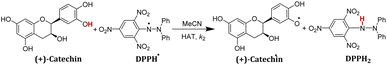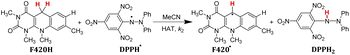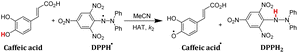Open Access Article
Open Access ArticleEvaluation and comparison of antioxidant abilities of five bioactive molecules with C–H and O–H bonds in thermodynamics and kinetics†
Yan-Hua Fu *a,
Zhen Wanga,
Kai Wang
*a,
Zhen Wanga,
Kai Wang a,
Guang-Bin Shen
a,
Guang-Bin Shen *b and
Xiao-Qing Zhu*c
*b and
Xiao-Qing Zhu*c
aCollege of Chemistry and Environmental Engineering, Anyang Institute of Technology, Anyang, Henan 455000, China. E-mail: 20180031@ayit.edu.cn
bSchool of Medical Engineering, Jining Medical University, Jining, Shandong 272000, P. R. China
cDepartment of Chemistry, Nankai University, Tianjin, 300071, China
First published on 27th September 2022
Abstract
In this work, the antioxidant abilities of NADH coenzyme analogue BNAH, F420 reduction prototype analogue F420H, vitamin C analogue iAscH−, caffeic acid, and (+)-catechin in acetonitrile in chemical reactions were studied and discussed. Three physical parameters of the antioxidant XH, homolytic bond dissociation free energy ΔG°(XH), self-exchange HAT reaction activation free energy ΔG≠XH/X, and thermo-kinetic parameter ΔG≠°(XH), were used to evaluate the antioxidant ability of XH in thermodynamics, kinetics, and thermo-kinetics. By comparing ΔG°(XH), ΔG≠XH/X and ΔG≠°(XH) of these five bioactive antioxidants to release hydrogen atoms, it is easy to find that iAscH− is the best hydrogen atom donor both thermodynamically and kinetically among these antioxidants. Caffeic acid is the worst hydrogen atom donor thermodynamically, and F420H is the worst hydrogen atom donor kinetically. In addition, the thermodynamic hydride donating abilities of BNAH, F420H, and iAscH− were also discussed, and the order of thermodynamic hydride donating abilities was BNAH > F420H > iAscH−. Four HAT reactions BNAH/DPPH˙, (+)-catechin/DPPH˙, F420H/DPPH˙, and caffeic acid/DPPH˙ in acetonitrile at 298 K were studied by the stopped-flow method. The actual order of H-donating abilities of these four antioxidants in the HAT reactions is consistent with the order predicted by thermo-kinetic parameters. It is feasible to predict accurately the antioxidant abilities of antioxidants using thermo-kinetic parameters.
Introduction
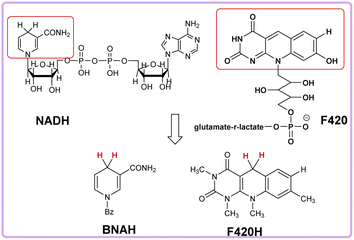
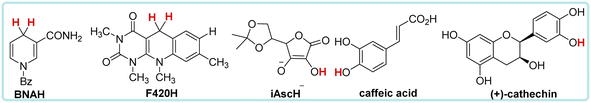
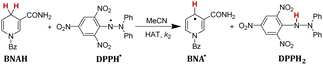
Nicotinamide coenzyme (NADH)1–3 and F420 coenzyme4–7 are the two extremely important natural redox cofactors, which exist extensively in vivo as effective hydride and electron sources taking part in a wide range of biochemical processes. They have similar functions and structures but perform unique functions, respectively.8,9 The active centres of NADH and F420 coenzyme are shown in Scheme 1. In this work, 1-benzyl-1,4-dihydro-nicotinamide (BNAH) and reductive F420 coenzyme (F420H) were investigated as the models of each coenzyme and used as the comparative research objects using thermodynamic and kinetic methods. The properties of the two coenzymes were studied and compared as hydrogen/hydride donors with C–H bonds. In addition to these two coenzyme analogues, another three organic bioactive molecules, vitamin C (iAscH−),10 caffeic acid,11 and (+)-catechin,12,13 were also studied as hydrogen atom donors with O–H bonds. These three bioactive molecules were widely used as antioxidants in the study of free radical activity, chemical mechanism study, etc.14 Two coenzyme derivatives with C–H bonds (BNAH and F420H) and three organic bioactive molecules with O–H bonds (iAscH−, caffeic acid, and (+)-catechin) (Scheme 2) were studied as antioxidants in hydrogen atom transfer (HAT) reaction (XH + Y → X + YH).Results and discussion
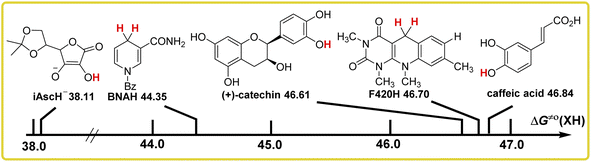
The evaluation and comparison of the antioxidant abilities of these five bioactive molecules were discussed carefully. Three physical parameters of these antioxidants were used to compare the antioxidant abilities using thermodynamic and kinetic methods in acetonitrile, which are the bond dissociation free energy [ΔG°(XH)], self-exchange HAT reaction activation free energy [ΔG≠XH/X], and thermo-kinetic parameter [ΔG≠°(XH)]. XH is the abbreviation of antioxidant, and H represents the transferred hydrogen atom. The data of these three parameters for BNAH, F420H, and iAscH− were derived from our previous work.15–18 The data source14 and calculation process of the parameters for caffeic acid and (+)-catechin are listed in ESI.† All data are listed in Table 1.| Antioxidant | C–H | O–H | |||
|---|---|---|---|---|---|
| Energya (kcal mol−1) | BNAH | F420H | iAscH− | Caffeic acidb | (+)-Catechinb |
| a The unit is kcal mol−1 ΔG≠°(XH) is the thermo-kinetic parameter of XH, which is proposed in previous publications,14–16 ΔG≠°(XH) = 1/2[ΔG≠XH/X + ΔG°(XH)].b The data source14,16 and calculation process of the parameters for caffeic acid and (+)-catechin were listed in ESI. | |||||
| ΔG°(XH) | 65.80 | 66.40 | 65.40 | 77.00 | 76.20 |
| ΔG≠XH/X | 22.90 | 26.99 | 10.81 | 16.68 | 17.02 |
| ΔG≠°(XH) | 44.35 | 46.70 | 38.11 | 46.84 | 46.61 |
Comparison of the bond dissociation free energies ΔG°(XH) of these antioxidants as hydrogen atom donors in acetonitrile
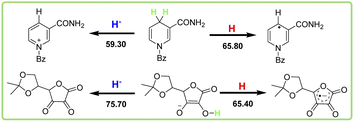
It is well known that the homolytic bond dissociation free energy ΔG°(XH) is usually used to access the potential H-donating ability of antioxidants in thermodynamics. The smaller the value of ΔG°(XH), the stronger the antioxidant ability for the antioxidant. According to Table 1, the order of ΔG°(XH) for these five antioxidants in acetonitrile is caffeic acid > (+)-catechin > F420H > BNAH > iAscH−, which indicates that iAscH− is the best hydrogen atom donor among them, thermodynamically. In order to facilitate the comparison of antioxidant abilities of these antioxidants, the bond dissociation free energies of the antioxidants to release hydrogen atoms are shown in Scheme 3 in descending order.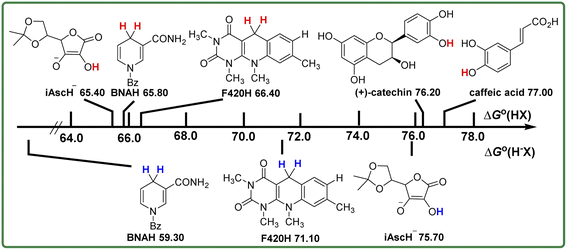 | ||
| Scheme 3 Comparison of homolytic bond dissociation free energies of XH and heterolytic bond dissociation free energies of BNAH, F420H, and iAscH−. | ||
BNAH and F420H both break the C–H bond at position 4 of the 1,4-dihydropyridine rings as antioxidants in HAT reactions. There is not much difference between ΔG°(XH) values (0.60 kcal mol−1) of these two coenzyme derivatives, even though there is a big difference in the structures. Caffeic acid and (+)-catechin both break the O–H bond on the phenol rings as antioxidants in HAT reactions. The difference between ΔG°(XH) values of these two phenol derivatives (0.80 kcal mol−1) is not large too. However, the ΔG°(XH) difference to break the O–H and C–H bonds is quite large. It is 9.80 kcal mol−1 between the values of ΔG°(XH) for F420 (C–H) and (+)-catechin (O–H), and is 11.20 kcal mol−1 between BNAH (C–H) and caffeic acid (O–H). For iAscH−, although it also breaks the O–H bond as an antioxidant in HAT reactions, the O–H bond is not on the benzene ring, but the alcohol O–H bond on the enol structure, so the bond dissociation free energy required to break the O–H bond is relatively small. The ΔG°(XH) of iAscH− is even 0.40 kcal mol−1 smaller than that of BNAH.
The above discussion shows that the bond dissociation free energy of antioxidant XH mainly depends on the central structure and type of the bond broken (C–H or O–H). Antioxidants with the same type of bond being broken and similar central structure have similar bond dissociation free energies. The type of chemical bond broken is crucial to the thermodynamic hydrogen atom donating ability of antioxidants.
BNAH, F420H, and iAscH− are not only good hydrogen atom donors, but also important hydride transporters in vivo. NAD coenzyme is hydrogenase as its main function,19 while F420 coenzyme can perform both dehydrogenase and hydrogenase functions in vivo.20,21 In Scheme 3, the heterolytic bond dissociation free energies ΔG°(H−X) of BNAH, F420H, and iAscH− in acetonitrile are also listed, which are 59.30 kcal mol−1 (BNAH), 71.10 kcal mol−1 (F420H), and 75.70 kcal mol−1 (iAscH−), respectively. The order of ΔG°(H−X) is iAscH− > F420H > BNAH, which indicates that BNAH is the best hydride donor among them thermodynamically. By comparing the bond dissociation free energy of BNAH as a hydride and hydrogen atom donor, it is clear that for BNAH, it is much easier (7.50 kcal mol−1) to release hydride than a hydrogen atom. For F420H, it is easier (4.70 kcal mol−1) to release a hydrogen atom than hydride. This is because the stable structure of pyridine positive ions is formed after the release of hydride ions for BNAH and F420H, and the electron deficient unstable structure of 1,4-dihydropyridine radicals is formed after the release of hydrogen atoms, as shown in Scheme 4. For iAscH−, however, the opposite phenomenon is found. It is much easier (10.30 kcal mol−1) to release a hydrogen atom than hydride, which indicates that iAscH− is a good hydrogen atom donor but a bad hydride donor thermodynamically. The reason for the large difference between homolytic and heterolytic bond dissociation free energies of O–H bond for iAscH− may be that the structures are quite different after homolytic and heterolytic cleavages (Scheme 4). It is not easy to take away an extra electron from the electron deficient system of α-β unsaturated ketone in iAsc−˙, so donating a hydride is much harder than only donating a hydrogen atom.
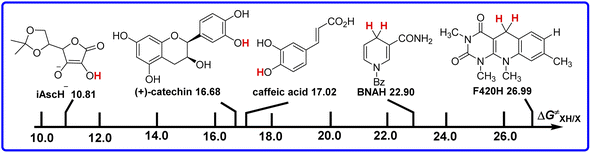
Comparison of the intrinsic kinetic barriers ΔG≠XH/X of these antioxidants as hydrogen atom donors in acetonitrile
For the self-exchange HAT transfer reaction of antioxidant XH (XH + X → X + XH), the thermodynamic driving force of the reaction is zero, and the activation free energy of the self-exchange HAT reaction (ΔG≠XH/X) can be regarded as the intrinsic kinetic barrier of XH as hydrogen atom donor in HAT reaction. The bigger the value of ΔG≠XH/X, the bigger the intrinsic kinetic barrier of XH in HAT reaction. This parameter is related to the spatial structure of the HAT reaction site of the antioxidant, the solvent effect in the reaction process and other kinetic factors, and it belongs to the characteristic kinetic parameter of the antioxidant. According to Table 1, the order of ΔG≠XH/X for these five antioxidants in acetonitrile is F420H > BNAH > caffeic acid > (+)-catechin > iAscH−, which indicates that iAscH− is the best hydrogen atom donor among them kinetically. The hydrogen atom donating abilities of antioxidants with C–H reaction sites (F420H > BNAH) are weaker than the antioxidants with phenol O–H reaction sites [caffeic acid > (+)-catechin] in kinetics, which are not consistent with the phenomena in thermodynamics. In order to facilitate the comparison of the antioxidant abilities of antioxidants, the intrinsic kinetic barriers of antioxidants to release hydrogen atoms are shown in Scheme 5 in descending order.As the reaction sites (O–H) on caffeic acid and (+)-catechin are relatively bare, the steric hindrance of HAT reactions is small. There is not much difference between ΔG≠XH/X values (0.34 kcal mol−1) of caffeic acid and (+)-catechin. For BNAH and F420H, the amide groups increase the steric hindrance of the reaction sites (C–H), resulting in higher activation free energies of self-exchange HAT reactions. Although the active sites in the HAT reaction are both C–H in BNAH and F420H, the difference between ΔG≠XH/X values is 4.09 kcal mol−1, which is bigger than the difference between ΔG≠XH/X values (0.34 kcal mol−1) of caffeic acid and (+)-catechin. This is because of the different structures between BNAH and F420H. Especially for F420H, except for the amide group, the tricycle molecular framework has a great influence on the steric hindrance of the reaction site. It results from the fact that F420H has the biggest kinetic intrinsic barrier in the HAT reaction. The ΔG≠XH/X difference between O–H broken and C–H broken is very large. It is 10.31 kcal mol−1 between the value of ΔG≠XH/X for F420H and (+)-catechin.
The small molecular structure and the exposure of the reaction site (O–H) lead to the smallest kinetic resistance of iAscH− in the HAT reaction. The value of ΔG≠XH/X for iAscH− is 10.81 kcal mol−1, even 5.87 kcal mol−1 less than that of (+)-catechin. It is 16.18 kcal mol−1 less than that of F420H, which has the largest ΔG≠XH/X.
According to the above analysis, it can be seen that the kinetic H-donating ability mainly depends on the steric hindrance of the hydrogen supply centre of the antioxidant XH and the overall structure of the compound. The difference in kinetic internal resistances of these five antioxidants in HAT reaction is greater than the difference in bond dissociation free energies. According to ΔG≠XH/X and ΔG°(HX) of these five antioxidants, the order of hydrogen atom donating abilities kinetically is not in line with their order thermodynamically, which indicates that the antioxidant ability cannot be determined only by thermodynamic parameters. Among these five antioxidants, only iAscH− is a good hydrogen atom donor both thermodynamically and kinetically.
Comparison of the thermo-kinetic parameters ΔG≠°(XH) of these antioxidants as hydrogen atom donors in acetonitrile
In our previous work,15–18 the thermo-kinetic parameter ΔG≠° is proposed to evaluate the actual ability of a compound in a chemical reaction during a certain reaction time, such as the ΔG≠°(XH) of antioxidant can be used to evaluate the actual antioxidant ability, and the ΔG≠°(X) of free radical can be used to evaluate the actual H-abstraction ability. ΔG≠°(XH) is defined as 1/2[ΔG≠XH/X + ΔG°(XH)], which consists of both bond dissociation free energy ΔG°(XH) of antioxidant and self-exchange HAT activation free energy ΔG≠XH/X. The higher the value of the thermo-kinetic parameter ΔG≠°(XH), the weaker the antioxidant ability of the antioxidant. From Table 1, the thermo-kinetic parameters ΔG≠°(XH) of these antioxidants in acetonitrile are in the order of caffeic acid > F420H > (+)-catechin > BNAH > iAscH−. The thermo-kinetic parameters ΔG≠°(XH) of antioxidants to release hydrogen atoms are shown in Scheme 6 in descending order.The order of thermo-kinetic parameters of these antioxidants as hydrogen atom donors in acetonitrile is the comprehensive result of thermodynamic and kinetic intrinsic barrier analysis, which is no longer simply dependent on the types of C–H and O–H bonds broken. The thermo-kinetic parameter of the antioxidant is derived from the actual HAT reaction rate, and it can truly reflect the hydrogen atom donating ability of antioxidants. From Scheme 6, the thermo-kinetic parameters of caffeic acid and (+)-catechin are very close [46.84 kcal mol−1 for caffeic acid, 46.61 kcal mol−1 for (+)-catechin], since their bond dissociation free energies and self-exchange HAT activation free energies are both close to each other. For F420H, although the ΔG°(XH) value is about 10 kcal mol−1 smaller than caffeic acid and (+)-catechin, the ΔG≠XH/X value is about 10 kcal mol−1 bigger than caffeic acid and (+)-catechin. Combining these two results, the thermo-kinetic parameter of F420H (46.70 kcal mol−1) is close to caffeic acid and (+)-catechin. For BNAH and iAscH−, although the difference in ΔG°(XH) between BNAH and iAscH− is only 0.4 kcal mol−1, the difference in ΔG≠XH/X is 12.24 kcal mol−1, resulting in the thermo-kinetic parameter of iAscH− being 6.24 kcal mol−1 smaller than that of BNAH. Therefore, the actual antioxidant ability of iAscH− is much bigger than BNAH. For example, for the HAT reactions BNAH/tBu3PhO˙ and iAscH−/tBu3PhO˙, the rate of the HAT reaction between BNAH and tBu3PhO˙ (kH is 8.85 × 101 M−1 s−1) is much slower than that of iAscH− and tBu3PhO˙ (kH is 3.39 × 106 M−1 s−1).15 As discussed above, iAscH− is the best antioxidant both thermodynamically and kinetically, which is also confirmed by the value of the thermo-kinetic parameter ΔG≠°(XH).
In order to verify whether the order of thermo-kinetic parameters of these five antioxidants is accurate, the following experiments were conducted. 2,2-diphenyl-1-picrylhydrazyl (DPPH˙) is a relatively stable neutral radical and is frequently used as reactive oxygen species (ROS) model to evaluate the radical-scavenging activity of antioxidants.22 It was chosen as the hydrogen atom acceptor, and the second-order rate constants kH (M−1 s−1) of the competitive HAT reactions BNAH/DPPH˙, (+)-catechin/DPPH˙, F420H/DPPH˙, and caffeic acid/DPPH˙ were researched using the stopped-flow technique by monitoring the absorbance decay of DPPH˙ at 518 nm using pseudo-first-order kinetic model. The kinetic absorbance decay curves of these four HAT reactions are shown in ESI.† The results of kH are listed in Table 2. For the same free radical, the actual order of hydrogen atom donating abilities of the four antioxidants in the HAT reactions is consistent with the order predicted by thermo-kinetic parameters. This phenomenon indicates that it is feasible and accurate to predict the antioxidant abilities of antioxidants using thermo-kinetic parameters.
Analysis of the actual antioxidant abilities of these antioxidants
In Table 3, the diagnoses of chemical properties as hydrogen atom donors for these five antioxidants in terms of thermodynamics, kinetics, and thermos-kinetics in acetonitrile are listed. iAscH− is the best hydrogen atom donor among these five bioactive antioxidants, both thermodynamically and kinetically. Since it has the smallest bond dissociation free energy and smallest self-exchange HAT activation free energy, it has the strongest actual antioxidant ability in the HAT reaction. Caffeic acid is a quite strong hydrogen atom donor kinetically, but the weakest hydrogen atom donor thermodynamically, and the hydrogen atom donating ability advantage in kinetics cannot cover the shortage of the hydrogen atom donating ability in thermodynamics, which results in the weakest antioxidant ability among these five antioxidants. For F420, although it is the weakest hydrogen atom donor kinetically, it is a mild hydrogen atom donor thermodynamically, and the hydrogen atom donating ability advantage in thermodynamics can cover the shortage of the hydrogen atom donating ability in kinetics to some extent, which results in quite weak actual antioxidant ability among these five antioxidants.| Compound | Diagnoses of the characteristic properties | ||
|---|---|---|---|
| Thermodynamics | Kinetics | Thermo-kinetic | |
| BNAH | Quite strong hydrogen atom donor | Quite weak hydrogen atom donor | Quite strong hydrogen atom donor |
| Strong hydride donor | |||
| F420H | Mild hydrogen atom donor | Weak hydrogen atom donor | Quite weak hydrogen atom donor |
| Mild hydride donor | |||
| iAscH− | Strong hydrogen atom donor | Strong hydrogen atom donor | Strong hydrogen atom donor |
| Weak hydride donor | |||
| Caffeic acid | Weak hydrogen atom donor | Quite strong hydrogen atom donor | Weak hydrogen atom donor |
| (+)-Catechin | Quite weak hydrogen atom donor | Mild hydrogen atom donor | Mild hydrogen atom donor |
For BNAH, F420H, and iAscH−, BNAH is the best hydride donor, followed by F420H, and iAscH− is the weakest hydride donor among these three antioxidants thermodynamically.
Conclusions
In this work, the abilities of five bioactive antioxidants with the breaking of C–H or O–H bonds and releasing hydrogen atoms in acetonitrile at 298 K were focused on and researched from the aspect of thermodynamics, kinetics, and thermo-kinetics. The following conclusions can be made:(1) The order of H-donating abilities of these five antioxidants thermodynamically is caffeic acid > (+)-catechin > F420H > BNAH > iAscH−. The bond dissociation free energy ΔG°(XH) of antioxidant XH mainly depends on the central structure and type of the bond broken (C–H or O–H). The type of the chemical bond broken is crucial to the thermodynamic H-donating ability of the antioxidant.
(2) The order of H-donating abilities of these five antioxidants kinetically is F420H > BNAH > caffeic acid > (+)-catechin > iAscH−. The kinetic H-donating ability mainly depends on the steric hindrance of the hydrogen supply centre of the antioxidant XH and the overall structure of the compound.
(3) The order of H-donating abilities of these five antioxidants in thermo-kinetics is caffeic acid > F420H > (+)-catechin > BNAH > iAscH−. The second-order rate constants of the four HAT reactions between XH and DPPH˙ in acetonitrile at 298 K were researched by the stopped-flow method. The actual order of H-donating abilities of the four antioxidants in the HAT reactions is consistent with the order predicted by thermo-kinetic parameters. It is feasible and accurate to predict the antioxidant abilities of antioxidants using thermo-kinetic parameters.
(4) It is inaccurate to estimate the antioxidant ability only using the thermodynamic parameter or kinetic parameter of the antioxidant. It is more accurate to evaluate the antioxidant ability by thermo-kinetic parameters in chemical reactions.
Experiment section
Materials
All reagents were of commercial quality from freshly opened containers or were purified before use. Reagent grade acetonitrile was refluxed over KMnO4 and K2CO3 for several hours and was doubly distilled over P2O5 under argon before use.23 All operations were carried out in an argon-filled glovebox. BNAH,16 F420H (ref. 9) and iAscH− (ref. 10c) were synthesized according to the conventional synthetic strategies. The typical synthetic routes of these compounds are provided in ESI.† Caffeic acid, (+)-catechin, and DPPH˙ were commercially available.Kinetic measurements
The kinetics of the HAT reactions, which were thermostated at 298 K under strictly anaerobic conditions in dry acetonitrile, were conveniently monitored by an Applied Photophysics SX.18MV-R stopped-flow method.8b The method of kinetic measurement was pseudo-first-order method. The concentration of the antioxidant was maintained at more than 20-fold excess of the oxidant to attain the pseudo-first-order condition. The second-order rate constants (k2) were derived from plots of the pseudo-first-order rate constants versus the concentrations of the excessive reactants. In each case, it was confirmed that the rate constants derived from three to five independent measurements agreed within an experimental error of ±5%.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
Financial support from PhD Research Startup Foundation of Anyang Institute of Technology (BSJ2019032).References
- (a) S. Immanuel, R. Sivasubramanian, R. Gul and M. Ahmad Dar, Chem.–Asian J., 2020, 15(24), 4256–4270 CrossRef CAS PubMed; (b) S. Zhang, J. Shi, Y. Chen, Q. Huo, W. Li, Y. Wu, Y. Sun, Y. Zhang, X. Wang and Z. Jiang, ACS Catal., 2020, 10(9), 4967–4972 CrossRef CAS; (c) J.-D. Yang, B.-L. Chen and X.-Q. Zhu, J. Phys. Chem. B, 2018, 122(27), 6888–6898 CrossRef CAS PubMed.
- T. Knaus, C. E. Paul, C. W. Levy, S. de Vries, F. G. Mutti, F. Hollmann and Ni. S. Scrutton, J. Am. Chem. Soc., 2016, 138(3), 1033–1039 CrossRef CAS PubMed.
- H. Wu, C. Tian, X. Song, C. Liu, D. Yang and Z. Jiang, Green Chem., 2013, 15(7), 1773–1790 RSC.
- R. Grinter and C. Greening, FEMS Microbiol. Rev., 2021, 45(5), fuab021 CrossRef CAS PubMed.
- H. Kumar, Q.-T. Nguyen, C. Binda, A. Mattevi and M. W. Fraaije, J. Biol. Chem., 2017, 292(24), 10123–10130 CrossRef CAS.
- D. Braga, D. Last, M. Hasan, H. Guo, D. Leichnitz, Z. Uzum, I. Richter, F. Schalk, C. Beemelmanns, C. Hertweck and G. Lackner, ACS Chem. Biol., 2019, 14(9), 2088–2094 CrossRef CAS.
- M. Wunderer, R. Markt, N. Lackner and A. O. Wagner, Sci. Total Environ., 2022, 809, 151112–151121 CrossRef CAS PubMed.
- (a) X.-Q. Zhu, Y. Liu, B.-J. Zhao and J.-P. Cheng, J. Org. Chem., 2001, 66, 370–375 CrossRef CAS PubMed; (b) X.-Q. Zhu, J.-Y. Zhang and J.-P. Cheng, J. Org. Chem., 2006, 71, 7007–7015 CrossRef CAS; (c) X.-Q. Zhu, Y. Tan and C.-T. Cao, J. Phys. Chem. B, 2010, 114, 2058–2075 CrossRef CAS PubMed; (d) G.-B. Shen, Y.-H. Fu and X.-Q. Zhu, J. Org. Chem., 2020, 85(19), 12535–12543 CrossRef CAS PubMed.
- K. Xia, G.-B. Shen and X.-Q. Zhu, Org. Biomol. Chem., 2015, 13, 6255–6268 RSC.
- (a) L. Aumailley, S. Bourassa, C. Gotti, A. Droit and M. Lebel, J. Proteome Res., 2021, 20(11), 5036–5053 CrossRef CAS PubMed; (b) H. Liu, W. Jia, X. Yu, X. Tang, X. Zeng, Y. Sun, T. Lei, H. Fang, T. Li and L. Lin, ACS Catal., 2021, 11, 137828–137844 Search PubMed; (c) X.-Q. Zhu, Y.-Y. Mu and X.-T. Li, J. Phys. Chem. B, 2011, 115, 14794–14811 CrossRef CAS PubMed.
- W. Zhao, J. Chao, R. Du and S. Huang, J. Inclusion Phenom. Macrocyclic Chem., 2011, 71, 25–34 CrossRef CAS.
- E. Laudadio, G. Mobbili, C. Minnelli, L. Massaccesi and R. Galeazzi, Mol. Inf., 2017, 36(11), 1700059–1700073 CrossRef PubMed.
- F. J. Hidalgo and R. Zamora, J. Agric. Food Chem., 2019, 67(7), 2043–2051 CrossRef CAS.
- M. Mazzonna, M. Bietti, G. A. DiLabio, O. Lanzalunga and M. Salamone, J. Org. Chem., 2014, 79(11), 5209–5218 CrossRef CAS.
- X.-Q. Zhu, F.-H. Deng, J.-D. Yang, X.-T. Li, Q. Chen, N.-P. Lei, F.-K. Meng, X.-P. Zhao, S.-H. Han, E.-J. Hao and Y.-Y. Mu, Org. Biomol. Chem., 2013, 11, 6071–6089 RSC.
- Y.-H. Fu, G.-B. Shen, Y. Li, L. Yuan, J.-L. Li, L. Li, A.-K. Fu, J. Chen, B.-L. Chen, L. Zhu and X.-Q. Zhu, ChemistrySelect, 2017, 2, 904–925 CrossRef CAS.
- Y.-H. Fu, K. Wang, G.-B. Shen and X.-Q. Zhu, J. Phys. Org. Chem., 2022, e4358 CAS.
- Y.-H. Fu, G.-B. Shen, K. Wang and X.-Q. Zhu, ChemistrySelect, 2021, 6, 8007–8010 CrossRef CAS.
- H. Yang, X. Jia and Y. Han, Trends Microbiol., 2022, 30(4), 318–321 CrossRef CAS PubMed.
- M. J. Nelson, D. P. Brown and J. G. Ferrym, Biochem. Biophys. Res. Commun., 1984, 120(3), 775–781 CrossRef CAS PubMed.
- N. L. Schauer, J. G. Ferry, J. F. Honek, W. H. Orme-Johnson and C. Walsh, Biochemistry, 1986, 25(22), 7163–7168 CrossRef CAS PubMed.
- (a) J. D. Yeo and F. Shahidi, J. Agric. Food Chem., 2019, 67(26), 7526–7529 CrossRef CAS; (b) Y. H. Lee, U. Chaudhuri and R. Mahendiran, J. Phys. Chem. C, 2021, 125(33), 18438–18443 CrossRef CAS.
- X.-Q. Zhu, M.-T. Zhang, A. Yu, C.-H. Wang and J.-P. Cheng, J. Am. Chem. Soc., 2008, 130, 2501–2516 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Syntheses of BNAH, F420H, and iAscH− and the 1H NMR spectra are provided. The calculation process of parameters ΔG≠XH/X and ΔG≠°(XH) for caffeic acid and (+)-catechin are available. The kinetic absorbance decay curves of four HAT reactions BNAH/DPPH˙, (+)-catechin/DPPH˙, F420H/DPPH˙, and caffeic acid/DPPH˙ in acetonitrile at 298 K are also shown. See https://doi.org/10.1039/d2ra04839f |
| This journal is © The Royal Society of Chemistry 2022 |