Open Access Article
Open Access ArticleColorimetric screening of elevated urinary mercury levels by a novel Hg2+-selective probe of resorufin phosphinothioate†
Myung Gil Choi ,
Byung Hoon Yun,
Hyeong Min Kim
,
Byung Hoon Yun,
Hyeong Min Kim ,
Sangdoo Ahn
,
Sangdoo Ahn * and
Suk-Kyu Chang
* and
Suk-Kyu Chang *
*
Department of Chemistry, Chung-Ang University, Seoul 06974, Republic of Korea. E-mail: sangdoo@cau.ac.kr; skchang@cau.ac.kr; Fax: +82 2 825 4736; Tel: +82 2 820 5199
First published on 24th August 2022
Abstract
Urinary mercury levels are the most reliable indicators of mercury exposure but identifying them requires complex techniques and heavy instruments. In this research, we reported a simple and convenient urinary mercury analysis method using a readily available office scanner. Probe MP-1 synthesized by the reaction of resorufin and dimethylthiophosphinoyl chloride revealed Hg2+-selective chromogenic and fluorescent signaling behavior. Signaling was realized through Hg2+-induced deprotection of the phosphinothioate protecting group in the resorufin-based probe MP-1 to yield the parent fluorochrome. A pronounced colorimetric response of color change from light yellow to pink alongside a turn-on type fluorescence enhancement was perceived exclusively toward Hg2+ ions over other metal ions and anions. The colorimetry provided a more advantageous ratiometric approach than the simple fluorometric analysis exhibiting an off–on type response, with a detection limit of 12 nM (2.4 ppb). The Hg2+ signaling of the MP-1 probe was not disturbed by the presence of coexisting metal ions and anions. The sensitive and convenient diagnosis of clinically important neurological symptoms and fatal inorganic mercury levels in urine was successfully demonstrated using a standard office scanner.
1. Introduction
Monitoring and removal of harmful water contaminants from polluted water are important since water pollution has become a serious environmental problem in our heavily industrialized society.1–3 Emissions of toxic pollutants, including inorganic, heavy metal, and organic contaminants, are increased substantially by various chemical and related fields such as refinery, smelter, fertilizer, pulp, and pharmaceutical industries.4 In particular, mercury is one of the most hazardous inorganic pollutants currently causing great concern in modern society and attracting much global attention for its negative impacts on our health and environment.5 It exhibits highly bio-accumulative properties in the food chain, producing mostly neurological and immune system damage.6 Mercury has many industrial applications, from the manufacturing of fluorescent light bulbs, jewelry, thermometers, and sphygmomanometers, in addition to cosmetics, insecticides, and pharmaceuticals.7 Currently, a pressing concern for workers in mercury-exposed sectors is the prevention and remediation of mercury poisoning.8 Mercury, which may be absorbed in inorganic and organic forms, is transformed into the most prevalent form of Hg2+ ions and can accumulate in the body of both humans and animals.9 Even modest levels of Hg2+ poisoning can harm human health by causing damage to the brain and kidneys, as well as endocrine and neurological systems.10,11 Consequently, the selective and sensitive quantification of trace mercury in both environmental and biological systems is a critical issue.Blood, urine, hair, or fingernail samples are commonly used for clinical mercury analyses.12 Commonly, urine samples have been widely employed as a viable biomarker for assessing the risk of exposure to mercurial species because their collection and management are non-invasive and simple.13 Since inorganic mercury species are absorbed and deposited mostly in the proximal tubules of the kidneys, urinary mercury levels are one of the most acceptable indicators of chronic and acute exposure.14 According to a recent study, the average mercury content in the urine of mercury-exposed miners was over 75 times higher than the control groups. The exposed miners suffered typical symptoms of mercury intoxication: digestive issues, hypomnesia, sleeping problems, tremors, and weight loss.15 It is well known that urinary mercury concentrations greater than 100 μg L−1 cause neurological symptoms, whereas concentrations exceeding 800 μg L−1 are frequently linked to mortality.16
Many standard analytical techniques have been employed to determine elevated mercury concentrations in urine samples, including cold-vapor atomic-absorption spectrometry,17 inductively coupled plasma mass spectrometry,18 surface-enhanced Raman scattering,19 bioluminescence,20 and voltammetry.21 Despite their great sensitivity and specificity for measuring mercurial species, these methods necessitate costly equipment, time-consuming sample preparation, and highly trained staff, and they can exhibit cross-sensitivity to other metal ions. Therefore, they might not be suitable for the convenient, selective, and sensitive on-site detection of Hg2+ ions. Consequently, it remains desirable to implement a simple, direct, and low-cost approach, such as optical sensors and molecular probes that create optical responses induced by the binding and/or reaction event for detecting Hg2+.22,23 In light of these, many colorimetric and/or fluorescence-based probes for the determination of urinary mercury levels have been reported. For example, carbothiohydrazide-appended diphenyl ether,24 thiocarbonate derivative of perylene diimide dye,25 dithioacetal derivative of bithiophene fluorophore,26 and terbium complex of 7-amino-4-methyl-2(1H)-quinolinone27 have been exploited for the urinary mercury detection. In addition, carbon dots,28 DNA templated gold nanoparticles, and gold nanoclusters have also been developed for the determination of Hg2+ ions in urine samples.29,30 A summary of the urinary mercury sensing system based on colorimetric and/or fluorescence signaling is presented in Table S1 (ESI†).
Recently, many elaborately designed optical sensors31 and reaction-based small molecule probes have been developed to selectively and sensitively determine Hg2+ ions.32,33 In particular, reaction-based probes relying on specific reactions with Hg2+ ions, such as Hg2+-induced ring-opening reactions of rhodamine and fluorescein dyes,34 protection–deprotection processes of phenolic dyes and aldehyde/ketone groups,35 and desulfurization of thiocarbonyl functions, have been consistently developed.36 We focused on the development of a reaction-based probe for Hg2+ ions, utilizing the specific and mild deprotection property of the phosphinothioate group. The phosphinothioate function has been exploited as a useful protection group for the phenol, thiol, and tryptophan groups in solid-phase peptide synthesis.37,38 Mercury, silver, and fluoride ions have been effectively employed in the deprotection of these phosphinothioate-protected derivatives.39 However, it has rarely been used to develop optical probes to analyze metal ions, anions, or other important industrial, environmental, and biological species.40–43 Interestingly, the thiophosphoryl-containing compounds have similar stability to their phosphoryl analogs, which contrasts the significantly enhanced reactivity of thiocarbonyl compounds when compared to their analogous carbonyl derivatives.44 Additionally, thiophosphoryl derivatives having the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S function possess the ability to form complexes with softer metal ions.45 We believe that the stability against spontaneous hydrolysis and softer ligating nature of the phosphinothioate group are advantageous features for a reaction-based probe to analyze thiophilic Hg2+ ions.
S function possess the ability to form complexes with softer metal ions.45 We believe that the stability against spontaneous hydrolysis and softer ligating nature of the phosphinothioate group are advantageous features for a reaction-based probe to analyze thiophilic Hg2+ ions.
Here, we developed a simple colorimetric method focusing on the easy determination of urinary mercury concentrations. For the convenient detection of Hg2+ ions, a novel reaction-based dual signaling probe was synthesized by employing well-established resorufin as a signaling fluorochrome and a phosphinothioate protection group as a signaling switch. The probe showed selective and sensitive colorimetric and fluorogenic signaling properties exclusively toward Hg2+ ions. As a practical application, we could easily determine the two clinically relevant urinary mercury levels causing neurological symptoms (>100 ppb of Hg2+) or leading to fatality (>800 ppb of Hg2+). Furthermore, the sensitive and convenient diagnosis of these clinically crucial inorganic mercury levels in urine was successfully demonstrated using a typical office scanner without resorting to complicated instruments.
2. Experimental
2.1 Synthesis of MP-1
Resorufin sodium salt (470 mg, 2.0 mmol) was dissolved in N,N-dimethylformamide (DMF, 24 mL), before triethylamine (TEA, 540 μL, 4.0 mmol) was added to the mixture. After 30 minutes, dimethylthiophosphinoyl chloride (380 μL, 3.0 mmol) was added to the reaction pot and mixed for 12 hours at room temperature. Deionized (DI) water (50 mL) was added to the reaction mixture, and the resulting precipitate was filtered. The solid was dissolved in dichloromethane (DCM) and washed with DI water several times. The DCM phase was collected and dried with anhydrous MgSO4. The solvent was evaporated under reduced pressure, and the remnant was purified by column chromatography (silica gel, eluent: DCM) to yield scarlet powder (0.50 g, 82%). 1H NMR (600 MHz, DMSO-d6) δ 7.87 (dd, J = 8.7, 0.7 Hz, 1H), 7.56 (d, J = 9.8 Hz, 1H), 7.39 (dd, J = 2.5, 1.6 Hz, 1H), 7.28 (ddd, J = 8.7, 2.6, 1.4 Hz, 1H), 6.83 (dd, J = 9.8, 2.1 Hz, 1H), 6.31 (d, J = 2.1 Hz, 1H), 2.11 (d, J = 13.5 Hz, 6H); 13C NMR (150 MHz, CDCl3) δ 186.1, 153.8 (d, J = 9.4 Hz), 149.9, 148.2, 144.6 (d, J = 1.5 Hz), 135.5, 135.0, 131.4 (d, J = 1.4 Hz), 130.9 (d, J = 1.9 Hz), 120.1 (d, J = 4.6 Hz), 109.9 (d, J = 5.0 Hz), 106.5, 23.9 (d, J = 71.7 Hz); HRMS (FAB); m/z calcd for C14H13NO3PS [M + H]+: 306.0348, found 306.0351.2.2 Preparation of stock solutions
A stock solution of probe MP-1 (0.50 mM) was prepared in acetonitrile. Stock solutions of metal ions of perchlorate salt and sodium salt-containing anions (10.0 mM) were made by dissolving in DI water. A phosphate-buffered saline (PBS) solution (pH 7.4) was made according to a previous method.462.3 Preparation of Hg2+ sensing samples
The analytes for the Hg2+ sensing of MP-1 were prepared in an optimized condition of PBS solution (pH 7.4) containing 20% acetonitrile. The metal ion or anion stock solution (30 μL, 10.0 mM) was added to a 15 mL vial and diluted successively with 2.22 mL of DI water and 0.57 mL acetonitrile. Then, solutions of PBS (0.15 mL, 0.20 M) and probe MP-1 (30 μL, 0.50 mM) were added to the vial and carefully mixed. The final concentrations of probe MP-1, analyte, and PBS in the solution (3.0 mL) were 5.0 μM, 100 μM, and 10 mM, respectively. Error bars were presented using the standard deviation of a triplicate measurement.2.4 Investigation of Hg2+ sensing mechanism
Probe MP-1 (31 mg, 100 μmol) was dissolved in acetonitrile (10 mL). Mercury(II) perchlorate (80 mg, 200 μmol) was dissolved in DI water (10 mL) and carefully mixed with the prepared solution. The Hg2+ signaling progress of MP-1 was observed by TLC measurement. After the reaction was completed, the mixture was acidified using 0.1 N HCl solution, and the signaling product was extracted by DCM. The volatiles were removed, and the residue was purified by column chromatography (silica gel, eluent: DCM/methanol = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The purified Hg2+ sensing product was characterized by mass spectral data.
1, v/v). The purified Hg2+ sensing product was characterized by mass spectral data.
2.5 HPLC analysis of Hg2+ signaling product
Sample solutions of MP-1 only, MP-1 + Hg2+, and standard resorufin sodium salt were prepared using a pH 7.4 PBS solution containing 20% acetonitrile. The MP-1 (or resorufin sodium salt) and Hg2+ ions were in the sample solutions at final concentrations of 5.0 μM and 100.0 μM, respectively. The HPLC experiment was performed using 50% aq. acetonitrile as the eluent and the C18 reverse phase column (SunFire, 4.6 × 150 mm). The sample injection volume was 20 μL, and the flow rate was 1.0 mL min−1. The column oven was maintained at room temperature, and the HPLC results were obtained with a UV detector (A254).2.6 Hg2+ analysis of artificial urine samples
The Hg2+ analysis of the artificial urine samples was conducted in 2-fold diluted artificial urine-based analytical solutions. The artificial urine solution (Sigmatrix Urine Diluent) consisted of CaCl2, MgCl2, KCl, NaCl, NaH2PO4, Na2SO3, urea, and creatinine, with NaN3 as a preservative. The sample solution was prepared via the addition of DI water (0.75 mL), acetonitrile (540 μL), PBS (150 μL, 0.20 M), artificial urine solution (1.5 mL), and Hg2+ ions (0, 0.75, 1.5, 3.0, 6.0, and 9.0 μL, 1.0 mM) in a 15 mL vial. Probe MP-1 (30 μL, 5.0 mM) was added to the mixture following careful mixing. The final concentrations of MP-1, Hg2+ ions, and PBS in the 3.0 mL analyte solution were 5.0 μM, 0–3.0 μM, and 10 mM, respectively. The Hg2+ calibration curve in the artificial urine solution was obtained by measuring the absorbance ratio (A576/A457) and the ratio of red channel/green channel (Red/Green) level of the sample images as a function of [Hg2+].3. Results and discussion
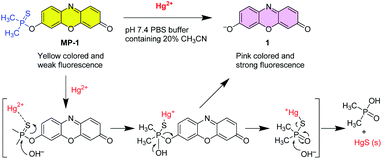
The Hg2+-selective probe was designed considering the selective and mild deprotection capabilities of the phosphinothioate protection group as a signaling switch. Phosphinothioate groups have been effectively used for the protection of phenolic functions and are easily deprotected under mild conditions via Hg2+- and F−-induced hydrolysis reactions.40–43 Resorufin was selected as the signaling fluorochrome because of its well-established chromogenic and fluorogenic responses toward various analytes through the analyte-induced regeneration of the parent dye.47 The designed probe MP-1 was easily prepared by the reaction of resorufin sodium salt with dimethylthiophosphinoyl chloride in DMF (yield: 82%) (Scheme 1). The probe structure was characterized by 1H NMR, 13C NMR, and mass spectral data. In its 1H NMR spectrum, four split resonances of characteristic nJP–H couplings were perceived for CH3 (13.5 Hz) and hydrogens of the resorufin moiety (0.7–1.6 Hz), owing to the couplings with the phosphorous atom of the dimethylphosphinothioate moiety. In the 13C NMR spectrum of MP-1, seven split resonances with characteristic nJP–C couplings were observed for CH3 (71.7 Hz) and carbons of the resorufin moiety (1.4–9.4 Hz), due to the coupling with the phosphorous atom of the dimethylphosphinothioate moiety.48The selective signaling behavior of the probe was assessed by UV-vis and fluorescence spectroscopy. A ‘bird's-eye’ view of the possible changes induced by the target analyte was obtained by measuring the spectral properties of the two relevant species of the designed signaling process, namely, probe MP-1 and its postulated signaling product resorufin 1 (vide infra). The two species showed quite contrasting spectral behaviors in an optimized condition of the PBS solution (pH 7.4) containing 20% acetonitrile. Probe MP-1 showed a strong absorption band at 457 nm in the UV-vis spectrum, while the expected signaling product 1 revealed a notably red-shifted and hyperchromic band at 576 nm (Fig. S1a, ESI†). The solution color changed from light yellow to pink. Meanwhile, probe MP-1 revealed a weak emission at 587 nm in the fluorescence spectrum under the same conditions, and the solution appeared nearly dark under illumination with a UV lamp (λex = 365 nm) (Fig. S1b, ESI†). Conversely, the expected signaling product 1 exhibited a strong emission at 592 nm with intense red fluorescence under the same UV illumination.
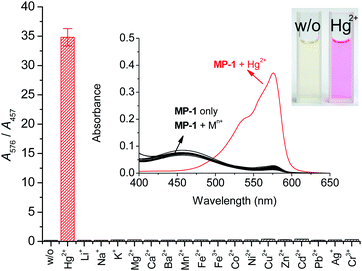
Firstly, we observed the UV-vis absorbance of probe MP-1 in the presence of representative thiophilic metal ions, such as Hg2+, Cu2+, and Ag+ ions. Probe MP-1 revealed prominent Hg2+ signaling behavior with moderate Cu2+ interference (Fig. S2, ESI†). To obtain exclusive Hg2+ selectivity of the probe, we employed a citrate additive as a masking agent for interfering Cu2+ ions in the measurement solutions.49 In this condition, the selective response of the probe toward targeting Hg2+ ions over common metal ions was elucidated (Fig. 1). Upon the treatment of MP-1 solution with various metal-containing analytes, only Hg2+ induced a marked color change from yellow to pink, which is a characteristic color of the resorufin fluorochrome (Fig. 1, inset). The absorbance enhancement of the probe at 576 nm (A/A0)576 was 63.4-fold. Most of the other tested metal ions revealed insignificant spectral changes; the ratio (A/A0)576 varied in a narrow range between 0.97 for Pb2+ and 1.67 for K+. Since MP-1 revealed considerable residual absorbance at 457 nm in the PBS solution (pH 7.4) containing 20% (v/v) acetonitrile, the selectivity toward Hg2+ ions was assessed through ratiometric treatment of the signaling data using two relevant absorbances at 576 and 457 nm. The ratio A576/A457 was 34.8 for Hg2+ and nearly constant for the rest of the metal ions (0.19–0.38) (Fig. 1). Ratiometric analysis revealed that the enhancement of the ratio A576/A457 reached 190-fold (0.19 for MP-1 only and 34.8 for MP-1 with Hg2+ ions). We also confirmed that probe MP-1 exhibited nearly perfect selectivity toward Hg2+ ions over common anions (Fig. S3, ESI†). The ratio A576/A457 for anions varied within a narrow range between 0.19 for Cl− and 0.25 for SO42− ions.
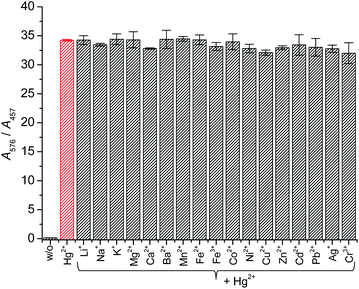
Next, the effect of foreign ions on the Hg2+ signaling of the probe was assessed to check the practical applicability. The Hg2+ signaling was not affected by the presence of commonly encountered metal ionic species. In the presence of background metal ions, the ratio (A576/A457) of the analytes after Hg2+ signaling by probe MP-1 changed marginally between 93.7% (for Cu2+) and 100.1% (for Mn2+) (Fig. 2). Similarly, the probe's Hg2+ signaling was not affected by the presence of commonly encountered anionic species (Fig. S4, ESI†).
Probe MP-1 showed negligible changes in UV-vis absorption behavior up to pH 8 (Fig. 3). Contrastingly, the absorbance ratio (A576/A457) of the Hg2+ signaling solution of MP-1 increased markedly from pH 5 and plateaued at around pH 8. The pH profile of Hg2+ signaling behavior by MP-1 (MP-1 + Hg2+) matched that of signaling product 1 exactly in the presence of Hg2+ ions (1 + Hg2+). From this result, we hypothesized that the Hg2+ signaling pH profile was due to the pH dependency of the absorption property of resorufin 1, rather than the pH-dependent Hg2+ signaling reaction by MP-1. Therefore, we chose the PBS solution (pH 7.4) as the working condition for the signaling experiments.
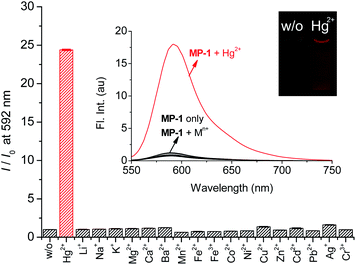
Next, the fluorogenic properties of the probe toward metal ions and anions were measured under the same optimal conditions. Probe MP-1 exhibited a weak emission at 587 nm under the measuring conditions (Fig. 4). Upon treatment with common metal ions, only Hg2+ induced a large increase in the emission band at 592 nm (I/I0 = 24.4). The remaining metal ions revealed no measurable fluorescence changes; I/I0 = 0.86 (for Pb2+) − 1.60 (for Ag+). The other tested thiophilic metal ions did not exhibit concerning responses under the measuring conditions. Moreover, probe MP-1 showed no measurable changes in fluorescence emission toward commonly encountered anions either; I/I0 = 0.87 (for S2−) − 1.60 (for F−) (Fig. S5, ESI†). These observations clearly demonstrated the potential of MP-1 for the selective analysis of Hg2+ ions in common chemical and environmental applications. However, the maximum signal enhancement expressed by the fluorometric enhancement I/I0 was rather small compared to the colorimetric results (I/I0 = 24.4 for fluorometry vs. (A576/A457)/(A576/A457)0 = 190 for colorimetry). This is due to the considerable residual fluorescence of probe MP-1 alone at 592 nm. Therefore, we investigated the colorimetric signaling behavior of probe MP-1, which results in more contrasting signaling and enables analysis with the more advantageous ratiometric approach.
The signaling route could be described by the Hg2+-assisted hydrolysis of the phosphinothioate protecting group of probe MP-1 to yield parent resorufin fluorochrome 1 (Scheme 2). The proposed sensing mechanism illustrates that the first step is conceived to be the complex formation process between the sulfur atom of the phosphinothioate group and thiophilic Hg2+ ions. The thus-formed complex hydrolyzed smoothly to yield resorufin dye and dimethylphosphinic acid as a byproduct, with the concomitant revival of the characteristic absorption and fluorescence signals. The postulated signaling reaction was ascertained by the NMR and mass measurements alongside HPLC monitoring. Upon treatment of probe MP-1 with Hg2+, the solution turned rapidly pink and revealed intense red fluorescence. The signaling reaction product between MP-1 and Hg2+ was characterized by NMR and mass spectral data. In the 1H NMR spectrum, probe MP-1 revealed six resonances of the resorufin moiety protons with typical splitting owing to JP–H couplings (Fig. 5). Alternatively, the reaction mixture (MP-1 + Hg2+) showed three resonances ascribable to resorufin 1 with no extra coupling due to JP–H couplings. In addition, as shown in Fig. S6 (ESI†), in the presence of Hg2+ ions, the resonance of methyl protons of the dimethylphosphinothioate moiety of probe MP-1 at 2.11 ppm disappeared, and a new doublet peak, which is ascribable to the resonance of the methyl protons of the byproduct dimethylphosphinic acid,50 at 1.32 ppm emerged. The mass spectrum of the purified signaling product confirmed a diagnostic peak at m/z = 213.1, which is in agreement with the calculated mass (calculated for C12H7NO3, 213.0) of signaling product 1 (Fig. S7, ESI†). Moreover, HPLC analysis provided additional supporting evidence. In the presence of Hg2+ ions (MP-1 + Hg2+), the elution time peak for probe MP-1 at 4.03 minutes disappeared, and a new peak evolved at 1.53 minutes for the reference compound 1 (Fig. S8, ESI†).
The quantitative analytical behavior of MP-1 for Hg2+ determination was studied by UV-vis titration (Fig. 6). The changes in the absorbance ratio (A576/A457) were linearly correlated with the increases in [Hg2+] (R2 = 0.9987). The limit of detection obtained from the concentration-dependent data as per the IUPAC recommendation (3sblk/m) was 12 nM. In addition, we confirmed that the signaling was complete within 5 minutes (Fig. S9, ESI†).
With this background, we tested the practical applicability of the probe for the easy determination of clinically relevant urinary mercury levels. Currently, the Hg2+ concentration in urine samples is determined by complicated instruments, such as inductively coupled plasma mass spectrometers and atomic absorption spectrometers. We presumed that one could diagnose elevated mercury levels in urine by applying probe MP-1 without resorting to these expensive and complicated instruments. Subsequently, the interference from major urine components was elucidated. Indeed, most of the metal ionic and anionic constituents showed no interference at all (Fig. 2 and S4, ESI†). Therefore, we measured the possible interference from other urine components of urea and creatinine. As shown in Fig. S10 (ESI†), there were no meaningful responses from these constituents, and the Hg2+ signaling of MP-1 was not disturbed by the presence of the two tested urine ingredients. Thus, the UV-vis titration of MP-1 with Hg2+ in artificial urine (Sigmatrix Urine Diluent) was performed to further elucidate the quantitative signaling potential of the probe. It is known that the Hg2+ levels in healthy human urine are less than 5 ng mL−1.8 Moreover, the facts that a urinary mercury concentration over 100 ppb (0.24 μM) induces neurological symptoms and that one above 800 ppb (1.96 μM) causes death are clinically important.12 The potential for the probe to diagnose these two clinically important levels was tested by conducting Hg2+ concentration-dependent experiments under the same conditions, except employing the 2-fold diluted commercially available artificial urine where no turbidity problem was observed. The calibration curve of Hg2+ analysis in artificial urine by probe MP-1, as plotted by the absorbance ratio (A576/A457), revealed a good linear relationship up to 3.0 μM (R2 = 0.9962) (Fig. S11, ESI†). The results showed that MP-1 could readily signify the fatal level (>800 ppb in urine) and even the neurological symptom level (>100 ppb in urine) for Hg2+ intoxication cases.
The colorimetric analytical behavior of MP-1 could be conveniently monitored by a scanner as a readily available detection tool under the same signaling conditions.51 The color change from yellow to pink of the signaling solutions was evident under the analysis conditions (Fig. 7a) and could be quantified by the changes in the green channel level as a function of the analytes' [Hg2+]. The correlation plot between the green channel level and [Hg2+] provided a good calibration curve (R2 = 0.9962) (Fig. S12, ESI†). Meanwhile, other color channel levels (red and blue) did not show meaningful changes with the increases in [Hg2+]. We also tried to obtain a more reliable approach for the assessment of these clinically important Hg2+ levels by employing the ratiometric analysis using the ratio of the two-color channel levels. Among these, the ratio between the red and green channel levels, Red/Green, afforded another useful calibration plot (R2 = 0.9974) (Fig. 7). The detection limit was calculated to be 91 nM. With this plot, the rapid and accurate diagnosis of fatal and even neurological symptom levels of Hg2+ intoxication cases, which were marked in red and blue, respectively, could be readily realized. From these results, we confirmed that the employed analytical technique could be applicable to the early clinical diagnosis of mercury poisoning cases using a standard UV-vis spectrophotometer or an office scanner.
4. Conclusions
We developed a novel Hg2+-selective colorimetric and fluorogenic probe to monitor clinically elevated urinary Hg2+ levels. Probe MP-1 showed the Hg2+-selective signaling behavior owing to the hydrolysis of phosphinothioate protecting group to yield the parent resorufin fluorochrome. Pronounced chromogenic (A576/A457 = 190-fold) and fluorescence ((I/I0)592 = 24.4-fold) responses were observed exclusively toward Hg2+ ions over other metal ions and anions. Hg2+ signaling was complete within 5 min, and the detection limit was calculated to be 12 nM. In particular, the convenient early diagnosis of fatal (1.96 μM) and even neurological symptom (0.24 μM) levels of Hg2+ intoxication cases could be readily realized using a common office scanner. The detection limit for Hg2+ ion in artificial urine samples using the office scanner was estimated to be 91 nM. These obtained results could be practically useful for the rapid diagnosis of elevated mercury levels in chronic and acute intoxication cases without resorting to complicated heavy instruments.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This research was supported by the Chung-Ang University Graduate Research Scholarship in 2021 (HMK) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2022R1F1A1072643).Notes and references
- A. Hojjati-Najafabadi, M. Mansoorianfar, T. Liang, K. Shahin and H. Karimi-Maleh, Sci. Total Environ., 2022, 824, 153844 CrossRef CAS PubMed.
- H. Karimi-Maleh, H. Beitollahi, P. S. Kumar, S. Tajik, P. M. Jahani, F. Karimi, C. Karaman, Y. Vasseghian, M. Baghayeri, J. Rouhi, P. L. Show, S. Rajendran, L. Fu and N. Zare, Food Chem. Toxicol., 2022, 164, 112961 CrossRef CAS PubMed.
- R. Guo, in Cross-Border Resource Management, Elsevier, Netherlands, 4th edn, 2021, Chapter 10 – Cross-border environmental pollution and human health, pp. 291–337 Search PubMed.
- K. L. Wasewar, S. Singh and S. K. Kansal, in Inorganic Pollutants in Water, ed. P. Devi, P. Singh and S. K. Kansal, Elsevier, Netherlands, 2020, Chapter 13 – Process intensification of treatment of inorganic water pollutants, pp. 245–271 Search PubMed.
- G. Bjørklund, M. Dadar, J. Mutter and J. Aaseth, Environ. Res., 2017, 159, 545–554 CrossRef PubMed.
- T. W. Clarkson, Crit. Rev. Clin. Lab. Sci., 1997, 34, 369–403 CrossRef CAS PubMed.
- F. D. Natale, A. Lancia, A. Molino, M. D. Natale, D. Karatza and D. Musmarra, J. Hazard. Mater., 2006, 132, 220–225 CrossRef PubMed.
- K. H. Kim, E. Kabir and S. A. Jahan, J. Hazard. Mater., 2016, 306, 376–385 CrossRef CAS PubMed.
- P. B. Tchounwou, W. K. Ayensu, N. Ninashvili and D. Sutton, Environ. Toxicol., 2003, 18, 149–175 CrossRef CAS PubMed.
- P. Kaur, M. Aschner and T. Syversen, Neurotoxicology, 2006, 27, 492–500 CrossRef CAS PubMed.
- K. Eto, H. Tokunaga, K. Nagashima and T. Takeuchi, Toxicol. Pathol., 2002, 30, 714–722 CrossRef CAS PubMed.
- B. J. Ye, B. G. Kim, M. J. Jeon, S. Y. Kim, H. C. Kim, T. W. Jang, H. J. Chae, W. J. Choi, M. Ha and Y. S. Hong, Ann. Occup. Environ. Med., 2016, 28, 5 CrossRef PubMed.
- W. Zheng, D. Foucher and H. Hintelmann, J. Anal. At. Spectrom., 2007, 22, 925–930 RSC.
- T. K. Chan, Clin. Toxicol., 2011, 49, 886–891 CrossRef CAS PubMed.
- C. Chen, H. Yu, J. Zhao, B. Li, L. Qu, S. Liu, P. Zhang and Z. Chai, Environ. Health Perspect., 2006, 114, 297–301 CrossRef CAS PubMed.
- M. Rafati-Rahimzadeh, M. Rafati-Rahimzadeh, S. Kazemi and A. Moghadamnia, Daru, J. Pharm. Sci., 2014, 22, 46 CrossRef PubMed.
- A. Sabouri and S. Nouroozi, RSC Adv., 2016, 6, 80354–80360 RSC.
- E. Kenduzler, M. Ates, Z. Arslan, M. McHenry and P. B. Tchounwou, Talanta, 2012, 93, 404–410 CrossRef CAS PubMed.
- X. Ding, L. Kong, J. Wang, F. Fang, D. Li and J. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 7072–7078 CrossRef CAS PubMed.
- A. Roda, P. Pasini, M. Mirasoli, M. Guardigli, C. Russo, M. Musiani and M. Baraldini, Anal. Lett., 2001, 34, 29–41 CrossRef CAS.
- M. S. Won, Y. J. Bae, S. S. Lee and Y. B. Shim, Electroanalysis, 2001, 13, 1003–1007 CrossRef CAS.
- M. Saleem, M. Rafiq and M. Hanif, Organic material based fluorescent sensor for Hg2+: A brief review on recent development, in Reviews in Fluorescence 2016, ed. C. D. Geddes and J. R. Lakowicz, Springer International Publishing AG, Switzerland, 2017, pp. 275–317 Search PubMed.
- P. Mahato, S. Saha, P. Das, H. Agarwalla and A. Das, RSC Adv., 2014, 4, 36140–36174 RSC.
- A. Kumar, D. Kumar and M. Chhibber, ChemistrySelect, 2020, 5, 13738–13747 CrossRef CAS.
- P. Singh and P. Sharma, J. Photochem. Photobiol., A, 2021, 408, 113096 CrossRef CAS.
- C. Li, Q. Niu, J. Wang, T. Wei, T. Li, J. Chen, X. Qin and Q. Yang, Spectrochim. Acta, Part A, 2020, 233, 118208 CrossRef CAS PubMed.
- H. Tan, Y. Zhang and Y. Chen, Sens. Actuators, B, 2011, 156, 120–125 CrossRef CAS.
- J. He, H. Zhang, J. Zou, Y. Liu, J. Zhuang, Y. Xiao and B. Lei, Biosens. Bioelectron., 2016, 79, 531–535 CrossRef CAS PubMed.
- M. Rana, M. Balcioglu, N. M. Robertson, M. S. Hizir, S. Yumakc and M. V. Yigit, Chem. Sci., 2017, 8, 1200–1208 RSC.
- S. Zhu, Y. Zhuo, H. Miao, D. Zhong and X. Yang, Luminescence, 2015, 30, 631–636 CrossRef CAS PubMed.
- T. Samanta and R. Shunmugam, Mater. Adv., 2021, 2, 64–95 RSC.
- H. Shuai, C. Xiang, L. Qian, F. Bin, L. Xiaohui, D. Jipeng, Z. Chang, L. Jiahui and Z. Wenbin, Dyes Pigm., 2021, 187, 109125 CrossRef CAS.
- E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443–3480 CrossRef CAS PubMed.
- (a) H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465–1472 RSC; (b) J. Hu, X. Yu, X. Zhang, C. Jing, T. Liu, X. Hu, S. Lu, K. Uvdal, H.-W. Gao and Z. Hu, Spectrochim. Acta, Part A, 2020, 241, 118657 CrossRef CAS PubMed; (c) A. Picard-Lafond, D. Larivière and D. Boudreau, ACS Omega, 2020, 5, 701–711 CrossRef CAS PubMed.
- (a) Y. Tang, D. Lee, J. Wang, G. Li, J. Yu, W. Lin and J. Yoon, Chem. Soc. Rev., 2015, 44, 5003–5015 RSC; (b) G. Yuan, H. Lv, H. Liu, H. He, Q. Sun, X. Zhang and S. Wang, Dyes Pigm., 2020, 183, 108674 CrossRef CAS; (c) J. Pan, J. Ma, L. Liu, D. Li, Y. Huo and H. Liu, J. Photochem. Photobiol., A, 2021, 416, 113322 CrossRef CAS.
- (a) D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105–7123 RSC; (b) S. De and G. Das, Dyes Pigm., 2021, 195, 109659 CrossRef CAS.
- M. Ueki and T. Inazu, Bull. Chem. Soc. Jpn., 1982, 55, 204–207 CrossRef CAS.
- M. Ueki and S. Kozo, Bull. Chem. Soc. Jpn., 1983, 56, 1187–1191 CrossRef CAS.
- P. G. M. Wuts, in Green's Protective Groups in Organic Synthesis, John Wiley & Sons, New Jersey, 5th edn, 2014, p. 540 Search PubMed.
- H. G. Im, H. Y. Kim and S.-K. Chang, Sens. Actuators, B, 2014, 191, 854–859 CrossRef CAS.
- L. Chen, S. J. Park, D. Wu, H. M. Kim and J. Yoon, Chem. Commun., 2019, 55, 1766–1769 RSC.
- H. Y. Kim, H. G. Im and S.-K. Chang, Dyes Pigm., 2015, 112, 170–175 CrossRef CAS.
- M. Du, B. Huo, J. Liu, M. Li, L. Fang and Y. Yang, Anal. Chim. Acta, 2018, 1030, 172–182 CrossRef CAS PubMed.
- K. Kuwabara, Y. Maekawa and T. Murai, Tetraherdon, 2020, 76, 131152 CrossRef CAS.
- D. J. Eisler and R. J. Puddephatt, Inorg. Chem., 2006, 45, 7295–7305 CrossRef CAS PubMed.
- D. D. Perin and B. Dempsey, in Buffers for pH and Metal Ion Control, Chapman and Hall Ltd, UK, 1979 Search PubMed.
- M. G. Choi, S. Y. Park, K. Y. Park and S.-K. Chang, Sci. Rep., 2019, 9, 3348 CrossRef PubMed.
- G. Mavel and M. J. Green, Chem. Commun., 1968, 742–743 RSC.
- E. M. M. Ferreira, R. J. A. L'Amour, J. M. N. Carmo, J. L. Mantovano and M. S. de Carvalho, Microchem. J., 2004, 78, 1–5 CrossRef.
- P. V. Ioannou, Z. Anorg. Allg. Chem., 2010, 636, 1347–1353 CrossRef CAS.
- D. C. Christodouleas, A. Nemiroski, A. A. Kumar and G. M. Whitesides, Anal. Chem., 2015, 87, 9170–9178 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: UV-vis, fluorescence, and HPLC data, Hg2+ assay in artificial urine sample, 1H and 13C NMR spectra of probe MP-1. See https://doi.org/10.1039/d2ra04093j |
| This journal is © The Royal Society of Chemistry 2022 |