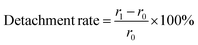Open Access Article
Open Access ArticlePolyethyleneimine-assisted co-deposition of polydopamine coating with enhanced stability and efficient secondary modification†
Chun-gong Li a,
Qinqin Yanga,
Dong Chena,
Hongliang Zhua,
Jiachen Chena,
Runjin Liua,
Qi Dang
a,
Qinqin Yanga,
Dong Chena,
Hongliang Zhua,
Jiachen Chena,
Runjin Liua,
Qi Dang *ab and
Xiang Wang
*ab and
Xiang Wang *a
*a
aKey Laboratory of Biorheological Science and Technology, Ministry of Education, College of Bioengineering, Chongqing University, Chongqing 400044, PR China. E-mail: qdangchn@cqu.edu.cn; xwangchn@vip.sina.com
bChongqing Engineering and Technology Research Center of Intelligent Rehabilitation and Eldercare, Chongqing City Management College, Chongqing 401331, PR China
First published on 7th December 2022
Abstract
The stability and grafting efficiency are important for polydopamine (pDA) coatings used as platforms for secondary grafting. In this work, polyethyleneimine (PEI) was co-deposited with dopamine on various materials (PP, PTFE and PVC), then immersed in a 1.0 M HCl solution or 1.0 M NaOH solution to investigate the detachment of the coatings using UV-vis spectroscopy, SEM, FTIR spectroscopy and XPS, and the effect of PEI molecular weight on the secondary grafting of heparin on the pDA/PEI coating was investigated through clotting time tests. The results showed that the detachment rates of the pDA/PEI coating (14.6%, 23.7%) co-deposited on PTFE in 1.0 M HCl or 1.0 M NaOH solutions were both lower than that of the pDA coating (35.0%, 74.6%), indicating that pDA/PEI coatings could better remain on substrates in a 1.0 M NaOH solution. Besides, pDA/PEI coatings on a PP membrane with both a higher deposition density and stability could be obtained when the mass ratio of DA/PEI was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and PEI molecular weight was 600 Da. After grafting heparin, it was found that the pDA/PEI coating with lower molecular weight (600 Da and 1800 Da) PEI could achieve a higher grafting density of heparin with a longer clotting time. Thus, the results provided better understanding about the stability of pDA/PEI coatings and efficiency of heparin grafting.
1 and PEI molecular weight was 600 Da. After grafting heparin, it was found that the pDA/PEI coating with lower molecular weight (600 Da and 1800 Da) PEI could achieve a higher grafting density of heparin with a longer clotting time. Thus, the results provided better understanding about the stability of pDA/PEI coatings and efficiency of heparin grafting.
Introduction
Polydopamine (pDA) coating inspired by mussels is a simple, universal and material-independent surface modification used to regulate the properties of virtually all material surfaces, avoiding one-to-one, complicated steps for surface modification,1 and could provide a platform for secondary grafting.2,3 Although pDA coatings can be tethered with molecules containing amine (–NH2) and thiol (–SH) groups through the Michael-type addition reactions and/or Schiff-base formations in Tris solutions of pH = 8.5,4,5 pDA coatings are unstable under some harsh conditions, especially in strong alkaline solutions and organic solvents (e.g., polar solvents), which has limited their practical application in secondary grafting and others.6–9 It was reported that the detachment percentage of a pDA coating on polypropylene (PP), polyvinylidene fluoride (PVDF) and nylon in 0.1 M NaOH was higher than that in 0.1 M HCl.10 Therefore, it is necessary to improve the chemical stability of pDA coatings for a variety of reactions, and this is a prerequisite for pDA coatings as a platform for secondary grafting. On the other hand, the reactive functional groups on pDA coatings when used as secondary graft coatings are equally important for efficient grafting. However, the amino groups available for secondary grafting are consumed due to the cyclization reaction of dopamine during the self-polymerization process, which results in insufficient reactive functional groups on the pDA coating for secondary grafting.11–13 Thus, it is important to retain as many functional groups as possible for secondary grafting while improving the stability of polydopamine (pDA) coatings.There has been some research devoted to improving the stability of polydopamine coatings. Increasing the oxidation of polydopamine could increase covalent interaction to enhance the stability of pDA coatings via oxidizing agents (e.g., NaIO4,14 (NH4)2S2O8,15 CuSO4/H2O2 (ref. 16) and so on), electrochemical actuation7 or plasma treatment.17 Although the further oxidative polymerization of PDA could enhance the stability of a pDA coating, oxidation would promotes the cyclization reaction of primary amino groups,7 which is not conducive to secondary grafting on the surface. A better way to introduce a polymer with amino groups into polydopamine might be through cross-linking, for example 1,6-hexanediamine, zwitterionic polymers, polyethyleneimine and so on.18–21 Polyethyleneimine (PEI) is a well-known cationic polyelectrolyte with rich primary, secondary and tertiary amino groups on its macromolecular chains.22 It was reported that PEI could react with pDA in Tris buffer (pH = 8.5) through Michael addition or Schiff base reaction to form a pDA/PEI coating, which might enhance the stability of the pDA coating.21 In addition, due to the rich primary, secondary and tertiary amino groups of macromolecular PEI, PEI co-deposited with pDA might provide the rich amino groups on the surface for secondary modification, such as heparin grafting.
Heparin, a common anticoagulant, is used widely in blood-contact materials and equipment to prevent coagulation.23,24 The immobilization of heparin on various materials could improve blood compatibility and reduce the amount of heparin injected,25,26 in which the grafting density and activity of heparin play key roles.27–29 PEI with rich amino groups could enhance the grafting amount of heparin. Liu et al.13 used PEI (1300 Da) co-deposited with catechol (CA) to obtain a CA/PEI coating with rich amino groups for heparin modification. However, different molecular weight PEI has different chain lengths and chain mobilities, which could cause various effects on the co-deposition and adsorption performance,30 and not much attention has been paid to the effect of PEI molecular weight on the secondary grafting of heparin on pDA/PEI coatings and its anticoagulation ability.
In this work, PEI was co-deposited with dopamine on polypropylene (PP), polytetrafluoroethylene (PTFE) and polyvinyl chloride (PVC), then immersed in a 1.0 M HCl solution and 1.0 M NaOH solution to investigate the stability of the coatings. The detachment of the coatings was detected using SEM, UV-vis spectrometry and water contact angle measurement, and the remaining coatings were evaluated using FT-IR spectroscopy and XPS. The effects of the amount of PEI added, the pH value, buffer solution and PEI molecular weight of the eluent on the detachment of the coatings were also investigated. Finally, the effects of PEI molecular weight on the secondary grafting of heparin on pDA/PEI coatings and its anticoagulation ability were evaluated through clotting time tests.
Materials and methods
Materials
Dopamine hydrochloride and polyethyleneimine (Mw = 600 Da, 1800 Da, 10000 Da and 70000 Da) were purchased from Aladdin (China). Sodium hydroxide (NaOH), hydrochloric acid (HCl, 38%) and ethanol were obtained from Chuandong Chemical Industry Co., Ltd (China). Tris-(hydroxymethyl)aminomethane (Tris) and heparin (≥160 U mg−1) were purchased from Beijing Solarbio Science & Technology Co., Ltd (China). Polypropylene (PP, 0.22 μm) membrane and Polytetrafluoroethylene (PTFE, 0.22 μm) membrane were provided by Shanghai Xinya Purification Device Factory. A polyvinyl chloride (PVC) sheet was obtained from Shandong Jintiancheng Plastic Products Co., Ltd. 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide (EDC), N-hydroxysuccinimide (NHS) and toluidine blue (TB) were purchased from Sigma-Aldrich Co., LtdPreparation of pDA/PEI coatings on substrates
PP membrane, PTFE membrane and PVC sheet were soaked in ethanol for 10 min and washed with distilled water 3 times. The washed PP, PTFE and PVC were immersed in a 2 mg mL−1 DA/PEI solution (molecular weight of PEI = 600 Da) with a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in Tris–HCl buffer solution (50 mM, pH = 8.5) in a vessel with a diameter of 35 mm, and shaken at 25 °C for 24 h with monitoring of oxygen levels during the first 900 s using a dissolved oxygen meter (JPSJ-605F, INESA Scientific instrument CO., Ltd, Shanghai). The obtained samples were named PP-DA/PEI, PTFE-DA/PEI and PVC-DA/PEI. At the same time, PP, PTFE and PVC were immersed in a 2 mg mL−1 DA solution in Tris buffer (50 mM, pH = 8.5) for 24 h and named PP-DA, PTFE-DA and PVC-DA.
1 in Tris–HCl buffer solution (50 mM, pH = 8.5) in a vessel with a diameter of 35 mm, and shaken at 25 °C for 24 h with monitoring of oxygen levels during the first 900 s using a dissolved oxygen meter (JPSJ-605F, INESA Scientific instrument CO., Ltd, Shanghai). The obtained samples were named PP-DA/PEI, PTFE-DA/PEI and PVC-DA/PEI. At the same time, PP, PTFE and PVC were immersed in a 2 mg mL−1 DA solution in Tris buffer (50 mM, pH = 8.5) for 24 h and named PP-DA, PTFE-DA and PVC-DA.
In order to investigate the effect of PEI mass proportion on the stability of pDA/PEI, the PEI concentration was varied from 0 to 8 mg mL−1 and the DA concentration was fixed at 2 mg mL−1 with DA/PEI ratios of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4
1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.
4.
In order to investigate the effect of molecular weight of PEI on deposition and stability, PP membranes were co-deposited with 600 Da, 1800 Da, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da and 70
000 Da and 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da PEI (2 mg mL−1) and DA (2 mg mL−1) in Tris buffer (50 mM, pH = 8.5) for 24 h, and named PP-D/P600, PP-D/P1800, PP-D/P10000 and PP-D/P70000.
000 Da PEI (2 mg mL−1) and DA (2 mg mL−1) in Tris buffer (50 mM, pH = 8.5) for 24 h, and named PP-D/P600, PP-D/P1800, PP-D/P10000 and PP-D/P70000.
Finally, the modified samples were washed with ethanol and distilled water 3 times respectively, and dried in an oven at 50 °C. The deposition density (DD) of the coatings on the substrate materials was calculated according to the following formula:
| DD = (m1 − m0)/S |
Evaluation of the stability of coatings on a surface
PP membrane, PTFE membrane and PVC sheet deposited with pDA and pDA/PEI were washed with a 1.0 M HCl solution or 1.0 M NaOH solution for 2 h, and the elution was monitored with a UV-visible spectrophotometer (Nanodrop One, Thermo Scientific, US). Then, the eluent was replaced with a 1.0 M HCl solution or 1.0 M NaOH solution every 2 h, and the absorbance of the eluent was detected at 280 nm.To investigate the effect of PEI mass proportion on the stability of pDA/PEI, different ratios of DA/PEI (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4
1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) were immersed in 1.0 M HCl or 1.0 M NaOH solutions for 2 h respectively. pDA-modified samples were used for comparison.
4) were immersed in 1.0 M HCl or 1.0 M NaOH solutions for 2 h respectively. pDA-modified samples were used for comparison.
The effect of different pH solutions on the stability of pDA/PEI coatings was studied by washing with NaOH or HCl solutions with different pH (1, 2, 4, 6, 8, 10, 12, and 14). pDA-modified samples were used for comparison.
In order to investigate the stability of the coating in buffer solutions with different pH, 50 mM citrate buffer (pH = 3, 4, 5), phosphate buffer (pH = 6, 7, 8), Tris buffer (pH = 9) and sodium bicarbonate buffer (pH = 10, 11) solutions were prepared, and 1/2 PP membranes or modified-PP membranes were immersed respectively into 0.5 mL of the above buffer solutions with different pH (3, 4, 5, 6, 7, 8, 9, 10, and 11) for 2 h. The eluent was measured using a UV-vis spectrophotometer (Nanodrop One, Thermo Scientific, US).
Surface heparinization
PP-D/P600, PP-D/P1800, PP-D/P10000 and PP-D/P70000 were immersed in citric acid buffer (pH = 4.7, EDC/NHS molar ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 containing 5 mg mL−1 heparin) respectively and shaken at 37 °C for 24 h. After the reaction was completed, samples were washed with PBS buffer (0.01 M, pH = 7.4), a 4 M NaCl solution and deionized water 3 times in turn, and dried at 50 °C to obtain PP-D/P600-H, PP-D/P1800-H, PP-D/P10000-H and PP-D/P70000-H.
1 containing 5 mg mL−1 heparin) respectively and shaken at 37 °C for 24 h. After the reaction was completed, samples were washed with PBS buffer (0.01 M, pH = 7.4), a 4 M NaCl solution and deionized water 3 times in turn, and dried at 50 °C to obtain PP-D/P600-H, PP-D/P1800-H, PP-D/P10000-H and PP-D/P70000-H.
The amount of heparin immobilized on the surfaces was quantified by TB assay as described in a previous study.31 Samples were immersed in 0.5 mL of 0.01 M HCl/0.2% NaCl aqueous solution containing 0.005% TB with shaking at 37 °C for 4 h, and rinsed three times with 0.01 M HCl/0.2% NaCl aqueous solution and deionized water, respectively. Then, the heparin–TB complexes on the surface were dissolved by 0.5 mL of an 80% ethanol/0.1 M NaOH (v/v = 4/1) mixed solution, and finally 150 μL was added to a 96-well plate to be measured at 540 nm by a microplate reader (Multiskan FC, Thermo Scientific, US). The heparin grafting density could be obtained from a standard curve.
Clotting time
Fresh blood containing sodium citrate as anticoagulant (vanticoagulant : vblood = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) was centrifuged at 3000 rpm for 10 min to obtain platelet-poor plasma (PPP). The sample was cut into 1/8 and was immersed into 600 μL of PPP and incubated at 37 °C for 60 min. The values of APTT and TT were measured by an automatic coagulation analyser (CA600, Sysmex Corporation, Kobe, Japan).
9) was centrifuged at 3000 rpm for 10 min to obtain platelet-poor plasma (PPP). The sample was cut into 1/8 and was immersed into 600 μL of PPP and incubated at 37 °C for 60 min. The values of APTT and TT were measured by an automatic coagulation analyser (CA600, Sysmex Corporation, Kobe, Japan).
Characterization
An attenuated total reflection infrared spectrometer (ATR-FTIR, iN10, Thermo Scientific, US) was used to detect the structural changes of samples. The probe material was germanium crystal, and the wave number range was 4000–700 cm−1 with a resolution of 8 cm−1 and number of scans of 64. X-ray photoelectron spectroscopy (XPS) spectra were recorded using a spectrometer (Escalab 250Xi, Thermo Scientific, US) with Al Kα excitation radiation (hv = 1486.6 eV). The wettability of samples was observed using a contact angle goniometer (MACT V6, GBX, France) equipped with video capture functionality. Scanning electron microscopy (SEM, EVO18, Zeiss, Germany and Gemini SEM 300, ZEISS, Germany) was used to observe the morphology and perform EDS mapping, respectively. Atomic force microscopy (AFM, Bruker Dimension Icon, Germany) was used to evaluate the surface roughness.Statistical analysis
The data in the experimental results are expressed as mean ± standard deviation (mean ± SD), and Student's t-test was used for statistical analysis. p < 0.05 represents a significant difference in the data.Results and discussion
The co-deposition of pDA/PEI coatings on substrates
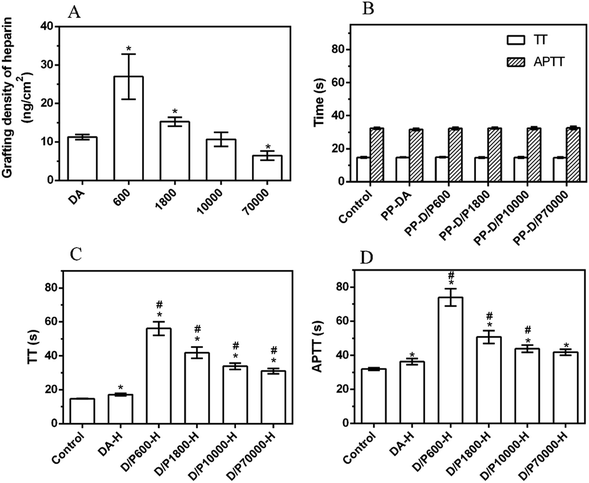
The color of the substrate material changed greatly after modification, as shown by the inset pictures of Fig. 1A. The color of the PP membrane and PTFE membrane was white and it changed to black after the deposition of the pDA coating, while the color of the surface co-deposited with DA and PEI became dark brown. The PVC sheet was colorless and transparent. After deposition with pDA or pDA/PEI, the transparency of PVC-DA and PVC-DA/PEI decreased slightly, and the color became light grey and light brown, respectively. These results indicated that PEI participated in the deposition process of polymerization of DA, which changed the color of the pDA coating. It could be observed from the SEM images (Fig. 1A) that a large number of particles or aggregates appeared on the surface when modified by pDA, but this was not observed on the surface deposited by pDA/PEI, which presented wrinkles or layers. It was reported that pDA could form aggregates and deposit on the surface due to non-covalent bond interactions between pDA molecules, such as π–π stacking and hydrogen bonding, etc.,32 while PEI covalently cross-linked with pDA molecules to weaken the non-covalent interaction between pDA molecules, thereby reducing the aggregation of pDA.33 There was no obvious difference in the pictures before and after the coating process on PTFE membranes, which was due to the morphology of PTFE membranes that affected the observation, but the color change of PTFE and modified-PTFE implied the different deposition of pDA and pDA/PEI coatings.34 | ||
| Fig. 1 (A) SEM images (insets are the corresponding photographs) of PP, PTFE and PVC before and after the modification. (B) The AFM and Rq values of PVC, PVC-DA and PVC-DA/PEI. | ||
In addition, the surface roughness before and after modification was calculated using AFM with PVC as an example. It could be seen from Fig. 1B that PVC-DA and PVC-DA/PEI had a rougher surface as compared with the PVC. Correspondingly, the root mean squared roughness (Rq) of PVC increased from 2.64 ± 0.31 nm to 9.82 ± 1.12 nm after being coated with pDA, and further increased to 22.45 ± 1.82 nm when modified with pDA/PEI, which might be attributed to polymer particulates grafted onto the surface.35
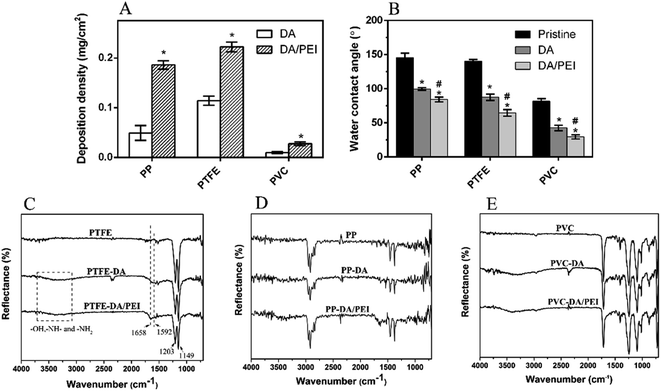
Then, the deposition density of the pDA/PEI coating on PP, PTFE and PVC was investigated, as shown in Fig. 2A. The deposition density of pDA/PEI on PP, PTFE and PVC was about 0.186, 0.222 and 0.028 mg cm−2, respectively, which demonstrated the versatility of pDA/PEI coatings that could be deposited on a variety of materials with different deposition densities. As a comparison, the deposition density of pDA on PP, PTFE and PVC was about 0.049, 0.114 and 0.010 mg cm−2, respectively, which was lower than that of the pDA/PEI coatings on each substrate due to the acceleration of the deposition process caused by PEI addition.21 At the same time, the wettability of the material surface also changed after modification, as shown in Fig. 2B. Compared with the pristine substrates, the water contact angle of the materials was decreased after modification with the pDA coating and was further reduced when the pDA/PEI coatings were deposited, which demonstrated that the introduction of PEI could improve the hydrophilicity of pDA coatings.
The chemical functional groups of the substrates before and after modification were characterized using ATR-FTIR. Sharp peaks at 1203 cm−1 and 1149 cm−1 were observed in the spectra of PTFE and modified PTFE (Fig. 2C), corresponding to the stretching vibration peak of –CF2– in PTFE,36 which was the characteristic absorption peak of PTFE. PTFE-DA and PTFE-DA/PEI had a broad absorption peak in the range of 3000–3750 cm−1, which was caused by the –OH, –NH– and –NH2 groups of pDA or PEI.36 Specifically, the new peaks at 1620 cm−1 and 1592 cm−1 in the PTFE-DA spectra were attributed to the vibration peaks of the benzene ring skeleton in DA and the stretching vibration peaks of N–H, which proved that pDA was successfully deposited on the surface of PTFE. In addition, a new absorption peak at 1658 cm−1 was observed in the spectra of PTFE-DA/PEI, corresponding to the absorption peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration,37 which suggested that pDA/PEI coatings were deposited successfully on PTFE, and PEI might be cross-linked with DA through the Schiff base reaction.
N stretching vibration,37 which suggested that pDA/PEI coatings were deposited successfully on PTFE, and PEI might be cross-linked with DA through the Schiff base reaction.
The changes of the FTIR spectra of PP (Fig. 2D) were mostly consistent with that of PTFE. The new peaks of PP-DA appeared at about 1590–1630 cm−1 compared with PP, while those of PP-DA/PEI appeared at 1670–1720 cm−1, suggesting the successful deposition of pDA and pDA/PEI on the surface of PP. However, there was an absorption peak at about 1700 cm−1 in the spectra of PVC due to the plasticizer in PVC (Fig. 2E), which overlapped with the characteristic absorption peaks of the pDA and pDA/PEI coatings. Although it was difficult to observe the difference in the spectra between PVC and modified-PVC due to the low deposition density, the change of color and roughness before and after modification confirmed the deposition of the coating on PVC from the side (Fig. 1).38
In order to better explain the interaction of DA and PEI, the possible reaction process between DA and PEI is shown in Fig. S1.† The polymerization process of pDA has been widely studied by many research groups.32 There are many views, and the recognized reaction mechanism is shown in Fig. S1.† DA is oxidized to dopamine quinone under alkaline conditions, and then forms 5,6-dihydroxyindole (DHI) and other intermediates through intermolecular cyclization, or stacks through non covalent interactions such as π–π or hydrogen bonding.39 The amino groups of PEI can react with the catechol structure of dopamine. Based on the above FTIR results, there were –NH– and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N absorption peaks in the FTIR spectra of the pDA/PEI coating. In combination with previous studies,40,41 it was speculated that PEI might be crosslinked with pDA through Michael addition and Schiff base reaction (Fig. S1†). Oxygen plays an important role in the oxidative polymerization of dopamine. It was reported that an increase of oxygen content could accelerate the polymerization of dopamine.42 Moreover, it was found that the oxygen content in the solution decreased faster after the addition of PEI (in ambient air), as shown in Fig. S2,† which confirmed that the addition of PEI could accelerate the polymerization of dopamine, consistent with the previous report. Therefore, we uniformly selected a reaction vessel with a diameter of 35 mm in the deposition experiment to reduce the influence of oxygen.
N absorption peaks in the FTIR spectra of the pDA/PEI coating. In combination with previous studies,40,41 it was speculated that PEI might be crosslinked with pDA through Michael addition and Schiff base reaction (Fig. S1†). Oxygen plays an important role in the oxidative polymerization of dopamine. It was reported that an increase of oxygen content could accelerate the polymerization of dopamine.42 Moreover, it was found that the oxygen content in the solution decreased faster after the addition of PEI (in ambient air), as shown in Fig. S2,† which confirmed that the addition of PEI could accelerate the polymerization of dopamine, consistent with the previous report. Therefore, we uniformly selected a reaction vessel with a diameter of 35 mm in the deposition experiment to reduce the influence of oxygen.
The detachment of coatings after elution
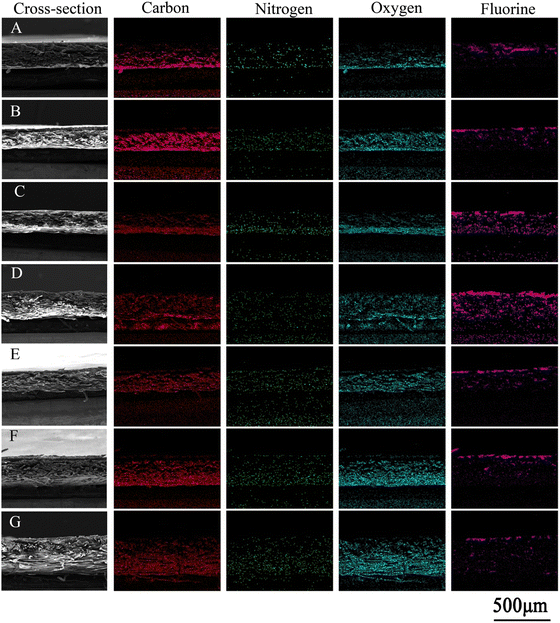
The addition of PEI increased the deposition of pDA coating, but what effect would the addition of PEI have on the stability? Fig. 3 shows the SEM images of substrates deposited with pDA and pDA/PEI coatings after elution with a 1.0 M HCl solution or 1.0 M NaOH solution. The morphologies of the pDA and pDA/PEI coated surfaces did not change significantly after elution with the 1.0 M HCl solution (compared with Fig. 1A), indicating that the pDA and pDA/PEI coatings could remain stable on substrates under strong acid conditions. After elution with the 1.0 M NaOH solution, the pDA-modified PP and PVC became smooth, and no aggregated particles were observed, indicating that the pDA coating completely detached under strong alkaline conditions, resulting in nudity of the substrates. However, the morphology of the pDA/PEI coating on PP and PVC did not change obviously after being eluted with the 1.0 M NaOH solution, indicating that the pDA/PEI coating was stable under strong alkaline conditions compared with the pDA coating. Due to the special morphology of PTFE, there was no obvious morphological change of PTFE-DA and PTFE-DA/PEI before and after the elution. So, the cross-section of PTFE and modified-PTFE was observed further, as shown in Fig. 4. The cross section of the coating on the material could not be well distinguished by SEM images, so the coating retention was preliminarily evaluated by EDS mapping. After elution with the 1.0 M HCl solution, the N and O signals of PTFE-DA were still strong, while after washing with the 1.0 M NaOH solution, the N and O signals for the surface of PTFE disappeared and only the background signal could be recorded, indicating that the pDA coating was not stable in the 1.0 M NaOH solution. After the introduction of PEI, the distribution of N and O signals was more uniform, and did not change obviously after elution with the 1.0 M HCl or 1.0 M NaOH solutions, which demonstrated that the introduction of PEI could make the coating more uniform, and make the pDA/PEI coating stable in 1.0 M HCl or 1.0 M NaOH solutions.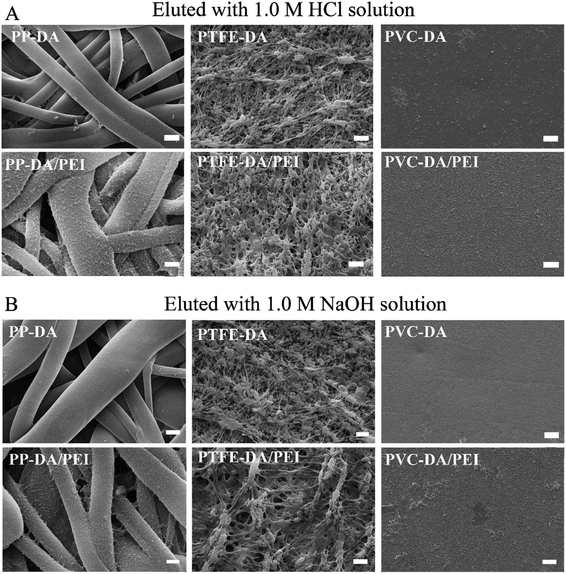 | ||
Fig. 3 SEM images of modified PP, PTFE and PVC eluted with 1.0 M HCl (A) or 1.0 M NaOH solutions (B) for 24 h, magnification = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00×, scale bar = 2 μm. 00×, scale bar = 2 μm. | ||
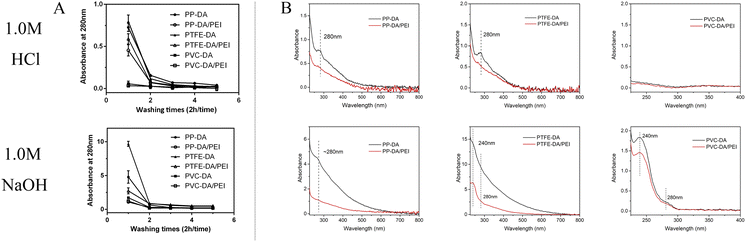
The eluent of the pDA/PEI and pDA coatings in 1.0 M HCl or 1.0 M NaOH solutions was measured using UV-vis absorbance to evaluate the stability of the coatings on different substrates. As shown in Fig. 5A, the absorbance of the eluent from all modified samples washed with 1.0 M HCl or 1.0 M NaOH solutions decreased rapidly to reach a threshold after the first washing, indicating that detachment of the pDA and pDA/PEI coatings mainly occurred in the first 2 h of elution. In addition, the absorbance of the eluent in the 1.0 M NaOH solution in the initial 2 h was higher than that in 1.0 M HCl, suggesting that the pDA/PEI coating and pDA coating were more stable in the 1.0 M HCl solution than the 1.0 M NaOH solution. Compared with the pDA coating, the absorbance of the eluent from the pDA/PEI coating immersed in the 1.0 M NaOH solution for 2 h was lower, suggesting that the introduction of PEI enhanced the stability of the pDA coating under strong acid conditions.
In addition, the absorbance of the coating deposited on different substrates was different, which might be attributed to the deposition amount of coating. Furthermore, the UV-vis spectra of the eluent of pDA and pDA/PEI coatings on different substrates immersed in 1.0 M NaOH or 1.0 M HCl solutions for 2 h were recorded. It could be seen from Fig. 5B that the peak shape of the UV-vis spectra of pDA and pDA/PEI coatings on the same substrate in the same solution were basically the same. It was speculated that the detachment of pDA/PEI coatings might be mainly due to the detachment of pDA or DA monomers.10 However, the UV-vis spectra of the eluent of the coatings on different substrates immersed in a 1.0 M NaOH solution were different. The eluent of PP-DA and PP-DA/PEI in the 1.0 M NaOH solution had an absorption peak at about 280 nm, while the eluent of pDA and pDA/PEI coatings on PTFE and PVC surfaces in the 1.0 M NaOH solution had an absorption peak not only at ∼280 nm, but also at 240 nm, implying that the structural properties of different material surfaces had different effects on the detachment of the coating. Different substrate materials have different chemical compositions, which might lead to different interactions with pDA, such as hydrogen bonding, π–π stacking, etc.,32,43 resulting in different structures or compositions of pDA on the surface, so the absorbance of the eluent was different when the pDA coating was decomposed in the 1.0 M NaOH solution.
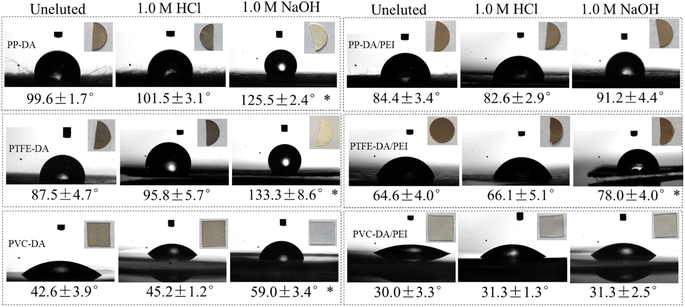
Subsequently, the water contact angle of samples before and after elution was measured to evaluate the hydrophilicity. It can be seen from Fig. 6 that the water contact angle and the color of pDA or pDA/PEI modified-samples did not change obviously after elution with the 1.0 M HCl solution, but the water contact angle of pDA-modified samples increased significantly after elution with the 1.0 M NaOH solution, and their color changed from gray–black to white mainly due to the almost complete detachment of the pDA coating in the 1.0 M NaOH solution, leading to a reduction of hydrophilicity. Whereas, the pDA/PEI coatings showed almost no change in color or water contact angle after the incorporation of PEI, which further confirmed that the addition of PEI could increase the stability of the pDA coating, and could maintain the hydrophilicity after elution with the 1.0 M HCl or 1.0 M NaOH solutions.
The remaining ability of coatings after elution
In order to evaluate quantitatively the remaining ability of the coatings on PTFE before and after elution, the peak area of the FTIR spectra was measured and analyzed, as shown by the dotted frame in Fig. S3A and B.† PTFE has characteristic peaks at 1203 cm−1 and 1149 cm−1 corresponding to –CF2– groups, whereas the signal of –CF2 in the spectra is covered when coatings are deposited on PTFE. Then, the remaining amount could be reflected by the ratio of the characteristic peak area of the coatings (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N or C
N or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and PTFE (–CF2–). The absorption peak areas derived from the C
C) and PTFE (–CF2–). The absorption peak areas derived from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N/C
N/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group and the –CF2– group were respectively defined as RA (1800–1400 cm−1) and RB (1270–1050 cm−1), and the ratio of RA and RB was used to evaluate the remaining amount of the coatings on the PTFE surface.17 Table 1 shows the RA/RB values of PTFE-DA and PTFE-DA/PEI before and after elution with the 1.0 M HCl or 1.0 M NaOH solutions. The RA/RB values of PTFE-DA and PTFE-DA/PEI were 0.197 and 0.384, respectively. RA/RB of the PTFE-DA/PEI coating obtained using the FTIR spectra was higher than that of PTFE-DA. On one hand, PEI stimulated deposition of the coatings; on the other, it also increased the formation of Schiff base structures (C
C group and the –CF2– group were respectively defined as RA (1800–1400 cm−1) and RB (1270–1050 cm−1), and the ratio of RA and RB was used to evaluate the remaining amount of the coatings on the PTFE surface.17 Table 1 shows the RA/RB values of PTFE-DA and PTFE-DA/PEI before and after elution with the 1.0 M HCl or 1.0 M NaOH solutions. The RA/RB values of PTFE-DA and PTFE-DA/PEI were 0.197 and 0.384, respectively. RA/RB of the PTFE-DA/PEI coating obtained using the FTIR spectra was higher than that of PTFE-DA. On one hand, PEI stimulated deposition of the coatings; on the other, it also increased the formation of Schiff base structures (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) produced by the covalent cross-linking of PEI and DA.
N) produced by the covalent cross-linking of PEI and DA.
| Substrates | RA/RB | ||
|---|---|---|---|
| As prepared | Eluted with 1.0 M HCl | Eluted with 1.0 M NaOH | |
| PTFE-DA | 0.197 ± 0.018 | 0.128 ± 0.027 | 0.050 ± 0.011 |
| PTFE-DA/PEI | 0.384 ± 0.017 | 0.328 ± 0.026 | 0.293 ± 0.050 |
Although there was much difference in the remaining amount of the pDA and pDA/PEI coatings, the remaining ability of the coatings could be evaluated by the detachment rate, as follows:
After elution with the 1.0 M HCl or 1.0 M NaOH solution, the remaining amount of all samples decreased, and the detachment rate of PTFE-DA was 35.0% and 74.6%, respectively. Whereas, the detachment rate of PTFE-DA/PEI was 14.6% and 23.7%, respectively, which was lower than that of the pDA coating. The results demonstrated that pDA/PEI coatings were still attached more to the PTFE after washing with the 1.0 M HCl or 1.0 M NaOH solutions compared with the pDA coatings.
Furthermore, the chemical composition of the pDA or pDA/PEI coatings after elution was characterized using XPS, as shown in Fig. S3C and D.† The N 1s and O 1s peaks of PP-DA were obviously weakened after washing with the 1.0 M NaOH solution and did not change after washing with the 1.0 M HCl solution, showing that PP-DA could not maintain its chemical composition after elution with 1 M NaOH. After co-deposition with DA and PEI, there was no obvious change in the spectra after washing with 1.0 M HCl or 1.0 M NaOH solutions, indicating that the pDA/PEI coating could retain its chemical composition after washing in 0.1 M HCl and in 0.1 M NaOH.
Subsequently, Table S1† summarizes the contents of C, N and O on PP-DA and PP-DA/PEI obtained from the XPS spectra. The content of N and O on PP-DA after washing with the 1.0 M NaOH solution decreased from 6.07% and 18.07% to 0.12% and 3.96%, respectively, in which the declining rate of N content was as high as 98.02%, indicating that the pDA coating was completely detached, and this led to a sharp drop in the content of amino groups and oxygen-containing functional groups. Because the pDA coating on the substrates was extremely unstable in the 1.0 M NaOH solution, this resulted in a large amount of detachment. However, the N and O content on the PP-DA/PEI surface remained at 7.73% and 11.54% after elution with the 1.0 M NaOH solution, and the declining rate of N content was only 26.59%, indicating that the pDA/PEI coating could keep a stable presence of amino groups and oxygen-containing functional groups under strong alkaline conditions. In the 1.0 M HCl solution, the changing rate of element content of the pDA and pDA/PEI coatings was within ±1.0%, demonstrating that the pDA and pDA/PEI coatings could maintain the stable existence of surface amino and oxygen-containing functional groups under strong acid conditions.
In summary, the pDA coating was extremely unstable after elution with the 1.0 M NaOH solution and prone to detach from substrates, which was consistent with previous reports.44 The –NH2 or –OH groups of the pDA coating were positively or negatively charged under strong acid or alkaline conditions. When the electrostatic repulsion exceeded the non-covalent interaction of the aggregates, the pDA coating composed of oligomer detached from the substrates.10 After the addition of PEI, PEI could covalently cross-link with pDA through Michael addition and Schiff base reaction, as proven by the FTIR spectra shown in Fig. 7. In addition, it was reported that cation–π interaction in the pDA/PEI coating could enhance the stability of the coating further.18 Based on those interactions, the introduction of PEI enhanced the chemical stability of the pDA coating, and could maintain the stable content of amino groups and oxygen-containing functional groups after the elution with extremely acid or alkaline solutions, which was proven by the XPS spectra (Table S1†), providing a more stable and universal platform for secondary grafting.
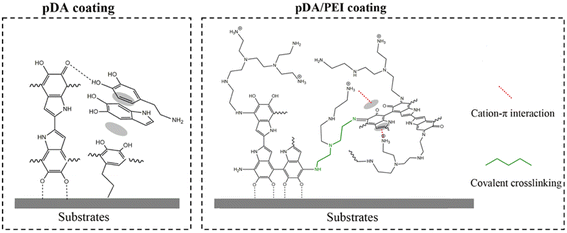 | ||
| Fig. 7 Schematic representation of interactions on the substrate surface with pDA and pDA/PEI coatings. | ||
From the above results, it is clear that the addition of PEI could improve the chemical stability of polydopamine coatings, regardless of the type of base material. Among these substrates, PP is one of the most common materials used in extracorporeal circulation due to its inertness and high stability,45 for example PP-based composite fibers or PP membrane used in blood purification,46 and polypropylene (PP) membranes for blood filtration, and it is necessary to improve the hemocompatibility through secondary modification,47 which is the research focus of our group. So, we selected PP membrane as a model substrate to study the influencing factors in pDA/PEI coating stability and heparin grafting further.
The stability of pDA/PEI coatings on PP membrane
The effects of ratio of DA/PEI and different solutions on the stability of pDA/PEI coatings were studied. The effect of PEI addition amount on the stability of the coatings was investigated further. Fig. 8A shows the absorbance at 280 nm of the eluent from pDA/PEI coatings with different mass ratios of DA and PEI soaked in a 1.0 M HCl solution or 1.0 M NaOH solution. The absorbance of the coating gradually decreased with the increase of PEI amount in the 1.0 M NaOH solution, which was attributed to an increase of the cross-linking between DA and PEI with the increased amount of PEI, indicating that PEI played an important role in the stability of pDA/PEI coatings. Besides, it was found from Fig. 8B that the deposition density of the pDA/PEI coatings with different amounts of PEI was different. When the mass ratio of DA/PEI was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the deposition density of the coating was maximum. This indicated that detachment of the coating was mainly due to the increase of cross-linking and cationic–π interaction with the increase of PEI amount when the DA/PEI mass ratio was greater than 1
1, the deposition density of the coating was maximum. This indicated that detachment of the coating was mainly due to the increase of cross-linking and cationic–π interaction with the increase of PEI amount when the DA/PEI mass ratio was greater than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, but when the amount of PEI exceeded that of DA, the decreased detachment of the coating was mainly caused by the decline of deposition density. Therefore, the mass ratio of DA/PEI should be fixed at 2
1, but when the amount of PEI exceeded that of DA, the decreased detachment of the coating was mainly caused by the decline of deposition density. Therefore, the mass ratio of DA/PEI should be fixed at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in order to make the coating more stable.
1 in order to make the coating more stable.
The stability of pDA/PEI coatings in strong acid or alkaline solutions has been studied, but the stability under other commonly used pH conditions remains unclear. Fig. 8C shows the absorbance at 280 nm of the eluents of PP-DA and PP-DA/PEI immersed in 1.0 M NaOH or HCl solutions with different pH values. It can be seen that the closer the pH of the solution is to neutral, the lower the absorbance of the eluent, indicating that the neutral solution was more conducive to stability of the pDA and pDA/PEI coatings on substrates. Specifically, the absorbance of the eluent of PP-DA was lower than 0.15 in the range of pH 4–8, and the absorbance of the eluent of PP-DA/PEI was lower than 0.16 in the range of pH 4–10, indicating that the pDA/PEI coating could remain stable in solutions with a wider pH range. Besides, the process of secondary grafting is performed with different buffer solutions, so the stability of the coatings immersed in different buffer solutions was investigated, as shown in Fig. 8D. It can be seen that there was no significant difference in the absorbance of eluent from PP-DA/PEI and PP-DA in the range of pH 3–8, and the absorbance of the eluent of PP-DA/PEI was significantly lower than that of PP-DA when pH = 9, 10, and 11 of the buffer solution, indicating that the stability of pDA and PDA/PEI coatings immersed in different pH buffer solutions was mainly affected by the pH of the buffer solution, not the type of buffer solution, and the introduction of PEI could enhance the stability of pDA coatings in buffer solutions when pH = 9, 10, and 11. In addition, the stability of coatings in DMSO was investigated. It can be observed from Fig. S4† that the UV-vis spectra of pDA/PEI in DMSO were below that of the pDA coatings, suggesting that the pDA/PEI coating was more stable in DMSO than the pDA coating. In short, the pDA/PEI coatings were stable over a wider range of pH than the pDA coatings and in a variety of solvents, which expands the application of surface modification for secondary grafting.
In the course of this experiment and according to previous reports, we also know that the molecular weight of PEI has an influence on the deposition density of DA/PEI coatings.48 The deposition density of the pDA/PEI coating was decreased with the increase of molecular weight of PEI (Fig. S5†), and, accordingly, the N and O contents gradually decreased (Fig. S6A and Table S2†). These results are consistent with a previous report. Interestingly, the high-resolution C 1s spectra of the co-deposited coatings with different molecular weights of PEI (Fig. S6B†) revealed that the percentage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N content that is mainly responsible for coating adhesion and cross-linking to form a stable structure gradually decreased with the increase of the molecular weight of PEI, implying that the number of adhesion sites of pDA and cross-linking sites was decreased with the increment of PEI molecular weight. Besides, the percentage of C
N content that is mainly responsible for coating adhesion and cross-linking to form a stable structure gradually decreased with the increase of the molecular weight of PEI, implying that the number of adhesion sites of pDA and cross-linking sites was decreased with the increment of PEI molecular weight. Besides, the percentage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N content was also decreased with the increase of PEI molecular weight, while the C content gradually increased, indicating that deposition of the pDA/PEI coating was decreased with the increase of PEI molecular weight, leading to exposure of the PP surface. These results confirmed that lower molecular weight PEI was more favorable for co-deposition with DA, where pDA adhesion sites and the formation of Schiff base structures played important roles in the process of co-deposition.
N content was also decreased with the increase of PEI molecular weight, while the C content gradually increased, indicating that deposition of the pDA/PEI coating was decreased with the increase of PEI molecular weight, leading to exposure of the PP surface. These results confirmed that lower molecular weight PEI was more favorable for co-deposition with DA, where pDA adhesion sites and the formation of Schiff base structures played important roles in the process of co-deposition.
Subsequently, the stability of a coating is important for secondary modification. Fig. 8E and F show the absorbance at 280 nm of the eluent from the pDA/PEI coating with different molecular weight PEI eluted with 1.0 M HCl and 1.0 M NaOH solutions. No matter whether under alkaline or acidic conditions, the absorbance of the eluent decreases with the increase of the molecular weight of PEI, and the absorbance of the PP-D/P70000 eluent was almost 0, indicating that detachment of the coating was gradually reduced with the increase of the molecular weight of PEI. This was mainly due to the decrease of deposition density.
Evaluation of heparin grafting efficiency on the coating
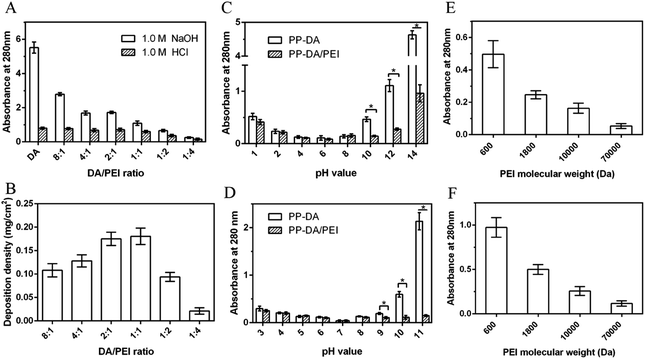
It is necessary to improve the blood compatibility of PP when used as a blood-contact material, and heparinization on a stable secondary graft coating is a common strategy.23 The graft efficiency of heparin largely determines the improvement of blood compatibility. Although the detachment of the pDA/PEI coating was decreased with the increase of molecular weight, the deposition density was also decreased, which would affect the graft efficiency of heparin. So, the grafting density of heparin on the pDA/PEI coating with different molecular weights of PEI was determined by the TB method, as shown in Fig. 9A. Compared with PP-DA-H, the heparin grafting density of PP-D/P600-H and PP-D/P1800-H was increased, and was 26.97 ng cm−2 and 15.25 ng cm−2, respectively, indicating that PEI (600 and 1800 Da) co-deposited with DA could improve the heparin grafting amount on the surface of pDA coatings, which was attributed to the introduction of PEI that could increase the content of amino groups. The heparin grafting density was lower than that of a previous report of Liu et al., which was attributed to the different methods used for measuring heparin density. Liu et al. used the quartz crystal microbalance-dissipation technique to monitor the heparin density,49 and we used the TB method to evaluate the heparin density through electrostatic interaction. But our research was focused on the PEI molecular weight effect on heparin grafting, and PEI with different molecular weights deposited with DA showed different heparin grafting densities, which could uncover the optimum PEI molecular weight for heparin grafting.When the molecular weight of PEI was increased to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da and 70
000 Da and 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, although the N content was still larger than that of the DA coating, the heparin grafting density of PP-D/P10000-H and PP-D/P70000-H was not as high as that of PP-DA-H. The main reason for this was that the increase of molecular weight of PEI reduced the deposition density of the pDA/PEI coating and reduced the number of reaction sites of primary amino groups, resulting in a reduction of heparin grafting. Overall, the co-deposition of the pDA/PEI coating with lower molecular weight PEI could increase the amount of heparin grafted on the surface, but the grafted amount of heparin was gradually decreased with the increase of molecular weight of PEI.
000 Da, although the N content was still larger than that of the DA coating, the heparin grafting density of PP-D/P10000-H and PP-D/P70000-H was not as high as that of PP-DA-H. The main reason for this was that the increase of molecular weight of PEI reduced the deposition density of the pDA/PEI coating and reduced the number of reaction sites of primary amino groups, resulting in a reduction of heparin grafting. Overall, the co-deposition of the pDA/PEI coating with lower molecular weight PEI could increase the amount of heparin grafted on the surface, but the grafted amount of heparin was gradually decreased with the increase of molecular weight of PEI.
The anticoagulant activity of heparin is usually monitored by measuring the coagulation time (TT) and activated partial thrombin time (APTT) of plasma. Therefore, the anticoagulant activity of the heparinized coated surface was investigated by detecting changes in APTT and TT. Fig. 9B shows the TT and APTT of the pDA and pDA/PEI coatings. The TT and APTT of plasma (control group) were 14.8 s and 32.4 s, respectively, while the TT and APTT of PP-DA and PP-DA/PEI did not change significantly, indicating that PP-DA, PP-D/P600, PP-D/P1800, PP-D/P10000 and PP-D/P70000 had no obvious inhibitory effect on the occurrence of coagulation. After grafting heparin on the coated surface (Fig. 9C and D), the TT and APTT in each group were significantly prolonged, indicating that the heparinized coated surface had an anticoagulant effect. Compared with PP-DA-H, the TT and APTT of PP-D/P600-H, PP-D/P1800-H and PP-D/P10000-H were prolonged, indicating that the introduction of PEI could enhance the anticoagulant ability of heparinized coatings due to the greater heparin grafting and the effect of spacers. However, the clotting time gradually decreased with the increase of molecular weight, which was attributed to the decrease of the grafting amount of heparin with the increase of molecular weight. These results suggested that PEI and DA co-deposited coatings could form heparinized coatings with better anticoagulant activity (compared with pDA), but the anticoagulant activity of the heparinized coatings was affected by the molecular weight of PEI. pDA co-deposited with lower molecular weight PEI was able to form an anticoagulant surface through grafting heparin.
Conclusions
In this study, we investigated the detachment and stability of pDA coatings and pDA/PEI coatings deposited on various materials (PP, PTFE and PVC). It was known by comparing pDA coatings that the introduction of PEI increased the chemical stability of the pDA coating on different substrate materials, especially enhancing stability in 1.0 M NaOH solutions. And the hydrophilicity of pDA/PEI was retained more effectively than that of the pDA coatings after elution with a 1.0 M NaOH solution. Furthermore, the detachment rates of pDA/PEI coatings deposited on PTFE in 1.0 M HCl or 1.0 M NaOH solutions (14.6%, 23.7%) were both lower than that of the pDA coating (35.0%, 74.6%). And pDA/PEI could maintain more stable amino and oxygen-containing group contents than pDA coatings after elution with a 1.0 M HCl solution or 1.0 M NaOH solution. Then, PP membrane was used to investigate the effects of amount of PEI, pH of solution, type of buffer and molecular weight of PEI on the stability of pDA/PEI coatings. It was found that pDA/PEI coatings with both a higher deposition density and better stability could be obtained when the mass ratio of DA/PEI was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the molecular weight of PEI was 600 Da. And detachment of pDA/PEI was decreased with the increment of molecular weight of PEI due to the decline of deposition density. Furthermore, PP-DA/PEI with a lower molecular weight could achieve a higher heparin grafting density with better anticoagulation. Thus, the study provided more understanding about the stability of pDA/PEI coatings for efficient secondary modification.
1 and the molecular weight of PEI was 600 Da. And detachment of pDA/PEI was decreased with the increment of molecular weight of PEI due to the decline of deposition density. Furthermore, PP-DA/PEI with a lower molecular weight could achieve a higher heparin grafting density with better anticoagulation. Thus, the study provided more understanding about the stability of pDA/PEI coatings for efficient secondary modification.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 31271229 and 12172071) and the Development, Clinical Trial and Registration of Hemoperfusion (Grant No. cstc2018jszx-cyzd0582). We also thank the Open Cooperation Program from the Key Laboratory of Extreme Environmental Medicine, Ministry of Education for its support of this study (Grant No. KL2019GY010).Notes and references
- H. A. Lee, Y. F. Ma, F. Zhou, S. Hong and H. Lee, Acc. Chem. Res., 2019, 52, 704–713 CrossRef CAS PubMed
.
- H. A. Lee, E. Park and H. Lee, Adv. Mater., 2020, 32, 2002357 CrossRef PubMed
.
- S. F. Wang, Y. Yu and Q. Y. Wu, Acta Polym. Sin., 2020, 51, 385–392 CAS
.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed
.
- J. H. Ryu, P. B. Messersmith and H. Lee, ACS Appl. Mater. Interfaces, 2018, 10, 7523–7540 CrossRef CAS PubMed
.
- F. Bernsmann, A. Ponche, C. Ringwald, J. Hemmerle, J. Raya, B. Bechinger, J. C. Voegel, P. Schaaf and V. Ball, J. Phys. Chem. C, 2009, 113, 8234–8242 CrossRef CAS
.
- X. L. Geng, J. Q. Wang, J. R. Ye, S. M. Yang, Q. Han, H. B. Lin and F. Liu, Sep. Purif. Technol., 2020, 244, 116857 CrossRef CAS
.
- W. Yang, C. Liu and Y. Chen, Langmuir, 2018, 34, 3565–3571 CrossRef CAS PubMed
.
- X. Geng, J. Wang, J. Ye, S. Yang, Q. Han, H. Lin and F. Liu, Sep. Purif. Technol., 2020, 244, 116857 CrossRef CAS
.
- H. Wei, J. Ren, B. Han, L. Xu, L. Han and L. Jia, Colloids Surf., B, 2013, 110, 22–28 CrossRef CAS PubMed
.
- Y. Yang, P. Qi, F. Wen, X. Li, Q. Xia, M. F. Maitz, Z. Yang, R. Shen, Q. Tu and N. Huang, ACS Appl. Mater. Interfaces, 2014, 6, 14608–14620 CrossRef CAS PubMed
.
- H. Lee, Y. Lee, A. R. Statz, J. Rho, T. G. Park and P. B. Messersmith, Adv. Mater., 2008, 20, 1619–1623 CrossRef CAS PubMed
.
- Y. J. Liu, R. F. Luo, F. Y. Shen, L. L. Tang, J. Wang and N. Huang, Appl. Surf. Sci., 2015, 328, 163–169 CrossRef CAS
.
- S. H. Hong, S. Hong, M. H. Ryou, J. W. Choi, S. M. Kang and H. Lee, Adv. Mater. Interfaces, 2016, 3, 1500857 CrossRef
.
- Q. Wei, F. L. Zhang, J. Li, B. J. Li and C. S. Zhao, Polym. Chem., 2010, 1, 1430–1433 RSC
.
- C. Zhang, Y. Ou, W. X. Lei, L. S. Wan, J. Ji and Z. K. Xu, Angew. Chem., Int. Ed., 2016, 55, 3054–3057 CrossRef CAS PubMed
.
- B. H. Cheng and K. Ishiha, Mater. Today Commun., 2020, 22, 100774 CrossRef CAS
.
- L. Yao, C. He, S. Chen, W. Zhao, Y. Xie, S. Sun, S. Nie and C. Zhao, Langmuir, 2019, 35, 1430–1439 CrossRef CAS PubMed
.
- Y. Xu, F. You, H. Sun and L. Shao, ACS Sustainable Chem. Eng., 2017, 5, 5520–5528 CrossRef CAS
.
- W. Z. Qiu, H. C. Yang and Z. K. Xu, Adv. Colloid Interface Sci., 2018, 256, 111–125 CrossRef CAS PubMed
.
- Y. Lv, S. J. Yang, Y. Du, H. C. Yang and Z. K. Xu, Langmuir, 2018, 34, 13123–13131 CrossRef CAS PubMed
.
- S. K. Samal, M. Dash, S. Van Vlierberghe, D. L. Kaplan, E. Chiellini, C. van Blitterswijk, L. Moroni and P. Dubruel, Chem. Soc. Rev., 2012, 41, 7147–7194 RSC
.
- L. Shan, Y. Sun, F. Shan, L. Li and Z. P. Xu, J. Mater. Chem. B, 2020, 8, 878–894 RSC
.
- R. Biran and D. Pond, Adv. Drug Delivery Rev., 2017, 112, 12–23 CrossRef CAS PubMed
.
- C. X. Nie, L. Ma, Y. Xia, C. He, J. Deng, L. R. Wang, C. Cheng, S. D. Sun and C. S. Zhao, J. Membr. Sci., 2015, 475, 455–468 CrossRef CAS
.
- Q. Dang, C. G. Li, X. X. Jin, Y. J. Zhao and X. Wang, Carbohydr. Polym., 2019, 205, 89–97 CrossRef CAS PubMed
.
- L. Wang, Y. Cai, Y. Jing, B. Zhu, L. Zhu and Y. Xu, J. Colloid Interface Sci., 2014, 422, 38–44 CrossRef CAS PubMed
.
- Z. Zheng, W. Wang, X. Huang, Q. Lv, W. Fan, W. Yu, L. Li and Z. Zhang, Artif. Organs, 2016, 40, E219–E229 CrossRef CAS PubMed
.
- W. Wang, Z. Zheng, X. Huang, W. Fan, W. Yu, Z. Zhang, L. Li and C. Mao, J. Biomed. Mater. Res., Part B, 2017, 105, 1737–1746 CrossRef CAS PubMed
.
- B. K. Kaang, N. Han, H. J. Lee and W. S. Choi, ACS Appl. Mater. Interfaces, 2018, 10, 1113–1124 CrossRef CAS PubMed
.
- R. Luo, J. Zhang, W. Zhuang, L. Deng, L. Li, H. Yu, J. Wang, N. Huang and Y. Wang, J. Mater. Chem. B, 2018, 6, 5582–5595 RSC
.
- H. A. Lee, E. Park and H. Lee, Adv. Mater., 2020, 32, e1907505 CrossRef PubMed
.
- H.-C. Yang, W. Xu, Y. Du, J. Wu and Z.-K. Xu, RSC Adv., 2014, 4, 45415–45418 RSC
.
- T. Bucher, J. I. Clodt, A. Grabowski, M. Hein and V. Filiz, Membranes, 2017, 7, 70 CrossRef PubMed
.
- H. Li, L. Peng, Y. Luo and P. Yu, RSC Adv., 2015, 5, 98566–98575 RSC
.
- M. Tang, D. Y. Hou, C. L. Ding, K. P. Wang, D. W. Wang and J. Wang, Sci. Total Environ., 2019, 696, 133883 CrossRef PubMed
.
- Z. L. Yang, S. Zhou, L. Lu, X. Wang, J. Wang and N. Huang, J. Biomed. Mater. Res., Part A, 2012, 100A, 3124–3133 CrossRef CAS PubMed
.
- T. Bucher, J. I. Clodt, A. Grabowski, M. Hein and V. Filiz, Membranes, 2017, 7, 70 CrossRef PubMed
.
- Y. Liu, K. Ai and L. Lu, Chem. Rev., 2014, 114, 5057–5115 CrossRef CAS PubMed
.
- C. Zhao, F. Zuo, Z. Liao, Z. Qin, S. Du and Z. Zhao, Macromol. Rapid Commun., 2015, 36, 909–915 CrossRef CAS PubMed
.
- H.-C. Yang, K.-J. Liao, H. Huang, Q.-Y. Wu, L.-S. Wan and Z.-K. Xu, J. Mater. Chem. A, 2014, 2, 10225–10230 RSC
.
- H. W. Kim, B. D. McCloskey, T. H. Choi, C. Lee, M. J. Kim, B. D. Freeman and H. B. Park, ACS Appl. Mater. Interfaces, 2013, 5, 233–238 CrossRef CAS PubMed
.
- B. Cheng and K. Ishihara, Mater. Today Commun., 2020, 22, 100774 CrossRef CAS
.
- H. L. Wei, J. Ren, B. Han, L. Xu, L. L. Han and L. Y. Jia, Colloids Surf., B, 2013, 110, 22–28 CrossRef CAS PubMed
.
- M. C. Lukowiak, S. Wettmarshausen, G. Hidde, P. Landsberger, V. Boenke, K. Rodenacker, U. Braun, J. F. Friedrich, A. A. Gorbushina and R. Haag, Polym. Chem., 2015, 6, 1350–1359 RSC
.
- K. Miwa, M. Fukuyama, N. Ida, H. Igarashi and T. Uchiyama, Int. J. Infect. Dis., 2003, 7, 21–26 CrossRef PubMed
.
- A. Venault, K. M. Trinh and Y. Chang, J. Membr. Sci., 2016, 501, 68–78 CrossRef CAS
.
- H. C. Yang, M. B. Wu, Y. J. Li, L. S. Wan and Z. K. Xu, J. Appl. Polym. Sci., 2016, 43792 Search PubMed
.
- Y. Liu, R. Luo, F. Shen, L. Tang, J. Wang and N. Huang, Appl. Surf. Sci., 2015, 328, 163–169 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: The ATR-FTIR spectra of PTFE-DA and PTFE-DA/PEI eluted with 1.0 M HCl or 1.0 M NaOH solutions for 24 h. XPS spectra of PP-DA and PP-DA/PEI before and after elution with 1.0 M HCl or 1.0 M NaOH solutions for 24 h. The content of surface elements determined by XPS analysis before and after elution with 1.0 M HCl or 1.0 M NaOH solutions for 24 h. XPS spectra recorded to evaluate the effect of PEI molecular weight on the coatings. See DOI: https://doi.org/10.1039/d2ra05130c |
| This journal is © The Royal Society of Chemistry 2022 |