Open Access Article
Open Access ArticleAggregation-induced polarization (AIP) of derivatives of BINOL and BINAP†
Yao Tang‡
a,
Qingkai Yuan‡a,
Yu Wang‡b,
Sai Zhanga,
Jia-Yin Wangbc,
Shengzhou Jinb,
Ting Xub,
Junyi Panb,
Collin Ray Guilbeau§
a,
Alyssa Jenae Pleasant§a and
Guigen Li *ab
*ab
aDepartment of Chemistry and Biochemistry, Texas Tech University, Lubbock 79409-1061, Texas, USA. E-mail: guigenli@nju.edu.cn; guigen.li@ttu.edu
bInstitute of Chemistry and BioMedical Sciences, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093, China
cContinuous Flow Engineering Laboratory of National Petroleum and Chemical Industry, Changzhou University, Changzhou, Jiangsu 213164, China
First published on 18th October 2022
Abstract
The relationship between optical rotations of small chiral molecules with water% in THF has been established. The typical aggregation co-solvent systems resulted in optical rotation amplification and adjustment, defined as aggregation-induced polarization (AIP). The AIP work can serve as a new tool to determine molecular aggregation, especially for those that cannot display aggregation-induced emission (AIE). Therefore, AIP and AIE are anticipated to complement each other. In addition, AIP can also serve as a new transmission tool providing adjusting right- or left-hand polarized lights of a series of individual wavelengths. Since chiral phosphine derivatives are among the most important ligands, this work would benefit research using chiral aggregates to control asymmetric synthesis and catalysts. Therefore, it will find many applications in chemical and materials sciences.
1 Introduction
For several decades, the research on chirality and chiral targets has been among the most active areas in science and technology communities.1–7 Controlling chirality has resulted in high potency and selectivity for chiral pharmaceuticals and drugs,8–11 challenging optical properties in radar, lasers, optical fibers, and wireless fiber telecommunications.12–17 In measuring some of these properties, circular light polarization and optical rotations have been utilized to evaluate the performance of chiral molecules, in which optical fields rotate in a plane at a constant rate when the waves travel through their solutions.18,19 The optical field is rotated toward the right- or left-hand direction during this measurement, called right- or left-circular polarization. It is noteworthy that circularly polarized luminescence (CPL) has become an active topic because of their potentials in display and optical storage devices, probes, and sensors.15,16,20,21 CPL molecules directly generate polarized lights via emissions in a broad range of wavelengths in most cases. In contrast, the solutions containing chiral compounds are not able to result in polarized lights directly, which are originally emitted from metal or metal filament cycle lamps in a single wavelength of laser beams.22,23In fact, aggregation-based CPL has attracted widespread attention since 2001, when Tang's group coined the concept of aggregation-induced emission (AIE) exhibiting strong fluorescence by aggregates states in solutions.24–27 AIE was made possible by restricting intramolecular rotation and vibration to limit or avoid aggregation-caused quenching of luminophores. During the course of our ongoing project on chirality and chiral GAP (Group-Assistant-Purification) reagents, we found that a series of chiral compounds, including multi-layer 3D folding chiral compounds, can form chiral aggregates displaying strong AIE activities by gradually increasing water fractions (fw) in THF–H2O co-solvents. In turn, the observation of strong AIE fluorescence has proven the formation of various chiral aggregates of the aforementioned compounds. We wonder if the co-existence of various chiral aggregates in solutions can systematically lead to optical rotation enhancement or adjustment. These chiral aggregates cover not only AIE molecules but also those of aggregation-caused quenching (ACQ). To the best of our knowledge, the study of aggregation-induced effects on optical rotation has not been well documented. Very recently, our group investigated the relationship between the optical rotation of those chiral aggregates in multilayered chiral folding oligomers and polymers with increasing H2O percentage in the co-solvent system, and we characterize this phenomenon as aggregation-induced polarization (AIP).28 However, little is known regarding the existence of this phenomenon in small chiral molecules. Herein, we would like to report our preliminary results of this study.
As mentioned above, the right- or left-polarized light beams via AIP transmission are originally emitted from metal or metal filament cycle lamps (external light sources). These polarized lights belong to laser beams of individual wavelengths. Therefore, AIP and AIE-based CPL would complement each other in studying chiral aggregates in solution and generating right- or left-polarized lights for future academic research and technological applications.
2 Results and discussion
2.1 Synthesis
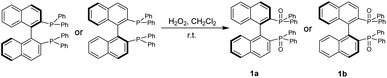
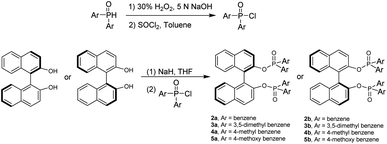
We started this project by taking advantage of GAP chemistry29–33 and derivatives of BINAP and BINOLs, which are probably the most widely used chiral categories in asymmetric synthesis and catalysis. The selection of the simplest GAP group is based on two factors: (1) it is more likely to form chiral aggregates in solutions in THF and water; (2) they are readily available and concise to synthesize. (S)- or (R)-[1,1′-binaphthalene]-2,2′-diylbis(diphenylphosphine oxide), 1a or 1b, was thus synthesized simply by subjecting it to oxidation with hydrogen peroxide in DCM at room temperature overnight to give both yields of 94% (Scheme 1).30The two-step preparation of BINOL derivatives 2a–5b was carried out in accordance with established methods and purified using GAP technology.34 The first step involves the oxidation reaction of diphenylphosphine oxide with hydrogen peroxide in aqueous 5 N NaOH from 95 °C to 100 °C for 1 hour. The resulting crude product was directly subjected to the reaction with SOCl2 in the presence of phosphonic acid in anhydrous toluene. The resulting solution was heated to 80 °C for 3 hours to give the protection crude product for work-up to give pure diphenylphosphinic chloride in a yield of 99%. The synthesis of (S)- or (R)-[1,1′-binaphthalene]-2,2′-diyl bis(diphenylphosphinate) was performed via deprotonation of BINOL with sodium hydride followed by dropwise addition of diphenylphosphinic chloride stirring at 0 °C for 15 minutes and then at room temperature overnight.35 The GAP work-up simply by washing afforded pure derivatives of 2a to 5b in yields from 65% to 90% (Scheme 2).
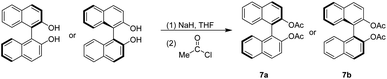
The generation of 7a and 7b is similar to that of 2a and 2b series by deprotonation of BINOL with sodium hydride, followed by adding acetyl chloride stirring at 0 °C for 15 minutes and then at room temperature overnight.31,35 The work-up and flash chromatography purification gave pure 7a and 7b with both yields of 96% (Scheme 3).
2.2 AIE and AIP determination
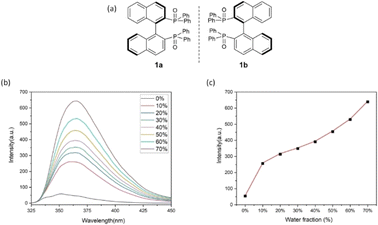
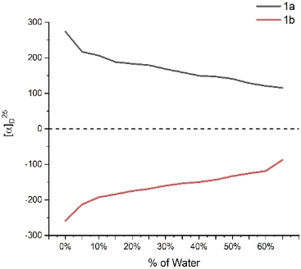
To confirm the formation of chiral aggregates, we first determined aggregation-induced emission (AIE) of (S)-[1,1′-binaphthalene]-2,2′-diylbis(diphenylphosphine oxide) 1a in THF and H2O as co-solvents. As shown in Fig. 1a, this compound displayed an obvious AIE indicating an efficient formation of aggregates in THF/H2O systems. The emission maxima of 1a were gradually increased as the water fractions (fw) were changed from 10% to 40%. There is a big jump in emission intensity from 0% to 10%. Relatively three large increments in emission intensity were observed when fw was increased from 40% to 70% (Fig. 1a). Usually, the big jump could attribute to the suppression of molecular motion via intermolecular packing of the chiral axial molecule. As fw continued to rise beyond 70%, the luminescence intensities were faintly weakened. A reasonable explanation was the motions between these individual molecules would decrease as the water fractions (fw) became larger in dilute environments, resulting in loosely packed chiral aggregates.The measurement of aggregation-induced polarization (AIP) was next planned on compound 1 under the above solvent systems. Rudolph polarimeter (Rudolph Research Analytical APIV/2W) was utilized to acquire optical rotation data at room temperature with a sodium lamp as the light source (wavelength = 589 nm). Measurements were performed in a vessel of 2 mL with consistent concentration (c = 4 mg mL−1) in THF and its co-solvents. The average data of three measurements for each sample was adopted in plotting the relationship curves. In this measurement, the water fraction (fw) was set at the component of 5% (v/v) on X-horizontal coordinate corresponding to specific rotation on the Y-vertical ordinate. As revealed in Fig. 2, under the standard aggregation co-solvents of THF and water, we observed a consistent relationship between the specific optical rotation of chiral compound 1 and water fractions (fw) by gradually increasing as water fractions (fw) in co-solvents of THF–H2O. When the fraction of water (fw) exceeded 65%, the optical rotation data showed a certain degree of instability in this case, which would be attributed to the fact that the glass surface tension at the two ends of the vessel is enlarged depending on contents of chiral aggregates.
While the GAP function-anchored 1a displayed strong aggregation-induced emission, other derivatives of chiral [1,1′-binaphthalene]-2,2′-diol (BINOL), 2a–5a, did not show obvious AIE, which is a little surprising. However, these molecules exhibited consistent relationships of aggregation-induced polarization (AIP). As described in Fig. 3–8, each pair of their enantiomers showed a nearly symmetric relationship in AIP curves toward opposite directions of optical rotations.
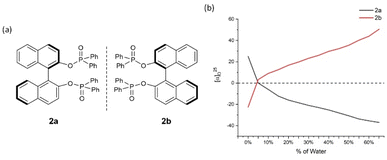 | ||
| Fig. 3 2a and 2b and their aggregation-induced polarization (AIP) in THF/water mixtures; c = 4 mg mL−1. | ||
In 2a/2b, there are consistent enhancements of specific rotation from 25.0 to −37.0 and −22.5 to 50.5, respectively, when % water (fw) was increased from 5% to 65% (Fig. 3). Specific rotation indicated an enhancement by a leap of over 20° between fw of 0% and 5%. In the cases of 3a/3b, a similar relationship to that of 2a/2b was observed (Fig. 4). However, AIP only has stable optical rotation data within % water (fw) range between 0% and 15%. The modest enhancement of aggregation-induced polarization (AIP) exists when % water (fw) is increased from 5% and 15%. This is a larger AIP enhancement within % water range between 0% and 5%. Compared with the following cases, the special steric effect by 3,5-dimethyl groups on the GAP function would be attributed to the complicated situation associated with this narrow fw range.
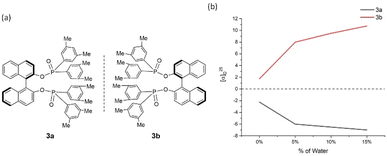 | ||
| Fig. 4 3a and 3b and their aggregation-induced polarization (AIP) in THF/water mixtures; c = 4 mg mL−1. | ||
Cases of 4a/4b and 5a/5b showed relatively less congested GAP function than 3a/3b and displayed AIP within a range of % water (fw) from 0% to 45% and 0% to 40%, respectively (Fig. 5 and 6). A similar consistent relationship of aggregation-induced polarization was observed in these cases. However, both led to specific rotation being adjusted decreasingly. This data is decreased to nearly zero when fw for 4a reaches 45% and for 5a reaches 40%, respectively. There are relatively bigger optical rotation changes in both cases when fw is increased from 0% to 5%.
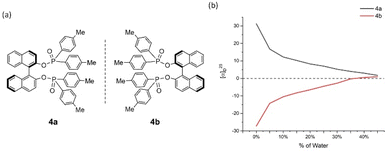 | ||
| Fig. 5 4a and 4b and their aggregation-induced polarization (AIP) in THF/water mixtures; c = 4 mg mL−1. | ||
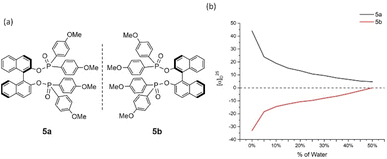 | ||
| Fig. 6 5a and 5b and their aggregation-induced polarization (AIP) in THF/water mixtures; c = 4 mg mL−1. | ||
After we successfully established the AIP-based GAP function of diarylphosphine oxides, the effect of concentrations on the AIP phenomenon was investigated. The AIP trends of diluted solutions remained nearly identical to those in 4 mg mL−1. These results indicated that adjusting the concentration of chiral samples might not influence AIP. (Fig. S15†) On the other hand, we went back to check original (S)- and (R)-[1,1′-binaphthalene]-2,2′-diol (BINOL), 6a/6b, and its derivative of [1,1′-binaphthalene]-2,2′-diyl diacetate, 7a/7b (Fig. 7 and 8). Interestingly, both of these cases displayed the AIP relationship, albeit the AIP in the latter cases is weaker than its former counterparts. A consistent relationship of aggregation-induced polarization was observed, similar to the above two cases. Specific rotation is adjusted decreasingly for both cases. This data is even decreased to nearly zero when fw for 6a reaches 45% and for 7a reaches 40%, respectively.
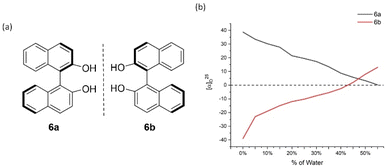 | ||
| Fig. 7 6a and 6b and their aggregation-induced polarization (AIP) in THF/water mixtures; c = 4 mg mL−1. | ||
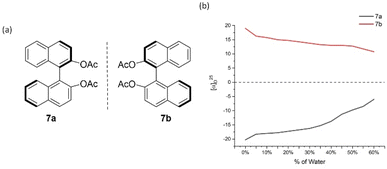 | ||
| Fig. 8 7a and 7b and their aggregation-induced polarization (AIP) in THF/water mixtures; c = 4 mg mL−1. | ||
3 Conclusions
In conclusion, we have established the relationship between optical rotations of small chiral molecules with water% in THF. The typical aggregation co-solvent systems resulted in optical rotation amplification and adjustment, also known as aggregation-induced polarization (AIP). The AIP work can serve as a new tool to determine molecular aggregation, especially for those that cannot display aggregation-induced emission (AIE). Therefore, AIP and AIE are anticipated to complement each other. In addition, AIP can also serve as a new tool providing enhanced right- or left-hand polarized lights with individual wavelengths. Since chiral phosphines are among the most important ligands, this work would lead to new chiral aggregate-based asymmetric synthesis and catalysts. This work demonstrated that many synthetic reactions, especially asymmetric reactions, may be about behaviors of aggregates instead of individual molecules. A new standard of reporting-specific rotation would be explored to avoid the inconsistency of reporting this data in the literature. Therefore, it will find many applications in chemical and materials sciences. Extensive AIP scope on other solvents and chiral compounds will be investigated in our lab in due course.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Robert A. Welch Foundation (D-1361-20210327, USA), and the National Natural Science Foundation of China (22071102 and 91956110).References
- K. Taniguchi, R. Maeda, T. Ando, T. Okumura, N. Nakazawa, R. Hatori, M. Nakamura, S. Hozumi, H. Fujiwara and K. Matsuno, Science, 2011, 333, 339–341 CrossRef CAS PubMed.
- A. H. Wang, S. Fujii, J. H. van Boom and A. Rich, Cold Spring Harbor Symp. Quant. Biol., 1983, 47, 33–44 CrossRef PubMed.
- S. Krautwald, D. Sarlah, M. A. Schafroth and E. M. Carreira, Science, 2013, 340, 1065–1068 CrossRef CAS PubMed.
- J. Zhang and L. Kürti, Natl. Sci. Rev., 2021, 8, nwaa205 CrossRef PubMed.
- K. P. Bryliakov, Research, 2020, 2020, 5689246 CrossRef CAS PubMed.
- I. Wagner and H. Musso, Angew. Chem., Int. Ed. Engl., 1983, 22, 816–828 CrossRef.
- J. D. Dunitz, Angew. Chem., Int. Ed. Engl., 2001, 40, 4167–4173 CrossRef CAS PubMed.
- V. J. Hruby, G. Li, C. Haskell-Luevano and M. Shenderovich, Pept. Sci., 1997, 43, 219–266 CrossRef CAS.
- V. K. Vemuri and A. Makriyannis, Clin. Pharmacol. Ther., 2015, 97, 553–558 CrossRef CAS PubMed.
- P. W. Schiller, Life Sci., 2010, 86, 598–603 CrossRef CAS PubMed.
- D. A. Smith and R. M. Jones, Curr. Opin. Drug Discovery Dev., 2008, 11, 72–79 CAS.
- T. Zhao, J. Han, P. Duan and M. Liu, Acc. Chem. Res., 2020, 53, 1279–1292 CrossRef CAS PubMed.
- G. Huang, R. Wen, Z. Wang, B. S. Li and B. Z. Tang, Mater. Chem. Front., 2018, 2, 1884–1892 RSC.
- H.-T. Feng, C. Liu, Q. Li, H. Zhang, J. W. Y. Lam and B. Z. Tang, ACS Mater. Lett., 2019, 1, 192–202 CrossRef CAS.
- O. Oki, C. Kulkarni, H. Yamagishi, S. C. J. Meskers, Z.-H. Lin, J.-S. Huang, E. W. Meijer and Y. Yamamoto, J. Am. Chem. Soc., 2021, 143, 8772–8779 CrossRef CAS PubMed.
- T.-T. Liu, Z.-P. Yan, J.-J. Hu, L. Yuan, X.-F. Luo, Z.-L. Tu and Y.-X. Zheng, ACS Appl. Mater. Interfaces, 2021, 13, 56413–56419 CrossRef CAS PubMed.
- J. Li, C. Hou, C. Huang, S. Xu, X. Peng, Q. Qi, W.-Y. Lai and W. Huang, Research, 2020, 2020, 3839160 CAS.
- T. W. Graham Solomons and C. B. Fryhle, Organic chemistry, eighth edition Solomons & fryhle Wiley student edition, 8th edition, Wiley, 2004 Search PubMed.
- P. J. Stephens, F. J. Devlin, J. R. Cheeseman, M. J. Frisch, O. Bortolini and P. Besse, Chirality, 2003, 15, S57–S64 CrossRef CAS PubMed.
- M. Hu, H.-T. Feng, Y.-X. Yuan, Y.-S. Zheng and B. Z. Tang, Coord. Chem. Rev., 2020, 416, 213329 CrossRef CAS.
- Y. Shen and C.-F. Chen, Chem. Rev., 2012, 112, 1463–1535 CrossRef CAS PubMed.
- Y. Sang, J. Han, T. Zhao, P. Duan and M. Liu, Adv. Mater., 2020, 32, e1900110 CrossRef PubMed.
- M. Hu, F.-Y. Ye, C. Du, W. Wang, W. Yu, M. Liu and Y.-S. Zheng, Angew. Chem., Int. Ed. Engl., 2022, 61, e202115216 CAS.
- J. Luo, Z. Xie, J. W. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- R. Hu, X. Chen, T. Zhou, H. Si, B. He, R. T. K. Kwok, A. Qin and B. Z. Tang, Sci. China: Chem., 2019, 62, 1198–1203 CrossRef CAS.
- X. Hu, X. Zhao, B. He, Z. Zhao, Z. Zheng, P. Zhang, X. Shi, R. T. K. Kwok, J. W. Y. Lam, A. Qin and B. Z. Tang, Research, 2018, 2018, 3152870 CrossRef PubMed.
- H. Rouh, Y. Tang, T. Xu, Q. Yuan, S. Zhang, J.-Y. Wang, S. Jin, Y. Wang, J. Pan, H. L. Wood, J. D. McDonald and G. Li, Research, 2022, 2022, 1–9 CrossRef PubMed.
- Y. Tang, S. Zhang, T. Xu, Q. Yuan, J.-Y. Wang, S. Jin, Y. Wang, J. Pan, I. Griffin, D. Chen and G. Li, Front. Chem., 2022, 10, 962638 CrossRef CAS PubMed.
- G. Wu, Y. Liu, Z. Yang, N. Katakam, H. Rouh, S. Ahmed, D. Unruh, K. Surowiec and G. Li, Research, 2019, 2019, 6717104 CAS.
- H. Zhang, Z. Yang, B. N. Zhao and G. Li, J. Org. Chem., 2018, 83, 644–655 CrossRef CAS PubMed.
- C. W. Seifert, A. Paniagua, G. A. White, L. Cai and G. Li, Eur. J. Org. Chem., 2016, 2016, 1714–1719 CrossRef CAS PubMed.
- G. An, C. Seifert and G. Li, Org. Biomol. Chem., 2015, 13, 1600–1617 RSC.
- C. Laye, J. Lusseau, F. Robert and Y. Landais, Adv. Synth. Catal., 2021, 363, 3035–3043 CrossRef CAS.
- S.-Z. Sun, M. Shang, H. Xu, T.-J. Cheng, M.-H. Li and H.-X. Dai, Chem. Commun., 2020, 56, 1444–1447 RSC.
- M. Hatano, T. Miyamoto and K. Ishihara, Adv. Synth. Catal., 2005, 347, 1561–1568 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra05597j |
| ‡ These authors contributed equally to this work. |
| § Undergraduate participant. |
| This journal is © The Royal Society of Chemistry 2022 |