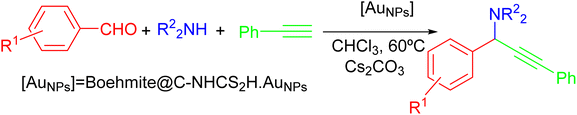Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Gold nanoparticle decorated dithiocarbamate modified natural boehmite as a catalyst for the synthesis of biologically essential propargylamines†
Elham Zarenezhad a,
Reza Taghavib,
Parisa Kamranib,
Mojtaba Farjama and
Sadegh Rostamnia
a,
Reza Taghavib,
Parisa Kamranib,
Mojtaba Farjama and
Sadegh Rostamnia *b
*b
aNoncommunicable Diseases Research Center, Fasa University of Medical Sciences, Fasa, Iran. E-mail: el.zarenezhad@fums.ac.ir
bOrganic and Nano Group (ONG), Department of Chemistry, Iran University of Science and Technology (IUST), PO Box 16846-13114, Tehran, Iran. E-mail: rostamnia@iust.ac.ir
First published on 7th November 2022
Abstract
Here, we prepare an Au NP decorated dithiocarbamate functionalized boehmite (γ-AlO(OH)@C-NHCS2H·AuNPs). This stepwise synthetic method gives an efficient, cost-effective, and green heterogenous Au-based nanocatalyst for the A3-coupling preparation of the biologically essential propargylamines. Different characterization methods, including FT-IR, XRD, SEM, TEM, EDX spectra, and elemental SEM-mapping, were employed to investigate the structure of the manufactured γ-AlO(OH)@C-NHCS2H·AuNPs. Then we used the prepared composite as a heterogeneous gold-based nanocatalyst for the one-pot A3-coupling preparation of propargyl amines by reacting a variety of aldehydes, amines, and phenylacetylene which exhibited promising results.
1 Introduction
The term A3-coupling reaction refers to the one-pot three-component reaction of an aldehyde, an amine, and a terminal alkyne in the presence of a transition metal to yield propargyl amines. Due to the broad application of such compounds in organic synthesis, especially as intermediates for preparing medicinally essential compounds, the A3-coupling preparation of propargyl amines has attracted a great deal of attention in the past two decades, and considerable progress has been achieved.1,2 This class of compounds is widely applied toward neurodegenerative disorders such as Alzheimer's and Parkinson's diseases. Many approved drugs, including rasagiline (I), pargyline (II), and selegiline (III) have a propargylamine framework. Such compounds possess monoamine oxidase (MAO) inhibitory properties, and the use of these compounds has been reported in mitochondrial protection and providing neuronal properties (Scheme 1).3–5 Utilization of metal NPs helps the progress of this reaction by increasing the acidity of the C–H bond of the terminal alkyne.6 To date, various transition metal NPs such as Au, Cu, and Ag have been employed to promote this reaction.7–9Recently, gold NPs were applied as catalysts to promote various reactions, including degradation of environmental pollution, hydrogen generation, organic transformation, etc.10–13 Such efforts help to disbelief the traditional idea that gold suffers from the lack of catalytic properties. There are various reports on preparing propargyl amines using Au NPs as catalysts.14–17 Gold NPs increases the selectivity and yield of the preparation of propargyl amines due to their small size, high surface-to-volume ratio, and intrinsic properties.6 The catalytic properties of the gold NPs are directly influenced by their size and shape. When the gold NPs grow to the micro size regime, their catalytic properties are entirely quenching.18 Therefore, various supports were employed to prevent the aggregation of gold NPs.
The critical factor for preparing stable metallic NPs and discovering their catalytic properties was using supports and stabilizers.19–21 To date, different supports, such as metal–organic frameworks, nanocrystalline magnesium oxide, alumina, cerium oxide, magnetic NPs, etc., are employed for stabilization and modification of the catalytic performance of Au NPs for the A3-coupling reaction.7,22–25 A metal NP supported on a nano-size heterogenous support benefits from the high selectivity, preventing the aggregation of the metal NPs, high dispersion in a liquid medium, and high reusability.18
Boehmite (γ-AlO(OH)), as a binary layer derivative of aluminum, finds its way into heterogeneous catalyst fields due to its insolubility, inexpensive precursors, commercial availability, non-toxicity, corrosion resistance, high chemical and mechanical stability, high dispersity, and accessible surface modification.26,27 The layers of boehmite are connected through hydrogen bonding, and a high-density layer of hydroxyl groups covers its surface. Such structure allows easy modification of its surface for desired applications.26
In this report, we modified the surface of the commercially available natural boehmite with the APTES ligand. Then, –NH2 modified boehmite was further mutated to the dithiocarbamate functional group decorated composite by adding CS2 and was utilized to support the stabilization of Au NPs. The resulting γ-AlO(OH)@C-NHCS2H·AuNPs catalyst was used as a heterogeneous catalyst for A3-coupling preparation of propargyl amines, which exhibited excellent results.
2 Experimental
2.1 Synthesis of γ-AlO(OH)@C-NHCS2H nanoparticles
In a 100 mL round-bottom flask, to 40 mL anhydrous toluene solution of boehmite (2 g), 4 mL of (3-aminopropyl) triethoxysilane (APTES) was added dropwise. The obtained solution was heated to 100 °C and stirred for 48 h. γ-AlO(OH)@C-NH2 was washed with H2O and dried in an oven overnight. Next, γ-AlO(OH)@C-NH2 was dispersed in 20 mL ethanol under sonication for 10 min 2.2 mL CS2 was added to the above solution and stirred for another 12 h. Dithiocarbamate functionalized boehmite (γ-AlO(OH)@C-NHCS2H) was washed with ethanol several times and dried at 70 °C overnight.2.2 Immobilization of AuNPs onto the surface of γ-AlO(OH)@C-NHCS2H
Immobilization of Au nanoparticles onto the surface of dithiocarbamate functionalized Boehmite (γ-AlO(OH)@C-NHCS2H) was performed based on the reported method in the literature.28 Generally, 0.3 g of γ-AlO(OH)@C-NHCS2H was added to 10 mL of 0.12 M aqueous solution of HAuCl4 gradually under sonication. This solution kept stirring for 24 h. The heterogeneous catalyst was separated from the solution by filtration and washed with ethanol (EtOH) for several times to gain γ-AlO(OH)@C-NHCS2H·AuCl3. Finally, 5 mL of freshly prepared NaBH4 in methanol (0.2 mol L−1) was added dropwise to the mixture of as prepared γ-AlO(OH)@C-NHCS2H·AuCl3. This solution was stirred for another 30 min, then filtered and washed with methanol to obtain pure γ-AlO(OH)@C-NHCS2H·AuNPs.2.3 General synthesis of propargyl amines
In a general process, one mmol of aromatic aldehyde, 1.2 mmol of amine, 1.3 mmol of phenylacetylene, and 0.4 g of γ-AlO(OH)@C-NHCS2H·AuNPs as a heterogeneous catalyst, and one mmol of Cs2CO3 as a base were added to 4 mL of CHCl3 at 60 °C and stirred for an appropriate time (Table 2). Next, the heterogeneous catalyst was removed from the solution mixture simply by filtration. Finally, the reaction solvent was evaporated, and corresponding propargyl amines were purified by plate-chromatography. The resulting propargyl amines were investigated by 1H NMR and 13C NMR (ESI†).3 Results and discussion
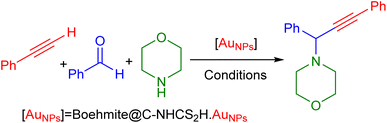
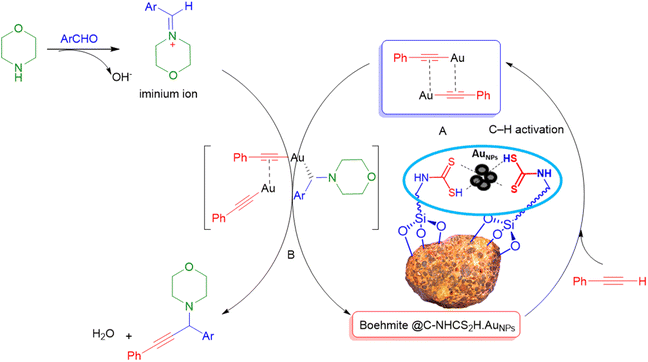
Due to our interest in developing green and sustainable methodologies for various organic synthesis reactions, herein we report a facile and efficient synthesis of propargyl amines via A3-coupling reaction using aldehyde, amine, and alkyne in the presence of the γ-AlO(OH)@C-NHCS2H·AuNPs nanocatalyst. First, natural boehmite (γ-AlO(OH)) was functionalized with the APTES to give an amine-rich surface. Then, CS2 was added to the amine-functionalized boehmite solution to cover its surface with a skin of dithiocarbamate (DTC). Finally, Au NPs were stabilized on the surface of the dithiocarbamate-functionalized boehmite to prepare the γ-AlO(OH)@C-NHCS2H·AuNPs catalyst. We employed the crafted nanocomposite as a catalyst for the A3-coupling reaction of aldehydes and amines with phenylacetylene under optimal conditions. A schematic illustration of the γ-AlO(OH)@C-NHCS2H·AuNPs synthesis is shown in Scheme 2.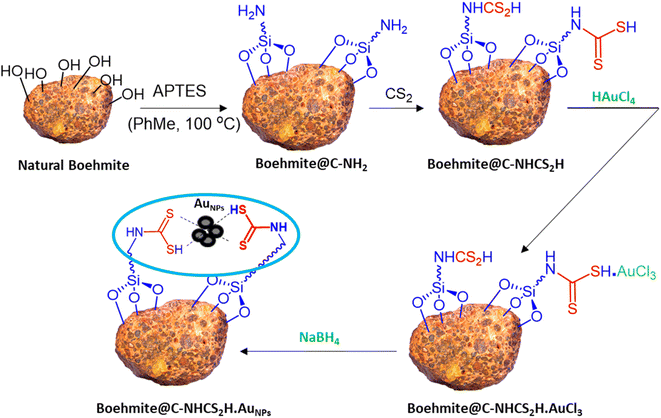 | ||
| Scheme 2 Schematic presentation of the immobilization of Au onto the surface of the γ-AlO(OH)@C-NHCS2H. | ||
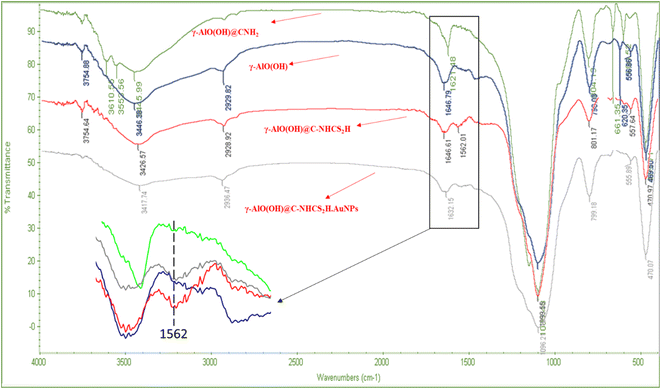
Fig. 1 represents the FT-IR spectra of as-synthesized bare γ-AlO(OH), γ-AlO(OH)@C-NH2, γ-AlO(OH)@C-NHCS2H, and γ-AlO(OH)@C-NHCS2H·AuNPs. For the bare γ-AlO(OH), the peak at 469 regarding octahedral morphology of Al. Bending vibration of Al–OH appeared at 1099 cm−1, and the stretching vibration of the OH group linked to the Al showed itself at 3446 cm−1. For the γ-AlO(OH)@C-NHCS2H, peaks in 2900–3000 cm−1 and 3426 cm−1 regions, probably regarding C–H of carbon groups of the APTES, while O–H functional groups probably attribute to the surface of boehmite. As shown in the spectra of γ-AlO(OH)@C-NHCS2H·AuNPs, the characteristic peak of –NH at 1646 cm−1 is red shifted to 1632 cm−1 due to the surface change of bifunctional hybrid nanomaterials. In this case, the characteristic of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S groups revealed itself at 1562 cm−1, and the broadband of the boehmite covers its other characteristics. These can be proof of the successful synthesis of γ-AlO(OH)@C-NHCS2H·AuNPs. To further prove the formation of the catalyst, EDX spectra and elemental SEM mapping were used to identify the presence of the elements in its structure (Fig. 2).
S groups revealed itself at 1562 cm−1, and the broadband of the boehmite covers its other characteristics. These can be proof of the successful synthesis of γ-AlO(OH)@C-NHCS2H·AuNPs. To further prove the formation of the catalyst, EDX spectra and elemental SEM mapping were used to identify the presence of the elements in its structure (Fig. 2).
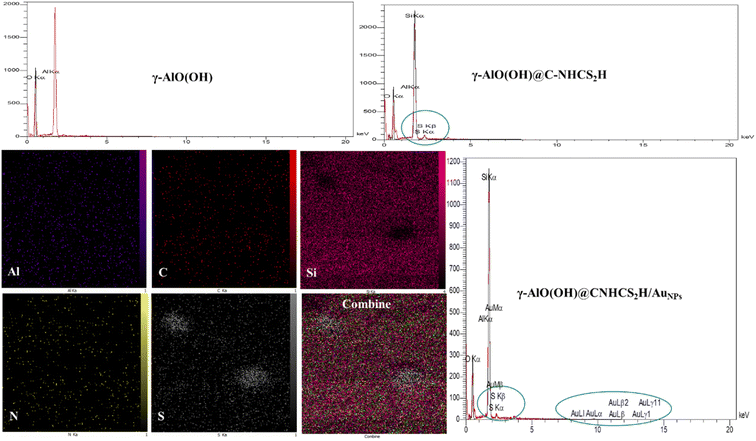
To determine the composition and the spatial elemental distribution of the synthesized γ-AlO(OH)@C-NHCS2H·AuNPs, EDX spectra and elemental SEM-mapping were hired (Fig. 2). The results revealed the characteristic peaks of Al, S, O, Si, and Au atoms in the spectrum, indicating the purity and successful deposition of Au onto the γ-AlO(OH)@C-NHCS2H support. wt% of Au in the catalyst was 1.36%. Since the attained results for wt% of Au were within the limit of detection for the EDX technique, atomic absorption spectrometry (AAS) was applied to confirm the accuracy of the obtained data. AAS confirms the result of EDX with a 2% percentage error.
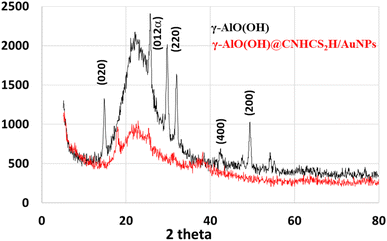
Fig. 3 represents the XRD pattern of the bare γ-AlO(OH) and γ-AlO(OH)@C-NHCS2H·AuNPs. The XRD pattern of the bare boehmite shows all the characteristics peaks with a good intensity which specifies its phase purity. The XRD pattern of the modified composite shows less intensity for the characteristic peaks of the boehmite, which is a result of the successful modification of the bare surface of the catalyst. No diffractions were detected for Au NP species from XRD patterns. The reason could be due to the amorphous structure, small size, or low concentration of gold NPs compared to the support (1.36 wt%).29
FESEM and TEM were employed to investigate the morphology of the synthesized catalyst (Fig. 4). The FESEM image (Fig. 4a) reveals agglomerated morphology of γ-AlO(OH)@C-NHCS2H·AuNPs catalyst. The TEM images provide a more detailed insight into the surface of the ensembled catalyst and prove that the functionalization of its surface with dithiocarbamate perfectly prevents aggregation of Au NPs (Fig. 4c and d).
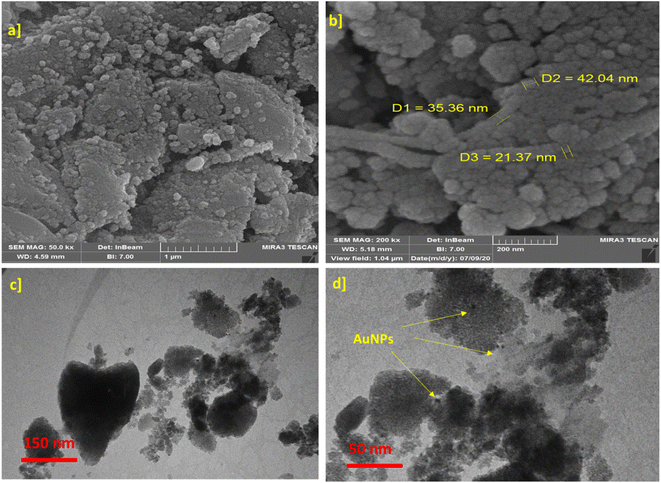 | ||
| Fig. 4 (a) SEM γ-AlO(OH), (b) SEM γ-AlO(OH)@C-NHCS2H·AuNPs. (c and d) TEM of γ-AlO(OH)@C-NHCS2H·AuNPs. | ||
3.1 Catalytic performances
We lunch the study of catalytic performance of the γ-AlO(OH)@C-NHCS2H·AuNPs toward the A3-coupling reaction by using the morpholine, benzaldehyde, and phenylacetylene as the precursors. In this context, we examine the effects of various parameters such as temperature, reaction solvents, reaction time, base, and amount of catalyst to reach the optimum condition. We initiated our study by probing the effect of various solvents on the progress of this reaction (Table 1). Results show that the EtOH and EG solvent restricted the progress of the reaction while other solvents such as acetonitrile and chloroform promoted the A3-coupling reaction in good yield. Table 1 indicates that 0.1 mol% of gold NPs onto the γ-AlO(OH)@C-NHCS2H·AuNPs in CHCl3 solvent gives the most satisfying results (Table 1, entry 5).| Entry | Solvent | Base | Temp. (°C) | Cat. (mol%) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1.2 mmol of morpholine, 1 mmol of benzaldehyde, and 1.3 mmol of phenylacetylene.b Isolated yields. | ||||||
| 1 | CH3CN | KOH | 60 | 0.05 | 10 | 75 |
| 2 | CH3CN | KOH | 60 | 0.1 | 10 | 75 |
| 3 | EtOH | KOH | 80 | 0.05 | 10 | 45 |
| 4 | EG | KOH | 100 | 0.05 | 10 | 61 |
| 5 | CHCl3 | K2CO3 | 60 | 0.1 | 10 | 92 |
| 6 | CHCl3 | Cs2CO3 | 60 | 0.1 | 10 | 96 |
| 7 | CHCl3 | KOH | 60 | 0.1 | 10 | 87 |
| 9 | CHCl3 | NaOH | 60 | 0.1 | 10 | 84 |
| 10 | CHCl3 | Et3N | 60 | 0.1 | 10 | 76 |
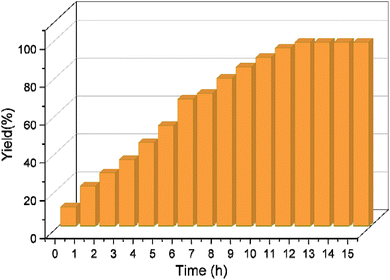
We further investigate the catalytic performance of boehmite@C-NHCS2H·AuNPs toward the A3-coupling of the model reaction by monitoring the effect of different reaction temperatures and bases. First, we investigate the impact of the temperature on the yield of the reaction. The results indicate that the reaction perfectly proceeds at 60 °C, and a further increase in temperature did not cause considerable changes in the yield of the reaction. Next, we studied the effect of various bases including Et3N, NaOH, KOH, K2CO3, and Cs2CO3 on the model A3-coupling reaction. This study showed that using Cs2CO3 and K2CO3 as bases yielded the best results (Table 1, entry 4–5). Fig. 5 represents the results of the studies of the optimum reaction time (0.1 mol% of gold NPs onto the γ-AlO(OH)@C-NHCS2H·AuNPs, Cs2CO3 as the base and 60 °C as reaction temperature). Here, the progress of the reaction was investigated by TLC. The maximum yield was achieved after 12 h, as is evident.
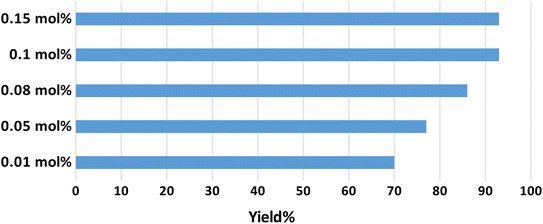
Optimization of the catalyst amount was performed by testing the catalytic performance of several amounts of γ-AlO(OH)@C-NHCS2H·AuNPs in the progress of the model reaction. The results indicate that using 0.1 mol% of γ-AlO(OH)@C-NHCS2H·AuNPs (per each mol of the substrate) leads to the maximum yield (Fig. 6).
After optimizing the condition of the model reaction (0.1 mol% of gold NPs onto the γ-AlO(OH)@C-NHCS2H·AuNPs, Cs2CO3 as the base and 60 °C as reaction temperature), the generality of the catalyst toward the A3-coupling reaction has been tested in the presence of a variety of aldehydes, amines, and phenylacetylene. All the subsequent reactions have been performed at the optimum condition in the presence of Cs2CO3 as the base. The results of this study are summarized in Table 2. These results indicate that the proposed catalyst can promote the A3-coupling reaction for various aldehydes with the electron-donor or electron-withdrawing groups in the ortho or meta position of the aromatic ring. Table 2 also specifies that the reaction's progress is influenced by the electronic characteristics of the aromatic ring of the aldehyde and the yield of the reaction differs from case to case.
| Entry | Time (h) | R22NH | R1 | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1.2 mmol of morpholine, 1 mmol of benzaldehyde, and 1.3 mmol of phenylacetylene.b Isolated yields in 4 mL of CHCl3. | ||||
| 1 | 12 | Piperidine | H | 96 |
| 2 | 15 | Morpholine | H | 80 |
| 3 | 18 | Piperidine | 4-Me | 75 |
| 4 | 18 | Morpholine | 4-Me | 73 |
| 5 | 16 | Piperidine | 2-Cl | 79 |
| 6 | 18 | Morpholine | 2-Cl | 68 |
| 7 | 18 | Morpholine | 4-OMe | 85 |
| 8 | 16 | Piperidine | 2-Me | 85 |
| 9 | 18 | Morpholine | 2-Me | 73 |
Reusability of the catalyst is a crucial feature for its scalable applications. To test the reusability of the proposed γ-AlO(OH)@C-NHCS2H·AuNPs catalyst for A3-coupling reaction of the model reaction, the catalyst was separated from the reaction medium and used for the same reaction at optimum condition for five times. After each run, the catalyst was thoroughly washed with the ethyl acetate several times to ensure the purity of the recycled catalyst, so the reaction kinetic be the same for all the cycles. As shown in Fig. 7a, the yield of the reaction remains almost the same for four cycles but it suddenly drops in the fifth cycle.
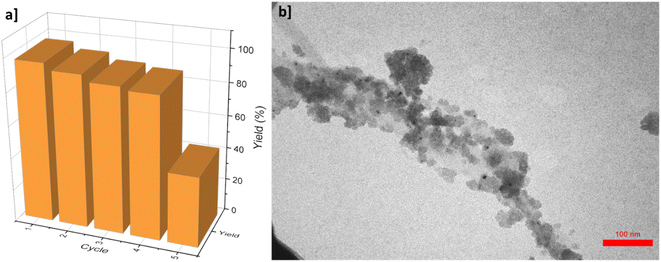 | ||
| Fig. 7 (a) Recyclability of γ-AlO(OH)@C-NHCS2H·AuNPs for model A3-coupling reaction (b) TEM image of the proposed catalyst after the 4th recycling period. | ||
Atomic absorption spectrometry (AAS) was employed to track the presence of the Au NPs in the reaction solution, which proves the existence of negligible amounts of the Au NPs in the solution medium, even after four cycles. TEM image of the catalyst after the 4th run reveals its formidable firmness under the reaction conditions (Fig. 7b).
It is appraises that the γ-AlO(OH)@C-NHCS2H·AuNPs catalyst helps the progress of the reaction via the Heaney and co-workers mechanism_ENREF_66. At first, gold NPs decorated catalyst helps the activation of C–H of phenylacetylene. Then, coordination of the obtained complex with iminium ion gives an intermediate. Finally, the addition of the alkynylide to iminium ion results in desired propargyl amines (Scheme 3).
4 Conclusion
In conclusion, we synthesis an Au NPs decorated dithiocarbamate functionalized boehmite as a cost-effective, green, and efficient heterogeneous catalyst for the one-pot three-component A3-coupling preparation of propargyl amines at 60 °C. The γ-AlO(OH)@C-NHCS2H·AuNPs catalyst managed to preserve its structure during the preparation procedure and successfully stabilizes monodispersed Au NPs perfectly. The proposed catalyst promotes the preparation of propargyl amines in good yields with a variety of aromatic aldehydes, possessing electron donor and electron-withdrawing groups in different positions of the aromatic ring and showing good recyclability up to four cycles.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors wish to thank the support of Noncommunicable Diseases Research Center, Fasa University of Medical Sciences (Grant No. 99226).References
- B. V. Rokade, J. Barker and P. J. Guiry, Chem. Soc. Rev., 2019, 48, 4766–4790 RSC
.
- B. Gholipour, S. Shojaei, S. Rostamnia, M. R. Naimi-Jamal, D. Kim, T. Kavetskyy, N. Nouruzi, H. W. Jang, R. S. Varma and M. Shokouhimehr, Green Chem., 2021, 23, 6223–6272 RSC
.
- R. Manujyothi, T. Aneeja and G. Anilkumar, RSC Adv., 2021, 11, 19433–19449 RSC
.
- O. Weinreb, T. Amit, O. Bar-Am and M. B. H. Youdim, Prog. Neurobiol., 2010, 92, 330–344 CrossRef CAS PubMed
.
- M. B. H. Youdim, O. B. Am, M. Yogev-Falach, O. Weinreb, W. Maruyama, M. Naoi and T. Amit, J. Neurosci. Res., 2005, 79, 172–179 CrossRef CAS PubMed
.
- M. Nasrollahzadeh, M. Sajjadi, F. Ghorbannezhad and S. M. Sajadi, Chem. Rec., 2018, 18, 1409–1473 CrossRef CAS PubMed
.
- K. O. Aghbash, H. Alamgholiloo, N. N. Pesyan, S. Khaksar and S. Rostamnia, Mol. Catal., 2021, 499, 111252 CrossRef CAS
.
- X. Liu, X. Tan, Y. Zhou, Y. Li and Z. Zhang, Res. Chem. Intermed., 2019, 45, 3359–3378 CrossRef CAS
.
- P. Akbarzadeh and N. Koukabi, Appl. Organomet. Chem., 2019, 34, e5395 Search PubMed
.
- H. Veisi, P. Abassi, P. Mohammadi, T. Tamoradi and B. Karmakar, Sci. Rep., 2021, 11, 1–17 CrossRef PubMed
.
- S. Mondal, L. Sahoo, C. P. Vinod and U. K. Gautam, Appl. Catal., B, 2021, 286, 119927 CrossRef CAS
.
- Z. Zhu, X. Gao, X. Wang, M. Yin, Q. Wang, W. Ren, B. Wang, H. Lü and W. Liao, J. Energy Chem., 2021, 62, 599–609 CrossRef CAS
.
- M. M. M. Naixin Kang, Q. Wang, R. Djeda, W. Wang, F. Fu, J.-L. P. Maria de los Angeles Ramirez, S. Moya, E. Coy, L. Salmon and D. Astruc, ACS Appl. Mater. Interfaces, 2020, 12, 53816–53826 CrossRef PubMed
.
- M. Nourmohammadi, S. Rouhani, S. Azizi, M. Maaza, T. A. M. Msagati, S. Rostamnia, M. Hatami, S. Khaksar, E. Zarenezhad, H. W. Jang and M. Shokouhimehr, Mater. Today Commun., 2021, 29, 102798 CrossRef CAS
.
- K. Lauder, A. Toscani, N. Scalacci and D. Castagnolo, Chem. Rev., 2017, 117, 14091–14200 CrossRef CAS PubMed
.
- G. B. María, K. Peters, G. L. Hallett-Tapley, M. Grenier and J. C. Scaiano, Chem. Commun., 2013, 49, 1732–1734 RSC
.
- G. Shore, W. J. Yoo, C. J. Li and M. G. Organ, Chem.–Eur. J., 2010, 16, 126–133 CrossRef CAS PubMed
.
- M. Sankar, Q. He, R. V. Engel, M. A. Sainna, A. J. Logsdail, A. Roldan, D. J. Willock, N. Agarwal, C. J. Kiely and G. J. Hutchings, Chem. Rev., 2020, 120, 3890–3938 CrossRef CAS PubMed
.
- H. Alamgholiloo, S. Rostamnia, A. Hassankhani, X. Liu, A. Eftekhari, A. Hasanzadeh, K. Zhang, H. Karimi-Maleh, S. Khaksar, R. S. Varma and M. Shokouhimehr, J. Colloid Interface Sci., 2020, 567, 126–135 CrossRef CAS PubMed
.
- A. Ahadi, H. Alamgholiloo, S. Rostamnia, X. Liu, M. Shokouhimehr, D. A. Alonso and R. Luque, ChemCatChem, 2019, 11, 4803–4809 CrossRef CAS
.
- H. Karimi-Maleh, M. Alizadeh, Y. Orooji, F. Karimi, M. Baghayeri, J. Rouhi, S. Tajik, H. Beitollahi, S. Agarwal, V. K. Gupta, S. Rajendran, S. Rostamnia, L. Fu, F. Saberi-Movahed and S. Malekmohammadi, Ind. Eng. Chem. Res., 2021, 60, 816–823 CrossRef CAS
.
- K. Layek, R. Chakravarti, M. Lakshmi Kantam, H. Maheswaran and A. Vinu, Green Chem., 2011, 13, 2878–2887 RSC
.
- A. Corma, J. Navas and M. J. Sabater, Chem.–Eur. J., 2012, 18, 14150–14156 CrossRef CAS PubMed
.
- L. Abahmane, J. M. Köhler and G. A. Grob, Chem.–Eur. J., 2011, 17, 3005–3010 CrossRef CAS PubMed
.
- L. Liu, X. Tai, G. Yu, H. Guo and Q. Meng, Chem. Res. Chin. Univ., 2016, 32, 443–450 CrossRef CAS
.
- M. Mohammadi, M. Khodamorady, B. Tahmasbi, K. Bahrami and A. Ghorbani-Choghamarani, J. Ind. Eng. Chem., 2021, 97, 1–78 CrossRef CAS
.
- P. Paluch, N. Potrzebowska, A. M. Ruppert and M. J. Potrzebowski, Solid State Nucl. Magn. Reson., 2017, 84, 111–117 CrossRef CAS PubMed
.
- J. Huang, D. G. Gray and C. Li, Beilstein J. Org. Chem., 2013, 9, 1388–1396 CrossRef PubMed
.
- H. L. Jiang, B. Liu, T. Akita, M. Haruta, H. Sakurai and Q. Xu, J. Am. Chem. Soc., 2009, 32, 11302–11303 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra03725d |
| This journal is © The Royal Society of Chemistry 2022 |