 8.3 and 8.1 μM, respectively.90
Further anthraquinone dimers including alterporriol N (117), F (124), G (125), Z1 (126), Z2 (127), and Z3 (128) were also isolated recently from another marine fungus Stemphylium sp. FJJ006.95 They showed neither antimicrobial activity against the Gram-positive and Gram-negative bacterial strains up to a concentration of 128.0 μg mL−1 nor antitumor activity against a panel of cancer cell lines with an IC50 value higher than 20.0 μM. Also, they did not show bioactivity against the microbial enzymes, isocitrate lyase, and sortase A with an IC50 value of more than 145.0 μM. However, the same study revealed that alterporriols N (117), F, G, and Z1–Z2 (124–127) had anti-inflammatory activity through their capability of suppressing the lipopolysaccharide-induced nitric oxide production in the murine macrophages RAW 264.7 cells with IC50 values of 8.4, 9.6, 10.7, 11.6, and 16.1 μM, respectively, whereas alterporriol Z3 (128) did not display any anti-inflammatory activity.95 On the other hand, another previous study demonstrated the potent cytotoxicity of alterporriol F (124) against the HeLa and KB human cell lines with IC50 values of 6.5 and 7.0 μg mL−1, respectively.96 In addition, alterporriol N (117) was presented in another study as a weak antimicrobial agent with a narrow spectrum of activity against only the Gram-positive bacteria, Enterococcus faecalis, MRSA, and Str. pneumoniae with MIC values of 15.63, 62.5, and 125.0 μg mL−1, respectively, while the same study revealed that alterporriol G (125) had a moderate cytotoxicity against the mouse cancer cell line, L5178Y97 (Fig. 6 and 7).
3.5. Anthraquinones from Alternaria sp.
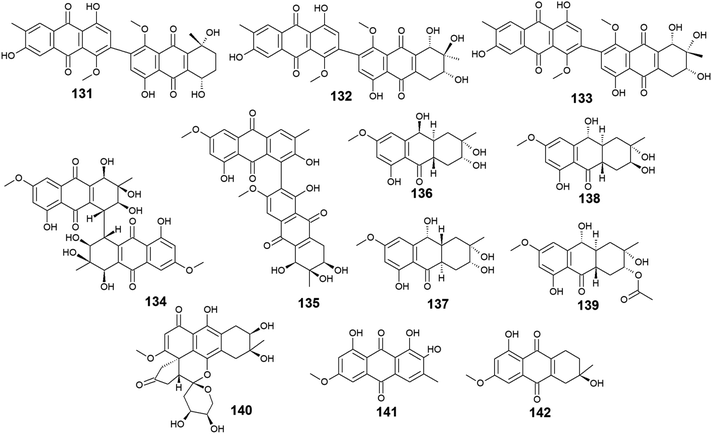
A list of twenty anthraquinones was isolated earlier from different species of Alternaria including the previously mentioned compounds, 100–102, 104, 105, 107, 108, and 114 as well as twelve anthraquinone derivatives, 131–142. Two bioactive bi-anthraquinones, named alterporriol K (131) and L (132) were isolated from the marine endophytic fungus Alternaria sp. ZJ9-6B ref. 98 and displayed moderate cytotoxic activity against the human breast cancer cells, MCF-7 and MDA-MB-435 with IC50 values of [29.11 and 26.97 μM] for alterporriol K (131) and [20.04 and 13.11 μM] for alterporriol L (132), respectively, while alterporriol M (133) was not evaluated for any biological activity in this study.98Further compounds including alterporriol O (134) and P (135) were isolated from the marine-derived Aspergillus sp. ZJ-2008003. Only alterporriol P (135) exhibited significant cytotoxicity against the human prostate cancer cell line, PC3, colon cancer cell line, HCT-116, liver hepatoma cell lines, Hep-G2 and Hep-3B in addition to the breast cancer cell line, MCF-7/ADR with IC50 values of 6.4, 8.6, 20.0, 21.0, and 23.0 μM, respectively. Unlikely, alterporriol O (134) did not demonstrate any bioactivity when it was evaluated for its cytotoxicity, antibacterial activity, and antiviral activities.93
Additional anthraquinones, tetrahydroaltersolanols C–F (136-139) were also isolated from the marine-derived Alternaria sp. ZJ-2008003.93 Only tetrahydroaltersolanol C (136) displayed moderate antiviral activity against the porcine reproductive and respiratory syndrome virus with an IC50 value of 65.0 μM.93
More anthraquinone derivatives 140–142 were reported recently from the marine fungus Alternaria tenuissima DFFSCS013.99 Anthrininone A (140) demonstrated selective protein tyrosine phosphatase inhibitory effect on indoleamine 2,3 dioxygenase 1 enzyme with an IC50 value of 32.3 μM as well as the stimulatory effect on the intracellular levels of calcium in HEK293 cells at a concentration of 10.0 μM.99 It is noteworthy that 6-O-methyl-alaternin (141) displayed a wide range of anti-protein tyrosine phosphatases activity including activity against TCPTP, SHP1, SHP2, and PTP-MEG2 enzymes with potent bioactivity against both indoleamine 2,3 dioxygenase 1 enzyme and PTP1B with IC50 values of 1.7 and 2.1 μM, respectively. On the other hand, compound 141 did not show a noticeable stimulatory effect on the intracellular levels of calcium in HEK293 cells at a concentration of 10.0 μM ref. 99 (Fig. 8).
3.6. Anthraquinones from Trichoderma sp.
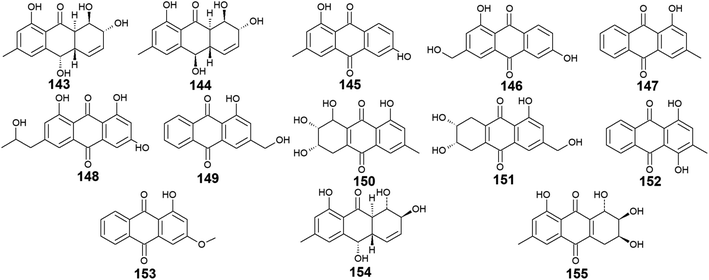
Trichoderma sp. is another prolific anthraquinones producer from which the previously discussed compounds 14, 83, and 84 were isolated as well as the anthraquinone derivatives, 143–155. Harzianumnones A and B (143 and 144) were reported earlier as new hydroxyanthraquinones from the marine fungus T. harzianum XS-20090075.100 They showed neither DNA Topo I inhibitory activity nor anti-acetylcholinesterase activity.100 The same study revealed that phomarin (145), ω-hydroxydigitoemodin (146), pachybasin (147), and (+)-2′S-isorhodoptilometrin (148) isolated also from T. harzianum XS-20090075, displayed a weak anti-acetylcholinesterase activity at a concentration of 100.0 μM.100Interestingly, pachybasin (147) also demonstrated potent cytotoxic activity against the human cancer cell lines, KB and KBv200 with IC50 values of 3.17 and 3.21 μM, respectively.40 In addition, its derivative, ω-hydroxypachybasin (149) as well as (+)-2′S-isorhodoptilometrin (148) exhibited moderate or good cytotoxicity against Hep-G2 and HeLa cancer cell lines showing IC50 values of [9.39 and 22.6 μM] for ω-hydroxypachybasin (149) and [2.10 and 8.59 μM] for (+)-2′S-isorhodoptilometrin (148), respectively, whilst only ω-hydroxypachybasin (149) exhibited cytotoxicity against the colon cancer cells, HCT-116 with an IC50 value of 29.8 μM.100 Also, compounds 148 and 149 revealed moderate DNA Topo I inhibitory activity with IC50 values of 100.0 and 50.0 μM, respectively, in addition to moderate selective antibacterial activity against the Gram-positive bacterium, S. aureus showing MIC value of 25.0 μM for both compounds.100
Moreover, another study demonstrated that compound 148 isolated from the marine-derived fungus T. aureoviride PSU-F95 showed good antibacterial activity against MRSA with a MIC value of 16.0 μg mL.54 Similarly, coniothranthraquinone 1 (150) displayed significant antibacterial activity against MRSA and S. aureus with MIC values of 8.0 and 16 μg mL−1, respectively.54 In the contrast, trichodermaquinone (151) which was also isolated from the marine fungus T. aureoviride PSU-F95 demonstrated very weak antibacterial activity against MRSA with a MIC value of 200.0 μg mL−1.54 However, compounds 152 and 153 which were recovered also from the marine fungus T. aureoviride PSU-F95, both were not evaluated for any bioactivity in this study.54
Additionally, coniothyrinone A (154) and lentisone (155) were previously isolated from another marine fungus, Trichoderma sp., and exhibited potent antibacterial activity against the Gram-negative bacteria, V. parahaemolyticus, V. anguillarum, and Pseudomonas putida with MIC values of [6.25, 1.56, 3.13 μM] for coniothyrinone A (154) and [12.5, 1.56, 6.25 μM] for lentisone (155), respectively60 (Fig. 9).
3.7. Anthraquinones from Eurotium sp.
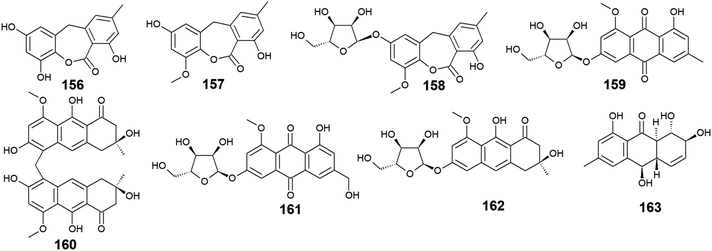
Seventeen anthraquinones and their derivatives were reported from species of the marine fungus Eurotium, including the previously mentioned compounds 14, 15, 18–20, 60, 62, 97, and 154 in addition to other eight congeners, 156–163. Compound 9-dehydroxyeurotinone (156) and its O-methyl derivative, 2-O-methyl-9-dehydroxyeurotinone (157) as well as its glycosidic derivative, 2-O-methyl-4-O-(α-D-ribofuranosyl)-9-dehydroxyeurotinone (158) were isolated from the marine-derived fungus Eu. rubrum.58,62 The parent compound, 9-dehydroxyeurotinone (156) exhibited weak antibacterial activity against the Gram-negative bacterium, E. coli showing a 7 mm zone of inhibition using 100.0 μg per disk. Also, it displayed selective cytotoxic activity against the human cholangiocarcinoma cells, SW1990 with an IC50 value of 25.0 μg mL−1.58 Another study revealed that compounds 157–159 had positive antioxidant activity through free radical scavenging activity against DPPH.62Furthermore, the same study showed that eurorubrin (160) demonstrated a potent free radical scavenging activity with an IC50 value of 44.0 μM with better antioxidant activity than the standard antioxidant, butylated hydroxytoluene which had an IC50 value of 82.6 μM.62 Interestingly, 3-O-(α-D-ribofuranosyl)-questin (159) and eurorubrin (160) were re-isolated also from the marine endophytic fungus Eu. cristatum EN 220. They displayed modest antibacterial activity against the Gram-negative bacterium, E. coli with MIC values of 32.0 and 64.0 μg mL−1, respectively.101 Notably, 3-O-(α-D-ribofuranosyl)questinol (161) which is an alcoholic derivative of the bioactive compound, 3-O-(α-D-ribofuranosyl)questin (159) showed no antibacterial activity against E. coli suggesting that this hydroxylation leads to loss of the antimicrobial activity.101
Furthermore, asperflavin ribofuranoside (162) which was isolated earlier from the marine fungus Eu. cristatum EN 220 ref. 101 and the marine-derived fungus Microsporum sp.,102 was reported as a potent free radical scavenging agent with an IC50 value of 14.2 μM with better antioxidant activity than the standard antioxidant, ascorbic acid which had an IC50 value of 20.0 μM.102 Also, it exhibited modest antibacterial activity against both MRSA and the multidrug-resistant S. aureus with MIC values of 50.0 and 50.0 μg mL−1, respectively.102 Moreover, rubrumol (163) was reported as a new anthraquinone derivative from the saline-alkali endophytic fungus Eu. rubrum with relaxation activity on Topo I with an IC50 value of 23.0 μM103 (Fig. 10).
3.8. Anthraquinones from Fusarium sp.
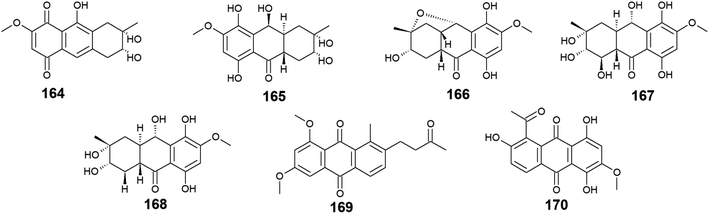
Twelve anthraquinone derivatives were isolated earlier from different species of the marine-derived fungus Fusarium sp. including the previously discussed compounds 5–8 and 10 along with other structurally related compounds 164–170. Although both nigrosporin A (164) and fusaranthraquinone (165) were recovered from the marine-derived fungus Fusarium sp. PSU-F14 ,39 only nigrosporin A (164) displayed promising inhibitory activity against photosynthesis and weak antibacterial activity against B. subtilis showing an inhibition zone of 14 mm at 200 ppm,44 whereas fusaranthraquinone (165) did not demonstrate any antibacterial activity when it was tested against both S. aureus and MRSA.39 Interestingly, additional bioactive fusaquinons A–C (166–168) were reported from the marine fungus Fusarium sp. ZH-210 and displayed weak cytotoxic activity against MCF-7, KB, and KBv200 cell lines with IC50 values of more than 50.0 μM.104It is noteworthy that nigrosporin A (164) and fusaquinon A (166) were also evaluated in another study for their antimalarial, anti-mycobacterial, antibacterial, and cytotoxic activity. Both compounds showed no antimalarial, antibacterial, or anti-mycobacterial activity, whereas they showed selective cytotoxicity.105 Nigrosporin A (164) displayed weak cytotoxic activity against the MCF-7 cell line with an IC50 value of 110.36 μM and good cytotoxicity against the NCI–H187 cell line with an IC50 value of 13.69 μM, while fusaquinon A (166) exhibited weak cytotoxicity against both human cancer cells, MCF-7, and monkey kidney cells, Vero cells with IC50 values of 84.38 and 44.46 μM, respectively. Also, fusaquinon A (166) displayed potent cytotoxicity against the NCI–H187 cell line with an IC50 value of 7.32 μM.105 Another bioactive anthraquinone derivative isolated from the mangrove-derived fungus Fusarium sp. ZZF60 was 6,8-dimethoxy-1-methyl-2-(3-oxobutyl)anthracene-9,10-dione (169).106 Notably, it demonstrated moderate cytotoxicity against Hep2 and Hep-G2 cells with IC50 values of 16.00 and 23.00, respectively (Fig. 11).
3.9. Anthraquinones from Engyodontium album
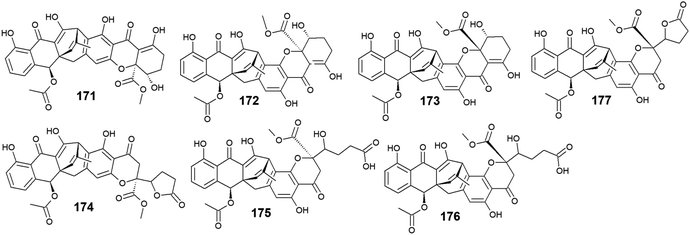
Six compounds 171–176 out of seven anthraquinone derivatives 171–177 isolated from the marine-derived fungus Engyodontium album LF069 were bioactive, while the anthraquinone derivative, Engyodontochone D (177) was not tested for any relevant biological activity.23 It is noteworthy that compounds 171–173 exhibited diverse bioactivities including antibacterial, antifungal, and cytotoxic activity. They demonstrated better antibacterial activity against S. epidermidis and MRSA than chloramphenicol with an IC50 values of [0.19 and 0.17 μM] for engyodontochone A (171), [0.21 and 0.25 μM] for JBIR-99 (172), and [0.22 and 0.24 μM] for engyodontochone B (173), respectively.23 On the other hand, they displayed weak to modest antifungal activity against the fungi, C. albicans, and T. rubrum with IC50 values ranging from 4.3 to 13.5 μM. Additionally, compounds 171–173 exhibited moderate cytotoxicity against the mouse fibroblasts cell line, NIH-3T3 with IC50 values of 11.0, 13.2, and 14.4 μM, respectively.23In addition, engyodontochone C (174) in the same study showed a good selective bioactivity against S. epidermidis and MRSA with IC50 values of 1.80 and 2.39 μM, respectively. In addition, it displayed weak cytotoxic activity against the cell line, NIH-3T3 with an IC50 value of 34.3 μM, whereas it did not show any antifungal activity against either, C. albicans or T. rubrum up to a concentration of 100.0 μM.23 Similarly, engyodontochone F (175) demonstrated promising selective antibacterial activity against both S. epidermidis and MRSA with IC50 values of 3.41 and 3.13 μM, respectively although it exhibited weak selective antifungal activity against T. rubrum with an IC50 value of 73.4 μM. In the contrast, engyodontochone E (176) has only showed potent antibacterial activity against S. epidermidis and MRSA with IC50 values of 6.77 and 6.74 μM, respectively with no antifungal or cytotoxic activity up to a concentration of 100.0 and 50.0 μM, respectively23 (Fig. 12).
3.10. Anthraquinones from Sporendonema casei
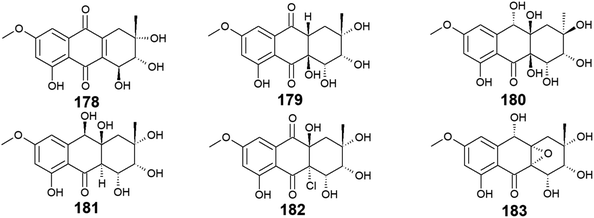
Seven bioactive anthraquinones named 4-dehydroxyaltersolanol A (178) and auxarthrols D–H (179–183) along with the previously discussed altersolanol B (101) were recovered from the marine fungus, Sporendonema casei HDN16-802.107 This group of anthraquinone derivatives 178–183 were evaluated for their antibacterial activity against M. phlei, B. subtilis, V. parahaemolyticus, E. coli, Pseudomonas aeruginosa, and Proteus sp. and for their antifungal activity against C. albicans. Interestingly, 4-dehydroxyaltersolanol A (178) exhibited the best antibacterial activity among this group of anthraquinones against M. phlei, B. subtilis, Pseudomonas aeruginosa, V. parahaemolyticus, and Proteus sp. with MIC values ranging from 25.0 to 50.0 μM.107 However, its parent altersolanol A (99) demonstrated potent antibacterial activity against S. aureus, E. coli, B. subtilis, and Micrococcus tetragenus with MIC values of 2.07, 4.1, 4.1, and 8.2 μM,90 suggesting that its dehydroxylation might lead to a decrease in its antimicrobial activity.In the contrast, auxarthrol E (180) and H (183) showed no antimicrobial activity against different indicator strains. However, auxarthrol F (181) only displayed very weak activity against M. phlei, B. subtilis, Pseudomonas aeruginosa, and Proteus sp. with a MIC value of 200.0 μM. Both auxarthrol D (179) and G (182) demonstrated a broad spectrum of antibacterial activity against M. phlei, B. subtilis, Pseudomonas aeruginosa, V. parahaemolyticus, and Proteus sp. with MIC values ranging from 25.0 to 100.0 μM, whereas compound 182 displayed very weak antifungal activity against C. albicans with a MIC value of 200.0 μM.107
Moreover, only compounds 179 and 181 were evaluated for their cytotoxicity against different cancer cell lines in the same study revealing modest cytotoxic activity against several cell lines. Compound 179 exhibited a selective cytotoxic effect on seven cell lines including HL-60, HCT-116, MGC-803, MDA-MB-231, SH-SY5Y, PC-3, and BEL-7402 with IC50 values ranging from 7.5 to 22.9 μM. In the contrast, compound 181 displayed a broad spectrum of cytotoxicity against the eleven tested cancer cell lines in this study with IC50 values ranging from 4.5 to 22.2 μM.107 In addition, all compounds 178–183 showed significant anticoagulant activity, meanwhile, they did not show any anti-mycobacterial activity107 (Fig. 13).
3.11. Anthraquinones from other marine fungi
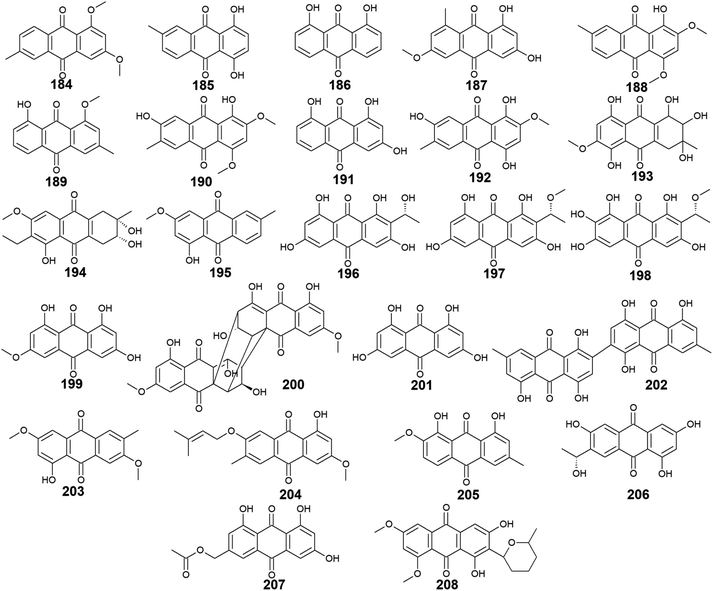
A considerable number of anthraquinones and their derivatives were isolated from other marine-derived fungi including compounds 184–208. Compounds 184–192, as well as previously discussed anthraquinone derivatives, 5, 41, 83, and 147, were reported from the mangrove endophytes, Halorosellinia sp. No. 1403 and Guignardia sp. No. 4382.40 Eight compounds from them, 184–191 showed weak cytotoxic activity, while 192 displayed no cytotoxicity up to a concentration of 500.0 μM.40 It is noteworthy that compounds 184–188 exhibited weak cytotoxicity against both tested cancer cell lines, KB and KBv200 with IC50 values ranging from 34.64 to 243.69 μM, whereas compounds 189–191 demonstrated a narrow spectrum of activity against only KBv200 cell line with IC50 values of 72.60, 185.68, and 301.47 μM, respectively. The best cytotoxicity was recorded for 1,3-dihydroxy-6-methoxy-8-methyl-anthracene-9,10-dione (187) which displayed activity against both KB and KBv200 cells lines with IC50 values of 38.05 and 34.64 μM, respectively.40Interestingly, SZ-685C (193) was isolated as a novel anthraquinone derivative from the marine endophytic fungus Halorosellinia sp. No. 1403 with anticancer potential.108–110 It was demonstrated that SZ-685C (193) had anticancer activity against the rat pituitary adenoma (MMQ) and human non-functioning pituitary adenoma cell lines with IC50 values of 14.51 and 18.76 μM, respectively, while it had an IC50 value of 56.09 μM against the normal cell line, rat pituitary cells.108 Another study revealed similar results of its cytotoxic activity against the MMQ and normal rat pituitary cell lines with IC50 values of 13.2 and 49.1 μM, respectively.110 Also, it showed good cytotoxicity against both human MCF-7 and MCF-7/ADR cancer cell lines with IC50 values of 7.38 and 4.17 μM, respectively.109
Additional anthraquinone derivatives, phomopsanthraquinone (194), and 1-hydroxy-3-methoxy-6-methyl-anthraquinone (195) were isolated from the marine-derived fungus, Phomopsis sp. PSU-MA214, besides the previously mentioned compounds 102, 107, 108, and 136.111 Phomopsanthraquinone (194) demonstrated cytotoxicity against MCF-7 and KB cancer cell lines with an IC50 value of 27.0 μg mL−1 for both cell lines. Also, it exhibited moderate antibacterial activity against both MRSA and S. aureus with MIC values of 64.0 and 128.0 μg mL−1, respectively. In the contrast, 1-hydroxy-3-methoxy-6-methyl-anthraquinone (195) neither showed antibacterial activity nor cytotoxicity.111
Further three anthraquinones, tetrahydroxyanthraquinone (196), methoxy-tetrahydroxyanthraquinone (197), and 1,2,3,6,8-pentahydroxy-7-[(1 R)-1-methoxyethyl]-9,10-anthraquinone (198) along with previously mentioned noraverufanin (36), were recorded from the sponge-associated fungus Microsphaeropsis sp.112 All those anthraquinones showed a broad spectrum of protein kinases' inhibitory activity against cyclin-dependent kinase 4 in complex with its activator cyclin D1, protein kinase C, and epidermal growth factor receptor with IC50 values ranging from 18.5 to 54.0 μM.112
Moreover, the anthraquinone, lunatin (199), and the anthraquinone dimer, cytoskyrin A (200) were reported earlier from the sponge-associated fungus Curvularia lunata with positive antibacterial activity.113 Both compounds exhibited antibacterial activity against B. subtilis, S. aureus, and E. coli using the disk diffusion method at a concentration of 5.0 μg per disk. Meanwhile, they showed no antifungal activity against C. albicans up to a concentration of 10.0 μg per disk.113
Furthermore, rheoemodin (201), 2, 2′-bis-(7-methyl-1,4,5-trihydroxy-anthracene-9,10-dione) (202), as well as the previously discussed compounds 62, 63, and 84, were isolated earlier from another sponge-associated fungus Talaromyces stipitatus KUFA 0207.82 Rheoemodin (201) displayed no significant anti-obesity activity, whereas 2, 2′-bis-(7-methyl-1,4,5-trihydroxy-anthracene-9,10-dione) (202) was not tested for any relevant activity.82
Additional two anthraquinones, 7-methoxymacrosporin (203) and 7-(γ,γ)-dimethyl-allyloxy-macrosporin (204) along with the previously discussed compounds 102, 105, 107 and 108, were isolated from the mangrove fungus, Phoma sp. L28.91 7-methoxymacrosporin (203) displayed weak antifungal activity against F. graminearum, F. oxysporum, P. italicum, Rhizoctonia solani, and Colletotrichum gloeosporioides with MIC values of 100.0, 100.0, 100.0, 150.0, and 200.0 μg mL−1, respectively. Also, 7-(γ,γ)-dimethyl-allyloxy-macrosporin (204) demonstrated weak selective antifungal activity against F. graminearum, Rhizoctonia solani, and Colletotrichum gloeosporioides with MIC values of 80.0, 150.0, and 200.0 μg mL−1, respectively.91 By comparing this weak antifungal activity of 203 and 204 to their parent macrosporin (108) which displayed potent antifungal activity against F. oxysporum and modest antifungal activity against Colletotrichum musae, F. graminearum, P. italicum, and Colletotrichum gloeosporioides,91 we can conclude that the structural modifications in both 203 and 204 have greatly affected their bioactivity.
Four additional bioactive anthraquinone derivatives were reported from the marine-derived fungus Monodictys sp. including the previously discussed compounds 14, 83, and 147 as well as monodictyquinone A (205). Compound 205 displayed promising antimicrobial activity against B. subtilis, E. coli, and C. albicans showing zones of inhibition with a diameter of 15.0, 15.0, and 11.0 mm, respectively at a concentration of 10.0 μg per disk.55
Two other anthraquinone derivatives, 1,3,6-trihydroxy-7-(1-hydroxyethyl) anthracene-9,10-dione (206) and phaseolorin I (207) were isolated earlier from the marine-derived fungi, Cladosporium sp. HNWSW-1 ref. 114 and Diaporthe phaseolorum FS431,115 respectively. Phaseolorin I (207) was inactive when it was tested for its cytotoxicity against the cell lines, MCF-7, Hep-G2, A549, and SF-268,115 whereas compound 206 did not demonstrate cytotoxicity against the cell lines, BEL-7042, HeLa, and K562 as well as the human papillomavirus-related endocervical adenocarcinoma SGC-7901 cell lines.114 However, anthraquinone 206 exhibited α-glycosidase inhibitory activity with an IC50 value of 49.3 μM compared to the standard agent, acarbose which had an IC50 value of 275.7 μM.114
Finally, 6,8-O,O′-dimethyl-averufanin (208) which is a derivative of the bioactive anthraquinone derivative, averufanin (35) was previously reported from the unidentified marine endophytic fungus ZSUH-36 as well as the previously mentioned compounds 27, 30, 32 and 33, 40, 43, and 80.116 Compound 208 demonstrated weak antifungal activity against the phytopathogenic fungi, Botrytis cinerea and Magnaporthe oryzae with MIC values of 50.0 and 100.0 μM, respectively.117 Also, it displayed good phytotoxicity on the hypocotyls of radish seedlings at a concentration of 100.0 μM with an inhibition rate of 30.6% compared to 28.1% for the standard, glyphosate117 (Fig. 14).
4 Drug likeness and pharmacokinetics of marine anthraquinones
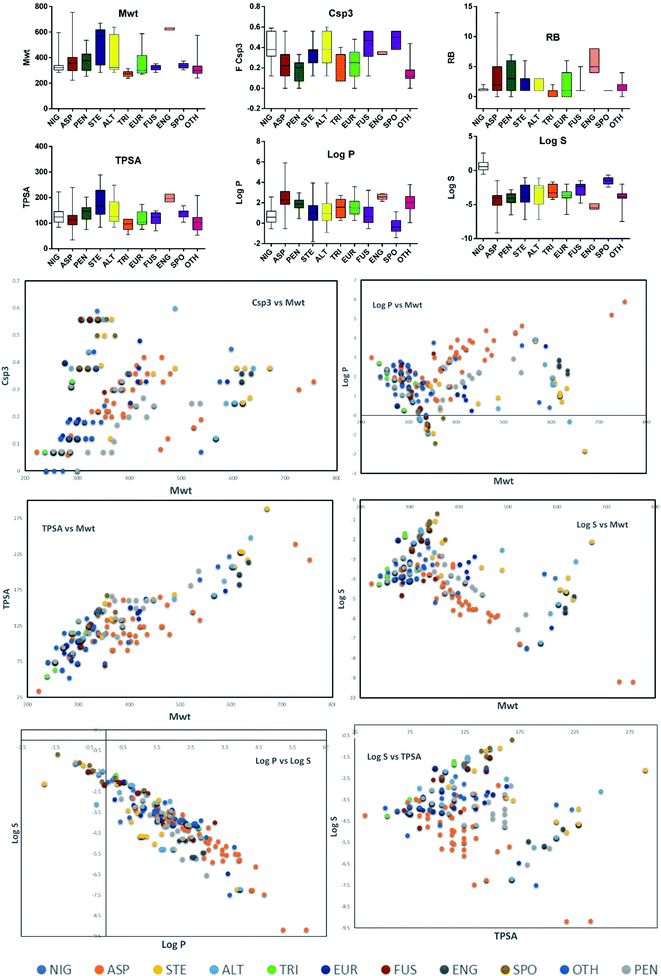
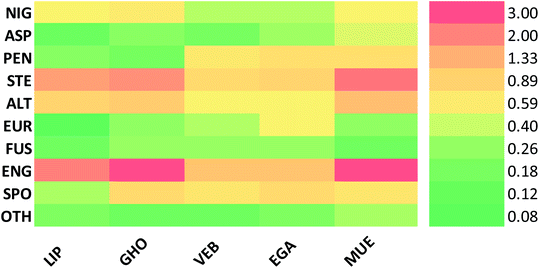
Altogether, 208 anthraquinones and their derivatives were characterized from 20 marine-derived fungal genera. These include Nigrospora, Aspergillus, Penicillium, Stemphylium, and Alternaria, among others. The identified anthraquinones revealed diverse biological and pharmacological activities including anticancer, antiviral, antimicrobial, antioxidant, and anti-inflammatory activities. Here, we attempted to highlight their potential as drug candidates via exploring their drug-likeness using several molecular descriptors including several drug-likeness rules (Muegge, Ghose, Veber, Egan, and Lipinski). Surprisingly, 133 anthraquinones satisfied all parameters of the 5 tested drug-likeness rules (7, 48, 7, 4, 10, 16, 10, 9, 2, and 20 anthraquinones from Nigrospora, Aspergillus, Penicillium, Stemphylium, Alternaria, Trichoderma, Eurotium, Fusarium, Sporendonema casei, and the other genera, respectively). Noteworthy, all anthraquinones identified from Trichoderma species fulfilled the 5 rules. On the other hand, all Engyodontium album derived compounds violated the 5 tested rules (Fig. 15, 16, and S† Table 1).| Compound | MF | Name | Bioactivity | Source | Ref. |
|---|---|---|---|---|---|
| 1 | C31H32O12 | Nigrodiquinone A | Displayed no antibacterial or antiviral activity | Zoanthid-derived fungus Nigrospora sp. | 37 |
| 2 | C17H22O7 | 4a-epi-9-methoxydihydrodeoxybostrycin | Antibacterial activity | Zoanthid-derived fungus Nigrospora sp. and sea anemone-derived fungus Nigrospora sp. | 37 and 38 |
| 3 | C16H16O7 | 10-Deoxybostrycin | Antibacterial and cytotoxic activities | Zoanthid-derived fungus Nigrospora sp. and sea anemone-derived fungus Nigrospora sp. | 37 and 38 |
| 4 | C16H12O6 | 3,5,8-Trihydroxy-7-methoxy-2-methyl-anthracene-9,10-dione | Antiviral activity | Zoanthid-derived fungus Nigrospora sp. and sea anemone-derived fungus Nigrospora sp. | 37 and 38 |
| 5 | C16H12O5 | Austrocortirubin | Antiviral and cytotoxic activities | Zoanthid-derived fungus Nigrospora sp., mangrove endophytic fungi Halorosellinia sp. (no. 1403), and Guignardia sp. (no. 4382), sea anemone-derived fungus Nigrospora sp., and sea fan-derived fungi Fusarium sp. PSU-F14 | 37–41 |
| 6 | C16H16O6 | Nigrosporin B | Antibacterial, anti-mycobacterial, cytotoxic, and phytotoxic activities | Sea anemone-derived fungus Nigrospora sp. and sea fan-derived fungi Fusarium sp. PSU-F14 | 38, 39, 43 and 44 |
| 7 | C16H20O7 | 1-Deoxytetrahydrobostrycin | Antibacterial and cytotoxic activities | Sea anemone-derived fungus Nigrospora sp., sea fan-derived fungi Fusarium sp. PSU-F14 and marine-derived fungus Aspergillus sp. | 38, 39, 42 |
| 8 | C16H20O8 | Tetrahydrobostrycin | Antibacterial, antimalarial, anti-mycobacterial, and cytotoxic activities | Sea anemone-derived fungus Nigrospora sp., sea fan-derived fungi Fusarium sp. PSU-F14, and marine-derived fungus Aspergillus sp. | 38, 39, 42 and 46 |
| 9 | C16H16O7 | 4-Deoxybostrycin | Antibacterial, anti-mycobacterial, and cytotoxic activities | Sea anemone-derived fungus Nigrospora sp. | 38, 43 and 45 |
| 10 | C16H16O8 | Bostrycin | Antibacterial, antimalarial, and cytotoxic activities | Sea anemone-derived fungus Nigrospora sp., sea fan-derived fungi Fusarium sp. PSU-F14, and marine-derived fungus Aspergillus sp. | 38, 39, 42 and 45 |
| 11 | C25H16O9 | Aspergiolide A | Cytotoxic activity | Marine-derived fungus A. glaucus | 47 and 48 |
| 12 | C26H18O9 | Aspergiolide B | Cytotoxic activity | Marine-derived fungus A. glaucus | 47 |
| 13 | C16H12O5 | Physcion | Antifungal, antioxidant, and cytotoxic activities | Marine-derived fungi Microsporum sp., A. glaucus, and halotolerant A. variecolor, and marine algae-derived fungus A. wentii EN-48 | 47 and 49–52 |
| 14 | C15H10O5 | Emodin | Antibacterial, antifungal, anti-HCV protease, anti-mycobacterial, and cytotoxic activities | Sea fan-derived fungus P. citrinum PSU-F51, marine-derived fungi T. aureoviride PSU-F95, Trichoderma sp., A. glaucus, and halotolerant A. variecolor, marine lichen-derived fungus Gliocladium sp. T31, sea urchin-derived fungus Monodictys sp., marine mangrove fungus Paecilomyces sp., and marine-derived endophytic fungus Eu. rubrum | 40, 47, 50, 53–61 and 63 |
| 15 | C16H16O5 | Asperflavin | Antioxidant activity | Marine-derived fungus A. glaucus and marine algae-derived endophytic fungus Eu. Cristatum EN-220 | 47, 62 and 101 |
| 16 | C16H16O5 | Isoasperflavin | Displayed no cytotoxic activity | Marine-derived fungus A. glaucus | 47 |
| 17 | C16H12O5 | Questin | Antioxidant activity | Marine-derived fungi A. glaucus and halotolerant A. variecolor, and mangrove-derived fungus P. citrinum HL-5126 | 47, 50, 62 and 84 |
| 18 | C15H10O6 | Catenarin | Antibacterial activity | Marine-derived fungi A. glaucus, Eu. Rubrum, and halotolerant A. variecolor | 47, 50, 63 and 103 |
| 19 | C16H12O6 | Rubrocristin | Displayed no antibacterial activity | Marine-derived fungi A. glaucus, Eu. Rubrum, and halotolerant A. variecolor | 47, 50, 63 and 103 |
| 20 | C20H18O10 | (+)-variecolorquinone A | Cytotoxic activity | Marine-derived fungi A. glaucus and halotolerant A. variecolor, and marine algae-derived endophytic fungus Eu. cristatum EN-220 | 47, 50 and 101 |
| 21 | C32H26O8 | Physcion-10,10′-bianthrone | Was not evaluated for any relevant bioactivity | Marine-derived fungus A. glaucus | 47 |
| 22 | C31H24O8 | (trans)-emodin-physcion bianthrone | Cytotoxic activity | Marine-derived fungus A. glaucus | 47 |
| 23 | C31H24O8 | (cis)-emodin-physcion bianthrone | Cytotoxic activity | Marine-derived fungus A. glaucus | 47 |
| 24 | C42H42O13 | 6,6′-oxybis(1,3,8-trihydroxy-2-((S)-1-methoxyhexyl)anthracene-9,10-dione) | Antibacterial and cytotoxic activities | Marine-derived fungus A. versicolor | 64 |
| 25 | C40H38O13 | 6,6′-oxybis(1,3,8-trihydroxy-2-((S)-1-hydroxyhexyl) anthracene-9,10-dione | Antibacterial activity | Marine-derived fungus A. versicolor | 64 |
| 26 | C20H20O7 | Averantin | Antibacterial, antioxidant, and cytotoxic activities | Marine-derived fungi A. versicolor and P. purpurogenum G59 | 64, 65, 67, 68 and 118 |
| 27 | C21H22O7 | 1′-O-methyl-averantin | Antibacterial, antioxidant, and cytotoxic activities | Marine-derived fungi A. versicolor and P. purpurogenum G59, and the mangrove endophytic fungus (ZSUH-36) | 64, 65, 67, 68 and 116 |
| 28 | C20H18O6 | Averythrin | Antioxidant and cytotoxic activities | Marine-derived fungi A. versicolor and Aspergillus sp. SCSIO F063 | 64, 66 and 67 |
| 29 | C22H24O7 | 6,8-O,O′-dimethyl-averantin | Antibacterial activity | Marine-derived fungus A. versicolor EN-7 | 69 |
| 30 | C20H16O7 | Averufin | Antibacterial, antioxidant, antiviral, and cytotoxic activities | Marine-derived fungi A. versicolor and A. niger (MF-16), mangrove endophytic fungi ZSUH-36 and (isolate 1850), and mangrove-derived endophytic fungus A. nidulans MA-143 | 67, 68, 70, 71, 116 and 119 |
| 31 | C21H18O7 | 6-O-methyl-averufin | Displayed no antimicrobial activity | Marine-derived fungus A. versicolor EN-7 | 69 |
| 32 | C22H20O7 | 6,8-O,O′-dimethyl-averufin | Displayed no anti-neuroinflammatory activity | Marine-derived fungi Aspergillus sp. SF-6796 and A. versicolor EN-7, and the mangrove endophytic fungus (ZSUH-36) | 69, 72 and 116 |
| 33 | C18H12O7 | Versicolorin B | Antioxidant activity | Marine-derived fungus A. versicolor and mangrove endophytic fungus ZSUH-36 | 67 |
| 34 | C18H12O8 | 1′-Hydroxyversicolorin B | Antioxidant and cytotoxic activities | Marine-derived fungus A. versicolor | 67 and 74 |
| 35 | C20H18O7 | Averufanin | Antioxidant activity and inhibitory activity of acyl-CoA: Cholesterol acyltransferase | Marine-derived fungus A. versicolor and mangrove-derived endophytic fungus A. nidulans MA-143 | 67 and 75 |
| 36 | C19H16O7 | Noraverufanin | Anti-HIV activity | Sponge-associated fungi Microsphaeropsis sp. and A. versicolor SCSIO 41016 | 73 and 112 |
| 37 | C20H16O8 | Nidurufin | Antibacterial, antioxidant, antiviral, and cytotoxic activities | Marine-derived fungi A. versicolor, A. niger (MF-16), and P. purpurogenum G59, and marine-derived mangrove endophytic fungus (isolate 1850) | 65, 67, 68, 70 and 119 |
| 38 | C22H20O8 | 6,8-O,O′-dimethyl-nidurufin | Antibacterial activity | Marine-derived fungus A. versicolor EN-7 | 69 |
| 39 | C18H16O8 | Versiconol | Cytotoxic activity | Marine-derived fungus A. versicolor | 68 |
| 40 | C20H20O8 | 6,8-O,O′-dimethyl-versiconol | Antibacterial activity | Mangrove endophytic fungus (ZSUH-36) and marine-derived fungus A. versicolor EN-7 | 69 and 120 |
| 41 | C16H12O5 | 1-Methyl-emodin | Anti-HCV protease and cytotoxic activities | Mangrove endophytic fungi Halorosellinia sp. (no. 1403) and Guignardia sp. (no. 4382), and red sea endophytic fungus A. versicolor | 40 and 76 |
| 42 | C18H16O6 | Isorhodoptilometrin-1-methyl-ether | Antibacterial and cytotoxic activities | Red sea endophytic fungus A. versicolor | 76 |
| 43 | C20H16O7 | Aversin | Displayed no antimicrobial activity | Mangrove endophytic fungus (ZSUH-36) and marine-derived fungus A. versicolor EN-7 | 69 and 120 |
| 44 | C20H14O7 | 6,8-O,O′-dimethyl-versicolorin A | Displayed no antimicrobial activity | Marine-derived fungus A. versicolor EN-7 | 69 |
| 45 | C16H12O6 | Evariquinone | Was not evaluated for any relevant bioactivity | Red sea endophytic fungus A. versicolor | 76 |
| 46 | C17H14O6 | 7-Hydroxyemodin 6,8-methyl-ether | Was not evaluated for any relevant bioactivity | Red sea endophytic fungus A. versicolor | 76 |
| 47 | C15H10O3 | 1-Hydroxy-2-methyl-anthraquinone | Anti-mosquito activity | Marine-derived fungus A. versicolor | 59 and 77 |
| 48 | C17H14O5 | 2-(Dimethoxy methyl)-1-hydroxy-9,10-anthraquinone | Antibacterial activity | Marine-derived fungus A. versicolor | 59 |
| 49 | C15H10O2 | Tectoquinone | Was not evaluated for any relevant bioactivity | Marine-derived fungus A. versicolor | 59 |
| 50 | C16H10O5 | Damnacanthal | Antibacterial and anti-mosquito activities | Marine-derived fungus A. versicolor | 59 and 77 |
| 51 | C14H8O4 | Xanthopurpurin | Antibacterial and anti-platelets aggregation activities | Marine-derived fungus A. versicolor | 59 and 78 |
| 52 | C15H10O4 | Rubiadin | Inhibitory activity on formation of advanced glycation end products | Marine-derived fungus A. versicolor | 59 and 79 |
| 53 | C15H10O5 | 6-Hydroxyrubiadin | Inhibitory effects on the release of β-hexosaminidase and inhibitory activity on phosphatase of regenerating liver-3 | Marine-derived fungus A. versicolor | 59 and 80 |
| 54 | C16H12O5 | Rubianthraquinone | Anti-inflammatory activity | Marine-derived fungus A. versicolor | 59 and 121 |
| 55 | C16H12O7 | Wentiquinone C | Displayed no antioxidant activity | Marine algae-derived fungus A. wentii EN-48 | 49 |
| 56 | C15H10O5 | Alatinone | Was not evaluated for any relevant bioactivity | Marine-derived endophytic fungus A. wentii pt-1 | 81 |
| 57 | C17H14O5 | 5-Hydroxy-1,3-dimethoxy-7-methyl-anthraquinone | Was not evaluated for any relevant bioactivity | Marine-derived endophytic fungus A. wentii pt-1 | 81 |
| 58 | C16H12O5 | 1,5-Dihydroxy-3-methoxy-7-methyl-anthraquinone | Was not evaluated for any relevant bioactivity | Marine-derived endophytic fungus A. wentii pt-1 | 81 |
| 59 | C15H12O6 | Eurotinone | Antioxidant activity and kinase insert domain receptor inhibitory activity | Marine-derived halotolerant fungus A. variecolor | 50 and 122 |
| 60 | C16H14O6 | 2-O-methyl-eurotinone | Antioxidant activity | Marine mangrove-derived endophytic fungus Eu. rubrum and marine-derived halotolerant fungus A. variecolor | 50 and 62 |
| 61 | C19H16O9 | (2S)-2,3-dihydroxypropyl 1,6,8-trihydroxy-3-methyl-9,10-dioxo-9,10-dihydro-2-anthracenecarboxylate | Was not evaluated for any relevant bioactivity | Marine-derived halotolerant fungus A. variecolor | 50 |
| 62 | C16H12O6 | Questinol | Anti-inflammatory and anti-obesity activities | Marine-derived halotolerant fungus A. variecolor and marine-derived fungi Eu. amstelodami and Talaromyces stipitatus KUFA 0207 | 50, 82 and 83 |
| 63 | C16H12O6 | Fallacinol | Displayed no significant anti-obesity activity | Marine-derived halotolerant fungus A. variecolor and marine algae-derived fungus Talaromyces stipitatus KUFA 0207 | 50 and 82 |
| 64 | C16H12O6 | Erythroglaucin | Displayed no antibacterial activity | Marine-derived halotolerant fungus A. variecolor | 50 and 63 |
| 65 | C18H12O7 | Versicolorin C | Antibacterial activity | Marine-derived mangrove endophytic fungus (isolate 1850) and mangrove-derived endophytic fungus A. nidulans MA-143 | 71 and 119 |
| 66 | C18H12O7 | Isoversicolorin C | Antibacterial activity | Mangrove-derived endophytic fungus A. nidulans MA-143 | 71 |
| 67 | C20H18O7 | Norsolorinic acid | Was not evaluated for any relevant bioactivity | Mangrove-derived endophytic fungus A. nidulans MA-143 | 71 |
| 68 | C21H22O7 | (1′S) 6-O-methyl-averantin | Displayed no cytotoxic activity | Marine-derived fungus Aspergillus sp. SCSIO F063 | 66 |
| 69 | C22H24O7 | (1′S) 6,1′-O,O′-dimethyl-averantin | Displayed no cytotoxic activity | Marine-derived fungus Aspergillus sp. SCSIO F063 | 66 |
| 70 | C24H28O7 | Averantin-1′-butyl ether | Cytotoxic activity | Marine-derived fungus Aspergillus sp. SCSIO F063 | 66 |
| 71 | C20H19ClO7 | (1′S)-7-chloroaverantin | Cytotoxic activity | Marine-derived fungus Aspergillus sp. SCSIO F063 | 66 |
| 72 | C21H21ClO7 | (1′S) 6-O-methyl-7-chloroaverantin | Cytotoxic activity | Marine-derived fungus Aspergillus sp. SCSIO F063 | 66 |
| 73 | C21H21ClO7 | (1′S) 1′-O-methyl-7-chloroaverantin | Cytotoxic activity | Marine-derived fungus Aspergillus sp. SCSIO F063 | 66 |
| 74 | C22H23ClO7 | (1′S) 6,1′-O,O′-dimethyl-7-chloroaverantin | Displayed no cytotoxic activity | Marine-derived fungus Aspergillus sp. SCSIO F063 | 66 |
| 75 | C24H27ClO7 | (1′S) 7-chloroaverantin-1′-butyl ether | Cytotoxic activity | Marine-derived fungus Aspergillus sp. SCSIO F063 | 66 |
| 76 | C20H17ClO6 | 7-Chloroaverythrin | Displayed no cytotoxic activity | Marine-derived fungus Aspergillus sp. SCSIO F063 | 66 |
| 77 | C21H19ClO6 | 6-O-methyl-7-chloroaverythrin | Cytotoxic activity | Marine-derived fungus Aspergillus sp. SCSIO F063 | 66 |
| 78 | C21H21ClO6 | (1′S) 6-O-methyl-7-bromoaverantin | Cytotoxic activity | Marine-derived fungus Aspergillus sp. SCSIO F063 | 66 |
| 79 | C22H23BrO7 | (1′S) 6,1′-O,O′-dimethyl-7-bromoaverantin | Displayed no cytotoxic activity | Marine-derived fungus Aspergillus sp. SCSIO F063 | 66 |
| 80 | C23H26O7 | 6,8,1′-O,O′,O′′-trimethyl-averantin | Anti-inflammatory activity | Marine-derived fungus Aspergillus sp. SF-6796 and mangrove endophytic fungus ZSUH-36 | 72 and 116 |
| 81 | C28H24O10 | Penicillanthranin A | Antibacterial and cytotoxic activities | Sea fan-derived fungus P. citrinum PSU-F51 | 53 |
| 82 | C28H24O11 | Penicillanthranin B | Displayed no cytotoxic activity | Sea fan-derived fungus P. citrinum PSU-F51 | 53 |
| 83 | C15H10O4 | Chrysophanol | Anti-acetylcholinesterase, antibacterial, and cytotoxic activities | Sea fan-derived fungus P. citrinum PSU-F51, marine-derived fungi T. aureoviride PSU-F95 and Trichoderma sp., mangrove endophytic fungi Halorosellinia sp. (no. 1403) and Guignardia sp. (no. 4382), sea urchin-derived fungus Monodictys sp., and marine mangrove fungus Paecilomyces sp. | 40, 53–55, 57 and 100 |
| 84 | C15H10O6 | ω-hydroxyemodin | Antibacterial, anti-mycobacterial, anti-obesity, and cytotoxic activities | Sea fan-derived fungus P. citrinum PSU-F51, mangrove-derived fungus P. citrinum HL-5126, marine-derived fungi T. aureoviride PSU-F95, and Talaromyces stipitatus KUFA 0207, and marine lichen-derived fungus Gliocladium sp. T31 | 53, 54, 56, 61, 82 and 84 |
| 85 | C18H13ClO7 | 2′-Acetoxy-7-chlorocitreorosein | Antibacterial activity | Mangrove-derived fungus P. citrinum HL-5126 | 84 |
| 86 | C15H10O9S | Citreorosein-3-O-sulphate | Was not evaluated for any relevant bioactivity | Marine-derived fungus P. oxalicum 2HL-M-6 | 85 |
| 87 | C15H10O8S | Emodin-3-O-sulphate | Was not evaluated for any relevant bioactivity | Marine-derived fungus P. oxalicum 2HL-M-6 | 85 |
| 88 | C15H10O5 | Aloe-emodin | Antibacterial and antimalarial activities | Marine-derived fungus P. oxalicum 2HL-M-6 | 85–87 |
| 89 | C21H19NO8 | Emodacidamide A | Immunomodulatory activity | Marine-derived fungus Penicillium sp. SCSIO sof101 | 88 |
| 90 | C20H17NO8 | Emodacidamide B | Immunomodulatory activity | Marine-derived fungus Penicillium sp. SCSIO sof101 | 88 |
| 91 | C20H16ClNO8 | Emodacidamide C | Immunomodulatory activity | Marine-derived fungus Penicillium sp. SCSIO sof101 | 88 |
| 92 | C22H21NO8 | Emodacidamide D | Immunomodulatory activity | Marine-derived fungus Penicillium sp. SCSIO sof101 | 88 |
| 93 | C21H19NO8 | Emodacidamide E | Immunomodulatory activity | Marine-derived fungus Penicillium sp. SCSIO sof101 | 88 |
| 94 | C21H18ClNO8 | Emodacidamide F | Immunomodulatory activity | Marine-derived fungus Penicillium sp. SCSIO sof101 | 88 |
| 95 | C21H18ClNO8 | Emodacidamide G | Immunomodulatory activity | Marine-derived fungus Penicillium sp. SCSIO sof101 | 88 |
| 96 | C18H13NO8 | Emodacidamide H | Immunomodulatory activity | Marine-derived fungus Penicillium sp. SCSIO sof101 | 88 |
| 97 | C15H8O7 | Emodic acid | Inhibitory activity on tyrosine kinase proteins | Marine-derived endophytic fungus Eu. rubrum and marine-derived fungus Penicillium sp. SCSIO sof101 | 58, 88 and 89 |
| 98 | C15H9ClO6 | 2-Chloro-1,3,8 trihydroxy-6 (hydroxymethyl)-anthracene-9,10 dione | Immunomodulatory activity | Marine-derived fungus Penicillium sp. SCSIO sof101 | 88 |
| 99 | C16H16O8 | Altersolanol A | Antibacterial and cytotoxic activities, as well as protein kinase inhibitory activity | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 and coral-associated fungus Stemphylium lycopersici 231 and coral-associated fungus Stemphylium lycopersici |
90, 92 and 94 |
| 100 | C16H18O7 | Dihydroaltersolanol A | Displayed no antibacterial or cytotoxic activity | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231, deep-sea-derived fungus Alternaria tenuissima DFFSCS013, and soft coral-derived Alternaria sp. ZJ-2008003 231, deep-sea-derived fungus Alternaria tenuissima DFFSCS013, and soft coral-derived Alternaria sp. ZJ-2008003 |
90, 93, 99 |
| 101 | C16H16O6 | Altersolanol B | Antibacterial, anticoagulant, anti-mycobacterial, and cytotoxic activities | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231, mangrove endophytic fungus Alternaria sp. ZJ9-6B, coral-associated fungus Stemphylium lycopersici and Alternaria sp. ZJ-2008003, deep sea-derived fungus Alternaria tenuissima DFFSCS013 and marine-derived fungus Sporendonema casei HDN16-802 231, mangrove endophytic fungus Alternaria sp. ZJ9-6B, coral-associated fungus Stemphylium lycopersici and Alternaria sp. ZJ-2008003, deep sea-derived fungus Alternaria tenuissima DFFSCS013 and marine-derived fungus Sporendonema casei HDN16-802 |
90, 92, 93, 98, 99 and 107 |
| 102 | C16H20O6 | Tetrahydroaltersolanol B | Antibacterial and antifungal activities | Mangrove-derived fungi Phomopsis sp.PSU-MA214, Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231, and Phoma sp. L28, mangrove endophytic fungus Alternaria sp. ZJ9-6B, and soft coral-derived Alternaria sp. ZJ-2008003 231, and Phoma sp. L28, mangrove endophytic fungus Alternaria sp. ZJ9-6B, and soft coral-derived Alternaria sp. ZJ-2008003 |
90, 91, 93, 98 and 111 |
| 103 | C18H18O7 | 2-O-acetylaltersolanol B | Antibacterial activity | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 231 |
90 |
| 104 | C16H16O7 | Altersolanol C | Antibacterial activity | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 and soft coral-derived Alternaria sp. ZJ-2008003 231 and soft coral-derived Alternaria sp. ZJ-2008003 |
90 and 93 |
| 105 | C16H20O7 | Altersolanol L | Antifungal and cytotoxic activities | Mangrove-derived fungi Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 and Phoma sp. L28, and deep-sea derived fungus Alternaria tenuissima DFFSCS013 and soft coral-derived Alternaria sp. ZJ-2008003 231 and Phoma sp. L28, and deep-sea derived fungus Alternaria tenuissima DFFSCS013 and soft coral-derived Alternaria sp. ZJ-2008003 |
90, 91, 93, 99 and 123 |
| 106 | C18H22O8 | 2-O-acetylaltersolanol L | Displayed no antibacterial or cytotoxic activity | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 231 |
90 |
| 107 | C16H20O8 | Ampelanol | Cytotoxic activity | Mangrove-derived fungi Phomopsis sp.PSU-MA214, Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231, and Phoma sp. L28, coral-associated fungus Stemphylium lycopersici and Alternaria sp. ZJ-2008003 and, deep-sea derived fungus Alternaria tenuissima DFFSCS013 231, and Phoma sp. L28, coral-associated fungus Stemphylium lycopersici and Alternaria sp. ZJ-2008003 and, deep-sea derived fungus Alternaria tenuissima DFFSCS013 |
90–94, 99 and 111 |
| 108 | C16H12O5 | Macrosporin | Antibacterial, antifungal, and cytotoxic activities as well as protein kinases' inhibitory activity | Mangrove-derived fungi Phomopsis sp.PSU-MA214, Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231, Alternaria sp. ZJ9-6B and Phoma sp. L28 and coral-associated fungus Stemphylium lycopersici and Alternaria sp. ZJ-2008003 231, Alternaria sp. ZJ9-6B and Phoma sp. L28 and coral-associated fungus Stemphylium lycopersici and Alternaria sp. ZJ-2008003 |
90–94, 98, 111 and 123 |
| 109 | C16H12O8S | Macrosporin-7-O-sulphate | Cytotoxic activity | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 231 |
90 and 94 |
| 110 | C24H24O11 | Macrosporin 2-O-(6′-acetyl)-α-D-glucopyranoside | Brine shrimp lethality | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 231 |
90 |
| 111 | C16H16O9 | Auxarthrol C | Antibacterial activity | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 and coral-associated fungus Stemphylium lycopersici 231 and coral-associated fungus Stemphylium lycopersici |
90 and 92 |
| 112 | C32H26O13 | Alterporriol A | Displayed no antibacterial or cytotoxic activity | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 231 |
90 |
| 113 | C32H26O13 | Alterporriol B | Antibacterial activity | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 231 |
90 |
| 114 | C32H22O10 | Alterporriol C | Antibacterial and antiviral activities | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 and soft coral-derived Alternaria sp. ZJ-2008003 231 and soft coral-derived Alternaria sp. ZJ-2008003 |
90 and 93 |
| 115 | C32H30O16 | Alterporriol D | Antibacterial and cytotoxic activities | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 231 |
90 and 94 |
| 116 | C32H22O10 | Alterporriol E | Antibacterial and cytotoxic activities | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 231 |
90 and 94 |
| 117 | C32H26O13 | Alterporriol N | Antibacterial and anti-inflammatory activities | Marine-derived fungus Stemphylium sp. FJJ006 and mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 231 |
90, 95 and 97 |
| 118 | C32H22O10 | Alterporriol Q | Antiviral activity | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 231 |
90 and 93 |
| 119 | C32H22O10 | Alterporriol R | Displayed no antiviral, antibacterial or cytotoxic activity | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 231 |
90 and 93 |
| 120 | C32H30O13 | Alterporriol T | Displayed no antibacterial activity | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 231 |
90 |
| 121 | C32H30O12 | Alterporriol U | Antibacterial activity | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 231 |
90 |
| 122 | C32H22O10 | Alterporriol V | Antibacterial activity | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 231 |
90 |
| 123 | C32H26O12 | Alterporriol W | Displayed no antibacterial or cytotoxic activity | Mangrove-derived fungus Stemphylium sp. 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 231 |
90 |
| 124 | C32H26O12 | Alterporriol F | Anti-inflammatory and cytotoxic activities | Marine-derived fungus Stemphylium sp. FJJ006 | 95 and 96 |
| 125 | C32H26O13 | Alterporriol G | Antibacterial, anti-inflammatory, and cytotoxic activities as well as protein kinase inhibitory activity | Marine-derived fungus Stemphylium sp. FJJ006 | 95, 97 and 123 |
| 126 | C32H26O13 | Alterporriol Z1 | Anti-inflammatory activity | Marine-derived fungus Stemphylium sp. FJJ006 | 95 |
| 127 | C32H26O13 | Alterporriol Z2 | Anti-inflammatory activity | Marine-derived fungus Stemphylium sp. FJJ006 | 95 |
| 128 | C33H28O13 | Alterporriol Z3 | Displayed no antibacterial or cytotoxic activity | Marine-derived fungus Stemphylium sp. FJJ006 | 95 |
| 129 | C22H22O10 | Macrosporin 2-O-α-D-glucopyranoside | Displayed no cytotoxic activity | Coral associated fungus Stemphylium lycopersici | 92 |
| 130 | C32H30O12 | Alterporriol Y | Displayed no cytotoxic activity | Coral associated fungus Stemphylium lycopersici | 92 |
| 131 | C32H26O11 | Alterporriol K | Cytotoxic activity | Mangrove endophytic fungus Alternaria sp. ZJ9-6B | 98 |
| 132 | C32H26O12 | Alterporriol L | Cytotoxic activity | Mangrove endophytic fungus Alternaria sp. ZJ9-6B | 98 |
| 133 | C32H26O12 | Alterporriol M | Was not evaluated for any relevant bioactivity | Mangrove endophytic fungus Alternaria sp. ZJ9-6B | 98 |
| 134 | C32H30O14 | Alterporriol O | Displayed no antibacterial, antiviral, or cytotoxic activity | Soft coral derived Alternaria sp. ZJ-2008003 | 93 |
| 135 | C32H26O12 | Alterporriol P | Cytotoxic activity | Soft coral derived Alternaria sp. ZJ-2008003 | 93 |
| 136 | C16H20O6 | Tetrahydroaltersolanol C | Antiviral activity | Mangrove-derived fungus Phomopsis sp.PSU-MA214 and soft coral-derived fungus Alternaria sp. ZJ-2008003 | 93 and 111 |
| 137 | C16H20O6 | Tetrahydroaltersolanol D | Displayed no antibacterial, antiviral, or cytotoxic activity | Soft coral derived Alternaria sp. ZJ-2008003 | 93 |
| 138 | C16H20O6 | Tetrahydroaltersolanol E | Displayed no antibacterial, antiviral, or cytotoxic activity | Soft coral derived Alternaria sp. ZJ-2008003 | 93 |
| 139 | C18H22O7 | Tetrahydroaltersolanol F | Displayed no antibacterial, antiviral, or cytotoxic activity | Soft coral derived Alternaria sp. ZJ-2008003 | 93 |
| 140 | C25H28O10 | Anthrininone A | Inhibitory activity on protein tyrosine phosphatases and stimulatory effect on intracellular calcium levels | Deep-sea derived fungus Alternaria tenuissima DFFSCS013 | 99 |
| 141 | C16H12O6 | 6-O-methyl-alaternin | Inhibitory activity on protein tyrosine phosphatases | Deep-sea derived fungus Alternaria tenuissima DFFSCS013 | 99 |
| 142 | C16H16O5 | (3R)-1-deoxyaustrocortilutein | Displayed no stimulation of intracellular calcium level | Deep-sea derived fungus Alternaria tenuissima DFFSCS013 | 99 |
| 143 | C15H16O5 | Harzianumnone A | Displayed no anti-acetylcholinesterase or DNA Topo I inhibitory activities | Coral-derived fungus T. harzianum XS-20090075 | 100 |
| 144 | C15H16O5 | Harzianumnone B | Displayed no anti-acetylcholinesterase or DNA Topo I inhibitory activities | Coral-derived fungus T. harzianum XS-20090075 | 100 |
| 145 | C15H10O4 | Phomarin | Anti-acetylcholinesterase activity | Coral-derived fungus T. harzianum XS-20090075 | 100 |
| 146 | C15H10O5 | ω-hydroxydigitoemodin | Anti-acetylcholinesterase activity | Coral-derived fungus T. harzianum XS-20090075 | 100 |
| 147 | C15H10O3 | Pachybasin | Anti-acetylcholinesterase and cytotoxic activities | Marine-derived fungus T. aureoviride PSU-F95 and mangrove endophytic fungi Halorosellinia sp. (no. 1403) and Guignardia sp. (no. 4382), and sea urchin-derived fungus Monodictys sp | 40, 41, 54, 55 and 100 |
| 148 | C17H14O6 | (+)-2′S-isorhodoptilometrin | Anti-acetylcholinesterase, antibacterial, and cytotoxic activities, as well as DNA Topo I inhibitory activity | Coral-derived fungus T. harzianum XS-20090075, marine lichen-derived fungus Gliocladium sp. T31, and marine-derived fungus T. aureoviride PSU-F95 | 54, 56 and 100 |
| 149 | C15H10O4 | ω-hydroxypachybasin | Antibacterial, and cytotoxic activities, as well as DNA Topo I inhibitory activity | Marine-derived fungus T. aureoviride PSU-F95 and coral-derived fungus T. harzianum XS-20090075 | 54 and 100 |
| 150 | C15H14O5 | Coniothranthraquinone 1 | Antibacterial activity | Marine-derived fungus T. aureoviride PSU-F95 | 54 |
| 151 | C15H14O6 | Trichodermaquinone | Antibacterial activity | Marine-derived fungus T. aureoviride PSU-F95 | 54 |
| 152 | C15H10O4 | 2-Methyl-quinizarin | Was not evaluated for any relevant bioactivity | Marine-derived fungus T. aureoviride PSU-F95 | 54 |
| 153 | C15H10O4 | 1-Hydroxy-3-methoxyanthraquinone | Was not evaluated for any relevant bioactivity | Marine-derived fungus T. aureoviride PSU-F95 | 54 |
| 154 | C15H16O5 | Coniothyrinone A | Antibacterial and antiangiogenetic activities | Marine-derived fungus Trichoderma sp. and saline-alkali plant endophytic fungus Eu. rubrum | 60 and 103 |
| 155 | C15H14O6 | Lentisone | Antibacterial and antiangiogenetic activities | Marine-derived fungus Trichoderma sp | 60 |
| 156 | C15H12O5 | 9-Dehydroxyeurotinone | Antibacterial and cytotoxic activities | Marine-derived endophytic fungus Eu. rubrum | 58 |
| 157 | C16H14O5 | 2-O-Methyl-9-dehydroxyeurotinone | Antioxidant activity | Marine-derived endophytic fungus Eu. rubrum and marine mangrove-derived endophytic fungus Eu. rubrum | 58 and 62 |
| 158 | C21H22O9 | 2-O-Methyl-4-O-(α-D-ribofuranosyl)-9-dehydroxyeurotinone | Antioxidant activity | Marine mangrove-derived endophytic fungus Eu. rubrum | 62 |
| 159 | C21H20O9 | 3-O-(α-D-ribofuranosyl)-questin | Antibacterial and antioxidant activities | Marine mangrove-derived endophytic fungus Eu. rubrum and marine algae-derived endophytic fungus Eu. cristatum EN-220 | 62 and 101 |
| 160 | C33H32O10 | Eurorubrin | Antibacterial and antioxidant activities as well as brine shrimp lethality | Marine mangrove-derived endophytic fungus Eu. rubrum and marine algae-derived endophytic fungus Eu. cristatum EN-220 | 62 and 101 |
| 161 | C21H20O10 | 3-O-(α-D-ribofuranosyl)questinol | Displayed no antibacterial activity or brine shrimp lethality | Marine algae-derived endophytic fungus Eu. cristatum EN-220 | 101 |
| 162 | C21H24O9 | Asperflavin ribofuranoside | Antibacterial and antioxidant activities | Marine algae-derived endophytic fungus Eu. cristatum EN-220 and marine-derived algicolous fungus Microsporum sp | 101 and 102 |
| 163 | C15H14O5 | Rubrumol | Relaxation activity on Topo I enzyme | Saline-alkali plant endophytic fungus Eu. rubrum | 103 |
| 164 | C16H16O6 | Nigrosporin A | Antibacterial, cytotoxic, and phytotoxic activities | Sea fan-derived fungus Fusarium sp. PSU-F14 | 39 and 44 |
| 165 | C16H20O7 | Fusaranthraquinone | Displayed no antibacterial activity | Sea fan-derived fungus Fusarium sp. PSU-F14 | 39 |
| 166 | C16H18O6 | Fusaquinon A | Cytotoxic activity | Marine-derived fungus Fusarium sp. ZH-210 | 104 and 105 |
| 167 | C16H20O8 | Fusaquinon B | Cytotoxic activity | Marine-derived fungus Fusarium sp. ZH-210 | 104 |
| 168 | C16H20O7 | Fusaquinon C | Cytotoxic activity | Marine-derived fungus Fusarium sp. ZH-210 | 104 |
| 169 | C21H20O5 | 6,8-Dimethoxy-1-methyl-2-(3-oxobutyl)anthracene-9,10-dione | Cytotoxic activity | Mangrove endophytic fungus Fusarium sp. ZZF60 | 106 |
| 170 | C17H12O7 | 5-Acetyl-2-methoxy-1,4,6-trihydroxy-anthraquinone | Was not evaluated for any relevant bioactivity | Marine endophytic fungus Fusarium sp. b77 | 124 |
| 171 | C33H28O12 | Engyodontochone A | Antibacterial, antifungal, and cytotoxic activities | Marine-derived fungus Engyodontium album strain LF069 | 23 |
| 172 | C33H28O12 | JBIR-99 | Antibacterial, antifungal, and cytotoxic activities | Marine-derived fungus Engyodontium album strain LF069 | 23 |
| 173 | C33H28O12 | Engyodontochone B | Antibacterial, antifungal, and cytotoxic activities | Marine-derived fungus Engyodontium album strain LF069 | 23 |
| 174 | C33H28O12 | Engyodontochone C | Antibacterial and cytotoxic activities | Marine-derived fungus Engyodontium album strain LF069 | 23 |
| 175 | C33H30O13 | Engyodontochone F | Antibacterial and antifungal activities | Marine-derived fungus Engyodontium album strain LF069 | 23 |
| 176 | C33H30O13 | Engyodontochone E | Antibacterial activity | Marine-derived fungus Engyodontium album strain LF069 | 23 |
| 177 | C33H28O12 | Engyodontochone D | Was not evaluated for any relevant bioactivity | Marine-derived fungus Engyodontium album strain LF069 | 23 |
| 178 | C16H16O7 | 4-Dehydroxyaltersolanol A | Antibacterial and anticoagulant activities | Marine-derived fungus Sporendonema casei HDN16-802 | 107 |
| 179 | C16H18O8 | Auxarthrol D | Antibacterial, anticoagulant, and cytotoxic activities | Marine-derived fungus Sporendonema casei HDN16-802 | 107 |
| 180 | C16H20O9 | Auxarthrol E | Anticoagulant activity | Marine-derived fungus Sporendonema casei HDN16-802 | 107 |
| 181 | C16H20O8 | Auxarthrol F | Antibacterial, anticoagulant, and cytotoxic activities | Marine-derived fungus Sporendonema casei HDN16-802 | 107 |
| 182 | C16H17ClO8 | Auxarthrol G | Antibacterial, anticoagulant, and antifungal activities | Marine-derived fungus Sporendonema casei HDN16-802 | 107 |
| 183 | C16H18O8 | Auxarthrol H | Anticoagulant activity | Marine-derived fungus Sporendonema casei HDN16-802 | 107 |
| 184 | C17H14O4 | 1,3-Dimethoxy-6-methyl-anthracene-9,10-dione | Cytotoxic activity | Mangrove endophytic fungi Halorosellinia sp. No. 1403 and Guignardia sp. No. 4382 | 40 |
| 185 | C15H10O4 | Demethoxyaustrocortirubin | Cytotoxic activity | Mangrove endophytic fungi Halorosellinia sp.No. 1403 and Guignardia sp. No. 4382 | 40 and 41 |
| 186 | C14H8O4 | Dantron | Cytotoxic activity | Mangrove endophytic fungi Halorosellinia sp.No. 1403 and Guignardia sp. No. 4382 | 40 |
| 187 | C16H12O5 | 1,3-Dihydroxy-6-methoxy-8-methyl-anthracene-9,10-dione | Cytotoxic activity | Mangrove endophytic fungi Halorosellinia sp.No. 1403 and Guignardia sp. No. 4382 | 40 |
| 188 | C17H14O5 | 1-Hydroxy-2,4-dimethoxy-7-methyl-anthracene-9,10-dione | Cytotoxic activity | Mangrove endophytic fungi Halorosellinia sp.No. 1403 and Guignardia sp. No. 4382 | 40 |
| 189 | C16H12O4 | 8-Hydroxy-1-methoxy-3-methyl-9,10-anthraquinone | Cytotoxic activity | Mangrove endophytic fungi Halorosellinia sp.No. 1403 and Guignardia sp. No. 4382 | 40 |
| 190 | C17H14O6 | 1,7-Dihydroxy-2,4-dimethoxy-6-methyl-anthracene-9,10-dione | Cytotoxic activity | Mangrove endophytic fungi Halorosellinia sp.No. 1403 and Guignardia sp. No. 4382 | 40 |
| 191 | C14H8O5 | 1,3,8-Trihydroxyanthraquinone | Cytotoxic activity | Mangrove endophytic fungi Halorosellinia sp.No. 1403 and Guignardia sp. No. 4382 | 40 |
| 192 | C16H12O6 | 1,4,7-Trihydroxy-2-methoxy-6-methyl-9,10-anthraquinone | Displayed no cytotoxic activity | Mangrove endophytic fungi Halorosellinia sp.No. 1403 and Guignardia sp. No. 4382 | 40 |
| 193 | C16H16O8 | SZ-685C | Cytotoxic activity | Mangrove endophytic fungus Halorosellinia sp. No. 1403 | 108–110 |
| 194 | C18H20O6 | Phomopsanthraquinone | Antibacterial and cytotoxic activities | Mangrove-derived fungus Phomopsis sp. PSU-MA214 | 111 |
| 195 | C16H12O4 | 1-Hydroxy-3-methoxy-6-methyl-anthraquinone | Displayed no antibacterial or cytotoxic activity | Mangrove-derived fungus Phomopsis sp. PSU-MA214 | 111 |
| 196 | C16H12O7 | Tetrahydroxyanthraquinone | Protein kinases' inhibitory activity | Sponge-associated fungus Microsphaeropsis sp | 112 |
| 197 | C17H14O7 | Methoxyl-tetrahydroxyanthraquinone | Protein kinases' inhibitory activity | Sponge-associated fungus Microsphaeropsis sp | 112 |
| 198 | C17H14O8 | 1,2,3,6,8-Pentahydroxy-7-[(1R)-1-methoxyethyl]-9,10-anthraquinone | Protein kinases' inhibitory activity | Sponge-associated fungus Microsphaeropsis sp | 112 |
| 199 | C15H10O6 | Lunatin | Antibacterial activity | Sponge-derived fungus Curvularia lunata | 113 |
| 200 | C30H22O12 | Cytoskyrin A | Antibacterial activity | Sponge-derived fungus Curvularia lunata | 113 |
| 201 | C14H8O6 | Rheoemodin | Displayed no significant anti-obesity activity | Marine sponge-associated fungus Talaromyces stipitatus KUFA 0207 | 82 |
| 202 | C30H18O10 | 2, 2′-Bis-(7-methyl-1,4,5-trihydroxy-anthracene-9,10-dione) | Was not evaluated for any relevant activity | Marine sponge-associated fungus Talaromyces stipitatus KUFA 0207 | 82 |
| 203 | C17H14O5 | 7-Methoxymacrosporin | Antifungal activity | Mangrove-derived fungus Phoma sp. L28 | 91 |
| 204 | C21H20O5 | 7-(γ,γ)-Dimethyl-allyloxy-macrosporin | Antifungal activity | Mangrove-derived fungus Phoma sp. L28 | 91 |
| 205 | C16H12O5 | Monodictyquinone A | Antibacterial and antifungal activities | Sea urchin-derived fungus Monodictys sp | 55 |
| 206 | C16H12O6 | 1,3,6-Trihydroxy-7-(1-hydroxyethyl) anthracene-9,10-dione | Inhibitory activity against α-glycosidase | Mangrove-derived fungus Cladosporium sp. HNWSW-1 | 114 |
| 207 | C17H12O7 | Phaseolorin I | Displayed no cytotoxic activity | Deep-sea sediment-derived fungus Diaporthe phaseolorum FS431 | 115 |
| 208 | C22H22O7 | 6,8-O,O′-Dimethyl-averufanin | Antifungal and phytotoxic activities, as well as brine shrimp lethality | Mangrove endophytic fungus ZSUH-36 | 116 and 117 |
Topological polar surface area (TPSA), another measure, is the sum of the surfaces of all the polar atoms present in a molecule. TPSA has a substantial effect on the potential of a compound to penetrate through the cell membranes and blood–brain barrier. Veber highlighted those compounds with TPSA ≤ 140 A2 tend to be well absorbed and able to reach their molecular target within the body cells. Egan stated that molecules with TPSA less than 132 A2 and log-P between −1 and 6 could be considered leads with high drug-likeness potential and good orally bioavailability. Muegge utilized a pharmacophore point filter based on very simple structural rules to differentiate between drug-like and nondrug-like molecules, among them TPSA not greater than 150 A2 as well as rotatable bonds (RB), not more than 15. All anthraquinones from Fusarium, Trichoderma, Nigrospora (except compound 1), Aspergillus (except compounds 11, 12, 20, 24, 25, 39, and 61), Penicillium (except compounds 81, 82, 86, and 89–96), Stemphylium (except compounds 110–130), Alternaria (except compounds 114, 131–135, and 140), Eurotium (except compounds 20, 160, and 161), Sporendonema casei (except compound 180), and the other genera (except compounds 200 and 202) had TPSA less than 150 A2. On the other hand, all Engyodontium album derived compounds had TPSA greater than 150 A2. All anthraquinones had RB less than 15 (Fig. 15 and ESI Table S1†).
Oral bioavailability, bioavailability score (BS), is another descriptor that indicates the possibility of a compound to be bioavailable with more than 10% in the absorption assays. Molecules obeying the Lipinski rule with BS of 0.55 are considered orally bioavailable. Interestingly, 166 anthraquinones showed a BS of 0.55. In alignment with other parameters, all Fusarium and Trichoderma derived anthraquinones showed a BS of 0.55 and all Engyodontium album derived compounds had a BS of 0.11. Compounds 3, 6, and 9, are of special interest as they showed a good BS of 0.56. Some other compounds, among them 10, 87, 97, and 109, showed good BS (0.56); however, violated one or more drug-likeness rules (Fig. 15 and ESI Table S1†).
Oral bioavailability relies as well on the degree of the molecular flexibility of the molecule. Candidates with an extreme degree of flexibility do not typically display acceptable bioavailability as they tend to be less planar and with very complex 3D shapes. The sp3 carbons fraction (Fraction Csp3) and the number of RB are two crucial measures for molecular flexibility. Csp3 is the ratio of the sp3 carbon atoms to the total carbons present in a given compound. It assigns the degree of carbon saturation, characterizes the space complexity, and correlates to the solubility of the compound. A Csp3 score between 0.25 and 1 is considered optimum for drug-likeness. One hundred anthraquinones, distributed in all marine fungi species, displayed a Csp3 score ranging between 0.28 and 0.6. The water solubility, expressed as log S, is another essential measure for drug bioavailability. Compounds with poor water solubility have poor absorption and oral bioavailability, as well as low formulation potential. Anthraquinones revealed different solubility orders as Sporendonema casei derived compounds were the most soluble (mean value of −1.46), followed by Nigrospora sp (mean value of −2.34), while Engyodontium album derived anthraquinones, as expected, were the most poorly soluble (mean value of −5.44) (Fig. 15 and ESI Table S1†).
Gastrointestinal (GI) absorption, blood–brain barrier (BBB) permeation, P-glycoprotein (P-gp) substrate and cytochrome P450 members inhibition potentials were also surveyed to draw insight about the pharmacokinetic behavior of the reviewed anthraquinones. Twenty anthraquinones (47, 48, 49, 51, 52, 57, 83, 147, 152, 153, 157, 169, 184, 185, 186, 188, 189, 195, 203, and 204) showed high GI absorption, passively crossed BBB and did not show any potential for P-gp substrate (ESI Table S2†). Surprisingly, compound 169 obeyed all the surveyed parameters (5 drug-likeness rules, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, Csp3, RB, TPSA, log
P, Csp3, RB, TPSA, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S, GI, BBB, Pgp) and the other 19 anthraquinones as well except for fraction Csp3. Noteworthy, all but one of the 20 anthraquinones have two benzenoid aromatic rings and two C
S, GI, BBB, Pgp) and the other 19 anthraquinones as well except for fraction Csp3. Noteworthy, all but one of the 20 anthraquinones have two benzenoid aromatic rings and two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. Also, several anthraquinones showed potential inhibition for some CYP 450 isoforms which necessitates awareness when co-administered with possible substrates of these enzymes (ESI Table S2†).
O groups. Also, several anthraquinones showed potential inhibition for some CYP 450 isoforms which necessitates awareness when co-administered with possible substrates of these enzymes (ESI Table S2†).
To sum up, marine fungi are a promising source of biologically active anthraquinones that obeyed all the criteria of several drug-likeness rules with promising pharmacokinetic behavior which promotes their utilization as well as further research to isolate their individual components and determine their pharmacological effects.
Conclusions and future prospective
The marine phoma is representing the most, the greatest and most diverse ecological structure on the planet. Over seven decades, marine natural products (MNPs) have owned credits and been privileged as a robust and sustainable supplier for pharmacologically active compounds that meet a huge interest in pharmaceutical and economical applications. Marine-derived fungi are valuable sources of structurally diverse MNPs due to their various habitats that range from the warm to the colder areas, and even at extreme temperatures and pressure like in hydrothermal outlets. One of the fascinating classes of fungal derived natural products is the anthraquinones. Herein, we presented a comprehensive literature review centered on marine-derived anthraquinones as a unique group of fungal polyketides over the period 2000–2020 from twenty marine fungal genera. A list of 208 anthraquinones have been reported from different marine fungi, featuring a myriad of structural and biological diversities. Investigating such extensive chemo-biological data has implied two remarkable points. First, it was clear that the marine fungi of the three genera Aspergillus sp., Stemphylium sp., and Penicillium sp., are the most creative fungal genera in terms of producing of anthraquinones. Secondly, the most common reported bioactivity was cytotoxicity, where a notable number of seventy-two compounds have been evaluated for their cytotoxic activity against planes of carcinoma cell lines, whilst the anthraquinones with antibacterial activity were the second on the list with sixty-nine compounds demonstrated bioactivity against a wide range of microorganisms. Meanwhile, an enormous spectrum of further biomedical potentialities exhibited by these compounds as (antioxidant, antiviral, antifungal, immunomodulatory, anti-inflammatory, …….etc.) have been documented. Such a massive connection between chemical spaces and bioactivities highlights the huge capacity of marine-derived fungi as an attractive biological source that is worth further exploitations with distinguished anticipations for the global pharmaceuticals industries. Additionally, recent advances in the level of sampling techniques, fermentation, synthetic biology, genetic engineering, genome mining, and total chemical synthesis, all are crucial to the success of fungal MNPs as future drug leads. Furthermore, all reported anthraquinones were extensively investigated for their in silico Drug-likeness and pharmacokinetics properties using SWISSADME online platform, which intriguingly highlighted a list of 20 anthraquinone containing compounds (ESI†) that could be considered as potential drug leads scaffolds (Fig. 17 and 18).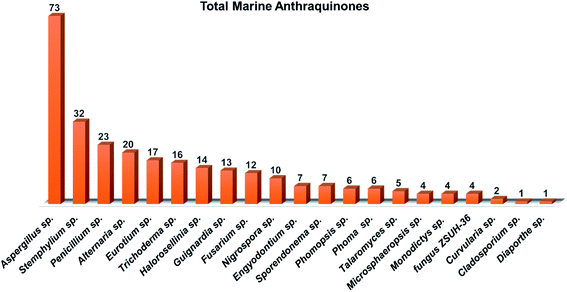 | ||
| Fig. 17 Distribution and total anthraquinones and their derivatives isolated from different species of marine-derived fungi. | ||
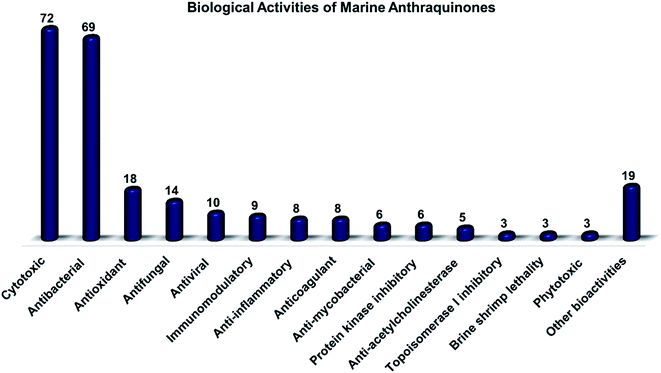 | ||
| Fig. 18 Total biological activities of various anthraquinones and their derivatives isolated from different species of marine-derived fungi. | ||
Author contributions
Conceptualization: Amr El-Demerdash. Validation: Amr El-Demerdash. Formal analysis: Mohamed Sebak, Fatma Molham, Claudio Greco Mohamed A. Tammam, Mansour Sobeh and Amr El-Demerdash. Investigation: Mohamed Sebak, Fatma Molham, Claudio Greco Mohamed A. Tammam, Mansour Sobeh and Amr El-Demerdash. Resources: Mohamed Sebak, Fatma Molham, Claudio Greco Mohamed A. Tammam, Mansour Sobeh and Amr El-Demerdash. Data curation: Mohamed Sebak, Fatma Molham, Claudio Greco Mohamed A. Tammam, Mansour Sobeh and Amr El-Demerdash. Writing original draft: Mohamed Sebak, Fatma Molham, Claudio Greco Mohamed A. Tammam, Mansour Sobeh and Amr El-Demerdash. Writing-review & editing: Mohamed Sebak, Fatma Molham, Claudio Greco Mohamed A. Tammam, Mansour Sobeh and Amr El-Demerdash.Conflicts of interest
The authors declare that they have no known competing commercial interests or personal relationships that could have appeared to influence the work reported in this paper.Funding
Amr El-Demerdash is immensely grateful to the John Innes Centre, Norwich Research Park, United Kingdom for the postdoctoral fellowship. Claudio Greco was supported by the BBSRC (BB/V005723/1).List of abbreviations
| A. | Aspergillus |
| ACP | acyl carrier protein |
| AT | acyl transferase |
| B. | Bacillus |
| BBB | blood–brain barrier |
| BS | bioavailability score |
| C | Candida |
| Cox-2 | cyclooxygenase-2 |
| Csp3 | sp3 carbons |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| E. | Escherichia |
| ED50 | median effective dose (the dose which produces a specified effect in 50% of the population in a study) |
| Eu. | Eurotium |
| F. | Fusarium |
| FCsp3 | fraction of sp3 carbons |
| HCV | Hepatitis C virus |
| GI | Gastrointestinal |
| KS | ketosynthase |
| IC50 | inhibitory concentration that causes a 50% reduction in cell viability |
| IL | interleukin |
| LD50 | lethal dose 50 (the dose which produces death in 50% of the population in a study) |
| Log P | lipophilicity |
| Log S | solubility |
| M. | Mycobacterium |
| MdpF | metallo-hydrolase protein |
| MIC | minimum inhibitory concentration |
| MNP | marine natural product |
| MRSA | methicillin-resistant Staphylococcus aureus |
| Mwt | molecular weight |
| nrPKS | non-reducing polyketide synthase |
| P. | Penicillium |
| P-gp | P-glycoprotein |
| PT | product template |
| RB | rotatable bond |
| S. | Staphylococcus |
| SAT | starter unit-ACP transacylase |
| Str. | Streptococcus |
| T. | Trichoderma |
| Topo | topoisomerase |
| TPSA | topological polar surface area |
| V. | Vibrio |
Acknowledgements
Amr El-Demerdash is immensely thankful to his home university, Mansoura University, Egypt for the unlimited support, inside and outside.References
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2016, 79, 629–661 CrossRef CAS PubMed.
- A. G. Atanasov, B. Waltenberger, E.-M. Pferschy-Wenzig, T. Linder, C. Wawrosch, P. Uhrin, V. Temml, L. Wang, S. Schwaiger and E. H. Heiss, Biotechnol. Adv., 2015, 33, 1582–1614 CrossRef CAS PubMed.
- M. A. Ghareeb, M. A. Tammam, A. El-Demerdash and A. G. Atanasov, Curr. Res. Biotechnol., 2020, 2, 88–102 CrossRef.
- A. El-Demerdash, D. Kumla and A. Kijjoa, Mar. Drugs, 2020, 18, 1–32 CrossRef PubMed.
- M. Sebak, A. E. Saafan, S. AbdelGhani, W. Bakeer, A. O. El-Gendy, L. C. Espriu, K. Duncan and R. Edrada-Ebel, PLoS One, 2019, 14, 1–29 CrossRef PubMed.
- N. Osama, W. Bakeer, M. Raslan, H. A. Soliman, U. R. Abdelmohsen and M. Sebak, R. Soc. Open Sci., 2022, 9, 211509 CrossRef CAS PubMed.
- W. Sun, W. Wu, X. Liu, D. A. Zaleta-Pinet and B. R. Clark, Mar. Drugs, 2019, 17, 339 CrossRef CAS PubMed.
- A. R. Carroll, B. R. Copp, R. A. Davis, R. A. Keyzers and M. R. Prinsep, Nat. Prod. Rep., 2019, 36, 122–173 RSC.
- P. Shinde, P. Banerjee and A. Mandhare, Expert Opin. Ther. Pat., 2019, 29, 283–309 CrossRef CAS PubMed.
- L. Macintyre, T. Zhang, C. Viegelmann, I. J. Martinez, C. Cheng, C. Dowdells, U. R. R. Abdelmohsen, C. Gernert, U. Hentschel and R. A. Edrada-Ebel, Mar. Drugs, 2014, 12, 3416–3448 CrossRef PubMed.
- L. Van Andel, H. Rosing, J. H. M. Schellens and J. H. Beijnen, Mar. Drugs, 2018, 16, 246 CrossRef PubMed.
- G. M. Cragg and D. J. Newman, Biochim. Biophys. Acta - Gen. Subj., 2013, 1830, 3670–3695 CrossRef CAS PubMed.
- J. W. Blunt, B. R. Copp, R. A. Keyzers, M. H. G. Munro and M. R. Prinsep, Nat. Prod. Rep., 2015, 32, 116–211 RSC.
- R. Montaser and H. Luesch, Future Med. Chem., 2011, 3, 1475–1489 CrossRef CAS.
- S. R. Damare, 2006.
- M. Fouillaud, M. Venkatachalam, E. Girard-Valenciennes, Y. Caro and L. Dufossé, Mar. Drugs, 2016, 14(4), 64 CrossRef.
- S. K. Deshmukh, V. Prakash and N. Ranjan, Front. Microbiol., 2018, 8, 1–24 CrossRef CAS PubMed.
- H. J. Shin, Mar. Drugs, 2020, 18, 230 CrossRef PubMed.
- C. Montagne, Durieu Maisonneuve, MC, Explor. Sci. l’Algerie pendant les annees 1840, 1841, 1842…, Sci. Phys. Bot. Cryptogam., 1846, 1, 1–197 Search PubMed.
- A. Amend, G. Burgaud, M. Cunliffe, V. P. Edgcomb, C. L. Ettinger, M. H. Gutiérrez, J. Heitman, E. F. Y. Hom, G. Ianiri, A. C. Jones, M. Kagami, K. T. Picard, C. A. Quandt, S. Raghukumar, M. Riquelme, J. Stajich, J. Vargas-Muñiz, A. K. Walker, O. Yarden and A. S. Gladfelter, MBio, 2019, 10(2) DOI:10.1128/mBio.01189-18.
- M. S. Butler, A. A. B. Robertson and M. A. Cooper, Nat. Prod. Rep., 2014, 31, 1612–1661 RSC.
- B. Wu, G. Chen, Z. Liu and Y. Pei, Rec. Nat. Prod., 2015, 9, 271 CAS.
- B. Wu, J. Wiese, A. Wenzel-Storjohann, S. Malien, R. Schmaljohann and J. F. Imhoff, Chem. - A Eur. J., 2016, 22, 7452–7462 CrossRef CAS PubMed.
- J. R. Hanson, Natural products: the secondary metabolites, Royal Society of Chemistry, 2003, vol. 17 Search PubMed.
- R. Ebel, in Comprehensive Natural Products II: Chemistry and Biology, Elsevier Ltd, 2010, pp. 223–262 Search PubMed.
- N. N. Gessler, A. S. Egorova and T. A. Belozerskaya, Appl. Biochem. Microbiol., 2013, 49, 85–99 CrossRef CAS.
- C. Greco, K. de Mattos-Shipley, A. M. Bailey, N. P. Mulholland, J. L. Vincent, C. L. Willis, R. J. Cox and T. J. Simpson, Chem. Sci., 2019, 10, 2930–2939 RSC.
- M. Masi and A. Evidente, Toxins, 2020, 12, 714 CrossRef CAS PubMed.
- G. Greco, E. Turrini, E. Catanzaro and C. Fimognari, Mar. Drugs, 2021, 19, 272 CrossRef CAS PubMed.
- A. El-Demerdash, J. Fungi, 2018, 4, 130 CrossRef CAS PubMed.
- A. El-Demerdash, G. Genta-Jouve, M. Bärenstrauch, C. Kunz, E. Baudouin and S. Prado, Phytochemistry, 2019, 166, 112056 CrossRef CAS PubMed.
- A. J. Birch, J. Baldas, J. R. Hlubucek, T. J. Simpson and P. W. Westerman, J. Chem. Soc. Perkin Trans., 1976, 1, 898–904 RSC.
- A. J. Birch and F. W. Donovan, Stud. Relat. to biosynthesis. I. Some possible routes ro Deriv. orcinol phloroglucinol, Aust. J. Chem., 1953, 6, 360–368 CrossRef CAS.
- Y.-M. Chiang, B. R. Oakley, N. P. Keller and C. C. C. Wang, Appl. Microbiol. Biotechnol., 2010, 86, 1719–1736 CrossRef CAS PubMed.
- T. J. Simpson, ChemBioChem, 2012, 13, 1680–1688 CrossRef CAS PubMed.
- S. Griffiths, C. H. Mesarich, B. Saccomanno, A. Vaisberg, P. J. G. M. De Wit, R. Cox and J. Collemare, Proc. Natl. Acad. Sci., 2016, 113, 6851–6856 CrossRef CAS PubMed.
- W. F. Xu, X. M. Hou, K. L. Yang, F. Cao, R. Y. Yang, C. Y. Wang and C. L. Shao, Mar. Drugs, 2016, 14, 51 CrossRef PubMed.
- K. L. Yang, M. Y. Wei, C. L. Shao, X. M. Fu, Z. Y. Guo, R. F. Xu, C. J. Zheng, Z. G. She, Y. C. Lin and C. Y. Wang, J. Nat. Prod., 2012, 75, 935–941 CrossRef CAS PubMed.
- K. Trisuwan, N. Khamthong, V. Rukachaisirikul, S. Phongpaichit, S. Preedanon and J. Sakayaroj, J. Nat. Prod., 2010, 73, 1507–1511 CrossRef CAS PubMed.
- J. Y. Zhang, L. Y. Tao, Y. J. Liang, L. M. Chen, Y. J. Mi, L. S. Zheng, F. Wang, Z. G. She, Y. C. Lin, K. K. W. To and L. W. Fu, Mar. Drugs, 2010, 8, 1469–1481 CrossRef CAS PubMed.
- X. K. Xia, H. R. Huang, Z. G. She, C. L. Shao, F. Liu, X. L. Cai, L. L. P. Vrijmoed and Y. C. Lin, Magn. Reson. Chem., 2007, 45, 1006–1009 CrossRef CAS.
- J. Xu, T. Nakazawa, K. Ukai, H. Kobayashi, R. E. P. Mangindaan, D. S. Wewengkang, H. Rotinsulu and M. Namikoshi, J. Antibiot., 2008, 61, 415–419 CrossRef CAS PubMed.
- C. Wang, J. Wang, Y. Huang, H. Chen, Y. Li, L. Zhong, Y. Chen, S. Chen, J. Wang, J. Kang, Y. Peng, B. Yang, Y. Lin, Z. She and X. Lai, Molecules, 2013, 18, 1728–1740 CrossRef CAS PubMed.
- M. Tanaka, T. Fukushima, Y. Tsujino and T. Fujimori, Biosci. Biotechnol. Biochem., 1997, 61, 1848–1852 CrossRef CAS PubMed.
- X. Xia, Q. Li, J. Li, C. Shao, J. Zhang, Y. Zhang, X. Liu, Y. Lin, C. Liu and Z. She, Planta Med., 2011, 77, 1735–1738 CrossRef CAS PubMed.
- U. Sommart, V. Rukachaisirikul, Y. Sukpondma, S. Phongpaichit, J. Sakayaroj and K. Kirtikara, Chem. Pharm. Bull., 2008, 56, 1687–1690 CrossRef CAS PubMed.
- L. Du, T. Zhu, H. Liu, Y. Fang, W. Zhu and Q. Gu, J. Nat. Prod., 2008, 71, 1837–1842 CrossRef CAS PubMed.
- L. Du, T. Zhu, Y. Fang, H. Liu, Q. Gu and W. Zhu, Tetrahedron, 2007, 63, 1085–1088 CrossRef CAS.
- X. Li, X. M. Li, G. M. Xu, C. S. Li and B. G. Wang, Phytochem. Lett., 2014, 7, 120–123 CrossRef CAS.
- W. Wang, T. Zhu, H. Tao, Z. Lu, Y. Fang, Q. Gu and W. Zhu, J. Antibiot., 2007, 60, 603–607 CrossRef CAS PubMed.
- I. Wijesekara, C. Zhang, Q. Van Ta, T. S. Vo, Y. X. Li and S. K. Kim, Microbiol. Res., 2014, 169, 255–261 CrossRef CAS PubMed.
- S. K. Agarwal, S. S. Singh, S. Verma and S. Kumar, J. Ethnopharmacol., 2000, 72, 43–46 CrossRef CAS PubMed.
- N. Khamthong, V. Rukachaisirikul, S. Phongpaichit, S. Preedanon and J. Sakayaroj, Tetrahedron, 2012, 68, 8245–8250 CrossRef CAS.
- N. Khamthong, V. Rukachaisirikul, K. Tadpetch, M. Kaewpet, S. Phongpaichit, S. Preedanon and J. Sakayaroj, Arch. Pharm. Res., 2012, 35, 461–468 CrossRef CAS PubMed.
- A. A. El-Beih, T. Kawabata, K. Koimaru, T. Ohta and S. Tsukamoto, Chem. Pharm. Bull., 2007, 55, 1097–1098 CrossRef CAS PubMed.
- H. Ren, L. Tian, Q. Gu and W. Zhu, Arch. Pharm. Res., 2006, 29, 59–63 CrossRef CAS PubMed.
- L. Wen, Y. C. Lin, Z. G. She, D. S. Du, W. L. Chan and Z. H. Zheng, J. Asian Nat. Prod. Res., 2008, 10, 133–137 CrossRef CAS PubMed.
- H. J. Yan, X. M. Li, C. S. Li and B. G. Wang, Helv. Chim. Acta, 2012, 95, 163–168 CrossRef CAS.
- W. Wang, R. Chen, Z. Luo, W. Wang and J. Chen, Nat. Prod. Res., 2018, 32, 558–563 CrossRef CAS PubMed.
- J. Qi, P. Zhao, L. Zhao, A. Jia, C. Liu, L. Zhang and X. Xia, Chem. Nat. Compd., 2020, 56, 112–114 CrossRef CAS.
- M. Isaka, P. Chinthanom, S. Veeranondha, S. Supothina and J. Jennifer Luangsa-ard, Tetrahedron, 2008, 64, 11028–11033 CrossRef CAS.
- D. L. Li, X. M. Li and B. G. Wang, J. Microbiol. Biotechnol., 2009, 19, 675–680 CAS.
- H. Anke, I. Kolthoum and H. Laatsch, Arch. Microbiol., 1980, 126, 231–236 CrossRef CAS PubMed.
- J. L. Li, X. Jiang, X. Liu, C. He, Y. Di, S. Lu, H. Huang, B. Lin, D. Wang and B. Fan, Fitoterapia, 2019, 133, 1–4 CrossRef CAS.
- C. J. Wu, C. W. Li and C. Bin Cui, Mar. Drugs, 2014, 12, 1815–1838 CrossRef PubMed.
- H. Huang, F. Wang, M. Luo, Y. Chen, Y. Song, W. Zhang, S. Zhang and J. Ju, J. Nat. Prod., 2012, 75, 1346–1352 CrossRef CAS PubMed.
- Z. H. WU, D. LIU, Y. XU, J. L. CHEN and W. H. LIN, Chin. J. Nat. Med., 2018, 16, 219–224 CAS.
- Y. M. Lee, H. Li, J. Hong, H. Y. Cho, K. S. Bae, M. A. Kim, D. K. Kim and J. H. Jung, Arch. Pharm. Res., 2010, 33, 231–235 CrossRef CAS PubMed.
- Y. Zhang, X. M. Li and B. G. Wang, Biosci. Biotechnol. Biochem., 2012, 76, 1774–1776 CrossRef CAS PubMed.
- Z. J. Wu, M. A. Ouyang, R. K. Su and Y. H. Kuo, Chinese J. Chem., 2008, 26, 759–764 CrossRef CAS.
- S. Q. Yang, X. M. Li, G. M. Xu, X. Li, C. Y. An and B. G. Wang, J. Antibiot., 2018, 71, 778–784 CrossRef CAS PubMed.
- K. W. Kim, H. J. Kim, J. H. Sohn, J. H. Yim, Y. C. Kim and H. Oh, Neurochem. Int., 2018, 113, 8–22 CrossRef CAS PubMed.
- X. W. Luo, H. M. Lu, X. Q. Chen, X. F. Zhou, C. H. Gao and Y. H. Liu, Chem. Nat. Compd., 2020, 56, 716–719 CrossRef CAS.
- A. Asai, Y. Yamashita, K. Ando, S. Kakita, K. Kita, Y. Suzuki, A. Mihara, T. Ashizawa, T. Mizukami and H. Nakano, J. Antibiot., 1999, 52, 1046–1049 CrossRef CAS PubMed.
- K. Sakai, S. Ohte, T. Ohshiro, D. Matsuda, R. Masuma, L. L. Rudel and H. Tomoda, J. Antibiot., 2008, 61, 568–572 CrossRef CAS PubMed.
- U. W. Hawas, A. A. El-Beih and A. M. El-Halawany, Arch. Pharm. Res., 2012, 35, 1749–1756 CrossRef CAS PubMed.
- G. C. L. Ee, Y. P. Wen, M. A. Sukari, R. Go and H. L. Lee, Nat. Prod. Res., 2009, 23, 1322–1329 CrossRef CAS PubMed.
- M.-I. Chung, S.-J. Jou, T.-H. Cheng, C.-N. Lin, F.-N. Ko and C.-M. Teng, J. Nat. Prod., 1994, 57, 313–316 CrossRef CAS PubMed.
- N. H. Yoo, D. S. Jang, Y. M. Lee, I. H. Jeong, J.-H. Cho, J.-H. Kim and J. S. Kim, Arch. Pharm. Res., 2010, 33, 209–214 CrossRef CAS PubMed.
- M. K. Moon, Y.-M. Han, Y.-J. Lee, L. H. Lee, J. H. Yang, B.-M. Kwon and D. K. Kim, Arch. Pharm. Res., 2010, 33, 1747–1751 CrossRef CAS PubMed.
- R. R. Sun, F. P. Miao, J. Zhang, G. Wang, X. L. Yin and N. Y. Ji, Magn. Reson. Chem., 2013, 51, 65–68 CrossRef CAS PubMed.
- J. Noinart, S. Buttachon, T. Dethoup, L. Gales, J. A. Pereira, R. Urbatzka, S. Freitas, M. Lee, A. M. S. Silva, M. M. M. Pinto, V. Vasconcelos and A. Kijjoa, Mar. Drugs, 2017, 15(5), 139 CrossRef PubMed.
- X. Yang, M. C. Kang, Y. Li, E. A. Kim, S. M. Kang and Y. J. Jeon, J. Microbiol. Biotechnol., 2014, 24, 1346–1353 CrossRef CAS PubMed.
- K. Y. He, C. Zhang, Y. R. Duan, G. L. Huang, C. Y. Yang, X. R. Lu, C. J. Zheng and G. Y. Chen, J. Antibiot., 2017, 70, 823–827 CrossRef CAS PubMed.
- P. Le Wang, D. Y. Li, L. R. Xie, X. Wu, H. M. Hua and Z. L. Li, Nat. Prod. Res., 2014, 28, 290–293 CrossRef PubMed.
- S. Kumar, M. Yadav, A. Yadav, P. Rohilla and J. P. Yadav, BMC Complement. Altern. Med., 2017, 1–10 Search PubMed.
- R. M. Coopoosamy and M. L. Magwa, Afr. J. Biotechnol., 2006, 5, 1092–1094 CAS.
- M. Luo, Z. Cui, H. Huang, X. Song, A. Sun, Y. Dang, L. Lu and J. Ju, J. Nat. Prod., 2017, 80, 1668–1673 CrossRef CAS PubMed.
- K. A. Alvi, B. Nair, C. Gallo and D. Baker, J. Antibiot., 1997, 50, 264–266 CrossRef CAS.
- X. M. Zhou, C. J. Zheng, G. Y. Chen, X. P. Song, C. R. Han, G. N. Li, Y. H. Fu, W. H. Chen and Z. G. Niu, J. Nat. Prod., 2014, 77, 2021–2028 CrossRef CAS PubMed.
- S. Huang, J. Xu, F. Li, D. Zhou, L. Xu and C. Li, Chem. Nat. Compd., 2017, 53, 237–240 CrossRef CAS.
- J. Li, Y. B. Zheng, T. Kurtán, M. X. Liu, H. Tang, C. L. Zhuang and W. Zhang, Nat. Prod. Res., 2020, 34, 2116–2123 CrossRef CAS PubMed.
- C. J. Zheng, C. L. Shao, Z. Y. Guo, J. F. Chen, D. S. Deng, K. L. Yang, Y. Y. Chen, X. M. Fu, Z. G. She, Y. C. Lin and C. Y. Wang, J. Nat. Prod., 2012, 75, 189–197 CrossRef CAS PubMed.
- A. H. Aly, R. A. Edrada-Ebel, V. Wray, W. E. G. Müller, S. Kozytska, U. Hentschel, P. Proksch and R. Ebel, Phytochemistry, 2008, 69, 1716–1725 CrossRef CAS PubMed.
- J.-Y. Hwang, S. C. Park, W. S. Byun, D.-C. Oh, S. K. Lee, K.-B. Oh and J. Shin, Mar. Drugs, 2020, 18, 436 CrossRef CAS PubMed.
- P. Phuwapraisirisan, J. Rangsan, P. Siripong and S. Tip-Pyang, Nat. Prod. Res., 2009, 23, 1063–1071 CrossRef CAS PubMed.
- A. Debbab, A. H. Aly, R. Edrada-Ebel, V. Wray, A. Pretsch, G. Pescitelli, T. Kurtan and P. Proksch, European J. Org. Chem., 2012, 1351–1359 CrossRef CAS.
- C. H. Huang, J. H. Pan, B. Chen, M. Yu, H. B. Huang, X. Zhu, Y. J. Lu, Z. G. She and Y. C. Lin, Mar. Drugs, 2011, 9, 832–843 CrossRef CAS PubMed.
- D. Pan, X. Zhang, H. Zheng, Z. Zheng, X. Nong, X. Liang, X. Ma and S. Qi, Org. Chem. Front., 2019, 6, 3252–3258 RSC.
- T. Shi, X. M. Hou, Z. Y. Li, F. Cao, Y. H. Zhang, J. Y. Yu, D. L. Zhao, C. L. Shao and C. Y. Wang, RSC Adv., 2018, 8, 27596–27601 RSC.
- F. Y. Du, X. M. Li, J. Y. Song, C. S. Li and B. G. Wang, Helv. Chim. Acta, 2014, 97, 973–978 CrossRef CAS.
- Y. Li, X. Li, U. Lee, S. K. Jung, D. C. Hong and W. S. Byeng, Chem. Pharm. Bull., 2006, 54, 882–883 CrossRef CAS PubMed.
- Y. Zhang, A. Jia, H. Chen, M. Wang, G. Ding, L. Sun, L. Li and M. Dai, J. Antibiot., 2017, 70, 1138–1141 CrossRef CAS PubMed.
- Y. Chen, X. Cai, J. Pan, J. Gao, J. Li, J. Yuan, L. Fu, Z. She and Y. Lin, Magn. Reson. Chem., 2009, 47, 362–365 CrossRef CAS PubMed.
- J. Kornsakulkarn, W. Choowong, P. Rachtawee, N. Boonyuen, S. Kongthong, M. Isaka and C. Thongpanchang, Phytochem. Lett., 2018, 24, 46–50 CrossRef CAS.
- H. Zhong-Jinga, Y. Run-Yunb, G. U. O. Zhi-Yongb, S. H. E. Zhi-Gangb and L. I. N. Yong-Chengb, Chinese J. Appl. Chem., 2010, 5 Search PubMed.
- X. Ge, C. Sun, Y. Feng, L. Wang, J. Peng, Q. Che, Q. Gu, T. Zhu, D. Li and G. Zhang, Mar. Drugs, 2019, 17, 1–11 CrossRef PubMed.
- X. Wang, T. Tan, Z. G. Mao, N. Lei, Z. M. Wang, B. Hu, Z. Y. Chen, Z. G. She, Y. H. Zhu and H. J. Wang, Mar. Drugs, 2015, 13, 1569–1580 CrossRef CAS PubMed.
- X. Zhu, Z. He, J. Wu, J. Yuan, W. Wen, Y. Hu, Y. Jiang, C. Lin, Q. Zhang, M. Lin, H. Zhang, W. Yang, H. Chen, L. Zhong, Z. She, S. Chen, Y. Lin and M. Li, Mar. Drugs, 2012, 10, 694–711 CrossRef CAS PubMed.
- C. Chen, W. Xiao, X. Jiang, J. Wang, Z. Mao, N. Lei, X. Fan, B. Song, C. Liao and H. Wang, Curr. Med. Chem., 2013, 20, 2145–2154 CrossRef CAS PubMed.
- S. Klaiklay, V. Rukachaisirikul, S. Phongpaichit, C. Pakawatchai, S. Saithong, J. Buatong, S. Preedanon and J. Sakayaroj, Phytochem. Lett., 2012, 5, 738–742 CrossRef CAS.
- G. Brauers, R. A. Edrada, R. Ebel, P. Proksch, V. Wray, A. Berg, U. Gräfe, C. Schächtele, F. Totzke, G. Finkenzeller, D. Marme, J. Kraus, M. Münchbach, M. Michel, G. Bringmann and K. Schaumann, J. Nat. Prod., 2000, 63, 739–745 CrossRef CAS PubMed.
- R. Jadulco, G. Brauers, R. A. Edrada, R. Ebel, V. Wray, Sudarsono and P. Proksch, J. Nat. Prod., 2002, 65, 730–733 CrossRef CAS PubMed.
- P. Wang, Y. Cui, C. Cai, H. Chen, Y. Dai, P. Chen, F. Kong, J. Yuan, X. Song, W. Mei and H. Dai, Mar. Drugs, 2019, 17, 1–9 Search PubMed.
- Z. Niu, Y. Chen, H. Guo, S. N. Li, H. H. Li, H. X. Liu, Z. Liu and W. Zhang, Molecules, 2019, 24, 1–9 Search PubMed.
- C. Shao, C. Wang, M. Wei, S. Li, Z. She, Y. Gu and Y. Lin, Magn. Reson. Chem., 2008, 46, 886–889 CrossRef CAS PubMed.
- H. Li, J. Wei, S. Y. Pan, J. M. Gao and J. M. Tian, Nat. Prod. Res., 2014, 28, 2358–2361 CrossRef CAS.
- R. P. Maskey, I. Grün-Wollny and H. Laatsch, J. Antibiot., 2003, 56, 459–463 CrossRef CAS PubMed.
- F. Zhu, G. Chen, X. Chen, Y. Yuan, M. Huang, W. Xiang and H. Sun, Biomed. Eng. Informatics New Dev. Futur. - Proc. 1st Int. Conf. Biomed. Eng. Informatics, BMEI 2008, 2008, 1, pp. 664–667 Search PubMed.
- C. Shao, Z. She, Z. Guo, H. Peng, X. Cai, S. Zhou, Y. Gu and Y. Lin, Magn. Reson. Chem., 2007, 45, 434–438 CrossRef CAS PubMed.
- J. Tao, T. Morikawa, S. Ando, H. Matsuda and M. Yoshikawa, Chem. Pharm. Bull., 2003, 51, 654–662 CrossRef CAS PubMed.
- C. Eder, H. Kogler and L. Toti, WO 2003002549, 2003.
- A. Debbab, A. H. Aly, R. A. Edrada-Ebel, V. Wray, W. E. G. Müller, F. Totzke, U. Zirrgiebel, C. Schächtele, M. H. G. Kubbutat, H. L. Wen, M. Mosaddak, A. Hakiki, P. Proksch and R. Ebel, J. Nat. Prod., 2009, 72, 626–631 CrossRef CAS PubMed.
- C. Shao, C. Wang, C. Zheng, Z. She, Y. Gu and Y. Lin, Nat. Prod. Res., 2010, 24, 81–85 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra03610j |
| This journal is © The Royal Society of Chemistry 2022 |