Open Access Article
Open Access ArticleA multi-functional zwitterionic hydrogel with unique micro-structure, high elasticity and low modulus†
Yueyang Wang a,
Qiao Wangb,
Xiaosai Hu
a,
Qiao Wangb,
Xiaosai Hu c,
Dan Hea,
Juan Zhao
c,
Dan Hea,
Juan Zhao *d and
Guoxing Sun*a
*d and
Guoxing Sun*a
aJoint Key Laboratory of the Ministry of Education, Institute of Applied Physics and Materials Engineering, University of Macau, Avenida da Universidade, Taipa, Macau SAR, China. E-mail: gxsun@um.edu.mo
bSchool of Civil and Transportation Engineering, Hebei University of Technology, 5340 Xiping Road, Beichen District, Tianjin 300401, China
cCollege of Textiles and Clothing, Yancheng Institute of Technology, Jiangsu Province, China
dSchool of Biotechnology and Health Sciences, Wuyi University, 529020, Guangdong, China. E-mail: wyuchemzj@126.com
First published on 30th September 2022
Abstract
Owing to their tissue-like softness and low modulus, hydrogels minimize the mechanical mismatch with biological tissues and have received wide attention as biomaterials. However, the development of soft hydrogels is often limited by their brittleness. Here, an ultra-soft and tough hydrogel based on zwitterionic poly(sulfobetaine methacrylate) (PSBMA) was designed and successfully prepared. The obtained PSBMA hydrogel exhibits a unique spike-like micro-structure, low modulus, good stretchability and excellent compressive elasticity, due to the formation of a dual-crosslinking structure. The obtained hydrogel also possesses self-healing properties and electromechanical responses to tensile and compressive deformations. Moreover, the hydrogel has good compatibility attributed to its outstanding anti-protein-adsorption properties.
Introduction
Hydrogels formed by infiltrating crosslinked polymer networks with water have gained increasing attention in tissue engineering,1,2 drug delivery,3,4 water treatment5,6 and so on. Owing to their tissue-like softness, high water-content and potential biocompatibility, hydrogels have growing applications as biomaterials7–9 or biosensors.10–12 However, there are still some tricky challenges waiting for solutions during their practical applications in human daily activities. For example, the modulus of a hydrogel should be tuned to match that of soft tissue, but most synthetic hydrogels exhibited a modulus in the range of 1–103 kPa, as compared with 0.1–30 kPa for human tissues like soft brain tissue.13,14 In addition, even if the hydrogels have achieved low modulus by refining the preparation procedure, they are often brittle and difficult to recover after deformation, which severely limit their applications.15 Unfortunately, the improvement of the toughness of hydrogels is often accompanied with an increase in modulus, leading to severe mismatch with the target objects and loss of the ability to function in the original application scenarios.16 Therefore, to achieve higher toughness with well-maintained low modulus, constructing a dual-crosslinking structure in the hydrogel was proposed according to the energy-dissipating mechanism.17Typically, the hydrogel with ionic crosslinks and covalent crosslinks had excellent toughness and outstanding stability.18 Among those promising ionic polymers for preparing functional hydrogel, zwitterionic polymer consisting of numerous cations and anions19,20 has wide applications, including antifouling blood contacted sensors,21–23 plasters for wound healing,24 drug delivery,25–27 etc. Taking advantage of the dynamic interactions between cations and anions in the side-chains of the zwitterionic polymer, poly(sulfobetaine methacrylate) (PSBMA) as one of the most widely used zwitterionic polymers is considered to be an ideal candidate to fabricate a soft and elastic hydrogel with dual-crosslinking structure, compared with the most studied poly(2-dimethylaminoethyl methacrylate) (PDMAEMA)28 and polyacrylamide (PAM)29 hydrogel. Here, we designed and prepared an ultra-soft but tough hydrogel with ionic and covalent crosslinks structure based on PSBMA polymer, which possesses excellent compressive elasticity, self-healablility, deformation stimuli-responsive property and good anti-protein adsorption property compared with other hydrogels. To our best knowledge, the synthesized gel is promising for various low modulus scenarios such as tissue repairing, healing assistance, etc.
Results and discussion
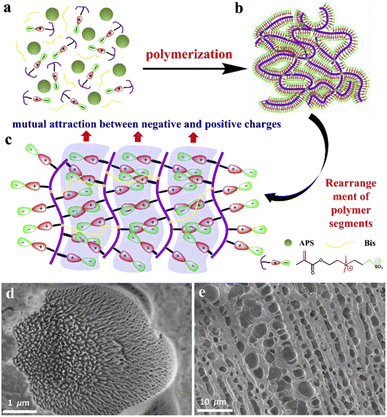
To prepare PSBMA hydrogel, the monomer sulfobetaine methacrylate (SBMA) was firstly synthesized from dimethylaminoethyl methacrylate (DMAEMA) and 1,3-propane sultone (PS) through a ring-opening reaction between tertiary amine and sultone (Fig. S1†). The molecular structure of the product was confirmed by 1H-NMR (Fig. S2†). The final product, a completely transparent hydrogel, was obtained through a typical in situ free-radical polymerization (for detailed information, see ESI†). During the polymerization, the mixture of crosslinker N,N-methylene-bisacrylamide (Bis), initiator ammonium peroxydisulfate (APS) and zwitterionic monomer SBMA was stood at 0 °C for 5 h, then the dual-crosslinking PSBMA hydrogel with ionic crosslinks and covalent crosslinks was produced (Fig. 1a and b). The covalent crosslinks were formed by the polymerization of SBMA with the crosslinkers of Bis and APS as the initiator. While the ionic crosslinks were generated due to the ionic interaction between quaternary ammonium and anionic sulfonate groups, resulting in the formation of a large number of ionic connections among the PSBMA chains (Fig. 1c). Owing to the existence of abundant ions on the side chains of PSBMA, hydrogel was fairly hydrophilic (Fig. 1d).The hydrophilicity can be proved by the microstructure of the freeze-dried PSBMA xerogel, which was clearly reflected in Fig. 1d and e shot by scanning electron microscope (SEM). A great number of micro-voids with diameter around 1–5 μm were parallelly distributed in the cross-section of PSBMA xerogel, while a partial image reflected a spike-like structures formed in the micro-void, which was extremely different from that of PDMAEMA hydrogel28 and the conventional PAM hydrogel.29 The specific rearranged combination of side chains may lead to the spike-like structure in PSBMA hydrogel, owing to electrostatic interaction between negative charges and positive charges on side chains of PSBMA, resulting in water accumulation among side chains. After removing the accumulated water via freeze-drying method, massive low-lying areas appeared at the position with aggregated side chains.
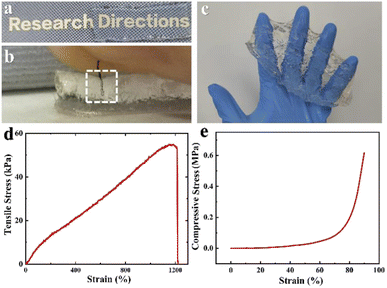
In terms of physical and mechanical properties, particularly, PSBMA hydrogel was relatively soft and exceptionally low modulus with almost absolute transparency (Fig. 2a). In fact, we found even a hair could be readily pierced into the synthesized PSBMA gel (Fig. 2b and c, Movie S1†). In addition, the obtained gel also exhibited good toughness of 369.78 kJ m−3, which was demonstrated by tensile test and compression test. From the tensile curve as shown in Fig. 2d, the hydrogel exhibited a maximum stretchability of 1211% under a tensile stress of 53 kPa. As expect, the compression elasticity of PSBMA hydrogel presented in Fig. 2e is comparatively attractive, the hydrogel could withstand 100% deformation with a low compressive modulus of 8.79 kPa, considering the low water content of ∼50%. Especially, it was able to recover to its original size after loading was removed with a rapid recovering speed (Movie S2†). For comparison, mechanical properties of the reported PDMAEMA hydrogel and PAM hydrogel were presented, and the data confirmed that the zwitterionic PSBMA hydrogel had better toughness and elasticity as well as relatively lower modulus (Table S1†).
The unique stretchability and compressive property of PSBMA hydrogel could be attributed to the dual-crosslinking structure consisting of electrostatic-force-coupled side chains and covalently crosslinked main chains. Ideally, in stress-free circumstances, side chains of contiguous polymers are one-to-one matched, thus forming a stable framework. When the PSBMA hydrogel suffers from tensile or compressive deformation, the matched pairs among the side chains are separated to dissipate energy firstly, thereby endowing the hydrogel with improved mechanic performances. Moreover, when loading was removed, the side chain couples would regenerate, partly restitute the elasticity of PSBMA hydrogel.18 Similar to the behaviour after unloading, the PSBMA hydrogel achieves self-healing through the movement of the polymer backbone and the re-pairing of side groups under high temperature. With the help of side chains, the healed gel still shows an elastic stage.
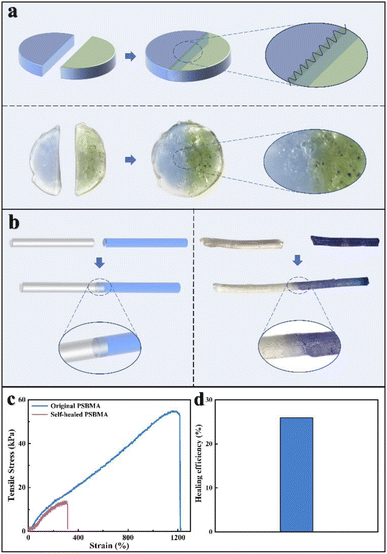
Apart from the mechanical properties, self-healing performance is another important property observed in hydrogel which is highly regarded in biological applications. As shown in Fig. 3a and b, the hydrogel was firstly cut into two pieces and stained with blue colour in one piece for better exhibition. We found the fractured hydrogels can be healed by compression and heating (90 °C for 12 h, for detailed information, see ESI†). And the recovered stretchable behaviour shown in Fig. 3c and d also suggests a relatively good healing effect.30
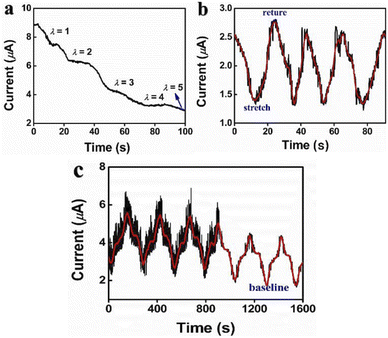
Abundant ions were formed in the zwitterionic PSBMA hydrogel, making it electro-conductive and deformation stimuli-responsive.34 Herein, the electro-mechanical responses of PSBMA hydrogel to tensile and compressive stimuli were tested (Fig. 4a–c). As shown in Fig. 4a, the responsive current decreased gradually under a stepwise incremental tensile deformation up to 500%, while later maintained a constant value as strain freeze to a certain point. Fig. 4b represented the electric response to cyclic deformation with a 100% stretching. It's confirmed that the hydrogel could accurately distinguish the strain stimulation through the clear intervals resulted from the rapid-recovering capability. On the other hand, for electric response of compressive deformation, the current variation of the hydrogel over 200 cycles of compression was presented in Fig. 4c, proving a high sensitivity to compressive pressure. This sensory behaviour was attributed to the geometry changes in PSBMA hydrogel and the electrical transmission of gel. When the hydrogels were subjected to stretch or compression, the electrical transmission path in hydrogels would change with the changes of the geometric shape, thus the resistance and current would change correspondingly while voltage value was hold. Finally, the electrical signals were transmitted to the signal receptor result from the transmitting action of numerous mobile ions in the hydrogel.31
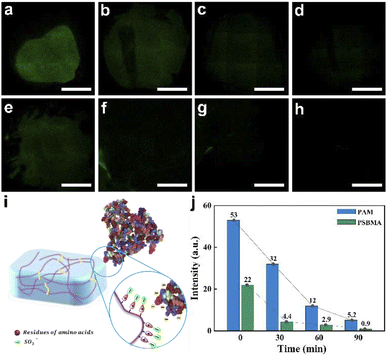
As a typical zwitterionic hydrogel, PSBMA has good anti-protein adsorption properties caused by electrostatic repulsions,32–34 which can be widely used in marine antifouling materials, biomedical materials, etc. Here, the anti-protein adsorption property of PSBMA hydrogel was studied by laser confocal fluorescence microscopy (Fig. 5a–e), using Bovine Serum Albumin (BSA) as a model protein for adsorption tests. At the same time, the conventional biomaterial PAM hydrogel was used for comparison. After taking the two different hydrogels incubated in fluorescein isothiocyanate–bovine serum albumin solutions (FITC–BSA) for 48 h (detailed information in the ESI†), the two hydrogels were reincubated in PBS solution. Then, we start to take fluorescence pictures and measure the fluorescence intensity of PSBMA hydrogel. In the beginning, the fluorescence intensity of PSBMA hydrogel was 22, which was nearly half of the value of PAM hydrogel with the value of 53, suggesting the weaker interactions of BSA with PSBMA hydrogel than with PAM hydrogel. As the re-incubated time increases, the fluorescence intensity of PSBMA drops rapidly with a higher decline rate than PAM hydrogel (Fig. 5j). Just after 30 min, the fluorescence dropped to 4.4, while the intensity of PAM hydrogel was 5.2 even after 90 min. In other words, the protein release rate of PSBMA hydrogel was quicker than that of PAM hydrogel.
Conclusions
To conclude, a dual-crosslinking hydrogel with ionic and covalent crosslinks based on zwitterionic polysulfobetaine methacrylate (PSBMA) was designed and successfully prepared via in situ free-radical polymerization. The obtained hydrogel exhibited unique microstructure due to the mutual attraction between negative charges and positive charges on the side chains of PSBMA. The synthesised PSBMA hydrogel was soft with a low modulus of 8.79 kPa. Besides, it had good stretchability which could be stretched to 12 times of its original length, and possessed excellent compression elasticity, which could be compressed up to 100% strain and recovered to its original size after unloading. Moreover, the PSBMA hydrogel is self-healable and electromechanically responsive to both tensile and compressive deformation. Owing to the over-all properties as well as the outstanding protein adsorption resistant property, the obtained hydrogel could be used as skin-like material, plaster for large-area wound healing, artificial tissue replacement, biosensor and so on.35Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by National Natural Science Foundation of China, Excellent Young Scientists Fund (HK&Macau) (File no. 5212290021); Shenzhen-Hong Kong-Macao Science and Technology Plan (c) (File no. SGDX2020110309360); The Science and Technology Development Fund, Macau SAR (File no. 0138/2020/A3); and Multi-Year Research Grant (File no. MYRG2022-00217-IAPME) from University of Macau.References
- K. Y. Lee and D. J. Mooney, Chem. Rev., 2001, 101, 1869 CrossRef CAS PubMed.
- Q. Yang, J. Peng, H. Xiao, X. Xu and Z. Qian, Carbohydr. Polym., 2022, 278, 118952 CrossRef CAS PubMed.
- Y. Qiu and K. Park, Adv. Drug Delivery Rev., 2001, 53, 321 CrossRef CAS.
- J. Zhu, H. Wu, Z. Li, X. Xu, H. Xing, M. Wang, H. Jia, L. Liang, C. Li, L. Sun, Y. Wang, F. Shen, D. Huang and T. Yang, Adv. Mater., 2022, 34, 2201651 CrossRef CAS PubMed.
- H. Lu, W. Shi, F. Zhao, W. Zhang, P. Zhang, C. Zhao and G. Yu, Adv. Funct. Mater., 2021, 31, 2101036 CrossRef CAS.
- X. Chen, Z. Song, B. Yuan, X. Li, S. Li, T. Nguyen, M. Guo and Z. Guo, Chem. Eng. J., 2022, 4(15), 133154 CrossRef.
- P. Huo, H. Ding, Z. Tang, X. Liang, J. Xu, M. Wang, R. Liang and G. Sun, Int. J. Biol. Macromol., 2022, 212, 1–10 CrossRef CAS PubMed.
- C. Larson, B. Peele, S. Li, S. Robinson, M. Totaro, L. Beccai, B. Mazzolai and R. Shepherd, Science, 2016, 351, 1071–1074 CrossRef CAS PubMed.
- Z. Tang, H. He, L. Zhu, Z. Liu, J. Yang, G. Qin, J. Wu, Y. Tang, D. Zhang, Q. Chen and J. Zheng, Adv. Sci., 2021, 9(5), 2102557 CrossRef PubMed.
- Q. Wang, H. Ding, X. Hu, X. Liang, M. Wang, Q. Liu, Z. Li and G. Sun, Mater. Horiz., 2020, 7, 2673–2682 RSC.
- Z. Xiong, S. Achavananthadith and S. Lian, et al., Sci. Adv., 2021, 7, 47 Search PubMed.
- H. Ding, X. Liang, J. Xu, Z. Tang, Z. Li and G. Sun, ACS Appl. Mater. Interfaces, 2021, 13(19), 22774–22784 CrossRef CAS PubMed.
- S. Park, H. Yuk and R. Zhao, et al., Nat. Commun., 2021, 12, 3435 CrossRef CAS PubMed.
- S. Choi, W. Guan and K. Chung, Cell, 2021, 184(76), 4115–4136 CrossRef CAS.
- M. A. Samp, N. C. Iovanac and A. J. Nolte, ACS Biomater. Sci. Eng., 2017, 3, 3176 CrossRef CAS PubMed.
- R. P. Behera and H. Le Ferrand, Matter, 2021, 4(9), 2831–2849 CrossRef CAS.
- Y. Huang, M. Zhong and Y. Huang, et al., Nat. Commun., 2015, 6, 10310 CrossRef CAS PubMed.
- B. Ying, R. Z. Chen and R. Zuo, et al., Adv. Funct. Mater., 2021, 31, 2104665 CrossRef CAS.
- L. Zheng, H. S. Sundaram, Z. Wei, C. Li and Z. Yuan, React. Funct. Polym., 2017, 118, 51 CrossRef CAS.
- J. Wu, Z. Xiao, C. He, J. Zhu, G. Ma, G. Wang, H. Zhang, J. Xiao and S. Chen, Acta Biomater., 2016, 40, 172 CrossRef CAS.
- M. Yao, H. Sun and Z. Guo, et al., Chem. Eng. J., 2021, 421(1), 129702 CrossRef CAS.
- F. Diehl, S. Hageneder and S. Fossati, et al., Chem. Soc. Rev., 2022, 51, 3926–3963 RSC.
- D. Chan, J. C. Chien and E. Axpe, et al., Adv. Mater., 2022, 34, 2109764 CrossRef CAS.
- A. A. Zahid, A. Chakraborty and Y. Shamiya, et al., Mater. Horiz., 2022, 9, 1850–1865 RSC.
- X. Yang, C. Zhang, D. Deng, Y. Gu, H. Wang and Q. Zhong, Small, 2022, 18, 2104368 CrossRef CAS.
- S. Zong, H. Wen and H. Lv, et al., Carbohydr. Polym., 2022, 278, 118943 CrossRef CAS PubMed.
- B. Mi, L. Chen and Y. Xiong, et al., ACS Nano, 2022, 16(1), 771–782 CrossRef CAS.
- J. Yin, W. Fan, Z. Xu, J. Duan, Y. Xia, Z. Nie and K. Sui, Small, 2022, 18, 2104440 CrossRef CAS PubMed.
- Z. Wang, Z. Liu and G. Zhao, et al., ACS Nano, 2022, 16(1), 1661–1670 CrossRef CAS PubMed.
- C. Cai, X. Zhang and Y. Li, et al., Adv. Mater., 2022, 34, 2106564 CrossRef CAS.
- Z. Lei and P. Wu, Nat. Commun., 2018, 9, 1134 CrossRef PubMed.
- J. Zhang, L. Chen, L. Chen, S. Qian, X. Mou and J. Feng, Carbohydr. Polym., 2021, 257, 117627 CrossRef CAS PubMed.
- H. Guo, M. Bai and Y. Zhu, et al., Adv. Funct. Mater., 2021, 31, 2106406 CrossRef CAS.
- M. Fujita, G. M. Policastro and A. Burdick, et al., Nat. Commun., 2021, 12, 3764 CrossRef CAS PubMed.
- D. Zhang, Q. Chen and C. Shi, et al., Adv. Funct. Mater., 2021, 31, 2007226 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra04915e |
| This journal is © The Royal Society of Chemistry 2022 |