Open Access Article
Open Access ArticleA green synthesis and antibacterial activity of ferrocene-based thiazole derivatives in choline chloride/glycerol eutectic solvent†
Di Zhaoa,
Yanying Liub,
Yang Li *a and
Yu Chen*c
*a and
Yu Chen*c
aCollege of Chemistry and Chemical Engineering, Bohai University, Jinzhou 121013, China. E-mail: bhuzh@163.com
bResearch Institute of Jinzhou Petrochemical Company, Jinzhou 121013, China
cSchool of Life Science and Biopharmaceutics, Shenyang Pharmaceutical University, Shenyang, 110016, China. E-mail: 569180398@qq.com
First published on 9th August 2022
Abstract
In this work, a green Hantzsch synthesis of 4-ferrocenylthiazole derivatives has been accomplished successfully. The Hantzsch reaction between bromoacetylferrocene and various aryl thioureas, 1-alkylindole-3- or 9-alkylcarbazole-3-carbothioamides proceeded efficiently in a deep eutectic solvent (DES) that is, choline chloride/glycerol (ChCl/Gly) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) at 80 °C, avoiding the use of common volatile organic solvents. Moreover, the DES media could be reused up to three times without any appreciable decrease in the yield. The synthetic strategy has the attractive features such as mild and environmentally benign reaction conditions, experimental simplicity, easy work-up procedure and good yields. Subsequently, a preliminary screening for in vitro antibacterial activities of all these newly-synthesized compounds revealed that the halo-substituted (F, Cl, Br) compounds 3f–h showed significant antibacterial activities against Gram (+) bacterial B. subtilis and Gram (−) E. coli, among which the fluoro-substituted 3f possessed the best activity with the MIC value of 7.8125 μg mL−1, being higher than the reference drug ciprofoxacin (15.625 μg mL−1).
2 molar ratio) at 80 °C, avoiding the use of common volatile organic solvents. Moreover, the DES media could be reused up to three times without any appreciable decrease in the yield. The synthetic strategy has the attractive features such as mild and environmentally benign reaction conditions, experimental simplicity, easy work-up procedure and good yields. Subsequently, a preliminary screening for in vitro antibacterial activities of all these newly-synthesized compounds revealed that the halo-substituted (F, Cl, Br) compounds 3f–h showed significant antibacterial activities against Gram (+) bacterial B. subtilis and Gram (−) E. coli, among which the fluoro-substituted 3f possessed the best activity with the MIC value of 7.8125 μg mL−1, being higher than the reference drug ciprofoxacin (15.625 μg mL−1).
Introduction
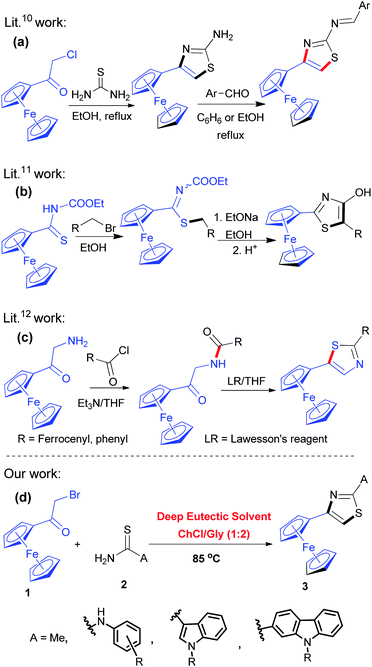
Thiazole is a valuable heterocyclic unit present in numerous pharmaceuticals.1,2 Derivatives of this family have exhibited a broad spectrum of biological activities such as antimicrobial,3 anticancer,4 antitubercular5 and anti-inflammatory properties,6 and have been extensively studied for current drug discovery. Consequently, considerable synthetic efforts have been invested surrounding the thiazole ring for further modification and functionalization to enhance the potency of this class of heterocycles.7 On the other hand, it has been well established that the introduction of ferrocene nucleus into organic heterocyclic compounds might often lead to a hybrid product with enhanced or unexpected activity compared to that of the parent compound.8 This could be rationalized as being due to the unique properties of the ferrocene nucleus such as membrane permeation, aqueous stability, anomalous metabolism, and redox behaviour.9 In light of these findings, the incorporation of ferrocene moiety and thiazole ring into a molecular framework would be recognized as an attractive way to endow important candidates for medicinal applications, though the related reports concerning the synthesis of such ferrocene–thiazole hybrids are very few so far. In this context, Ma et al.10 reported the synthesis of ferrocene-based thiazole Schiff bases with potential antibacterial activity, involving the Hantzsch reaction of chloroacetylferrocene with thiourea followed by the condensation with aromatic aldehydes (eqn (a), Scheme 1). Wrona et al.11 described the synthesis of 2-ferrocenyl-4-hydroxythiazoles by cyclization of ferrocenyl thioimidates in the presence of sodium ethoxide (eqn (b), Scheme 1). Tarraga et al.12 reported the synthesis of ferrocenylthiazoles and 2,5-bis(ferrocenyl)thiazoles via acylation of α-aminoacetylferrocene with benzoyl chloride or chlorocarbonyl ferrocene followed by the action of the Lawesson's reagent (LR) (eqn (c), Scheme 1).In light of the current stringent environmental requirements and safety consideration in the chemical production, increasing research effort has been focused on the development of sustainable and environmentally benign reaction procedures to replace those efficient but somewhat outdated methods, particularly, to replace hazardous volatile organic solvents.13 During the past decade, deep eutectic solvents (DESs), as an emerging class of unconventional solvents derived from the combination of two or three safe and cheap components of Lewis or Brønsted acids and bases through hydrogen bond formation, have attracted enormous attention due to their unique properties including wide liquid range, negligible vapor pressure, low toxicity, non-flammability, high biodegradability, low cost of components and convenient preparation,14 thus rendering them widely acknowledged as an excellent alternative to volatile organic solvents in the development of environmentally friendly organic reactions.15 Against this background and in continuation of our interest toward the development of new efficient synthetic methodologies for the construction of heterocycles,16,17 we would like to report, herein, a new and green Hantzsch thiazole synthesis of various structurally intriguing ferrocene-based derivatives (3) by using the DES choline chloride/glycerol (ChCl/Gly) as a safe, eco-friendly and unconventional solvent as outlined in eqn (d) of Scheme 1. As far as we know, no related reports are available concerning the application of the DES for the green synthesis of ferrocene-based thiazole derivatives.
Results and discussion
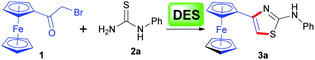
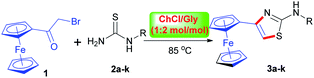
As well known, the classical and well-established Hantzsch reaction protocol is a preferred approach for the construction of thiazole derivatives with the use of α-haloketones and thiourea as the reactants in refluxing organic solvents such as methanol or ethanol.18,19 Currently, DESs have been increasingly applied as possible alternative ‘green’ and bio-renewable solvents for organic transformations, avoiding many disadvantages of common hazardous volatile organic solvents.20 Thus, following the line of green chemistry we envisioned that the application of deep eutectic solvents in the Hantzsch thiazole synthesis of ferrocene-based derivatives would attract significant interest.Accordingly, we initially conducted the model reaction of bromoacetylferrocene (1) with 1-phenylthiourea (2a) by using the widely used DES, namely ChCl–urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the media at 80 °C, which was reported to yield good results in the heterocyclodehydration reaction between α-chloroketones and guanidine derivatives.21 However, we observed that in our case a mixture of products was produced as shown by TLC analysis after completion of the reaction, from which the desired product was isolated only in a low yield of 19% (entry 1, Table 1), while the unexpected by-product, identified as 4-ferrocenyloxazol-2-amine, was formed as a major product in 63% yield. The reason for this attributed presumably to the fact that α-bromoketone 1 might be more inclined to react with the urea component of the ChCl/urea to give the corresponding 2-aminooxazole. It was worthy to mention that in consistent with our observations, Singh et al.22 also described a similar reaction, wherein phenacyl bromide reacted with the urea component of ChCl–urea to deliver the 4-phenyloxazol-2-amine as a final product. Switching ChCl–urea to ChCl/thiourea was also to no avail (entry 2, Table 1). Recently, Xiao et al.23 reported the use of ChCl/ZnCl2 as solvent for the efficient Hantzsch dihydropyridine synthesis. However, our attempt to follow the purported approach was frustrated as well by a poor yield with a number of side-products being evident (entry 3, Table 1). In order to pick a suitable DES for the model reaction, a series of ChCl-based DESs were screened. Upon using ChCl/glucose (ChCl/Glu),24 no formation of the desired product was observed and the starting materials were recovered unchanged (entry 4, Table 1). We also attempted to use ChCl/oxalic acid (ChCl/OA)25 or ChCl/polyethylene glycol (ChCl/PEG)26 as solvent, but the results were still not satisfactory as we expected (entries 5 and 6, Table 1). After these fruitless attempts, we were delighted to find that ChCl/Gly27 could be used as an efficient solvent, in which the reaction proceeded smoothly, giving the desired compound 3a in a remarkably high yield of 73% (entry 7, Table 1). The reason for the effectiveness of ChCl/Gly solvent might be due to its relatively low viscosity, good solubility, high stability and positive synergic effect on the reaction through extensive hydrogen bonding in comparison with other DESs. Further, in order to determine the optimum reaction conditions, the effect of different reaction temperature and molar ratio of the ChCl/Gly was examined. We observed that the best result could be achieved when the reaction was carried out at 85 °C (entry 10, Table 1), but altering the molar ratio resulted in no further improvement in the product yield (entries 13 and 14, Table 1). In addition, in order to highlight the advantages of the DES solvent, we also carried out the model reaction only in glycerol media at 85 °C or in organic solvent such as ethanol at refluxing temperature as described in literature.10 We found that the yield of the product obtained from the reaction in glycerol was only 38% after 12 hours (entry 15, Table 1), which suggested that the reaction gave a good yield due to ChCl/Gly as solvent and not due to its individual glycerol component. Likewise, the model reaction in ethanol medium delivered the product 3a in a moderate yield of 65% after 12 hours (entry 16, Table 1). Thus, we could conclude that the application of ChCl/Gly is superior over the conventional solvent procedure.
2) as the media at 80 °C, which was reported to yield good results in the heterocyclodehydration reaction between α-chloroketones and guanidine derivatives.21 However, we observed that in our case a mixture of products was produced as shown by TLC analysis after completion of the reaction, from which the desired product was isolated only in a low yield of 19% (entry 1, Table 1), while the unexpected by-product, identified as 4-ferrocenyloxazol-2-amine, was formed as a major product in 63% yield. The reason for this attributed presumably to the fact that α-bromoketone 1 might be more inclined to react with the urea component of the ChCl/urea to give the corresponding 2-aminooxazole. It was worthy to mention that in consistent with our observations, Singh et al.22 also described a similar reaction, wherein phenacyl bromide reacted with the urea component of ChCl–urea to deliver the 4-phenyloxazol-2-amine as a final product. Switching ChCl–urea to ChCl/thiourea was also to no avail (entry 2, Table 1). Recently, Xiao et al.23 reported the use of ChCl/ZnCl2 as solvent for the efficient Hantzsch dihydropyridine synthesis. However, our attempt to follow the purported approach was frustrated as well by a poor yield with a number of side-products being evident (entry 3, Table 1). In order to pick a suitable DES for the model reaction, a series of ChCl-based DESs were screened. Upon using ChCl/glucose (ChCl/Glu),24 no formation of the desired product was observed and the starting materials were recovered unchanged (entry 4, Table 1). We also attempted to use ChCl/oxalic acid (ChCl/OA)25 or ChCl/polyethylene glycol (ChCl/PEG)26 as solvent, but the results were still not satisfactory as we expected (entries 5 and 6, Table 1). After these fruitless attempts, we were delighted to find that ChCl/Gly27 could be used as an efficient solvent, in which the reaction proceeded smoothly, giving the desired compound 3a in a remarkably high yield of 73% (entry 7, Table 1). The reason for the effectiveness of ChCl/Gly solvent might be due to its relatively low viscosity, good solubility, high stability and positive synergic effect on the reaction through extensive hydrogen bonding in comparison with other DESs. Further, in order to determine the optimum reaction conditions, the effect of different reaction temperature and molar ratio of the ChCl/Gly was examined. We observed that the best result could be achieved when the reaction was carried out at 85 °C (entry 10, Table 1), but altering the molar ratio resulted in no further improvement in the product yield (entries 13 and 14, Table 1). In addition, in order to highlight the advantages of the DES solvent, we also carried out the model reaction only in glycerol media at 85 °C or in organic solvent such as ethanol at refluxing temperature as described in literature.10 We found that the yield of the product obtained from the reaction in glycerol was only 38% after 12 hours (entry 15, Table 1), which suggested that the reaction gave a good yield due to ChCl/Gly as solvent and not due to its individual glycerol component. Likewise, the model reaction in ethanol medium delivered the product 3a in a moderate yield of 65% after 12 hours (entry 16, Table 1). Thus, we could conclude that the application of ChCl/Gly is superior over the conventional solvent procedure.
| Entry | Solvent | Temp./°C | Time/h | Yieldb/% |
|---|---|---|---|---|
| a Reaction conditions: bromoacetylferrocene 1 (0.5 mmol) and 1-phenylthiourea (2a) (0.55 mmol).b Isolated yield.c NR means no reaction.d Reaction in the 1st recovered DES solvent.e Reaction in the 2nd recovered DES solvent.f Reaction in the 3rd recovered DES solvent.g Reaction in the 4th recovered DES solvent. | ||||
| 1 | ChCl/urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
80 | 10 | 19 |
| 2 | ChCl/thiourea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
80 | 10 | 13 |
| 3 | ChCl/ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
80 | 10 | 27 |
| 4 | ChCl/Glu (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 10 | NRc |
| 5 | ChCl/OA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 10 | 21 |
| 6 | ChCl/PEG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
80 | 10 | 44 |
| 7 | ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
80 | 6 | 73 |
| 8 | ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
70 | 6 | 61 |
| 9 | ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
75 | 6 | 66 |
| 10 | ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
85 | 6 | 82 |
| 11 | ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
90 | 6 | 78 |
| 12 | ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
95 | 6 | 76 |
| 13 | ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
85 | 6 | 38 |
| 14 | ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
85 | 6 | 53 |
| 15 | Glycerol | 85 | 12 | 38 |
| 16 | EtOH | Reflux | 12 | 65 |
| 17 | ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)d 2)d |
85 | 6 | 82 |
| 18 | ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)e 2)e |
85 | 6 | 81 |
| 19 | ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)f 2)f |
85 | 6 | 79 |
| 20 | ChCl/Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)g 2)g |
85 | 6 | 67 |
Finally, the recyclability of the ChCl/Gly for the model reaction was investigated under the optimized conditions. After completion of the reaction, an equal volume of water was added to the reaction mixture to completely precipitate out the resulting product, which was then extracted using dichloromethane. And the DES ChCl/Gly could be recovered by removing water from the aqueous layer under vacuum and reused for the next run. Thus, the fresh substrates 1 and 2a were added to the recovered ChCl/Gly to repeat the model reaction. We found that the recovered ChCl/Gly could be re-used up to three consecutive runs without significant decrease in 3a yield (entries 17–19, Table 1), though a slight darkening of the eutectic mixture was observed after recycling. However, starting from the fourth cycle, a significant drop in the product yield was noticed (entry 20, Table 1).
To demonstrate the synthetic potential by using ChCl/Gly as the privileged reaction medium in the Hantzsch thiazole synthesis, we extended the reaction to other substituted 1-phenylthioureas in a similar fashion. Satisfactorily, these substances were equally amenable to the reaction process without any experimental difficulties, successfully furnishing the corresponding 3b–k in satisfactory yields of 76–91% as listed in Table 2. Moreover, in all cases, the recyclability of the DES system could be successfully achieved. It appeared that the nature of the substituent present in the benzene ring did not exert an obvious influence on the product yields. For example, products 3b–e with electron-donating methyl, methoxyl or ethyl substituent (entries 2–5, Table 2) on the benzene ring were obtained in comparable yields with products 3f–h bearing electron-withdrawing groups such as fluoro, chloro, and bromo substituent (entries 6–8, Table 2). Further, we attempted the reaction with 1-(2-hydroxyphenyl)thiourea, 1-methylthiourea and 1-(benzo[d][1,3]dioxol-5-yl)thiourea under the same reaction conditions. As expected, these species were also viable substrate for this transformation, invariably giving the expected products 3i, 3j and 3k in satisfactory yields of 74%, 76% and 81%, respectively (entries 9–11, Table 2).
| Entry | Compd | R | Yielda/% | Mp/°C |
|---|---|---|---|---|
| a Isolated yield. | ||||
| 1 | 3a | Phenyl | 82 | 161–162 |
| 2 | 3b | o-Tolyl | 79 | 167–168 |
| 3 | 3c | p-Tolyl | 87 | 159–160 |
| 4 | 3d | 4-Methoxyphenyl | 91 | 168–169 |
| 5 | 3e | 4-Ethylphenyl | 85 | 139–141 |
| 6 | 3f | 4-Fluorophenyl | 76 | 145–146 |
| 7 | 3g | 4-Chlorophenyl | 80 | 177–178 |
| 8 | 3h | 4-Bromophenyl | 83 | 163–164 |
| 9 | 3i | o-Hydroxyphenyl | 74 | 189–190 |
| 10 | 3j | Methyl | 76 | 144–145 |
| 11 | 3k | Piperonyl | 81 | 175–176 |
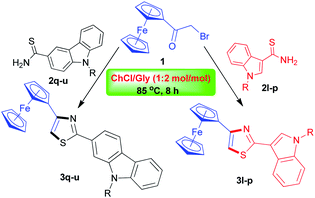
Recently, the synthesis of biologically interesting indole- or carbazolo-based thiazole hybrids have been frequently reported through molecular hybridization approach,28–30 and these hybrids have showed potent antimicrobial, antioxidant and anticancer activities.31–33 Prompted by these reports and with the aim of further diversifying our work to give rise to a new dimension of structural diversity as potential candidates for biological evaluation, we became very interested in the synthesis of the novel ferrocene-based indole/carbazolo-thiazole hybrids. To this purpose, an analogous series of reactions were performed using the readily synthesized 1-alkylindole-3- (2l–p) or 9-alkylcarbazole-3-substituted carbothioamides (2q–u)34 as the reactants in place of aryl thioureas under the same reaction conditions. To our delight, their Hantzsch reaction proceeded smoothly as well, furnishing the expected 2-(1-alkyl-1H-indol-3-yl)-4-ferrocenylthiazole (3l–p) and 2-(9-alkyl-9H-carbazol-2-yl)-4-ferrocenylthiazoles (3q–u) as listed in Table 3. From the experiments of Table 3, we found that in the Hantzsch reaction with bromoacetylferrocene the reactivity of 1-alkylindole-3-carbothioamides and 9-alkylcarbazole-3-carbothioamides was slightly lower in comparison with those of aryl thioureas, thus requiring slightly longer reaction time of 8 hours with somewhat lower product yields of 65–74%.
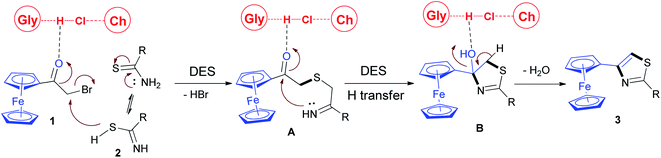
On the basis of a recent report concerning the reactivity of α-chloro oximes in DESs,27 a proposed reaction mechanism for the synthesis of the title compounds in ChCl/Gly was outlined in Scheme 2. The reaction might first involved in the nucleophilic attack of the thiourea or thioamide sulfur atom on the α-carbon of the bromoacetyl group to form the intermediate A with the elimination of an equivalent of HBr. In this reaction process, the interaction of ChCl/Gly with the oxygen atom of the α-bromoketone could enhance the reactivity of the bromoacetyl moiety and facilitate the nucleophilic attack by the sulfur atom. Subsequently, this conversion was followed by the in situ intramolecular nucleophilic cyclization reaction involving the participation of the imine N-atom and the carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group to form the intermediate B.35 In the cyclisation reaction step the activation of intermediate A by extensive hydrogen bonding with DES played a key role, increasing the electrophilicity of the carbonyl group and enhanced the rate of the cyclisation.36 Subsequently, the generated hydroxyl group formed hydrogen bonded with the DES, thus facilitating a loss of water to produce the final products 3.
O group to form the intermediate B.35 In the cyclisation reaction step the activation of intermediate A by extensive hydrogen bonding with DES played a key role, increasing the electrophilicity of the carbonyl group and enhanced the rate of the cyclisation.36 Subsequently, the generated hydroxyl group formed hydrogen bonded with the DES, thus facilitating a loss of water to produce the final products 3.
Currently, due to the serious concern related to the resistance of pathogenic bacteria towards the clinically used antibacterial drugs, screening new class of compounds for development of new antibacterial drugs is an urgent priority to overcome the increasing danger of drug-resistant problems. Thus, considering thiazole ring being a characteristic component of numerous antibacterial reagents,37 a preliminary evaluation for their in vitro antibacterial activities against Bacillus subtilis (B. subtilis) and Staphylococcus aureus (S. aureus) as Gram (+), Escherichia coli (E. coli) and Pseudomonas aeruginosa (P. aeruginosa) as Gram (−) was assayed by measuring minimum inhibitory concentrations (MICs), and the test results were recorded in Table 4.
| Entry | Compd | B. subtilis | S. aureus | E. coli | P. aeruginosa |
|---|---|---|---|---|---|
| 1 | 3a | 31.25 | 62.5 | 31.25 | 125 |
| 2 | 3b | 125 | 62.5 | 125 | 125 |
| 3 | 3c | 125 | 250 | 125 | 250 |
| 4 | 3d | 31.25 | 62.5 | 62.5 | 125 |
| 5 | 3e | 125 | 125 | 62.5 | 250 |
| 6 | 3f | 7.8215 | 62.5 | 7.8125 | 125 |
| 7 | 3g | 15.625 | 31.25 | 15.625 | 62.5 |
| 8 | 3h | 7.8215 | 31.25 | 15.625 | 125 |
| 9 | 3i | 31.25 | 15.625 | 31.25 | 125 |
| 10 | 3j | 31.25 | 125 | 31.25 | 62.5 |
| 11 | 3k | 62.5 | 31.25 | 31.25 | 62.5 |
| 12 | 3l | 62.5 | 125 | 31.25 | 125 |
| 13 | 3m | 250 | 250 | 125 | 125 |
| 14 | 3n | 250 | 125 | 125 | 125 |
| 15 | 3o | 62.5 | 125 | 125 | 125 |
| 16 | 3p | 62.5 | 125 | 31.25 | 125 |
| 17 | 3q | 250 | 250 | 250 | 250 |
| 18 | 3r | 62.5 | 125 | 125 | 125 |
| 19 | 3s | 62.5 | 125 | 31.25 | 125 |
| 20 | 3t | 250 | 250 | 125 | 125 |
| 21 | 3u | 250 | 125 | 125 | 125 |
| Ref. | Ciprofoxacin | 15.625 | 15.625 | 15.625 | 15.625 |
As shown in Table 4, the series of N-substituted-4-ferrocenylthiazol-2-amines (3a–k) exhibited moderate to good antibacterial activity against the Gram (+) bacterial B. subtilis and Gram (−) E. coli., whereas 2-(indol-3-yl)- (3l–p) and 2-(carbazol-2-yl)-4-ferrocenylthiazoles (3q–u) showed poor antibacterial activities against all the tested pathogenic bacteria. In the series of 3a–k, compound N-phenyl-4-ferrocenylthiazol-2-amine (3a) showed moderate activities against B. subtilis and E. coli (entry 1, Table 4). The introduction of electron-donating substituents such as methyl, methoxyl and ethyl group into phenyl moiety as in compounds 3b–e, resulted in much lower activities against the two bacterial strains (entries 2–5, Table 4), which demonstrated that the introduction of the electron-donating substituents was not conducive to their inhibitory activities. Interestingly, we observed that the presence of halo group such as F, Cl and Br gave an significant improvement of the inhibitory activities, increasing the antibacterial potential with the order of F > Br > Cl (entries 6–8, Table 4). Especially, the most active fluoro-substituted 3f with the MIC value of 7.8215 μg mL−1 against B. subtilis and E. coli was much superior to the reference drug ciprofoxacin (entry 6, Table 4), and thus might be interesting and promising candidates for further biological research. In addition, it has been found that the o-hydroxyphenyl-substituted product 3i exhibited a good antibacterial effect against S. aureus with the MIC value of 15.625 μg mL−1, being comparable with the reference drug (entry 9, Table 4). These insights from the in vitro antibacterial activity might provide valuable information for further optimization of the series of derivatives, and hopefully have the potential to further exploitation in new antibacterial drug discovery. Our next efforts will mainly focus on the structural activity relationship study by structural optimization towards the ultimate goal of providing intriguing lead compounds for the development of novel and effective antibacterial agents.
Conclusions
In conclusion, a green and facile synthesis of structurally intriguing ferrocene-based thiazoles using the readily available, environmentally benign and non-toxic DES ChCl/Gly as reaction medium has been accomplished. Our synthetic strategy have the attractive advantages of mild reaction conditions, simple experimental operation, wide range of applicable substrates, easy purification procedure, and good product yields. The recyclability and biodegradability of the ChCl/Gly make this methodology highly sustainable and reliable. These synthesized compounds belong to a new class of ferrocene–thiazole hybrids and a preliminary evaluation for their in vitro antibacterial bioassay revealed that compounds bearing halogen substitution showed potent and promising activity against Gram (+) bacterial B. subtilis and Gram (−) E. coli with the MIC value being equipotent or even better than the reference drug ciprofoxacin. These results might give an important insight to future optimization of the series of ferrocene–thiazole hybrids. Currently, work is ongoing, mainly focusing on the further elaboration and application of these compounds, which represent an intriguing goal that we are contemplating, and these results will be a part of future reports.Author contributions
Y. Li and Y. Chen conceptualization, D. Zhao and Y. Liu methodology, investigation and synthesis, Y. Chen antibacterial activity assay, Y. Li writing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the Doctoral Start-up Foundation of Liaoning Province (grant no. 2019-BS-004) and the Scientific Research Foundation of the Education Department of Liaoning Province (grant no. LQ2019006).Notes and references
- S. H. Ali and A. R. Sayed, Synth. Commun., 2021, 51, 670 CrossRef CAS.
- A. Petrou, M. Fesatidou and A. Geronikaki, Molecules, 2021, 26, 3166 CrossRef CAS PubMed.
- P. Vicini, A. Geronikaki, K. Anastasia, M. Incerti and F. Zani, Bioorg. Med. Chem., 2006, 14, 3859 CrossRef CAS PubMed.
- Y. WWan, J. Long, H. Gao and Z. Tang, Eur. J. Med. Chem., 2021, 210, 112953 CrossRef PubMed.
- M. Pieroni, B. Wan, S. Cho, S. G. Franzblau and G. Costantino, Eur. J. Med. Chem., 2014, 72, 26 CrossRef CAS PubMed.
- M. H. M. Helal, M. A. Salem, M. S. A. El-Gaby and M. Aljahdali, Eur. J. Med. Chem., 2013, 65, 517 CrossRef CAS PubMed.
- A. Singh, D. Malhotra, K. Singh, R. Chadha and P. M. S. Bedi, J. Mol. Struct., 2022, 1266, 133479 CrossRef CAS.
- B. Sharma and V. Kumar, J. Med. Chem., 2021, 64, 16865 CrossRef CAS PubMed.
- P. Štěpnička, Dalton Trans., 2022, 51, 8085 RSC.
- H. Ma, Y. Hou, Y. Bai, J. Lu and B. Yang, J. Organomet. Chem., 2001, 637, 742 CrossRef.
- A. Wrona, J. Zakrzewski, L. Jerzykiewicz and K. Nakatani, J. Organomet. Chem., 2008, 693, 2982 CrossRef CAS.
- A. Tarraga, P. Molina, D. Curiel and M. D. Velasco, Tetrahedron Lett., 2002, 43, 8453 CrossRef CAS.
- R. A. Sheldon, Green Chem., 2005, 7, 267 RSC.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060 CrossRef CAS PubMed.
- D. A. Alonso, A. Baeza, R. Chinchilla, G. Guillena, I. M. Pastor and D. J. Ramón, Eur. J. Org. Chem., 2016, 612 CrossRef CAS.
- W. Gao, X. Fu, X. Zhang, Y. Zhao, D. Wang and Y. Li, Tetrahedron Lett., 2016, 57, 4145 CrossRef CAS.
- Y. Li, B. Tang, S. Dong, W. Gao, W. Jiang and Y. Chen, ChemistrySelect, 2020, 5, 2746 CrossRef CAS.
- A. Dondoni and A. Marra, Chem. Rev., 2004, 104, 2557 CrossRef CAS PubMed.
- J. M. Guernon and Y. J. Wu, Tetrahedron Lett., 2011, 52, 3633 CrossRef CAS.
- P. Liu, J.-W. Hao, L.-P. Mo and Z.-H. Zhang, RSC Adv., 2015, 5, 48675 RSC.
- M. Capua, S. Perrone, F. M. Perna, P. Vitale, L. Troisi, A. Salomone and V. Capriati, Molecules, 2016, 21, 924 CrossRef PubMed.
- B. S. Singh, H. R. Lobo, D. V. Pinjari, K. J. Jarag, A. B. Pandit and G. S. Shankarling, Ultrason. Sonochem., 2013, 20, 633 CrossRef CAS PubMed.
- L. Xiao, G. Liu, Z. Li, P. Ren, L. Ren and J. Kong, Chin. J. Org. Chem., 2020, 40, 2988 CrossRef CAS.
- Z. Xie, H. Li, B. Wang, Z. Wu and Z. Le, J. Heterocycl. Chem., 2021, 58, 1588 CrossRef CAS.
- A. Gholami, M. Mokhtary and M. Nikpassand, Dyes Pigm., 2020, 180, 108453 CrossRef CAS.
- S. Srivastava, ChemistrySelect, 2020, 5, 799 CrossRef CAS.
- S. Perrone, M. Capua, F. Messa, A. Salomone and L. Troisi, Tetrahedron, 2017, 73, 6193 CrossRef CAS.
- J. U. Song, S. P. Choi, T. H. Kim, C. K. Jung, J. Y. Lee, S. H. Jung and G. T. Kim, Bioorg. Med. Chem. Lett., 2015, 25, 1254 CrossRef CAS PubMed.
- M. S. Shaikh, M. B. Palkar, H. M. Patel, R. A. Rane, W. S. Alwan, M. M. Shaikh, I. M. Shaikh, G. A. Hampannavar and R. Karpoormath, RSC Adv., 2014, 4, 62308 RSC.
- M. A. T. Nguyen, A. K. Mungara, J. A. Kim, K. D. Lee and S. Park, Phosphorus, Sulfur Silicon Relat. Elem., 2015, 190, 191 CrossRef CAS.
- J. E. F. Alves, J. F. de Oliveira, T. R. C. de Lima Souza, R. O. de Moura, L. B. de Carvalho Junior, M. D. C. A. de Lima and S. M. V. de Almeida, Int. J. Biol. Macromol., 2021, 170, 622 CrossRef CAS PubMed.
- A. O'Dea, C. Sondergard, P. Sweeney and C. K. Arnatt, ACS Med. Chem. Lett., 2018, 9, 901 CrossRef PubMed.
- M. A. T. Nguyen, A. K. Mungara, J. A. Kim, K. D. Lee and S. Park, Phosphorus, Sulfur Silicon Relat. Elem., 2015, 190, 191 CrossRef CAS.
- S. Aki, T. Fujioka, M. Ishigami and J. Minamikawa, Bioorg. Med. Chem. Lett., 2002, 12, 2317 CrossRef CAS PubMed.
- R. S. Egan, J. Tadanier, D. L. Garmaise and A. P. Gaunce, J. Org. Chem., 1968, 33, 4422 CrossRef CAS.
- D. B. Yedage and D. V. Patil, ChemistrySelect, 2018, 3, 3611 CrossRef CAS.
- P. M. Jadhav, S. Kantevari, A. B. Tekale, S. V. Bhosale, R. P. Pawar and S. U. Tekale, Phosphorus, Sulfur Silicon Relat. Elem., 2021, 196, 879 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra04587g |
| This journal is © The Royal Society of Chemistry 2022 |